Abstract
Nucleophosmin1 (NPM1) mutations are the most frequently detected gene mutations in acute myeloid leukemia (AML) and are considered a favorable prognostic factor. We retrospectively analyzed the prognosis of 605 Japanese patients with de novo AML, including 174 patients with NPM1‐mutated AML. Although patients with NPM1‐mutated AML showed a high remission rate, this was not a favorable prognostic factor for overall survival (OS); this is contrary to generally accepted guidelines. Comprehensive gene mutation analysis showed that mutations in codon R882 of DNA methyltransferase 3A (DNMT3A R882 mutations) were a strong predicative factor indicating poor prognosis in all AML (p < 0.0001) and NPM1‐mutated AML cases (p = 0.0020). Furthermore, multivariate analysis of all AML cases showed that DNMT3A R882 mutations and the co‐occurrence of internal tandem duplication in FMS‐like tyrosine kinase 3 (FLT3‐ITD), NPM1 mutations, and DNMT3A R882 mutations (triple mutations) were independent factors predicting a poor prognosis related to OS, with NPM1 mutations being an independent factor for a favorable prognosis (hazard ratios: DNMT3A R882 mutations, 1.946; triple mutations, 1.992, NPM1 mutations, 0.548). Considering the effects of DNMT3A R882 mutations and triple mutations on prognosis and according to the classification of NPM1‐mutated AML into three risk groups based on DNMT3A R882/FLT3‐ITD genotypes, we achieved the improved stratification of prognosis (p < 0.0001). We showed that DNMT3A R882 mutations are an independent factor for poor prognosis; moreover, when confounding factors that include DNMT3A R882 mutations were excluded, NPM1 mutations were a favorable prognostic factor. This revealed that ethnological prognostic discrepancies in NPM1 mutations might be corrected through prognostic stratification based on the DNMT3A status.
Keywords: acute myeloid leukemia, DNMT3A, NPM1, prognostic factor, triple mutation
Our new stratification system using FLT3‐ITD/DNMT3A R882 genotypes predicted the recurrence of NPM1 mutation‐positive acute myeloid leukemia more clearly than did the ELN2017 classification and succeeded in extracting patients with a poor prognosis.
Abbreviations
- allo‐HSCT
allogeneic hematopoietic stem cell transplantation
- CEBPA
CCAAT Enhancer Binding Protein Alpha
- CR
complete remission
- CRR
rate of CR in the induction phase
- DNMT3A
DNA methyltransferase 3A
- EFS
event‐free survival
- FLT3‐ITD
FMS‐like tyrosine kinase 3
- GS‐JAML
multicenter collaborative program for gene sequencing of Japanese AML
- ITD‐AR
FLT3‐ITD allelic ratio
- NPM1
Nucleophosmin1
- OS
overall survival
1. INTRODUCTION
Exon12 frameshift mutations in NPM1 are found in ~35% of de novo AML patients. This NPM1 mutation is frequently detected in an overlapping manner with internal tandem duplications of FLT3‐ITD and mutations in DNMT3A. 1 , 2 Multiple large cohort trials conducted in Europe and the USA showed that NPM1 mutations are a strong factor indicating a favorable prognosis. 2 , 3 , 4 , 5 , 6 In addition, according to the European LeukemiaNet 2017 (ELN 2017) classification, which is a commonly used prognostic model, this mutation is considered an absolute factor of a favorable prognosis that complements the prognostic value of the FLT3‐ITD allelic ratio (AR) and lowers the one‐step risk category. 7 , 8 Meanwhile, the clinical significance of NPM1 mutations in Asia and the Middle East remains unclear. Although several studies have targeted cohorts in Asia and the Middle East, most of them have not been able to show the effect of NPM1 mutations based on univariate analyses. 9 , 10 , 11 , 12 , 13 , 14
DNMT3A mutations are genetic abnormalities detected in 10%–30% of de novo AML cases, with ~60% of them occurring at the R882 site. Their frequent detection in AML was first reported in 2010 15 and, since then, they have been reported as a factor indicative of a poor prognosis in many cohort analyses. 16 , 17 , 18 However, their ability to serve as an independent prognostic factor has been debated because they are detected in an overlapping manner with various genetic abnormalities, and in fact, DNMT3A mutations were excluded among major prognostic factors even in the ELN 2017 classification. 7 Interestingly, mutations at the R882 site of DNMT3A (DNMT3A R882; which are the most common among DNMT3A mutations) 19 overlap with other genetic abnormalities. Alternatively, overlapping DNMT3A mutations with NPM1 mutations or FLT3‐ITD have been suggested to be stronger factors indicating a poor prognosis, 2 , 20 and suggesting the need for a more detailed subgroup analysis.
We previously conducted a retrospective prognostic analysis of 744 patients with AML enrolled between 2001 and 2019 based on the data from the GS‐JAML, which was a clinical sequencing program that was conducted as a multicenter joint study in Japan. 21 However, patients with NPM1‐mutated AML did not show a favorable prognosis. In this study, we sought to clarify the factors that influence this “unexpected prognosis of NPM1‐mutated AML” by targeting only patients aged 70 years or younger who were diagnosed after 2010 and who received standard induction therapy based on GS‐JAML data. We also analyzed the effects of NPM1 and concurrent mutations, particularly DNMT3A mutations, on the prognosis.
2. METHODS
2.1. Study population
All patients in this analysis were enrolled and selected by the GS‐JAML and conducted by the Nippon Medical School. Briefly, Japanese residents aged ≥16 years with de novo AML since 2001 were enrolled in this observational study after obtaining their consent. The following test results of patients with AML, since 2009, were provided by the investigators to the physicians within 1 month: FLT3‐ITD (PCR assay from 2009, fragment analysis from 2018), NPM1 exon12 (from 2009), CEBPA (from 2009), and DNMT3A R882 (from 2017). This analysis included patients with de novo AML (excluding the FAB‐M3 subtype) enrolled in the GS‐JAML study from 2010 to 2019.
2.2. Mutation screening and target‐capture sequencing of the AML gene panel
Patient samples were collected at the time of diagnosis, and genomic DNA was extracted from patients with ≥20% blasts in bone marrow or peripheral blood. Screening for cytogenetic abnormalities was performed through G‐band analysis, and fluorescence in situ hybridization analysis was used to additionally search for t(8;21)(q22;q22.1), t(15;17)(q22;q21), and inv(16)(p13;q22)/t(16;16)(p13;q22). The cytogenetic prognosis was then designated according to the ELN 2017 classification. Screening for FLT3‐ITD, mutations in exon 12 of NPM1, DNMT3A R882 mutations, and the entire exon of CEBPA, was performed as previously described. 21 , 22 For this study, high‐AR and low‐AR cases were defined as ITD‐AR >0.5 and <0.5, respectively. Using cryopreserved samples, target‐capture sequencing of the AML gene panel (Table S1) was performed retrospectively, as previously described. 21 The sequences of all exons of DNMT3A were confirmed through this target‐capture sequencing.
2.3. Statistical analysis
Definitions of response criteria were in accordance with the system recommended by the ELN 2017. 7 The primary outcome was OS. Secondary outcomes included OS censored at the time of allo‐HSCT and EFS. The induction phase was defined as the period of 60 days after the start of initial remission induction therapy. Induction failure was defined as failure to achieve CR in the induction phase. OS was calculated from the time of diagnosis until death from any cause. Events related to EFS included induction failure, first relapse, and death resulting from any cause. For patients without an event, all survival end‐points were censored at the date of the last follow‐up.
Chi‐squared and Fisher's exact tests were used to test the association between categorical variables and the presence or absence of mutations. The non‐parametric Mann–Whitney U‐test was used to assess the difference in the distributions of a non‐proportional continuous variable. All statistical tests were two‐sided. The Kaplan–Meier method and log‐rank test were used to analyze OS and EFS. Regarding the prognostic factors, multivariate analysis was conducted using the Cox proportional hazards model. A backward and forward stepwise procedure selection model using the Akaike information criterion was used to identify independent prognostic factors. Statistical analyses were performed using GraphPad Prism version 9.00 for Windows (GraphPad Software) and EZR (version 1.36; Saitama Medical Center, Jichi Medical University). 23
3. RESULTS
3.1. Patient characteristics
Among 919 patients who registered with the GS‐JAML from 2010 to 2019 and who were 70 years of age or younger, we targeted those who underwent the 7 + 3 induction regimen, which is a standard induction therapy consisting of 7 days of standard‐dose cytarabine (100–200 mg/m2 continuous infusion) and 3 days of an anthracycline antibiotic infusion (idarubicin 12 mg/m2 or daunorubicin 60–90 mg/m2), and evaluated the results of their comprehensive gene analysis. Patients who used FLT3 inhibitors for induction therapy, low‐dose cytarabine treatment (for unfit patients), or azacitidine were excluded because these comprised a small number of cases. In total, 605 patients were included in this analysis. Table S2 shows the clinical backgrounds of patients. The median observation period for all 605 patients was 830.5 days. Among all patients, we found that 127 (20.9%) exhibited FLT3‐ITD and, of these, 44 were low‐AR, whereas 80 were high‐AR cases. We detected ITD alleles in three patients by PCR, but fragment analysis via capillary electrophoresis was difficult, and the ITD‐AR was unknown. We also detected NPM1 mutations in 174 patients (28.8%), whereas DNMT3A mutations were detected in 125 patients (20.7%). Of the 125 DNMT3A mutation‐positive patients, 67 had a DNMT3A R882 mutation, whereas 58 had a DNMT3A non‐R882 mutation; there was no overlap between DNMT3A R882 mutations and DNMT3A non‐R882 mutations. We identified 83 patients who had both NPM1 and DNMT3A mutations, of whom 49 had both NPM1 and DNMT3A R882 mutations, whereas 34 had both NPM1 and DNMT3A non‐R882 mutations. Figure S1 shows the gene abnormality distribution, including the overlap in mutations between AML‐related genes included in the ELN 2017 classification and DNMT3A mutations. We mainly detected FLT3‐ITD and mutations in NPM1 and DNMT3A in the intermediate‐risk karyotype group, especially in patients with a normal karyotype, in agreement with previous reports. Additionally, we observed that these gene mutations had little overlap with mutations in ASXL1, RUNX1, and TP53.
3.2. Rate of complete remission in the induction phase
Table S3 shows the CRR in each subgroup classified based on FLT3‐ITD, NPM1, and DNMT3A mutations. The overall CRR was 74.5%. In particular, we detected that the CRRs for the chromosomal risk groups were 92.4% for the favorable‐risk karyotype, 75.4% for the intermediate‐risk karyotype, and 50.0% for the adverse‐risk karyotype, resulting in clear stratification based on chromosomal prognostic classification. We also found that the CRRs based on the FLT3‐ITD status were 77.1% for FLT3‐ITD‐negative, 72.1% for low‐AR, and 60.0% for high‐AR cases. Meanwhile, the CRRs based on the normal karyotype only were 80.8% for FLT3‐ITD‐negative, 72.7% for low‐AR, and 59.7% for high‐AR cases, with CRR gradually decreasing. Furthermore, we noticed that for the FLT3‐ITD negative, ITD low‐AR, and ITD high‐AR groups, the NPM1 mutation‐positive cases tended to have a higher CRR than that of the negative cases (ITD negative: NPM1 mutation positive vs. negative = 87.1% vs. 74.6%; low‐AR: NPM1 mutation positive vs. negative = 76.9% vs. 64.7%; high‐AR: NPM1 mutation positive vs. negative = 68.8% vs. 46.9%).
3.3. Prognostic value of FLT3‐ITD, NPM1, and DNMT3A mutations
Kaplan–Meier plots of OS, OS censored at allo‐HSCT, and EFS for the entire cohort are shown in Figure S2. Kaplan–Meier plots comparing the following are shown in Figure S3 with OS, Figure S4 with OS censored at allo‐HSCT, and Figure S5 with EFS, respectively: (a) all patients within the subgroups were classified based on the FLT3‐ITD AR (FLT3‐ITD‐negative, low‐AR, or high‐AR), (b) all patients with NPM1 mutation‐positive or ‐negative disease, (c) all patients with DNMT3A wild‐type, DNMT3A R882 mutation positive, or DNMT3A non‐R882 mutation positive, (d) normal karyotype AML patients with subgroups classified based on the FLT3‐ITD AR (FLT3‐ITD‐negative, low‐AR, or high‐AR), (e) normal karyotype AML patients with NPM1 mutation‐positive or ‐negative disease, (f) normal karyotype AML patients with DNMT3A wild‐type, DNMT3A R882 mutation‐positive, or DNMT3A non‐R882 mutation‐positive disease. As shown in Figures S3–S5a,d, we identified the FLT3‐ITD high‐AR status as a strong factor indicative of a poor prognosis related to the OS, OS censored at allo‐HSCT, and EFS in the analysis of all patients and those with a normal karyotype. Meanwhile, as shown in Figures S3–S5b,e, we could not establish the NPM1 mutation as a factor indicating a favorable prognosis for OS, OS censored at allo‐HSCT, or EFS in analyses of all patients or those with a normal karyotype. Likewise, NPM1 mutations were not a prognostic factor in subgroup analysis based on FLT3‐ITD (data not shown).
As shown in Figures S3–S5c,f, we identified DNMT3A mutations as strong factors indicative of a poor prognosis. In particular, we found that, compared with the wild‐type DNMT3A, the DNMT3A R882 mutation was strongly correlated with a worse OS, OS censored at allo‐HSCT, and EFS for all patients and for those with a normal karyotype. Meanwhile, the DNMT3A non‐R882 mutation‐positive group showed an intermediate prognosis between that of the DNMT3A wild‐type group and that of the DNMT3A R882 mutation‐positive group. Further, when comparing the DNMT3A non‐R882 mutation‐positive group and DNMT3A wild‐type group, we found that DNMT3A non‐R882 mutations were significantly correlated with a poor OS, OS censored at allo‐HSCT, and EFS in the analysis of patients with a normal karyotype.
3.4. Different prognostic values of DNMT3A mutations in NPM1‐mutated and NPM1 wild‐type AML
We assumed that the profiles of concurrent genetic abnormalities were involved in the “unexpected prognosis of NPM1‐mutated AML.” Therefore, we performed univariate analyses of OS, OS censored at allo‐HSCT, and EFS according to the mutation status of the markedly recurring mutated genes in NPM1‐mutated AML. DNMT3A mutations were frequently detected in NPM1‐mutated AML (47.7%; 83/174) and were strongly associated with a poor prognosis (Table S4). Figure 1A–C shows Kaplan–Meier plots comparing the DNMT3A genotype subgroups (DNMT3A wild‐type group, DNMT3A R882 mutation‐positive group, and DNMT3A non‐R882 mutation‐positive group) for all karyotypes/NPM1‐mutated AML (Figure 1A: OS; Figure 1B: OS censored at allo‐HSCT; Figure 1C: EFS), whereas Figure 1D–F shows the Kaplan–Meier plots comparing the DNMT3A genotype subgroups for normal karyotypes/NPM1‐mutated AML (Figure 1D: OS; Figure 1D: OS censored at allo‐HSCT; Figure 1F: EFS). We observed that for patients with all karyotypes/NPM1‐mutated AML and normal karyotypes/NPM1‐mutated AML, DNMT3A R882 mutations were strong predictive factors of a poor prognosis and were associated with a shortened OS, OS censored at allo‐HSCT, and EFS. Furthermore, we found that DNMT3A non‐R882 mutation‐positive patients tended to have a shorter OS censored at allo‐HSCT and EFS but a similar OS compared with those of the DNMT3A wild‐type patients. These results suggested that the presence of DNMT3A R882 mutations was primarily related to the “unexpected poor prognosis of NPM1‐mutated AML” tendency observed in the present study cohort.
FIGURE 1.
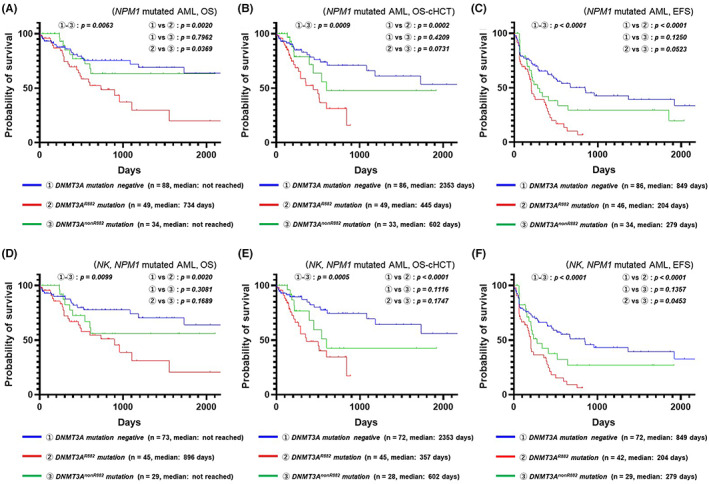
Effect of DNMT3A mutations on the prognosis of NPM1‐mutated acute myeloid leukemia (AML). (A) Kaplan–Meier plots (overall survival; OS) comparing DNMT3A genotype subgroups for all karyotypes/NPM1‐mutated AML. (B) Kaplan–Meier plots (OS censored at allo‐HSCT) comparing DNMT3A genotype subgroups for all karyotypes/NPM1‐mutated AML. (C) Kaplan–Meier plots (event‐free survival; EFS) comparing DNMT3A genotype subgroups for all karyotypes/NPM1‐mutated AML. (D) Kaplan–Meier plots (OS) comparing DNMT3A genotype subgroups for normal karyotype (NK)/NPM1‐mutated AML. (E) Kaplan–Meier plots (OS censored at allo‐HSCT) comparing DNMT3A genotype subgroups for NK/NPM1‐mutated AML. (F) Kaplan–Meier plots (EFS) comparing DNMT3A genotype subgroups for NK/NPM1‐mutated AML
We further found that the combined rate of DNMT3A mutations in NPM1 wild‐type AML was 9.74% (42/431). Figure 2A–C shows Kaplan–Meier plots comparing DNMT3A genotype subgroups for all karyotypes/NPM1 wild‐type AML (Figure 2A: OS; Figure 2B: OS censored at allo‐HSCT; Figure 2C: EFS), whereas Figure 2D–F shows Kaplan–Meier plots comparing DNMT3A genotype subgroups for normal karyotypes/NPM1 wild‐type AML (Figure 2D: OS; Figure 2E: OS censored at allo‐HSCT; Figure 2F: EFS). We determined that DNMT3A R882 mutations were a significant factor indicating a poor prognosis with respect to the OS, OS censored at allo‐HSCT, and EFS for NPM1 wild‐type AML and with respect to the OS and EFS for normal karyotype/NPM1 wild‐type AML. Meanwhile, we found that DNMT3A non‐R882 mutations were a significant factor indicating a poor prognosis with respect to the OS and EFS for normal karyotype/NPM1 wild‐type AML. As the sample size of DNMT3A mutation‐positive cases among those with NPM1 wild‐type AML was small and the genetic background was diverse, the ability of DNMT3A mutations to serve as independent prognostic factors needs to be further investigated.
FIGURE 2.
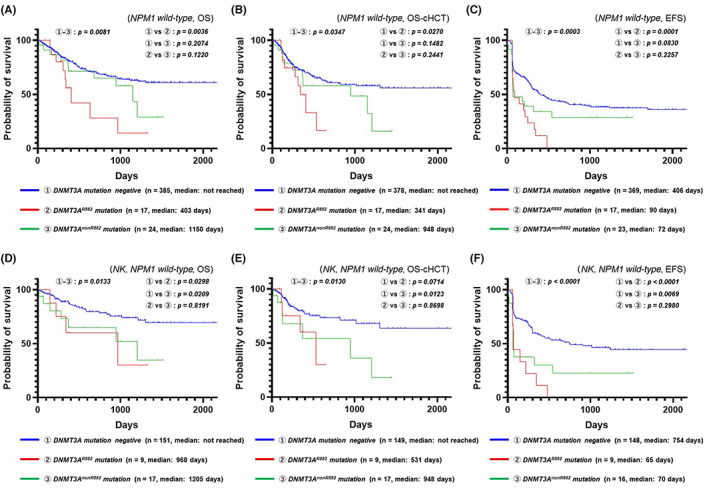
Effect of DNMT3A mutations on the prognosis of NPM1 wild‐type acute myeloid leukemia (AML). (A) Kaplan–Meier plots (overall survival; OS) comparing DNMT3A genotype subgroups for all karyotypes/NPM1 wild‐type AML. (B) Kaplan–Meier plots (OS censored at allo‐HSCT) comparing DNMT3A genotype subgroups for all karyotypes/NPM1 wild‐type AML. (C) Kaplan–Meier plots (event‐free survival; EFS) comparing DNMT3A genotype subgroups for all karyotypes/NPM1 wild‐type AML. (D) Kaplan–Meier plots (OS) comparing DNMT3A genotype subgroups for normal karyotype (NK)/NPM1 wild‐type AML. (E) Kaplan–Meier plots (OS censored at allo‐HSCT) comparing DNMT3A genotype subgroups for NK/NPM1 wild‐type AML. (F) Kaplan–Meier plots (EFS) comparing DNMT3A genotype subgroups for NK/NPM1 wild‐type AML
3.5. Identification of prognostic factors through multivariate analysis
To investigate the effect of DNMT3A mutations on the prognosis of AML, we targeted all patients with AML, as well as those patients with NPM1‐mutated and NPM1 wild‐type disease, and conducted a Cox regression analysis of OS using the following factors as input: age (>60 or ≤60 years old); allo‐HSCT at first CR (yes or no); favorable‐risk karyotype (yes or no); adverse‐risk karyotype (yes or no); FLT3‐ITD high‐AR (positive or negative); FLT3‐ITD (regardless of ITD‐AR, positive or negative); NPM1 mutation (positive or negative); CEBPA double mutation (positive or negative); ASXL1 mutations (positive or negative); RUNX1 mutations (positive or negative); TP53 mutations (positive or negative); DNMT3A R882 mutations (positive or negative); DNMT3A mutations (regardless of position, positive or negative); triple mutation in NPM1, FLT3‐ITD, and DNMT3A (yes or no); and triple mutation in NPM1, FLT3‐ITD, and DNMT3A R882 (yes or no). A backward and forward stepwise procedure selection model using the Akaike information criterion was used to extract independent events. Table 1 shows a summary of the analysis. Using Cox regression, we extracted the DNMT3A R882 mutation and triple mutation in NPM1, FLT‐ITD, and DNMT3A R882 as independent factors of poor prognosis. Simultaneously, removing these confounding factors resulted in the NPM1 mutation being an independent factor for a favorable prognosis. Using Cox regression analysis for only NPM1‐mutated AML, we identified the DNMT3A R882 mutation, FLT3‐ITD mutation, and age >60 years as factors indicative of a poor prognosis, whereas allo‐HSCT at first CR was identified as a factor associated with a favorable prognosis. Meanwhile, using Cox regression analysis for only NPM1 wild‐type AML, we identified TP53 mutations, the adverse‐risk karyotype, FLT3‐ITD high‐AR, DNMT3A mutations, and age >60 years as factors related to a poor prognosis. Interestingly, all DNMT3A mutations were identified as independent factors correlated with OS in NPM1 wild‐type AML, regardless of the mutation site.
TABLE 1.
Cox proportional hazards regression analysis on OS
| Overall survival | Hazard ratio | Lower 95% CI | Upper 95% CI | p‐value |
|---|---|---|---|---|
| All cases | n = 597, number of events = 177 (8 observations deleted due to missingness) Concordance index = 0.728 | |||
| TP53 mutation | 2.802 | 1.668 | 4.705 | <0.001 |
| Adverse karyotype | 2.249 | 1.435 | 3.525 | <0.001 |
| Triple mutation in NPM1, FLT3‐ITD, and DNMT3A R882 | 1.992 | 0.925 | 4.288 | 0.078 |
| Age > 60 years old | 1.95 | 1.427 | 2.665 | <0.001 |
| DNMT3A R882 mutation | 1.946 | 1.133 | 3.341 | 0.016 |
| FLT3‐ITD | 1.835 | 1.209 | 2.786 | 0.004 |
| Favorable karyotype | 0.548 | 0.316 | 0.95 | 0.032 |
| NPM1 mutation | 0.548 | 0.356 | 0.842 | 0.006 |
| Allo‐HSCT at 1st CR | 0.498 | 0.333 | 0.744 | 0.001 |
| CEBPA double mutation | 0.321 | 0.162 | 0.633 | 0.001 |
| NPM1‐mutated AML | n = 171, number of events = 53 (3 observations deleted due to missingness) Concordance index = 0.687 | |||
|---|---|---|---|---|
| DNMT3A R882 mutation | 2.823 | 1.583 | 5.035 | <0.001 |
| FLT3‐ITD | 2.678 | 1.495 | 4.797 | 0.001 |
| Age > 60 years old | 2.227 | 1.239 | 4.004 | 0.007 |
| Allo‐HSCT at 1st CR | 0.543 | 0.256 | 1.151 | 0.111 |
| NPM1 wild‐type AML | n = 426, number of events = 124 (5 observations deleted due to missingness) Concordance index: 0.742 | |||
|---|---|---|---|---|
| TP53 mutation | 2.899 | 1.674 | 5.022 | <0.001 |
| Adverse karyotype | 2.401 | 1.504 | 3.834 | <0.001 |
| FLT3‐ITD high‐AR | 1.862 | 1.056 | 3.281 | 0.032 |
| DNMT3A mutation | 1.803 | 1.076 | 3.021 | 0.025 |
| Age > 60 years old | 1.757 | 1.213 | 2.544 | 0.003 |
| Favorable karyotype | 0.56 | 0.319 | 0.982 | 0.043 |
| Allo‐HSCT at 1st CR | 0.493 | 0.307 | 0.792 | 0.003 |
| CEBPA double mutation | 0.332 | 0.167 | 0.662 | 0.002 |
3.6. Effects of FLT3‐ITD and DNMT3A R882 mutations on the prognosis of NPM1‐mutated AML
From our multivariate analysis, we speculated that treating DNMT3A R882 mutation‐positive patients as a separate prognostic group would correct the discrepancy between the NPM1‐mutated AML cohort and the conventional prognostic model. To show the effect of DNMT3A R882 mutation/FLT3‐ITD AR genotypes on the prognosis of NPM1‐mutated AML, we demonstrated the prognosis of each FLT3‐ITD subgroup in terms of NPM1 mutation‐positive/DNMT3A R882 wild‐type and NPM1 mutation‐positive/DNMT3A R882 mutation‐positive patients using Kaplan–Meier plots (NPM1 mutation‐positive/DNMT3A R882 wild‐type: OS, Figure 3A; OS censored at allo‐HSCT, Figure 3B; EFS, Figure 3C; NPM1 mutation‐positive/DNMT3A R882 mutation‐positive: OS, Figure 3D, OS censored at allo‐HSCT, Figure 3E; EFS, Figure 3F). As shown in Figure 3A–C, NPM1 mutation‐positive/DNMT3A R882 wild‐type patients tended to have a favorable prognosis in all FLT3‐ITD subgroups. Focusing on the prognostic value of the FLT3‐ITD AR for NPM1 mutation‐positive/DNMT3A R882 wild‐type patients, a comparison of OS and EFS showed more early deaths and early events in FLT3‐ITD high‐AR patients than in FLT3‐ITD‐negative or low‐AR patients (OS: FLT3‐ITD high‐AR vs. FLT3‐ITD‐negative or low‐AR, p = 0.1045; EFS: FLT3‐ITD high‐AR vs. FLT3‐ITD‐negative or low‐AR, p = 0.0558). Furthermore, a comparison of OS censored at allo‐HSCT among NPM1 mutation‐positive/DNMT3A R882 wild‐type patients showed that FLT3‐ITD‐negative patients had increased survival compared with FLT3‐ITD‐positive patients (FLT3‐ITD‐negative vs. FLT3‐ITD‐positive, p = 0.0086). These facts suggested that FLT3‐ITD‐positive cases should routinely be assigned to allo‐HSCT even for patients with FLT3‐ITD low‐AR disease. Meanwhile, as shown in Figure 3D–F, NPM1 mutation‐positive/DNMT3A R882 mutation‐positive patients showed a poor prognosis for all subgroups classified based on FLT3‐ITD. In particular, we noticed that FLT3‐ITD‐positive patients had an extremely poor prognosis with respect to all prognostic indices, namely OS, OS censored at allo‐HSCT, and EFS, regardless of the ITD‐AR. This result suggests the importance of triple mutations for a short‐term prognosis.
FIGURE 3.
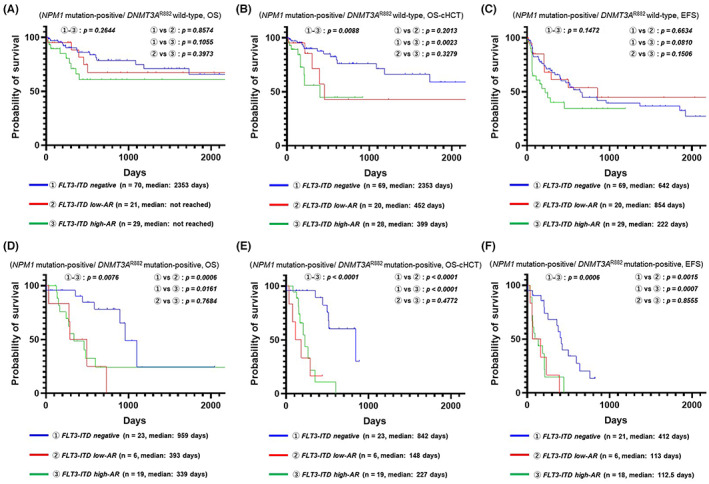
Effect of FLT3‐ITD allelic ratio on the prognosis of NPM1 mutation‐positive/DNMT3A R882 wild‐type acute myeloid leukemia (AML) and NPM1 mutation‐positive/DNMT3A R882 mutation‐positive AML. (A) Kaplan–Meier plots (overall survival; OS) comparing FLT3‐ITD subgroups for NPM1 mutation‐positive/DNMT3A R882 wild‐type patients. (B) Kaplan–Meier plots (OS censored at allo‐HSCT) comparing FLT3‐ITD subgroups for NPM1 mutation‐positive/DNMT3AR882 wild‐type patients. (C) Kaplan–Meier plots (event‐free survival; EFS) comparing FLT3‐ITD subgroups for NPM1 mutation‐positive/DNMT3AR882 wild‐type patients. (D) Kaplan–Meier plots (OS) comparing FLT3‐ITD subgroups for NPM1 mutation‐positive/DNMT3AR882 mutation‐positive patients. (E) Kaplan–Meier plots (OS censored at allo‐HSCT) comparing FLT3‐ITD subgroups for NPM1 mutation‐positive/DNMT3AR882 mutation‐positive patients. (F) Kaplan–Meier plots (EFS) comparing FLT3‐ITD subgroups for NPM1 mutation‐positive/DNMT3AR882 mutation‐positive patients
3.7. Prognostic model for NPM1‐mutated AML
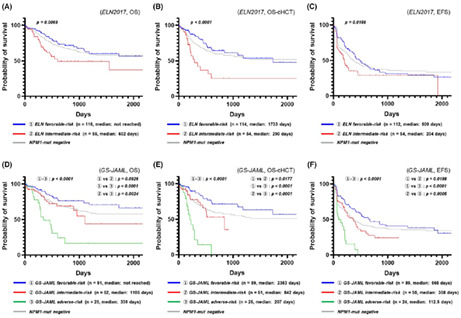
We attempted to modify the conventional prognostic stratification system using the FLT3‐ITD status for NPM1‐mutated AML. Table 2 shows a newly modified risk classification, as well as the 2‐year OS, OS censored at allo‐HSCT, and EFS of each subgroup, sorted based on the DNMT3A R882 mutation/NPM1 mutation/FLT3‐ITD AR genotype. As with the ELN 2017 classification, we classified DNMT3A R882 wild‐type patients into a favorable prognosis group if they were FLT3‐ITD negative or had a low AR and into an intermediate prognosis group if they had a high AR. Meanwhile, we classified DNMT3A R882 mutation‐positive patients into an intermediate prognosis group if they were FLT3‐ITD negative and into a poor prognosis group if they were FLT3‐ITD positive, regardless of their low‐ or high‐AR status. Notably, there were only six patients in the poor prognosis group (i.e., patients with triple‐mutated AML) of the 25 patients who underwent transplantation during the first CR. The 19 patients who did not undergo transplantation comprised a higher number of patients who were resistant to the initial treatment or had an early relapse; thus, the low rate of transplantation seemed to reflect unsuccessful bridging therapy to the transplantation.
TABLE 2.
Risk stratification by DNMT3A R882 mutation/NPM1 mutation/FLT3‐ITD allelic ratio genotypes
| NPM1 mutation a | DNMT3A R882 mutation | FLT3‐ITD | Patient number (HSCT at 1st CR) | 2‐year OS | 2‐year OS censored at HSCT | 2‐year EFS | ELN 2017 | GS‐JAML (2022) |
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Negative | 73 (4) | 78.7% | 76.0% | 44.8% | Favorable | Favorable |
| Low AR | 21 (10) | 67.4% | 42.8% | 53.6% | Favorable | Favorable b | ||
| High AR | 29 (13) | 60.9% | 44.8% | 34.3% | Intermediate | Intermediate | ||
| Positive | Negative | 23 (4) | 78.2% | 60.4% | 20.5% | Favorable | Intermediate | |
| Low AR | 6 (2) | 25.0% | 16.7% | 0.0% | Favorable | Adverse | ||
| High AR | 19 (4) | 24.3% | 0.0% | 0.0% | Intermediate | Adverse |
TP53 mutation was detected in one patient with normal karyotype/NPM1 mutation positive/FLT3‐ITD negative/DNMT3A R882 mutation positive and in one patient with normal karyotype/NPM1 mutation positive/FLT3‐ITD negative/DNMT3A mutation negative. Furthermore, a complex karyotype was detected in one patient with NPM1 mutation positive/FLT3‐ITD high‐AR/DNMT3A mutation negative. These patients are included in the NPM1 mutation‐positive AML analysis data.
The NPM1 mutation positive/DNMT3A R882 negative/FLT3‐ITD low‐AR group exhibited a short survival in the analysis of OS censored at HSCT, and allogeneic HSCT should be actively indicated when FLT3 inhibitors are not used.
Figure 4A–C shows the Kaplan–Meier plots when patients with NPM1‐mutated AML were stratified according to the ELN 2017 classification (OS: Figure 4A; OS censored at allo‐HSCT: Figure 4B, EFS: Figure 4C), whereas Figure 4D–F shows the Kaplan–Meier plots when patients with NPM1‐mutated AML were stratified according to the new system that was reorganized based on information of the GS‐JAML cohort (OS: Figure 4D; OS censored at allo‐HSCT: Figure 4E; EFS: Figure 4F). We found that the new stratification system could predict the recurrence of NPM1‐mutated AML more clearly than the ELN 2017 classification and succeeded in identifying the patients with a poor prognosis.
FIGURE 4.
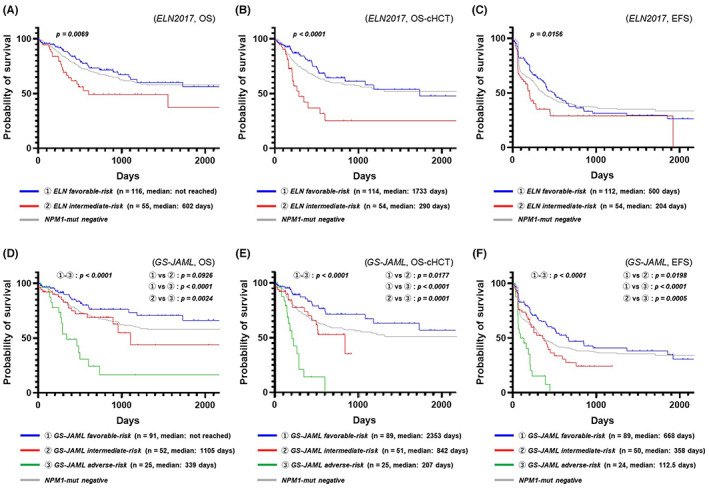
Prognostic model based on DNMT3A R882 mutation/NPM1 mutation/FLT3‐ITD allelic ratio genotypes. (A) Prognostic stratification using NPM1/FLT3‐ITD genotypes (overall survival; OS) according to the European LeukemiaNet 2017 (ELN 2017) classification of NPM1‐mutated acute myeloid leukemia (AML). (B) Prognostic stratification using NPM1/FLT3‐ITD genotypes (OS censored at allo‐HSCT) according to the ELN 2017 classification of NPM1‐mutated AML. (C) Prognostic stratification using NPM1/FLT3‐ITD genotypes (event‐free survival; EFS) according to the ELN 2017 classification of NPM1‐mutated AML. (D) Prognostic stratification using DNMT3AR882/NPM1/FLT3‐ITD genotypes (OS) according to the multicenter collaborative program for gene sequencing of Japanese AML (GS‐JAML) classification of NPM1‐mutated AML. (E) Prognostic stratification using DNMT3AR882/NPM1/FLT3‐ITD genotypes (OS censored at allo‐HSCT) according to the GS‐JAML classification of NPM1‐mutated AML (F) Prognostic stratification using DNMT3AR882/NPM1/FLT3‐ITD genotypes (EFS) according to the GS‐JAML classification of NPM1‐mutated AML
4. DISCUSSION
In this study, we investigated the effect of NPM1 mutations on the prognosis of 605 patients with AML, aged ≤70 years, who underwent standard induction therapy. Although patients with NPM1‐mutated AML showed a high CRR, this was not a factor predicting a favorable prognosis in terms of OS, in contrast with that shown in previous studies and key guidelines. Furthermore, we demonstrated that DNMT3A R882 mutations were an independent factor for poor prognosis; when confounding factors that include DNMT3A R882 mutations were excluded, NPM1 mutations were a strong factor predicting a favorable prognosis. As DNMT3A mutations frequently occur in patients with NPM1 mutations and FLT3‐ITD, which are strong predictive factors of prognosis, it is often affected by Simpson's Paradox in univariate analysis, which presumably explains how its effects on prognosis and its importance are underestimated. 24 In addition, the stratification of prognosis by ITD‐AR introduced in the ELN 2017 classification has resulted in the subclassification of NPM1‐mutated AML, obscuring the value of DNMT3A mutations in NPM1‐mutated AML. The results of this study demonstrate that DNMT3A mutations are a factor indicative of a poor prognosis even with stratification based on ITD‐AR, proving its clinical significance.
Moreover, the results of this study call attention to the importance of DNMT3A mutations in our Japanese cohort. Interestingly, JALSG conducted a comprehensive gene mutation analysis of 197 patients with de novo AML who were registered in the JALSG AML201 in 2014. NPM1 mutations were not identified as a prognostic factor in this analysis, either in univariate or multivariate analyses in which abundant gene data were inputted. 20 Moreover, they demonstrated that the prognosis of NPM1‐mutated AML patients could be more clearly stratified by including the DNMT3A mutation status compared with that with the original ELN system. These findings were in agreement with those of our study. The fact that JALSG, which is the largest prospective cohort study group in Japan, and our study, which is the largest retrospective cohort study conducted in Japan, were in agreement is particularly important. The current study and the JALSG study indicated that ethnological prognostic discrepancies in NPM1 mutations might be corrected by stratifying prognosis based on the DNMT3A status.
We also highlighted the importance of DNMT3A R882 mutations as a factor predicting a poor prognosis in NPM1‐mutated AML. However, we did not manage to establish the clinical significance of DNMT3A non‐R882 mutations. An analysis of the OS for NPM1‐mutated AML showed that the DNMT3A non‐R882 mutation‐positive group had an intermediate prognosis between that of the DNMT3A wild‐type and that of DNMT3A R882 mutation‐positive group, and analysis of OS for NPM1 wild‐type patients with normal karyotypes showed a tendency for a poor prognosis, similar to that observed in the DNMT3A R882 mutation‐positive group. Previously, the AML Study Group (AMLSG) analyzed 1770 patients with de novo AML and reported that, among DNMT3A mutations, DNMT3A R882 mutations were often detected in AML with a normal karyotype and NPM1‐mutated AML and were factors indicative of a poor prognosis related to OS. In contrast, DNMT3A non‐R882 mutations had a low detection frequency, overlapped with chromosomal abnormalities, and had little association with the prognosis of NPM1‐mutated AML patients. 19 This reported difference in clinical significance depending on the site of the DNMT3A mutation is in good agreement with our findings.
We would like to propose a rearrangement of the genetic risk stratification that includes DNMT3A mutations. Recently, Herold et al. analyzed the long‐term follow‐up data of the target cohort of ELN 2017 by adding an extension cohort and reported that DNMT3A mutations were associated with a worse OS and relapse‐free survival in each stratified risk group according to NPM1/FLT3‐ITD genotypes. 25 The importance of DNMT3A mutations has been reassessed as a factor predicting a poor prognosis. Based on our results, we created a newly modified risk classification. This new prognostic model clarifies the importance of DNMT3A R882 mutations and emphasizes the importance of triple‐mutated AML with duplicate FLT3‐ITD, NPM1 mutations, and DNMT3A mutations. In recent years, several large cohort studies have reported a poor prognosis in triple‐mutated AML. 2 , 26 , 27 , 28 Our study further supports these studies. The recently released ELN 2022 risk classification did not adopt the DNMT3A mutation as a poor prognostic factor, because it might have been thought that the efficacy of an FLT3 inhibitor combined with chemotherapy must have overcome the poor prognosis associated with the triple‐mutant type. 29 The Ratify trial revealed that midostaurin, as an adjunct to daunorubicin‐cytarabine induction and high‐dose cytarabine consolidation therapy, improved the long‐term survival rate. 30 Based on this result, it has become standard to incorporate the FLT3 inhibitor midostaurin into first‐line therapy for patients with FLT3‐mutated AML. Certainly, combination chemotherapy using FLT3 inhibitors and subsequent bridging to allo‐HSCT might be the key to treatment success for triple‐mutated AML that is treatment refractory in the early treatment phase, but there is no evidence that FLT3 inhibitors can improve the prognosis of triple‐mutated AML to date. In fact, considering that the prognosis of triple‐mutated AML is extremely poor, as reflected by the data of this study, the use of FLT3 inhibitors would most likely also result in inadequate therapeutic outcomes, hence implying the importance of thoroughly validating the recommendation proposed in the ELN 2022 guidelines. Early prediction using subsequent molecular responses to NPM1 mutations can help to develop improved strategies for the treatment of NPM1‐mutated AML harboring DNMT3A mutations. Recently, Onate et al. analyzed 164 patients diagnosed with NPM1‐mutated AML enrolled in the Spanish Cooperative Group for the Diagnosis and Treatment of Acute Myeloid Leukemia and Myelodysplastic Syndromes (CETLAM) protocols. The authors reported that DNMT3A mutations did not modify the prognostic value of FLT3‐ITD AR in NPM1‐mutated AML, although patients with the DNMT3A mutation had a worse clearance of measurable residual disease (MRD) for NPM1 mutation. 31 The adverse effect of the DNMT3 mutation may be counteracted by a closer follow‐up of NPM1‐mutation MRD and a pre‐emptive therapeutic intervention.
In conclusion, we showed that DNMT3A R882 mutations are an independent factor that can predict a poor prognosis; when confounding factors that include DNMT3A R882 mutations were excluded, NPM1 mutations were a strong factor associated with a favorable prognosis. These findings indicate that ethnological prognostic discrepancies in NPM1 mutations might be corrected by stratifying prognosis based on the DNMT3A status. In addition, the triple‐mutant disease was associated with an inferior response to early‐phase AML therapy, which could provide new insights for individualized AML chemotherapy.
AUTHOR CONTRIBUTIONS
Satoshi Wakita and Atsushi Marumo were the principal investigators and take primary responsibility for the paper. Hiroki Yamaguchi designed the study and supervised the experiments; Satoshi Wakita and Atsushi Marumo designed and performed the experiments, interpreted the data, and wrote the manuscript; Kaoru Morita, Shinichi Kako, Takashi Toya, Yuho Najima, Noriko Doki, Junya Kanda, Junya Kuroda, Shinichiro Mori, Kensuke Usuki, Toshimitsu Ueki, Nobuhiko Uoshima, Yutaka Kobayashi, Eri Kawata, Kazutaka Nakayama, Yuhei Nagao, Katsuhiro Shono, Motoharu Sibusawa, Jiro Tadokoro, Masao Hagihara, Hitoji Uchiyama, Naoyuki Uchida, Yasushi Kubota, Shinya Kimura, Hisao Nagoshi, Tatsuo Ichinohe, Saiko Kurosawa, Sayuri Motomura, Akiko Hashimoto, Hideharu Muto, Eriko Sato, Masao Ogata, Kenjiro Mitsuhashi, Jun Ando, Haruko Tashiro, Takahiro Fukuda, and Yoshinobu Kanda contributed to patient recruitment and data extraction; Kunihito Arai, Tomoaki Kitano, Miho Miyata, Haruka Arai, Masayuki Kanda, and Kako Itabashi performed the laboratory work for the study; Masahiro Sakaguchi and Shunsuke Yui interpreted molecular data and performed statistical analysis. All authors reviewed and approved the manuscript.
CONFLICT OF INTEREST
No authors have a financial interest with respect to the conduction or reporting of the study. S. Kimura is an editorial board member of Cancer Science.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study was reviewed and approved by the Human Subjects Institutional Review Board (project approval number 29‐07‐783) of the Nippon Medical School (Tokyo, Japan).
Informed Consent: Informed consent was obtained in accordance with the Declaration of Helsinki from all participants.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
The authors are very grateful to all physicians who cared for patients and collected clinical data for this study.
Wakita S, Marumo A, Morita K, et al. Mutational analysis of DNMT3A improves the prognostic stratification of patients with acute myeloid leukemia. Cancer Sci. 2023;114:1297‐1308. doi: 10.1111/cas.15720
Satoshi Wakita and Atsushi Marumo contributed equally to this work.
REFERENCES
- 1. Cancer Genome Atlas Research Network . Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209‐2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254‐266. [DOI] [PubMed] [Google Scholar]
- 4. Schnittger S, Schoch C, Kern W, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106(12):3733‐3739. [DOI] [PubMed] [Google Scholar]
- 5. Döhner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106(12):3740‐3746. [DOI] [PubMed] [Google Scholar]
- 6. Verhaak RGW, Goudswaard CS, van Putten W, et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood. 2005;106(12):3747‐3754. [DOI] [PubMed] [Google Scholar]
- 7. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamaguchi H. Significance of gene diagnosis in acute myeloid leukemia with the emergence of new molecular target drug treatment. J Nippon Med Sch. 2022;89(5):470‐478. [DOI] [PubMed] [Google Scholar]
- 9. Chou WC, Tang JL, Lin LI, et al. Nucleophosmin mutations in de novo acute myeloid leukemia: the age‐dependent incidences and the stability during disease evolution. Cancer Res. 2006;66(6):3310‐3316. [DOI] [PubMed] [Google Scholar]
- 10. Yan L, Chen S, Liang J, et al. Analysis of NPM1 gene mutations in Chinese adults with acute myeloid leukemia. Int J Hematol. 2007;86(2):143‐146. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki T, Kiyoi H, Ozeki K, et al. Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood. 2005;106(8):2854‐2861. [DOI] [PubMed] [Google Scholar]
- 12. Boonthimat C, Thongnoppakhun W, Auewarakul CU. Nucleophosmin mutation in southeast Asian acute myeloid leukemia: eight novel variants, FLT3 coexistence and prognostic impact of NPM1/FLT3 mutations. Haematologica. 2008;93(10):1565‐1569. [DOI] [PubMed] [Google Scholar]
- 13. Koh Y, Park J, Bae EK, et al. Non‐a type nucleophosmin 1 gene mutation predicts poor clinical outcome in de novo adult acute myeloid leukemia: differential clinical importance of NPM1 mutation according to subtype. Int J Hematol. 2009;90(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 14. Shamaa S, Laimon N, Aladle DA, et al. Prognostic implications of NPM1 mutations and FLT3 internal tandem duplications in Egyptian patients with cytogenetically normal acute myeloid leukemia. Hematology. 2014;19(1):22‐30. [DOI] [PubMed] [Google Scholar]
- 15. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363(25):2424‐2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thol F, Damm F, Lüdeking A, et al. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol. 2011;29(21):2889‐2896. [DOI] [PubMed] [Google Scholar]
- 17. Ostronoff F, Othus M, Ho PA, et al. Mutations in the DNMT3A exon 23 independently predict poor outcome in older patients with acute myeloid leukemia: a SWOG report. Leukemia. 2013;27(1):238‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahn JS, Kim HJ, Kim YK, et al. DNMT3A R882 mutation with FLT3‐ITD positivity is an extremely poor prognostic factor in patients with normal‐karyotype acute myeloid leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(1):61‐70. [DOI] [PubMed] [Google Scholar]
- 19. Gaidzik VI, Schlenk RF, Paschka P, et al. Clinical impact of DNMT3A mutations in younger adult patients with acute myeloid leukemia: results of the AML Study Group (AMLSG). Blood. 2013;121(23):4769‐4777. [DOI] [PubMed] [Google Scholar]
- 20. Kihara R, Nagata Y, Kiyoi H, et al. Comprehensive analysis of genetic alterations and their prognostic impacts in adult acute myeloid leukemia patients. Leukemia. 2014;28(8):1586‐1595. [DOI] [PubMed] [Google Scholar]
- 21. Wakita S, Sakaguchi M, Oh I, et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022;6(1):238‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakaguchi M, Yamaguchi H, Najima Y, et al. Prognostic impact of low allelic ratio FLT3‐ITD and NPM1 mutation in acute myeloid leukemia. Blood Adv. 2018;2(20):2744‐2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gale RE, Lamb K, Allen C, et al. Simpson's paradox and the impact of different DNMT3A mutations on outcome in younger adults with acute myeloid leukemia. J Clin Oncol. 2015;33(18):2072‐2083. [DOI] [PubMed] [Google Scholar]
- 25. Loghavi S, Zuo Z, Ravandi F, et al. Clinical features of de novo acute myeloid leukemia with concurrent DNMT3A, FLT3 and NPM1 mutations. J Hematol Oncol. 2014;4(7):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bezerra MF, Lima AS, Piqué‐Borràs MR, et al. Co‐occurrence of DNMT3A, NPM1, FLT3 mutations identifies a subset of acute myeloid leukemia with adverse prognosis. Blood. 2020;135(11):870‐875. [DOI] [PubMed] [Google Scholar]
- 27. Elrhman HAEA, El‐Meligui YM, Elalawi SM. Prognostic impact of concurrent DNMT3A, FLT3 and NPM1 gene mutations in acute myeloid leukemia patients. Clin Lymphoma Myeloma Leuk. 2021;21(12):e960‐e969. [DOI] [PubMed] [Google Scholar]
- 28. Herold T, Rothenberg‐Thurley M, Grunwald VV, et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia. 2020;34(12):3161‐3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345‐1377. [DOI] [PubMed] [Google Scholar]
- 30. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oñate G, Bataller A, Garrido A, et al. Prognostic impact of DNMT3A mutation in acute myeloid leukemia with mutated NPM1. Blood Adv. 2022;6(3):882‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


