Abstract
Purpose
Helicobacter pylori is associated with the development of gastrointestinal diseases. However, its eradication is challenged by an increased rate of drug resistance. AlgC and GalU are important for the synthesis of UDP-glucose, which is a substrate for the synthesis of lipopolysaccharide (LPS) in H. pylori. In this study, we investigated the role of UDP-glucose in the intrinsic drug resistance in H. pylori.
Methods
Gene knockout strains or complementation strains, including ΔalgC, ΔgalU, ΔgalE, Δhp0045, ΔalgC/algC* and ΔgalU/galU* were constructed in Hp26695; and ΔalgC and ΔgalU were also constructed in two clinical drug-resistant strains, Hp008 and Hp135. The minimum inhibitory concentrations (MIC) of H. pylori to amoxicillin (AMO), tetracycline (TET), clarithromycin (CLA), metronidazole (MNZ), levofloxacin (LEV), and rifampicin (RIF) were measured using MIC Test Strips. Silver staining was performed to examine the role of AlgC and GalU in LPS synthesis. Ethidium bromide (EB) accumulation assay was performed to assess the outer membrane permeability of H. pylori strains.
Results
Knockout of algC and galU in H. pylori resulted in increased drug sensitivity to AMO, MNZ, CLA, LEV, and RIF; whereas knockout of hp0045 and galE, which are involved in GDP-fucose and UDP-galactose synthesis, respectively, did not significantly alter the drug sensitivity of H. pylori. Knockout of algC and galU in clinically drug-resistant strains resulted in significantly increased drug sensitivity to all the antibiotics, except MNZ. The lipid A-core structure was altered in ΔalgC and ΔgalU when their EB accumulation was higher than that in the wild type and complementation strains.
Conclusion
UDP-glucose may play an important role in increasing drug resistance to AMO, MNZ, CLA, LEV, TET, and RIF by maintaining the lipid A-core structure and decreasing membrane permeability. AlgC and GalU may serve as potential drug targets for decreasing antibiotic resistance in clinical isolates.
Keywords: H. pylori, UDP-glucose, algC, galU, intrinsic multidrug resistance
Introduction
Helicobacter pylori is a gram-negative, spiral-shaped, microaerophilic bacterium that infects more than half of the world’s population.1 H. pylori infection is characterized by the development of gastrointestinal diseases, including chronic atrophic gastritis, peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoma.2 Furthermore, a H. pylori infection lasts a lifetime, unless eradicated with antibiotics.3 H. pylori infection can be eradication by administering triple or quadruple therapy using at least two different antibiotics, clarithromycin (CLA) and amoxicillin (AMO).4 However, the eradication rate has decreased to < 80% due to bacterial drug resistance, particularly to CLA.5
Lipopolysaccharide (LPS), one of the main components of the bacterial outer membrane, is typically composed of lipid A, core oligosaccharides, and O antigens. The distribution of LPS on the outer leaf of the outer membrane provides a potent barrier to antibiotics.6 UDP-glucose is one of the major building blocks of polysaccharide.7 Notably, the UDP-glucose synthetic pathway is highly conserved in gram-negative bacteria. The de novo biosynthesis of UDP-glucose involves three steps: (a) generation of α-D-glucose-6-phosphate by a glucokinase, (b) conversion of glucose-6-phosphate into glucose-1-phosphate by phosphoglucomutase (PGM), and (c) transformation of α-D-glucose-1-phosphate and UTP into UDP-glucose by UDP-Glc pyrophosphorylase (UGP), also known as GalU. PGM plays an important role in the synthesis of LPS and in the antibiotic resistance of various pathogens, such as Salmonella Typhimurium, Yersinia pestis, and Stenotrophomonas maltophilia.8–10 UGP is associated with LPS synthesis, drug resistance, and bacterial virulence in pathogens, such as Haemophilus parasuis, Proteus mirabilis, and Vibrio cholerae.11–13 Therefore, UDP-glucose may play an important role in the intrinsic drug resistance of gram-negative bacteria. However, the role of UDP-glucose in the drug resistance of H. pylori has not been elucidated yet.
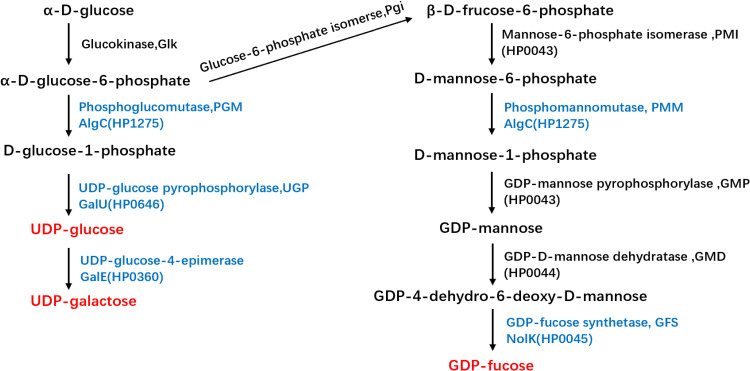
H. pylori harbors two ortholog genes, hp1275(algC) and hp0646(galU) that encode PGM and UGP, respectively. In H. pylori, AlgC was suggested to have phosphomannomutase (PMM) activity, in addition to PGM activity (Figure 1). Furthermore, algC inactivation can lead to the disruption of UDP-galactose and GDP-fucose synthesis, both of which are active glycosyl donors for LPS. Moreover, the disruption of GDP-fucose synthesis resulted in LPS truncation, thereby increasing the susceptibility of H. pylori to SDS and novobiocin.14 In this study, we constructed ΔalgC and ΔgalU mutants of H. pylori and investigated their drug sensitivity, LPS structure, and outer membrane permeability. We found that AlgC and GalU were critical for the intrinsic resistance of H. pylori. Knockout of either algC or galU resulted in the alteration of the lipid A-core structure and higher outer membrane permeability. Furthermore, knockout of either algC or galU in two clinically drug-resistant strains significantly increased the antibiotic sensitivity of H. pylori, especially to CLA, rifampicin (RIF), and levofloxacin (LEV).
Figure 1.
Biosynthetic pathways for UDP-glucose, UDP-galactose, and GDP-fucose synthesis in H. pylori. The three sugar nucleotides are marked in red and the enzymes inactivated in this study are marked in blue.
Materials and Methods
Bacterial Strains and Growth Conditions
The H. pylori strains Hp26695, Hp008, Hp135, and their isogenic mutant strains were cultured on Columbia blood agar (OXOID, Basingstoke, UK) plates supplemented with 5% sheep blood or in Brucella broth (BD, Sparks, MD, USA) supplemented with 10% fetal bovine serum (PAN Seratech, Aidenbach, Germany) (BB+FBS) with gentle agitation (120 rpm) at 37 °C under microaerophilic conditions (5% O2, 10% CO2, and 85% N2). Kanamycin (5 µg/mL; MP Biomedicals LLC, Solon, OH, USA) or chloramphenicol (4 µg/mL; Inalco S.p.A., Milano, Italy) was added to H. pylori isogenic or complemented mutant strains when constructing and screening positive mutants.
Construction of algC, galU, hp0045, or galE Knockout Mutants in Hp26695, Hp008 and Hp135, and Complementation of algC or galU in Δalgc and Δgalu
To knockout algC from the chromosomes of Hp26695, Hp008, and Hp135, the upstream sequence of algC was amplified using the primers algC-up-FI and algC-up-RI, or algC-up-FII and algC-up-RII; downstream sequence of algC was amplified using algC-down-FI and algC-down-RI, or algC-down-FII and algC-down-RII; and a nonpolar kanamycin resistance cassette was amplified using aphA-F and aphA-R (primer sequences are listed in Supplementary Table 1). The plasmid pbluescript SK II (-) was ligated with the upstream and downstream sequence of algC flanked via a nonpolar kanamycin resistance cassette using ClonExpress® Ultra One Step Cloning Kit (C115, Vazyme Biotech, Nanjing, China). The resulting plasmid, pBluescript-algCKO, was transformed into E. coli DH5α, and plasmid construction was confirmed via DNA sequencing. ΔalgC strains were obtained by transforming pBluescript-algCKO into Hp26695, Hp008, and Hp135 through electroporation, as described previously.15 The construction of ΔgalU, Δhp0045, and ΔgalE was performed using a similar method, except for the primers used (Supplementary Table 1).
To complement algC in ΔalgC, a pBlcom-hp0203-hp0204-cat vector constructed previously was used as template and amplified using the primers algC-com-inverse-F and algC-com-inverse-R.16 The coding sequence of algC was amplified using the algC-COM-F and algC-COM-R. Subsequently, these fragments were ligated, generating pBlue-Com-AlgC, which was further transformed into E. coli DH5α.The complementation strain ΔalgC/algC* was obtained by transforming pBLue-Com-AlgC into ΔalgC, as described previously.16 ΔgalU/galU* was obtained using a similar method except for the primers listed in Supplementary Table 1.
Antibiotic Susceptibility Test
The minimum inhibitory concentrations (MIC) of H. pylori against amoxicillin (AMO), tetracycline (TET), clarithromycin (CLA), metronidazole (MNZ), levofloxacin (LEV), and rifampicin (RIF) for all strains were determined using the diffusion MIC test strips (Liofilchem, Roseto degli Abruzzi, Italy). MIC values were measured as previously described,17 with slight modifications. Briefly, H. pylori strains were cultured on Columbia agar plates for 3 d, and colonies were collected and resuspended in Brucella broth to a turbidity value of 2 McFarland units measured using a DensiCHEKTM plus Instrument (BioMrieux Inc, Loveland, CO, USA). Subsequently, the samples were spread evenly on Mueller–Hinton blood agar (OXOID, Basingstoke, UK) using sterile cotton swabs, and MIC Test Strips were placed on the surface of the agar. MIC was determined after 3 d of incubation under 37 °C according to the concentration of the strips at the intersection of the growth inhibition zone. Each experiment was repeated at least thrice.
Growth Curves and Growth Inhibition Curves
To monitor the growth curve, H. pylori strains were cultured in Brucella broth supplemented with 10% fetal bovine serum, with an initial OD600 of 0.05 in the presence or absence of antibiotics at the indicated concentrations, and cell density was measured every 12 h. The results are the average means of at least three biological replicates.
LPS Extraction and Silver Staining
LPS extraction was performed as described previously.18 Briefly, bacterial cells harvested from Columbia agar plates were harvested and resuspended in 1 mL of Brucella broth with OD600 of approximately 3.0; and subsequently washed by PBS (137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 2mM KH2PO4; pH 7.4), and resuspended in of 100 μL LPS lysis buffer (2% SDS, 4% β-mercaptoethanol, 0.1% bromophenol blue, 10% glycerol, and 1 M Tris-HCl; pH 6.8). Each sample was then heated at 100 °C for 10 min. The cooled samples were incubated overnight at 55 °C with 5 μL of proteinase K (20 mg/mL). The samples were then subjected to 15.5% Tricine-SDS-PAGE. The LPS profile was visualized using a Fast Silver Stain Kit (Beyotime, Shanghai, China), according to the manufacturer’s instructions.
Ethidium Bromide Accumulation Assay
H. pylori cultured on Columbia blood agar was harvested, and the bacterial cells were washed twice and resuspended in PBS (137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 2mM KH2PO4; pH 7.4) at a density of OD600~0.4. Subsequently, 10 μM of carbonyl cyanidem-chlorophenylhydrazine (CCCP; C2759, Sigma-Aldrich, St-Louis, Missouri, USA) was added, and the samples were incubated for 30 min at 37 °C to deplete metabolic energy. Subsequently, 2 μM of EB was added and EB fluorescence intensity was measured for 30 min at an interval of 60s using an EnSightTM multimode plate reader (Perkin Elmer, Waltham, MA, USA), with an excitation wavelength of 545 nm and an emission wavelength of 590 nm.
Statistical Analysis
All experiments were performed at least three times, and the results were analyzed using GraphPad Prism version 7.00 for Windows (GraphPad Software, San Diego, CA, United States). Student’s t-test was used to evaluate the significance of the differences. A P-value < 0.05 was considered statistically significant.
Results
Knockout of Either algC or galU Results in Higher Antibiotic Susceptibility in H. pylori
To determine whether UDP-glucose synthesis is involved in the antibiotic susceptibility in H. pylori, we constructed Hp26695ΔalgC and Hp26695ΔgalU and the complementation strains Hp26695ΔalgC/algC* and Hp26695ΔgalU/galU*. The MIC values of each strain for AMO, MNZ, CLA, LEV, TET, and RIF were determined using the MIC Test Strip. The MIC values of both ΔalgC and ΔgalU for AMO, MNZ, CLA, LEV, TET, and RIF were 2-, 2.7-, 16.5-, 6-, 4-, and 4-fold, respectively, lower than those in the wild-type Hp26695 (Table 1). Furthermore, the complementation strains Hp26695ΔalgC/algC* and Hp26695ΔgalU/galU* showed MIC values similar to those of the wild-type strain. These results suggest that AlgC and GalU contribute to the intrinsic drug resistance of H. pylori.
Table 1.
MICs (µg/mL) of Hp26695 Strains to Antibiotics
| Strain Antibiotic |
Hp26695 | ||||||
|---|---|---|---|---|---|---|---|
| WT | ΔalgC | ΔalgC/algC* | ΔgalU | ΔgalU/galU* | ΔgalE | Δhp0045 | |
| Amoxicillin | 0.125 | 0.064 | 0.125 | 0.064 | 0.125 | 0.094 | 0.094 |
| Metronidazole | 4 | 1.5 | 4 | 1.5 | 4 | 3 | 4 |
| Clarithromycin | 0.38 | 0.023 | 0.38 | 0.023 | 0.38 | 0.125 | 0.094 |
| Levofloxacin | 0.25 | 0.047 | 0.25 | 0.047 | 0.25 | 0.19 | 0.19 |
| Tetracycline | 0.25 | 0.064 | 0.25 | 0.064 | 0.25 | 0.25 | 0.25 |
| Rifampicin | 0.38 | 0.094 | 0.38 | 0.094 | 0.38 | 0.19 | 0.25 |
Abbreviations: WT, wild type strain; ΔalgC, algC knockout strain; ΔalgC/algC*, algC complementation strain; ΔgalU, galU knockout strain; ΔgalU/galU*, galU complementation strain; ΔgalE, galE knockout strain; Δhp0045, hp0045 knockout strain.
Furthermore, AlgC participates in the synthesis of GDP-fucose, which is a substrate for the O antigen.19,20 While GalE is a UDP-glucose-4-epimerase that transfers UDP-glucose to UDP-galactose, another substrate incorporated into the O-side chain of LPS (Figure 1).21 Therefore, we constructed Hp26695Δhp0045 and Hp26695ΔgalE to disrupt the synthesis of GDP-fucose and UDP-galactose, respectively. We then investigated the role of GalE and HP0045 in the drug sensitivity of H. pylori (Table 1). The results showed that the MIC values of ΔgalE and Δhp0045 for AMO, MNZ, CLA, LEV, TET, and RIF were significantly higher than those for ΔalgC and ΔgalU, suggesting that UDP-glucose synthesis plays a more vital role in the intrinsic resistance of H. pylori to antibiotics, compared to UDP-galactose or GDP-fucose.
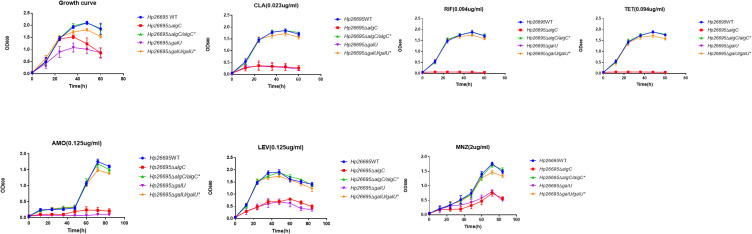
We also compared the growth curves of H. pylori wild type, ΔalgC, and ΔgalU in the presence or absence of antibiotics to confirm the role of UDP-glucose synthesis in the antibiotic resistance of H. pylori (Figure 2). In the presence of antibiotics, the growth of ΔgalU and ΔalgC was completely inhibited by CLA, RIF, TET, and AMO, whereas it was significantly reduced by LEV and MNZ, compared to that of the wild-type and complementation strains. These results suggest that UDP-glucose is critical for the intrinsic bacterial resistance of H. pylori to various antibiotics.
Figure 2.
Influence of AlgC and GalU in drug resistance of H. pylori. Growth curves of Hp26695, ΔalgC, ΔalgC/algC*, ΔgalU and ΔgalU/galU* with CLA (0.023 µg/mL), RIF (0.094 µg/mL), TET (0.094 µg/mL), AMO (0.125 µg/mL), LEV (0.125 µg/mL) and MNZ (2 µg/mL). The data represents the mean ± SEM of three independent experiments.
Disruption of AlgC or GalU Increases Drug Sensitivity of Clinical Drug-Resistant Isolates
We also investigated whether the disruption of UDP-glucose synthesis restores the drug sensitivity of clinical drug-resistant isolates. Two clinical drug-resistant H. pylori isolates, Hp135 and Hp008, were used in this study. Hp008 is resistant to MNZ, CLA, LEV, and RIF; and Hp135 is resistant to CLA and MNZ. Resistance of Hp135 and Hp008 to antibiotics was due to drug resistance-associated point mutations, which were confirmed via DNA sequencing (data not shown). We constructed a knockout mutation of algC and galU in Hp135 and Hp008, and investigated the drug sensitivity of these strains using MIC test strips (Table 2). Except for MNZ, knockout of either algC or galU reduced the MIC of Hp008 to CLA from >256 to 8 μg/mL, and that of Hp135 to CLA from >256 to 0.016 μg/mL. The MICs of ΔalgC and ΔgalU against AMO, LEV, TET, and RIF were 2-, 8-, 2.9-, and 32-fold, respectively, lower than those of the wild-type strain in Hp008, whereas the MICs of ΔalgC and ΔgalU against AMO, LEV, TET, and RIF were 2-, 4-, 4.1-, and 8.1-fold, respectively, lower than those of the wild-type strain in Hp135. These results suggest that the inactivation of UDP-glucose synthesis resulted in a significant increase in the drug sensitivity of the clinical isolates to CLA, TET, RIF, LEV, and AMO.
Table 2.
MICs (µg/mL) of Two Clinical Drug-Resistant H. pylori Isolates
| Strain Antibiotic |
Hp008 | Hp135 | ||||
|---|---|---|---|---|---|---|
| WT | ΔalgC | ΔgalU | WT | ΔalgC | ΔgalU | |
| Amoxicillin | 0.047 | 0.023 | 0.023 | 0.032 | <0.016 | <0.016 |
| Metronidazole | >256 | >256 | >256 | >256 | >256 | >256 |
| Clarithromycin | >256 | 8 | 8 | >256 | 0.016 | 0.016 |
| Levofloxacin | >32 | 4 | 4 | 0.5 | 0.125 | 0.125 |
| Tetracycline | 0.047 | 0.016 | 0.016 | 0.094 | 0.023 | 0.023 |
| Rifampicin | 4 | 0.125 | 0.125 | 0.38 | 0.047 | 0.047 |
Abbreviations: WT, wild type strain; ΔalgC, algC knockout strain; ΔgalU, galU knockout strain.
Knockout of Either algC or galU Altered LPS Structure
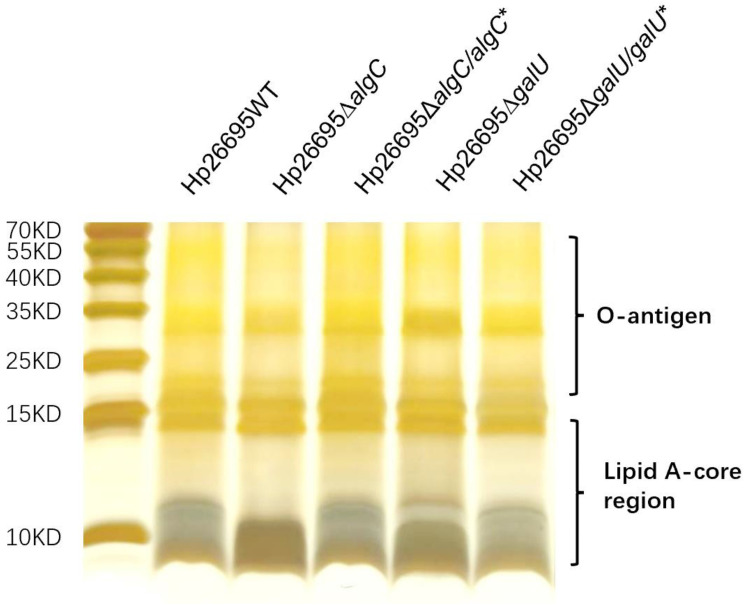
The structure of LPS is important for maintaining drug sensitivity. Therefore, we verified whether loss of algC and galU affected LPS structure. LPS from wild-type, ΔalgC, ΔalgC/algC*ΔgalU, and ΔgalU/galU* strains was isolated and visualized via silver staining (Figure 3). The results showed that the lipid A-core in ΔalgC and ΔgalU were significantly different from that in the wild-type strains. Moreover, complementation of either algC or galU in the cognate mutant strain restored the lipid A-core profile, thereby confirming that AlgC and GalU participated in the synthesis or structure of the lipid A-core.
Figure 3.
LPS profile of H. pylori. Silver-stained Tricine-SDS-PAGE gel (15.5%) depicting the LPS profiles from wild type, algC knockout, algC complementation, galU knockout, and galU complementation in the strain Hp26695. This experiment has been repeated independently for at least three times with similar results.
Knockout of algC and galU Increases Bacterial Outer Membrane Permeability
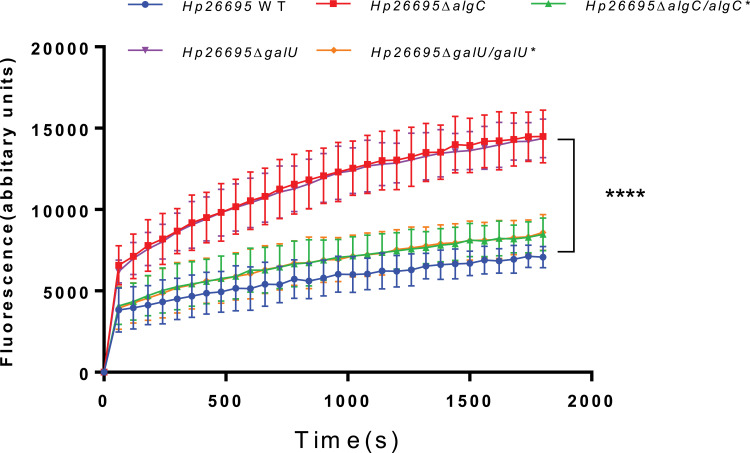
LPS establishes a membrane barrier for the entry of several antibiotics.22,23 Therefore, we further investigated whether AlgC and GalU influenced outer membrane permeability by comparing the ethidium bromide (EB) accumulation among the strains wild type, ΔalgC, ΔgalU, ΔalgC/algC*and ΔgalU/galU* (Figure 4). CCCP, an efflux inhibitor, was added to eliminate the effects of efflux pumps harbored by H. pylori. In the presence of EB, the fluorescence of the wild type increased at a significantly slower rate than that of ΔalgC or ΔgalU, suggesting ΔalgC or ΔgalU habors a higher outer membrane permeability comparing to wild-type strain. Complementation of algC or galU reduced the fluorescence of ΔalgC or ΔgalU to a level similar to that of the wild type, suggesting that AlgC and GalU are important for outer membrane permeability of H. pylori.
Figure 4.
Comparison of EB accumulation in various types of H. pylori strains. The fluorescence intensity was recorded at excitation and emission wavelengths of 545 and 590 nm, respectively, over a 30-min incubation period. The data represents the mean ± SEM of three independent experiments. A paired Student’s t test was performed for comparing the EB accumulation between the WT and gene knockout strains (ΔalgC and ΔgalU). ****P<0.0001.
Discussion
The rise in antibiotic resistance in H. pylori has led to a declining eradication rate, thereby necessitating new antimicrobial strategies.4,24 LPS is a major component of the outer leaflet of the outer membrane, and is considered to be of great importance in the intrinsic resistance of gram-negative bacteria.25 An outer membrane-penetrating agent to disrupt the outer membrane can be used to increase antimicrobial activity and broaden antimicrobial spectrum, even in clinical drug-resistant strains.26 Targeting the enzymes involved in LPS synthesis is also a prospective strategy to increase bacterial sensitivity to antibiotics.
UDP-glucose is involved in cell envelope biosynthesis, and its role in LPS synthesis has been confirmed in gram-negative bacteria, such as Pseudomonas aeruginosa, Neisseria gonorrhoeae, Bordetella bronchiseptica, Stenotrophomonas maltophilia, and Proteus mirabilis etc.10,12,27–29 In this study, we found that AlgC and GalU are important for maintaining LPS structure and intrinsic drug sensitivity. This result is in agreement with previous studies on other gram-negative bacteria. In Bordetella bronchiseptica, loss of PGM caused apparent physical changes in the electrophoretic profiles of LPS, making bacteria more vulnerable to treatment with antimicrobial peptides and oxidative stress.29 In Stenotrophomonas maltophilia, PGM mutant strains displayed shorter O-polysaccharide chains and higher sensitivity to nalidixic acid, gentamicin, polymyxin B, and polymyxin E than the wild-type strains.10 Similarly, disruption of GalU in Vibrio cholerae and Proteus mirabilis increased the sensitivity to novobiocin, SDS, bile salts etc.12,13
We also found that the disruption of GalE (encoding UDP-galactose 4-epimerase) and HP0045 (encoding GDP-l-fucose synthetase) had no significant effect on the regulation of bacterial drug sensitivity in H. pylori (Table 1). These results suggest that GDP-fucose and UDP-galactose play less important roles in intrinsic drug resistance of H. pylori. A similar result was reported in a study on Vibrio cholerae, where the disruption of galU increased sensitivity to antibiotics, such as novobiocin, polymyxin, and the hydrophilic agent SDS, whereas the disruption of galE played no significant role.13
CLA-resistant H. pylori has been listed as a high-priority pathogen for the development of new drugs.30 In the present study, the knockout of either algC or galU, in the two clinical antibiotic-resistant isolates, greatly improved antibiotic sensitivity, especially for CLA (Table 2). Hp008 carries the drug resistance point mutations 2143A>G and 2182T>C, whereas Hp135 carries 2182T>C in 23S rRNA, as confirmed by sequencing.31,32 The loss of either algC or galU leads to an increase in the membrane permeability of bacteria, thereby increasing the bactericidal efficacy of CLA. However, unlike CLA, knockout of algC and galU did not change high resistance to MNZ in these clinical isolates (Table 2). MNZ resistance in Hp008 and Hp135 was due to mutations in rdxA, which was also confirmed via sequencing. Hp008 carries R16C, H53R, and V144A point mutations, whereas Hp135 carries a Q50-stop mutation. MNZ is a prodrug that requires a nitroreductase, encoded by rdxA, to converts MNZ to a bactericidal agent.33,34 This explains why a higher outer membrane permeability in algC and galU mutants did not increase bacterial sensitivity to MNZ.
Lipid A, the anchor for LPS molecules on the outer membrane, directly influences membrane properties.35 Inhibition of lipid A biosynthesis is lethal to most gram-negative bacteria, whereas reduced lipid A biosynthesis leads to hypersensitivity to a wide range of antibiotics.36,37 In this study, LPS structural analysis, via silver staining, indicated that the loss of algC or galU caused changes in the lipid A-core region, while not altering the O-antigen (Figure 3). This result is also consistent with reports on other gram-negative bacteria. In Pseudomonas aeruginosa, loss of algC altered the LPS core region.10,27 Jutta et al reported that LPS in the mutant of galU showed altered core oligosaccharide, but with intact O antigen in Vibrio cholerae.13 Similar to our results from a recent study, algC knockout also led to an aberrant core region in H. pylori.14 However, mutations in LPS synthesis-related genes do not always increase membrane permeability. Our previous study showed that the loss of rfaF encoding lipopolysaccharide heptosyltransferase II, which transfers a second heptose residue into the LPS core, reduced membrane permeability.38 Therefore, we conclude that the structure of lipid A-core region plays an important role in the outer membrane permeability and intrinsic resistance of H. pylori, and the UDP-glucose synthesis pathway can serve as a good drug target to increase the sensitivity of clinical drug-resistant strains.
In addition to LPS synthesis, AlgC and GalU are also involved in bacterial adaptability, growth, and metabolism.7,39 In H. pylori, UDP-glucose is also required for the synthesis of cholesterol glucosides, which are vital components of the cell envelope involved in host immune escape and resistance to toxic compounds.40,41 In addition, bacterial PGM showed low homology with eukaryotes.39,42 Bacterial UGPs, encoded by galU, are unrelated to their eukaryotic counterparts.43 AlgC and GalU are potential targets for developing bacteria-targeted drugs that can reduce the drug resistance of clinical multidrug-resistant H. pylori strains. This could be a useful strategy as an antibiotic adjuvant to overcome the challenge of drug resistance. However, the limitation of this study is that the findings rely on in vitro experiments. Since the host environment is complex, whether similar results can be obtained in vivo requires further investigation.
Conclusion
Taken together, our results suggest that AlgC and GalU might influence LPS structure and outer membrane permeability through the synthesis of UDP-glucose. Since we have confirmed that the disruption of the synthesis of UDP-galactose and GDP-fucose exhibited no significant effect on antibiotic resistance, we speculate that UDP-glucose is important for the intrinsic drug resistance of H. pylori. AlgC and GalU might serve as drug targets for clinical multidrug-resistant strains of H. pylori.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82072316), the Natural Science Foundation of Fujian Province, China (Grant Nos. 2020Y9003, 2021Y9101), Key Projects of the Natural Science Foundation of Fujian Province, China (Grant No. 2020J02019), Fujian Provincial Financial Special Plan (Grant No. 22SCZZX021).
Disclosure
The authors declare no competing interests.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 2.Naumann M, Crabtree JE. Helicobacter pylori-induced epithelial cell signalling in gastric carcinogenesis. Trends Microbiol. 2004;12(1):29–36. doi: 10.1016/j.tim.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Kocsmár É, Buzás GM, Szirtes I, et al. Primary and secondary clarithromycin resistance in Helicobacter pylori and mathematical modeling of the role of macrolides. Nat Commun. 2021;12(1):2255. doi: 10.1038/s41467-021-22557-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022. doi: 10.1136/gutjnl-2022-327745 [DOI] [PubMed] [Google Scholar]
- 5.Liou JM, Lin JT, Chang CY, et al. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59(5):572–578. doi: 10.1136/gut.2009.198309 [DOI] [PubMed] [Google Scholar]
- 6.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berbís M, Sánchez-Puelles JM, Cañada FJ, Jiménez-Barbero J. Structure and Function of Prokaryotic UDP-Glucose Pyrophosphorylase, A Drug Target Candidate. Curr Med Chem. 2015;22(14):1687–1697. doi: 10.2174/0929867322666150114151248 [DOI] [PubMed] [Google Scholar]
- 8.Paterson GK, Cone DB, Peters SE, Maskell DJ. The enzyme phosphoglucomutase (Pgm) is required by Salmonella enterica serovar Typhimurium for O-antigen production, resistance to antimicrobial peptides and in vivo fitness. Microbiology. 2009;155(Pt 10):3403–3410. doi: 10.1099/mic.0.029553-0 [DOI] [PubMed] [Google Scholar]
- 9.Felek S, Muszyński A, Carlson RW, Tsang TM, Hinnebusch BJ, Krukonis ES. Phosphoglucomutase of Yersinia pestis is required for autoaggregation and polymyxin B resistance. Infect Immun. 2010;78(3):1163–1175. doi: 10.1128/iai.00997-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKay GA, Woods DE, MacDonald KL, Poole K. Role of phosphoglucomutase of Stenotrophomonas maltophilia in lipopolysaccharide biosynthesis, virulence, and antibiotic resistance. Infect Immun. 2003;71(6):3068–3075. doi: 10.1128/iai.71.6.3068-3075.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou Y, Feng S, Xu C, et al. The role of galU and galE of Haemophilus parasuis SC096 in serum resistance and biofilm formation. Vet Microbiol. 2013;162(1):278–284. doi: 10.1016/j.vetmic.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Jiang SS, Lin TY, Wang WB, Liu MC, Hsueh PR, Liaw SJ. Characterization of UDP-Glucose Dehydrogenase and UDP-Glucose Pyrophosphorylase Mutants of Proteus mirabilis: defectiveness in Polymyxin B Resistance, Swarming, and Virulence. Antimicrob Agents Chemother. 2010;54(5):2000–2009. doi: 10.1128/aac.01384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nesper J, Lauriano CM, Klose KE, Kapfhammer D, Kraiss A, Reidl J. Characterization of Vibrio cholerae O1 El tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect Immun. 2001;69(1):435–445. doi: 10.1128/iai.69.1.435-445.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu AN, Teng KW, Chew Y, Wang PC, Nguyen TTH, Kao MC. The Effects of HP0044 and HP1275 Knockout Mutations on the Structure and Function of Lipopolysaccharide in Helicobacter pylori Strain 26695. Biomedicines. 2022;10:1. doi: 10.3390/biomedicines10010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen Y, Huang H, Tang T, et al. AI-2 represses CagA expression and bacterial adhesion, attenuating the Helicobacter pylori-induced inflammatory response of gastric epithelial cells. Helicobacter. 2021:e12778. doi: 10.1111/hel.12778 [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Huang X, Zhang X, et al. AI-2 Induces Urease Expression Through Downregulation of Orphan Response Regulator HP1021 in Helicobacter pylori. Front Med. 2022;9. doi: 10.3389/fmed.2022.790994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cammarota G, Martino A, Pirozzi G, et al. High efficacy of 1-week doxycycline- and amoxicillin-based quadruple regimen in a culture-guided, third-line treatment approach for Helicobacter pylori infection. Aliment Pharmacol Ther. 2004;19(7):789–795. doi: 10.1111/j.1365-2036.2004.01910.x [DOI] [PubMed] [Google Scholar]
- 18.Li H, Yang T, Liao T, et al. The redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. PLoS Pathog. 2017;13(3):e1006280. doi: 10.1371/journal.ppat.1006280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Järvinen N, Mäki M, Räbinä J, Roos C, Mattila P, Renkonen R. Cloning and expression of Helicobacter pylori GDP-l-fucose synthesizing enzymes (GMD and GMER) in Saccharomyces cerevisiae. Eur j Biochem. 2001;268(24):6458–6464. doi: 10.1046/j.0014-2956.2001.02601.x [DOI] [PubMed] [Google Scholar]
- 20.Wu B, Zhang Y, Wang PG. Identification and characterization of GDP-d-mannose 4,6-dehydratase and GDP-l-fucose synthetase in a GDP-l-fucose biosynthetic gene cluster from Helicobacter pylori. Biochem Biophys Res Commun. 2001;285(2):364–371. doi: 10.1006/bbrc.2001.5137 [DOI] [PubMed] [Google Scholar]
- 21.Kwon DH, Woo JS, Perng CL, Go MF, Graham DY, El-Zaatari FA. The effect of galE gene inactivation on lipopolysaccharide profile of Helicobacter pylori. Curr Microbiol. 1998;37(2):144–148. doi: 10.1007/s002849900354 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Liao T, Debowski AW, et al. Lipopolysaccharide Structure and Biosynthesis in Helicobacter pylori. Helicobacter. 2016;21(6):445–461. doi: 10.1111/hel.12301 [DOI] [PubMed] [Google Scholar]
- 23.Bertani B, Ruiz N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus. 2018;8:1. doi: 10.1128/ecosalplus.ESP-0001-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Tang G, Pan L, Zhu H, Zhou S, Wei Z. Clinical factors associated with initial Helicobacter pylori eradication therapy: a retrospective study in China. Sci Rep. 2020;10(1):15403. doi: 10.1038/s41598-020-72400-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox G, Wright GD. Intrinsic antibiotic resistance: mechanisms, origins, challenges and solutions. Int J Med Microbiol. 2013;303(6–7):287–292. doi: 10.1016/j.ijmm.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 26.MacNair CR, Brown ED. Outer Membrane Disruption Overcomes Intrinsic, Acquired, and Spontaneous Antibiotic Resistance. mBio. 2020;11(5). doi: 10.1128/mBio.01615-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coyne MJ, Russell KS, Coyle CL, Goldberg JB. The Pseudomonas aeruginosa algC gene encodes phosphoglucomutase, required for the synthesis of a complete lipopolysaccharide core. J Bacteriol. 1994;176(12):3500–3507. doi: 10.1128/jb.176.12.3500-3507.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou D, Stephens D, Gibson B, et al. Lipooligosaccharide biosynthesis in pathogenic Neisseria. Cloning, identification, and characterization of the phosphoglucomutase gene. Proteins. 1994;269(15):11162–11169. doi: 10.1016/S0021-9258(19)78105-8 [DOI] [PubMed] [Google Scholar]
- 29.West NP, Jungnitz H, Fitter JT, McArthur JD, Guzmán CA, Walker MJ. Role of phosphoglucomutase of Bordetella bronchiseptica in lipopolysaccharide biosynthesis and virulence. Infect Immun. 2000;68(8):4673–4680. doi: 10.1128/iai.68.8.4673-4680.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/s1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 31.Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41(12):2621–2628. doi: 10.1128/aac.41.12.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan R, Nahar S, Sultana J, Ahmad MM, Rahman M. T2182C mutation in 23S rRNA is associated with clarithromycin resistance in Helicobacter pylori isolates obtained in Bangladesh. Antimicrob Agents Chemother. 2004;48(9):3567–3569. doi: 10.1128/aac.48.9.3567-3569.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards DI. Nitroimidazole drugs--action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31(1):9–20. doi: 10.1093/jac/31.1.9 [DOI] [PubMed] [Google Scholar]
- 34.Jeong JY, Mukhopadhyay AK, Dailidiene D, et al. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori. J Bacteriol. 2000;182(18):5082–5090. doi: 10.1128/jb.182.18.5082-5090.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna E, Kim S, Gao Y, Widmalm G, Im W. Influences of Vibrio cholerae Lipid A Types on LPS Bilayer Properties. J Phys Chem B. 2021;125(8):2105–2112. doi: 10.1021/acs.jpcb.0c09144 [DOI] [PubMed] [Google Scholar]
- 36.Vuorio R, Vaara M. The lipid A biosynthesis mutation lpxA2 of Escherichia coli results in drastic antibiotic supersusceptibility. Antimicrob Agents Chemother. 1992;36(4):826–829. doi: 10.1128/aac.36.4.826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CR, Lee JH, Jeong BC, Lee SH. Lipid a biosynthesis of multidrug-resistant pathogens - a novel drug target. Curr Pharm Des. 2013;19(36):6534–6550. doi: 10.2174/13816128113199990494 [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Zhang X, Wen Y, Chen H, She F, Newly Discovered A. Drug Resistance Gene rfaF In Helicobacter pylori. Infect Drug Resist. 2019;12:3507–3514. doi: 10.2147/IDR.S231152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiers KM, Muenks AG, Beamer LJ. Biology, Mechanism, and Structure of Enzymes in the alpha-d-Phosphohexomutase Superfamily. Adv Protein Chem Struct Biol. 2017;109:265–304. doi: 10.1016/bs.apcsb.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qaria MA, Kumar N, Hussain A, et al. Roles of Cholesteryl-α-Glucoside Transferase and Cholesteryl Glucosides in Maintenance of Helicobacter pylori Morphology, Cell Wall Integrity, and Resistance to Antibiotics. mBio. 2018;9:6. doi: 10.1128/mBio.01523-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi J, Kawakubo M, Fujii C, et al. Cholestenone functions as an antibiotic against Helicobacter pylori by inhibiting biosynthesis of the cell wall component CGL. Proc Natl Acad Sci U S A. 2021;118:16. doi: 10.1073/pnas.2016469118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehra-Chaudhary R, Mick J, Tanner JJ, Henzl MT, Beamer LJ. Crystal structure of a bacterial phosphoglucomutase, an enzyme involved in the virulence of multiple human pathogens. Proteins. 2011;79(4):1215–1229. doi: 10.1002/prot.22957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai X, Wu J, Chen S, Zhang X, Wang H. Expression, purification, and characterization of a functionally active Mycobacterium tuberculosis UDP-glucose pyrophosphorylase. Protein Expr Purif. 2008;61(1):50–56. doi: 10.1016/j.pep.2008.05.015 [DOI] [PubMed] [Google Scholar]