Abstract
Background
Poor prognosis is linked to peripheral blood levels of preoperative platelet‐lymphocyte ratio (PLR) and neutrophil‐lymphocyte ratio (NLR) in many advanced cancers. Nevertheless, whether the correlation exists in resected early‐stage cases with non‐small cell lung cancer (NSCLC) stays controversial. Consequently, we performed a meta‐analysis to explore the preoperative NLR and PLR's prognostic significance in early‐stage patients with NSCLC undergoing curative surgery.
Methods
Relevant studies that validated the link between preoperative NLR or PLR and survival results were found via the proceeding databases: PubMed, Embase, Cochrane Library, and Web of Science. The merged 95% confidence interval (CI) and hazard ratio (HR) was employed to validate the link between the NLR or PLR's index and overall survival (OS) and disease‐free survival (DFS) in resected NSCLC cases. We used sensitivity and subgroup analyses to assess the studies' heterogeneity.
Results
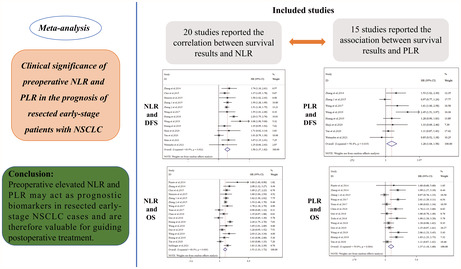
An overall of 21 studies were attributed to the meta‐analysis. The findings indicated that great preoperative NLR was considerably correlated with poor DFS (HR = 1.58, 95% CI: 1.37–1.82, p < 0.001) and poor OS (HR = 1.51, 95% CI: 1.33–1.72, p < 0.001), respectively. Subgroup analyses were in line with the pooled findings. In aspect of PLR, raised PLR was indicative of inferior DFS (HR = 1.28, 95% CI: 1.04–1.58, p = 0.021) and OS (HR = 1.37, 95% CI: 1.18–1.60, p < 0.001). In the subgroup analyses between PLR and DFS, only subgroups with a sample size <300 (HR = 1.67, 95% CI: 1.15–2.43, p = 0.008) and TNM staging of mixed (I‐II) (HR = 1.47, 95% CI: 1.04–2.07, p = 0.028) showed that the link between high PLR and poor DFS was significant.
Conclusions
Preoperative elevated NLR and PLR may act as prognostic biomarkers in resected early‐stage NSCLC cases and are therefore valuable for guiding postoperative adjuvant treatment.
Keywords: meta‐analysis, neutrophil‐lymphocyte ratio, non‐small cell lung cancer, operation, platelet‐lymphocyte ratio, prognosis
The meta‐analysis investigated the prognostic significance of preoperative NLR and PLR in early‐stage patients with NSCLC undergoing curative surgery. A total of 21 studies were enrolled in the metaanalysis. Summarizing the available data from the included studies, our results confirmed that high preoperative NLR and PLR were significantly associated with inferior DFS and OS. Therefore, the levels of preoperative NLR and PLR may serve as prognostic biomarkers in the clinic and are valuable measures for guiding postoperative adjuvant treatment.
1. INTRODUCTION
Non‐small cell lung cancer (NSCLC), which makes up about 85% of lung malignancies, is the most repeatedly diagnosed cancer globally and the leading mortality reason among all other cancers. 1 Notwithstanding the recent rapid advancements in molecular therapeutic strategies and immunotherapy, surgery is still the go‐to treatment for people with early‐stage NSCLC. However, when contrasted to thoracotomy, minimally invasive surgery (MIS), including video‐assisted thoracoscopic surgery (VATS), can significantly reduce the time patients need to stay in the hospital and the number of postoperative complications they experience. 2 , 3
Furthermore, the tumor‐node‐metastasis (TNM) classification system can be utilized to some extent for determining treatment strategy and prognostic evaluation in patients with NSCLC. 4 , 5 This is valuable for clinicians, for whom the prognosis of cases with lung cancer has always been the focus. Nevertheless, patients with similar TNM staging continuously have different clinic survival outcomes.
Due to accumulating studies, it has become increasingly evident that inflammation can affect the progression and invasion of lung cancer. 6 , 7 Systematic inflammatory cells, along with their calculated ratios, including platelet‐lymphocyte ratio (PLR), lymphocyte‐monocyte ratio (LMR), and neutrophil‐lymphocyte ratio (NLR), act as independent factors which influence the cases' prognosis with different cancers, involving lung, 8 breast, 9 and nasopharyngeal malignancies. 10 A published meta‐analysis, involving 244 studies of cases with resected pan‐cancers, demonstrated that a close association exists between raised NLR/PLR and poor OS or cancer‐specific survival (CSS) and between raised LMR and poor OS. 11 Another meta‐analysis, including cases with NSCLC medicated with immune checkpoint inhibitors (ICIs), also indicated that raised PLR and NLR were related to inferior progression‐free survival (PFS) and OS. 12 Nevertheless, the link between NLR/PLR and survival outcomes in cases with resected NSCLC stays indefinite. Therefore, a comprehensive meta‐analysis is warranted to validate the NLR/PLR's prognostic significance in patients with NSCLC.
Accordingly, this meta‐analysis includes numerous relevant studies to explore the link between peripheral blood NLR/PLR and survival results in resected early‐stage NSCLC cases.
2. MATERIALS AND METHODS
2.1. Search strategy
We searched the literature from the four following databases: PubMed, Embase, Cochrane Library, and Web of Science. The primary deadline for literature retrieval was March 2022. The search strategy was performed using the following terms: (“Lung neoplasms” OR “pulmonary neoplasms” OR “lung cancer” OR “Carcinoma, Non‐Small‐Cell Lung” OR “non‐small cell lung cancer” OR “NSCLC”) AND (“Surgical Procedures, Operative” OR “surgery” OR “operative therapy” OR “operation” OR “operative procedures” OR “invasive procedures”) AND ((“neutrophil lymphocyte ratio” OR “neutrophil‐to‐lymphocyte ratio” OR “NLR”) OR (“platelet lymphocyte ratio” OR “platelet‐to‐lymphocyte ratio” OR “PLR”)). The specific retrieval strategy can be found in Supplementary Text S1.
2.2. Exclusion and inclusion criteria
Studies gathering the following requirements were involved: (a) populations involving early‐stage NSCLC patients; (b) populations of NSCLC patients for whom curative surgical resection was performed; (c) the link between NLR/PLR and prognostic indicators of OS and/or disease‐free survival (DFS) were investigated; (d) the 95% confidence interval (CI) and hazard ratio (HR) could be obtained from the original studies; (e) the literature was written in English.
The exclusion criteria were as follows: (a) reviews, case reports, abstracts, letters, and expert opinions; (b) populations of patients with other primary tumors; (c) surgical resection was not performed; (d) the studies had insufficient data to conclude on the HR and 95% CI; (e) literature with Newcastle‐Ottawa Scale (NOS) scores <6; (f) the literature was not published in English.
2.3. Literature's data extraction and quality validation
Two reviewers (Weibo Cao and Haochuan Yu) independently extracted relevant data and evaluated the studies' quality. Any disagreements were then resolved through discussion and consensus.
The first author's name, the year of publication, study design, country of origin, study design, sample size, pathological category of lung cancer, TNM, median follow‐up (months), NLR/PLR cut‐off values, and survival data (DFS and/or OS) were all extracted from each study. When both multivariate and univariate analyses were run, the multivariate analysis's HRs were extracted first. Our way of determining cut‐off values for subgroup analysis was exploited according to the median value from all of NLR or PLR cut‐off in the studies recruited in this meta‐analysis.
We evaluated the literature's quality involved according to the scoring system of the NOS. The NOS contains three aspects: patient selection, comparability, and outcome assessment. Literature with scores of ≥6 was considered of high quality. Studies with lower scores were regarded as low quality and therefore excluded from the analysis.
2.4. Statistical analysis
Higgins I2 and Cochran's Q test statistics were utilized to evaluate the studies' heterogeneity. p ≤ 0.10 or I2 ≥ 50% were significant heterogeneity indicative, and in such cases, a random‐effect model was utilized. Conversely, a fixed‐effect model was utilized to evaluate studies that did not present significant heterogeneity. Subgroup analyses dependent on the sample size, TNM, and cut‐off values were also performed to investigate potential factors influencing the NLR/PLR's prognostic significance. Sensitivity analyses were then performed to determine any heterogeneity's source and assess the results' stability. Publication bias assessment was conducted via Funnel plots and Egger's test, in which p < 0.05 was deemed statistically significant. All statistical analyses were done utilizing STATA 15.0 (Stata Corporation, College Station, TX).
3. RESULTS
3.1. Literature search and study characteristics
As per the search approach, 1006 studies were initially identified, of which 290 were ruled out owing to duplication. By screening literature titles and abstracts, a further 634 studies were excluded based upon the exclusion criteria, resulting in 82 full‐text studies. Of these, 21 studies met the inclusion criteria and were subsequently involved in the meta‐analysis. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The literature selection process is outlined in Figure 1.
FIGURE 1.
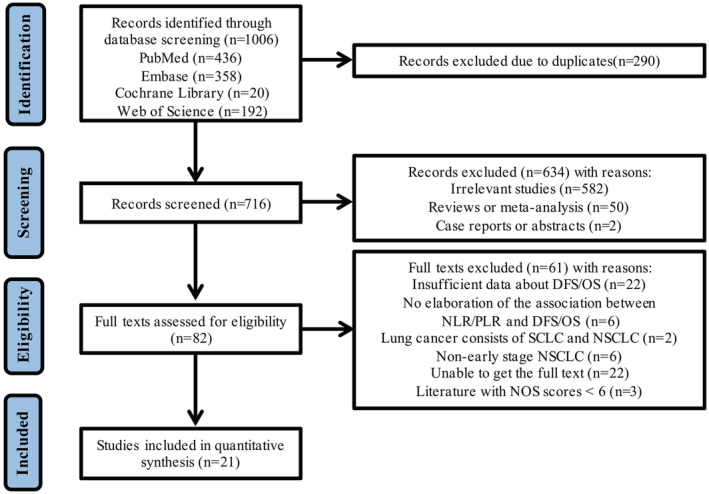
The flow diagram of literature selection.
Table 1 lists the studies' primary traits that made up the meta‐analysis. In conclusion, case sample sizes in the studies ranged from 134 to 2027, and the studies were all published between 2014 and 2021. Out of the included studies, six focused on NLR only, one concentrated on PLR only, and 14 evaluated both NLR and PLR. We had 20 NLR studies with 13,915 lung cancer cases and 15 PLR studies with 7484 lung cancer cases in the meta‐analysis. NOS scores for the quality of the involved literature varied from 6 to 9, demonstrating a high quality of literature among the 21 studies. Quality assessment of involved studies can be found in Supplementary Table S1.
TABLE 1.
Main characteristics of the studies included in the meta‐analysis
| Author | Year | Region | Study design | Sample size | Histology | TNM | Postoperative adjuvant therapy | Median follow‐up (months) | Prognostic value | NLR cut‐off | PLR cut‐off | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pinato et al. | 2014 | Europe | P | 220 | NSCLC | I‐III | 7% adjuvant CT; 7% adjuvant RT; 4% adjuvant CRT | NR | NLR, PLR | 5 | 300 | DFS, OS |
| Zhang et al. | 2014 | China | R | 400 | NSCLC | I‐II | NP | 46 | NLR, PLR | 3.3 | 171 | DFS, OS |
| Choi et al. | 2015 | America | R | 1139 | NSCLC | I‐III | 25% adjuvant CT; 15% adjuvant RT | NR | NLR | 5 | NR | DFS, OS |
| Shimizu et al. | 2015 | Japan | R | 334 | NSCLC | I‐III | NR | 32 | NLR | 2.5 | NR | DFS, OS |
| Zhang 1 et al. | 2015 | China | R | 678 | NSCLC | I‐III | Adjuvant therapy a | 43.5 | NLR, PLR | 2.3 | 106 | DFS, OS |
| Zhang 2 et al. | 2015 | China | R | 1238 | NSCLC | I‐III | Adjuvant therapy a | 45 | NLR | 2.3 | NR | DFS, OS |
| Wang et al. | 2017 | China | R | 134 | SCC | I‐III | 58% adjuvant CT | 22 | NLR, PLR | 2.16 | 145 | DFS, OS |
| Yuan et al. | 2017 | China | R | 1466 | NSCLC | I‐III | 44% adjuvant CT | 69.9 | NLR, PLR | 2.06 | 204 | OS |
| Chen et al. | 2018 | China | R | 577 | NSCLC | I | 16% adjuvant CT | 93.77 | NLR, PLR | 3.13 | 81.07 | OS |
| Gao et al. | 2018 | China | R | 410 | NSCLC | I‐III | NR | 54 | NLR, PLR | 1.90 | 108.8 | OS |
| Huang et al. | 2018 | China | R | 589 | NSCLC | I‐III | NR | 44 | NLR | 2.3 | NR | DFS, OS |
| Toda et al. | 2018 | Japan | R | 327 | NSCLC | I‐III | 24% adjuvant CT | NR | PLR | NR | 162 | DFS, OS |
| Wang et al. | 2018 | China | R | 952 | NSCLC | I‐III | 51% adjuvant CT | 40 | NLR, PLR | 3.1 | 170.58 | OS |
| Guo et al. | 2019 | China | R | 569 | NSCLC | I‐III | NR | 60.3 | NLR, PLR | 1.74 | 88.7 | OS |
| Wang et al. | 2019 | China | R | 261 | NSCLC | I‐III | NR | 38 | NLR, PLR | 2.12 | 92.9 | DFS, OS |
| Huang et al. | 2019 | China | R | 254 | NSCLC | I‐III | 54% adjuvant CT; 7% adjuvant RT | 48 | NLR, PLR | 3.18 | 122 | DFS, OS |
| Shoji et al. | 2020 | Japan | R | 311 | NSCLC | I | NP | 63 | NLR, PLR | 1.5 | 184 | DFS |
| Yan et al. | 2020 | China | R | 538 | NSCLC | I‐III | 58% adjuvant CT | 54 | NLR, PLR | 2.35 | 150.95 | DFS, OS |
| Shen et al. | 2021 | China | R | 1431 | AD | I | 13% adjuvant CT | 63 | NLR | 2.606 | NR | DFS |
| Watanabe et al. | 2021 | Japan | R | 387 | NSCLC | I‐III | 28% adjuvant CT | 39.2 | NLR, PLR | 2.90 | 231 | DFS |
| Seitlinger et al. | 2021 | Europe | R | 2027 | NSCLC | I‐III | 32% adjuvant CT; 2% adjuvant RT; 7% adjuvant CRT | 69 | NLR | 4.07 | NR | OS |
Abbreviations: AD, adenocarcinoma; CRT, chemo/radiotherapy; CT, chemotherapy; DFS, disease‐free survival; NLR, neutrophil‐lymphocyte ratio; NP, not provided; NR, not reported; NSCLC, non‐small cell lung cancer; OS, overall survival; P, prospective study; PLR, platelet‐lymphocyte ratio; R, retrospective study; RT, radiotherapy; SCC, squamous cell carcinoma.
The exact means of treatment (CT, RT, or CRT) are not mentioned.
3.2. NLR's impact on DFS
Of the 21 studies, 20 reported the correlation between survival results and NLR in resected early‐stage NSCLC cases. Of these, 13 studies provided information on DFS (Figure 2A), which recommended that raised NLR was correlated with poor DFS (HR = 1.58, 95% CI: 1.37–1.82, p < 0.001). Owing to the great heterogeneity detected (I 2 = 60.90%, p = 0.002), a random‐effect model was utilized to evaluate the studies. From the subgroup analyses, a strong association between raised NLR and poor DFS was noted for all subgroups, indicating the reliability of the meta‐analysis. The findings of this can be found in Table 2.
FIGURE 2.
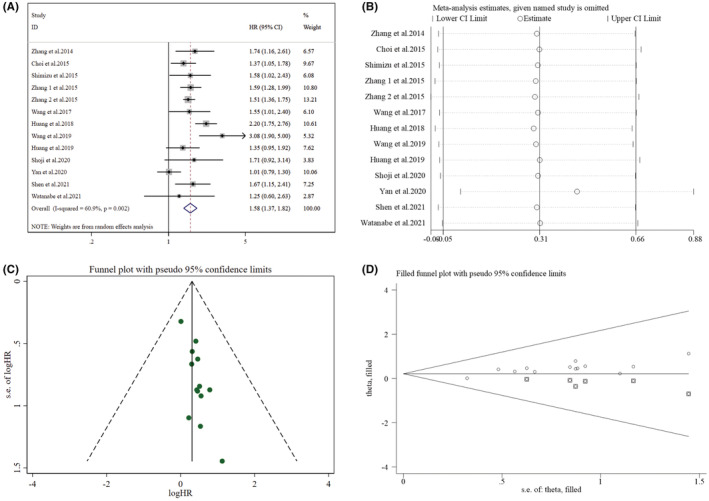
Pooled analyses of the association between preoperative NLR and DFS in resected early‐stage NSCLC patients. (A) Forest plot of the correlation between preoperative NLR and DFS. (B) Sensitivity analysis for DFS after excluding each study. (C) Funnel plot of publication bias regarding DFS. (D) Funnel plot adjusted by the trim and fill method regarding DFS.
TABLE 2.
Subgroup analyses of the association between preoperative NLR and survival outcomes
| Variables | N | DFS | Heterogeneity | N | OS | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p‐value | I 2 | p‐value | HR (95% CI) | p‐value | I 2 | p‐value | |||
| Total | 13 | 1.58(1.37,1.82) | <0.001 | 60.90% | 0.002 | 17 | 1.51(1.33,1.72) | <0.001 | 68.00% | <0.001 |
| Sample size | ||||||||||
| <300 | 4 | 1.79(1.28,2.49) | 0.001 | 60.60% | 0.055 | 4 | 1.85(1.36,2.51) | <0.001 | 36.20% | 0.195 |
| ≥300 | 9 | 1.44(1.15,1.79) | <0.001 | 63.50% | 0.005 | 13 | 1.45(1.26,1.67) | <0.001 | 72.20% | <0.001 |
| TNM | ||||||||||
| I‐III | 10 | 1.56(1.32,1.84) | <0.001 | 70.10% | <0.001 | 15 | 1.50(1.31,1.72) | <0.001 | 70.60% | <0.001 |
| Mixed (I‐II) | 3 | 1.70(1.33,2.19) | <0.001 | 0.00% | 0.989 | 2 | 1.63(1.07,2.48) | 0.023 | 56.10% | 0.131 |
| Cut‐off value | ||||||||||
| ≥2.5 | 6 | 1.48(1.27,1.73) | <0.001 | 0.00% | 0.863 | 8 | 1.67(1.46,1.91) | <0.001 | 1.80% | 0.416 |
| <2.5 | 7 | 1.66(1.33,2.07) | <0.001 | 78.90% | <0.001 | 9 | 1.40(1.16,1.68) | <0.001 | 79.90% | <0.001 |
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; NLR, neutrophil‐lymphocyte ratio; OS, overall survival.
From sensitivity analyses (Figure 2B, Supplementary Table S2), it was conducted that a single study's omission could not statistically affect the NLR's impact on DFS. Nevertheless, after excluding the study of Yan et al. (2020), the heterogeneity was found to decrease significantly (I 2 = 39.60%, p = 0.077), with a pooled HR of 1.65 (95% CI: 1.47–1.86, p < 0.001).
3.3. Influence of NLR on OS
An overall of 17 studies investigated the NLR's impact on OS, among which high heterogeneity was detected (I 2 = 68.00%, p < 0.001). Thus, the random‐effect model was again utilized, resulting in a pooled HR of 1.51 (95% CI: 1.33–1.72, p < 0.001). Overall, results from these studies revealed that great NLR was linked with worse OS in cases with NSCLC (Figure 3A). This can be seen from the subgroup analyses between OS and NLR outlined in Table 2, indicating a significant association between raised NLR and poor OS.
FIGURE 3.
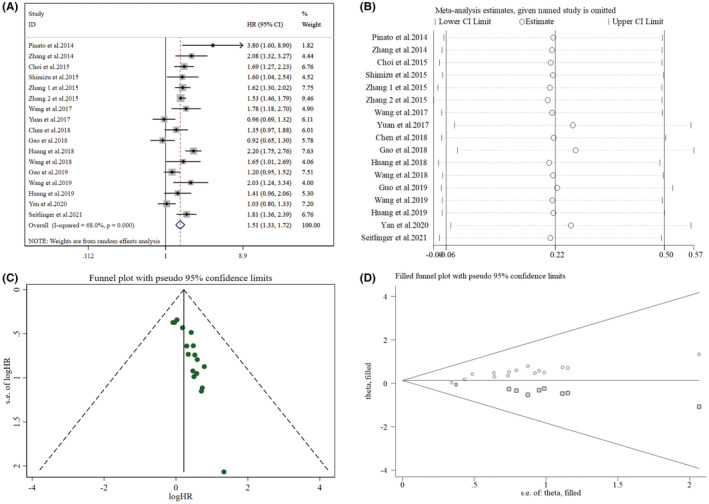
Pooled analyses of the correlation between preoperative NLR and OS in resected early‐stage NSCLC patients. (A) Forest plot of the association between preoperative NLR and OS. (B) Sensitivity analysis for OS after excluding each study. (C) Funnel plot of publication bias regarding OS. (D) Funnel plot adjusted by the trim and fill method regarding OS.
Furthermore, sensitivity analyses exploring the potential heterogeneity of OS (Figure 3B) suggested that the omission of a single study would not be able to statistically affect the NLR's impact on OS in the meta‐analysis. In terms of OS (Supplementary Table S3), it was found that heterogeneity decreased to some extent (I 2 = 61.00%, p = 0.001) after excluding the study of Huang et al. (2018), with a merged HR of 1.46 (95% CI: 1.29–1.65, p < 0.001).
3.4. Effect of PLR on DFS
Eight studies reported the correlation between DFS and PLR (Figure 4A) and were evaluated utilizing a random‐effect model owing to high heterogeneity (I 2 = 58.40%, p = 0.019). The pooled findings revealed that raised PLR was correlated with poorer DFS (HR = 1.28, 95% CI: 1.04–1.58, p = 0.021). As listed in Table 3, only subgroups with sample size <300 (HR = 1.67, 95% CI: 1.15–2.43, p = 0.008) and TNM staging of mixed (I‐II) (HR = 1.47, 95% CI: 1.04–2.07, p = 0.028) demonstrated a significant link between raised PLR and poor DFS.
FIGURE 4.
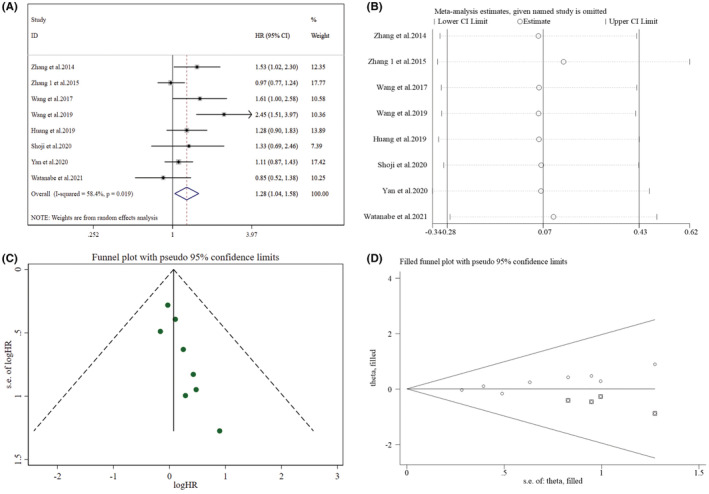
Pooled analyses of the relationship between preoperative PLR and DFS in resected early‐stage NSCLC patients. (A) Forest plot of the link between preoperative PLR and DFS. (B) Sensitivity analysis for DFS after excluding each study. (C) Funnel plot of publication bias regarding DFS. (D) Funnel plot adjusted by the trim and fill method regarding DFS.
TABLE 3.
Subgroup analyses of the association between preoperative PLR and survival outcomes
| Variables | N | DFS | Heterogeneity | N | OS | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p‐value | I2 | p‐value | HR (95% CI) | p‐value | I2 | p‐value | |||
| Total | 8 | 1.28(1.04,1.58) | 0.021 | 58.40% | 0.019 | 13 | 1.37(1.18,1.60) | < 0.001 | 59.00% | 0.004 |
| Sample size | ||||||||||
| <300 | 3 | 1.67(1.15,2.43) | 0.008 | 55.60% | 0.105 | 4 | 1.93(1.51,2.48) | < 0.001 | 0.00% | 0.970 |
| ≥300 | 5 | 1.09(0.92,1.30) | 0.308 | 19.70% | 0.289 | 9 | 1.24(1.07,1.43) | 0.004 | 51.10% | 0.037 |
| TNM | ||||||||||
| I‐III | 6 | 1.25(0.97,1.61) | 0.085 | 67.00% | 0.010 | 11 | 1.30(1.12,1.52) | 0.001 | 55.60% | 0.013 |
| Mixed (I‐II) | 2 | 1.47(1.04,2.07) | 0.028 | 0.00% | 0.716 | 2 | 1.87(1.37,2.54) | < 0.001 | 0.00% | 0.695 |
| Cut‐off value | ||||||||||
| ≥150 | 4 | 1.17(0.94,1.45) | 0.168 | 17.60% | 0.303 | 6 | 1.31(1.06,1.61) | 0.012 | 49.40% | 0.079 |
| <150 | 4 | 1.43(0.97,2.10) | 0.069 | 76.80% | 0.005 | 7 | 1.44(1.14,1.82) | 0.002 | 68.30% | 0.004 |
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; OS, overall survival; PLR, platelet‐lymphocyte ratio.
In terms of DFS (Figure 4B, Supplementary Table S4), the heterogeneity decreased significantly (I 2 = 23.90%, p = 0.247) after ruling out the study of Wang et al. (2019), yet no association was found between preoperative PLR and DFS (HR = 1.16, 95% CI: 0.99–1.36, p = 0.060). Moreover, after excluding the studies of Zhang et al. (2014) (HR = 1.25, 95% CI: 0.99–1.57, p = 0.056) and Wang et al. (2017) (HR = 1.25, 95% CI: 1.00–1.56, p = 0.054), preoperative PLR and DFS remained uncorrelated.
3.5. PLR's influence on OS
Data relating to the PLR's effect on OS was provided by 13 studies (Figure 5A). High heterogeneity (I 2 = 59.00%, p = 0.004) was found among these studies. Thus, a meta‐analysis was conducted utilizing the random‐effect model, from which a strong relation was found between high PLR and worse OS (HR = 1.37, 95% CI: 1.18–1.60, p < 0.001). For the subgroup analyses between PLR and OS (Table 3), all subgroups indicated a significant link between higher PLR and shorter OS.
FIGURE 5.
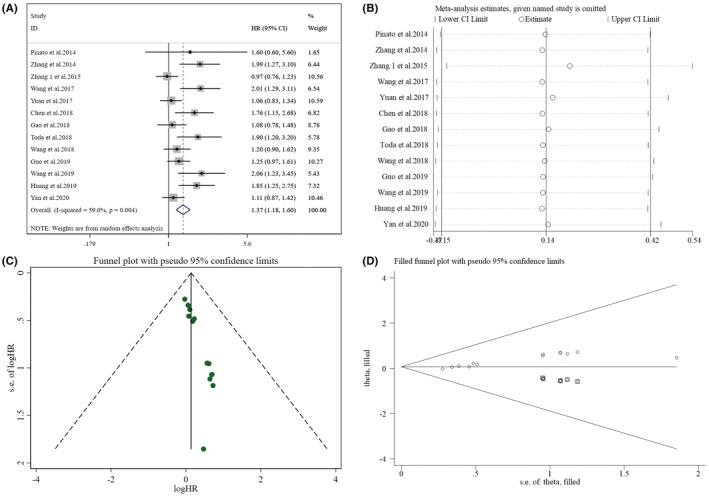
Pooled analyses of the link between preoperative PLR and OS in resected early‐stage NSCLC patients. (A) Forest plot of the relationship between preoperative PLR and OS. (B) Sensitivity analysis for OS after excluding each study. (C) Funnel plot of publication bias regarding OS. (D) Funnel plot adjusted by the trim and fill method regarding OS.
As for the sensitivity analyses of OS (Figure 5B, Supplementary Table S5), after excluding the study of Zhang 1 et al. (2015), heterogeneity decreased to some extent (I2 = 53.50%, p = 0.014), with a combined HR of 1.43 (95% CI: 1.22–1.66, p < 0.001), suggesting that raised PLR was still linked with poor OS.
3.6. Publication bias
Funnel plots were found not visually asymmetric as per the influence of NLR on DFS (Figure 2C) and OS (Figure 3C), indicating a significant risk of publication bias. This observation was further validated by the findings of Egger's test for DFS (p = 0.001) and OS (p < 0.001). Subsequently, trim and fill methods were done to explore the publication bias's impact on effect estimates. No statistically significant alterations were noticed in the findings (Figures 2D and 3D). As per the PLR's influence on DFS (Figure 4C) and OS (Figure 5C), the p values for DFS (Egger's test, p = 0.012) and OS (Egger's test, p < 0.001) showed significant publication bias, accommodated by funnel plots which were not asymmetrical from visual inspection. Moreover, trim and fill methods again indicated no statistically significant changes in the results (Figures 4D and 5D).
4. DISCUSSION
Surgery has continuity as the radical medication strategy preferred for cases with early‐stage NSCLC. It is also essential in the comprehensive modality of several cases with advanced‐stage NSCLC. 34 In recent decades, several independent prognostic biomarkers have been discovered in patients with resected NSCLC. 35 , 36 , 37 Previous studies have demonstrated that NLR and PLR, as host inflammatory markers, are often used as valuable prognostic indicators for some cancers. 38 , 39 , 40 , 41 However, it remains unclear if NLR and PLR still have a similar effect in cases with resected NSCLC.
In the present study, we merged 20 NLR studies with 13,915 lung cancer cases and 15 PLR studies with 7484 lung cancer cases to further find out the NLR and PLR's prognostic impact in resected early‐stage cases with NSCLC. The meta‐analysis found that increased preoperative NLR in peripheral blood was correlated with poor DFS and OS. Despite high heterogeneity, the prognostic effect of NLR was not weakened by all subgroup analyses dependent on sample size, TNM, and cut‐off value, which supports the reliability of the meta‐analysis. Our combined findings regarding PLR still showed that high PLR was connected to poor DFS and OS. In terms of OS, each subgroup revealed a connection between longer OS and higher PLR. For the subgroup analyses between PLR and DFS, only subgroups with sample size <300 and TNM staging of mixed (I‐II) indicated a significant correlation between raised PLR and poor DFS. These results suggest that the pooled results between PLR and DFS lack stability, which may be attributed to the limited studies in the meta‐analysis.
The underlying mechanisms of the link between great preoperative NLR/PLR and poor survival results in NSCLC cases remain unknown. It has been noticed that tumor‐linked neutrophils can produce IL‐17A, promoting epithelial‐mesenchymal transition (EMT) in gastric cancer via JAK2/STAT3 signaling cascade. 42 Additionally, the study by Mishalian et al. (2014) demonstrated that neutrophils promote regulatory T‐cells into the tumor by secreting CCL17. Regulatory T‐cells possess the function of immunosuppression and thus impair anti‐tumor immune function. 43 Furthermore, inflammatory neutrophils can promote tumor angiogenesis and tumor progression by releasing vascular endothelial growth factor (VEGF) and Gelatinase A (MMP‐2). 44 , 45 Thus, raised neutrophils are often correlated with poor prognosis in cancer cases. Several studies have shown that absolute lymphocytes can anticipate long‐term survival outcomes in cancer patients. 46 , 47 Consequently, lymphocytes play an essential role in immune defense, and reduced lymphocytes are often associated with poorer survival in patients. Platelets were also often associated with biological processes that allowed tumors to escape immune defense, thereby promoting tumor progression and dissemination. 48 It may therefore be concluded that NLR and PLR act essential functions in the inflammatory and anti‐inflammatory balance in the human immune response.
As a literature‐dependent meta‐analysis, several restrictions of this meta‐analysis should be noted. First, most of the literature involved were retrospective studies; hence, selection bias is present. More prospective studies are therefore required to affirm our findings. Second, only literature published in English was included in our study, with studies in other languages and unpublished studies not included, which reduced the data available for analysis. It is also possible that unpublished studies contain results considered ‘unfavorable’ or ‘negative’ by the researcher or the publisher, which may otherwise have contributed to this meta‐analysis. Third, characteristics such as sample size, region, and TNM differed throughout studies, which may also be the source of high heterogeneity. Fourth, we cannot rule out the possibility that non‐tumor‐related factors influence the blood markers of patients. Moreover, 19 out of 21 studies did not specify the pathological types of NSCLC and we could not perform subgroup analyses based on pathological type. Finally, there was a lack of standardization of cut‐off values for NLR or PLR in the included studies in the meta‐analysis. The cut‐off values of NLR (range: 1.5–5.0) and PLR (range: 81.07–300) differed throughout the involved studies. These cut‐off values were defined in different ways. For instance, in the study of Yuan et al. (2017), the appropriate cut‐off values for NLR and PLR were termed by X‐tile software. 20 In contrast, in the study of Chen et al. (2018), the optimal values of PLR and LMR were determined by receiver operating characteristic (ROC) curve analysis. 21 Therefore, the lack of standardization of NLR and PLR cut‐off values limits the translation of study results to clinical application. It was necessary to standardize the cut‐off values of preoperative NLR and PLR.
5. CONCLUSIONS
This meta‐analysis concludes that poor DFS and OS in resected early‐stage cases of NSCLC are strongly correlated with preoperative peripheral blood high NLR or PLR. This suggests that levels of NLR and PLR may serve as prognostic biomarkers in the clinic and are valuable measures for guiding patients' postoperative adjuvant treatment. In the future, more prospective, multi‐center, and large‐sample studies are needed to affirm the findings of this meta‐analysis and promote its clinical application, especially in combination with other prognostic biomarkers.
AUTHOR CONTRIBUTIONS
The authors all participated in designing the meta analysis. Weibo Cao, Shuai Zhu, and Ning Zhou participated in the literature search. Weibo Cao and Haochuan Yu independently extrated relevant data and evaluated the quality of the studies. Tong Li, Fan Ren, and Xi Lei performed the statistical analysis. Weibo Cao wrote the first draft. Song Xu and Lingling Zu revised the article. All authors agreed on the final version of the manuscript.
FUNDING INFORMATION
The present study was funded by the National Natural Science Foundation of China (82172776), Tianjin Science and Technology Plan Project (19ZXDBSY00060) and (303078100412), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK‐061B), and Diversified Input Project of Tianjin National Natural Science Foundation (21JCYBJC01770).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Ethical approval doesn't apply in the meta‐analysis.
Supporting information
Text S1. The specific retrieval strategy in each database.
Table S1. Quality assessment by the Newcastle‐Ottawa Scale.
Table S2. Sensitivity analysis of the relationship between NLR and DFS.
Table S3. Sensitivity analysis of the association between NLR and OS.
Table S4. Sensitivity analysis of the link between PLR and DFS.
Table S5. Sensitivity analysis of the correlation between PLR and OS.
Cao W, Yu H, Zhu S, et al. Clinical significance of preoperative neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio in the prognosis of resected early‐stage patients with non‐small cell lung cancer: A meta‐analysis. Cancer Med. 2023;12:7065‐7076. doi: 10.1002/cam4.5505
Weibo Cao and Haochuan Yu contributed equally to this work.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during the meta‐analysis are included in the published article and its supplementary materials.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non‐small‐cell lung cancer. Nat Med. 2021;27(8):1345‐1356. [DOI] [PubMed] [Google Scholar]
- 3. Ujiie H, Gregor A, Yasufuku K. Minimally invasive surgical approaches for lung cancer. Expert Rev Respir Med. 2019;13(6):571‐578. [DOI] [PubMed] [Google Scholar]
- 4. Donnem T, Kilvaer TK, Andersen S, et al. Strategies for clinical implementation of TNM‐Immunoscore in resected nonsmall‐cell lung cancer. Ann Oncol. 2016;27(2):225‐232. [DOI] [PubMed] [Google Scholar]
- 5. Shin J, Keam B, Kim M, et al. Prognostic impact of newly proposed M descriptors in TNM classification of non‐small cell lung cancer. J thorac Oncol. 2017;12(3):520‐528. [DOI] [PubMed] [Google Scholar]
- 6. Lu C‐H, Yeh D‐W, Lai C‐Y, et al. USP17 mediates macrophage‐promoted inflammation and stemness in lung cancer cells by regulating TRAF2/TRAF3 complex formation. Oncogene. 2018;37(49):6327‐6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carpagnano GE, Palladino GP, Lacedonia D, Koutelou A, Orlando S, Foschino‐Barbaro MP. Neutrophilic airways inflammation in lung cancer: the role of exhaled LTB‐4 and IL‐8. BMC Cancer. 2011;11:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li A, Mu X, He K, et al. Prognostic value of lymphocyte‐to‐monocyte ratio and systemic immune‐inflammation index in non‐small‐cell lung cancer patients with brain metastases. Future Oncol. 2020;16(30):2433‐2444. [DOI] [PubMed] [Google Scholar]
- 9. Ethier J‐L, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil‐to‐lymphocyte ratio in breast cancer: a systematic review and meta‐analysis. Breast Cancer Res. 2017;19(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takenaka Y, Kitamura T, Oya R, et al. Prognostic role of neutrophil‐lymphocyte ratio in nasopharyngeal carcinoma: a meta‐analysis. PloS One. 2017;12(7):e0181478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta‐analysis. Sci Rep. 2017;7(1):16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio in non‐small cell lung cancer patients treated with immune checkpoint inhibitors: a meta‐analysis. Int Immunopharmacol. 2020;85:106677. [DOI] [PubMed] [Google Scholar]
- 13. Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation‐based prognostic indices in primary operable non‐small cell lung cancer. Br J Cancer. 2014;110(8):1930‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang T, Jiang Y, Qu X, Shen H, Liu Q, Du J. Evaluation of preoperative hematologic markers as prognostic factors and establishment of novel risk stratification in resected pN0 non‐small‐cell lung cancer. PloS One. 2014;9(10):e111494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi JE, Villarreal J, Lasala J, et al. Perioperative neutrophil:lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer Med. 2015;4(6):825‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimizu K, Okita R, Saisho S, Maeda A, Nojima Y, Nakata M. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non‐small cell lung cancer. World J Surg Oncol. 2015;13:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Xia H, Zhang L, Zhang B, Yue D, Wang C. Clinical significance of preoperative neutrophil‐lymphocyte vs platelet‐lymphocyte ratio in primary operable patients with non‐small cell lung cancer. Am J Surg. 2015;210(3):526‐535. [DOI] [PubMed] [Google Scholar]
- 18. Zhang H, Zhang L, Zhu K, et al. Prognostic significance of combination of preoperative platelet count and neutrophil‐lymphocyte ratio (COP‐NLR) in patients with non‐small cell lung cancer: based on a large cohort study. PloS One. 2015;10(5):e0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y‐Q, Zhi Q‐J, Wang X‐Y, Yue D‐S, Li K, Jiang R‐C. Prognostic value of combined platelet, fibrinogen, neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with lung adenosquamous cancer. Oncol Lett. 2017;14(4):4331‐4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuan C, Li N, Mao X, Liu Z, Ou W, Wang S‐Y. Elevated pretreatment neutrophil/white blood cell ratio and monocyte/lymphocyte ratio predict poor survival in patients with curatively resected non‐small cell lung cancer: results from a large cohort. Thoracic Cancer. 2017;8(4):350‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Wang W, Zhang X, et al. Prognostic significance of combined preoperative platelet‐to‐lymphocyte ratio and lymphocyte‐to‐monocyte ratio in patients undergoing surgery with stage IB non‐small‐cell lung cancer. Cancer Manag Res. 2018;10:5411‐5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune‐inflammation index predicts poor prognosis in patients with operable non‐small cell lung cancer. Clinica Chimica Acta. 2018;484:272‐277. [DOI] [PubMed] [Google Scholar]
- 23. Huang W, Wang S, Zhang H, Zhang B, Wang C. Prognostic significance of combined fibrinogen concentration and neutrophil‐to‐lymphocyte ratio in patients with resectable non‐small cell lung cancer. Cancer Biol Med. 2018;15(1):88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toda M, Tsukioka T, Izumi N, et al. Platelet‐to‐lymphocyte ratio predicts the prognosis of patients with non‐small cell lung cancer treated with surgery and postoperative adjuvant chemotherapy. Thoracic Cancer. 2018;9(1):112‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Qu X, Kam N‐W, et al. An inflammation‐related nomogram for predicting the survival of patients with non‐small cell lung cancer after pulmonary lobectomy. BMC Cancer. 2018;18(1):692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo W, Cai S, Zhang F, et al. Systemic immune‐inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non‐small cell lung cancer. Thoracic Cancer. 2019;10(4):761‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Hu X, Xu W, Wang H, Huang Y, Che G. Prognostic value of a novel scoring system using inflammatory response biomarkers in non‐small cell lung cancer: a retrospective study. Thoracic Cancer. 2019;10(6):1402‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Q, Diao P, Li C‐L, et al. Preoperative platelet‐lymphocyte ratio is a superior prognostic biomarker to other systemic inflammatory response markers in non‐small cell lung cancer. Medicine. 2020;99(4):e18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shoji F, Kozuma Y, Toyokawa G, Yamazaki K, Takeo S. Complete blood cell count‐derived inflammatory biomarkers in early‐stage non‐small‐cell lung cancer. Annals Thorac Cardiovasc Surg. 2020;26(5):248‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan X, Li G. Preoperative systemic immune‐inflammation index predicts prognosis and guides clinical treatment in patients with non‐small cell lung cancer. Biosci Rep. 2020;40(3):BSR20200352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen Y‐J, Qian L‐Q, Ding Z‐P, et al. Prognostic value of inflammatory biomarkers in patients with stage I lung adenocarcinoma treated with surgical dissection. Front Oncol. 2021;11:711206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe K, Noma D, Masuda H, Masuda M. Preoperative inflammation‐based scores predict early recurrence after lung cancer resection. J Thorac Dis. 2021;13(5):2812‐2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seitlinger J, Prieto M, Guerrera F, et al. Neutrophil‐to‐lymphocyte ratio is correlated to driver gene mutations in surgically‐resected non‐small cell lung cancer and its post‐operative evolution impacts outcomes. Clin Lung Cancer. 2022;23(1):e29‐e42. [DOI] [PubMed] [Google Scholar]
- 34. Lang‐Lazdunski L. Surgery for nonsmall cell lung cancer. Eur Respir Rev. 2013;22(129):382‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Renaud S, Falcoz P‐E, Schaëffer M, et al. Prognostic value of the KRAS G12V mutation in 841 surgically resected Caucasian lung adenocarcinoma cases. Br J Cancer. 2015;113(8):1206‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheng L, Luo M, Sun X, Lin N, Mao W, Su D. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer. 2013;133(11):2720‐2725. [DOI] [PubMed] [Google Scholar]
- 37. Kim S‐H, Go S‐I, Song DH, et al. Prognostic impact of CD8 and programmed death‐ligand 1 expression in patients with resectable non‐small cell lung cancer. Br J Cancer. 2019;120(5):547‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil‐lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: a systematic review and meta‐analysis. Int J Surg. 2018;55:73‐80. [DOI] [PubMed] [Google Scholar]
- 39. Hu G, Xu F, Zhong K, et al. The prognostic role of preoperative circulating neutrophil‐lymphocyte ratio in primary bladder cancer patients undergoing radical cystectomy: a meta‐analysis. World J Urol. 2019;37(9):1817‐1825. [DOI] [PubMed] [Google Scholar]
- 40. Hamid HKS, Davis GN, Trejo‐Avila M, Igwe PO, Garcia‐Marín A. Prognostic and predictive value of neutrophil‐to‐lymphocyte ratio after curative rectal cancer resection: a systematic review and meta‐analysis. Surg Oncol. 2021;37:101556. [DOI] [PubMed] [Google Scholar]
- 41. Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta‐analysis. Ann Surg Oncol. 2016;23(2):646‐654. [DOI] [PubMed] [Google Scholar]
- 42. Li S, Cong X, Gao H, et al. Tumor‐associated neutrophils induce EMT by IL‐17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mishalian I, Bayuh R, Eruslanov E, et al. Neutrophils recruit regulatory T‐cells into tumors via secretion of CCL17—a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135(5):1178‐1186. [DOI] [PubMed] [Google Scholar]
- 44. Gong Y, Koh D‐R. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010;339(2):437‐448. [DOI] [PubMed] [Google Scholar]
- 45. Shamamian P, Schwartz JD, Pocock BJ, et al. Activation of progelatinase a (MMP‐2) by neutrophil elastase, cathepsin G, and proteinase‐3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189(2):197‐206. [DOI] [PubMed] [Google Scholar]
- 46. Miyoshi Y, Yoshimura Y, Saito K, et al. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin‐but not with treatment of physician's choice‐in the EMBRACE study. Breast Cancer. 2020;27(4):706‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Behl D, Porrata LF, Markovic SN, et al. Absolute lymphocyte count recovery after induction chemotherapy predicts superior survival in acute myelogenous leukemia. Leukemia. 2006;20(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 48. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237‐249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. The specific retrieval strategy in each database.
Table S1. Quality assessment by the Newcastle‐Ottawa Scale.
Table S2. Sensitivity analysis of the relationship between NLR and DFS.
Table S3. Sensitivity analysis of the association between NLR and OS.
Table S4. Sensitivity analysis of the link between PLR and DFS.
Table S5. Sensitivity analysis of the correlation between PLR and OS.
Data Availability Statement
All data generated or analyzed during the meta‐analysis are included in the published article and its supplementary materials.


