Abstract
Background
The purpose of this study was to evaluate the accuracy of automatic cephalometric landmark localization and measurements using cephalometric analysis via artificial intelligence (AI) compared with computer-assisted manual analysis.
Methods
Reconstructed lateral cephalograms (RLCs) from cone-beam computed tomography (CBCT) in 85 patients were selected. Computer-assisted manual analysis (Dolphin Imaging 11.9) and AI automatic analysis (Planmeca Romexis 6.2) were used to locate 19 landmarks and obtain 23 measurements. Mean radial error (MRE) and successful detection rate (SDR) values were calculated to assess the accuracy of automatic landmark digitization. Paired t tests and Bland‒Altman plots were used to compare the differences and consistencies in cephalometric measurements between manual and automatic analysis programs.
Results
The MRE for 19 cephalometric landmarks was 2.07 ± 1.35 mm with the automatic program. The average SDR within 1 mm, 2 mm, 2.5 mm, 3 and 4 mm were 18.82%, 58.58%, 71.70%, 82.04% and 91.39%, respectively. Soft tissue landmarks (1.54 ± 0.85 mm) had the most consistency, while dental landmarks (2.37 ± 1.55 mm) had the most variation. In total, 15 out of 23 measurements were within the clinically acceptable level of accuracy, 2 mm or 2°. The rates of consistency within the 95% limits of agreement were all above 90% for all measurement parameters.
Conclusion
Automatic analysis software collects cephalometric measurements almost effectively enough to be acceptable in clinical work. Nevertheless, automatic cephalometry is not capable of completely replacing manual tracing. Additional manual supervision and adjustment for automatic programs can increase accuracy and efficiency.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-023-02881-8.
Keywords: Cephalometric analysis, Automatic identification, Cone-beam CT, Artificial intelligent
Background
Lateral cephalometry has consistently played an important role in orthodontic diagnosis and treatment protocol design for decades. It can not only clarify the dentofacial morphology and the anatomic basis for malocclusion but also evaluate the growth patterns in the craniofacial complex. Thus, it is significant for orthodontists to locate cephalometric landmarks precisely [1, 2].
Currently, cephalometric analysis is commonly carried out by manual tracing with computer-assisted analysis software [3–5]. Precise definitions of landmarks, calibration of operators and replication of tracings are all important in cephalometric analysis. However, repeat work of landmark localization may spare a great deal of time but do little to improve the accuracy of analysis [6]. The precision, reliability and time demands of cephalometric analysis depend to a great extent on the experience of orthodontists, who must spend a great deal of time training and accumulating experience before they can perform the analysis reliably.
In recent years, a large number of artificial intelligence (AI) studies have been conducted to explore automated landmark locations [7–14]. Compared with traditional cephalometric analysis, AI landmark identification shows superiority in repeatability and efficiency [11, 15]. Although there were several studies comparing the difference of cephalometric analysis between human and AI [16–20], the various automated cephalometric software available to practitioners still need to be evaluated with the development of AI application in orthodontics. Planmeca Romexis software is widely used for cephalometric analysis, and 2-dimensional automatic tracing is feasible and practical with this program. Rather than spending valuable time on training, supporting and monitoring the work of others, clinicians only need a few seconds to obtain the results of cephalometric analysis from it. Therefore, it is important to evaluate the accuracy of this program as a potentially time-saving tool. Our study was designed to compare the automatic localization program with one of the most frequently used computer-assisted cephalometric analysis programs, Dolphin software. Moreover, as far as we know that there have been only a few studies involving both cephalometric landmark and measurement analysis, the accuracy of landmarks and measurements in terms of bone, teeth and soft tissue were evaluated respectively in this study to explore more comprehensively the potential application of AI cephalometric analysis in clinical work.
Methods
The study was approved by the Ethical Committee Department of the Affiliated Hospital of Stomatology, Nanjing Medical University (PJ2022-030-01).
Data collection
In this observational study, reconstructed lateral cephalograms (RLCs) processed from cone-beam computed tomography (CBCT) scans were assessed. The retrospective CBCT images obtained from the patient database at the Affiliated Hospital of Stomatology, Nanjing Medical University were collected for the purposes of clinical diagnosis and analysis including airway volume analysis, temporomandibular joint or alveolar bone assessment and so on. A statistical power of 80% at a significance level (alpha) of 0.05 using a two-sided paired t test was assumed by the G*power software (version 3.1, Heinrich-Heine-University Dusseldorf, Germany). The calculation showed that 35 images were required with an effect size of 0.49 according to the previous study [17]. For this study, we searched the patient database and selected 85 patients who underwent CBCT from 2015 to 2020.
The sample inclusion criteria were as follows: (1) all CBCT images were taken in a supine position using the same machine and fully included all relevant anatomical structures, and (2) no central incisors were missing or defective. Additionally, the factors of age, gender and skeletal classification were considered during sample inclusion to increase the representativeness of the sample and therefore the applicability of the conclusion. The exclusion criteria were patients who had (1) impacted teeth in the anterior region, (2) prosthetic restoration of the central incisors, (3) previous orthodontic treatment or orthognathic surgery, or (4) cleft lip and palate syndromes.
All CBCT images were obtained with the same CBCT machine (NewTom 5G; Quantitative Radiology, Verona, Italy) with a standard acquisition protocol ( 18 × 16 cm field of view, 110 kV, 1–20 mA pulsed mode, and 0.3-mm voxel size).
RLC acquisition and processing
CBCT images were reconstructed into RLCs by using Dolphin software (version 11.9, Chatsworth, California, USA). The orthogonal projection type was chosen to create non-distorted images. All RLCs were generated with optimal opacity of hard tissue visualization and adjusted with the slider to sharpen the images. Then, the RLCs were entered into the computer-assisted manual analysis program (Dolphin software) and the automatic cephalometric analysis software (Planmeca Romexis software, version 6.2, Helsinki, Finland).
Cephalometric analysis
Totally 19 landmarks and 23 measurements (Fig. 1) were chosen on the basis of Steiner, Downs, Tweed cephalometric analysis and some other measurements commonly used in clinical practice. All RLCs were analysed twice by a well-trained orthodontic clinician. The procedure was supervised by two experienced orthodontists (BY and LL, with 17 and 10 years of experience) to ensure the accuracy of manual analysis. No more than 20 images were traced in a single day, and repeat analyses were separated by a two-week interval. The time required for manual tracing was also recorded. Then, the landmark coordinate information and cephalometric measurement values were obtained. To verify the inter-operator precision, another operator (also an orthodontic clinician having undergone the cephalometric training) performed the same process, and the cephalometric measurement values were compared with those of the first operator. The operators identified the landmarks on the same computer.
Fig. 1.
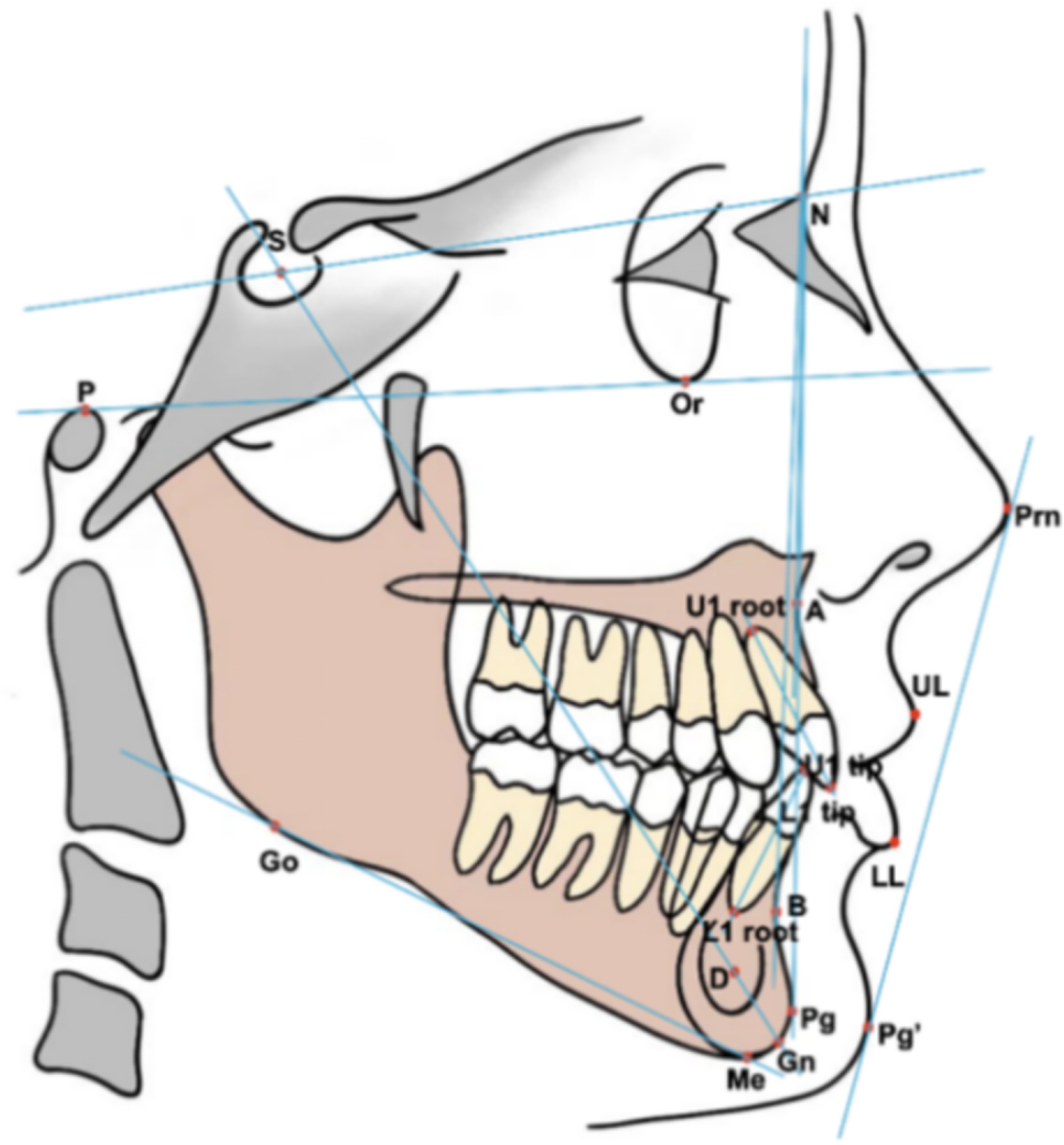
Cephalometric landmarks and measurements in terms of bone, teeth and soft tissue. The definitions of the landmarks and measurements are enumerated in Additional file 1 and Additional file 2
Statistical analysis
Analyses were conducted using IBM SPSS, version 23 (IBM Corp., Armonk, NY). Statistical significance was defined as a two-sided P value < 0.05. The Shapiro–Wilk test was used to test the normality of the distributions.
The error of landmark positions was identified by superimposing ruler points using the x and y coordinates. The radial error R was calculated between manually detected and AI-detected landmark coordinates:
The mean radial error (MRE), standard deviation (SD) and successful detection rate (SDR) were calculated as follows (in the following equations, N denotes the sample size, and z refers to the precision ranges of 1, 2, 2.5, 3, and 4 mm.)
The MRE; SD; and SDR at 1, 2, 2.5, 3, and 4 mm for 19 landmarks were analysed to evaluate the accuracy of AI-driven localization performance. Moreover, the scatter plots with 95% confidence ellipses were depicted to visualize and evaluate the error pattern in 2-dimensional directions.
The intraclass correlation coefficient (ICC) was analysed to confirm the consistency of the results among multiple measurements. Paired t tests; proportion within 95% limits of agreement; and Bland‒Altman plots were used to assess the consistency of manual and AI cephalometric measurements.
Results
In this study, we analysed 85 CBCT images, taking into account baseline variables including gender, age, and skeletal classification. The distributions of specific characteristics within the sample are described in Table 1.
Table 1.
Characteristics of the sample
| Variables | N (%) | |
|---|---|---|
| Total | 85 (100%) | |
| Gender | Female | 44 (52%) |
| Male | 41 (48%) | |
| Age | Mixed dentition (11.26 ± 0.79 y) | 43 (51%) |
| Permanent dentition (19.93 ± 5.82 y) | 42 (49%) | |
| Skeletal classification | Class I | 39 (46%) |
| Class II | 15 (18%) | |
| Class III | 31 (36%) |
The ICC values showed mean intra-operator reproducibility of 0.97 (0.91–0.99) and 0.96 (0.90–0.99). The ICC of the mean measurement values between the two operators was 0.94 (0.86–0.99) on average. Both the intra-operator and inter-operator comparisons [see Additional file 3] indicated excellent reliability [21]. Given the high consistency between operators, the mean values of operator 1’s analysis were used in this study. The average time required for cephalometry was 157 s for manual tracing and 2 s for AI analysis.
Accuracy in detecting landmarks
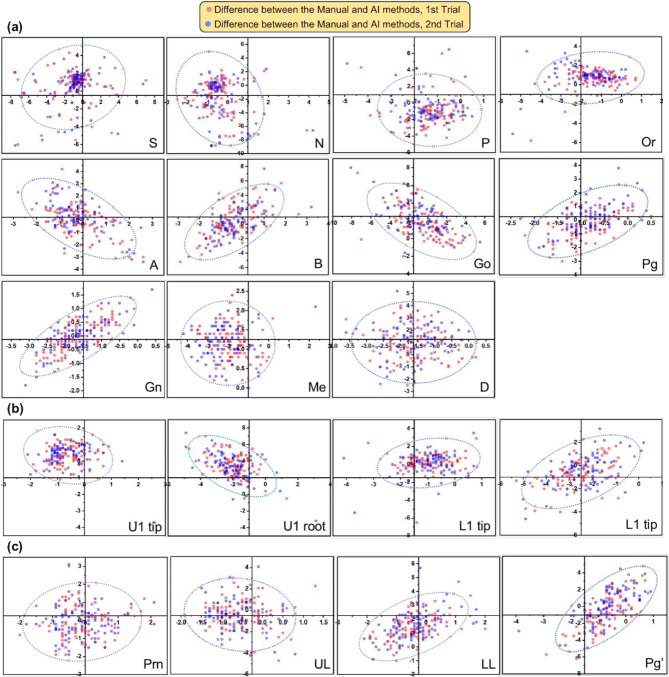
The differences in landmark tracing between manual and AI detection and the SDR of automatic detection are shown in Table 2. The detection accuracy of landmarks varied over a large range. The average variation of 19 landmarks was 2.07 ± 1.35 mm. In a comparison of results among the three landmark categories, soft tissue landmarks had the highest consistency (1.54 ± 0.85 mm), while dental landmarks had the lowest consistency (2.37 ± 1.55 mm). Among the 19 landmarks, the Prn point (1.01 ± 0.48 mm) had the highest consistency and the best performance in terms of SDR. Meanwhile, the Pg (1.31 ± 0.57 mm) and U1 tip (1.06 ± 0.49 mm) points almost had the lowest MRE and highest SDR among the skeletal and dental landmarks. The scatter plots (Fig. 2) suggest the distribution of landmark position differences.
Table 2.
The bias between manual and automatic detection and the SDR of automatic detection
| Landmarks | MRE (mm) | SD (mm) | 95% CI (mm) | SDR (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | < 1 mm | < 2 mm | < 2.5 mm | < 3 mm | < 4 mm | ||||
| Skeletal landmarks (11) | ||||||||||
| S | 2.61 | 1.88 | -1.07 | 6.29 | 7.06 | 57.65 | 63.53 | 71.76 | 81.18 | |
| N | 2.63 | 2.31 | -1.90 | 7.16 | 28.24 | 55.29 | 62.35 | 63.53 | 77.65 | |
| P | 2.24 | 1.14 | 0.01 | 4.47 | 11.76 | 51.76 | 62.35 | 78.82 | 95.29 | |
| Or | 1.97 | 1.07 | -0.13 | 4.07 | 16.47 | 58.82 | 72.94 | 87.06 | 96.47 | |
| A | 1.39 | 0.87 | -0.32 | 3.10 | 35.29 | 77.65 | 85.88 | 95.29 | 98.82 | |
| B | 1.96 | 1.19 | -0.37 | 4.29 | 20.00 | 61.18 | 71.76 | 80.00 | 94.12 | |
| Go | 3.08 | 1.67 | -0.19 | 6.35 | 3.53 | 25.88 | 48.24 | 58.82 | 75.29 | |
| Pg | 1.31 | 0.57 | 0.19 | 2.43 | 32.94 | 87.06 | 96.47 | 98.82 | 100.00 | |
| Gn | 1.77 | 0.53 | 0.73 | 2.81 | 4.71 | 65.88 | 89.41 | 97.65 | 100.00 | |
| Me | 2.41 | 0.65 | 1.14 | 3.68 | 3.53 | 20.00 | 54.12 | 85.88 | 98.82 | |
| D | 2.27 | 0.86 | 0.58 | 3.96 | 5.88 | 41.18 | 64.71 | 83.53 | 96.47 | |
| Average | 2.15 | 1.37 | -0.54 | 4.84 | 15.40 | 54.76 | 70.16 | 81.92 | 92.19 | |
| Dental landmarks (4) | ||||||||||
| U1 tip | 1.06 | 0.49 | 0.10 | 2.02 | 45.88 | 97.65 | 98.82 | 98.82 | 98.82 | |
| U1 root | 4.14 | 1.33 | 1.53 | 6.75 | 1.18 | 4.71 | 9.41 | 22.35 | 43.53 | |
| L1 tip | 1.41 | 0.86 | -0.28 | 3.10 | 31.76 | 87.06 | 94.12 | 96.47 | 97.65 | |
| L1 root | 2.85 | 0.93 | 1.03 | 4.67 | 0.00 | 17.65 | 35.29 | 62.35 | 90.59 | |
| Average | 2.37 | 1.55 | -0.67 | 5.41 | 19.71 | 51.77 | 59.41 | 70.00 | 82.65 | |
| Soft tissue landmarks (4) | ||||||||||
| Prn | 1.01 | 0.48 | 0.07 | 1.95 | 51.76 | 97.65 | 98.82 | 98.82 | 100.00 | |
| UL | 1.40 | 0.78 | -0.13 | 2.93 | 34.12 | 83.53 | 91.76 | 95.29 | 98.82 | |
| LL | 1.80 | 0.74 | 0.35 | 3.25 | 9.41 | 64.71 | 85.88 | 94.12 | 98.82 | |
| Pg′ | 1.97 | 0.98 | 0.05 | 3.89 | 14.12 | 57.65 | 76.47 | 89.41 | 94.12 | |
| Average | 1.54 | 0.85 | -0.13 | 3.21 | 27.35 | 75.89 | 88.23 | 94.41 | 97.94 | |
| Total average | 2.07 | 1.35 | -0.58 | 4.72 | 18.82 | 58.58 | 71.70 | 82.04 | 91.39 | |
SD Standard deviation; CI Confidence interval
Fig. 2.
The scatter plots with 95% confidence ellipses show the difference in the coordinates of each landmark between the manual and AI methods. a Skeletal landmarks, b dental landmarks, c soft tissue landmarks. The origin points (0,0) are located by the AI program for different landmarks. The Red and blue points represent the difference between two repetitions of manual digitization and AI detection, respectively
Accuracy in acquiring measurements
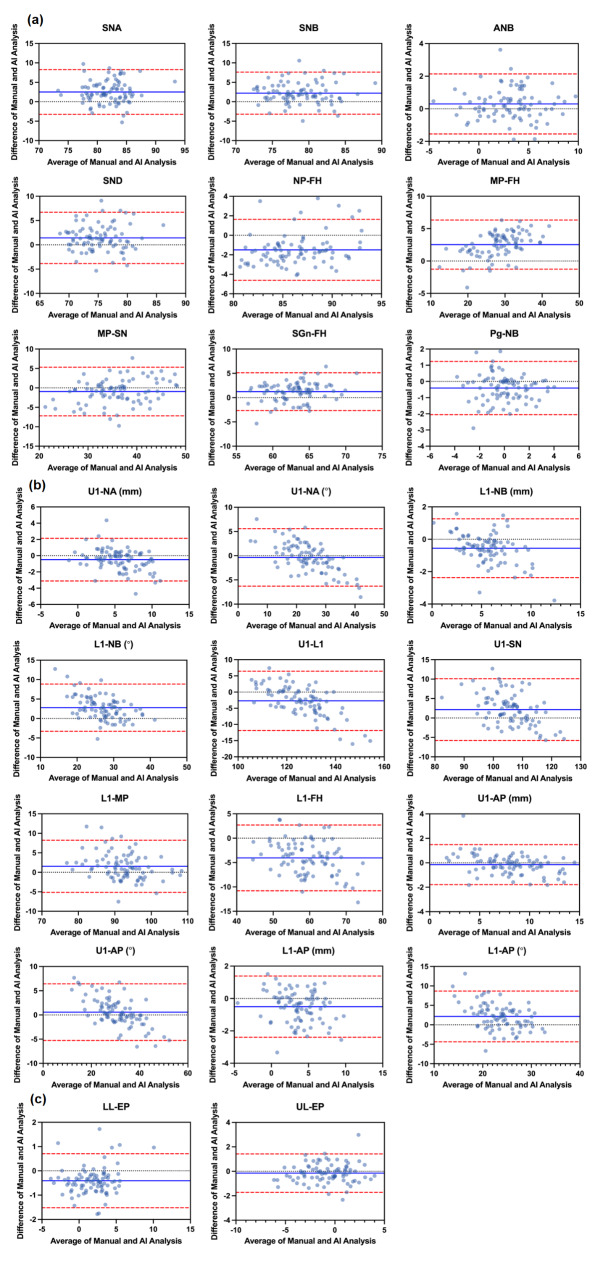
Except for 4 cephalometric measurements, U1-NA (°), U1-AP (°), U1-AP (°) and UL-EP (mm), the other difference values of 19 measurements between manual and AI detection were statistically significant (P < 0.05) according to a paired t test (Table 3). Considering the clinically acceptable limit of 2 mm or 2°, 8 measurements [20], L1-FH (°) (-4.05 ± 3.44), L1-NB (°) (2.78 ± 3.10), U1-L1 (°) (-2.72 ± 4.67), MP-FH (°) (2.53 ± 1.93), SNA (°) (2.50 ± 2.94), SNB (°) (2.21 ± 2.76), L1-AP (°) (2.15 ± 3.33), and U1-SN (°) (2.14 ± 4.06), were discrepant from manual tracing. The Bland‒Altman plots (Fig. 3) represent the consistency of the results between the manual and AI methods. Measurement bias was within the 95% limits of agreement in more than 90% of instances (Table 3).
Table 3.
Comparison of the cephalometric measurements between manual and AI analyses
| Bias | Mean (mm) | SD (mm) |
95% limits of agreement (mm) | P valuea | Proportion within 95% limits of agreement (%) |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Skeletal measurements (9) | ||||||
| SNA (°) | 2.50 | 2.94 | -3.25 | 8.26 | 0.00 | 95.29 |
| SNB (°) | 2.21 | 2.76 | -3.21 | 7.62 | 0.00 | 95.29 |
| ANB (°) | 0.30 | 0.94 | -1.55 | 2.14 | 0.01 | 95.29 |
| SND (°) | 1.42 | 2.69 | -3.86 | 6.69 | 0.00 | 94.12 |
| NP-FH (°) | -1.50 | 1.60 | -4.63 | 1.63 | 0.00 | 91.76 |
| MP-FH (°) | 2.53 | 1.93 | -1.26 | 6.32 | 0.00 | 96.47 |
| MP-SN (°) | -0.94 | 3.19 | -7.19 | 5.31 | 0.01 | 96.47 |
| Y axis (°) | 1.22 | 1.98 | -2.66 | 5.11 | 0.00 | 95.29 |
| Pg-NB (mm) | -0.41 | 0.84 | -2.06 | 1.23 | 0.00 | 95.29 |
| Dental measurements (12) | ||||||
| U1-NA (mm) | -0.49 | 1.34 | -3.12 | 2.13 | 0.00 | 95.29 |
| U1-NA (°) | -0.36 | 3.03 | -6.29 | 5.58 | 0.28 | 95.29 |
| L1-NB (mm) | -0.56 | 0.93 | -2.37 | 1.26 | 0.00 | 95.29 |
| L1-NB (°) | 2.78 | 3.10 | -3.29 | 8.85 | 0.00 | 94.12 |
| U1-L1 (°) | -2.72 | 4.67 | -11.87 | 6.44 | 0.00 | 94.12 |
| U1-SN (°) | 2.14 | 4.06 | -5.81 | 10.09 | 0.00 | 98.82 |
| L1-MP (°) | 1.52 | 3.41 | -5.17 | 8.21 | 0.00 | 92.94 |
| L1-FH (°) | -4.05 | 3.44 | -10.80 | 2.70 | 0.00 | 95.29 |
| U1-AP (mm) | -0.15 | 0.84 | -1.79 | 1.48 | 0.10 | 96.47 |
| U1-AP (°) | 0.57 | 2.98 | -5.28 | 6.42 | 0.08 | 94.12 |
| L1-AP (mm) | -0.51 | 0.97 | -2.40 | 1.39 | 0.00 | 96.47 |
| L1-AP (°) | 2.15 | 3.33 | -4.38 | 8.67 | 0.00 | 96.47 |
| Soft tissue measurements (2) | ||||||
| LL-EP (mm) | -0.41 | 0.57 | -1.52 | 0.70 | 0.00 | 91.76 |
| UL-EP (mm) | -0.15 | 0.80 | -1.72 | 1.42 | 0.09 | 95.29 |
SD Standard deviation
aResults of a paired t test between manual and AI detection. All data were found to be normally distributed according to the Shapiro‒Wilk test
Fig. 3.
Bland‒Altman plots of cephalometric measurements. a Skeletal measurements, b dental measurements, c soft tissue measurements. For each plot, the x-axis presents the average of manual and AI results, while the y-axis presents the difference between the paired measurements. The blue line represents the mean bias, and the red dashed lines represent the upper and lower 95% limits of agreement. The scale values on the x- and y-axis vary according to the mean and bias of each measurement comparison
Discussion
Cephalometric analysis is essential in every stage of orthodontic treatment. However, manual analysis has low consistency between repetitions and takes a great deal of time. With the rapid development of technology, computer vision allows machines to understand and work with images for cephalometric landmark detection after being trained with a given dataset. In contrast to conventional manual methods, AI can assess images within seconds, helping users save time and increasing the efficiency of repetitive work [11, 15]. According to our study, humans take an average of nearly 80 times as long as the AI program to complete the task. Using an automatic program saves a great deal of time and frees up orthodontists for other tasks. Additionally, the accuracy of cephalometric landmark digitization depends largely on the operator. The results of tracings by different operators or even repeated tracings by the same operator are likely to be significantly different [22]. In contrast to computer-assisted manual tracings, the results obtained from the same automatic program are fairly stable, showing its superiority in terms of repeatability [15]. Nevertheless, whether the accuracy of manual and AI cephalometric analyses is acceptable is always an important concern. To date, growing numbers of studies have focused on the accuracy of the automated cephalometric software, which is more accessible to the practicing orthodontist than in-house algorithms [16–20]. Therefore, our study is practical and meaningful for both the users of automatic programs and the engineers who seek to improve the algorithms.
The results regarding accuracy in landmark detection are consistent with published studies [9, 10]. The most accurately defined points, such as Prn, U1 tip and Pg, are on the edges of anatomical structures with sharp margins on CBCT images. Automatic programs are capable of identifying them with a high degree of precision similar to that of humans. The MREs of the U1 root (4.14 ± 1.33 mm), Go (3.08 ± 1.67 mm), L1 root (2.85 ± 0.93 mm), N (2.63 ± 2.31 mm), S (2.61 ± 1.88 mm), Me (2.41 ± 0.65 mm), D (2.27 ± 0.86 mm) and P points (2.24 ± 1.14 mm) exceeded the average value (2.07 ± 1.35 mm). The great deviation of the U1 root and L1 root points may be due to the indistinct outlines of the incisor roots [10, 23]. The recognition of incisor roots is obstructed by other dental roots when patients have anterior tooth crowding or overbite malocclusion, which is also a challenge for orthodontists in clinical work. The mixed density of incisor roots on imaging makes them difficult to identify by manual or automatic analysis. The reason for the significant errors in the Go, Me and P points is probably the asymmetrical structure of the lower mandible margin and external auditory canal, which varies over a wide range in the population. Midpoints of bilateral structures are chosen when the left and right sides cannot overlap, while asymmetrical structures increase the complexity of the region. Localizing these points relies heavily on the personal judgement of the operator. Obstruction by adjacent anatomical structures may also affect the localization of the Go point, which increases the difficulty of detection. As for the S, N and D points, the relevant structures are sometimes vague and quite ambiguous in RLCs, which makes them difficult for automatic programs to locate. The distance in landmark position between manual localization and automatic localization is interpreted as follows: <2 mm is correct, and < 4 mm is acceptable [24]. In this study, the average SDR as defined by MRE within 1 mm, 2 mm, 2.5 mm, 3 and 4 mm were 18.82%, 58.58%, 71.70%, 82.04% and 91.39%, respectively. Thus, more than half of the points were localized correctly, and more than 90% of the points were localized acceptably well.
The scatter plots (Fig. 2) indicate the distribution of landmark localization error by distance and direction. The 95% confidence ellipse shows a two-dimensional expansion of the Bland-Altman plot to observe the correlation between the x- and y-axis errors in the shape of the ellipse [15]. Points such as Or, UL and LL were distributed along the x-axis, which implied mainly horizontal bias in automatic detection. Similarly, points such as N, B, Gn and Pg’ illustrated mainly vertical bias. The anatomical positions of cephalometric landmarks are subject to bias in both the x and y coordinates. Points with considerable horizontal bias or vertical bias are usually ambiguous to locate in the corresponding direction to a certain extent. The results of AI detection are consistent with those of manual detection.
For cephalometric measurements, the rate of consistency within the 95% limits of agreement was between 91.76% and 98.82% (Table 3), which indicated high consistency in these measurements. Also, the Bland-Altman plots (Fig. 3) visualize the consistency between paired measurements. The results essentially corroborate those of a previous study [17]. The range between the limits of agreement is wider for dental measurements than for skeletal or soft tissue measurements. This conclusion is consistent with our conclusion regarding landmark identification. There were a total of 8 measurements that were both statistically significant (P<0.05) and had mean differences above 2 mm or 2°. Dental measurements have more discrepancy in comparison with manual tracing. Soft tissue measurements are basically equivalent in the two methods. Since the RLCs selected in this study were all from people who had not yet undergone orthodontic treatment, the precision of dental measurements might increase if the automatic program were applied to post-treatment images.
Generally, the consistency of AI cephalometric measurements is almost at an acceptable level, considering that it is nearly 80 times faster than manual cephalometric analysis. The main results that were of interest to us proved fairly good precision and consistency with manual analysis. Only a few measurements showed significant bias. Thus, human supervision is still necessary for automatic landmark identification. The solutions for increasing the accuracy and efficiency of automatic cephalometric analysis may be to improve the quality of the image like sharpening and algorithm approving and to implement an outlier detection and feedback system [23]. Orthodontists would need to spend only a short time checking the results of automatic localization, which could save them a great deal of time during clinical work.
Despite its strengths, this study also has several limitations. We used initial diagnosis RLCs to assess the automatic program. Diverse and complex intraoral conditions, such as overlapping teeth, overjet and overbite, are complex and make it difficult for AI to recognize relevant landmarks and obtain measurements. More detailed classification of image samples would increase the comprehensiveness of the conclusions. In addition, we selected fewer soft tissue landmarks than skeletal or dental landmarks, and the same was true of soft tissue measurements versus skeletal and dental measurements. The results would be more reliable if more soft tissue indicators were included.
Conclusion
Cephalometric measurements obtained from automatic analysis software are almost reliable enough to be acceptable in clinical work. Nevertheless, automatic cephalometry is not capable of completely replacing manual tracing. There still exist differences in localization, especially for some dental landmarks. Additional manual supervision and adjustment of automatic programs will help increase their accuracy, consistency and efficiency.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Jiangsu Province Capability Improvement Project through Science, Technology and Education-Jiangsu Provincial Research Hospital Cultivation Unit (YJXYYJSDW4) and Jiangsu Provincial Medical Innovation Center (CXZX202227) for their contribution to this study.
Abbreviations
- CBCT
Cone beam computer tomography
- RLC
Reconstructed lateral cephalogram
- MRE
Mean radial error
- SDR
Successful detection rate
- AI
Artificial intelligence
- SD
Standard deviation
- ICC
Intraclass correlation coefficient
- CI
Confidence interval.
Authors’ contributions
H.B. and K.Z. performed the experiments and did the manuscript writing. C.Y. did the data collection. H.L., D.C. and H.S. carried out statistical analysis and interpretation of the results. B.Y. and L.L. conceived and supervised the whole project. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (82071143), National Key Research and Development Program of China (2022YFC2402103), Key Medical Research Projects of Jiangsu Health Commission (ZDA2020003), Key Research and Development Program of Jiangsu Province (BE2022795) and COS Clinical Research Fund (COS-C2021-07).
Data availability
The data analysed in this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee Department of the Affiliated Hospital of Stomatology, Nanjing Medical University (PJ022-030-01). All methods were carried out in accordance with relevant guidelines and regulations of the Ethical Committee Department of the Affiliated Hospital of Stomatology, Nanjing Medical University. This is an observational study. Imaging data used in this study were collected for clinical diagnosis and analysis, which were obtained from the patient database in the Affiliated Hospital of Stomatology, Nanjing Medical University. Written informed consents of using x-ray imaging data were obtained from the patients and their parents/Guardians, respectively, before treatment.
Consent for publication
Not Applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
The manuscript has been edited for proper English language, grammar, punctuation, spelling, and overall style by one or more of the highly qualified native English-speaking editors at Springer Nature Author Services (SNAS).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Han Bao and Kejia Zhang contributed equally as the first author.
Contributor Information
Luwei Liu, Email: liuluwei_orth@njmu.edu.cn.
Bin Yan, Email: byan@njmu.edu.cn.
References
- 1.Steiner CC. The use of cephalometrics as an aid to planning and assessing orthodontic treatment. Am J Orthod. 1960;46:721–35. doi: 10.1016/0002-9416(60)90145-7. [DOI] [Google Scholar]
- 2.Moyers RE, Bookstein FL, Guire KE. The concept of pattern in craniofacial growth. Am J Orthod. 1979;76(2):136–48. doi: 10.1016/0002-9416(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 3.Doberschütz PH, Schwahn C, Krey KF. Cephalometric analyses for cleft patients: a statistical approach to compare the variables of Delaire’s craniofacial analysis to Bergen analysis. Clin Oral Investig. 2022;26(1):353–64. doi: 10.1007/s00784-021-04006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B, Zhang Z, Lin X, et al. Sagittal cephalometric evaluation without Point Nasion: Sagittal G-Triangle analysis. J Craniofac Surg. 2022;33(2):521–5. doi: 10.1097/SCS.0000000000008290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luca L, Francesca C, Daniela G, et al. Cephalometric analysis of dental and skeletal effects of Carriere Motion 3D appliance for class II malocclusion. Am J Orthod Dentofacial Orthop. 2022;161(5):659–65. doi: 10.1016/j.ajodo.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Houston WJ, Maher RE, McElroy D, Sherriff M. Sources of error in measurements from cephalometric radiographs. Eur J Orthod. 1986;8(3):149–51. doi: 10.1093/ejo/8.3.149. [DOI] [PubMed] [Google Scholar]
- 7.Arık S, Ibragimov B, Xing L. Fully automated quantitative cephalometry using convolutional neural networks. J Med Imaging (Bellingham) 2017;4(1):014501. doi: 10.1117/1.JMI.4.1.014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu G, Zhang Y, Kong Y, Zhang C, Coatrieux JL, Shu H. Landmark localization for cephalometric analysis using Multiscale Image Patch-Based graph Convolutional Networks. IEEE J Biomed Health Inform. 2022;26(7):3015–24. doi: 10.1109/JBHI.2022.3157722. [DOI] [PubMed] [Google Scholar]
- 9.Le VNT, Kang J, Oh IS, Kim JG, Yang YM, Lee DW. Effectiveness of Human-Artificial intelligence collaboration in Cephalometric Landmark Detection. J Pers Med. 2022;12(3):387. doi: 10.3390/jpm12030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J, Zeng W, He T, et al. Automatic localization of cephalometric landmarks based on convolutional neural network. Am J Orthod Dentofacial Orthop. 2022;161(3):e250–9. doi: 10.1016/j.ajodo.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Hwang HW, Moon JH, et al. Automated identification of cephalometric landmarks: part 1-Comparisons between the latest deep-learning methods YOLOV3 and SSD. Angle Orthod. 2019;89(6):903–9. doi: 10.2319/022019-127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunz F, Stellzig-Eisenhauer A, Zeman F, Boldt J. Artificial intelligence in orthodontics: evaluation of a fully automated cephalometric analysis using a customized convolutional neural network. J Orofac Orthop. 2020;81(1):52–68. doi: 10.1007/s00056-019-00203-8. [DOI] [PubMed] [Google Scholar]
- 13.Hwang HW, Moon JH, Kim MG, Donatelli RE, Lee SJ. Evaluation of automated cephalometric analysis based on the latest deep learning method. Angle Orthod. 2021;91(3):329–35. doi: 10.2319/021220-100.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimoto S, Sotsuka Y, Kawai K, Ishise H, Kakibuchi M. Personal Computer-Based Cephalometric Landmark Detection with Deep Learning, using Cephalograms on the internet. J Craniofac Surg. 2019;30(1):91–5. doi: 10.1097/SCS.0000000000004901. [DOI] [PubMed] [Google Scholar]
- 15.Hwang HW, Park JH, Moon JH, et al. Automated identification of cephalometric landmarks: part 2-Might it be better than human? Angle Orthod. 2020;90(1):69–76. doi: 10.2319/022019-129.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahto RK, Kafle D, Giri A, et al. Evaluation of fully automated cephalometric measurements obtained from web-based artificial intelligence driven platform. BMC Oral Health. 2022;22(1):132. doi: 10.1186/s12903-022-02170-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon S, Lee KC. Comparison of cephalometric measurements between conventional and automatic cephalometric analysis using convolutional neural network. Prog Orthod. 2021;22(1):14. doi: 10.1186/s40510-021-00358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meriç P, Naoumova J. Web-based fully automated cephalometric analysis: comparisons between App-aided, computerized, and manual tracings. Turk J Orthod. 2020;33(3):142–9. doi: 10.5152/TurkJOrthod.2020.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alqahtani H. Evaluation of an online website-based platform for cephalometric analysis. J Stomatol Oral Maxillofac Surg. 2020;121(1):53–7. doi: 10.1016/j.jormas.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Yassir YA, Salman AR, Nabbat SA. The accuracy and reliability of WebCeph for cephalometric analysis. J Taibah Univ Med Sci. 2021;17(1):57–66. doi: 10.1016/j.jtumed.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cicchetti DV. Guidelines, Criteria, and rules of Thumb for evaluating normed and standardized Assessment Instruments in psychology. Psychol Assess. 1994;6(4):284–90. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- 22.Chen YJ, Chen SK, Yao JC, Chang HF. The effects of differences in landmark identification on the cephalometric measurements in traditional versus digitized cephalometry. Angle Orthod. 2004;74(2):155–61. doi: 10.1043/0003-3219(2004)074<0155:TEODIL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Ghowsi A, Hatcher D, Suh H, et al. Automated landmark identification on cone-beam computed tomography: accuracy and reliability. Angle Orthod. 2022;92(5):642–54. doi: 10.2319/122121-928.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue W, Yin D, Li C, Wang G, Xu T. Automated 2-D cephalometric analysis on X-ray images by a model-based approach. IEEE Trans Biomed Eng. 2006;53(8):1615–23. doi: 10.1109/TBME.2006.876638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analysed in this study are available from the corresponding author on reasonable request.