Abstract
There are no established regulations governing patient selection for simultaneous heart-kidney transplantation (SHK), creating the potential for significant center-level variations in clinical practice. Using the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) file we examined practice trends and variations in patient selection for SHK at the center level between Jan 1, 2004 and March 31, 2019. Overall, SHK is becoming more common with most centers performing heart transplants. Among patients who underwent heart transplant who were receiving dialysis, the rate of SHK varied from 22% to 86% at the center level. Among patients not on dialysis, the median eGFR of patients receiving SHK varied between 19–59mL/min/1.73m2. When adjusting for other factors, the odds of SHK varied 57-fold between the highest and lowest SHK performing centers. Variation in SHK at the center level suggests the need for national guidelines around the selection of patients for SHK.
Introduction
No national eligibility criteria for simultaneous heart-kidney (SHK) transplantation exist for heart transplant candidates with kidney dysfunction. Absent a national policy, patient selection for SHK has been determined by individual transplant centers, with or without institutional standardization. This policy void sets the stage for potential wide variability in center-level patient selection for SHK. Indeed, center level differences pertaining to patient selection in solid-organ transplantation are well-described1–11, including in simultaneous liver-kidney transplantation (SLK)12 prior to the implementation of standardized SLK criteria in 201713. To our knowledge, differences in center level practice of SHK transplantation have not yet been described.
Identifying center-level differences in patient selection for SHK is important for several reasons. First, differences may reflect inequities in access to multi-organ transplant at the center level, in direct contradiction to The Final Rule14. Second, wide variation in patient selection for SHK may highlight uncertainties regarding the level of kidney dysfunction at which patients could benefit from SHK. Describing glomerular filtration rates (GFR) in SHK candidates and recipients will permit more detailed study of outcomes after SHK and heart-alone (HA) transplantation15–18. Finally, describing center-level variability provides a “baseline” description of national practice that may be used to measure the impact of future SHK policies.
We sought to describe center-level practice in patient selection for SHK, with particular interest in defining the center-level variability after accounting for patient-level factors and differences between centers with high rates of SHK transplantation vs. centers with lower performance rates. Additionally, we examined the relationship between center-level waitlist mortality and proportion of SHK performed to explore the hypothesis that centers performing more SHK are waitlisting sicker patients for heart transplant.
Materials and Methods
Cohort Definition
We utilized the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) file for both heart and kidney transplants. This file contains data on all transplant registrants and candidates from October 1, 1987 to March 31, 2019. We defined our cohort as heart transplant recipients from Jan 1, 2004 to March 31, 2019 (n=38,650), who were 18 years of age or greater (n=32,848), who underwent no multi-organ transplants other than SHK (n=32,581). SHK were defined as patients who received a kidney from the same donor as the heart for a total of n=1,422 SHK and 31,159 HA transplants (Supplemental Figure 1). For analysis at the center level, we included only centers that performed at least 1 SHK per year on average over the study period, or 16 SHK in total (n=35). For SHK recipients, many variables related to patient demographics and comorbidity overlap in the kidney and heart (THORACIC) STAR files. Given its central importance, we examined the distribution of the creatinine values at transplant (used to calculate eGFR) in both files and did not note large discrepancies between the two files (Supplemental Figure 2). There was no formal sample size calculation performed given the relatively low event rate of SHK at a center level.
Statistical Analysis
The main analysis was conducted at the patient level. We examined center variability using a fixed effects model for each of the outcomes examined. We limited our main analyses to the 35 centers with the performing at least one SHK per year during the whole study period, representing >50% of all SHK volume in the study period.
Our primary endpoint was receipt of SHK (vs HA alone), with specific interest in the association between transplant center and patient selection for SHK. We used logistic regression to assess the center-level effect, adjusting for a priori factors including patient age (categorized as <40, 40–49, 50–59, 60–69, and 70 or greater), era (categorized as 2004–2008, 2009–2013, or 2014 and beyond), ventricular assist device (VAD) usage, diabetes, and eGFR at transplant (as defined by the creatinine value available in the Thoracic STAR file at the time of transplant). These factors were chosen based upon a previous analysis demonstrating their statistically significant relationship with patient selection for HA vs. SHK17. We then estimated the probability of SHK at varying eGFR to determine the effect of eGFR on the chance receipt of SHK at each center as varied by eGFR.
We explored differences between centers that performed a high or low proportion of SHK, by dividing centers into terciles based upon the proportion of SHK transplants performed to the total number of heart transplants performed, and then comparing transplant centers in the first versus the third tercile. We performed summary statistics on their patient populations using Χ2 to compare categorical and Wilcoxon Rank-Sum tests to compare continuous variables.
Finally, we examined the association between center-level waitlist mortality and the likelihood of receiving SHK. To calculate waitlist mortality, we divided the study period into 5-year eras and calculated the death rate as total death over the waitlist time accrued by each HA/SHK candidate both on the waitlist on the first day of the 5-year era or added to the waitlist during the 5-year era. We represented waitlist mortality as the number of deaths per 100 person years at each center in each 5-year era. We examined the relationship between the proportion of patients receiving SHKs with waitlist mortality using linear regression.
All analyses were performed using STATA v15 (Statacorp, College Station, TX).
Results
SHK is increasing over time
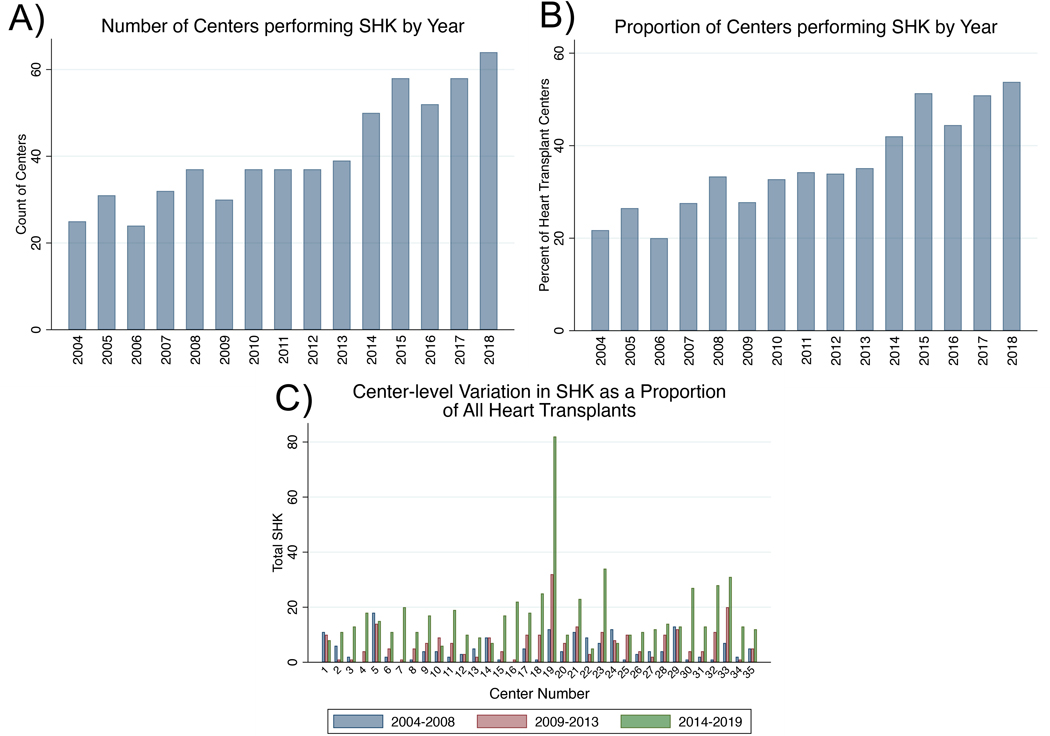
We first examined the number of centers performing SHK and the incidence of SHK by era. Overall, both the number (25 in 2004 to 64 in 2018) and proportion of centers performing SHK increased over time (21.7% in 2004 to 53.8% in 2018; Figure 1 A & B), while the total number of heart transplant centers remained relatively stable (115 in 2004 vs 119 in 2018). Additionally, the number and proportions of SHKs increased in most centers by era, with 63% of centers performing their highest proportion of SHK in the era 2014–2019 (Figure 1C). Of the 32 centers that performed any SHK between 2004–2008, the relative number of SHK at each center increased by a median of 263% (IQR 81%−566%) by the 2014–2019 era, while the increase in HA at the same group of centers from 2004–2008 to 2014–2019 was 51% (IQR 4.0%−130%).
Figure 1 :
The number of centers performing SHK and the incidence of SHK at individual centers is increasing. A) Number of centers performing SHK from 2004–2018. B) Proportion of heart transplant centers performing SHK from 2004–2018. C) Proportion of transplants that are SHK at the individual center level among the top 35 SHK performing centers among 3 eras (2004–2008, 2009–2103, and 2014–2019).
There is wide center-variability in the kidney function of patients selected for SHK
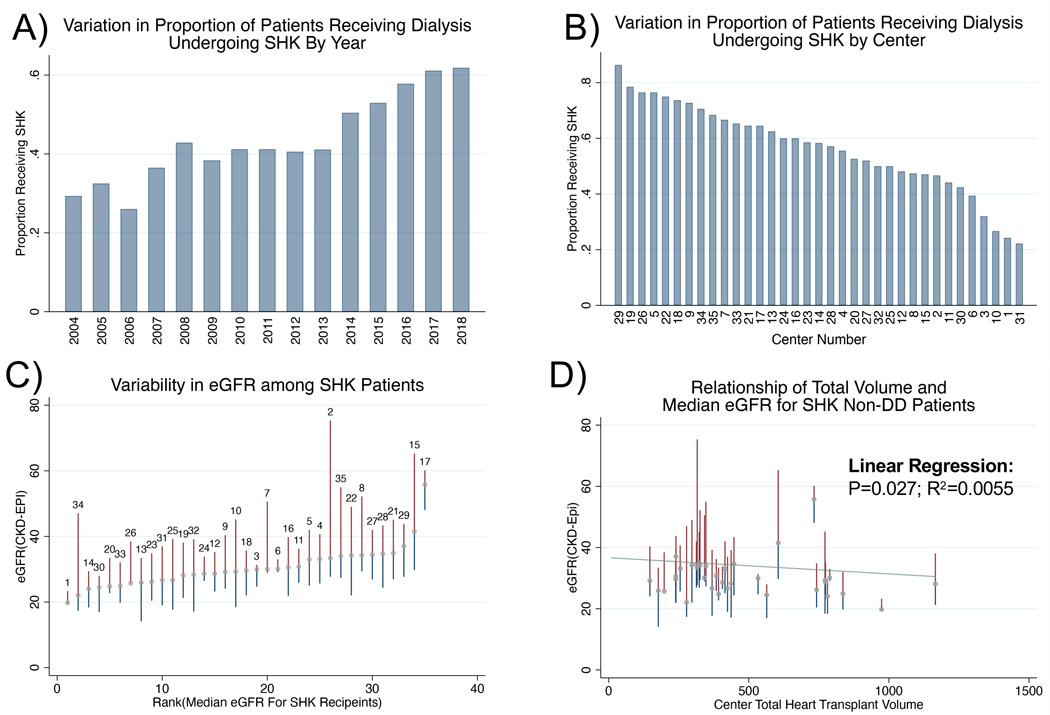
Of the patients receiving dialysis at the time of heart transplant, the proportion of those undergoing SHK increased from 30% in 2004 to 63% in 2018 (Figure 2A), with significant variation by center (22% to 86%, Figure 2B). Centers also varied in the eGFR of SHK recipients not receiving dialysis prior to transplant (Figure 2C) with the median eGFR ranging from 20 to 56 ml/min/1.73m2. This variation was similar if the analysis was restricted to era 3 (2014–2019), with the median eGFR ranging from 19 to 59 ml/min/1.73m2. There was a weak inverse relationship between overall heart transplant volume (as measured over the whole study period) and the median eGFR at which patients underwent SHK at the center level (p=0.027, Adjusted R2=0.0055, Figure 2D). Twenty-two centers (62% of all centers) performed SHK in patients with relatively normal kidney function (eGFR>60 mL/min/1.73m2), five centers performed greater than 10% of their SHK in such patients and contributed more than 1/3 of all such SHK while 13 centers never performed transplants in patients with eGFR>60 mL/min/1.73m2.
Figure 2 :
SHK is variably implemented among both patients receiving dialysis and those with residual renal function. A) Proportion of patients receiving dialysis who underwent SHK over time. B) Variation in proportion of patients receiving dialysis who underwent SHK by center among top 35 centers performing SHK. C) Variation in eGFR among non-dialysis dependent patients among the top 35 centers performing SHK (Median and IQR; labeled with center number). D) Correlation of SHK proportion with center volume among non-dialysis dependent patients with slight negative correlation.
Difference in centers that perform high and low proportions of SHK
We next examined the differences in patient populations between centers that performed a high versus low proportion of SHK (highest vs. lowest tercile, Table 1). Examining all patients undergoing transplant at centers in the top tercile of SHK, these patients were more likely to be nonwhite (49% nonwhite vs. 42%, p<0.001), to be on dialysis(8% vs. 4% p<0.001), to be listed as Status 1A in the pre-2018 allocation system (64% vs. 57%, p<0.001, no differences in the post-2018 allocation system), to have had a prior transplant(5% vs. 3% p<0.001) and to have a slightly lower median eGFR (Median 64 IQR (48–85) vs. 66(49–88) p=0.005). They were less likely to have a VAD prior to transplant (30% vs. 40%, p<0.001). Examining center level differences, centers in the highest tercile of SHK had lower overall volumes (240 [198–415] vs. 669.0 [478.5–777.5], p=0.003 by Wilcoxon Rank-Sum) but higher SHK specific volumes (36 [22–47] vs. 20 [17.5–27], p=0.031 by Wilcoxon Rank-Sum). Regions 5 (n=7) and 7 (n=4) contributed nearly 1/3 of all centers in the highest tercile, with no centers in the lowest tercile. Finally, it worth noting that among all patients transplanted at high SHK performing centers, wait times were substantially lower (Med IQR 59 [20–186] vs. 82 [21–247] days, p<0.001).
Table 1:
Patient and Center Characteristics in Centers Performing the Highest vs. Lowest Proportion of Simultaneous Heart Kidney Transplants (by Tercile).
| Lowest Tercile SHK Utilizing Centers N=7,669 | Highest Tercile SHK Utilizing Centers N=3,837 | p-value | |
|---|---|---|---|
|
| |||
| Gender(F)-n(%) | 1,914 (25%) | 962 (25%) | 0.89 |
| Recipient Age-Med(IQR) | 56.0 (46.0–63.0) | 57.0 (48.0–64.0) | <0.001 |
| Race-n(%) | <0.001 | ||
| White | 5,217 (68%) | 2,337 (61%) | |
| Black | 1,676 (22%) | 826 (22%) | |
| Asian | 503 ( 7%) | 383 (10%) | |
| Native American | 214 ( 3%) | 225 ( 6%) | |
| Native Hawaiian/PI | 27 ( 0%) | 7 ( 0%) | |
| Multiracial | 16 ( 0%) | 37 ( 1%) | |
| Unknown | 16 ( 0%) | 22 ( 1%) | |
| Payor Type-n(%)* | <0.001 | ||
| Private | 3,985 (52%) | 1,920 (50%) | |
| Public | 3,580 (47%) | 1,837 (48%) | |
| Self | 81 ( 1%) | 77 ( 2%) | |
| eGFR, CKD-EPI Formula-Med(IQR)* | 66 (49–88) | 64 (48–85) | 0.005 |
| Pre-Transplant Dialysis-n(%)* | 342 ( 4%) | 301 ( 8%) | <0.001 |
| Pre-Transplant Albumin(mg/dL)-Med(IQR)& | 3.7 (3.2–4.1) | 3.9 (3.4–4.3) | <0.001 |
| Post-2018 Status-n(%) | 0.30 | ||
| Status 1 | 17 ( 6%) | 17 ( 8%) | |
| Status 2 | 116 (41%) | 96 (48%) | |
| Status 3 | 82 (29%) | 46 (23%) | |
| Status 4 | 54 (19%) | 34 (17%) | |
| Status 5 | 1 ( 0%) | 1 ( 0%) | |
| Status 6 | 16 ( 6%) | 6 ( 3%) | |
| Pre-2018 Status-n(%) | <0.001 | ||
| Status 1A | 4,172 (57%) | 2,343 (64%) | |
| Status 1B | 2,709 (37%) | 884 (24%) | |
| Status 2 | 500 ( 7%) | 409 (11%) | |
| Diabetes-n(%)* | 2,121 (28%) | 1,035 (27%) | 0.45 |
| Calculated Recipient BMI-Med(IQR)* | 26.9 (23.7–30.5) | 26.3 (23.2–30.0) | <0.001 |
| ECMO-n(%) | 86 ( 1%) | 46 ( 1%) | 0.71 |
| Ventilator-n(%) | 124 ( 2%) | 51 ( 1%) | 0.23 |
| Prior Transplant-n(%) | 248 ( 3%) | 173 ( 5%) | <0.001 |
| VAD-n(%)* | 3,027 (40%) | 1,167 (30%) | <0.001 |
| Total Days On Waiting List-Med(IQR) | 82.0 (21.0–247.0) | 59.0 (20.0–186.0) | <0.001 |
0–5% missing
5–10% missing
>10% missing
Center-level use of SHK is not associated with waitlist mortality
We next examined whether the rate at which a center chooses SHK over HA is associated with HA/SHK waitlist mortality. Overall, there was no association across the 3 eras (era 1:rho2=0.0092, p=0.589; era 2: rho2=0.023, p=0.387; era 3: rho2=0.092, p=0.076).
The odds of undergoing SHK varies by center but is predictably influenced by patient factors
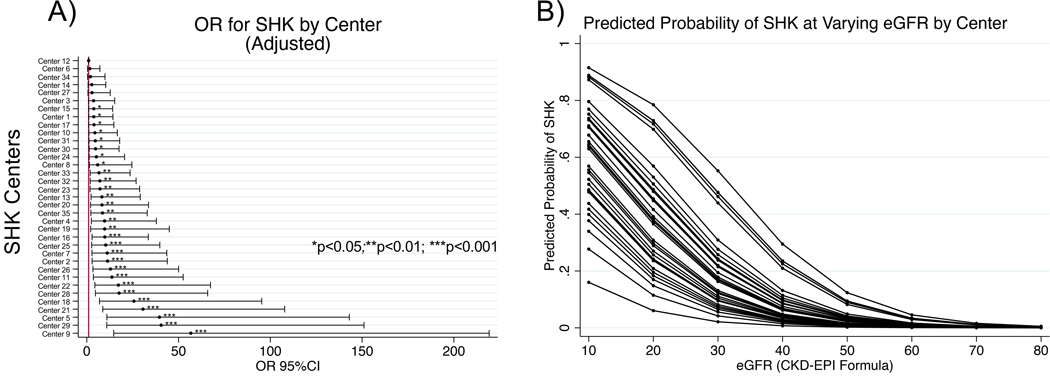
We next sought to determine the odds of undergoing SHK vs. HA among non-dialysis dependent patients for a given center, using the center with the lowest odds (center 12) of SHK as the referent. Note, we found that the proportion of patients who were waitlisted for SHK by center was highly correlated with the proportion of patients who were transplanted with SHK (p<0.001, R2=0.844), and therefore elected to examine only transplanted patients, for whom more data is available. Relative to the center with the lowest odds of performing SHK, a patient at the center with the highest odds (center 9) had a 42 times greater odds of undergoing SHK (OR 42.8 95% CI 12.5–146.2, p<0.001): whereas center 12 performed SHK in only 0.4% of non-dialysis-dependent patients, center 9 performed SHK in 14.7% of non-dialysis-dependent patients. Adjusting for a priori specified patient factors (age, era, ventricular assist device usage, diabetes, and eGFR at transplant) did not alter the results substantially: the center with the highest odds of SHK had 57 times the odds of performing an SHK compared to the referent (OR 56.7 95% CI 14.7–219.2; Figure 3A). While eGFR was a powerful predictor of SHK, the predicted probability for an SHK as eGFR decreased still varied substantially (Figure 3B).
Figure 3 :
Variation in risk of SHK receipt by center. A) Adjusted odds of SHK by center. B) Predicted probability of SHK at various eGFR. Each line represents a single center varied over eGFR at intervals of 10 from 10–80 mL/min/1.73m2.
Discussion
Our study describes the practice of SHK at the center level. Despite the limitations inherent in registry-based studies, three findings are worth highlighting. Firstly, SHK is increasing in overall incidence with a growing number of centers performing SHK. Secondly, SHK practice varies considerably between centers, even after accounting for available patient-level factors known to contribute to patient selection for SHK. Finally, there are differences in patient and transplant center characteristics between centers performing a higher rate versus lower rate of SHK transplants.
SHK is an increasingly common practice, a finding that is consistent with other publications16, 17. Between 2004 and 2018 the number of heart transplant centers performing SHK increased from 25 to 64 and the median number of SHK at each center increased by 263%, compared to the 151% median increase in HA at the same group of centers. Without a systematic description of all heart failure patients being considered for heart transplant (including those not added to the waitlist) and more granular characterization of their kidney disease, these numbers may represent either a national trend towards greater consideration of heart transplant in heart failure patients with concomitant kidney failure or a greater tendency to perform SHK at any given level of kidney function – or perhaps both. Additionally, there may be unintentional incentives within current UNOS policy to perform more multi-organ transplants including an increase in prioritization (patients listed for multiple organs move from Status 6 to Status 5) and differential reporting of mortality as multi-organ transplants are not captured in the Scientific Registry of Transplant Recipients Program Specific Reports 19, 20. Whatever the reason, the increasing number of SHK requires further investigation, as others have recently described important ramifications for patients on the waitlist for kidneys alone, including removal of high quality kidneys from the donor pool21 and longer wait times and increased odds of death for kidney alone recipients22. At present, the impact of increasing SHK numbers on patients being consider for heart transplantation is unknown.
Arguably the most significant finding in our study is the extent of center level variability in patient selection for SHK that is not explained by observed patient characteristics, both in dialysis-dependent and in non-dialysis dependent patients. Indeed, we find that a single patient had more than a 40-fold difference in the odds of being selected for SHK vs. HA based on the center where they received transplant. Figure 3B demonstrates the wide center-level variability of eGFR “threshold” for SHK. Additionally, 6 individuals, 3% of all SHK recipients, received an SHK despite a recorded eGFR >60ml/kg/1.73m2.
The variability in center-level practice occurs in the absence of policy governing patient selection for SHK. Published opinions have recommended against HA in patients with eGFR less than 40ml/kg/1.73m2 23 and a recent consensus conference proposed a threshold eGFR of 30 when CKD is not present15. However, there are no formal or enforceable requirements for SHK listing. Our data are congruent with previously published studies in simultaneous liver-kidney (SLK) which showed the percentage of eligible SLK candidates listed varied from 9% to 70.7%12, even after adjusting for both patient and center level characteristics. Though the SLK study cited was performed as a baseline study prior to the implementation of a standardized SLK policy, it remains to be seen whether the implementation of these rules has changed listing practices at the center level. Since policy implementation, SLK listings have stabilized (i.e. stopped increasing) and the use of kidney-after-liver transplantation via the safety net policy has increased24, 25. We would support a similar safety-net policy in SHK to help reduce any pressures to proceed with SHK in persons likely to have good renal outcomes.16, 17.
Also of interest was the finding that SHK is performed in a varying proportion of patients that are receiving dialysis at the time of heart transplant. This may stem from our inability to fully describe the clinical circumstances of those receiving dialysis prior to transplant, as the STAR file does not distinguish between those receiving dialysis briefly for acute kidney injury (AKI) from those with end-stage kidney disease (ESKD). However, the wide degree of variability suggests that centers are not evaluating patients on dialysis for SHK equally.
Comparing centers with a high rate versus low rate of SHK performance, we observed higher performing centers more often transplanted patients on dialysis, who had undergone a prior transplant, and who were listed as Status 1A. This suggests that while centers with the highest rate of SHK transplantation may be more comfortable transplanting ‘sicker’ patients, it comes at the expense of utilizing more kidney allografts. It is possible that these centers are listing patients later in their clinical course as time on the waiting list was substantially lower among high SHK performing centers. As mentioned, center-level variability in patient selection for SHK remained in our analysis despite controlling for patient level factors. This raises questions about whether center ‘culture’ and/or ‘clinical practice’ simply vary between institutions and providers working in these centers. For example, if a center transplanting very sick patients has the experience of many HA recipients ending up on dialysis, might they may be more apt to list patients for SHK?
Unfortunately, while our analysis shows great differences in center level practice, due to inherent shortcoming in our data, we are unable to fully explain the origin. Nonetheless, we believe that understanding these differences is important to ensuring equitable organ allocation and to eliminate potential unsupported biases in patient selection. In our study, we are unable to rule out the possibility that patients’ clinical differences not captured in the UNOS registry explain much of the variability seen in center-level practices. These uncaptured patient level factors include duration of kidney disease prior to heart transplant, or other assessments of kidney function, such as proteinuria, variables that are central to risks for unrecoverable kidney injury that might benefit from SHK. However, the uncaptured data are unlikely to be able to explain the extent of center variability we described. Broader collection of patient level data by UNOS would facilitate better understanding center-level decision making.
Other potential sources of variability include uncertainty about candidate selection, a point that has been highlighted by many experts in the field, and differences in internal processes, such as early involvement of nephrology services in candidate selection, a topic discussed at a recent national consensus conference15. It also may reflect center-level variability in clinical expertise and comfort in pursuing single- versus dual-organ transplant, as mentioned above. Indeed, prior work in kidney transplantation has shown differences in outcomes based on factors such as clinician experience6. We attempted to mitigate this by restricting our primary analysis to only SHK-performing centers, as these centers likely have at least some comfort and expertise with dual-organ transplantation. Additionally, we calculated eGFR using a version of the CKD-EPI formula which includes race. As such calculations have been shown to be problematic27, it may be necessary to repeat this study using a non-race adjusted formula.
Regardless of origin, wide variability in SHK transplantation raises concerns about equitable access to heart transplantation among those with kidney failure. This could be investigated by examining the characteristics of all patients considered for heart transplantation, regardless of whether they were able to access the waitlist or transplant. Access disparities in those pursuing kidney transplant alone has been easier to investigate because almost the entire end-stage kidney disease population is included in the United States Renal Data System28, 29. By comparison, there is no analogous registry of patients being considered for heart transplant who do not go on to be listed for transplant. Were there such a registry, we would likely see a magnification of the disparity observed in this paper.
We also tested the hypothesis that programs performing SHK are pushing the limits of transplantation by accepting sicker patients, which ought to be reflected by a higher waitlist mortality. We found no correlation between waitlist mortality and the proportion of recipients undergoing SHK and hence nothing to support that hypothesis.
Our study has important limitations. As pointed out, insufficient patient-level clinical data is a major limitation30. We chose not to adjust for center-level factors in our model, such as transplant volume, as we posited that center-level characteristics themselves would contribute to center-level differences in meaningful ways and our goal was to capture these differences that were attributable to practice variation and organizational differences. For the same reason, we chose not to control for socioeconomic factors and race, known to affect listing for and receipt of organ transplantation31, 32. Finally, we explicitly decided to focus on patients who received SHK and not those listed for SHK, therefore not capturing the true variation in listing practices. However, a sensitivity analysis did show a strong correlation between listing for and receipt of SHK, indicating that these results may extrapolate to the listed population as well. As waitlist times in heart transplant are short but waitlist mortality is high33, we chose to examine only those patients that were successfully transplanted as some patients listed for SHK may progress into multi-organ system failure, a population very likely to die and unlikely to undergo successful transplant.
In summary, SHK is an increasingly common practice with a wide center variability in patient selection for SHK vs HA across United States heart transplant programs. Variability in practice may reflect important uncertainties regarding candidate selection for SHK or perhaps differences in center culture and process but are unlikely to be solely attributable to the medical conditions of patients. The absence of national SHK eligibility criteria therefore constitutes a potential violation of The Final Rule. A national effort to understand candidate selection and to develop national guidelines around kidney allocation for SHK—and the potential creation of a safety-net for patients who develop renal failure post-operatively—is warranted to assure proper use of, and fair access to, available organs.
Supplementary Material
Abbreviations
- eGFR
Estimated Glomerular Filtration Rate
- HA
Heart Alone
- UNOS
United Network for Organ Sharing
- SHK
Simultaneous Heart Kidney
- SLK
Simultaneous Liver Kidney
- STAR
Standard Transplant Analysis and Research
- VAD
Ventricular Assist Device
Footnotes
Authorship
Brian I Shaw participated in research design, writing of the paper, performance of the research, and data analysis. No relevant disclosures are noted. Brian I Shaw was supported by NIAID R38AI140297 during this research.
Mariya L Samoylova participated in research design, writing of the paper, performance of the research, and data analysis. No relevant disclosures are noted.
Andrew S Barbas participated in research design and writing of the paper. No relevant disclosures are noted.
Xingxing S Chen participated in the writing of the paper. No relevant disclosures are noted.
Yee Lu participated in the writing of the paper. No relevant disclosures are noted.
Lisa M McElroy participated in research design and writing of the paper. No relevant disclosures are noted.
Scott Sanoff participated in research design and writing of the paper. No relevant disclosures are noted.
References
- 1.Goldberg DS, French B, Lewis JD, et al. Liver transplant center variability in accepting organ offers and its impact on patient survival. Journal of Hepatology. 2016/04/01/ 2016;64(4):843–851. doi: 10.1016/j.jhep.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazzal M, Lentine KL, Naik AS, et al. Center-driven and Clinically Driven Variation in US Liver Transplant Maintenance Immunosuppression Therapy: A National Practice Patterns Analysis. Transplantation Direct. 2018;4(7):e364. doi: 10.1097/txd.0000000000000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong AJ, Flores A, Saracino G, et al. Center Variation in Intention-to-Treat Survival Among Patients Listed for Liver Transplant. Liver Transpl. Dec 2020;26(12):1582–1593. doi: 10.1002/lt.25852 [DOI] [PubMed] [Google Scholar]
- 4.Mooney JJ, Weill D, Boyd JH, Nicolls MR, Bhattacharya J, Dhillon GS. Effect of Transplant Center Volume on Cost and Readmissions in Medicare Lung Transplant Recipients. Ann Am Thorac Soc. Jul 2016;13(7):1034–41. doi: 10.1513/AnnalsATS.201601-017OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival Differences Following Lung Transplantation Among US Transplant Centers. JAMA. 2010;304(1):53–60. doi: 10.1001/jama.2010.885 [DOI] [PubMed] [Google Scholar]
- 6.Tsampalieros A, Knoll GA, Fergusson N, Bennett A, Taljaard M, Fergusson D. Center Variation and the Effect of Center and Provider Characteristics on Clinical Outcomes in Kidney Transplantation: A Systematic Review of the Evidence. Can J Kidney Health Dis. 2017;4:2054358117735523. doi: 10.1177/2054358117735523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King KL, Husain SA, Schold JD, et al. Major Variation across Local Transplant Centers in Probability of Kidney Transplant for Wait-Listed Patients. Journal of the American Society of Nephrology. 2020;31(12):2900–2911. doi: 10.1681/asn.2020030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker WF, Anderson AS, Hedeker D, et al. Geographic Variation in the Treatment of U.S. Adult Heart Transplant Candidates. J Am Coll Cardiol. Apr 24 2018;71(16):1715–1725. doi: 10.1016/j.jacc.2018.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akintoye E, Shin D, Alvarez P, Briasoulis A. State-Level Variation in Waitlist Mortality and Transplant Outcomes Among Patients Listed for Heart Transplantation in the US From 2011 to 2016. JAMA network open. 2020;3(12):e2028459-e2028459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpern SE, McConnell A, Peskoe SB, et al. A three-tier system for evaluation of organ procurement organizations’ willingness to pursue and utilize nonideal donor lungs. Am J Transplant. Mar 2021;21(3):1269–1277. doi: 10.1111/ajt.16347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi AY, Mulvihill MS, Lee H-J, et al. Transplant Center Variability in Organ Offer Acceptance and Mortality Among US Patients on the Heart Transplant Waitlist. JAMA Cardiology. 2020;5(6):660–668. doi: 10.1001/jamacardio.2020.0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X, Massie AB, Haugen CE, et al. Baseline and Center-Level Variation in Simultaneous Liver-Kidney Listing in the United States. Transplantation. Apr 2018;102(4):609–615. doi: 10.1097/tp.0000000000001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNOS/OPTN. Simultaneous Liver Kidney (SLK) Policy. https://optn.transplant.hrsa.gov/media/1192/0815-12_SLK_Allocation.pdf
- 14.The Final Rule. Accessed April 30, 2021, https://www.ecfr.gov/cgi-bin/text-idx?SID=bb60e0a7222f4086a88c31211cac77d1&mc=true&node=pt42.1.121&rgn=div5
- 15.Kobashigawa J, Dadhania DM, Farr M, et al. Consensus conference on heart-kidney transplantation. Am J Transplant. Feb 2 2021;doi: 10.1111/ajt.16512 [DOI] [PubMed] [Google Scholar]
- 16.Shaw BI, Sudan DL, Boulware LE, McElroy LM. Striking a Balance in Simultaneous Heart Kidney Transplant: Optimizing Outcomes for All Wait-Listed Patients. J Am Soc Nephrol. Aug 2020;31(8):1661–1664. doi: 10.1681/asn.2020030336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw BI, Samoylova ML, Sanoff S, et al. Need for improvements in simultaneous heart-kidney allocation: The limitation of pretransplant glomerular filtration rate. Am J Transplant. Dec 22 2020;doi: 10.1111/ajt.16466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng XS, Han J, Stedman MR, Chertow GM, Tan JC. And Then There Were Three: Effects of Pretransplant Dialysis on Multiorgan Transplantation. Transplantation Direct. 2021;7(2):e657. doi: 10.1097/txd.0000000000001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recipients SRoT. FAQs: For Transplant Professionals: Program Specific Report Methodology - Posttransplant. Accessed December 4, 2021, https://www.srtr.org/faqs/for-transplant-center-professionals/#ptxcohortlisting
- 20.OPTN. Policy 6: Allocation of Hearts and Heart-Lungs. Accessed Dec. 4, 2021, https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf
- 21.Reese PP, Veatch RM, Abt PL, Amaral S. Revisiting Multi-Organ Transplantation in the Setting of Scarcity. American Journal of Transplantation. 2014;14(1):21–26. doi: 10.1111/ajt.12557 [DOI] [PubMed] [Google Scholar]
- 22.Westphal SG, Langewisch ED, Robinson AM, et al. The impact of multi-organ transplant allocation priority on waitlisted kidney transplant candidates. American Journal of Transplantation. n/a(n/a)doi: 10.1111/ajt.16390 [DOI] [PubMed] [Google Scholar]
- 23.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant. Jan 2016;35(1):1–23. doi: 10.1016/j.healun.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 24.Wilk AR, Booker SE, Stewart DE, et al. Developing simultaneous liver-kidney transplant medical eligibility criteria while providing a safety net: A 2-year review of the OPTN’s allocation policy. American Journal of Transplantation. n/a(n/a)doi: 10.1111/ajt.16761 [DOI] [PubMed] [Google Scholar]
- 25.Samoylova ML, Wegermann K, Shaw BI, et al. The Impact of the 2017 Kidney Allocation Policy Change on Simultaneous Liver-Kidney Utilization and Outcomes. Liver Transplantation. 2021;27(8):1106–1115. doi: 10.1002/lt.26053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolsrud O, Karason K, Holmberg E, et al. Renal function and outcome after heart transplantation. J Thorac Cardiovasc Surg. Apr 2018;155(4):1593–1604.e1. doi: 10.1016/j.jtcvs.2017.11.087 [DOI] [PubMed] [Google Scholar]
- 27.Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. Nov 4 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gander JC, Zhang X, Ross K, et al. Notice of Retraction and Replacement. Gander et al. Association Between Dialysis Facility Ownership and Access to Kidney Transplantation. JAMA. 2019;322(10):957–973. Jama. Apr 21 2020;323(15):1509–1510. doi: 10.1001/jama.2020.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gander JC, Zhang X, Plantinga L, et al. Racial disparities in preemptive referral for kidney transplantation in Georgia. Clin Transplant. Sep 2018;32(9):e13380. doi: 10.1111/ctr.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laskey HL, Schomaker N, Hung KW, et al. Predicting renal recovery after liver transplant with severe pretransplant subacute kidney injury: The impact of warm ischemia time. Liver Transpl. Aug 2016;22(8):1085–91. doi: 10.1002/lt.24488 [DOI] [PubMed] [Google Scholar]
- 31.Wesselman H, Ford CG, Leyva Y, et al. Social Determinants of Health and Race Disparities in Kidney Transplant. Clinical Journal of the American Society of Nephrology. 2021;16(2):262–274. doi: 10.2215/cjn.04860420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng Y-H, Pankratz VS, Leyva Y, et al. Does Racial Disparity in Kidney Transplant Waitlisting Persist After Accounting for Social Determinants of Health? Transplantation. 2020;104(7):1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein BA, Thomas L, Zaroff JG, Nguyen J, Menza R, Khush KK. Assessment of Heart Transplant Waitlist Time and Pre- and Post-transplant Failure: A Mixed Methods Approach. Epidemiology. Jul 2016;27(4):469–76. doi: 10.1097/ede.0000000000000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.