Abstract
Background:
Ultrarapid-acting insulin analogs that could improve or even prevent postprandial hyperglycemia are now available for both research and clinical care. However, clear glycemic benefits remain elusive, especially when combined with automated insulin delivery (AID) systems. In this work, we study two insulin formulations in silico and highlight adjustments of both open-loop and closed-loop insulin delivery therapies as a critical step to achieve clinically meaningful improvements.
Methods:
Subcutaneous insulin transport models for two faster analogs, Fiasp (Novo Nordisk, Bagsværd, Denmark) and AT247 (Arecor, Saffron Walden, United Kingdom), were identified using data collected from prior clamp experiments, and integrated into the UVA/Padova type 1 diabetes simulator (adult cohort, N = 100). Pump therapy parameters and the aggressiveness of our full closed-loop algorithm were adapted to the new insulin pharmacokinetic and pharmacodynamic profiles through a sequence of in silico studies. Finally, we assessed these analogs' glycemic impact with and without modified therapy parameters in simulated conditions designed to match clinical trial data.
Results:
Simply switching to faster insulin analogs shows limited improvements in glycemic outcomes. However, when insulin acceleration is accompanied by therapy adaptation, clinical significance is found comparing time-in-range (70–180 mg/dL) with Aspart versus AT247 in open-loop (+5.1%); and Aspart versus Fiasp (+5.4%) or AT247 (+10.6%) in full closed-loop with no clinically significant differences in the exposure to hypoglycemia.
Conclusion:
In silico results suggest that properly adjusting intensive insulin therapy profiles, or AID tuning, to faster insulin analogs is necessary to obtain clinically significant improvements in glucose control.
Keywords: Automated insulin delivery, Pharmacokinetic/pharmacodynamic profile, Therapy optimization, Type 1 diabetes, Ultrarapid insulin
Introduction
People with type 1 diabetes (T1D) require exogenous insulin to maintain their glucose levels within safe ranges.1 Even with rapidly improving drugs and technology, safe and effective glucose control remains challenging for people with T1D, in part, due to the relatively slow absorption and action of current insulin analogs.2 Although automated insulin delivery (AID) systems have helped simplify and improve glucose control overall,3 they are also limited by modern analogs' pharmacokinetic and pharmacodynamic (PK/PD) profiles,4–6 highlighting an unmet need for new faster analogs.7
All AID systems that are currently on the US market (Medtronic Minimed 670G and 770G,8,9 Tandem t:slim X2 with Control-IQ,10 and Insulet Omnipod 511) or that have received CE-Mark approval in Europe (Medtronic 780G,12 DiabeLoop,13 CamDiab CamAPS FX14) represent hybrid closed-loop solutions that work best when users enter manual doses at mealtimes to compensate for slow insulin PK/PD profiles. Despite high user involvement and technological advances such as safety modules to protect against hypoglycemia and automatic correction doses to mitigate prevailing hyperglycemia, percentage of time in range (TIR: 70–180 mg/dL) remains around 70%, on average, under free-living conditions.15–17 This indicates a potential for improvement and also highlights the compromise imposed by current insulin analogs.18
From a control engineering viewpoint, trying to gain performance by making the controller more aggressive should be avoided for systems with time delays, since it can easily lead to oscillations and instability. For AID systems, aggressive controllers increase chances of insulin stacking, and consequently, of late hypoglycemia.19
New faster insulin formulations have been produced to improve prandial glycemic control and potentially allay the constraints on AID performances.20,21 A key innovation behind new insulin formulations is to meet the rapidly increasing insulin needs after meals by including excipients that accelerate the rate of insulin absorption from the subcutaneous injection site. Among these formulations, four insulin analogs with faster PK/PD profiles can be highlighted: two approved by the Food and Drug Administration (FDA) for marketing: Fast Acting Insulin Aspart22 (FIASP, manufactured by Novo Nordisk, Bagsværd, Denmark) and Ultra Rapid Lispro23 (URLi, manufactured by Eli Lilly, Indianapolis IN, USA), and two currently under development: BioChaperone Lispro24 and AT247,25 manufactured by Adocia (Lyon, France) and Arecor (Saffron Walden, United Kingdom), respectively.
Different studies have been published in the literature presenting the specifications of these insulin formulations and the comparison of their PK/PD profiles with respect to current insulin analogs.25–27 According to these results, insulin analogs with faster PK/PD profiles seem to be promising candidates in the search for better postprandial glycemic control.
The difficulties in demonstrating clinically relevant benefits for these new formulations may indicate that insulin-specific titration is necessary to maximize the glycemic impact of faster insulin analogs. By way of illustration, in a recent study in people with T1D on continuous subcutaneous insulin infusion (CSII) therapy, improved postprandial glucose control was obtained with URLi and dose titration.28 In case of AID systems, although insulin acceleration seems a natural solution to increase TIR, recent studies have shown that simply switching to an insulin with faster PK/PD profiles (e.g., URLi and Fiasp) fails to show clinically significant improvements in glycemic outcomes.29,30 This was demonstrated in a double-blind, randomized, crossover trial in 20 participants with T1D using a full closed loop system where no superiority in glycemic control was found using Fiasp compared to using standard insulin Aspart (TIR: 53.3% vs. 57.9%, P = 0.170).31
The conclusion of this study was that the closed-loop algorithm was better adjusted to Aspart. Similarly, Aleppo et al29 presented the results of a head-to-head trial with insulin pump users, in which it was shown that there is no superiority in overall glycemic control of Fiasp versus insulin Aspart when pump settings are not optimized. However, in the same study, the benefit of using Fiasp for postprandial control is shown in a particular case study. Similar results were also presented in Ozer et al32 where the authors showed that overall TIR with Fiasp was only 1.81% higher than with insulin Aspart, but with a greater reduction in the 1 h postmeal glucose excursion.
Moreover, two clinical trials in adults and children with T1D are underway to evaluate the safety of URLi with two commercially available AID systems. The first one using Control-IQ technology to obtain updates on the labeling of URLi and the t:slim X2 insulin pump (NCT 05403502) and, the second one using the MiniMed™ 780 G System to support product and system labeling (NCT 05325294). Results of both studies are expected in May 2023.
The aim of this work is to identify potential benefits of faster PK/PD profiles when insulin therapy is optimized to the insulin analog in both open- and closed-loop settings. To this end, we first update our simulation testing platform by identifying subcutaneous insulin transport models for two insulin analogs (Fiasp and AT247) with PK/PD profiles faster than the default insulin Aspart already included in the FDA-accepted UVA/Padova T1D simulator.33 We then use this new platform to optimize insulin therapy parameters for each insulin analog. Both open- and closed-loop therapies are then evaluated through simulations that resemble real-life conditions, comparing baseline and adjusted approaches.
Materials and Methods
PK models of subcutaneous insulin transport
Model parameters of the subcutaneous insulin transport model included in the UVA/Padova T1D simulator34 (details presented in Supplementary Appendix SA1) were identified for Fiasp and AT247 using data collected from 8 h euglycemic clamp experiments conducted in 19 subjects with T1D,25 and following a maximum a posteriori approach that minimizes differences between data and modeled insulin concentration, penalizing deviations from an a priori probability distribution:
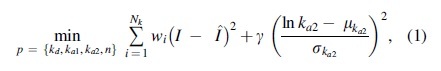
where is the set of parameters to be identified and described in the Supplementary Appendix SA1, Nk is the number of data points taken per subject during the clamp, I is the real insulin concentration, 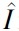 is the modeled insulin concentration, and wi and are weighting factors.
is the modeled insulin concentration, and wi and are weighting factors.
Considering that new insulin formulations focus on increasing availability and diffusion of insulin monomers, for example using vasodilation,35,36 we penalize deviations of the estimated rate of absorption of insulin in the second compartment into plasma () from its original probability distribution function reported in Schiavon et al34 (mean and standard deviation [SD] ), forcing model-fitting solutions that reflect changes in the first compartment. Factor wi is built to heavily weight tail errors and thus capture insulin clearance from data properly, while is defined to balance the two terms in Equation (1).
Finally, affine transformations in the logarithmic space are calculated to adjust mean (translation) and SD (scaling) of parameter distributions for Aspart to match parameter distributions for Fiasp and AT247; maintaining the correlation structure within the UVA/Padova T1D Simulator. All simulations in the following sections are performed on this updated version of the simulator, using the FDA-accepted N = 100 adult cohort (age = 33.8 ± 9.59 years; body weight [BW] = 75.25 ± 12.07 kg).
Open-loop therapy optimization
For patients who are on CSII therapy, basal insulin is delivered as continuous micro boluses that follow a preprogrammed profile, while a manual bolus (MB) is usually calculated as37:

where CHO is the estimated amount of carbohydrates in grams, CR is the correction ratio in g/U, is the current BG concentration in mg/dL, is the predefined BG target in mg/dL, CF is the correction factor in mg/dL/U, and IOB (or insulin on board) is the amount of insulin in U that is still active in the body.
Thus, to adjust insulin therapy to faster insulin, one might think of adjusting all insulin-dosing parameters (basal rate [BR], CR, CF, and IOB) to allow for greater insulin infusion when using faster insulin analogs. But, while increasing the dose of prandial bolus would appear logical (the faster kinetics allowing to reduce the insulin action on the tail end of the disturbance, therefore creating an opportunity to increase the dose without increasing the risk for hypoglycemia), it is less clear that increased BR would be beneficial (in our model, the BR controls the glucose steady state, a construct that is not impacted by the insulin diffusion time constants).
Although the underlying model used to estimate IOB could be also adjusted to reflect PK changes, this was not explored in this work as the simulation experiments performed were designed with sufficiently separated meals that the estimated IOB would be approximately zero at mealtimes. Given the above reasoning, in this work, it is considered that optimization of open-loop therapy will concern the setting of insulin-dosing parameters CR and CF.
Estimation of optimal CR
To obtain an analog-specific, personalized CR parameter, 12 h simulations are performed with all in silico adults stabilized at 110 mg/dL (i.e., ) and receiving a standard meal of 0.8 g/kg of BW at t = 2 h. The optimal insulin dose MB to cover the meal is determined by solving the following optimization problem that minimizes the squared difference between the glucose concentration premeal () and the minimum glucose concentration reached from 4 h after the meal intake to the end of the simulation ():
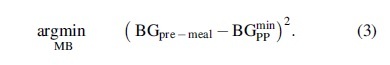
Assuming IOB = 0 U, the optimal insulin dose can be defined as according to Equation (2). The optimization problem in Equation (3) is solved iteratively following a closed- loop based approach, where MB was initialized as 0.04 U/kg (MB0) and then adjusted at each iteration step k as:
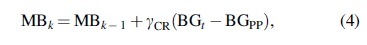
with being the empirical controller's gain and considering a tolerance band of 3%. It should be noted that optimal CR values were also computed for insulin Aspart to establish an optimized baseline to compare against. Thus, based on the for each subject obtained by solving Equation (3), the optimal CR was computed as .
Estimation of optimal CF
A variation of the previous experiment is considered to estimate CF. Similarly, 12 h simulations are performed with all virtual subjects receiving a standard meal of 0.8 g/kg of BW at t = 2 h but setting the premeal glucose concentration () at the default fasting glucose concentration of each virtual subject (i.e., ). Then, the optimal prandial dose, , is computed by solving Equation (3), considering IOB = 0 U and obtained from the experiment presented in Estimation of Optimal CR section.
Once is obtained, the optimal correction factor is computed from Equation (2) as 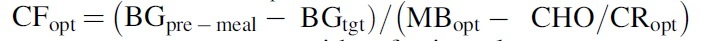 . Note that for the subjects with a fasting glucose concentration lower than (i.e., ), is computed based on and total daily insulin (TDI) as follows:
. Note that for the subjects with a fasting glucose concentration lower than (i.e., ), is computed based on and total daily insulin (TDI) as follows:

In this regard, a set of personalized (CR, CF) per subject and for each insulin analog (Aspart, Fiasp, and AT247) is obtained.
Adapting RocketAP to faster insulin analogs
To understand how controller tuning may impact full closed-loop AID performances with novel insulin analogs, we selected the novel RocketAP algorithm developed at UVA. This AID system integrates a model predictive control (MPC) law with ad-hoc dosing modules in the UVA modular architecture.38
In a previous article,18 we adapted the MPC formulation of RocketAP to theoretical α-insulins that were generated by accelerating α times () the PK parameters of the subcutaneous insulin transport model within the UVA/Padova T1D simulator. Duration of insulin action (DIA) was defined as a function of α and integrated into the cost function of the MPC problem to shape its responsiveness to glucose deviations. To this end, parameter , which penalizes difference between two consecutive doses, and , which enforces the maximum allowable difference between control actions, were reformulated as functions of DIA. While decreases as DIA decreases and vice versa, the opposite behavior was defined for . For further details about the tuning procedure, the reader is referred to the cited work.18
In this work, we follow a similar approach. Each insulin analog under study (Aspart, Fiasp, and AT247) was matched to a value of through simulated euglycemic clamp experiments where a 0.2 U/kg single dose of insulin was administered.18 For each analog, an average glucose infusion rate (GIR) was obtained across virtual subjects and the area under the curve (AUC) was computed for the first 4 h of the clamp experiment. Average AUCs for Fiasp and AT247 were matched to AUCs for -insulins, considering that Aspart is matched to by design. Finally, the procedure described in Colmegna et al18 was used to compute DIA as a function of , and define new and values for each analog. Details regarding the integration of into the MPC cost function are presented in Supplementary Appendix SA2.
In silico testing: Simulation scenarios
In silico simulations considering the 100 adult cohort of the UVA/Padova T1D Simulator are performed to evaluate whether adjusting open- and closed-loop insulin delivery settings maximizes potential glycemic control benefits associated with faster insulins. To this end, each insulin analog is tested under both baseline and adjusted settings, in which dosing parameters or controller aggressiveness are modified as described above. We therefore present six cases in open loop: three analogs, times two settings (adjusted and baseline), and five cases in closed loop since the baseline condition is already adjusted for the Aspart analog (i.e., the two settings are identical in this case).
Simulations are performed over 10 days considering interday variability in insulin sensitivity parameters, which can randomly vary up to 40% around their nominal values. Three meals per day were included with meal size calculated based on BW ([0.77 0.77 0.65]*BW for breakfast, lunch, and dinner, respectively) and mealtime variability around ±30 min. In addition, hypoglycemic treatments are administered whenever blood glucose drops below 60 mg/dL. It is worth remarking that meals are announced only in the open-loop therapy. The entire resulting glucose trace is then used to compute the outcomes for each in silico individual in each tested scenario.
Outcomes and statistical analysis
Two-sample t-tests are performed to compare real and estimated insulin PK features. All glycemic outcomes are computed based on simulated BG values. The primary outcomes are the percentages of time between 70 and 180 mg/dL (TIR), above 180 mg/dL (TAR), and below 70 mg/dL (TBR). Additional outcomes are mean BG level, percent of time-in-tight-range 70–140 mg/dL (TTR), total insulin delivery, and coefficient of variation (CV) of BG. All glycemic results are presented as mean and SD. Following Battelino et al,39 we define clinical significance for changes in TIR and TBR as being greater than 5% and 1%, respectively. Data formatting and preparation were carried out with MATLAB R2020a (MATLAB; MathWorks, Natick, MA, USA). Finally, paired t-tests with significance level at 0.05 were performed to address differences in overall glycemic control when the insulin therapy is adjusted to use faster insulin analogs.
Results
Subcutaneous insulin dynamics into the UVA/Padova simulator
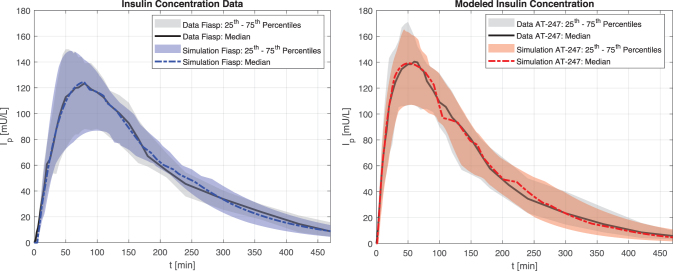
Model parameters and n were identified by solving the optimization problem in Equation (1) using data collected from the clamp experiments described in Svehlikova et al25 for Fiasp and AT247. Identified model parameters are reported in the Supplementary Appendix SA1 as the median and the 25th–75th percentiles with their corresponding CV values. To confirm that differences observed between the PK profiles for Fiasp and AT247 were well captured by the identified models, clamp experiments with these two analogs were reproduced in simulation, and real and simulated insulin concentration values were compared (see Fig. 1).
FIG. 1.
Comparison of real insulin concentration versus simulated insulin concentration for Fiasp (left) and AT247 (right). Data presented as median and range interquartile, which represents the intersubject variability of the population that performed the clamp experiments.
Peak insulin concentration (), time to peak insulin concentration (), and time to reach 50% of peak concentration () were computed for each insulin analog using real and simulated insulin data. All insulin characteristics were statistically indistinguishable between data and simulation and differences between insulin analogs were captured accurately, see Table 1. Once model parameters were identified, transformations between probability distribution functions for each analog were determined to easily switch from one analog to another within the UVA/Padova T1D Simulator. Details are presented in the Supplementary Appendix SA1.
Table 1.
Insulin Kinetic Overall Characteristics (Data Vs. Simulation)
| Fiasp (mean ± SD)a |
AT247 (mean ± SD)a |
Treatment differencesbAT247-Fiasp |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Data | Simulation | P | Data | Simulation | P | Data | Simulation | P | |
| Imax (mU/L) | 128.7 (45.7) | 126.6 (49.4) | 0.89 | 148.1 (52.5) | 144.1 (54.6) | 0.81 | 1.1 [0.9 to 1.4] | 1.1 [0.9 to 1.4] | 0.83 |
| tI,max (min) | 74.2 (17.3) | 80.1 (21.4) | 0.38 | 52.1 (17.5) | 55.7 (16.4) | 0.51 | −18 [−45 to −5] | −22 [−34 to −11] | 0.69 |
| tI,50% (min) | 24.6 (6.0) | 23.2 (5.0) | 0.48 | 12.7 (4.7) | 12.0 (4.0) | 0.63 | −11 [−18 to −9] | −11 [−16 to −6] | 0.75 |
Data are presented as mean ± 1 SDa or median [25th percentile–75th percentile] bfor normally or non-normally distributed time series, respectively.
SD, standard deviation.
Glycemic control using open-loop insulin therapy with faster insulin analogs
Estimation of optimal CR and CF
was estimated for each in silico subject by solving the optimization problem in Equation (3) for each insulin analog. Average across-subject results suggest a reduction in (increase in prandial dosing) of ∼3% and 8% when switching from Aspart to Fiasp and AT247, respectively (Aspart: 14.9 ± 8.3 g/U vs. Fiasp: 14.5 ± 7.9 g/U vs. AT247: 13.7 ± 7.3 g/U). In this sense, a higher meal dose (MB) can be provided when switching to Fiasp or AT247 without increasing the risk of hypoglycemia.
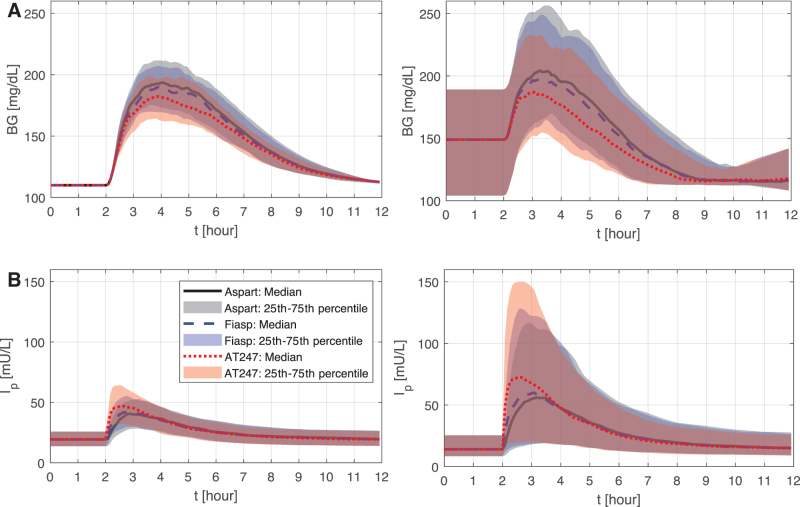
A comparison of the resulting BG envelopes using is presented in Figure 2A. As shown, slight adjustments of the prandial doses (Aspart/Fiasp: +0.11 [0.01 0.23] U; Aspart/AT247: +0.31 [0.06 0.58] U) helped further reduce the postprandial glucose peak by 4 and 11.5 mg/dL when switching from Aspart to Fiasp and AT247, respectively. Thus, when adjusting for CR, peak glucose was lower by 2 and 4 mg/dL for Fiasp and AT247, respectively, with respect to the case in which the same CR was used with all insulins (results not shown).
FIG. 2.
Average glucose responses (A) and circulating insulin concentration (B) using the optimized CR (left) and CF (right) for Aspart, Fiasp, and AT247. CF, correction factor; CR, correction ratio.
Once an optimal was obtained for each subject and insulin analog, the optimization problem in Equation (3) was solved again but considering premeal glucose different to 110 mg/dL to determine optimal CF (Fig. 2B). It is worth mentioning that for the adult cohort considered in this analysis, 14 virtual subjects (of the 100 in silico subjects) had a fasting glucose concentration below 110 mg/dL, and for them was calculated according to Equations (5) and (6).
Obtained results suggest that increments in the correction dose about 2% and 7% when switching from Aspart to Fiasp and AT247, respectively (Aspart: 8.3 [5.6 12.3] mg/dL/U vs. Fiasp: 8.1 [5.4 11.9] mg/dL/U vs. AT247: 7.7 [5.3 11.7] mg/dL/U) could lead to reductions in the postprandial glucose peak by 7.2 and 17.2 mg/dL as shown in Figure 3 (Aspart/Fiasp: +0.11 [0.02 0.3] U; Aspart/AT247: +0.27 [0.02 0.65] U).
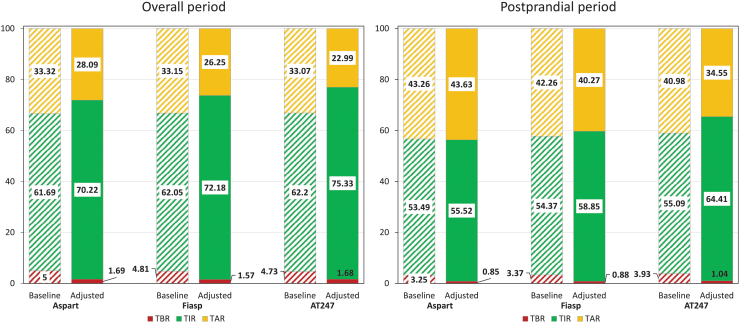
FIG. 3.
Glycemic metrics in open-loop insulin therapy under baseline (striped) and adjusted (solid) scenarios for overall (left) and postprandial (right) periods.
Simulation scenario
Simulation results for the baseline scenario are shown on the left-hand bars of Figure 3 for both overall (O) and postprandial periods (PP, 4 h following meals). Note that glycemic metrics obtained with Aspart closely resemble glycemic metrics reported for people with T1D on sensor augmented pump under free-living conditions.10 If baseline therapy is not optimized, no substantial differences in overall glycemic outcomes are observed when switching from Aspart to Fiasp or AT247 (TBR: 5% vs. 4.81% vs. 4.73%; TIR: 61.69% vs. 62.05% vs. 62.2%), beyond some small improvements in TIR during the postprandial period (TIR: 53.49% vs. 54.37% vs. 55.09%) that cannot be considered clinically significant39 (ΔTIR >5% or ΔTBR >1%).
Results of optimizing therapy and then using for each analog are presented on the right-hand bars of Figure 3. Comparing adjusted scenarios (optimized CR) across analogs, TIR increased 2.0% and 5.1% overall (Aspart: 70.2% vs. Fiasp: 72.2% vs. AT247: 75.3%) and 3.3% and 8.9% in the postprandial period (Aspart: 55.5% vs. Fiasp: 58.9% vs. AT247: 64.4%). All glycemic metrics under baseline and adjusted settings are reported in Table 2. As shown, glycemic metrics improved without increasing the risk for hypoglycemia (TBR—Aspart: 1.7% vs. Fiasp: 1.6% vs. AT247: 1.7%).
Table 2.
Glycemic Outcomes in the Open-Loop Therapy Using Different Insulin Analogs for the Baseline and Adjusted In Silico Scenarios
| Baseline—Open-loop therapy |
Adjusted— Open-loop therapy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postprandial |
Overall |
Postprandial |
Overall |
|||||||||
| Aspart | Fiasp | AT247 | Aspart | Fiasp | AT-247 | Aspart | Fiasp | AT247 | Aspart | Fiasp | AT-247 | |
| Average of BG levels (mg/dL) | 173.3 (61.4) | 171.4 (60.9) | 168.5 (60.4) | 157.6 (59.5) | 157.2 (58.9) | 156.8 (58.1) | 175.4 (44.1) | 171.8 (42.5) | 166.2 (40.5) | 156.8 (44.5) | 155.1 (42.5) | 152.1 (40.2) |
| Time spent <54 mg/dL (%) | 0.7 (2.1) | 0.6 (1.8) | 0.6 (1.8) | 1.1 (2.3) | 1 (2.1)a | 0.9 (2)b | 0.2 (0.7) | 0.2 (0.8) | 0.2 (0.7) | 0.3 (0.9) | 0.2 (0.8)a | 0.3 (0.9)a |
| Time spent <70 mg/dL (%) | 3.2 (7.2) | 3.4 (7.6) | 3.9 (8.7) | 5 (9.5) | 4.8 (9.5)a | 4.7 (9.6)a | 0.9 (2.5) | 0.9 (2.5) | 1 (3) | 1.7 (4.1) | 1.6 (3.9)a | 1.7 (4.2)a |
| Time spent in 70–140 mg/dL (%) | 28.8 (31.4) | 29.7 (32.6) | 30.7 (33.8) | 37 (32.7) | 37.3 (33.8)a | 37.4 (35)a | 18.8 (20.8) | 20.7 (23) | 23.7 (26.2) | 35.4 (24.6) | 36.3 (25.9)b | 38.2 (27.8)b |
| Time spent in 70–180 mg/dL (%) | 53.5 (37.9) | 54.4 (38.3) | 55.1 (38.6) | 61.7 (33.1) | 62 (34)a | 62.2 (34.9)a | 55.5 (33.3) | 58.9 (33.8) | 64.4 (34) | 70.2 (25) | 72.2 (25.6)b | 75.3 (26.2)b |
| Time spent >180 mg/dL (%) | 43.3 (40.6) | 42.3 (41) | 41 (41.4) | 33.3 (36.4) | 33.1 (37)a | 33.1 (37.8)a | 43.6 (33.9) | 40.3 (34.4) | 34.5 (34.6) | 28.1 (25.8) | 26.2 (26.4)b | 23 (26.9)b |
| Time spent >250 mg/dL (%) | 11.5 (25.4) | 10.9 (25.1) | 10.1 (24.6) | 7.4 (17.9) | 7.2 (17.9)b | 6.9 (18.3)b | 5.2 (14.1) | 4 (12.6) | 2.6 (9.9) | 2.8 (8.1) | 2.2 (7.2)b | 1.4 (5.7)b |
| Coefficient of variation of BG levels (%) | 16.1 (7.1) | 15.4 (7.1) | 14.7 (7.8) | 19.5 (7.4) | 18.4 (7.3) | 17 (7.4) | 15.7 (6.1) | 15 (6) | 14 (6.6) | 20.5 (7.2) | 19.3 (7.1) | 17.6 (7.4) |
| Insulin delivered (U) | 38.7 (17.2) | 38.7 (17.3) | 38.9 (17.4) | 51.3 (22.9) | 51.4 (22.9) | 51.5 (23) | 38.5 (22.5) | 39.1 (22.8) | 40.4 (23.9) | 51.1 (27.2) | 51.7 (27.6) | 53 (28.5) |
Results presented as mean (standard deviation).
No statistically significant differences in glycemic metrics when switching from Aspart to Fiasp or AT247 for the overall period (P > 0.05).
Statistically significant differences in glycemic metrics when switching from Aspart to Fiasp or AT247 for the overall period (P < 0.05).
BG.
In addition, clinically significant improvement was obtained only when switching from Aspart to AT247 with an increase in TIR greater than 5% (ΔTIR >5%). Differences in primary glycemic metrics (i.e., TBR, TIR, and TAR) were not statistically significant when comparing Aspart with Fiasp or with AT247 (P > 0.05) in the baseline scenario. In the adjusted scenario, significance is found for TIR and TAR, but not for TBR.
Glycemic control using closed-loop insulin therapy with faster insulin analogs: RocketAP
Estimation of
According to the methodology proposed in Colmegna et al,18 clamp experiments were simulated for the virtual population using the identified subcutaneous insulin transport models for Fiasp and AT247 to relate them to the -insulins. In this regard, if faster PK profiles are associated with faster appearance and action, the GIR of the faster analogs will be higher during the first few hours after bolus injection but the total area under the GIR curve should be the same for all insulin analogs.
Therefore, to reflect the main differences in the PK properties of the faster insulin analogs (Fiasp and AT247), the area under the GIR curve for the first 4 h (postprandial period) after bolus injection (AUGIR|0–4h) was considered as a metric to compare Fiasp and AT247 with the α-insulins reported in Colmegna et al.18 Thus, based on the average across-subject AUGIR|0–4h resulting from the simulated clamp experiments, α = 1 was obtained for Aspart (default insulin), and to α = 1.15 and α = 1.4 for Fiasp and AT247, respectively (details are presented in Supplementary Appendix SA2). In this regard, as α increases, DIA decreases and decreases while increases, allowing the controller to take more aggressive actions.
Simulation scenario
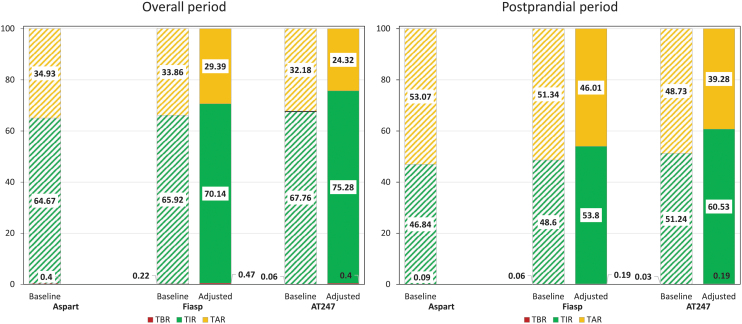
Primary glycemic outcomes for the 10-day simulation and postprandial period under baseline and adjusted scenarios are presented and compared in Figure 4. For the baseline scenario (left-hand bars in Fig. 4), no clinically significant improvements in TIR were obtained overall and in the postprandial period when switching from Aspart to Fiasp (O: +1.2%, PP: +1.8%) or AT247 (O: +3.1%, PP: +4.4%). Of note, TBR did not increase either. For the adjusted scenario (right-hand bars in Fig. 4), TIR for the overall period increased 5.4% and 10.6% when switching from Aspart to Fiasp and AT247, respectively (TIR: 64.7% vs. 70.1% vs. 75.3%), while the TBR remained unaffected (0.4% vs. 0.5% vs. 0.4%). Similar performance was obtained in the postprandial period, for which TAR decreased 7.1% and 13.8% (TAR: 53.1% vs. 46% vs. 39.3%), driving improvements in TIR (PP: 46.8% vs. 53.8% vs. 60.5%) without increasing the risk for hypoglycemia.
FIG. 4.
Glycemic metrics for the full closed-loop insulin therapy (RocketAP) under baseline (striped) and adjusted (solid) scenario for overall (left) and postprandial (right) periods.
All glycemic metrics under baseline and adjusted scenarios are reported in Table 3. Minor changes in total insulin delivery were observed for the baseline scenario (+0.1% vs. +0.4%), in which the same control algorithm setting was used for all insulins tested here. Of note, differences in the primary glycemic metrics were statistically significant when comparing Aspart with Fiasp or AT247 in the baseline scenario. Similarly, in the adjusted scenario, statistically significant differences when switching from Aspart to Fiasp or AT247 were obtained for TIR and TAR, again with no statistically significant differences in times below range (% time <70 mg/dL and % time <54 mg/dL).
Table 3.
Glycemic Outcomes in the Full Closed-Loop Therapy (RocketAP) Using Different Insulin Analogs for the Baseline and Adjusted In Silico Scenarios
| Baseline—RocketAP |
Adjusted—RocketAP |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postprandial |
Overall |
Postprandial |
Overall |
|||||||||
| Asparta | Fiasp | AT-247 | Asparta | Fiasp | AT-247 | Asparta | Fiasp | AT-247 | Asparta | Fiasp | AT-247 | |
| Average of BG levels (mg/dL) | 186.6 (46.4) | 185.1 (45.6) | 182.8 (44.4) | 162.7 (50.6) | 162 (49.3) | 161 (47.5) | 186.6 (46.4) | 178.4 (43.5) | 171.7 (40) | 162.7 (50.6) | 155.8 (46.6) | 150.5 (42.3) |
| Time spent <54 mg/dL (%) | 0 (0.1) | 0 (0) | 0 (0) | 0 (0.2) | 0 (0.1)b | 0 (0)b | 0 (0.1) | 0 (0.1) | 0 (0.2) | 0 (0.2) | 0.1 (0.4) b | 0.1 (0.5) b |
| Time spent <70 mg/dL (%) | 0.1 (0.5) | 0.1 (0.4) | 0 (0.2) | 0.4 (1.6) | 0.2 (1.1)c | 0.1 (0.4)c | 0.1 (0.5) | 0.2 (0.9) | 0.2 (1) | 0.4 (1.6) | 0.5 (1.9) b | 0.4 (1.9) b |
| Time spent in 70–140 mg/dL (%) | 15.5 (13.5) | 15.8 (13.9) | 16.4 (14.9) | 39.4 (21) | 40.1 (21.4)c | 41 (22.1)c | 15.5 (13.5) | 18.9 (15.7) | 21.6 (17.6) | 39.4 (21) | 43.8 (21.5)c | 47.4 (21.8)c |
| Time spent in 70–180 mg/dL (%) | 46.8 (30.1) | 48.6 (31.2) | 51.2 (33) | 64.7 (25.7) | 65.9 (26.3)c | 67.8 (27.2)c | 46.8 (30.1) | 53.8 (31.2) | 60.5 (32.2) | 64.7 (25.7) | 70.1 (24.8)c | 75.3 (23.6)c |
| Time spent >180 mg/dL (%) | 53.1 (30.2) | 51.3 (31.2) | 48.7 (33.1) | 34.9 (25.8) | 33.9 (26.3)c | 32.2 (27.3)c | 53.1 (30.1) | 46 (31.3) | 39.3 (32.3) | 34.9 (25.8) | 29.4 (25)c | 24.3 (23.8)c |
| Time spent >250 mg/dL (%) | 9.2 (21.1) | 8.6 (20.8) | 7.9 (20.4) | 5.7 (14.3) | 5.4 (14.2)c | 5 (14)c | 9.2 (21.1) | 6.2 (17.3) | 3.8 (12.9) | 5.7 (14.4) | 3.7 (11)c | 2.1 (7.7)c |
| Coefficient of variation of BG levels (%) | 16.4 (4.1) | 15.7 (3.9) | 14.7 (3.3) | 23.5 (5.4) | 22.5 (5) | 21 (4.4) | 16.4 (4.2) | 16.3 (4.4) | 15.5 (4.3) | 23.5 (5.4) | 22.8 (5.4) | 21.6 (5.2) |
| Insulin delivered (U) | 33.4 (16.8) | 33.4 (16.8) | 33.3 (16.8) | 49.3 (25.9) | 49.4 (26) | 49.7 (26.1) | 33.4 (16.8) | 35.3 (17.7) | 36.8 (18) | 49.3 (25.9) | 51.3 (26.8) | 52.9 (27.5) |
Results presented as mean (standard deviation).
Aspart in both baseline and adjusted scenario were the same since (Aspart) corresponds to the optimal tuning for this insulin analog. However, the metrics are repeated to facilitate comparison in both cases.
No statistically significant differences in glycemic metrics when switching from Aspart to Fiasp or AT247 for the overall period (P > 0.05).
Statistically significant differences in glycemic metrics when switching from Aspart to Fiasp or AT247 for the overall period (P < 0.05).
Discussion
In T1D, the use of ultrarapid-acting insulins has been shown to improve 1- and 2 h postprandial glycemic control in comparison to conventional rapid-acting insulins.40 However, integration of those faster insulin analogs with closed-loop AID systems has not shown clinically significant improvements () in glycemic control when compared to conventional insulin analogs.30 One reason could be that available insulin therapies are tuned for insulins with PK/PD profiles slower than the new ultrarapid insulins, and, therefore, the benefits of using faster insulin analogs cannot be maximized. Thus, properly tuning of AID system according to the PK/PD properties of new developed faster insulins may be necessary to maximize benefits of those insulins.
In this work, subcutaneous insulin transport models were identified based on data collected during clamp experiments performed in 19 subjects with T1D.25 Results from the clamp experiments showed faster PK/PD profiles for AT247 than Fiasp and Aspart, and those differences were well represented with the identified models. Then, transformations of the probability distribution functions of the model parameters for Aspart were introduced into the UVA/Padova simulator to match those identified for Fiasp and AT247 (see Supplementary Appendix SA1). It is worth mentioning that the proposed methodology can be applied to any insulin analog for which clamp data are available. For the case of URLis, an alternative approach is proposed by Colmegna et al,41 where mean profiles were extracted from published data, and an exploration about how to adjust insulin therapy profiles used by USS-Virginia Closed-Loop system (academic version of Control-IQ) when switching to URLi is presented.
Subsequently, in silico testing of both open- and closed-loop insulin therapies with and without adjusting them to the use of faster insulin analogs were performed. The results presented in this work showed how benefits of faster insulin analogs (e.g., AT247) could be maximized for both open- and closed-loop insulin settings when insulin therapy parameters are adequately adjusted. For the open-loop insulin therapy, differences in the optimal insulin-dosing parameters (i.e., CR and CF) suggested that increasing meal boluses by ∼3% and ∼8% or correction doses by ∼2% and ∼7% when switching from Aspart to Fiasp and AT247, respectively, lead to improved glycemic control with an increase in TIR of ∼2.0 and 5.1 percent points.
Thus, switching to AT247 in open-loop insulin therapy showed a clinically significant improvement in TIR, without increasing the percent of time below 70 mg/dL. This result is a consequence of the differences in the onset of insulin action, time to peak, and maximum insulin concentration observed in the PK profiles between Fiasp and AT247 (see Fig. 2).
Our full closed-loop control algorithm (RocketAP) showed minor improvements when switching to faster insulin analogs. However, when faster analogs were used and the controller was tuned accordingly (see Supplementary Appendix SA2), overall glycemic control was improved with clinical significance ( and , from Aspart to Fiasp and AT247, respectively) without increasing the risk for hypoglycemia. Therefore, adjusting insulin therapies when switching from conventional to ultrarapid insulin analogs appears to be a viable step to leverage the faster PK/PD properties of these insulins, observe clinically significant changes in glycemic metrics, and improve both postprandial and overall glycemic control. It should be noted that we use a specific, outcome driven, definition of clinical significance in this article, following recently published consensus guidelines; this definition may not reflect what patients and/or their clinical team consider significant in the care and management of the disease.
Thus, the main limitation of this work is related to the fact that the results presented were obtained by in silico testing and depend, to some extent, on the predefined simulation conditions for each simulation scenario (baseline and adjusted), in which although interday variability of insulin sensitivity parameters was considered, the error in carbohydrate counting or the delay/anticipation in meal announcement for the case of open-loop therapy was not included. In addition, quantization of optimal CR and CF should be analyzed to obtain values of these parameters in the format required for commercially available insulin pumps when the suggested relative changes in CR and CF are to be applied, as it has not been considered here; finally, application of these results to multiple daily injection may be difficult due to the precision of manual injections and the potentially small changes in prandial doses considered here (8% of 5 U would only be 0.4 U,m below the resolution of most insulin pens).
Therefore, as further work, the results obtained in this work should be confirmed in a human clinical trial. However, it should be noted that since this work deals mainly with the relative differences and changes when switching to faster insulin analogs, the general conclusions about the benefits of using faster insulin analogs to improve glycemic control may be maintained.
Conclusion
In silico experiments predict significant improvements in TIR without increasing TBR when insulin therapy parameters are properly adjusted to the new analog's PK/PD properties.
Supplementary Material
Authors' Contributions
J.L.D. analyzed the data and wrote the first draft of the article. J.L.D. and P.C. proposed the idea for the presented method. P.C. supported data analysis, contributed to discussion, and reviewed the article. M.B. supported J.L.D. in the design of the experimental conditions and the analysis, contributed to discussion, and reviewed the article. J.L.D. is the guarantor of this work and, as such, had full access to all the data in study and takes responsibility for the integrity of the data and the accuracy of the analysis.
Author Disclosure Statement
The authors declared the following potential conflicts of interest. J.L.D. and P.C. receive research support and royalties from Dexcom handled by the University of Virginia's Licensing and Ventures Group. M.B. consults for Roche Diagnostics, Dexcom, and Tandem Diabetes Care; receives research support from Dexcom, Tandem Diabetes Care, and Novo Nordisk. M.B. is an inventor on over 20 patents in the field of diabetes technologies and receives royalties from licensees through the University of Virginia Licensing and Ventures Group.
Funding Information
This work was supported by the NIH NIDDK R01DK129553 and a research support from Arecor Limited, Little Chesterford, United Kingdom.
Supplementary Material
References
- 1. Chiang JL, Kirkman MS, Laffel LMB, et al. Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care 2014;37(7):2034–2054; doi: 10.2337/DC14-DC1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathieu C, Gillard P, Benhalima K. Insulin analogues in type 1 diabetes mellitus: Getting better all the time. Nat Rev Endocrinol 2017;13(7):385–399; doi: 10.1038/nrendo.2017.39 [DOI] [PubMed] [Google Scholar]
- 3. Berget C, Messer LH, Forlenza GP. A clinical overview of insulin pump therapy for the management of diabetes: Past, present, and future of intensive therapy. Diabetes Spectr 2019;32(3):194–204; doi: 10.2337/DS18-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: A multicentre, 12-week randomised trial. Lancet 2018;392(10155):1321–1329; doi: 10.1016/S0140-6736(18)31947-0/ATTACHMENT/62D6321D-1D42-499B-99AE-6A93D0FFDB4F/MMC1.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sherr JL, Buckingham BA, Forlenza GP, et al. Safety and performance of the omnipod hybrid closed-loop system in adults, adolescents, and children with type 1 diabetes over 5 days under free-living conditions. Diabetes Technol Ther 2020;22(3):174–184; doi: 10.1089/DIA.2019.0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Tirado J, Brown SA, Laichuthai N, et al. Anticipation of historical exercise patterns by a novel artificial pancreas system reduces hypoglycemia during and after moderate-intensity physical activity in people with type 1 diabetes. Diabetes Technol Ther 2021;23(4):277–285; doi: 10.1089/dia.2020.0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Senior P, Hramiak I. Fast-acting insulin aspart and the need for new mealtime insulin analogues in adults with type 1 and type 2 diabetes: A Canadian Perspective. Can J Diabetes 2019;43(7):515–523; doi: 10.1016/J.JCJD.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 8. Forlenza GP, Ekhlaspour L, DiMeglio LA, et al. Glycemic outcomes of children 2–6 years of age with type 1 diabetes during the pediatric MiniMed™ 670G system trial. Pediatr Diabetes 2022;23(3):324–329; doi: 10.1111/PEDI.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garg SK, Weinzimer SA, Tamborlane Wv, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19(3):155–163; doi: 10.1089/DIA.2016.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381(18):1707–1717; doi: 10.1056/NEJMOA1907863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44(7):1630–1640; doi: 10.2337/DC21-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlson AL, Sherr JL, Shulman DI, et al. Safety and glycemic outcomes during the MiniMed™ Advanced Hybrid Closed-Loop System pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24(3):178–189; doi: 10.1089/DIA.2021.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amadou C, Franc S, Benhamou PY, et al. Diabeloop DBLG1 closed-loop system enables patients with type 1 diabetes to significantly improve their glycemic control in real-life situations without serious adverse events: 6-Month follow-up. Diabetes Care 2021;44(3):844–846; doi: 10.2337/DC20-1809 [DOI] [PubMed] [Google Scholar]
- 14. Chen NS, Boughton CK, Hartnell S, et al. User engagement with the CamAPS FX Hybrid Closed-Loop App according to age and user characteristics. Diabetes Care 2021;44(7):e148–e150; doi: 10.2337/DC20-2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 2021;23(9):601–608; doi: 10.1089/DIA.2021.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forlenza GP, Lal RA. Current status and emerging options for automated insulin delivery systems. Diabetes Technol Ther 2022;24(5):362–371; doi: 10.1089/DIA.2021.0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kovatchev BP, Singh H, Mueller L, et al. Biobehavioral changes following transition to automated insulin delivery: A large real-life database analysis. Diabetes Care 2022;45(11):2636–2643; doi: 10.2337/DC22-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colmegna P, Cengiz E, Garcia-Tirado J, et al. Impact of accelerating insulin on an artificial pancreas system without meal announcement: An in silico examination. J Diabetes Sci Technol 2021;15(4):833–841; doi: 10.1177/1932296820928067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heise T, Meneghini L. Insulin stacking versus therapeutic accumulation: Understanding the differences. Endocr Pract 2014;20(1):75–83; doi: 10.4158/EP13090.RA [DOI] [PubMed] [Google Scholar]
- 20. Haahr H, Heise T. Fast-acting insulin aspart: A review of its pharmacokinetic and pharmacodynamic properties and the clinical consequences. Clin Pharmacokinet 2020;59(2):155–172; doi: 10.1007/s40262-019-00834-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heinemann L, Muchmore DB. Ultrafast insulins: Ultrafast-acting insulins: State of the art. J Diabetes Sci Technol 2012;6(4):728; doi: 10.1177/193229681200600402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klonoff DC, Evans ML, Lane W, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast-acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab 2019;21(4):961–967; doi: 10.1111/dom.13610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leohr J, Dellva MA, Carter K, et al. Ultra Rapid Lispro (URLi) accelerates insulin lispro absorption and insulin action vs Humalog® consistently across study populations: A pooled analysis of pharmacokinetic and glucodynamic data. Clin Pharmacokinet 2021;60(11):1423–1434; doi: 10.1007/S40262-021-01030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andersen G, Meiffren G, Lamers D, et al. Ultra-rapid BioChaperone Lispro improves postprandial blood glucose excursions vs insulin lispro in a 14-day crossover treatment study in people with type 1 diabetes. Diabetes Obes Metab 2018;20(11):2627–2632; doi: 10.1111/dom.13442 [DOI] [PubMed] [Google Scholar]
- 25. Svehlikova E, Mursic I, Augustin T, et al. Pharmacokinetics and pharmacodynamics of three different formulations of insulin aspart: A randomized, double-blind, crossover study in men with type 1 diabetes. Diabetes Care 2020;44(2):448–455; doi: 10.2337/DC20-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heise T, Pieber TR, Danne T, et al. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet 2017;56(5):551–559; doi: 10.1007/s40262-017-0514-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linnebjerg H, Zhang Q, LaBell E, et al. Pharmacokinetics and glucodynamics of Ultra Rapid Lispro (URLi) versus Humalog® (Lispro) in younger adults and elderly patients with type 1 diabetes mellitus: A randomised controlled trial. Clin Pharmacokinet 2020;59(12):1589–1599; doi: 10.1007/s40262-020-00903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warren M, Bode B, Cho JI, et al. Improved postprandial glucose control with ultra rapid lispro versus lispro with continuous subcutaneous insulin infusion in type 1 diabetes: PRONTO-Pump-2. Diabetes Obes Metab 2021;23(7):1552–1561; doi: 10.1111/dom.14368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aleppo G, Bode B, Carlson AL. Can faster aspart be used to optimize glycemic control with insulin pump therapy? From expectations to lessons learned after a year of use in the United States. Clin Diabetes 2022;40(4):413–424; doi: 10.2337/CD21-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bode B, Carlson A, Liu R, et al. Ultrarapid Lispro demonstrates similar time in target range to Lispro with a hybrid closed-loop system. Diabetes Technol Ther 2021;23(12):828–836; doi: 10.1089/dia.2021.0184 [DOI] [PubMed] [Google Scholar]
- 31. Dovc K, Piona C, Mutlu GY, et al. Faster compared with standard insulin Aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: A double-blind randomized crossover trial. Diabetes Care 2019;43(1):29–36; doi: 10.2337/DC19-0895 [DOI] [PubMed] [Google Scholar]
- 32. Ozer K, Cooper AM, Ahn LP, et al. Fast Acting insulin aspart compared with insulin aspart in the Medtronic 670G hybrid closed loop system in type 1 diabetes: An open label crossover study. Diabetes Technol Ther 2021;23(4):286–292; doi: 10.1089/dia.2020.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Visentin R, Campos-Náñez E, Schiavon M, et al. The UVA/Padova type 1 diabetes simulator goes from single meal to single day. J Diabetes Sci Technol 2018;12(2):273–281; doi: 10.1177/1932296818757747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schiavon M, Dalla Man C, Cobelli C. Modeling subcutaneous absorption of fast-acting insulin in type 1 diabetes. IEEE Trans Biomed Eng 2018;65(9):2079–2086; doi: 10.1109/TBME.2017.2784101 [DOI] [PubMed] [Google Scholar]
- 35. Hirsch IB, Juneja R, Beals JM, et al. The Evolution of insulin and how it informs therapy and treatment choices. Endocr Rev 2020;41(5):733–755; doi: 10.1210/ENDREV/BNAA015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kildegaard J, Buckley ST, Nielsen RH, et al. Elucidating the mechanism of absorption of fast-acting insulin aspart: The role of niacinamide. Pharm Res 2019;36(3):49; doi: 10.1007/s11095-019-2578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmidt S, Norgaard K.. Bolus calculators. J Diabetes Sci Technol 2014;8(5):1035; doi: 10.1177/1932296814532906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia-Tirado J, Lv D, Corbett JP, et al. Advanced hybrid artificial pancreas system improves on unannounced meal response—In silico comparison to currently available system. Comput Methods Programs Biomed 2021;211:106401; doi: 10.1016/J.CMPB.2021.106401 [DOI] [PubMed] [Google Scholar]
- 39. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019;42(8):1593–1603; doi: 10.2337/DCI19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Avgerinos I, Papanastasiou G, Karagiannis T, et al. Ultra-rapid-acting insulins for adults with diabetes: A systematic review and meta-analysis. Diabetes Obes Metab 2021;23(10):2395–2401; doi: 10.1111/dom.14461 [DOI] [PubMed] [Google Scholar]
- 41. Colmegna P, Diaz C JL, Garcia-Tirado J, et al. Adjusting therapy profiles when switching to ultra-rapid Lispro in an advanced hybrid closed-loop system: An in silico study 2022; In Press; doi: 10.1177/19322968221140401 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.