Abstract
Symptomatic Uncomplicated Diverticular Disease (SUDD) is a syndrome within the diverticular disease spectrum, characterized by local abdominal pain with bowel movement changes but without systemic inflammation. This narrative review reports current knowledge, delivers practical guidance, and reveals challenges for the clinical management of SUDD. A broad and common consensus on the definition of SUDD is still needed. However, it is mainly considered a chronic condition that impairs quality of life (QoL) and is characterized by persistent left lower quadrant abdominal pain with bowel movement changes (eg, diarrhea) and low-grade inflammation (eg, elevated calprotectin) but without systemic inflammation. Age, genetic predisposition, obesity, physical inactivity, low-fiber diet, and smoking are considered risk factors. The pathogenesis of SUDD is not entirely clarified. It seems to result from an interaction between fecal microbiota alterations, neuro-immune enteric interactions, and muscular system dysfunction associated with a low-grade and local inflammatory state. At diagnosis, it is essential to assess baseline clinical and Quality of Life (QoL) scores to evaluate treatment efficacy and, ideally, to enroll patients in cohort studies, clinical trials, or registries. SUDD treatments aim to improve symptoms and QoL, prevent recurrence, and avoid disease progression and complications. An overall healthy lifestyle – physical activity and a high-fiber diet, with a focus on whole grains, fruits, and vegetables – is encouraged. Probiotics could effectively reduce symptoms in patients with SUDD, but their utility is missing adequate evidence. Using Rifaximin plus fiber and Mesalazine offers potential in controlling symptoms in patients with SUDD and might prevent acute diverticulitis. Surgery could be considered in patients with medical treatment failure and persistently impaired QoL. Still, studies with well-defined diagnostic criteria for SUDD that evaluate the safety, QoL, effectiveness, and cost-effectiveness of these interventions using standard scores and comparable outcomes are needed.
Keywords: colonic diverticulosis, diverticulitis, irritable bowel syndrome, microbiota, treatment, probiotic
Introduction
Colonic diverticulosis represents the most frequent finding during colonoscopy,1 and diverticular disease represents a common gastrointestinal condition and a worldwide burden on health-care systems.2 Symptomatic Uncomplicated Diverticular Disease (SUDD) is a syndrome within the diverticular disease spectrum. It is characterized by localized and persistent abdominal pain with bowel movement changes and low-grade inflammation but without systemic inflammation.3–7 SUDD is a recurrent chronic condition that negatively affects the quality of life (QoL).8–10 As similar symptoms are present in irritable bowel syndrome (IBS), it is debated whether SUDD has to be considered a disease of its own or whether it represents the coexistence of IBS and diverticulosis.3–7 This narrative review aims to explore the current knowledge, deliver practical guidance, and show challenges for clinical management of SUDD.
Methods
This is a narrative review in which we report current knowledge, deliver practical guidance, and show challenges for the clinical management of SUDD. The SANRA scale has been used to structure the present narrative review.11 Literature search was conducted in October 2022 on MEDLINE, searching for MeSH and keywords including diverticulosis, colonic diverticula, diverticular disease, diverticulitis, symptomatic uncomplicated diverticular disease, and irritable bowel syndrome. After the first selection, only scientific articles related to the aim of the present review were considered. Then, treatment recommendations are classified according to the evidence level (EL) and recommendation grade (RG) or according to GRADE (Grading quality of evidence and strength of recommendations) level of evidence as they are reported in literature.12,13 Herein, we report the last evidence on SUDD in chronological order and present this review with an organized framework: SUDD definition, epidemiology, natural history, etiology, diagnosis, treatment, summary, and conclusion.
Symptomatic Uncomplicated Diverticular Disease
Definition
In recent years, there has been an evolution in the taxonomic classification of diverticular diseases: diverticulitis (complicated/uncomplicated diverticulitis, acute resolving diverticulitis, smoldering diverticulitis), segmental colitis associated with diverticulosis (SCAD) and symptomatic uncomplicated diverticular disease (SUDD). Multiple definitions of SUDD exist, and there is no uniform consensus mainly because of its similarity with IBS. Early in 2014, the Italian consensus conference of the Italian Study Group of Diverticular Disease (GRIMAD) defined SUDD as a syndrome characterized by recurrent abdominal symptoms resembling or overlapping IBS symptoms, attributed to diverticula in the absence of macroscopically evident alterations other than the presence of diverticula.5 Similarly, in 2015, the Italian Society of Colorectal Surgery released practice guidelines on diverticular diseases. Symptomatic uncomplicated diverticular disease (SUDD) is defined as mild, recurring abdominal pain attributed to diverticula and may be difficult to distinguish from irritable bowel syndrome (IBS).6 However, results from the symposium at United European Gastroenterology Week in 2017 and the international consensus from the 3rd International Symposium on Diverticular Disease in 2019 characterized SUDD as a distinct clinical syndrome characterized by recurrent abdominal symptoms attributed to diverticula in the absence of clinical, laboratory, or radiographic markers of overt inflammation.3,4 In addition, according to the 2020 European Society of Coloproctology (ESCP) guidelines for managing colon diverticular disease, SUDD has not been generally accepted as patients with diverticula who experience abdominal symptoms and changes in bowel habits with an overlap with IBS.7 However, both guidelines clarify as recent studies showed differences between SUDD and IBS other than colonic diverticula. Indeed, SUDD more frequently interests older males while IBS younger females.3,13,14 Also, in SUDD, diarrhea is predominant rather than constipation, and pain is not relieved by defecation or flatulence.3 In addition, pain patterns and localization are different between SUDD and IBS. Pain is usually prolonged (>24h) with long remission periods and localized in the left lower quadrant in SUDD, while in IBS it is shorter, with frequent recurrences, and diffuses to all the abdomen.14–16 Besides, while both diseases are not interested by systemic inflammation (no fever, white blood cell count, and C-reactive protein within the limits), fecal calprotectin is usually increased in SUDD and its concentration is correlated with the severity of abdominal pain and the extension of diverticulosis.15,17 In addition, cohort studies showed that SUDD has a chronic course,10,18,19 and negatively affects QoL.8–10 Some previous studies defined SUDD as atypical diverticular disease or atypical uncomplicated diverticulitis when occurred post diverticulitis, also described as PD-SUDD.20–23 In summary, SUDD might be defined as a chronic condition that impairs QoL characterized by persistent left-lower quadrant abdominal pain with bowel movement changes and low-grade inflammation but without systemic inflammation.
Epidemiology
The prevalence and incidence of SUDD remain challenging to assess, mainly because of different definitions between studies and the controversy of considering SUDD a distinct clinical syndrome. The prevalence of colonic diverticula increases with age, and it is primarily a life-long asymptomatic condition, with only 10–20% of patients that will develop diverticular disease.23,24 Few studies explored SUDD prevalence, and the results are contrasting. In an Italian case–control study including 5451 patients with colonic diverticula, Tursi et al found a prevalence of SUDD in 6.8% of patients.23 Interestingly, abdominal pain was recorded in almost 21% of patients, diarrhea in 5.3%, and constipation in 9.8% among patients with colonic diverticulosis.23 In a second Italian prospective observational multicenter cohort study (the REMAD Registry), Carabotti et al found that 6.3% of the 490 patients with baseline diverticulosis developed SUDD at one-year follow-up.19 Differently, in a cohort of 310 participants during a mean follow-up time of 6.8 years at the University of North Carolina, Peery et al found that only 1% of participants with diverticulosis reported a protracted left lower quadrant abdominal pain associated with SUDD.25 However, this lower prevalence might be the result of a selection bias for the smaller number of participants and different inclusion criteria in the cohort of Peery et al. Indeed, a previous study on an overlapping cohort of participants found that neither colonic diverticulosis, chronic gastrointestinal symptoms nor mucosal inflammation were associated with mucosal inflammation in individuals with diverticulosis and chronic gastrointestinal symptoms (considered as patients with SUDD).26 Indeed, some concerns were raised about the validity of these results, and more studies are needed to outline the prevalence of SUDD.16
Natural History
SUDD is a chronic condition with recurrent abdominal pain and bowel movement changes. SUDD’s natural history has been explored by three studies with a follow-up period of at least 5 years after diagnosis.10,18,19 As reported by Salem et al, among 163 patients with SUDD, acute diverticulitis occurred in 1.7%, and surgery was required in 0.9% after a mean follow-up time of 5 years.18 While the REMAD Registry reports among 300 patients with SUDD, a 1.6% incidence of acute diverticulitis after only 1 year of follow-up.19 Moreover, Tursi et al reported that among 185 patients with SUDD, acute diverticulitis occurred in 7.6% and surgery was required in 3.2% after a median follow-up time of 13 years.10 The difference in acute diverticulitis rate between the studies is consistent with the more rigorous inclusion criteria to define SUDD used by Tursi et al and with different follow-up periods. Importantly, all three studies demonstrated that SUDD is a chronic and distinct clinical entity. Indeed, Tursi et al showed how QoL scores were negatively affected by SUDD and were unmodified during the study period despite patients requiring multiple courses of treatment. Still, only 8.6% achieved symptom resolution after SUDD diagnosis.10 Also, previous studies found lower QoL scores in patients with SUDD with associated negative psychological, social, and physical symptoms, both during and after acute attacks.8,9 To assess QoL properly in patients with SUDD, a specific disease-targeted health-related QoL questionnaire, the DV-QOL, has been developed and validated.9
Etiology
Research exploring risk factors and pathogenesis of SUDD report overlapping results with other diverticular diseases (such as diverticulitis and SCAD). There are no specific studies on SUDD risk factors. Therefore, since formation of colonic diverticula is the primary step in order to develop SUDD, herein we briefly list risk factors associated with colonic diverticula formation and diverticular disease. However, specific studies of risk factors for development of SUDD are warranted.
Risk Factors
Risk factors include age, genetic predisposition, obesity, physical inactivity, diet, and smoking.
Age is considered a risk factor for diverticular disease as the presence of colonic diverticula increases in prevalence with age.1,24
Genetic predisposition to diverticular disease has been demonstrated in twin and sibling studies.27,28 In addition, genes involved in immunity, extracellular matrix biology, cell adhesion, membrane transport, and intestinal motility have been related to diverticular disease.29–31
Obesity and physical inactivity are major risk factors for diverticular disease, while a higher level of physical activity may be a protective factor.32–34
A diet high in red meat, fat dairy, and refined grains and low in fiber is considered a risk factor as it is associated with diverticular disease.7,35 However, multiple studies explored the association of low fiber diet with diverticular disease and diverticulitis, finding no causative relation between low fiber diet and diverticular disease.36,37 Nevertheless, large epidemiological studies indicate that a healthy lifestyle (high fiber, with a focus on whole grains, fruits, and vegetables) has a protective effect against the development of diverticulosis and diverticulitis.38–40 Also, nuts, grains, corn, and popcorn – traditionally excluded – have been shown not to increase the risk of diverticular disease or diverticulitis.41
Smoking has also been considered a possible risk factor for symptomatic diverticular disease, both in men and women.42,43 Commonly used drugs, such as nonsteroidal anti-inflammatory drugs (NSAIDs), acetylsalicylic acid, opioids, and steroids, significantly increase the risk of diverticular bleeding and complicated diverticulitis, but specific data on SUDD are limited.35,44
Pathogenesis
Pathogenesis of colonic diverticula into diverticular disease or SUDD is complex and not wholly understood by several theories and interdependent mechanisms.36 Low-grade inflammation, alteration of the fecal microbiota, abnormal colonic motility, colonic mucosal ischemia, and neuro-immune interaction have been identified as potential factors leading to symptom development.
Low-grade inflammation within the diverticular colonic mucosa has been considered a symptom-developing mechanism associated with SUDD.35,45,46 Indeed, while low-grade inflammation has no association with diverticulosis,26 multiple studies showed how diverticular disease, particularly SUDD, is related to low-grade mucosal inflammation and neuro-immune interaction (so-called visceral hypersensitivity).35,45–47 Interestingly, studies have shown elevated fecal calprotectin and tumor necrosis factor-alpha (TNF α) mRNA expression in patients with SUDD.15,17,47 Also, elevated fecal calprotectin and TNF α seem to help differentiate the diagnosis of SUDD from IBS and correlate with symptoms severity.3,48 In particular, fecal calprotectin, as it is widely available as a laboratory test and is already used in clinical practice.
Fecal microbiota alteration is considered a central mechanism in the pathogenesis of SUDD. Studies on microbiota in patients with SUDD have found decreases in bacteria that produce short-chain fatty acids and in Akkermansia muciniphila, a mucin-degrading bacterium that promotes epithelial barrier integrity and suppresses inflammation.45,48,49 In addition, a pilot study found clinical and symptom scores correlated with fecal microbiota features like abundance in Ruminococcus, Roseburia, Lactobacillus, and Cyanobacterium in SUDD.48 Also, Barbara et al reported depletion of microbiota members with anti-inflammatory properties, including Clostridium cluster IV, Clostridium cluster IX, Fusobacterium, and Lactobacillaceae, with microbiota changes being related to mucosal immune activation.45 Other studies showed that different microbiota compositions were associated with SUDD.50 Therefore, colonic microbiota has been considered a therapeutic target for probiotics, fibers, and non-absorbable antibiotics.
Abnormal colonic motility and mucosal ischemia are also possible mechanisms for developing the prolonged abdominal pain typical of SUDD. This could be produced by the sustained spastic state of the bowel wall, which predisposes to mucosal ischemia in the diverticulum.51,52 In addition, abnormal segmentation of colon motility might explain the higher frequency of diverticular disease in the sigmoid colon and, secondly, in the right colon compared to the other segment.53 Also, altered colonic motility is associated with impaired intestinal permeability, assuming that altered mucosal permeability could be responsible for symptoms genesis in SUDD.54 However, these hypotheses are theoretical and based mainly on biological plausibility rather than evidence. Nevertheless, abnormal colonic motility and colonic mucosal ischemia might be considered part of a dysfunction in the neuro-immune interaction.
Neuro-immune interaction has been considered a key element in the pathophysiological pathway of diverticular disease and especially of SUDD. Numerous studies have demonstrated that patients with diverticulosis have differences within myenteric plexuses and ganglions, with a reduction in the number of colonic interstitial cells in Cajal (ICCs) and enteric glial cells and an increase in mast cells and myenteric plexitis.55–57 The above modifications result in an abnormal neuromuscular function and propulsive activity of the colon, especially in the diverticula segments.58 More recent studies have explored neuro-immune interaction between muscularis macrophages and ICCs, enteric neurons, smooth muscle, fibroblast-like, and enteric glial cells.59 This results in an imbalance in neuro-immune interactions that may sustain low-grade inflammation and abnormal colonic motility in many different gastrointestinal diseases.36,45,55,56,58,59
Although not completely clarified, SUDD’s pathogenesis seems to be related to an interaction between fecal microbiota alterations, neuro-immune enteric interaction, and muscular system dysfunction associated with a low-grade inflammation state. As described above, these factors lead to symptom development, providing a pathophysiological basis for therapeutic options.
Diagnosis
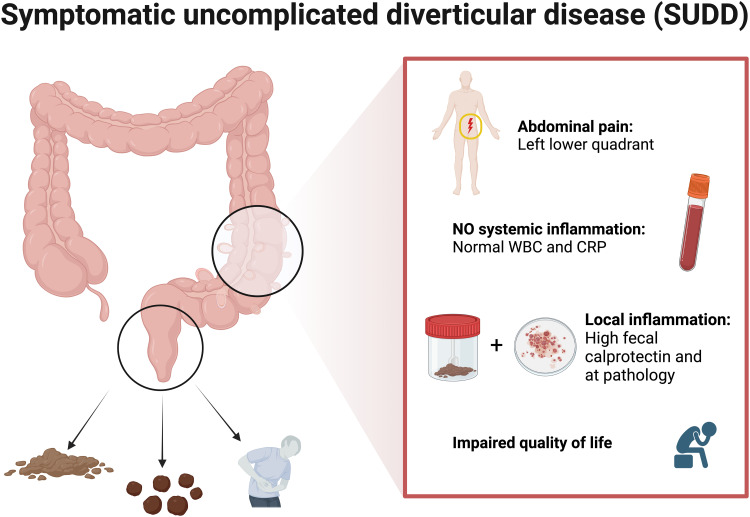
The clinical presentation of SUDD is characterized by left lower quadrant abdominal pain associated with bowel movement changes in patients with diverticular disease without systemic inflammation.3,14–16,60 Abdominal pain is located in the left lower quadrant. It is a chronic and prolonged pain (sometimes >24h) that not improves with bowel movement.10 It is reported to be moderate and recurrent, with typically long remission periods.14–16 Associated bowel movement changes include diarrhea, constipation, and bloating.3 No systemic inflammation, such as elevated white blood count (WBC) and C-reactive protein (CRP), nor complication of diverticular diseases, such as bleeding, abscess, perforation, fistula, colitis, and diverticulitis, should be present. In addition, fecal calprotectin can be used as a biomarker for SUDD as it is typically increased, and its concentration is correlated with the severity of abdominal pain and the extension of diverticulosis.15,17 Studies detecting fecal calprotectin in SUDD have used rapid (positive/negative) or semi-quantitative tests.15,17 Therefore, a specific threshold is still missing for patients with SUDD. Therefore, differential diagnosis with IBS is based on pain location, pain pattern, and fecal calprotectin.14–16 Diagnostic features are displayed in Figure 1.
Figure 1.
Diagnosis of SUDD. SUDD is mainly considered a chronic condition that impairs quality of life and is characterized by persistent left lower quadrant abdominal pain with bowel movement changes (diarrhea, constipation, and bloating) and local inflammation (elevated calprotectin and at pathology) but without systemic inflammation. Figure created with BioRender.com.
Abbreviations: WBC, white blood count; CRP, C-reactive protein.
There are no specific indications and guidelines on the diagnostic workup for SUDD. When a patient presents with abdominal pain consistent with SUDD, we recommend performing a complete physical exam with a rectal exam and blood tests with total blood count and CRP. In case of WBC and CRP within the limits, and if SUDD is suspected, further analyses might be considered case by case, especially in patients with long-lasting SUDD and in a research setting, for example, fecal calprotectin and fecal microbiota analysis. Still, imaging should be considered according to symptoms severity and lab test results to exclude misdiagnosed diverticulitis or diverticular disease complications such as abscess or micro-perforation. Intestinal ultrasonography (US) and Computed Tomography (CT) could be performed according to local practice and availability.61 Where there is no previous diagnosis, colonoscopy is recommended for patients in remission to confirm the presence of colonic diverticula and perform biopsy to rule out other colonic diseases.7 Within this workup, we consider it essential to assess QoL at baseline to evaluate QoL improvement after treatments. In particular, the Gastrointestinal Quality of Life Index (GIQLI) is a well-known QoL score used in clinical practice and research. In addition, a specific diverticular disease QoL instrument (DV-QOL) for patients with SUDD has been developed as a patient-reported outcome measure (PROM) and recently used to define the thresholds of minimal clinically significant difference and patient-acceptable symptom state.9,62 Also, a diverticular clinical score (DICS) for SUDD has been developed and validated in a small prospective cohort.63 GIQLI, DV-QOL, and DICS are helpful in the diagnosis, and monitoring, of SUDD in clinical practice and research settings, and they facilitate patient stratification and therapeutic decision.63 However, their use is still scarce because of their recent development. Finally, a pilot study presented an electronic multisensorial system capable of detecting fecal microbiota changes to predict clinical response in patients with SUDD.64 Clinical monitoring might benefit from this new technology. However, further studies need to confirm its replicability and usability in clinical practice.
Treatment
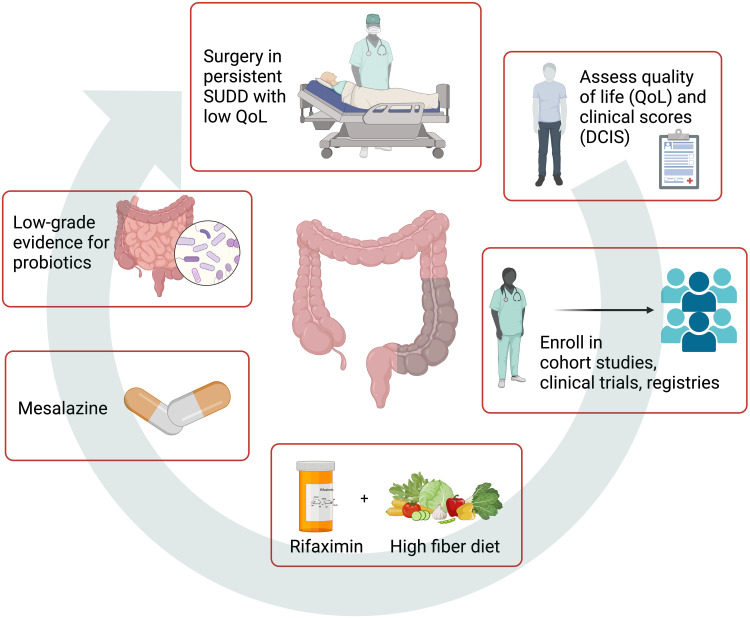
SUDD treatment aims to improve symptoms and QoL, prevent recurrence, and avoid disease progression and complications. Therefore, we consider it pivotal to determine QoL at baseline to measure the treatment’s response and define a long follow-up assessment to evaluate recurrence, disease progression, and complication. In particular, the diverticular disease QoL instrument (DV-QOL) establishes a threshold for minimal clinically significant difference and acceptable symptom state in patients with SUDD.9,62 Also, the diverticular clinical score (DICS) has been recently validated in patients with SUDD, and it helps comparing treatments in clinical practice and research settings.63 Ideally, the best management of SUDD is enrolling patients in cohort studies, clinical trials, or registries.65,66 Symptoms control and decrease progression to complications (eg, diverticulitis) include probiotics, high-fiber diet, gut-directed antibiotics, and mesalazine; elective surgery might be considered for patients with medical treatment failure and persistent low QoL.8–10,67,68 Management of SUDD is displayed in Figure 2. Unfortunately, treatment recommendations are weakened because of the scarcity of studies and their heterogeneity produced by the lack of a global consensus in defining SUDD, comparable clinical scores used, and QoL assessment.
Figure 2.
Management of patients with SUDD. SUDD management aims to improve symptoms and QoL, prevent a recurrence, and avoid diverticulitis and complications. Therefore, it is pivotal to determine QoL (DV-QOL) and clinical scores (DCIS) at baseline and enroll patients in cohort studies, clinical trials, or registries to ensure an adequate follow-up and fill the knowledge gap. Treatments: cyclic Rifaximin (400 mg/12 h) 7 days/month plus High-fiber diet ± supplemental fiber to reduce symptoms and improve remission [EL: 2b; RG: B]; Mesalazine 800 mg/12 h to enhance remission [EL: 1b; RG: A]; low-grade evidence for probiotics [EL: 3a; RG: B]; surgery in chronic persistent SUDD with low QoL [EL 3; RG C]; no evidence for nutraceutical formulation, anticholinergic or antispasmodic drug. Evidence Level (EL) and Recommendation Grade (RG) are reported according to OCEBM.12 DV-QOL is a specific diverticular disease QoL instrument for patients with SUDD.9,62 DCIS is a diverticular clinical score developed and validated for patients with SUDD.63 Figure created with BioRender.com.
Diet
Food and lifestyle are among the commonly discussed controllable risk factors, particularly dietary fiber.7 An overall healthy lifestyle – physical activity and a high-fiber diet, with a focus on whole grains, fruits, and vegetables – is encouraged in case of diverticular disease.32,69 High fiber diet and dietary supplements have been the mainstay of SUDD treatment despite insufficient evidence3,60 and are often associated with poor-absorbable antibiotics.4 In a recent systematic review and meta-analysis, Eberhardt et al reported with a low level of evidence that a high dietary fiber intake (>29g/day) may improve gastrointestinal function in patients with SUDD and that dietary fiber supplementation should be considered on an individualized basis.70 However, the GRADE (Grading quality of evidence and strength of recommendations) level of evidence was low. Indeed, substantial methodological limitations, the heterogeneity of the therapeutic regimens employed, and the lack of ad hoc designed studies limit the validity of fiber alone in SUDD, as another systematic review highlighted.71 Current indications recommend a high fiber diet and diet supplement only in association with cyclic Rifaximin.4–6,72–74
Probiotics
The rationale for probiotics in SUDD is based on their microbiota modulation ability and anti-inflammatory effects. In a randomized, double-blind, placebo-controlled trial with 120 patients, Kvasnovsky et al found that a multi-strain probiotic improved constipation and diarrhea but not bloating or abdominal pain. In addition, they discovered that fecal calprotectin at 3 months did not differ from baseline.75 Results from three clinical trials demonstrate that patients with SUDD benefit from improved maintenance of remission when treated with Lactobacillus cases, especially when in combination with Mesalazine.76–78 Further studies investigated the efficacy of Lactobacillus acidophilus, L. helveticus, and Bifidobacterium spp. 420 or Lactobacillus paracasei in patients with uncomplicated DD, observing a reduction in abdominal pain and bloating.79–81 These studies suggest that probiotics may be part of managing SUDD. Indeed, the 2019 international consensus on diverticulosis and diverticular disease reached >95% of agreement that there is some evidence that probiotics could effectively reduce symptoms in patients with SUDD [EL: 3a; RG: B].4 However, a systematic review could not demonstrate the efficacy of probiotics in diverticular disease or SUDD due to the high heterogeneity and poor quality of data retrieved,82 and other guidelines do not recommend their use in SUDD.5,83 Therefore, more extensive randomized, placebo-controlled studies are still needed before probiotics can be recommended for managing SUDD with adequate evidence.6
Mesalazine
Mesalazine was proposed as a treatment for SUDD’s low-grade inflammation of the colonic mucosa. Three meta-analyses of randomized controlled trials have been published so far about treatment with mesalazine.84–86 Table 1 summarizes and compares the different study populations, controls, and outcomes of these meta-analyses. Khan et al evaluated the effect of mesalazine in patients with SUDD using wider inclusion criteria.84 In particular, they included patients with diverticular disease after an episode of diverticulitis and patients with SUDD after at least one episode of uncomplicated diverticulitis (in other studies this condition has been defined as PD-SUDD).20–23,84 Their pooled analysis estimating the effect of mesalazine compared to placebo showed no difference in overall recurrence.84 Indeed, in subgroup analysis based on the Mesalazine dose, patients who received >2g per day had a higher recurrence.84 Iannone et al found that Mesalazine may reduce recurrence in SUDD, but they could not prove the effect of Mesalazine in achieving remission. Nevertheless, Mesalazine reduced symptoms compared to controls such as a placebo, probiotics, and rifaximin.85 Picchio et al found that mesalazine achieved a higher remission rate and decreased the incidence of diverticulitis in a patient with SUDD compared to a placebo.86 To date, data comparing mesalazine to other treatment options, such as probiotics or rifaximin, is inconclusive. Given these results and according to the 2019 international consensus on diverticulosis and diverticular disease, Mesalazine effectively reduces symptoms for patients with SUDD [EL: 1b; RG: A].4 However, long-term treatment is not recommended. The dose recommended is 800mg/12h, which is more frequently reported in RCTs. However, the length (3weeks-1year) and modality (continuous vs 7/10 days/month) of treatment or superiority of one regimen versus another have not been tested in clinical trials. Therefore, treatment should be adapted to the individual patient based on symptom resolution.
Table 1.
Meta-Analyses on Mesalazine Use in SUDD
| Study | Population | Control Group | Outcome |
|---|---|---|---|
| Khan, 201884 | SUDD after at least one episode of acute uncomplicated diverticulitis. (PD-SUDD) | Placebo | Recurrence:
- Mesalazine dose ≤ 2g/day: No difference (Five RCTs, OR 1.1, 95% CI 0.7–1.5), I2=27%. - Mesalazine dose > 2g/day: higher recurrence with Mesalazine (Four RCTs, OR 1.28, 95% CI 1.02–1.62), I2=0% |
| Iannone, 201885 | SUDD and acute uncomplicated diverticulitis.
Excluded patients with acute complicated diverticulitis. |
Probiotics, Rifaximin, placebo, no treatment, or both probiotic and placebo | Achievement of remission:
(One RCT, 81 patients, OR 2.67; 95% CI 1.05–6.79), Disease recurrence:
|
| Picchio, 201886 | SUDD | Placebo | Symptom relief (achievement of remission): (Three RCTs, OR 0.4, 95% CI 0.24–0.75, I2=65%) Diverticulitis occurrence: (Two RCTs, OR 0.35; 95% CI 0.17–0.70, I2=38%) |
Abbreviations: SUDD, Symptomatic Uncomplicated Diverticular Disease; RCT, Randomized Controlled Trial; OR, Odd Ratio; CI, Confidence Interval.
Rifaximin
Rifaximin is a poorly absorbable oral antibiotic with a broad-spectrum activity, which decreases intestinal flora’s metabolic activity, increases fecal mass, and reduces bacterial overgrowth.60 Its safety and tolerability are high because of its low absorption rates, implying that non-enteric pathogens are not exposed to selective pressure and the risk of bacterial resistance.3,60 Rifaximin acts through different mechanisms: inhibition of bacterial growth; better resistance to bacterial infection; modulatory effects on some bacterial species, such as Lactobacillus spp and Bifidobacterium spp, leading to the so-called eubiotic effect; modulation of bacterial metabolism; and anti-inflammatory activity.87 Different guidelines and international consensus recommend using Rifaximin (plus fiber) in SUDD.4–6,72–74 Evidence about the efficacy of Rifaximin for SUDD (summarized in Table 2) originates mainly from two studies: a meta-analysis and a systematic review.88,89
Table 2.
Studies on Rifaximin Use in Symptomatic Uncomplicated Diverticular Disease (SUDD)
| Study | Population | Control Group | Outcome |
|---|---|---|---|
| Maconi, 201188 Systematic review |
Heterogeneous: SUDD, PD-SUDD, asymptomatic DD after SUDD. 31 studies |
Placebo, fiber, fiber supplement, Mesalazine, probiotics, and combinations | High heterogeneity (inclusion criteria, treatments and outcomes). Symptoms reduction in 21 studies
Acute diverticulitis (4 RCT, Rifaximin + fiber vs fiber only, 1 y follow-up):
|
| Bianchi, 201189 Meta-analysis |
SUDD 1660 patients |
Fiber supplement (+ placebo) or fiber only. | Symptom relief:
(Four RCTs, 95% CI 24.5% - 33.6%; p<0.0001; NNT=3; I2=0%) Acute diverticulitis occurrence:
(Four RCTs, 95% CI −3.4% - −0.57%; p = 0.0057; NNT=50; I2=0%) |
| Papi, 199595 DB PC RCT |
SUDD 168 patients |
Both fiber supplement and placebo | Cumulative symptoms score:
The effect is particularly evident on abdominal pain and bloating. |
| Di Mario, 201998 Retrospective cohort study |
SUDD 816 patients |
Group A: Rifaximin 7d/mo. Group B: symptomatic treatment only. |
Symptoms (median VAS at 8 years follow-up):
Acute diverticulitis occurrence:
Surgery rate:
|
| Stallinger, 201499 Observational, prospective study |
SUDD 1003 patients. |
None | Symptoms improvement:
|
Abbreviations: DD, Diverticular Disease; DB, Double-Blind; CI, Confidence Interval; OR, Odd Ratio; PC, Placebo-Controlled; PD-SUDD, Post-Diverticulitis SUDD; RCT, Randomized Controlled Trial; RD, Rate Difference; VAS, Visual Analogue Scale.
In a systematic review, Maconi et al examined the evidence for medical therapy in reducing symptoms and preventing acute diverticulitis. They analyzed 31 prospective clinical trials reporting a high heterogeneity in study design, inclusion criteria, patients’ characteristics, regimens and a combination of studied treatment, and outcome reporting that precluded quantitative pooling of results and limited interpretation. However, in all the nine randomized trials with symptoms reduction as an outcome,8,90–97 a 400mg/12h or a higher dose of Rifaximin succeeded in reducing symptoms. And cumulative data from the four randomized trials90,94–96 evaluating the prevention of acute diverticulitis with Rifaximin plus fiber vs fiber alone demonstrated significant benefit following Rifaximin and fiber (1-year rate of acute diverticulitis: 11/970 (1.1%) vs 20/690 (2.9%); P = 0.012), but with a number needed to treat of 57 to prevent an attack of acute diverticulitis.88
A meta-analysis of 1660 patients from four RCTs compared long-term efficacy administration of Rifaximin plus fiber supplementation vs supplementation alone.89 Bianchi et al found a pooled risk difference (RD) for symptom relief of 29.0% (Rifaximin versus control; 95% CI 24.5–33.6%; p < 0.0001) with a number needed to treat (NNT) of three. And a pooled RD for a complication rate of −1.7% in favor of Rifaximin (95% CI −3.2 to −0.1%; P=0.03; NNT=59). Therefore, Bianchi et al concluded that in symptomatic uncomplicated diverticular disease, treatment with Rifaximin plus fiber supplementation is effective in obtaining symptom relief and preventing complications at 1 year.89
There is only a single multicenter, double-blind, placebo-controlled trial on Rifaximin.95 In 1995, Papi et al monitored 168 patients treated with fiber supplementation plus Rifaximin 400 mg every 12 h for 7 days every month (84 patients) or fiber supplementation plus placebo at three-month intervals for 12 months. After 3 months, both treatments effectively reduced the symptoms (lower abdominal pain, bloating, and diarrhea). Rifaximin plus fiber showed improved symptom scores for lower abdominal pain and bloating compared to placebo plus fiber at 6, 9, and 12 months. In addition, both treatments showed similar acute diverticular rates (2.4% vs 2.4%) during the study period.
A cohort of patients with SUDD treated with Rifaximin was analyzed during an 8-year follow-up.98 Overall, Di Mario et al compared 436 patients treated with Rifaximin 800 mg/d for 7 days every month to 470 taking any other treatment on demand (symptomatic treatment only). Left abdominal pain with visual analog scale (VAS), bloating, daily bowel movements, acute diverticulitis, surgery, mortality, and side effects were analyzed. They found that the Rifaximin group had improved left abdominal pain compared to baseline (VAS 3, IQR [3–4] vs VAS 6, IQR [5–7]) and to control group (VAS 3, IQR [3–4] vs VAS 6, IQR [5–7]) at 8 years of follow-up (p<0.000). In addition, both bloating and daily bowel movements were significantly reduced in the Rifaximin group. Acute diverticulitis was not statistically different (n. 9, 2.6% vs n. 21, 4.5%; p=0.155). Also, surgery and disease-related mortality were similar, with no side effects recorded during the entire study period.
Similarly, a multicentric, non-interventional prospective study carried out in Austria by Stallinger et al confirmed the above results.99 Among 1003 patients over a period of 3 months, Rifaximin achieved improved symptoms (lower abdominal pain, flatulence, tenesmus, diarrhea, abdominal tenderness), with good compliance (84%) and a low rate of adverse events (0.6%).
Although the studies of Di Mario et al and Stallinger et al have a high risk of selection bias and retrospective nature of the analysis, they are essential in evaluating real-world, long-term results in patients with SUDD treated with Rifaximin. Multiple guidelines recommend that Rifaximin plus fiber supplementation be used in patients with SUDD for symptom relief.5,6,72,74 Similarly, the international consensus from the 3rd International Symposium on Diverticular Disease concurs with >98% of agreement that Rifaximin plus fiber is effective in reducing symptoms in SUDD patients compared to fiber alone [EL: 2b; RG: B].4
Therefore, a prolonged treatment with Rifaximin 400gm/12 h for 7 days/months plus a high-fiber diet with/without supplemental fiber is recommended as it reduces symptoms and improves remission.4,60,73 QoL improvement and cost-efficacy of Rifaximin for the treatment of SUDD still need to be determined.
Other Medical Treatment
There is no sufficient evidence to recommend nutraceutical formulations, anticholinergic and antispasmodic drugs in SUDD.60 Preliminary studies on nutraceutical formulation reported positive results in improving symptoms in SUDD, but more studies are needed to confirm these findings.100
Surgery
SUDD is a chronic condition with recurrent abdominal pain and bowel movement changes that has a long-term negative impact on health-related QoL, low rate of symptom resolution, and limited evidence about QoL improvement with medical therapy.7,8,10,18,19,92,101 Patients might experience chronic discomfort, medical treatment failure, and persistently impaired QoL. In such patients, surgery can considered case by case in a shared decision-making setting, after patient expectation discussion and informed consent.102 This discussion is necessary as few data regarding long-term outcome and rate of recurrence have been published so far. In addition, some clarifications are needed as SUDD might affect patients in differently. For example, a recent study showed that SUDD occurred after an acute diverticulitis episode as a different clinical condition named post-diverticulitis SUDD (PD-SUDD),23 and found it associated with an unexpected abscess in almost half of the patients post asymptomatic uncomplicated diverticulitis and 63% with PD-SUDD.22 Also, an overlapping preoperative diagnosis of IBS has been associated with postoperative recurrence of SUDD, analogously to diverticulitis.21,103 Therefore, excluding a diagnosis of IBS is mandatory before surgery.
Sigmoidectomy is safe and effective in improving QoL and symptoms in patients with SUDD.20,21,68,104 According to ESCP guidelines, elective colon resections should be performed with a minimally invasive surgery approach to benefit from better short-term outcomes7 Still, minimally invasive sigmoidectomy carries a burden in postoperative complications, with 9.6% of patients developing a Clavien-Dindo ≥ II.68 In addition, despite QoL improvement, 7.4–12% of patients report symptom persistence after sigmoidectomy.20,21 According to Danish national guidelines for the treatment of diverticular disease, in chronic SUDD not amenable to conservative measures 3 of 4 benefits from resection, and chronic SUDD or by frequent relapse, resection can be considered if the condition is intolerable [EL 3; RG C].72 Minimally invasive elective sigmoidectomy might be proposed according to patients’ needs and expectations whenever medical treatment fails, and QoL improvement is needed.
Summary and Conclusion
This review provided practical guidance about the clinical management of patients with SUDD and showed the current challenges and the missing knowledge gap. Even if broad consensus on the SUDD definition is still needed, SUDD might be defined as a chronic condition that impairs QoL, characterized by persistent left lower quadrant abdominal pain with bowel movement changes and diverticula but without systemic inflammation. Risk factors for SUDD include age, genetic predisposition, obesity, physical inactivity, diet, and smoking. Pathogenesis of SUDD is still unclear: low-grade inflammation, alteration of the fecal microbiota, abnormal colonic motility, colonic mucosal ischemia, and neuro-immune interaction have been identified as potential factors leading to symptom development. At diagnosis, it is crucial to assess baseline clinical and Quality of Life (QoL) scores to evaluate treatment efficacy and, ideally, to enroll patients in cohort studies, clinical trials, or registries. Using Rifaximin plus fiber and mesalazine shows potential in controlling symptoms in patients with SUDD and might prevent acute diverticulitis. Surgery could be considered in patients with medical treatment failure and persistently impaired QoL. Still, better-quality studies with well-defined diagnostic criteria for SUDD that evaluate these interventions’ safety, QoL, effectiveness, and cost-effectiveness using standard scores and comparable outcomes are needed.
Acknowledgments
Figures were created with BioRender.com.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the united states part ii: lower gastrointestinal diseases. Gastroenterology. 2009;136(3):741–754. doi: 10.1053/j.gastro.2009.01.015 [DOI] [PubMed] [Google Scholar]
- 2.Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018;12(2):125–132. doi: 10.5009/gnl16552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarpignato C, Barbara G, Lanas A, Strate LL. Management of colonic diverticular disease in the third millennium: highlights from a symposium held during the United European Gastroenterology Week 2017. Therap Adv Gastroenterol. 2018;11:1756284818771305. doi: 10.1177/1756284818771305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tursi A, Brandimarte G, Di Mario F, et al. International consensus on diverticulosis and diverticular disease. statements from the 3rd international symposium on diverticular disease. J Gastrointestin Liver Dis. 2019;28(suppl. 4):57–66. doi: 10.15403/jgld-562 [DOI] [PubMed] [Google Scholar]
- 5.Cuomo R, Barbara G, Pace F, et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United Eur Gastroenterol J. 2014;2(5):413–442. doi: 10.1177/2050640614547068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binda GA, Cuomo R, Laghi A, et al. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Tech Coloproctol. 2015;19(10):615–626. doi: 10.1007/s10151-015-1370-x [DOI] [PubMed] [Google Scholar]
- 7.Schultz JK, Azhar N, Binda GA, et al. European Society of Coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis. 2020;22(Suppl 2):5–28. doi: 10.1111/codi.15140 [DOI] [PubMed] [Google Scholar]
- 8.Comparato G, Fanigliulo L, Aragona G, et al. Quality of life in uncomplicated symptomatic diverticular disease: is it another good reason for treatment? Dig Dis. 2007;25(3):252–259. doi: 10.1159/000103896 [DOI] [PubMed] [Google Scholar]
- 9.Spiegel BMR, Reid MW, Bolus R, et al. Development and validation of a disease-targeted quality of life instrument for chronic diverticular disease: the DV-QOL. Qual Life Res. 2015;24(1):163–179. doi: 10.1007/s11136-014-0753-1 [DOI] [PubMed] [Google Scholar]
- 10.Tursi A, Franceschi M, Elisei W, Picchio M, Di Mario F, Brandimarte G. The natural history of symptomatic uncomplicated diverticular disease: a long-term follow-up study. AOG. 2020. doi: 10.20524/aog.2020.0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4(1):5. doi: 10.1186/s41073-019-0064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxford Centre for Evidence-Based Medicine. OCEBM Levels of Evidence Working Group*. The oxford levels of evidence 2. OCEBM - Oxford Centre for Evidence-Based Medicine. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed December 3, 2022.
- 13.Jung HK, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Diarrhea-predominant irritable bowel syndrome is associated with diverticular disease: a population-based study. Am J Gastroenterol. 2010;105(3):652–661. doi: 10.1038/ajg.2009.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuomo R, Barbara G, Andreozzi P, et al. Symptom patterns can distinguish diverticular disease from irritable bowel syndrome. Eur J Clin Invest. 2013;43(11):1147–1155. doi: 10.1111/eci.12152 [DOI] [PubMed] [Google Scholar]
- 15.Tursi A, Picchio M, Giorgetti GM, Brandimarte G. Moderate to severe and prolonged left lower-abdominal pain is the best symptom characterizing symptomatic uncomplicated diverticular disease of the colon: a comparison with fecal calprotectin in clinical setting. J Clin Gastroenterol. 2015;49(3):218–221. doi: 10.1097/MCG.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 16.Tursi A, Scarpignato C. Symptomatic uncomplicated diverticular disease: chronic abdominal pain in diverticulosis is not enough to make the diagnosis. Clin Gastroenterol Hepatol. 2018;16(12):2001–2002. doi: 10.1016/j.cgh.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 17.Tursi A, Brandimarte G, Elisei W, Giorgetti GM, Inchingolo CD, Aiello F. Faecal calprotectin in colonic diverticular disease: a case–control study. Int J Colorectal Dis. 2009;24(1):49–55. doi: 10.1007/s00384-008-0595-9 [DOI] [PubMed] [Google Scholar]
- 18.Salem TA, Molloy RG, O’Dwyer PJ. Prospective, five-year follow-up study of patients with symptomatic uncomplicated diverticular disease. Dis Colon Rectum. 2007;50(9):1460–1464. doi: 10.1007/s10350-007-0226-5 [DOI] [PubMed] [Google Scholar]
- 19.Carabotti M, Cuomo R, Barbara G, Pace F, Andreozzi P, Annibale B. Five-year Italian Registry of Diverticulosis and Diverticular Disease (Remad): a low progression rate of disease during the first year of follow-up. EMJ. 2017;6(1):42–44. [Google Scholar]
- 20.Horgan AF, McConnell EJ, Wolff BG, The S, Paterson C. Atypical diverticular disease: surgical results. Dis Colon Rectum. 2001;44(9):1315–1318. doi: 10.1007/BF02234790 [DOI] [PubMed] [Google Scholar]
- 21.Boostrom SY, Wolff BG, Cima RR, Merchea A, Dozois EJ, Larson DW. Uncomplicated diverticulitis, more complicated than we thought. J Gastrointest Surg. 2012;16(9):1744–1749. doi: 10.1007/s11605-012-1924-4 [DOI] [PubMed] [Google Scholar]
- 22.Mari GM, Crippa J, Borroni G, et al. Symptomatic uncomplicated diverticular disease and incidence of unexpected abscess during sigmoidectomy: a multicenter prospective observational study. Dig Surg. 2020;37(3):199–204. doi: 10.1159/000500084 [DOI] [PubMed] [Google Scholar]
- 23.Tursi A, Elisei W, Franceschi M, Picchio M, Di Mario F, Brandimarte G. The prevalence of symptomatic uncomplicated diverticular disease could be lower than expected: a single-center colonoscopy-based cohort study. Eur J Gastroenterol Hepatol. 2021;33(1S):e478. doi: 10.1097/MEG.0000000000002142 [DOI] [PubMed] [Google Scholar]
- 24.Stollman N, Raskin JB. Diverticular disease of the colon. Lancet. 2004;363(9409):631–639. doi: 10.1016/S0140-6736(04)15597-9 [DOI] [PubMed] [Google Scholar]
- 25.Peery AF, To K, Ja G, Rs S. Colonic diverticulosis is not associated with painful abdominal symptoms in a US population. Gastro Hep Adv. 2022;1(4):659–665. doi: 10.1016/j.gastha.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peery AF, Keku TO, Addamo C, et al. Colonic diverticula are not associated with mucosal inflammation or chronic gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2018;16(6):884–891.e1. doi: 10.1016/j.cgh.2017.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease--a twin study. Aliment Pharmacol Ther. 2012;35(9):1103–1107. doi: 10.1111/j.1365-2036.2012.05069.x [DOI] [PubMed] [Google Scholar]
- 28.Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology. 2013;144(4):736–742.e1; quiz e14. doi: 10.1053/j.gastro.2012.12.030 [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Sandler RS, Peery AF. Etiopathogenetic mechanisms in diverticular disease of the colon. Cell Mol Gastroenterol Hepatol. 2020;9(1):15–32. doi: 10.1016/j.jcmgh.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire LH, Handelman SK, Du X, Chen Y, Pers TH, Speliotes EK. Genome-wide association analyses identify 39 new susceptibility loci for diverticular disease. Nat Genet. 2018;50(10):1359–1365. doi: 10.1038/s41588-018-0203-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigurdsson S, Alexandersson KF, Sulem P, et al. Sequence variants in ARHGAP15, COLQ and FAM155A associate with diverticular disease and diverticulitis. Nat Commun. 2017;8:15789. doi: 10.1038/ncomms15789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldoori WH, Giovannucci EL, Rimm EB, et al. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut. 1995;36(2):276–282. doi: 10.1136/gut.36.2.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aune D, Sen A, Leitzmann MF, Norat T, Tonstad S, Vatten LJ. Body mass index and physical activity and the risk of diverticular disease: a systematic review and meta-analysis of prospective studies. Eur J Nutr. 2017;56(8):2423–2438. doi: 10.1007/s00394-017-1443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PT. Incident diverticular disease is inversely related to vigorous physical activity. Med Sci Sports Exerc. 2009;41(5):1042–1047. doi: 10.1249/MSS.0b013e318192d02d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strate LL, Morris AM. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology. 2019;156(5):1282–1298.e1. doi: 10.1053/j.gastro.2018.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbaro MR, Cremon C, Fuschi D, et al. Pathophysiology of diverticular disease: from diverticula formation to symptom generation. Int J Mol Sci. 2022;23(12):6698. doi: 10.3390/ijms23126698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622–1627. doi: 10.1016/j.cgh.2013.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldoori WH, Giovannucci EL, Rockett HR, Sampson L, Rimm EB, Willett WC. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714–719. doi: 10.1093/jn/128.4.714 [DOI] [PubMed] [Google Scholar]
- 39.Crowe FL, Balkwill A, Cairns BJ, et al. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63(9):1450–1456. doi: 10.1136/gutjnl-2013-304644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowe FL, Appleby PN, Allen NE, Key TJ. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ. 2011;343:d4131. doi: 10.1136/bmj.d4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strate LL, Liu YL, Syngal S, Aldoori WH, Giovannucci EL. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907–914. doi: 10.1001/jama.300.8.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aldoori WH, Giovannucci EL, Rimm EB, Wing AL, Trichopoulos DV, Willett WC. A prospective study of alcohol, smoking, caffeine, and the risk of symptomatic diverticular disease in men. Ann Epidemiol. 1995;5(3):221–228. doi: 10.1016/1047-2797(94)00109-7 [DOI] [PubMed] [Google Scholar]
- 43.Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98(7):997–1002. doi: 10.1002/bjs.7477 [DOI] [PubMed] [Google Scholar]
- 44.Longo S, Altobelli E, Castellini C, et al. Non-steroidal anti-inflammatory drugs and acetylsalicylic acid increase the risk of complications of diverticular disease: a meta-analysis of case-control and cohort studies. Int J Colorectal Dis. 2022;37(3):521–529. doi: 10.1007/s00384-021-04088-1 [DOI] [PubMed] [Google Scholar]
- 45.Barbara G, Scaioli E, Barbaro MR, et al. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut. 2017;66(7):1252–1261. doi: 10.1136/gutjnl-2016-312377 [DOI] [PubMed] [Google Scholar]
- 46.Scaioli E, Colecchia A, Marasco G, Schiumerini R, Festi D. Pathophysiology and therapeutic strategies for symptomatic uncomplicated diverticular disease of the colon. Dig Dis Sci. 2016;61(3):673–683. doi: 10.1007/s10620-015-3925-0 [DOI] [PubMed] [Google Scholar]
- 47.Humes DJ, Simpson J, Smith J, et al. Visceral hypersensitivity in symptomatic diverticular disease and the role of neuropeptides and low grade inflammation. Neurogastroenterol Motil. 2012;24(4):318–e163. doi: 10.1111/j.1365-2982.2011.01863.x [DOI] [PubMed] [Google Scholar]
- 48.Kvasnovsky CL, Leong LEX, Choo JM, et al. Clinical and symptom scores are significantly correlated with fecal microbiota features in patients with symptomatic uncomplicated diverticular disease: a pilot study. Eur J Gastroenterol Hepatol. 2018;30(1):107–112. doi: 10.1097/MEG.0000000000000995 [DOI] [PubMed] [Google Scholar]
- 49.Tursi A, Mastromarino P, Capobianco D, et al. Assessment of fecal microbiota and fecal metabolome in symptomatic uncomplicated diverticular disease of the colon. J Clin Gastroenterol. 2016;50(Suppl 1):S9–S12. doi: 10.1097/MCG.0000000000000626 [DOI] [PubMed] [Google Scholar]
- 50.Tursi A, Papa V, Lopetuso LR, Settanni CR, Gasbarrini A, Papa A. Microbiota composition in diverticular disease: implications for therapy. Int J Mol Sci. 2022;23(23):14799. doi: 10.3390/ijms232314799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piscopo N, Ellul P. Diverticular disease: a review on pathophysiology and recent evidence. Ulster Med J. 2020;89(2):83–88. doi: 10.1136/bmj.d4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zullo A. Medical hypothesis: speculating on the pathogenesis of acute diverticulitis. Ann Gastroenterol. 2018;31(6):747–749. doi: 10.20524/aog.2018.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golder M, Ster IC, Babu P, Sharma A, Bayat M, Farah A. Demographic determinants of risk, colon distribution and density scores of diverticular disease. World J Gastroenterol. 2011;17(8):1009–1017. doi: 10.3748/wjg.v17.i8.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altomare A, Gori M, Cocca S, et al. Impaired Colonic Contractility and Intestinal Permeability in Symptomatic Uncomplicated Diverticular Disease. J Neurogastroenterol Motil. 2021;27(2):292–301. doi: 10.5056/jnm20110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bassotti G, Villanacci V, Nascimbeni R, et al. The role of colonic mast cells and myenteric plexitis in patients with diverticular disease. Int J Colorectal Dis. 2013;28(2):267–272. doi: 10.1007/s00384-012-1554-z [DOI] [PubMed] [Google Scholar]
- 56.Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol. 2005;58(9):973–977. doi: 10.1136/jcp.2005.026112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwase H, Sadahiro S, Mukoyama S, Makuuchi H, Yasuda M. Morphology of myenteric plexuses in the human large intestine: comparison between large intestines with and without colonic diverticula. J Clin Gastroenterol. 2005;39(8):674–678. doi: 10.1097/01.mcg.0000173856.84814.37 [DOI] [PubMed] [Google Scholar]
- 58.Bassotti G, Villanacci V, Bernardini N, Dore MP. Diverticular disease of the colon: neuromuscular function abnormalities. J Clin Gastroenterol. 2016;50:S6. doi: 10.1097/MCG.0000000000000578 [DOI] [PubMed] [Google Scholar]
- 59.Mischopoulou M, D’Ambrosio M, Bigagli E, Luceri C, Farrugia G, Cipriani G. Role of macrophages and mast cells as key players in the maintenance of gastrointestinal smooth muscle homeostasis and disease. Cell Mol Gastroenterol Hepatol. 2022;13(6):1849–1862. doi: 10.1016/j.jcmgh.2022.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanas A, Abad-Baroja D, Lanas-Gimeno A. Progress and challenges in the management of diverticular disease: which treatment? Therap Adv Gastroenterol. 2018;11:1756284818789055. doi: 10.1177/1756284818789055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maconi G, Carmagnola S, Guzowski T. Intestinal Ultrasonography in the diagnosis and management of colonic diverticular disease. J Clin Gastroenterol. 2016;50(Suppl 1):S20–22. doi: 10.1097/MCG.0000000000000657 [DOI] [PubMed] [Google Scholar]
- 62.Khor S, Flum DR, Strate LL, et al. Establishing clinically significant patient-reported outcomes for diverticular disease. J Surg Res. 2021;264:20–29. doi: 10.1016/j.jss.2021.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lahat A, Fidder HH, Ben-Horin S. Development and validation of a diverticular clinical score for symptomatic uncomplicated diverticular disease after acute diverticulitis in a prospective patient cohort. Therap Adv Gastroenterol. 2020;13:1756284820913210. doi: 10.1177/1756284820913210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Vincentis A, Santonico M, Del Chierico F, et al. Gut microbiota and related electronic multisensorial system changes in subjects with symptomatic uncomplicated diverticular disease undergoing rifaximin therapy. Front Med. 2021;8:655474. doi: 10.3389/fmed.2021.655474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cuomo R. Diverticular disease register (REMAD). clinicaltrials.gov; 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03325829. Accessed December 6, 2022.
- 66.Origi M, Achilli P, Calini G, et al. The Diverticular Disease Registry (DDR Trial) by the advanced international mini-invasive surgery academy clinical research network: protocol for a multicenter, prospective observational study. Int J Surg Protoc. 2021;25(1):194–200. doi: 10.29337/ijsp.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Justin V, Uranues S, Rabl H, Fingerhut A. Quality of life in uncomplicated recurrent diverticulitis: surgical vs. conservative treatment. Sci Rep. 2020;10(1):10261. doi: 10.1038/s41598-020-67094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mari GM, Crippa J, Roscio F, et al. Quality of life after elective laparoscopic sigmoidectomy for symptomatic uncomplicated diverticular disease. Surg Laparosc Endosc Percutan Tech. 2021;31(2):193–195. doi: 10.1097/SLE.0000000000000860 [DOI] [PubMed] [Google Scholar]
- 69.Hawkins AT, Wise PE, Chan T, et al. Diverticulitis: an update from the age old paradigm. Curr Probl Surg. 2020;57(10):100862. doi: 10.1016/j.cpsurg.2020.100862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eberhardt F, Crichton M, Dahl C, et al. Role of dietary fibre in older adults with asymptomatic (AS) or symptomatic uncomplicated diverticular disease (SUDD): systematic review and meta-analysis. Maturitas. 2019;130:57–67. doi: 10.1016/j.maturitas.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 71.Carabotti M, Annibale B, Severi C, Lahner E. Role of fiber in symptomatic uncomplicated diverticular disease: a systematic review. Nutrients. 2017;9(2):161. doi: 10.3390/nu9020161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andersen JC, Bundgaard L, Elbrønd H, et al. Danish national guidelines for treatment of diverticular disease. Dan Med J. 2012;59(5):C4453. [PubMed] [Google Scholar]
- 73.Cuomo R, Barbara G, Annibale B. Rifaximin and diverticular disease: position paper of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2017;49(6):595–603. doi: 10.1016/j.dld.2017.01.164 [DOI] [PubMed] [Google Scholar]
- 74.Pietrzak A, Bartnik W, Szczepkowski M, et al. Polish interdisciplinary consensus on diagnostics and treatment of colonic diverticulosis (2015). Pol Przegl Chir. 2015;87(4):203–220. doi: 10.1515/pjs-2015-0045 [DOI] [PubMed] [Google Scholar]
- 75.Kvasnovsky CL, Bjarnason I, Donaldson AN, Sherwood RA, Papagrigoriadis S. A randomized double-blind placebo-controlled trial of a multi-strain probiotic in treatment of symptomatic uncomplicated diverticular disease. Inflammopharmacology. 2017;25(5):499–509. doi: 10.1007/s10787-017-0363-y [DOI] [PubMed] [Google Scholar]
- 76.Tursi A, Brandimarte G, Elisei W, et al. Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease – a double-blind, randomised, placebo-controlled study. Aliment Pharmacol Ther. 2013;38(7):741–751. doi: 10.1111/apt.12463 [DOI] [PubMed] [Google Scholar]
- 77.Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Mesalazine and/or Lactobacillus casei in maintaining long-term remission of symptomatic uncomplicated diverticular disease of the colon. Hepatogastroenterology. 2008;55(84):916–920. [PubMed] [Google Scholar]
- 78.Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Mesalazine and/or lactobacillus casei in preventing recurrence of symptomatic uncomplicated diverticular disease of the colon: a prospective, randomized, open-label study. J Clin Gastroenterol. 2006;40(4):312–316. doi: 10.1097/01.mcg.0000210092.77296.6d [DOI] [PubMed] [Google Scholar]
- 79.Annibale B, Maconi G, Lahner E, Giorgi F, Cuomo R. Efficacy of Lactobacillus paracasei sub. paracasei F19 on abdominal symptoms in patients with symptomatic uncomplicated diverticular disease: a pilot study. Minerva Gastroenterol Dietol. 2011;57:13–22. [PubMed] [Google Scholar]
- 80.Lahner E, Esposito G, Zullo A, et al. High-fibre diet and Lactobacillus paracasei B21060 in symptomatic uncomplicated diverticular disease. World J Gastroenterol. 2012;18(41):5918–5924. doi: 10.3748/wjg.v18.i41.5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamiki P, Tsuchiya J, Pathak S, et al. Probiotics in diverticular disease of the colon: an open label study. J Gastrointestin Liver Dis. 2010;19(1):31–36. [PubMed] [Google Scholar]
- 82.Lahner E, Bellisario C, Hassan C, Zullo A, Esposito G, Annibale B. Probiotics in the treatment of diverticular disease. a systematic review. J Gastrointestin Liver Dis. 2016;25(1):79–86. doi: 10.15403/jgld.2014.1121.251.srw [DOI] [PubMed] [Google Scholar]
- 83.Hall J, Hardiman K, Lee S, et al. The American Society of colon and rectal surgeons clinical practice guidelines for the treatment of left-sided colonic diverticulitis. Dis Colon Rectum. 2020;63(6):728–747. doi: 10.1097/DCR.0000000000001679 [DOI] [PubMed] [Google Scholar]
- 84.Khan RMA, Ali B, Hajibandeh S, Hajibandeh S. Effect of mesalazine on recurrence of diverticulitis in patients with symptomatic uncomplicated diverticular disease: a meta-analysis with trial sequential analysis of randomized controlled trials. Colorectal Dis. 2018;20(6):469–478. doi: 10.1111/codi.14064 [DOI] [PubMed] [Google Scholar]
- 85.Iannone A, Ruospo M, Wong G, et al. Mesalazine for people with diverticular disease: a systematic review of randomized controlled trials. Can J Gastroenterol Hepatol. 2018;2018:5437135. doi: 10.1155/2018/5437135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Picchio M. Mesalazine to treat symptomatic uncomplicated diverticular disease and to prevent acute diverticulitis occurrence. A systematic review with meta-analysis of randomized, placebo-controlled trials. J Gastrointest Liver Dis. 2018;27(3). doi: 10.15403/jgld.2014.1121.273.pic [DOI] [PubMed] [Google Scholar]
- 87.Carabotti M, Annibale B. Treatment of diverticular disease: an update on latest evidence and clinical implications. Drugs Context. 2018;7:212526. doi: 10.7573/dic.212526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maconi G, Barbara G, Bosetti C, Cuomo R, Annibale B. Treatment of diverticular disease of the colon and prevention of acute diverticulitis: a systematic review. Dis Colon Rectum. 2011;54(10):1326–1338. doi: 10.1097/DCR.0b013e318223cb2b [DOI] [PubMed] [Google Scholar]
- 89.Bianchi M, Festa V, Moretti A, et al. Meta-analysis: long-term therapy with rifaximin in the management of uncomplicated diverticular disease. Aliment Pharmacol Ther. 2011;33(8):902–910. doi: 10.1111/j.1365-2036.2011.04606.x [DOI] [PubMed] [Google Scholar]
- 90.Colecchia A, Vestito A, Pasqui F, et al. Efficacy of long term cyclic administration of the poorly absorbed antibiotic Rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol. 2007;13(2):264–269. doi: 10.3748/wjg.v13.i2.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Comparato G, Fanigliulo L, Cavallaro LG, et al. Prevention of complications and symptomatic recurrences in diverticular disease with mesalazine: a 12-month follow-up. Dig Dis Sci. 2007;52(11):2934–2941. doi: 10.1007/s10620-007-9766-8 [DOI] [PubMed] [Google Scholar]
- 92.Di Mario F, Aragona G, Leandro G, et al. Efficacy of mesalazine in the treatment of symptomatic diverticular disease. Dig Dis Sci. 2005;50(3):581–586. doi: 10.1007/s10620-005-2478-z [DOI] [PubMed] [Google Scholar]
- 93.D’Incà R, Pomerri F, Vettorato MG, et al. Interaction between rifaximin and dietary fibre in patients with diverticular disease. Aliment Pharmacol Ther. 2007;25(7):771–779. doi: 10.1111/j.1365-2036.2007.03266.x [DOI] [PubMed] [Google Scholar]
- 94.Latella G, Pimpo MT, Sottili S, et al. Rifaximin improves symptoms of acquired uncomplicated diverticular disease of the colon. Int J Colorectal Dis. 2003;18(1):55–62. doi: 10.1007/s00384-002-0396-5 [DOI] [PubMed] [Google Scholar]
- 95.Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. A multicentre double-blind placebo-controlled trial. Aliment Pharmacol Ther. 1995;9(1):33–39. doi: 10.1111/j.1365-2036.1995.tb00348.x [DOI] [PubMed] [Google Scholar]
- 96.Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin on symptoms of uncomplicated diverticular disease of the colon. A pilot multicentre open trial. Diverticular Disease Study Group. Ital J Gastroenterol. 1992;24(8):452–456. [PubMed] [Google Scholar]
- 97.Tursi A, Brandimarte G, Daffinà R. Long-term treatment with mesalazine and rifaximin versus rifaximin alone for patients with recurrent attacks of acute diverticulitis of colon. Dig Liver Dis. 2002;34(7):510–515. doi: 10.1016/s1590-8658(02)80110-4 [DOI] [PubMed] [Google Scholar]
- 98.Di Mario F, Miraglia C, Cambiè G, et al. Long-term efficacy of rifaximin to manage the symptomatic uncomplicated diverticular disease of the colon. J Investig Med. 2019;67(4):767–770. doi: 10.1136/jim-2018-000901 [DOI] [PubMed] [Google Scholar]
- 99.Stallinger S, Eller N, Högenauer C. Non-interventional study evaluating efficacy and tolerability of rifaximin for treatment of uncomplicated diverticular disease. Wien Klin Wochenschr. 2014;126(1–2):9–14. doi: 10.1007/s00508-013-0447-7 [DOI] [PubMed] [Google Scholar]
- 100.D’Amico F, Fiorini G, Tursi A, et al. Efficacy of a new nutraceutical formulation in patients with Symptomatic Uncomplicated Diverticular Disease (SUDD): a prospective observational study. JGLD. 2020;28:49–52. doi: 10.15403/jgld-560 [DOI] [PubMed] [Google Scholar]
- 101.Carabotti M, Cuomo R, Barbara G, et al. Demographic and clinical features distinguish subgroups of diverticular disease patients: results from an Italian nationwide registry. United Eur Gastroenterol J. 2018;6(6):926–934. doi: 10.1177/2050640618764953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Friso Lock* J, Galata* C, Reißfelder C, Ritz JP, Schiedeck T, Germer CT. The indications for and timing of surgery for diverticular disease. Dtsch Arztebl Int. 2020;117(35–36):591–596. doi: 10.3238/arztebl.2020.0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mari GM, Gaia S, Andrea C, et al. Recurrent diverticulitis after elective surgery. Int J Colorectal Dis. 2022;37(10):2149–2155. doi: 10.1007/s00384-022-04248-x [DOI] [PubMed] [Google Scholar]
- 104.van de Wall BJM, Draaisma WA, van Iersel JJ, Consten ECJ, Wiezer MJ, Broeders IAMJ. Elective resection for ongoing diverticular disease significantly improves quality of life. Dig Surg. 2013;30(3):190–197. doi: 10.1159/000346482 [DOI] [PubMed] [Google Scholar]