Abstract
With mounting preclinical and clinical evidences on the prominent roles of the tumor microenvironment (TME) played during carcinogenesis, the TME has been recognized and used as an important onco‐therapeutic target during the past decade. Delineating our current knowledge on TME components and their functionalities can help us recognize novel onco‐therapeutic opportunities and establish treatment modalities towards desirable anti‐cancer outcome. By identifying and focusing on primary cellular components in the TME, that is, tumor‐infiltrating lymphocytes, tumor‐associated macrophages, cancer‐associated fibroblasts and mesenchymal stem cells, we decomposed their primary functionalities during carcinogenesis, categorized current therapeutic approaches utilizing traits of these components, and forecasted possible benefits that cold atmospheric plasma, a redox modulating tool with selectivity against cancer cells, may convey by targeting the TME. Our insights may open a novel therapeutic avenue for cancer control taking advantages of redox homeostasis and immunostasis.
Keywords: cancer‐associated fibroblast, cold atmospheric plasma, mesenchymal stem cell, tumor microenvironment, tumor‐associated macrophage, tumor‐infiltrating lymphocyte
CAP can possibly enhance tumor antigen secretion and enhance CD8+ TIL cytotoxicity, potentially repolarize TAMs from the M2 to the M1 state, modulate p53‐driven CAF hierarchy towards enhanced drug sensitivity, block MSCs differentiation to CAFs that is associated with reduced cancer stemness, and function as the cargo of MSCs or their derived exosomes for enhanced delivery to the tumor loci.
1. INTRODUCTION
Tumorigenesis is a complicated process not only involving genetic and epigenetic alterations of tumor cells, but also their surrounding non‐malignant cells, interactions between transformed and non‐transformed cells, as well as communications among these cellular components through the secretion of extracellular molecules. With our incremental knowledge on cancer initiation and progression, the roles of non‐transformed cells in nourishing cancer cells and cancer stemness have been recognized. The term “tumor microenvironment” (TME) has thus emerged to describe these cells and the buffering environment they foster. 1 The TME is known to facilitate uncontrolled proliferation, 2 accelerate tumor angiogenesis, 3 develop cancer invasion/metastasis, 4 promote cancer‐associated inflammation, 5 help cancer cells escape immune surveillance, 6 and contribute to metabolic reprogramming. 7 With these demonstrated impacts on cancer hallmarks, 8 the TME has been considered as the driving force and therapeutic avenue for conquering many clinical challenges such as cancer relapse and drug resistance. 9 , 10
Through categorizing the primary TME components and their associated onco‐therapeutic targeting modalities, we identify major TME‐modulating mechanisms that existing anti‐cancer strategies used and, accordingly, propose possible opportunities that cold atmospheric plasma (CAP) may have in the battle against cancers as an emerging TME editing tool.
2. PRIMARY CELLS IN TME AND THEIR ROLES IN CANCER
Primary TME components include immune cells such as tumor‐infiltrating lymphocytes (TILs) and tumor‐associated macrophages (TAMs), stromal cells such as cancer‐associated fibroblasts (CAFs) and mesenchymal stem cells (MSCs), and extracellular components such as cytokines, growth factors, hormones and extracellular matrix (ECM).
2.1. Immune cells in the TME
Immune cells residing in the TME include both players in the adaptive (i.e., T cells, B cells) and innate (e.g., natural killer [NK] cells, macrophages) immune responses. Here, we focus on TILs and TAMs that are dominant types of immune cells infiltrated to the TME.
2.1.1. TIL
Tumor‐infiltrating lymphocytes, composed of CD8+ T cells, CD4+ T cells, B lymphocytes and NK cells, are lymphocytes infiltrated to the TME from the blood. 11 CD8+ T cells, also known as cytotoxic T cells, are the main anti‐cancer immune cells. CD4+ T cells are represented by helper T cells type I (Th1), type II (Th2) and regulatory T (Treg) cells, where Th1 cells promote CD8+ T cell and NK cell proliferation by secreting IL2 and interferon, Th2 cells enhance the proliferation and maturation of B cells by releasing cytokines such as IL4 and IL6, and Treg cells suppress the cytotoxicity of CD8+ T and NK cells. 12 , 13 TILs can also be classified by disease specificity in the context of cancer immunity, where TILs recognizing non‐cancer peptides or being cancer ignorant are called “bystander TILs”. 14 There is emerging evidence that bystander TILs may represent dominant TILs in the TME. 15 , 16 , 17 Bystander TILs can also be sub‐grouped into “inactive bystander TILs”, “active bystander TILs”, and “false bystander TILs”, where inactive bystander TILs recognize tumor‐unrelated antigens and do not contribute to the anti‐cancer immunity, active bystander TILs recognize tumor‐unrelated antigens but are activated in response to concurrent infection or in a T‐cell receptor (TCR)‐independent manner, and false bystander TILs recognize both cancer‐specific and cancer‐unrelated targets such as viral or bacterial antigens due to the presence of dual TCRs or cross‐reactivity 14 (Figure 1). Thus, cancer‐specific TILs and false bystander TILs are truly functional entities in the TME contributing to the anti‐cancer immunity, with most types of TILs being tumor suppressive except for Treg.
FIGURE 1.
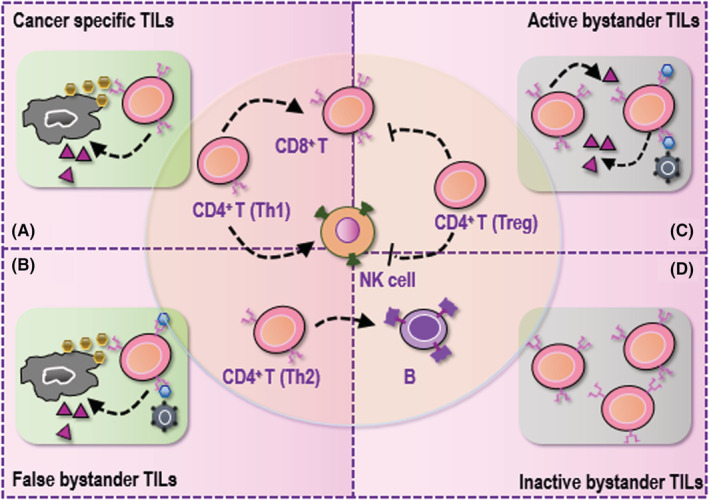
Types of tumor infiltrating lymphocytes and their primary roles in cancer. Primary tumor‐infiltrating lymphocytes (TILs) include CD8+ T cells, CD4+ T cells, B cells and natural killer (NK) cells, where CD4+ T cells are sub‐categorized into Th1, Th2 and Treg cells. CD8+ T cells are the primary TILs taking on the cytotoxicity function against cancer cells. Th1 cells and Treg cells take opposite roles, i.e., while Th1 cells activate CD8+ T and NK cells, Treg cells suppress them. Th2 cells activate B cells. These TILs can be categorized into four subclasses based on their contributions to anti‐cancer immunity. (A) Cancer‐specific T cells are activated in a T‐cell receptor (TCR)‐dependent way and kill tumor cells upon TCR binding of major histocompatibility complex‐presented antigens. (B) False bystander TILs recognize antigens from both cancer cells and cancer‐unrelated pathogens as they have dual TCRs and viral or bacterial antigens may also be present in tumor cells. (C) Active bystander TILs recognize tumor‐unrelated antigens in response to concurrent infection or in a TCR‐independent manner. (D) Inactive bystander TILs recognize tumor‐unrelated antigens. Both active and inactive bystander TILs do not contribute to the anti‐cancer immunity.
The differential roles of TILs in cancer have profound clinical implications. Sufficient tumor site infiltration of immune cells including, e.g., CD8+ cytotoxic T cells and CD4+ helper T cells, has been associated with inflamed TME that is characteristic of increased immune‐modulating chemokines, 18 where intratumoral CD8+ T cell dysfunction has been proposed as a therapeutic avenue for immune‐therapies. 19 Increased CD8+ cytotoxic T cells and suppressed Treg activity as triggered by curcumin was reported to be associated with halted head and neck cancer cell invasion. 20 On the contrary, decreased CD8+ T cell density coupled with elevated Treg TME infiltration resulted in impaired IFNγ release from TILs and consequently a suppressive T cell contexture and accelerated colorectal cancer progression. 21 Accordingly, low CD8+ T cell and high Treg density was suggested as an useful index prognostic of poor lung adenocarcinoma outcome, alone or coupled with other biomarkers. 22 In addition, overproducing Treg‐induced cytokines generated an immune‐suppressive TME in IKKα‐deficient lung adenocarcinomas, 23 decreasing the survival of Treg cells enhanced the anti‐tumor activity of TILs without disrupting the immune homeostasis, 24 and suppressing Treg differentiation and infiltration was proposed as a promising approach in breast cancer immunotherapy. 25
2.1.2. TAM
Tumor‐associated macrophages, derived from monocyte TME infiltration and macrophage differentiation, are the most abundant immune cells residing in the TME. Macrophages have two main states, that is, M1 and M2. While the M1 state is tumor suppressive by releasing pro‐inflammatory cytokines such as TNFα, IL1, IL12, and participating in Th1 cell responses, the M2 state is tumor promotive by expressing anti‐inflammatory cytokines such as TGFβ and IL10. 26 TAMs can be viewed as macrophages attracted at the M2 state that provide tumors with an immunosuppressive microenvironment by inhibiting T‐cell‐mediated anti‐tumor immunity.
Tumor‐associated macrophages can promote tumor progression by secreting factors such as chemokines, cytokines, proteases, and growth factors, 27 , 28 , 29 and establish an immune‐suppressive TME by interplaying with Tregs. 30 This has been demonstrated to involve many canonical cancer‐associated pathways and in varied types of tumors. Take studies in gastric cancers as an example, TAMs were shown capable of promoting cancer growth by activating the Wnt signaling, 27 promoting tumor angiogenesis by enhancing VEGF expression, 31 increasing cancer cell invasiveness by stimulating the NFκB pathway, 32 among the varied molecular mechanisms reported. The promotive role of TAMs has been well‐documented in other malignancies such as bladder 28 and lung 29 cancers for accelerated cancer cell growth, melanoma, 33 prostate 34 and lung 35 carcinomas for elevated tumor‐associated angiogenesis, and ovarian, 36 , 37 , 38 breast, 39 and lung 29 , 40 , 41 , 42 cancers for enhanced metastasis.
2.2. Stromal cells in the TME
Stromal cells in the TME are non‐transformed cells that develop crosstalk with tumor cells and participate in tumor progression. Here we focus on CAFs and MSCs, two primary forms of TME stromal cells responsible for therapeutic hurdles such as drug resistance and cancer stemness.
2.2.1. CAF
Cancer‐associated fibroblasts (CAFs), stromal cells with a mesenchymal fibroblast‐like phenotype, are originated from a variety of cells such as normal fibroblasts, CSCs, bone marrow‐derived cells, and epithelial cells undergoing the epithelial‐mesenchymal transition (EMT) process. 1 They represent the most abundant stromal cells in the TME that accounts for approximately 50% cells in a tumor tissue. 43 CAFs are inducted from their normal tissue‐resident fibroblasts or non‐fibroblastic mesenchymal elements by tumor cells via varied molecular mechanisms including, for example, direct contact between cancer cells and fibroblasts via Notch signaling, JAK–STAT signaling, inflammatory signaling as mediated via pro‐inflammatory cytokines (such as TNFα, IL1, IL6), TGFβ family ligands, RTK ligands such as FGF and PDGF, physical or chemical ECM alterations, DNA damages triggered by chemo‐ or radio‐therapies, stresses as imposed by metabolic or redox alterations, fibroblast stretching, epigenetic alterations such as histone acetylation, and SRF‐ or YAP1‐dependent transcriptional programs. 44 , 45 The diversified original and inductive modes of CAFs foster their heterogeneous nature, as exemplified by the existence of at least three CAP sub‐cohorts, that is, inflammatory CAFs (iCAFs), myofibroblastic CAFs (myCAFs) and antigen‐presenting CAFs (apCAFs). 46 , 47 , 48 Given the aforementioned complexity of CAF, the concept of stromagenesis emerges that refers to a dynamic pro‐tumorigenesis stromal ECM editing process comprised of varied bi‐directional stromal fibroblastic crosstalks through the secretion of a variety of cytokines and metabolites in, mostly, a paracrine manner. 49 Such a temporal–spatial heterogeneity of CAFs and the co‐evolvement of CAFs with tumor cells towards stromagenesis and tumorigenesis make CAF a critical contributor to cancer hallmarks and one possible determinant of many clinical challenges such as drug resistance, and thereby been considered as a critical roadblock in solid cancer therapy. 43 Accumulated evidence has suggested the roles of CAFs in developing solid tumor therapeutic resistance. For example, CAFs were intrinsically resistant to gemcitabine, a standard of care for pancreatic cancer patients, and capable of secreting exosomes accelerating such a chemo‐resistance on gemcitabine exposure. 50 A CD10+GPR77+ CAF cohort defined a chemo‐resistant lung cancer population due to persistent NFkB activation. 51 Suppressed CAF proliferation reduced the resistance of pancreatic ductal adenocarcinomas to oxidative stress and the growth of these tumor cells. 52
2.2.2. MSC
Mesenchymal stem cells are stromal cells capable of self‐renew and multi‐lineage differentiation. MSCs can differentiate into CAFs with compelling supportive evidences favoring their pro‐tumorigenic roles, among which maintaining cancer stemness through the secretion of a variety of regulatory factors is the most frequently reported. 53 , 54 Specifically, CSCs can recruit and activate cells including MSCs that, in turn, modify the stroma to establish a unique microenvironment favorable for CSC maintenance and transit cancer cells from the bulk tumor state to the CSC state through the establishment of a crosstalk with cancer cells. 55 For instance, TGFβ‐stimulated MSCs induced EMT and a CSC phenotype by activating Notch signaling in pancreatic cancers 56 and hepatocellular carcinomas 57 ; MSCs from the TME increased cancer stemness and the metastatic phenotype of prostate cancer cells through altering the CCL5‐androgen receptor pathway, 58 promoted the tumorigenic phenotype of glioma CSCs through activating IL6/STAT3 signaling, 59 increased the number of CSCs in ovarian tumor cells via altering bone morphogenetic protein signaling, 60 enhanced the stem‐like properties of gastric cancer cells by upregulating Tregs, 61 and polarized macrophages to the M2 phenotype in gastric cancers. 62
3. EXISTING ONCO‐THERAPIES TARGETING TME
3.1. Onco‐therapeutic strategies relying on immune cells in the TME
3.1.1. Targeting immune checkpoints towards restored immune surveillance
Immune checkpoints are signals capable of suppressing the immune response through regulating the antigen recognition of TCR. Cancer cells take advantages of immune checkpoints to reduce the efficacies of cytotoxic CD8+ T cells in the TME and thus evade the immune surveillance for uncontrolled cancer progression. Such an immune‐suppressive TME arrests many solid tumors in the “cold” state and imposes a great challenge to immune‐therapies in treating solid tumors.
Antibodies against programmed cell death 1 (PD1) and PD1 ligand (PD‐L1) have shown great promises in fighting against cancers and thus attracted much attention during recent years 61 , 63 , 64 (Figure 2). PD1 is a transmembrane protein expressed on T cell surface, and CD8+ T cells loose cytotoxicity when PD1 binds to PD‐L1 that is expressed on the surface of cancer cells. Antibodies of PD1 and PD‐L1 allow CD8+ T cells to kill cancer cells by blocking interactions between PD1 and PD‐L1. 65 Several onco‐therapeutics of this kind have been made commercially available. For instance, pembrolizumab (PD1 antibody) was shown effective in treating many types of malignancies such as triple negative breast cancers, 66 cervical cancers, 67 prostate cancers, 68 gastric cancers, 69 , 70 esophageal cancers, 71 gastroesophageal junction cancers, 70 bladder cancers, 72 pancreatic cancers, 73 non‐small lung cancers, 74 , 75 melanomas, 75 head and neck cancers, 76 endometrial cancers, 77 colorectal cancers, 78 urothelial cancers 79 ; and was approved by the USA Food and Drug Administration (FDA) for treating tumor mutational burden‐high solid tumors, 80 microsatellite instability‐high solid tumors, 81 advanced urothelial carcinomas ineligible for cisplatin‐containing chemotherapy, 82 recurrent or metastatic head and neck squamous cell carcinomas with disease progression on or after platinum‐containing chemotherapies, 83 recurrent locally advanced or metastatic merkel cell carcinomas, 84 recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinomas expressing PD‐L1, 85 cervical cancers expressing PD‐L1, 86 BCG‐unresponsive non‐muscle invasive bladder cancers, 87 metastatic non‐small cell lung cancers expressing PD‐L1 (as a first‐line therapy), 88 , 89 , 90 MSI‐H/dMMR advanced unresectable or metastatic colorectal carcinomas (as a first‐line therapy), 91 metastatic melanomas (as a second‐line therapy), 92 and locally recurrent unresectable or metastatic triple negative breast cancers through combined use with chemotherapies. 93 As an example of PD‐L1 antibodies, nivolumab was shown effective for treating recurrent squamous‐cell carcinomas of the head and neck, 94 , 95 advanced renal‐cell carcinomas, 96 metastatic melanomas, 97 advanced squamous‐cell non‐small cell lung cancers 98 ; and was approved by FDA in the treatment of relapsed or progressive classical Hodgkin lymphomas, 99 advanced renal cell carcinomas, 100 metastatic non‐small cell lung cancers with progression on or after platinum‐based chemotherapies, 101 bladder cancers, 102 BRAF(V600) wild‐type unresectable or metastatic melanomas (as a first‐line therapy), 103 advanced hepatocellular carcinomas, 104 , 105 and unresectable malignant pleural mesotheliomas when combined with lpilimumab (antibody of CTLA4). 106
FIGURE 2.
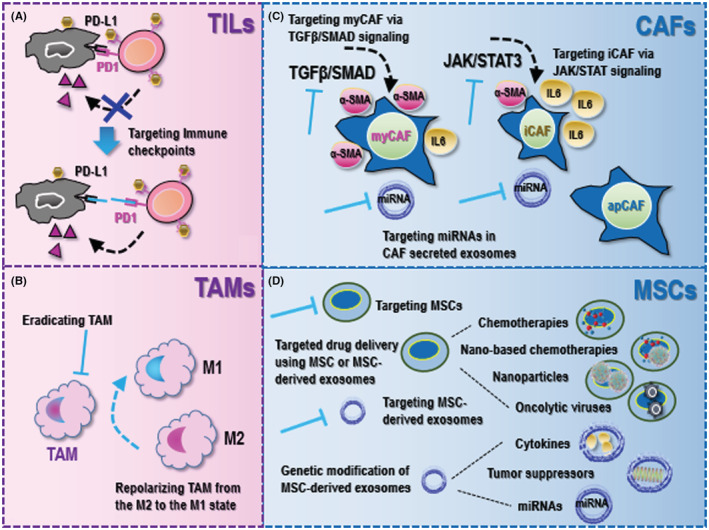
Current onco‐therapeutic strategies utilizing properties of primary tumor microenvironment (TME) cellular components. (A) Onco‐therapeutic strategies utilizing tumor‐infiltrating lymphocyte (TIL) properties largely rely on blocking immune checkpoints. (B) Onco‐therapeutic strategies targeting tumor‐associated macrophages (TAMs) either eradicate TAMs or repolarize TAMs from the M2 to the M1 state. (C) Onco‐therapeutic strategies targeting cancer‐associated fibroblasts (CAFs) mainly target myofibroblastic CAFs (myCAFs) and inflammatory CAFs (iCAFs). As myCAFs are featured by ‘high α‐SMA and low IL6’ and are activated by TGFβ/SMAD signaling, therapeutics against myCAFs are designed to target the TGFβ/SMAD axis. As iCAFs are characterized by ‘low α‐SMA and high IL6’ and are activated by JAK/STAT signaling, therapeutics killing these cells are designed to target the JAK/STAT axis. Therapeutics have also been proposed to target miRNAs in CAF‐derived exosomes. (D) Onco‐therapeutic strategies targeting mesenchymal stem cells (MSCs) can be either targeting MSCs or their derived exosomes. MSCs of different origins and their derived exosomes can be used for delivering drugs, including chemotherapies, nano‐based chemotherapies, nanoparticles, and oncolytic viruses. MSC‐derived exosomes can also be genetically modified to deliver cytokines, tumor suppressors, or miRNAs to tumors or the TME towards desirable therapeutic outcome.
3.1.2. Targeting TAM
Being an essential TME component, TAMs are tumor‐promotive. As the M2 state of TAMs is responsible for promoted tumor growth, current strategies targeting TAMs largely rely on eradicating TAMs or converting TAMs from the M2 state to the M1 state (Figure 2).
Consecutive efforts have been devoted to develop technologies targeting TAMs taking advantages of nanotechnologies. For example, desirable therapeutic outcome has been achieved in triple negative breast cancers by delivering doxorubicin, a chemotherapeutic agent, to TAMs using DOX‐AS‐M‐PLGA‐NPs (surface‐functionalized by acid‐sensitive sheddable PEGylation and modified with mannose). 107 As another example, PLGA nanoparticles encapsulating baicalin and melanoma antigen Hgp peptide fragment 25–33 were fabricated and further loaded with CpG fragments to conjugate M2pep and α‐pep peptides on their surfaces, and the fabricated nano‐complexes were capable of transforming the M2‐like TAMs into the M1‐like phenotype. 108 Also, the M1/M2 ratio was increased by over four folds through dual transfection of polyplexes into both tumors and TAMs in pancreatic cancer cell models. 109 Several other approaches for TAM repolarization have also been proposed including, for example, m@Au‐D/B nanoparticle (a cancer cell membrane‐camouflaged gold nanocage loading doxorubicin and l‐buthionine sulfoximine)‐mediated photothermal therapy combined with ROS production, 110 TAM‐targeted delivery of microRNAs with redox/pH dual‐responsive sPEG/GLC nanovectors, 111 Ru‐based nanoparticles (Ru@ICG‐BLZ NPs), 112 and iron chelated melanin‐like nanoparticles (Fe@PDA‐PEG). 113
Several Chinese herb medications have also been proposed to repolarize TAMs. For instance, Astragaloside IV, a main component of nontoxic Chinese herb, was shown capable of rewiring M2 TAMs to the M1 phenotype, and thus been proposed to be combined with immune checkpoint inhibitors for colorectal cancer management. 114 Hydrazinocurcumin repolarized TAMs to the M1 phenotype via blocking STAT3 signaling in breast cancers. 115 Glycyrrhiza Radix et Rhizome prevented TAM M2 polarization in murine breast cancer cells via, partially, suppressing STAT6 signaling. 116 Exosomes derived from Epigallocatechin gallate (EGCG) decreased TAM infiltration and M2 polarization in breast cancers by down‐regulating IL6 and TGFβ. 117 Resveratrol inhibited lung cancer cell growth via suppressing STAT3‐triggered M2 polarization. 118 HangAmDan‐B attenuated the growth of Lewis lung carcinoma (LLC) cells via inhibiting M1 polarization of TAMs. 119 The water extract of ginseng and astragalus (WEGA) inhibited LLC cell growth by promoting M1 polarization of TAMs. 120 PHY906, a four‐herb Chinese medicine formula (Scutellaria baicalensis Georgi, Paeonia lactiflora Pall, Ziziphus jujuba Mill, Glycyrrhiza uralensis Fisch), improved the efficacy of Sorafenib in triggering lung cancer cell apoptosis in vivo by increasing M1 TAMs. 121
3.2. Onco‐therapeutic strategies relying on stroma cells in the TME
3.2.1. Targeting CAF
Cancer‐associated fibroblasts are recognized players in cancer progression, with the primary contribution to carcinogenesis, among others, being therapeutic resistance. CAFs have been shown to convey resistance to radiotherapies in colorectal cancers, 24 , 26 , 122 , 123 nasopharyngeal carcinomas, 124 and esophageal squamous cell carcinomas 125 , 126 ; to promote chemotherapeutic resistance in breast cancers, 127 gastric cancers, 42 , 128 , 129 , 130 , 131 head and neck cancers, 132 pancreatic cancers, 133 lung cancers, 134 , 135 bladder cancers, 136 gastric cancers, 42 , 128 , 129 , 130 , 131 colorectal cancers, 137 , 138 and ovarian cancers 139 , 140 ; to contribute to targeted therapeutic resistance in breast cancers, 141 , 142 prostate cancers, 143 hepatocellular carcinomas, 144 melanomas, 145 , 146 , 147 , 148 , 149 , 150 , 151 and lung cancers 152 , 153 ; to enhance immunotherapeutic resistance in pancreatic cancers, 48 , 154 , 155 lung cancers, 154 , 156 breast cancers, 157 melanomas, 158 intrahepatic cholangiocarcinomas, 159 , 160 urothelial cancers, 161 esophageal cancers, 162 and hepatocellular carcinomas. 163
Cancer‐associated fibroblasts are heterogeneous that include myCAFs, 47 iCAFs, 47 and apCAFs. 48 The myCAF cohort resides in the peri‐glandular region and is featured by high level of α‐SMA and low IL6 expression. The iCAF cells are located away from tumor cells and are characteristic of α‐SMA low and IL6 high expression. The apCAF cells are featured by the presence of major histocompatibility complex (MHC) class II (MHC II) family genes such as CD74, H2‐Aa, and H2‐Ab1 for antigen processing and presentation. While the first two subcategories of CAFs are tumor‐promotive, apCAFs play a tumor‐suppressive role. Thus, out of the three CAF forms, myCAF and iCAFs are the primary onco‐therapeutic targets.
As myCAFs are activated by TGFβ/SMAD signaling with elevated expression of α‐SMA, Ctgf, Col1α1, TAGLN, MYL9 and TPM1, therapeutic design against myCAFs largely relies on targeting TGFβ signaling (Figure 2). Galunisertib was the first oral inhibitor of TGFβ receptor with demonstrated efficacy in substantially enhancing the overall survival of unresectable pancreatic cancer patients receiving gemcitabline. 164 M7824 was a double‐fusion protein against tumorigenesis that took action by blocking both TGFβ and PD‐L1 signalings. 165 Several herbal medicines were reported with suppressive roles on myCAFs via blocking α‐SMA expression including, e.g., docosahexaenoic acid, 166 resveratrol, 167 curcumin, 168 and silibinin. 169
Since iCAFs are stimulated by the JAK/STAT3 axis and are featured by up‐regulated expression of IL6, IL8, IL11, CXCL1, CXCL2, CXCL12, and LIF, current strategies killing iCAFs include targeting the JAK/STAT3 axis as well as chemokines/cytokines elevated in these CAFs (Figure 2). Ruxolitinib, an inhibitor of the JAK/STAT pathway, has been shown capable of overcoming cisplatin resistance in non‐small cell lung cancers, 170 sensitizing pancreatic cancer cells to oncolytic vesicular stomatitis viruses when coupled with polycation, 171 restoring the sensitivity of tamoxifen‐resistant breast cancer cells, 172 and thus been undergoing clinical trials for the treatment of metastatic HER2‐positive breast cancers 173 and metastatic triple negative breast cancers. 174 Blocking IL6 signaling was shown capable of rewiring the chemotherapeutic resistance of pancreatic cancers in vivo, 175 with a clinical trial involving 140 advanced pancreatic cancer patients being launched to examine the efficacy of tocilizumab (an IL6R inhibitor) in improving the chemotherapeutic outcome (NCT02767557). In addition, combined blockage of IL6 and PD‐L1 signalings reduced pancreatic cancer progression in vivo, 176 with the efficacy being clinically investigated (NCT04191421). Anakinra, an IL1R antagonist, improved the overall survival of pancreatic cancers in vivo, 177 and is now under clinical investigation (NCT02021422). IL1β blockage rewired the drug resistance of pancreatic tumors in vivo, 178 and IL1β inhibitors are being actively examined in clinics (NCT04581343). In addition, suppressing TGFβ receptors decreased STAT3 activation in pancreatic tumors in vivo, 179 suggestive of the crosstalk between myCAFs and iCAFs as well as the possibility of concomitantly suppressing both cell cohorts using one agent.
Emerging therapeutics have been established to target exosomal microRNAs secreted by CAFs (Figure 2). For example, CAFs suppressed gastric cancer cell ferroptosis by secreting exosomal microRNA‐522, and cancer cells developed chemo‐resistance to cisplatin and paclitaxel as a result of increased exosome secretion in response to these two drugs. 129
3.2.2. Therapeutics relying on MSC
Mesenchymal stem cells, another important component in the TME, orchestrate pro‐tumor responses by supporting CSCs and interacting with non‐malignant TME components. Accumulated evidences have indicated the contribution of MSCs to cancer progression and chemotherapy resistance by maintaining cancer stemness. For instance, MSCs enhanced the self‐renewal ability of gastric cancer cells and promoted their chemo‐resistance both in vivo and in vitro through fatty acid oxidation (FAO), suggesting the feasibility of combining FAO inhibitors with chemotherapy regimens in restoring cell drug sensitivity. 180 , 181 MSC‐derived exosomes prevented 5‐FU triggered gastric cancer cell apoptosis both in vivo and in vitro via calcium/calmodulin‐dependent protein kinases and Raf/MEK/ERK signaling, 182 suggestive of a promising anti‐cancer strategy by targeting MSC‐derived exosomes coupled with conventional chemotherapies (Figure 2).
The story of applying MSCs in cancer treatment is not restricted to direct targeting. One of the earliest interactions between MSCs and cancer cells is the natural homing of MSCs to the cancer milieu. 183 The high tropism of MSCs to tumors has enabled them to be a promising tool for delivering onco‐therapeutics such as chemotherapies, nanoparticles, and oncolytic viruses 184 (Figure 2). For example, by delivering paclitaxel using MSCs, the proliferation capacity of multiple myeloma cells was remarkably hampered, 185 and the tumor angiogenetic ability of acute lymphoblastic leukemia was substantially reduced in vivo. 186 The high anti‐cancer activity of nanoparticles has once attracted lots of focus in cancer treatment that, however, suffers from low tumor‐homing efficiency. Loading nano‐based chemotherapies on MSCs showed a great promise in cancer treatment. Increased drug access to the tumor site was observed by loading nano‐docetaxel on MSCs that led to potent induction of lung cancer cell death. 187 In agreement with this, enhanced quantum dots uptake by breast cancer cells was observed when they were loaded on MSCs, 188 and 37‐fold increased tendency of gold nanoparticles to the tumor site was reported when delivered by MSCs. 189 Oncolytic viruses such as herpes simplex virus (HSV), adenovirus and lentivirus have been used to deliver anti‐cancer agents. Manipulated MSCs were shown capable of delivering HSV thymidine kinase (HSV‐TK) to the tumor site and significantly reducing the size and progression of glioma in vivo, 190 suggestive of the efficacy and safety this onco‐therapeutic approach. MSCs expressing HSV‐TK have also been shown to reinforce the therapeutic value of some agents such as fluorouracil (5‐FU) in a prostate cancer xenograft model, 191 implicative of a potential therapeutic synergy.
In addition, MSCs can secrete exosomes that possess similar properties to the source MSCs. Since exosomes can readily fuse with and evacuate cargos into the target tumor cells, they have been considered as an ideal tool for anti‐cancer agent delivery 184 (Figure 2). For example, through incubating MSC‐derived exosomes with Dox·HCl, the drug‐loaded exosomes (Exo‐Dox) showed higher cellular uptake and anti‐tumor efficiency in osteosarcoma cells without observable cytotoxicity to normal cells. 192 Besides, by genetically manipulating MSCs, exosomes capable of reconstructing the TME towards an unfavorable environment for the survival of neoplastic cells can be obtained. For instance, engineered MSCs with amplified INFβ expression reduced the angiogenesis capacity of prostate cancer cells by releasing INFβ to cancer cells that suppressed VEGF expression. 193 Similarly, MSCs with enhanced INFγ expression induced glioma cell death, 194 and hampered the proliferation of chronic myeloid leukemia cells. 195 Apart from cytokines, attempts have also been made to express tumor suppressor genes in MSCs. For instance, MSC‐derived exosomes over‐expressing Pten eliminated glioblastoma cells 196 ; exosomes originated from MSCs and over‐expressing apoptin substantially reduced the metabolic activity and remarkably diminished the size of liver tumors in vivo. 197 Lastly is the modification of microRNA contents of MSCs. For example, through co‐delivery of microRNA‐124 and microRNA‐145 to glioblastoma cells via MSC‐derived exosomes, significant reduction of cancer cells was observed due to concomitant suppression on Sox2 and Oct4. 198 , 199
Intensive clinical efforts have been devoted to MSC‐based onco‐therapeutics, most of which focused on tissue‐derived MSCs (over 50%) followed by engineered MSCs (approximately 23%) and only 1 trial was designated to evaluate the safety and efficacy of MSC‐derived exosomes. 200
4. CAP AS AN EMERGING TME EDITING TOOL
Cold atmospheric plasma is composed of varied reactive oxygen and nitrogen species (RONS) including short‐lived species such as hydroxyl radical (OH·), singlet oxygen (O), superoxide (O2−), and nitric oxide (NO·), and long‐lived species such as hydrogen peroxide (H2O2), ozone (O3), anionic (OONO−), and protonated (ONOOH) forms of peroxynitrite. Since the first discovery on the anti‐cancer efficacy of CAP in 2007, consecutive efforts have been devoted to investigate its onco‐therapeutic impacts in varied types of cancers with demonstrated efficacies already been proven in, for example, triple negative breast cancers, 201 bladder cancers, 202 prostate cancers, 203 melanomas, 204 and pancreatic cancers. 205 Differential cell death events can be triggered by CAP in a dose‐dependent manner 206 that include, for example, cell cycle arrest, 203 autophagy, 207 apoptosis, 201 ferroptosis, 208 immunogenic cell death (ICD), 209 and necrotic cell death. 210 It has also been proposed that CAP can modulate the immunogenic response 211 and drug sensitivity 212 of cancer cells, halt cancer invasion and metastasis, 202 and rewire the metabolic reprogramming of malignant cells, 213 among others. With accumulated evidences on the selectivity of CAP against cancers and its diversified anti‐cancer properties keep being discovered, CAP has been proposed as an emerging onco‐therapeutics 214 capable of controlling cancer cell states. 215
Besides these preclinical studies showing the efficacy 201 , 202 , 203 , 215 , 216 , 217 , 218 and safety 219 of CAP in cancer treatment both in vitro and in vivo, the first clinical trial using CAP as an oncotherapy had been approved by FDA on July 30, 2019, in the USA (NCT04267575). Among the 20 stage IV solid tumor patients recruited in this trial, 17 patients were still alive by the study completion on 14 April 2021, suggestive of the safety and efficacy of CAP as a novel onco‐therapeutic modality. 220
Before introducing the roles of CAP relevant to TME immune cells, we need to firstly review the cancer immunity cycle. Cancer cells in a healthy individual can be effectively killed by the cancer immunity cycle. Specifically, neoantigens are secreted by dying cells and captured by DCs, where immunogenic signals including, e.g., proinflammatory cytokines, are also released in accompany. Then, DCs present these captured antigens on MHC class I (MHCI) and MHCII molecules to T cells, which are primed to recognize and kill malignant cells carrying cancer‐specific antigens. Activated T cells then home to tumor sites and infiltrate to the TME to recognize cancer cells and take on the cytotoxic effect, where the killing of cancer cells releases additional tumor‐associated antigens to sustain the cancer immunity cycle. In cancer patients, this cycle may fail at any step. For instance, tumor antigens may not be detected, DCs may fail in presenting these antigens to T cells, T cells may not treat cancer antigens as foreign materials and thus not activated, T cells may not properly traffic to tumors, succeed in infiltrating the TME, or take on the cell killing effect due to various suppressive factors residing in the TME such as M2 TAM and CAF. 221 Below, we characterize the possible roles of CAP in fixing the abnormal cancer immunity cycle in cancer patients by focusing on the impact of CAP on primary components aforementioned in the TME.
4.1. Onco‐therapeutic opportunities of CAP relevant to TME immune cells
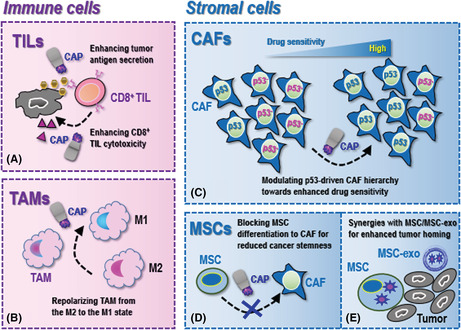
4.1.1. CAP enhances tumor antigen release and CD8 + T cell priming
The presentation of cancer antigens by MHCI is essential for CD8+ T cells to take on their anti‐cancer cytotoxicity, where elevated intracellular ROS production can promote antigen cross presentation. 222 CAP is a known redox modulating tool capable of enhancing cellular ROS level, and thus is possible to sensitize CD8+ T cells towards improved anti‐cancer activities (Figure 3). Indeed, several studies have already reported the ability of CAP in triggering ICD that is featured by enhanced cancer cell emission of danger associated molecular patterns and CD8+ T cell priming. 223
FIGURE 3.
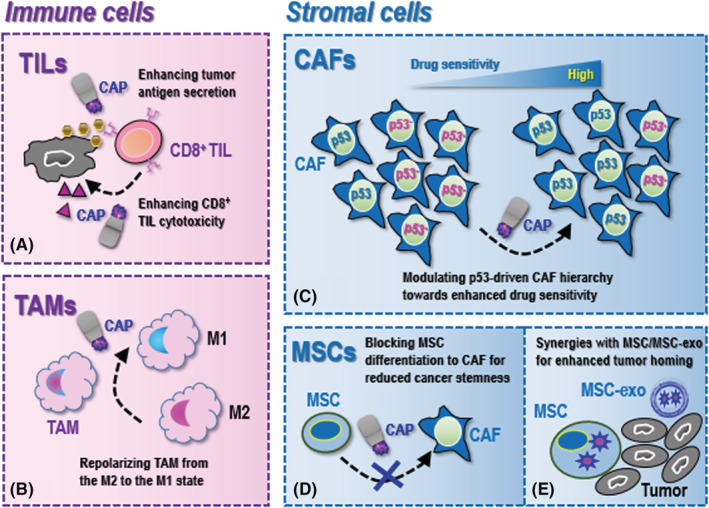
Onco‐therapeutic opportunities of cold atmospheric plasma (CAP) utilizing properties of primary tumor microenvironment (TME) cellular components. (A) For tumor‐infiltrating lymphocytes (TILs), CAP can possibly enhance tumor antigen secretion and enhance CD8+ TIL cytotoxicity. (B) For tumor‐associated macrophages (TAMs), CAP can potentially repolarize TAMs from the M2 to the M1 state. (C) For cancer‐associated fibroblasts (CAFs), CAP may modulate p53‐driven CAF hierarchy towards enhanced drug sensitivity. (D) For mesenchymal stem cells (MSCs), CAP may block MSCs differentiation to CAFs that is associated with reduced cancer stemness. (E) CAP can function as the cargo of MSCs or their derived exosomes for enhanced delivery to the tumor loci, where MSCs are not necessarily originated from the TME.
4.1.2. CAP repolarizes TAM from the M2 to the M1 state
It has been long and well‐acknowledged that the M1/M2 polarization of TAMs is a dynamic process in response to multiple physical factors as exemplified by oxygen tension, and the M1 TAMs are featured by an enhanced RONS forming capacity for tumoricidal activities. 224 CAP, by definition, is a RONS generator. Thus, it is natural to assume that CAP may function as an excellent tool for TAM repolarization toward the M2 state, the study of which deserves intensive efforts (Figure 3).
4.2. Onco‐therapeutic opportunities of CAP relevant to TME stromal cells
4.2.1. CAP restores drug sensitivity by modulating p53‐driven CAF hierarchy
Before we can understand how CAP may restore the drug sensitivity of resistant cancer cells via modulating the TME, we should firstly be acknowledged with the role of p53 mutation on CAF functionalities. The p53‐driven CAF hierarchy of pancreatic cancer cells toward a pro‐metastatic and chemo‐resistant TME has been established. 225 Specifically, cancer cells with a gain‐of‐function p53‐mutant educated a dominant CAF cohort for a pro‐metastatic microenvironment that delayed cancer cell response to gemcitabine/abraxane, and reprogrammed the rest CAF populations towards the acquisition of more invasive features. 225
Cold atmospheric plasma has been demonstrated capable of activating genes involved in p53 signaling in cancer cells 226 and modulating p53 in keratinocytes. 227 Given the essential roles played by p53 in maintaining the therapeutic‐responsive CAF hierarchy and thus cancer cell drug sensitivity, it is plausible to assume that the demonstrated efficacy of CAP in restoring the therapeutic response of many resistant cancer cells is, at least partially, attributable to the remodeled p53‐driven CAF hierarchy in the TME (Figure 3).
4.2.2. CAP blocks the differentiation of MSC to CAF
Mesenchymal stem cells can be considered as the nourishing cells of CSCs and can differentiate into CAFs that are known capable of promoting tumorigenesis. 228 The transition of MSCs into CAFs is at least partially attributable to the active secretome in the TME that includes, for example, pro‐angiogenetic factors such as VEGF and PDGF, pro‐metastatic factors such as TGFβ, pro‐inflammatory factors such as CXCL12 and IL6, and ECM modulators such as matrix metalloproteases (MMPs). 229 , 230
Accumulated evidences have suggested the selectivity of CAP against triple negative breast cancers, 201 , 216 , 231 where significantly reduced expression of MMP1, MT‐MMP and uPA (a critical player in the plasminogen activation system that activates MMPs and degrades most ECM proteins) in response to CAP treatment was reported, 232 suggestive of the causal relationship between the suppressive role of CAP on MMPs and its anti‐cancer effects. Interestingly, this study also reported retarded CD44 expression, 232 the high level of which is characteristic of CSCs, associating the blocked transition from MSCs to CAFs (as indicated by reduced MMPs) with reduced cancer stemness. In agreement with this, our previous investigations in triple negative breast cancers also embraced the suppressive role of CAP on cancer stemness. 202 In addition, another study reported reduced expression of MMP2/9 and VEGF on CAP exposure that restored the chemo‐sensitivity of breast cancer cells, 233 implicative of a less retarded drug response as a result of blocked differentiation from MSCs to CAFs (Figure 3).
4.2.3. CAP creates synergies with MSC or MSC‐derived exosomes for enhanced tumor homing
Although having been considered as a promising onco‐therapeutic strategy, the clinical application of CAP was hindered by the limited lifespan of its short‐lived species. CAP can be prepared in the form of liquid, for example, plasma activated Ringer emulsion, and can be made as the cargo of delivery vehicles alone or mixed with, e.g., hyaluronic acid 234 for improved stability or with, e.g., hydrogel 235 for extended release. MSCs (not necessarily originated from the TME) and their derived exosomes may function as the ideal vehicle for CAP delivery given their excellent tumor‐homing and cargo protection properties, which is expected to concentrate CAP in the tumor milieu or the TME for improved drug utility (Figure 3). Besides, as exosomes can easily pass through the blood brain barrier, MSC‐derived exosomes may offer additional benefits by delivering CAP to the brain tissues to kill tumor cells originated from or metastasized to the brain that currently lack effective and safe cure (Figure 3). In addition, it is worthwhile to explore the potential of delivering CAP in the form of oral capsules with the aid of MSC‐derived exosomes for cancer treatment that can tolerate gastric acidity (Figure 3).
5. CONCLUSION
This paper delineates the functionalities of the TME in tumorigenesis by classifying their primary cellular components into “immune cells” (as represented by TILs and TAMs) and “stromal cells” (as exemplified by CAFs and MSCs), reviewing current onco‐therapeutic strategies targeting these components as well as the existing clinical endeavors. Importantly, we advocate the possible roles of CAP in modulating the TME towards an environment favorable for cancer management, and identify possible molecular mechanisms driving the demonstrated selectivity of CAP against cancer hallmarks.
Cold atmospheric plasma has been proposed as an emerging tool for TME editing given its role in modulating key indexes of the TME, that is, hypoxia, acidosis, hypo‐nutrition, and inflammation. 236 From a complementary perspective, we focus on potential impacts of CAP on the primary cellular components in the TME here. We identify and forecast the functions of CAP in enhancing tumor antigen secretion and CD8+ T cell cytotoxicity, repolarizing TAM from the M2 to the M1 state, modulating p53‐driven CAF hierarchy toward enhanced drug sensitivity, and blocking the differentiation of MSCs to CAFs for reduced cancer stemness. We also propose possible synergies between CAP and MSCs (not restricted to those residing in the TME) for efficient drug delivery and tumor homing. These insights may offer additional views on what redox modulation can do to resolve tumors that calls for experimental validations and deserves future attention. We do not exclude other possible impacts of CAP on the TME that may be unveiled in the future given our incremental understandings on the cellular system and the properties of CAP.
AUTHOR CONTRIBUTIONS
Xiaofeng Dai conceived the idea and drafted the manuscript, prepared the figures, and conducted literature searching.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (Grant No. 81972789), Fundamental Research Funds for the Central Universities (Grant No. JUSRP22011). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Dai X, Zhu K. Cold atmospheric plasma: Novel opportunities for tumor microenvironment targeting. Cancer Med. 2023;12:7189‐7206. doi: 10.1002/cam4.5491
DATA AVAILABILITY STATEMENT
N/A.
REFERENCES
- 1. Yang Y, Meng WJ, Wang ZQ. Cancer stem cells and the tumor microenvironment in gastric cancer. Front Oncol. 2021;11:803974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jia Q, Liao X, Zhang Y, et al. Anti‐tumor role of CAMK2B in remodeling the stromal microenvironment and inhibiting proliferation in papillary renal cell carcinoma. Front Oncol. 2022;12:740051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jang B, Song HK, Hwang J, et al. Shed syndecan‐2 enhances colon cancer progression by increasing cooperative angiogenesis in the tumor microenvironment. Matrix Biol. 2022;107:40‐58. [DOI] [PubMed] [Google Scholar]
- 4. Malla RR, Kiran P. Tumor microenvironment pathways: cross regulation in breast cancer metastasis. Genes Dis. 2022;9(2):310‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mashukov A, Shapochka D, Seleznov O, et al. Histological differentiation impacts the tumor immune microenvironment in gastric carcinoma: relation to the immune cycle. World J Gastroenterol. 2021;27(31):5259‐5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang H, Luo K, Guan Z, et al. Identification of the crucial role of CCL22 in F. nucleatum‐related colorectal tumorigenesis that correlates with tumor microenvironment and immune checkpoint therapy. Front Genet. 2022;13:811900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan Y, Li H, Pu W, et al. Cancer metabolism and tumor microenvironment: fostering each other? Sci China Life Sci. 2022;65(2):236‐279. [DOI] [PubMed] [Google Scholar]
- 8. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 9. Dou Z, Berger SL. Senescence elicits stemness: a surprising mechanism for cancer relapse. Cell Metab. 2018;27(4):710‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehraj U, Ganai RA, Macha MA, et al. The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: new challenges and therapeutic opportunities. Cell Oncol. 2021;44(6):1209‐1229. [DOI] [PubMed] [Google Scholar]
- 11. Han J, Khatwani N, Searles TG, Turk MJ, Angeles CV. Memory CD8+ T cell responses to cancer. Semin Immunol. 2020;49:101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu S, Wang X. The role of helper innate lymphoid cells in cancer. Immunotherapy. 2019;11(12):1067‐1081. [DOI] [PubMed] [Google Scholar]
- 13. Dong C. Helper T cells and cancer‐associated inflammation: a new direction for immunotherapy? J Interferon Cytokine Res. 2017;37(9):383‐385. [DOI] [PubMed] [Google Scholar]
- 14. Meier SL, Satpathy AT, Wells DK. Bystander T cells in cancer immunology and therapy. Nat Cancer. 2022;3(2):143‐155. [DOI] [PubMed] [Google Scholar]
- 15. Simoni Y, Becht E, Fehlings M, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575‐579. [DOI] [PubMed] [Google Scholar]
- 16. Scheper W, Kelderman S, Fanchi LF, et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med. 2019;25(1):89‐94. [DOI] [PubMed] [Google Scholar]
- 17. Liu Z, Li JP, Chen M, et al. Detecting tumor antigen‐specific T cells via interaction‐dependent fucosyl‐biotinylation. Cell. 2020;183(4):1117‐1133.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park HS, Kim YM, Kim S, et al. High endothelial venule is a surrogate biomarker for T‐cell inflamed tumor microenvironment and prognosis in gastric cancer. J Immunother Cancer. 2021;9(10):e003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu S, Chaudhary O, Rodríguez‐Morales P, et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity. 2021;54(7):1561‐1577.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu L, Lim MA, Jung SN, et al. The effect of curcumin on multi‐level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine. 2021;92:153758. [DOI] [PubMed] [Google Scholar]
- 21. Yang Z, Gao A, Shi W, et al. ILT4 in colorectal cancer cells induces suppressive T cell contexture and disease progression. Onco Targets Ther. 2021;14:4239‐4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Q, Li J, Wang S, et al. Overexpressed immunoglobulin‐like transcript (ILT) 4 in lung adenocarcinoma is correlated with immunosuppressive T cell subset infiltration and poor patient outcomes. Biomark Res. 2020;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song NY, Li X, Ma B, et al. IKKalpha‐deficient lung adenocarcinomas generate an immunosuppressive microenvironment by overproducing Treg‐inducing cytokines. Proc Natl Acad Sci U S A. 2022;119(6):e2120956119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Franco F, Tsui YC, et al. CD36‐mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21(3):298‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li J, Wang S, Wang N, et al. Aiduqing formula inhibits breast cancer metastasis by suppressing TAM/CXCL1‐induced Treg differentiation and infiltration. Cell Commun Signal. 2021;19(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor‐associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10(11):2156‐2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oguma K, Oshima H, Aoki M, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor‐alpha in gastric tumour cells. EMBO J. 2008;27(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu S, Deng L, Liao X, et al. Tumor‐associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci. 2019;110(7):2110‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang ZW, Ge XX, Xu MD, et al. Tumor‐associated macrophages promote the metastasis and growth of non‐small‐cell lung cancer cells through NF‐kappaB/PP2Ac‐positive feedback loop. Cancer Sci. 2021;112(6):2140‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campesato LF, Budhu S, Tchaicha J, et al. Blockade of the AHR restricts a Treg‐macrophage suppressive axis induced by L‐kynurenine. Nat Commun. 2020;11(1):4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu H, Xu JB, He YL, et al. Tumor‐associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol. 2012;106(4):462‐468. [DOI] [PubMed] [Google Scholar]
- 32. Yamanaka N, Morisaki T, Nakashima H, et al. Interleukin 1beta enhances invasive ability of gastric carcinoma through nuclear factor‐kappaB activation. Clin Cancer Res. 2004;10(5):1853‐1859. [DOI] [PubMed] [Google Scholar]
- 33. Chen P, Huang Y, Bong R, et al. Tumor‐associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res. 2011;17(23):7230‐7239. [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Xu L, Hu Y, et al. miRNA let‐7b modulates macrophage polarization and enhances tumor‐associated macrophages to promote angiogenesis and mobility in prostate cancer. Sci Rep. 2016;6:25602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kong X, Bu J, Chen J, et al. PIGF and Flt‐1 on the surface of macrophages induces the production of TGF‐beta1 by polarized tumor‐associated macrophages to promote lung cancer angiogenesis. Eur J Pharmacol. 2021;912:174550. [DOI] [PubMed] [Google Scholar]
- 36. Steitz AM, Steffes A, Finkernagel F, et al. Tumor‐associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C. Cell Death Dis. 2020;11(4):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hong L, Wang S, Li W, Wu D, Chen W. Tumor‐associated macrophages promote the metastasis of ovarian carcinoma cells by enhancing CXCL16/CXCR6 expression. Pathol Res Pract. 2018;214(9):1345‐1351. [DOI] [PubMed] [Google Scholar]
- 38. Long L, Hu Y, Long T, et al. Tumor‐associated macrophages induced spheroid formation by CCL18‐ZEB1‐M‐CSF feedback loop to promote transcoelomic metastasis of ovarian cancer. J Immunother Cancer. 2021;9(12):e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang W, Liu Y, Guo J, et al. miR‐100 maintains phenotype of tumor‐associated macrophages by targeting mTOR to promote tumor metastasis via Stat5a/IL‐1ra pathway in mouse breast cancer. Oncogenesis. 2018;7(12):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang S, Che D, Yang F, et al. Tumor‐associated macrophages promote tumor metastasis via the TGF‐beta/SOX9 axis in non‐small cell lung cancer. Oncotarget. 2017;8(59):99801‐99815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, Chen Z, Ni Y, et al. Tumor‐associated macrophages secret exosomal miR‐155 and miR‐196a‐5p to promote metastasis of non‐small‐cell lung cancer. Transl Lung Cancer Res. 2021;10(3):1338‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ham IH, Oh HJ, Jin H, et al. Targeting interleukin‐6 as a strategy to overcome stroma‐induced resistance to chemotherapy in gastric cancer. Mol Cancer. 2019;18(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De P, Aske J, Dey N. Cancer‐associated fibroblast functions as a road‐block in cancer therapy. Cancers. 2021;13(20):5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer‐associated fibroblasts. Nat Rev Cancer. 2020;20:174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen T, Li Y, Zhu S, et al. YAP1 plays a key role of the conversion of normal fibroblasts into cancer‐associated fibroblasts that contribute to prostate cancer progression. J Exp Clin Cancer Res. 2020;39(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ganguly D, Chandra R, Karalis J, et al. Cancer‐associated fibroblasts: versatile players in the tumor microenvironment. Cancers. 2020;12(9):2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ohlund D, Handly‐Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elyada E, Bolisetty M, Laise P, et al. Cross‐species single‐cell analysis of pancreatic ductal adenocarcinoma reveals antigen‐presenting cancer‐associated fibroblasts. Cancer Discov. 2019;9(8):1102‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 2005;15(5):329‐341. [DOI] [PubMed] [Google Scholar]
- 50. Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer‐associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36(13):1770‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Su S, Chen J, Yao H, et al. CD10+GPR77+ cancer‐associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer Stemness. Cell. 2018;172(4):841‐856.e16. [DOI] [PubMed] [Google Scholar]
- 52. Sharbeen G, McCarroll J, Akerman A, et al. Cancer‐associated fibroblasts in pancreatic ductal adenocarcinoma determine response to SLC7A11 inhibition. Cancer Res. 2021;81(13):3461‐3479. [DOI] [PubMed] [Google Scholar]
- 53. Qian H, Ding X, Zhang J, et al. Cancer stemness and metastatic potential of the novel tumor cell line K3: an inner mutated cell of bone marrow‐derived mesenchymal stem cells. Oncotarget. 2017;8(24):39522‐39533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheng JW, Duan LX, Yu Y, et al. Bone marrow mesenchymal stem cells promote prostate cancer cell stemness via cell‐cell contact to activate the Jagged1/Notch1 pathway. Cell Biosci. 2021;11(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jing Y, Liang W, Zhang L, Tang J, Huang Z. The role of mesenchymal stem cells in the induction of cancer‐stem cell phenotype. Front Oncol. 2022;12:817971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kabashima‐Niibe A, Higuchi H, Takaishi H, et al. Mesenchymal stem cells regulate epithelial‐mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013;104(2):157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jing Y, Han Z, Liu Y, et al. Mesenchymal stem cells in inflammation microenvironment accelerates hepatocellular carcinoma metastasis by inducing epithelial‐mesenchymal transition. PLoS One. 2012;7(8):e43272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luo J, Ok Lee S, Liang L, et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014;33(21):2768‐2778. [DOI] [PubMed] [Google Scholar]
- 59. Hossain A, Hossain A, Shinojima N, Gumin J, Feng G, Lang FF. Tumor‐associated mesenchymal stromal cells increase proliferation and maintain stemness of glioma stem cells through the Il6/Stat3 pathway. Neuro Oncol. 2011;13:19. [Google Scholar]
- 60. McLean K, Gong Y, Choi Y, et al. Human ovarian carcinoma‐associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121(8):3206‐3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun L, Wang Q, Chen B, et al. Human gastric cancer mesenchymal stem cell‐derived IL15 contributes to tumor cell epithelial‐mesenchymal transition via upregulation Tregs ratio and PD‐1 expression in CD4+T cell. Stem Cells Dev. 2018;27(17):1203‐1214. [DOI] [PubMed] [Google Scholar]
- 62. Li W, Zhang X, Wu F, et al. Gastric cancer‐derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019;10(12):918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Francis DM, Manspeaker MP, Schudel A, et al. Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci Transl Med. 2020;12(563):3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Isazadeh A, Hajazimian S, Garshasbi H, et al. Resistance mechanisms to immune checkpoints blockade by monoclonal antibody drugs in cancer immunotherapy: focus on myeloma. J Cell Physiol. 2021;236(2):791‐805. [DOI] [PubMed] [Google Scholar]
- 65. Wu X, Gu Z, Chen Y, et al. Application of PD‐1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Latif F, Bint Abdul Jabbar H, Malik H, et al. Atezolizumab and pembrolizumab in triple‐negative breast cancer: a meta‐analysis. Expert Rev Anticancer Ther. 2022;22(2):229‐235. [DOI] [PubMed] [Google Scholar]
- 67. Kahraman S, Erbas UE, Yalcin B. Who should be treated with pembrolizumab in addition to standard of care in advanced cervical cancer? Med Oncol. 2022;39(3):33. [DOI] [PubMed] [Google Scholar]
- 68. McNeel DG, Eickhoff JC, Wargowski E, et al. Phase 2 trial of T‐cell activation using MVI‐816 and pembrolizumab in patients with metastatic, castration‐resistant prostate cancer (mCRPC). J Immunother Cancer. 2022;10(3):e004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Killock D. Pembrolizumab for HER2+ gastric cancer. Nat Rev Clin Oncol. 2022;19(3):150. [DOI] [PubMed] [Google Scholar]
- 70. Fuchs CS, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated PD‐L1‐positive advanced gastric or gastroesophageal junction cancer: 2‐year update of the randomized phase 3 KEYNOTE‐061 trial. Gastric Cancer. 2022;25(1):197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Muro K, Kojima T, Moriwaki T, et al. Second‐line pembrolizumab versus chemotherapy in Japanese patients with advanced esophageal cancer: subgroup analysis from KEYNOTE‐181. Esophagus. 2022;19(1):137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nakasato T, Inoue T, Kato R, et al. A case of complete response following the administration of pembrolizumab and metastasectomy for lung and bone metastases of bladder cancer. IJU Case Rep. 2022;5(2):92‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mafei K, Shengyuan X, Jieqiong S. Pembrolizumab enhances the anti‐pancreatic cancer activity of anlotinib. Asian J Surg. 2022;45(3):881‐882. [DOI] [PubMed] [Google Scholar]
- 74. Tong BC, Gu L, Wang X, et al. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non‐small cell lung cancer. J Thorac Cardiovasc Surg. 2022;163(2):427‐436. [DOI] [PubMed] [Google Scholar]
- 75. Kartolo A, Holstead R, Hopman W, Young L, Baetz T. Safety and efficacy analysis of pembrolizumab dosing patterns in patients with advanced melanoma and non‐small cell lung cancer. J Oncol Pharm Pract. 2022;28(1):87‐95. [DOI] [PubMed] [Google Scholar]
- 76. Fuereder T, Minichsdorfer C, Mittlboeck M, et al. Pembrolizumab plus docetaxel for the treatment of recurrent/metastatic head and neck cancer: a prospective phase I/II study. Oral Oncol. 2022;124:105634. [DOI] [PubMed] [Google Scholar]
- 77. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jin H, Amonkar M, Aguiar‐Ibáñez R, Thosar M, Chase M, Keeping S. Systematic literature review and network meta‐analysis of pembrolizumab versus other interventions for previously untreated, unresectable or metastatic, microsatellite instability‐high or mismatch repair‐deficient colorectal cancer. Future Oncol. 2022;18:1633‐2171. [DOI] [PubMed] [Google Scholar]
- 79. Kobayashi T, Ito K, Kojima T, et al. Pre‐pembrolizumab neutrophil‐to‐lymphocyte ratio (NLR) predicts the efficacy of second‐line pembrolizumab treatment in urothelial cancer regardless of the pre‐chemo NLR. Cancer Immunol Immunother. 2022;71(2):461‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marcus L, Fashoyin‐Aje LA, Donoghue M, et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden‐high solid tumors. Clin Cancer Res. 2021;27(17):4685‐4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability‐high solid tumors. Clin Cancer Res. 2019;25(13):3753‐3758. [DOI] [PubMed] [Google Scholar]
- 82. Suzman DL, Agrawal S, Ning YM, et al. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin‐containing chemotherapy. Oncologist. 2019;24(4):563‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Larkins E, Blumenthal GM, Yuan W, et al. FDA approval summary: pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum‐containing chemotherapy. Oncologist. 2017;22(7):873‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bradford D, Demko S, Jin S, et al. FDA accelerated approval of pembrolizumab for recurrent locally advanced or metastatic Merkel cell carcinoma. Oncologist. 2020;25(7):e1077‐e1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fashoyin‐Aje L, Donoghue M, Chen H, et al. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD‐L1. Oncologist. 2019;24(1):103‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Borcoman E, Le Tourneau C. Keynote‐158 study, FDA granted accelerated approval of pembrolizumab for the treatment of patients with advanced PD‐L1‐positive cervical cancer. Ann Transl Med. 2020;8(23):1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wright KM. FDA approves pembrolizumab for BCG‐unresponsive NMIBC. Oncology. 2020;34(2):44. [PubMed] [Google Scholar]
- 88. Pai‐Scherf L, Blumenthal GM, Li H, et al. FDA approval summary: pembrolizumab for treatment of metastatic non‐small cell lung cancer: first‐line therapy and beyond. Oncologist. 2017;22(11):1392‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non‐small cell lung cancer whose tumors express programmed death‐ligand 1. Oncologist. 2016;21(5):643‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Akinboro O, Larkins E, Pai‐Scherf LH, et al. FDA approval summary: pembrolizumab, atezolizumab, and cemiplimab‐rwlc as single agents for first‐line treatment of advanced/metastatic PD‐L1 high NSCLC. Clin Cancer Res. 2022;28(11):2221‐2228. [DOI] [PubMed] [Google Scholar]
- 91. Casak SJ, Marcus L, Fashoyin‐Aje L, et al. FDA approval summary: pembrolizumab for the first‐line treatment of patients with MSI‐H/dMMR advanced unresectable or metastatic colorectal carcinoma. Clin Cancer Res. 2021;27(17):4680‐4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chuk MK, Chang JT, Theoret MR, et al. FDA approval summary: accelerated approval of pembrolizumab for second‐line treatment of metastatic melanoma. Clin Cancer Res. 2017;23(19):5666‐5670. [DOI] [PubMed] [Google Scholar]
- 93. Slater H. FDA approves pembrolizumab + chemotherapy combination for locally recurrent unresectable or metastatic TNBC. Oncology. 2020;34(12):547. [DOI] [PubMed] [Google Scholar]
- 94. Economopoulou P, Kotsantis I, Papaxoinis G, et al. Association of autoimmunity with survival in patients with recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. 2020;111:105013. [DOI] [PubMed] [Google Scholar]
- 95. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Santoni M, Aurilio G, Massari F, et al. Nivolumab versus cabozantinib as second‐line therapy in patients with advanced renal cell carcinoma: a real‐world comparison. Clin Genitourin Cancer. 2022: p. ahead of print;20:285‐295. [DOI] [PubMed] [Google Scholar]
- 97. Rizzo A. Nivolumab plus ipilimumab in melanoma brain metastases. Lancet Oncol. 2022;23(2):e52. [DOI] [PubMed] [Google Scholar]
- 98. Krefting F, Basara N, Schütte W, et al. Clinical experience of immunotherapy treatment: efficacy and toxicity analysis of the compassionate use program of nivolumab in patients with advanced squamous cell non‐small cell lung cancer. Oncol Res Treat. 2019;42(5):243‐255. [DOI] [PubMed] [Google Scholar]
- 99. Kasamon YL, de Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncologist. 2017;22(5):585‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu JX, Maher VE, Zhang L, et al. FDA approval summary: nivolumab in advanced renal cell carcinoma after anti‐Angiogenic therapy and exploratory predictive biomarker analysis. Oncologist. 2017;22(3):311‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non‐small cell lung cancer with progression on or after platinum‐based chemotherapy. Oncologist. 2016;21(5):634‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nivolumab gets FDA nod for bladder cancer. Cancer Discov. 2017;7(4):OF7. [DOI] [PubMed]
- 103. Beaver JA, Theoret MR, Mushti S, et al. FDA approval of nivolumab for the first‐line treatment of patients with BRAF(V600) wild‐type unresectable or metastatic melanoma. Clin Cancer Res. 2017;23(14):3479‐3483. [DOI] [PubMed] [Google Scholar]
- 104. Wright K. FDA approves nivolumab plus ipilimumab for the treatment of advanced HCC. Oncology (Williston Park). 2020;34(4):693606. [PubMed] [Google Scholar]
- 105. Saung MT, Pelosof L, Casak S, et al. FDA approval summary: nivolumab plus ipilimumab for the treatment of patients with hepatocellular carcinoma previously treated with Sorafenib. Oncologist. 2021;26(9):797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wright K. FDA approves nivolumab plus ipilimumab for previously untreated unresectable malignant pleural mesothelioma. Oncology. 2020;34(11):502‐503. [DOI] [PubMed] [Google Scholar]
- 107. Niu M, Valdes S, Naguib YW, Hursting SD, Cui Z. Tumor‐associated macrophage‐mediated targeted therapy of triple‐negative breast cancer. Mol Pharm. 2016;13(6):1833‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Han S, Wang W, Wang S, et al. Tumor microenvironment remodeling and tumor therapy based on M2‐like tumor associated macrophage‐targeting nano‐complexes. Theranostics. 2021;11(6):2892‐2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Qiu N, Wang G, Wang J, et al. Tumor‐associated macrophage and tumor‐cell dually transfecting polyplexes for efficient interleukin‐12 cancer gene therapy. Adv Mater. 2021;33(2):e2006189. [DOI] [PubMed] [Google Scholar]
- 110. Wei Y, Wang Z, Yang J, et al. Reactive oxygen species/photothermal therapy dual‐triggered biomimetic gold nanocages nanoplatform for combination cancer therapy via ferroptosis and tumor‐associated macrophage repolarization mechanism. J Colloid Interface Sci. 2022;606(Pt 2):1950‐1965. [DOI] [PubMed] [Google Scholar]
- 111. Liu L, Yi H, He H, Pan H, Cai L, Ma Y. Tumor associated macrophage‐targeted microRNA delivery with dual‐responsive polypeptide nanovectors for anti‐cancer therapy. Biomaterials. 2017;134:166‐179. [DOI] [PubMed] [Google Scholar]
- 112. Liu Y, Wen Y, Chen X, et al. Inflammation‐responsive functional Ru nanoparticles combining a tumor‐associated macrophage repolarization strategy with phototherapy for colorectal cancer therapy. J Mater Chem B. 2019;7(40):6210‐6223. [DOI] [PubMed] [Google Scholar]
- 113. Rong L, Zhang Y, Li WS, Su Z, Fadhil JI, Zhang C. Iron chelated melanin‐like nanoparticles for tumor‐associated macrophage repolarization and cancer therapy. Biomaterials. 2019;225:119515. [DOI] [PubMed] [Google Scholar]
- 114. Liu F, Ran F, He H, Chen L. Astragaloside IV exerts anti‐tumor effect on murine colorectal cancer by re‐educating tumor‐associated macrophage. Arch Immunol Ther Exp. 2020;68(6):33. [DOI] [PubMed] [Google Scholar]
- 115. Zhang X, Tian W, Cai X, et al. Hydrazinocurcumin encapsuled nanoparticles "re‐educate" tumor‐associated macrophages and exhibit anti‐tumor effects on breast cancer following STAT3 suppression. PLoS One. 2013;8(6):e65896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jiang YX, Chen Y, Yang Y, Chen XX, Zhang DD. Screening five qi‐tonifying herbs on M2 phenotype macrophages. Evid Based Complement Alternat Med. 2019;2019:9549315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor‐associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sun L, Chen B, Jiang R, Li J, Wang B. Resveratrol inhibits lung cancer growth by suppressing M2‐like polarization of tumor associated macrophages. Cell Immunol. 2017;311:86‐93. [DOI] [PubMed] [Google Scholar]
- 119. Park HR, Lee EJ, Moon SC, et al. Inhibition of lung cancer growth by HangAmDan‐B is mediated by macrophage activation to M1 subtype. Oncol Lett. 2017;13(4):2330‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen Y, Bi L, Luo H, et al. Water extract of ginseng and astragalus regulates macrophage polarization and synergistically enhances DDP's anticancer effect. J Ethnopharmacol. 2019;232:11‐20. [DOI] [PubMed] [Google Scholar]
- 121. Lam W, Jiang Z, Guan F, et al. PHY906(KD018), an adjuvant based on a 1800‐year‐old Chinese medicine, enhanced the anti‐tumor activity of Sorafenib by changing the tumor microenvironment. Sci Rep. 2015;5:9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tommelein J, de Vlieghere E, Verset L, et al. Radiotherapy‐activated cancer‐associated fibroblasts promote tumor progression through paracrine IGF1R activation. Cancer Res. 2018;78(3):659‐670. [DOI] [PubMed] [Google Scholar]
- 123. Chen X, Liu Y, Zhang Q, et al. Exosomal miR‐590‐3p derived from cancer‐associated fibroblasts confers radioresistance in colorectal cancer. Mol Ther Nucleic Acids. 2021;24:113‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Huang W, Zhang L, Yang M, et al. Cancer‐associated fibroblasts promote the survival of irradiated nasopharyngeal carcinoma cells via the NF‐kappaB pathway. J Exp Clin Cancer Res. 2021;40(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang H, Hua Y, Jiang Z, et al. Cancer‐associated fibroblast‐promoted LncRNA DNM3OS confers radioresistance by regulating DNA damage response in esophageal squamous cell carcinoma. Clin Cancer Res. 2019;25(6):1989‐2000. [DOI] [PubMed] [Google Scholar]
- 126. Zhang H, Yue J, Jiang Z, et al. CAF‐secreted CXCL1 conferred radioresistance by regulating DNA damage response in a ROS‐dependent manner in esophageal squamous cell carcinoma. Cell Death Dis. 2017;8(5):e2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ruocco MR, Avagliano A, Granato G, et al. Involvement of breast cancer‐associated fibroblasts in tumor development, therapy resistance and evaluation of potential therapeutic strategies. Curr Med Chem. 2018;25(29):3414‐3434. [DOI] [PubMed] [Google Scholar]
- 128. Zhai J, Shen J, Xie G, et al. Cancer‐associated fibroblasts‐derived IL‐8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37‐43. [DOI] [PubMed] [Google Scholar]
- 129. Zhang H, Deng T, Liu R, et al. CAF secreted miR‐522 suppresses ferroptosis and promotes acquired chemo‐resistance in gastric cancer. Mol Cancer. 2020;19(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ma J, Song X, Xu X, Mou Y. Cancer‐associated fibroblasts promote the chemo‐resistance in gastric cancer through secreting IL‐11 targeting JAK/STAT3/Bcl2 pathway. Cancer Res Treat. 2019;51(1):194‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Uchihara T, Miyake K, Yonemura A, et al. Extracellular vesicles from cancer‐associated fibroblasts containing annexin A6 induces FAK‐YAP activation by stabilizing beta1 integrin. Enhancing Drug Resistance Cancer Res. 2020;80(16):3222‐3235. [DOI] [PubMed] [Google Scholar]
- 132. Qin X, Guo H, Wang X, et al. Exosomal miR‐196a derived from cancer‐associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wei L, Ye H, Li G, et al. Correction: cancer‐associated fibroblasts promote progression and gemcitabine resistance via the SDF‐1/SATB‐1 pathway in pancreatic cancer. Cell Death Dis. 2021;12(3):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang L, Li X, Ren Y, et al. Cancer‐associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019;110(5):1609‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Masuda T, Nakashima T, Namba M, et al. Inhibition of PAI‐1 limits chemotherapy resistance in lung cancer through suppressing myofibroblast characteristics of cancer‐associated fibroblasts. J Cell Mol Med. 2019;23(4):2984‐2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Long X, Xiong W, Zeng X, et al. Cancer‐associated fibroblasts promote cisplatin resistance in bladder cancer cells by increasing IGF‐1/ERbeta/Bcl‐2 signalling. Cell Death Dis. 2019;10(5):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Deng X, Ruan H, Zhang X, et al. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int J Cancer. 2020;146(6):1700‐1716. [DOI] [PubMed] [Google Scholar]
- 138. Hu JL, Wang W, Lan XL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial‐mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Leung CS, Yeung TL, Yip KP, et al. Cancer‐associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J Clin Invest. 2018;128(2):589‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Guo H, Ha C, Dong H, Yang Z, Ma Y, Ding Y. Cancer‐associated fibroblast‐derived exosomal microRNA‐98‐5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell Int. 2019;19:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gao Y, Li X, Zeng C, et al. CD63+ cancer‐associated fibroblasts confer tamoxifen resistance to breast cancer cells through Exosomal miR‐22. Adv Sci. 2020;7(21):2002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fernandez‐Nogueira P, Mancino M, Fuster G, et al. Tumor‐associated fibroblasts promote HER2‐targeted therapy resistance through FGFR2 activation. Clin Cancer Res. 2020;26(6):1432‐1448. [DOI] [PubMed] [Google Scholar]
- 143. Zhang Z, Karthaus WR, Lee YS, et al. Tumor microenvironment‐derived NRG1 promotes antiandrogen resistance in prostate cancer. Cancer Cell. 2020;38(2):279‐296 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Liu J, Li P, Wang L, et al. Cancer‐associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell Mol Gastroenterol Hepatol. 2021;11(2):407‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Leask A. A centralized communication network: recent insights into the role of the cancer associated fibroblast in the development of drug resistance in tumors. Semin Cell Dev Biol. 2020;101:111‐114. [DOI] [PubMed] [Google Scholar]
- 146. Hirata E, Girotti MR, Viros A, et al. Intravital imaging reveals how BRAF inhibition generates drug‐tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27(4):574‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Frame MC, Serrels A. FAK to the rescue: activated stroma promotes a "safe haven" for BRAF‐mutant melanoma cells by inducing FAK signaling. Cancer Cell. 2015;27(4):429‐431. [DOI] [PubMed] [Google Scholar]
- 148. Alderton GK. Therapeutic resistance: fibroblasts restrain drug sensitivity. Nat Rev Cancer. 2015;15(6):318‐319. [DOI] [PubMed] [Google Scholar]
- 149. Anderson KI. Fibroblasts keep melanoma safe from harm. Dermatol Int. 2015;4(2):e1074788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Leask A. A centralized communication network: recent insights into the role of the cancer associated fib'roblast in the development of drug resistance in tumors. Semin Cell Dev Biol. 2020;101:111‐114. [DOI] [PubMed] [Google Scholar]
- 151. Flach EH, Rebecca VW, Herlyn M, Smalley KSM, Anderson ARA. Fibroblasts contribute to melanoma tumor growth and drug resistance. Mol Pharm. 2011;8(6):2039‐2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Apicella M, Giannoni E, Fiore S, et al. Increased lactate secretion by cancer cells sustains non‐cell‐autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab. 2018;28(6):848‐865 e6. [DOI] [PubMed] [Google Scholar]
- 153. Suzuki E, Yamazaki S, Naito T, et al. Secretion of high amounts of hepatocyte growth factor is a characteristic feature of cancer‐associated fibroblasts with EGFR‐TKI resistance‐promoting phenotype: a study of 18 cases of cancer‐associated fibroblasts. Pathol Int. 2019;69(8):472‐480. [DOI] [PubMed] [Google Scholar]
- 154. Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein‐alpha. Science. 2010;330(6005):827‐830. [DOI] [PubMed] [Google Scholar]
- 155. Gorchs L, Kaipe H. Interactions between cancer‐associated fibroblasts and T cells in the pancreatic tumor microenvironment and the role of chemokines. Cancers. 2021;13(12):2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Xiang H, Ramil CP, Hai J, et al. Cancer‐associated fibroblasts promote immunosuppression by inducing ROS‐generating monocytic MDSCs in lung squamous cell carcinoma. Cancer Immunol Res. 2020;8(4):436‐450. [DOI] [PubMed] [Google Scholar]
- 157. Silzle T, Kreutz M, Dobler MA, Brockhoff G, Knuechel R, Kunz‐Schughart LA. Tumor‐associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol. 2003;33(5):1311‐1320. [DOI] [PubMed] [Google Scholar]
- 158. Balsamo M, Scordamaglia F, Pietra G, et al. Melanoma‐associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(49):20847‐20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yang X, Lin Y, Shi Y, et al. FAP promotes immunosuppression by cancer‐associated fibroblasts in the tumor microenvironment via STAT3‐CCL2 signaling. Cancer Res. 2016;76(14):4124‐4135. [DOI] [PubMed] [Google Scholar]
- 160. Ford K, Hanley CJ, Mellone M, et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer‐associated fibroblast‐mediated CD8 T‐cell exclusion from tumors. Cancer Res. 2020;80(9):1846‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kato T, Noma K, Ohara T, et al. Cancer‐associated fibroblasts affect Intratumoral CD8+ and FoxP3+ T cells via IL6 in the tumor microenvironment. Clin Cancer Res. 2018;24(19):4820‐4833. [DOI] [PubMed] [Google Scholar]
- 163. Yu L, Liu Q, Huo J, Wei F, Guo W. Cancer‐associated fibroblasts induce immunotherapy resistance in hepatocellular carcinoma animal model. Cell Mol Biol. 2020;66(2):36‐40. [PubMed] [Google Scholar]
- 164. Melisi D, Garcia‐Carbonero R, Macarulla T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first‐line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119(10):1208‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD‐L1 and TGF‐beta. Sci Transl Med. 2018;10(424):eaan5488. [DOI] [PubMed] [Google Scholar]
- 166. Bianchini F, Giannoni E, Serni S, Chiarugi P, Calorini L. 22:6 n‐3 DHA inhibits differentiation of prostate fibroblasts into myofibroblasts and tumorigenesis. Br J Nutr. 2012;108(12):2129‐2137. [DOI] [PubMed] [Google Scholar]
- 167. Godichaud S, Krisa S, Couronné B, et al. Deactivation of cultured human liver myofibroblasts by trans‐resveratrol, a grapevine‐derived polyphenol. Hepatology. 2000;31(4):922‐931. [DOI] [PubMed] [Google Scholar]
- 168. Avasarala S, Zhang F, Liu G, Wang R, London SD, London L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral‐induced acute respiratory distress syndrome. PLoS One. 2013;8(2):e57285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ting H, Deep G, Kumar S, Jain AK, Agarwal C, Agarwal R. Beneficial effects of the naturally occurring flavonoid silibinin on the prostate cancer microenvironment: role of monocyte chemotactic protein‐1 and immune cell recruitment. Carcinogenesis. 2016;37(6):589‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hu Y, Hong Y, Xu YJ, Liu P, Guo DH, Chen Y. Inhibition of the JAK/STAT pathway with ruxolitinib overcomes cisplatin resistance in non‐small‐cell lung cancer NSCLC. Apoptosis. 2014;19(11):1627‐1636. [DOI] [PubMed] [Google Scholar]
- 171. Felt SA, Droby GN, Grdzelishvili VZ. Ruxolitinib and Polycation combination treatment overcomes multiple mechanisms of resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus. J Virol. 2017;91(16):e00461‐00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kim JW, Gautam J, Kim JE, Kim JA, Kang KW. Inhibition of tumor growth and angiogenesis of tamoxifen‐resistant breast cancer cells by ruxolitinib, a selective JAK2 inhibitor. Oncol Lett. 2019;17(4):3981‐3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kearney M, Franks L, Lee S, et al. Phase I/II trial of ruxolitinib in combination with trastuzumab in metastatic HER2 positive breast cancer. Breast Cancer Res Treat. 2021;189(1):177‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Stover DG, Gil del Alcazar CR, Brock J, et al. Phase II study of ruxolitinib, a selective JAK1/2 inhibitor, in patients with metastatic triple‐negative breast cancer. NPJ Breast Cancer. 2018;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Long KB, Tooker G, Tooker E, et al. IL6 receptor blockade enhances chemotherapy efficacy in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16(9):1898‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mace TA, Shakya R, Pitarresi JR, et al. IL‐6 and PD‐L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67(2):320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Brunetto E, de Monte L, Balzano G, et al. The IL‐1/IL‐1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer. J Immunother Cancer. 2019;7(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhang D, Li L, Jiang H, et al. Tumor‐stroma IL1beta‐IRAK4 feedforward circuitry drives tumor fibrosis, chemoresistance, and poor prognosis in pancreatic cancer. Cancer Res. 2018;78(7):1700‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Huang H, Zhang Y, Gallegos V, et al. Targeting TGFbetaR2‐mutant tumors exposes vulnerabilities to stromal TGFbeta blockade in pancreatic cancer. EMBO Mol Med. 2019;11(11):e10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sun L, Huang C, Zhu M, et al. Gastric cancer mesenchymal stem cells regulate PD‐L1‐CTCF enhancing cancer stem cell‐like properties and tumorigenesis. Theranostics. 2020;10(26):11950‐11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. He W, Liang B, Wang C, et al. MSC‐regulated lncRNA MACC1‐AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38(23):4637‐4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ji R, Zhang B, Zhang X, et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14(15):2473‐2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Stagg J. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008;4(2):119‐124. [DOI] [PubMed] [Google Scholar]
- 184. Xie M, Tao L, Zhang Z, Wang W. Mesenchymal stem cells mediated drug delivery in tumor‐targeted therapy. Curr Drug Deliv. 2021;18(7):876‐891. [DOI] [PubMed] [Google Scholar]
- 185. Bonomi A, Steimberg N, Benetti A, et al. Paclitaxel‐releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematol Oncol. 2017;35(4):693‐702. [DOI] [PubMed] [Google Scholar]
- 186. Pessina A, Coccè V, Pascucci L, et al. Mesenchymal stromal cells primed with paclitaxel attract and kill leukaemia cells, inhibit angiogenesis and improve survival of leukaemia‐bearing mice. Br J Haematol. 2013;160(6):766‐778. [DOI] [PubMed] [Google Scholar]
- 187. Wang X, Chen H, Zeng X, et al. Efficient lung cancer‐targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin B. 2019;9(1):167‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Saulite L, Pleiko K, Popena I, Dapkute D, Rotomskis R, Riekstina U. Nanoparticle delivery to metastatic breast cancer cells by nanoengineered mesenchymal stem cells. Beilstein J Nanotechnol. 2018;9:321‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kang S, Bhang SH, Hwang S, et al. Mesenchymal stem cells aggregate and deliver gold nanoparticles to tumors for photothermal therapy. ACS Nano. 2015;9(10):9678‐9690. [DOI] [PubMed] [Google Scholar]
- 190. Uchibori R, Okada T, Ito T, et al. Retroviral vector‐producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med. 2009;11(5):373‐381. [DOI] [PubMed] [Google Scholar]
- 191. Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue‐derived mesenchymal stem cells expressing prodrug‐converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18(1):223‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wei H, Chen J, Wang S, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment In vitro. Int J Nanomedicine. 2019;14:8603‐8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ren C, Kumar S, Chanda D, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon‐beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15(21):1446‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Nakamizo A, Marini F, Amano T, et al. Human bone marrow‐derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307‐3318. [DOI] [PubMed] [Google Scholar]
- 195. Li X, Lu Y, Huang WL, et al. In vitro effect of adenovirus‐mediated human gamma interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematol Oncol. 2006;24(3):151‐158. [DOI] [PubMed] [Google Scholar]
- 196. Yang ZS, Tang XJ, Guo XR, et al. Cancer cell‐oriented migration of mesenchymal stem cells engineered with an anticancer gene (PTEN): an imaging demonstration. Onco Targets Ther. 2014;7:441‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Du J, Zhang Y, Xu C, Xu X. Apoptin‐modified human mesenchymal stem cells inhibit growth of lung carcinoma in nude mice. Mol Med Rep. 2015;12(1):1023‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sharif S, Ghahremani MH, Soleimani M. Delivery of exogenous miR‐124 to glioblastoma multiform cells by Wharton's jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev Rep. 2018;14(2):236‐246. [DOI] [PubMed] [Google Scholar]
- 199. Lee HK, Finniss S, Cazacu S, et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self‐renewal. Oncotarget. 2013;4(2):346‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Xiang L, Xu X, Zhang S, Cai D, Dai X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic Biol Med. 2018;124:205‐213. [DOI] [PubMed] [Google Scholar]
- 202. Wang P, Zhou R, Thomas P, et al. Epithelial‐to‐mesenchymal transition enhances cancer cell sensitivity to cytotoxic effects of cold atmospheric plasmas in breast and bladder cancer systems. Cancers. 2021;13(12):2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Hua D, Cai D, Ning M, et al. Cold atmospheric plasma selectively induces G0/G1 cell cycle arrest and apoptosis in AR‐independent prostate cancer cells. J Cancer. 2021;12(19):5977‐5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Golpour M, Alimohammadi M, Sohbatzadeh F, Fattahi S, Bekeschus S, Rafiei A. Cold atmospheric pressure plasma treatment combined with starvation increases autophagy and apoptosis in melanoma in vitro and in vivo. Exp Dermatol. 2022;31:1016‐1028. [DOI] [PubMed] [Google Scholar]
- 205. Privat‐Maldonado A, Verloy R, Cardenas Delahoz E, et al. Cold atmospheric plasma does not affect stellate cells phenotype in pancreatic cancer tissue in Ovo. Int J Mol Sci. 2022;23(4):1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Dai X, Zhang Z, Zhang J, Ostrikov KK. Dosing: the key to precision plasma oncology. Plasma Processes Polym. 2020;17:e1900178. [Google Scholar]
- 207. Miao Y, Han P, Hua D, et al. Cold atmospheric plasma increases IBRV titer in MDBK cells by orchestrating the host cell network. Virulence. 2021;12(1):679‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Furuta T, Shi L, Toyokuni S. Non‐thermal plasma as a simple ferroptosis inducer in cancer cells: a possible role of ferritin. Pathol Int. 2018;68(7):442‐443. [DOI] [PubMed] [Google Scholar]
- 209. Miller V, Lin A, Fridman A. Why target immune cells for plasma treatment of cancer. Plasma Chem Plasma Process. 2015;36:259‐268. [Google Scholar]
- 210. Virard F, Cousty S, Cambus JP, Valentin A, Kémoun P, Clément F. Cold atmospheric plasma induces a predominantly necrotic cell death via the microenvironment. PLoS One. 2015;10(8):e0133120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Van Loenhout J, Freire Boullosa L, Quatannens D, et al. Auranofin and cold atmospheric plasma synergize to trigger distinct cell death mechanisms and immunogenic responses in glioblastoma. Cell. 2021;10(11):2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lee S, Lee H, Jeong D, et al. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF‐7 breast cancer cell. Free Radic Biol Med. 2017;110:280‐290. [DOI] [PubMed] [Google Scholar]
- 213. Nitsch A, Strakeljahn S, Jacoby JM, et al. New approach against chondrosoma cells‐cold plasma treatment inhibits cell motility and metabolism, and leads to apoptosis. Biomedicine. 2022;10(3):1183‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Dai X, Bazaka K, Richard DJ, Thompson ER, Ostrikov KK. The emerging role of gas plasma in oncotherapy. Trends Biotechnol. 2018;36(11):1183‐1198. [DOI] [PubMed] [Google Scholar]
- 215. Dai X, Bazaka K, Thompson E, Ostrikov K. Cold atmospheric plasma: a promising controller of cancer cell states. Cancers. 2020;12(11):3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Zhou X, Cai D, Xiao S, et al. InvivoPen: a novel plasma source for in vivo cancer treatment. J Cancer. 2020;11(8):2273‐2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Schneider C, Gebhardt L, Arndt S, et al. Acidification is an essential process of cold atmospheric plasma and promotes the anti‐cancer effect on malignant melanoma cells. Cancers. 2019;11(5):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Bekeschus S, Freund E, Spadola C, et al. Risk assessment of kINPen plasma treatment of four human pancreatic cancer cell lines with respect to metastasis. Cancers. 2019;11(9):1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Evert K, Kocher T, Schindler A, et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci Rep. 2021;11:20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. FDA . Summary FDA IDE #G190165 clinical Trials.gov identifier: NCT04267575 Canady Helios cold plasma scalpel treatment at the surgical margin & macroscopic tumor site. 2021.
- 221. Chen DS, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity. 2013;39(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 222. Wang C, Li P, Liu L, et al. Self‐adjuvanted nanovaccine for cancer immunotherapy: role of lysosomal rupture‐induced ROS in MHC class I antigen presentation. Biomaterials. 2016;79:88‐100. [DOI] [PubMed] [Google Scholar]
- 223. Lin A, Gorbanev Y, de Backer J, et al. Non‐thermal plasma as a unique delivery system of short‐lived reactive oxygen and nitrogen species for immunogenic cell death in melanoma cells. Adv Sci. 2019;6(6):1802062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Espey MG. Tumor macrophage redox and effector mechanisms associated with hypoxia. Free Radic Biol Med. 2006;41(11):1621‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Vennin C, Mélénec P, Rouet R, et al. CAF hierarchy driven by pancreatic cancer cell p53‐status creates a pro‐metastatic and chemoresistant environment via perlecan. Nat Commun. 2019;10(1):3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shi L, Yu L, Zou F, Hu H, Liu K, Lin Z. Gene expression profiling and functional analysis reveals that p53 pathway‐related gene expression is highly activated in cancer cells treated by cold atmospheric plasma‐activated medium. PeerJ. 2017;5:e3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Schmidt A, Bekeschus S, Jarick K, Hasse S, von Woedtke T, Wende K. Cold physical plasma modulates p53 and mitogen‐activated protein kinase signaling in keratinocytes. Oxid Med Cell Longev. 2019;2019:7017363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma‐associated fibroblast‐like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68(11):4331‐4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582‐598. [DOI] [PubMed] [Google Scholar]
- 230. Barcellos‐de‐Souza P, Comito G, Pons‐Segura C, et al. Mesenchymal stem cells are recruited and activated into carcinoma‐associated fibroblasts by prostate cancer microenvironment‐derived TGF‐beta1. Stem Cells. 2016;34(10):2536‐2547. [DOI] [PubMed] [Google Scholar]
- 231. Xu X, Dai X, Xiang L, Cai D, Xiao S, Ostrikov K. Quantitative assessment of cold atmospheric plasma anti‐cancer efficacy in triple‐negative breast cancers. Plasma Processes Polym. 2018;15(8):e1800052. [Google Scholar]
- 232. Aggelopoulos CA, Christodoulou AM, Tachliabouri M, et al. Cold atmospheric plasma attenuates breast cancer cell growth through regulation of cell microenvironment effectors. Front Oncol. 2021;11:826865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Zhu W, Lee SJ, Castro NJ, Yan D, Keidar M, Zhang LG. Synergistic effect of cold atmospheric plasma and drug loaded core‐shell nanoparticles on inhibiting breast cancer cell growth. Sci Rep. 2016;6:21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Liu Z, Zheng Y, Dang J, et al. A novel antifungal plasma‐activated hydrogel. ACS Appl Mater Interfaces. 2019;11(26):22941‐22949. [DOI] [PubMed] [Google Scholar]
- 235. Xu Y, Niu Y, Wu B, et al. Extended‐release of therapeutic microRNA via a host‐guest supramolecular hydrogel to locally alleviate renal interstitial fibrosis. Biomaterials. 2021;275:120902. [DOI] [PubMed] [Google Scholar]
- 236. Dai X, Luo Y, Xu Y, Zhang J. Key indexes and the emerging tool for tumor microenvironment editing. Am J Cancer Res. 2019;9(5):1027‐1042. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.


