Abstract
Age-related decline in visual search performance has been associated with differential patterns of activation in frontoparietal regions using functional magnetic resonance imaging (fMRI), but whether these age-related effects represent specific influences of target and distractor processing is unclear. Therefore, we acquired event-related fMRI data from 68 healthy, community-dwelling adults ages 18–78 years, during both conjunction (T/F target among rotated Ts and Fs) and feature (T/F target among Os) search. Some displays contained a color singleton that could correspond to either the target or a distractor. A diffusion decision analysis indicated age-related increases in sensorimotor response time across all task conditions, but an age-related decrease in the rate of evidence accumulation (drift rate) was specific to conjunction search. Moreover, the color singleton facilitated search performance when occurring as a target and disrupted performance when occurring as a distractor, but only during conjunction search, and these effects were independent of age. The fMRI data indicated that decreased search efficiency for conjunction relative to feature search was evident as widespread frontoparietal activation. Activation within the left insula mediated the age-related decrease in drift rate for conjunction search, whereas this relation in the FEF and parietal cortex was significant only for individuals younger than 30 or 44 years, respectively. Finally, distractor singletons were associated with significant parietal activation, whereas target singletons were associated with significant frontoparietal deactivation, and this latter effect increased with adult age. Age-related differences in frontoparietal activation therefore reflect both the overall efficiency of search and the enhancement from salient targets.
Case studies of patients with brain damage (Eglin et al., 1991; Friedman-Hill et al., 2003) and functional magnetic resonance imaging (fMRI) studies of healthy adults (Corbetta et al., 2008; Corbetta & Shulman, 2002; Nobre & Mesulam, 2014; Shulman et al., 2003; Vossel et al., 2014) have implicated widely distributed frontoparietal brain regions in attentional control during visual search. These regions include a dorsal network, including the frontal eye field (FEF), posterior parietal cortex, and intraparietal sulcus, involved in top-down guidance based on task goals, and a ventral network, including the anterior cingulate cortex, anterior insula, and temporoparietal junction, which responds to unexpected events and triggers shifts of attention. Several studies have reported increased activation of dorsal frontoparietal regions during conjunction search (when targets and distractors share features) relative to feature search (when targets and distractors do not share features; Corbetta et al., 1995; Donner et al., 2000; Wei et al., 2011). Dorsal frontoparietal activation may reflect specific feature binding processes (Donner et al., 2002; Kristjánsson & Egeth, 2020; Pollmann et al., 2014), but also the more general dimension of task difficulty and the continuum of search efficiency between the feature and conjunction search conditions (Duncan & Humphreys, 1989; Ischebeck et al., 2021; Nobre et al., 2003; Remington et al., 2021; Wei et al., 2009; Wolfe, 2014; Wolfe & Horowitz, 2004).
Target identification can be enhanced when the target has increased salience, from either bottom-up (featural) or top-down (cognitive) sources (Proulx, 2007; Theeuwes, 2010), and this improvement in target identification is associated with significant deactivation, particularly in FEF (Liu & Pleskac, 2011; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al., 2017; Madden, Siciliano, et al., 2020), analogous to priming effects (Henson, 2003; Lustig & Buckner, 2004; Schacter et al., 2007). Whereas a salient target improves performance, salient distractor items often interfere with performance, by capturing attention that could otherwise be devoted to target identification (Theeuwes, 2014; Yantis, 1996). The interfering effects of a distractor can be suppressed, especially when targets are predictable, and when spatial filtering or other top-down strategies are available (Geng et al., 2019; Leber & Egeth, 2006; Ruthruff & Gaspelin, 2018; Stilwell & Gaspelin, 2021; Wöstmann et al., 2022). But suppression is less effective when distractors are either previously presented targets (Gaspelin et al., 2019) or occur with an abrupt onset (Adams et al., 2022). Functional neuroimaging studies indicate that, in contrast to target enhancement, distractor interference is typically associated with increased dorsal frontoparietal activation (Akyurek et al., 2010; de Fockert et al., 2004; Geng et al., 2006).
The current investigation aimed to assess age-related differences (and similarities) in the functional brain activation associated with target enhancement and distractor interference during visual search. The efficiency of visual attention, and the associated pattern of functional brain activation, varies as a function of adult age (Kramer & Madden, 2008; Madden, 2007; Madden & Monge, 2019). Behavioral studies have established that visual search performance declines as a function of increasing age, especially during conjunction search, which relies on the discrimination of target and distractor features, as well as the inhibition of information not relevant to target identification (Hommel et al., 2004; Humphrey & Kramer, 1997; Madden et al., 1996; Plude & Doussard-Roosevelt, 1989; Rabbitt, 2017). Functional MRI studies have further established that decreasing search efficiency (e.g., dividing attention among spatial locations, increasing the difficulty of target identification) typically leads to greater activation of dorsal frontoparietal regions for older adults, relative to younger adults (Dennis & Cabeza, 2008; Eyler et al., 2011; Madden & Monge, 2019; Madden et al., 2005; Spreng et al., 2010). Age-related increases in frontoparietal activation may represent a compensatory recruitment of neural resources or a more general level of task difficulty and effort (Cabeza et al., 2018; Dennis & Cabeza, 2008; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Park, 2014).
Age-related differences in the component processes of visual search, however, such as target enhancement and distractor interference, are less consistent. Age-related decline in fluid cognition, the ability to process and integrate novel information in a speed-dependent manner, has long been a theme of behavioral studies of attention and working memory (Lustig et al., 2007; Rey-Mermet & Gade, 2018; Salthouse, 1996; Salthouse & Madden, 2007). Consistent with this, some behavioral indices of distraction or attentional capture from irrelevant information are magnified for older adults (Cashdollar et al., 2013; Kane et al., 1994; Kramer et al., 2000; Mevorach et al., 2016). But in other reports, the specific effects for target and distractor features in visual search performance are comparable across adult age (Madden, 2007; McAvinue et al., 2012; Pratt & Bellomo, 1999; Rey-Mermet & Gade, 2018; Wiegand & Wolfe, 2020). Similarly, frontoparietal activation associated with distracting information, during visual target identification, has been found to be increased for older relative to younger adults (Geerligs et al., 2014; Nielson et al., 2002). In contrast, activation for other target- and distractor-related components of search performance do not vary significantly as a function of adult age (Ashinoff et al., 2020; Madden et al., 2014; Madden, Spaniol, Whiting, et al., 2007).
A complicating, but theoretically important, issue is that the relation between behavioral target and distractor effects and activation may vary with age, even when the individual behavioral or activation effect is constant with age. Madden et al. (2017), for example, found that in a conjunction search task (with color x orientation targets), the addition of a salient feature (size) to the target was associated with both faster target identification and greater target-related frontoparietal deactivation. This enhancement of target identification for size-singleton targets, in both reaction time (RT) and activation, was constant as a function of adult age, but increasing age was associated with an increased correlation between the size singleton effect in FEF activation and search RT. In a feature search task (Madden, Siciliano, et al., 2020), the presence of a response-compatible distractor was associated with FEF/parietal deactivation and improved target identification, consistent with the Madden et al. (2017) finding for conjunction search. The correlation between activation and search performance, however, for target enhancement during feature search, did not vary as a function of adult age in Madden et al. (2020). Thus, the coupling between search-related activation and search performance may vary across the adult lifespan, depending on the complexity of task demands.
A challenge in interpreting these previous investigations of fMRI activation and visual search is that the previous studies vary along several dimensions, including the nature of the task (e.g., present/absent target detection vs. target discrimination), the efficiency of the target identification (e.g., conjunction vs. feature search) and the nature of the salient features (e.g., size vs. response compatibility). Here, we investigated age-related differences in target enhancement and distractor interference, when the target discrimination (T vs. F) was constant across different levels of search efficiency (conjunction vs. feature search), and within each search condition, a salient display item (a color singleton) could contribute to either target enhancement or distractor interference. Displays without a color singleton were also included to better isolate the target- and distractor-related effects. The different display types derived from these independent variables were presented randomly within fMRI scan runs, so that the effects would not be influenced by expectation for a particular display type. We assessed visual search performance using the diffusion decision model of RT in two-choice response tasks (Dutilh et al., 2019; Ratcliff & McKoon, 2008; Ratcliff et al., 2016; Voss, Nagler, et al., 2013; Wagenmakers et al., 2007), which distinguishes the rate of evidence accumulation (drift rate) from visual sensory encoding and motor processes (nondecision time) and cautiousness (boundary separation). The application of mediation analyses, within ordinary linear regression (Hayes, 2013; Hayes & Rockwood, 2017), allowed us to estimate the influence of activation on the relation between age and search performance. By sampling age as a continuous variable from 18 to 78 years of age, we could also estimate a particular point within the age span at which potentially moderating effects of age may occur.
This study represents the first direct comparison of age-related differences in fMRI activation for conjunction search relative to feature search. Extending prior reports of age-related differences in frontoparietal activation (Dennis & Cabeza, 2008; Eyler et al., 2011; Madden & Monge, 2019; Madden et al., 2005; Spreng et al., 2010), we expected to observe an age-related increase in conjunction search-related activation, reflecting older adults’ allocation of additional attentional resources in response to decreased search efficiency. In addition, we hypothesized that frontoparietal activation would mediate the relation between age and search performance, particularly the age-related decrease in the rate of evidence accumulation (drift rate) associated with conjunction search relative to feature search.
Finally, we anticipated that specific effects of the color singleton in both the behavioral and activation measures of target enhancement and distractor interference, as well as the relation between these measures, would be particularly evident during conjunction search. Color singleton targets should decrease activation, representing the enhancement of behavioral target identification (Liu & Pleskac, 2011; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al., 2017; Madden, Siciliano, et al., 2020), whereas attentional capture from distractor singletons should increase activation and impair search performance (Akyurek et al., 2010; de Fockert et al., 2004; Geng et al., 2006). These specific components of search may not vary significantly with adult age (Ashinoff et al., 2020; Madden et al., 2014; Madden, Spaniol, Whiting, et al., 2007). However, we hypothesized that the relation between the activation effects for the target and distractor singletons and the drift diffusion measures of search performance, especially drift rate, would increase as a function of adult age (Geerligs et al., 2014; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al., 2017).
Materials and Methods
Participants
This study was conducted in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and with the Institutional Review Board for Duke University Medical Center. Each participant provided informed consent and was compensated for their participation.
All participants reported that they had completed at least a high school education and were free of major neurological (e.g., epilepsy, stroke) or medical (e.g., diabetes, emphysema, uncontrolled hypertension) conditions. Testing comprised two sessions, the first of which was sensory and psychometric testing, and the second of which was the MRI scanning, completed approximately one month later (median time between sessions = 30 days). Ninety-eight healthy, community-dwelling individuals between 18 and 84 years of age completed the initial screening session. This first session was approximately 2 hr. in duration and included a battery of cognitive, neuropsychological, and visual tests to screen for cognitive impairment, depression, and visual acuity, as well as a practice version of the visual search task to be completed during MRI scanning. Exclusion criteria for participation in the MRI scanning session were any one of the following: visual acuity < 20/40 Snellen on the Freiburg Visual Acuity test (Bach, 1996); Dvorine (1963) color plate score < 12; Mini-Mental State Exam (MMSE; Folstein et al., 1975) score < 27; Beck Depression Inventory (BDI; Beck, 1978) score > 15, or Wechsler Adult Intelligence Scale-III (WAIS; Wechsler, 1997) vocabulary subtest score < 50th percentile.
The cognitive battery included a total of 12 computerized RT and standardized psychometric tests, with four tests administered for each of three domains of fluid cognition: perceptual-motor speed, executive function, and episodic memory (Howard et al., 2022; Madden, Jain, et al., 2020; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Packard, et al., 2017). Each domain included one test from the cognition section of the National Institutes of Health (NIH) Toolbox (Gershon et al., 2013). Perceptual-motor speed was measured from three computer-administered RT tests (simple RT and two versions of choice RT), and number correct in 85 s from the NIH Toolbox Pattern Comparison Test. Executive function was measured from two computer-administered tests (two-choice digit symbol comparison RT and flanker task incompatible RT divided by compatible RT), a standardized psychometric test (Trails B minus Trails A; Reitan, 1971), and the computed score on the NIH Toolbox Dimensional Change Card Sort Test. Episodic memory was measured from two computer-administered tests (a 6-item shape change detection task; Saults & Cowan, 2007) and 20-min delayed recall of 16 words, one psychometric test (WAIS logical memory delayed; Wechsler, 1997), and the computed score for the NIH Toolbox Picture Sequence Memory Test. We used the unrotated first factor from a factor analysis of all 12 tests as a measure of general fluid cognition, and the first factor from each set of four tests for perceptual speed, executive function, and episodic memory as indicators of these cognitive domains (Hedden et al., 2016; Hedden et al., 2012; Madden, Jain, et al., 2020; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Packard, et al., 2017; Salthouse et al., 2015).
Of the 98 participants who completed the first session, 20 participants were excluded prior to MRI scanning. Of these, five participants were lost to follow up, nine scored below the cutoffs for the sensory and psychometric screening criteria, four did not meet MRI safety requirements (e.g., claustrophobia, body size, or metal implant), and two were outliers (> 3 standard deviations) on one or more of the RT cognitive tests. The remaining 78 participants completed MRI scanning, but 10 individuals were excluded because of excessive motion during scanning, MRI artifacts or image processing issues, or overall search performance accuracy (during scanning) < 75%. Demographic and psychometric data for the final sample of 68 participants ages 18–78 years (36 females) are presented in Table 1. The distribution of participants within each age decades is as follows: 18–30 years (n = 15), 30s (n = 13), 40s (n = 12), 50s (n = 8), 60s (n = 11), and 70s (n = 9).
Table 1.
Participant Characteristics
| M (SD) | r with age | |
|---|---|---|
| % Female | 52.94 | 0.050 |
| Education (years) | 17.412 (2.294) | 0.006 |
| MMSE | 29.588 (0.604) | 0.004 |
| BDI | 2.647 (3.203) | 0.077 |
| Vocabulary | 58.529 (4.530) | −0.018 |
| Color Vision | 13.875 (0.476) | −0.253* |
| Visual Acuity (log MAR) | −0.009 (0.085) | 0.355** |
| General Fluid Cognition | 0.0 (0.969) | −0.859*** |
| Perceptual Speed | 0.0 (0.947) | −0.808*** |
| Executive Function | 0.0 (0.795) | −0.784*** |
| Memory | 0.0 (0.810) | −0.679*** |
Note. N = 68; values are presented as mean (standard deviation, SD) or percent (% Female); MMSE = raw score on Mini-Mental State Exam (Folstein et al., 1975); BDI = score on the Beck Depression Inventory (Beck, 1978); Vocabulary = raw score on the vocabulary subtest of the Wechsler Adult Intelligence Scale III (Wechsler, 1997); Color Vision = score on Dvorine color plates (Dvorine, 1963); Visual Acuity = logarithm of the minimum angle of resolution (MAR), for the Freiburg Visual Acuity Test (Bach, 1996). Log MAR of 0 corresponds to Snellen 20/20, with negative values corresponding to better resolution. Thus, the positive correlation for acuity represents age-related decline in this measure. General Fluid Cognition = factor score for 12 reaction time (RT) and psychometric tests sampling the three cognitive domains of perceptual speed, executive function, and memory, with four tests per domain. Perceptual Speed = factor score for four RT and psychometric tests of perceptual speed. Executive Function = factor score for four RRT and psychometric tests of executive function. Memory = factor score for four RT and psychometric tests of memory. Factor score correlations with age are covaried for sex and WAIS vocabulary.
p < 0.05,
p < 0.01,
p < 0.001
Visual Search Task
During event-related fMRI data acquisition, participants completed a two-choice discrimination version of visual search, with letter T and F targets (Figure 1). A normally oriented T or F was present in each display. On each trial, a visual display of five items (one target and four distractors) was presented on a 1920 × 1080 resolution screen for 350 ms. In the conjunction search condition, the distractor items included one rotated T or F and three T-F hybrids, whereas in the feature search condition the distractors were Os. Critically, on some trials, one of the display items could also be a color singleton (a red item among the dark gray display items, against a white background), which could correspond to either the target or one of the distractors. On each trial, participants responded as to which target (T or F) was present, using their right index and middle fingers resting on two buttons on a fiber optic response box (Current Designs, Philadelphia, PA, USA). Participants were instructed to respond as quickly as possible while still being correct. (Feedback regarding response accuracy was provided during the practice version of the task, in the screening session, but not during the fMRI session.) Six task conditions comprised two search conditions (conjunction, feature) combined with three color singleton conditions (distractor, target, or none). Participants completed a brief (12 trials) practice block in the scanner, and then 60 test trials during each of the four event-related fMRI runs (240 trials).
Figure 1.
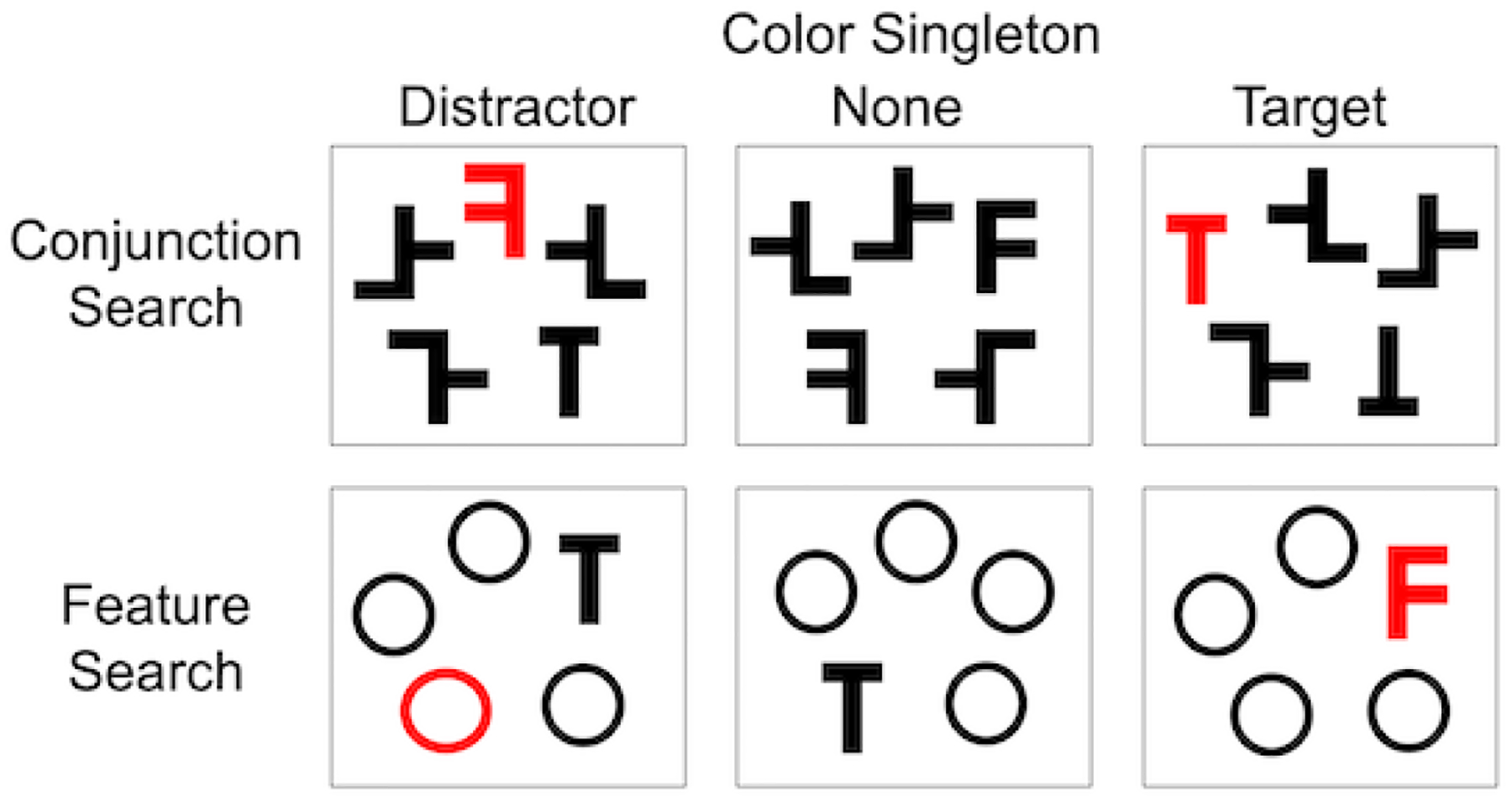
Visual search displays. During fMRI data acquisition, participants completed a visual search task where they decided which target letter (upright T or F) was present on each 350 ms display. On each display, one of six unique trial types were possible, including one of two types of search condition (conjunction, feature) and one of three types of salient color singleton (distractor, none, target). In the feature search condition, the distractors were all Os. In the conjunction search condition, the distractors were rotated Ts, Fs, and T-F hybrids, with three hybrids and one rotated T or F present in each display.
Across the four runs, there were 40 trials (20 per T/F target) for each of the six task conditions, with the exception of one trial in the conjunction distractor condition that was miscoded and corrected, yielding 39 conjunction-distractor trials and 41 conjunction-none trials. Across the 120 test trials for each search condition (conjunction, feature), each of the T and F targets occurred 12 times at each of the five display locations. The 60 trials within each fMRI run contained 9–11 instances for each combination of search condition and color singleton, distributed randomly. Each trial began with a fixation cross with variable duration (jitter), followed by the search display for a duration of 350 ms, then a 2650 ms response screen, during which the display was white. We measured RT from display onset, allowing a total of 3000 ms for the response. Following the response period, the fixation cross returned to begin the next trial. The jitter duration was varied among values of 1500, 3000, 4500 ms, and 6000 ms defined by multiples of the fMRI repetition time (TR) value (1500 ms). The average jitter across trials was 3000 ms. The jitter values and trial order across conditions were randomized and optimized using the Optseq2 program (Dale, 1999; https://surfer.nmr.mgh.harvard.edu/optseq). We constructed two different sequential orders (forward and reverse) of the four blocks of test trials and combined those with the two assignments of the targets to the response buttons, for a total of four test list versions that were varied across participants.
Display presentation and response acquisition were controlled by a Windows-based computer running E-Prime 2.0 (Psychology Software Tools, Sharpsburg, PA, USA). Displays were rear projected to a mirror that was 71 cm from the projector and 11 cm from the participant’s eyes. The five display items were isoluminant red and dark gray, on a white background, and were located at approximately the 12, 2, 5, 7, and 10 o’clock positions. The available space for each display item was approximately 1o square. Due to the nature of the stimuli, the height and width of individual items ranged from 0.56o to 0.90o, with the nontarget circles (0.90o × 0.90o) being slightly larger than the letter T (0.58o × 0.90o) and F (0.56o × 0.88o) targets during feature search. The midpoints of the five display items were arranged along the diameter of a circle approximately 3.0o in size. The center of each display location was varied slightly (10–20 pixels) across trials, to reduce adaptation effects, and the edges of adjacent letters were separated by at least 0.83o.
Diffusion Decision Model Parameters
In the diffusion decision model (Figure 2), overall RT for each participant comprises several decisional components that can be identified from analyses of the complete distributions of RT for correct and incorrect responses, and error rate (Dutilh et al., 2019; Ratcliff & McKoon, 2008; Ratcliff et al., 2016; Voss, Nagler, et al., 2013; Wagenmakers et al., 2007). The diffusion decision model has been applied to a wide range of two-choice response tasks, ranging from psychophysical studies of brightness discrimination (Ratcliff et al., 2003) to visual search (Madden, Siciliano, et al., 2020), flanker tasks (Servant & Evans, 2020), lexical and semantic category decisions (Madden et al., 2010; Ratcliff et al., 2004; Voss, Rothermund, et al., 2013), and recognition memory (Ratcliff & McKoon, 2015; Spaniol et al., 2006). Here, we focus on three parameters: the rate of evidence accumulation towards one of the two response boundaries (drift rate; v), sensory and motor response processes (nondecision time; t0), and cautiousness (boundary separation; a). Drift rate and nondecision time can vary across trials, but boundary separation is assumed to remain constant across trials. Previous applications of the diffusion decision model in aging have consistently found age-related increases in nondecision time and cautiousness (Madden, Siciliano, et al., 2020; Ratcliff, 2008; Ratcliff et al., 2004; Ratcliff et al., 2003; Servant & Evans, 2020). Age-related decline in drift rate has also been observed but is more variable and task-dependent (Madden et al., 2010; Spaniol et al., 2006; Thapar et al., 2003). We estimated these decisional components with the EZ-diffusion model (Wagenmakers et al., 2007). This method uses a mathematically simplified algorithm that estimates fewer parameters than other versions of the diffusion decision model, but the EZ-diffusion model is as successful as more complex methods in recovering the underlying parameters (Dutilh et al., 2019; van Ravenzwaaij & Oberauer, 2009).
Figure 2.
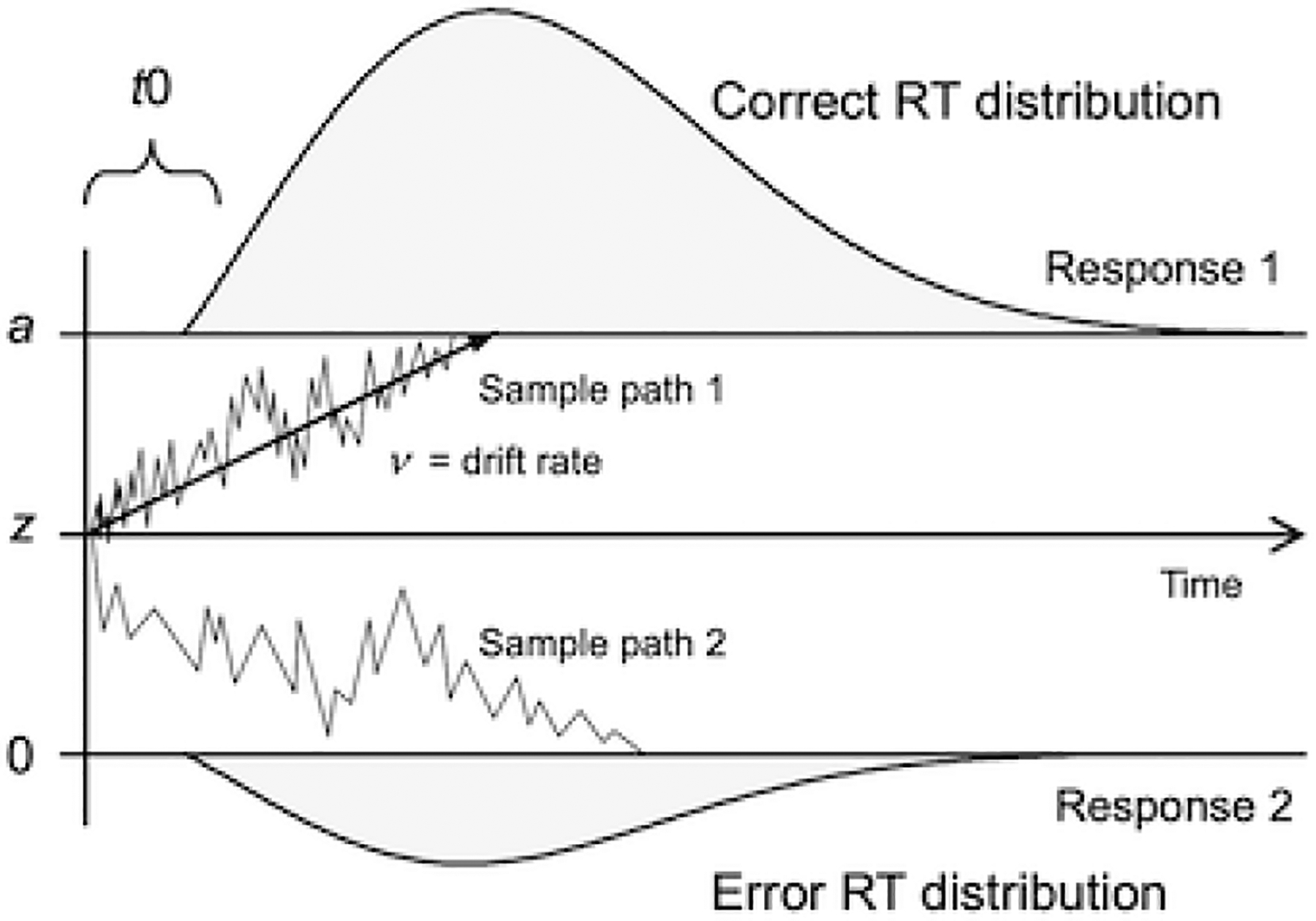
The diffusion decision model. Information from each participant’s reaction time (RT) distributions for correct and incorrect responses provides estimates of drift rate (v), which is the rate at which information is accumulated to make a decision; nondecision time (t0), which is the time spent on processes such as encoding the display items and selecting a response; and boundary separation (a), which is the amount of information required for a decision (i.e., cautiousness).
We conducted linear regression and analysis of variance (ANOVA) of drift rate, nondecision time, and boundary separation with SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). Power analyses (Faul et al., 2007) indicated that with 68 participants, a Pearson correlation r value of 0.35 (r2 = 0.123) would be detected as significant at alpha = 0.05 (two-tailed), with a power of 0.84. For the ANOVAs, the difference between two within-subjects conditions, corresponding to a small-to-medium effect size f of 0.20 (Cohen, 1988) would be detected at alpha = 0.05 (two-tailed) with a power of 0.90.
Imaging Data Acquisition
We collected the imaging data at Duke University Medical Center on a 3T GE Signa Ultra High Performance whole-body 60 cm bore MRI scanner (GE Healthcare, Waukesha, WI, USA) equipped with a 48 channel receive-only head coil. Participants wore earplugs to reduce scanner noise, and foam pads were used to minimize head motion.
Structural imaging was a single T1-weighted 3D fast inverse-recovery-prepared spoiled gradient recalled sequence acquired as 292 axial slices with TR = 2203.5 ms, echo time (TE) = 3.076 ms, inversion recovery time (TI) = 900 ms, field of view (FOV) = 240 × 240 mm, flip angle = 8o, voxel size = 1 mm3, acquisition matrix = 240 × 240 mm, and a sensitivity encoding (SENSE) factor = 2.
The event-related fMRI of the visual search task was conducted over four runs of a T2*-weighted echo-planar imaging (EPI) sequence sensitive to the blood oxygen-level dependent (BOLD) signal. Each of the task-related runs included 264 brain volumes collected over 6.60 min. Fifty contiguous axial slices were acquired parallel to the plane connecting the anterior and posterior commissures, with TR = 1500 ms, TE = 30 ms, FOV = 256 mm × 256 mm, flip angle = 60o, voxel size = 2 mm3, acquisition matrix = 128 × 128 mm, and a SENSE factor = 2.
During the scanning session, data from several other imaging modalities were acquired, including resting-state fMRI, susceptibility-weighted angiography, diffusion-weighted imaging, and fluid attenuated inversion recovery imaging, to be reported separately.
Functional MRI Data Analysis
We used tools from fMRIPrep (Esteban et al., 2019) and FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) for processing of the fMRI data. In fMRIPrep, we conducted motion correction using MCFLIRT (Jenkinson et al., 2002), slice timing correction using AFNI’s 3dTshift (Analysis of Functional NeuroImages; Cox, 1996), and susceptibility-induced distortion correction using the Advanced Normalization Tools (ANTs) symmetric normalization technique (Treiber et al., 2016). We then completed several steps in FSL’s FEAT: removal of the first four brain volumes (disabled data acquisitions; disdaqs) for each task run, skull stripping using the brain extraction tool (Smith, 2002), spatial smoothing using a Gaussian kernel with a full width at half maximum of 4 mm, high-pass filtering (cutoff = 90 s), and grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor. Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al., 2001). We then registered the functional data to each participant’s T1-weighted image using FLIRT, and then to a study-specific template aligned to a Montreal Neurological Institute (MNI) 152 template (2 mm3 resolution) using a combination of FLIRT and FNIRT (Jenkinson et al., 2002; Jenkinson & Smith, 2001). The study-specific template was a composite structural image averaged across these 68 participants, created within the ANTs script for multivariate construction. The final preprocessed data were used as input for the lower-level analyses. Across the 68 participants, we excluded the data for five runs (two runs for one participant and one run for each of three other participants), in which visual search accuracy was < 75%.
For each participant and each run, we constructed a model of the BOLD signal for each trial type in FSL’s FEAT. Each model included six explanatory variables representing correct responses to the six task conditions (two search conditions x three types of singletons), plus errors and RT outlier trials combined in a seventh explanatory variable, as well as each event’s temporal derivative. Voxelwise modeling was conducted with a stick function (duration = 0 s) convolved with double γ hemodynamic response function and contrasted against the implicit baseline (jitter). The modeled data were then averaged across the four task runs for each participant using fixed effects modeling.
We focused on several contrasts to investigate specific effects within the visual search task. The all trials > implicit baseline contrast compared all trials, to the blank inter-trial interval (jitter), without designating task condition, with the goal of estimating the activation for all processes shared between conjunction and feature search. To limit our primary analyses to voxels with positive activation, we used the resulting cluster map from this contrast as a pre-threshold mask for the following task condition contrasts, which were defined based on a prior report that increases in BOLD activation correspond to increases in RT (Yarkoni et al., 2009). The conjunction > feature search contrast reflects the decreased search efficiency and increased activation for conjunction search relative to feature search, averaged across singleton type. Displays with a salient target, if in fact an enhancement effect, should reduce RT and activation, relative to displays without a color singleton (Liu & Pleskac, 2011; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al., 2017; Madden, Siciliano, et al., 2020). Thus, we defined this contrast as none > target singleton. On the other hand, relative to displays without a color singleton, displays with a salient distractor should increase RT and the corresponding activation needed for target identification, as a result of attentional capture by a salient nontarget. We thus defined this contrast as distractor singleton > none. We conducted the target and distractor singleton contrasts separately within the conjunction and feature search conditions and examined the reverse contrasts (i.e., feature > conjunction search, target > none, and none > distractor). We conducted group-level one sample t-tests on the above outputs using FMRIB’s Local Analysis of Mixed Effects Stage 1 (Beckmann et al., 2003; Woolrich et al., 2004). Significant clusters were identified using corrected cluster thresholding with false discovery rate (FDR) procedures to correct for multiple comparisons and nonparametric statistical thresholds of z > 3.1, p < 0.05 for trials > implicit baseline and z > 2.3, p < 0.05 for all other contrasts.
To assess relations among search-related activation, search performance, and chronological age, we used FSL’s featquery to extract the average parameter estimates from 5 mm spheres centered on the peak voxel of each significant cluster. We then conducted separate linear regressions with activation from each sphere predicting drift rate, nondecision time, and age, corrected for multiple comparisons using FDR procedures (Benjamini & Hochberg, 1995). Finally, we tested for moderation and mediation effects associated with the activation in each sphere using PROCESS (Hayes & Rockwood, 2017). We created a model with paths for the relations among predictor variables (age and activation) and the outcome variable (search performance). For each sphere, regardless of its relation to search performance across the sample, we tested whether age moderated the relation between activation and visual search performance (i.e., an interaction between predictor variables). For each sphere with a significant relation to search performance after FDR correction, we tested whether activation mediated the relation between age and visual search performance (i.e., an interaction between model paths). Moderation and mediation effects were considered significant if the 95% confidence intervals (CIs) did not contain zero after 10,000 bootstrap replacements. Significant moderation effects were probed using the Johnson-Neyman technique (Bauer & Curran, 2005; Johnson & Fay, 1950). All analyses with drift rate and nondecision time were covaried for visual acuity.
Results
Visual Search Performance
Following the exclusion of five runs with accuracy < 75% (two runs for one participant and one run for each of three other participants), 16,020 trials remained. Following the additional exclusion of 879 incorrect trials and 17 trials exceeding our RT outlier thresholds of < 250 ms and > 2500 ms, 15,124 trials were included in the final behavioral and fMRI analyses. Mean RT for correct responses and mean accuracy for these trials are presented in Table 2.
Table 2.
Mean Reaction Time and Accuracy
| Search Condition | Singleton | RT | Accuracy |
|---|---|---|---|
| Conjunction | Distractor | 994 (159) | 0.931 (0.057) |
| Conjunction | None | 907 (153) | 0.946 (0.052) |
| Conjunction | Target | 792 (146) | 0.964 (0.037) |
| Feature | Distractor | 695 (109) | 0.981 (0.026) |
| Feature | None | 690 (108) | 0.978 (0.026) |
| Feature | Target | 688 (115) | 0.972 (0.037) |
Note. n = 68. Values are presented as mean ms (standard deviation, SD) for reaction time (RT) and mean proportion correct (SD) for accuracy.
Diffusion Decision Model Parameters.
Our application of the diffusion decision model (Wagenmakers et al., 2007) assumes that the starting point of evidence accumulation is located equidistantly between the response boundaries (T vs. F). Preliminary analyses of the mean RT and accuracy data indicated that errors were slower than correct responses (Supplementary Table 1), which suggests that the errors were driven more by variation in drift rate than by variation in the starting point across trials (Wagenmakers et al., 2007). Importantly, the starting point for evidence accumulation was not biased towards one of the response boundaries and did not vary significantly with age (Supplementary Table 2).
Because cautiousness (boundary separation; a) is assumed to be constant across trials unless manipulated explicitly, the a parameter was treated as a participant-level variable. Boundary separation increased with age, r = 0.363, p < 0.01, indicating a more conservative decision threshold as a function of increasing age.
Search Condition and Singleton Effects.
Mean values for drift rate (v) and nondecision time (t0) are presented in Figure 3. We conducted a univariate ANOVA on each of these two outcome variables, with Search Condition (conjunction, feature) and Singleton (distractor, none, target) as within-person variables. The main effects of Search Condition and Singleton, and the Search Condition x Singleton interaction, were significant for both drift rate and nondecision time (Table 3). To follow up the Condition x Singleton interactions, we used paired t-tests to examine the specific effects of the target and distractor singletons, relative to the no-singleton condition, within each search condition, separately for drift rate and nondecision time (Bonferroni corrected for four comparisons, p < 0.013). These values (Table 4) were defined such that the difference between singleton conditions would be numerically positive. For drift rate, higher values reflect faster evidence accumulation; thus, the target singleton effect was defined as target minus none, and the distractor singleton effect was defined as none minus distractor. For nondecision time, higher values reflect additional time for encoding or response implementation; thus, the target singleton effect was defined as none minus target, and the distractor singleton effect was defined as distractor minus none.
Figure 3.
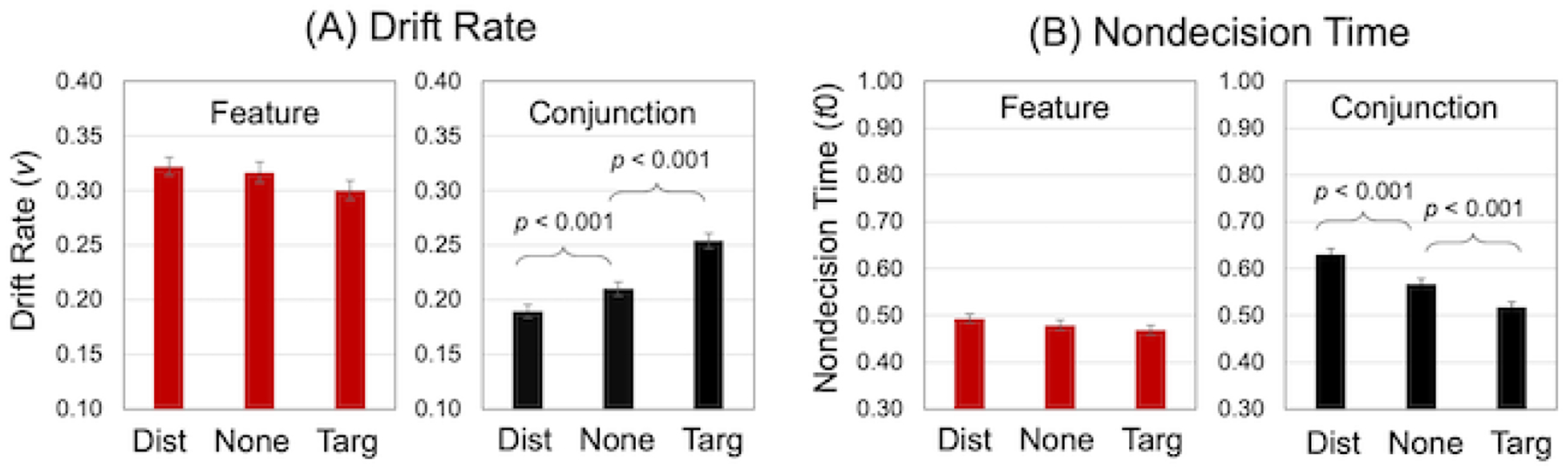
Visual search performance. Bar graphs display Drift Rate (Panel A) and Nondecision Time (Panel B) as a function of search condition and singleton. Higher values for drift rate and lower values for nondecision time indicate better performance. Error bars represent the standard error of the mean. Dist = distractor; Targ = target.
Table 3.
Analysis of Variance (ANOVA) Effects for Drift Rate and Nondecision Time
| df num | df dem | F | p < | |
|---|---|---|---|---|
| Drift Rate | ||||
| Task | 1 | 67 | 259.75 | 0.0001 |
| Singleton | 2 | 134 | 8.41 | 0.0004 |
| Task x Singleton | 2 | 134 | 37.11 | 0.0001 |
| Nondecision Time | ||||
| Task | 1 | 67 | 253.83 | 0.0001 |
| Singleton | 2 | 134 | 61.97 | 0.0001 |
| Task x Singleton | 2 | 134 | 25.91 | 0.0001 |
Table 4.
Target and Distractor Singleton Effects for Drift Rate and Nondecision Time as a Function of Age
| M | SD | t | r with age | |
|---|---|---|---|---|
| Feature Search: Target Singleton vs. None | ||||
| Drift Rate | −0.016 | (0.070) | −1.93 | −0.049 |
| Nondecision Time | 0.010 | (0.066) | 1.31 | −0.084 |
| Feature Search: Distractor Singleton vs. None | ||||
| Drift Rate | −0.006 | (0.071) | −0.65 | 0.125 |
| Nondecision Time | 0.014 | (0.064) | 1.79 | −0.067 |
| Conjunction Search: Target Singleton vs. None | ||||
| Drift Rate | 0.044 | (0.050) | 7.19*** | 0.198 |
| Nondecision Time | 0.049 | (0.071) | 5.74*** | −0.076 |
| Conjunction Search: Distractor Singleton vs. None | ||||
| Drift Rate | 0.021 | (0.043) | 3.96*** | −0.194 |
| Nondecision Time | 0.062 | (0.072) | 7.11*** | 0.033 |
Note. N = 68. Correlations are covaried for visual acuity. None of the correlations were significant at p < 0.05, uncorrected. SD = standard deviation; t = difference between singleton and none conditions. M = differences between singleton and non-singleton (none) conditions, defined so that the outcome variable would usually be positive. For Target Singleton vs. None, the drift rate effect was defined as Target minus None, whereas the nondecision time effect was defined as None minus Target. For Distractor Singleton vs. None, the drift rate effect was defined as None minus Distractor, and the nondecision time effect was defined as Distractor minus None.
p < 0.0001 (corrected)
Within the conjunction search condition, both the target and distractor singleton effects were significant, for both drift rate and nondecision time (Table 4). Within the feature search condition, neither the target nor the distractor singleton effect was significant, for either drift rate or nondecision time.
Age-Related Effects.
Drift rate and nondecision time are presented as a function of age in Figure 4. For both outcome variables, we first assessed age-related differences separately within the feature search and conjunction search conditions, averaged across singletons. In these age-related correlations (Bonferroni corrected for two comparisons, p < 0.025), we covaried the visual acuity measure obtained during Session 1. We found that the average drift rate decreased significantly with age for conjunction search, r = −0.367, p < 0.01, but not for feature search, r = −0.113, ns, and the difference between these two correlations was significant by Steiger’s z (Steiger, 1980), z = 2.588, p < 0.01. The age-related effect for nondecision time, in contrast, increased significantly with age for both conjunction search, r = 0.611, p < 0.001, and feature search, r = 0.639, p < 0.001, and these correlations did not differ significantly by Steiger’s z.
Figure 4.
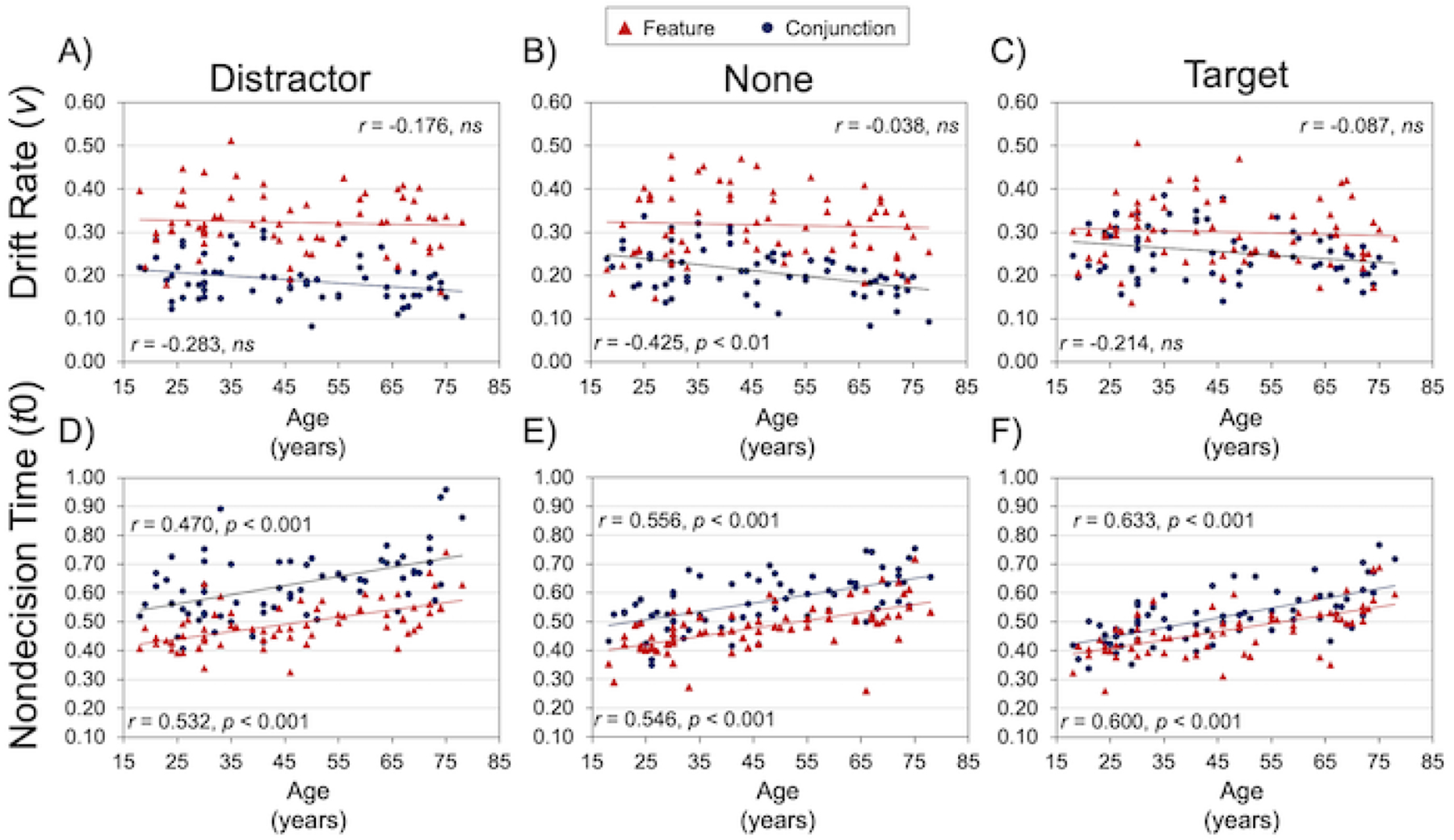
Age-related differences in visual search performance. Scatterplots display drift rate (Panels A-C) and nondecision time (Panels D-F) as a function of age, separately for each search condition and singleton and while covarying for visual acuity.
Correlations with age for the target and distractor singleton effects, for drift rate and nondecision time, are presented in Table 4 as a function of search condition. Covarying for visual acuity, neither the target nor distractor singleton effect within either search condition was correlated significantly with age, even at a more liberal threshold of p < 0.05 (uncorrected).
Task-Related Activation and Visual Search Performance
All Trials versus Baseline.
We observed an extensive pattern of overall task-related activation that spanned subcortical and frontal, parietal, and occipital regions (Figure 5A and Table 5). As noted previously (Functional MRI Data Analysis), we used this cluster map as a pre-threshold mask for each subsequent task condition contrast.
Figure 5.
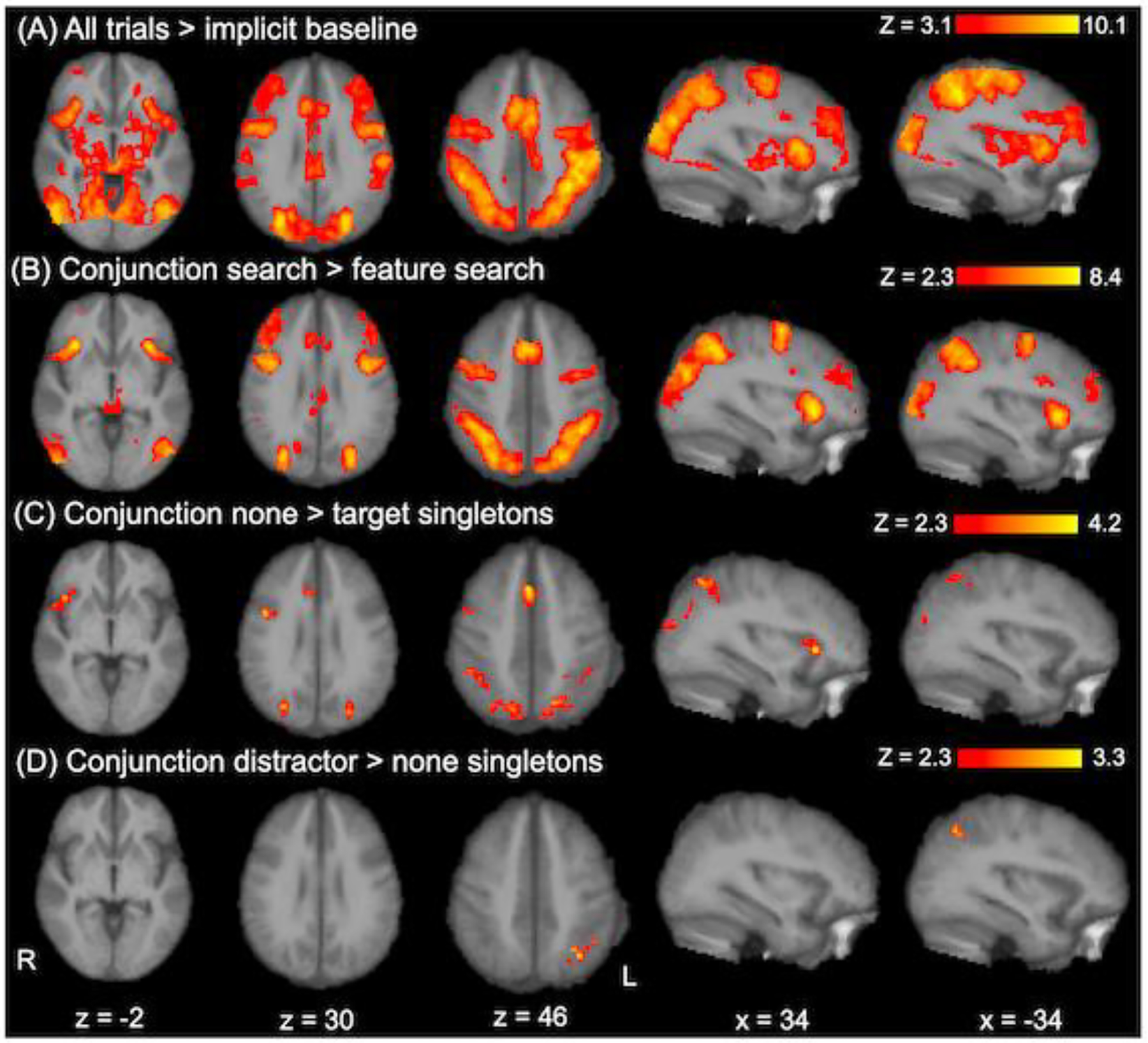
Task-related fMRI activation. Significant clusters are displayed for all trials relative to the implicit baseline (Panel A), conjunction search relative to feature search, averaged across singleton conditions (Panel B), none relative to target singletons within conjunction search (Panel C), and distractors relative to none singletons within conjunction search (Panel D). Activation was false discovery rate (FDR) corrected at p < 0.05 and thresholded at z > 3.1 for all trials > implicit baseline or z > 2.3 for all other contrasts. Activation is displayed in Montreal Neurological Institute (MNI) 152 space, with a study-specific anatomical image as the underlay, in radiological orientation (left [L] = right [R]).
Table 5.
Significant Clusters for Each Contrast of Interest
| Cluster | x | y | z | Max z | Size (voxels) | BA |
|---|---|---|---|---|---|---|
| All trials > baseline | ||||||
| L inferior parietal cortex | −40 | −38 | 40 | 10.10 | 47823 | 40 |
| R posterior cingulate | 4 | −32 | 26 | 8.15 | 484 | 23 |
| L orbital frontal cortex | −20 | 42 | −6 | 4.79 | 89 | 10 |
| R middle temporal gyrus | 48 | −28 | −6 | 4.88 | 78 | 21 |
| R putamen | 18 | 12 | −4 | 4.33 | 72 | 49 |
| Conjunction search > feature search | ||||||
| L inferior parietal cortex | −42 | −44 | 42 | 8.15 | 9272 | 40 |
| R insula | 34 | 24 | −2 | 8.30 | 5757 | 13 |
| L frontal eye field | −44 | 0 | 34 | 7.73 | 2377 | 6 |
| L primary visual cortex | −12 | −76 | 10 | 5.04 | 849 | 17 |
| L insula | −34 | 22 | 0 | 7.98 | 595 | 13 |
| L thalamus | −2 | −32 | −2 | 3.80 | 200 | 50 |
| L posterior cingulate | 0 | −30 | 26 | 4.72 | 177 | 23 |
| R thalamus | 10 | −20 | 12 | 3.75 | 113 | 50 |
| Conjunction none > target singletons | ||||||
| R superior parietal cortex | 22 | −68 | 54 | 3.95 | 1169 | 7 |
| L superior parietal cortex | −18 | −66 | 46 | 3.68 | 703 | 7 |
| R supplemental motor area | 4 | 20 | 46 | 4.21 | 315 | 8 |
| R insula | 34 | 24 | 2 | 4.21 | 228 | 13 |
| R frontal eye field | 46 | 6 | 28 | 4.28 | 211 | 6 |
| Conjunction distractor > none singletons | ||||||
| L inferior parietal cortex | −38 | −52 | 54 | 3.28 | 134 | 40 |
Note. Clusters that exhibited a significant voxelwise main effect (z > 3.1, p < 0.05 for all trials > implicit baseline; z > 2.3, p < 0.05 for all other contrasts) are described with their peak voxel (x, y, z coordinates in Montreal Neurological Institute [MNI] 152 space), maximum z-statistic (z-max), and size (number of voxels). Clusters were labeled using automated anatomical labeling (https://github.com/yunshiuan/label4MRI/). R = right; L = left; BA = Brodmann’s Area.
Conjunction Search versus Feature Search.
As predicted, the conjunction > feature search contrast yielded eight significant clusters representing extensive frontoparietal activation, with local maxima in the left inferior parietal cortex, left FEF, left primary visual cortex, left posterior cingulate, bilateral insula, and bilateral thalamus (Figure 5B and Table 5). We did not observe any significant clusters for the reverse contrast (feature > conjunction search).
Linear regression analyses indicated that increased activation from spheres around the peak voxels of the bilateral insula clusters significantly predicted the decrease in drift rate for conjunction search relative to feature search, r2 ≥ 0.178, pFDR ≤ 0.036 (Figure 6A and 6B). Activation in these clusters did not significantly predict the increase in nondecision time for conjunction search relative to feature search.
Figure 6.
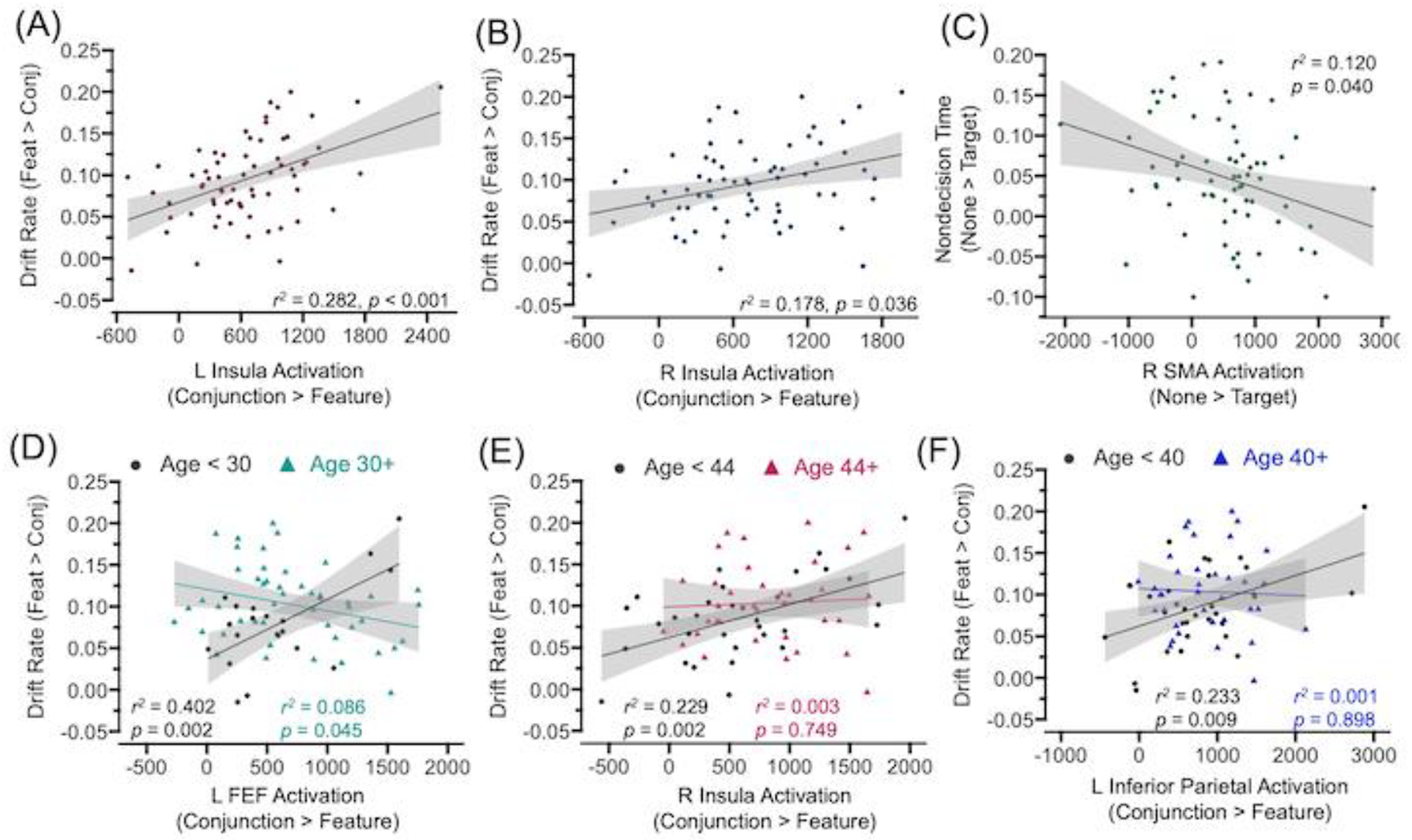
Activation-performance relations. For select clusters in which the corresponding trial type difference was significant, activation parameter estimates from spheres around their peak voxels are plotted against visual search performance (drift rate or nondecision time), separately for the conjunction search versus feature search contrast (Panels A, B) or the conjunction none versus target singletons contrast (Panel C). For clusters in which age moderated the relation between performance and activation in the conjunction > feature search contrast, parameter estimates from those spheres are plotted against drift rate (Panels D-F). Reported r2 values and their corresponding p-values were corrected for multiple comparisons using FDR procedures and covaried for visual acuity. The shaded gray area around the regression line represents 95% confidence intervals. L = Left; R = Right; FEF = Frontal Eye Field; SMA = supplemental motor area; Conj = Conjunction Search; Feat = Feature Search.
Target Singleton Effects.
As predicted, the target singleton contrast (none > target), within conjunction search, yielded five significant clusters of deactivation for target singletons, in the bilateral superior parietal cortex, right FEF, right supplemental motor area (SMA), and right insula (Figure 5C and Table 5). We did not observe any significant clusters for the reverse contrast (target > none) within conjunction search or for either contrast within feature search.
Linear regression analyses indicated that increased target-related deactivation, in a sphere around the peak voxel of the right SMA, significantly predicted a smaller difference in nondecision time for displays with a salient target relative to displays without a color singleton, r2 = 0.120, pFDR = 0.040 (Figure 6C). Activation in this cluster did not significantly predict the target singleton effect for drift rate.
Distractor Singleton Effects.
The distractor singleton contrast (distractor > none), within conjunction search, yielded a significant cluster of activation in the left inferior parietal cortex, as expected (Figure 5D and Table 5). We did not observe any significant clusters for the reverse contrast (none > distractor) within conjunction search or for either contrast within feature search.
In contrast to our predictions, linear regression analyses indicated that activation in a sphere around the peak voxel of the left inferior parietal cluster did not significantly predict the distractor singleton effect for either drift rate or nondecision time.
Age-Related Differences in Task-Related Activation
Conjunction Search versus Feature Search.
We assessed age-related differences in the parameter estimates of activation from spheres around the eight significant peak voxels identified by the conjunction > feature search contrast (Figure 5B and Table 5). Linear regressions analyses indicated significant age-related increases in activation for the bilateral insula, r2 ≥ 0.142, pFDR ≤ 0.004 (Figure 7A and 7B).
Figure 7.
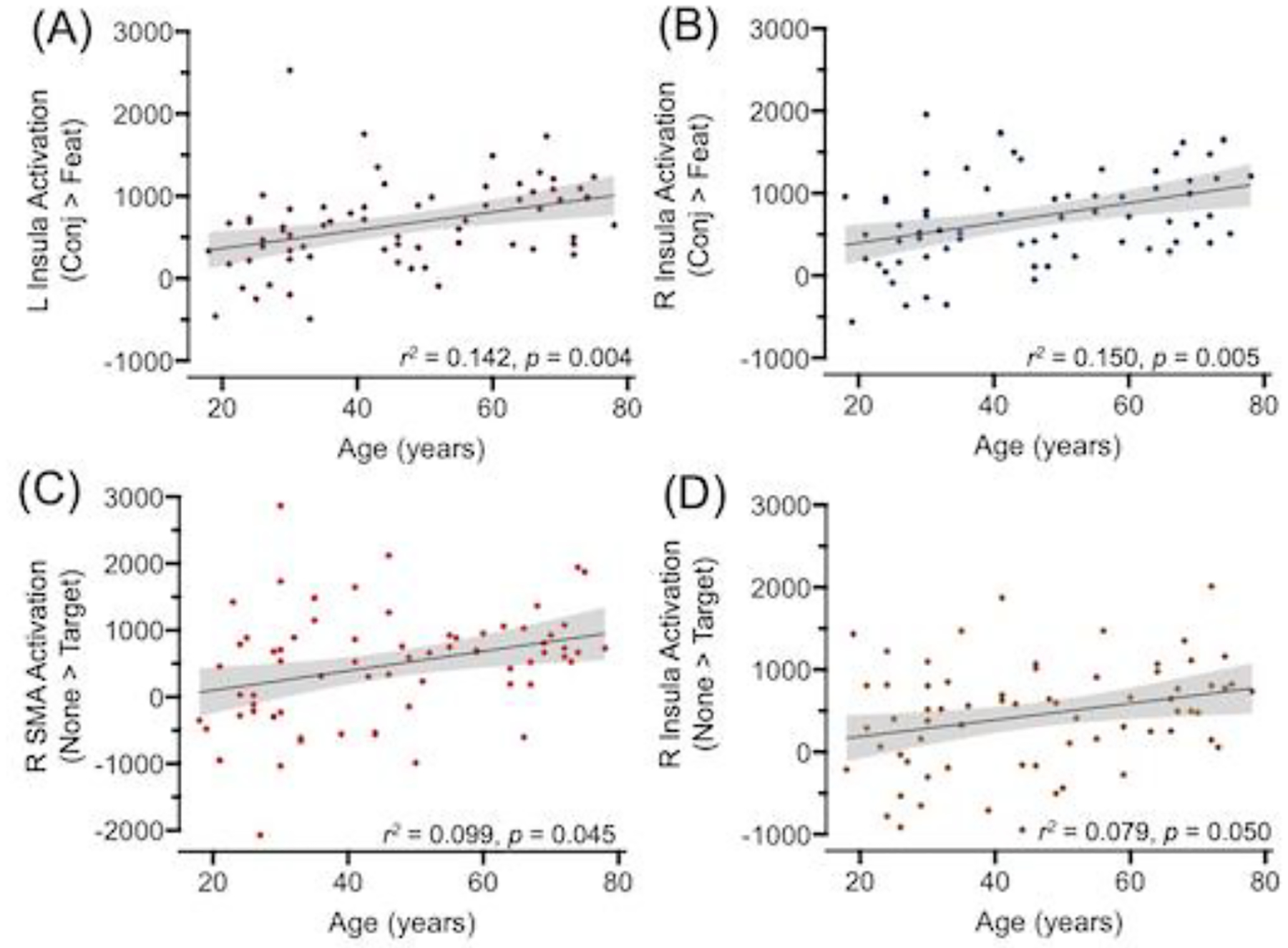
Age-related differences in activation. For select clusters in which the activation difference between conjunction search and feature search (conj > feat; Panels A-B) or conjunction none versus target singletons (none > target; Panels C-D) was significant, activation parameter estimates from spheres around their peak voxels are plotted against chronological age (years). Reported r2 values and their corresponding p-values were corrected for multiple comparisons using FDR procedures. The shaded gray area around the regression line represents 95% confidence intervals. L = Left; R = Right, SMA = supplemental motor area.
We then conducted mediation (for the bilateral insula spheres related to drift rate) and moderation (for all eight significant spheres) analyses (Hayes, 2013; Hayes & Rockwood, 2017), to clarify the relation of the activation variables to the relation between age and search performance. In these models, the outcome variables were the difference between conjunction search and feature search for drift rate or nondecision time.
Mediation analyses indicated that increased activation in the left insula significantly mediated the relation between age and the decrease in drift rate for conjunction search relative to feature search (Table 6, Model 1). To confirm that this mediation effect does not simply reflect the shared variance among the variables, we examined an alternative model in which the decline in drift rate for conjunction search, relative to feature search, was the mediator of the relation between age and the left insula activation. Mediation in this alternative model was not significant (Table 6, Model 2).
Table 6.
Mediation Models of Age, Activation, and Drift Rate
| β | SE | t | p | Lower CI | Upper CI | |
|---|---|---|---|---|---|---|
| Model 1: x = Age; m = Left insula activation (conj > feature); y = Drift rate (feature > conj) | ||||||
| Age (a path) | 11.574 | 3.569 | 3.218 | 0.002 | 4.392 | 18.756 |
| L insula activation (b path) | 0.00004 | 0.00001 | 3.762 | <0.001 | 0.00002 | 0.00006 |
| Total effect for age (c path) | 0.0005 | 0.0003 | 1.602 | 0.114 | −0.0001 | 0.0012 |
| Direct effect for age (c’ path) | 0.0008 | 0.0003 | 0.236 | 0.814 | −0.0006 | 0.0007 |
| Mediation effect (ab interaction) | 0.0005 | 0.0002 | - | - | 0.00011 | 0.00084 |
| Model 2: x = Age; m = Drift rate (feature > conj); y = Left insula activation (conj > feature) | ||||||
| Age (a path) | 0.0005 | 0.0003 | 1.602 | 0.114 | −0.0001 | 0.0012 |
| Drift rate (b path) | 4549.98 | 1209.18 | 3.762 | <0.001 | 2134.36 | 6965.61 |
| Total effect for age (c path) | 11.574 | 3.569 | 3.218 | 0.002 | 4.392 | 18.756 |
| Direct effect for age (c’ path) | 9.122 | 3.344 | 2.728 | 0.008 | 2.442 | 15.802 |
| Mediation effect (ab interaction) | 2.452 | 1.897 | - | - | −0.514 | 6.837 |
Note. a, b, c, paths in mediation model, with x as predictor variable, y as outcome variable, and m as mediator; a = path from predictor to mediator; b = path from mediator to outcome, controlling for a path; c = total effect of predictor; c’ = direct effect of predictor, controlling for mediator; ab = interaction of a and b paths representing indirect influence of x as mediated by m; β = unstandardized regression coefficient; SE = standard error; Lower/Upper CI = lower/upper bounds of 95% confidence intervals, estimated from bootstrap sampling with 10,000 samples, Conj = Conjunction. Visual acuity was modeled as a covariate and significant effects are bolded.
Moderation analyses indicated that the relation between activation and the decrease in drift rate for conjunction search relative to feature search varied with age in the left FEF, t(63) = −2.229, p = 0.029 (CI = −2.70-e5, −1.00-e6), right insula, t(63) = −2.229, p = 0.029 (CI = −2.40-e5, −1.00-e6), and left inferior parietal cortex, t(63) = −2.027, p = 0.046 (CI = −2.10-e5, −2.00-e7). Johnson-Neyman analyses (Johnson & Fay, 1950) indicated that activation significantly predicted drift rate only for individuals younger than 30, 44, or 40 years of age, respectively, r2 ≥ 0.229, p ≤ 0.009 (Figure 6D–6F).
Target Singleton Effects.
We assessed age-related differences in the activation from spheres around the five significant peak voxels identified in the conjunction none > target contrast (Figure 5C and Table 5). Linear regressions analyses indicated significant age-related increases in the magnitude of target-related deactivation in the right insula and right SMA, r2 ≥ 0.079 pFDR ≤ 0.050 (Figure 7C and 7D). We did not observe significant evidence of moderation or mediation for the target singleton effect for either drift rate or nondecision time.
Distractor Singleton Effects.
Finally, we assessed age-related differences in the activation from a sphere around the peak voxel of the left inferior parietal cluster identified in the conjunction distractor > none contrast (Figure 5D and Table 5). In contrast to our expectation, linear regression analyses indicated that age did not significantly predict activation in this cluster. We also did not observe significant evidence of moderation for the distractor singleton effect for either drift rate or nondecision time.
Discussion
The decline in fluid cognition observed during healthy aging includes an increased vulnerability to the attentional demands of conjunction search, as well as increased activation within regions of the frontoparietal network. The distracting and beneficial effects of salient display items in search performance, however, are often constant with adult age. Direct comparisons of these effects across previous fMRI studies of visual search are difficult because previous studies differ in the nature of the target identification response, the difficulty of the search task, and the type of salience. Here, we used event-related fMRI and diffusion decision modeling to understand age-related differences in target enhancement and distractor interference from a color singleton, in feature search and conjunction search, with a two-choice version of target discrimination. Our findings extend several previously reported effects in the literature, but also suggest that age-related differences both in frontoparietal activation, and in the effects of salient target and distractor items on search performance, are related to the increased attentional demands imposed by conjunction search.
Visual Search Performance
The present study is, to our knowledge, the first direct comparison of feature search and conjunction search with the diffusion decision model. Consistent with the extant search literature based on mean RT (Duncan & Humphreys, 1989; Wolfe, 2014; Wolfe & Horowitz, 2004), we found that search performance was substantially less efficient during conjunction search than during feature search, expressed here in Figure 3 as a decline in the rate of evidence accumulation (v; drift rate). This difference in drift rate was expected due to differences in the featural composition of the displays, where the T and F targets had high similarity to distractors during conjunction search (rotated Ts and Fs, and T-F hybrids) but low similarity to the distractors during feature search (Os). The t0 nondecision time parameter, however, also varied between the search conditions, with a relatively higher nondecision time during conjunction search (Figure 3 and Table 4). This latter effect is surprising because feature search and conjunction search have generally similar nondecision components, including the initial visual encoding of a five-item display and selection of a two-choice, button press response. It is possible that the increased visual complexity of the conjunction search displays led to additional time required for visual encoding. Alternatively, as Voss et al. (2013) noted, nondecision time can also include response selection as well as response execution, and the selection process may involve re-checking in the more difficult conjunction search condition.
We also found that, relative to a neutral (no-singleton) display, search performance improved when the color singleton corresponded to the target, and performance was disrupted when the color singleton corresponded to a distractor. These effects, evident in both drift rate and nondecision time, are consistent with the previous literature on attentional capture from a salient display item (Theeuwes, 2014; Yantis, 1996). A novel contribution from this analysis is that the effects of the target and distractor singletons were significant only within conjunction search (Figure 3), where target-distractor similarity may have limited the ability to focus on specific target features or suppress distractors (Geng et al., 2019; Leber & Egeth, 2006; Ruthruff & Gaspelin, 2018; Stilwell & Gaspelin, 2021; Theeuwes, 2014; Wöstmann et al., 2022). Whereas visual salience is a highly influential determinant of search performance (Theeuwes, 2013), the color singleton in this task was not associated with significant differences in feature search performance. It is possible that participants could suppress attentional capture from the feature search distractor singletons (Geng et al., 2019; Ruthruff & Gaspelin, 2018; Stilwell & Gaspelin, 2021; Wöstmann et al., 2022), although performance for displays with a distractor was not significantly better than the neutral (no-singleton) displays. It is more likely that the present design allowed participants to adopt a top-down, preparatory set for the features of the T/F targets in the feature search condition, which enabled them to ignore the irrelevant dimension of color (Leber & Egeth, 2006). Although top-down strategies are less effective when distractors are either previously presented targets (Gaspelin et al., 2019) or occur with an abrupt onset (Adams et al., 2022), neither of these conditions were used in the current design. Instead, the current observation of significant performance disruptions from salient distractors during conjunction search, but not feature search, suggests that increased target-distractor similarity may further hinder the successful use of top-down strategies.
Age-Related Differences.
Previous reports of larger age effects on conjunction search, relative to feature search, have relied on mean RT and accuracy as outcome measures (Bennett et al., 2012; Hommel et al., 2004; Humphrey & Kramer, 1997; Müller-Oehring et al., 2014; Plude & Doussard-Roosevelt, 1989). Here, using a novel application of the diffusion decision model of RT, we demonstrated similar age-related slowing in nondecision time for conjunction search and feature search, but an age-related decline in the rate of evidence accumulation (drift rate) that was specific to conjunction search (Figure 3 and Table 4). Madden et al. (2020) also observed that drift rate in a feature search task did not vary significantly with adult age, whereas age-related slowing was prominent in nondecision time. We further found that boundary separation increased with age, representing increased cautiousness, although the task conditions could not be compared on this parameter because they were randomized across trials. Age-related increases in nondecision time and cautiousness are the most reliable findings across various applications of the diffusion decision model to aging (Madden, Siciliano, et al., 2020; Ratcliff, 2008; Ratcliff et al., 2004; Ratcliff et al., 2003; Servant & Evans, 2020). Age-related decline in drift rate has also been reported (Madden et al., 2010; Madden et al., 2009; Spaniol et al., 2006; Thapar et al., 2003), but appears to require a threshold level of task complexity or attentional demand, as in the present version of conjunction search.
The enhancement of search performance from target singletons, and interference from distractor singletons, did not vary significantly as a function of adult age (Figure 4). Although older adults have exhibited increased vulnerability to distraction in other tasks (Cashdollar et al., 2013; Kane et al., 1994; Kramer et al., 2000; Mevorach et al., 2016), we did not detect any age-related differences in the specific effects of the color singletons. This result, along with the comparable level of feature search drift rates across adult age, are consistent with the idea that at least some forms of attentional capture and target facilitation remain preserved during healthy aging (Madden, 2007; Madden & Monge, 2019; Madden, Spaniol, Bucur, et al., 2007; Rey-Mermet & Gade, 2018; Wiegand & Wolfe, 2020). From the design of this study, however, we cannot determine the relative contribution of top-down and bottom-up attentional processes to this age constancy of the target and distractor singleton effects. One possible explanation is the use of isoluminant distractor singletons, which has been associated with smaller attentional capture effects than bright onset distractors in both older (Kramer et al., 2000) and younger (Adams et al., 2022) adults.
Search-Related Activation
In the fMRI data, our initial hypotheses regarding search-related activation were largely supported. Across the feature and conjunction search trials (Figure 5A and Table 5), overall search performance was accompanied by activation in broadly distributed dorsal and ventral frontoparietal regions, as predicted, consistent with previous studies of visual search and target identification (Corbetta et al., 2008; Corbetta & Shulman, 2002; Nobre & Mesulam, 2014; Shulman et al., 2003; Vossel et al., 2014). When comparing these search conditions, we observed that a subset of these subcortical, frontal, parietal, and occipital regions exhibited increased activation for conjunction search relative to feature search (Figure 5B and Table 5). This finding supports the previously observed pattern of regional activation observed for conjunction search relative to feature search (Corbetta et al., 1995; Donner et al., 2002; Nobre et al., 2003; Remington et al., 2021; Wei et al., 2011). A novel finding, from the drift diffusion analyses, was that increasing activation in the bilateral anterior insula for conjunction > feature search was related to the decrease in drift rate associated with conjunction search (Figures 6A and 6B), whereas the activation-performance correlation was not significant for nondecision time. Activation in the anterior insula, a component of the ventral frontoparietal network, has previously been associated with the identification of search targets (Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al., 2017; Shulman et al., 2003). But the anterior insula may function in a broader manner, as a hub within a midcingulate-insular salience network, operating as a gatekeeper to orchestrate and drive activity of other major functional brain networks (Eckert et al., 2009; Molnar-Szakacs & Uddin, 2022). Although this correlation with drift rate is new evidence highlighting the role of feature-extraction processes in conjunction search, we cannot determine whether these processes reflect feature binding specifically, rather than the more general dimension of search difficulty or efficiency (Wolfe & Horowitz, 2004).
As hypothesized, the additional salience afforded by the color singleton, when coinciding with the target, was associated with reduced frontoparietal activation relative to neutral (no-singleton) displays (Figure 5C and Table 5). This finding supports the idea that increased salience will facilitate target identification (Liu & Pleskac, 2011; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al., 2017; Madden, Siciliano, et al., 2020), as in the case of priming effects (Henson, 2003; Lustig & Buckner, 2004; Schacter et al., 2007). As expected, this target enhancement-related deactivation was evident only in the conjunction search condition. It is likely that evidence from feature search targets reaches a decision threshold before the additional information from the color singleton provides assistance (Shulman et al., 2003). We further observed that greater target-related deactivation during conjunction search was associated with a smaller difference in nondecision time, but not drift rate, in the right SMA (Figure 6C). In a previous study of target absent versus present displays during conjunction search, Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al. (2017) also observed a relation between target-related decreases in RT and activation in the FEF, but the relation was positive. The current finding of a negative relation in the SMA is a relatively small effect (r2 = 0.12), and is difficult to interpret. It is possible that this activation-behavioral correlation reflects the role of this region in response-related processes (Nachev et al., 2008). For example, if the selection process involves re-checking the target identification response in the more difficult conjunction search condition, this would decrease the magnitude of the enhancement effect in nondecision time.
Finally, as predicted, we observed significant increases in activation for distractor-singleton displays, relative to no-singleton displays. The location of this distractor-related activation was in the left inferior parietal cortex (Figure 5D and Table 5). We had expected to observe more robust increases in frontoparietal activation for distractor singletons (Akyurek et al., 2010; de Fockert et al., 2004; Geng et al., 2006), in view of the fact that no spatial filtering or top-down strategies were available as a basis for distractor suppression (Geng et al., 2019; Leber & Egeth, 2006; Ruthruff & Gaspelin, 2018; Stilwell & Gaspelin, 2021; Wöstmann et al., 2022). Although distractor singletons were associated with substantial decrements in both drift rate and nondecision time for conjunction search (Figure 3), we did not observe significant relations between these behavioral effects and activation in the left inferior parietal cluster. Wöstmann et al. (2022) do mention that distractor-related effects are often weaker than target-related effects, and we did have relatively fewer trials in this contrast (40) than the conjunction versus feature search contrast (120). However, Huettel and McCarthy (2001) demonstrated that, with signal averaging, the estimation of the hemodynamic response function (HRF) is stable with 25 trials per condition, suggesting that there were a sufficient amount of trials for reliable HRF estimation. An alternative explanation is that distractor-related activation relies on successful distractor suppression (Cosman et al., 2018). Because all display items in the current design were relevant as potential targets, complete suppression of distractors was not possible here.
Age-Related Differences.
The age-related increase in frontoparietal activation for conjunction search relative to feature search (Figure 7A and 7B) replicates and extends previous findings from perceptual and cognitive tasks (Dennis & Cabeza, 2008; Eyler et al., 2011; Madden & Monge, 2019; Madden et al., 2005; Spreng et al., 2010). A novel finding from these analyses is that activation in the left anterior insula mediated the relation between age and the decrease in drift rate for conjunction search relative to feature search (Table 6), as hypothesized. Thus, the effect of age on the decline in drift rate for conjunction search was indirect, operating through age-related increases in activation in the left insula. The relation between activation and the magnitude of the drift rate effect was positive (i.e., the b path in Table 6, Model 1), suggesting that this allocation is more likely to reflect the increased effort or processing resources associated with visual feature extraction, than a compensatory strategy.
Moderation analyses further indicated that activation in the left FEF, right insula, and left inferior parietal cortex was significantly related to the decrease in drift rate for conjunction search, but only for individuals younger than 30, 44, or 40 years of age, respectively (Figure 6D–6F). The association between conjunction search-related activation and drift rate in the left FEF confirm previous fMRI findings suggesting a prominent role for this region in visual target identification (Madden et al., 2014; Madden, Parks, Tallman, Boylan, Hoagey, Cocjin, Johnson, et al., 2017). Related findings from intracortical microstimulation (Moore & Armstrong, 2003), neurotransmitter modulation (Noudoost & Moore, 2011), and cortical lesions (Rossi et al., 2007), raise the possibility that FEF is a specific site of top-down signals enhancing the activity within visual cortical regions (Cosman et al., 2018; Miller & Buschman, 2013; Noudoost et al., 2010). However, finding that activation in the FEF, as well as the right insula and left inferior parietal cortex, was only related to drift rate for individuals younger than 30–44 years of age suggests that age-related decline in search efficiency arises from decreased coupling between frontoparietal activation and search performance.
Within conjunction search, the deactivation for target singleton displays, relative to no-singleton displays, in the right insula and right SMA exhibited an increase as a function of age (Figure 7C and 7D), even though there was no significant age-related effect in the target enhancement behavioral measures. Age-related increases in the magnitude of this target enhancement effect may reflect age-related differences in the top-down emphasis of target-relevant features (Geerligs et al., 2014; Madden, Spaniol, Bucur, et al., 2007; Madden et al., 2004; Müller-Oehring et al., 2014; Whiting et al., 2005). However, activation the right SMA was related to nondecision time, but not drift rate, across the sample, suggesting that activation in this region is more reflective of age-related differences in either encoding or response execution than decision-making processes. Taken together, these findings together suggest that age-related differences in components of the dorsal and ventral frontoparietal networks reflect both the overall efficiency of search and the facilitation from salient target singletons.
Strengths and Limitations
One strength of this study is the diffusion decision modeling, which allowed us to identify unique cognitive and perceptual-motor contributions, and search-related activation, for conjunction search relative to feature search. Ideally, applying the diffusion decision model should be based on 200 or more trials per experimental condition (Wagenmakers et al., 2007). We thus used a modified version of the model (Wagenmakers et al., 2007) that is designed for studies with a smaller number of observations, but has been as successful as the more complete models in recovering the underlying parameters (Dutilh et al., 2019; van Ravenzwaaij & Oberauer, 2009). The length of fMRI scanning imposes some practical limitations on data collection. Nevertheless, with our limited data set of 40 trials per experimental condition, we detected pronounced differences between feature search and conjunction search in both drift rate and nondecision time (Table 3). We also replicated previously observed age-related increases in nondecision time and cautiousness (Madden, Siciliano, et al., 2020; Ratcliff, 2008; Ratcliff et al., 2004; Ratcliff et al., 2003; Servant & Evans, 2020), and the age-related decline in drift rate that we observed for conjunction search is consistent with previous findings (Madden et al., 2010; Spaniol et al., 2006; Thapar et al., 2003). Extending these findings in fMRI studies with other search tasks, with larger numbers of observations per condition, would be valuable.
The continuous age range of the sample participants, spanning the second through the eighth decades of life, is also a strength. An important future direction will be to test whether increased activation in response to increased visual search demands is similarly observed across later ages (e.g., 80+ years) and in older adults with cognitive impairment, where deficits in feature search performance may become more apparent. As a necessary first step, we characterized age-related differences in the neural substrates of conjunction search and feature search, and their relation to salient targets and distractors, across healthy adults. In particular, we used mediation and moderation analyses to characterize the relations among variables, but the variables we used as mediators and moderators may represent the effects of other, unmeasured variables, and thus the obtained results must be interpreted with caution. While our cross-sectional design does not allow us to satisfy the temporal precedence requirement for causal interpretations, these results do provide preliminary evidence that can be used to form hypotheses in future longitudinal work. Finally, while activation studies provide essential information about the overall magnitude of task-related activation and help identify task-relevant regions, it would be valuable to investigate age-related differences in the functional connectivity among these brain regions in relation to visual search (Geerligs et al., 2014; Grady, 2017; Madden & Monge, 2019; Monge et al., 2017).
Conclusions
From this direct comparison of conjunction search and feature search, diffusion decision model analyses revealed an age-related slowing of perceptual-motor processing (nondecision time) within both task conditions, consistent with age-related decline in speed-based (fluid) cognition. The age-related decline in the rate of evidence accumulation (drift rate), however, occurs only when attentional demands are sufficiently challenging, as in conjunction search. Attentional capture from salient color singletons either facilitates or impairs performance, depending on whether the salient display item is a target or a distractor, respectively. Whereas age-related slowing contributes to visual search performance, especially when search is inefficient, we find that components of visual attention related to the processing of salient target and distractor items are relatively constant as a function of adult age. The fMRI data suggest that among the widespread activation of frontoparietal regions during conjunction search, relative to feature search, increasing activation of the left anterior insula reflects the slowing rate of evidence accumulation (drift rate) for conjunction search targets across the adult lifespan. However, other frontoparietal regions (FEF, inferior parietal cortex) were only related to drift rate for individuals younger than 30–44 years of age, suggesting age-related decreases in the coupling between frontoparietal activation and search performance. Finally, we find that target enhancement, from the addition of a salient but irrelevant feature (color), is expressed as an improvement in search performance and reduced frontoparietal activation, whereas attentional capture from salient distractors was associated with increased parietal activation. Age-related differences in frontoparietal activation therefore reflect declines in the overall efficiency of search, the facilitation from salient target singletons, and the coupling between activation and search performance.
Supplementary Material
Acknowledgements:
This work was supported by R01 AG039684 (DJM). We thank Ragini Singh, Cortney Howard, Shivangi Jain, Alexa Putka, Angela Cook, Matthew Wang, and Nicole Stepovich for their assistance.
Footnotes
Open Practices Statement: The data presented in this paper are not readily available on the internet due to privacy or ethical restrictions. The data that support the findings of this study are available upon request from the corresponding author. A formal data sharing agreement may be required.
References
- Adams OJ, Ruthruff E, & Gaspelin N (2022). Oculomotor suppression of abrupt onsets versus color singletons. Attention, Perception, & Psychophysics. 10.3758/s13414-022-02524-0 [DOI] [PubMed] [Google Scholar]
- Akyurek EG, Vallines I, Lin EJ, & Schubo A (2010). Distraction and target selection in the brain: An fMRI study. Neuropsychologia, 48(11), 3335–3342. 10.1016/j.neuropsychologia.2010.07.019 [DOI] [PubMed] [Google Scholar]
- Ashinoff BK, Mayhew SD, & Mevorach C (2020). The same, but different: Preserved distractor suppression in old age is implemented through an age-specific reactive ventral fronto-parietal network. Human Brain Mapping, 41, 3938–3955. 10.1002/HBM.25097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M (1996). The Freiburg Visual Acuity test--automatic measurement of visual acuity. Optometry and Vision Science, 73(1), 49–53. 10.1097/00006324-199601000-00008 [DOI] [PubMed] [Google Scholar]
- Bauer DJ, & Curran PJ (2005). Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research, 40(3), 373–400. 10.1207/s15327906mbr4003_5 [DOI] [PubMed] [Google Scholar]
- Beck AT (1978). The Beck Depression Inventory. Psychological Corporation. [Google Scholar]
- Beckmann CF, Jenkinson M, & Smith SM (2003). General multilevel linear modeling for group analysis in FMRI. Neuroimage, 20(2), 1052–1063. 10.1016/S1053-8119(03)00435-X [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bennett IJ, Motes MA, Rao NK, & Rypma B (2012). White matter tract integrity predicts visual search performance in young and older adults. Neurobiology of Aging, 33(2), 433.e421–433.e431. 10.1016/j.neurobiolaging.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, & Rajah MN (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 19(11), 701–710. 10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar N, Fukuda K, Bocklage A, Aurtenetxe S, Vogel EK, & Gazzaley A (2013). Prolonged disengagement from attentional capture in normal aging. Psychology and Aging, 28(1), 77–86. 10.1037/a0029899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. Erlbaum. [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, & Petersen SE (1995). Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science, 270, 802–805. 10.1126/science.270.5237.802 [DOI] [PubMed] [Google Scholar]
- Cosman JD, Lowe KA, Zinke W, Woodman GF, & Schall JD (2018). Prefrontal control of visual distraction. Current Biology, 28(3), 414–420.e413. 10.1016/j.cub.2017.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Dale AM (1999). Optimal experimental design for event-related fMRI. Human Brain Mapping, 8, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert J, Rees G, Frith C, & Lavie N (2004). Neural correlates of attentional capture in visual search. Journal of Cognitive Neuroscience, 16(5), 751–759. 10.1162/089892904970762 [DOI] [PubMed] [Google Scholar]
- Dennis NA, & Cabeza R (2008). Neuroimaging of healthy cognitive aging. In Craik FIM & Salthouse TA (Eds.), The handbook of aging and cognition (3rd ed., pp. 1–54). Psychology Press. [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, & Brandt SA (2000). Involvement of the human frontal eye field and multiple parietal areas in covert visual selection during conjunction search. European Journal of Neuroscience, 12(9), 3407–3414. 10.1046/j.1460-9568.2000.00223.x [DOI] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, & Brandt SA (2002). Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage, 15(1), 16–25. 10.1006/nimg.2001.0951 [DOI] [PubMed] [Google Scholar]
- Duncan J, & Humphreys GW (1989). Visual search and stimulus similarity. Psychological Review, 96(3), 433–458. 10.1037/0033-295x.96.3.433 [DOI] [PubMed] [Google Scholar]
- Dutilh G, Annis J, Brown SD, Cassey P, Evans NJ, Grasman R, Hawkins GE, Heathcote A, Holmes WR, Krypotos AM, Kupitz CN, Leite FP, Lerche V, Lin YS, Logan GD, Palmeri TJ, Starns JJ, Trueblood JS, van Maanen L, … Donkin C (2019). The quality of response time data inference: A blinded, collaborative assessment of the validity of cognitive models. Psychonomic Bulletin & Review, 26(4), 1051–1069. 10.3758/s13423-017-1417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorine I (1963). Dvorine pseudo-isochromatic plates (2nd ed.). Harcourt. [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, & Dubno JR (2009). At the heart of the ventral attention system: The right anterior insula. Human Brain Mapping, 30(8), 2530–2541. 10.1002/hbm.20688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglin M, Robertson LC, & Knight RT (1991). Cortical substrates supporting visual search in humans. Cerebral Cortex, 1, 262–272. 10.1093/cercor/1.3.262 [DOI] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, & Gorgolewski KJ (2019). fMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16(1), 111–116. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Sherzai A, Kaup AR, & Jeste DV (2011). A review of functional brain imaging correlates of successful cognitive aging. Biological Psychiatry, 70(2), 115–122. 10.1016/j.biopsych.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. Journal of Psychiatric Research, 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Desimone R, & Ungerleider LG (2003). Posterior parietal cortex and the filtering of distractors. Proceedings of the National Academy of Sciences, 100(7), 4263–4268. 10.1073/pnas.0730772100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Gaspar JM, & Luck SJ (2019). Oculomotor inhibition of salient distractors: Voluntary inhibition cannot override selection history. Visual Cognition, 27(3–4), 227–246. 10.1080/13506285.2019.1600090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Saliasi E, Maurits NM, Renken RJ, & Lorist MM (2014). Brain mechanisms underlying the effects of aging on different aspects of selective attention. NeuroImage, 91, 52–62. 10.1016/J.NEUROIMAGE.2014.01.029 [DOI] [PubMed] [Google Scholar]
- Geng JJ, Eger E, Ruff CC, Kristjansson A, Rotshtein P, & Driver J (2006). On-line attentional selection from competing stimuli in opposite visual fields: effects on human visual cortex and control processes. Journal of Neurophysiology, 96(5), 2601–2612. 10.1152/jn.01245.2005 [DOI] [PubMed] [Google Scholar]
- Geng JJ, Won B-Y, & Carlisle NB (2019). Distractor ignoring: Strategies, learning, and passive filtering. Current Directions in Psychological Science, 28(6), 600–606. 10.1177/0963721419867099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, & Nowinski CJ (2013). NIH Toolbox for assessment of neurological and behavioral function. Neurology, 80, S2–S6. 10.1212/WNL.0B013E3182872E5F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL (2017). Age differences in functional connectivity at rest and during cognitive tasks. In Cognitive neuroscience of aging: Linking cognitive and cerebral aging (pp. 105–130). Oxford University Press. 10.1093/acprof:oso/9780199372935.003.0005 [DOI] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis. Guilford. [DOI] [PubMed] [Google Scholar]
- Hayes AF, & Rockwood NJ (2017). Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behaviour Research and Therapy, 98, 39–57. 10.1016/j.brat.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, & Buckner RL (2016). Multiple brain markers are linked to age-related variation in cognition. Cerebral Cortex, 26(4), 1388–1400. 10.1093/cercor/bhu238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Shire EH, Sperling RA, Johnson KA, & Buckner RL (2012). Failure to modulate attentional control in advanced aging linked to white matter pathology. Cerebral Cortex, 22(5), 1038–1051. 10.1093/cercor/bhr172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN (2003). Neuroimaging studies of priming. Progress in Neurobiology, 70(1), 53–81. 10.1016/s0301-0082(03)00086-8 [DOI] [PubMed] [Google Scholar]
- Hommel B, Li KZ, & Li SC (2004). Visual search across the life span. Developmental Psychology, 40(4), 545–558. 10.1037/0012-1649.40.4.545 [DOI] [PubMed] [Google Scholar]
- Howard CM, Jain S, Cook AD, Packard LE, Mullin HA, Chen N. k., Liu C, Song AW, & Madden DJ (2022). Cortical iron mediates age-related decline in fluid cognition. Human Brain Mapping(43), 1047–1060. 10.1002/HBM.25706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, & McCarthy G (2001). The effects of single-trial averaging upon the spatial extent of fMRI activation. Neuroreport, 12(11), 2411–2416. 10.1097/00001756-200108080-00025 [DOI] [PubMed] [Google Scholar]
- Humphrey DG, & Kramer AF (1997). Age differences in visual search for feature, conjunction, and triple-conjunction targets. Psychology and Aging, 12(4), 704–717. 10.1037//0882-7974.12.4.704 [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Hiebel H, Miller J, Höfler M, Gilchrist ID, & Körner C (2021). Target processing in overt serial visual search involves the dorsal attention network: A fixation-based event-related fMRI study. Neuropsychologia, 153. 10.1016/j.neuropsychologia.2021.107763 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–156. 10.1016/S1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Johnson PO, & Fay LC (1950). The Johnson-Neyman technique, its theory and application. Psychometrika, 15(4), 349–367. 10.1007/BF02288864 [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hasher L, Stoltzfus ER, Zacks RT, & Connelly SL (1994). Inhibitory attentional mechanisms and aging. Psychology and Aging, 9, 103–112. 10.1037/0882-7974.9.1.103 [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Irwin DE, & Theeuwes J (2000). Age differences in the control of looking behavior: Do you know where your eyes have been? Psychological Science, 11(3), 210–217. 10.1111/1467-9280.00243 [DOI] [PubMed] [Google Scholar]
- Kramer AF, & Madden DJ (2008). Attention. In Craik FIM & Salthouse TA (Eds.), The handbook of aging and cognition (3rd ed., pp. 189–249). Psychology Press. [Google Scholar]
- Kristjánsson Á, & Egeth H (2020). How feature integration theory integrated cognitive psychology, neurophysiology, and psychophysics. Attention, Perception, & Psychophysics, 82(1), 7–23. 10.3758/s13414-019-01803-7 [DOI] [PubMed] [Google Scholar]
- Leber AB, & Egeth HE (2006). It’s under control: top-down search strategies can override attentional capture. Psychonomic Bulletin & Review, 13(1), 132–138. 10.3758/bf03193824 [DOI] [PubMed] [Google Scholar]
- Liu T, & Pleskac TJ (2011). Neural correlates of evidence accumulation in a perceptual decision task. Journal of Neurophysiology, 106(5), 2383–2398. 10.1152/jn.00413.2011 [DOI] [PubMed] [Google Scholar]
- Lustig C, & Buckner RL (2004). Preserved neural correlates of priming in old age and dementia. Neuron, 42(5), 865–875. 10.1016/j.neuron.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, & Zacks RT (2007). Inhibitory deficit theory: Recent developments in a “new view”. In Inhibition in cognition. (pp. 145–162). American Psychological Association. 10.1037/11587-008 [DOI] [Google Scholar]
- Madden DJ (2007). Aging and visual attention. Current Directions in Psychological Science, 16, 70–74. 10.1111/j.1467-8721.2007.00478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, & Cabeza R (2010). Adult age differences in functional connectivity during executive control. Neuroimage, 52(2), 643–657 10.1016/j.neuroimage.2010.04.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Jain S, Monge ZA, Cook AD, Lee A, Huang H, Howard CM, & Cohen JR (2020). Influence of structural and functional brain connectivity on age-related differences in fluid cognition. Neurobiology of Aging, 96, 205–222. 10.1016/j.neurobiolaging.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, & Monge ZA (2019). Visual attention with cognitive aging. In Oxford research encyclopedia of psychology. Oxford University Press. 10.1093/acrefore/9780190236557.013.369 [DOI] [Google Scholar]
- Madden DJ, Parks EL, Davis SW, Diaz MT, Potter GG, Chou Y. h., Chen N. k., & Cabeza R (2014). Age mediation of frontoparietal activation during visual feature search. NeuroImage, 102, Part 2(0), 262–274. 10.1016/j.neuroimage.2014.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Parks EL, Tallman CW, Boylan MA, Hoagey DA, Cocjin SB, Johnson MA, Chou Y. h., Potter GG, Chen N. k., Packard LE, Siciliano RE, Monge ZA, & Diaz MT (2017). Frontoparietal activation during visual conjunction search: Effects of bottom-up guidance and adult age. Human Brain Mapping, 38(4), 2128–2149. 10.1002/hbm.23509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Parks EL, Tallman CW, Boylan MA, Hoagey DA, Cocjin SB, Packard LE, Johnson MA, Chou Y. h., Potter GG, Chen N. k., Siciliano RE, Monge ZA, Honig JA, & Diaz MT (2017). Sources of disconnection in neurocognitive aging: Cerebral white matter integrity, resting-state functional connectivity, and white matter hyperintensity volume Neurobiology of Aging, 54, 199–213. 10.1016/j.neurobiolaging.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Pierce TW, & Allen PA (1996). Adult age differences in the use of distractor homogeneity during visual search. Psychology and Aging, 11(3), 454–474. 10.1037//0882-7974.11.3.454 [DOI] [PubMed] [Google Scholar]
- Madden DJ, Siciliano RE, Tallman CW, Monge ZA, Voss A, & Cohen JR (2020). Response-level processing during visual feature search: Effects of frontoparietal activation and adult age. Attention, Perception, & Psychophysics, 82(1), 330–349. 10.3758/s13414-019-01823-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Bucur B, & Whiting WL (2007). Age-related increase in top-down activation of visual features. Quarterly Journal of Experimental Psychology (Hove), 60(5), 644–651. 10.1080/17470210601154347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, & Huettel SA (2009). Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal of Cognitive Neuroscience, 21(2), 289–302 10.1162/jocn.2009.21047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, & Huettel SA (2007). Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiology of Aging, 28(3), 459–476. 10.1016/j.neurobiolaging.2006.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Cabeza R, & Huettel SA (2004). Age-related preservation of top-down attentional guidance during visual search. Psychology and Aging, 19(2), 304–309. 10.1037/0882-7974.19.2.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, & Huettel SA (2005). Age-related changes in neural activity during visual perception and attention. In Cabeza R, Nyberg L, & Park D (Eds.), Cognitive neuroscience of aging: Linking cognitive and cerebral aging (pp. 157–185). Oxford University Press. [Google Scholar]
- McAvinue LP, Habekost T, Johnson KA, Kyllingsbaek S, Vangkilde S, Bundesen C, & Robertson IH (2012). Sustained attention, attentional selectivity, and attentional capacity across the lifespan. Attention, Perception, & Psychophysics, 74(8), 1570–1582. 10.3758/s13414-012-0352-6 [DOI] [PubMed] [Google Scholar]
- Mevorach C, Spaniol MM, Soden M, & Galea JM (2016). Age-dependent distractor suppression across the vision and motor domain. Journal of Vision, 16, 27–27. 10.1167/16.11.27 [DOI] [PubMed] [Google Scholar]
- Miller EK, & Buschman TJ (2013). Cortical circuits for the control of attention. Current Opinion in Neurobiology, 23(2), 216–222. 10.1016/j.conb.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Szakacs I, & Uddin LQ (2022). Anterior insula as a gatekeeper of executive control. Neuroscience & Biobehavioral Reviews, 139, 104736. 10.1016/j.neubiorev.2022.104736 [DOI] [PubMed] [Google Scholar]
- Monge ZA, Geib BR, Siciliano RE, Packard LE, Tallman CW, & Madden DJ (2017). Functional modular architecture underlying attentional control in aging. Neuroimage, 155, 257–270. 10.1016/j.neuroimage.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, & Armstrong KM (2003). Selective gating of visual signals by microstimulation of frontal cortex. Nature, 421(6921), 370–373. 10.1038/nature01341 [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Schulte T, Rohlfing T, Pfefferbaum A, & Sullivan EV (2014). Visual search and the aging brain: Discerning the effects of age-related brain volume shrinkage on alertness, feature binding, and attentional control. Neuropsychology, 27, 48–59. 10.1037/a0030921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, & Husain M (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience, 9(11), 856–869. 10.1038/nrn2478 [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, & Garavan H (2002). Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging, 17(1), 56–71. 10.1037//0882-7974.17.1.56 [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Walsh V, & Frith CD (2003). Brain activations during visual search: contributions of search efficiency versus feature binding. Neuroimage, 18(1), 91–103. 10.1006/nimg.2002.1329 [DOI] [PubMed] [Google Scholar]
- Nobre AC, & Mesulam M-M (2014). Large-scale networks for attentional biases. In Nobre AC & Kastner S (Eds.), The Oxford handbook of attention (pp. 105–151). Oxford University Press. [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, & Moore T (2010). Top-down control of visual attention. Current Opinion in Neurobiology, 20(2), 183–190. 10.1016/j.conb.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, & Moore T (2011). Control of visual cortical signals by prefrontal dopamine. Nature, 474(7351), 372–375. 10.1038/nature09995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, & Reuter-Lorenz P (2009). The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196. 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plude DJ, & Doussard-Roosevelt JA (1989). Aging, selective attention, and feature integration. Psychology and Aging, 4(1), 98–105. 10.1037/0882-7974.4.1.98 [DOI] [PubMed] [Google Scholar]
- Pollmann S, Zinke W, Baumgartner F, Geringswald F, & Hanke M (2014). The right temporo-parietal junction contributes to visual feature binding. Neuroimage, 101, 289–297. 10.1016/j.neuroimage.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Pratt J, & Bellomo CN (1999). Attentional capture in younger and older adults. Aging, Neuropsychology, and Cognition, 6(1), 19–31. 10.1076/anec.6.1.19.792 [DOI] [Google Scholar]
- Proulx MJ (2007). Bottom-up guidance in visual search for conjunctions. Journal of Experimental Psychology: Human Perception and Performance, 33(1), 48–56. 10.1037/0096-1523.33.1.48 [DOI] [PubMed] [Google Scholar]
- Rabbitt P (2017). Speed of visual search in old age: 1950 to 2016. The Journals of Gerontology: Series B, 72(1), 51–60. 10.1093/geronb/gbw097 [DOI] [PubMed] [Google Scholar]
- Ratcliff R (2008). Modeling aging effects on two-choice tasks: response signal and response time data. Psychology and Aging, 23(4), 900–916. 10.1037/a0013930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, & McKoon G (2008). The diffusion decision model: Theory and data for two-choice decision tasks. Neural Computation, 20(4), 873–922. 10.1162/neco.2008.12-06-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, & McKoon G (2015). Aging effects in item and associative recognition memory for pictures and words. Psychology and Aging, 30(3), 669–674. 10.1037/pag0000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, & McKoon G (2016). Diffusion decision model: Current issues and history. Trends in Cognitive Sciences, 20(4), 260–281. 10.1016/j.tics.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, Gomez P, & McKoon G (2004). A diffusion model analysis of the effects of aging in the lexical-decision task. Psychology and Aging, 19(2), 278–289. 10.1037/0882-7974.19.2.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, & McKoon G (2003). A diffusion model analysis of the effects of aging on brightness discrimination. Perception & Psychophysics, 65(4), 523–535. 10.3758/bf03194580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1971). Trail making test results for normal and brain-damaged children. Perceptual and Motor Skills, 33, 575–581. 10.2466/pms.1971.33.2.575 [DOI] [PubMed] [Google Scholar]
- Remington RW, Vromen JMG, Becker SI, Baumann O, & Mattingley JB (2021). The role of frontoparietal cortex across the functional stages of visual search. Journal of Cognitive Neuroscience, 33(1), 63–76. 10.1162/jocn_a_01632 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Park DC (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology Review, 24(3), 355–370. 10.1007/s11065-014-9270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Mermet A, & Gade M (2018). Inhibition in aging: What is preserved? What declines? A meta-analysis. Psychonomic Bulletin & Review, 25(5), 1695–1716. 10.3758/s13423-017-1384-7 [DOI] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, & Ungerleider LG (2007). Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. Journal of Neuroscience, 27(42), 11306–11314. 10.1523/JNEUROSCI.2939-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthruff E, & Gaspelin N (2018). Immunity to attentional capture at ignored locations. Attention, Perception, & Psychophysics, 80(2), 325–336. 10.3758/s13414-017-1440-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403–428. 10.1037/0033-295x.103.3.403 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Habeck C, Razlighi Q, Barulli D, Gazes Y, & Stern Y (2015). Breadth and age-dependency of relations between cortical thickness and cognition. Neurobiology of Aging, 36(11), 3020–3028. 10.1016/j.neurobiolaging.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, & Madden DJ (2007). Information processing speed and aging. In Deluca J & Kalmar J (Eds.), Information processing speed in clinical populations (pp. 221–241). Psychology Press. [Google Scholar]
- Saults JS, & Cowan N (2007). A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. Journal of Experimental Psychology: General, 136, 663–684. 10.1037/0096-3445.136.4.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, & Stevens WD (2007). Reductions in cortical activity during priming. Current Opinion in Neurobiology, 17, 171–176. 10.1016/J.CONB.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Servant M, & Evans NJ (2020). A diffusion model analysis of the effects of aging in the Flanker Task. Psychology and Aging, 35, 831–849. 10.1037/pag0000546 [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d’Avossa G, & Corbetta M (2003). Quantitative analysis of attention and detection signals during visual search. Journal of Neurophysiology, 90(5), 3384–3397. 10.1152/jn.00343.2003 [DOI] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Madden DJ, & Voss A (2006). A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition, 32(1), 101–117. 10.1037/0278-7393.32.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, & Grady CL (2010). Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neuroscience & Biobehavioral Reviews, 34(8), 1178–1194. 10.1016/j.neubiorev.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Steiger JH (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 87, 245–251. 10.1037/0033-2909.87.2.245 [DOI] [Google Scholar]
- Stilwell BT, & Gaspelin N (2021). Attentional suppression of highly salient color singletons. Journal of Experimental Psychology: Human Perception and Performance, 47(10), 1313–1328. 10.1037/xhp0000948 [DOI] [PubMed] [Google Scholar]
- Thapar A, Ratcliff R, & McKoon G (2003). A diffusion model analysis of the effects of aging on letter discrimination. Psychology and Aging, 18(3), 415–429. 10.1037/0882-7974.18.3.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J (2010). Top-down and bottom-up control of visual selection. Acta Psychologica (Amst), 135(2), 77–99. 10.1016/j.actpsy.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2013). Feature-based attention: it is all bottom-up priming. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 368(1628), 20130055. 10.1098/rstb.2013.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J (2014). Spatial orienting and attentional capture. In Nobre AC & Kastner S (Eds.), The Oxford handbook of attention (pp. 231–252). Oxford. 10.1093/oxfordhb/9780199675111.013.005 [DOI] [Google Scholar]
- Treiber JM, White NS, Steed TC, Bartsch H, Holland D, Farid N, McDonald CR, Carter BS, Dale AM, & Chen CC (2016). Characterization and correction of geometric distortions in 814 diffusion weighted images. PLoS One, 11(3), e0152472. 10.1371/journal.pone.0152472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ravenzwaaij D, & Oberauer K (2009). How to use the diffusion model: Parameter recovery of three methods: EZ, fast-dm, and DMAT. Journal of Mathematical Psychology, 53(6), 463–473. 10.1016/j.jmp.2009.09.004 [DOI] [Google Scholar]
- Voss A, Nagler M, & Lerche V (2013). Diffusion models in experimental psychology: A practical introduction. Experimental Psychology, 60, 385–402. 10.1027/1618-3169/a000218 [DOI] [PubMed] [Google Scholar]
- Voss A, Rothermund K, Gast A, & Wentura D (2013). Cognitive processes in associative and categorical priming: a diffusion model analysis. Journal of Experimental Psychology: General, 142(2), 536–559. 10.1037/a0029459 [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, & Fink GR (2014). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist, 20, 150–159. 10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers E-J, Van Der Maas HLJ, & Grasman RPPP (2007). An EZ-diffusion model for response time and accuracy. Psychonomic Bulletin & Review, 14(1), 3–22. 10.3758/BF03194023 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale (WAIS-III): Administration and scoring manual. The Psychological Corporation. [Google Scholar]
- Wei P, Muller HJ, Pollmann S, & Zhou X (2011). Neural correlates of binding features within- or cross-dimensions in visual conjunction search: an fMRI study. Neuroimage, 57(1), 235–241. 10.1016/j.neuroimage.2011.04.024 [DOI] [PubMed] [Google Scholar]
- Wei P, Müller HJ, Pollmann S, & Zhou X (2009). Neural basis of interaction between target presence and display homogeneity in visual search: An fMRI study. NeuroImage, 45(3), 993–1001. 10.1016/j.neuroimage.2008.12.053 [DOI] [PubMed] [Google Scholar]
- Whiting WL, Madden DJ, Pierce TW, & Allen PA (2005). Searching from the top down: Ageing and attentional guidance during singleton detection. The Quarterly journal of experimental psychology. A, Human experimental psychology, 58(1), 72–97. 10.1080/02724980443000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I, & Wolfe JM (2020). Age doesn’t matter much: hybrid visual and memory search is preserved in older adults. Aging, Neuropsychology, and Cognition, 27(2), 220–253. 10.1080/13825585.2019.1604941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM (2014). Approaches to visual search. In Nobre AC & Kastner S (Eds.), The Oxford handbook of attention (pp. 1–40). Oxford University Press. 10.1093/oxfordhb/9780199675111.013.002 [DOI] [Google Scholar]
- Wolfe JM, & Horowitz TS (2004). What attributes guide the deployment of visual attention and how do they do it? Nature Reviews Neuroscience, 5, 495–501. 10.1038/nrn1411 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, & Smith SM (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage, 21(4), 1732–1747. 10.1016/j.neuroimage.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, & Smith SM (2001). Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage, 14, 1370–1386. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- Wöstmann M, Störmer VS, Obleser J, Addleman DA, Andersen SK, Gaspelin N, Geng JJ, Luck SJ, Noonan MP, Slagter HA, & Theeuwes J (2022). Ten simple rules to study distractor suppression. Progress in Neurobiology, 213, 102269. 10.1016/j.pneurobio.2022.102269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S (1996). Attentional capture in vision. In Kramer AF, Coles GH, & Logan GD (Eds.), Coverging operations in the study of visual selective attention (pp. 45–76). American Psychological Association. [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, & Braver TS (2009). BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLOS ONE, 4(1), e4257–e4257. 10.1371/JOURNAL.PONE.0004257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


