Abstract
People with schizophrenia (SZ) are at increased risk for type-2 diabetes (DM), its complications, depression, and disability. However, little is known about the interrelationships of these three factors in adults with SZ and DM.
Purpose:
We assessed the number of diabetic complications and depressive symptom severity as predictors of disability and evaluated depressive symptom severity as a mediator of the relationship between diabetic complications and disability in a sample of 62 adults with SZ and DM.
Methods:
Two- and three-step sequential regression models were used to evaluate depression and the number of diabetic complications as predictors of disability. Path analysis with bootstrapping was used to evaluate depressive symptom severity as a mediator of the relationship between complications and disability.
Results:
Diabetic complications significantly predicted disability scores when controlling for age, gender, socioeconomic status, hemoglobin A1C, positive symptom severity, and negative symptom severity. The addition of depression severity scores resulted in a significant increase in explained variance in disability scores. In the final model, only depression severity scores were significantly associated with disability scores. The full- model accounted for 56.2% of the variance in disability scores. Path analysis revealed a significant indirect association of complications on disability through depression severity scores while controlling for all covariates. The association between complications and disability was non-significant when depression symptom severity was included in the model.
Discussion:
Relative to diabetes complications, depressive symptoms may present a more effective and tractable target for interventions aimed at reducing disability in people with SZ and DM.
Keywords: Schizophrenia, Depression, Diabetes, Complications, Disability
Introduction
Schizophrenia and other psychotic disorders are among the most severe and disabling psychiatric conditions (1). Globally, schizophrenia is the eighth leading contributor to Disability Adjusted Life Years lost for people between ages 15 and 44 (2). The disease course for schizophrenia is associated with a high rate of symptom relapse (e.g., acute psychosis) (3), and even those who achieve sustained clinical remission often experience protracted functional impairment (4). Consequently, disability among people with schizophrenia is characterized by poor social and vocational outcomes, as well as low rates of independent living (5).
In addition to the social and functional burden of disease, people with schizophrenia are at increased risk for comorbid physical and mental health conditions, such as diabetes (6, 7) and depression (8), which may further contribute to high levels of disability. For example, chronic medical complications associated with the course of diabetes (e.g., heart disease, vascular disease, peripheral neuropathy, kidney failure, and ocular disease) have been associated with greater rates of physical disability (9, 10) among the general population (i.e., a non-psychiatric population). Similarly, depressive symptoms are related to high rates of disability among individuals in the general population (11, 12) and people with schizophrenia (13).
While major depression and diabetes are frequently comorbid (14), little is known about the relative contribution of these factors to disability. Research has shown that the co-occurrence of these disorders is associated with the number of lost work days (15) and risk for functional disability within the general population (16). Furthermore, some research has suggested that, compared to the complications of diabetes, depression may have a stronger relationship to lost work days (17) and global level of disability (18) among those with diabetes.
Findings from other research have suggested that depressive symptoms may partially account for the disabling effect of diabetic complications (19). For example, diabetes and the burden of its complications have been associated with greater experience of depressive symptoms (20–23). In turn, high depressive symptom severity has been associated with high levels of disability among people with diabetes (15, 16). At least one recent study has also shown that depressive symptoms actually precipitate the development of disability among people with diabetes in the general population (24). Given these associations, it is possible that diabetic complications may impact disability through depressive symptoms. However, few studies have simultaneously examined the relationships of diabetic complications, depressive symptom severity, and disability in the general population or in unique subpopulations. Additional research is particularly needed to improve our understanding of the relationship among depressive symptoms, diabetes complications, and disability in high risk populations such as those with serious mental illness and type-2 diabetes.
The purpose of this study was to examine the role of depression within the relationship between diabetes complications and disability among adults with schizophrenia and type-2 diabetes. First, we hypothesized that diabetic complications and depressive symptom severity would be positively associated with disability among members of this population. Second, we expected that depressive symptom severity would account for the variance in disability above and beyond the total number of diabetic complications when controlling for potential covariates of disability (i.e., age, gender, socioeconomic status, positive symptoms, negative symptoms, and hemoglobin A1C). Third, we expected that depressive symptom severity would mediate the putative relationship between number of diabetic complications and disability.
Methods
Participants
The current study used baseline data from a pilot intervention which aimed to improve health outcomes in adults with serious mental illness and comorbid type-2 diabetes. Participants who were over the age of 18, demonstrated the capacity to provide informed consent, and had a chart diagnosis of schizophrenia or schizoaffective disorder and comorbid type-2 diabetes were included in this study. Participants with a diagnosis of dementia were excluded.
Procedure
Study participants were recruited at board-and-care facilities, day treatment programs, and community club houses in San Diego County. Following receipt of informed consent, participants were invited to take part in an assessment with a trained interviewer. Interviews lasted approximately 2.5 hours, and participants were compensated 10 dollars for completing the interview. All participants were treated in a manner consistent with the American Psychological Association Ethical Principles of Psychologists and Code of Conduct. All methods were approved by the appropriate IRB boards.
Measures
Sociodemographic and diabetes-related variables.
Sociodemographic measures were used to gather self-report data on participants’ age and gender. Medical and psychiatric chart abstraction was used to verify participants’ psychiatric diagnosis (i.e., schizophrenia or schizoaffective disorder) and diabetes status.
Hollingshead Four Factor Index of Social Status.
The Hollingshead Four Factor Index of Social Status is a measure of socioeconomic status based on highest level of education, occupation, marital status, and sex (25). The measure has demonstrated convergent validity with yearly income (r = .78 for men and r = .67 for women) (25).Weighted scores for participants’ current occupation and highest level of education are summed to generate a social status score. Scores range from 11 to 84 with higher scores indicating higher socioeconomic status.
Positive and Negative Syndrome Scale.
Participants’ psychiatric symptom severity was measured using the Positive and Negative Syndrome Scale (PANSS). The PANSS is a 30-item, semi-structured interview that is administered by a trained rater. Item scores are based on verbal responses, behavioral observation, and collateral reports. Individual item scores are aggregated to yield three subscales that reflect positive, negative, and general psychiatric symptom severity. The positive and negative symptom severity scales were used for the purpose of this study. The PANSS positive and negative subscales have demonstrated acceptable to good internal reliability (α = .73 and α = .83, respectively) and convergent validity with other measures of positive and negative symptom severity (26). Higher scores reflect greater symptom severity.
Hemoglobin A1C.
Hemoglobin A1C (A1C) is a measure of participants’ glucose control during the previous 2 to 3 months (27). Fasting blood samples of 3 mL were collected from each participant at the baseline time point. Blood samples were submitted to high performance liquid chromatography (28). Values for A1C are expressed as a ratio of glycosolated hemoglobin to total hemoglobin (28). Higher values reflect higher average blood glucose levels.
Hamilton Depression Rating Scale.
Depressive symptom severity was measured using the 17-item Hamilton Depression Rating Scale (HAM-D) (29). Ratings for each item are made by a trained interviewer. The HAM-D is a commonly used measure of depressive symptom severity that has demonstrated good reliability (i.e., internal reliability, inter-rater reliability, test-rest reliability) and the ability to accurately differentiate among depressed and non-depressed patients with schizophrenia (sensitivity = 79%, specificity = 83%) (30). Higher scores reflect greater depressive symptom severity.
Diabetic Complications.
Section IV of the Diabetes History Form Version 2.0 (DMHV2) was used to collect self-report data on 27 diabetes-related complications. Established diabetic complications were identified using the 2011 Diabetes Fact Sheet (31). Participants’ responses (i.e., Yes/No) on these items were used to quantify total number of diabetic complications. No studies have assessed the reliability or validity of the DMH-V2; however, this instrument has been used in published studies (32).
World Health Organization Disability Assessment Scale – Second Version.
Total disability was assessed using the Word Health Organization Disability Assessment Schedule – Second Version (WHODAS-II). The WHODAS-II includes 36 items spread across five subscales (i.e., understanding and communicating, getting around, self-care, getting along with other people, and life activities). Participants rate their disability on 5-point Likert-type scales (1 = none, 5 = extreme or cannot do). The WHODAS-II has shown good internal and test-retest reliability, as well as good convergent validity with other measures of self-reported quality of life among people with schizophrenia (13). A total percent disability (0%–100%) was calculated using the Item Response Theory algorithm provided by the World Health Organization. Percent disability, as measured by the WHODAS-II, was the dependent variable of interest in this study. Higher scores indicate a greater level of disability.
Statistical Analysis
The sample was characterized using descriptive statistics. To test the first hypothesis, a two-step sequential linear regression analysis was used to evaluate number of diabetic complications as a predictor of WHODAS-II scores. Age, gender, socioeconomic status, psychiatric symptom severity (i.e., PANSS positive and negative scores), and A1C were entered in the first block of the model to statistically control for the potential effects of these variables on WHODAS-II scores. Number of diabetic complications was entered into the second block. To test the second hypothesis, a separate sequential linear regression model added HAM-D scores in a third block to the model specified for hypothesis one. Pearson product-moment correlations were used to assess for colinearity between predictor variables. The order of variable entry was determined a priori in the case of both models. Regression analyses were performed using SPSS, version 19.0. The third hypothesis was tested using a path model with bootstrapping (5,000 replications) to calculate the effect size and confidence intervals for the indirect path of total diabetic complications on disability through depressive symptom severity. This path model was tested using the PROCESS macro for SPSS (33). Hayes has suggested that partially standardized measures of indirect effect size (reported here) are stable given small samples (34). PROCESS calculates partially standardized coefficients by rescaling the product of the a and b paths to the standard deviation of the Y variable (i.e., WHODAS-II scores). These effect sizes are expressed in terms of the X scale metric (i.e., number of diabetic complications). All other coefficients reported for the path model (i.e., a path, b path, direct, and total effects) are expressed as unstandardized coefficients. An alternative path model was used to test the indirect association of depressive symptom severity on disability via diabetic complications. The practice of testing competing path models within a specified set of variables is consistent with methods described by Hayes (34). Alpha was set at p < .05 and all tests were two-tailed.
Results
Participants
Baseline data for 64 participants with chart diagnoses of schizophrenia or schizoaffective disorder and type-2 diabetes were available for analysis in the present study. Of these participants, one was excluded due to missing diabetes complications data and another was excluded for missing A1C data. Therefore, the final sample consisted of 62 adults with schizophrenia (n = 52, 83.8%) or schizoaffective disorder (n = 10, 16.1%) and type-2 diabetes. The sample was predominately male (n = 36, 58.1%) and Caucasian (n = 36, 58.1%) with an average age of 52.5 (SD = 8.9) years. The average score on the Four Factor Index of Social Position was 24.0 (SD = 4.5) indicating relatively low socioeconomic status (25). PANSS positive (M =14.8, SD = 5.6) and negative (M = 15.3, SD = 5.6) symptom scores were somewhat lower than those reported in a PANSS validation study by Kay et al. (26). Internal consistency was acceptable for the PANSS positive (α = .78) and PANSS negative (α = .77) subscales. Participants’ mean A1C (M = 7.1%, SD = 2.4%) was slightly above the maximum threshold recommended by the American Diabetes Association (i.e., > 7.0) for glycemic control (35).
The average depression severity score for the sample (i.e., HAM-D) was 9.6 (SD = 7.0) and somewhat lower than the cutoff score (i.e., >12) suggested to maximize specificity and sensitivity for depression among people with schizophrenia (30). Internal reliability for the HAM-D was adequate (α = .79). The average participant had more than two diabetic complications according to the DMH-V2. Cardiovascular problems (e.g., heart attack, stroke, or high blood pressure) were the most frequently reported complications, followed by lower extremity complications (e.g., peripheral vascular disease and peripheral neuropathy), ocular complications (e.g., retinopathy, blindness, and macular edema), and renal complications (i.e., kidney failure). See Table 1.
Table 1.
Means and Frequencies of Diabetic Complications (n = 62)
| Diabetic Complication | Frequency n (%) | Mean Number of Complications M (SD) |
|---|---|---|
|
| ||
| Cardiovascular | 0.84 (0.81) | |
| Heart Attack | 5 (8.0) | |
| Heart Failure | 3 (34.8) | |
| Hypertension | 33 (53.2) | |
| Angina | 4 (6.5) | |
| Transient Ischemic Attack | 4 (6.5) | |
| Stroke | 4 (6.5) | |
| Ocular | 0.08 (0.37) | |
| Retinopathy | 0 (0.0) | |
| Blindness | 3 (4.8) | |
| Macular Edema | 0 (0.0) | |
| Renal/Bladder | 0.37 (0.72) | |
| Kidney Failure | 3 (4.8) | |
| Bladder/Kidney Infection | 14 (22.6) | |
| Protein in Urine | 6 (9.7) | |
| Lower Extremity | 0.89 (1.16) | |
| Peripheral Vascular Disease | 11 (17.7) | |
| Intermittent Claudication | 6 (9.7) | |
| Peripheral Neuropathy | 14 (22.6) | |
| Gangrene | 1 (1.6) | |
| Foot Ulcers | 2 (3.2) | |
| Athletes Foot | 22 (35.4) | |
| Amputation | 0 (0.0) | |
| Total | 2.29 (1.90) | |
Factors Associated with Disability
Pearson correlations showed significant positive relationships between WHODAS-II scores, number of diabetic complications, r = .42, p = .001, and HAM-D scores, r = .63, p < .001. A two-step sequential linear regression model (Model 1) was used to evaluate number of diabetic complications as a predictor of WHODAS-II scores while controlling for age, gender, socioeconomic status, psychiatric symptom severity (i.e., positive and negative symptoms) and A1C. The first block of Model 1 included all four covariates and accounted for a significant amount of variance in WHODAS-II scores, F(6,55) = 2.90, R2 = .24, p = .016. Of these covariates, only female gender was significantly associated with WHODAS-II scores, B = −13.13, SE = 4.34, t = −3.03, p = .004. As expected, the addition of diabetic complications in the second block of Model 1 accounted for a significant increase in explained variance above the first block (i.e., age, gender, socioeconomic status, PANSS positive scores, PANSS negative scores, and A1C), ΔF(1,54) = 15.25, ΔR2 = .17, p < .001. Number of diabetic complications was significantly associated with disability while controlling for covariates entered in the first block, B = 3.97, SE = 1.01, t = 3.91, p < .001. See Table 2.
Table 2.
Two and three-step sequential linear regression models predicting WHODAS-II total scores
| Block/Predictors | Model 1a (β) |
Model 2b (β) |
ΔR2 |
|---|---|---|---|
|
| |||
| Block 1 | .24* | ||
| SES | −.15 | −.12 | |
| Age | −.07 | .00 | |
| Gender | −.32* | −.30* | |
| PANSS Positive | .19 | .09 | |
| PANSS Negative | .01 | −.13 | |
| A1C | −.13 | −.15 | |
| Block 2 | .17** | ||
| Diabetic Complications | .43** | .20 | |
| Block 3 | .16** | ||
| HAM-D | .50** | ||
Note: SES = Socioeconomic Status, PANSS = Positive and Negative Syndrome Scale, HAM-D = Hamilton Depressive Rating Scale, A1C = Hemoglobin A1C
Blocks 1 and 2
Blocks 1, 2, and 3
p < .05
p < .001
A separate three-step sequential linear regression model (Model 2) added HAM-D scores in a third block to the previous two-step model. This model showed that HAM-D scores accounted for a significant increase in variance above and beyond the combined variance of blocks one (i.e., age, gender, socioeconomic status, positive symptoms, negative symptoms, and A1C) and two (i.e., diabetic complications), ΔF(1,53) = 18.70, ΔR2 = .16, p < .001. Only gender, B = −10.49, SE = 3.38, t = −3.10, p = .003, and HAM-D scores, B = 1.24, SE =.29, t =4.33, p < .001, remained significant predictors of WHODAS-II scores in the full model. The full model (Model 2), including diabetic complications, HAM-D scores, and all six covariates, accounted for 56.2% of the variance in WHODAS-II total scores. See Table 2.
The Mediating Role of Depressive Symptom Severity
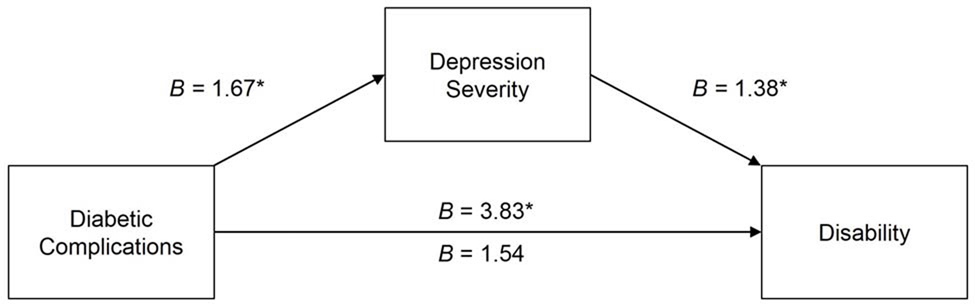
As hypothesized, a path model showed a significant indirect association for total number of diabetic complications with WHODAS-II scores through HAM-D scores, B = 0.13, SE = 0.04, CI.95 [0.07, 0.22], p < .05. Number of diabetic complications was positively associated with depressive symptom severity, B = 1.67, SE = .43, t = 3.87, p < .001, and depressive symptom severity was in turn positively associated with increased level of disability, B = 1.38, SE = 0.27, t = 5.05, p < .001. The total association between number of diabetic complications and disability was significant, B = 3.83, SE = 1.08, t = 3.56, p < .001; however, the direct association was not significant, B = 1.54, SE = 1.01, t = 1.52, p = .13. See Figure 1. This result indicates that depressive symptom severity fully accounted for the effect of diabetic complications on disability. The hypothesized indirect relationship remained significant (p < .05) while controlling for age, gender, socioeconomic status, PANSS positive scores, PANSS negative scores, and A1C. An alternative path model showed no significant indirect effect of depressive symptom severity on disability through diabetic complications, B = .01, SE = .01, CI.95 [−0.03, 0.21], p > .05.
FIGURE.
The Indirect Association of Total Number of Diabetic Complications on Disability (i.e., WHODAS-II Scores) Through Depressive Symptom Severity (i.e., HAM-D Scores). Note: B Depicted in the Figure is an Unstandardized Regression Coefficient. The Indirect Effect of the Total Diabetic Complications on WHODAS-II Scores (Reported as a Partially Standardized Regression Coefficient) was Significant (B = 0.13, SE = 0.04, CI.95 [0.7, 0.22], p « 0.05). the Coefficient Above the c Path is the Total Association and the Coefficient Below the c Path Represents the Direct Association, *p < 0.001.
Discussion
The current study is one of the first to examine predictors of disability among people with schizophrenia and comorbid type-2 diabetes. Results showed that both number of diabetic complications and depressive symptom severity are significantly associated with level of disability. However, depressive symptom severity accounted for variance in disability scores above and beyond participants’ number of diabetic complications. This result is consistent with other findings, which have shown a stronger association of disability with depression than with diabetic complications among the general population (i.e., a non-psychiatric population) (18). Interestingly, the relationship between depressive symptom severity and disability existed in the present sample when controlling for variance due to diabetic complications as well as sociodemographic (i.e., age, gender, socioeconomic status), psychiatric (i.e., positive and negative symptoms severity), and clinical factors (i.e., A1C). This finding indicates that depressive symptom severity has a robust relationship to disability which is not fully accounted for by other potentially important demographic, psychiatric, and clinical factors among people with schizophrenia and comorbid diabetes.
These results build on previous findings in two important ways. First, this study simultaneously assessed the relationships of diabetic complications and depressive symptoms to disability in a population at risk for both depression and diabetes (i.e., adults diagnosed with schizophrenia). Second, the current results, which utilized continuous measures of depression severity and disability level, extend previous findings that have relied on dichotomous variables (e.g., major depression vs. no depression, disabled vs. non-disabled). This study consequently provides evidence for a strong linear relationship between depressive symptom severity and disability in this population.
Of particular interest, path analyses in this study provided some support to the mediating role of depressive symptom severity on the relationship between diabetic complications and disability. Specifically, the path model demonstrated that a high number of diabetic complications is associated with a greater depressive symptom severity, which is, in turn, associated with a high level of disability. These findings suggest that depressive symptom severity may partially explain the association between burden of diabetic complications and disability among people with serious mental illness such as schizophrenia. Notably, a separate path model showed that diabetic complications did not mediate the relationship between depressive symptom severity and disability.
These results are supported by prior literature, which has demonstrated that the burden associated with diabetes complications (i.e., illness intrusiveness and associated distress) contributes to higher levels of depressive symptoms (36–38), that are, in turn, associated with high levels of self-reported disability among non-psychiatric populations with diabetes (16, 24). However, other possible causal relationships among these variables should be noted. For example, high depressive symptom severity may simultaneously increase the risk for both complications and disability. Other important variables should be considered as well. For example, depression may lead to greater diabetic complications through poor self-care behaviors (i.e., diet and exercise) and associated long-term glycemic dysregulation (39). Alternatively, all three variables may interact via bidirectional relationships.
Limitations
Results of this study should be considered in the context of four important limitations. First, all data included in the current study were derived from participants who elected to participate in a baseline assessment for a healthy lifestyle program; however, no restriction of range was observed among the study variables. Second, this study used a cross-sectional design, and, as a result, the causal effects among the variables within the path model cannot be known with certainty. For example, extant literature provides support for a bidirectional causal relationship between diabetic complications and depressive symptoms (i.e., the a path specified with the current model) (40). However, an alternative path model, tested in the current study, showed no indirect relationship between depressive symptoms and disability via number of diabetic complications. Additional studies using longitudinal designs and lagged analyses are needed to better quantify the causal direction of the interrelationships among these variables. Third, the clinical sample used for the current study (n = 62) was relatively small. Nevertheless, the relationships between disability and the variables of interest (i.e., depressive symptom severity and number of diabetic complications) were highly significant (p < .001) and accounted for a substantial amount of variance in disability. Fourth, the HAM-D contains a number of items that assess somatic symptoms of depression. As a result, participants with a greater number of diabetic complications may have endorsed more items on this scale.
Clinical Implications
Findings from the present study suggest that depression may be an important clinical target for practitioners or program developers attempting to reduce the burden of disability among adults with schizophrenia and diabetes. Namely, results indicate that depressive symptoms (i.e., a psychological factor) may have a stronger relationship to overall self-reported disability than number of diabetic complications (i.e., a physical health factor), and may in fact at least partially account for the relationship between diabetic complications and disability. Before applying these findings to clinical cases, additional research using other factors of interest and employing prospective designs and lagged analyses is needed to better specify the nature of the interrelationships of depression, diabetes complications, and disability. Subsequent work may be needed to examine the efficacy of current empirically supported psychotherapy and psychopharmaceutical treatments to reduce depressive symptom severity in adults with serious mental illness and type-2 diabetes.
Acknowledgement:
This work was supported, in part, by the National Institute of Mental Health grants MH063139, MH66248, MH62554, RR00827, and by the Department of Veterans Affairs. This work was also supported by the National Center for Research Resources (P20RR016474) and the National Institute of General Medical Sciences (P20GM103432) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rossler W, Salize HJ, van Os J, Riecher-Rossler A. Size of burden of schizophrenia and psychotic disorders. European Neuropsychopharmacology. 2005; 15(4): 399–409. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. The World Health Report 2001–Mental health: New Understanding, New Hope. Geneva, Switzerland. World Health Organization. 2001. [Google Scholar]
- 3.Robinson DG, Woerner MG, Alvir JMJ, Geisler S, Koreen A, Sheitman B, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 1999; 156(4): 544–9. [DOI] [PubMed] [Google Scholar]
- 4.Hert MD, van Winkel R, Wampers M, Kane J, van Os J, Peuskens J. Remission criteria for schizophrenia: Evaluation in a large naturalistic cohort. Schizophrenia Research. 2007; 92(1): 68–73. [DOI] [PubMed] [Google Scholar]
- 5.Harvey PD, Helldin L, Bowie CR, Heaton RK, Olsson A-K, Hjärthag F, et al. Performance-based measurement of functional disability in schizophrenia: A cross-national study in the United States and Sweden. The American Journal of Psychiatry. 2009; 166(7): 821–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon L, Weiden P, Delahanty J, Goldberg R, Postrado L, Lucksted A, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophrenia Bulletin. 2000; 26(4): 903–12. [DOI] [PubMed] [Google Scholar]
- 7.De Hert M, Mauri M, Shaw K, Wetterling T, Doble A, Giudicelli A, et al. The METEOR study of diabetes and other metabolic disorders in patients with schizophrenia treated with antipsychotic drugs. I. Methodology. International Journal of Methods in Psychiatric Research. 2010; 19(4): 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophrenia Bulletin. 2009; 35(2): 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce DG, Davis WA, Davis TM. Longitudinal predictors of reduced mobility and physical disability in patients with type 2 diabetes: The Fremantle Diabetes Study. Diabetes Care. 2005; 28(10): 2441–7. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz N, Messier L, Nitka D, Ivanova A, Gariepy G, Wang J, et al. Factors associated with disability and depressive symptoms among individuals with diabetes: A community study in Quebec. Psychosomatics. 2011; 52(2): 167–77. [DOI] [PubMed] [Google Scholar]
- 11.Pagán-Rodríguez R, Pérez S. Depression and self-reported disability among older people in western Europe. Journal of Aging and Health. 2012; 24(7): 1131–56. [DOI] [PubMed] [Google Scholar]
- 12.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012; 380: 2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKibbin C, Patterson TL, Jeste DV. Assessing disability in older patients with schizophrenia: Results from the WHODAS-II. The Journal of Nervous and Mental Disease. 2004; 192(6): 405–13. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes a meta-analysis. Diabetes Care. 2001; 24(6): 1069–78. [DOI] [PubMed] [Google Scholar]
- 15.Egede LE. Effects of depression on work loss and disability bed days in individuals with diabetes. Diabetes Care. 2004; 27(7): 1751–3. [DOI] [PubMed] [Google Scholar]
- 16.Egede LE. Diabetes, major depression, and functional disability among U.S. adults. Diabetes Care. 2004; 27(2): 421–8. [DOI] [PubMed] [Google Scholar]
- 17.Von Korff M, Katon W, Lin EH, Simon G, Ciechanowski P, Ludman E, et al. Work disability among individuals with diabetes. Diabetes Care. 2005; 28(6): 1326–32. [DOI] [PubMed] [Google Scholar]
- 18.Von Korff M, Katon W, Lin EH, Simon G, Ludman E, Oliver M, et al. Potentially modifiable factors associated with disability among people with diabetes. Psychosomatic Medicine. 2005; 67(2): 233–40. [DOI] [PubMed] [Google Scholar]
- 19.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care. 2010; 33(5): 1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins M, Corcoran P, Perry I. Anxiety and depression symptoms in patients with diabetes. Diabetic Medicine. 2009; 26(2): 153–61. [DOI] [PubMed] [Google Scholar]
- 21.De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosomatic Medicine. 2001; 63(4): 619–30. [DOI] [PubMed] [Google Scholar]
- 22.Peyrot M, Rubin RR. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997; 20(4): 585–90. [DOI] [PubMed] [Google Scholar]
- 23.Pouwer F, Beekman A, Nijpels G, Dekker J, Snoek F, Kostense P, et al. Rates and risks for co-morbid depression in patients with Type 2 diabetes mellitus: Results from a community-based study. Diabetologia. 2003; 46(7): 892–8. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Russo J, Von Korff M, Ciechanowski P, Lin E, Ludman E, et al. The effect of changes in depressive symptoms on disability status in patients with diabetes. Psychosomatics. 2012; 53(1): 21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollingshead AB (1975) Four-factor index of social status. Working Paper. New Haven, CT: Yale University. [Google Scholar]
- 26.Kay SR, Opler LA, Lindenmayer J-P. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Research. 1988; 23(1): 99–110. [DOI] [PubMed] [Google Scholar]
- 27.Bunn HF. Nonenzymatic glycosylation of protein: relevance to diabetes. The American Journal of Medicine. 1981; 70(2): 325–30. [DOI] [PubMed] [Google Scholar]
- 28.Ellis G, Diamandis EP, Giesbrecht EE, Daneman D, Allen L. An automated “high-pressure” liquid-chromatographic assay for hemoglobin A1C. Clinical Chemistry. 1984; 30(11): 1746–52. [PubMed] [Google Scholar]
- 29.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960; 23(1): 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lako IM, Bruggeman R, Knegtering H, Wiersma D, Schoevers R, Slooff C, et al. A systematic review of instruments to measure depressive symptoms in patients with schizophrenia. Journal of Affective Disorders. 2012; 140(1): 38–47. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. U.S. Department of Health and Human Services. 2011. [Google Scholar]
- 32.Johnson CL, Nicholas A, Divine H, Perrier DG, Blumenschein K, Steinke DT. Outcomes from Diabetes CARE: A pharmacist-provided diabetes management service. Journal of the American Pharmacists Association. 2008; 48(6): 722–30. [DOI] [PubMed] [Google Scholar]
- 33.Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [Whiter paper] 2012. [cited 2013 Oct 23]. Available from: http://www.afhayes.com/public/process2012.pdf.
- 34.Hayes AF. Introduction to Mediation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guildford; 2013. [Google Scholar]
- 35.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013; 36 (Supplement 1): S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, et al. Depression and diabetes symptom burden. General Hospital Psychiatry. 2004. 26(6): 430–6. [DOI] [PubMed] [Google Scholar]
- 37.Talbot F, Nouwen A, Gingras J, Bélanger A, Audet J. Relations of diabetes intrusiveness and personal control to symptoms of depression among adults with diabetes. Health Psychology. 1999; 18(5): 537–42. [DOI] [PubMed] [Google Scholar]
- 38.Karlson B, Agardh CD. Burden of illness, metabolic control, and complications in relation to depressive symptoms in IDDM patients. Diabetic Medicine. 1997; 14(12): 1066–72. [DOI] [PubMed] [Google Scholar]
- 39.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. General Hospital Psychiatry. 2003; 25(4): 246–52. [DOI] [PubMed] [Google Scholar]
- 40.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan a meta-analysis. Diabetes Care. 2008; 31(12): 2383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]