Abstract
Aims
Levels of growth differentiation factor 15 (GDF-15), a cytokine secreted in response to cellular stress and inflammation, have been associated with multiple types of cardiovascular (CV) events. However, its comparative prognostic performance across different presentations of atherosclerotic cardiovascular disease (ASCVD) remains unknown.
Methods and results
An individual patient meta-analysis was performed using data pooled from eight trials including 53 486 patients. Baseline GDF-15 concentration was analyzed as a continuous variable and using established cutpoints (<1200 ng/L, 1200–1800 ng/L, > 1800 ng/L) to evaluate its prognostic performance for CV death/hospitalization for heart failure (HHF), major adverse cardiovascular events (MACE), and their components using Cox models adjusted for clinical variables and established CV biomarkers. Analyses were further stratified on ASCVD status: acute coronary syndrome (ACS), stabilized after recent ACS, and stable ASCVD. Overall, higher GDF-15 concentration was significantly and independently associated with an increased rate of CV death/HHF and MACE (P < 0.001 for each). However, while GDF-15 showed a robust and consistent independent association with CV death and HHF across all presentations of ASCVD, its prognostic association with future myocardial infarction (MI) and stroke only remained significant in patients stabilized after recent ACS or with stable ASCVD [hazard ratio (HR): 1.24, 95% confidence interval (CI): 1.17–1.31 and HR: 1.16, 95% CI: 1.05–1.28 for MI and stroke, respectively] and not in ACS (HR: 0.98, 95% CI: 0.90–1.06 and HR: 0.87, 95% CI: 0.39–1.92, respectively).
Conclusion
Growth differentiation factor 15 consistently adds prognostic information for CV death and HHF across the spectrum of ASCVD. GDF-15 also adds prognostic information for MI and stroke beyond clinical risk factors and cardiac biomarkers but not in the setting of ACS.
Keywords: GDF-15, Biomarker, ASCVD, MI, Stroke
Structured Graphical Abstract
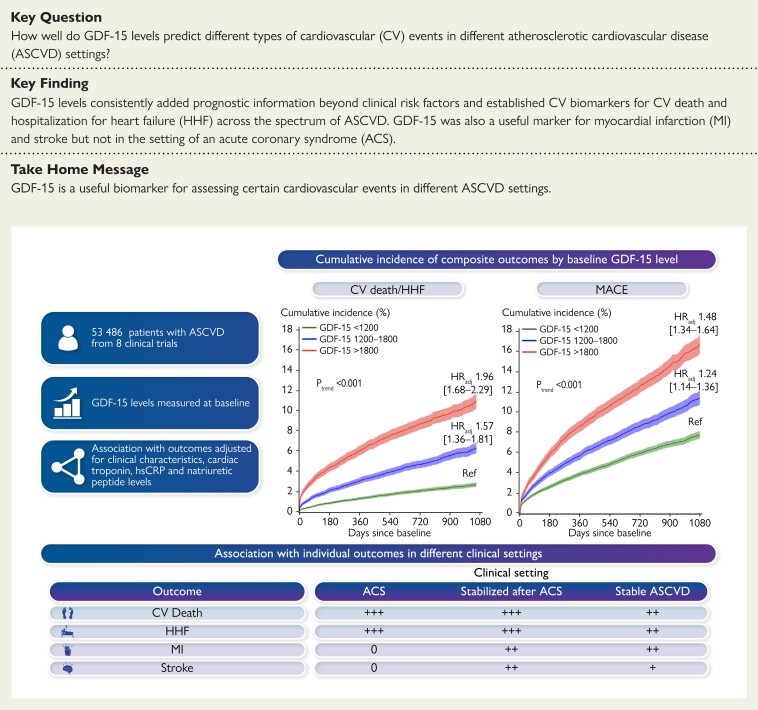
Structured Graphical Abstract.
Growth differentiation factor 15 added prognostic information beyond clinical risk factors and cardiac biomarkers for CV death and HHF across the spectrum of ASCVD and for MI and stroke outside of ACS.
See the editorial comment for this article ‘Growth differentiation factor 15: a biomarker searching for an indication’, by C. Mueller et al., https://doi.org/10.1093/eurheartj/ehac681.
Introduction
Circulating protein biomarkers can be used to facilitate diagnosis, assess prognosis, guide intervention, and help manage cardiovascular (CV) diseases.1 Growth differentiation factor 15 (GDF-15) is a member of the transforming growth factor beta superfamily that is weakly expressed in most organs under healthy conditions. In human disease, GDF-15 is strongly upregulated in response to hypoxic, mechanical, oxidative or inflammatory stress.2 Therefore, GDF-15 has been explored as a prognostic biomarker in multiple disease entities, including ischaemic heart disease, heart failure (HF), atrial fibrillation, diabetes mellitus, and cancer.
In seminal studies, higher GDF-15 concentrations in atherosclerotic cardiovascular disease (ASCVD) were associated with atherosclerotic risk factors, CV disease burden, and HF.3–7 Prior evidence has established a strong direct association between GDF-15 concentration and the future risk of death and HF. In contrast, the relationship between GDF-15 and future ischaemic events has been inconsistent across studies of patients with ASCVD,8–10 leaving its value as a prognostic marker for future ischaemic events uncertain.
Therefore, we performed an individual patient meta-analysis in 53 486 patients with ASCVD with data pooled across 8 clinical trials to evaluate the prognostic performance of GDF-15 with respect to various CV outcomes across differing presenting syndromes of ASCVD.
Methods
Patient-level data from 8 trials of patients with unstable or stable ASCVD with available measurements of baseline GDF-15 were pooled for the analysis: Aggrastat-to-Zocor (A2Z) Thrombolysis in Myocardial Infarction (TIMI) 21,11 Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE IT)-TIMI 22,6,7 Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes (MERLIN)-TIMI 36,12 Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome (EARLY-ACS)-TIMI 39,13 Stabilization of pLaques usIng Darapladib (SOLID)-TIMI 52,14 Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE),15 Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin (PEGASUS)-TIMI 54,16 and Further cardiovascular Outcomes Research with PCSK9 Inhibition in subjects with Elevated Risk (FOURIER)-TIMI 59.17 The details of each trial are included in Supplementary material online, Table S1. These multicentre randomized controlled trials enrolled patients across the spectrum of ASCVD, from patients presenting with an acute coronary syndrome (ACS), to those stabilized after a recent ACS, to those with stable ASCVD. Patients stabilized after ACS represented in these trials included patients clinically stable for at least 24–48 h after acute presentation with ACS to no more than 30 days after hospitalization for ACS. The protocol, including the biomarker substudy, for each trial was approved by the Institutional Review Board or Ethics Committee of each participating center and all study participants provided written informed consent.
Biomarker samples
In each trial, baseline blood samples were collected at the time of randomization and were centrifuged on site. Isolated serum or plasma was stored at −20°C or colder at the site and was shipped frozen to the TIMI Clinical Trials Laboratory (Boston, MA) where samples were stored at −80°C or colder. The assay used for determination of GDF-15 concentration in each trial is specified in Supplementary material online, Table S2.18 Other CV biomarkers, including brain natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein (hsCRP), and cardiac troponin I were measured using established commercially available immunoassays (Supplementary material online, Table S2).
Endpoints
On the basis of prior work with GDF-15 in patients with ASCVD,4,7,19 we focused on the following CV events as our outcomes of interest for this study: a composite of CV death or hospitalization for HF (HHF) and its individual endpoints as well as major adverse cardiovascular events [MACE: CV death, myocardial infarction (MI), or stroke] and its components as available. As a further exploratory analysis, we also examined the association of GDF-15 with non-CV death. All personnel performing biomarker testing were blinded to clinical outcomes.
Statistical analyses
Demographics and other baseline characteristics are reported as median and 25th and 75th percentiles for continuous variables and as counts and percentages for categorical variables. The χ2 test was used for categorical variables and the Kruskal–Wallis test was used for continuous variables to compare differences among groups. A general linear model with forward selection method with a threshold value of 0.05 was used to identify the baseline characteristics that were independently and most strongly associated with GDF-15 levels.
GDF-15 concentration was analyzed both as a log-transformed continuous variable and categorically using previously established cutpoints (low range: < 1200 ng/L, intermediate range: 1200–1800 ng/L, and high range: > 1800 ng/L).3,7 The cumulative incidence function (CIF) was used for estimation of the incidence of the occurrence of CV death or HHF (or MACE) while accounting for a competing risk of non-CV death. For a single non-fatal endpoint such as MI or stroke or HHF, CIF was used while accounting for a competing risk of all-cause death.20 The association between GDF-15 concentration and each clinical outcome was analyzed using Cox proportional-hazards modeling to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) by treating those subjects who experience a competing event without having had an event of interest as being censored at the time of the occurrence of the competing event. These regression coefficients here onward can be interpreted as the relative effect of the corresponding covariate on the relative increase in the rate of occurrence of the event of interest in subjects who are currently event free.21
In the main analysis, the Cox regression models were adjusted for trial, age, sex, body mass index (BMI), current smoking, hypertension, diabetes mellitus, hypercholesterolaemia (defined as either low-density lipoprotein cholesterol ≥100 mg/dL or apolipoprotein B ≥ 90 mg/dL), estimated glomerular filtration rate (eGFR), history of MI, history of stroke or transient ischaemic attack (TIA), history of HF, prior coronary artery bypass grafting or percutaneous coronary intervention and index event [not ACS vs. non-ST-elevation ACS (NSTE-ACS) vs. ST-elevation MI (STEMI)]. In a secondary analysis, the Cox regression models were also adjusted for hsCRP, cardiac troponin I, and BNP or NT-proBNP in addition to the variables used in the main analysis. NT-proBNP and BNP, as well as cardiac troponin I were normalized relative to the 99th percentile of upper reference limit of normal population reported by manufacturers (Supplementary material online, Table S2). All biomarkers were further log-transformed. In sensitivity analyses, the five trials that had NT-proBNP and the three trials that had BNP were analyzed separately, which enabled us to model the natriuretic peptide using the raw values. In addition, the evaluation of the incremental value of the GDF-15 when added to clinical information and established biomarkers was tested by the use of the likelihood ratio test. Concordance indices were also examined across these models.
We categorized each of the trials based on the setting of enrollment of their target patient population into one of three presentations of ASCVD: (i) acute ACS; (ii) stabilized after recent ACS (within the past 30 days); and (iii) stable ASCVD to permit analysis of the prognostic performance of GDF-15 in each of these presentations.
Statistical analyses were performed using SAS System V9.4 (SAS Institute, Cary, NC). Cumulative incidence function and 95% CI were estimated in R software (www.r-project.org) using the cuminc function in the cmprsk package. All tests were two-sided and a P-value of <0.05 was considered significant.
Results
A total of 53 486 patients with ASCVD from 8 clinical trials were included in the analysis. The median follow-up was 2.2 years. The demographics of the overall combined population and each randomized controlled trial population are summarized in Table 1 and Supplementary material online, Table S1, respectively. Overall, the median age was 64 [interquartile range (IQR): 56–70) years, 25.1% were female, 49.7% had history of MI, and 11.8% had history of stroke/TIA. Patients with higher GDF-15 levels tended to be women, older, and have a higher prevalence of ASCVD risk factors, such as diabetes mellitus, hypertension, and worse eGFR, and were more likely to have a history of MI, stroke/TIA, coronary revascularization, or HF. The top three clinical characteristics that were independently associated with higher baseline values of GDF-15 were history of diabetes, age, and baseline eGFR. The correlation between GDF-15 levels and age was 0.37 (P < 0.0001). The correlations with the other CV biomarkers were all weak to moderate, although all statistically significant (P < 0.001), given the very large sample size (r = 0.10 for troponin; r = 0.15 for hsCRP; r = 0.31 for NT-proBNP; r = 0.18 for BNP). Median (IQR) GDF-15 levels in patients with ACS, stabilized after a recent ACS, and with stable ASCVD were 1058.0 (790.5–1478.1), 1104.0 (804.2–1569.0), and 1176.0 (858.6–1694.0) ng/L, respectively (Supplementary material online, Figure S1).
Table 1.
Baseline demographics
| Characteristics | Total (N = 53 486) |
Enrollment GDF-15 concentration | ||
|---|---|---|---|---|
| <1200 ng/L (N = 28 928) | 1200–1800 ng/L (N = 13 891) | >1800 ng/L (N = 10 667) | ||
| Age (years), median (IQR) | 64.0 (56.0–70.0) | 61.0 (54.0–67.0) | 66.0 (59.0–72.0) | 68.0 (62.0–74.0) |
| Female sex | 13 397 (25.1) | 6787 (23.5) | 3553 (25.6) | 3057 (28.7) |
| BMI (kg/m2), median (IQR) | 28.4 (25.6–31.9) | 28.4 (25.7–31.6) | 28.4 (25.6–32.0) | 28.7 (25.5–32.5) |
| Current smoker | 13 967 (26.1) | 7648 (26.5) | 3725 (26.8) | 2594 (24.3) |
| eGFR (mL/min/1.73 m2), median (IQR) | 74.0 (62.6–86.7) | 78.2 (68.2–90.0) | 71.5 (60.5–83.4) | 62.1 (49.1–75.9) |
| Hypertension | 38 488 (72.0) | 19 306 (66.8) | 10 422 (75.0) | 8760 (82.2) |
| Diabetes mellitus | 16 052 (30.0) | 5831 (20.2) | 4569 (32.9) | 5652 (53.0) |
| Dyslipidemia | 22 117 (42.6) | 13 059 (46.5) | 5515 (40.8) | 3543 (34.2) |
| History of MI | 26 537 (49.7) | 13 943 (48.2) | 6955 (50.1) | 5639 (52.9) |
| History of stroke/TIA | 6327 (11.8) | 2681 (9.3) | 1881 (13.5) | 1765 (16.6) |
| History of HF | 9237 (18.5) | 4321 (16.6) | 2421 (18.2) | 2495 (23.8) |
| Prior CABG/PCI | 29 870 (55.9) | 15 568 (53.9) | 7891 (56.8) | 6411 (60.2) |
Data are shown as numbers and percentage, unless indicated otherwise.
BMI, body mass index; eGFR, estimated glomerular filtration rate; GDF-15, growth differentiation factor 15; IQR, interquartile range; MI, myocardial infarction; TIA, transient ischaemic attack; HF, heart failure; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting. P for trend <0.0001 for each baseline characteristic across GDF-15 categories.
Association between GDF-15 concentration and CV events
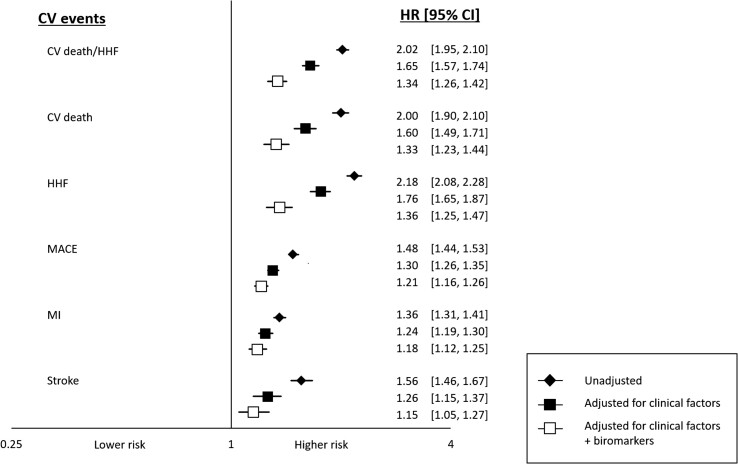
Overall, a higher GDF-15 concentration was associated with a higher rate for all of the CV events examined, and this association remained significant after adjusting for clinical factors and established CV biomarkers (Figure 1, P < 0.005 for each). Particularly notable was the independent association between GDF-15 levels and CV death or HHF, with an adjusted HR per 1 SD log GDF-15 (which equates to rate increase seen with GDF-15 increasing by 2165 ng/L) of 1.34 (95% CI: 1.26–1.42). The results for each of the components were very similar with HR of 1.33 (95% CI: 1.23–1.44, P < 0.0001) and 1.36 (95% CI: 1.25–1.47, P < 0.0001), respectively, for CV death and HHF. In sensitivity analyses, the results for GDF-15 were similar for hospitalization for HF when data were analyzed separately for patients in whom the natriuretic peptide measured was NT-proBNP (adjusted HR per 1 SD log GDF-15 of 1.32 (95% CI: 1.21–1.44), P < 0.0001) or BNP [adjusted HR per 1 SD log GDF-15 of 1.48 (95% CI: 1.24–1.75), P < 0.0001]. The adjusted HR for MACE was 1.21 (95% CI: 1.16–1.26) and the HRs for MI and for stroke were 1.18 (95% CI: 1.12–1.25, P < 0.0001) and 1.15 (95% CI: 1.05–1.27, P = 0.004), respectively. Of note, GDF-15 levels were also associated with an increased rate of non-CV death (HR: 1.73, 95% CI: 1.57–1.90, P < 0.0001). There was no heterogeneity in the strength of the prognostic association by treatment arm (Pinteraction 0.63 for CV death/HHF and 0.74 for MACE).
Figure 1.
Association of baseline log-transformed growth differentiation factor 15 (per 1 standard deviation) with cardiovascular outcomes. MACE, major adverse cardiac events; CV, cardiovascular; HHF, hospitalization for heart failure; MI, myocardial infarction; HR, hazard ratio; CI, confidence interval. Hazard ratio adjusted for clinical factors and other biomarkers. An increase in 1 standard deviation of log growth differentiation factor 15 was independently associated with all the cardiovascular endpoints (P-value all <0.005).
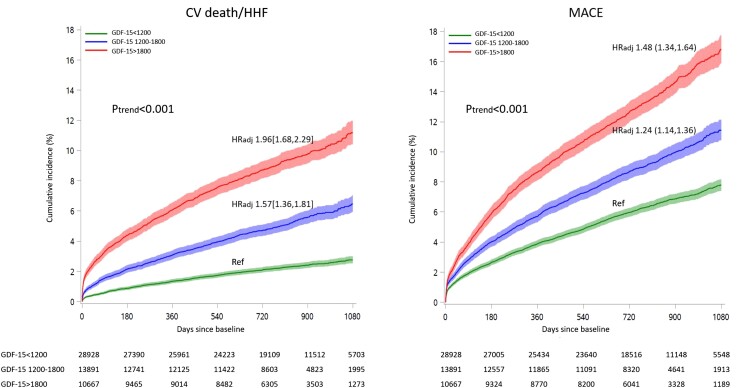
In the pooled categorical analysis, both intermediate and high GDF-15 values were independently associated with all of the prespecified endpoints, with a graded increase in event rate with a higher range of GDF-15 (Ptrend < 0.001 for each; Figure 2, Supplementary material online, Figure S2). At 3 years, the cumulative incidences of CV death/HF in patients with GDF-15 levels <1200 ng/L, 1200–1800 ng/L, and >1800 ng/L were 2.8%, 6.5%, and 11.2%, respectively. Likewise for MACE, the cumulative incidences were 7.8%, 11.4%, and 16.8%, respectively. The differences in event rates appeared to emerge within 6 months and continued to diverge throughout the studied period.
Figure 2.
Growth differentiation factor 15 concentration (categorical) and cardiovascular events. Cumulative incidence curves of cardiovascular events by growth differentiation factor 15 categories while treating non-cardiovascular death as a competing risk. CV, cardiovascular; HHF, hospitalization for heart failure; MACE, major adverse cardiac events; HRadj, adjusted hazard ratio; Ref, reference. Each hazard ratio is compared with the low range growth differentiation factor 15 (growth differentiation factor 15 < 1200ng/L) after adjusting for clinical factors and established cardiovascular biomarkers.
Different presenting syndromes of atherosclerotic vascular disease
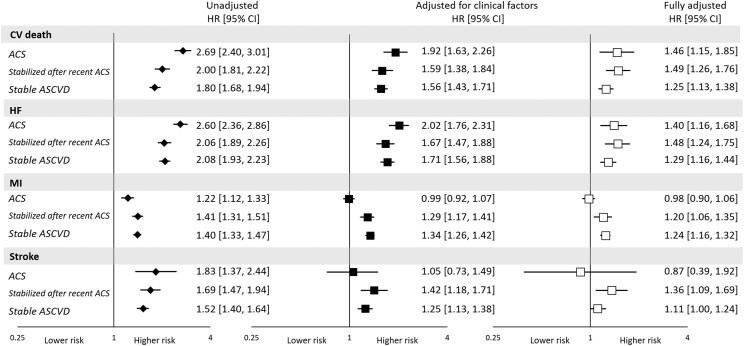
An elevated concentration of GDF-15 was consistently associated with CV death, HHF, and the composite regardless of the presenting ASCVD syndrome (P < 0.05 for all), with a numerically lesser magnitude of association in patients with stable ASCVD (Figure 3). In contrast, the association with rate of MI and stroke differed by ASCVD syndrome. In patients with ACS, after adjusting for the specified clinical factors and established CV biomarkers, GDF-15 was not associated with the rate of MI (HR: 0.98, 95% CI: 0.90–1.06; Figure 3). In contrast, in patients stabilized after recent ACS [median time (IQR) from ACS was 8.0 (4.0–17.0) days] or with stable ASCVD, GDF-15 was independently associated with the rate of future MI beyond clinical and established CV biomarkers (HR: 1.24, 95% CI: 1.17–1.31), with a P-interaction of <0.001 compared with the association seen in ACS. Separately considering those patients stabilized after recent ACS and with stable ASCVD, the HRs (95% CI) were 1.20 (1.06–1.35) and 1.24 (1.16–1.32), respectively. There was a similar pattern for rate of future stroke, with no association in patients with ACS (HR: 0.87, 95% CI: 0.39–1.92), whereas there was a significant association in the pooled cohort of patients stabilized after recent ACS or with stable ASCVD (HR: 1.16, 95% CI: 1.05–1.28); the interaction P-value was 0.67, likely reflecting the broader CIs for rate of stroke in patients with ACS. In the separate subgroup of patients stabilized after recent ACS and with stable ASCVD, the HR (95% CI) were 1.36 (1.09–1.69) and 1.11 (1.00–1.24), respectively.
Figure 3.
Association between log-transformed growth differentiation factor 15 (per 1 standard deviation) and cardiovascular events stratified by trial category. ACS, acute coronary syndrome; SD, standard deviation; ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; HHF, hospitalization for heart failure; MI, myocardial infarction; HR, hazard ratio. Hazard ratios are adjusted for clinical factors and other established cardiovascular biomarkers. Growth differentiation factor 15 is independently associated with myocardial infarction and stroke with exception among patients withacute coronary syndrome. *P < 0.05 except for MI in the acute acute coronary syndrome cohort after adjustment for clinical factors (P = 0.88), and after adjustment for clinical and cardiovascular biomarkers (P = 0.58) and stroke in the ACS cohort after adjustment for clinical factors (P = 0.80) and clinical and cardiovascular biomarkers (P = 0.72), in the stable ASCVD cohort after adjustment for clinical and cardiovascular biomarkers (P = 0.052).
Similar to the analysis of GDF-15 as a continuous variable, the a priori categories of GDF-15 showed a consistent pattern of higher rate of CV death, and HHF across the different presenting ASCVD syndromes in those with higher GDF-15 levels (Supplementary material online, Table S3). In contrast, higher GDF-15 levels were associated with an increased rate of MI and stroke in patients stabilized after recent ACS and with stable ASCVD but not in those with ACS (Supplementary material online, Figure S3).
The C-indices for the full clinical model and the full clinical model plus the established CV biomarkers with and without GDF-15 are shown in Supplementary material online, Table S4.
Discussion
In this large individual patient meta-analysis of pooled data from multiple cohorts of patients across different presenting ASCVD syndromes, we observed strong associations between GDF-15 and multiple types of CV events. The strength of our study is its robust samples size with >34 000 patients with stable ASCVD, > 8000 patients recently stabilized after an ACS, and >10 000 patients with ACS, which enabled us to provide new insight into the prognostic capacity of GDF-15 across different presentations of ASCVD and examine clinically meaningful cutpoints that can be referenced for prognostic assessment in these vulnerable populations.
The main finding of our analysis is that prognostic associations of GDF-15 appear to differ among presentations of ASCVD. While GDF-15 showed a robust and consistent independent association with CV death and HHF across all presentations of ASCVD, an association between GDF-15 and the rate of future MI and stroke independent of clinical characteristics and established CV biomarkers was not apparent when measured early after an ACS (Structured Graphical Abstract).
GDF-15 and cardiovascular outcomes
GDF-15 first emerged as a promising biomarker for prognostication of death and HHF in apparently healthy individuals in the community and in patients with ACS or chronic HF.22–25 GDF-15 and BNP (or NT-proBNP) share some of the same pathophysiological triggers, including hemodynamic overload and myocardial stress. However, unlike the natriuretic peptides, which are cardiac-specific hormones secreted from the heart, various stressors induce GDF-15 also in endothelial cells, adipocytes, macrophages, and in human atherosclerotic plaque tissue.26,27 We posit that this very fact enables GDF-15 to add significant prognostic information to the cardiomyocyte-derived natriuretic peptide. Moreover, experimental data have raised a possible mediating role of GDF-15 in glucose metabolism.28 Therefore, it was reasonable to hypothesize that a high serum concentration of GDF-15 might be associated with a higher rate of MI or stroke. However, in contrast to the strong relationship between GDF-15 with HHF, CV death, and all-cause mortality,3,29 the association between GDF-15 and ischemic events has been less certain.3,4,9,29,30 Some studies in patients with stable ASCVD or ACS showed GDF-15 as an independent predictor of subsequent MI and stroke, 8,31 whereas other studies that enrolled patients with ACS, those stabilized after ACS, or those with stable angina found no such associations.4,9,32 However, these studies were relatively small.
Unlike troponin or BNP, acute myocardial injury induces only a modest increase of GDF-15 in the circulation (approximately 5% median increase), which occurs in the first week after an uncomplicated ACS event, and gradually returns back to baseline within 30 days.32,33 This observation is supported by studies that suggest only a weak association between GDF-15 and extent of myocardial damage.3,19,33 Similarly, HF studies have also reported low intraindividual and a higher interindividual biological variation with GDF-15.34,35
Based on these previous pathophysiological findings which support GDF-15 as a marker of integrated underlying CV disease burden, rather than a marker of acute instability of ASCVD, we analyzed our clinical data by different presentation of ASCVD. Importantly, our data showed a strong relationship between GDF-15 and CV death and HHF across the entire spectrum of ASCVD, whereas GDF-15 levels were associated with the risk of future MI and stroke in patients stabilized after recent ACS and with stable ASCVD but not in those with ACS. While demonstrating this important epidemiology, our results do not directly interrogate the pathobiology underlying this observation. We can speculate that in the acute presentation of ACS factors related to nature of the culprit lesion may dominate the risk of recurrent ischemic events, whereas with time other underlying chronic factors better reflected by GDF-15 predominate as drivers of the risk of new ischemic events. Also, it is possible that in the acute phase GDF-15 elevation may be protective in manner that counterbalances adverse processes reflected by GDF-15 expression. Given the paucity of established biomarkers that robustly evaluate the risk of ischemic events in stable ASCVD, our results provide important evidence suggesting GDF-15 as a potentially useful marker that is incremental to clinical factors and established cardiac markers. For example, GDF-15 is now approved in Europe as an aid in risk stratification of patients with ACS. Looking forward, proteomic and metabolomic profiling may identify additional useful biomarkers.36
Limitations
This analysis was performed in selected patients participating in clinical trials rather than from the general population and, therefore, there are potential limitations to its generalizability. This limitation is particularly relevant to GDF-15 as a biomarker because GDF-15 is elevated in association with other disease processes, such as cancer, that are seen in clinical practice but would be excluded from patients selected for enrollment in clinical trials. The median follow-up was only 2.2 years. While it is important to consider whether analytical imprecision or lack of reproducibility might adversely affect the prognostic performance of GDF-15 in some cohorts, prior studies of the analytical precision of GDF-15 demonstrate within-laboratory and between-laboratory imprecision <10% over relevant concentrations.18 Moreover, reported low biological variation of GDF-15 (over repeated measurements in the same individual) supports that it may be useful for prognostication in individual subjects.37 It is not known whether GDF-15 is useful in identifying groups of patients who will benefit from specific cardiac interventions. Although there was statistically significant heterogeneity in the HR for MI with higher GDF-15 levels in patients with ACS vs. in those without, it should be noted that there were only three ACS trials in this dataset and validation of this observation in additional data sets will be helpful.
We did not adjust for multiple hypothesis testing. The P-values for the associations between GDF-15 levels and the two primary composite endpoints, CV death/HHF and MACE, were both <0.0001. Additional analyses, including components of the composite endpoints and subgroups of the overall population, were supportive analyses. Finally, although we have adjusted for clinical factors and other CV biomarkers, residual confounders may exist due to incompletely measured or unmeasured factors.
Conclusion
In this large study across multiple populations with ASCVD, we found that GDF-15 is a robust biomarker for assessing the likelihood of CV death and HHF across the full spectrum of patients with ASCVD, regardless of the type of presentation. GDF-15 is also a useful marker for MI and stroke beyond established clinical factors and biomarkers in patients with stabilized or stable ASCVD but not in the acute setting of ACS.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Contributor Information
Eri Toda Kato, Department of Cardiovascular Medicine and Department of Clinical Laboratory, Kyoto University Hospital, 54 Shogoin-kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan.
David A Morrow, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Jianping Guo, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
David D Berg, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Michael A Blazing, Duke Clinical Research Institute, Duke University, 300 W. Morris Street, Durham, NC 27701, USA.
Erin A Bohula, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Marc P Bonaca, Cardiovascular Division, Department of Medicine, University of Colorado School of Medicine, 13001 East 17th PIace, Aurora, CO 80045, USA.
Christopher P Cannon, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
James A de Lemos, Division of Cardiology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-9003, USA.
Robert P Giugliano, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Petr Jarolim, Department of Pathology, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Tibor Kempf, Division of Molecular and Translational Cardiology, Department of Cardiology and Angiology, Hannover Medical School, Carl-Neuberg-Str, 1. D-30625 Hannover, Germany.
L Kristin Newby, Duke Clinical Research Institute, Duke University, 300 W. Morris Street, Durham, NC 27701, USA.
Michelle L O’Donoghue, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Marc A Pfeffer, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Nader Rifai, Department of Pathology, Boston Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115, USA.
Stephen D Wiviott, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Kai C Wollert, Division of Molecular and Translational Cardiology, Department of Cardiology and Angiology, Hannover Medical School, Carl-Neuberg-Str, 1. D-30625 Hannover, Germany.
Eugene Braunwald, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Marc S Sabatine, TIMI Study Group, 60 Fenwood Road, 7th floor, Boston, MA 02115, USA; Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA.
Data availability
The study data cannot be shared but we encourage individuals interested in collaboration to contact us directly.
References
- 1. Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation 2007;115:949–952. 10.1161/CIRCULATIONAHA.106.683110 [DOI] [PubMed] [Google Scholar]
- 2. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem 2017;63:140–151. 10.1373/clinchem.2016.255174 [DOI] [PubMed] [Google Scholar]
- 3. Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation 2007;115:962–971. doi: CIRCULATIONAHA.106.650846 [pii] 10.1161/CIRCULATIONAHA.106.650846 [DOI] [PubMed] [Google Scholar]
- 4. Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation 2007;116:1540–1548. doi: CIRCULATIONAHA.107.697714 [pii] 10.1161/CIRCULATIONAHA.107.697714 [DOI] [PubMed] [Google Scholar]
- 5. Eggers KM, Kempf T, Allhoff T, Lindahl B, Wallentin L, Wollert KC. Growth-differentiation factor-15 for early risk stratification in patients with acute chest pain. Eur Heart J 2008;29:2327–2335. 10.1093/eurheartj/ehn339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kempf T, Wollert KC. Growth differentiation factor-15: a new biomarker in cardiovascular disease. Herz 2009;34:594–599. 10.1007/s00059-009-3317-3 [DOI] [PubMed] [Google Scholar]
- 7. Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, et al. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscl Thromb Vasc Biol 2011;31:203–210. 10.1161/ATVBAHA.110.213512 [DOI] [PubMed] [Google Scholar]
- 8. Schopfer DW, Ku IA, Regan M, Whooley MA. Growth differentiation factor 15 and cardiovascular events in patients with stable ischemic heart disease (the heart and soul study). Am Heart J 2014;167:186–192.e1. 10.1016/j.ahj.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 9. Kempf T, Sinning JM, Quint A, Bickel C, Sinning C, Wild PS, et al. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: results from the AtheroGene study. Circ Cardiovasc Genet 2009;2:286–292. 10.1161/CIRCGENETICS.108.824870 [DOI] [PubMed] [Google Scholar]
- 10. Hagstrom E, Held C, Stewart RA, Aylward PE, Budaj A, Cannon CP, et al. Growth differentiation factor 15 predicts all-cause morbidity and mortality in stable coronary heart disease. Clin Chem 2017;63:325–333. 10.1373/clinchem.2016.260570 [DOI] [PubMed] [Google Scholar]
- 11. de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004;292:1307–1316. 10.1001/jama.292.11.1307 [DOI] [PubMed] [Google Scholar]
- 12. Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA 2007;297:1775–1783. 10.1001/jama.297.16.1775 [DOI] [PubMed] [Google Scholar]
- 13. Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009;360:2176–2190. 10.1056/NEJMoa0901316 [DOI] [PubMed] [Google Scholar]
- 14. O’Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014;312:1006–1015. 10.1001/jama.2014.11061 [DOI] [PubMed] [Google Scholar]
- 15. Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med 2004;351:2058–2068. 10.1056/NEJMoa042739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–1800. 10.1056/NEJMoa1500857 [DOI] [PubMed] [Google Scholar]
- 17. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 18. Wollert KC, Kempf T, Giannitsis E, Bertsch T, Braun SL, Maier H, et al. An automated assay for growth differentiation factor 15. J Appl Lab Med 2017;1:510–521. 10.1373/jalm.2016.022376 [DOI] [PubMed] [Google Scholar]
- 19. Eitel I, Blase P, Adams V, Hildebrand L, Desch S, Schuler G, et al. Growth-differentiation factor 15 as predictor of mortality in acute reperfused ST-elevation myocardial infarction: insights from cardiovascular magnetic resonance. Heart 2011;97:632–640. 10.1136/hrt.2010.219543 [DOI] [PubMed] [Google Scholar]
- 20. Gray RJ. A class of $K$-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–1154, 1114. [Google Scholar]
- 21. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eggers KM, Kempf T, Wallentin L, Wollert KC, Lind L. Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community-dwelling elderly individuals. Clin Chem 2013;59:1091–1098. doi: clinchem.2012.201210 [pii] 10.1373/clinchem.2012.201210 [DOI] [PubMed] [Google Scholar]
- 23. Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 2006;98:351–360. doi: 01.RES.0000202805.73038.48 [pii] 10.1161/01.RES.0000202805.73038.48 [DOI] [PubMed] [Google Scholar]
- 24. Ho JE, Hwang SJ, Wollert KC, Larson MG, Cheng S, Kempf T, et al. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin Chem 2013;59:1613–1620. doi: clinchem.2013.205716 [pii] 10.1373/clinchem.2013.205716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho JE, Mahajan A, Chen MH, Larson MG, McCabe EL, Ghorbani A, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem 2012;58:1582–1591. 10.1373/clinchem.2012.190322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res 2004;318:325–333. 10.1007/s00441-004-0986-3 [DOI] [PubMed] [Google Scholar]
- 27. de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet 2003;362:316–322. doi: S0140-6736(03)13976-1 [pii] 10.1016/S0140-6736(03)13976-1 [DOI] [PubMed] [Google Scholar]
- 28. Lee SE, Kang SG, Choi MJ, Jung SB, Ryu MJ, Chung HK, et al. Growth differentiation factor 15 mediates systemic glucose regulatory action of T-helper type 2 cytokines. Diabetes 2017;66:2774–2788. 10.2337/db17-0333 [DOI] [PubMed] [Google Scholar]
- 29. Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, et al. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J 2007;28:2858–2865. 10.1093/eurheartj/ehm465 [DOI] [PubMed] [Google Scholar]
- 30. Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, et al. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J 2009;30:1057–1065. 10.1093/eurheartj/ehn600 [DOI] [PubMed] [Google Scholar]
- 31. Hagstrom E, James SK, Bertilsson M, Becker RC, Himmelmann A, Husted S, et al. Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur Heart J 2016;37:1325–1333. 10.1093/eurheartj/ehv491 [DOI] [PubMed] [Google Scholar]
- 32. Eggers KM, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Jantzen F, et al. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non-ST-segment-elevation acute coronary syndrome. Circ Cardiovasc Genet 2010;3:88–96. doi: CIRCGENETICS.109.877456[pii] 10.1161/CIRCGENETICS.109.877456 [DOI] [PubMed] [Google Scholar]
- 33. Buljubasic N, Vroegindewey MM, Oemrawsingh RM, Asselbergs FW, Cramer E, Liem A, et al. Temporal pattern of growth differentiation factor-15 protein after acute coronary syndrome (from the BIOMArCS study). Am J Cardiol 2019;124:8–13. 10.1016/j.amjcard.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 34. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, et al. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the valsartan heart failure trial. Circulation 2010;122:1387–1395. doi: CIRCULATIONAHA.109.928846[pii] 10.1161/CIRCULATIONAHA.109.928846 [DOI] [PubMed] [Google Scholar]
- 35. Bouabdallaoui N, Claggett B, Zile MR, McMurray JJV, O’Meara E, Packer M, et al. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail 2018;20:1701–1709. 10.1002/ejhf.1301 [DOI] [PubMed] [Google Scholar]
- 36. Nurmohamed NS, Belo Pereira JP, Hoogeveen RM, Kroon J, Kraaijenhof JM, Waissi F, et al. Targeted proteomics improves cardiovascular risk prediction in secondary prevention. Eur Heart J 2022;43:1569–1577. 10.1093/eurheartj/ehac055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sithiravel C, Roysland R, Alaour B, Sylte MS, Torsvik J, Strand H, et al. Biological variation, reference change values and index of individuality of GDF-15. Clin Chem Lab Med 2022;60:593–596. 10.1515/cclm-2021-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data cannot be shared but we encourage individuals interested in collaboration to contact us directly.