Abstract
Background:
Estimation of insulin sensitivity (SI) and its daily variation are key for optimizing insulin therapy in patients with type 1 diabetes (T1D). We recently developed a method for SI estimation from continuous glucose monitoring (CGM) and continuous subcutaneous insulin infusion (CSII) data in adults with T1D (SISP) and validated it under restrained experimental conditions. Herein, we validate in vivo a new version of SISP performing well in daily life unrestrained conditions.
Methods:
The new SISP was tested in both simulated and real data. The simulated dataset consists of 100 virtual adults of the UVa/Padova T1D Simulator monitored during an open-loop experiment, whereas the real dataset consists of 10 youths with T1D monitored during a hybrid closed-loop meal study. In both datasets, participants underwent two consecutive meals (breakfast and lunch, at 7 and 11 am) with the same carbohydrate content (70 g). Plasma glucose and insulin were measured during each meal to estimate the oral glucose minimal model SI (SIMM). CGM and CSII data were used for SISP calculation, which was then validated against the gold standard SIMM.
Results:
SISP was estimated with good precision (median coefficient of variation <20%) in 100% of the real and 91% of the simulated meals. SISP and SIMM were highly correlated, both in the simulated and real datasets (R = 0.82 and R = 0.83, P < 0.001), and exhibited a similar intraday pattern.
Conclusions:
SISP is suitable for estimating SI in both closed- and open-loop settings, provided that the subject wears a CGM sensor and a subcutaneous insulin pump.
Keywords: CGM, CSII, Decision support system, Outpatient, Mathematical models
Introduction
Insulin sensitivity quantifies the ability of insulin to simultaneously suppress endogenous glucose production and stimulate glucose disposal. The “gold standard” method to quantify insulin sensitivity (SI) is the hyperinsulinemic/euglycemic clamp,1 which requires to infuse insulin and glucose intravenously, in a laboratory setting, and frequently measure plasma glucose and insulin concentrations. Such a method is rather invasive and nonphysiological since glucose is administered intravenously and both glucose and insulin are artificially maintained almost constant. Other methods have been proposed and validated to partially overcome the above limitations, for example, the intravenous2 and oral glucose or meal tolerance tests interpreted with mathematical models.3,4 Empiric surrogate indices,5,6 or methods based on basal/nonstimulated conditions,7–9 are also available. However, a drawback of all the above methodologies is the need to measure both plasma glucose and insulin concentrations. This precludes their use in outpatient settings.
So far, a few methodologies have been developed for the estimation of SI from outpatient data and thus potentially usable to quantify daily SI variations.10,11 Fabris et al proposed a method based on Kalman filter and an extended oral glucose minimal model (OGMM) to track real-time changes of SI10 from continuous glucose monitoring (CGM) and continuous subcutaneous insulin infusion (CSII) data, assuming minimal model parameters to be either fixed to population values or estimated from historical patient data. The method proved to be effective at adjusting insulin to carbohydrate ratio (CR) after a single bout of aerobic exercise12 but, to the best of our knowledge, it has never been validated against other inpatient-derived SI indices available in the literature.
In a previous work we used a different approach based on an algebraic formula to calculate, for each meal, an index of SI from CGM and CSII data (SISP).11 The method was validated against the OGMM SI and recently used to optimize CR both in subjects wearing a sensor-augmented insulin pump13,14 and artificial pancreas.15,16 However, the SISP method was originally developed and validated on data collected in strictly controlled experimental conditions, that is, with well-spaced consecutive meals (meal-to-meal interval of at least 6 h) and no occurrence of hypoglycemic events,11 and thus its performances are likely to degrade outside that domain of validity.
In this study, we propose a new algorithm to assess SI from CGM and CSII data in patients with type 1 diabetes (T1D) under real-life conditions. The new method is tested and validated both in silico and in vivo, against the OGMM index of SI (minimal model SI [SIMM])3 derived from plasma glucose and insulin data.
Materials and Methods
Database
In silico dataset
The virtual dataset was generated using the most recent version of the UVa/Padova T1D simulator (T1DS),17 a tool accepted by U.S. Food and Drug Administration as substitute for preclinical trials of certain insulin treatments such as artificial pancreas,18 insulin analogs,19 and glucose sensors.20 The simulator consists of a model of glucose–insulin–glucagon dynamics and a population of in silico T1D subjects (100 adults, 100 adolescents, and 100 children). In particular, in this study, the 100 in silico T1D adults were used (mean ± standard deviation [SD]: age = 34 ± 10 years, body weight = 75.2 ± 12.1 kg), for each of whom the optimal daily pattern of basal insulin rate and CR were available and usable for calculating the optimal basal insulin infusion and prandial insulin boluses, respectively. Of note, the latest version of the simulator incorporates a series of novelties useful for the purpose of this work, including a model of diurnal SI variability.21
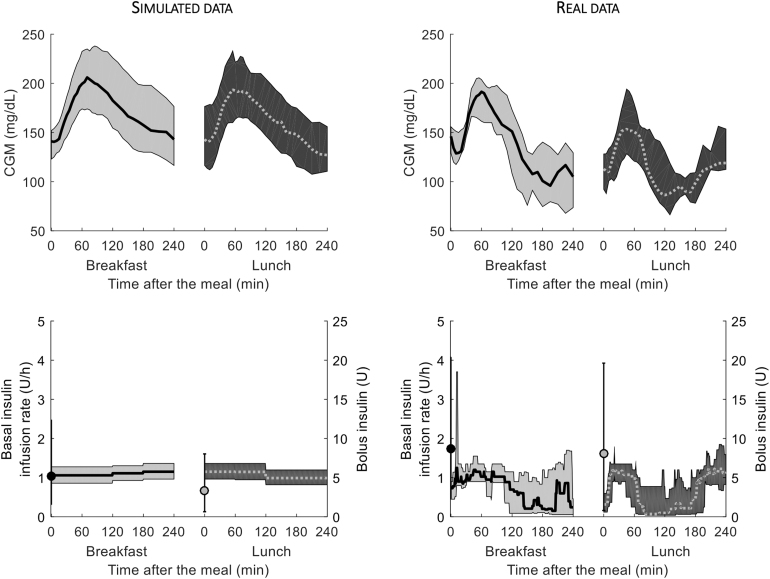
Each in silico subject received 70 g of carbohydrate (CHO) both at breakfast and lunch, these being separated by 4 h, with premeal insulin bolus calculated based on subject's optimal CR and subject's specific basal insulin infusion, mimicking the protocol performed by the real subjects (see below). Simulated CGM and CSII data are reported in Figure 1, left panels (top and bottom, respectively) for both meals, whereas simulated plasma glucose and insulin concentration data are shown in Supplementary Figure S1 (left panels, top and bottom, respectively). Of note, to simulate realistic data, we superimposed noise to simulated CGM traces as described in Visentin et al17; an independent, Gaussian noise, with zero mean and coefficient of variation (CV) equal to 2% to plasma glucose3; and an independent, Gaussian noise, with zero mean and known variance to plasma insulin data.22
FIG. 1.
Median (line) and interquartile ranges (shaded area) of CGM (top) and insulin pump (bottom) data, during breakfast (black continuous line) and lunch (gray dashed line) meals, for the simulated and real datasets (left and right panels, respectively). CGM, continuous glucose monitoring.
Real dataset
The real dataset was obtained from a larger study (NCT03234491) and composed of 11 youths with T1D (6 males; mean ± SD: age = 21 ± 4 years, BW = 64.8 ± 8.1 kg, HbA1c = 7.3% ± 0.6%), who underwent an open-label, randomized three-way crossover study comparing glucose control during hybrid closed-loop (HCL) therapy, using fast-acting insulin analog, with premeal insulin bolus given either through the subcutaneous route (control) or Afrezza® (MannKind, Danbury, CT) at two different doses.23 Here we used only data coming from the control visit of this study. One of the 11 subjects had incomplete pump data record, and, therefore, was excluded from the analysis.
The real subjects underwent the same experimental scenario as the virtual ones, consisting of two consecutive meals (breakfast and lunch), administered around 7 and 11 am, respectively. In particular, the ingested meals were designed by the metabolic kitchen to be identical in terms of CHO and nutrient content (total CHO content of 70–80 g per meal, lipid content of 14–15 g, protein content of 25–30 g, and energy 540–570 kcal). Premeal insulin bolus was calculated based on subject's CR for that meal, whereas basal insulin and/or correction boluses were calculated by the HCL platform (Diabetes Assistant, DiAs).24 The HCL platform included the Dexcom G5 sensor (Dexcom, San Diego, CA) and the t:slim insulin pump (Tandem Diabetes Care, San Diego, CA). Plasma glucose and insulin levels were frequently measured for 8 h using YSI 2300 glucose analyzer (YSI Life Sciences, Yellow Springs, OH) and Millipore ELISA assay (EMD Millipore Corporation, Burlington, MA), respectively.
Hypoglycemia (YSI glucose values below 80 mg/dL) was treated with 16 g fast-acting CHO if participants were experiencing symptoms. For YSI glucose values below 70 mg/dL, all subjects received a rescue oral glucose treatment regardless of the symptoms. The study protocol was approved by the Human Investigations Committee of the Yale School of Medicine (NCT03234491). The details of the original study are available elsewhere.23
Measured CGM and insulin pump data are reported in Figure 1, right panels (top and bottom, respectively), while frequently measured plasma glucose and insulin concentrations are reported in Supplementary Figure S1 (right panels, top and bottom, respectively).
Calculations
Assessment of SI from CGM and CSII data
Paralleling what already presented in a previous work,11 we made some approximations about how to use CGM sensor and CSII device as surrogates for the glucose and insulin signals, respectively, and we calculated the amount of carbs absorbed during the meal (AoC) [mg], possibly accounting for residual CHOs from previous meals (Supplementary Data). Thus, one obtains:
| (1) |
where BW is subject's body weight [kg], AUC area under the curve obtained using the trapezoidal rule, Δt time between meal ingestion () and the end of experiment (), Basal and Bolus the amount of insulin administered by the pump through basal infusion and premeal/correction boluses during the observation period, possibly accounting for residual active insulin (Insulin On Board, IOB)26 (Supplementary Data), CL plasma insulin clearance (L/min) calculated using a population model,27 and risk the function describing the behavior of insulin action below basal glucose levels. Precision of SISP estimation was assessed by propagating the measurement error on CGM traces to SISP.
Since the Dexcom G5 sensor was used in both real and simulated datasets, a mean absolute relative deviation equal to 9% was assumed.28 Finally, it is important to clearly define the domain of validity of Eq. (1). Specifically, SISP cannot be calculated if one or more of the following conditions occur:
-
(i)
is higher than 150 mg/dL 6 h after meal ingestion, since this makes it difficult for the quantification of insulin action and possibly leads to negative SISP estimates;
-
(i
i) CGM at meal time is below 60 mg/dL or above 200 mg/dL, since they are far from target glucose levels;
-
(i
ii) absolute glucose rate of change at meal time is higher than 2 mg/dL/min, since this reveals unstable glucose levels at the time of meal ingestion;
-
(i
v) the ratio between glucose excursion above and below the basal glucose value, as quantified by AUC, is below 60% and the precision of SISP, expressed as CV (CV %), is higher than 50%.
Validation
The above-described method was validated by comparing with the index of SI derived from the OGMM (), which employs plasma glucose and insulin concentration data,3 instead of CGM and insulin infusion by the pump.
In particular, the model is similar to the OGMM3 (Eqs. S1–S3 in Supplementary Data) but, here, the meal glucose rate of appearance (RaG) (mg/kg/min) was described with the model of gastrointestinal tract reported in another work,29 to facilitate model identification in the case of temporally close meals.
The model was identified from plasma glucose and insulin concentration data using a Bayesian Maximum a Posteriori estimator to help numerical identifiability. Measurement error on plasma glucose concentration was assumed to be independent, Gaussian, with zero mean and known SD (CV = 2%).3 Precision of model parameters was obtained from the Fisher Information matrix.30
Statistical analysis
Data are presented as median and interquartile range, unless otherwise specified. Two-sample comparisons were done by paired Student's t-test, for normally distributed variables, or Wilcoxon signed rank test, otherwise. Normality of the distributions was assessed by the Lilliefors test. Pearson's correlation was used to evaluate univariate linear correlation.
Results
Simulated dataset
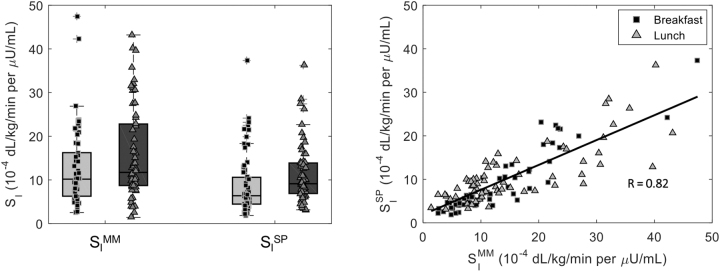
We were able to estimate SISP in about 91% of the simulated meals. The remaining ones fell outside the domain of validity of the method, described above, and were removed from the analysis. SISP was 6.4 [4.5, 10.6] and 9.1 [6.9, 13.9] 10–4 dL/kg/min per μU/mL at breakfast and lunch (CV = 15%), respectively, while SIMM was 10.2 [6.3, 16.2] and 11.7 [8.7, 22.8] 10–4 dL/kg/min per μU/mL in the two meals (CV = 5%) (Fig. 2, left panel). Both SISP and SIMM resulted significantly lower at breakfast than lunch (P = 0.019 and P = 0.003, respectively). The overall correlation between the two indices (Fig. 2, right panel) was high (R = 0.82, P < 0.001), as it was the meal-specific correlation for breakfast (RB = 0.86, P < 0.001) and lunch (RL = 0.78, P < 0.001). We also assessed the performance of SISP if plasma glucose was used instead of CGM: as expected, this led to a significant improvement in the overall correlation (R = 0.89, P < 0.001), and the correlations at breakfast (RB = 0.94, P < 0.001) and lunch (RL = 0.86, P < 0.001) alone (Supplementary Fig. S2).
FIG. 2.
Boxplots (left panel) and correlation (right panel) of SI indices obtained with the oral MM, which exploits plasma glucose and insulin data, versus SP, which exploits CGM sensor and insulin pump data, at breakfast (black squares) and lunch (gray triangles) meals for the simulated dataset. Pearson's correlation (R) was used to evaluate univariate linear correlation. MM, minimal model; SI, insulin sensitivity; SP, sensor and pump.
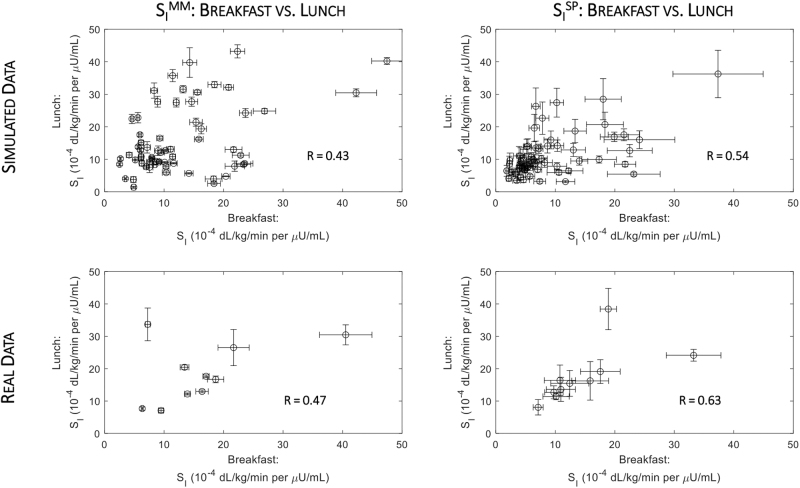
In addition, a correlation between SI at breakfast and lunch was shown for both the MM and the sensor and pump method (R = 0.43 and R = 0.54, P < 0.001, respectively; Fig. 4, top panels), but its extent is influenced by a nonnegligible intersubject variability. The ratio between lunch and breakfast indices obtained from the MM (lunch SIMM/breakfast SIMM) positively correlated with that obtained with the sensor and pump (lunch SISP/breakfast SISP) method (R = 0.54, P < 0.001) (Supplementary Fig. S4, left panel), which rose (R = 0.76, P < 0.001) if plasma glucose was used instead of CGM (Supplementary Fig. S5, left panel).
FIG. 4.
Correlation between SI indices at breakfast and lunch meals obtained either with the oral MM (left panels), which exploits plasma glucose and insulin data, and SP (right panels), which exploits CGM sensor and insulin pump data, for the simulated (top panels) and real datasets (right panels). Pearson's correlation (R) was used to evaluate univariate linear correlation.
Real dataset
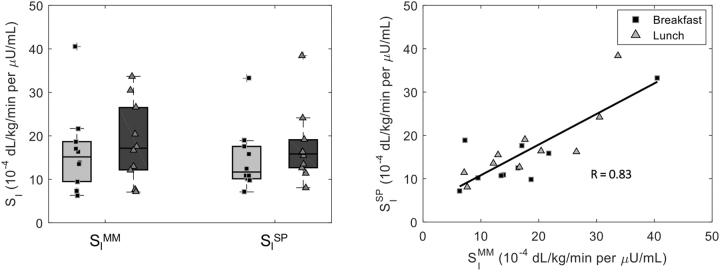
In real patients, SISP was 11.7 [10.1, 17.6] and 15.8 [12.7, 19.1] 10–4 dL/kg/min per μU/mL at breakfast and lunch (CV = 19%), respectively, whereas SIMM was 15.2 [10.5, 18.3] and 17.2 [12.4, 25.0] 10–4 dL/kg/min per μU/mL (CV = 6%) (Fig. 3, left panel). Of note, SISP resulted slightly significantly lower at breakfast than lunch (P = 0.02), whereas this was not the case for SIMM. The correlation between the two indices (Fig. 2, right panel) was good both overall (R = 0.83, P < 0.001), and by meal (breakfast: RB = 0.81, P < 0.001; lunch: RL = 0.85, P < 0.001). In agreement with the simulated results, we found a significant improvement in the correlation, both overall (R = 0.89, P < 0.001) and at breakfast (RB = 0.94, P < 0.001) and lunch (RL = 0.85, P < 0.001), whenever CGM data were substituted with plasma glucose (Supplementary Fig. S3). In addition, a correlation between SI at breakfast and lunch was shown for both the MM and the sensor and pump method (R = 0.47 and R = 0.63, respectively; Fig. 4, bottom panels). Such correlations were not statistically significant, probably due to both the nonnegligible intersubject variability and the small sample size (n = 10).
FIG. 3.
Boxplots (left panel) and correlation (right panel) of SI indices obtained with the oral MM, which exploits plasma glucose and insulin data, versus SP, which exploits CGM sensor and insulin pump data, at breakfast (black squares) and lunch (gray triangles) meals for the real dataset. Pearson's correlation (R) was used to evaluate univariate linear correlation.
The lunch SIMM/breakfast SIMM well correlated with lunch SISP/breakfast SISP (R = 0.85, P = 0.002) (Supplementary Fig. S4, right panel), which rose (R = 0.97, P < 0.001) if plasma glucose was used instead of CGM (Supplementary Fig. S5, right panel).
Discussion
We presented a novel SI index, derived from CGM and CSII data in daily life conditions. The index has been validated during two consecutive meal scenarios in a simulation framework, using the adult population of the UVa/Padova T1DS,17 and in real youths with T1D wearing a HCL system.23 The validation was performed by comparing, for each meal, SISP with the reference SIMM estimated using the OGMM.3
The results showed a good agreement between SISP and SIMM, with a high correlation between the two indices as well as a similar breakfast versus lunch pattern in both the simulated (Fig. 2) and real (Fig. 3) datasets. Of note, to facilitate model identification in the case of meals that are too close to each other (4 h), we coupled a model of the gastrointestinal tract to the model of glucose kinetics.3 This was necessary to obtain the best possible estimate of the reference SIMM since, after only 4 h, not all the ingested glucose might be fully absorbed into the circulation and this could affect the SIMM calculation. However, this is not facilitating the comparison between SIMM versus SISP, since the latter adopted an integral approach to compute the amount of glucose absorbed during the meal. On the contrary, the fact that the absorption model used in the OGMM is the same used to generate the (in silico) data would make SIMM as accurate as possible, thus making the validation of the integral method even more challenging.
Results also showed a nonnegligible intersubject variability in breakfast versus lunch SI, both for the MM and the SP method (Fig. 4). Hence, using the proposed method, one might wonder how many SI estimates would be required to detect a statistically significant variation in SI: assuming an average precision of SI estimation equal to 20%, a type I error equal to 5%, and a statistical power of 80%, the number of SI estimates required to detect a 20% (or 10%) variation in SI is 8 (or 32). In addition, we also calculated the ratio of SI estimated at lunch and breakfast with both the reference MM and sensor and pump methods and compared them (Supplementary Fig. S4). The correlation between the two was similar to, or slightly lower than, that obtained when comparing breakfast and lunch SI values separately. This is partially expected since SI estimates at breakfast and lunch are affected by an estimation error and this propagated (sometime badly) to ratios. Nevertheless, when comparing the same ratios obtained using plasma glucose instead of CGM, the correlation indices improved in both datasets (Supplementary Fig. S5).
The new SISP index overcomes some of the limitations of the one previously proposed by our group,11 for example, it works even in case of not well-spaced consecutive meals and/or in the presence of hypoglycemic events, which can occur in daily life conditions of individuals with T1D. This was achieved, thanks to the two modifications incorporated in the new formula: (1) the ability to describe the peculiar dynamic of insulin action in the hypoglycemic range and (2) the possibility to separately account for basal and bolus insulin in determining insulin action, which may also be beneficial for analyzing data coming from patients under multiple daily injection therapy.
To better grasp the positive impact of such modifications, we also calculated SISP using the previous approach11 and compared it with SIMM in our data: correlation between the model-based and sensor-based indices were lower with the previous method (from 0.82 and 0.83 of the newly proposed to 0.66 and 0.67 of the previous one, in simulated and real dataset, respectively). Moreover, the previously published method provided values of SISP more than doubled with respect to those estimated with the MM and the new formula (20.8 [11.0, 41.6] and 40.9 [35.0, 54.5] 10–4 dL/kg/min per μU/mL with the previous formula, in simulated and real dataset, respectively).
We also tested the proposed methodology in the adult population with T1D used to develop the previous version of the SISP methodology11: both SISP indices provided a good correlation when compared with SIMM (R = 0.79 with the new SISP, R = 0.82 with the previously published one). This proves that, in strictly controlled experimental conditions, that is, when consecutive meals are well spaced (meal-to-meal interval of at least 6 h) and there are not hypoglycemic events, both methodologies provide good results in terms of correlation with the reference SIMM.
We previously showed how SISP index can be used to optimize the insulin to CHO ratio (CR)13,14 Hereafter, we tested in silico the ability of the new SISP formula to optimize CR, and compared the performance against the one based on the previous SISP formulation.13 In particular, we used the virtual adult population of the UVa/Padova T1D Simulator17 to perform an in silico scenario, consisting of a 70 g CHO meal administered three times: in the first experiment, the meal insulin bolus was calculated based on patient-specific CR; whereas in the second and third experiments the meal insulin bolus was calculated using the CR based on the previous SISP calculation13 and the adapted CR derived from the new SISP methodology, both calculated from the CGM and insulin pump data of the first experiment. Moreover, to test the efficacy and robustness of the CR method against suboptimal CR therapy, three in silico scenarios were performed differing by the patient-specific CR used in the first experiment: nominal CR, CR underestimated by 20% and CR overestimated by 20%.
Results showed that, for each in silico scenario, the performance of the new SISP index in optimizing CR was almost identical to the ones previously reported,13 especially in protecting from hypoglycemia (results not shown). Furthermore, using the in silico data described above, we also assessed the robustness of the new SISP methodology to suboptimal CR: a good correlation was shown between SIMM versus the new SISP in all the analyzed scenarios (overall R = 0.90). Of note, as assessed by repeated measurements ANOVA followed by post-hoc analysis, both SIMM and SISP showed slightly (but statistically significant) higher SI values in the scenario with CR underestimated by 20%, and slightly (but statistically significant) lower SI in the scenario with CR overestimated by 20%, with respect to the scenario with nominal CR. This can be explained by the well-known nonlinear relationship between insulin-dependent glucose disposal and glucose levels, which results in an apparent reduction in SI (or increase in insulin resistance) when glucose levels achieve high values.31
Needless to say, before adapting patient's insulin therapy parameters, like CR, in real life condition of individuals with T1D, one should manage the within- and between-day variability in SI.32,33 As an example, one can iteratively update SI and CR estimates, let say every week, using a run-to-run approach, as done in,16 where the previous version of SI11 was used to optimize CR during a closed-loop study.
Differently from other approaches for SI estimation, for example,10,12 our integral approach does not require any assumptions on glucose absorption and subcutaneous insulin infusion. On the other hand, our approach provides an estimate of SI with a lower time resolution (every few hours—e.g., after each meal—instead of almost every 5 min10). However, given the slow dynamics of the glucose–insulin system, the impact of fast SI variations on glucose outcomes is expected to be modest.34,35
The methodology proposed in this study still has some limitations. Our study was validated in a limited number of real youths and adults with T1D, therefore additional studies are needed to extend the domain of validity of the method to populations of different ages. Moreover, the method requires dynamic data, particularly after a meal ingestion, and thus it cannot be used to assess SI during night-time and/or in the postabsorptive state. Future work will also focus on the extension of the methodology to assess SI in these conditions and/or to assess if the administration of a small insulin bolus, able to properly induce a glucose excursion without causing hypoglycemia, would allow to estimate SISP even overnight. Finally, the method was not designed to assess SI in subjects with type 2 diabetes on CGM and CSII, as the endogenous insulin secretion, which is not modeled in the method proposed in this study, might represent a confounding factor in the quantification of the effect of exogenous insulin on glucose kinetics.
As discussed, SISP index cannot be calculated when CGM readings are too high or too low for a long-time frame after the meal (Methods section) since, in such conditions, nonlinearities in insulin action or counterregulatory mechanisms may occur, which are not accounted by the method. Nevertheless, these limits apply also to the OGMM. To this end, the use of in silico data was fundamental to assess the constraints cited above and to strengthen the validation of the methodology. Another limitation concerns the need to fix some parameters (GEZI, VG, r1, and r2) to population values3,25,36 and to calculate others from population models (CL) using anthropometric data.27 Nevertheless, these values were consistently fixed in both SISP and SIMM and, according to what was reported in a previous work,11 the sensitivity of the method to the chosen values was modest. Finally, the accuracy of SISP relies on the quality of the data provided by CGM devices, which luckily has greatly improved in the last decade. Another important information, which affects the estimation of SISP, as well SIMM, is the knowledge of the amount of CHOs entering the circulation.
We also acknowledge that macronutrient composition or other surrogate indices, like the glycemic index, can be helpful in the calculation of SISP, especially in the presence of multiple meals close to each other. To deal with this, we have previously developed and validated the concept of the CHOs on Board (COB, Supplementary Data),11 using model-based estimations of glucose postprandial rate of absorption. Future work will focus on assessing how different types of CHOs,37 macronutrient intake, and/or food glycemic indices modulate COB, as well as how these affect SI. In fact, it has been shown that meal composition may affect the estimate of SI, with an apparent reduction by 20%–30% in the presence of fat and proteins (e.g., oral glucose vs. mixed meal test).38 In addition, learning techniques can also be applied to classify meal compositions based on CGM readings, and this information may be used to tune the SI estimation algorithm. This, however, would require the availability of data collected in real-life conditions, in which meal doses and compositions are known.
The current version of the proposed methodology can be used by individuals with T1D using either sensor-augmented pump (SAP) therapy or closed-loop systems. Future works will include the extension of SISP to the broader population of T1D on multiple daily injection therapy, as well as subjects with T1D with different degrees of insulin resistance. Finally, it would be interesting to assess the accuracy and limitations of the methodology in other conditions like exercise, sick-days, use of drug modifying the SI (e.g., steroids), menstrual cycle, as well as intra- and interday variations of SI due to habits and/or behavioral factors, as apparently shown also in well-controlled experimental conditions.32,33 However, these require additional data not available to date.
Conclusions
We propose a new index of SI, estimated from glucose sensor and insulin pump data, and thus valuable to quantify SI in real-life conditions in subjects with T1D. The method was validated, both in silico and in vivo, against the OGMM. This methodology would additionally permit, once the repeatability of the method is assessed, the quantification of the intra- and interday variability of SI, and correlation with patient's daily activities. This, in turn, would forecast an automatic optimization of insulin treatment for patients on SAP or HCL that includes adjustments of insulin-to-CR and/or insulin correction factor.
Supplementary Material
Authors' Contributions
M.S. developed the method, performed the analysis, contributed to the discussion, and wrote the article. C.D.M. developed the method, supervised the analysis, contributed to the discussion, and edited the article. A.G. and E.C. designed and conducted the clinical study in youths, contributed to the discussion, and critically revised the article. A.B. and Y.C.K. provided the data from the adult cohort and critically revised the article. C.D.M. is the guarantor of this work and, as such, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work supported by MIUR (Italian Minister for Education) under the initiative, “Departments of Excellence” (Law 232/2016) (to M.S. and C.D.M.); Institute for Pediatric Research, Padova, Italy (StarG); ISPAD Research Fellowship (to A.G.); UCSF Benioff Professor in Children's Health Endowed Chair Fund, San Francisco, CA, USA (to E.C.); and the National Institutes of Health K-R01-085516 and DK-DP3-094331 (to A.B. and Y.C.K.).
Supplementary Material
References
- 1. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223. [DOI] [PubMed] [Google Scholar]
- 2. Bergman RN, Ider YZ, Bowden CR, et al. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677. [DOI] [PubMed] [Google Scholar]
- 3. Dalla Man C, Caumo A, Basu R, et al. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: Validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643. [DOI] [PubMed] [Google Scholar]
- 4. Mari A, Pacini G, Murphy E, et al. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–548. [DOI] [PubMed] [Google Scholar]
- 5. Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301. [DOI] [PubMed] [Google Scholar]
- 6. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 7. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 9. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 10. Fabris C, Ozslan B, Breton MD. Continuous glucose monitors and activity trackers to inform insulin dosing in type 1 diabetes: The University of Virginia contribution. Sensors 2019;5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schiavon M, Dalla Man C, Kudva YC, et al. Quantitative estimation of insulin sensitivity in type 1 diabetic subjects wearing a sensor-augmented insulin pump. Diabetes Care 2014;37:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fabris C, Nass RM, Pinnata J, et al. The use of a smart bolus calculator informed by real-time insulin sensitivity assessments reduces postprandial hypoglycemia following aerobic exercise session in individuals with type 1 diabetes. Diabetes Care 2020;43:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiavon M, Dalla Man C, Cobelli C. Insulin sensitivity index-based optimization of insulin to carbohydrate ratio: In silico study shows efficacious protection against hypoglycemic events caused by suboptima therapy. Diabetes Technol Ther 2018;20:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schiavon M, Dalla Man C, Cobelli C. Physiology-based run-to-run adaptation of insulin to carbohydrate ratio improves type1 diabetes therapy: Results from an in silico study. 2019. American Control Conference. [Google Scholar]
- 15. Dassau E, Brown SA, Basu A, et al. Adjustment of open-loop settings to improve closed-loop results in type 1 diabetes: A multicenter randomized trial. J Clin Endocrinol Metab 2015;100(10):3878–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dassau E, Pinsker JE, Kudva YC, et al. 12 week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery setting: Effect on hemoglobin A1C and hypoglycemia. Diabetes Care 2017;40:1719–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Visentin R, Campos-Nàñez E, Schiavon M, et al. The UVA/Padova type 1 diabetes simulator goes from single meal to single day. J Diabetes Sci Technol 2018;12:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cobelli C, Renard E, Kovatchev B. Artificial pancreas: Past, present, future. Diabetes 2011;60:2672–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiavon M, Visentin R, Giegerich C, et al. In silico head-to-head comparison of insulin glargine 300 U/mL and insulin degludec 100 U/mL in type 1 diabetes. Diabetes Technol Ther 2020;22:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edelman SV. Regulation catches up to reality: Nonadjunctive use of continuous glucose monitoring data. J Diabetes Sci Technol 2017;11:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Visentin R, Dalla Man C, Kudva YC, et al. Circadian variability of insulin sensitivity: Physiological input for in silico artificial pancreas. Diabetes Technol Ther 2015;17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toffolo G, Campioni M, Basu R, et al. A minimal model of insulin secretion and kinetics to assess hepatic insulin extraction. Am J Physiol Endocrinol Metab 2006;290(1):E169–E176. [DOI] [PubMed] [Google Scholar]
- 23. Galderisi A, Cohen N, Calhoun P, et al. Effect of Afrezza on glucose dynamics during HCL treatment. Diabetes Care 2020;43:2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovatchev BP, Cheng P, Anderson SM, et al. Feasibility of long-term closed-loop control: A multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24. [DOI] [PubMed] [Google Scholar]
- 25. Dalla Man C, Micheletto F, Lv D, et al. The UVA/Padova type 1 diabetes simulator: New features. J Diabetes Sci Technol 2014;8:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patek SD, Magni L, Dassau E, et al. Modular closed-loop control in diabetes. IEEE Trans Biomed Eng 2012;59:2986–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campioni M, Toffolo G, Basu R, et al. Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetics parameters. Am J Physiol Endocrinol Metab 2009;297:E941–E948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol 2015;9(2):2019–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dalla Man C, Camilleri M, Cobelli C. A system model of oral glucose absorption: Validation on gold standard data. IEEE Trans Biomed Eng 2006;53:2472–2478. [DOI] [PubMed] [Google Scholar]
- 30. Cobelli C, Carson ER. Introduction to Modeling in Physiology and Medicine. Academic Press: San Diego, CA, USA; 2008. [Google Scholar]
- 31. Yki-Jarvinen H, Young AA, Lamkin C, et al. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest 1987;79:1713–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hinshaw L, Dalla Man C, Nandy DK, et al. Diurnal pattern of insulin action in type 1 diabetes: Implications for a closed-loop system. Diabetes 2013;62(7):2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalla Man C, Pillonetto G, Riz M, et al. An index of parameter reproducibility accounting for estimation uncertainty: Theory and case study on β-cell responsivity and insulin sensitivity. Am J Physiol Endocrinol Metab 2015;308(11):E971–E977. [DOI] [PubMed] [Google Scholar]
- 34. Kanderian SS, Weinzimer S, Voskanyan G, et al. Identification of intraday metabolic profiles during closed-loop glucose control in individuals with type 1 diabetes. J Diabetes Sci Technol 2009;3(5):1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996;45(8):1044–1050. [DOI] [PubMed] [Google Scholar]
- 36. Basu A, Dalla Man C, Basu R, et al. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness and postprandial glucose metabolism. Diabetes Care 2009;32:866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Basu R, Schiavon M, Petterson XM, et al. A novel natural tracer method to measure complex carbohydrates metabolism. Am J Physiol Endocrinol Metab 2019;17(3):483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bock G, Dalla Man C, Campioni M, et al. Effects of nonglucose nutrients on insulin secretion and action in people with pre-diabetes. Diabetes 2007;56:1113–1119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.