Abstract
Immunogenetic mechanisms operating within the immune system are known to influence cytokine profiles and disease susceptibility. Yet the role of the individual’s neurohormonal background in these processes remains undefined. Hormonal imbalances are documented in immune-related diseases, but it is unclear whether this represents a secondary phenomenon or a primary “defect” related to specific neurohormonal immune phenotype(s). We report that in a large subpopulation of healthy humans the baseline epinephrine output (but not cortisol and sex steroid hormones) correlated inversely with proinflammatory and positively with anti-inflammatory cytokine production. Thus, low vs high epinephrine excretors had a 2- to 5-fold higher TNF-α and IL-12 production but 2-fold lower IL-10 production induced by LPS ex vivo. In alternative settings, we found low baseline levels and profoundly blunted stress-induced epinephrine responses but high TNF-α levels in Lewis vs Fischer inbred rats. Additionally, isoproterenol, a β adrenoreceptor agonist suppressed LPS-induced TNF-α production, with more pronounced effect in Lewis than in Fischer rats. In human monocytes, epinephrine and the β2 adrenoreceptor agonist fenoterol potently inhibited LPS-induced TNF-α and IL-12, but stimulated IL-10 production. The order of potency for hormones able to inhibit IL-12 production ex vivo was: epinephrine > norepinephrine > = 1,25-(OH)2 vitamin D3, > hydrocortisone. This indicates that baseline epinephrine conditions cytokine responsiveness and through this mechanism intrinsic hypo- or hyperactive adrenal medullas in some individuals may shape opposite cytokine profiles. Since Lewis and Fischer rats have opposite susceptibility to experimental immunological diseases, this suggests that the parallel human phenotypes could be linked to differing responsiveness and susceptibility to infections and immune/inflammatory-related conditions.
The production of cytokines associated with innate immunity shows large interindividual variations (1-4). This phenomenon may have an important clinical relevance since recent evidence indicates that individuals with relatively high or low pro- and anti-inflammatory cytokine profiles have an increased risk or resistance to a number of immune-related diseases (4-8). Factors that control cytokine phenotypes are traditionally linked to the effects of immunogenetic mechanisms operating exclusively within the immune system. The cytokine milieu and the genetic background are considered major factors that control the individual’s cytokine responsiveness and profiles. For example, IL-12 production is enhanced by TNF-α, but both IL-12 and TNF-α production are down-regulated by IL-10. At the genetic level, single nucleotide polymorphisms (SNPs)2 of cytokine genes or TLRs determine, at least in part, the interindividual cytokine variability and phenotypes, which in turn affect disease susceptibility (4, 7-9).
Influences of factors outside the immune system such as the neurohormonal milieu remain poorly understood. The genetic background undoubtedly controls both the neuroendocrine and the immune system’s phenotypes, but these complex traits are also modified in the long term by hormonal and environmental factors during the individual’s fetal and early postnatal development (10, 11). However, the influence of an established adult neuroendocrine phenotype on the individual’s cytokine profiles remains undefined.
Supraphysiological or low levels of hormones such as catecholamines (CAs), glucocorticoids (GCs), 1,25-(OH)2 vitamin D3, and sex steroid hormones are commonly associated with stress, pregnancy/postpartum period, or pathologies such as infections, myocardial infarction, stroke, traumatic brain injury, and rheumatoid arthritis. An increasing body of evidence indicates that the above-mentioned hormonal changes contribute to the altered cytokine and Th1/Th2 balance in these conditions (12-19). In general, high systemic levels of these hormones inhibit proinflammatory/Th1 responses but up-regulate anti-inflammatory/Th2 responses through complex effects on innate/adaptive immune cells (12, 20-25).
These studies strongly suggest an interactive nature between neurohormonal and cytokine systems, under abnormal or in vitro conditions, but still do not link interindividual differences in unstimulated baseline hormone levels to the above-mentioned large interindividual variations in cytokine expression. Addressing this issue may help answer two fundamental and clinically important questions: are the baseline hormones that are part of the natural microenvironment of cytokine-secreting cells “innocent bystanders” or are they actively involved in shaping the individual’s cytokine responsiveness and profiles? In chronic inflammatory diseases, is hormonal dysfunction secondary to the ongoing inflammatory process, or is it a primary defect in some individuals that has a role in disease susceptibility and pro-/anti-inflammatory cytokine imbalances?
A helpful model for studying neurohormonal immune phenotypic interactions is the Lewis (LEW)/Fischer (F344) paradigm. LEW and F344 inbred rats share the same MHC class II Ags, but have opposite susceptibility to experimentally induced models of autoimmune diseases, infections, and tumors (19, 26). LEW rats express high proinflammatory/Th1, whereas F344 express low proinflammatory/Th1 responses, as indicated by high vs low IL-12 and IFN-γ cytokine expression phenotypes (27, 28). These differences have been linked to the previously described low corticosterone levels in LEW as compared with F344 rats (19, 29) and may explain, at least in part, their opposite disease susceptibility phenotypes. The possibility that LEW-like and F344-like neuroendocrine immune phenotypes exist in the human population has not been previously explored.
Therefore, we addressed the question of whether interindividual differences in baseline hormonal levels would predict differences in innate cytokine profiles in a subpopulation of healthy humans and looked for analogous associations in LEW and F344 inbred rats. Our data indicate that relatively low or high baseline levels of the adrenomedullary hormone epinephrine (EPI, adrenaline) condition the activity of cytokine-secreting cells toward relatively high or low proinflammatory cytokine profiles, respectively, both in rodents and humans.
Materials and Methods
Study participants
One hundred seven healthy subjects (49 females, 58 males) between the ages of 20 and 40 participated in the study, which was approved by the institutional review board of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (NIH, Bethesda, MD). We screened each participant at the National Institutes of Health Clinical Center using medical history, physical examination, and routine laboratory tests. All participants signed informed consent forms. Female participants had their tests during the early and mid-follicular phase of their menstrual cycle (days 3–8). All participants abstained from taking medications the week before the study. The subjects visited the outpatient clinic of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH) and blood specimens for hormone and cytokine measurements were drawn after 1 h of rest between 1 and 2 p.m.
Drugs and reagents
LPS from Escherichia coli, serotype K-235, dobutamine HCl, fenoterol hydrobromide, dexamethasone, and hydrocortisone were purchased from Sigma-Aldrich. Epinephrine bitartrate and norepinephrine bitartrate were purchased from Research Biochemicals.
Whole blood cultures
Ex vivo whole blood cytokine production assays were performed as described elsewhere (1, 4, 30). Blood was drawn into sodium-heparin-containing sterile tubes and processed within 45 min. The blood, diluted 1:5 with RPMI 1640 (supplemented with 1% glutamine and 50 μg/ml gentamicin), was stimulated with bacterial LPS at 1 μg/ml final concentration, and the samples were incubated in 5% CO2 at 37°C for 18 h. Hormones or drugs were tested at 10−5–10−11 M concentrations. EPI, norepinephrine (NE), dobutamine, and fenoterol were added 10 min before LPS. All other hormones or drugs were added simultaneously with LPS. The whole-blood ex vivo cytokine assay offers the opportunity to induce and detect cytokine production in small blood samples and avoids the isolation of PBMCs that may alter the lymphocyte:monocyte ratio and/or cause potential activation and artifactual differences not present in vivo. In whole blood not only are natural cell-cell interactions preserved, but circulating stimulatory or inhibitory mediators (including hormones) are also present in their normal proportions. This demonstrates that this assay forms a good basis for the study of genetically or hormonally defined variation. Because monocytes are the main IL-12-, TNF-α-, and IL-10-producing cells in LPS-stimulated whole blood (1, 30) and to study more accurately how hormonal variations affected the capacity of monocytes to produce cytokines, the whole-blood cytokine production was corrected for monocyte/macrophage counts (picograms per 106 monocytes/macrophages).
Cytokine assays
Human and rat cytokines were measured using ELISA (R&D Systems) methods. Because at the present there is no protein-based assay to detect rat IL-12, we were unable to measure this cytokine in our experiments. The detection limits for the human IL-12p70 and the high-sensitivity IL-12p70 ELISA were 7.5 and 0.5 pg/ml, respectively, while they were 15.0 and 2.0 pg/ml for the TNF-α and IL-10 ELISA, respectively. The detection limits for the rat TNF-α and IL-10 ELISA were 5.0 and 31.2 pg/ml, respectively.
Experimental animals
Male, specific-pathogen-free, LEW and F344 rats (280–340 g) were purchased from Harlan Sprague Dawley and Charles River Farm. Animals were given food and water ad libitum. All animal procedures conformed to institutional guidelines and the Association for Research in Vision and Ophthalmology Resolution on Use of Animals in Research.
Experimental design for the study of rat cytokines
Rats were weighed 1 day before the experiment. On the day of the experiment, they were challenged i.p. with 1 ml of LPS (0.05 mg/kg). In the case of drug treatment, isoproterenol or propranolol (5 mg/kg) was injected i.p. 30 min before the LPS challenge. Control animals received the same volume of saline. Animals were killed at 90 min after LPS treatment and blood was collected in ice-cold heparinized Eppendorf tubes and centrifuged at 4°C for 10 min. The plasma was stored at —70°C until assayed.
Experimental design for the study of rat CAs
Blood for plasma CA determinations was sampled from awake rats via a chronically indwelling polyethylene catheter inserted into the tail artery 20–24 h before the experiment. After surgery, each rat was housed in an individual plastic cage, with the catheter extending out of the cage. Blood samples (0.5 ml) were collected via the catheter, and the same volume of heparinized saline (50 IU ml−1) was administered intra-arterially after each blood sample was obtained. Immobilization was done by taping all four limbs of the rat to metal mounts attached to a board.
TNF-α RNA Northern blot analysis
Total RNA was prepared from freshly isolated spleens from LEW and F344 rats after LPS injections. Twenty micrograms of total RNA was electrophoresed through a 1.2% agarose-formaldehyde gel, and transferred to Genescreen Plus nylon membrane (DuPont-NEN). The membranes were hybridized with a 32P-labeled cDNA probe at 42°C overnight in 50% formamide, 10% dextran sulfate, 7% SDS, 0.25 M NaCl, 0.25 M NaHPO4, and 1 mM EDTA. The membranes were washed twice with 0.2× SSC and 0.2% SDS at 55°C for 15 min.
Hormonal measurements
The 24-h urinary excretion of NE is a fairly reliable index of the overall sympathetic activity, whereas the 24-h urinary output of EPI and free cortisol specifically reflect the activity of the adrenal medulla and cortex, respectively. CAs and cortisol were assessed in 24-h urine collections on 2 consecutive days, i.e., prior to the blood draw. The mean urinary output of NE, EPI, and cortisol from the two consecutive collections was expressed in micrograms per 24 h. Twenty-four-hour urinary excretion of EPI and NE was measured by HPLC with electrochemical detection. Normal values are 0–20 μg/24 h and 15–80 μg/24 h, respectively (Mayo Clinic Laboratories). Twenty-four-hour urinary excretion of free cortisol was measured after extraction by a chemiluminescent competitive protein-binding assay. Normal values are 24–108 μg/24 h (Mayo Clinic Laboratories). 1,25-Dihydroxyvitamin D3 was measured by cartridge extraction and radioreceptor assay. Normal values are 15–60 pg/ml (Mayo Clinical Laboratories). Rat plasma NE and EPI were measured by the radioenzymatic method. The detection limit for NE and EPI was 1 pg/μl plasma.
Statistical analysis
All data are presented as mean ± SEM. Cytokine and catecholamine levels were compared using one-way ANOVA. The Pearson product-moment correlation was used to examine relationships between variables. Stepwise multiple regression analysis was used to examine the independent relationships among different variables. All statistical analyses were done using Statistica (version 5.5; StatSoft).
Results
Interindividual variations and correlations of IL-12, TNF-α, and IL-10 production
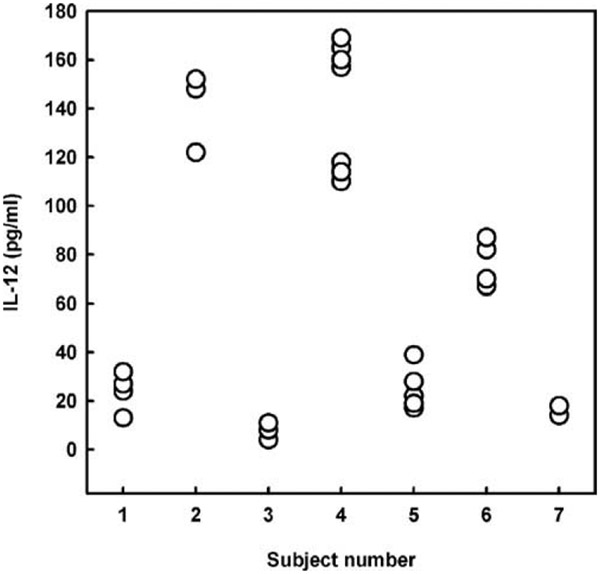
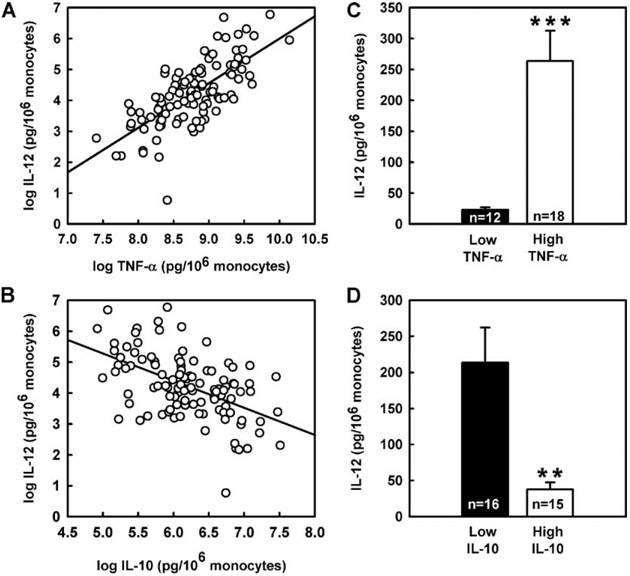
In our study cohort consisting of 107 healthy humans, ex vivo innate cytokine production exhibited marked interindividual differences. LPS-induced IL-12p70, TNF-α, and IL-10 values ranged from 1 to 350, 445 to 9638, and 44 to 949 pg/ml, respectively. Previous studies have shown stable and reproducible interindividual differences of the ex vivo TNF-α and IL-10 production with <15% variation when studied longitudinally (1, 4). To verify that the IL-12 production variability is a constant trait, we repeatedly measured LPS-induced IL-12 production in seven healthy subjects at intervals varying from 2 wk to 2 years. We observed a substantial interindividual variation as opposed to <15% intraindividual variation of the ex vivo IL-12 production (Fig. 1). A strong positive correlation was found between IL-12 and TNF-α cytokine production (r = 0.71, p < 0.0001; Fig. 2A) and an inverse association between IL-10 and IL-12 (r = −0.52, p < 0.0001; Fig. 2B) and TNF-α production (r = −0.37, p < 0.0001; data not shown). These correlations are consistent with the known immunoregulatory interactions of these cytokines. Thus, low TNF-α and IL-10 producers had ~11-fold lower IL-12 production and 5-fold higher IL-12 production, respectively, than high TNF-α and IL-10 producers (Fig. 2, C and D).
FIGURE 1.
Reproducibility of human IL-12 production interindividual variations. LPS-induced IL-12p70 production in whole blood cultures ex vivo from seven healthy subjects, as determined by ELISA and described in Materials and Methods. Experiments with whole blood cultures from different individuals were repeated two to seven times within several weeks, months, and, in some cases, within 2–3 years. To avoid circadian variations of cytokine production, experiments were performed between 2 and 3 p.m. Raw data for the cytokine production in pg/ml are depicted for each subject.
FIGURE 2.
Correlations among IL-12, TNF-α, and IL-10 production ex vivo in 107 healthy human subjects. A, Plot of IL-12 vs TNF-α. B, Plot of IL-12 vs IL-10. C, Ex vivo IL-12 production in low TNF-α (≤3,423 pg/106 monocytes) vs high TNF-α producers (≥11,063 pg/106 monocytes). D, Ex vivo IL-12 production in low IL-10 (≤236 pg/106 monocytes) vs high IL-10 producers (≥954 pg/106 monocytes). Low vs high TNF-α and IL-10 producers were estimated by subtracting or adding 1 SD from the means of the population estimates of TNF-α and IL-10 production values. Data are expressed as means ± SE. *** and ** designate significance at p < 0.001 and 0.01, respectively. Cytokine production was corrected for monocyte number and expressed as pg/106 monocytes. The means of the population estimates for the ex vivo IL-12, TNF-α, and IL-10 production were 113, 7243, and 595 pg/106 monocytes, respectively.
Correlation between adrenomedullary activity and innate cytokine production
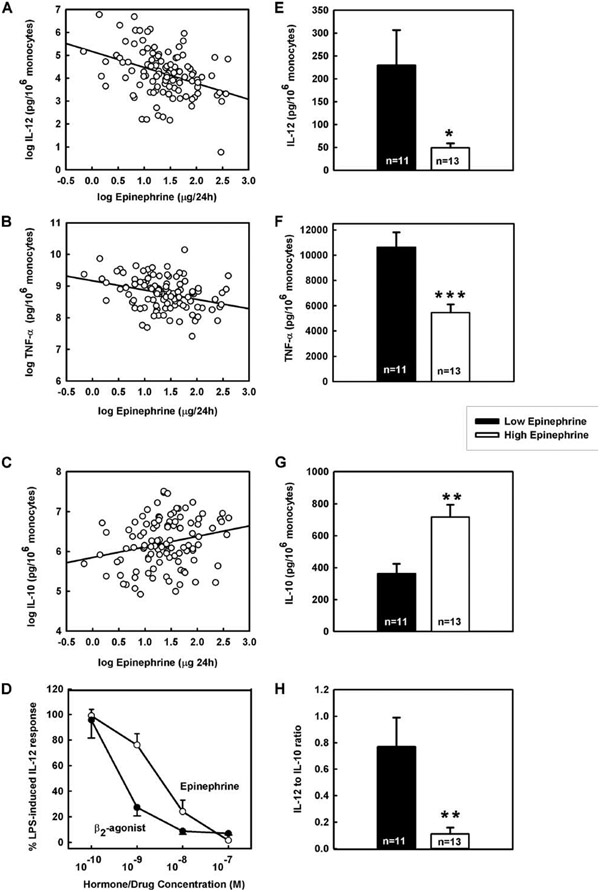
To determine whether differences in innate cytokine profiles are linked to the interindividual baseline hormonal variability in humans, we analyzed the production of cytokines ex vivo as a function of the baseline 24-h urinary output or serum levels of seven different hormones known to have immunomodulatory properties: cortisol, EPI, NE, estradiol, progesterone, testosterone, and 1,25-(OH)2 vitamin D3. We found that the 24 h urinary EPI output correlated inversely and significantly with the ex vivo production of IL-12 and TNF-α and positively with IL-10 production, corrected for monocyte numbers (Fig. 3, A-C, and Table I). Similar correlations between the urinary EPI excretion and IL-12, TNF-α, and IL-10 were found when cytokine production was not corrected for monocyte numbers and was expressed as picograms per milliliter (r = −0.36, −0.27, 0.24, respectively, p < 0.05; data not shown). The IL-12 production also correlated inversely, but to a lesser extent, with the levels of 1,25-(OH)2 vitamin D3 (Table I).
FIGURE 3.
Correlations between cytokines ex vivo and baseline EPI output in vivo in 107 healthy human subjects. A, Plot of IL-12 vs 24-h EPI excretion. B, Plot of TNF-α vs 24-h EPI excretion. C, Plot of IL-10 vs 24-h EPI excretion. D, Dose-dependent inhibitory effect of EPI and fenoterol on human IL-12 production in whole blood cultures. Ex vivo IL-12 (E), TNF-α (F), IL-10 (G), and IL-12:IL-10 ratio (H) in low (≤1.94 μg/24 h) vs high EPI excretors (≥7.19 μg/24 h). Low vs high EPI excretors were estimated by subtracting and adding 1 SD from the means of the population estimates of 24-h urinary EPI excretion. Data are expressed as means ± SE. ***, **, and * designate significance at p < 0.001, 0.0,1 and 0.05, respectively. Cytokine production was corrected for monocyte number and expressed as pg/106 monocytes. The means of the urinary EPI excretion (μg/24 h ± SD) in low and high EPI excretors were 1.49 ± 0.34 and 10.13 ± 2.21, respectively. The means ± SD of the monocyte percentages and absolute monocyte count in low vs high EPI excretors were 7.39 ± 1.85% and 0.41 ± 0.14 × 103/μl vs 7.44 ± 1.56% and 0.47 ± 0.12 × 103/μl, respectively. The means of the population estimates for monocyte percentages and absolute monocyte count were 7.73% and 0.43 × 103/μl. The means of the population estimates for 24-h urinary EPI excretion, the ex vivo IL-12, TNF-α, and IL-10 production and IL-12: IL-10 ratio were 4.57 μg/24 h, 113, 7243, and 595 pg/106 monocytes and 0.37, respectively.
Table I.
Correlation between hormones in vivo and cytokines ex vivo (r values)
| Hormone | IL-12 | TNF-α | IL-10 |
|---|---|---|---|
| 24-h EPI | −0.37*** | −0.32** | 0.24* |
| 24-h NE | −0.18 | −0.09 | 0.10 |
| 24-h Urinary-free Cortisol | 0.03 | 0.04 | −0.09 |
| 1,25-(OH)2 vitamin D3 | −0.20** | −0.19 | 0.12 |
| Estradiol | −0.11 | −0.03 | −0.10 |
| Progesterone | −0.15 | −0.12 | 0.13 |
| Testosterone | −0.03 | 0.09 | 0.10 |
***, **, and *, r value significant at p < 0.001, 0.01, and 0.05, respectively, the r values for estradiol and progesterone are from 49 female subjects, and the r values for testosterone from 58 male subjects.
The 24-h urinary EPI excretion did not correlate with the monocyte percentages and absolute monocyte count (r = −0.07 and 0.01, respectively; data not shown), suggesting that baseline EPI affects monocytic activity but not monocyte number. In our study cohort, the urinary EPI output ranged from 0.85 to 13.5 μg/24 h. Similarly to a previous study (31), the 24-h urinary EPI excretion assessed on 2 consecutive days showed relatively wide but stable interindividual variability (r = 0.70, p < 0.05, EPI excretion on day 1 vs EPI excretion on day 2; data not shown). The 24-h urinary EPI excretion correlated positively and significantly with the 24-h urinary-free cortisol excretion (r = 0.22, p < 0.05; data not shown), indicating an association between the baseline activity of the adrenal medulla and cortex in healthy subjects.
The 24-h urinary-free cortisol excretion, however, did not correlate with the ex vivo production of IL-12, TNF-α, and IL-10. Similarly, serum levels of estradiol, progesterone, and testosterone did not correlate with the production of any of the cytokines examined (Table I). Using multiple forward stepwise regression analysis to test how age, gender, cortisol, NE, and 1,25-(OH)2 vitamin D3 may have influenced the effect of EPI, we found that EPI was the only independent variable significantly related to the variation of IL-12, TNF-α, and IL-10 production (explaining 8.1, 5.2, and 5.8% of the variability, p < 0.001, p < 0.01, p < 0.01, respectively).
Marked differences in the ex vivo cytokine production in low vs high EPI excretors
Consistent with the above-reported results, it was estimated that low EPI excretors had ~5-fold higher IL-12 (p < 0.05; Fig. 3E), 2-fold higher TNF-α (p < 0.001; Fig. 3F) but 2-fold lower IL-10 (p < 0.01; Fig. 3G) production than high EPI excretors. Thus, low EPI excretors had ~4-fold higher TNF-α IL-10 ratio (36.6 ± 5.4 vs 9.2 ± 1.8, p < 0.0001) and 7-fold higher IL-12:IL-10 ratio than high EPI excretors (p < 0.01; Fig. 3H).
Sympathetic nervous system activity and cytokine profiles in LEW vs F344 rats
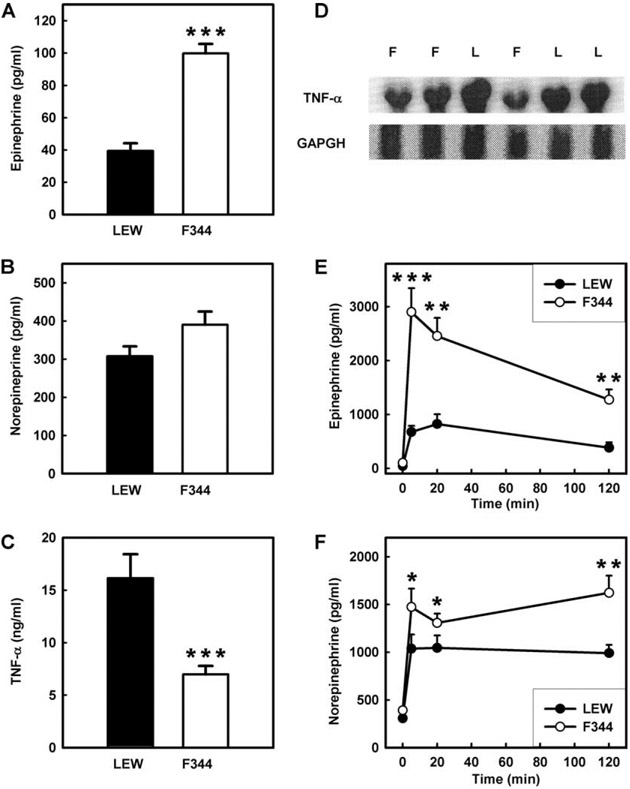
To look for analogous CA-cytokine interactions in animal models, we measured CA levels in LEW and F344 inbred rats. We found that LEW rats had significantly lower baseline EPI levels than F344 rats and lower baseline NE levels that did not achieve significance (Fig. 4, A and B). Compared with F344 rats, LEW had higher LPS-induced plasma TNF-α levels and splenic TNF-α RNA expression (Fig. 4, C and D) and slightly higher plasma IL-10, but the difference was not statistically significant (data not shown). LEW rats also had impaired plasma EPI and NE in response to a substantial stressor, such as the immobilization test, while F344 expressed a robust EPI response, compared with the profoundly blunted EPI response in LEW rats (Fig. 4, E and F).
FIGURE 4.
Cytokine production, CA levels and responses in LEW vs F344 inbred rats. A, EPI baseline plasma levels. B, NE baseline plasma levels. C, LPS-induced TNF-α plasma levels. D, Splenic total RNA as assessed by Northern blot probed with TNF-α. E, EPI plasma levels after immobilization. F, NE plasma levels after immobilization. Data are expressed as means ± SE, five to seven animals per group. ***, **, and * designate significance at p < 0.001, 0.01, and 0.05, respectively. F, F344 rats; L, LEW rats.
Effect of β adrenoreceptor (AR) agents on TNF-α production in inbred rats
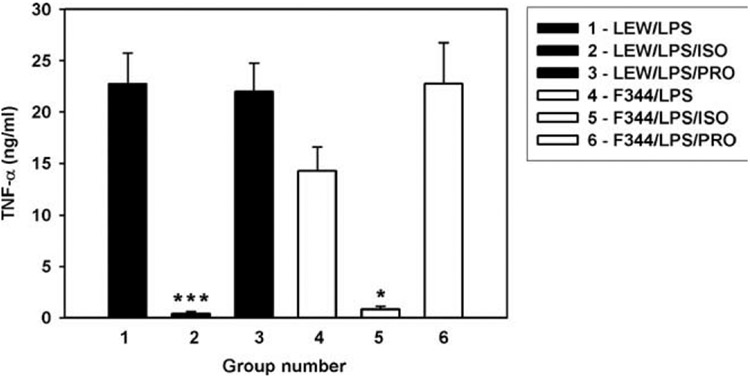
In vitro and in mice in vivo, CAs are known to inhibit the production of TNF-α through stimulation of β-ARs (32). To examine whether this mechanism also operated in LEW and F344 rats, we studied the effect of isoproterenol, a β-AR agonist. Isoproterenol substantially suppressed LPS-induced TNF-α production in both rat strains, with more significant inhibition in LEW than in F344 rats (p < 0.001 and 0.05, respectively). The β-AR antagonist propranolol potentiated the LPS-induced TNF-α production in F344 but not in LEW rats (Fig. 5).
FIGURE 5.
Effect of isoproterenol and propranolol on LPS-induced TNF-α production in LEW and F344 inbred rats. Data are expressed as means and SE, five to seven animals per group. *** and * designate significance at p < 0.001 and 0.05, respectively. ISO, Isoproterenol; PRO, propranolol.
Potency of hormonal effects ex vivo
To verify the responsiveness of human monocytes (the main IL-12-, TNF-α-, and IL-10-producing cells in LPS-stimulated whole blood (1, 30)) to hormones and to support the concept that the above-described correlations are causal, we studied the effect of hormones and their analogs ex vivo. Consistent with these correlations, EPI expressed much higher potency in modulating monocytic cytokine production than did NE, 1,25-(OH)2 vitamin D3, and hydrocortisone. Estradiol, progesterone, and testosterone did not influence innate cytokine production, whereas 1,25-(OH)2 vitamin D3 did not affect IL-10, but dose-dependently inhibited LPS-induced IL-12 production (Table II).
Table II.
Effect and potency (EC50) of hormones or their analogs on the ex vivo LPS-induced cytokine production in human whole blood from five to six healthy subjects
| Hormone or Drug | Inhibition (EC50) |
Potentiation (EC50) |
|
|---|---|---|---|
| IL-12 | TNF-α | IL-10 | |
| EPI | 5 × 10−9 M | 2 × 1−8 M | 10−8 M |
| NE | 3 × 10−8 M | 10−7 M | 10−7 M |
| Hydrocortisone | 10−7 M | 6 × 10−7 M | — |
| 1,25-(OH)2 vitamin D3 | 6 × 10−8 M | ND | — |
| Estradiol | —a | — | — |
| Progesterone | — | — | — |
| Testosterone | — | ND | ND |
| Dexamethasone | 10−8 M | 2 × 10−8 M | — |
| Dobutamine (β1 agonist) | 5 × 10−7 M | 10−6 M | 10−6 M |
| Fenoterol (β2 agonist) | 6 × 10−10 M | 10−9 M | 10−9 M |
—, No effect.
Monocytes express high-density β2 ARs, while EPI has a much higher affinity for β2 ARs than for β1 ARs and NE has a higher affinity for β1 ARs than for β2 ARs (32, 33). Also, the synthetic glucocorticoid dexamethasone, commonly used for in vitro studies, exerts more potent immunomodulatory effects than hydrocortisone (cortisol). Consistent with this, in our study, EPI and the β2 AR agonist fenoterol expressed much higher potency in modulating monocytic cytokine production than did NE and the β1 AR agonist dobutamine, whereas hydrocortisone was an order of magnitude less effective than dexamethasone. The order of potency for hormones/drugs modulating IL-12 production was: fenoterol > EPI > dexamethasone >NE ≧ 1,25-(OH)2 vitamin D3 > hydrocortisone > dobutamine (Fig. 3D and Table II).
Discussion
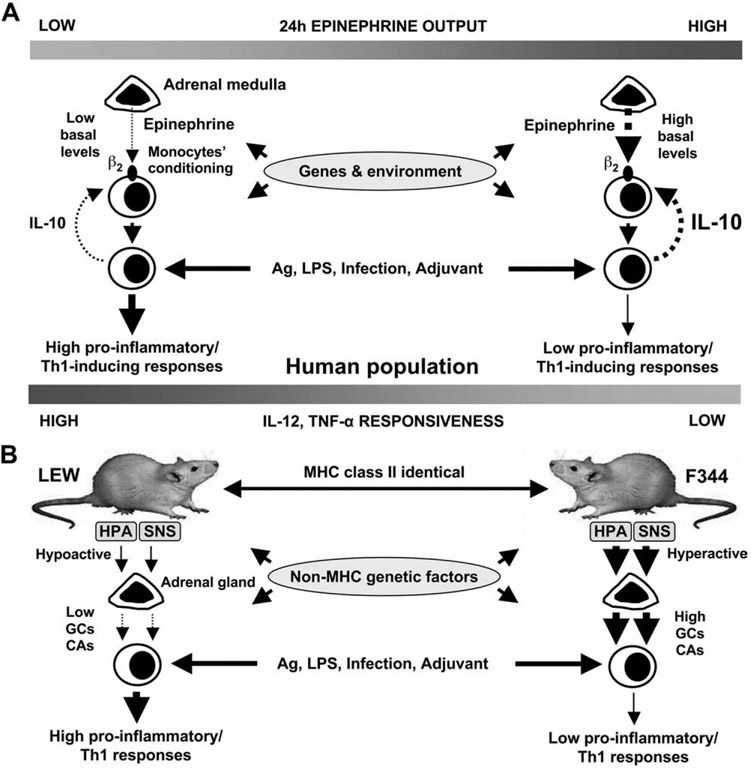
The present study identifies a strong influence of the individual’s neurohormonal background on the production of innate cytokines ex vivo. This study 1) establishes a link between adrenomedullary EPI and innate cytokine profiles in humans, 2) indicates that baseline EPI is involved in immune homeostasis by conditioning the activity and responsiveness of cytokine-secreting cells, and 3) identifies that subgroups of humans with intrinsic hypoactive or hyperactive adrenal medullas express opposite innate cytokine profiles. Our study suggests that hormonal factors such as the baseline EPI superimposes upon and interweaves with immunogenetic and environmental factors that control the individual’s innate cytokine responsiveness and variability (9, 10). Thus, upon antigenic challenge, systemic concentrations of EPI along with local concentrations of immunoregulatory cytokines may shape the individual’s innate cytokine phenotypes (Fig. 6A).
FIGURE 6.
A schematic interpretation of the role of baseline EPI (adrenaline) in shaping innate cytokine responsiveness and cytokine profiles. A, In humans, a relative low activity of the adrenal medulla in some individuals provides low tonic inhibitory input on proinflammatory cytokine production, exerted by endogenous basal EPI and thus conditions peripheral monocytes to produce more IL-12 and TNF-α but less IL-10 upon antigenic stimulation than monocytes in individuals with relatively high adrenomedullary activity. B, Genetically determined hypoactive and hyperactive hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS) result in low vs high tonic inhibitory input on proinflammatory cytokine production exerted by endogenous basal GCs and CAs and thus determine to a great extend high vs low proinflammatory/Th1 responses in LEW and F344 rats, respectively. Solid lines represent stimulation, while dashed lines represent inhibition.
The modulatory role of EPI is further suggested by our observations in inbred rats, where LEW rats had a 2- to 3-fold lower baseline and 3–5-fold lower stress-induced EPI levels than F344 rats. Sympathetic/adrenomedullary activity is among the major factors that control the expression of peripheral ARs in target tissues, including β ARs on immune cells (32, 34, 35). In our study, isoproterenol inhibited TNF-α production more effectively in LEW than F344 rats, whereas propranolol, by blocking the inhibitory input of endogenous CAs, potentiated the TNF-α response of F344 rats. These data suggest low endogenous CAs with respective physiological up-regulation of β ARs in LEW rats vs high endogenous CAs with down-regulation of β ARs in F344 rats. Thus, taken as a whole, our study indicates that LEW, compared with F344 rats, have low adrenomedullary activity and responsiveness. Our study might also suggest that the LEW rat phenotype could be converted to that of F344 rats with β ARs agonist treatment. This is substantiated by previous studies showing that β AR agonists suppress the expression of experimental allergic encephalomyelitis and collagen- or adjuvant-induced arthritis in LEW rats and mice (36-38).
It has been previously shown that LEW rats express higher levels of IL-12 and IFN-γ mRNA and produce more IFN-γ at the protein level than F344, but both F344 and LEW have similar IL-10 mRNA levels (27, 28, 39). Therefore, as recently discussed by Caspi et al. (27), the F344 cannot be called a Th2-responder in the conventional sense, keeping in mind that the Th1 and Th2 cytokine profiles in the rat may not be identical to those in the mouse and humans. Our observation that LEW had higher TNF-α but similar IL-10 production compared to F344 rats further supports the concept that a high proinflammatory/Th1 response in LEW and a low proinflammatory/Th1 response rather than a high Th2 protective response is specific for F344 rats (27, 39) that may control disease susceptibility in LEW vs the resistance in F344. Previous studies have shown that LEW, compared with F344 rats, are relatively low corticosterone producers and have smaller adrenal glands (29, 40). Our study indicates that the combination of genetically determined low vs high adrenocortical/adrenomedullary activity may determine, at least in part, the high vs low proinflammatory/Th1 cytokine responses in these inbred rats (Fig. 6B).
In humans, however, under baseline physiological conditions, it appears that systemic EPI (adrenomedullary) but not cortisol (adrenocortical) is an important regulatory hormone that affects both pro- and anti-inflammatory cytokine profiles. Comparing the EC50 of hydrocortisone (Table II) with the free cortisol concentrations (in the range of 11–43 nM) may help explain the lack of correlation between baseline cortisol and innate cytokine profiles. This may also indicate that, in contrast to baseline EPI, the baseline cortisol levels do not affect innate cytokine production and variability, simply because they do not reach the necessary threshold for an effect on peripheral monocytes. It should be pointed out, however, that baseline physiological conditions might be different from stressful conditions, where the combination of high supraphysiological levels of both cortisol and EPI may suppress proinflammatory/Th1 but up-regulate anti-inflammatory/Th2 responses, thus inducing a systemic Th2 shift (12). Recent evidence indicates that besides the adrenomedullary chromaffin cells and postganglionic sympathetic nerve terminals, lymphocytes and phagocytes may represent an additional source of CAs (41, 42). It appears that, locally, in an autocrine/paracrine manner these CAs may be involved in immunoregulation, particularly during inflammation. However, it is not known whether, and to what extent, immune cell-derived CAs may contribute to baseline or stress-induced systemic levels of CAs and, alternatively, peripheral cytokine profiles in humans.
The lack of correlation between sex steroid levels and IL-12, TNF-α, and IL-10 production is in accord with their inability to modulate monocytic cytokine production ex vivo (Table I). This issue may also reflect differences in the cell/tissue (or systemic vs local(-specific effects of different hormones (12, 32, 43). For example, sex steroids may up-regulate the cytokine production at the level of Th2 cells (22-24), whereas estradiol may up-regulate IL-12 production in bone marrow-derived dendritic cells but not in CD11c+ splenocytes (44). Our study, however, suggests that 1,25-(OH)2 vitamin D3 is another hormonal factor that may affect interindividual variations of proinflammatory cytokine production. The negative correlation between 1,25-(OH)2 vitamin D3 and IL-12 levels is consistent with the inhibitory effect of this hormone observed in vitro (25) and ex vivo (Table I). Whether baseline hormonal variations, including CAs and steroids, may affect directly adaptive Th1 and Th2 cell-related cytokine profiles was beyond the scope of the present study and needs further investigation.
Several lines of evidence suggest a causal link between variations of baseline EPI and innate cytokine responsiveness. A dose-response effect is typically considered a major criterion for causality (45). Both EPI and fenoterol had potent and dose-dependent effects (Fig. 3D and Table II). Importantly, there were parallels between the presence/absence of correlation and r values and between the presence/absence of an ex vivo effect and the effective concentration (EC50) of all hormones tested (Tables I and II). The data in humans suggesting EPI-cytokine profile interactions showed consistency in replication in alternative settings (data in inbred rats) and they are consistent with the existing knowledge: monocytes, in contrast to T cells, are extremely sensitive to CAs, expressing high-density β2 ARs, with higher affinity for EPI than for NE; CAs inhibit proinflammatory but potentiate anti-inflammatory cytokine production via the β2 cAMP-protein kinase A pathway; and β2 agonists administered to humans inhibit IL-12 production (16, 20, 21, 32).
The identification of two subgroups in healthy individuals with relatively low and high EPI output that had opposite innate cytokine profiles provides a clear link with the LEW/F344 paradigm (Fig. 6) and poses several questions: Are these two opposite neurohormonal immune phenotypes parallels of LEW and F344 in humans? Are individuals with 5- to 7-fold differences in IL-12 production and IL-12:IL-10 ratio high and low Th1 responders similar to LEW and F344 inbred rats? Apart from EPI and, to a lesser extent, from 1,25-(OH)2 vitamin D3, what might be the role of other hormones, neurotransmitters, and neuropeptides present in the microenvironment of cytokine-secreting cells in vivo? Since LEW and F344 rats have opposite susceptibility to experimental immunological diseases, are the analogous phenotypes in humans linked to differing responsiveness or susceptibility to infections and immune-related diseases? These questions should be answered in future studies.
In fact, recent evidence suggests that low vs high pro- and anti-inflammatory cytokine profiles may play a role in the susceptibility of the individual to various immune disorders. Thus, families with low TNF-α whole blood production have a 10-fold increased risk for fatal meningococcal disease, whereas high IL-10 production increases the risk to 20-fold (4). In addition, unaffected family members of patients with disseminated Mycobacterium avium infection, systemic lupus erythematosus patients, or type 1 diabetes have abnormal IL-12 and IL-10 production (5, 6, 9). Recent evidence also indicates “clustering” (18 distinct clusters) of non-MHC susceptibility candidate loci in autoimmune, infectious, and atopic diseases (46). Since any SNP of a cytokine gene cannot fully explain cytokine variability and a complex phenotype such as disease susceptibility (4, 9, 47), our study suggests that loci influencing regulatory pathways affecting multiple cytokine gene expression further upstream at the neurohormonal immune interface may be part of these clusters. Given that the neuroendocrine and the immune systems are highly interconnected, our study also suggests that better understanding of the neurohormonal immune phenotypes and their link to genotypes and regulatory pathways may provide insights into susceptibility to and mechanisms underlying common human immune-related diseases. This approach may also lead to identification of candidate genes/SNPs for these diseases and to the detection of biomarkers of disease risk in unaffected individuals.
In conclusion, we have shown that EPI-mediated conditioning of the innate cytokine secretion might represent a previously unrecognized hormonal, nonimmunological mechanism that is involved in shaping cytokine responsiveness, variability, and profiles. This neuroendocrine mechanism may play a critical role in driving opposite innate cytokine profiles in humans with intrinsic hypo- and hyperactive adrenal medullas. Thus, in the human population, certain individuals may express opposite LEW- and F344-like neurohormonal immune phenotypes. Our findings have implications for the hormonal immune phenotypic interactions and for the understanding of the mechanisms controlling the differing susceptibility to infections and immune/inflammatory-related conditions in subgroups of humans.
Acknowledgments
We thank Drs. Jeannine Majde, Salvatore Alesci, and Rita Businaro for critical reading of this manuscript, and Kevin Barnes for assistance with the preparation of figures.
Footnotes
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: SNP, single nucleotide polymorphism; AR, adrenoreceptor; CA, catecholamine; GC, glucocorticoid; EPI, epinephrine (adrenaline); NE, norepinephrine.
References
- 1.Molvig J, Baek L, Christensen P, Manogue KR, Vlassara H, Platz P, Nielsen LS, Svejgaard A, and Nerup J. 1988. Endotoxin-stimulated human monocyte secretion of interleukin 1, tumour necrosis factor α, and prostaglandin E2 shows stable interindividual differences. Scand. J. Immunol 27: 705–716. [DOI] [PubMed] [Google Scholar]
- 2.de Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, and Dehart I. 1992. Direct stimulation of cytokines (IL-1β, TNF-α, IL-6, IL-2, IFN-γ, and GM-CSF) in whole blood: I. Comparison with isolated PBMC stimulation. Cytokine 4: 239–248. [DOI] [PubMed] [Google Scholar]
- 3.Entzian P, Linnemann K, Schlaak M, and Zabel P. 1996. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am. J. Respir. Crit Care Med 153: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 4.Westendorp RG, Langermans JA, Huizinga TW, Elouali AH,Verweij CL, Boomsma DI, Vandenbroucke JP, and Vandenbrouke JP. 1997. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 349: 170–173. [DOI] [PubMed] [Google Scholar]
- 5.Frucht DM, and Holland SM. 1996. Defective monocyte costimulation for IFN-γ production in familial disseminated Mycobacterium avium complex infection: abnormal IL-12 regulation. J. Immunol 157: 411–416. [PubMed] [Google Scholar]
- 6.Szelachowska M, Kretowski A, and Kinalska I. 1997. Increased in vitro interleukin-12 production by peripheral blood in high-risk IDDM first degree relatives. Horm. Metab. Res 29: 168–171. [DOI] [PubMed] [Google Scholar]
- 7.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenger F, Bonora E, Willeit J, and Schwartz DA. 2002. Toll-like receptor 4 polymorphisms and atherosclerosis. N. Engl. J. Med 347: 185–192. [DOI] [PubMed] [Google Scholar]
- 8.Gibson AW, Edberg JC, Wu J, Westendorp RG, Huizinga TW, and Kimberly RP. 2001. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J. Immunol 166: 3915–3922. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus R, Vercelli D, Palmer LJ, Klimecki WJ, Silverman EK, Richter B, Riva A, Ramoni M, Martinez FD, Weiss ST, and Kwiatkowski DJ. 2002. Single nucleotide polymorphisms in innate immunity genes: abundant variation and potential role in complex human disease. Immunol. Rev 190: 9–25. [DOI] [PubMed] [Google Scholar]
- 10.Shanks N, and Lightman SL. 2001. The maternal-neonatal neuro-immune interface: are there long-term implications for inflammatory or stress-related disease? J. Clin. Invest 108: 1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Hertzen LC 2002. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J. Allergy Clin. Immunol 109: 923–928. [DOI] [PubMed] [Google Scholar]
- 12.Elenkov IJ, and Chrousos GP. 1999. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metab 10: 359–368. [DOI] [PubMed] [Google Scholar]
- 13.Elenkov IJ, Wilder RL, Bakalov VK, Link AA, Dimitrov MA, Fisher S, Crane M, Kanik KS, and Chrousos GP. 2001. IL-12, TNF-α, and hormonal changes during late pregnancy and early postpartum: implications for autoimmune disease activity during these times. J. Clin. Endocrinol. Metab 86: 4933–4938. [DOI] [PubMed] [Google Scholar]
- 14.Cole SW, Naliboff BD, Kemeny ME, Griswold MP, Fahey JL, and Zack JA. 2001. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proc. Natl. Acad. Sci. USA 98: 12695–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rook GA, Lightman SL, and Heijnen CJ. 2002. Can nerve damage disrupt neuroendocrine immune homeostasis? Leprosy as a case in point. Trends Immunol. 23: 18–22. [DOI] [PubMed] [Google Scholar]
- 16.Riese U, Brenner S, Docke WD, Prosch S, Reinke P, Oppert M, Volk HD, and Platzer C. 2000. Catecholamines induce IL-10 release in patients suffering from acute myocardial infarction by transactivating its promoter in monocytic but not in T-cells. Mol. Cell. Biochem 212: 45–50. [PubMed] [Google Scholar]
- 17.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K,Victorov IV, Priller J, Dirnagl U, et al. 2003. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation: reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med 198: 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woiciechowsky C, Asadullah K, Nestler D, Eberhardt B, Platzer C, Schoning B, Glockner F, Lanksch WR, Volk HD, and Docke WD. 1998. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat. Med 4: 808–813. [DOI] [PubMed] [Google Scholar]
- 19.Wilder RL 1995. Neuroendocrine-immune system interactions and autoimmunity. Annu. Rev. Immunol 13: 307–338. [DOI] [PubMed] [Google Scholar]
- 20.Elenkov IJ, Papanicolaou DA, Wilder RL, and Chrousos GP. 1996. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc. Assoc. Am. Physicians 108: 374–381. [PubMed] [Google Scholar]
- 21.Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, and Sinigaglia F. 1997. β2-Agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Invest 100: 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilmore W, Weiner LP, and Correale J. 1997. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol 158: 446–451. [PubMed] [Google Scholar]
- 23.Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, and Livi C. 1995. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol 155: 128–133. [PubMed] [Google Scholar]
- 24.Liva SM, and Voskuhl RR. 2001. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol 167: 2060–2067. [DOI] [PubMed] [Google Scholar]
- 25.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, and Panina-Bordignon P. 1998. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3: involvement of NF-κB downregulation in transcriptional repression of the p40 gene. J. Clin. Invest 101: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kempf M, Cesbron-Delauw M, Grob D, Herrmann T, and Sutton P. 1999. Different manifestations of Toxoplasma gondii infection in F344 and LEW rats. Med. Microbiol. Immunol 187: 137–142. [DOI] [PubMed] [Google Scholar]
- 27.Caspi RR, Silver PB, Chan CC, Sun B, Agarwal RK, Wells J, Oddo S, Fujino Y, Najafian F, and Wilder RL. 1996. Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J. Immunol 157: 2668–2675. [PubMed] [Google Scholar]
- 28.Sakamoto S, Fukushima A, Ozaki A, Ueno H, Kamakura M, and Taniguchi T. 2001. Mechanism for maintenance of dominant T helper 1 immune responses in Lewis rats. Microbiol. Immunol 45: 373–381. [DOI] [PubMed] [Google Scholar]
- 29.Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, and Wilder RL. 1989. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis- susceptible Lewis rats. Proc. Natl. Acad. Sci. USA 86: 2374–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, and Wilder RL. 1998. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J. Immunol 161: 2586–2593. [PubMed] [Google Scholar]
- 31.Curtin F, Walker JP, and Schulz P. 1996. Day-to-day intraindividual reliability and interindividual differences in monoamines excretion. J. Affect. Disord 38: 173–178. [DOI] [PubMed] [Google Scholar]
- 32.Elenkov IJ, Wilder RL, Chrousos GP, and Vizi ES. 2000. The sympathetic nerve-an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev 52: 595–638. [PubMed] [Google Scholar]
- 33.Guimaraes S, and Moura D. 2001. Vascular adrenoceptors: an update. Pharmacol. Rev 53: 319–356. [PubMed] [Google Scholar]
- 34.Pende A, Musso NR, Vergassola C, Ioverno A, Galbariggi G, and Lotti G. 1991. Intraindividual variation of β 2-adrenergic receptors on mononuclear leukocytes and their relationships with endogenous plasma catecholamines in man. Horm. Metab. Res 23: 438–441. [DOI] [PubMed] [Google Scholar]
- 35.Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, and Wallin BG. 2006. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J. Physiol 572: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiegmann K, Muthyala S, Kim DH, Arnason BG, and Chelmicka-Schorr E. 1995. β-adrenergic agonists suppress chronic/relapsing experimental allergic encephalomyelitis (CREAE) in Lewis rats. J. Neuroimmunol 56: 201–206. [DOI] [PubMed] [Google Scholar]
- 37.Cobelens PM, Kavelaars A, Vroon A, Ringeling M, van der Zee R, van Eden W, and Heijnen CJ. 2002. The β2-adrenergic agonist salbutamol potentiates oral induction of tolerance, suppressing adjuvant arthritis and antigen-specific immunity. J. Immunol 169: 5028–5035. [DOI] [PubMed] [Google Scholar]
- 38.Malfait AM, Malik AS, Marinova-Mutafchieva L, Butler DM, Maini RN, and Feldmann M. 1999. The β2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J. Immunol 162: 6278–6283. [PubMed] [Google Scholar]
- 39.Sun B, Sun SH, Chan CC, and Caspi RR. 2000. Evaluation of in vivo cytokine expression in EAU-susceptible and resistant rats: a role for IL-10 in resistance? Exp. Eye Res 70: 493–502. [DOI] [PubMed] [Google Scholar]
- 40.Sternberg EM, Young WS, Bernardini R, Calogero AE, Chrousos GP, Gold PW, and Wilder RL. 1989. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc. Natl. Acad. Sci. USA 86: 4771–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, et al. 2007. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 449: 721–725. [DOI] [PubMed] [Google Scholar]
- 42.Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, and Lecchini S. 2007. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 109: 632–642. [DOI] [PubMed] [Google Scholar]
- 43.Elenkov IJ, Iezzoni DJ, Daly A, Harris AJ, and Chrousos GP. 2005. Cytokine dysregulation, inflammation, and well-being. Neuroimmunomodulation 12: 255–269. [DOI] [PubMed] [Google Scholar]
- 44.Siracusa MC, Overstreet MG, Housseau F, Scott AL, and Klein SL. 2008. 17β-estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J. Immunol 180: 1423–1431. [DOI] [PubMed] [Google Scholar]
- 45.Holberg CJ, and Halonen M. 2003. Cytokines, atopy, and asthma. Lancet 362: 1166–1167. [DOI] [PubMed] [Google Scholar]
- 46.Becker KG, Simon RM, Bailey-Wilson JE, Freidlin B, Biddison WE, McFarland HF, and Trent JM. 1998. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc. Natl. Acad. Sci. USA 95: 9979–9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormack RM, Maxwell AP, Carson DJ, Patterson CC, Middleton D, and Savage DA. 2002. The IL12B 3′ untranslated region DNA polymorphism is not associated with early-onset type 1 diabetes. Genes Immun. 3: 433–435. [DOI] [PubMed] [Google Scholar]