Abstract
Esophageal cancer (EC) is the sixth leading cause of cancer‐related death worldwide. Recently, neoadjuvant chemotherapy (NAC) before curative surgery has become a standard treatment for clinical stage II or III EC patients. Some EC patients receive a complete response (CR) by NAC; thus, curative surgery may be unnecessary for such patients. MicroRNA levels in plasma have the potential to be a predictor of response to NAC. In the present study, we focused on miR‐192‐5p, which is highly expressed in EC tissue. The purpose was to investigate the correlations between levels of plasma miR‐192‐5p and the response to NAC. Furthermore, molecular functions of miR‐192‐5p associated with chemosensitivity were examined using EC cell lines. The levels of miR‐192‐5p in plasma before surgery were evaluated in 113 EC patients. Sixty‐nine patients received NAC. miR‐192‐5p levels in the CR group were significantly higher than in the other groups (p = 0.002). The downregulation of miR‐192‐5p in the EC cell line inhibited sensitivity to cisplatin, and the overexpression of miR‐192‐5p in the EC cell line promoted sensitivity to cisplatin. miR‐192‐5p regulated sensitivity to cisplatin by targeting ERCC3 and ERCC4. Plasma miR‐192‐5p could be used as a predictor of response to chemotherapy and prognosis in EC patients.
Keywords: chemosensitivity, ERCC3, ERCC4, esophageal cancer, miR‐192‐5p
Expression of miR‐192 in esophageal cancer regulated sensitivity to cisplatin by targeting ERCC3 and ERCC4. Plasma miR‐192 may be used as a predictor of response to chemotherapy in esophageal cancer patients.
Abbreviation
- CR
complete response
- CT
computed tomography
- DCF
docetaxel+cisplatin+5‐FU
- EC
esophageal cancer
- EUS
endoscopic ultrasound
- FP
cisplatin+5‐FU
- HV
healthy volunteers
- miRNAs
MicroRNAs
- NAC
neoadjuvant chemotherapy
- NER
nucleotide excision repair.
- OS
overall survival
- PI
propidium iodide
- qRT‐PCR
quantitative RT‐PCR
- RFS
recurrence‐free survival
- TBST
TBS with Tween‐20
- WB
western blotting
1. INTRODUCTION
Esophageal cancer was the seventh most diagnosed malignancy and the sixth leading cause of cancer‐related death worldwide in 2018, with ~572,000 new patients and 509,000 deaths. 1 In the United States, 40% of EC were found with distant metastases, and another 32% were found to spread to regional lymph nodes. 2 EC has a high mortality rate (5‐year survival rate is 46.7%) and further improvements in treatment are needed. 2 For advanced EC, esophagectomy with mediastinal lymph node dissection is generally selected as a radical operation. 3
Recently, NAC before curative surgery was revealed to improve postoperative prognosis, and has become a standard treatment in Japan for clinical stage II or III EC patients. 4 , 5 However, overall complications occur in 59% of patients after esophagectomy, 6 and the presence of NAC is mentioned as a risk factor for postoperative complications. 7 Pathological findings of resected specimens have revealed that 6%–29% of EC patients obtained CR by NAC. 4 , 8 These patients have a good prognosis, and additional esophagectomy and lymphadenectomy are considered unnecessary if CR by NAC is diagnosed accurately before surgery. Various methods for the diagnosis of CR have been investigated, but there is still no solid indicator. Didi et al. reported that image inspections, such as CT, PET, and EUS, may be useful indicators for the diagnosis of CR, but they concluded that these imaging techniques were insufficient for an accurate diagnosis. 9
miRNAs are endogenous, small non‐coding RNAs that consist of ~22 nucleotides. 10 miRNAs regulate target gene expression through binding to the 3′UTR of mRNAs, and affect various cell functions. 10 One miRNA can regulate the expression of other target genes that have different functions. Previous studies have reported an association between miRNAs and chemosensitivity in various cancers. 11 Thus, some miRNAs may affect the chemosensitivity of EC and have the potential to predict CR by NAC.
We searched PubMed (National Institutes of Health; Bethesda, MD, USA) for candidate miRNAs that might be used to predict the effects of NAC for EC. To the best of our knowledge, between 2009 and 2019, there were 94 studies reporting miRNAs associated with EC progression or chemosensitivity. In these reports, at least three papers reported miR‐192‐5p as a prognostic indicator of EC, 12 , 13 , 14 and Shujun et al. reported that expression of miR‐192‐5p in EC tissue was associated with a poor prognosis and promoted tumor progression. 15 In addition, high expression of miR‐192‐5p in biopsied tumor tissues before NAC was associated with sensitivity for NAC. 16 Therefore, in the present study, we focused on miR‐192‐5p as a predictor of the effect of NAC for EC.
There are various fluids suitable for miRNA detection, of which plasma is one of the most suitable samples for the prediction of miRNA levels in terms of stableness and non‐invasiveness. 17 The purpose of the present study was to investigate any correlations between the levels of plasma miR‐192‐5p after NAC and the response to NAC or prognosis. Furthermore, molecular functions of miR‐192‐5p associated with chemosensitivity were examined using EC cell lines.
2. MATERIALS AND METHODS
2.1. Patients and clinical plasma samples
In total, 113 patients who had undergone curative surgery for EC between 2014 and 2020 at the hospital of Kyoto Prefectural University of Medicine were reviewed retrospectively. All patients underwent curative surgery for EC, and NAC was performed on 69 patients. The regimen of NAC was cisplatin‐based therapy consisting of DCF (docetaxel + cisplatin + 5‐FU) or FP. Here, 7 ml of peripheral blood samples were collected into sodium heparin tubes (BD Vacutainer) before curative surgery regardless of NAC.
The plasma was extracted from the collected blood using a three‐spin protocol (1500 rpm, 4°C for 30 min; 3000 rep., 4°C for 5 min; and 4500 rpm 4°C for 5 min) to reduce contamination by cellular nucleic acids. Plasma was stored in a freezer at −80°C before further processing. Tumor staging and clinicopathological factors were classified according to the Japanese Classification of Esophageal Cancer, 11th edition, 18 and pathological features were diagnosed by pathologists. The treatment policy was decided according to the Esophageal Cancer Practice Guidelines 2017. 19 The response to NAC was assessed by histopathological findings according to the Japanese Classification of Esophageal Cancer, 11th edition 18 : Grade 1 was defined as ≥33% of residual tumor cells, grade 2 as <33% of residual tumor cells, and grade 3 as no residual tumor cells. Plasma samples of 55 HV were collected using the same method before surgery for non‐cancerous diseases.
The present study was conducted in accordance with the principles of the Declaration of Helsinki and written informed consent about the therapy and participation in the research work was obtained from all patients before surgery. This study was reviewed and approved by the Institutional Ethics Review Board (approval no. ERB‐C‐1414‐1).
2.2. Cell culture
Human EC cell lines, KYSE170 and KYSE70, were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). TE‐15, TE‐11, TE‐8, and TE‐2 were obtained from RIKEN Bioresource Center (Ibaraki, Japan). HUVEC was purchased from Promocell (Heidelberg, Germany). The human normal esophageal squamous epithelial cell line, HET‐1A, and human mesothelial cell line, MeT‐5A, were purchased from the ATCC (Rockville, MD, USA). These non‐tumor cell lines were used as the control. All EC cell lines, MeT‐5A, and HET‐1A, were maintained in RPMI medium (Nakalai Tisque), supplemented with 10%FBS (System Biosciences). HUVEC was cultured in an endothelial basal medium (Lonza) with endothelial growth supplement Single Quots (Lonza). All cell lines were cultured in an humidified 37°C incubator with 5% carbon dioxide.
2.3. RNA extraction and quantification of the expression of miRNA and mRNA
Total RNA was extracted from 400 μl of plasma sample using a mirVana PARIS Kit (Ambion) according to the manufacturer's protocol. In accordance with a previous study, we selected miR‐1228‐3p as a stable endogenous control for plasma samples. 20 The same protocol was used to extract cell‐free RNA from the medium of the KYSE170 cell line.
Total RNA was extracted from the cultured cells using an miRNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. The reverse transcription reaction was performed using a High‐capacity cDNA Reverse Transcription Kit (Applied Biosystems). miRNA and mRNA expression levels were measured by qRT‐PCR using a StepOnePlus PCR system (Applied Biosystems), and the cycle threshold (Ct) value was calculated using StepOne Software v2.0 (Applied Biosystems). TaqMan MicroRNA Assays (hsa‐miR‐192‐5p [Assay ID: 000491], hsa‐miR‐1228‐3p [Assay ID: 002919], and RNU6B [Assay ID: 001093]) and TaqMan Gene Expression Assays (ERCC3 [Assay ID: Hs01554457_m1], ERCC4 [Assay ID: Hs00193342_m1], and β‐actin [Assay ID: Hs01060665_g1]) were used as the primer sets (Thermo Fisher Scientific). In plasma samples, the RNA extraction technique and sample quality were evaluated using a spike control, cel‐miR‐39. Results were corrected using the 2−ΔΔCt method relative to the levels of miR‐1228‐3p in the plasma, with RNU6B for miRNA expression and β‐actin for mRNA expression in cultured cells.
2.4. Transfection of miRNA mimic and inhibitor
We used the miR‐192‐5p mimic (Assay ID: MC10456; Thermo Fisher Scientific), miR‐192‐5p inhibitor (Assay ID: MH10456; Thermo Fisher Scientific; Waltham, MA, USA), a control vector for the mimic (negative control #1; Thermo Fisher Scientific), and a control vector for the inhibitor (negative control #1; Thermo Fisher Scientific). The inhibitor was transfected into KYSE170 cells at a final concentration of 6 nM using a Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the manufacturer's protocol. In the same methods, the mimic was transfected into TE‐15 cells at a final concentration of 3 nM. The alterations of miR‐192‐5p expression for each concentration of the inhibitor or mimic are shown in Figure S1. The final concentration of the inhibitor and mimic was determined as 6 nM and 3 nM, according to these results and the recommended concentration by the products. miR‐192‐5p expression was confirmed by qRT‐PCR in each experiment.
2.5. Proliferation assay
KYSE170 (2.5 × 103 cells/well) and TE‐15 (1.0 × 104 cells/well) cells were seeded onto a 24‐well plate. The viable cells were evaluated by colorimetric water‐soluble tetrazolium salt assay (Cell Counting Kit‐8; Dojindo Laboratories). The measurements were assessed at 0, 24, 48, and 72 h after transfection of miR‐192‐5p inhibitor or mimic. The absorbance value was measured at a wavelength of 450 nm.
2.6. Cell cycle assay
In total, 50,000 KYSE170 or TE‐15 cells per well were seeded onto a 6‐well plate. The proportion of each cell cycle was analyzed 48 h after transfection of the miR‐192‐5p inhibitor or mimic using flow cytometry with the BD Accuri C6 system (BD Biosciences). Cells were detached from the plate using trypsin–EDTA, treated with 0.2% Triton X‐100, and stained with PI/RNase staining buffer (Becton‐Dickinson Biosciences). Next, 10,000 cells were measured in each sample.
2.7. Apoptosis assay
Here, 50,000 KYSE170 or TE‐15 cells per well were seeded onto a 6‐well plate. Apoptosis rates were evaluated 48 h after transfection of the miR‐192‐5p inhibitor or mimic using an Annexin V‐FITC Kit (Beckman Coulter). The proportions of early and late apoptotic cells were measured by flow cytometry (BD Accuri C6 system). Next, 10,000 cells were measured in each sample.
2.8. Assessment of chemosensitivity to cisplatin
KYSE170 and TE‐15 cells were seeded at 1.0 × 103 cells/well on a 96‐well plate. The miR‐192‐5p inhibitor was transfected into KYSE170 cells at a final concentration of 6 nM, and the mimic was transfected into TE‐15 cells at a final concentration of 3 nM. At 24 h after the transfection of the inhibitor or mimic, cisplatin was applied to each well at final concentrations of 0, 2, 4, 6, 8, 12, 16, or 24 μM. Cells were incubated for 48 h at each cisplatin concentration, and the viable cells were evaluated using the Cell Counting Kit‐8. To compare the chemosensitivity, half maximal inhibitory concentration was calculated using JMP 10 software (SAS Institute).
2.9. Western blotting
Protein samples were extracted using Mammalian Protein Extraction Reagent (Thermo Fisher Scientific). Protein concentration was measured using the Protein Assay Rapid Kit Wako II (Wako) and adjusted to 20 μg per sample. Each protein sample was separated by SDS‐PAGE and subsequently transferred onto PVDF membranes (GE Healthcare). The membranes were blocked in TBST containing 5% BSA for 1 h and then incubated with the primary antibodies at 4°C overnight. The membranes were washed with TBST to remove excess primary antibodies and incubated with anti‐rabbit (#7074S) or anti‐mouse (#7076S) secondary antibodies (both from Cell Signaling Technology) at room temperature for 1 h; proteins were detected using the ECL Plus Western Blotting Detection System (GE Healthcare).
The antibodies used in the present study were anti‐ERCC3 (1:1000 dilution, anti‐rabbit, ab190698), anti‐ERCC4 (1:2000 dilution, anti‐rabbit, ab76948), and anti‐β‐actin (1:20,000 dilution, anti‐mouse, #A2228‐200UL). Antibodies for ERCC3 and ERCC4 were purchased from Abcam. An anti‐β‐actin antibody was purchased from Sigma‐Aldrich.
2.10. Statistical analysis
The Kaplan–Meier method was used to analyze OS and RFS, and the differences were evaluated using the log‐rank test. An unpaired t‐test was used to evaluate the differences among the unpaired parametric variables, and the Mann–Whitney U test among unpaired non‐parametric variables. The Wilcoxon t‐test was used for paired non‐parametric variables. The chi‐squared test was used for categorical variables. Statistical tests used in the present study were two sided, and a p‐value <0.05 was considered significant. Data are presented in the figures as the mean ± SD. All statistical analyses were performed using JMP 10 software.
3. RESULTS
3.1. Functional analysis of miR‐192‐5p in EC cell line
The expression of miR‐192‐5p in EC cell lines is shown in Figure 1A. It was highest in the KTSE170 cell line. miR‐192‐5p expression in the KYSE170 cell was significantly suppressed by the transfection of miR‐192‐5p inhibitor (p = 0.032), and proliferation ability was significantly inhibited (Figure 1B). There was no significant difference in cell cycle analysis by suppressing miR‐192‐5p expression, but the population of late apoptosis significantly increased (p = 0.001, Figure 1C). Conversely, proliferation was confirmed by the overexpression of miR‐192‐5p in TE15 cells with low miR‐192‐5p expression (Figure S2A). Moreover, both early apoptosis and late apoptosis were significantly reduced (Figure S2B).
FIGURE 1.
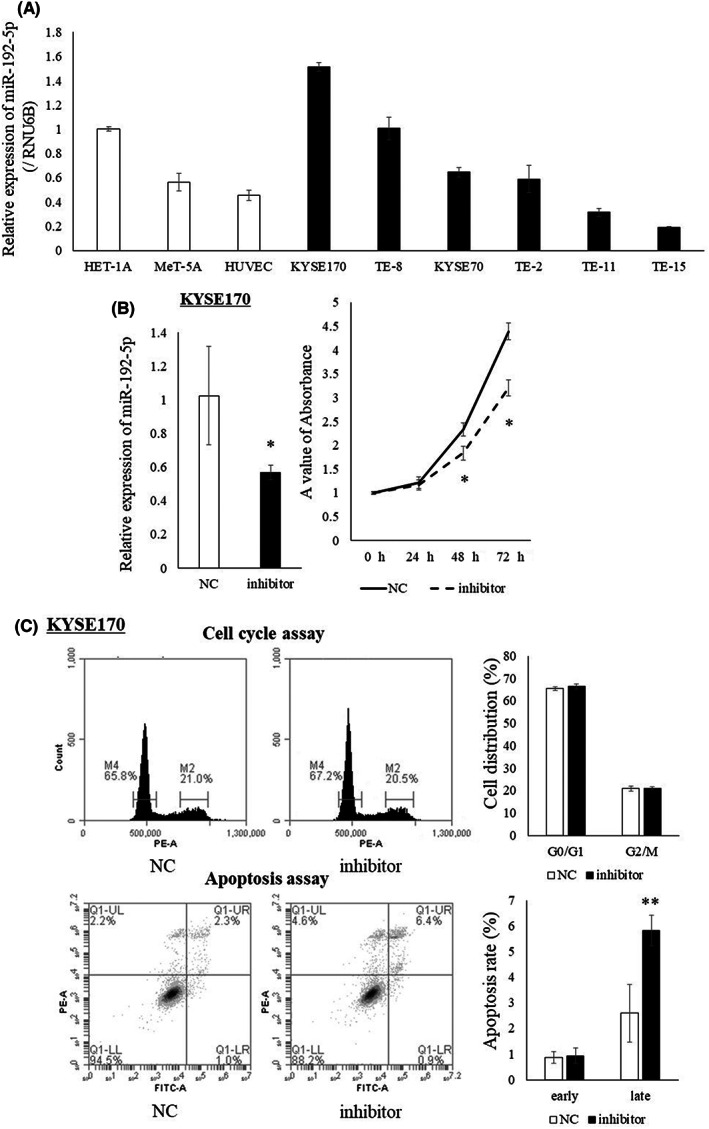
(A) Expression of miR‐192‐5p in EC cell lines and normal cell lines. Expression of miR‐192‐5p was highest in KYSE170 cells and lowest in TE‐15 cells. (B) Expression of miR‐192‐5p in KYSE170 cells was downregulated to 60% using the miR‐192‐5p inhibitor. Proliferation ability of KYSE170 cells was significantly suppressed by the miR‐192‐5p inhibitor. (C) There was no change in cell cycle analysis by the miR‐192‐5p inhibitor. In apoptosis assay, the number of late apoptosis cells was increased by miR‐192‐5p inhibition. All experiments were performed in triplicate and results are shown as mean ± SD. Unpaired t‐test was used to analyze the data (*p < 0.05; **p < 0.01). NC, negative control.
3.2. Levels of miR‐192‐5p in plasma
Figure 2A shows the plasma miR‐192‐5p levels of EC patients without NAC (n = 44) and HV (n = 55). miR‐192‐5p levels were significantly higher in EC patients (p < 0.001). miR‐192‐5p levels were also examined in patients with NAC before surgery using plasma samples (n = 69). miR‐192‐5p levels were significantly higher in the group with a grade 3 response to chemotherapy than the other groups (Figure 2B).
FIGURE 2.
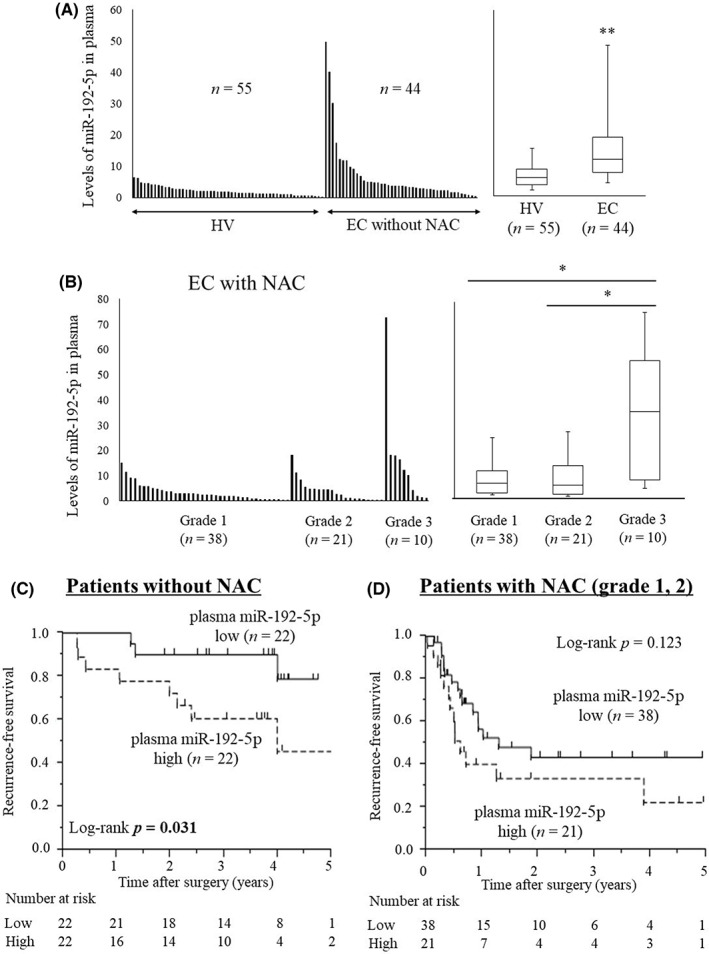
(A) Comparison of miR‐192‐5p levels in plasma between healthy volunteers (HV) and esophageal cancer (EC) patients without neoadjuvant chemotherapy (NAC). miR‐192‐5p levels were significantly higher in EC patients than HV. Mann–Whitney U test was used to analyze the data (**p < 0.01). (B) miR‐192‐5p levels in plasma from EC patients after NAC (plasma before surgery). Patients were distributed by response of NAC (grade 1, grade 2, and grade 3), and miR‐192‐5p levels among the three groups were compared. miR‐192‐5p level was significantly higher in EC patients with grade 3 cancer compared with the other grades. Mann–Whitney U test was used to analyze the data (*p < 0.05). (C) Recurrence‐free survival (RFS) curves for EC patients without NAC. A high level of miR‐192‐5p in plasma was associated with a poor prognosis in RFS (p = 0.007). (D) RFS curves of EC patients with NAC and with the response of NAC were grade 1 or 2. A high level of miR‐192‐5p was associated with poor prognosis in RFS (p = 0.010).
3.3. Association between plasma miR‐192‐5p level and clinicopathological factors
The cutoff value for miR‐192‐5p was determined as 3.5 by the median value of plasma miR‐192‐5p levels of all 113 patients, and patients were divided into two groups (Low and High). Table 1 shows the association between plasma miR‐192‐5p level of EC patients without NAC and clinicopathological factors. A high level of plasma miR‐192‐5p was significantly correlated with the progression of pathological T factor (p = 0.039), lymphatic invasion (p = 0.006), venous invasion (p < 0.001), and pathological stage (p = 0.015). Conversely, in EC patients with NAC, patients with high‐grade pathological responses to NAC had higher levels of plasma miR‐192‐5p (p = 0.040, Table 2). However, there was no significant association between plasma miR‐192‐5p levels and other clinicopathological factors.
TABLE 1.
Association between plasma miR‐192‐5p and clinicopathological factors in EC without NAC
| Variables | n = 44 | Plasma miR‐192‐5p before treatment | p‐value a | ||
|---|---|---|---|---|---|
| Low (≤3.5) | High (>3.5) | ||||
| n = 22 | n = 22 | ||||
| Age | >65 | 30 | 13 (43%) | 17 (57%) | 0.195 |
| ≤65 | 14 | 9 (64%) | 5 (36%) | ||
| Sex | Male | 38 | 18 (47%) | 20 (53%) | 0.379 |
| Female | 6 | 4 (67%) | 2 (33%) | ||
| Tumor size (mm) | >40 | 19 | 7 (37%) | 12 (63%) | 0.128 |
| ≤40 | 25 | 15 (60%) | 10 (40%) | ||
| Pathological T factor b | T3,4 | 7 | 1 (5%) | 6 (95%) | 0.039 |
| T1,2 | 37 | 21 (56%) | 16 (44%) | ||
| Pathological N factor b | N1‐4 | 16 | 5 (31%) | 11 (69%) | 0.060 |
| N0 | 28 | 17 (61%) | 11 (39%) | ||
| Lymphatic invasion b | + | 21 | 6 (29%) | 15 (71%) | 0.006 |
| − | 23 | 16 (69%) | 7 (31%) | ||
| Venous invasion b | + | 22 | 4 (18%) | 18 (82%) | <0.001 |
| − | 22 | 18 (82%) | 4 (18%) | ||
| Pathological stage b | 2, 3 | 20 | 6 (30%) | 14 (70%) | 0.015 |
| 1 | 24 | 16 (67%) | 8 (33%) | ||
p‐values were calculated using the chi‐squared test.
According to the 11th edition of the Japanese Classification of Esophageal Cancer.
TABLE 2.
Association between plasma miR‐192‐5p after NAC and clinicopathological factors in EC with NAC
| Variables | n = 69 | Plasma miR‐192‐5p after NAC | p‐value a | ||
|---|---|---|---|---|---|
| Low (≤3.5) | High (>3.5) | ||||
| n = 41 | n = 28 | ||||
| Age | >65 | 42 | 24 (57%) | 18 (43%) | 0.630 |
| ≤65 | 27 | 17 (63%) | 10 (37%) | ||
| Sex | Male | 56 | 31 (55%) | 25 (45%) | 0.153 |
| Female | 13 | 10 (77%) | 3 (23%) | ||
| Tumor size (mm) | >40 | 31 | 20 (65%) | 11 (35%) | 0.436 |
| ≤40 | 38 | 21 (55%) | 17 (45%) | ||
| Regimen of NAC | DCF | 42 | 24 (57%) | 18 (43%) | 0.630 |
| FP | 27 | 17 (63%) | 10 (37%) | ||
| Pathological T factor b | T3,4 | 36 | 25 (69%) | 11 (31%) | 0.076 |
| T0‐2 | 33 | 16 (48%) | 17 (52%) | ||
| Pathological N factor b | N1‐4 | 33 | 23 (70%) | 10 (30%) | 0.096 |
| N0 | 36 | 18 (50%) | 18 (50%) | ||
| Lymphatic invasion b | + | 18 | 10 (56%) | 8 (44%) | 0.697 |
| − | 51 | 31 (61%) | 20 (39%) | ||
| Venous invasion b | + | 26 | 18 (69%) | 8 (31%) | 0.196 |
| − | 43 | 23 (53%) | 20 (47%) | ||
| Pathological stage b | 2, 3 | 40 | 27 (67%) | 13 (33%) | 0.138 |
| 0, 1 | 29 | 14 (48%) | 15 (52%) | ||
| Pathological grade b | 1, 2 | 59 | 38 (65%) | 21 (35%) | 0.040 |
| 3 | 10 | 3 (30%) | 7 (70%) | ||
Abbreviations: DCF, docetaxel + cisplatin + 5‐FU; FP, cisplatin + 5‐FU; NAC, neoadjuvant chemotherapy.
p‐values were calculated using the chi‐squared test.
According to the 11th edition of the Japanese Classification of Esophageal Cancer.
In the survival analysis of patients without NAC, RFS was worse in patients with a high level of plasma miR‐192‐5p (p = 0.031; Figure 2C). Furthermore, in the patients with NAC, RFS was worse in the patients with a high level of plasma miR‐192‐5p but not significant (p = 0.123; Figure 2D).
3.4. Effects of miR‐192‐5p in chemosensitivity and candidate target genes
In the present study, a high level of miR‐192‐5p was associated with chemosensitivity, which is similar to the findings of a previous study. 16 Sensitivity to cisplatin was suppressed by the downregulation of miR‐192‐5p expression in KYSE170 cells and promoted by the upregulation of miR‐192‐5p expression in TE‐15 cells (Figure 3A).
FIGURE 3.
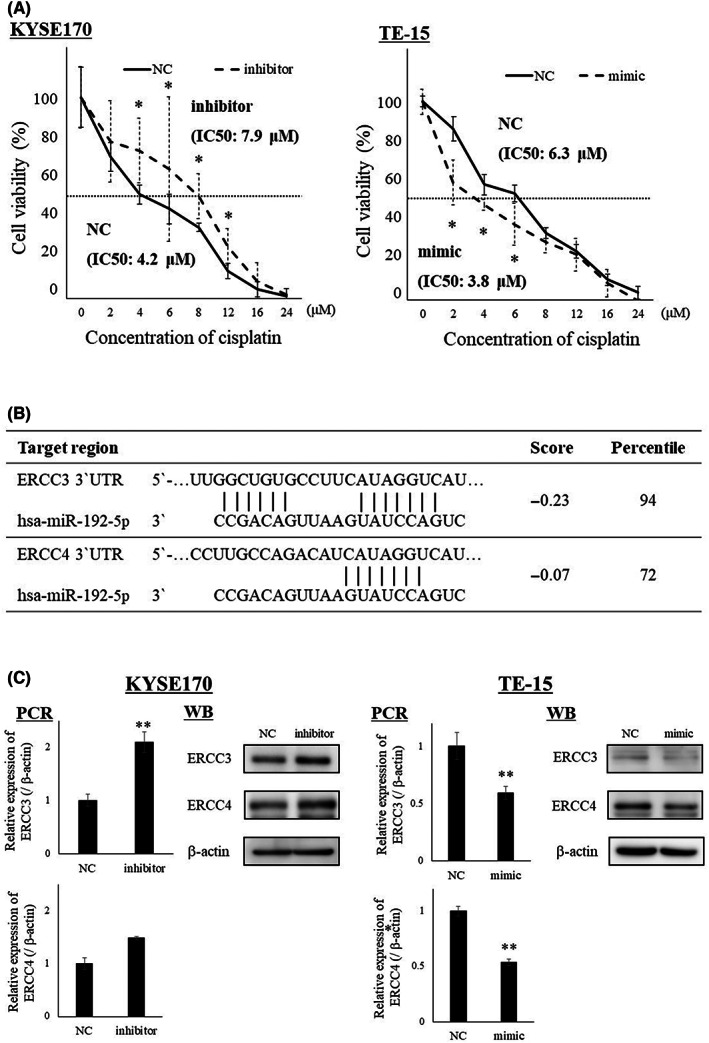
(A) Sensitivity of cisplatin was suppressed by decreasing the expression of miR‐192‐5p in KYSE170 cells, and sensitivity of cisplatin was promoted by the overexpression of miR‐192‐5p in TE‐15 cells. Experiments were performed in octuplicate and results are shown as mean ± SD. Unpaired t‐test was used to analyze the data (*p < 0.05). (B) Predictive miR‐192‐5p binding sites for ERCC3 and ERCC4 according to TargetScan. (C) Expression levels of ERCC3 and ERCC4 in EC cell lines were evaluated by quantitative (q)RT‐PCR and western blotting (WB). Transfection of miR‐192‐5p inhibitor in KYSE170 cells upregulated the expression of ERCC3 and ERCC4. Conversely, the overexpression of miR‐192‐5p in TE‐15 cells downregulated the expression of ERCC3 and ERCC4. Experiments were performed in triplicate and results are shown as mean ± SD. Unpaired t‐test was used to analyze the data (*p < 0.05; **p < 0.01).
ERCC3 and ERCC4 were selected as candidate target genes of miR‐192‐5p using the public database TargetScan (http://www.targetscan.org) (Figure 3B), and by their association with resistance to cisplatin in various cancers. 21 , 22 Expression levels of ERCC3 and ERCC4 in EC cell lines are shown in Figure S3. Transfection of the miR‐192‐5p inhibitor in KYSE170 cell upregulated the mRNA and protein expression of ERCC3 and ERCC4 as shown in the results of qRT‐PCR and WB (Figure 3C). Conversely, the overexpression of miR‐192‐5p in TE‐15 cells downregulated the expression of ERCC3 and ERCC4 (Figure 3C). These findings suggest that ERCC3 and ERCC4 are associated with cisplatin resistance as target genes of miR‐192‐5p.
3.5. Extracellular miR‐192‐5p levels and clinical course
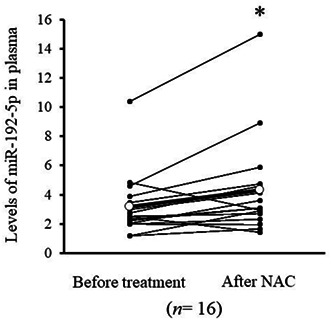
In vitro assay revealed that the level of miR‐192‐5p in the medium of KYSE170 cells increased with the concentration of administered cisplatin (Figure 4A). The level of plasma miR‐192‐5p in 16 patients with NAC was also compared before and after NAC. As shown in Figure 4B, the level of plasma miR‐192‐5p significantly increased after NAC (p = 0.015). A representative profile of the level of plasma miR‐192‐5p in a clinical assay is shown in Figure 4C. In the patient with grade 3 pathological response, levels of plasma miR‐192‐5p were as high as the cutoff value over the entire period, especially after NAC. These results reflect a high sensitivity to cisplatin and high therapeutic effects. Conversely, in the patient with a grade 1a pathological response, the levels of plasma miR‐192‐5p were constantly below the cutoff value and did not increase obviously after NAC (Figure 4C).
FIGURE 4.
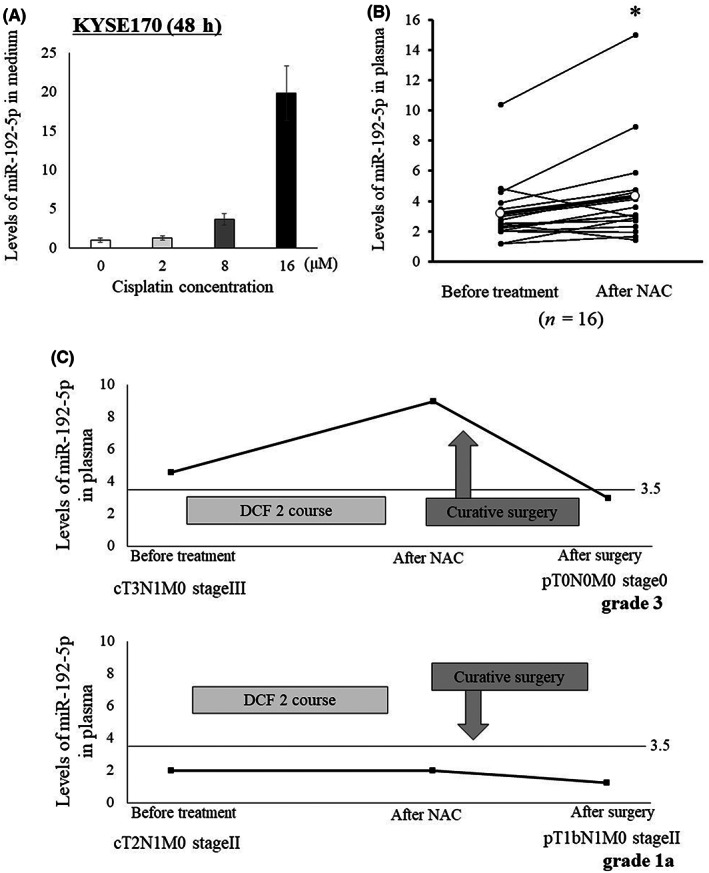
(A) miR‐192‐5p levels in medium 48 h after cisplatin concentration to KYSE170 cells. The release of miR‐192‐5p to medium increased as the concentration of cisplatin was increased. (B) Comparison of miR‐192‐5p level in plasma between before and after neoadjuvant chemotherapy (NAC). The levels of miR‐192‐5p significantly increased after NAC (p = 0.015). (C) miR‐192‐5p levels in plasma before NAC, after NAC, and after surgery were examined for grades 1 (n = 1) and 3 (n = 1). In the patient with grade 3, the level of miR‐192‐5p was higher in all periods, especially after NAC. Cutoff value of miR‐192‐5p was determined as 3.5 by median value of whole cohort. DCF, docetaxel + cisplatin + 5‐FU.
4. DISCUSSION
Neoadjuvant treatment, such as chemoradiation therapy, has become the standard treatment for EC patients worldwide, 4 and cisplatin‐based chemotherapy is the main regimen for the neoadjuvant treatment of advanced EC patients in Japan. 5 In these treatment strategies, one of the important issues is to accurately evaluate a response to NAC before surgery, which may be able to prevent overtreatment for EC patients. Conversely, it is well known that many miRNAs contribute to the initiation and progression of EC. 23 A previous study reported the roles of miRNAs in the sensitivity or resistance to chemotherapy in various cancers. 11 In the present study, plasma miR‐192‐5p levels were higher in EC patients than in HV. A high level of miR‐192‐5p in plasma after NAC correlated with a CR (grade 3) in EC patients who had undergone NAC. Although miR‐192‐5p promoted cancer progression in EC cells, the expression of miR‐192‐5p also promoted sensitivity to cisplatin by suppressing its target genes, ERCC3 and ERCC4. Furthermore, miR‐192‐5p levels in culture medium increased in a cisplatin concentration‐dependent manner in an in vitro assay. We hypothesize that miR‐192‐5p will be released from EC tissue to the extracellular environment according to the effects of chemotherapy and be a useful predictor of the effect of NAC.
ERCC3 and ERCC4 are proteins related to the NER pathway. 24 NER reaction is a DNA repair pathway that is associated with the repair of genes damaged by chemotherapy or radiotherapy. 24 After recognizing the distortion of the double helix structure of DNA, the damaged single‐stranded DNA region is removed, and the generated single‐stranded gap is repaired by DNA polymerase. The NER reaction is completed by sealing the final nick with DNA ligase. In this pathway, ERCC3 forms a complex that binds to damaged single‐stranded DNA, and ERCC4 is involved in DNA repair. Therefore, the NER pathway is activated by the overexpression of ERCC3, and ERCC4 promotes DNA repair and leads to cisplatin resistance. A similar function of miR‐192‐5p and ERCC was reported in gastric cancer, 21 and miR‐192‐5p and the NER pathway were associated with radiosensitivity in hepatocellular carcinoma. 22 In EC cells with upregulated miR‐192‐5p expression, chemosensitivity will be promoted due to suppression of ERCC3 and ERCC4 expression.
miR‐192‐5p has been reported as a cancer‐promoting miRNA in various cancers, including EC. 13 , 15 , 25 , 26 , 27 In the present study, inhibition of miR‐192‐5p suppressed proliferation ability and promoted apoptosis in EC cells, and the opposite results were confirmed by the overexpression of miR‐192‐5p. However, miR‐192‐5p also has a cancer‐suppressing effect via suppression of ERCC expression and increase of sensitivity to cisplatin. 21 A previous study reported that miRNA targeted some other genes with various functions. 10 Thus, miR‐192‐5p will have exerted tumor‐promoting effects in EC cells as in previous reports. However, in limited situations such as chemotherapy, miR‐192‐5p may show a tumor‐suppressing effect, such as by increasing sensitivity to chemotherapy. Further examinations are needed on the two‐sided effects of miRNAs including miR‐192‐5p in various cancers.
The collection of a peripheral blood sample is an easy procedure and minimally invasive for patients. Furthermore, miRNAs in plasma are markedly stable because they are protected from endogenous RNase activity by binding to proteins or being included in small vesicles. 17 miR‐192‐5p was reported to be contained in extracellular vesicles. 13 Therefore, cell‐free circulating miRNA is a useful biomarker for cancer detection or the evaluation of cancer progression. Previous studies have reported plasma miR‐744 as a diagnostic marker in pancreatic cancer, miR‐320 in gastric cancer, and miR‐1207‐5p in colorectal cancer. 28 , 29 , 30 Furthermore, some miRNAs in plasma have been identified for their application in minimally invasive diagnosis or therapeutic monitoring of EC. 13 In the present study, we focused on miR‐192‐5p. A higher level of miR‐192‐5p in plasma was associated with prognosis and a high response to NAC. 16 We also found that EC cells treated with higher concentrations of cisplatin released higher levels of miR‐192‐5p in the medium. Thus, plasma miR‐192‐5p is a useful biomarker of EC that reflects the condition of the tumor status and response to chemotherapy.
In the present study, there were 10 EC patients diagnosed with CR in histopathological findings. Only endoscopy without biopsy and contrast‐enhanced CT scan were performed after NAC to evaluate the response to NAC. It is true that diagnosis by PET or EUS is not performed in our institute, but none of the patients was accurately diagnosed with CR before surgery. Conversely, the level of miR‐192‐5p in plasma was higher than the cutoff value in 7 out of 10 CR patients (70%). Although 36% (21/ 59) of patients with pathological grades 1 and 2 showed a high level of plasma miR‐192‐5p, the level of miR‐192‐5p in plasma may be a more sensitive predictor of the good effects of NAC, including CR response, than existing clinical examinations. It is necessary to evaluate more patients with NAC in a clinical trial for further investigation.
There were some limitations in the present study. This study was a single‐institutional retrospective analysis, and the sample size was small; thus, further analysis with a larger sample size is necessary. miR‐192‐5p expression in tumor tissue before NAC, such as a biopsy sample, could not be evaluated due to the difficulty of sample collection. Therefore, we used the results of a previous study that reported that miR‐192‐5p expression in EC tissue before NAC affected chemosensitivity. 16 In addition, the present study revealed the usefulness of measurements of plasma miR‐192‐5p for conflicting functions of tumor promotion and suppression in EC. In many reports on cancer, the one‐way function of miRNAs, such as tumor promotion or suppression, was reported because usual comprehensive examinations, such as microarray analysis, could only detect one‐way changes and the candidates that had conflicting functions were excluded. Such analyses are difficult when examining only intracellularly or extracellularly; however, in clinical practice, many miRNAs are expected to perform conflicting functions in cancer cells. It is necessary to clarify the complex function of miRNA based on past reports in order to understand cancer biology and detect more useful miRNA biomarkers.
In conclusion, a high miR‐192‐5p level in plasma is associated with prognosis and CR to NAC in EC patients, and the expression of miR‐192‐5p in EC cells enhances cancer progression and sensitivity to cisplatin through reduced expression of ERCC3 and ERCC4. Plasma miR‐192‐5p may be a useful predictor of response to chemotherapy and prognosis in EC patients.
AUTHOR CONTRIBUTIONS
HF and HK conceived the study design, performed the experiments, and wrote the initial manuscript drafts. HK, TA, SK, JS, KT, WT, and EO discussed the progress and results of this research and performed critical editing of the manuscript. HS, YY, SK, AS, and HF collected clinical samples and carefully considered the research plans and contents. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
We have no acknowledgement to declare.
FUNDING INFORMATION
The authors declare that we have no source of financial grants or other funding.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENTS
Approval of the research protocol by an Institutional Reviewer Board: This study was reviewed and approved by the Institutional Ethics Review Board (approval no. ERB‐C‐1414‐1).
Informed Consent: The present study was conducted in accordance with the principles of the Declaration of Helsinki and written informed consent about the therapy and participation in the research work was obtained from all patients before surgery.
Registry and Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Figure S1.
Figure S2.
Figure S3.
Figure 3C
Furuke H, Konishi H, Arita T, et al. Plasma microRNA‐192‐5p can predict the response to neoadjuvant chemotherapy and prognosis in esophageal cancer. Cancer Sci. 2023;114:1686‐1696. doi: 10.1111/cas.15703
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010‐1021. [DOI] [PubMed] [Google Scholar]
- 3. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383‐2396. [DOI] [PubMed] [Google Scholar]
- 4. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074‐2084. [DOI] [PubMed] [Google Scholar]
- 5. Kakeji Y, Oshikiri T, Takiguchi G, et al. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus. 2021;18:25‐32. [DOI] [PubMed] [Google Scholar]
- 6. Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking complications associated with esophagectomy. Ann Surg. 2019;269:291‐298. [DOI] [PubMed] [Google Scholar]
- 7. Holakouie‐Naieni K, Mansournia MA, Doosti‐Irani A, Rahimi‐Foroushani A, Haddad P. Treatment‐related complications in patients with esophageal cancer: a systematic review and network meta‐analysis. Surgeon. 2021;19:37‐48. [DOI] [PubMed] [Google Scholar]
- 8. Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32:2983‐2990. [DOI] [PubMed] [Google Scholar]
- 9. de Gouw D, Klarenbeek BR, Driessen M, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta‐analysis. J Thorac Oncol. 2019;14:1156‐1171. [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281‐297. [DOI] [PubMed] [Google Scholar]
- 11. Taheri M, Shoorei H, Tondro Anamag F, Ghafouri‐Fard S, Dinger ME. LncRNAs and miRNAs participate in determination of sensitivity of cancer cells to cisplatin. Exp Mol Pathol. 2021;123:104602. [DOI] [PubMed] [Google Scholar]
- 12. Huang Z, Zhang L, Zhu D, et al. A novel serum microRNA signature to screen esophageal squamous cell carcinoma. Cancer Med. 2017;6:109‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warnecke‐Eberz U, Chon SH, Holscher AH, Drebber U, Bollschweiler E. Exosomal onco‐miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor. Tumour Biol. 2015;36:4643‐4653. [DOI] [PubMed] [Google Scholar]
- 14. Saad R, Chen Z, Zhu S, et al. Deciphering the unique microRNA signature in human esophageal adenocarcinoma. PLoS One. 2013;8:e64463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li S, Li F, Niu R, et al. Mir‐192 suppresses apoptosis and promotes proliferation in esophageal aquamous cell caicinoma by targeting Bim. Int J Clin Exp Pathol. 2015;8:8048‐8056. [PMC free article] [PubMed] [Google Scholar]
- 16. Odenthal M, Bollschweiler E, Grimminger PP, et al. MicroRNA profiling in locally advanced esophageal cancer indicates a high potential of miR‐192 in prediction of multimodality therapy response. Int J Cancer. 2013;133:2454‐2463. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513‐10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Japan Esophageal Society . Japanese classification of esophageal cancer, 11th edition: part I. Esophagus. 2017;14:1‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu J, Wang Z, Liao BY, et al. Human miR‐1228 as a stable endogenous control for the quantification of circulating microRNAs in cancer patients. Int J Cancer. 2014;135:1187‐1194. [DOI] [PubMed] [Google Scholar]
- 21. Xie X, Huang N, Zhang Y, et al. MiR‐192‐5p reverses cisplatin resistance by targeting ERCC3 and ERCC4 in SGC7901/DDP cells. J Cancer. 2019;10:1039‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie QH, He XX, Chang Y, et al. MiR‐192 inhibits nucleotide excision repair by targeting ERCC3 and ERCC4 in HepG2.2.15 cells. Biochem Biophys Res Commun. 2011;410:440‐445. [DOI] [PubMed] [Google Scholar]
- 23. Harada K, Baba Y, Ishimoto T, et al. The role of microRNA in esophageal squamous cell carcinoma. J Gastroenterol. 2016;51:520‐530. [DOI] [PubMed] [Google Scholar]
- 24. Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 2014;15:465‐481. [DOI] [PubMed] [Google Scholar]
- 25. Jin Z, Selaru FM, Cheng Y, et al. MicroRNA‐192 and ‐215 are upregulated in human gastric cancer in vivo and suppress ALCAM expression in vitro. Oncogene. 2011;30:1577‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen ZJ, Yan YJ, Shen H, et al. miR‐192 is overexpressed and promotes cell proliferation in prostate cancer. Med Princ Pract. 2019;28:124‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Q, Ge X, Zhang Y, et al. Plasma miR‐122 and miR‐192 as potential novel biomarkers for the early detection of distant metastasis of gastric cancer. Oncol Rep. 2014;31:1863‐1870. [DOI] [PubMed] [Google Scholar]
- 28. Miyamae M, Komatsu S, Ichikawa D, et al. Plasma microRNA profiles: identification of miR‐744 as a novel diagnostic and prognostic biomarker in pancreatic cancer. Br J Cancer. 2015;113:1467‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li B, Zhang H. Plasma microRNA‐320 is a potential diagnostic and prognostic bio‐marker in gastric cancer. Int J Clin Exp Pathol. 2017;10:7356‐7361. [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Li L, Xiao W, Xu Q. Plasma microRNA‐1207‐5p as a potential biomarker for diagnosis and prognosis of colorectal cancer. Clin Lab. 2020;66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Figure S3.
Figure 3C


