Abstract
Endothelial cell (EC) dysfunction is a characteristic complication of coronavirus-19 (COVID-19). This review discusses the role of the endothelium during the pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with a focus on different vascular beds, possible routes of infectivity and the impact of EC dysfunction across multiple organ systems. It is now known that COVID-19 disease elicits a distinct transcriptomic and molecular profile that is different to other viral infections, such as Influenza A (H1N1). Interestingly, there is also a suggested interplay between the heart and lungs that promotes the amplification of inflammatory cascades, leading to an exacerbation in disease severity. Multiomic studies have informed common pathways that may be responsible for endothelial activation while also highlighting key differences in COVID-19 pathogenesis between organ systems. At a pathological level, endothelialitis is an endpoint result regardless of either a direct viral infection or via indirect effects independent of infection. Understanding if ECs are directly targeted by SARS-CoV-2 or are collaterally damaged amid a cytokine storm originating from other cells and organs can provide novel insights into disease progression and may highlight possible new therapeutic opportunities targeted at the damaged endothelium.
Current Opinion in Physiology 2023, 34:100670
This review comes from a themed issue on Endothelium
Edited by Jeremy Pearson and Paul C Evans
https://doi.org/10.1016/j.cophys.2023.100670
2468–8673/© 2023 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resultant coronavirus-19 (COVID-19) disease pandemic has caused widespread mortality and disrupted life at an unprecedented rate [1]. The pathophysiological consequences of COVID-19 disease are still being gradually uncovered through ongoing investigation; however, endothelial cell (EC) dysfunction has been observed to play a key role in disease progression and severity 2, 3••. The primary organ affected is the lung, with 42% of patients hospitalised with COVID-19 pneumonia progressing to acute respiratory distress syndrome (ARDS), and a further 61–81% requiring subsequent intensive care treatments [4]. Although COVID-19 is primarily a pulmonary disease, systemic consequences that indicate the contribution of multiple organ systems are also now well documented [5]. During COVID-19, endogenous inflammatory factors from the lung can be disseminated from the pulmonary circulation into the systemic circulation via the heart, leading to SARS-CoV-2 pathogenesis in other parts of the body [6]. While systemic manifestations are usually organ-specific, the dysregulated endothelium itself can contribute to COVID-19 disease exacerbation by promoting damage within the ubiquitous vasculature through an increase in nitric oxide (NO) bioavailability, glycocalyx/barrier disruption, hyperpermeability, hypercoagulability and thrombosis 7, 8.
Here, we discuss the pathophysiological understanding of multi-systemic COVID-19 disease within the EC compartment and how the ubiquity of the vascular endothelium uniquely proposes a pan-organ model to study disease outcomes across different vascular beds, as well as proposed targets for therapeutic intervention [9].
Cardiovascular complications in the lungs
The leading cause of mortality in COVID-19 patients is hypoxic respiratory failure as a result of ARDS and viral pneumonia [4]. However, further pulmonary complications such as thromboembolism, deep vein thrombosis and intussusceptive angiogenesis are also substantially higher in patients following disseminated intravascular coagulation, leading to widespread morbidity and mortality 10, 11.
At a transcriptional level, changes to the vascular endothelium can inform wider manifestations of COVID-19 disease through the identification of dysregulated genes and pathways. Pulmonary ECs of deceased COVID-19 patients showed an upregulation of genes involved in cellular stress and interferon cascades, and downregulation in immunomodulation and vessel wall integrity markers. This suggests that viral exposure may promote inflammation and cell permeability causing immune cell infiltration that exacerbates disease and can cause ARDS or viral pneumonia [12]. A study using immunostained lung samples from patients with diffuse alveolar damage (DAD) as a result of COVID-19 showed a significant increase in the number of pneumocytes, macrophages and CD3+ T cells compared with healthy controls and non-DAD-COVID patients [13]. An increased presence of macrophages and fewer CD8+ T cells were further associated with poor outcomes in all COVID-19 patients suggesting a role between the overactivated innate immune system and disease exacerbation [14]. This was similarly observed in a study mapping the spatial landscape of the SARS-CoV-2-infected lung, which showed a marked increase in the interactions between macrophages, fibroblasts and dendritic cells, suggesting preferential macrophage interactions with fibroblasts in the alveoli that may contribute to subsequent fibrosis and the thickening of the alveolar wall in late COVID-19 [15].
Moreover, fibrosis has also proven to be a key driver in the establishment of severe COVID-19 disease, as seen through shared similarities in patients with late-stage Idiopathic pulmonary fibrosis (IPF). Melms et al. identified fibroblast subtypes that were present in higher proportions in the lungs of the COVID-19 cohort compared with controls [16]. The hallmark genes that defined these cells were Collagen type I alpha 1 chain (COL1A1) and Collagen type III alpha 1 chain (COL3A1) that are known key drivers of lung fibrosis in mouse models and in patients with Idiopathic pulmonary fibrosis (IPF) and scleroderma [17]. This was further stratified by de Rooij et al., through the identification of 30 genes that were common between circulating ECs in COVID-19 and IPF patients, cumulatively these genes were representative of pathways promoting increased stress, altered immune signalling fibrosis and perturbed barrier integrity in both disease groups [18]. A further EC-interactome analysis by the group identified perturbed crosstalk between vascular and non-vascular compartments suggesting an infiltration of immune cells into pulmonary tissue types, as discussed earlier, which may be indicative of disease exacerbation as well as possible compensatory-induced repair mechanisms [19]. Findings from the group also suggest a role of fibroblasts, as well as ECs, in COVID-19- induced pulmonary fibrosis and explain the underlying similarities between patients with COVID-19 disease and IPF.
Coronavirus-19 and H1N1 have different transcriptional profiles and pathogenesis
While similar in terms of its fibrotic manifestation in IPF, SARS-CoV-2 infection is markedly different in terms of its pathological and transcriptional presentation compared with other severe respiratory infections such as H1N1. A comparative examination of patients with lethal COVID-19 disease and H1N1 showed only COVID-19 autopsies to present with severe endothelial injury and a marked presence of intracellular virus within ECs. The group also reported a higher incidence of thrombosis and macroangiopathy in the COVID-19 disease cohort — thrombi were 9 times more frequent in lungs of COVID-19 patients with intussusceptive angiogenesis being 2.7 times more likely, suggesting thrombogenesis to be a unique characteristic in SARS-CoV-2 compared with other viral infections [20].
At spatial resolution, Margaroli et al. compared pulmonary regions that showed advanced ARDS in H1N1 and SARS-CoV-2-infected lungs [21]. The SARS-CoV-2-infected lung showed increased expression of the extracellular matrix and coagulation pathways compared with upregulated immune signalling pathways in H1N1. Specifically, genes related to complement activation, platelet biology and endothelial injury were greatly upregulated in SARS-CoV-2 vascular beds demonstrating transcriptionally regulated responses to promoting hypercoagulopathy and inflammasome activation. Similarly, spatial transcriptomics analysis of SARS-CoV-2 compared with H1N1 identified a group of 7 genes that were specific to the SARS-CoV-2 transcriptome, 3 of which were responsible for the activation of type-1 interferon responses Lymphocyte antigen 6 complex, locus E, Interferon alpha subtype 127, Interferon alpha inducible protein 6 (LY6E, IF127 and IFI6) [22]. A common interferon upregulation has been further reported by several other groups, including aforementioned Margaroli et al., suggesting hyperinflammation and hypercoagulation to play a more deterministic role in COVID-19 disease outcomes compared with H1N1 23, 24.
Cardiovascular complications in the heart
COVID-19 is also well known to induce significant cardiac complications, even in patients with no underlying cardiovascular disease. The burden of cardiac comorbidities such as myocardial infarction has been shown to increase COVID-19 progression to ARDS at a rate of 57.7% compared with 11.9% in patients without cardiac complications [25]. A single-centre retrospective study analysed demographic data of patients hospitalised with COVID-19 disease to find 27.8% of patients had acute myocardial injury (MI), which subsequently resulted in cardiac dysfunction and arrhythmias [25]. MI also had a significant association with fatal outcomes in COVID-19, while the prognosis of patients without MI seemed more favourable. Instances of cardiac sequelae following COVID-19 infection have been shown to persist and lead to secondary outcome events, including heart failure [26], myocarditis [27] and dysrhythmias [28].
With respect to the cardiac system, in vitro systems using stem cell-derived cardiomyocytes (hiPSC-CM) and EC models have been used to describe the impact of SARS-CoV-2 directly on the cardiac vasculature. One study has shown when hiPSC-CMs were infected with SARS-CoV-2; a marked dysregulation was observed, such as an increase in levels of cardiac troponin. This suggests high cardiac stress and damage responses, disorganisation of myofibrils implying muscular weakness and deterioration and alterations in co-ordinated beating [29]. Similarly, another group tested the effects of SARS-CoV-2 on cardiac tissue using a cardiosphere model — a 3D co-culture system comprising of ECs, CM and fibroblasts — as well as endomyocardial biopsies of patients with COVID-19 30, 31. The results from the cardiosphere models showed spheroid shrinkage five days post viral induction, which was indicative of spheroid death and apoptosis. In the biopsied sections, virus particles were detected within CM and associated with visible cytotoxic effects, such as the loss of myofibrils showing muscle deterioration in fresh tissue as a result of severe COVID-19 disease [30].
The crosstalk between the heart and lungs may be essential in understanding coronavirus-19
COVID-19 can directly affect cardiac and pulmonary function by promoting damage to both organs. Liu et al. suggested that a decrease in barrier function paired with cytokine storms may lead to acute coronary syndrome, which may permeate into the systemic circulation causing intravascular blockage and thrombosis [32]. This, in turn, is thought to promote circulatory disorders and dead space ventilation, further exacerbating pulmonary hypoxia in a constant loop. While this knowledge is still being slowly decoded in the literature in the context of COVID-19 disease, Puri et al. have described several pathways that may inform the crosstalk between the heart–lung axis as seen in other severe infections. The first is due to a disruption of the electrolyte balance as a result of the angiotensin-converting enzyme-2 receptor (ACE2) complex binding competitively to SARS-CoV-2. This dysregulation can lead to high blood pressure and vasoconstriction promoting fluid imbalance within the heart and lungs to cause disease exacerbation. The group then identified hypoxia and immune dysregulation to play a pivotal role in understanding the interplay between the lungs and heart. Hypoxaemia caused by acute respiratory illness results in decreased oxygen in cardiac tissues directly impairing cardiofunction. When further paired with the high cardiometabolic demand of the body during severe illness and added stress due to immune cell infiltration, an imbalance of myocardial oxygen may be a key contributor to ischaemia and further systemic vascular dysfunction.
Moreover, the crosstalk between the heart and lung has been transcriptionally supported by compelling evidence from a multi-organ single-cell study by Delorey et al., that profiled the cardiac and pulmonary compartments during SARS-CoV-2 infection [33]. The group reported several pathways upregulated in the heart during COVID, such as oxidative stress-induced apoptosis, cell adhesion, immunomodulation and cell differentiation, which were similar to findings from the lung that showed higher expression levels for pathways responsible for apoptosis, adaptive immune responses and cell differentiation.
Cardiovascular complications in the systemic vasculature
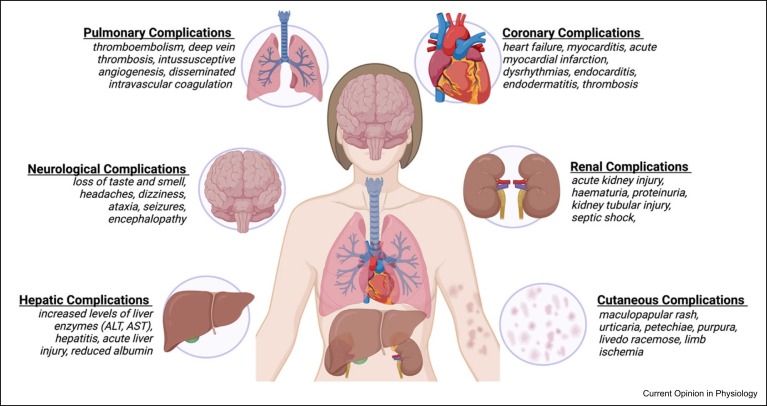
Several other organ systems are also susceptible to both acute and long-term pathological consequences of COVID-19 ( Figure 1). For example, in the brain, numerous neurological symptoms have been documented throughout the pandemic with the most common being a loss of taste and smell, headaches and dizziness in acute cases; with ataxia, seizures, stroke, encephalopathy in more severe cases and also in persistent long-COVID cases [34]. RNA sequencing of human brain microvascular endothelial cells (HBMEC) following SARS-CoV-2 infection revealed upregulation of multiple cytokines Tumor necrosis factor, Interleukin 1 beta, Interleukin 6, Interleukin 32 (e.g. TNF, IL1B, IL6 and IL32) and chemokines Chemokine (C-X-C) ligand 8, Chemokine (C-X-C) ligand 1, Chemokine (C-C) ligand 1 (e.g. CXCL8, CXCL1 and CCL1) that might associate with dysfunction in the blood–brain barrier by causing increased vascular permeability through the downregulation and remodelling of intercellular tight junctions to increase neuroinflammation [35]. This is supported by in vitro and in vivo characterisation of SARS-CoV-2-infected HBMECs, which showed an increase in the presence of string vessels — empty basement membrane tubes formed as a result of HBMEC death — due to cleavage by the SARS-CoV-2 proteases leading to disruption of the blood–brain barrier and allowing viral entry into the brain [31].
Figure 1.
Systemic sequelae as a result of COVID-19 infection described in the brain, lung, heart, liver, skin and kidney.
In the kidney, microvascular dysfunction can manifest as thrombotic microangiopathy resulting from COVID-19 disease [36]. Renal inflammation and immune cell infiltration can lead to the development of acute kidney injury (AKI) in patients with SARS-CoV-2 infection [37]. The results from a multicentre retrospective study showed that approximately 20.6% of critically ill COVID-19 patients admitted for intensive care developed AKI, with the risk of mortality increasing with the need for kidney replacement therapy and cardiovascular comorbidities [38].
Coronavirus-19 disease and the endothelium
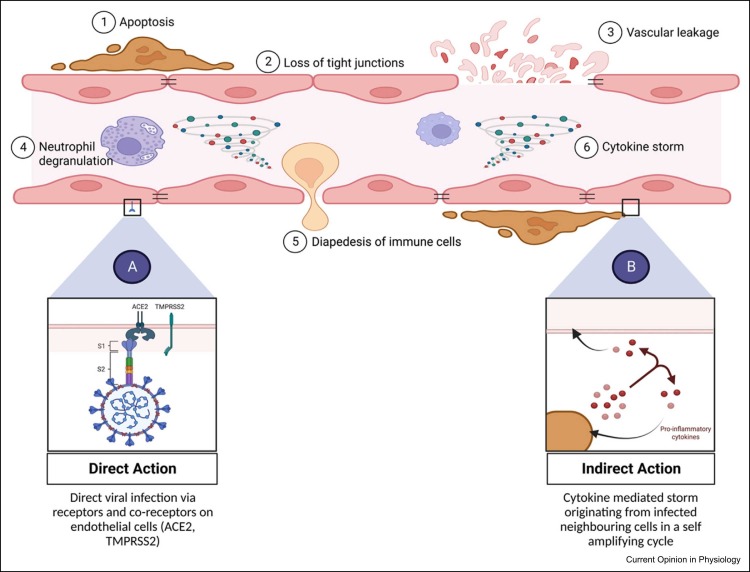
From the aforementioned discussion, it is clear that endothelial damage is a key hallmark of COVID-19 disease pathogenesis across multiple body systems. However, there is still contention over how the damage to the endothelium is mediated [39]. Dupont et al. assessed markers of endothelial damage (e.g. Von Willebrand factor, syndecan-1, complement etc.) and histologically profiled thrombi from patients hospitalised with severe COVID-19 disease. The results showed significantly higher levels of damage-associated markers and neutrophil extracellular traps in COVID thrombi compared with controls [40]. There remains disparity regarding whether SARS-CoV-2 infects the endothelium in a direct or indirect manner, or possibly both. While the eventual outcome from either route is EC dysfunction and the establishment of a pro-inflammatory and pro-coagulative state, through systemic toxicity such as cytokine storms leading to EC damage, a better understanding of primary entry points and the mechanism of viral action may better inform disease modelling and therapeutic opportunities 41, 42 ( Figure 2).
Figure 2.
Endothelial dysfunction following SARS-CoV-2 infection. (a) Direct action of viral particles infecting ECs. (b) Indirect action through cytokine-mediated storm originating from infected neighbouring cells.
The route of action in determining severe acute respiratory syndrome coronavirus 2 pathogenesis
Direct viral action refers to the active uptake of SARS-CoV-2 into ECs through binding with the ACE2 and co-receptors: transmembrane serine protease 2, cathepsin L1 or neuropilin 1 43, 44. ACE2 is the cell receptor responsible for SARS-CoV-2 entry, with binding affinity being 10–20-fold higher when compared with SARS-CoV-1 binding to ACE2 [45]. ACE2 is the most representative bioactive peptide in the renin–angiotensin system (RAS) that participates in the progression of cardiovascular diseases such as hypertension and MI [46]. Meanwhile, the RAS is a complex network that plays an important role in the maintenance of blood pressure and electrolyte homoeostasis across the body. Hence, the formation of an ACE2–SARS-CoV-2 complex may cause dysregulation across the entire RAS system resulting in a disruption of cardiovascular homoeostasis. On the other hand, an indirect mechanism of action suggests endotheliopathy is a result of cytokine-mediated storms that originate from virally infected cells, either locally or peripherally at areas of acute infection, that possess the receptors and co-receptors for active viral uptake [47].
It has been shown through transcriptional profiling that cells within the naso-oral and pulmonary mucosa — such as alveolar cells and bronchial epithelial cells — are directly infected by the virus through uptake by ACE2 48, 49. However, reports regarding direct infection of ECs tend to be inconsistent. RNA sequencing and epigenomic data analysed through Encyclopedia of DNA Elements (ENCODE) by McCracken et al. showed minimal or no expression of ACE2 in ECs across arterial, venous or microvascular beds, neither at the onset of infection nor activated later upon exposure to inflammatory cytokines in the plasma of patients with severe COVID-19 [50]. Such studies suggest that infected neighbouring cells are responsible for initiating a cytokine cascade following direct infection with SARS-CoV-2 causing damage to the endothelium downstream [47].
Conversely, the presence of live virus has been observed within the endothelium through histopathological examination of autopsied pulmonary tissues from COVID-19 patients 51, 52. This could potentially be due to the advanced disease state of these patients where increased cell permeability may have facilitated viral entry into ECs regardless of the cell type possessing receptor and co-receptor activity 47, 53.
Potential treatment opportunities
While there is still much contention regarding the mechanisms underpinning SARS-CoV-2 effects on the vascular endothelium, the major effect can be endothelial dysfunction and the promotion of pro-thrombotic, pro-coagulative and pro-inflammatory cascades within the vasculature [54]. Currently, the literature shows treatment efforts focused on supplying supplemental oxygen, a cocktail of antiviral drugs (ritonavir, remdesivir etc.) and low doses of heparin to patients in intensive care 55, 56. While this approach does directly target the virus and attain a level of symptomatic management, further combination therapies that can target inflammation, coagulopathy, thrombocytopathy and endotheliopathy together may be advantageous in tackling COVID-19-associated complications. Sriram et al. have proposed that proteinase-activated and purinergic receptors (PAPR) that are present on multiple vascular cell types, including ECs, are key drivers of inflammation and thrombosis in COVID-19 [57]. The group has tabulated various drugs approved by the Food and Drug Administration that target PAPR receptors directly at the endothelium, which can be repurposed to treat symptoms associated with COVID-19 disease. Two examples of this are vorapaxar and dabigatran that target the activation of platelets (during MI) and inhibit thrombin (responsible for thromboembolism), respectively. Both drugs are proposed to be active within the vasculature and can be used in tandem to reduce the contributions of inflammation and thrombogenesis in the pathophysiology of severe COVID-19 disease.
Additionally, the endothelium has a pronounced role in immune surveillance, which can be useful in the determination of COVID-19 disease severity classification. Ma et al. ectopically expressed ACE2 in human ECs in culture, and observed an increase in pro-inflammatory markers that predominantly promoted monocyte adhesion and NO production [58]. Single-cell RNA sequencing by the group has revealed the toll-like receptor-4 (TLR4) pathway to be crucial in the endothelial response to SARS-CoV-2. Treatment with TLR4 inhibitors, resatorvid (CLI095) and dexamethasone, aborted virus-induced monocyte adhesion and NO production in HUVECs and showed a pronounced role in blocking p38 mitogen-activated protein kinase, nuclear factor κB MAPK/NF-κB/ interleukin-1β in the endothelium.
Interestingly, TLR4 blockade via specific inhibitors, such as CLI095, could also minimise side effects from general immunosuppression as a result of dexamethasone treatment to great effect as seen by a reduction in 28-day mortality in hospitalised patients receiving respiratory support [59]. Furthermore, the use of in vitro models that simulate endothelial dysfunction has numerous possibilities to be modelled in the COVID-19 disease context, such as through the use of ectopic ACE2 expression, exposure to patient plasma or infection via pseudoviruses 60, 61. Other than the usual benefits of using an in vitro-based approach, added value can be found in the identification of prognostic biomarkers as well as transcriptional profiling of pathways responsible for COVID-19 disease pathogenesis. Furthermore, ECs may have a pronounced role in the regulation of immune cascades and thrombosis, especially during chronic inflammation, where ECs respond to angiogenic factors to form new blood vessels that are required to sustain inflammatory neo-tissue 62, 63. A deeper understanding of the mediators and signalling pathways that are involved in EC-mediated inflammatory functions may allow for cell-specific targeting at an early stage for the treatment of thrombosis and cytokine storms in severe COVID-19 disease.
In conclusion, the endothelium is important in improving our understanding of SARS-CoV-2 pathogenesis and defining its effects on the systemic vasculature and multi-organ injury. Here, we have summarised the role of the endothelium in SARS-CoV-2 pathogenesis, modes of infection and potential therapeutic targeting. Within the literature, there is still a divide between the routes of viral action through either direct infection or indirect toxicity. Identification of receptors that may be responsible for endocytic or phagocytic uptake of virus and virus particles may better drive therapeutic targeting and prevent EC dysfunction downstream, however, there is still uncompelling evidence of such receptors contributing to disease pathogenesis. Furthermore, an analysis of current multiomic studies has shown a distinct transcriptional signature in SARS-CoV-2 compared with other viral infections, such as H1N1, but similar to other severe pulmonary diseases, such as PAH. Moreover, we have outlined how the interplay between the lungs and heart contributes to the systemic dissemination of SARS-CoV-2 and how the pulmonary and systemic circulation may be responsible for disease exacerbation. It is possible that a shared spatial niche exists between the lungs and heart during COVID-19 disease as seen by the common upregulation of key transcriptional signatures in previous publications. Studying both organs at spatial resolution may provide an indication of the pathways and genes that contribute to the systemic pathogenesis and widespread dissemination of COVID-19. However, further investigation will be required to uncover the exact nature and extent of the crosstalk between heart and lung through both multiomic and functional characterisation.
Acknowledgements
R.P is supported by the University of Edinburgh-KU Leuven Global PhD Partnership (Grant G33781); M.B is supported by the British Heart Foundation Intermediate Research Fellowship (FS/16/4/31831); A.H.B is supported by the British Heart Foundation Chair of Translational Cardiovascular Sciences (CH/11/2/28733) and the European Union Horizon 2020 project COVIRNA (Grant Agreement 101016072).
Data Availability
No data were used for the research described in the article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloch S., Baloch M.A., Zheng T., Pei X. The coronavirus disease 2019 (COVID-19) pandemic. Tohoku J Exp Med. 2020;250:271–278. doi: 10.1620/tjem.250.271. [DOI] [PubMed] [Google Scholar]
- 3••.Huertas A., Montani D., Savale L., Pichon J., Tu L., Parent F., et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review describes the role of EC dysfunction and impairment of microcirculatory networks across the body and its contribution to the life-threatening complications of COVID-19.
- 4.Gibson P.G., Qin L., Puah S.H. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213:54–56. doi: 10.5694/mja2.50674. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A., Zaheer S., Kumar N., Singla T., Ranga S. Covid19, beyond just the lungs: a review of multisystemic involvement by Covid19. Pathol Res Pract. 2021;224 doi: 10.1016/j.prp.2021.153384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mejia-Renteria H., Travieso A., Sagir A., Martinez-Gomez E., Carrascosa-Granada A., Toya T., et al. In-vivo evidence of systemic endothelial vascular dysfunction in COVID-19. Int J Cardiol. 2021;345:153–155. doi: 10.1016/j.ijcard.2021.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancini D.M., Brunjes D.L., Lala A., Trivieri M.G., Contreras J.P., Natelson B.H. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9:927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S.W., Ilyas I., Weng J.P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44:695–709. doi: 10.1038/s41401-022-00998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruhashi T., Higashi Y. Pathophysiological association of endothelial dysfunction with fatal outcome in COVID-19. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez C., Luque N., Blanco I., Sebastian L., Barbera J.A., Peinado V.I., et al. Pulmonary endothelial dysfunction and thrombotic complications in patients with COVID-19. Am J Respir Cell Mol Biol. 2021;64:407–415. doi: 10.1165/rcmb.2020-0359PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao W., Birkenbach M., Chen S. Patterns of inflammatory cell infiltration and expression of STAT6 in the lungs of patients with COVID-19: an autopsy study. Appl Immunohistochem Mol Morphol. 2022;30:350–357. doi: 10.1097/PAI.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chioh F.W., Fong S.W., Young B.E., Wu K.X., Siau A., Krishnan S., et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife. 2021;10 doi: 10.7554/eLife.64909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rendeiro A.F., Ravichandran H., Bram Y., Chandar V., Kim J., Meydan C., et al. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021;593:564–569. doi: 10.1038/s41586-021-03475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melms J.C., Biermann J., Huang H., Wang Y., Nair A., Tagore S., et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021;595:114–119. doi: 10.1038/s41586-021-03569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukui T., Sun K.H., Wetter J.B., Wilson-Kanamori J.R., Hazelwood L.A., Henderson N.C., Adams T.S., Schupp J.C., Poli S.D., Rosas I.O., Kaminski N., Matthay M.A., Wolters P.J., Sheppard D. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 2020;11:1920. doi: 10.1038/s41467-020-15647-5. PMID: 32317643, PMCID: PMC7174390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.de Rooij L., Becker L.M., Teuwen L.A., Boeckx B., Jansen S., Feys S., et al. The pulmonary vasculature in lethal COVID-19 and idiopathic pulmonary fibrosis at single cell resolution. Cardiovasc Res. 2022;119:520–535. doi: 10.1093/cvr/cvac139. [DOI] [PMC free article] [PubMed] [Google Scholar]; This articleidentifies 30 common genes between patients with COVID-19 and IPF while providing compelling evidence of receptor-ligand crosstalk influencing the communication between ECs and non-vascular cell types.
- 19.Higashi Y., Sukhanov S., Shai S.Y., Danchuk S., Snarski P., Li Z., et al. Endothelial deficiency of insulin-like growth factor-1 receptor reduces endothelial barrier function and promotes atherosclerosis in Apoe-deficient mice. Am J Physiol Heart Circ Physiol. 2020;319:H730–H743. doi: 10.1152/ajpheart.00064.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margaroli C., Benson P., Sharma N.S., Madison M.C., Robison S.W., Arora N., et al. Spatial mapping of SARS-CoV-2 and H1N1 lung injury identifies differential transcriptional signatures. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Kulasinghe A., Tan C.W., Ribeiro Dos Santos Miggiolaro A.F., Monkman J., SadeghiRad H., Bhuva D.D., et al. Profiling of lung SARS-CoV-2 and influenza virus infection dissects virus-specific host responses and gene signatures. Eur Respir J. 2022;59 doi: 10.1183/13993003.01881-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study attempts to spatially resolve the COVID lung with the H1N1 lung, the paper highlights the similarities and differences between disease profiles and characterises differentially expressed genes common to both conditions.
- 23.Desai N., Neyaz A., Szabolcs A., Shih A.R., Chen J.H., Thapar V., et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. Nat Commun. 2020;11 doi: 10.1038/s41467-020-20139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler D., Mozsary C., Meydan C., et al. Shotgun transcriptome, spatial omics, and isothermal profiling of SARS-CoV-2 infection reveals unique host responses, viral diversification, and drug interactions. Nat Commun. 2021;12:1660. doi: 10.1038/s41467-021-21361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornowski R., Witberg G. Acute myocarditis caused by COVID-19 disease and following COVID-19 vaccination. Open Heart. 2022;9 doi: 10.1136/openhrt-2021-001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiq M.M., Chan A.T., Miorin L., Yadaw A.S., Beaumont K.G., Kehrer T., et al. Physiology of cardiomyocyte injury in COVID-19. medRxiv. 2020 doi: 10.1101/2020.11.10.20229294. [DOI] [Google Scholar]
- 30.Bojkova D., Wagner J.U.G., Shumliakivska M., Aslan G.S., Saleem U., Hansen A., et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020;116:2207–2215. doi: 10.1093/cvr/cvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenzel J., Lampe J., Muller-Fielitz H., Schuster R., Zille M., Muller K., et al. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021;24:1522–1533. doi: 10.1038/s41593-021-00926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L., Jing H., Wu X., Xiang M., Novakovic V.A., Wang S., et al. The cross-talk of lung and heart complications in COVID-19: endothelial cells dysfunction, thrombosis, and treatment. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.957006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Delorey T.M., Ziegler C.G.K., Heimberg G., Normand R., Yang Y., Segerstolpe A., et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595:107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This seminal multiomic study utilises spatial transcriptomics and single cell RNA sequencing to elucidate the role of multiple cell types across a varity of organs in patients with lethal COVID-19.
- 34.Romero-Sanchez C.M., Diaz-Maroto I., Fernandez-Diaz E., Sanchez-Larsen A., Layos-Romero A., Garcia-Garcia J., et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang R.C., Huang K., Zhang H.P., et al. SARS-CoV-2 productively infects human brain microvascular endothelial cells. J Neuroinflammation. 2022;19:149. doi: 10.1186/s12974-022-02514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Stang M.B., Desenclos J., Flamant M., Chousterman B.G., Tabibzadeh N. The good treatment, the bad virus, and the ugly inflammation: pathophysiology of kidney involvement during COVID-19. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.613019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legrand M., Bell S., Forni L., Joannidis M., Koyner J.L., Liu K., et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17:751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y.C., Fang J.T., Yang C.W. Endothelial dysfunction for acute kidney injury in coronavirus disease 2019: how concerned should we be? Nephron. 2021;145:513–517. doi: 10.1159/000515919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Jin Y., Ji W., Yang H. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Sig Transduct Target Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review eloquently summarises the basic cellular and molecular mechanisms of SARS-CoV-2 infection and its progression towards endothelial dysfunction. Furthermore, the authors highlight potential ediators of dysfunction and therapeutic implications.
- 40•.Dupont A., Rauch A., Staessens S., Moussa M., Rosa M., Corseaux D., et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:1760–1773. doi: 10.1161/ATVBAHA.120.315595. [DOI] [PubMed] [Google Scholar]; This article describes endothelial dysfunction through circulating factors isolated from the plasma of patients with severe COVID-19 and characerises dysfunction without the need for live virus while also highlighting the role of endotheliopathy in multiorgan failure.
- 41.Guervilly C., Burtey S., Sabatier F., Cauchois R., Lano G., Abdili E., et al. Circulating endothelial cells as a marker of endothelial injury in severe COVID -19. J Infect Dis. 2020;222:1789–1793. doi: 10.1093/infdis/jiaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung F., Kruger-Genge A., Franke R.P., Hufert F., Kupper J.H. COVID-19 and the endothelium. Clin Hemorheol Micro. 2020;75:7–11. doi: 10.3233/CH-209007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hossain M.G., Akter S., Uddin M.J. Emerging role of neuropilin-1 and angiotensin-converting enzyme-2 in renal carcinoma-associated COVID-19 pathogenesis. Infect Dis Rep. 2021;13:902–909. doi: 10.3390/idr13040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao M.M., Yang W.L., Yang F.Y., Zhang L., Huang W.J., Hou W., Fan C.F., Jin R.H., Feng Y.M., Wang Y.C., Yang J.K. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct Target Ther. 2021;6(1):134. doi: 10.1038/s41392-021-00558-8. PMID: 33774649, PMCID: PMC7997800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 46.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., Hou C., Wang H., Liu J., Yang D., Xu Y., Cao Z., Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. PMID: 32660650, PMCID: PMC7356137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicosia R.F., Ligresti G., Caporarello N., Akilesh S., Ribatti D. COVID-19 vasculopathy: mounting evidence for an indirect mechanism of endothelial injury. Am J Pathol. 2021;191:1374–1384. doi: 10.1016/j.ajpath.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12 doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.McCracken I.R., Saginc G., He L., Huseynov A., Daniels A., Fletcher S., et al. Lack of evidence of angiotensin-converting enzyme 2 expression and replicative infection by SARS-CoV-2 in human endothelial cells. Circulation. 2021;143:865–868. doi: 10.1161/CIRCULATIONAHA.120.052824. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper uses human transcriptomic data derived from RNA sequencing and in vitro assays to show the lack of ACE2 expression in ECs, suggesting the ‘indirect’ route damage to the endothelium.
- 51.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar A., Narayan R.K., Kumari C., Faiq M.A., Kulandhasamy M., Kant K., et al. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dandel M. Heart-lung interactions in COVID-19: prognostic impact and usefulness of bedside echocardiography for monitoring of the right ventricle involvement. Heart Fail Rev. 2022;27:1325–1339. doi: 10.1007/s10741-021-10108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norooznezhad A.H., Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19) Micro Res. 2021;137 doi: 10.1016/j.mvr.2021.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gul M.H., Htun Z.M., Shaukat N., Imran M., Khan A. Potential specific therapies in COVID-19. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620926853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sriram K., Insel P.A. Inflammation and thrombosis in COVID-19 pathophysiology: proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets. Physiol Rev. 2021;101:545–567. doi: 10.1152/physrev.00035.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Z., Li X., Fan R.L.Y., Yang K.Y., Ng C.S.H., Lau R.W.H., et al. A human pluripotent stem cell-based model of SARS-CoV-2 infection reveals an ACE2-independent inflammatory activation of vascular endothelial cells through TLR4. Stem Cell Rep. 2022;17:538–555. doi: 10.1016/j.stemcr.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmetaj-Shala B., Peacock T.P., Baillon L., Swann O.C., Gashaw H., Barclay W.S., Mitchell J.A. Resistance of endothelial cells to SARS-CoV-2 infection in vitro Blerina Ahmetaj-Shala. bioRxiv. 2020 doi: 10.1101/2020.11.08.372581. [DOI] [Google Scholar]
- 61.Beltran-Camacho L., Eslava-Alcon S., Rojas-Torres M., Sanchez-Morillo D., Martinez-Nicolas M.P., Martin-Bermejo V., et al. The serum of COVID-19 asymptomatic patients up-regulates proteins related to endothelial dysfunction and viral response in circulating angiogenic cells ex-vivo. Mol Med. 2022;28 doi: 10.1186/s10020-022-00465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pober J., Sessa W. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 63.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D'Angelo A., De Cobelli F., Rovere-Querini P., Tresoldi M., Dagna L., Zangrillo A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.