Abstract
Circulating tumor cells (CTCs) derived from any tumor tissue could contribute to metastasis and resistance to cancer treatments. In this study, we performed single‐cell next‐generation sequencing of CTCs and evaluated their usefulness for characterizing tumor biology and the mechanisms of metastasis in neuroblastomas (NB). We aimed to isolate CTCs from 10 patients with NB at diagnosis before any treatments and four patients at relapse. GD2+CD90+CD45−CD235a−DAPI− cells were isolated as neuroblastoma CTCs using fluorescence‐activated cell sorting. In five patients with advanced stages (M stage), DNA and RNA sequencing of CTCs at single‐cell level were performed. NB CTCs were isolated from eight of the 10 patients at diagnosis and three of the four patients at relapse. More CTCs could be isolated from patients with advanced stages. In one patient, ALK mutation (p.F1174L), was identified in both tumor tissue and a CTC. In patients with MYCN amplification, this gene was amplified in 12 of 13 CTCs. Using single‐cell RNA sequencing, angiogenesis‐related and cell cycle‐related genes together with CCND1 and TUBA1A genes were found to be upregulated in CTCs. In one patient, CTCs were divided into two subgroups showing different gene expression profiles. In one subgroup, cell cycle‐related and proliferation‐related genes were differentially upregulated compared with the other group. In conclusion, next‐generation sequencing of CTCs at single‐cell level might help to characterize the tumor biology and the mechanisms of metastasis in NB.
Keywords: circulating tumor cells, liquid biopsy, neuroblastoma, next‐generation sequencing, single‐cell sequencing
Circulating tumor cells (CTCs) might be a group of tumor cells with high malignancy in neuroblastoma. The genetic information of CTCs obtained by single‐cell next‐generation sequencing might help to characterize tumor biology in neuroblastoma as well as mechanisms of metastasis and resistance to cancer treatments.
Abbreviations
- CTC
circulating tumor cell
- DEG
differentially expressed gene
- FACS
fluorescence‐activated cell sorting
- GD2
disialoganglioside
- NB
neuroblastoma
- PTC
primary tumor cell
1. INTRODUCTION
Neuroblastoma (NB) is the most common extracranial solid tumor in children. In NB, both intratumor and clinical heterogeneity have been extensively reported, with some tumors regressing spontaneously, while others progressing aggressively. 1 , 2 , 3 To evaluate tumor biology, open or endoscopic biopsies are performed under general anesthesia. However, the information obtained is sometimes not informative enough, as it reflects a transient state of the tumor and is only derived from a specific part of it. Therefore, because of the frequent heterogeneity found in NB, the data obtained from a single biopsy might not reflect the biological characteristics of the whole tumor. Furthermore, multiple or serial biopsies would be too invasive to be considered as an alternative option to evaluate tumor characteristics and evolution.
Liquid biopsy of CTCs has been proposed to be a valuable tool for cancer diagnosis, treatment, and research. 4 CTCs can be collected noninvasively and used for the assessment of malignancy, prediction of prognosis, and early detection of relapse. 4 Moreover, serial sampling of CTCs can provide insights into therapeutic responses and contribute to advances in personalized medicine development. 4
Importantly, some of CTCs are derived from actively invading tumor cells such as cancer stem cells. 5 Given these characteristics, single‐cell next‐generation sequencing of CTCs may provide an insight into tumor malignancy and cancer treatments including targeted therapy.
Previous studies on neuroblastoma CTCs using immunocytology and flow cytometry have focused mainly on their cellular characterization. In contrast, genetic information on neuroblastoma CTCs is limited. 5 , 6 In this study, we aimed to isolate CTCs from patients with neuroblastoma and characterize their CTC features using single‐cell next‐generation sequencing.
2. MATERIALS AND METHODS
2.1. Patients and samples
We aimed to isolate neuroblastoma CTCs from 5–10 mL peripheral blood of 10 patients at diagnosis before any treatment, among whom two, one, and seven patients were staged as L1, L2, and M, respectively, according to the International Neuroblastoma Risk Group (INRGSS) staging system (cases 1–10) and four patients at relapse (cases 11–14). In one patient (case 6), we intended to isolate CTCs both at diagnosis and relapse and in another patient (case 4), we attempted to isolate CTCs both at diagnosis and after intensive chemotherapy. The clinical features of the patients are listed in Table S1. In five patients with neuroblastoma stage M at diagnosis (cases 6–10), CTCs were characterized using single‐cell next‐generation sequencing.
2.2. Isolation of neuroblastoma CTCs
Neuroblastoma CTCs were isolated from peripheral blood using multiparametric fluorescence‐activated cell sorting (FACS) as previously described. 7 Briefly, GD2+CD90+CD45−CD235a−DAPI− cells were isolated as neuroblastoma CTCs from blood using FACS Aria II cell sorter (BD Biosciences, Flanklin Lakes, NJ). 7
2.3. Extraction of DNAs from biopsied tumor tissue and whole blood cells
DNA was extracted from biopsied tumor tissues from five patients with neuroblastoma (cases 6–10) as previously described. 8 Also, DNA from peripheral blood mononuclear cells (PBMCs) was extracted as a reference for characterization using single‐nucleotide polymorphisms (SNPs) by DNA Extractor WB Kit (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan).
2.4. Whole genome amplification of single CTC
DNA from single CTCs from five patients with neuroblastoma (cases 6–10) was amplified using REPLI‐g Advanced DNA Single Cell Kit (Qiagen, Hilden, Germany). In total, the DNA from 45 single CTCs was amplified; specifically, 15, 10, 5, 6, and 9 amplified DNA datasets for single CTCs were obtained from cases 6, 7, 8, 9, and 10, respectively. Quality check (QC) was performed using multiplex qPCR for eight cancer‐related genes: BRAF, EGFR, KIT, KRAS, NRAS, PIK3CA, PTEN, and TP53. The QC standard was based on the number of threshold cycles (Ct) ≤ 20 obtained for these genes based on our previous study. 7
2.5. DNA sequencing of tumor tissues and CTCs at single‐cell level
The DNA extracted from tumor tissue and single CTCs of five patients with neuroblastoma (cases 6–10) was sequenced using both Ion AmpliSeq™ Cancer Hotspot Panel v2 (Thermo Fisher Scientific Waltham, MA) targeting 50 most common cancer‐related genes, and Ion PGM system (Thermo Fisher Scientific). FASTQ files of DNA sequencing data were processed using IonTorrent Suite (Thermo Fisher Scientific) and the sequenced reads were aligned to University of California Santa Cruz (UCSC) hg19 reference genome. Variant calling was performed using IonReporter (Thermo Fisher Scientific) with pre‐set parameters (variant allele frequency = single‐nucleotide variants [SNV], multinucleotide variants [MNV] ≥ 1–2%, InDel ≥5% in tumor tissue, SNV, MNV, and InDel ≥10% in single CTC). We confirmed the existence of the identified mutations in single CTCs and tumor tissue using Integrative Genomics Viewer (IGV) software and annotated the identified mutations using Catalogue Of Somatic Mutations In Cancer (COSMIC) and International Cancer Genome Consortium (ICGC) databases.
2.6. Evaluation of MYCN status in CTCs at single‐cell level
We evaluated MYCN gene status in tumor tissue and single CTCs from five patients with neuroblastoma (cases 6–10). As MYCN is not targeted by Ion AmpliSeq™ Cancer Hotspot Panel v2, the evaluation of MYCN status was performed using QX100 Droplet Digital PCR system(Bio‐Rad, Hercules, CA) according to our previous study. 8 MYCN/ N‐acetyl‐d‐glucosamine kinase (NAGK) ratio was measured as MYCN copy number and MYCN/NAGK ratio ≥2.5 was considered as amplified MYCN in accordance with our previous study. 8
2.7. Whole transcriptome amplification of single neuroblastoma cell from biopsied tumor tissue and single CTC
We isolated single neuroblastoma cells from biopsied tumor tissue (primary tumor cells: PTCs) from five patients with neuroblastoma (cases 6–10), to compare their gene expression profile with that of single CTCs. After dissociation of tissue specimen using a cell strainer, GD2+CD90+CD45−CD235a−DAPI− cells were isolated using FACS as previously described. 7 In total, mRNA from 30 single PTCs and 48 single CTCs was amplified; specifically, 5, 10, 5, 5 and 5 amplified mRNA datasets for single PTCs, and 8, 10, 5, 5 and 20 amplified mRNA datasets for single CTCs were obtained from cases 6, 7, 8, 9, and 10, respectively, using SMART‐Seq HT Kit (TaKaRa Bio, Shiga, Japan).
2.8. RNA sequencing of PTCs and CTCs at single‐cell level
The RNA collected from PTCs and CTCs from five patients with neuroblastoma (cases 6–10) was sequenced at single‐cell level as previously described. 7 Processing of FASTQ files and alignment of sequenced reads were performed in accordance with our previous study. 7 In total, 21 single PTCs and 42 single CTCs with over 500,000 reads from transcriptome were selected for data analysis; specifically, 3, 3, 5, 5, and 5 mRNA datasets for single PTCs, and 6, 6, 5, 5, and 20 mRNA datasets for single CTCs were analyzed from cases 6, 7, 8, 9, and 10, respectively. The quantification of reads from transcripts and their normalization between samples were performed in accordance with our previous study. 7 Gene expression profiles were compared between PTCs and CTCs to identify differentially expressed genes (DEGs). Whole tumor characteristics of a single patient could be assessed based on the data of at least 20 CTCs. 9 Therefore, in a patient with more than 20 analyzed CTCs (case 10), we performed hierarchical clustering of CTCs based on normalized read counts of each gene and compared gene expression profiles between CTC subgroups. Pathway analysis was performed using WikiPathways database. Pathways were identified as significantly over‐represented using hypergeometric test with a p‐value ≤ 0.01.
2.9. Statistical analysis
DEGs were identified using moderated t test following Benjamini–Hochberg method for false discovery rate (FDR) correction of p‐values. We considered genes with a p‐value ≤0.01 and fold change (FC) ≥2 as statistically significant, and differentially expressed. Other data were reported as mean ± SD, and analyses were performed using Microsoft Excel software.
3. RESULTS
3.1. Isolation of neuroblastoma CTCs
Cells with the following expression profile: GD2+, CD90+, CD45−, CD235a−, and DAPI−, were isolated as neuroblastoma CTCs from eight out of the 10 patients with neuroblastoma at diagnosis, and three out of the four patients at relapse (mean = 2003/mL, range = 0–7471/mL at diagnosis, mean = 723/mL, range = 0–2751/mL at relapse). In one patient (case 6), CTCs were isolated at both diagnosis (1202/mL) and relapse (8/mL). The numbers of CTCs were highest in patients with neuroblastoma stage M (L1, 0/mL; L2, 21/mL; M, 2858/mL [mean]). In one patient (case 4), we found that the numbers of CTCs at diagnosis were 4800/ml, and CTCs were no longer detectable at 2 months after myeloablative chemotherapy. (Figure 1).
FIGURE 1.
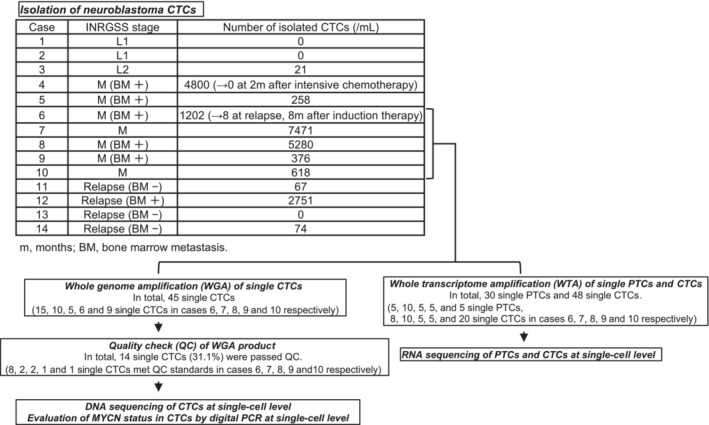
Study workflow. Neuroblastoma circulating tumor cells (CTC) were isolated from eight out of 10 patients with neuroblastoma at diagnosis and three out of four patients at relapse. In one patient (case 6), CTCs were isolated at both diagnosis and relapse. In another patient (case 4), CTCs were isolated at diagnosis before any treatment but not after intensive chemotherapy. Furthermore, whole genome amplifications were performed in 45 single CTCs from the five patients with neuroblastoma (cases 6–10). Among them, 14 single CTCs (31.1%) met the quality check (QC) standards. DNA sequencing targeting 50 cancer‐related genes and MYCN status evaluation using digital PCR at single‐cell level were performed. In total, whole transcriptome amplifications were performed on 30 single neuroblastoma cells from biopsied tumor tissues (primary tumor cells: PTC) and 48 single CTCs. Then, RNA sequencing of PTCs and CTCs at single‐cell level was performed.
3.2. Quality check for single CTCs
In case 6, Ct values (mean ± SD) of 15 single CTCs were the following: BRAF 17.8 ± 2.8, EGFR 16.9 ± 3.6, KIT 17.9 ± 3.4, KRAS 18.7 ± 2.9, NRAS 17.7 ± 3.1, PIK3CA 18.6 ± 2.9, PTEN 18.6 ± 2.7, and TP53 15.1 ± 2.8. Eight of 15 single CTCs complied with the QC standards with Ct values ≤20 in all these genes.
In case 7, Ct values (mean ± SD) of 10 single CTCs were 17.9 ± 4.4 in BRAF, 19.4 ± 4.6 in EGFR, 18.8 ± 5.4 in KIT, 20.3 ± 2.9 in KRAS, 18.1 ± 4.1 in NRAS, 19.6 ± 4.3 in PIK3CA, 19.9 ± 2.1 in PTEN, and 17.2 ± 3.2 in TP53. Two of 10 single CTCs complied with the QC standards with Ct values ≤20 in all these genes.
In case 8, Ct values (mean ± SD) of five single CTCs were 18.7 ± 3.5 in BRAF, 18.1 ± 3.9 in EGFR, 20.6 ± 3.0 in KIT, 17.1 ± 2.7 in KRAS, 17.7 ± 1.9 in NRAS, 18.4 ± 4.1 in PIK3CA, 20.4 ± 2.5 in PTEN, and 17.6 ± 2.6 in TP53. Two of five single CTCs complied with QC standards with Ct values ≤20 in all these genes.
In case 9, Ct values (mean ± SD) of six single CTCs were 15.6 ± 2.8 in BRAF, 18.8 ± 3.4 in EGFR, 18.6 ± 3.7 in KIT, 17.6 ± 3.7 in KRAS, 17.0 ± 3.6 in NRAS, 21.1 ± 6.5 in PIK3CA, 19.2 ± 3.1 in PTEN, and 17.5 ± 3.5 in TP53. One of six single CTCs complied with the QC standards with Ct values ≤20 in all these genes.
In case 10, Ct values (mean ± SD) of nine single CTCs were 19.0 ± 3.1 in BRAF, 19.7 ± 3.9 in EGFR, 20.0 ± 3.8 in KIT, 18.3 ± 3.3 in KRAS, 19.1 ± 2.9 in NRAS, 18.1 ± 3.7 in PIK3CA, 20.7 ± 3.9 in PTEN, and 18.3 ± 2.7 in TP53. One of nine single CTCs complied with the QC standards with Ct values ≤20 in all these genes.
In total, 14 out of 45 single CTCs (31.1%), specifically 8, 2, 2, 1, and 1 single CTCs from cases 6, 7, 8, 9, and 10, respectively, complied with the QC standards; their DNA was extracted and sequenced (Figure 1). Detailed information on the QCs of single CTCs is shown in Table S2.
3.3. DNA sequencing of tumor tissues and CTCs at single‐cell level
The mean depths of coverage (mean ± SD) were 814 ± 1110 in tumor tissues and 2603 ± 528 in single CTCs. The coverages within the target regions (mean ± SD) were 98.9 ± 2.4% in tumor tissues and 95.4 ± 5.3% in single CTCs. The uniformities of coverage (mean ± SD) were 99.6 ± 0.5% in tumor tissues and 70.1 ± 9.9% in single CTCs.
In case 6, we identified mutations in the following genes: APC (frameshift deletion), EZH2 (frameshift deletion), RET (mutation in intron) and TP53 (synonymous mutation) in tumor tissue. Conversely, mutations were identified in the following genes: STK11 (mutation in intron), APC (synonymous mutation), PTPN11 (missense mutation), ERBB2 (synonymous mutation), and PIK3CA (missense mutation) in single CTCs (Table 1).
TABLE 1.
The identified mutations and MYCN status in tumor tissues and single CTCs from the recent five patients with neuroblastoma
| Case | Sample | MYCN status | Mutated gene (allele frequency) | Amino acid change |
|---|---|---|---|---|
| 6 | Tumor tissue (primary site) | Amp (×123) | APC (2.9%) | p.S1495Mfs*19 |
| EZH2 (1.8%) | p.N640Mfs*35 | |||
| RET (90.3%) | Mutation in intron | |||
| TP53 (1.3%) | p.H193= | |||
| CTC1 | Amp (×279) | ‐ | ‐ | |
| CTC2 | Amp (×163) | STK11 (10.2%) | Mutation in intron | |
| CTC3 | Amp (× >1000) | ‐ | ‐ | |
| CTC4 | Amp (×39) | APC (77.9%) | p.S1436= | |
| CTC5 | Amp (× >1000) | ‐ | ‐ | |
| CTC6 | Amp (×111) | PTPN11 (14.8%) | p.R498W | |
| CTC7 | Amp (×83) | ERBB2 (30.6%) | p.H858= | |
| CTC8 | Amp (×30) | PIK3CA (14.2%) | p.L113F | |
| 7 | Tumor tissue (primary site) | Amp (×220) | ALK (1.8%) | p.F1174L |
| CTC1 | Amp (×47) | ALK (100%) | p.F1174L | |
| CTC2 | Amp (×95) | ‐ | ‐ | |
| 8 | Tumor tissue (primary site) | Amp (×45) | ‐(RB1 p.E365 = (0.03%)) (SMAD4 p.Q169 = (0.05%)) | ‐ |
| CTC1 | Amp (× >1000) | RB1 (61.9%) | p.E365= | |
| CTC2 | Not amp (×2) | SMAD4 (35.1%) | p.Q169= | |
| 9 | Tumor tissue (primary site) | Not amp (×1) | ‐ | ‐ |
| CTC1 | Not amp (×0.2) | ‐ | ‐ | |
| 10 | Tumor tissue (primary site) | Amp (×46) | ‐ | ‐ |
| CTC1 | Amp (×36) | ‐ | ‐ |
Abbreviations: Amp, amplification (MYCN/NAGK ratio >2.5); fs*, insertion‐frameshift.
In case 7, we identified the same mutations in ALK gene (p.F1174L) in both tumor tissue and a single CTC. Mutations other than ALK gene were not identified in this case (Table 1).
In case 8, no mutation was identified in tumor tissue. In single CTCs, we identified mutations in RB1 gene (synonymous mutation) and SMAD4 gene (synonymous mutation). The mutations in RB1 and SMAD4 genes were validated with very low frequency (0.03 and 0.05%) in tumor tissue (Table 1).
In cases 9 and 10, no mutation was identified in tumor tissue or single CTCs (Table 1).
3.4. Evaluation of MYCN status in CTCs at single‐cell level
MYCN was amplified in tumor tissue of cases 6, 7, 8, and 10, and also in 12 out of 13 CTCs, except for one CTC of case 8. MYCN/NAGK ratio as MYCN copy number varied among the CTCs of each patient. In case 9, MYCN was neither amplified in tumor tissue nor in CTC (Table 1).
3.5. RNA sequencing of PTCs and CTCs at single‐cell level
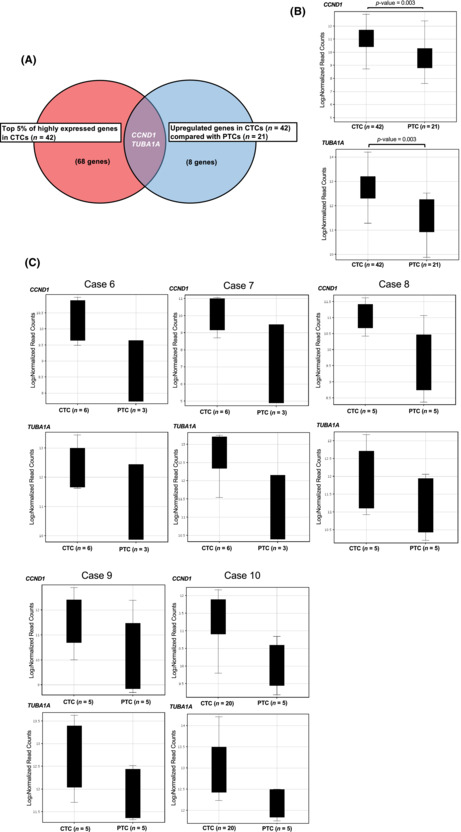
Pathway analysis revealed that angiogenesis‐related and cell cycle‐related genes ACTG1, CCND1, CFL1, GAPDH, HSP90AA1, PTMA, RACK1, RPL5, RPS6, TMSB10, YWHAE (pathway name: VEGFA–VEGFR2 signaling pathway) and CCND1, HDAC2, SKP1, YWHAE, YWHAQ, YWHAZ (pathway name: Cell cycle) were over‐represented in the top 5% of highly expressed genes (68 genes) in CTCs. These 68 genes are listed in Table S3. Within this group of genes, CCND1 and TUBA1A were shown to be significantly (p‐value = 0.003) upregulated compared with PTCs (Figure 2A,B). The complete list of genes that were upregulated in CTCs compared with PTCs (eight genes) is shown in Table S4. Although statistically not significant because of the small number of samples, there was also a trend for upregulation of CCND1 and TUBA1A in CTCs compared with PTCs in each case (cases 6–10). (Figure 2C).
FIGURE 2.
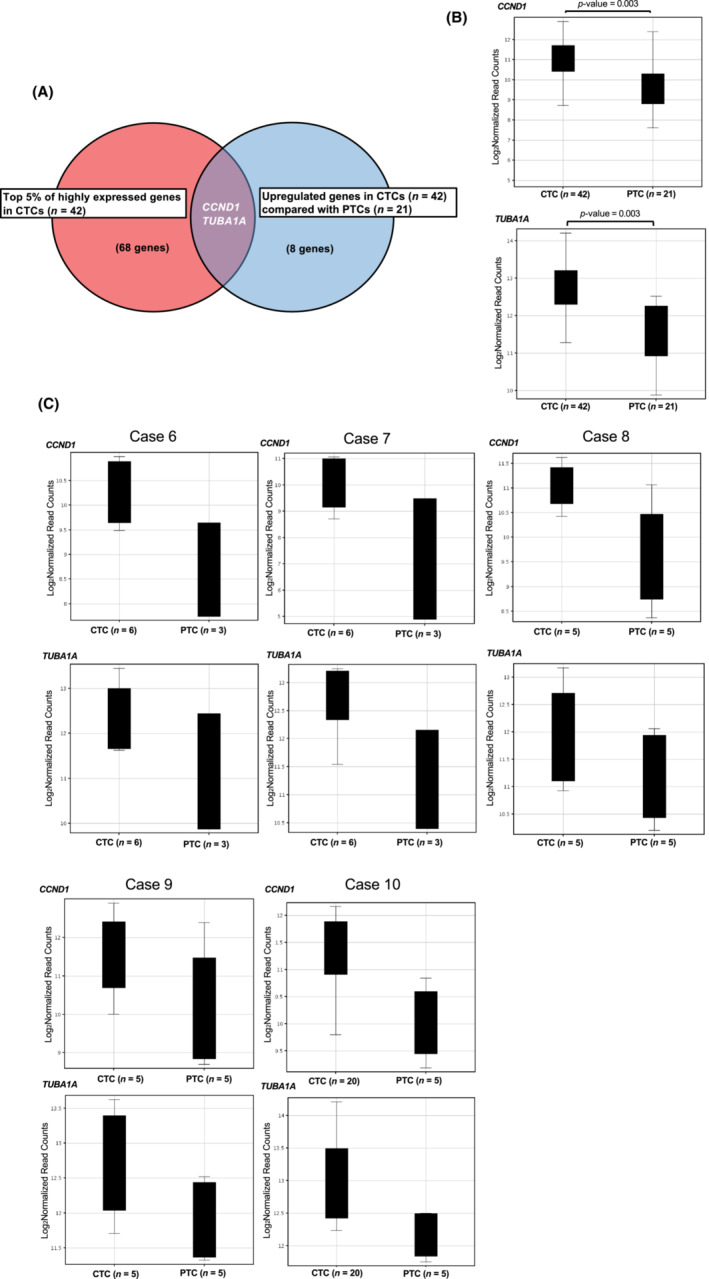
RNA sequencing of primary tumor cells (PTCs) and circulating tumor cells (CTCs) at single‐cell level. (A, B) RNA sequencing was performed on 30 single neuroblastoma cells from biopsied tumor tissue (PTCs) and 48 single CTCs from five patients with neuroblastoma. In total, 21 single PTCs and 42 single CTCs were analyzed with over 500,000 reads from transcriptome. CCND1 and TUBA1A genes were in the top 5% of highly expressed genes in the CTCs and significantly upregulated compared with PTCs. (C) The trend of upregulation of CCND1 and TUBA1A in CTCs compared with PTCs was also observed in each case (cases 6–10).
In case 10, 20 CTCs were clustered into two subgroups using hierarchical clustering based on gene expression profiles (Figure 3A), one consisting of four CTCs (subgroup 1) and the other consisting of 16 CTCs (subgroup 2). Gene expression profiles were compared between the two subgroups, which resulted in 62 genes significantly upregulated (p‐value ≤0.01) in subgroup 1 compared with subgroup 2 (Table S5). Pathway analysis revealed that cell cycle‐related and proliferation‐related genes (CCNA2, CCNB2, CDC6, CDC45, PKMYT1 (pathway name: Cell cycle) and CDC6, CDC45, POLD1, MCM10 (pathway name: DNA replication)) were over‐represented within the upregulated genes in subgroup 1 (Figure 3B). Conversely, pathway analysis in the top 5% of highly expressed genes in subgroup 2 revealed specifically over‐representation of neuronal injury‐related genes FOS, RHOA, MIF (pathway name: Spinal cord injury) compared with that of subgroup 1.
FIGURE 3.
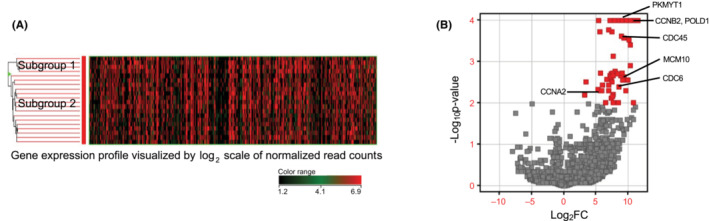
RNA sequencing of 20 circulating tumor cells (CTCs) at single‐cell level in the representative case (case 10). (A, B). Hierarchical clustering based on gene expression profiles of 20 CTCs was performed for case 10. CTCs were divided into two subgroups. Comparison of gene expression profiles between the subgroups showed that cell cycle‐related and proliferation‐related genes (CCNA2, CCNB2, CDC6, CDC45, MCM10, PKMYT1, and POLD1) were significantly upregulated in the CTC subgroup that consisted of four CTCs (subgroup 1).
4. DISCUSSION
Here, we isolated GD2‐expressing cells as neuroblastoma CTCs in accordance with previous studies. 5 , 6 GD2 is specifically and strongly expressed in neuroblastoma cells and used as a target of immunotherapy for patients with neuroblastoma. 10 However, the complete or partial lack of GD2 expression on neuroblastoma cells in bone marrow metastasis in ~10% of patients with advanced neuroblastomas has been shown and there was the possibility of missing a population of neuroblastoma CTCs that do not express GD2. 11 It is known that the isolation method based on cell diameter is not suitable for neuroblastoma CTCs. 6 However, GD2‐independent isolation using immunostaining–fluorescence in situ hybridization (i‐FISH) has shown to accurately detect neuroblastoma CTCs. 12 In the future, to avoid the isolation bias, we should detect and measure neuroblastoma CTCs using the combination of GD2‐dependent and ‐independent procedures.
The number of isolated neuroblastoma CTCs was higher in patients with advanced stages of neuroblastoma. Therefore, the number of CTCs could well represent the tumor burden in a patient. We could isolate CTCs at relapse; monitoring the number of CTCs during the course of the disease might be useful for cancer management in patients with neuroblastoma.
We identified the same ALK mutations (p.F1174L) in both tumor tissue and single CTC in one patient (case 7). ALK is the most frequently mutated gene in patients with neuroblastoma (7–10%). 13 Additionally, mutations in ALK gene are strongly associated with poor prognosis. 13 , 14 In particular, the p.F1174L mutation in ALK gene is one of the hotspot mutations and leads to the resistance to ALK kinase inhibitor. 13 , 14 The ALK mutation was identified with about 100% allele frequency in the CTC; this might show homogenous mutations in ALK or be caused by allele dropout, the disadvantage of single‐cell DNA sequencing. In this case, single‐cell sequencing of not only CTCs but also PTCs would provide more insights into tumor biology in neuroblastoma with ALK mutation. In another patient (case 6), various mutations were identified only in single CTCs and not in tumor tissue. Conversely, other mutations present in tumor tissue were not found in single CTCs. This patient relapsed within 1 year after induction chemotherapy, showing high chemoresistance, and died 2 years and 5 months after diagnosis. Clinical sequencing using Foundation One® CDx (Foundation Medicine, Cambridge, MA) targeting 324 cancer‐related gene mutations was performed using this patient's sample. The results revealed a high tumor mutation burden (three mutations/Mb) and a particular mutation in DNA repair‐related gene POLD1 (data not shown). 15 The overall results indicated high genome instability in the patient that contributed to the poor prognosis. In the patient, we also identified a PTPN11 mutation in a CTC. PTPN11 mutations are the second most frequent in patients with neuroblastoma (2.9%), 13 and have been associated with chemoresistance and neuroblastoma relapse. 16 The identified PTPN11 mutation (p.R498W) was not reported previously and could be related to the tumor progression of the patient. 13 In another patient with MYCN‐amplified tumor tissue (case 8), we identified a CTC with a not‐amplified MYCN, which might reflect the intratumoral heterogeneity of MYCN status in neuroblastoma. 17 Therefore, the genetic information of CTCs at single‐cell level might be useful for cancer treatment and provide insights into tumor biology in patients with neuroblastoma. However, in this study, we performed single‐cell DNA sequencing on a restricted number of CTCs per case, so there would be the possibility of missing rare mutations in tumors. Therefore, if we could get reliable genetic information on CTCs for cancer treatment and research in neuroblastoma, we should apply high‐throughput methods such as droplet microfluidics for single‐cell DNA sequencing into CTCs. 18
RNA sequencing of CTCs and PTCs at single‐cell level revealed that angiogenesis‐related and cell cycle‐related genes were upregulated in CTCs and the downstream core factor involved in these processes, CCND1, was significantly upregulated in CTCs compared with PTCs. Angiogenesis‐related genes together with CCND1 are related to tumor progression in neuroblastoma. 19 , 20 Moreover, TUBA1A was significantly upregulated in CTCs compared with PTCs and related to tumor progression mechanisms in neuroblastoma. 21 In one patient (case 10), CTCs were divided into two subgroups, and one of them showed higher proliferative features than the other group. CCNA2, CDC6, MCM10, and PKMYT1 genes associated with tumor progression of neuroblastoma, were significantly upregulated in the subgroup. 22 , 23 , 24 , 25 These results contributed to the idea that CTCs are a group of tumor cells with high malignancy that are involved in metastasis and suggest the existence of a subgroup of highly malignant tumor cells, such as cancer stem cells, that are resistant to conventional therapies. However, to confirm these ideas, we should perform single‐cell RNA sequencing on many more CTCs per case and compare the features of not only CTCs in a patient but also CTCs from a larger cohort of patients, including with or without metastatic neuroblastoma. Furthermore, to clarify the role of CTCs in the mechanism of metastasis, we should investigate the overlap of the genetic features between CTCs and metastatic sites and perform functional assays using the ex vivo cell culture of CTCs or xenografts.
The limitation of this study is that we performed next‐generation sequencing at single‐cell level on some of the CTCs in a patient. Moreover, the CTCs were isolated from a restricted number of patients with neuroblastoma. Further investigation with a larger cohort of patients and the investigation of many more CTCs with various phenotypes and at several points during the course of the disease will provide additional, potentially useful, information for the development of novel cancer treatments.
In conclusion, although there were some limitations in this study and further investigations are needed in the future, the genetic information for CTCs obtained using next‐generation sequencing at single‐cell level might have the possibility to characterize tumor biology in neuroblastoma, as well as the mechanisms of metastasis and resistance to cancer treatment.
AUTHOR CONTRIBUTIONS
M.K. and E.H. conceived and designed the experiments. M.K. and T.H. conducted the experiments, collected the data, and analyzed the results. M.K. wrote the first draft of the manuscript, and T.F., S.K., R.T, I.S., S.T., and E.H. supervised and revised the manuscript.
FUNDING INFORMATION
This research was supported partially by JSPS Kakenhi Grant Numbers JP20K08927, JP 22H031315, JP22KK0133 and by AMED (Japan Agency for Medical Research and Development) Grant numbers JP22ck0106609, JP22ama221403.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
The study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Approval of the research protocol by an Institutional Reviewer Board: Ethical approval was obtained from the Ethics Committee of Hiroshima University.
Informed Consent: Informed consent was obtained from all patients or from their parents.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
ACKNOWLEDGMENTS
We would like to thank all staff of the institutes that submitted samples to this study. We thank Y. Hayashi, A. Nimura, N. Morihara, S. Hirano and F. Irisuna for technical assistance. We also thank the Analysis Center of Life Science, the Natural Science Center for Basic Research and Development for the use of their facilities.
Kojima M, Harada T, Fukazawa T, et al. Single‐cell next‐generation sequencing of circulating tumor cells in patients with neuroblastoma. Cancer Sci. 2023;114:1616‐1624. doi: 10.1111/cas.15707
DATA AVAILABILITY
The data from next‐generation sequencing analyzed in this study are not publicly available, however it can be requested from the corresponding author.
REFERENCES
- 1. Johnsen JI, Dyberg C, Wickstrim M. Neuroblastoma‐A neural crest derived embryonal Malignacy. Front Mol Neurosci. 2019;12:1‐11. doi: 10.3389/fnmol.2019.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villamón E, Berbegall AP, Piqueras M, et al. Genetic instability and intratumoral heterogeneity in neuroblastoma with MYCN amplification plus 11q deletion. PLoS One. 2013;8(1):e53740. doi: 10.1371/journal.pone.0053740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schleiermacher G, Javanmardi N, Bernard V, et al. Emergence of new ALK mutations at relapse of neuroblastoma. J Clin Oncol. 2014;32(25):2727‐2734. doi: 10.1200/JCO.2013.54.0674 [DOI] [PubMed] [Google Scholar]
- 4. Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells‐biology and biomarkers. Nat Rev Clin Oncol. 2014;11(3):129‐144. doi: 10.1038/nrclinonc.2013.253 [DOI] [PubMed] [Google Scholar]
- 5. Uemura S, Ishida T, Thwin KKM, et al. Dynamics of minimal residual disease in neuroblastoma patients. Front Oncol. 2019;9:455. doi: 10.3389/fonc.2019.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merugu S, Chen L, Gavens E, et al. Detection of circulating and disseminated neuroblastoma cells using the ImageStream flow cytometer for use as predictive and pharmacodynamic biomarkers. Clin Cancer Res. 2020;26(1):122‐134. doi: 10.1158/1078-0432.CCR-19-0656 [DOI] [PubMed] [Google Scholar]
- 7. Kojima M, Harada T, Fukazawa T, et al. Single‐cell DNA and RNA sequencing of circulating tumor cells. Sci Rep. 2021;11(1):22864. doi: 10.1038/s41598-021-02165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kojima M, Hiyama E, Fukuba I, et al. Detection of MYCN amplification using blood plasma: noninvasive therapy evaluation and prediction of prognosis in neuroblastoma. Pediatr Surg Int. 2013;29(11):1139‐1145. doi: 10.1007/s00383-013-3374-9 [DOI] [PubMed] [Google Scholar]
- 9. Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110‐1122. doi: 10.1016/j.cell.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sait S, Modak S. Anti‐GD2 immunotherapy for neuroblastoma. Expert Rev Anticancer Ther. 2017;17(10):889‐904. doi: 10.1080/14737140.2017.1364995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schumacher‐Kuckelkorn R, Volland R, Gradehandt A, Hero B, Simon T, Berthold F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr Blood Cancer. 2017;64(1):46‐56. doi: 10.1002/pbc.26184 [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Zhang Z, Zhang B, et al. Circulating tumor cells detection in neuroblastoma patients by EpCAM‐independent enrichment and immunostaining‐fluorescence in situ hybridization. EBioMedicine. 2018;35:244‐250. doi: 10.1016/j.ebiom.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high‐risk neuroblastoma. Nat Genet. 2013;45(3):279‐284. doi: 10.1038/ng.2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sasaki T, Okuda K, Zheng W, et al. The neuroblastoma‐associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK‐translocated cancers. Cancer Res. 2010;70(24):10038‐10043. doi: 10.1158/0008-5472.CAN-10-2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang F, Zhao Q, Wang YN, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019;5(10):1504‐1506. doi: 10.1001/jamaoncol.2019.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eleveld TF, Oldridge DA, Bernard V, et al. Relapsed neuroblastomas show frequent RAS‐MAPK pathway mutations. Nat Genet. 2015;47(8):864‐871. doi: 10.1038/ng.3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marrano P, Irwin MS, Thorner PS. Heterogeneity of MYCN amplification in neuroblastoma at diagnosis, treatment, relapse, and metastasis. Genes Chromosomes Cancer. 2017;56(1):28‐41. doi: 10.1002/gcc.22398 [DOI] [PubMed] [Google Scholar]
- 18. Pellegrino M, Sciambi A, Treusch S, et al. High‐throughput single‐cell DNA sequencing of acute myeloid leukemia tumors with droplet microfluidics. Genome Res. 2018;28(9):1345‐1352. doi: 10.1101/gr.232272.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shusterman S, Maris JM. Prospects for therapeutic inhibition of neuroblastoma angiogenesis. Cancer Lett. 2005;228(1–2):171‐179. doi: 10.1016/j.canlet.2005.01.049 [DOI] [PubMed] [Google Scholar]
- 20. Cheung IY, Feng Y, Vickers A, Gerald W, Cheung NK. Cyclin D1, a novel molecular marker of minimal residual disease, in metastatic neuroblastoma. J Mol Diagn. 2007;9(2):237‐241. doi: 10.2353/jmoldx.2007.060130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato C, Miyazaki K, Nakagawa A, et al. Low expression of human tubulin tyrosine ligase and suppressed tubulin tyrosination/detyrosination cycle are associated with impaired neuronal differentiation in neuroblastomas with poor prognosis. Int J Cancer. 2004;112(3):365‐375. doi: 10.1002/ijc.20431 [DOI] [PubMed] [Google Scholar]
- 22. Kramer M, Ribeiro D, Arsenian‐Henriksson M, Deller T, Rohrer H. Proliferation and survival of embryonic sympathetic neuroblasts by MYCN and activated ALK signaling. J Neurosci. 2016;36(40):10425‐10439. doi: 10.1523/JNEUROSCI.0183-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng L, Barnhart JR, Seeger RC, et al. Cdc6 knockdown inhibits human neuroblastoma cell proliferation. Mol Cell Biochem. 2008;311(1–2):189‐197. doi: 10.1007/s11010-008-9709-5 [DOI] [PubMed] [Google Scholar]
- 24. Koppen A, Ait‐Aissa R, Koster J, et al. Direct regulation of the minichromosome maintenance complex by MYCN in neuroblastoma. Eur J Cancer. 2007;43(16):2413‐2422. doi: 10.1016/j.ejca.2007.07.024 [DOI] [PubMed] [Google Scholar]
- 25. Chayka O, D'Acunto CW, Middleton O, Arab M, Sala A. Identification and pharmacological inactivation of the MYCN gene network as a therapeutic strategy for neuroblastic tumor cells. J Biol Chem. 2015;290(4):2198‐2212. doi: 10.1074/jbc.M114.624056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
Data Availability Statement
The data from next‐generation sequencing analyzed in this study are not publicly available, however it can be requested from the corresponding author.


