Abstract
Empirical evidence addressing the association between SARS-CoV-2 vaccination and long COVID would guide public health priorities and inform personal health decisions. Herein, the co-primary objectives are to determine the differential risk of long COVID in vaccinated versus unvaccinated patients, and the trajectory of long COVID following vaccination. Of 2775 articles identified via systematic search, 17 were included, and 6 were meta-analyzed. Meta-analytic results determined that at least one vaccine dose was associated with a protective effect against long COVID (OR 0.539, 95% CI 0.295–0.987, p = 0.045, N = 257 817). Qualitative analysis revealed that trajectories of pre-existing long COVID following vaccination were mixed, with most patients reporting no changes. The evidence herein supports SARS-CoV-2 vaccination for the prevention of long COVID, and recommends long COVID patients adhere to standard SARS-CoV-2 vaccination schedules.
Keywords: COVID-19, SARS-CoV-2, Long COVID, Post-COVID-19 condition, Post-COVID-19 syndrome, Post-acute sequelae of COVID-19 PASC, Vaccination, Inflammation, Cognition, Prevention, Treatment, Population health, Depression, Bipolar disorder, Mood disorders
1. Introduction
It is well established that COVID-19 is associated with significant mortality, as well as morbidity, and that the latter encompasses persons experiencing post-COVID conditions. Long COVID, differentially defined as symptoms persisting for a minimum of 4 weeks to 12 weeks following SARS-CoV-2 infection, is rapidly emerging as a global health priority. The World Health Organization (WHO) defines ‘post-COVID-19 condition’ as “occurring in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis”. (Soriano et al., 2021) Ten to 30% of infected individuals exhibit enduring symptoms, including, but not limited to, fatigue, cognitive impairment, dyspnea, and mental disorders. (Soriano et al., 2021, Ceban et al., 2021b, Renaud-Charest et al., 2021) Although numerous clinical trials are ongoing, (Ceban et al., 2022) there are currently no safe and effective treatments for long COVID. Recent estimates quantifying the economic burden associated with long COVID suggest that the condition could account for over 15% of labour shortages in the United States. (Bach, 2022).
The accelerated development and approval of multiple vaccines against SARS-CoV-2 has proven a preeminent strategy in altering the trajectory of the COVID-19 pandemic. There are currently 50 SARS-CoV-2 vaccines approved across the globe in different jurisdictions, and 242 vaccine candidates in Phase I-III trials. (COVID19 vaccine tracker, 2022) Although SARS-CoV-2 vaccination reduces the risk of infection and severe disease, (McDonald et al., 2021) breakthrough cases (i.e., infection following vaccination[s]) are increasingly frequent. (Gupta and Topol, 2021) Cases of long COVID have been reported to occur following breakthrough infections (Bergwerk et al., 2021) and the Omicron variant(s), (Ayoubkhani and Bosworth, 2022) however, the differential risk of long COVID in breakthrough infections remains to be determined.
A related but separate consideration is the effect of SARS-CoV-2 vaccination on pre-existing long COVID. Fear of exacerbating long COVID symptoms has contributed to vaccine hesitancy, which may put individuals at undue risk. (Gaber et al., 2021, Scherlinger et al., 2021) Moreover, evidence suggests that individuals with long COVID may be at increased risk of re-infection due to immune system dysfunction, further underscoring the need to determine the safety of SARS-CoV-2 vaccination in individuals with long COVID. (Sun et al., 2021, Su et al., 2022) Taken together, a review of the extant literature concerning the trajectory of pre-existing long COVID following SARS-CoV-2 vaccination is warranted in order to enable patients to make informed health decisions.
The co-primary objectives of the present review are to 1) determine the differential risk of long COVID in vaccinated versus unvaccinated patients, as well as 2) to establish the trajectory of pre-existing long COVID following SARS-CoV-2 vaccination(s). We hypothesize that SARS-CoV-2 vaccination will have a protective effect against the development of long COVID and will not exacerbate pre-existing long COVID symptoms.
2. Methods
2.1. Data sources and searches
The protocol pertaining to this review was registered on PROSPERO (CRD42022307220). A systematic search was conducted on PubMed/MEDLINE, PsycInfo, EMBASE, Web of Science, and Scopus from database inception to January 27, 2022. The search string was: “long COVID [MeSH] AND vaccines [MeSH]”. We additionally performed manual searches on Google Scholar and medRxiv incorporating the search term “breakthrough infection”, as well as manually searched the references of relevant articles. No language or publication date restrictions were imposed.
Titles and abstracts were independently screened by two review authors (FC and DK) using the Covidence platform. (Better systematic review management, 2022) Articles identified as potentially relevant by at least one reviewer were retrieved, and duplicates were removed. Full text-articles were independently screened by two reviewers (FC and DK), with discrepancies resolved through discussion. Authors of potentially eligible studies were contacted to provide clarification and/or supplementary data where necessary. This review adhered to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) (Stroup et al., 2000) and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. (Moher et al., 2009).
2.2. Study selection
We sought articles reporting on the association of prior SARS-CoV-2 vaccination and occurrence of long COVID in breakthrough infections (i.e., co-primary outcome one), and/or the symptom trajectory of pre-existing long COVID following SARS-CoV-2 vaccination (i.e., co-primary outcome two). Inclusion criteria were established prior to article review and were as follows:
-
1.
Qualitative or quantitative data pertaining to at least one of the two co-primary outcomes, as defined previously.
-
2.
‘Long COVID’ defined as symptoms persisting beyond the acute phase of COVID-19. All established definitions of long COVID are permissible, including proprietary definitions proposed by study authors.
-
3.
Median/mean follow-up of at least 4 weeks (28 days) since SARS-CoV-2 infection.
-
4.
Studies reporting on the development/occurrence of long COVID following breakthrough infection must include a control group of individuals which did not receive the SARS-CoV-2 vaccine prior to infection, derived from the same population as the vaccinated group, or a control group of individuals that received fewer doses of the SARS-CoV-2 vaccine(s).
-
5.
Primary research.
-
6.
Presentation as full-text article, including preprints.
The exclusion criteria were:
-
1.
Study does not report data pertaining to the co-primary outcomes.
-
2.
Outcomes reported in the general population, or in persons without a prior COVID-19 diagnosis and/or SARS-CoV-2 vaccination.
-
3.
Long COVID symptom reporting occurs at a median/mean follow-up time of less than 4 weeks (28 days) since SARS-CoV-2 infection/COVID-19 diagnosis; this is the rate-limiting symptom duration across all the established definitions of long COVID.
-
4.
Mathematical models/projections intended to predict future caseloads. Due to the evolving and complex nature of the COVID-19 pandemic, as well as the large variety of statistical models possible, such forecasts may not be accurate.
-
5.
Non-primary research, unpublished data, abstract, case report, study with a sample size of less than 10, or protocol. Case series including >10 individuals are eligible for inclusion.
2.3. Data extraction
Published summary data were independently extracted by two reviewers (FC and DK) using a piloted data extraction form, then corroborated, with discrepancies resolved through discussion. Information to be extracted was established a priori and included study characteristics, participant characteristics and subgroups, sample size and source, treatment (i.e., type and manufacturer of SARS-CoV-2 vaccine), summary data of vaccinated and unvaccinated individuals, number of vaccine doses received, summary data of infected and uninfected individuals, modes of ascertainment, follow-up period/symptom duration, long COVID definition, symptoms, and associated functional outcomes, and additional quantitative and qualitative results pertaining to the two co-primary outcome measures, as defined previously.
2.4. Quality assessment
Methodological quality and risk of bias were assessed using the Newcastle-Ottawa Scale (NOS), (Stang, 2010) modified for cohort and case-control studies, as well as adapted for cross-sectional studies (as previously utilized in meta-analyses by our group) (Ceban et al., 2021a, Ceban et al., 2021b), and the Joanna Briggs Institute (JBI) Checklist for Case Series was employed for quality appraisal of case series. All component studies were independently rated by two reviewers (FC and DK) and results were corroborated, with discrepancies resolved through discussion. Modified NOSs, the JBI checklist, and methodological quality rankings for each study organized by design are provided in the Supplement.
2.5. Data synthesis and analysis
A meta-analysis of pre-calculated odds ratios (ORs) was undertaken to determine whether prior SARS-CoV-2 vaccination was associated with a protective effect against the development/occurrence of long COVID (i.e., ‘prevention meta-analysis’). Complete statistical methods are described in the Supplement.
Qualitative analysis via narrative synthesis was undertaken for all component studies, including those captured in the meta-analyses. A quantitative analysis was not undertaken to examine the nature of the association between SARS-CoV-2 vaccination and pre-existing long COVID due to a high degree of inter-study heterogeneity insofar as study design and data reporting, as well as the low methodological quality ratings of many studies reporting data on the foregoing outcome. Although a meta-analysis was conducted to investigate the association between SARS-CoV-2 and protective effects against long COVID, we chose to include a narrative analysis to describe additional and/or nuanced results which were not captured by the meta-analysis.
3. Results
3.1. Overview of component studies
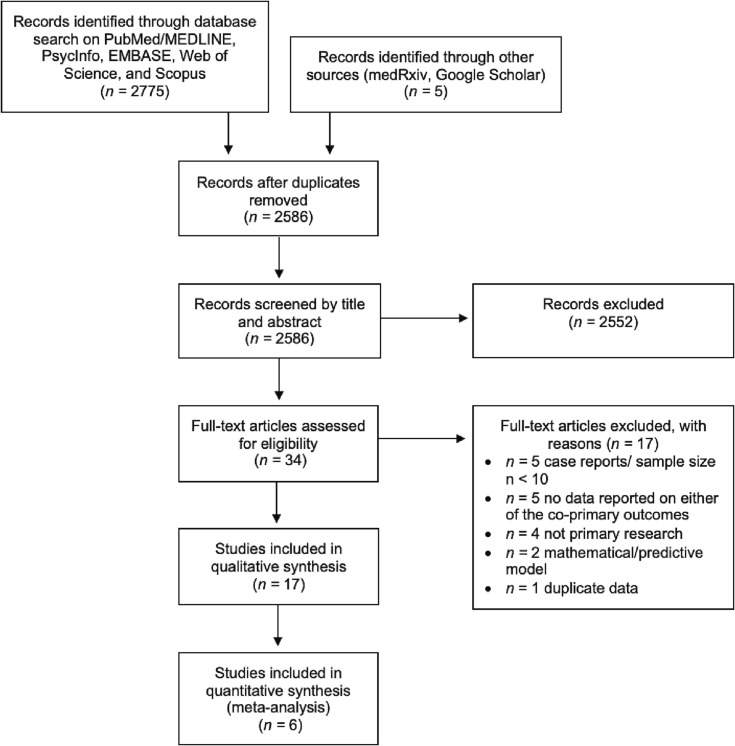
The combined search yielded 2775 articles, of which 34 were eligible following the removal of duplicates and screening of titles and abstracts. Seventeen studies were further excluded following full-text screening. Details of study selection are provided in Fig. 1 . Seventeen studies were included in the present review: 7 cross-sectional studies, (Gaber et al., 2021, Scherlinger et al., 2021, Strain et al., 2022, Wanga et al., 2021, Kuodi et al., 2022, Schultheiß et al., 2021, Senjam et al., 2021) 3 prospective cohort studies, (Ayoubkhani et al., 2021, Ayoubkhani et al., 2022, Tran et al., 2021), 5 retrospective cohort studies, (Arjun et al., 2022, Taquet et al., 2021, Simon et al., 2021, Al-Aly et al., 2021, Herman et al., 2022) 1 case-control study, (Antonelli et al., 2022) and 1 case series with prospective enrolment. (Arnold et al., 2021) Four studies analyzed data from the United States, (Taquet et al., 2021, Simon et al., 2021, Wanga et al., 2021, Al-Aly et al., 2021), 3 from the United Kingdom (UK), (Antonelli et al., 2022, Ayoubkhani et al., 2022, Ayoubkhani et al., 2021), 2 from England, (Gaber et al., 2021, Arnold et al., 2021), 2 from France (Scherlinger et al., 2021, Tran et al., 2021) and India, (Senjam et al., 2021, Arjun et al., 2022), and one from Israel, (Kuodi et al., 2022) Germany, (Schultheiß et al., 2021) and Indonesia, (Herman et al., 2022) respectively. Strain et al. (Strain et al., 2022) included data predominantly from the UK, but also from respondents in Israel, Russia, India, and South Africa. The sample sizes ranged from 36 to 240 648. Respondents were vaccinated with Pfizer-BioNTech BNT162b2, Moderna mRNA-1273, Janssen Ad26.COV2.S, AstraZeneca ChAdOx1 nCoV-19, and/or Novavax-Serum Institute of India Covovax NVX-CoV2373. Table 1 provides detailed characteristics and summaries of applicable findings pertaining to SARS-CoV-2 vaccination for the prevention of long COVID, and Table 2 provides detailed characteristics and summaries of applicable findings pertaining to SARS-CoV-2 vaccination for the treatment of long COVID. Simon et al. (Simon et al., 2021) reported both on vaccination prior to the development of long COVID and vaccination post-infection and is thus included in both tables. The terms ‘long COVID’ and ‘post-COVID-19 condition’ are used interchangeably throughout the manuscript; the favoured patient term “long COVID” is preferentially used in order to increase the accessibility of the present review to the public.
Fig. 1.
PRISMA flow diagram of study selection.
Table 1.
Studies investigating SARS-CoV-2 vaccination for the prevention of long COVID (n = 9).
| Study | Country | Study Design | Sample Source | Total Sample | Vaccinated (Treatment) Group | Control Group | Long COVID Definition/Persistent COVID-19 Symptoms and Frequency | Summary of Findings |
|---|---|---|---|---|---|---|---|---|
| Al-Aly et al., 2021* | United States | Retrospective Cohort | United States Veterans Health Administration (VHA) EHR |
n = 64 571 Age range: ≥18 Mean (SD) age*: 66.62 (13.79) Mean (SD) age**: 56.07 (15.72) Sex (%F/%M)*: 8.68/91.32 Sex (%F/%M)**: 14.15/85.85 *breakthrough cases, prior to weighting **no prior SARS-CoV-2 vaccination, prior to weighting |
|
|
|
Those with breakthrough COVID-19 exhibited a lower risk of post-acute sequelae and burden (-30.60; 95% CI −42.25, −18.49) compared to those with COVID-19 and no prior history of SARS-CoV-2 vaccination. The risk of post-acute sequelae in the cardiovascular, coagulation, metabolic, and pulmonary organ systems, as well as risk of fatigue, was lower in those with breakthrough COVID-19 vs. those with COVID-19 and without prior SARS-CoV-2 vaccination. The lower risk was not statistically significant for post-acute sequelae in affecting the kidney, gastrointestinal, mental health, and neurologic organ systems. |
| Antonelli et al., 2022 | United Kingdom | Case-control | COVID Symptom Study mobile phone app |
n = 16,800 Age range: ≥18 Mean (SD) age*: 50.2 (14.1) Mean (SD) age**: 52.9 (13.5) Mean (SD) age***: 51.7 (14.5) Mean (SD) age****: 54.0 (13.1) Sex (%F/%M)*: 62.5/37.5 Sex (%F/%M)**: 61.2/38.8 Sex (%F/%M)***: 62.5/37.5 Sex (%F/%M)****: 61.2/38.8 *cases 1 **cases 2 ***controls 1 ****controls 2 |
|
|
|
The odds of reporting persistent symptoms were approximately halved (OR 0.51, 95% CI 0.32–0.82, p = 0.0015) by having two SARS-CoV-2 vaccine doses. The odds of reporting persistent symptoms were not significantly associated with one prior SARS-CoV-2 vaccine dose (OR 1.04, 95% CI 0.86–1.25, p = 0.691). Reported rates of persistent symptoms lasting ≥ 28 days post-infection are as follows: cases 3: 229/2479 (9.2%) cases 4: 31/592 (5.2%) controls 3: 296/2762 (10.7%) controls 4: 55/482 (4%) |
| Arjun et al., 2022* | India | Retrospective Cohort |
Department of Community Medicine and Family Medicine, All India Institute of Medical Sciences Bhubaneswar |
n = 487 Age range: ≥18 Mean (SD) age: 39 (15) Sex (%F/%M): 40.9/59.1 |
|
|
|
Having received 2 doses of SARS-CoV-2 vaccination was significantly associated with long COVID (aOR 2.32, 95% CI 1.17–4.58, p = 0.01). One dose was not significantly associated with long COVID. |
| Herman et al., 2022* | Indonesia | Retrospective cohort | Indonesian POST-COVID retrospective longitudinal data (online questionnaire) |
n = 442 Mean (SD) age*: 31.60 (8.58) Mean (SD) age**: 32.49 (10.39) (%F/%M)*: 50.2/49.8 Sex (%F/%M)**: 49.8/50.2 *double vaccinated, after matching **unvaccinated, after matching |
|
|
|
Breakthrough infections occurring > 14 days following full SARS-CoV-2 vaccination were associated with a 69% lower odds of developing olfactory dysfunction (aOR 0.31, 95% CI 0.102–0.941). The greater the interval between the second dose and SARS-CoV-2 infection, the greater the odds of developing long COVID (aOR 1.012 95% CI 1.002–1.022, p = 0.015). |
| Kuodi et al., 2022* | Israel | Cross-sectional (nested in prospective cohort) | Ziv Medical Centre, Padeh-Poriya Medical Centre, and Galilee Medical Centre |
n = 3388 Age range: >18 Mean age: N/A Sex (%F/%M): N/A |
|
|
|
After adjusting for follow-up time and baseline symptoms, those who received two SARS-CoV-2 vaccine doses were less likely than unvaccinated individuals to report post-COVID fatigue by 64%, headache by 54%, arm or leg weakness by 57%, and muscle pain by 68% (RRs 0.36, 0.46, 0.43, 0.32; p < 0.04 in the listed sequence). Those who received two SARS-CoV-2 vaccine doses were no more likely to report any of these symptoms than individuals reporting no previous SARS-CoV-2 infection. Adjusted RR for recovery from COVID-19 following two doses 0.981 (0.798–1.206, p = 0.856). The foregoing associations were largely not seen amongst individuals who received a single dose of a SARS-CoV-2 vaccine, who were in most cases likely to have been infected prior to vaccination within this study (recovery from COVID-19 unadjusted RR 1.019, 95% CI 0.893–1.163, p = 0.778). |
| Senjam et al., 2021* | India | Cross-sectional | Tertiary healthcare institute in Delhi |
n = 773 Age range: >18 Median age: 34 (IQR 27–44) Sex (%F/%M): 43.6/56.4 |
|
|
|
Receiving two doses of a SARS-CoV-2 vaccine prior to infection was associated with a reduction in the odds of self-reported long COVID (aOR 0.55; 95 %CI 0.37–0.85). One dose was not associated with a protective effect against the development of long COVID (aOR 1.00, 95% CI 0.66–1.49). |
| Simon et al., 2021** | United States | Retrospective Cohort | Arcadia Data Research |
n = 240 648 Age range: N/A Mean (SD) age: N/A Sex (%F/%M): 59.9/40.1 |
|
|
|
Individuals who received one dose of a SARS-CoV-2 vaccine prior to COVID-19 infection were 4.5x less likely to report any long COVID symptom (OR 0.220, 95% CI 0.196–0.245, p < 0.005) and 8.8x less likely to report 2 + long COVID symptoms. The foregoing result applies regardless of the manufacturer of the vaccine. |
| Taquet et al., 2021* | United States | Retrospective Cohort | TriNetX EHR network |
n = 18 958 Age range: N/A Mean (SD) age*: 56.5 (18.0) Mean (SD) age**: 57.6 (20.6) Sex (%F/%M)*:59.9/40.1 Sex (%F/%M)**:60.8/39.2 71.6% white* 72.5% white** *vaccinated (matched) **unvaccinated (matched) |
|
|
|
Receiving at least one SARS-CoV-2 vaccine dose prior to infection was not significantly associated with decreased of reporting any long COVID features (HR 1.01, 95% CI 0.96–1.05, p = 0.83, Bonferroni-corrected p = 1.0), The risk of several individual long COVID features were negatively associated with prior SARS-CoV-2 vaccination, but did not survive correction for multiple comparisons: myalgia (HR 0.78, 332 95% CI 0.67–0.91), fatigue (HR 0.89, 95% CI 0.81–0.97), and pain (HR 0.90, 95% CI 0.81–0.99), with potentially additional protection after a second dose of the SARS-CoV-2 vaccine against abnormal breathing (HR 0.89, 95% CI 0.81–0.98) and cognitive symptoms (HR 0.87, 95% CI 0.76–0.99). |
| Office of National Statistics Data Ayoubkhani et al., 2022 |
United Kingdom | Prospective Cohort | UK Coronavirus (COVID-19) Infection Survey [CIS]) data to November 2021 |
n = 6 180 Age range: 18–69 Mean (SD) age*: 49.0 (12.0) Mean (SD) age**: 46.7 (11.2) Sex (%F/%M)*: 54.2/45.8 Sex (%F/%M)**: 53.7/46.3 91.8% white* 91.2% white** *double vaccinated, after matching **unvaccinated, after matching |
|
|
|
Receiving two doses of a SARS-COV-2 vaccine prior to infection was associated with a 41.1% decrease in the odds of self-reported long COVID, relative to socio-demographically similar study participants who were not vaccinated when infected (aOR 0.589, 95% CI 0.501–0.691). 9.5% (95% CI 8.5–10.6%) of double vaccinated individuals reported long COVID symptoms of any severity vs.14.6% (95% CI 13.4–15.9%) unvaccinated individuals. The corresponding estimates for long COVID symptoms severe enough to result in limitation to day-to-day activities were 5.5% (95% CI 4.8–6.4%) and 8.7% (95% CI 7.7–9.7%), respectively. There was no statistically significant difference in outcomes between adenovirus vector (aOR 0.623, 95% CI 0.514–0.714) vs. mRNA (aOR 0.504, 95% CI 0.370–0.685) vaccines. |
*preprint article (not peer-reviewed) as of January 28, 2022.
**Simon et al. reported on both vaccination prior to development of long COVID, and vaccination post factum.
Age is reported in years. ‘Doses’ refer to SARS-CoV-2 vaccine doses.
Acronyms: N/A: Not Available, SF-36: Short Form-36 Health Survey, WEMWBS: Warwick–Edinburgh Mental Wellbeing Scale, 95% CI: 95% confidence interval, OR: odds ratio, aOR: adjusted OR, HR: hazard ratio, RR: relative risk, COVID-19: coronavirus 2019.
Table 2.
Studies investigating SARS-CoV-2 vaccination for the treatment of long COVID (n = 9).
| Study | Country | Study Design | Sample Source | Total Sample | Vaccination Status | Long COVID Definition/Persistent COVID-19 Symptoms | Summary of Findings |
|---|---|---|---|---|---|---|---|
| Arnold et al., 2021 | England | Case Series (with prospective enrolment) | North Bristol NHS Trust |
n = 36 Age range: ≥18 Median (IQR) age: 64 (53–73) Sex (%F/%M): 42/58 |
|
|
Among the 159 long COVID symptoms reported prior to vaccination, 37/159 (23.2%) had improved, 9/159 (5.6%) had worsened, and 113/159 (71.1%) were unchanged at a median of 30 days (IQR 26–36) post vaccination. There was no significant worsening in quality-of-life metrics before vs. after vaccination (t test p > 0.1 for all SF-36 comparisons). Mental well-being (ascertained via the WEMWBS) was stable in vaccinated participants before and after vaccination (median, 49 [IQR 42–54] vs. 50 [IQR 40–59], respectively). There was no difference in outcome measure between the Pfizer-BioNTech vs. Oxford-AstraZeneca vaccines (t test p > 0.1). |
| Ayoubkhani et al., 2021* | United Kingdom | Prospective Cohort (uncontrolled) | Office for National Statistics (ONS) COVID-19 Infection Survey (CIS) |
n = 28 356 Age range: 18–69 Mean (SD) age*: 46 (14) Sex (%F/%M)*: 55.6/44.4 88.7% white* *at the last study visit |
|
|
First SARS-CoV-2 vaccination was associated with an initial 12.8% decrease (95% CI −18.6% to −6.6%) in the odds of Long COVID but increasing by 0.3% (95% CI −0.6% to + 1.2%) per week after the date of first vaccination. Second SARS-CoV-2 vaccination was associated with an 8.8% decrease (95% CI −14.1% to −3.1%) in the odds of Long COVID, with the odds subsequently decreasing by 0.8% (-1.2% to −0.4%) per week after the date of second vaccination. First vaccination was associated with an initial 12.3% decrease (95% CI −19.5% to −4.5%) in the odds of activity-limiting Long COVID, followed by an increase of 0.9% (-0.2% to + 1.9%) per week until receiving the second dose. Second vaccination was associated with an initial 9.1% decrease (-15.6% to −2.1%) in the odds, followed by a decrease of 0.5% (-1.0% to + 0.05%) per week. The odds of experiencing most symptoms, as well as more than 3 or 5 symptoms together, initially numerically decreased after each vaccination. After first vaccination, the largest numerical decreases were observed for loss of smell (-12.5%, 95% CI −21.5% to −2.5%), loss of taste (-9.2%, 95% CI −19.8% to + 2.7%), and trouble sleeping (-8.8%, 95% CI −19.4% to + 3.3%). After second vaccination, the largest numerical decreases were observed for fatigue (-9.7%, 95% CI −16.5% to −2.4%), headache (-9.0%, 85% CI −18.1% to + 1.0%), and trouble sleeping (-9.0%, 95% CI −18.2% to + 1.2%). Trends were generally upwards between the first and second vaccinations, with most returning to a declining or flat trend after the second dose. There were no statistical differences in post-vaccination Long COVID trajectories between participants that received a first dose of the adenoviral vector vs. mRNA COVID-19 vaccines (initial 14.9% decrease [95% CI −21.8% to −7.5%] for adenoviral vector vs. initial 8.9% decrease [95% CI −18.2% to + 1.4%] for mRNA). Similarly, the decreases in the long COVID odds following second dose were numerically similar between vaccine types, at 8.7% decrease (-15.4% to −1.4%) for adenoviral vector and 8.9% decrease (-17.6% to + 0.7%) for mRNA SARS-CoV-2 vaccines. |
| Gaber et al., 2021 | England | Cross-sectional | Wrightington, Wigan, and Leigh NHS Trust Hospitals |
n = 67 Age range: 18–65 Mean age: N/A Sex (%F/%M)*: 91/9 Healthcare workers *includes individuals who refused the vaccine (n = 10) |
|
|
(45/67) 67% of respondents did not report any changes in long COVID symptoms several weeks following SARS-CoV-2 vaccination, (14/67) 21% reported improvement of symptoms and, (8/67) 12% reported worsening of symptoms. The symptom which was most frequently reported to have worsened following SARS-CoV-2 vaccination was fatigue (reported by 3/67). |
| Scherlinger et al., 2021 | France | Cross-sectional | French social media platforms and patient associations |
n = 567 Age range: ≥18 Median (IQR) age: 44 (25–75) Sex (%F/%M): 83.4/16.6 |
|
|
201/380 (52.8%) PASC patients reported an impact on symptoms following SARS-CoV-2 vaccination. A global worsening of symptom severity was reported by 117/380 (31%) of PASC patients, mostly represented by worsening of fever/chills (74%), gastro-intestinal symptoms (70%), paresthesia (64%) and arthralgia (63%). Conversely, a global improvement was reported by 83/380 (21.8%) PASC patients and was mainly driven by the improvement of anosmia (62%) and brain fog (51%). The SARS-CoV-2 vaccine impact on PASC symptoms lasted more than 2 weeks in 72.6% of patients reporting improvement and 63.7% of patients reporting worsening. The impact of SARS-CoV-2 vaccination on PASC was not different depending on the vaccine administered (p = 0.60). Amongst unvaccinated participants with pre-existing PASC (170/567 [30%]), the most cited reasons for postponing the COVID-19 vaccine were fear of worsening PASC symptoms (55.9%) and the belief that vaccination was contraindicated because of PASC (15.6%). |
| Schultheiß et al., 2021 | Germany | Cross-sectional | DigiHero cohort (recruited via direct mailing to citizens of Halle, Germany) |
n = 294 Age range: >14 Median (IQR) age*: 51.2 (15–83) Median (IQR) age**: 50 (17–81) Sex (%F/%M)*: 62.4/37.6 Sex (%F/%M)**:61.1/38.9 *n = 258 (87.8%) participants with confirmed prior COVID-19 **n = 36 (12.2%) without prior COVID-19 |
|
|
Post-infection vaccination was not associated with resolution of PASC. 80 (40.8%) vaccinated individuals reported ongoing symptoms, whereas 24 (38.7%) unvaccinated individuals reported ongoing symptoms. Furthermore, the percentage of post-infection vaccinations was identical in patients with PASC that experienced resolution of their symptoms and in those that reported ongoing PASC. From the 175 individuals with reported PASC, 104 individuals still had ongoing symptoms at the time of analysis, while 71 had resolved PASC. Out of the 104 individuals with ongoing symptoms, 80 (76.9%) were vaccinated post-infection, whereas out of the 71 individuals with resolved PASC, 54 (76.1%) were vaccinated post-infection. |
| Simon et al., 2021** | United States | Retrospective Cohort | Arcadia Data Research |
n = 240 648 Age range: N/A Mean (SD) age: N/A Sex (%F/%M): 59.9/40.1 |
|
|
Individuals whose first SARS-CoV-2 vaccination occurred within 12 weeks following COVID-19 diagnosis were significantly less likely to report long COVID symptoms than if they had remained unvaccinated, with earlier vaccine administration post-diagnosis associated with a greater likelihood of not reporting long COVID. Parenthetically, those who received their first within four weeks of infection were 4–6 times less likely to report multiple long COVID symptoms, and those who received their first dose 4–8 weeks after diagnosis were 3 times less likely to report multiple long COVID symptoms compared to those who remained unvaccinated. |
| Strain et al., 2022* | United Kingdom, Israel, Russia,India, South Africa | Cross-sectional | Social media and/or direct mailing to long COVID support groups |
n = 812 Age range: N/A Mean (SD) age: N/A Sex (%F/%M): 80.3/19.7 90.8% white |
|
|
470/812 (57.9%) of participants reported improvements in long COVID symptoms at least one week following first SARS-CoV-2 vaccination, 145/812 (17.9%) reported deterioration, and 197/812 (24.3%) reported no change. 24/812 (3%) reported that all of their long COVID symptoms deteriorated, compared to 221/812 (27.2%) that all their symptoms improved. 424/812 (52.3%) reported that the improvement of symptoms had abated by the time they completed the survey, with the median duration of improvement between 14 and 21 days. For those who experienced worsening, 406/412 (50%) had recovered by the time of the survey, with the median time to improvement being 3–7 days, suggesting the deterioration was a vaccination reaction rather than true exacerbation of long COVID. Larger improvements in symptom severity scores were seen in those receiving mRNA vaccines compared to adenoviral vector vaccines (58% improvement vs. 19% deterioration for AstraZeneca, 56% improvement vs. 18% deterioration for Pfizer, and 66% improvement vs. 12% deterioration for Moderna vaccine, with the rest reporting no difference). The average improvement across all symptoms was 22.6% of the baseline symptom score after the AstraZeneca/Oxford vaccine, 24.4% after the Pfizer/BioNTech vaccine, and 31.0% in recipients of the Moderna (p = 0.003 compared to the AZ/Oxford vaccine and p = 0.01 compared to Pfizer/BioNTech). The second SARS-CoV-2 vaccine dose was associated with a modest further improvement in symptoms or maintenance of benefit, but results were not statistically significant. |
| Tran et al., 2021 | France | ProspectiveCohort (target trial emulation) | ComPaRe long COVID cohort |
n = 910 Age range: ≥ 18 Median (IQR) age: 47 (40–54) Sex (%F/%M)*: 80.5/19.5 Hospitalized/non-hospitalized: 8.9/91.1 |
|
|
At the 120 day follow-up, long COVID symptoms were less severe in the vaccinated group (mean [SD] ST score in the vaccination group 13.0 [9.4] vs. 14.8 [9.8] in the control group; mean difference: −1.8, 95% CI −2.5 to −1.0), and had double the rate of patients in complete remission (remission rate 16.6% vaccinated vs. 7.5% unvaccinated, HR: 1.97, 95% CI 1.23–3.15). Furthermore, the impact of long COVID on patients’ lives was significantly lower in the vaccination group than in the control group. The mean (SD) long COVID IT score was 24.3 (16.7) in the vaccinated group and 27.6 (16.7) in the unvaccinated group (mean difference: −3.3, 95% CI −6.2 to −0.5). |
| Wanga et al., 2021 | United States | Cross-sectional | Non-probability based internet panel survey conducted by Porter Novelli Public Services and ENGINE Insights |
n = 3126 Age range: ≥ 18 Mean (SD) age: N/A Sex (%F/%M)*: 48.5/51.5 Sex (%F/%M)**: 51.5/48.5 *positive COVID-19 test result **negative COVID-19 test result |
|
|
Among those who reported any long-term symptom(s), more respondents who received a positive test result than those who received a negative test result reported that having long-term symptoms motivated them to receive or consider receiving a COVID-19 vaccine (11.0% vs. 7.0%), and believed that receiving the vaccine made their long-term symptoms better (28.7% vs. 15.7%; p = 0.023), or that their symptoms were gone before receiving the vaccine (28.4% versus 13.1%). A similar percentage of respondents who received a positive test result (16.1%) and those who received a negative test result (11.2%) reported that receiving the vaccine made their long-term symptoms worse (p = 0.271), whereas 26.4% of respondents who received a positive test result and 59.2% of those who received a negative test result believed that receiving a vaccine did not affect their symptoms (p < 0.001). |
**Simon et al. reported on both vaccination prior to development of long COVID, and vaccination post factum.
Acronyms: N/A: Not Available, PASC: Post-Acute Sequelae of COVID-19.
3.2. Methodological quality and risk of bias
The methodological quality of the included studies varied markedly depending on study design. The mean NOS score for prospective cohort studies was 5.33 out of 9 (moderate), 5.60 out of 6 (high) for retrospective studies, and 3.29 out of 9 (low) for cross-sectional studies. The component case-control study (Antonelli et al., 2022) scored 6 out of 8 (moderate) on the NOS, and the case series (Arnold et al., 2021) fulfilled 8 out of 9 criteria on the JBI checklist. NOS rankings within each category for individual studies organized by design as well as the completed JBI checklist are provided in Table 1 of the Supplement.
3.3. Meta-Analysis
3.3.1. SARS-CoV-2 vaccination for the prevention of long COVID
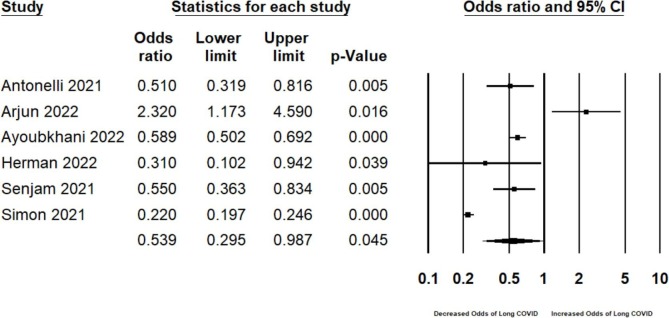
At least one dose of a SARS-CoV-2 vaccine was associated with a protective effect against the development of long COVID, relative to individuals who did not receive the SARS-CoV-2 vaccine prior to infection, or those who received fewer doses (OR 0.539, 95% CI 0.295–0.987, p = 0.045, I2 = 96.46, N = 257, 817; Fig. 2a ). Although visual inspection of the funnel plot suggested some asymmetry (Supplement Fig. 1), both the Egger’s (Intercept = 4.325, 1-tailed p = 0.127) and Begg and Mazumdar rank correlation test (1-tailed p = 0.425) were not statistically significant.
Fig. 2a.
Pooled odds ratio for developing long COVID in individuals receiving at least one COVID-19 vaccine dose compared to those who did not receive a COVID-19 vaccine (i.e., ‘prevention meta-analysis’).
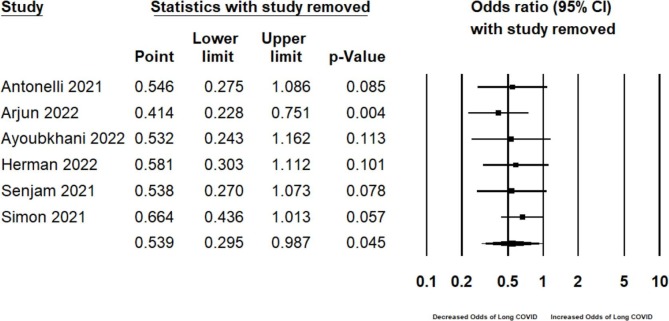
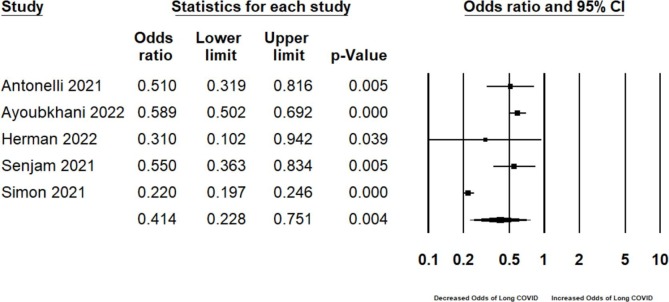
One-study-removed sensitivity analysis did not markedly influence the effect size or heterogeneity (Fig. 2b ). Parenthetically, following the removal of Arjun et al., the only study reporting an increased odds of long COVID following SARS-CoV-2 vaccination (aOR 2.32, 95% CI 1.17–4.58, p = 0.01, N = 487) (Arjun et al., 2022), the OR further decreased to 0.414, (95% CI 0.228, 0.751; I2 = 96.33, N = 257, 330; Fig. 2c ), now representing a greater than 50% reduction in the odds of presenting with long COVID following breakthrough infection.
Fig. 2b.
One-study-removed sensitivity analyses for the prevention meta-analysis.
Fig. 2c.
Sensitivity analysis excluding Arjun et al. from the prevention meta-analysis.
3.4. Narrative synthesis
3.4.1. SARS-CoV-2 vaccination for the prevention of long COVID
Taken together, 7 out of 9 studies investigating the frequency of long COVID in breakthrough COVID-19 infections reported that SARS-CoV-2 vaccination prior to infection was associated with a lower incidence of long COVID when compared to unvaccinated individuals (Table 1). Among the 2 studies which did not cite protective effects of SARS-CoV-2 vaccination, Taquet et al. reported that one dose of the BNT162b2 Pfizer-BioNTech, mRNA90-1273 Moderna, or Ad26.COV2.S Janssen SARS-CoV-2 vaccines was not associated with a decrease in reporting long COVID features post-infection (HR 1.01, 95% CI 0.96–1.05, p = 0.83, Bonferroni-corrected p > 0.99, N = 18 958), (Taquet et al., 2021) whereas Arjun et al. determined that two doses of the Novavax-Serum Institute India Covovax vaccine were associated with an increased odds of long COVID (aOR 2.32, 95% CI 1.17–4.58, p = 0.01, N = 487). (Arjun et al., 2022) The association for one dose was not statistically significant (aOR 1.88, 95% CI 0.84–4.22, p = 0.13). (Arjun et al., 2022)
Amongst studies which reported a statistically significant association between prior SARS-CoV-2 vaccination and a reduced risk of long COVID, the magnitude of the protective effect varied. Prior vaccination was associated with a 78.0% to 41.1% decrease in the odds or risk of self-reported long COVID, (Antonelli et al., 2022, Herman et al., 2022, Ayoubkhani et al., 2022, Senjam et al., 2021) and Al-Aly et al. (N = 64 571) reported that the hazard rate of post-acute sequelae was 13% lower in the vaccinated group. (Al-Aly et al., 2021)
Results were inconclusive as to whether one dose of a SARS-CoV-2 vaccine was sufficient to reduce the risk of developing long COVID. Two studies reported a statistically significant association between one dose of a SARS-CoV-2 vaccine and a reduced incidence of long COVID. (Simon et al., 2021, Al-Aly et al., 2021) Conversely, 4 studies reported no significant association between one dose of a SARS-CoV-2 vaccine and decreased reporting of long COVID. (Antonelli et al., 2022, Taquet et al., 2021, Kuodi et al., 2022, Arjun et al., 2022) Notably, both Herman et al. (N = 442) and Ayoubkhani et al. (N = 6180) found that the protective effects of vaccination against the development of long COVID decreased as the interval between vaccination and infection increased. (Herman et al., 2022, Ayoubkhani et al., 2022)
Where studies reported on individual long COVID symptoms, protective effects of vaccination tended to vary by long COVID symptom category. Al-Aly et al. reported that the risk of post-acute sequelae in the cardiovascular, coagulation, metabolic, and pulmonary organ systems, as well as risk of fatigue, was significantly lower in those with breakthrough COVID-19 compared with those with COVID-19 but without prior SARS-CoV-2 vaccination. Conversely, the lower risk was not statistically significant for post-acute sequelae affecting the kidney, gastrointestinal, mental health, and neurologic organ systems. (Al-Aly et al., 2021) Furthermore, Kuodi et al. (N = 3388) determined that those who received two SARS-CoV-2 vaccine doses were less likely than unvaccinated individuals to report post-COVID fatigue by 64%, headache by 54%, arm or leg weakness by 57%, and muscle pain by 68% (RRs 0.36, 0.46, 0.43, 0.32; p < 0.04 for each, respectively). (Kuodi et al., 2022) The reported associations for other long COVID symptoms were not statistically significant. Herman et al., which delimited their analysis to olfactory dysfunction, reported that breakthrough infections occurring >14 days following full SARS-CoV-2 vaccination were associated with a 69% lower odds of developing olfactory dysfunction (aOR 0.31, 95% CI 0.102–0.941, N = 442). (Herman et al., 2022)
Taken together, associations between SARS-CoV-2 vaccination and the development/occurrence of long COVID did not tend to vary by vaccine type or manufacturer, with the exception of results reported by Arjun et al., the only study wherein participants received the Novavax-Serum Institute of India Covovax. (Arjun et al., 2022) The majority of studies included individuals vaccinated with more than one vaccine brand, and protective effects were reported for Pfizer-BioNTech BNT162b2, Oxford-AstraZeneca ChAdOx1 nCoV-19, Moderna mRNA-1273, and Janssen Ad26.COV2.S. Furthermore, several studies stated that intra-study results did not vary by vaccine manufacturer and/or type. (Simon et al., 2021, Ayoubkhani et al., 2022)
3.4.2. SARS-CoV-2 vaccination for the treatment of long COVID
Seven of 9 studies determined that the majority of individuals presenting with long COVID at baseline did not experience changes in their symptoms following 1 or more SARS-CoV-2 vaccine doses. (Gaber et al., 2021, Scherlinger et al., 2021, Schultheiß et al., 2021, Wanga et al., 2021, Ayoubkhani et al., 2021, Tran et al., 2021, Arnold et al., 2021) Conversely, Strain et al. reported that 57.9% of individuals (470 of 812 respondents, N = 812) experienced improvement in symptoms following vaccination, compared to 24.3% reporting no change, and 17.9% reporting deterioration. (Strain et al., 2022) Moreover, amongst studies which reported post-vaccination long COVID outcomes as 3 dichotomous categories (i.e., ‘improved’, ‘worsened’, or ‘stayed the same’), the proportion of individuals reporting improvements exceeded the proportion reporting worsening in all but one study; (Arnold et al., 2021, Gaber et al., 2021, Strain et al., 2022, Wanga et al., 2021, Tran et al., 2021) Scherlinger et al. reported that 21.8% experienced global improvement and 31% experienced global worsening of long COVID symptoms (N = 567). (Scherlinger et al., 2021) Furthermore, Simon et al. reported that individuals whose first SARS-CoV-2 vaccination occurred within 12 weeks following COVID-19 diagnosis were significantly less likely to report long COVID symptoms than if they had remained unvaccinated, with earlier vaccine administration associated with a greater likelihood of not reporting long COVID (N = 240 648). (Simon et al., 2021)
The component studies did not report marked differences regarding the association of one versus two SARS-CoV-2 vaccine doses and changes in pre-existing long COVID symptoms. One and two doses were associated with similar proportions of individuals reporting improvement and worsening of symptoms, with the majority citing no changes. However, Ayoubkhani et al. reported an initial 12.8% (95% CI −18.6% to −6.6%) decrease in the reporting of long COVID for first vaccination compared to an 8.8% decrease (95% CI −14.1% to −3.1%) in the odds of long COVID following the second dose, (Ayoubkhani et al., 2021) suggesting that the first dose may produce a greater initial reduction as compared to the second (i.e., ceiling effect of symptom resolution).
Only one study described differences in the post-vaccination trajectory of pre-existing long COVID between vaccine types. Strain et al. noted a 58% improvement vs. 19% deterioration for Oxford-AstraZeneca ChAdOx1 nCoV-19, 56% improvement vs. 18% deterioration for Pfizer-BioNTech BNT162b2, and 66% improvement vs. 12% deterioration for the Moderna mRNA-1273, with the rest of respondents reporting no difference in long COVID symptoms pre and post vaccination. (Strain et al., 2022) As such, mRNA vaccines were superior to the adenoviral vaccine in the foregoing study. Conversely, 3 studies noted no statistically significant differences between vaccine types and/or manufacturers, (Scherlinger et al., 2021, Ayoubkhani et al., 2021, Arnold et al., 2021) with the remainder component studies either including only one make of vaccine or not stratifying results by vaccine type/manufacturer. (Gaber et al., 2021, Schultheiß et al., 2021, Wanga et al., 2021, Tran et al., 2021).
Three studies stratified changes in long COVID by symptoms. Ayoubkhani et al. reported that following the first SARS-CoV-2 vaccination, the largest numerical decreases were observed for loss of smell (−12.5%, 95% CI −21.5% to −2.5%), loss of taste (-9.2%, 95% CI −19.8% to +2.7%), and trouble sleeping (-8.8%, 95% CI −19.4% to +3.3%). (Ayoubkhani et al., 2021) After the second vaccination, the largest numerical decreases were observed for fatigue (-9.7%, 95% CI −16.5% to −2.4%), headache (-9.0%, 85% CI −18.1% to +1.0%), and trouble sleeping (-9.0%, 95% CI −18.2% to +1.2%). (Ayoubkhani et al., 2021) Gaber et al. reported that 8/14 (57%) experienced improvement of respiratory sequelae, 4/14 (28.6%) reported improvements in fatigue, 5/14 (36%) of anxiety, and 2/14 (14.3%) of other symptoms (N = 67). (Gaber et al., 2021) In terms of long COVID deterioration, 3/8 (37.5%) reported worsening of fatigue, 1/8 (12.5%) of respiratory symptoms, and 2/8 (25%) of anxiety. (Gaber et al., 2021) Scherlinger et al. reported that long COVID deterioration was mostly represented by worsening of fever/chills (74%), gastro-intestinal symptoms (70%), paresthesia (64%), and arthralgia (63%). (Scherlinger et al., 2021) Conversely, improvements were most frequently exhibited in anosmia (62%) and brain fog (51%). (Scherlinger et al., 2021)
4. Discussion
4.1. Summary of results
This systematic review and meta-analysis identified that at least one dose of a SARS-CoV-2 vaccine may be protective against the development of long COVID in breakthrough infection. Furthermore, in most cases, vaccination did not affect the symptom trajectory of pre-existing long COVID, and a greater number of individuals experienced improvement versus deterioration of pre-existing post-COVID symptoms after vaccination. Notably, there was a lack of evidence that SARS-CoV-2 vaccination exacerbates pre-existing long COVID symptoms. As such, it is recommended that long COVID patients adhere to standard SARS-CoV-2 vaccination schedules.
It stands to reason that SARS-CoV-2 vaccination can prevent the development of long COVID by preventing SARS-CoV-2 infection, which is not accounted for in the present analysis. However, the mechanisms whereby SARS-CoV-2 vaccination may be able to thwart or decrease severity of long COVID following breakthrough infection are more nuanced. The pathophysiology underlying long COVID can be broadly categorized as 1) direct viral effects of SARS-CoV-2, 2) secondary inflammatory effects of infection, 3) post-critical illness, and 4) other (e.g., psychological or ‘nocebo’ factors). (Crook et al., 2021)
In keeping with the foregoing paradigm, vaccination may decrease the intensity of the acute phase immune response and enable faster clearance of SARS-CoV-2, preventing or lessening the extent of organ damage, immune dysfunction, and exacerbation of pre-existing disease, as well as enabling the clearance of persistent post-acute viral reservoirs. It is well documented that breakthrough SARS-CoV-2 infections are phenotypically milder compared to infections in unvaccinated persons, as well as associated with less immune dysregulation. (Rovida et al., 2021, Chia et al., 2021) Furthermore, previous work has determined that vaccination may prevent the development of autoantibodies following breakthrough infections, (Arunachalam et al., 2021) which may partly account for the protective effects against long COVID. Moreover, SARS-CoV-2 vaccination has been shown to counter SARS-CoV-2- induced pathogenic double memory B cell subsets, the presence of which may correlate with the production of autoantibodies possibly implicated in the development of long COVID symptoms. (Mishra et al., 2021)
Variation in the symptom trajectory of pre-existing long COVID following vaccination may reflect underlying biological heterogeneity and/or differential etiology. Multiple risk factors for long COVID at the time of COVID-19 diagnosis have been determined, including type 2 diabetes, SARS-CoV-2 RNAemia, Epstein-Barr virus viremia, and specific autoantibodies. (Su et al., 2022) Therefore, severity of acute episode, as well as effect of vaccination, may vary depending on the unique combination of genetic, environmental, and behavioral traits of the individual. Similarly, long COVID symptoms may affect SARS-CoV-2 vaccine efficacy; for example, individuals with mental disorders such as depression and anxiety – features of long COVID – (Renaud-Charest et al., 2021) have been shown to exhibit attenuated post-vaccination immune responses. (Xiao et al., 2022) The foregoing associations may be attributable to immunological dysregulation, including a persistent proinflammatory state, and alterations in both humoral and cell-mediated immunity, which, in addition to decreasing the host response against infection, may compromise the generation of immunological memory.
5. Limitations
The results presented herein are subject to several limitations. First, the present review does not consider the prevention of COVID-19 infection as part of the protective effect against long COVID, thus, the results likely underestimate the effect size. Second, heterogeneity resulting from differences in study design and reporting, as well as methodological quality, precluded a quantitative summary of the data pertaining to SARS-CoV-2 vaccination post factum. Third, the cross-sectional nature of many studies does not allow for the determination of the temporality of changes in the presentation of long COVID; the foregoing is an important consideration in light of the ‘waxing and waning’ presentation of many long COVID symptoms. Fourth, previous work has shown that some individuals experience natural improvement of long COVID symptoms over time; as such, improvement post-vaccination may not have been affected by the vaccine(s) in some cases. Fifth, there was an underrepresentation of inactivated virus SARS-CoV-2 vaccines as compared to mRNA COVID-19 vaccines in the primary studies; the inactivated virus vaccine is more frequently administered in developing countries. Moreover, only one study (Taquet et al., 2021) controlled for the potential confounder that remaining unvaccinated for SARS-CoV-2 may be associated with other health behaviours which may affect primary outcomes, (Latkin et al., 2021) and it was not possible to determine whether there are confounding factors that may make vaccine hesitant or unvaccinated individuals more likely to report long COVID symptoms. Additionally, no data on the effect of booster doses was available.
6. Conclusions
The evidence presented herein recommends SARS-CoV-2 vaccination for the prevention of long COVID in breakthrough cases. Furthermore, evidence does not support that SARS-CoV-2 vaccination exacerbates long COVID symptoms. Thus, most patients with long COVID should be vaccinated for SARS-CoV-2. Future research should investigate factors which affect the trajectory of long COVID symptoms, as well as the temporality of changes following SARS-CoV-2 vaccination, the effects of boosters, and how different long COVID patient subgroups respond to SARS-CoV-2 vaccination.
Declaration of Competing Interest
Dr. Roger S. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim,Sage,Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, IntraCellular, NewBridge Pharmaceuticals, Viatris, Abbvie, Atai Life Sciences. Dr. Roger S. McIntyre is a CEO of Braxia Scientific Corp.
Dr. Roger Ho received funding from NUS Department of Psychological Medicine (R-177-000-100-001/R-177-000-003-001) and NUS iHeathtech Other Operating Expenses (R-722-000-004-731). Leanna M. W. Lui has received personal fees from Braxia Scientific Corp and honoraria from Medscape.
Dr. Joshua D. Rosenblat is the medical director of Braxia Health (formally known as the Canadian Rapid Treatment Center of Excellence and is a fully owned subsidiary of Braxia Scientific Corp) which provides ketamine and esketamine treatment for depression; he has received research grant support from the American Psychiatric Association, the American Society of Psychopharmacology, the Canadian Cancer Society, the Canadian Psychiatric Association, the Joseph M. West Family Memorial Fund, the Timeposters Fellowship, the University Health Network Centre for Mental Health, and the University of Toronto and speaking, consultation, or research fees from Allergan, COMPASS, Janssen, Lundbeck, and Sunovion.
Leanna Lui has received personal fees from Braxia Scientific Corp and honoraria Medscape.
Acknowledgments
Acknowledgements
Author contributions: FC and RSM conceptualized and designed study. FC and DK conducted literature search, study selection, data extraction, and quality assessment of component studies. FC conducted statistical analyses, interpreted statistical results, conducted qualitative analyses, and drafted the manuscript. All authors reviewed and approved the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2023.03.022.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Al-Aly Z., Bowe B., Xie Y. Long Covid after Breakthrough COVID-19: the post-acute sequelae of breakthrough COVID-19. Research Square. 2021 doi: 10.21203/rs.3.rs-1062160/v1. published online Nov 15. [DOI] [Google Scholar]
- Antonelli M., Penfold R.S., Merino J., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjun M.C., Singh A.K., Pal D., et al. Prevalence, characteristics, and predictors of Long COVID among diagnosed cases of COVID-19. bioRxiv. 2022 doi: 10.1101/2022.01.04.21268536. published online Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D.T., Milne A., Samms E., Stadon L., Maskell N.A., Hamilton F.W. Symptoms After COVID-19 Vaccination in Patients With Persistent Symptoms After Acute Infection: A Case Series. Ann Intern Med. 2021;174:1334–1336. doi: 10.7326/M21-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam P.S., Scott M.K.D., Hagan T., et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021;596:410–416. doi: 10.1038/s41586-021-03791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubkhani, D., Bosworth, M. Self-reported long COVID after infection with the Omicron variant in the UK - Office for National Statistics. 2022; published online May 5. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidafterinfectionwiththeomicronvariant/6may2022 (accessed May 15, 2022).
- Ayoubkhani D., Bermingham C., Pouwels K.B., et al. Changes in the trajectory of Long Covid symptoms following COVID-19 vaccination: community-based cohort study. bioRxiv. 2021 doi: 10.1101/2021.12.09.21267516. published online Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubkhani D, Bosworth M, King S. Self-reported long COVID after two doses of a coronavirus (COVID-19) vaccine in the UK. 2022; published online Jan 26. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/selfreportedlongcovidaftertwodosesofacoronaviruscovid19vaccineintheuk (accessed Feb 13, 2022).
- Bach K. Is ‘long Covid’ worsening the labor shortage? Brookings. 2022; published online Jan 11. https://www.brookings.edu/research/is-long-covid-worsening-the-labor-shortage/(accessed Feb 15, 2022).
- Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better systematic review management. Covidence. 2020; published online June 11. https://www.covidence.org (accessed Feb 15, 2022).
- Ceban F, Nogo D, Carvalho IP, et al. Association between mood disorders and risk of COVID-19 infection, hospitalization, and death: A systematic review and meta-analysis. JAMA Psychiatry 2021; published online July 28. DOI:10.1001/jamapsychiatry.2021.1818. [DOI] [PMC free article] [PubMed]
- Ceban F., Ling S., Lui L.M.W., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2021;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Leber A., Jawad M.Y., et al. Registered clinical trials investigating treatment of long COVID: a scoping review and recommendations for research. Infect Dis. 2022:1–11. doi: 10.1080/23744235.2022.2043560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Ong S.W.X., Chiew C.J., et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.11.010. published online Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID19 vaccine tracker. https://covid19.trackvaccines.org (accessed Feb 7, 2022).
- Crook H., Raza S., Nowell J., Young M., Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021:374. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- Gaber T.A.Z.K., Ashish A., Unsworth A., Martindale J. Are mRNA covid 19 vaccines safe in long covid patients? A health care workers perspective. British Journal of Medical Practitioners. 2021;14 [Google Scholar]
- Gupta R.K., Topol E.J. COVID-19 vaccine breakthrough infections. Science. 2021;374:1561–1562. doi: 10.1126/science.abl8487. [DOI] [PubMed] [Google Scholar]
- Herman B, Viwattanakulvanid P, Dzulhadj A, Oo AC, Patricia K, Pongpanich S. Effect of full vaccination and post-covid olfactory dysfunction in recovered covid-19 patient. A retrospective longitudinal study with propensity matching. bioRxiv. 2022; published online Jan 11. DOI:10.1101/2022.01.10.22269007.
- Kuodi, P., Gorelik, Y., Zayyad, H., et al., 2022. Association between vaccination status and reported incidence of post-acute COVID-19 symptoms in Israel: a cross-sectional study of patients tested between March 2020 and November 2021. medRxiv; 2022.01.05.22268800.
- Latkin C.A., Dayton L., Yi G., Konstantopoulos A., Boodram B. Trust in a COVID-19 vaccine in the U.S.: A social-ecological perspective. Soc Sci Med. 2021;270 doi: 10.1016/j.socscimed.2021.113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Bruiners N, Ukey R, et al. Vaccination boosts protective responses and counters SARS-CoV-2-induced pathogenic memory B cells. medRxiv 2021; published online May 12. DOI:10.1101/2021.04.11.21255153.
- Moher D., Liberati A., Tetzlaff J., Altman DG D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- Renaud-Charest O., Lui L.M.W., Eskander S., et al. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J Psychiatr Res. 2021;144:129–137. doi: 10.1016/j.jpsychires.2021.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovida F., Cassaniti I., Paolucci S., et al. SARS-CoV-2 vaccine breakthrough infections with the alpha variant are asymptomatic or mildly symptomatic among health care workers. Nat Commun. 2021;12:6032. doi: 10.1038/s41467-021-26154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherlinger M., Pijnenburg L., Chatelus E., et al. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines (Basel) 2021:10. doi: 10.3390/vaccines10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiß C, Willscher E, Paschold L, et al. From online data collection to identification of disease mechanisms: The IL-1ß, IL-6 and TNF-α cytokine triad is associated with post-acute sequelae of COVID-19 in a digital research cohort. medRxiv 2021; : 2021.11.16.21266391.
- Senjam SS, Balhara YPS, Kumar P, et al. Assessment of Post COVID-19 Health Problems and its Determinants in North India: A descriptive cross section study. medRxiv 2021; : 2021.10.03.21264490.
- Simon MA, Luginbuhl RD, Parker R. Reduced incidence of long-COVID symptoms related to administration of COVID-19 vaccines both before COVID-19 diagnosis and up to 12 weeks after. bioRxiv. 2021; published online Nov 18. DOI:10.1101/2021.11.17.21263608.
- Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00703-9. published online Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Strain W.D., Sherwood O., Banerjee A., van der Togt V., Hishmeh L., Rossman J. The Impact of COVID Vaccination on Symptoms of Long COVID. An International Survey of People with Lived Experience of Long COVID. Vaccines. 2022;10(5):652. doi: 10.2139/ssrn.3868856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Su Y., Yuan D., Chen D.G., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022 doi: 10.1016/j.cell.2022.01.014. published online Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zheng Q., Madhira V., et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2021;182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M, Dercon Q, Harrison PJ. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. bioRxiv. 2021; published online Oct 26. DOI:10.1101/2021.10.26.21265508. [DOI] [PMC free article] [PubMed]
- Tran V-T, Perrodeau E, Saldanha J, Pane I, Ravaud P. Efficacy of COVID-19 Vaccination on the Symptoms of Patients With Long COVID: A Target Trial Emulation Using Data From the ComPaRe e-Cohort in France. 2021; published online Sept 29. DOI:10.2139/ssrn.3932953.
- Wanga V., Chevinsky J.R., Dimitrov L.V., et al. Long-Term Symptoms Among Adults Tested for SARS-CoV-2 — United States, January 2020–April 2021. MMWR. Morbidity and Mortality Weekly Report. 2021;70:1235–1241. doi: 10.15585/mmwr.mm7036a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Gillissie E.S., Lui L.M.W., et al. Immune Response to Vaccination in Adults with Mental Disorders: A Systematic Review. J Affect Disord. 2022 doi: 10.1016/j.jad.2022.02.025. published online Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.