Abstract
Background:
Cystic fibrosis (CF) is a progressive genetic disease characterized by multisystem symptom burden. Specialist palliative care (PC), as a model of care, has been shown to be effective in improving quality of life and reducing symptom burden in other conditions, but has not been tested in CF.
Objectives:
To develop and test the feasibility and acceptability of a specialist PC intervention embedded within an outpatient CF clinic.
Design:
Single-site, equal-allocation randomized pilot study comparing usual care with addition of four protocolized quarterly visits with a PC nurse practitioner.
Participants:
Adults with CF age ≥18 years with any of the following: FEV1% predicted ≤50, ≥2 CF-related hospitalizations in the past 12 months, supplemental oxygen use, or noninvasive mechanical ventilation use, and moderate-or-greater severity of any symptoms on the Edmonton Symptom Assessment Scale.
Measurements:
Randomization rate, intervention visit completion, data completements, participant ratings of intervention acceptability and benefit, and intervention delivery fidelity.
Results:
We randomized 50 adults with CF of 65 approached (77% randomization rate) to intervention (n = 25) or usual care (n = 25), mean age 38, baseline mean FEV1% predicted 41.8 (usual care), and 41.2 (intervention). No participants withdrew, five were lost to follow-up, and two died (88% retention). In the intervention group, 23 of 25 completed all study visits; 94% stated the intervention was not burdensome, and 97.6% would recommend the intervention to others with CF. More than 90% of study visits addressed topics prescribed by intervention manual.
Conclusions:
Adding specialist PC to standard clinic visits for adults with CF is feasible and acceptable.
Keywords: adult, cystic fibrosis, outpatient, palliative, pilot
Introduction
Cystic fibrosis (CF) is a chronic, fatal, and life-limiting genetic disease affecting >30,000 people in the United States and leads to severe lung, endocrine, and other multisystem organ dysfunction.1,2 CF is characterized by high physical and emotional symptom burden and extensive treatment burden, which collectively lead to impaired quality of life (QoL).1 In addition, high rates of anxiety and depression3–7 can predispose people with CF to poorer outcomes, including decreased lung function, decreased medical adherence, and increased hospitalization rates.8 Yet, people living with CF report that they feel their symptoms, including pain, are sometimes overlooked or inadequately managed by clinicians.9
While specialist-delivered palliative care (PC) has been proved effective in improving QoL and reducing symptom burden in serious illnesses such as cancer and heart failure, no evidence exists regarding the efficacy of PC interventions in genetic disorders, including CF.10,11 Using evidence by analogy from conditions, in which PC interventions have been shown to be effective, in 2021, the Cystic Fibrosis Foundation recommended specialist PC consultation for adults with CF whose palliative needs could not be met by CF care teams or who are considering organ transplantation.12 Clinicians and adults attending a CF clinic found integrated specialist PC within an outpatient CF team helpful in one service evaluation,13 but there is little evidence about how and when to add specialist PC to usual CF care.
Therefore, we conducted a pilot randomized controlled trial of longitudinal specialist PC added to routine CF clinic care versus usual CF care (NCT02668575). Our primary outcomes were the feasibility, acceptability, and perceived efficacy of the intervention, and intervention fidelity.
Methods
Intervention development
We developed the InSPIRe:CF (Integrating Specialist Palliative care to Improve care and Reduce suffering) intervention based on qualitative research with adults living with CF, CF clinicians, and caregivers,3–5,14 and other stakeholder feedback, as well as review of existing models of PC delivery.10 InSPIRe:CF is a multicomponent intervention, informed by the Chronic Care Model15 and National Consensus Project for Quality Palliative Care,16 that integrates a PC specialist within the existing interdisciplinary care team of an outpatient adult CF clinic. A core feature of the intervention is embedding the PC clinician as a member of the CF care team to enhance both collaboration and quality of care, as well as to increase rapport and trust with people living with CF.
The intervention comprises at least four visits with the PC clinician during participants' regular visits to the CF clinic, which typically occur quarterly. Protocolized PC visits address physical symptoms, emotional symptoms, existential support, social roles and function, and goals of care and advance care planning (Table 1). Before each appointment, participants complete an Edmonton Symptom Assessment Scale (ESAS)17 and Patient Health Questionnaire-9 (PHQ9),18 which the PC clinician reviewed before the visit. Two weeks after each intervention visit, the clinician called patients to reinforce intervention content and to elicit incident needs. Caregivers were encouraged but not required to attend. All visits were documented using the hospital's electronic medical record.
Table 1.
Protocolized Topics for Intervention Visits
| Visit | Topics |
|---|---|
| 1 | Build rapport Demystify PC Set visit agenda Focused physical and emotional symptom management Explore prognostic awareness Introduce advance care planning and PREPARE website |
| 2 | Set visit agenda Focused physical and emotional symptom management Check in regarding proxy and advance directive status Explain that advance care planning will be focus of visit 3 |
| 3 | Set visit agenda Physical and emotional symptom management Goals of care and advance care planning using advance directive pamphlet Prognostic understanding |
| 4 | Set visit agenda Assess coping strategy and resilience resources Physical and emotional symptom assessment Advance care planning follow-up Provide contact information for outpatient PC |
PC, palliative care.
Participants and randomization
We recruited participants from the adult CF clinic at the University of Pittsburgh Medical Center from March 2016 to December 2017. Study coordinators screened medical records to identify potential candidates and attended the CF interdisciplinary team preclinic meeting to confirm eligibility before approaching patients during that afternoon's clinic session. After providing informed consent and completing baseline measures, participants were randomized either to the intervention or usual care group in a 1:1 allocation ratio using a computer-generated permuted block sequence with random block sizes stratified by sex and FEV1% (<25% vs. ≥25%) loaded into the REDCap database system. The study protocol was approved by the University of Pittsburgh's Institutional Review Board.
Initially, inclusion criteria were age 18 years or older, intent to receive primary CF care at [redacted] for the next 12 months, FEV1% predicted ≤50, plus any of the following: ≥2 CF-related hospitalizations in the past 12 months, supplemental oxygen use, or noninvasive mechanical ventilation use (Table 2). Approximately halfway through recruitment, discussions among the study team revealed that despite significant pulmonary impairment, trial participants did not consistently report high levels of distress; interim analyses of baseline data of the first 25 participants supported this observation that FEV1% predicted was not correlated with patient-reported symptom burden or QoL.19
Table 2.
Inclusion and Exclusion Criteria
| Phase 1 | Phase 2 |
|---|---|
| Inclusion: | Inclusion: |
| Age ≥18 years | Age ≥18 years |
| Intent to receive primary CF care at site for next 12 months | Intent to receive primary CF care at site for next 12 months |
| Any of the following: | Any of the following: |
| FEV1% predicted ≤50 | FEV1% predicted ≤50 |
| ≥2 CF-related hospitalizations in the past 12 months | ≥2 CF-related hospitalizations in the past 12 months |
| Supplemental oxygen use | Supplemental oxygen use |
| Noninvasive mechanical ventilation use | Noninvasive mechanical ventilation use |
| >3/10 on ESAS for any one symptom | |
| Exclusion: | Exclusion: |
| Cognitive impairment | Cognitive impairment |
| Unreliable access to telephone | Unreliable access to telephone |
| Inability to speak English fluently | Inability to speak English fluently |
| Received specialty PC in past year | Received specialty PC in past year |
CF, cystic fibrosis; ESAS, Edmonton Symptom Assessment Scale.
As such, we revised our inclusion criteria after the first 25 participants (Phase 1) to identify individuals for whom PC might be appropriate: adults who scored a >3/10 on the ESAS for any one symptom (indicating moderate or greater severity), independent of their FEV1% predicted (Phase 2). Exclusion criteria throughout the study were underlying cognitive impairment, unreliable access to a telephone, or inability to speak English, or receipt of specialist PC within the past year.
Usual care
Individuals randomized to usual care received standard interdisciplinary CF care, including care from pulmonology, nursing, respiratory therapy, pharmacy, dietary, and social work. Usual care arm participants could receive specialist PC consultation if requested by the participant or the CF care team.
Intervention
Participants in the intervention group received quarterly visits with one PC clinician during regular outpatient CF clinic appointments in person; intervention visits could be completed by telephone if necessary. The PC clinician received ∼10 hours of training on the CF life course, symptom management, and palliative communication in the CF context. If intervention participants were hospitalized, the PC specialist recommended consultation by the inpatient PC team and coordinated with the inpatient CF team on the patient's status and overarching goals of care to help ensure continuity.
Data collection and measures
To assess feasibility of data collection for a subsequent full-scale trial, we collected a variety of patient-reported outcome measures. We evaluated CF-related QoL using the Cystic Fibrosis Questionnaire-Revised, whose range is 0–100, where higher scores indicate better QoL.20 We evaluated disease-generic QoL using the PROMIS Global Health measure (range 4–40; higher scores indicate better health-related QoL).21 Psychological symptoms were evaluated using the Hospital Anxiety and Depression Scale instrument (range 0–21, lower scores indicate lower distress),22 and the PHQ-9 (scores ≥10 indicate moderate or greater depression).18 We evaluated symptom prevalence and burden using the ESAS (range 0–900, higher scores indicate greater symptom burden),23 and whether the participant had documented health care wishes, such as a living will or identification of a surrogate decision maker.
To evaluate the intervention, we asked several questions only of intervention participants at each of four survey points, including whether the intervention was burdensome and whether they would recommend the intervention to others; to capture overall perception of the intervention, we averaged their responses across all encounters. Participants completed surveys in person at baseline and by telephone with research staff within two weeks after the three-, six-, and nine-month appointments. Participants received $20 for completing each assessment timepoint, and an additional $20 for completing all four timepoints.
For a subset (n = 11, 44%) of intervention participants, we conducted semistructured qualitative interviews at nine months. Interviews addressed participants' experience with the PC interventionist, and at each visit, their perception of whether PC visits affected their life with CF, and recommendations for whether and how to integrate PC into routine CF care. A trained research assistant conducted the interviews, which were audio-recorded and transcribed verbatim. An experienced qualitative researcher [redacted] coded the transcripts inductively to construct preliminary themes that were reviewed by the PI.
Sample size
A sample size of 50 patients was primarily based on clinical and practical rather than statistical considerations and should provide preliminary estimates of feasibility, feasibility, and patient- and caregiver reported outcomes. The half-width of the 95% confidence interval (CI) for recruitment rate was 8%. Assuming that the retention and acceptability rates were 80%, a sample of 50 patients would have achieved 95% CIs with half-width equal to 11%. For the patient- and caregiver-reported outcomes, the probability was 0.863 that the estimate of the standard deviation (SD) would be within 15% of the true population SD.
Analysis
The intent of this pilot trial was to evaluate the feasibility and acceptability of embedded specialist PC within usual outpatient CF care. As per best practices in intervention science, we did not test for differences between trial arms regarding outcomes.24 As appropriate for pilot trials, analyses focused on estimating proportions of binary feasibility, acceptability, and fidelity endpoints along with 95% CIs. Analyses of patient- and caregiver-reported outcomes primarily are descriptive. We evaluated intervention feasibility with randomization rate, intervention visit completion, and data completeness. We assessed acceptability and perceived effectiveness based on proportion of participants' endorsement of the intervention as acceptable and beneficial; intervention participants also reported their perception of whether the intervention had affected their mood, physical symptoms, or QoL.
As part of fidelity monitoring, the interventionist completed checklists of topics covered during each visit and recorded all visits. An independent reviewer assessed fidelity comparing visit audio recordings against using a checklist based on the study manual.
Results
Sample characteristics
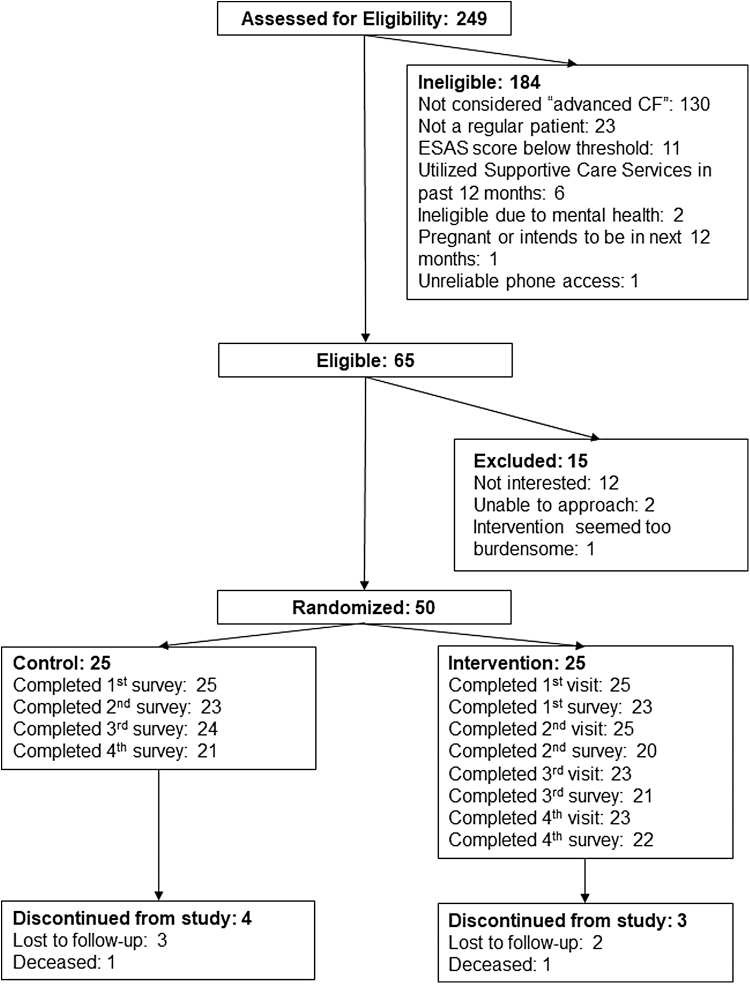
We evaluated 65 individuals in CF clinic for eligibility (Fig. 1); of those, we enrolled 50 for a randomization rate of 77% (95% CI, 65%–86%). The most common reason for refusing trial participation was lack of interest (n = 12, 18%). Two participants died during the study (one in intervention, one in usual care); five were lost to follow-up (three in usual care, two in intervention).
FIG. 1.
Study enrollment and retention diagram.
In the intervention group, 12 were randomized in Phase 1, and 13 were randomized after inclusion criteria were changed to include ESAS symptom scores >3 (indicating moderate or greater severity). Mean age was 31.2 (SD, 9.9) and 38 (SD, 12.5) years for the combined intervention groups and usual care group, respectively; 60% of intervention and 52% of usual care participants were male (Table 3). At baseline, mean FEV1% predicted was 41.2 (SD 14.3) in the combined intervention groups (mean 38.8 (9.1) for Phase 1; 43.5 (17.8) for Phase 2), and 41.8 (SD 16.6) in the usual care group.
Table 3.
Baseline Sample Characteristics
| Variable | Control (n = 25) n (%) | Intervention overall (n = 25) n (%) | Intervention Phase 1 (n = 12) n (%) | Intervention, Phase 2 (n = 13) n (%) |
|---|---|---|---|---|
| Age, mean (SD) | 38.0 (12.5)* | 31.2 (9.9)* | 28.6 (5.0) | 33.5 (12.8) |
| Male gender | 13 (52) | 15 (60) | 6 (50) | 9 (69) |
| White race | 25 (100) | 25 (100) | 12 (100) | 13 (100) |
| Education | ||||
| Some high school | 0 (0) | 2 (8) | 0 (0) | 2 (15) |
| High school diploma | 10 (40) | 13 (52) | 7 (58) | 6 (46) |
| College degree | 15 (60) | 10 (40) | 5 (42) | 5 (38 |
| Professional or graduate degree | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Financial status at end of month | ||||
| Money left over | 16 (64) | 11 (44) | 5 (42) | 6 (46) |
| Just enough money | 8 (32) | 11 (44) | 5 (42) | 6 (46) |
| Not enough money | 1 (4) | 3 (12) | 2 (17) | 1 (8) |
| FEV1% predicted (most recent), mean (SD) | 41.8 (16.6) | 41.2 (14.3) | 38.8 (9.1) | 43.5 (17.8) |
| BMI (most recent), mean (SD) | 21.0 (2.7) | 21.0 (3.1) | 21.6 (2.5) | 20.6 (3.6) |
| Supplemental oxygen use, n (%) | 5 (20) | 3 (12) | 2 (17) | 1 (8) |
| No. of CF-related hospitalizations in past 12 months, mean (IQR) | 1.0 (1.0) | 1.7 (1.7) | 1.6 (1.6) | 1.8 (1.9) |
| No. of CF clinic outpatient visits in past 12 months, mean (IQR) | 5.7 (2.4) | 5.7 (1.6) | 5.3 (1.7) | 6.0 (1.5) |
| Quality of life | ||||
| CFQ-R (mean, SD) | ||||
| Physical functioning | 54.3 (25.2) | 61.9 (24.9) | 63.1 (20.2) | 60.8 (29.3) |
| Vitality | 54.0 (17.2) | 54.3 (18.5) | 58.3 (14.7) | 50.6 (21.4) |
| Health perception | 56.0 (23.2) | 52.0 (25.2) | 54.6 (23.9) | 49.6 (27.1) |
| Respiratory symptoms | 56.8 (15.6) | 59.4 (18.2) | 62.0 (18.0) | 56.9 (18.8) |
| Treatment burden | 49.8 (14.0) | 52.9 (21.8) | 60.2 (21.4) | 46.2 (20.7) |
| Role functioning | 72.3 (24.3) | 66.0 (22.6) | 74.3 (19.6) | 58.3 (23.1) |
| Emotional functioning | 79.7 (18.0) | 75.5 (20.5) | 82.8 (14.6) | 68.7 (23.3) |
| Social functioning | 62.7 (20.2) | 63.3 (15.8) | 69.4 (14.5) | 57.7 (15.3) |
| PROMIS Global Health (mean, SD) | ||||
| Physical health | 42.7 (4.1) | 42.2 (4.3) | 44.1 (3.1)* | 40.5 (4.5)* |
| Mental health | 41.7 (4.0) | 42.4 (4.5) | 41.9 (3.5) | 42.8 (5.4) |
| Mental health | ||||
| HADS (mean, SD) | ||||
| Anxiety | 4.9 (4.4) | 5.2 (3.4) | 4.7 (2.4) | 5.8 (4.2) |
| Depression | 3.4 (3.0) | 3.3 (3.1) | 2.5 (2.1) | 4.1 (3.7) |
| PHQ-9 (mean, SD) | 4.4 (3.5) | 4.4 (3.5) | 3.8 (2.9) | 4.8 (4.0) |
| Symptoms | ||||
| ESAS Global Distress Score (mean, SD) | 207.6 (136.0) | 187.6 (126.7) | 141.7 (78.8) | 230.0 (149.5) |
| Advance care planning | ||||
| Living will signed | 5 (20) | 8 (32) | 3 (25) | 5 (38) |
| Advance directive signed | 6 (24) | 6 (24) | 3 (25) | 3 (28) |
| Surrogate decision-maker identified to primary physician | 5 (20) | 6 (24) | 4 (33) | 2 (18) |
| End of life medical preferences discussed | 6 (24) | 4 (16) | 2 (17) | 2 (15) |
BMI, body mass index; CFQ-R, Cystic Fibrosis Questionnaire-Revised; HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; PHQ-9, Patient Health Questionnaire-9; SD, standard deviation.
At baseline, mean ESAS global distress score was 187.6 (SD 126.7) among intervention group participants and 207.6 (SD 136) among the usual care group. Changing the eligibility criteria resulted in enrolling participants with higher symptom burden in Phase 2: mean ESAS for intervention participants in Phase 1 was 147.7 (SD 78.8), compared with 230 (SD 149.5) in Phase 2.
Feasibility
Ninety-two percent of intervention participants (23/25) completed all study visits (96 of 100 visits completed); five visits (5.2%) were completed by phone. No participants withdrew, one died, and two were lost to follow-up. Among intervention group participants, 94% (80/85 surveys completed by intervention group participants) stated that InSPIRe:CF was not burdensome, a notable finding because InSPIRe:CF added an average of 47 minutes (range 11–70) to outpatient CF clinic visits. One participant in the intervention group died and two were lost to follow-up; one control group participant died during the study (Fig.1 [CONSORT]).
Intervention fidelity
Of visits, 90.6% contained ≥80% of topics prescribed in the intervention manual.
Acceptability
Participants found the intervention to be acceptable; in 97.6% (83/85) of completed surveys over the course of the intervention period, participants stated that they would recommend PC to others with CF. Importantly, in 98.9% (84/85) of surveys, participants indicated that they were satisfied with the care received.
Perceived efficacy
The intervention was perceived to be effective as measured by patient-reported outcomes in symptom burden and QoL, with 90.9% (77/85) of intervention participant surveys reporting moderate or greater improvement in physical symptoms, mood, or QoL. In addition, 72% (61/85) of surveys indicated the intervention improved participants' understanding of their illness or what their future holds regarding their chronic disease process and their ability to cope with their CF.
Qualitative results
We identified two predominant themes in analysis of participant qualitative interviews.
Embedded PC reduces barriers to access
In interviews, participants focused both on the ease of accessing PC during an already-scheduled visit and the likelihood that adults with CF would feel more comfortable with someone already familiar with the condition. “I think all CF patients should have [someone] they can talk to. Because I don't think we realize, a lot of people don't do it on their own, and with it set up like that, it was so easy and convenient, and she did such a nice job. I think it's a good thing to incorporate in the clinic because it's very beneficial to us. … I think they'd feel more comfortable with her being a part of the CF team… If you go to a counselor outside of that, they really don't understand the full dynamics [of living with CF].” (White female, age 31)
Focus on nonphysical needs
Recalling their PC visits, interviewed intervention participants noted that PC helped address the emotional and psychological aspects of living with CF, such as improving reframing and coping skills. “I think CF patients need more help with the emotional part than the physical part. I can deal with the physical part, like I do everything I need to do, and sometimes stuff goes wrong. But the emotional part is the hardest because it doesn't only affect you, it affects my family, it affects everybody.” (White male, age 23)
Discussion
This pilot trial demonstrated that specialist PC embedded into routine CF care is feasible and well regarded by adults with CF. Participants did not find intervention visits burdensome and felt that the visits enhanced QoL, physical symptoms, or mood. Almost all were satisfied with their care from the PC clinician. Among the more surprising findings was the impact of the intervention on participants' perceived understanding of their illness, particularly given that most individuals with CF are diagnosed in early childhood. These findings help demonstrate that specialist PC's value lies not only in expert symptom management but also in helping people understand and cope with serious illness, regardless of onset.
This study, conducted before widespread use of CF transmembrane conductanse regulator (CFTR) modulator therapies, has implications for future interventions that measure and treat subjective symptoms in CF. In analysis of baseline screening data, we found FEV1% predicted, the standard clinical measure of disease progression, and correlated with shortness of breath and fatigue, but not anxiety, depression, pain, nausea, drowsiness, appetite, or overall sense of well-being.19,25 These symptoms, however, are among the most prevalent in CF.4 One hypothesis could be that people with CF are able to adapt to some changes in physiological function over time, rendering a gap between clinical markers of disease progression and the nature and the perception of symptoms as burdensome.
This observation is particularly salient given the increasing use of modulator therapies that often improve pulmonary function but not gastrointestinal or other symptom clusters.1 The lack of correlation between traditional markers of disease progression and symptom burden suggests the need for further investigation into physical, emotional, and existential symptom clusters in CF and their associations with disease progression, particuarly in the current era of CFTR modulator therapies.
This study is remarkable in that it is one of very few interventions to implement specialist PC in a serious genetic illness, such as CF. To date, the majority of research regarding PC has been conducted in cancer and heart failure.10 Yet, for several key factors, it is likely inappropriate to assume that existing thinking and approaches to PC would be transferrable to genetic conditions like CF.
First, individuals with CF are typically much younger than the patient populations classically studied in PC interventions. Across 43 randomized clinical trials included in a 2016 meta-analysis of PC interventions, the average participant was 67 years old,10 whereas the mean age at death in CF is 30.6 years.1 This stark difference in age may affect important factors such as treatment decision-making. For example, while spouses are most often surrogates for individuals with cancer, individuals with CF are more likely to rely on parents or other nonspouse family members, altering patient-caregiver dynamics.26 Second, the natural history and trajectory of CF is different than of most cancers. As opposed to the gradual and predictable declines observed in cancers, CF is characterized by unpredictable and repeated exacerbations, rendering prognostication difficult.
Third, while people living with cancer must “adjust” to a new normal postdiagnosis, most people with CF have lived with their illness and its treatment burden for the majority of, if not their entire lives, their lives. As such, concepts of illness understanding, coping, and prognostic awareness—all hallmarks of most PC interventions—may need to be reconceptualized when applied to a genetic, often lifelong disease such as CF. Fourth, articulating goals of care and participating in advance care planning are nuanced in CF, as high-risk therapies such as lung transplantation are available. These differences in patient populations, needs, and experience with illness, require a fundamental rethinking of both what PC is for people with CF, as well as when and how it should be integrated into their care.
In the context of “early” PC, is there perhaps too early a point for introduction of longitudinal specialty PC if a patient may live for years or decades. Several important questions remain: Can PC teams absorb this volume? How do we demonstrate value of PC over such a long course of illness? Also, from a scientific perspective, what is the optimal dose and duration of a specialist PC intervention in a slowly progressive genetic illness, and can this be studied in traditional grant funding cycles (e.g., five years)?
This study has several important limitations. As a pilot of intervention feasibility and acceptability, the study was neither designed nor powered to assess efficacy per best practices in intervention development.24,27,28 Second, this was a single site study; results may differ in more heterogeneous treatment settings and populations. The intervention did not include patients who had already undergone lung transplantation, for whom care more often is coordinated through transplant clinics. While transplant is a life-extending therapy for CF, posttransplant complications, anti-rejection therapy and its side effects, and associated emotional concerns all represent unmet palliative needs for patients considering or living with lung transplant.29–31 Third, this study was conducted before the wide availability of high effective CFTR modulators. In addition, PC clinician time was supported by the study; resources may differ outside of research.
Finally, the study was limited to adults aged 18 years or older. As CF is often diagnosed early in childhood, future studies should examine the effect of introducing PC concepts much earlier in the disease course.
Conclusion
Embedded specialist PC is a feasible and acceptable model for enhancing regular clinic care for adults with CF. Future efficacy trials are warranted to examine its role in improving QoL, coping with illness and managing symptoms of this complex disease process, while serving as a model for how to approach implementation of PC in other genetic illnesses.
Acknowledgments
The authors sincerely thank the participants in this trial who volunteered their time and perspectives. We also thank Rachel Hess, MD, MSc, Laura Obregon, BS, and Adelina Malito, MSW, for their assistance with study conceptualization and startup.
Funding Information
This study was supported by the Cystic Fibrosis Foundation (PILEWS14QI0). D.K. was supported by the National Heart, Lung, and Blood Institute (K01HL133466), and the Cystic Fibrosis Foundation (KAVAL18QI0).
Author Disclosure Statement
D.K. co-chairs the PC clinical practice guidelines committee at the US Cystic Fibrosis Foundation. All other authors: none.
References
- 1. Cystic Fibrosis Foundation. 2020 Patient Registry and Annual Report. n.d. https://www.cff.org/medical-professionals/patient-registry (Last accessed November 4, 2022).
- 2. Castellani C, Assael BM. Cystic fibrosis: A clinical view. Cell Mol Life Sci CMLS 2017;74(1):129–140; doi: 10.1007/s00018-016-2393-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trandel ET, Pilewski JM, Dellon EP, et al. Prevalence of unmet palliative care needs in adults with cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc 2020;19(3):394–401; doi: 10.1016/j.jcf.2019.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trandel ET, Pilewski JM, Dellon EP, et al. Symptom burden and unmet existential needs in adults with cystic fibrosis. West J Nurs Res 2019;41(10):1448–1464; doi: 10.1177/0193945919852585 [DOI] [PubMed] [Google Scholar]
- 5. Obregon LL, Jeong K, Hoydich ZP, et al. Associations between demographic characteristics and unmet supportive care needs in adults with cystic fibrosis. BMJ Support Palliat Care 2019;bmjspcare-2019-001819; doi: 10.1136/bmjspcare-2019-001819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dellon EP, Basile M, Hobler MR, et al. Palliative care needs of individuals with cystic fibrosis: Perspectives of multiple stakeholders. J Palliat Med 2020;23(7):957–963; doi: 10.1089/jpm.2019.0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sawicki GS, Dill EJ, Asher D, et al. Advance care planning in adults with cystic fibrosis. J Palliat Med 2008;11(8):1135–1141; doi: 10.1089/jpm.2008.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quittner AL, Saez-Flores E, Barton JD. The psychological burden of cystic fibrosis. Curr Opin Pulm Med 2016;22(2):187–191; doi: 10.1097/MCP.0000000000000244 [DOI] [PubMed] [Google Scholar]
- 9. Dubin E, Lowers J, Dellon EP, et al. Prevalence of unmet pain and symptom management needs in adults with cystic fibrosis. J Cyst Fibros Off J Eur Cyst Fibros Soc 2022;S1569-1993(22)00643-9; doi: 10.1016/j.jcf.2022.08.006 [DOI] [PubMed] [Google Scholar]
- 10. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA 2016;316(20):2104–2114; doi: 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quinn KL, Shurrab M, Gitau K, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: A systematic review and meta-analysis. JAMA 2020;324(14):1439–1450; doi: 10.1001/jama.2020.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kavalieratos D, Georgiopoulos AM, Dhingra L, et al. Models of palliative care delivery for individuals with cystic fibrosis: Cystic Fibrosis Foundation evidence-informed consensus guidelines. J Palliat Med 2021;24(1):18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bourke SJ, Booth Z, Doe S, et al. A service evaluation of an integrated model of palliative care of cystic fibrosis. Palliat Med 2016;30(7):698–702; doi: 10.1177/0269216315626658 [DOI] [PubMed] [Google Scholar]
- 14. Dellon EP, Goggin J, Chen E, et al. Defining palliative care in cystic fibrosis: A Delphi study. J Cyst Fibros 2018;17(3):416–421; doi: 10.1016/j.jcf.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 15. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA 2002;288(14):1775–1779. [DOI] [PubMed] [Google Scholar]
- 16. National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care; 2013. https://www.nationalcoalitionhpc.org/ncp/ (Last accessed November 4, 2022).
- 17. Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol Tor Ont 2009;16(1):55; doi: 10.3747/co.v16i1.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tessier R, Pilewski J, Tycon L, et al. Discordance between pulmonary function and symptom burden in patients with cystic fibrosis. Pediatr Pulmonol 2017;52:S502–S502. [Google Scholar]
- 20. Quittner AL, Modi AC, Wainwright C, et al. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610–1618; doi: 10.1378/chest.08-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 2009;18(7):873–880; doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52(2):69–77; doi: 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 23. Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991;7(2):6–9. [PubMed] [Google Scholar]
- 24. Moore CG, Carter RE, Nietert PJ, et al. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci 2011;4(5):332–337; doi: 10.1111/j.1752-8062.2011.00347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Griese M, Costa S, Linnemann RW, et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for 24 weeks or longer in people with cystic fibrosis and one or more F508del alleles: Interim results of an open-label phase 3 clinical trial. Am J Respir Crit Care Med 2021;203(3); doi: 10.1164/rccm.202008-3176LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGuffie K, Sellers DE, Sawicki GS, et al. Self-reported involvement of family members in the care of adults with CF. J Cyst Fibros Off J Eur Cyst Fibros Soc 2008;7(2):95–101; doi: 10.1016/j.jcf.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee EC, Whitehead AL, Jacques RM, et al. The statistical interpretation of pilot trials: Should significance thresholds be reconsidered? BMC Med Res Methodol 2014;14:41; doi: 10.1186/1471-2288-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res 2011;45(5):626–629; doi: 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Floreth T, Bhorade SM. Current trends in immunosuppression for lung transplantation. Semin Respir Crit Care Med 2010;31(2):172–178; doi: 10.1055/s-0030-1249112 [DOI] [PubMed] [Google Scholar]
- 30. Dew MA, Rosenberger EM, Myaskovsky L, et al. Depression and anxiety as risk factors for morbidity and mortality after organ transplantation: A systematic review and meta-analysis. Transplantation 2015;100(5):988–1003; doi: 10.1097/TP.0000000000000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenberger EM, DiMartini AF, DeVito Dabbs AJ, et al. Psychiatric predictors of long-term transplant-related outcomes in lung transplant recipients. Transplantation 2016;100(1):239–247; doi: 10.1097/TP.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]