Abstract
The semi-essential amino acid, cysteine, plays important roles in both essential cellular processes as well as in modulation of signaling cascades. Cysteine is obtained both from the diet as well as generated endogenously via the transsulfuration pathway. Cysteine is further utilized in protein synthesis and biosynthesis of various sulfur containing molecules. One of the products of cysteine catabolism, hydrogen sulfide (H2S), is a gaseous signaling molecule, which regulates a multitude of cellular processes. Cysteine metabolism is dysregulated in several neurodegenerative diseases and during aging. This minireview focuses on aberrant cysteine and H2S metabolism in Huntington’s disease, a neurodegenerative disease caused by expansion of polyglutamine encoding repeats in the gene huntingtin, which leads to motor and cognitive deficits.
Keywords: Huntington’s disease, Cysteine, Transsulfuration, Golgi stress response, Cystathionine γ-lyase, Hydrogen sulfide
1. Introduction
Huntington’s disease (HD) is a neurodegenerative disease triggered by the expansion of polyglutamine (CAG) repeats in the gene huntingtin [1]. In HD, the corpus striatum of the brain is profoundly affected and atrophies during disease progression. Symptoms of HD include involuntary movements, muscle wastage, motor and cognitive deficits among others [2-4]. The length of the CAG repeat has been linked to disease progression and pathology in several studies [5-7]. Additionally, genetic modifiers of HD have been identified, that influence the onset or severity of the disease [8,9]. A molecular hallmark of HD is the aggregation of mutant huntingtin (mHtt), which disrupts several cellular processes ranging from transcriptional and translational regulation, nuclear cytoplasmic import, amino acid homeostasis and nutrient sensing, antioxidant and stress responses, second messenger signaling, DNA repair and autophagy, to name a few [10-22].
At the molecular level, redox imbalance and oxidative damage are characteristic features of HD [14,23-25]. While reactive oxygen species (ROS) are essential for certain physiological processes, excess production impairs cellular stress responses and elicits neurotoxicity in several neurodegenerative disorders such as HD [17,23]. It has been observed that changes in redox signaling processes, some of which are reversible, often precede neurodegeneration. Production of ROS, reactive nitrogen species (RNS) and reactive nitrogen species (RSS) occur both during normal cellular processes such as mitochondrial function and immune responses, however in pathological conditions, the production of these free radicals and oxidants may exceed the cellular capacity to neutralize them [17]. Accordingly, cells have evolved a diverse array of small molecules and proteins to counteract the damaging effects of these reactive species and free radicals, which are regulated at multiple levels, both during basal conditions and during stress responses.
Central to the maintenance of redox balance in cells, is the reverse transsulfuration pathway, which leads to the biosynthesis of the amino acid cysteine and the antioxidant glutathione (GSH) (Fig. 1A). GSH is one of the most abundant thiols, which functions as a cofactor for several enzymes involved in maintenance of redox homeostasis. Both cysteine and GSH are potent antioxidants and protect cells from a wide variety of damaging stimuli. Besides its essential function as a component of protein synthesis, cysteine serves a precursor of several sulfur containing molecules including GSH, coenzyme A, lanthionine, cysteamine and taurine (Fig. 1B). Cysteine also plays a central role in iron sulfur (Fe–S) cluster protein biogenesis, which sustains several cellular processes [26]. Although cysteine is one of the scarcest amino acids, comprising only 2% of the proteome, it undergoes the largest number of post-translational modifications, which include persulfidation/sulfhydration, nitrosylation, glutathionylation, palmitoylation and sumoylation among others [27,28]. Thus, perturbations in these modifications and/or metabolism of cysteine itself can alter cellular functions. We have shown that disrupted cysteine and H2S metabolism occurs in several neurodegenerative diseases [14,15,29]. Cysteine metabolism is affected in neurodegenerative diseases in multiple ways as discussed below.
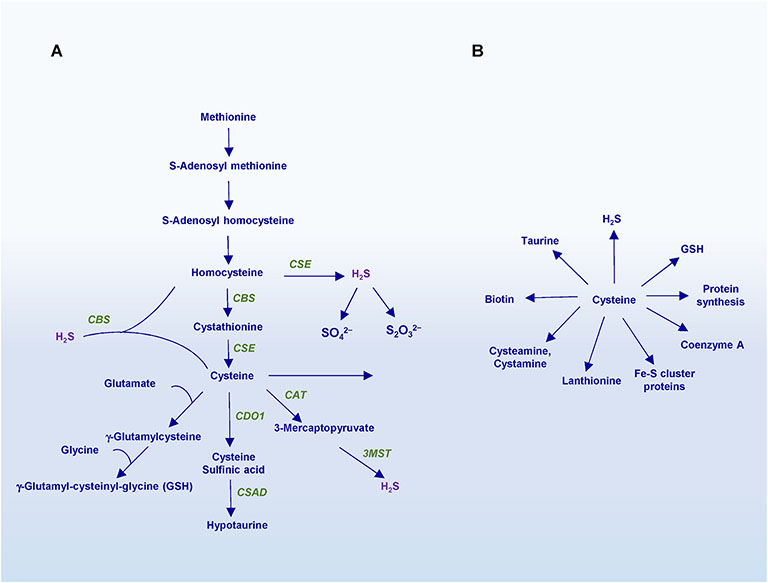
Fig. 1. Biosynthesis of cysteine and its downstream products.
A) The transsulfuration pathway. Dietary methionine is utilized through a series of reactions to generate homocysteine, which is condensed with serine to generate cystathionine by cystathionine β-synthase (CBS). Cystathionine γ-lyase (CSE) then utilizes cystathionine as a substrate to produce cysteine. Excess cysteine may be degraded by cysteine dioxygenase 1 (CDO1) to generate cysteine sulfinic acid, which is further acted on by cysteine sulfinic acid decarboxylase (CSAD) to produce hypotaurine. Cysteine may be used to generate glutathione via γ-glutamylcysteine. Cysteine is also utilized to produce other sulfur containing molecules and Fe–S cluster proteins or used as a substrate to generate hydrogen sulfide (H2S). Both homocysteine and cysteine serve as substrates for the generation of H2S. While CSE may synthesize H2S from either cysteine or homocysteine, CBS generates H2S using a combination of cysteine and homocysteine. A third enzyme, 3-mercaptopyruvate sulfur transferase (3-MST), in conjunction with cysteine amino transferase (CAT), also produces H2S using 3- mercaptopyruvate (3-MP) as a substrate. H2S is also subject to degradation by the sulfide oxidation unit (SOU) in the mitochondria to produce sulfate and thiosulfate. B) Products and derivatives of cysteine. In addition to its essential role in protein synthesis, cysteine is the precursor of sulfur containing molecules such as glutathione, taurine, lanthionine, coenzyme A, biotin and the gasotransmitter hydrogen sulfide (H2S).
2. Biosynthesis and uptake of cysteine in the brain
Cysteine is a semi-essential amino acid and its availability is the rate limiting step for GSH biosynthesis. Cysteine is obtained both from the diet and also endogenously through the reverse transsulfuration pathway, which refers to the transfer of sulfur from homocysteine to cysteine. Homocysteine, in turn is derived from dietary methionine. In bacteria, the pathway operates in the opposite direction and the biosynthesis of methionine and was referred to as transsulfuration. For the sake of clarity, we will refer to the pathway as transsulfuration. Cystathionine γ-lyase (CSE) is the key enzyme in the pathway and the sole biosynthetic enzyme for de novo synthesis of cysteine in vivo in mammals, generating the amino acid from cystathionine. Mice lacking CSE die within two weeks on a cysteine-free diet [30,31]. In addition to its essential role as the biosynthetic enzyme for cysteine, CSE utilizes cysteine as a substrate to generate hydrogen sulfide (H2S), which modulates several physiological processes [32-34].
Cysteine is taken up from the diet through several transporters, which include the system , which imports the oxidized form, cystine in a Na+-independent but chloride-dependent manner. The transporter also takes up cystathionine, the precursor for cysteine [28,35]. Other transporters include the excitatory amino acid transporter 3 (EAAT3/EAAC1) and the alanine, serine, cysteine transporters (ASCTs) [28]. Both cysteine and H2S levels are tightly regulated in cells to avoid toxicity. Excess cysteine may be utilized in the biosynthesis of sulfur containing molecules or degraded by cysteine dioxygenase 1 (CDO1) to form cysteine sulfinic acid, which is further converted to hypotaurine [36,37]. H2S is catabolized in the mitochondria by the sulfide oxidation unit to thiosulfate and sulfate, with the rate varying in different tissues [33,38,39].
3. Deficiencies in CSE and CBS are linked to disease
Several mutations have been identified in CBS, which alter its folding and/or activity [40]. Mutations in the CBS or CBS deficiency, leads to hyperhomocysteinemia (HHcy) and thromboembolism, which are linked to lifespan and healthspan. CBS deficiency has been linked to hypertension and cardiovascular abnormalities. Elevated homocysteine caused by CBS deficiency has also been linked to ocular defects and lens dislocation and skeletal defects and resembles Marfan syndrome [41-43]. In stark contrast, elevation of CBS, which resides on chromosome 21, is linked to excess H2S and associated symptoms in Down syndrome [44-46]. Mutations in Cth, the gene encoding CSE, although not as frequent as those in Cbs, has been linked to cystathionuria and homocystinuria, which may cause vascular abnormalities [47,48]. Abnormalities in the transsulfuration pathway have also been linked to Alzheimer’s disease and Parkinson’s disease, along with decreased persulfidation [49-51].
4. Dysregulation of cysteine and H2S metabolism in HD
Disruption of cysteine metabolism results in altered GSH levels and H2S signaling, which impacts almost all cellular processes. We and others have shown that both cysteine uptake and biosynthesis is compromised in HD. Studies conducted earlier identified CBS as a huntingtin interacting protein, which may affect H2S production by CBS [52]. We identified a profound depletion of CSE in cell culture and mouse models of HD as well as human postmortem striatal tissue [14]. The dysregulation stems from the influence of mHtt on transcription factors regulating expression of CSE [53]. The diminished expression of CSE in HD was also confirmed by other studies, where expression of CSE was linked to CAG repeats [54]. Furthermore, elevated cystathionine, the precursor of cysteine, has been reported in the R6/2 mouse model, which may arise from depletion of CSE [55]. Under basal conditions, CSE is regulated by specificity protein 1 (SP1) and by the activating transcription factor 4 (ATF4) in response to stress stimuli [56]. SP1 is sequestered by mHtt, leading to decreased transcription of CSE. As a result, the striatal progenitor cell line STHdhQ111/Q111 (Q111), which harbors 111 polyglutamine repeats cannot grow in cysteine free medium or produce sufficient H2S in response to stress [14,15]. Transfecting SP1 and its coactivator, TAF4, in Q111 cells elevated CSE expression and restored growth during cysteine deprivation. In addition to the biosynthetic deficit, the cysteine and cystine transporters are also suboptimally expressed at the cell surface, leading to an overall cysteine deficit [57, 58](Fig. 2). The cysteine homeostatic machinery in response to stress is also derailed in HD, exhibiting altered ATF4 dynamics. While the striatal STHdhQ7/Q7 (Q7) express ATF4 in response to cysteine deprivation, the Q111 cells fail to do so. Intriguingly, the Q7 cells upregulated ATF4 and CSE in response to endoplasmic reticulum (ER) stress and deprivation of other amino acids. The inability to induce ATF4 in response to cysteine deprivation occurs due to the elevated oxidative stress associated with HD, which prevents the optimal stress-induced expression of ATF4. This disruption culminates in a vicious cycle that leads to cell death. As a proof of concept, inducing oxidative stress in Q7 cells prevented induction and decreasing oxidative stress in Q111 cells promoted induction of ATF4 in response to cysteine deprivation [15].
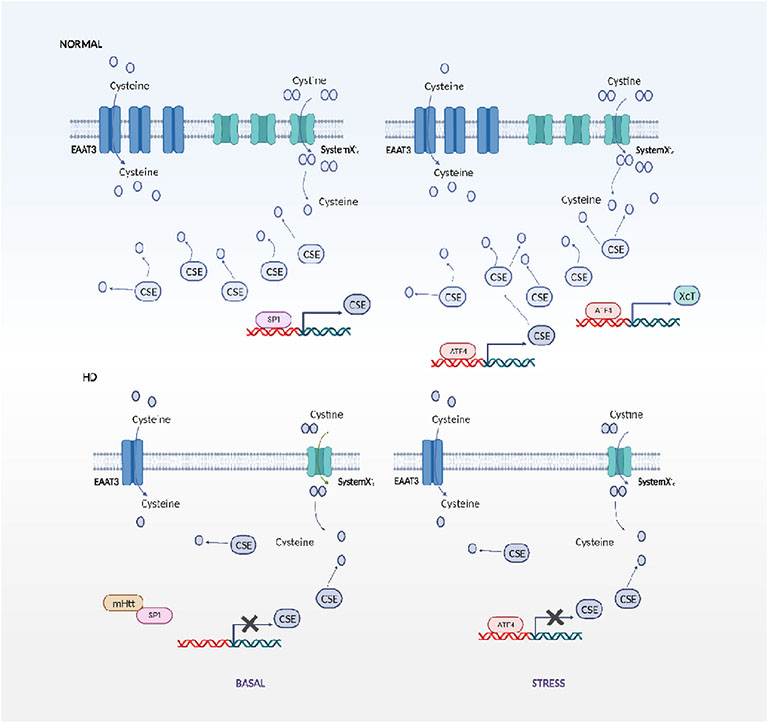
Fig. 2. Disrupted cysteine and H2S metabolism in Huntington’s disease.
The biosynthetic enzyme for cysteine and H2S, cystathionine γ-lyase (CSE) is regulated by specificity protein 1 (SP1) under basal conditions and by activating transcription factor 4 (ATF4) during response to stress in normal cells. In striatal HD cell lines, SP1 is sequestered by mutant huntingtin (mHtt) leading to decreased transcription of CSE, which leads to decreased cysteine and H2S production. Additionally the neuronal cysteine transporter, excitatory amino acid transporter 3 (EAAT3/EAAC1) and system Xc− are dysregulated in HD under basal conditions, leading to an overall cysteine deficit. During stress, in normal cells, ATF4 is upregulated, which stimulates expression of CSE. In HD cells, this induction is blunted, further contributing to the derailment of cysteine metabolism.
One of the modes by which the transsulfuration pathway plays a role in regulation of signaling events is through sulfhydration/persulfidation. Sulfhydration is a posttranslational modification mediated by H2S and occurs on reactive cysteine residues on target proteins [34,59,60]. Sulfhydration modulates several aspects of mammalian physiology, which includes regulation of vascular functions and neuroprotection [49,50,61]. The diminished levels of CSE also translated to a decrease in overall levels of sulfhydration in the Q111 cells [62]. Additionally, this study also reported that levels of sulfhydration decline during aging, the biggest risk factor for several neurodegenerative diseases.
Another benefit resulting from upregulation of the transsulfuration pathway is inhibition of ferroptosis. Ferroptosis is a form of cell death involving oxidative damage by iron and elevated lipid peroxidation [63]. Numerous studies have shown that depletion of cysteine leads to ferroptosis [64,65]. Interestingly, depletion of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by erastin, an inhibitor of the XcT/SLC7A11 transporter, which imports cystine into cells [66]. It is likely that in HD, ferroptosis may play a role in disease progression. Indeed, the tumor suppressor, p53 has been reported to inhibit the cystine transporter and induce ferroptosis [67]. Dysregulation of p53 has been observed in HD and deletion of p53 has proved beneficial in mouse models of HD [68]. Thus, the transsulfuration pathway intersects with several cell death and cell survival pathways.
5. Upregulation of the transsulfuration pathway, stress responses and neuroprotection
As the sulfur metabolites generated via the transsulfuration pathway are beneficial, supplementing these metabolites or augmenting the flux through this pathway was tested in several studies. Supplementing cysteine in the diet and N-acetylcysteine in the drinking water of the R6/2 model of HD delayed striatal shrinkage, improved motor deficits and survival [57]. At the molecular level, restoring the induction of ATF4 by the reduction of oxidative stress, also improved survival of the Q111 cells [15]. ATF4 is induced by several stress response pathways, which constitute the integrated stress response. For instance, the amino acid and ER stress induce ATF4 [15,69]. More recently, we demonstrated that ATF4 is also induced in response to Golgi stress, which leads to increased expression of CSE and H2S protection in the Q7 and Q111 cell lines. The Golgi apparatus is vital for glycosylation of proteins and its resident glycosyltransferases, glycosidases, and nucleotide sugar transporters regulate addition of various sugars that result in a mature glycan [70]. When the capacity of the Golgi is crossed, Golgi stress ensues in a manner analogous to ER stress. In order to mitigate this stress, cells mount signaling cascades which constitute the Golgi stress response [71]. The Golgi stress response has several different arms and is not as well studied as the ER stress response [72].
Similar to ER stress response, the Golgi stress response also acts via the PKR-like ER kinase (PERK) to induce ATF4 and CSE (Fig. 3). Both ATF4 and CSE were not induced in PERK −/− cells. However, the ER stress response protein, binding Ig protein (BiP/GRP78) is not induced in response to monensin, a Golgi stressor, indicating differences in the two stress responses [29,72]. Golgi stress induced by monensin and nigericin upregulated both ATF4 and CSE and supported growth of Q111 cells in cysteine-free media. Thus, it appears that the CSE/H2S pathway is induced in response to various stressors. The induction of ATF4 is responsible for induction of CSE and xCT, a subunit of the cystine transporter [15,29,69]. Together, cysteine levels are boosted, leading to upregulation of H2S production [29]. Although low concentrations of Golgi stressors were protective in cell models of HD, effects in mouse models of HD and the minimal effective dose required to mitigate symptoms remain to be determined.
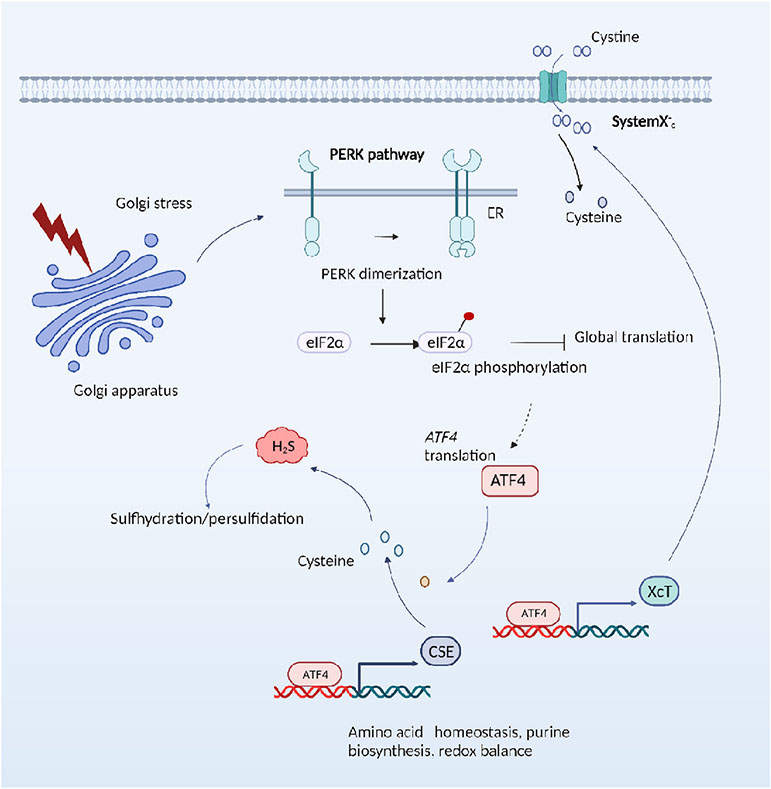
Fig. 3. Mild Golgi stress confers cytoprotection in Huntington’s disease.
Treatment of striatal HD cells with Golgi stressors upregulates ATF4 via the protein kinase R (PKR)-like ER kinase (PERK) pathway and stimulates expression of CSE and cysteine biosynthesis via the reverse transsulfuration pathway. Additionally, the cystine transporters (xCT) may also be upregulated by ATF4, in addition to other targets, leading to enhanced cysteine and H2S levels, and growth in cysteine-deprived conditions.
These findings show that enhancement of the transsulfuration pathway as a whole may be therapeutic in HD. Accordingly, stimulation of the ATF4/CSE axis may be harnessed to upregulate cellular antioxidant defense mechanisms to combat neurodegeneration. Hence molecules that stimulate the transsulfuration pathway may confer adaptive benefits in HD as well as other conditions involving redox imbalance. These findings may be relevant to other neurodegenerative states associated with deficits in cysteine metabolism.
Acknowledgements
B.D.P. was supported by the American Heart Association (AHA)/Paul Allen Frontiers Group; grant number 19PABH134580006 and National Institutes of Health, NIA grant 1R21AG073684-01, NIA grant R01AG071512-01A1 and NIDA grant DA044123. Illustrations were generated using Biorender.
List of Abbreviations
- 3-MP
3-mercapto pyruvate
- 3-MST
3-mercapto pyruvate sulfur transferase
- ASCT
alanine, serine, cysteine transporter
- ATF4
activating transcription factor 4
- BiP
binding Ig protein
- CARS
cysteinyl-tRNA synthetase
- CAT
cysteine amino transferase
- CBS
cystathionine β-synthase
- CDO1
cysteine dioxygenase 1
- CSAD
cysteine sulfinic acid decarboxylase
- CSE
cystathionine γ-lyase
- eIF2α
eukaryotic translation initiation factor 2 subunit-α
- EAAT3/EAAC1
excitatory amino acid transporter 3
- ER
endoplasmic reticulum
- GSH
glutathione
- HD
Huntington’s Disease
- HHcy
hyperhomocysteinemia
- mHtt
mutant huntingtin
- PERK
protein kinase R protein kinase R (PKR)-like ER kinase
- SP1
specificity protein 1
- xCT/SLC7A11
cystine/glutamate antiporter
Footnotes
This is part of a Special Issue - Neurodegeneration and regeneration: antioxidant and redox signalingGuest Editors: Neven Zarkovic, David Butterfield
References
- [1].A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group, Cell 72 (6) (1993) 971–983. [DOI] [PubMed] [Google Scholar]
- [2].Bates GP, et al. , Huntington disease, Nat Rev Dis Primers 1 (2015) 15005. [DOI] [PubMed] [Google Scholar]
- [3].McColgan P, Tabrizi SJ, Huntington’s disease: a clinical review, Eur J Neurol 25 (1) (2018) 24–34. [DOI] [PubMed] [Google Scholar]
- [4].Tabrizi SJ, et al. , Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities, Nat Rev Neurol 16 (10) (2020) 529–546. [DOI] [PubMed] [Google Scholar]
- [5].Langbehn DR, Registry N, Investigators of the European Huntington Disease, Longer CAG repeat length is associated with shorter survival after disease onset in Huntington disease, Am J Hum Genet 109 (1) (2022) 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ament SA, et al. , Transcriptional regulatory networks underlying gene expression changes in Huntington’s disease, Mol Syst Biol 14 (3) (2018) e7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Keum JW, et al. , The HTT CAG-expansion mutation determines age at death but not disease duration in Huntington disease, Am J Hum Genet 98 (2) (2016) 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].C. Genetic Modifiers of Huntington’s Disease, Identification of genetic factors that modify clinical onset of huntington’s disease, Cell 162 (3) (2015) 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gusella JF, Lee JM, MacDonald ME, Huntington’s disease: nearly four decades of human molecular genetics, Hum Mol Genet 30 (R2) (2021) R254–R263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zsindely N, Siagi F, Bodai L, DNA methylation in huntington’s disease, Int J Mol Sci 22 (23) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grima JC, et al. , Mutant huntingtin disrupts the nuclear pore complex, Neuron 94 (1) (2017) 93–107 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cha JH, Transcriptional signatures in Huntington’s disease, Prog Neurobiol 83 (4) (2007) 228–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gasset-Rosa F, et al. , Polyglutamine-Expanded huntingtin exacerbates age-related disruption of nuclear integrity and nucleocytoplasmic transport, Neuron 94 (1) (2017) 48–57 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paul BD, et al. , Cystathionine gamma-lyase deficiency mediates neurodegeneration in Huntington’s disease, Nature 509 (7498) (2014) 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sbodio JI, Snyder SH, Paul BD, Transcriptional control of amino acid homeostasis is disrupted in Huntington’s disease, Proc Natl Acad Sci U S A 113 (31) (2016) 8843–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ahmed I, et al. , Huntington’s disease: neural dysfunction linked to inositol polyphosphate multikinase, Proc Natl Acad Sci U S A 112 (31) (2015) 9751–9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sbodio JI, Snyder SH, Paul BD, Redox mechanisms in neurodegeneration: from disease outcomes to therapeutic opportunities, Antioxid Redox Signal 30 (11) (2019) 1450–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sharma M, et al. , Cyclic GMP-AMP synthase promotes the inflammatory and autophagy responses in Huntington disease, Proc Natl Acad Sci U S A 117 (27) (2020) 15989–15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Beaumont V, et al. , Phosphodiesterase 10A inhibition improves cortico-basal ganglia function in huntington’s disease models, Neuron 92 (6) (2016) 1220–1237. [DOI] [PubMed] [Google Scholar]
- [20].Eshraghi M, et al. , Mutant Huntingtin stalls ribosomes and represses protein synthesis in a cellular model of Huntington disease, Nat Cornmun 12 (1) (2021) 1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Seredenina T, Luthi-Carter R, What have we learned from gene expression profiles in Huntington’s disease? Neurobiol Dis 45 (1) (2012) 83–98. [DOI] [PubMed] [Google Scholar]
- [22].Maiuri T, et al. , DNA repair in huntington’s disease and spinocerebellar ataxias: somatic instability and alternative hypotheses, J Huntingtons Dis 10 (1) (2021) 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Paul BD, Snyder SH, Impaired redox signaling in huntington’s disease: therapeutic implications, Front Mol Neurosci 12 (2019) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perluigi M, et al. , Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: a model of Huntington disease, Mol Cell Proteomics 4 (12) (2005) 1849–1861. [DOI] [PubMed] [Google Scholar]
- [25].Stack EC, Matson WR, Ferrante RJ, Evidence of oxidant damage in Huntington’s disease: translational strategies using antioxidants, Ann N Y Acad Sci 1147 (2008) 79–92. [DOI] [PubMed] [Google Scholar]
- [26].Johnson DC, et al. , Structure, function, and formation of biological iron-sulfur clusters, Annu Rev Biochem 74 (2005) 247–281. [DOI] [PubMed] [Google Scholar]
- [27].Miseta A, Csutora P, Relationship between the occurrence of cysteine in proteins and the complexity of organisms, Mol Biol Evol 17 (8) (2000) 1232–1239. [DOI] [PubMed] [Google Scholar]
- [28].Paul BD, Sbodio JI, Snyder SH, Cysteine metabolism in neuronal redox homeostasis, Trends Pharmacol Sci 39 (5) (2018) 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sbodio JI, Snyder SH, Paul BD, Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease, Proc Natl Acad Sci U S A 115 (4) (2018) 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mani S, Yang G, Wang R, A critical life-supporting role for cystathionine gamma-lyase in the absence of dietary cysteine supply, Free Radic Biol Med 50 (10) (2011) 1280–1287. [DOI] [PubMed] [Google Scholar]
- [31].Ishii I, et al. , Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury, J Biol Chem 285 (34) (2010) 26358–26368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang R, Physiological implications of hydrogen sulfide: a whiff exploration that blossomed, Physiol Rev 92 (2) (2012) 791–896. [DOI] [PubMed] [Google Scholar]
- [33].Paul BD, Snyder SH, Kashfi K, Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics, Redox Biol 38 (2021) 101772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Paul BD, Snyder SH, H2S: a novel gasotransmitter that signals by sulfhydration, Trends Biochem Sci 40 (11) (2015) 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kobayashi S, et al. , Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function, J Biol Chem 290 (14) (2015) 8778–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Paul BD, Snyder SH, Therapeutic applications of cysteamine and cystamine in neurodegenerative and neuropsychiatric diseases, Front Neurol 10 (2019) 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stipanuk MH, Metabolism of sulfur-containing amino acids: how the body copes with excess methionine, cysteine, and sulfide, J Nutr 150 (Suppl 1) (2020) 2494S–2505S. [DOI] [PubMed] [Google Scholar]
- [38].Bartholomew TC, et al. , Oxidation of sodium sulphide by rat liver, lungs and kidney, Biochem Pharmacol 29 (18) (1980) 2431–2437. [DOI] [PubMed] [Google Scholar]
- [39].Abou-Hamdan A, et al. , Oxidation of H2S in mammalian cells and mitochondria, Methods Enzymol 554 (2015) 201–228. [DOI] [PubMed] [Google Scholar]
- [40].Kozich V, et al. , Cystathionine beta-synthase mutations: effect of mutation topology on folding and activity, Hum Mutat 31 (7) (2010) 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Paradkar MU, et al. , Association of genetic variants with hyperhomocysteinemia in Indian patients with thrombosis, Indian J Clin Biochem 35 (4) (2020) 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hua N, et al. , Recurrent dislocation of binocular crystal lenses in a patient with cystathionine beta-synthase deficiency, BMC Ophthalmol 21 (1) (2021) 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brenton DP, et al. , Homocystinuria and Marfan’s syndrome. A comparison, J Bone Joint Surg Br 54 (2) (1972) 277–298. [PubMed] [Google Scholar]
- [44].Ichinohe A, et al. , Cystathionine beta-synthase is enriched in the brains of Down’s patients, Biochem Biophys Res Commun 338 (3) (2005) 1547–1550. [DOI] [PubMed] [Google Scholar]
- [45].Panagaki T, et al. , Overproduction of hydrogen sulfide, generated by cystathionine beta-synthase, disrupts brain wave patterns and contributes to neurobehavioral dysfunction in a rat model of down syndrome, Redox Biol (2022) 102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Panagaki T, et al. , Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome, Proc Natl Acad Sci U S A 116 (38) (2019) 18769–18771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang J, Hegele RA, Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH), Hum Genet 112 (4) (2003) 404–408. [DOI] [PubMed] [Google Scholar]
- [48].Wang J, et al. , Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration, Clin Genet 65 (6) (2004) 483–486. [DOI] [PubMed] [Google Scholar]
- [49].Giovinazzo D, et al. , Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3beta and inhibiting Tau hyperphosphorylation, Proc Natl Acad Sci U S A 118 (4) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vandiver MS, et al. , Sulfhydration mediates neuroprotective actions of parkin, Nat Commun 4 (2013) 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Paul BD, Neuroprotective roles of the reverse transsulfuration pathway in alzheimer’s disease, Front Aging Neurosci 13 (2021) 659402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Boutell JM, et al. , Huntingtin interacts with cystathionine beta-synthase, Hum Mol Genet 7 (3) (1998) 371–378. [DOI] [PubMed] [Google Scholar]
- [53].Dunah AW, et al. , Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease, Science 296 (5576) (2002) 2238–2243. [DOI] [PubMed] [Google Scholar]
- [54].Li L, et al. , Real-time imaging of Huntingtin aggregates diverting target search and gene transcription, Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hashimoto M, et al. , Multiplatform metabolomic analysis of the R6/2 mouse model of Huntington’s disease, FEBS Open Bio 11 (10) (2021) 2807–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ishii I, et al. , Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression, Biochem J 381 (Pt 1) (2004) 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Frederick NM, et al. , Dysregulation of system xc(−) expression induced by mutant huntingtin in a striatal neuronal cell line and in R6/2 mice, Neurochem Int 76 (2014) 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li X, et al. , Aberrant Rab11-dependent trafficking of the neuronal glutamate transporter EAAC1 causes oxidative stress and cell death in Huntington’s disease, J Neurosci 30 (13) (2010) 4552–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Paul BD, Snyder SH, Protein sulfhydration, Methods Enzymol 555 (2015) 79–90. [DOI] [PubMed] [Google Scholar]
- [60].Paul BD, Snyder SH, H(2)S signalling through protein sulfhydration and beyond, Nat Rev Mol Cell Biol 13 (8) (2012) 499–507. [DOI] [PubMed] [Google Scholar]
- [61].Yang G, et al. , H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase, Science 322 (5901) (2008) 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zivanovic J, et al. , Selective persulfide detection reveals evolutionarily conserved antiaging effects of S-sulfhydration, Cell Metab 30 (6) (2019) 1152–1170 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jiang X, Stockwell BR, Conrad M, Ferroptosis: mechanisms, biology and role in disease, Nat Rev Mol Cell Biol 22 (4) (2021) 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Badgley MA, et al. , Cysteine depletion induces pancreatic tumor ferroptosis in mice, Science 368 (6486) (2020) 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Stockwell BR, et al. , Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease, Cell 171 (2) (2017) 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hayano M, et al. , Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation, Cell Death Differ 23 (2) (2016) 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jiang L, et al. , Ferroptosis as a p53-mediated activity during tumour suppression, Nature 520 (7545) (2015) 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bae BI, et al. , p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease, Neuron 47 (1) (2005) 29–41. [DOI] [PubMed] [Google Scholar]
- [69].Harding HP, et al. , An integrated stress response regulates amino acid metabolism and resistance to oxidative stress, Mol Cell 11 (3) (2003) 619–633. [DOI] [PubMed] [Google Scholar]
- [70].Stanley P, Golgi glycosylation, Cold Spring Harb Perspect Biol 3 (4) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sasaki K, Yoshida H, Organelle autoregulation-stress responses in the ER, Golgi, mitochondria and lysosome, J Biochem 157 (4) (2015) 185–195. [DOI] [PubMed] [Google Scholar]
- [72].Paul BD, Signaling overlap between the Golgi stress response and cysteine metabolism in huntington’s disease, Antioxidants (Basel) 10 (9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]