Abstract
Background:
High-risk gestational trophoblastic neoplasia (GTN) is rare and treated with diverse approaches. Limited published institutional data has yet to be systematically reviewed.
Objectives:
To compile global high-risk GTN (prognostic score ≥7) cohorts to summarize treatments and outcomes by disease characteristics and primary chemotherapy.
Search Strategy:
MEDLINE, Embase, Scopus, ClinicalTrials.gov, and Cochrane were searched through March 2021.
Selection Criteria:
Full-text manuscripts reporting mortality among ≥10 high-risk GTN patients.
Data Collection and Analysis:
Binomial proportions were summed, and random-effects meta-analyses performed.
Main Results:
From 1,137 records, we included 35 studies, representing 20 countries. Among 2,276 unique high-risk GTN patients, 99.7% received chemotherapy, 35.8% surgery, and 4.9% radiation. Mortality was 10.9% (243/2,236; meta-analysis: 10%, 95%CI 7-12%), and likelihood of complete response to primary chemotherapy was 79.7% (1,506/1,890; meta-analysis: 78%, 95%CI 74-83%). Across 24 reporting studies, modern preferred chemotherapy (EMA/CO or EMA/EP) was associated with lower mortality (overall: 8.8% vs. 9.5%; comparative meta-analysis: 8.1% vs. 12.4%, OR=0.42, 95%CI 0.20-0.90, 14 studies) and higher likelihood of complete response (overall: 76.6% vs. 72.8%; comparative meta-analysis: 75.9% vs. 60.7%, OR=2.98, 95%CI 1.06-8.35, 14 studies), though studies focused on non-preferred regimens reported comparable outcomes. Mortality was increased for ultra-high-risk disease (30% vs. 7.5% high-risk; meta-analysis OR=7.44; 95%CI 4.29-12.9) and disease following term delivery (20.8% versus 7.3% following molar pregnancy; meta-analysis OR=2.64; 95%CI 1.10-6.31). Relapse rate estimates ranged 3-6%.
Conclusions:
High-risk GTN is responsive to several chemotherapy regimens, with EMA/CO or EMA/EP associated with improved outcomes. Mortality is increased in patients with ultra-high-risk, relapsed, and post-term pregnancy disease.
Funding:
This study received no direct funding.
Keywords: gestational trophoblastic disease, gestational trophoblastic disease, choriocarcinoma, systematic reviews, meta-analysis
Introduction
Gestational trophoblastic neoplasia (GTN) is defined by malignant proliferations of placental trophoblastic cells.1 GTN is diagnosed most commonly following molar pregnancy, by abnormal trends in human chorionic gonadotropin (hCG) levels during routine screening following uterine evacuation.2 Less commonly, gestational choriocarcinomas sometimes present months to years following spontaneous abortions or normal pregnancy.1,3 The exquisite chemo-sensitivity of these diseases made them foundational in the development of the first chemotherapeutics for solid malignancies in the 1950s.4 Methotrexate remains a mainstay of GTN treatment today, leading to cures even in some cases of widespread metastatic disease.3,5
However, single-agent therapy does not offer a universal cure for GTN. Research from leading referral centers identified high-risk features associated with chemoresistance and mortality,6–8 which were compiled into scoring systems for standardized risk assessment. The first was published by the World Health Organization (WHO) in 1983,9 and subsequently other scores were developed.10 General consensus was achieved with the publication of guidelines by the International Federation of Gynecology and Obstetrics (FIGO) in 2000.11 These guidelines instituted a new staging and scoring system (the modified WHO Prognostic Scoring System as adopted by FIGO), recently re-affirmed in 2021.12
High-risk disease (prognostic score ≥7) is typically treated with multi-agent chemotherapy, with some growing consensus on the preferred first-line regimen (EMA/CO or EMA/EP; etoposide, methotrexate, and actinomycin alternating with either cyclophosphamide and vincristine, or etoposide and cisplatin).5,13–15 However, due to the rarity of high-risk GTN, variation in treatment approach remains across different centers around the globe. Randomized comparative studies are lacking,16 and published institutional cohorts to date have yet to be systematically reviewed.
The objectives of our study were to compile global reports of high-risk GTN to (1) summarize treatments and outcomes since publication of the FIGO 2000 prognostic scoring guidelines, and (2) compare outcomes by disease characteristics and primary chemotherapy, particularly to assess for benefit of modern preferred chemotherapy with EMA/CO or EMA/EP.
Methods
Study Selection
We followed standard methodology for systematic review and meta-analysis, according to Cochrane, MOOSE (meta-analysis of Observational Studies in Epidemiology) and PRISMA (Preferred Reporting In Systematic review and Meta-Analysis) guidelines.17–19 A biomedical librarian (SJK) led the development of the search strategy in coordination with the multiple content experts on the authorship team. The MEDLINE (via PubMed), Embase, Scopus, ClinicalTrials.gov, and Cochrane library databases were queried from inception to March 4th, 2021. We used a combination of Medical Subject Heading (MeSH) terms and keywords to populate sets related to the themes of ‘gestational trophoblastic neoplasia’ and ‘high-risk,’ then the Boolean term “AND” was applied to identify the intersection in search results. Limits or restrictions on dates and language were not applied during the search process. Due to the breadth of the initial search and high number of eligible papers identified, additional hand searching of reference lists was not performed. The general search strategy and search details and outputs for each database are detailed in Appendix S1. The study protocol was prospectively submitted for registration on the PROSPERO international registry (CRD42021254677).
For inclusion, we required studies to meet the following eligibility criteria: (1) the study was published as a English language full-length manuscript, (2) the study was a retrospective or prospective trial, cohort, or case series (study type), (3) the study included cases of high-risk GTN as determined by the modified WHO Prognostic Scoring System as adopted by FIGO published in 2000 (referred to henceforth as ‘prognostic score’) and not limited to a particular GTN histology, surgical treatment, or specific site of metastatic disease (study population), (4) the study reported on overall mortality among the high-risk GTN cases (study outcome), and (5) the study reported data on ≥10 patients with high-risk GTN, excluding placental site trophoblastic and epithelioid trophoblastic tumors. In instances of multiple studies from the same institution with suspected overlap in represented patients, we selected the study with the most complete data reporting for inclusion.
Study selection was performed within Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Search results were first purged of duplicates. Studies titles and abstracts were then screened in duplicate by two independent reviewers (one of BBA or BAD, and one of HEK, TE, ECG, or KAM). Disagreements were resolved via consultation with a third independent reviewer (HAM, RAP, or LJH). Every search result was screened by a gynecologic oncology attending or fellow. Full-text manuscripts were accessed, then reviewed by two independent reviewers (BBA and BAD), with disagreements resolved by consensus discussion with two additional reviewers (RAP, LJH). Given the geographic diversity of the GTN literature and the age of some of the data for included cohorts, we did not contact study authors to obtain unpublished data.
Data extraction and quality assessment
Data were then extracted onto a standardized form by the primary reviewer (BBA) using Microsoft Excel v14.7.7 (Microsoft Corporation, Redmond, WA, USA), with any uncertainties resolved by consensus discussion (HAM, RAP, LJH, BAD). We designed the form based on goals of the study, seeking to maximize what could be summarized and what comparisons could be made, recognizing that every study would not report on every data point.
Data extraction included descriptive data on study characteristics, including the country, city, and facility of origin, years of data, and any cohort restrictions. We next collected descriptive data on patient characteristics including antecedent pregnancy (molar pregnancy, term delivery, abortion/ectopic/unknown), prognostic score (high-risk score 7-11 or 7-12 vs. ultra-high-risk score ≥12 or ≥13), FIGO stage, and metastatic sites (lung, vagina, brain, liver, or other), extracting data specific to high-risk GTN cases whenever reported. We then extracted treatment characteristics for high-risk GTN cases including prior failed single-agent treatment of low-risk disease with subsequent high-risk re-scoring, primary chemotherapy for high-risk disease, and utilization of surgery (excluding dilation and curettage) and radiation therapy.
Lastly, we extracted data on patient outcomes specific to cases of high-risk GTN. Our primary outcome was overall mortality (deaths / cases high-risk GTN), which was required to be reported for study inclusion. We recorded additional outcome data specific to high-risk cases when reported, including overall complete response rate to primary chemotherapy (complete responses / cases high-risk GTN) and relapse risk (relapses after complete response / total complete responses). Mortality and complete responses specific to primary chemotherapy regimen were also extracted when reported. We further described deaths by multiple characteristics when reported, including early (≤1 month from presentation), ultra-high-risk disease, stage IV disease, antecedent pregnancy, and following relapse.
As our outcomes of mortality and complete response (study definition, typically hCG normalization sustained for ≥3 weeks) were objective, assessment would only be biased by limitations to patient follow up. Due to the observational nature of the included studies and the objectiveness of outcomes, existing quality scoring mechanisms were not considered useful and, therefore, not applied. Instead, we summarized the extent a of follow-up, and the completeness of data reporting on key elements by each study.
Data Synthesis
We first summarized treatments and outcomes for high-risk GTN by summing binomial proportions across reporting studies. We then performed meta-analyses overall across all studies and in subgroups by study characteristics, utilizing random effects modeling to minimize the risk of type 1 error and account for the expected heterogeneity in the underlying studies due to significant variation in underlying populations and utilized treatments from studies compiled across numerous countries from around the world.19 Heterogeneity between studies was assessed with Higgins I2. Publication bias was assessed graphically using funnel plots.
We first ran meta-analyses of binomial proportions of utilized treatments and disease outcome. We primarily utilized the score (Wilson) confidence intervals,20 which were recommended by a statistical comparison of methods of confidence interval estimation for binomial proportions.21 We utilized the Freeman-Tukey transformation to allow inclusion of studies with no recorded deaths.22,23 We also trialed exact (Clopper-Pearson) confidence intervals as a sensitivity analyses,24 but found no meaningful difference in estimated confidence intervals (results not presented).
We then performed comparative random-effects meta-analyses of mortality risk and complete response rate by treatments and disease characteristics. We assessed both outcomes comparing modern preferred chemotherapy (EMA/CO or EMA/EP)5,13–15 to other treatments. We also compared mortality risk by disease characteristics of ultra-high-risk disease (versus high-risk), stage IV disease (versus stages I-III), and antecedent term pregnancy (versus molar pregnancy).
We assessed robustness of results with sensitivity analyses limiting to studies with high quality of data reporting. We first performed analyses limited to studies with <10% of patients lost to follow up, then limiting to studies reporting median or mean length of follow up of at least 18 months, then a combination of the two criteria.
Data synthesis, analysis, and figure generation was conducted with STATA v17.0 (Statacorp, College Station, TX, USA), using the imported ‘metaprop’ package for summaries of binomial proportions,22 and the internal ‘meta esize’ package for two-group comparisons of binary outcomes.
Results
After combining search results from all databases, 2,153 total records were identified, with about half excluded as duplicates (n=1,016). Among the 1,137 remaining results, 936 were deemed ineligible by title and abstract review, and an additional 140 were found ineligible upon full text review. A total of 60 studies met all inclusion criteria, 25 of which were excluded for suspected overlapping patient data, reported from the same or overlapping institutions in overlapping time periods (Table S1). Ultimately, 35 studies were included.25–59 See Figure S1 for PRISMA flow diagram of study selection.
The 35 included studies (Tables S2–S3) reported on 2,276 unique patients with high-risk GTN, representing 20 countries spanning 5 continents. India (7 studies) and China (5 studies) were the most frequently represented countries by study count. Most studies included ≥10 years of compiled data from one or several centers, though only four reported on any patients prior to 1990, and just two included no patients from the last 15 years. Some studies included patients treated prior to publication of the modern prognostic scoring system in 2000, but all patients were re-scored by these guidelines.
Among the 35 included studies, 17 included patients with low-risk disease, with data specific to the high-risk cohort extracted for meta-analysis. Among the 18 reporting only on high-risk patients, four were limited to cases with metastatic disease (excluding high-risk stage I disease), and one was limited to ultra-high-risk disease. A total of 10 studies reported on patients receiving a specific chemotherapy regimen (3 EMA/CO, 2 EMA/EP, and 5 other), either in the primary or salvage setting.
The characteristics of included patients are summarized in Table S3, Table S4, Table S5, and Figure S2. The lung and pelvis/vagina were the most common sites of metastatic disease spread at presentation reported across included studies. Nearly all patients received chemotherapy (99.7%), with 19 studies reporting ≥80% patients received one of the modern preferred regimens, EMA/CO or EMA/EP, while 5 studies included no patients treated on one of these regimens. Utilization of surgery in treatment of high-risk GTN varied greatly. While 8 studies reported surgery in ≥50% of patients, and another 8 studies reported surgical utilization <10%, with overall utilization in 35.8% of patients. Radiation was less commonly utilized, with only 8 studies reporting use in >10% of patients, and an overall utilization rate of 4.9%.
The quality and completeness of data reporting by included studies is summarized in Table S6. Only 10 studies reported any patients lost to follow-up, with just 4 reporting ≥10% of patients lost, and 40 of 2,276 patients lost to follow-up in total (1.8%). While 12 studies failed to report on follow up time, just 3 of 23 reporting studies reported mean/median follow up <18 months. Primary chemotherapy and associated complete response (CR) rates were reported in 33 and 31 of 35 studies, respectively, with a standard CR definition of hCG normalization for ≥3 weeks specified in 28 studies.
Mortality results are summarized in Table 1. Overall, 243 of 2,236 high-risk GTN patients died, for mortality of 10.9%. The meta-analysis estimate of mortality risk was 10% (95%CI 7-12%; I2=68%; Figure 1). Among 24 studies specifying timing of deaths, 37 of 102 deaths (36.3%) occurred ≤1 month from presentation. Our secondary outcome of CR to primary chemotherapy was observed in 1,506 of 1,890 patients (79.7%) across 31 reporting studies, with meta-analysis estimate of 78% (95%CI 74-83%; I2=80%; Figure 2).
Table 1.
Summary of compiled outcomes across included studies for high-risk gestational trophoblastic neoplasia.
| Reporting Studies | Total N | N with outcome | Percent with outcome | Meta-analysis* Estimate (95%CI) | |
|---|---|---|---|---|---|
| Complete Response | |||||
| Overall | 31 | 1890 | 1506 | 79.7% | 78% (73-83%) |
| Preferred chemotherapy | 24 | 806 | 617 | 76.6% | 78% (72-84%) |
| Non-preferred treatment | 20 | 599 | 436 | 72.8% | 60% (43-76%) |
| Relapse after complete response | 27 | 901 | 37 | 4.1% | 3% (1-5%) |
| Mortality | |||||
| Overall | 35 | 2236 | 243 | 10.9% | 10% (7-12%) |
| Preferred chemotherapy | 24 | 897 | 79 | 8.8% | 7% (4-10%) |
| EMA/CO | 22 | 834 | 74 | 8.9% | 7% (4-11%) |
| EMA/EP | 5 | 63 | 5 | 7.9% | 2% (0-13%) |
| Non-preferred treatment | 20 | 558 | 53 | 9.5% | 8% (2-17%) |
| Early death (<1 month) | 24 | 37 | 1253 | 3.0% | 1% (0-3%) |
| High-risk (excluding ultra-high-risk) | 9 | 810 | 61 | 7.5% | 3% (0-7%) |
| Ultra-high-risk | 9 | 210 | 63 | 30.0% | 25% (15-36%) |
| Stage IV | 7 | 51 | 16 | 31.3% | 28% (14-43%) |
| After molar pregnancy | 5 | 137 | 10 | 7.3% | 4% (1-9%) |
| After term delivery | 5 | 77 | 16 | 20.8% | 14% (3-28%) |
| Prior single-agent chemotherapy for low-risk disease | 10 | 96 | 9 | 9.4% | 7% (1-14%) |
| Death after relapse | 14 | 37 | 10 | 27.0% | 18% (3-37%) |
Preferred chemotherapy = EMA/CO (etoposide, methotrexate, and actinomycin D, alternating with cyclophosphamide, and vincristine) or EMA/EP (etoposide, methotrexate, and actinomycin D, alternating with etoposide and cisplatin); score refers to modified WHO Prognostic Scoring System as adopted by FIGO, first published in 2000 FIGO guidelines
Random-effects analysis using ‘metaprop’ package in Stata v17.0
Figure 1.
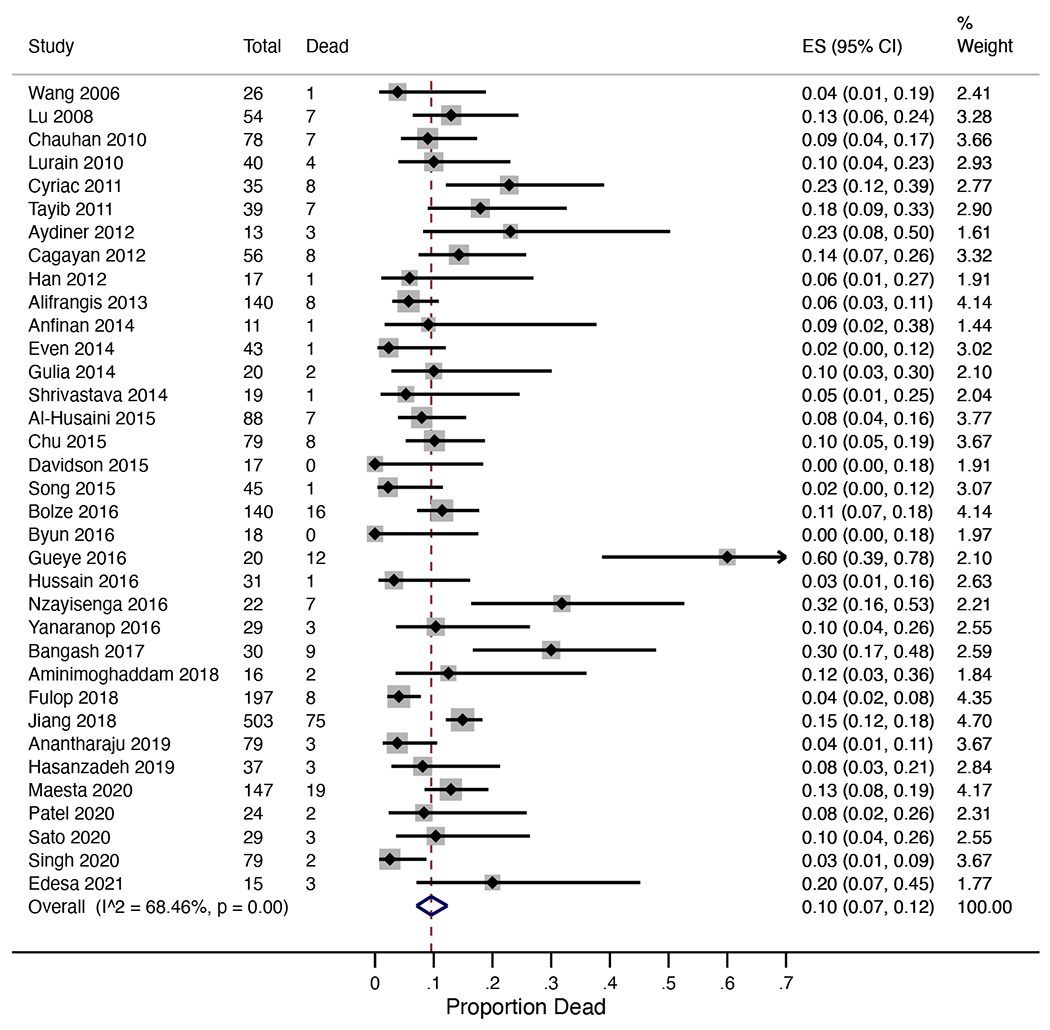
Forest plot for meta-analysis of overall mortality from high-risk gestational trophoblastic neoplasia.
Figure 2.

Forest plot for meta-analysis of complete response to primary multi-agent chemotherapy from high-risk gestational trophoblastic neoplasia.
Use of modern preferred EMA/CO or EMA/EP chemotherapy was reported in 897 patients across 24 studies (93.0% EMA/CO) with 79 deaths (8.8%; meta-analysis estimate 7%, 95%CI 4-10%, I2=47%). Mortality among those receiving non-preferred treatments was 9.5% (53/558 patients; meta-analysis estimate 8%, 95%CI 2-17%, I2=77%). Overall CR rate was 76.6% with EMA/CO or EMA/EP (617/806 patients) versus 72.8% for patients receiving other treatments (436/599).
Across 14 studies reporting ≥1 patient with both preferred and non-preferred treatments, comparative meta-analysis showed EMA/CO or EMA/EP chemotherapy was associated with significantly lower mortality (8.1% vs 12.4%; OR=0.42; 95%CI 0.20-0.90; p=0.03; I2=25%; Figure 3A), and significantly higher likelihood of CR (75.9% vs 60.7%; OR=2.98; 95%CI 1.06-8.35; p=0.04; I2=65%; Figure 3B). When limiting to studies with >1 patient in each arm (Figure S3), preferred regimens had statistically lower mortality but improvement in CR rate did not retain statistical significance. Among 9 studies reporting primarily on non-preferred chemotherapy, outcomes were comparable (85% and 5% respectively, Table S7).
Figure 3.
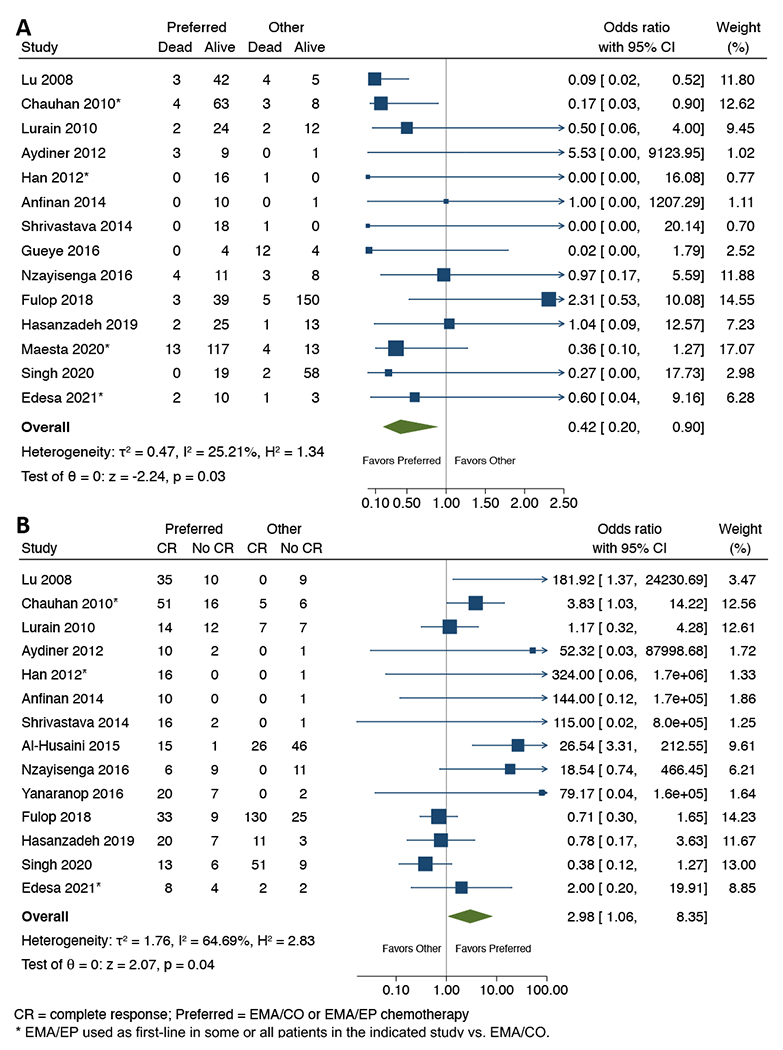
Forest plot for comparative meta-analyses of (A) overall mortality, and (B) complete response (CR) rate with preferred (EMA/CO or EMA/EP) vs. other chemotherapy in high-risk gestational trophoblastic neoplasia, among the 14 included studies reporting on at least one patient receiving ‘preferred’ EMA/CO or EMA/EP and one patient receiving an alternative ‘other’ regimen. *EMA/EP used as first-line in some or all patients in the indicated study vs. EMA/CO; see Table S3 for details of chemotherapy utilization.
We found considerably higher mortality among patients with ultra-high-risk disease, with 63 of 210 patients reported dead (30%) across 9 reporting studies (meta-analysis estimate 25%; 95%CI 15-36%; I2=47%; Figure S4). Estimated mortality was higher in four studies using a threshold of prognostic score ≥13 (35%; 95%CI 15-45%). In comparative meta-analysis, we estimated a statistically significant difference (32.7% vs 7.5% for ultra-high-risk vs high-risk; OR=7.44; 95%CI 4.29-12.89; p<0.01; I2=14%). Risk of death for stage IV disease was similarly high, with 16 of 51 patients reported dead (31.3%) across 7 studies specifying mortality by stage. We also found high mortality for disease following term delivery, with 16 of 77 patients dying (20.8%) across 5 reporting studies, versus 10 of 137 patients (7.3%) following molar pregnancy (OR=2.64; 95%CI 1.10-6.31; p=0.03; I2=0%). Across 10 studies specifying prior single-agent chemotherapy for initially low-risk disease, we found similar mortality (9.4%, 9/96 patients) to the overall risk.
Across 27 reporting studies, we identified 37 cases of relapse among 901 complete responders, for 4.1% overall risk. Meta-analysis of this outcome estimated 3% risk of relapse (95%CI 1-6%; I2=74%; Figure S5). Among the 14 studies reporting deaths specific to relapse, we estimated 27% risk of death after relapse (10 of 37 patients) and relapse deaths accounted for 10 of 94 total deaths (11%).
Sensitivity analyses were performed limiting to highest quality studies: those with <10% loss to follow-up (31 studies), ≥18 months mean/median length of follow-up (19 studies), and a combination of both criteria (18 studies). Meta-analysis estimates of overall mortality (10%, 8%, and 9%, respectively) and CR rate were (79%, 82%, and 81%, respectively) did not differ significantly from our original results. For comparative meta-analyses of highest quality studies, estimated mortality was significantly lower with EMA/CO or EMA/EP chemotherapy (OR=0.22; 95%CI 0.07-0.68), and no significant difference was found in CR rate, though only 3 studies were included in these analyses. Estimated relapse rate of 3% did not change when limiting to studies with standard definition of CR, though increased to 6% when further limiting to highest quality studies on follow-up.
Our assessment for publication bias with funnel plots can be seen in Figure S6. While we saw no clear evidence of publication bias in our primary outcome of overall mortality, asymmetry in the funnel plot for complete response rate indicates that smaller comparative series favoring chemotherapy regimens besides EMA/CO or EMA/EP may be under-represented in the published literature for that outcome.
Discussion
Main Findings
This study presents a global perspective on treatment and outcomes of high-risk GTN according to the most recent definition of high-risk disease.11 Systematic review and meta-analysis are important tools for the study of rare diseases and rare outcomes.60 The only prior systematic review of high-risk GTN, published in 2013,16 sought randomized trials, formally including only one 1989 study.61 The authors did summarize some of the existing retrospective data, but without any formal meta-analysis, and including only 6 studies that overlap with data presented here.
We found an overall mortality risk from high-risk GTN of 10%. Mortality is strongly related to the extent and risk of disease at presentation, with considerably higher mortality rates (around 30%) for patients with ultra-high-risk or stage IV disease. Additionally, antecedent term pregnancy, which is included in the prognostic scoring system, was specifically associated with increased mortality risk. We found complete response rate of 80% to primary treatment, and low relapse risk of <5% after complete response. Although high-risk GTN typically presents with widespread metastases, it remains highly treatable and curable in most cases. Importantly, given the lack of prospective comparative trials, we found that primary treatment with modern preferred EMA/CO or EMA/EP chemotherapy was associated with statistically significant improvements in both mortality and complete response rate in comparative analysis.
Strengths and Limitations
The strengths of this study begin with our broad search, with screening of over 1,100 unique results, and inclusion of 35 non-overlapping studies, representing 20 countries across 5 continents. We used a rigorous systematic review methodology, including selection of studies independently in duplicate.19 Our study had an objective primary outcome, and generally complete data reporting for adequate assessment of our main questions, and our primary outcome lacked evidence for any publication bias in assessment via funnel plot.
The weakness of our study is primarily related to the rarity of high-risk GTN. All identified studies were retrospective and observational, with variation in the length and completeness of follow up, and selection bias in chemotherapy treatment may have influenced observed differences in outcomes. Our comparative analysis by primary chemotherapy was limited by the fact that most centers adopt standard practices, and therefore, many studies only reported on a single treatment regimen. Those including patients treated with different regimens may not represent contemporaneous practices, but rather different eras of evolving standard practice or centralized referral after different or failed treatment locally. Reporting of outcomes by subgroups varied from study to study, limiting our ability to adjust for confounding and to include all identified papers for most analyses. Many included studies come from regional referral centers, with potential for higher concentrations of cases with advanced or higher risk disease, possibly upwardly biasing our estimates for mortality.
While we chose to exclude studies lacking English language manuscripts, this led to exclusion of only 7 total studies, with a total of 307 patients. All included English language abstracts, with full-text manuscripts representing 5 different languages (3 Chinese, 1 each of Czech, French, Polish, and Spanish). None of these studies reported on mortality specific to high-risk GTN in the abstract and it is unclear if any of the studies would have otherwise qualified for inclusion. Additionally, exclusion of non-English works has not been shown to represent a significant bias in reported results.62
Interpretation
While our results do not radically change the overall understanding of high-risk GTN, they do provide a formal and novel summary of the global experience with high-risk GTN in the modern era since the FIGO 2000 guidelines, which validates our current understanding and treatment paradigms for this uncommon disease.
EMA/CO was first described by the Charing Cross group in 1986.63 Over time, this regimen has become the most common and preferred first-line chemotherapy.5,64 It is now explicitly described in the most recent Royal College of Obstetrics and Gynecology, Society of Gynecologic Oncology, and FIGO guidelines,12,14,15 and formally recommended by the National Comprehensive Cancer Network,13 though no randomized comparative trials evaluating its efficacy against alternatives have been published.65 EMA/EP was developed initially as a second line regimen and is most often reserved for first-line in ultra-high-risk GTN due to greater relative toxicity.66,67 Our results offer supportive evidence for EMA/CO as first-line therapy for high-risk disease, in absence of prospective randomized data.
However, EMA/CO and EMA/EP are costly and toxic regimens,68,69 requiring one to two nights of hospitalization per cycle,70 which contributes to both financial and psychosocial costs for patients.71 High-risk GTN is responsive to a wide range of multi-agent chemotherapies. Our review identified 9 studies focused on 6 other regimens with generally comparable outcomes.25,28,31,32,34,36,45,51,53 The efficacy of multiple different regimens is also an important takeaway from this global data, as EMA/CO or EMA/EP may not be accessible or practically feasible to offer to patients in all cases around the world, given differences in available resources. Future research on the tradeoff between clinical benefits and costs of various chemotherapy regimens is warranted.
Conclusion
We found that high-risk GTN is a rare malignancy, described mostly by observational and retrospective institutional series spanning many years. Despite its high likelihood to present with widely metastatic disease, high-risk GTN remains highly curable, with much of the mortality limited to ultra-high-risk disease. Numerous chemotherapy regimens have been shown to lead to high response rates and durable cures, with modern preferred EMA/CO or EMA/EP having the best observational data to support its choice as first-line therapy. While randomized evidence would be ideal to support the choice of this intensive, costly, and sometimes toxic regimen, international collaboration would likely be required to accrue adequate numbers of patients or an appropriately powered study, which may not be practical or feasible given the nature of this disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors have no acknowledgements to report.
FUNDING
This study received no direct funding or financial support. RAP was supported by early career funding from NIH grant K12HD103083–01. Other authors have no organizational/institutional funding to report.
Footnotes
DISCLOSURE OF INTERESTS
The authors report no conflicts of interest or financial interests.
DETAILS OF ETHICS APPROVAL
This study involved only literature review of previously published studies and the contained data. It involved no primary research on human or animal subjects, or medical records. As such, this work was considered exempt from ethical review.
REFERENCES
- 1.Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010. Dec 1;203(6):531–9. [DOI] [PubMed] [Google Scholar]
- 2.Albright BB, Shorter JM, Mastroyannis SA, Ko EM, Schreiber CA, Sonalkar S. Gestational Trophoblastic Neoplasia After Human Chorionic Gonadotropin Normalization Following Molar Pregnancy: A Systematic Review and Meta-analysis. Obstet Gynecol. 2020. Jan 1;135(1):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soper JT. Gestational Trophoblastic Disease: Current Evaluation and Management. Obstet Gynecol. 2021. Feb 1;137(2):355–70. Available from: https://pubmed.ncbi.nlm.nih.gov/33416290/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertz R, Lewis J, Lipsett MB. Five years’ experience with the chemotherapy of metastatic choriocarcinoma and related trophoblastic tumors in women. Am J Obstet Gynecol. 1961. Sep 1;82(3):631–40. [DOI] [PubMed] [Google Scholar]
- 5.Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011. Jan 1;204(1):11–8. [DOI] [PubMed] [Google Scholar]
- 6.DuBeshter B, Berkowitz RS, Goldstein DP, Cramer DW, Bernstein MR. Metastatic gestational trophoblastic disease: experience at the New England Trophoblastic Disease Center, 1965 to 1985. Obstet Gynecol. 1987;69(3):390–5. [PubMed] [Google Scholar]
- 7.Lurain JR, Brewer JI, Torok EE, Halpern B. Gestational trophoblastic disease: treatment results at the Brewer Trophoblastic Disease Center. Obstet Gynecol. 1982. Sep 1;60(3):354–60. Available from: https://europepmc.org/article/med/6289207 [PubMed] [Google Scholar]
- 8.Bagshawe KD. Risk and prognostic factors in trophoblastic neoplasia. Cancer. 1976. Sep 1;38(3):1373–85. Available from: [DOI] [PubMed] [Google Scholar]
- 9.WHO Scientific Group on Gestational Trophoblastic Diseases & World Health Organization. Gestational trophoblastic diseases: report of a WHO scientific group [meeting held in Geneva from 6 to 10 December 1982]. 1983. Available from: https://apps.who.int/iris/handle/10665/39169
- 10.Kohorn EI. The trophoblastic Tower of Babel: classification systems for metastatic gestational trophoblastic neoplasia. Gynecol Oncol. 1995;56(2):280–8. Available from: https://pubmed.ncbi.nlm.nih.gov/7896199/ [DOI] [PubMed] [Google Scholar]
- 11.Ngan HYS, Bender H, Benedet JL, Jones H, Montruccoli GC, Pecorelli S. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification. Int J Gynecol Obstet. 2003;83(SUPPL. 1):175–7. Available from: https://pubmed.ncbi.nlm.nih.gov/14763174/ [DOI] [PubMed] [Google Scholar]
- 12.Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynecol Obstet. 2021. Oct 1;155(S1):86–93. Available from: 10.1002/ijgo.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Sook Chon H, et al. Gestational trophoblastic neoplasia, version 2.2019. Vol. 17, JNCCN Journal of the National Comprehensive Cancer Network. Harborside Press; 2019. p. 1374–91. Available from: https://jnccn.org/view/journals/jnccn/17/11/article-p1374.xml [DOI] [PubMed] [Google Scholar]
- 14.Management of Gestational Trophoblastic Disease. BJOG An Int J Obstet Gynaecol. 2021. Feb 1;128(3):e1–27. Available from: 10.1111/1471-0528.16266 [DOI] [PubMed] [Google Scholar]
- 15.Horowitz NS, Eskander RN, Adelman MR, Burke W. Epidemiology, diagnosis, and treatment of gestational trophoblastic disease: A Society of Gynecologic Oncology evidenced-based review and recommendation. Gynecol Oncol. 2021. Dec 1;163(3):605–13. Available from: http://www.gynecologiconcology-online.net/article/S0090825821014219/fulltext [DOI] [PubMed] [Google Scholar]
- 16.Deng L, Zhang J, Wu T, Lawrie TA. Combination chemotherapy for primary treatment of high-risk gestational trophoblastic tumour. Cochrane Database Syst Rev. 2013;(1). Available from: 10.1002/14651858.CD005196.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009. Jul 21;6(7):e1000097. Available from: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. J Am Med Assoc. 2000. Apr 19;283(15):2008–12. Available from: https://jamanetwork.com/journals/jama/fullarticle/192614 [DOI] [PubMed] [Google Scholar]
- 19.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane. 2020. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 20.Wilson EB. Probable Inference, the Law of Succession, and Statistical Inference. J Am Stat Assoc. 1927;22(158):209–12. [Google Scholar]
- 21.Brown LD, Cai TT, Das Gupta A. Interval Estimation for a Binomial Proportion. 10.1214/ss/1009213286. 2001. May 1;16(2):101–33. Available from: https://projecteuclid.org/journals/statistical-science/volume-16/issue-2/Interval-Estimation-for-a-Binomial-Proportion/10.1214/ss/1009213286.full [DOI] [Google Scholar]
- 22.Nyaga VN, Arbyn M, Aerts M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch Public Heal. 2014. Nov 10;72(1):1–10. Available from: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JJ. The Inverse of the Freeman – Tukey Double Arcsine Transformation. 10.1080/00031305197810479283. 2012;32(4):138. Available from: [DOI] [Google Scholar]
- 24.Clopper CJ, Pearson ES. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika. 1934. Dec;26(4):404. [Google Scholar]
- 25.Wang S, An R, Han X, Zhu K, Xue Y. Combination chemotherapy with 5-fluorouracil, methotrexate and etoposide for patients with high-risk gestational trophoblastic tumors: A report based on our 11-year clinical experiences. Gynecol Oncol. 2006. Dec 1;103(3):1105–8. [DOI] [PubMed] [Google Scholar]
- 26.Lu WG, Ye F, Shen YM, Fu YF, Chen HZ, Wan XY, et al. EMA-CO chemotherapy for high-risk gestational trophoblastic neoplasia: a clinical analysis of 54 patients. Int J Gynecol Cancer. 2008. Mar;18(2):357–62. Available from: https://pubmed.ncbi.nlm.nih.gov/17711444/ [DOI] [PubMed] [Google Scholar]
- 27.Anfinan N, Sait K, Sait H. Gestational trophoblastic disease in the western region of Saudi Arabia (single-institute experience). Eur J Obstet Gynecol Reprod Biol. 2014. Sep 1;180(1):8–11. Available from: http://www.ejog.org/article/S0301211514003303/fulltext [DOI] [PubMed] [Google Scholar]
- 28.Even C, Pautier P, Duvillard P, Floquet A, Kerbrat P, Troalen F, et al. Actinomycin D, cisplatin, and etoposide regimen is associated with almost universal cure in patients with high-risk gestational trophoblastic neoplasia. Eur J Cancer. 2014. Aug 1;50(12):2082–9. Available from: http://www.ejcancer.com/article/S0959804914006510/fulltext [DOI] [PubMed] [Google Scholar]
- 29.Gulia S, Bajpai J, Gupta S, Maheshwari A, Deodhar K, Kerkar RA, et al. Outcome of Gestational Trophoblastic Neoplasia: Experience from a Tertiary Cancer Centre in India. Clin Oncol. 2014. Jan 1;26(1):39–44. Available from: http://www.clinicaloncologyonline.net/article/S0936655513003282/fulltext [DOI] [PubMed] [Google Scholar]
- 30.Shrivastava S, Kataki A, Barmon D, Deka P, Bhuyan C, Bhargav S. Gestational trophoblastic neoplasia: A 6 year retrospective study. South Asian J Cancer. 2014;3(1):33. Available from: /pmc/articles/PMC3961865/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Husaini H, Soudy H, Darwish A, Ahmed M, Eltigani A, Edesa W, et al. Gestational trophoblastic neoplasia: Treatment outcomes from a single institutional experience. Clin Transl Oncol. 2015. May 1;17(5):409–15. Available from: 10.1007/s12094-014-1251-1 [DOI] [PubMed] [Google Scholar]
- 32.Chu MMY, Ma Y, Tse KY, Chan KKL, Ngan HYS. Cyclophosphamide, Hydroxyurea, Actinomycin D, Methotrexate, and Vincristine in the Treatment of Gestational Trophoblastic Neoplasia. Int J Gynecol Cancer. 2015. Mar 1;25(3):498–503. Available from: https://ijgc.bmj.com/content/25/3/498 [DOI] [PubMed] [Google Scholar]
- 33.Davidson BA, Nagel CI, Richardson DL, Kehoe SM, Miller DS, Lea JS. Outcomes of Treatment of Gestational Trophoblastic Neoplasia in a Primarily Indigent Urban Population. J Reprod Med. 2015;60(5–6):243–8. [PubMed] [Google Scholar]
- 34.Song SQ, Wang C, Zhang GN, Shi Y, Zhu Y, Hu T, et al. BEP for high-risk gestational trophoblastic tumor: results from a cohort of 45 patients. Eur J Gynaecol Oncol. 2015;36(6):726–9. [PubMed] [Google Scholar]
- 35.Bolze PA, Riedl C, Massardier J, Lotz JP, You B, Schott AM, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am J Obstet Gynecol. 2016. Mar 1;214(3):390.e1–390.e8. Available from: http://www.ajog.org/article/S0002937815011977/fulltext [DOI] [PubMed] [Google Scholar]
- 36.Byun SW, Park TC, Bae SN. Conservative Chemotherapy in Gestational Trophoblastic Disease: Experience With Etoposide, Methotrexate, and Dactinomycin Chemotherapy. Int J Gynecol Cancer. 2016. May 1;26(4):790–5. Available from: https://pubmed.ncbi.nlm.nih.gov/27057813/ [DOI] [PubMed] [Google Scholar]
- 37.Chauhan A, Dave K, Desai A, Mankad M, Patel S, Dave P. High-risk gestational trophoblastic neoplasia at Gujarat Cancer and Research Institute: thirteen years of experience. J Reprod Med. 2010. Jul 1;55(7–8):333–40. Available from: https://europepmc.org/article/med/20795348 [PubMed] [Google Scholar]
- 38.Gueye M, Ndiaye-Gueye MD, Kane-Gueye SM, Gassama O, Diallo M, Moreau JC. Diagnosis, Treatment and Outcome of Gestational Trophoblastic Neoplasia in a Low Resource Income Country. Int J MCH AIDS. 2016;5(2):112. Available from: /pmc/articles/PMC5187643/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain A, Shiekh AA, Bhat GM, Lone AR. Gestational trophoblastic neoplasia, management as per risk stratification in a developing country. Indian J Med Paediatr Oncol. 2016. Jan 1;37(1):28. Available from: /pmc/articles/PMC4795371/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nzayisenga I, Segal R, Pritchett N, Xu MJ, Park PH, Mpanumusingo EV, et al. Gestational Trophoblastic Neoplasia Treatment at the Butaro Cancer Center of Excellence in Rwanda. J Glob Oncol. 2016. Dec 13;2(6):365–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28717722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanaranop M, Potikul C, Tuipae S. A 10-Year Clinical Experience of Gestational Trophoblastic Disease at Rajavithi Hospital, 2001–2010. J Med Assoc Thail. 2016;99(Feb):S17–27. [PubMed] [Google Scholar]
- 42.Bangash AG, Sadaf R, Nisa M un. Gestational trophoblast neoplasia and mortality risk factors. J Med Sci. 2017;25(1):19–23. [Google Scholar]
- 43.Jiang F, Wan X run, Xu T, Feng F zhi, Ren T, Yang J jun, et al. Evaluation and suggestions for improving the FIGO 2000 staging criteria for gestational trophoblastic neoplasia: A ten-year review of 1420 patients. Gynecol Oncol. 2018. Jun 1;149(3):539–44. Available from: https://pubmed.ncbi.nlm.nih.gov/29653688/ [DOI] [PubMed] [Google Scholar]
- 44.Aminimoghaddam S, Nezhadisalami F, Anjidani S, Tond SB. Outcome of treatment with EMA/EP (etoposide methotrexate and actinomycin-D/etoposide and cisplatin) regimen in gestational trophoblastic neoplasia. Med J Islam Repub Iran. 2018;32(1):36. Available from: /pmc/articles/PMC6108260/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fulop V, Szigetvari I, Szepesi J, Vegh G, Demeter J, Berkowitz RS. Diagnosis and Treatment of High-Risk Metastatic Gestational Trophoblastic Neoplasia in Hungary: 40 Years of Experience. J Reprod Med. 2018;63(5–6):240–8. [Google Scholar]
- 46.Anantharaju A, Pallavi VR, Bafna UD, Rathod PS, Vijay CR, Shobha K, et al. Role of salvage therapy in chemo resistant or recurrent high-risk gestational trophoblastic neoplasm. Int J Gynecol Cancer. 2019. Mar 1;29(3):547–53. Available from: https://ijgc.bmj.com/content/29/3/547 [DOI] [PubMed] [Google Scholar]
- 47.Hasanzadeh M, Samadi F, Seresht LM, Shandiz FH. Evaluation of the relation between treatment results and predictive factors in metastatic and high risk gestational trophoblastic neoplasia. Middle East J Cancer. 2019. Jul 1;10(3):214–20. [Google Scholar]
- 48.Lurain JR, Singh DK, Schink JC. Management of Metastatic High-Risk Gestational Trophoblastic Neoplasia: FIGO Stages II-IV: Risk Factor Score ≥ 7. J Reprod Med. 2010;55(5–6):199–207. [PubMed] [Google Scholar]
- 49.Maestá I, De Freitas Segalla Moreira M, Rezende-Filho J, Bianconi MI, Jankilevich G, Otero S, et al. Outcomes in the management of high-risk gestational trophoblastic neoplasia in trophoblastic disease centers in South America. Int J Gynecol Cancer. 2020. Sep 1;30(9):1366–71. Available from: https://ijgc.bmj.com/content/30/9/1366 [DOI] [PubMed] [Google Scholar]
- 50.Patel S, Arora R, Tiwari R, Poddar P, Desai A, Mankad M, et al. Management of “Ultra-High Risk” gestational trophoblastic neoplasia at a tertiary center in India. Indian J Med Paediatr Oncol. 2020. May 1;41(3):345–50. [Google Scholar]
- 51.Sato S, Yamamoto E, Niimi K, Ino K, Nishino K, Suzuki S, et al. The efficacy and toxicity of 4-day chemotherapy with methotrexate, etoposide and actinomycin D in patients with choriocarcinoma and high-risk gestational trophoblastic neoplasia. Int J Clin Oncol. 2020. Jan 1;25(1):203–9. Available from: 10.1007/s10147-019-01540-9 [DOI] [PubMed] [Google Scholar]
- 52.Edesa WA, Ayad NN, Mounir AM, Haggag MH. Treatment outcome of gestational trophoblastic neoplasia patients in Egypt. Indian J Cancer. 2021. Jan 6; Available from: https://pubmed.ncbi.nlm.nih.gov/33402570/ [DOI] [PubMed] [Google Scholar]
- 53.Singh K, Gillett S, Ireson J, Hills A, Tidy JA, Coleman RE, et al. M-EA (methotrexate, etoposide, dactinomycin) and EMA-CO (methotrexate, etoposide, dactinomycin / cyclophosphamide, vincristine) regimens as first-line treatment of high-risk gestational trophoblastic neoplasia. Int J Cancer. 2021. May 1;148(9):2335–44. Available from: 10.1002/ijc.33403 [DOI] [PubMed] [Google Scholar]
- 54.Cyriac S, Rajendranath R, Sridevi V, Sagar TG. Management of high-risk gestational trophoblastic neoplasia with etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine chemotherapy. J Reprod Med. 2011;56(5–6):219–23. [PubMed] [Google Scholar]
- 55.Tayib S, Van Wijk L, Denny L. Gestational Trophoblastic Neoplasia and Human Immunodeficiency Virus Infection: A 10-Year Review. Int J Gynecol Cancer. 2011. Nov 1;21(9):1681–91. Available from: https://ijgc.bmj.com/content/21/9/1684 [DOI] [PubMed] [Google Scholar]
- 56.Aydiner A, Keskin S, Berkman S, Bengisu E, İlhan HR, Tas F, et al. The roles of surgery and EMA/CO chemotherapy regimen in primary refractory and non-refractory gestational trophoblastic neoplasia. J Cancer Res Clin Oncol. 2012;138(6):971–7. Available from: https://pubmed.ncbi.nlm.nih.gov/22358303/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cagayan MSFS. High-risk metastatic gestational trophoblastic neoplasia. Primary management with EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine) chemotherapy. J Reprod Med. 2012;57(5–6):231–6. [PubMed] [Google Scholar]
- 58.Han SN, Amant F, Leunen K, Devi UK, Neven P, Vergote I. EP-EMA Regimen (Etoposide and Cisplatin With Etoposide, Methotrexate, and Dactinomycin) in a Series of 18 Women With Gestational Trophoblastic Neoplasia. Int J Gynecol Cancer. 2012. Jun 1;22(5):875–80. Available from: https://ijgc.bmj.com/content/22/5/875 [DOI] [PubMed] [Google Scholar]
- 59.Alifrangis C, Agarwal R, Short D, Fisher RA, Sebire NJ, Harvey R, et al. EMA/CO for high-risk gestational trophoblastic neoplasia: Good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol. 2013. Jan 10;31(2):280–6. [DOI] [PubMed] [Google Scholar]
- 60.Atalaia A, Thompson R, Corvo A, Carmody L, Piscia D, Matalonga L, et al. A guide to writing systematic reviews of rare disease treatments to generate FAIR-compliant datasets: Building a Treatabolome. Orphanet J Rare Dis. 2020. Aug 12;15(1):1–11. Available from: 10.1186/s13023-020-01493-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curry SL, Blessing JA, Disaia PJ, Soper JT, Twiggs LB. A prospective randomized comparison of methotrexate, dactinomycin, and chlorambucil versus methotrexate, dactinomycin, cyclophosphamide, doxorubicin, melphalan, hydroxyurea, and vincristine in “poor prognosis” metastatic gestational trophoblastic disease: Obstet Gynecol. 1989;73(3):357–62. [PubMed] [Google Scholar]
- 62.Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of english-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Vol. 28, International Journal of Technology Assessment in Health Care. Int J Technol Assess Health Care; 2012. p. 138–44. Available from: https://pubmed.ncbi.nlm.nih.gov/22559755/ [DOI] [PubMed] [Google Scholar]
- 63.Newlands ES, Bagshawe KD, Begent RHJ, Rustin GJS, Holden L, Dent J. Developments in chemotherapy for medium- and high-risk patients with gestational trophoblastic tumours (1979–1984). BJOG An Int J Obstet Gynaecol. 1986. Jan 1;93(1):63–9. Available from: 10.1111/j.1471-0528.1986.tb07815.x [DOI] [PubMed] [Google Scholar]
- 64.Alazzam M, Tidy J, Osborne R, Coleman R, Hancock BW, Lawrie TA. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016;(1). Available from: 10.1002/14651858.CD008891.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng L, Zhang J, Wu T, Lawrie TA. Combination chemotherapy for primary treatment of high-risk gestational trophoblastic tumour. Vol. 2013, Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd; 2013. Available from: 10.1002/14651858.CD005196.pub4/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newlands ES, Mulholland PJ, Holden L, Seckl MJ, Rustin GJS. Etoposide and cisplatin/etoposide, methotrexate, and actinomycin D (EMA) chemotherapy for patients with high-risk gestational trophoblastic tumors refractory to EMA/cyclophosphamide and vincristine chemotherapy and patients presenting with metastatic placental site trophoblastic tumors. J Clin Oncol. 2000. Sep 21;18(4):854–9. [DOI] [PubMed] [Google Scholar]
- 67.Surwit EA, Childers JM. High-risk metastatic gestational trophoblastic disease. A new dose-intensive, multiagent chemotherapeutic regimen. J Reprod Med. 1991;36(1):45–8. [PubMed] [Google Scholar]
- 68.Miller CR, Chappell NP, Sledge C, Leath CA, Phippen NT, Havrilesky LJ, et al. Are different methotrexate regimens as first line therapy for low risk gestational trophoblastic neoplasia more cost effective than the dactinomycin regimen used in GOG 0174? Gynecol Oncol. 2017. Jan 1;144(1):125–9. Available from: 10.1016/j.ygyno.2016.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albright BB, Myers ER, Moss HA, Ko EM, Sonalkar S, Havrilesky LJ. Surveillance for gestational trophoblastic neoplasia following molar pregnancy: a cost-effectiveness analysis. Am J Obstet Gynecol. 2021. Nov 1;225(5):513.e1–513.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jareemit N, Horowitz NS, Goldstein DP, Berkowitz RS, Elias KM. EMA vs EMACO in the treatment of gestational trophoblastic neoplasia. Gynecol Oncol. 2020. Jul 1;158(1):99–104. [DOI] [PubMed] [Google Scholar]
- 71.Chambers LM, Chalif J, Vargas R. Analysis of patient experiences with gestational trophoblastic neoplasia reported on Instagram social media. Gynecol Oncol. 2022. Jun 1;165(3):603–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0090825822002116 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


