Abstract
OBJECTIVE:
To examine the association of low fetal fraction of cell-free DNA (cfDNA) with placental compromise and adverse perinatal outcomes.
MATERIALS AND METHODS:
This was a retrospective cohort utilizing a sample of convenience including 639 women undergoing cfDNA screening at our institution from January 2013 to January 2017. Low fetal fraction was defined as less than the 25th percentile. Indicators of placental compromise were examined individually and as a composite outcome, including hypertensive disease of pregnancy, intrauterine growth restriction, abruption, and oligohydramnios. Neonatal outcomes, including preterm delivery, low Apgar scores, and small for gestational age, also were examined. We calculated risk ratios (RR) and 95% confidence intervals (CI).
RESULTS:
Low fetal fraction was associated with placental compromise (RR 1.6 [CI 1.1–2.2]), hypertensive disease of pregnancy (RR 1.6 [CI 1.003–2.6]), and preeclampsia with severe features (RR 3.3 [CI 1.2–8.9]). Low fetal faction was not associated with preterm delivery, low Apgar scores, or small for gestational age.
CONCLUSIONS:
Low fetal fraction of cfDNA among asymptomatic women may serve as a predictor of subsequent placental dysfunction and hypertensive disease.
Keywords: cell-free DNA, fetal fraction, placental dysfunction, hypertensive disease in pregnancy
INTRODUCTION
The villous trophoblast of the placenta undergoes continuous turnover throughout gestation. This physiologic process results in release of apoptotic debris and cell-free DNA (cfDNA) into the maternal circulation. These short genomic DNA fragments often are referred to as fetal, despite their placental origin (1–4). The growing pregnancy contributes approximately 3–13% of the total cfDNA in the maternal circulation, which is referred to as the fetal fraction (5,6). This genetic material increases with gestational age and may be affected by aneuploidy, maternal obesity, race, number of gestations, treatment with low molecular weight heparin, states of high cell turnover such as malignancy, and consanguinity (7–12).
Identification of cfDNA in the maternal serum in 1997 launched a scientific pursuit into its clinical significance and potential applications (13–17). This technology employs next generation sequencing to measure cfDNA in the maternal circulation and screens for common fetal aneuploidies with high sensitivity and specificity. Given limited evidence supporting the use of cfDNA screening for aneuploidy in low risk populations, professional organizations currently recommend that this modality be reserved for patients considered to be high risk, including those age 35 years or greater at the time of delivery (18–20). Thus, the majority of data correlating cfDNA measurements to pregnancy outcomes are limited to women who are at least 35 years old.
While cfDNA screening has emerged as a screening modality for common aneuploidies, research suggests that concentrations of cfDNA also may reflect placental pathology (21). The fetal fraction has been linked to adverse perinatal outcomes, though the directionality of this relationship remains unclear. With respect to hypertensive disease in pregnancy, a higher fetal fraction has been reported among patients with preeclampsia, postulating that oxidative stress and ischemia promote trophoblast turnover, while maternal endothelial damage results in reduced clearance of cfDNA from the maternal circulation (12,22–30). Nakib et al. demonstrated that increased fetal fraction was observed more commonly among patients with intrauterine growth restriction (IUGR) independent of preeclampsia (31). High fetal fraction also has been associated with spontaneous preterm delivery (32,33).
Others have refuted these observations (34–36), and recent data have linked low fetal fraction to hypertensive disorders, as well as other adverse perinatal outcomes including miscarriage, fetal demise, neonatal death, preterm birth, placental abruption, and low birth weight (37–39). The relationship between timing of cfDNA screening during pregnancy and onset of pregnancy-related complications remains unclear. The utility of fetal fraction among asymptomatic women is also of uncertain significance.
This objective of this study was to determine the association between low fetal fraction of cfDNA and adverse perinatal outcomes among asymptomatic women undergoing routine cfDNA aneuploidy screening, with the specific aim of assessing the ability of fetal fraction to serve as a marker of placental compromise.
MATERIALS AND METHODS
Study design and data collection
We conducted a retrospective cohort study of all women undergoing cfDNA screening for aneuploidy at our institution from January 2013 to January 2017. Routine aneuploidy screening was offered to pregnant women considered to be at high risk of aneuploidy, as defined by multiple professional organizations, including age 35 or greater at the time of delivery (18–20). Women were eligible if they were at least 18 years of age; had a singleton gestation; were screened in the first or second trimester; and delivered at our institution. We excluded women with failed cfDNA screening or cfDNA results indicating elevated risk for aneuploidy, as well as women for whom cfDNA screening results or demographic data were unavailable. Women were ineligible if they had pre-gestational hypertension, abnormal ultrasound findings suggestive of aneuploidy or other genetic syndrome, or cfDNA screening indicated by abnormal first- or second-trimester serum screening (Fig. 1). Our institution uses two platforms for cfDNA—Harmony® (Pleasanton, CA) and Panorama® (San Carlos, CA). The test offered was specific to the clinical site at which the screening was performed. We abstracted maternal demographic characteristics, fetal fraction, obstetric outcomes, and neonatal outcomes from the medical record.
Figure 1:
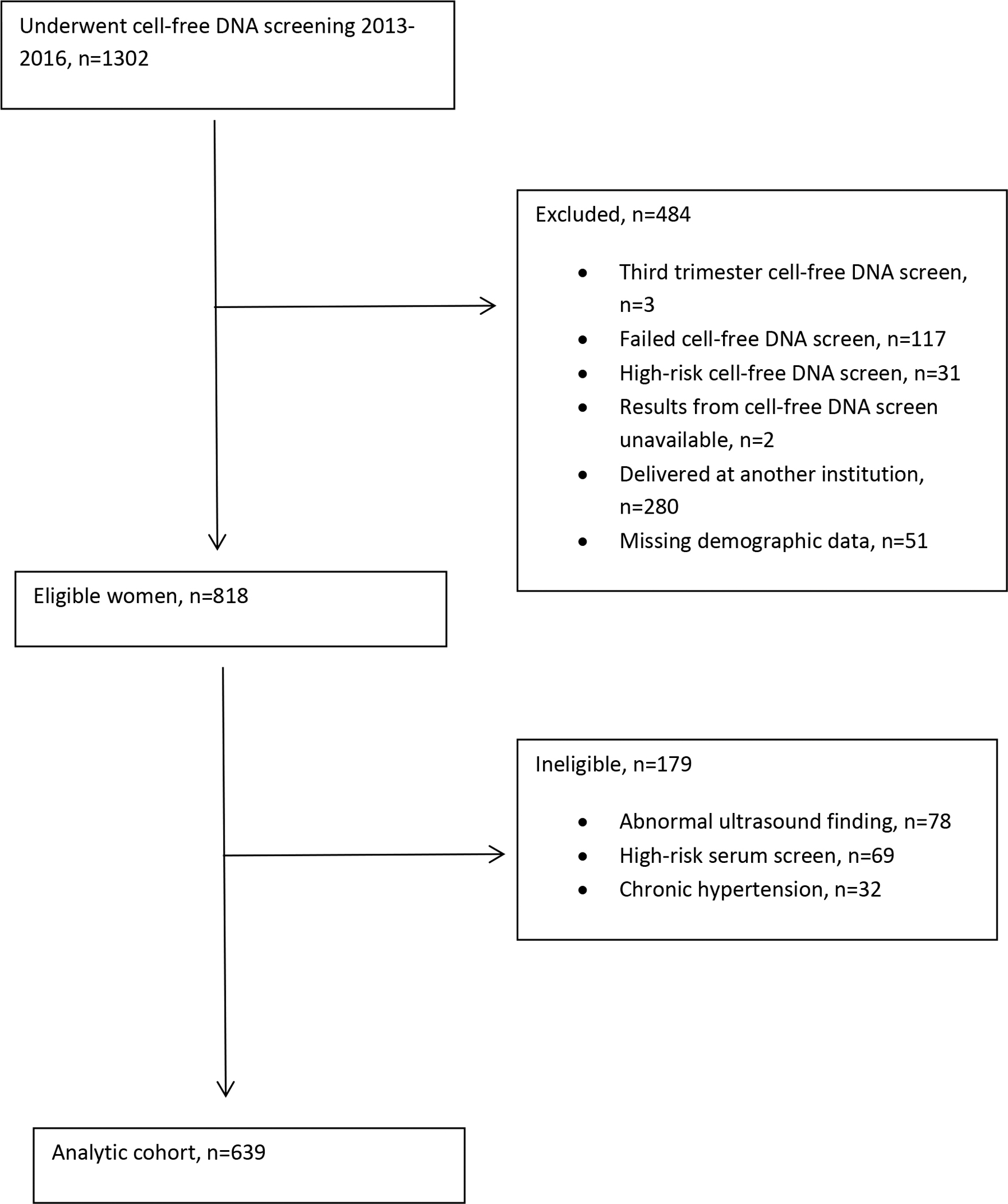
Flow chart defining cohort
The primary outcome was a composite outcome of placental compromise, defined as hypertensive disease of pregnancy, IUGR, abruption, or oligohydramnios. Hypertensive disease of pregnancy included gestational hypertension, preeclampsia, preeclampsia with severe features, and hemolysis, elevated liver enzymes and low platelets (HELLP). Hypertensive diseases of pregnancy were classified based on the 2013 American College of Obstetrician and Gynecologists consensus guidelines and recommendations from the International Society for the Study of Hypertension in Pregnancy (40,41). Gestational hypertension was defined as systolic blood pressure greater than 140 mmHg or diastolic blood pressure greater than 90 mmHg on two occasions at least four hours apart after 20 weeks of gestation without evidence of preeclampsia. Preeclampsia was defined as the aforementioned blood pressure criteria plus proteinuria greater than or equal to 300 mg per 24-hour urine collection or protein/creatinine ratio greater than or equal to 0.3. In the absence of proteinuria, a diagnosis of preeclampsia was made when blood pressure criteria were met with evidence of severe features, including systolic blood pressure of 160 mmHg or higher or diastolic blood pressure of 110 mmHg or higher on two occasions at least four hours apart, thrombocytopenia less than 100,000/μL, impaired liver function (liver enzymes twice normal), severe persistent right upper quadrant or epigastric pain, renal insufficiency (serum creatinine greater than 1.1 mg/dL or doubling of baseline in absence of underlying renal disease), pulmonary edema, or new-onset cerebral or visual disturbances. Participants with both early onset (less than 34 weeks of gestation) and late onset (34 weeks of gestation or later) preeclampsia were included in this study. IUGR was defined as estimated fetal weight of less than the 10th percentile for gestational age. Abruption was defined clinically as vaginal bleeding in pregnancy not attributed to placenta previa, cervical change, or conditions of the lower genital tract. Oligohydramnios was defined as maximum vertical pocket less than two cm on transabdominal ultrasound.
Secondary outcomes included birth weight, small for gestational age (birth weight less than the 10th percentile for gestational age), preterm birth (all pregnancies delivered prior to 37 weeks 0 days of gestation), poor Apgar scores defined as less than five at one minute or less than seven at five minutes, neonatal intensive care unit admission, and morbidity of prematurity (including bronchopulmonary dysplasia, retinopathy of prematurity, intraventricular hemorrhage, and respiratory distress syndrome).
Statistical analysis
Data were stored in REDCap (42) and analyzed using SAS 9.4 (SAS Institute. Cary, NC). Fetal fraction below the 25th percentile was considered low, based on the distribution within the cohort and consideration of clinical relevance. We compared categorical data using chi-square or Fisher’s exact tests and continuous data using a t-test. We calculated risk ratios (RR) and 95% confidence intervals (CI) using Poisson regression with robust error variances (43). We considered both maternal age at time of blood draw and body mass index (BMI) as potential confounders, though only BMI appreciably altered the effect estimates and was retained in the final models. After initial analyses, we dichotomized fetal fraction at the 75th percentile to examine outcomes among women in the top quartile using the aforementioned statistical approaches.
RESULTS
A total of 639 women were eligible for the cohort. The cut-off for low fetal fraction after dichotomization at the 25th percentile was 8.4. Women with both low and normal fetal fraction were approximately 37 years old, and the timing of screening was similar between groups at 12 to 13 weeks of gestation (Table 1). The distributions of race and Hispanic ethnicity were similar between women with normal and low fetal fraction, with non-Hispanic white women representing the majority in both groups. The prevalence of medical morbidities also was comparable between groups. Mean BMI was 29 kg/m2 among women with low fetal fraction compared to 25 kg/m2 among those with normal fetal fraction. Demographic characteristics are reported in Table 1.
Table 1:
Demographic and clinical characteristics
| Low Fetal Fraction n=157 |
Normal Fetal Fraction n=482 |
|
|---|---|---|
|
| ||
| Maternal age | 37 ± 3 | 37 ± 3 |
| Gestational age at blood draw (weeks) | 13 ± 2 | 12 ± 2 |
| Body mass index (kg/m2) | 29 ± 7 | 25 ± 4 |
| Race | ||
| Non-Hispanic white | 98 (62) | 317 (66) |
| Non-Hispanic black | 22 (14) | 44 (9) |
| Asian | 19 (12) | 83 (17) |
| Other | 18 (11) | 38 (8) |
| Hispanic | 18 (11) | 52 (11) |
| Nulliparous | 69 (44) | 169 (35) |
| Current tobacco use | 4 (3) | 7 (1) |
| Diabetes mellitus (preexisting or gestational) | 22 (14) | 31 (6) |
| Renal disease | 1 (1) | 3 (1) |
| Preeclampsia in a prior pregnancy | 10 (6) | 13 (3) |
| Type of cell-free DNA test | ||
| Harmony | 10 (6) | 46 (10) |
| Panorama | 147 (94) | 436 (90) |
Data are presented as mean ± standard deviation and n (%)
Gestational age at the time of delivery was approximately 39 weeks in both groups. Mode of delivery was not significantly different; the cesarean delivery rate was 41% for women with low fetal fraction and 33% for women with normal fetal fraction. The incidence of placental compromise was significantly higher among women with low fetal fraction (29%) compared to women with normal fetal fraction (17%; p<0.001). Hypertensive disease in pregnancy also occurred more frequently among women with low fetal fraction (20%) than normal fetal fraction (10%; p<0.001). Other indicators of placental compromise were not significantly different between groups. Obstetric outcomes are shown in Table 2.
Table 2:
Obstetric outcomes stratified by fetal fraction
| Low Fetal Fraction n=157 |
Normal Fetal Fraction n=482 |
p | |
|---|---|---|---|
|
| |||
| Gestational age at delivery (weeks) | 39 ± 2 | 39 ± 2 | 0.30 |
| Mode of delivery | 0.19 | ||
| Spontaneous vaginal | 89 (57) | 311 (65) | |
| Operative vaginal | 4 (3) | 13 (3) | |
| Cesarean | 64 (41) | 158 (33) | |
| Composite outcome for placental compromise | 45 (29) | 80 (17) | <0.001 |
| Hypertensive disease of pregnancy | 32 (20) | 48 (10) | <0.001 |
| Gestational hypertension | 12 (8) | 20 (4) | 0.08 |
| Preeclampsia | 11 (7) | 14 (3) | 0.02 |
| Preeclampsia with severe features | 9 (6) | 11 (2) | 0.06 |
| HELLP syndrome | 0 (0) | 3 (1) | 1.0 |
| Intrauterine growth restriction | 4 (3) | 7 (1.5) | 0.48 |
| Abruption | 3 (2) | 18 (4) | 0.27 |
| Oligohydramnios | 10 (6) | 15 (3) | 0.07 |
| Placenta accreta | 1 (1) | 6 (1) | 1.0 |
| Postpartum hemorrhage | 10 (6) | 25 (5) | 0.57 |
| Transfusion | 2 (1) | 9 (2) | 1.0 |
| Abnormal placental pathology | 31 (20) | 83 (17) | 0.47 |
The mean birth weight and incidence of small for gestational age neonates were similar between women with low and normal fetal fraction (both p≥0.29). Preterm birth was not associated with fetal fraction and occurred in approximately 9 to 10% of pregnancies in both groups. Women in the low fetal fraction group were more likely to have a neonate with an Apgar score less than seven at five minutes (p=0.004). Neonatal intensive care unit (NICU) admission occurred in 14% of neonates born to mothers with low fetal fraction compared to 11% of neonates born to mothers with normal fetal fraction (p=0.24). There did not appear to be a difference in morbidity of prematurity across groups. Neonatal outcomes are shown in Table 3.
Table 3:
Neonatal outcomes stratified by fetal fraction
| Low Fetal Fraction n=157 |
Normal Fetal Fraction n=482 |
p | |
|---|---|---|---|
|
| |||
| Birth weight | 3334 ± 628 | 3300 ± 566 | 0.53 |
| Small for gestational age | 15 (10) | 61 (13) | 0.29 |
| Preterm birth | 15 (10) | 45 (9) | 0.94 |
| Spontaneous | 9 (60) | 26 (58) | 0.88 |
| Medically indicated | 6 (40) | 19 (42) | |
| Poor Apgar | 8 (5) | 11 (2) | 0.10 |
| Apgar <5 at one minute | 7 (4) | 11 (2) | 0.17 |
| Apgar <7 at five minutes | 5 (3) | 1 (0) | 0.004 |
| Neonatal intensive care unit admission | 22 (14) | 51 (11) | 0.24 |
| Morbidity of prematurity | |||
| Bronchopulmonary dysplasia | 0 (0) | 0 (0) | - |
| Retinopathy of prematurity | 0 (0) | 4 (1) | 0.58 |
| Intraventricular hemorrhage | 3 (2) | 2 (0) | 0.10 |
| Respiratory distress syndrome | 6 (4) | 14 (3) | 0.60 |
After adjusting for BMI, women in the low fetal fraction group were significantly more likely to develop placental compromise (RR: 1.6; 95% CI: 1.1–2.2) than women with normal fetal fraction. The association also was significant for hypertensive diseases collectively (Table 4). Among individual components, low fetal fraction was associated with development of preeclampsia with severe features (BMI-adjusted RR: 3.3; 95% CI: 1.2, 8.8). Although the risk of developing oligohydramnios and IUGR also appeared to be greater among women with low fetal fraction, these findings were not statistically significant. The crude and BMI-adjusted risks of adverse perinatal outcomes are reported in Table 4.
Table 4:
Risk of adverse perinatal outcomes comparing women with low fetal fraction to those with normal fetal fraction
| Crude Risk Ratio (95% Confidence Interval) |
Adjusted* Risk Ratio (95% Confidence Interval) |
|||
|---|---|---|---|---|
|
| ||||
| Low Fetal Fraction |
Normal Fetal Fraction |
Low Fetal Fraction |
Normal Fetal Fraction |
|
|
| ||||
| Placental compromise | 1.7 (1.3, 2.4) | Ref | 1.6 (1.1, 2.2) | Ref |
| Hypertensive disease of pregnancy | 2.3 (1.6, 3.3) | Ref | 1.6 (1.003, 2.6) | Ref |
| Gestational hypertension | 2.0 (0.996, 4.0) | Ref | 0.97 (0.44, 2.1) | Ref |
| Preeclampsia | 2.6 (1.2, 5.4) | Ref | 2.3 (0.99, 5.5) | Ref |
| Preeclampsia with severe features | 2.7 (1.1, 6.4) | Ref | 3.3 (1.2, 8.9) | Ref |
| Intrauterine growth restriction | 1.8 (0.52, 5.9) | Ref | 2.9 (0.78, 10.7) | Ref |
| Abruption | 0.51 (0.15, 1.7) | Ref | 0.75 (0.22, 2.5) | Ref |
| Oligohydramnios | 2.0 (0.94, 4.5) | Ref | 1.9 (0.80, 4.0) | Ref |
| Small for gestational age | 0.75 (0.44, 1.3) | Ref | 0.82 (0.44, 1.5) | Ref |
| Preterm birth | 1.02 (0.57, 1.8) | Ref | 0.93 (0.52, 1.7) | Ref |
| Spontaneous | 1.06 (0.48, 2.3) | Ref | 0.80 (0.38, 1.7) | Ref |
| Medically indicated | 0.97 (0.40, 2.4) | Ref | 1.1 (0.43, 3.0) | Ref |
| Poor Apgar score | 2.2 (0.92, 5.4) | Ref | 1.6 (0.73, 3.7) | Ref |
| Neonatal intensive care unit admission | 1.3 (0.83, 2.1) | Ref | 1.1 (0.65, 1.8) | Ref |
| Respiratory distress syndrome | 1.3 (0.51, 3.4) | Ref | 0.997 (0.37, 2.7) | Ref |
Adjusted for body mass index
After initial analyses, we considered the possibility that risk of complications may be inherent to both extremes (i.e. low or high fetal fraction), and that values approaching the median may be protective. To test this possibility, we performed similar analyses after dichotomizing fetal fraction at the 75th percentile to examine outcomes among women in the top quartile; however, we did not detect elevated risk for our outcomes of interest (data not shown).
DISCUSSION
This study demonstrates that low fetal fraction of cfDNA among asymptomatic women undergoing routine aneuploidy screening in the first and second trimesters may function as a marker of subsequent placental compromise and hypertensive disease of pregnancy. Our data comprise the largest reported cohort of asymptomatic women linking low fetal fraction to clinical markers of placental dysfunction. Prior reports have grouped various perinatal complications, including miscarriage, hypertension, fetal demise, and preterm birth into a single composite outcome, raising questions as to the plausibility of a common underlying mechanism given their diverse biologic etiologies (37,44).
While our findings corroborate smaller studies in the literature (37–39), they contradict early data implicating high fetal fraction in adverse perinatal outcomes (22–29,31). Discrepancies between early reports and our findings may reflect limitations of the screening test, which captures an isolated picture in time; meanwhile, the placenta remains a dynamic organ throughout pregnancy, and fetal fraction may fluctuate with the development of complications. Placental compromise may manifest well after inciting events that involve insufficient invasion of cytotrophoblasts into maternal spiral arterioles in early pregnancy. When examined in the first and second trimesters among asymptomatic women, fetal fraction may be low due to shallow placentation with relatively poor access to maternal blood supply. As placental or hypertensive disease progresses, endothelial damage occurs and placental debris may gain entry more readily into the maternal circulation and accumulate peripherally.
This notion suggests that morbidly invasive placentation may result in higher fetal fraction; however, pilot studies have not identified a link between patients with placenta accreta and high fetal faction, rendering this theory less plausible (45). Such studies were performed in the third trimester and significantly limited by sample size. Others have explored the relationship between placental function and fetal fraction, proposing that placental insufficiency results in diminished placental volume with less extravasation of placental debris into the maternal circulation. This concept has since been refuted by observations that fetal fraction is independent of placental size (44,46).
While the preterm birth rate in this cohort was consistent with national trends in the United States, our data did not substantiate findings from other studies linking either low or high fetal fraction to increased incidence of preterm delivery (32,33,37,44). One explanation for this difference is that we excluded women at potentially higher risk of this clinical outcome, including those with chronic hypertension, abnormal serum screening or ultrasound findings, and high risk cfDNA results, in contrast to previously published literature. Moreover, previous studies are limited by the fact that preterm birth is heterogeneous, resulting from a wide range of maternal, genetic, and infectious causes.
Strengths of this study include restriction to asymptomatic women, the ability to adjust for potential confounders, such as BMI, and exclusion of participants with pre-existing hypertension given their increased risk of developing the primary outcome of interest. While our study had a relatively large sample size compared with existing literature, we did not have sufficient power to detect potentially meaningful differences in various uncommon outcomes, such as HELLP syndrome or specific morbidities of prematurity. While the directionality of the relationship between fetal fraction and some individual outcomes of interest was congruent with that of the composite outcome (i.e. abruption and oligohydramnios), these associations were not statistically significant likely due to sample size. In addition, our cohort of asymptomatic women was comprised of women of advanced maternal age who are at increased risk of many adverse perinatal outcomes; this is the result of screening practices for aneuploidy, which are tightly linked to maternal age. Nonetheless, we anticipate that our findings may be generalizable to younger women given that the utility of cfDNA screening for aneuploidy in average risk (younger) women was recently shown comparable to its previously demonstrated performance in high risk (older) women (47). A final limitation of this study was our inability to capture serial measurements of cfDNA, as this is not the standard of clinical care.
CONCLUSIONS
In summary, low fetal fraction of cfDNA among asymptomatic women in early to mid pregnancy may serve as a biomarker for subsequent placental compromise and hypertensive disease. The clinical implications of our findings require further investigation. It is possible that cfDNA screening may offer greater diagnostic value if combined with traditional serum analogues, such as PAPP-A, to define individual risk. From a biologic standpoint, additional research should focus on the trajectory of fetal fraction over time among women who ultimately develop placental dysfunction and hypertensive disease. Future studies also should evaluate these observations in younger women and in larger prospective cohorts that are powered to detect potential differences in neonatal outcomes.
HIGHLIGHTS.
Low fetal fraction of cfDNA may serve as a biomarker of placental compromise in pregnancy
Low fetal fraction of cfDNA is associated with increased risk of developing hypertensive disease in pregnancy
Larger studies are required to determine association of fetal fraction of cfDNA with neonatal outcomes
Fetal fraction of cfDNA may offer greater diagnostic value if combined with traditional serum analogues to define individual risk
FUNDING SUPPORT:
This work was supported by Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Footnotes
DECLARATION OF INTEREST: None.
Contributor Information
Kristin D. Gerson, Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Boston, MA; Department Obstetrics, Gynecology, and Reproductive Biology, Harvard Medical School, Boston, MA; Maternal and Child Health Research Center, Department of Obstetrics and Gynecology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Samantha Truong, Harvard Medical School, Boston, MA.
Miriam J. Haviland, Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Boston, MA; Department Obstetrics, Gynecology, and Reproductive Biology, Harvard Medical School, Boston, MA.
Barbara M. O’Brien, Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Boston, MA; Department Obstetrics, Gynecology, and Reproductive Biology, Harvard Medical School, Boston, MA.
Michele R. Hacker, Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Boston, MA; Department Obstetrics, Gynecology, and Reproductive Biology, Harvard Medical School, Boston, MA.
Melissa H. Spiel, Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Boston, MA; Department Obstetrics, Gynecology, and Reproductive Biology, Harvard Medical School, Boston, MA; 330 Brookline Avenue, Beth Israel Deaconess Medical Center, East Campus, Kirstein 3, Boston, MA 02215.
REFERENCES
- 1.Lui YYN, Chik KW, Chiu RWK, Ho CY, Lam CWK, Lo YMD. Predominant hematopoietic origin of cell-free dna in plasma and serum after sex-mismatched bone marrow transplantation. Clin Chem. 2002;48(3):421–7. [PubMed] [Google Scholar]
- 2.Alberry M, Maddocks D, Jones M, Abdel Hadi M, Abdel-Fattah S, Avent N, et al. Free fetal DNA in maternal plasma in anembryonic pregnancies: Confirmation that the origin is the trophoblast. Prenat Diagn. 2007;27(5):415–8. [DOI] [PubMed] [Google Scholar]
- 3.Chan KCA, Zhang J, Hui ABY, Wong N, Lau TK, Leung TN, et al. Size Distributions of Maternal and Fetal DNA in Maternal Plasma. Clin Chem. 2004;50(1):88–92. [DOI] [PubMed] [Google Scholar]
- 4.Fan H, Blumenfeld Y, Chitkara U, Hudgins L, Quake S. Analysis of the size distributions of fetal and maternal cell-free DNA by paired-end sequencing. Clin Chem. 2010;56(8):1279–86. [DOI] [PubMed] [Google Scholar]
- 5.Ashoor G, Syngelaki A, Poon LCY, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11–13 weeks’ gestation: Relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41(1):26–32. [DOI] [PubMed] [Google Scholar]
- 6.ACOG. Cell- free DNA Screening for Fetal Aneuploidy. Obs Gynecol. 2015;126(3):e31–37. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Zhu Z, Gao Y, Yuan Y, Guo Y, Zhou L, et al. Effects of Maternal and Fetal Characteristics on Cell-Free Fetal DNA Fraction in Maternal Plasma. Reprod Sci. 2015;22(11):1429–35. [DOI] [PubMed] [Google Scholar]
- 8.Grömminger S, Erkan S, Schöck U, Stangier K, Bonnet J, Schloo R, et al. The influence of low molecular weight heparin medication on plasma DNA in pregnant women. Vol. 35, Prenatal Diagnosis. 2015. p. 1155–7. [DOI] [PubMed] [Google Scholar]
- 9.Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, et al. Cell-free DNA Analysis for Noninvasive Examination of Trisomy. N Engl J Med [Internet]. 2015;372(17):1589–97. Available from: 10.1056/NEJMoa1407349 [DOI] [PubMed] [Google Scholar]
- 10.Livergood MC, LeChien KA, Trudell AS. Obesity and cell-free DNA “no calls”: is there an optimal gestational age at time of sampling? In: American Journal of Obstetrics and Gynecology. 2017. p. 413.e1–413.e9. [DOI] [PubMed] [Google Scholar]
- 11.Burns W, Koelper N, Barberio A, DeAgostino-Kelly M, Mennuti M, Sammel MD, et al. The association between anticoagulation therapy, maternal characteristics, and a failed cfDNA test due to a low fetal fraction. Prenat Diagn [Internet]. 2017; Available from: 10.1002/pd.5152 http://www.ncbi.nlm.nih.gov/pubmed/28881030 [DOI] [PubMed]
- 12.Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet [Internet]. 1999;64(1):218–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9915961%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1377720%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1377720&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzenberg L a Bianchi DW, Schröder J Cann HM, Iverson GM. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1979;76(3):1453–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo YM, Patel P, Wainscoat JS, Sampietro M, Gillmer MD, Fleming KA. Prenatal sex determination by DNA amplification from maternal peripheral blood. Lancet. 1989;2(8676):1363–5. [DOI] [PubMed] [Google Scholar]
- 15.Simpson JL, Elias S. Isolating fetal cells in maternal circulation for prenatal diagnosis. Prenat Diagn. 1994;14(13):1229–42. [DOI] [PubMed] [Google Scholar]
- 16.Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood [Internet]. 1996;88(11):4390–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8943877 [PubMed] [Google Scholar]
- 17.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet [Internet]. 1997;350(9076):485–7. Available from: http://www.thelancet.com/journals/a/article/PIIS0140-6736(97)02174-0/fulltext%5Cnhttp://www.thelancet.com/journals/lancet/article/PIIS0140-6736(97)02174-0/abstract [DOI] [PubMed] [Google Scholar]
- 18.Benn P, Borrell A, Crossley J, Cuckle H, Dugoff L, Gross S, et al. Aneuploidy screening: A position statement from a committee on behalf of the Board of the International Society for Prenatal Diagnosis, January 2011. Prenat Diagn. 2011;31(6):519–22. [DOI] [PubMed] [Google Scholar]
- 19.Committee opinion no. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstetrics and Gynecology. 2012. [DOI] [PubMed] [Google Scholar]
- 20.Wilson KL, Czerwinski JL, Hoskovec JM, Noblin SJ, Sullivan CM, Harbison A, et al. NSGC practice guideline: Prenatal screening and diagnostic testing options for chromosome aneuploidy. In: Journal of Genetic Counseling. 2013. [DOI] [PubMed] [Google Scholar]
- 21.Taglauer ES, Wilkins-Haug L, Bianchi DW. Review: Cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Vol. 35, Placenta. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn S, Rusterholz C, Hösli I, Lapaire O. Cell-free nucleic acids as potential markers for preeclampsia. Placenta. 2011; [DOI] [PubMed] [Google Scholar]
- 23.Knight M, Redman CWG, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. BJOG An Int J Obstet Gynaecol. 1998; [DOI] [PubMed] [Google Scholar]
- 24.Smid M, Vassallo A, Lagona F, Valsecchi L, Maniscalco L, Danti L, et al. Quantitative analysis of fetal DNA in maternal plasma in pathological conditions associated with placental abnormalities. Ann N Y Acad Sci. 2001; [DOI] [PubMed] [Google Scholar]
- 25.Zhong XY, Laivuori H, Livingston JC, Ylikorkala O, Sibai BM, Holzgreve W, et al. Elevation of both maternal and fetal extracellular circulating deoxyribonucleic acid concentrations in the plasma of pregnant women with preeclampsia. Am J Obstet Gynecol. 2001; [DOI] [PubMed] [Google Scholar]
- 26.Farina A, Sekizawa A, Rizzo N, Concu M, Banzola I, Carinci P, et al. Cell-free fetal DNA (SRY locus) concentration in maternal plasma is directly correlated to the time elapsed from the onset of preeclampsia to the collection of blood. Prenat Diagn. 2004; [DOI] [PubMed] [Google Scholar]
- 27.Alberry MS, Maddocks DG, Hadi MA, Metawi H, Hunt LP, Abdel-Fattah SA, et al. Quantification of cell free fetal DNA in maternal plasma in normal pregnancies and in pregnancies with placental dysfunction. Am J Obstet Gynecol. 2009; [DOI] [PubMed] [Google Scholar]
- 28.Miranda ML, Macher HC, Muñoz-Hernández R, Vallejo-Vaz A, Moreno-Luna R, Villar J, et al. Role of circulating cell-free DNA levels in patients with severe preeclampsia and HELLP syndrome. Am J Hypertens. 2013; [DOI] [PubMed] [Google Scholar]
- 29.Zeybek YG, Gunel T, Benian A, Aydinli K, Kaleli S. Clinical evaluations of cell-free fetal DNA quantities in pre-eclamptic pregnancies. J Obs Gynaecol Res. 2013; [DOI] [PubMed] [Google Scholar]
- 30.Lazar L, Rigó J, Nagy B, Balogh K, Makó V, Cervenak L, et al. Relationship of circulating cell-free DNA levels to cell-free fetal DNA levels, clinical characteristics and laboratory parameters in preeclampsia. BMC Med Genet. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al Nakib M, Desbriere R, Bonello N, Bretelle F, Boubli L, Gabert J, et al. Total and fetal cell-free DNA analysis in maternal blood as markers of placental insufficiency in intrauterine growth restriction. Fetal Diagn Ther. 2009; [DOI] [PubMed] [Google Scholar]
- 32.Farina A, LeShane ES, Romero R, Gomez R, Chaiworapongsa T, Rizzo N, et al. High levels of fetal cell-free DNA in maternal serum: A risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2005; [DOI] [PubMed] [Google Scholar]
- 33.Dugoff L, Barberio A, Whittaker PG, Schwartz N, Sehdev H, Bastek JA. Cell-free DNA fetal fraction and preterm birth. Am J Obstet Gynecol. 2016; [DOI] [PubMed] [Google Scholar]
- 34.Rolnik DL, O’Gorman N, Fiolna M, Van Den Boom D, Nicolaides KH, Poon LC. Maternal plasma cell-free DNA in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2015; [DOI] [PubMed] [Google Scholar]
- 35.Quezada MS, Francisco C, Dumitrascu-Biris D, Nicolaides KH, Poon LC. Fetal fraction of cell-free DNA in maternal plasma in the prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2015; [DOI] [PubMed] [Google Scholar]
- 36.Bender W, Koelper N, Sammel M, Dugoff L. Association of Fetal Fraction of Cell-Free DNA and Hypertensive Disorders of Pregnancy. Am J Perinatol. 2018; [DOI] [PubMed] [Google Scholar]
- 37.Krishna I, Badell M, Loucks TL, Lindsay M, Samuel A. Adverse perinatal outcomes are more frequent in pregnancies with a low fetal fraction result on noninvasive prenatal testing. Prenat Diagn. 2016; [DOI] [PubMed] [Google Scholar]
- 38.Rolnik DL, da Silva Costa F, Lee TJ, Schmid M, McLennan AC. Association between fetal fraction on cell-free DNA testing and first trimester markers for pre-eclampsia. Ultrasound Obstet Gynecol. 2018; [DOI] [PubMed] [Google Scholar]
- 39.Suzumori N, Sekizawa A, Ebara T, Samura O, Sasaki A, Akaishi R, et al. Fetal cell-free DNA fraction in maternal plasma for the prediction of hypertensive disorders of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2018; [DOI] [PubMed] [Google Scholar]
- 40.C A. ACOG Guidelines: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. 2013. [DOI] [PubMed] [Google Scholar]
- 41.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018. [DOI] [PubMed] [Google Scholar]
- 42.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou G A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004; [DOI] [PubMed] [Google Scholar]
- 44.Manokhina I, Singh TK, Robinson WP. Cell-Free Placental DNA in Maternal Plasma in Relation to Placental Health and Function. Fetal Diagn Ther. 2017; [DOI] [PubMed] [Google Scholar]
- 45.Samuel A, Bonanno C, Oliphant A, Batey A, Wright JD. Fraction of cell-free fetal DNA in the maternal serum as a predictor of abnormal placental invasion-a pilot study. Prenat Diagn. 2013; [DOI] [PubMed] [Google Scholar]
- 46.Wataganara T, Metzenbauer M, Peter I, Johnson KL, Bianchi DW. Placental volume, as measured by 3-dimensional sonography and levels of maternal plasma cell-free fetal DNA. Am J Obstet Gynecol. 2005; [DOI] [PubMed] [Google Scholar]
- 47.Bianchi DW, Lamar Parker R, Wentworth J, Madankumar R, Saffer C, Das AF, et al. DNA sequencing versus standard prenatal aneuploidy screening. Obstetrical and Gynecological Survey. 2014. [DOI] [PubMed] [Google Scholar]


