Abstract
In spite of its central role in biology and disease, protein turnover is a largely understudied aspect of most proteomic studies due to the complexity of computational workflows that analyze in vivo turnover rates. To address this need, we developed a new computational tool, TurnoveR, to accurately calculate protein turnover rates from mass spectrometric analysis of metabolic labeling experiments in Skyline, a free and open-source proteomics software platform. TurnoveR is a straightforward graphical interface that enables seamless integration of protein turnover analysis into a traditional proteomics workflow in Skyline, allowing users to take advantage of the advanced and flexible data visualization and curation features built into the software. The computational pipeline of TurnoveR performs critical steps to determine protein turnover rates, including isotopologue demultiplexing, precursor-pool correction, statistical analysis, and generation of data reports and visualizations. This workflow is compatible with many mass spectrometric platforms and recapitulates turnover rates and differential changes in turnover rates between treatment groups calculated in previous studies. We expect that the addition of TurnoveR to the widely used Skyline proteomics software will facilitate wider utilization of protein turnover analysis in highly relevant biological models, including aging, neurodegeneration, and skeletal muscle atrophy.
Keywords: metabolic labeling, stable isotope labeling, Skyline, proteostasis, protein turnover, aging, quantitative proteomics, mass spectrometry, protein synthesis, protein degradation
Graphical Abstract

INTRODUCTION
Dysfunctional proteostasis and altered protein turnover are features of aging and a wide array of diseases,1-5 and characteristic alterations in protein turnover, such as extended protein half-lives, are strongly linked to health and long life.6-12 While disruptions of the protein turnover machinery, such as autophagy and the ubiquitin-proteasome system, are often associated with aging and associated pathologies, turnover rates of proteins do not necessarily reflect changes in these processes. Therefore, methods to measure the turnover rates of individual proteins directly, rather than surrogate measurements of translation and degradation machinery, are critically needed to accurately examine the stability of the proteome during aging and disease.7,13 Large-scale proteomic studies assessing protein turnover have provided insights into the complexity and heterogeneity of protein turnover changes.14 While the proteomic measurement of protein turnover is relevant in many biological settings, a protein turnover study typically requires stable isotope labeling in vivo, and remains computationally complex and challenging for most scientists. Versatile computational tools on widely accessible, open-source platforms are needed to make this approach more user-friendly.
Modern mass spectrometry (MS) and computational pipelines have enabled the measurement of protein turnover rates with superior granularity, multiplexing, and throughput over other methods. Importantly, MS-based proteomic workflows to measure protein turnover rates report thousands of individual proteins changes, rather than bulk changes in the proteome, enabling a detailed molecular view of temporal proteomic turnover changes. The experimental design of a typical protein turnover workflow consists of a metabolic labeling phase and a data acquisition and analysis phase.3,15 During the first phase, a cell or an entire organism is metabolically labeled with a synthetic, stable isotope-enriched diet or drinking water, and its cells or tissues are subsequently collected at multiple time points. However, the bottleneck for most laboratories in conducting a protein turnover experiment occurs during the data analysis phase. In that phase, replicate tissue samples from each time point are typically analyzed by MS and subsequently processed through one of various specialized data analysis processing or software pipelines to determine protein turnover rates.16-22 While there are many effective tools available for calculating protein turnover from MS data, many scientists find it difficult to navigate existing tools and to fit them into their data-processing pipeline, due to vendor-specific file formats, the requirement for custom formatting of data prior to input, or prerequisite familiarity with coding. Additionally, most tools do not incorporate a module for statistical analysis of differences between treatment groups.
To overcome these bottlenecks, we created TurnoveR, an R-based external bioinformatic tool for analyzing protein turnover experiments integrated in the quantitative proteomics Skyline software platform. Skyline is a widely used, freely available, and open-source software for MS data analysis and quantification, data curation, method development, and visualization.23,24 The modular external tool system integrated into Skyline allows specialized MS analysis workflows to be conducted within the highly customizable, dynamic, and curatable Skyline workspace while making use of its features, such as advanced peak picking and scoring and chromatographic retention time alignment. Using the TurnoveR external tool, the full data-analysis pipeline of a turnover experiment can be performed within a Skyline document for amino acid metabolic-labeling experiments. Thus, these workflows become more accessible to the larger community of researchers who are already familiar with the Skyline proteomics platform. The straightforward graphical user interface of the TurnoveR tool simplifies turnover data analysis by requiring few user inputs and calling settings already specified within the endogenous Skyline document.
The computational workflow of TurnoveR incorporates many complex and critical computational steps associated with accurate calculation of protein turnover rates, including deconvolution of overlapping isotope envelopes between unlabeled and labeled precursor ion peaks,25,26 calculation of relative isotope abundance (RIA) of the amino acid precursor pool,13,27 turnover regressions, statistical comparisons between treatment groups, and visualization of data. Importantly, due to its integration within Skyline, the analysis can be curated and customized through the dynamic and user-friendly Skyline graphical interface.
We analyzed the in vivo protein turnover rates in two independent mouse experiments with data collected on different instrument platforms from different vendors. We found that TurnoveR accurately reproduced results from a published protein turnover study12 as a performance benchmark for this novel workflow and bioinformatic tool. The advantages of this pipeline include the automated generation of an extensive array of reports and visualizations that include detailed numerical reports, statistical analysis of differential changes between treatment groups, protein-by-protein regressions plots, and summary visualizations of global changes in the enrichment of isotope in the amino acid precursor pool as well as newly synthesized protein enrichments. The user-friendly TurnoveR tool is available for download within the External Tool Store in Skyline, and a step-by-step tutorial is provided (Supplemental Note S1).
EXPERIMENTAL SECTION
Mouse Studies
Two independent mouse experiments were analyzed in this study: (i) data from a calorie-restriction liver study12 and (ii) selected data from mouse skeletal muscle. The details of the calorie-restriction study were published.10,12 Briefly, 25-month-old C57BL/6 female mice were placed on a synthetic diet (Teklad diet TD.99366; Harlan Laboratories, Madison, WI) that was nutritionally similar to the NIH-31 standard diet for rodents for 3 weeks. After acclimation to the synthetic diet for 14 days, mice were switched to a 40% calorie restriction or an ad libitum diet for 10 weeks, after which dietary leucine was substituted with [5,5,5-2H3]-labeled leucine for metabolic labeling. Mice on each diet were metabolically labeled for 3, 7, 12, and 17 days prior to collecting liver tissue for MS sample processing. For the second study, mice were metabolically labeled with heavy leucine over 30 days. Quadricep muscles were collected after 3, 7, 15, and 30 days of metabolic labeling.
Proteomic Sample Preparation
Chemicals.
Acetonitrile (#AH015) and water (#AH365) were from Burdick and Jackson (Muskegon, MI). Iodoacetamide (IAA, #I1149), dithiothreitol (DTT, #D9779), formic acid (FA, #94318-50 ML-F), and triethylammonium bicarbonate buffer 1.0 M, pH 8.5 (#T7408) were from Sigma-Aldrich (St. Louis, MO), urea (#29700) was from Thermo Scientific (Waltham, MA), sequencing grade trypsin (#V5113) was from Promega (San Luis Obispo, CA), and HLB Oasis SPE cartridges (#186003908) were from Waters (Milford, MA).
Homogenization.
Sample preparation procedures and MS acquisition details for the calorie restriction liver study12 were performed on isolated mitochondria from fresh collected liver tissues as described.28 Briefly, liver tissues were Dounce-homogenized in mitochondrial isolation buffer (250 mm sucrose, 1 mM EGTA, 10 mM HEPES, 10 mM Tris-HCl pH 7.4) and centrifuged at 800g for 10 min. The supernatants were centrifuged at 4000g for 30 min at 4 °C to generate a crude mitochondrial pellet. Pellets were resuspended in a 19% Percoll solution, layered in a stepped Percoll gradient of 30% and 60%, and centrifuged at 10 000g for 20 min. Purified mitochondria were recovered from the interface between two layers. Protein quantitation was then performed using a BCA Protein Assay Kit (#23225, Pierce, Waltham, MA). The sample number breakdown per treatment group and time point (number of days fed heavy leucine) is as follows: 13 total calorie-restricted mice from four different metabolic labeling time points (3 days (n = 2), 7 days (n = 4), 12 days (n = 3), 17 days (n = 4)) and 13 total ad libitum fed mice (3 days (n = 3), 7 days (n = 4), 12 days (n = 3), 17 days (n = 3)).
Digestion.
Mitochondrial fractions were solubilized in 0.1% Rapigest (Waters Corporation, Milford, MA) and boiled for 5 min. Disulfide bonds were reduced (5 mM dithiothreitol and 15 mM iodoacetamide) and trypsin digested (1:100 μg trypsin/μg protein). Subsequently, samples were quenched with 200 mM HCl and centrifuged at 20 000g for 10 min. Supernatants were saved for subsequent desalting. In the second study, muscle samples were processed and digested using a Suspension-traps (S-Traps) procedure (#C02-micro-80, Protifi, Huntington, NY), according to the manufacturer’s protocol.
Desalting.
Peptide samples were desalted with Oasis HLB 30-mg Sorbent Cartridges (Waters, Milford, MA; #186003908), concentrated, and resuspended in a solution containing 0.2% formic acid in water.
Mass Spectrometry Acquisition
Liver peptides were analyzed by LC-MS/MS on a Waters nanoAcquity UPLC coupled to a Thermo Scientific LTQ Orbitrap Velos. For each sample, 1 μg was loaded onto a 4 cm long, 100-μm inner diameter trap column and then loaded onto a 35 cm long, 75-μm inner diameter analytical column. Gradient elution was performed with buffer A (0.1% formic acid in 99.9% water) and organic buffer B (0.1% formic acid in 99.9% acetonitrile). Peptides were eluted over a linear gradient of 2–32% of organic buffer B at a flow rate of 250 nL/min. Peptides were analyzed on an LTQ Orbitrap Velos in data-dependent acquisition (DDA) mode (see below).
Muscle samples were analyzed by reverse-phase HPLC-ESI-MS/MS on an orthogonal quadrupole time-of-flight SCIEX TripleTOF 6600 MS (SCIEX, Redwood City, CA). Typically, mass resolution in precursor scans was approximately 45 000, and the fragment ion resolution was approximately 15 000. After injection, peptide mixtures were transferred onto a C18 precolumn chip (200 μm × 6 mm ChromXP C18-CL chip, 3 μm, 300 Å; SCIEX, Redwood City, CA) and washed at 2 μL/min for 10 min with the loading solvent (H2O/0.1% formic acid) for desalting. Peptides were transferred to the 75 μm × 15 cm ChromXP C18-CL chip, 3 μm, 300 Å (SCIEX, Redwood City, CA) and eluted at 300 nL/min with a 2-h gradient using aqueous and acetonitrile solvent buffers for DDA analysis. Solvents were prepared as follows: mobile phase A, 2% acetonitrile/98% of 0.1% formic acid (v/v) in water; mobile phase B, 98% acetonitrile/2% of 0.1% formic acid (v/v) in water.
All samples were analyzed by DDA downstream for protein turnover. On the Orbitrap platform, MS1 survey scans were followed by five dependent MS2 scans, with a dynamic exclusion duration of 30 s. For the muscle study, DDA was used for MS/MS collection on the TripleTOF 6600 to obtain MS/MS spectra for the 30 most-abundant parent ions after each survey MS1 scan. Dynamic exclusion features were based on value M not m/z and were set to an exclusion mass width of 50 mDa and an exclusion duration of 15–20 s.
Database Searching
Orbitrap RAW files (Thermo) from the published study of liver turnover12 from old mice on ad libitum diet (Ctl), and old mice on calorie restriction (CR) were database searched. Biological replicates from each diet at four different labeling time points were analyzed: 3 days (Ctl n = 3, CR n = 2), 7 days (Ctl n = 4, CR n = 4), 12 days (Ctl n = 3, CR n = 3), and 17 days (Ctl n = 3, CR n = 4). Raw data files were each searched in the trans-proteomic pipeline with the Comet (2018.01 rev. 4) algorithm with enzyme specified as trypsin, allowing for two internal cleavage sites. Cysteine carbamidomethylation (+57.021464) was set as a fixed modification. Methionine oxidation (+15.994900) and 2H3-leucine (+3.018832) were set as variable modification and maximum variable modifications allowed per peptide were 5. Search results were combined PeptideProphet (TPP v5.2.0 Flammagenitus, Build 201903130949-7900).
MS DDA raw files (wiff files, SCIEX) from muscle tissues were database searched using ProteinPilot Software 5.0 (revision 4769, Paragon Algorithm 5.0.0.0.4767, AB SCIEX). All data files were searched using the UniProt Mus musculus Proteome (downloaded December 2016). The following sample parameters were used in Protein Pilot: sample type set to SILAC (Leu +3), trypsin digestion, cysteine alkylation set to iodoacetamide, urea denaturation, and acetylation emphasis. Processing parameters were set to “Biological modification”, and a thorough ID search effort was used. A global FDR of 1% was chosen using the Protein Pilot FDR analysis tool.
Data Availability
Raw MS data files, database search results, quantitative reports, spectral libraries, protein databases, and other supplementary files are available on MassIVE (MassIVE MSV000088893, ftp://massive.ucsd.edu/MSV000088893/; or https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=67595c1e1d424cd6b0230a85c675530b) and ProteomeX-change (PXD031832).
TurnoveR source code is available on GitHub: https://github.com/ProteoWizard/pwiz/tree/master/pwiz_tools/Skyline/Executables/Tools/Turnover.
Skyline workspaces are downloadable from Panorama. Orbitrap data is available at https://panoramaweb.org/Schilling/protein%20turnover_tool_2020/targetedms-showPrecursorList.view?fileName=2022_0102_OCR_ORP_Turnover_permuted_decoys_mprophet_allfiles_v3_2022-01-02_15-33-52.sky.zip. TripleTOF data is available at https://panoramaweb.org/Schilling/protein%20turnover_tool_2020/targetedms-showPrecursorList.view?fileName=CA9_dagmqlqgyr_v1_2022-01-02_17-12-02.sky.zip.
A step-by-step tutorial for performing an analysis with TurnoveR and Skyline is provided (Supplemental Note S1).
RESULTS AND DISCUSSION
Quantitative Analysis of Protein Turnover Rates with TurnoveR and Skyline
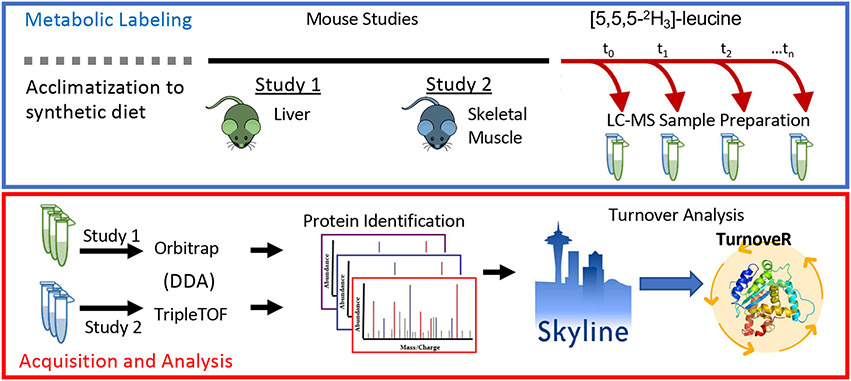
To estimate protein turnover rates in the mouse liver and skeletal muscle, we metabolically labeled mice with deuterated leucine supplemented in the diets of mice in two independent experiments (Figure 1). For the Orbitrap workflow, old mice (25 months old) either calorie-restricted (CR) or fed ad libitum for 10 weeks and switched to a diet containing deuterated leucine for 3, 7, 12, or 17 days before liver tissues were harvested. In the second experiment, mice were fed a diet containing deuterated leucine for 3, 7, 15, or 30 days prior to the collection of quadricep muscles. Samples from both experiments were homogenized, trypsin-digested, and desalted for MS analysis. Liver samples were analyzed by DDA analysis on an Orbitrap Velos (Thermo) MS, and muscle samples were analyzed by DDA on a TripleTOF 6600 (SCIEX). The resulting raw files and search results (see Experimental Section) were loaded into Skyline for analysis with the TurnoveR external tool. Quantitative MS1 peak areas were extracted from DDA raw files in the Skyline-daily v21.1.9.353 (5be4398d4) software platform. ProteinPilot or Comet search results were imported into Skyline to build a spectral library, followed by import of raw MS files for extraction of chromatographic peak areas. Files were processed retention time alignment calculated based on iRT peptides (Biognosys, Switzerland) and peak scoring using the mProphet model. We used a new feature in Skyline, the “permute isotope modification” function to populate Skyline with isotopologues of peptides containing leucine, which are required because isotopologue peak area ratios are required to calculate precursor enrichment for stable isotopes and the protein-turnover rates in downstream analysis. The TurnoveR tool was then launched within Skyline and used to perform the full analysis of protein turnover with information already contained within the Skyline document: all peptide and protein characteristics, annotations, and quantitative information, including isotopologue peak.
Figure 1.
TurnoveR external tool enables comprehensive measurement of protein turnover rates in vivo from metabolic-labeling experiments in Skyline. A typical workflow for a metabolic-labeling experiment for the calculation of protein-turnover rates. Treated or transgenic mice and their respective control mice are metabolically labeled with deuterated leucine. Protein lysates are analyzed by untargeted data-dependent acquisitions (DDA) MS. MS data are database searched, processed, and imported into Skyline. After setting up a Skyline document, the TurnoveR external tool is used to perform protein-turnover analysis.
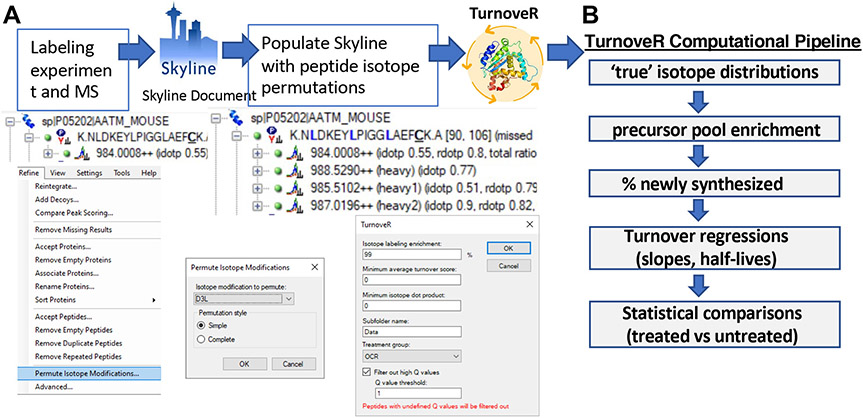
The workflow for calculating turnover rates with Skyline and TurnoveR is summarized in Figure 2. In the setup of a Skyline document, users can curate the data going into the subsequent turnover analysis. An optional but recommended procedure is to perform chromatographic alignment and peak scoring with the retention time calculator within Skyline and advanced peak scoring features, respectively, to improve confidence in peak identifications. In addition, advanced peak picking and scoring with mProphet29 will generate Q-values that are valuable for the filtering of high-quality peptide peaks, which result in turnover regressions with less variability. Prior to analysis with the TurnoveR tool, deuterated leucine ([5,5,5-2H3]-leucine) was specified as a heavy label, and treatment groups are specified within the document grid of Skyline. A critical prerequisite to calculating the relative isotopic enrichment in the amino acid precursor pool is the relative quantification of signal from all isotopologues of peptides containing two or more heavy leucines (as described further below). To enable this calculation, we recently implemented a new Skyline feature, “Permute Isotope Modifications”, available under the “Refine” menu in Skyline (Figure 2A). This feature, a necessary requirement for accurate calculation of protein turnover rates with TurnoveR, populates the Skyline document with extracted ion chromatograms from all isotopologues of heavy-label-containing peptides (Figure S1). For example, for peptides containing two leucines, Skyline will by default populate the document with the light (0 heavy leucines) and heavy (two heavy leucines) versions of the peptide. After permuting isotope modifications, the Skyline document will be populated with extracted ion chromatograms for the 0 heavy leucine, one heavy leucine, and two heavy leucine-containing permutations of the peptide (Figure S1). Notably, this feature is independent of the TurnoveR tool and, therefore, can be used more broadly to accommodate any workflow in Skyline that would benefit from isotope permutation. After the setup of a Skyline document and permutation of modifications, the TurnoveR external tool is loaded, and several parameters are specified for filtering of peptides, subfolder name, and specification of a control treatment group. Otherwise, all data required for the full analysis of protein turnover are directly retrieved from Skyline document, including treatment groups, time points, peptide and protein annotations, mProphet Q-values, isotope dot products, and isotopologue peak areas. The TurnoveR pipeline (Figure 2B) then runs a series of computations to calculate accurate protein half-lives and generate the associated visualizations and statistical calculations.
Figure 2.
Workflow for analysis of metabolic-labeling experiments in Skyline using the TurnoveR external tool. (A) First, a Skyline document is created and loaded with MS data from a metabolic labeling experiment. A new Skyline feature “Permute Isotope Modifications” is then used to populate leucine-containing peptides in Skyline with all isotopic permutations of heavy and light leucine (one heavy leucine, two heavy leucine, three heavy leucine, etc.). TurnoveR is then loaded and run with a few user-specified settings. (B) TurnoveR performs a series of computational analyses, calculates protein-turnover rates, and produces detailed reports of protein half-lives, statistical analysis, and figures.
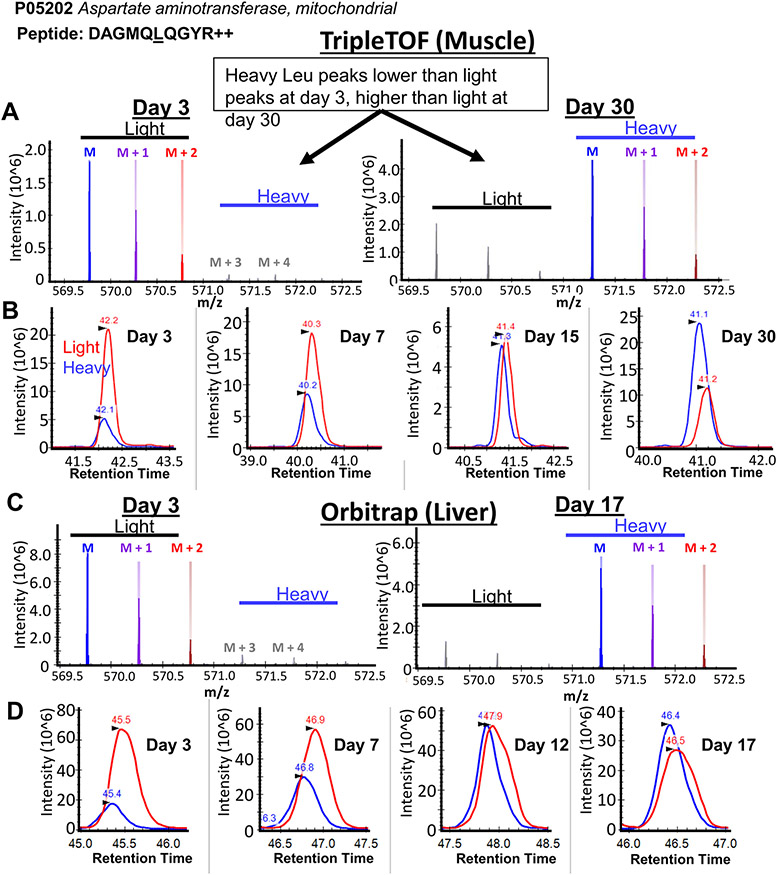
As with any Skyline workflow, we recommend that users manually spot-check the data to ensure correct peak picking, and to check for increasing proportion of heavy peptides over time points using the visualizations available in the Skyline interface (Figure S2). To demonstrate the incorporation of deuterated leucine into peptides in Skyline, we loaded data from two tissues and different mass analyzer platforms into Skyline and looked for changes in isotopologue envelopes and chromatographic peak areas of leucine-containing peptides over multiple labeling times (Figure 3). In data from mouse skeletal muscle proteins acquired on a triple quadrupole time-of-flight instrument, we observed a clear shift in total MS1 signal toward heavy isotope peaks after 30 days of metabolic labeling, compared with 3 days (Figure 3A). The ratio of heavy-to-light chromatographic peak areas accordingly increased over all time points (Figure 3B). The same trends were seen in representative peptide isotopic envelopes (Figure 3C) and chromatographic peak areas (Figure 3B) from mouse livers measured on an Orbitrap mass analyzer.
Figure 3.
Heavy leu incorporation is observable in Skyline software. (A) The isotope envelope of the representative peptide DAGMQLQGYR from aspartate aminotransferase, in mouse skeletal muscle detected on a triple quadrupole time-of-flight instrument after 3 (left) or 30 days (right) on a synthetic diet. Incorporation of heavy leucine is clearly visible in Skyline by the increase in abundance of the “heavy” leucine isotopic peaks. (B) Extracted ion chromatograms of the peptide DAGMQLQGYR over four time points shows an increase in the peak area of deuterated heavy leucine (blue, 571.2785 m/z), relative to light leucine (red, 569.7691 m/z), over time. The equivalent (C) isotopic envelope and (D) extracted ion chromatograms for DAGMQLQGYR in liver lysates from an Orbitrap are shown.
Deconvolution of Signal Intensity from Overlapping Isotopologue Envelopes
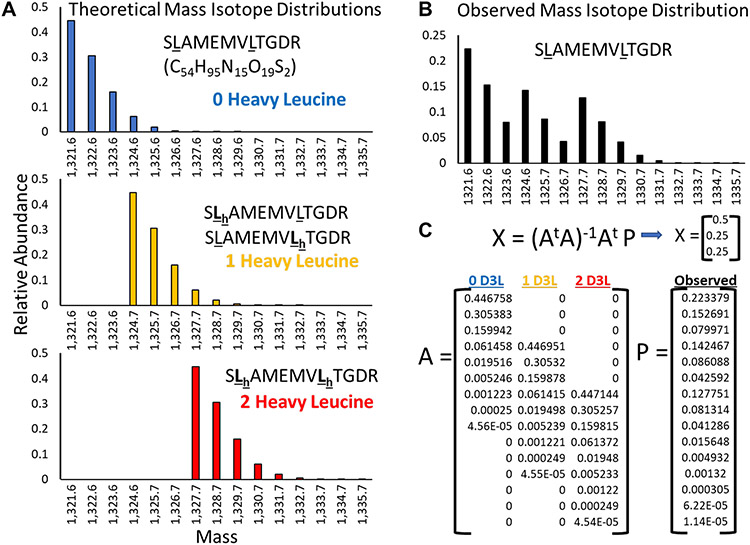
Precursor-pool corrected protein turnover rates were calculated in TurnoveR using an approach similar to that in previous studies with the Topograph software platform.3,9,19 An important step in the accurate calculation of protein turnover is to determine the true relative signal intensity contributed by light and heavy peptide isotopologue peaks. In an early step of the TurnoveR computational pipeline (Figure 2B), peptide mass isotope distributions are analyzed to deconvolute the relative abundances of light and heavy peptide isotopologues after correcting for the contribution of natural isotope abundances (Figure 4), which occur at a natural frequency in biological systems.30 For every isotopologue of a leucine-containing peptide, a theoretical isotope distribution was generated by calculating the list of m/z bin intensities for all possible isotope peaks expected to contribute at least 1% of the total isotope distribution signal. The observed isotopologue envelopes for each leucine containing peptide were determined, based on the relative peak areas of each isotope peak integrated by Skyline. To perform the natural isotope abundance correction, TurnoveR first calculates the theoretical mass isotope distribution for all isotopologues of leucine-containing peptides, as illustrated for the example peptide isotopologues of SLAMEMVLTGDR (C14H95N15O19S2) at a mass of 1321.6 Da, which may contain zero (1321.6 Da), one (1324.7 Da), or two heavy leucines (1327.7 Da) (Figure 4A). The observed mass isotope distribution for that peptide (Figure 4B) contains some combination of signal from each of the individual peptide isotopologues. To calculate the relative contribution of each isotopologue, the fractional amount of each theoretical isotopologue distribution that best fits the observed signal is computed using a published skewed least-squares analysis,25,26 applying a matrix calculation (Figure 4C). Larger peptides, or peptides containing a greater number of Leucines, will produce larger and more complex isotope envelopes. These computations run in the background of the Skyline document after initiating the TurnoveR tool.
Figure 4.
Correction for naturally occurring heavy isotope signals in TurnoveR. Based on the molecular formula and the natural proportion of heavy isotopes of carbon, hydrogen, nitrogen, oxygen, and sulfur, the theoretical isotope envelopes of every peptide are calculated for every leucine-containing peptide isotopologue (every permutation of light/heavy leucine). (A) For example, the theoretical isotope envelopes of SLAMEMVLTGDR containing zero, one, or two heavy leucines are shown. The isotope envelopes have overlapping mass. (B) In a theoretically observed distribution of SLAMEMVLTGDR, 50% of total MS1 intensity was contributed by the unlabeled peptide isotopologue, 25% from the singly labeled peptide isotopologue, and 25% from the fully labeled peptide isotopologue. (C) Matrix math is used to solve for the relative contribution of signal from each peptide isotopologue. Matrix A is defined by the fractional intensity of each isotopologue (columns) at different molecular masses (rows). Matrix P is the observed intensity at each molecular mass.
TurnoveR Calculates the Fraction of Newly Synthesized Protein Corrected to Precursor Pool Enrichment
The TurnoveR tool also calculates relative isotope enrichment or the enrichment of deuterated leucine in the intracellular amino acid precursor pool. Correcting to precursor pool enrichment is critical for the accurate calculation of protein turnover rates in whole organisms.19 Stable isotope labeling of amino acids in cell culture (SILAC) studies is relatively simple: the precursor pool is saturated and turnover rates can be calculated based solely on the proportion of heavy-label-containing peptides. However, it is extremely challenging to completely saturate the precursor pool with heavy isotope in vivo in complex organisms, such as mice. Analysis of mouse livers with the TurnoveR tool revealed that enrichment of deuterated leucine in the intracellular amino acid precursor pools increased over consecutive time points and reached a maximum of 80% in old control-fed mice after 17 days of metabolic labeling. The incorporation of heavy label in the precursor pool of calorie-restricted mice is lower than in fully fed mice at 7, 12, and 17 days (Figure 5). Moreover, the global distribution of newly synthesized proteins increased over time and were significantly lower in calorie-restricted mice, as reported (Figure 6A, Figure S3).12 Boxplots and histograms summarizing precursor enrichments and global percentage of newly synthesized protein are automatically generated by the TurnoveR tool with every analysis.
Figure 5.
TurnoveR calculates and visualizes precursor pool enrichments. (A) Box plot of the cellular precursor pool enrichment of heavy leucine at each time point in control (CTL) and calorie-restricted (CR) mice. Precursor pools increase during labeling period and reduced in CR mice at 3, 12, and 17 days of metabolic labeling. (B) Histogram and density plot of precursor pool enrichment in each condition and time point. All plots are automatically generated by the TurnoveR tool.
Figure 6.
TurnoveR generates summary reports and regressions. (A) Summary boxplot depicting the percentage of newly synthesized proteins across the proteome in control (CTL) and calorie-restricted (CR) mice across each time point. The global fraction of newly synthesized proteins was significantly lower in CR mice at each time point. (B) The incorporation of newly synthesized protein in CTL (red) and CR (blue) mice in Echs1, Got2, Mdh2, and Hint2. TurnoveR generates PDF reports containing regressions of all proteins for which half-lives are calculated.
The calculated relative abundances of peptide isotopologues from peptides containing two or more leucine’s is used to compute the relative isotope abundance (RIA) of the amino acid precursor pool as described.13,27 For peptides containing more than one leucine, the theoretical isotopologue distribution generated by any RIA is unique. Therefore, the isotopologue patterns from these peptides were used to back-calculate the enrichment of heavy leucine in the amino acid precursor pools. We used the same strategy as Hsieh et al.19 to solve the precursor enrichment for all peptides containing two to five leucine residues and subsequently used the median precursor enrichment calculated across all peptides in a sample as the precursor enrichment for downstream estimation of newly synthesized protein. The precursor RIA was calculated separately for each genotype and time point. Median precursor pool enrichments were then applied to recalculate the fractional abundances of newly synthesized peptides vs pre-existing peptides.
The fraction of newly synthesized peptide, , is defined by the following equation:
The fraction of newly synthesized peptide is equal to the total signal (chromatographic area) contribution from new peptide divided by the total chromatographic peak area of new plus pre-existing peptide. The total area of newly synthesized peptide is equal to the total contribution of new peptide to the “light” leucine isotopologue chromatographic peak area (not containing heavy leucines), , plus the sum of all “heavy” leucine-containing isotopologue peaks, , which are presumed to be newly synthesized. The total newly synthesized area, , is divided by the sum total of all heavy and light isotopologue peak areas, , to calculate the fraction of newly synthesized protein. Observed isotopologue peak areas are defined as , where n is the number of heavy leucines corresponding to the peak. With algebraic rearrangement and substitution, the component of this equation, or the contribution of newly synthesized protein to the light leucine peak area, can be written as follows:
where corresponds to the theoretically expected peak area of a given isotopologue peak representing newly synthesized peptide, calculated based on the closest fitting simulated mass isotopologue distribution, and corresponds to the number of heavy leucines in the corresponding peak. For example, corresponds to the theoretical peak area containing no heavy leucines, corresponds to the theoretical peak containing a single heavy leucine, and so on.
TurnoveR Calculates of Protein Half-Lives and Statistical Analysis of Changes between Treatment Groups
For each protein, a turnover rate was calculated by fitting the “fraction newly synthesized” of all its unique peptides to the following exponential growth equation and solving for the rate constant.
where , the fractional amount of newly synthesized protein, is equal to one minus the fraction of pre-existing, unlabeled peptide , where is the time of initiation of metabolic labeling, zero. The rate constant, , represents the turnover rate of peptides. The turnover rate constant was solved by determining the nonlinear least-squares estimates of the exponential rate constant parameter, , in R.
This yielded protein turnover rates that were transformed into half-lives using a simple conversion:
The fraction of newly synthesized protein determined at each time point is corrected to the enrichment of label in the precursor pool. The TurnoveR tool modeled the data to first-order kinetics to generate regressions of the fraction of newly synthesized protein over time for every protein. The rate of incorporation of newly synthesized proteins over time reflects rates of protein turnover. Finally, to calculate statistical differences between treatment groups, the exponential regression model was natural log-transformed, creating linear relationship between the log-transformed fraction of newly synthesized proteins and time. We then used linear modeling statistics to determine whether the interaction of the log-transformed values and time were different between treatment groups. The model was written as follows in R:
where log.Perc.New.Synth is defined as the natural log of the fraction of newly synthesized protein and Cohort*Time is the interaction term for treatment group and time. A p-value less than 0.05 for the Cohort*Time interaction term is considered a statistically significant difference in protein turnover rate between treatment groups.
Comprehensive reports of protein turnover rates, half-lives, fold-changes, and statistical differences between treatment groups are automatically generated by the TurnoveR tool. Additionally, multiple graphical visualizations of data, including changes in the global precursor pool, fractional of newly synthesized proteins (Figure 6A, Figure S3), and individual regressions of newly synthesized proteins (Figure 6B) generated for all proteins in the originating Skyline document, are generated by the TurnoveR tool.
For statistical comparison of turnover rates between treatment groups, first-order equations were natural log transformed, making a linear relationship between the log of percent newly synthesized proteins and time. Then linear modeling statistics were applied to determine if the interaction between the log-transformed percent newly synthesized values and time are different between treatment groups. Interaction p-values were used to determine if turnover rates were statistically different between treatment groups. A full report of protein turnover rates, annotations, variance, statistical analysis, and other quantitative information for the calorie restriction study is provided in Table S2.
The TurnoveR Pipeline Recapitulates Computations from Existing Tools
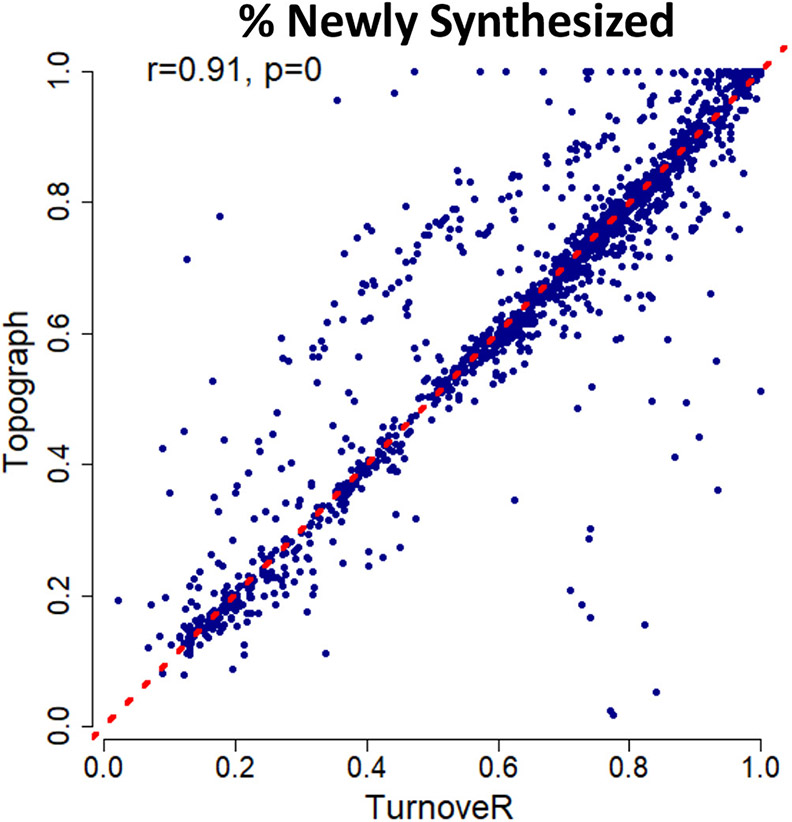
To benchmark the TurnoveR against an existing gold-standard tool for the analysis of protein turnover in whole organisms, we performed a side-by-side analysis of protein turnover with the existing Topograph tool19 on the mouse liver data set. Our analysis revealed a high degree of correlation between the fractional abundance of newly synthesized protein calculated by each tool (r = 0.91, Figure 7, Table S3). We ascribe small differences to the calculations by Topograph and TurnoveR to differences in the computational pipelines in Topograph and Skyline. Table S3 contains the metadata on each of the peptides included in the correlation, including the turnover scores produced by both software platforms, the number of heavy leucines, isotope dot products, and the percentage of label enrichment. These parameters, as well as abundance, are distributed similarly among outlying peptides as well as correlated peptides from the correlation of TurnoveR vs Topograph. Based on the lack of a clear association with any of these parameters, we believe the differences in the calculated values likely stem from differences in the peak-picking algorithms of the two software platforms. For example, the TurnoveR workflow utilized indexed retention time alignment and mProphet peak scoring algorithms available in Skyline but not in Topograph. Overall, these results suggest that TurnoveR can be used to perform large-scale analysis of protein turnover within the Skyline software platform.
Figure 7.
TurnoveR recapitulates results calculated by Topograph software. Scatter plot showing the calculated values of percent newly synthesized protein in TurnoveR versus Topograph. Peptides containing two or more leucines are plotted. Values are significantly correlated (Pearson r = 0.91, and p < 10−12). Topograph is an available open-source software tool for calculating precursor-pool corrected turnover rates.3 The red line depicts a slope of 1 (y = x).
CONCLUSIONS
While several excellent options are available for determining protein turnover based on metabolic labeling, no existing tools calculate protein turnover in the Skyline software environment. For example, the gold standard tool includes Topograph, a stand-alone software that calculates precursor pool corrected turnover rates from multiple types of isotopic labels.19 A modular analysis pipeline calculates protein turnover rates in N15 labeling studies.17 SILACtor determines protein turnover for studies with stable isotope labeling of amino acids in cell culture.18 The Python-based DeuteRater software package and the java-based ProTurn software calculate protein turnover after heavy water labeling. ProteinTurnover, an R package, analyzes protein turnover in isotopically labeled plants.16 D2ome, a freely available tool, determines in vivo protein turnover in heavy water experiments and is compatible with the Human Proteome Organization’s standard file formats.21 Notably, the KNIME working environment, a recently described approach utilizing a fully light carbon source, enables the calculation of protein turnover rates in organisms using simple light isotope metabolic labeling (SLIM) and a simplified calculation utilizing two mass isotopologue peaks.31 A similar “two-isotopologue” approach has been described for heavy water labeling.32 Recently, a data-independent acquisition workflow that uses open-source tools (EncyclopeDIA and Skyline) for analysis of SILAC-DIA data was introduced.33 One interesting caveat not addressed by any of the current tools available, including TurnoveR, is the possibility that certain proteins fall into two distinct protein pools. An example hypothetical scenario would be the existence of a highly soluble and pool of Tau that is easily turned over and a highly insoluble fraction of Tau which is completely sequestered from turnover machinery. In such a case, the fraction of newly synthesized protein would truly be asymptotic at a value less than 100% newly synthesized protein. However, the modeling applied by many tools, including TurnoveR, assumes a 100% maximum and does not clearly identify these cases. In these scenarios, it is more likely that the regression of newly synthesized protein over time will be noisy, and as a result, will return a lower-confidence regression. To identify such cases, users of the TurnoveR tool can refer to the “Step 3” output file, which is automatically generated after running the tool. The p-value produced in this file represents the confidence or “goodness of fit” of the regression and can be used to identify noisier proteins, and potentially those that are separated into multiple pools.
One of the challenges of the existing tools is that incorporating them into a workflow for the analysis of proteomic data. Input files and differences have to be custom fitted into workflows between vendor file formats. Some tools, such as the R-based packages, require knowledge of coding. Of all available tools, our novel algorithm TurnoveR is uniquely capable of performing protein turnover analysis seamlessly within the Skyline analysis workflow. Additionally, the TurnoveR tool is unique in its integration of a statistical analysis module that calculates significant changes in protein turnover between conditions and treatment groups. The TurnoveR tool is available for download within the External Tool Store in Skyline. Accompanying tutorials are available in Supplemental Note S1.
Our hope in creating an external tool within the Skyline ecosystem is to enable scientists greater control over the data going into the protein turnover analysis. Skyline is a widely used, user-friendly, platform-independent, and vendor-agnostic proteomic analysis platform that grants users great control in curating data prior to downstream quantitative analysis. The advanced features of the Skyline ecosystem enable users to easily produce a highly curated data set for subsequent analysis of protein-turnover rates. With the TurnoveR external tool, the global analysis of protein turnover rates is integrated into the Skyline document workspace and bridges the gap between the creation of a Skyline document and the very advanced set of computations required for protein turnover analysis. This seamless integration into the Skyline ecosystem will help make the analysis of protein turnover more accessible to proteomic researchers and facilitate future studies examining the role of in vivo protein turnover in the context of aging and diseases.
Supplementary Material
Table S3: TurnoveR and Topograph underlying data for the percentage of newly synthesized proteins for calorie restricted and control-fed mice (XLSX)
Table S2: Quantitative report of protein turnover rates, half-lives, and associated statistics generated by the TurnoveR tool (XLSX)
Table S1: Full custom Skyline report containing all relevant data to TurnoveR analysis in table format (ZIP)
Supplemental Note S1: Tutorial document with detailed step-by-step instructions for performing protein turnover analysis with TurnoveR and Skyline (PDF)
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH) and the Intramural Research Program. Specifically, we acknowledge support of a Nathan Shock Pilot Award (5P30 AG013280 PI: Kaeberlein/Rabinovitch, Pilot award to Schilling), R01 AR071762 and R01 AG060637 (NIH, PI: Adams), K99 AG065484 (NIH, PI: Basisty), S10 OD016281 (NIH, Buck Institute), U01 AG060906 (NIH, PI: Schilling), R24 GM141156 (NIH, PI: MacCoss), P30 AG013280 (NIH, PI: Rabinovitch), and P41 GM 103533 (NIH, PI: MacCoss).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.2c00173.
The authors declare the following competing financial interest(s): Drs. Scott Ebert and Christopher M. Adams are part of Emmyon, Inc.
Contributor Information
Nathan Basisty, Buck Institute for Research on Aging, Novato, California 94945, United States; Translational Gerontology Branch, National Institute on Aging, NIH, Baltimore, Maryland 21224, United States.
Nicholas Shulman, Department of Genome Sciences, University of Washington, Seattle, Washington 98195, United States.
Cameron Wehrfritz, Buck Institute for Research on Aging, Novato, California 94945, United States.
Alexandra N. Marsh, Department of Genome Sciences, University of Washington, Seattle, Washington 98195, United States
Samah Shah, Buck Institute for Research on Aging, Novato, California 94945, United States.
Jacob Rose, Buck Institute for Research on Aging, Novato, California 94945, United States.
Scott Ebert, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, Minnesota 55905, United States; Emmyon, Inc., Rochester, Minnesota 55902, United States.
Matthew Miller, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, Minnesota 55905, United States; Medical Scientist Training Program, University of Iowa, Iowa City, Iowa 52242, United States.
Dao-Fu Dai, Department of Pathology, University of Iowa, Iowa City, Iowa 52242, United States.
Peter S. Rabinovitch, Department of Pathology, University of Washington, Seattle, Washington 98195, United States
Christopher M. Adams, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, Minnesota 55905, United States; Emmyon, Inc., Rochester, Minnesota 55902, United States
Michael J. MacCoss, Department of Genome Sciences, University of Washington, Seattle, Washington 98195, United States
Brendan MacLean, Department of Genome Sciences, University of Washington, Seattle, Washington 98195, United States.
Birgit Schilling, Buck Institute for Research on Aging, Novato, California 94945, United States.
REFERENCES
- (1).Abbott CB; Lawrence MM; Kobak KA; Lopes EBP; Peelor FF III; Donald EJ; Van Remmen H; Griffin TM; Miller BF A Novel Stable Isotope Approach Demonstrates Surprising Degree of Age-Related Decline in Skeletal Muscle Collagen Proteostasis. Function 2021, 2 (4), zqab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Toyama BH; Savas JN; Park SK; Harris MS; Ingolia NT; Yates JR; Hetzer MW Identification of Long-Lived Proteins Reveals Exceptional Stability of Essential Cellular Structures. Cell 2013, 154 (5), 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Basisty N; Meyer JG; Schilling B Protein Turnover in Aging and Longevity. Proteomics 2018, 18 (5—6), No. e1700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Basisty N; Schilling B A Need for Proteomics: Untangling the Relationship between Protein Turnover, Aging, and Longevity. J. Data Mining Genomics Proteomics 2021, 12 (237), 2. [Google Scholar]
- (5).Basisty N; Holtz A; Schilling B Accumulation of “Old Proteins” and the Critical Need for MS-Based Protein Turnover Measurements in Aging and Longevity. Proteomics 2020, 20 (5—6), No. e1800403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Swovick K; Firsanov D; Welle KA; Hryhorenko JR; Wise JP; George C; Sformo TL; Seluanov A; Gorbunova V; Ghaemmaghami S Interspecies Differences in Proteome Turnover Kinetics Are Correlated With Life Spans and Energetic Demands. Molecular & Cellular Proteomics 2021, 20, 100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Miller BF; Drake JC; Naylor B; Price JC; Hamilton KL The Measurement of Protein Synthesis for Assessing Proteostasis in Studies of Slowed Aging. Ageing Res. Rev 2014, 18, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Thompson AC; Bruss MD; Price JC; Khambatta CF; Holmes WE; Colangelo M; Dalidd M; Roberts LS; Astle CM; Harrison DE; Hellerstein MK Reduced in Vivo Hepatic Proteome Replacement Rates but Not Cell Proliferation Rates Predict Maximum Lifespan Extension in Mice. Aging Cell 2016, 15 (1), 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Basisty N; Dai DF; Gagnidze A; Gitari L; Fredrickson J; Maina Y; Beyer RP; Emond MJ; Hsieh EJ; MacCoss MJ; Martin GM; Rabinovitch PS Mitochondrial-Targeted Catalase Is Good for the Old Mouse Proteome, but Not for the Young: “reverse” Antagonistic Pleiotropy? Aging Cell 2016, 15 (4), 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Basisty NB; Liu Y; Reynolds J; Karunadharma PP; Dai D-F; Fredrickson J; Beyer RP; MacCoss MJ; Rabinovitch PS Stable Isotope Labeling Reveals Novel Insights Into Ubiquitin-Mediated Protein Aggregation With Age, Calorie Restriction, and Rapamycin Treatment. J. Gerontol. A Biol. Sci. Med. Sci 2018, 73 (5), 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Dai DF; Karunadharma PP; Chiao YA; Basisty N; Crispin D; Hsieh EJ; Chen T; Gu H; Djukovic D; Raftery D; Beyer RP; MacCoss MJ; Rabinovitch PS Altered Proteome Turnover and Remodeling by Short-Term Caloric Restriction or Rapamycin Rejuvenate the Aging Heart. Aging Cell 2014, 13 (3), 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Karunadharma PP; Basisty N; Dai DF; Chiao YA; Quarles EK; Hsieh EJ; Crispin D; Bielas JH; Ericson NG; Beyer RP; MacKay VL; MacCoss MJ; Rabinovitch PS Subacute Calorie Restriction and Rapamycin Discordantly Alter Mouse Liver Proteome Homeostasis and Reverse Aging Effects. Aging Cell 2015, 14 (4), 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Doherty MK; Whitehead C; McCormack H; Gaskell SJ; Beynon RJ Proteome Dynamics in Complex Organisms: Using Stable Isotopes to Monitor Individual Protein Turnover Rates. Proteomics 2005, 5 (2), 522–533. [DOI] [PubMed] [Google Scholar]
- (14).Pratt JM; Petty J; Riba-Garcia I; Robertson DHL; Gaskell SJ; Oliver SG; Beynon RJ Dynamics of Protein Turnover, a Missing Dimension in Proteomics*. Molecular & Cellular Proteomics 2002, 1 (8), 579–591. [DOI] [PubMed] [Google Scholar]
- (15).Beynon RJ; Pratt JM Metabolic Labeling of Proteins for Proteomics*. Molecular & Cellular Proteomics 2005, 4 (7), 857–872. [DOI] [PubMed] [Google Scholar]
- (16).Fan KT; Rendahl AK; Chen WP; Freund DM; Gray WM; Cohen JD; Hegeman AD Proteome Scale-Protein Turnover Analysis Using High Resolution Mass Spectrometric Data from Stable-Isotope Labeled Plants. J. Proteome Res 2016, 15 (3), 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Guan S; Price JC; Prusiner SB; Ghaemmaghami S; Burlingame AL A Data Processing Pipeline for Mammalian Proteome Dynamics Studies Using Stable Isotope Metabolic Labeling. Mol. Cell Proteomics 2011, 10 (12), M111.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hoopmann MR; Chavez JD; Bruce JE SILACtor: Software to Enable Dynamic SILAC Studies. Anal. Chem 2011, 83 (22), 8403–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hsieh EJ; Shulman NJ; Dai DF; Vincow ES; Karunadharma PP; Pallanck L; Rabinovitch PS; MacCoss MJ Topograph, a Software Platform for Precursor Enrichment Corrected Global Protein Turnover Measurements. Mol. Cell Proteomics 2012, 11 (11), 1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Naylor BC; Porter MT; Wilson E; Herring A; Lofthouse S; Hannemann A; Piccolo SR; Rockwood AL; Price JC DeuteRater: A Tool for Quantifying Peptide Isotope Precision and Kinetic Proteomics. Bioinformatics 2017, 33 (10), 1514–1520. [DOI] [PubMed] [Google Scholar]
- (21).Sadygov RG; Avva J; Rahman M; Lee K; Ilchenko S; Kasumov T; Borzou A D2ome, Software for in Vivo Protein Turnover Analysis Using Heavy Water Labeling and LC-MS, Reveals Alterations of Hepatic Proteome Dynamics in a Mouse Model of NAFLD. J. Proteome Res 2018, 17 (11), 3740–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Alevra M; Mandad S; Ischebeck T; Urlaub H; Rizzoli SO; Fornasiero EF A Mass Spectrometry Workflow for Measuring Protein Turnover Rates in Vivo. Nat. Protoc 2019, 14 (12), 3333–3365. [DOI] [PubMed] [Google Scholar]
- (23).MacLean B; Tomazela DM; Shulman N; Chambers M; Finney GL; Frewen B; Kern R; Tabb DL; Liebler DC; MacCoss MJ Skyline: An Open Source Document Editor for Creating and Analyzing Targeted Proteomics Experiments. Bioinformatics 2010, 26 (7), 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Pino LK; Searle BC; Bollinger JG; Nunn B; MacLean B; MacCoss MJ The Skyline Ecosystem: Informatics for Quantitative Mass Spectrometry Proteomics. Mass Spectrom Rev. 2020, 39 (3), 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Brauman JI Least Squares Analysis and Simplification of Multi-Isotope Mass Spectra. Anal. Chem 1966, 38 (4), 607–610. [Google Scholar]
- (26).Jennings ME; Matthews DE Determination of Complex Isotopomer Patterns in Isotopically Labeled Compounds by Mass Spectrometry. Anal. Chem 2005, 77 (19), 6435–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hellerstein MK; Neese RA Mass Isotopomer Distribution Analysis: A Technique for Measuring Biosynthesis and Turnover of Polymers. Am. J. Physiol 1992, 263 (5), E988–E1001. [DOI] [PubMed] [Google Scholar]
- (28).Zhang J; Li X; Mueller M; Wang Y; Zong C; Deng N; Vondriska TM; Liem DA; Yang JI; Korge P; Honda H; Weiss JN; Apweiler R; Ping P Systematic Characterization of the Murine Mitochondrial Proteome Using Functionally Validated Cardiac Mitochondria. Proteomics 2008, 8 (8), 1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Reiter L; Rinner O; Picotti P; Hüttenhain R; Beck M; Brusniak M-Y; Hengartner MO; Aebersold R MProphet: Automated Data Processing and Statistical Validation for Large-Scale SRM Experiments. Nat. Methods 2011, 8 (5), 430–435. [DOI] [PubMed] [Google Scholar]
- (30).Beavis RC Chemical Mass of Carbon in Proteins. Anal. Chem 1993, 65 (4), 496–497. [Google Scholar]
- (31).Sénécaut N; Alves G; Weisser H; Lignières L; Terrier S; Yang-Crosson L; Poulain P; Lelandais G; Yu Y-K; Camadro J-M Novel Insights into Quantitative Proteomics from an Innovative Bottom-Up Simple Light Isotope Metabolic (BSLIM) Labeling Data Processing Strategy. J. Proteome Res 2021, 20 (3), 1476–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Sadygov RG Partial Isotope Profiles Are Sufficient for Protein Turnover Analysis Using Closed-Form Equations of Mass Isotopomer Dynamics. Anal. Chem 2020, 92 (21), 14747–14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Pino LK; Baeza J; Lauman R; Schilling B; Garcia BA Improved SILAC Quantification with Data-Independent Acquisition to Investigate Bortezomib-Induced Protein Degradation. J. Proteome Res 2021, 20 (4), 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S3: TurnoveR and Topograph underlying data for the percentage of newly synthesized proteins for calorie restricted and control-fed mice (XLSX)
Table S2: Quantitative report of protein turnover rates, half-lives, and associated statistics generated by the TurnoveR tool (XLSX)
Table S1: Full custom Skyline report containing all relevant data to TurnoveR analysis in table format (ZIP)
Supplemental Note S1: Tutorial document with detailed step-by-step instructions for performing protein turnover analysis with TurnoveR and Skyline (PDF)
Data Availability Statement
Raw MS data files, database search results, quantitative reports, spectral libraries, protein databases, and other supplementary files are available on MassIVE (MassIVE MSV000088893, ftp://massive.ucsd.edu/MSV000088893/; or https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=67595c1e1d424cd6b0230a85c675530b) and ProteomeX-change (PXD031832).
TurnoveR source code is available on GitHub: https://github.com/ProteoWizard/pwiz/tree/master/pwiz_tools/Skyline/Executables/Tools/Turnover.
Skyline workspaces are downloadable from Panorama. Orbitrap data is available at https://panoramaweb.org/Schilling/protein%20turnover_tool_2020/targetedms-showPrecursorList.view?fileName=2022_0102_OCR_ORP_Turnover_permuted_decoys_mprophet_allfiles_v3_2022-01-02_15-33-52.sky.zip. TripleTOF data is available at https://panoramaweb.org/Schilling/protein%20turnover_tool_2020/targetedms-showPrecursorList.view?fileName=CA9_dagmqlqgyr_v1_2022-01-02_17-12-02.sky.zip.
A step-by-step tutorial for performing an analysis with TurnoveR and Skyline is provided (Supplemental Note S1).