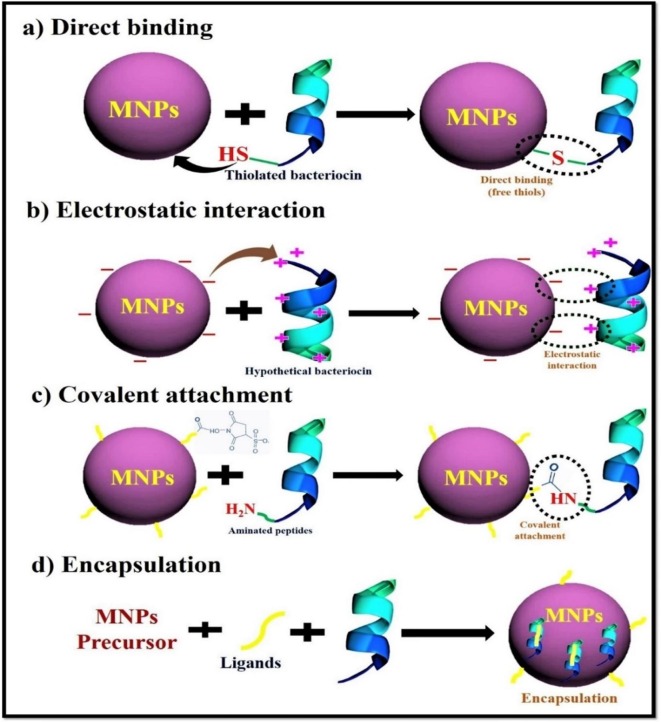
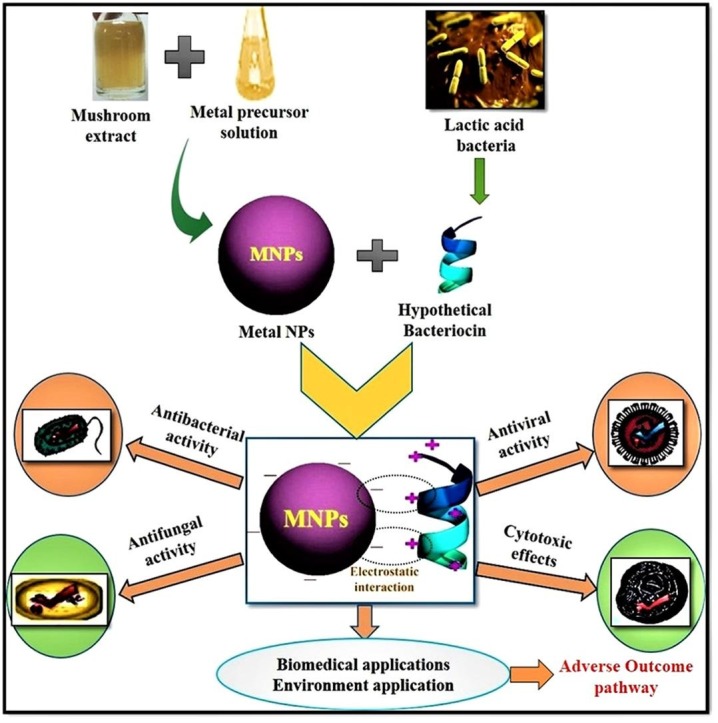
Graphical abstract
Keywords: Multi drug resistant, COVID-19, Metal NPs, Mycosynthesis, Bacteriocin, Nanocomposite
Abstract
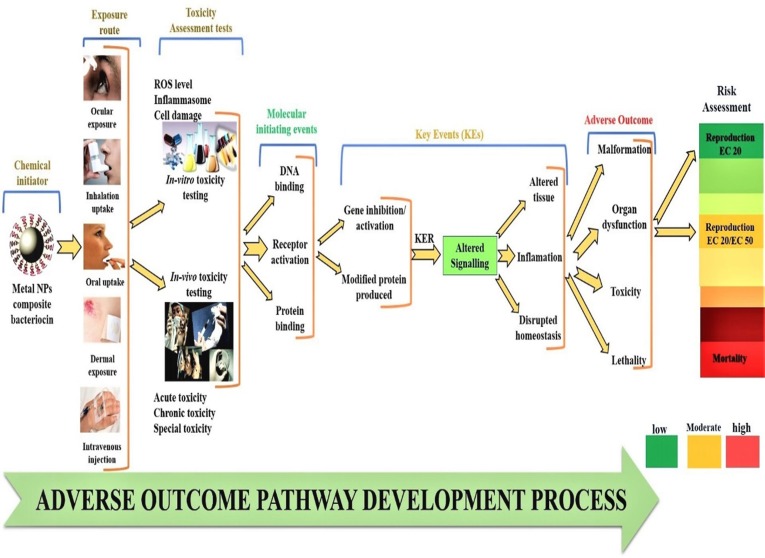
Multidrug resistant (MDR) pathogens have become a major global health challenge and have severely threatened the health of society. Current conditions have become worse as a result of the COVID-19 pandemic, and infection rates in the future will rise. It is necessary to design, respond effectively, and take action to address these challenges by investigating new avenues. In this regard, the fabrication of metal NPs utilized by various methods, including green synthesis using mushroom, is highly versatile, cost-effective, eco-compatible, and superior. In contrast, biofabrication of metal NPs can be employed as a powerful weapon against MDR pathogens and have immense biomedical applications. In addition, the advancement in nanotechnology has made possible to modify the nanomaterials and enhance their activities. Metal NPs with biomolecules composite prevent the microbial adhesion and kills the microbial pathogens through biofilm formation. Bacteriocin is an excellent antimicrobial peptide that works well as an augmentation substance to boost the antimicrobial effects. As a result, we concentrate on the creation of new, eco-compatible mycosynthesized metal NPs with bacteriocin nanocomposite via electrostatic, covalent, or non-covalent bindings. The synergistic benefits of metal NPs with bacteriocin to combat MDR pathogens and COVID-19, as well as other biomedical applications, are discussed in this review. Moreover, the importance of the adverse outcome pathway (AOP) in risk analysis of manufactured metal nanocomposite nanomaterial and their future possibilities were also discussed.
1. Introduction
MDR Pathogenic bacteria and other microbes induce numerous pathogen associated infections and the sickness in human globally reported by world health organization (WHO) in 2016 [1]. Microbial infections are becoming a serious threat due to the development of resistance. There are several factors responsible for the development of MDR. In past, the introduction of the first antibiotic, penicillin unlocked a new era for treating the infectious diseases which were known as “golden age” in the field of antibiotic research during 1940–1962. The timeline followed the introduction of other antibiotics like streptomycin, chloramphenicol, and tetracycline and much more. Initially, antibiotics were able to cure many of the common bacterial infections and played an important role during 2nd World War [2], [3]. The increasing use of antibiotics in infectious diseases resulted in the development of MDR microbes. The MDR microbial pathogens are able to produce a different kind of hydrolyzing enzymes which mainly target the active sites of antibiotics to make them non-functional. β-lactamases are one of the major enzymes made by many Gram-positive and negative bacterial cells that plays a key role in the development of resistance. The developing countries are leading the microbial disease burden in the world [3]. MDR is a major concern in developing nations like India, where microbial infections impose a significant cost burden on healthcare and pharmaceutical industries. In India, according to a recent survey performed by the Center for Disease Dynamics, Economics, and Policy (CDDEP), 80–100 percent of E. coli can develop resistance by creating Extended-spectrum-lactamases (ESBLs) enzymes, while 50–61 percent of K. pneumonia are MBL-producing organisms [4]. ESBLs producing cells target antibiotics like penicillin as well as third generation cephalosporin [5], [6].
MDR organisms are able to modify the functional group of antibiotics to render them inactive. Bacteria of this category deactivate the antibiotics mostly by O -acetylation, and N –acetylation. Some resistant organisms can oxidize or reduce the antibiotics to make them non-functional. Alteration in the active site of antibiotics can play an key role in the development of resistance. In S. aureus, Mec A gene-encoded penicillin- binding protein 2a transpeptidase can help in the development of methicillin resistance [methicillin- resistant Staphylococcus aureus (MRSA)] [3], [7]. If no action is taken, the number of deaths caused annually by harmful microbes would rise to 10 million by 2050, according to the MDR research 2016 report [8]. Coronavirus infections are currently a serious global pandemic. There have also been two more recent CoV outbreaks. Severe Acute Respiratory Syndrome (SARS-CoV) transmission from civet cats to people was discovered in 2002. It was discovered that the Middle East Respiratory Syndrome (MERS-CoV) of 2012 can spread from dromedary camels to people. Despite the fact that COVID-19 has already demonstrated some parallels to current coronavirus outbreaks. Novel beta-corona virus responsible for COVID-19 likely occurred in a “wet market” in Wuhan, central China [8], [9]. The unique coronavirus-related illness was formally referred to as Coronavirus Disease 2019 on February 12, 2020, by WHO (COVID-19). An overall the COVID-19 infections were affected by 292.5 million and 5.4 million deaths occurred globally till December 2021. In particular, first wave of the COVID-19 pandemic conditions arise on 19 April 2020 as reported by WHO, there after increasing the corona cases nearly 152 million confirmed by July 2020 over with 3 million deaths occurred in worldwide [10]. The 2nd wave of COVID-19 pandemic arise in April and May month of 2021 by Delta variant, while 29.27 million cases were confirmed and 1.19 million deaths were occurred up to October 2021 reported by WHO and Institute for Health Metrics and Evaluation (IHME). After the devastating 2nd wave of COVID-19, the SARS CoV-2 has more variants or mutations at globally in the way of silent mutations, deletion, point mutation, synonymous, and non-synonymous due to environmental factors, climatic changes and various circumstance during December 2019 to December 2021. A new mutated version of the Delta variant has made its appearance. It was 1st detected in India. However, this variant named as Delta Plus or B.1.617.2. Scientists have discovered a novel strain of the virus that causes COVID-19 in Southern France, as the rest of the globe struggles with the severely mutated Omicron variety of SARS-CoV-2. Recent research indicates that the Omicron variant, which triggered the horrific second wave of the pandemic, is substantially more contagious than the Delta variety. Over a hundred nations have so far reported finding the Omicron variant. Over the course of 23 states and Union Territories in India, 1,892 instances of the Omicron variant have thus far been identified. India is currently experiencing the third COVID-19 wave, during which the number of cases has actually doubled over the last three days from the original 27,553 patients to 58,097 patients on January 5th 2022, with an increase in Omicron variant cases as well. The COVID-19 variant B.1.640.2 has been identified by researchers at the institution IHU Mediterranean Infection in at least 12 cases and it has been connected to travel to the African nation Cameroon. This discovery comes as COVID-19 cases are once again on the rise around the world. IHU is not well understood, however it is hypothesized to have more mutations than the Omicron variant. 30 amino acid changes and 12 deletions, caused by 46 mutations and 37 deletions, have been recorded by WHO 2022, according to the novel variant IHU. On the theory of this virus variant leading to an ‘Endemic’ stage, but still we are not yet over the 'Pandemic’.
In order to save thousands of lives, it is important that hospitals are quickly established in a temporary setting. The public must be able to deal with the crisis in the most circumspect and safest possible way because there is no end in sight for it in the coming years. It is obvious that the prevention of this disease is preferable to its treatment, so all practical precautions must be taken, along with implicating and imposing public participation in disease control, strict adherence to COVID-19 appropriate behaviors (such as hand hygiene, the wearing of face masks, and social restriction), and the execution of mini-lockdowns, nighttime curfews, and micro-contained areas. The pandemic situations have significantly affected the economy, safety, health and well-being of both individual and communities. To date, so many COVID-19 vaccines are used for SARS CoV-2 and most of the people are vaccinated. Despite an available COVID-19 vaccine, SARS CoV-2 not lost their virulence and also mutated due to circumstance, to date pandemic is still continuous, so WHO does not conclude that perfect vaccine for COVID-19 and also associated MDR pathogens.
The issue is both pandemics and epidemics that arise rapidly, most probably due to mutation of the viral genome. A change in a single nucleic acid can have a wide destructive effect on the human population. The commonly spread viral diseases can cost billions of dollars, whereas the sudden occurrence and rapid proliferation of a novel extremely virulent viruses result in high mortality. Therefore, rapid and reliable diagnostics have to be part of any successful defense against any kind of pandemic. Globally, conventional molecular diagnostic techniques are generally used in laboratories in order to detect microbes with a high degree of sensitivity and reproducibility. The short shelf half-life of some reagents (enzymes and DNA primers) as well as high cost; limit the relevance of conventional pathogen detection methods in developing nations. In addition to this, despite their sensitivity, current technologies (e.g. PCR and ELISA), require a wide-range of sample preparations and have long readout times, that ends in delayed response as well as disease containment [11], [12]. Conventional molecular diagnosis is a consuming time process with lower specificity and accuracy, which is considered a major drawback in clinical diagnosis. Also, most of these methods are not applicable in the fields such as aerodrome and food courts [13]. At present, there are no potential vaccines or drugs that have been shown to prevent or treat COVID-19 effectively, and most countries are currently trying to prevent the spreading of the SARS-CoV-2 virus by implementing control and preventive strategies. Microbiologists and clinicians are recently struggling with increasing drug resistance pathogenic microbes [14]. So, an urgent need to develop novel drug or antimicrobial materials with various chemical compositions and novel mechanisms to combat COVID-19 and MDR pathogens exists.
1.1. Need for novel approach to control the MDR pathogens and COVID-19
Recent studies have focused on developing novel antimicrobial drugs by constructing new or changing the presence of existing compounds and techniques to successfully cope with emerging MDR pathogens because of the obstacles [15]. In this regard, Nanotechnology provides a platform to fabricate and improve bioactive nanomaterials. Nanoparticles (NPs) at the nanoscale aspects attract the researcher's attention because of their unique size and shape explored in the field of advanced bio-nanotechnology [16]. Nanomaterials with at least 1–100 nm size are considered as the initial building blocks of nanotechnology [17]. CRISPR-Cas9 gene editing by nanotechnology is a new golden age in the realm of medical science. The ultimate goal of nano-carrier design is to examine the basic working strategy of CRISPR-Cas9 using NPs, cells, or tissues, and the application of laboratory findings to the clinics [18], [19]. NPs have a broad range of potential uses in physics, chemistry, biology, material science, and medicine [20]. Metal NPs, such as gold (Au), silver (Ag) [21], [22], platinum [23], titanium [24], copper [25], and zinc [26] have piqued the interest of numerous researchers in recent years, and are being employed for a variety of applications. Among them, Ag and Au were shown to be more important in biological applications than the other metals. Antimicrobials, antioxidants, catalysts, anticancer, and other characteristics of Au and Ag NPs have been documented [27], [28], [29].
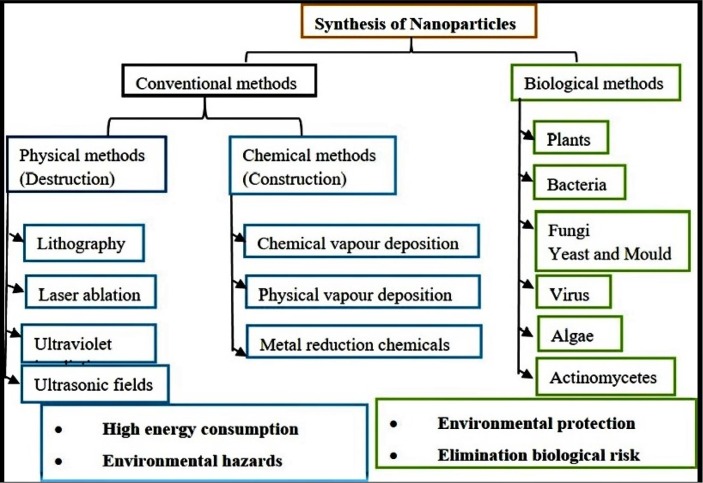
NPs can be prepared by top-down (physical) and bottom-up (chemical and biological) methods (Fig. 1 ). The physico-chemical processes of NPs fabrication are costly and lead to the release of toxic by-products. Green chemistry production of metal NPs utilising biological systems has emerged as a feasible remedy to these drawbacks. The biological technique is economical, simple to produce, less toxic to the environment, and does not require extra processing during synthesis [30]. Biological methods of synthesis consisting of the principle of ‘Nature’ with maximum societal benefits and minimal negative impact on the ecosystem. The green synthesis of metal NPs mediated by biological agents such as bacteria [31], [32], fungi [33], [34], yeast [35], [36], algae [37], [38], actinomycetes [39], [40], and plants [41], [42], [43], which containing bioactive chemicals that to play a key role as reductants in the conversion of metal ions to NPs as well as provide capping agents to stabilize them. However, green synthesized NPs with small size and the high surface area could easily adhere to the cell membrane and enter into the plasma membrane [44].
Fig. 1.
Different approaches used for synthesis of metal NPs.
Among all the type of biological systems, fungi could be a better choice for the mycosynthesis of metal NPs. In the past, people have used mushrooms, which are macro-fungal biological organisms, as both food and medicine since they are a rich source of vitamins, minerals, carbohydrates, and proteins, fibres [45]. Moreover, they have polyphenols, terpenoids, and lectins which are also known for their various biological activities [46]. Mushrooms are an attractive natural system for the green synthesis of metal NPs, because they offer high tolerance to metals; easy to grow, and fungal mycelia provide them with a larger surface area. The mycelial mass of mushrooms is more resistant to agitation and pressure, making them more suitable for large-scale NPs synthesis [47]. Besides, they revealed simple downstream processing during NPs synthesis. Mushroom possess antioxidant, anti-inflammatory, antiviral, antifungal, antibacterial, cardiovascular, hepatoprotective, and lowering hypotensive activities [46]. Edible mushroom extracts contain various biomolecules such as proteins, amino acids, polysaccharides, and vitamins. These molecules contribute to the better reduction of metal ions, biocompatibility, long-term stability and capping actions during NPs synthesis. Additionally, these capping layers offer a surface that is active for the interaction of higher affinity functional groups with biomolecules [48], [49]. Furthermore, Ag and Au NPs synthesis utilizing the aqueous extract of edible mushrooms is easy to use, effective, affordable, safe, and doesn't need complicated equipment.
Bacteriocins are ribosomally produced peptides or proteins by many bacterial species, including probiotic strains. Exerting their antimicrobial property versus other microbes, either belonging to the same species (narrow spectrum) or various genera (broad-spectrum) [50], [51]. Bacteriocins are, colorless, heat-stable and odorless, having a much amount of biomedical applications [52]. They are generally tiny molecular weight of proteins that enter the target cells through binding to cell surface specific receptors. Recently, bacteriocin-secreting lactic acid bacteria (LAB) has attracted significant attention because they are generally recognized as safe (GRAS) status and effective antimicrobial substance to replace an antibiotics [53]. Bacteriocin act as a perfect augmentation substance for increasing the antimicrobial properties. Ag NPs and Au NPs by themselves have better antimicrobial property, yet increasing of their antimicrobial property is desirable. Moreover, conjugation of metal NPs with effective antimicrobial compounds like bacteriocin helps to prevent their microbial adhesion and kills the microbial pathogens through biofilm formation. Generally, Ag and Au are positively charged with large surface area, which facilitates their binding to the negatively charged bacterial membrane [54]. However, metal NPs were identified as a potential weapon against viral pathogens [12]. Metallic and non-metallic nano-materials showed anti-viral activity by
(i) directly interacting with the viral membranes, (ii) controlled drug delivery, (iii) interacting deleteriously with viral genomic material and proteins, (iv) recruiting host immune cells, and (v) generating reactive oxygen species (ROS). Furthermore, Metallic and non-metallic nanomaterials were used to develop materials with a broad spectrum of chemical, mechanical, magnetic, and electrical properties for biomedical applications such as drugs, anti-viral surface and viral diagnostics [12], [55]. A wide array of nano bases applications are under investigation to fight against the COVID 19 [56]. Therefore, there is a need to investigate the synergistic effect of antimicrobial effects of bacteriocins in combinations with metal NPs. This review will focus on novel approaches of mycofabricated Ag-Au NPs bionanocomposite with bacteriocin to enhance the antimicrobial potential & less cytotoxic effects in humans. This review will also elucidate the synthesis of metallic NPs and its effects on MDR pathogens & COVID-19. An adverse outcome pathway (AOP) process development relevance to risk assessment of nanomaterials and their prospects is critically reviewed.
2. NPs Synthesis: Approaches and their importance
Metal NPs are being fabricated using the different approaches viz., physical, chemical, and biological (Fig. 1).
2.1. Conventional approaches of NPs synthesis
The physical and chemical approaches are much popular and widely adopted for metal NPs synthesis (Fig. 1). Different physical approach are employed for synthesizing NPs viz., ultraviolet irradiation [57], evaporation condensation, lithography, laser ablation, ultrasonic fields [58], high energy ball milling, pyrolysis, sputter deposition, plasma arcing, diffusion, electrolysis [59]. Moreover, fabricating metal NPs are cost-intensive and require high energy consumption by using physical approach methods.
On the other hand, metal NPs are fabricated through different chemical approaches, viz., chemical reduction, microemulsion, electrochemical, and thermal decomposition has been limited by its various factors like costly and lead to the production of toxic by-products as they involve hazardous chemicals that are harmful to the environment [60]. In order to overcome these disadvantages, green chemistry approach has emerged as novel method as they are safe, eco-compatible, cost-effective, non-toxic, and easily scaled up for metal NPs synthesis. Further, the green chemistry approach does not involve toxic chemical, high temperature, energy, and pressure.
2.2. Biological approaches of NPs synthesis
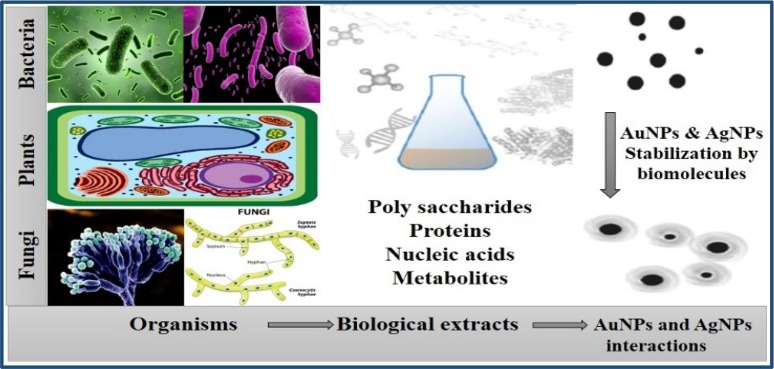
The biological fabrication of NPs is deemed to be a green chemistry approach that interconnects biotechnology and nanotechnology. The biological mode of NPs synthesis used extracts from various organisms including bacteria [32], fungi [33], actinomycetes [61], yeast [62], algae [63], and wide range of plants [64]. Different biological substances are being utilized to synthesize metal NPs (Fig. 2 ).
Fig. 2.
Biological synthesis of metal NPs involving proteins, polysaccharides, nucleic acids and other metabolites from bacteria, plants and fungi.
2.2.1. Biosynthesis of Ag and Au NPs using plant extracts
Ag NPs synthesis from different parts of plants [41], [42], [43]. So far there are a large number of studies regarding the use of plant extract for NPs synthesis [65], [66]. According to geographical variation, the plant metabolites may get changed and so the same plant from different parts may give different results. Identification of a single compound which mediates reduction is very tedious as the plant contains a large number of metabolites compared to microbes. Plants contain many medically important metabolites which can initiate the green synthesis of Ag NPs. Plant extracts mediate one-step synthesis of Ag NPs in a rapid, cost effective method [67]. The plant extracts could mediate the green synthesis of Au NPs from reacting solution containing gold ions, which indicates the solution turns red [68]. The green procedure employs extracts from leaves of Cistus incanus [69] and Mentha piperita [70] to reduce chloroauric acid to Au NPs.
2.2.2. Biosynthesis of Ag and Au NPs using bacteria
Microorganisms are beneficial than plants towards NPs synthesis because they can be easily cultivated and preserved for further use. Plants, when used, are vulnerable to the risk of getting endangered. Bacteria can be used as nanofactories for the synthesis of metal NPs because it is very easy to handle them. The bacteria can effectively use to synthesis NPs by the intracellular or extracellular method. The extracellular method is the most adopted one as it is beneficial compared to intracellular. Extracellular production offers effortless purification steps compared to the intracellular synthesis. The scale up process is easy with the extracellular method and handling of the cell free filtrate is less laborious than managing biomass [31], [32]. Various researchers were reported that the important challenges must be addressed before moving into the industrial production of NPs using bacteria [32], [44], [71]. The main disadvantages associated with the biological system includes comprehensive knowledge on the mechanism involved in the green synthesis of NPs by the organism, control of the size of NPs, time taken for synthesis is more compared to other chemical and physical methods [59].
2.2.3. Biosynthesis of Ag and Au NPs using fungi
Synthesis of NPs by fungi is an exciting and new aspect of current nanotechnology. In recent year’s myco-nanotechnology attained considerable popularity. Fungi can bear up to many tough environmental conditions compared to other biological resources used for NPs synthesis. Fungi secrete plenty of extracellular enzymes, which contribute much into synthesis and stabilization of NPs [27], [33]. When, proceeding to green synthesis with fungi, the total quantity of NPs obtained is extended to a greater extent than bacteria; the fact behind it is the extracellular enzymes and proteins produced by fungi (Mukherjee et al., 2008). The synthesis arbitrated by fungi can be extracellular or intracellular, extracellular method of synthesis is easy and commonly used. Intracellular production is tedious as it needs additional steps to obtain NPs as it is intracellularly produced [72], [73]. Commonly used salt AgNO3 is reduced to Ag NPs when it is treated with fungal biomass and the previous studies showed that the particles are visible close to the cell wall and it rationalizes the presence of reducing an enzyme in the cell membrane. The role of nitrate reductase enzyme in reducing AgNO3 to Ag NPs was well studied with Fusarium oxysporum [74]. The roles of polyphenols/ flavonoids, proteins, terpenoids, tannins as reducing, capping and stabilizing agents were well documented [75]. El-sonbaty et al., (2013) studied the intracellular synthesis of Ag NPs where they observed a high dispersity and perfect shaped particles [76].
2.2.4. Biosynthesis of Ag and Au NPs using algae
Biosynthesis of Ag NPs by algae is an area with clear prospects. Usage of algae is more convenient because it eliminates the risk of serial sub-culturing and sophisticated storage [38]. Commonly followed the method of NPs synthesis is extracellular in case of algae. The reaction could be carried out in algal extract and the preparation of extract and the crucial process in NPs synthesis. The extract can be prepared either in water or in a corresponding solvent [77]. Considerable studies on marine algae on this aspect have been reported. Various freshwater algae and microalgae also used for the synthesis of Ag NPs. Algal synthesis is eco-friendly, rapid and stable [37], [38]. The main advantage of NPs production by algae is its easy availability and effortless cultivation [77]. Algae and its extract are generally known for the extended bioactive potential and it is witnessed in kinds of literature. Algae possess antimicrobial, anti-inflammatory, antimitotic, antineoplastic, antioxidant and antifouling activities. Algae are effective producers of hydroxyl, carboxyl and amino functional groups, alkaloids, phenols, polysaccharides, saponins, flavonoids steroids. These molecules can contribute well for capping, stabilization and reduction reactions [78]. Phytobioreactors can be designed to establish the commercial production of metal NPs. All the scale-up strategies should be strictly followed to obtain optimum production [37], [79].
Compared with different biomaterials utilized in green synthesis approach, the mushroom mediated metal NPs has more advantages like safe to handle, one-step process, environmentally friendly, a rapid rate of synthesis, more stable NPs, and better control over the size and shape of NPs. Owing to these advantages, many researchers aimed to explore the various mushroom species for synthesis of metal NPs.
3. Mycosynthesis of Ag NPs and Au NPs
3.1. Mushroom biology
Mushrooms are eukaryotic organisms classified under macrofungi with rigid cell walls and are non-photosynthetic. They vary from all other groups of organisms of this earth based on their peculiar physiology, lifestyle, biochemistry, and exclusively absorptive mode of nutrition [80]. It grows best in moist and shady places and has a cosmopolitan distribution. Mushrooms are both edible and non-edible fungi. They represent up to 41,000 species, of which about 850 species are recorded from various areas of India, in particular Himalayas and the Western Ghats. However, only a less numbers are commercially cultivated [81]. The most farmed mushrooms are the oyster mushroom (Pleurotus florida), straw mushroom (Volvariella volvacea), winter mushroom (Flammulina velutipes), black forest mushroom (Lentinus edodes), button mushroom (Agaricus bisporus). Historically, mushrooms have been served as a special kind of functional food and referred to as a storehouse of biological compounds. In this view, many mushrooms are revealed to have high nutritional value with more quantity of proteins, fibers, low or no cholesterol, minerals, and trace elements [82]. Mushrooms are consumed as folk medicine, and it comprises a vast and mostly untapped source of suitable new pharmaceutical materials. Mushrooms are well known for their anti-oxidant, anti-tumor, anti-viral, anti-yeast, anti-bacterial, anti-diabetic, anti-hypercholesterolic, anti-cancer, anti-arthritic, and anti-fungal activities [83], [84].
3.2. Mushroom based fabrication of Ag NPs and Au NPs
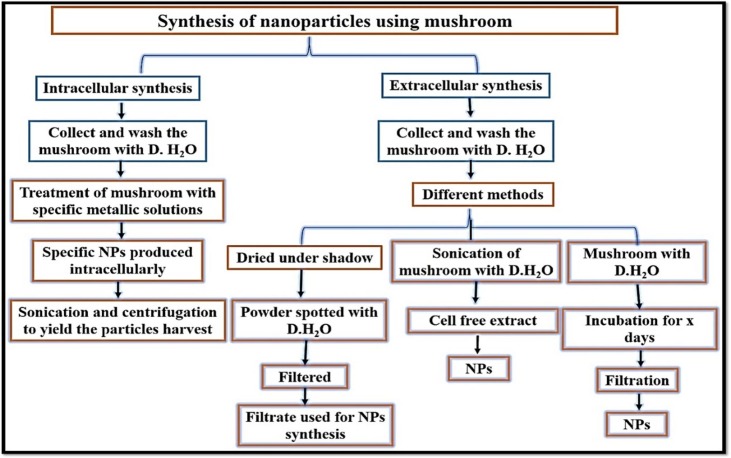
Many bioactive chemicals with various biological activities may be found in many species of edible and medicinal mushrooms. When compared to chemical synthesis, the rate of mycosynthesis of Ag NPs and Au NPs was substantially faster. The outline protocol employed for NPs synthesis utilize mushroom extracellularly or intracellularly is displayed in Fig. 3 .
Fig. 3.
Steps involved in the mycosynthesis of NPs using mushroom.
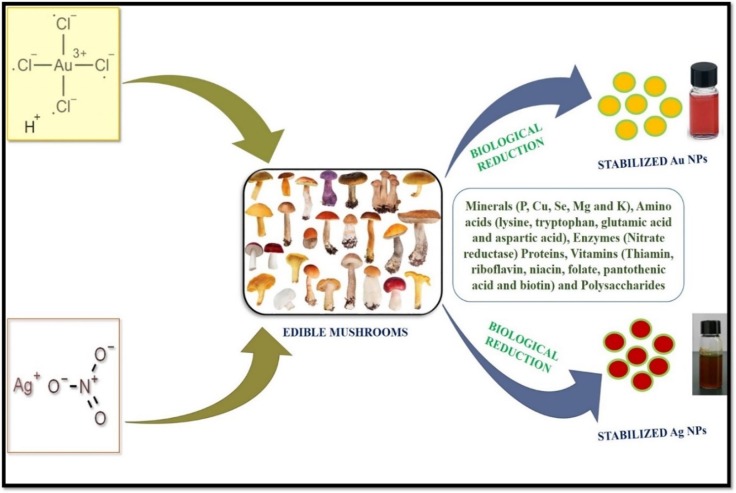
The mushroom extract has inspired consideration for the biological development of metal NPs due to their tolerance and capability to accumulate metals [85], [86]. Further, it was revealed that mushroom contains rich polysaccharides and proteins that attribute to the intracellular and extracellular fabrication of Au/Ag NPs [45]. Ag NPs and Au NPs have been extensively prepared through the biological reduction of Ag NO3 to Ag NPs and HAuCl4 to Au NPs using different mushroom species extracts viz., A. bisporus, L. edodes, Agrocybe aegerita, Ganoderma lucidium, V. volvaceae, and P. sajor caju, etc. [87]. Another exciting aspects of mushroom fungi as a perfect substance for fabricating NPs is the intracellular metal uptake capacities and high metal binding to the cell walls surface. Besides, mushroom fungi acquires one or more metal tolerance strategies viz., precipitation, intracellular sequestration, complexation, extracellular metal sequestration, increased metal efflux, and suppressed influx, [88].
Furthermore, mushrooms have the capability to synthesize metal NPs with nanoscale structures by the influence of their reducing enzymes and biomimetic mineralization (Fig. 4 ). The mycelial mesh used for NPs synthesis can bear agitation, other bioreactors conditions, flow pressure and compared with bacteria [89]. It can be easy to handle in downstream method of metal NPs synthesis as the reducing agents like proteins and polysaccharides are readily secreted by mushroom fungi [90]. A wide variety of mushroom species have been explored in many research for the green chemistry synthesis of both Ag and Au NPs through intracellular as well as extracellular processes, and their NPs shape and size are well examined (Table 1 ). Moreover, the mushroom extracts has been considered as cost-effective, much reliable, and compatible with biomedical research fields [33].
Fig. 4.
Mycosynthesis of Ag and Au NPs using edible mushroom.
Table 1.
List of mushrooms explored for the synthesis of Ag NPs and Au NPs.
| NPs type | Mushroom species | Mode of synthesis | Size | Shape | Reference |
|---|---|---|---|---|---|
| Au | Pleurotus florida | Extracellular | 12–15 nm | Crystalline spherical | [91] |
| Au | Pleurotus florida | Extracellular | 14.93 ± 2.88 nm | Crystalline spherical | [92] |
| Au | Volvariella volvacea | Extracellular | 20–150 nm | Triangular | [21] |
| Au | Pleurotus florida | Extracellular | 10–50 nm | Spherical | [91] |
| Au | Pleurotus florida | Extracellular | 10–50 nm | Triangular | [91] |
| Au | Volvariella volvacea | Extracellular | 20–150 nm | Spherical | [21] |
| Au | Volvariella volvacea | Extracellular | 20–150 nm | Prism | [21] |
| Au | Agaricus bisporus | Intracellular | 10–50 nm | Spherical | [93] |
| Au | Lentinus edodes | Intracellular | 5–15 nm | Spherical | [73] |
| Au | Pleurotus ostreatus | Intracellular | 5–15 nm | Spherical | [73] |
| Au | Pleurotus ostreatus | Intracellular | 22–39 nm | – | [72] |
| Au | Grifola frondosa | Intracellular | 5–15 nm | Spherical | [73] |
| Au | Ganoderma lucidum | Intracellular | 5–15 nm | Spherical | [73] |
| Au | Pleurotus ostreatus | Intracellular | 7.0 ± 0.5 nm | Crystalline spherical | [94] |
| Au | P. cornucopiae var. citrinopileatus | Intracellular | 16–91 nm | Spherical | [95] |
| Au | Lentinula edodes | Intracellular | 72 nm | Irregular | [96] |
| Au | Flammulina velutipes | Intracellular | 10–80 nm | Spherical | [97] |
| Ag | Agaricus bisporous | Extracellular | 30 nm | Spherical | [98] |
| Ag | Agaricus bisporous | Extracellular | 10–20 nm | Spherical | [99] |
| Ag | Fomes fomenterieus | Extracellular | 8–50 nm | – | [100] |
| Ag | Lentinula edodes | Extracellular | – | – | [100] |
| Ag | Agaricus bisporous | Extracellular | 20–44 nm | Dispersed spherical | [98] |
| Ag | Agaricus bisporous | Extracellular | – | – | [99] |
| Ag | Ganoderma appalanatum | Extracellular | 15–20 nm | – | [21] |
| Ag | Pleurotus florida | Extracellular | 20–50 nm | – | [101] |
| Ag | Pleurotus citrinopileatus | Extracellular | 6–10 nm | Core-shell spherical | [102] |
| Ag | Pleurotus florida | Extracellular | 20 ± 5 nm | – | [21] |
| Ag | Volvariella volvacea | Extracellular | 5 nm | – | [103] |
| Ag | Pleurotus ostreatus (white) | Extracellular | – | – | [95] |
| Ag | P.salmoneostramineus (pink) | Extracellular | – | – | [45] |
| Ag | Agaricus bisporous | Extracellular | 5–50 nm | Spherical | [100] |
| Ag | Calocybe indica | Extracellular | 5–50 nm | Spherical | [100] |
| Ag | Pleurotus florida | Extracellular | 5–50 nm | Spherical | [100] |
| Ag | Pleurotus florida | Extracellular | 20 ± 5 nm | Spherical | [90] |
| Ag | Tricholoma matsutake | Extracellular | 10–20 nm | – | [104] |
| Ag | Schizophyllum commune | Extracellular | – | – | [105] |
| Ag | Pleurotus florida | Extracellular | 20 ± 5 nm | Spherical | [91] |
| Ag | Agaricus bisporous | Intracellular | 80–100 nm | Spherical | [106] |
| Ag | Agaricus bisporous | Intracellular | 40–60 nm | Spherical | [86] |
| Ag | Coriolus Versicolor | Intracellular | 10 nm | Spherical | [107] |
| Ag | P. comucopiae var. citrinopileatus (bright yellow) | Intracellular | 10–20 nm | Spherical | [83] |
| Ag | Calocybe indica | Intracellular | 100 nm | Spherical | [106] |
| Ag | Pleurotus ostreatus (grey) | Intracellular | – | – | [45] |
4. Mechanisms of Ag and Au NPs synthesis using mushroom
The mushroom mediated NPs synthesis mechanism involves three steps of nucleation, growth and stabilization. Firstly, a portion of metal ions in a solution is reduced to metal atoms by the available reducing agents. These atoms act as nucleation centers and catalyze the remaining metal ions which are present in the reaction solution. Clusters are formed via atoms aggregation. Secondly, the surface ions are frequently reduced in order to attain the high values of nuclei leading to the formation of larger particles during the process. Finally, the capping agents in the solution function as stabilizer and prevent the further aggregation of the particles.
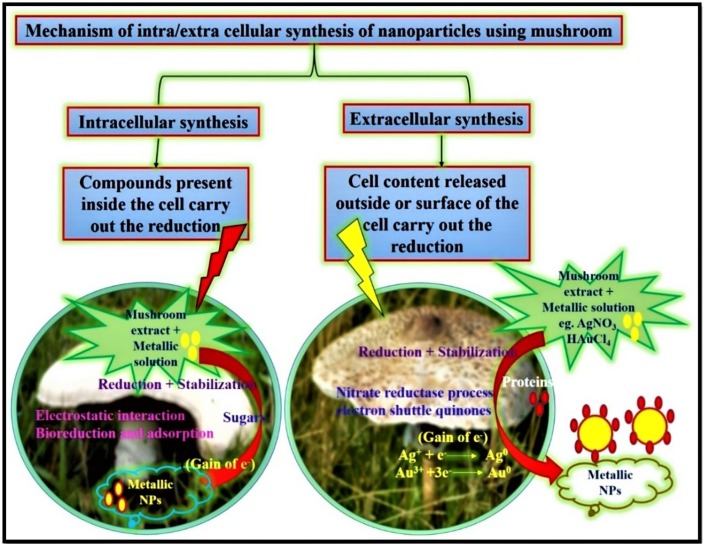
Various types of mushrooms display diverse kinds of mechanisms; moreover, the reduction response is the main principle for NPs development (Fig. 5 ). However, the development of NPs occurs by the impact of an enzyme reductase, which exists in the culture extract mixed with metal ions [85]. Mushroom-mediated NPs synthesis can be intracellular or extracellular as stated in the area of NPs development. In general, extracellular fabrication of NPs involved nitrate reductase action and electron shuttle quinones. Nitrate reductase start off NPs development in numerous fungi, including mushrooms. Some enzymes, such as nitrate-dependent reductases, α-NADPH-dependent reductases, and an extracellular shuttle quinone, are correlated with Ag NPs fabrication [108]. It was revealed that the development of NPs was due to the shift an electrons from free amino acids to metal ions [109]. The ninhydrin analysis could be proved the existence of free amino acids in the heat-denatured fungal extract, which may be produce for the NPs synthesis.
Fig. 5.
Mechanisms of intra/extra cellular synthesis of NPs using mushroom.
Mandal et al. (2006) revealed that the enzymes present in mushroom filtrate could play a crucial part in reducing metal ions by oxidating aldehyde groups to carboxylic acids [110] . A latest findings display that a glucan polysaccharide isolated from P. florida mushroom serves as a reducing and stabilizing material [92]. The mushroom extract of Tricholoma matsutake recommended the potentiality of reducing metal ions to metal NPs due to the existence of proteins [104]. Flavo-proteins present in the mushroom filtrate also play a key role for reducing Ag ions into Ag NPs [103].
The extracellular fabrication of Ag NPs and Au NPs by mushroom is due to the high existence of nitrate reductase enzymes in mushroom cytoplasm, which greatly assist the reduction of Ag and Au ions into Ag NPs and Au NPs, respectively. Molnar et al. (2018) recommended that the fabrication of Au NPs might contain two main concept affair that include stabilization by capping the ligands of NPs and reducing Au3+ into Au0 fabricate nuclei later the growth of NPs [111]. The safety ligands avert the additional development and agglomeration of NPs due to electrostatic stabilization and in the end promote well solid Au NPs.
In the intracellular development of NPs, the microbial cell serves as a particular ion transport process, where the negatively charged cell wall energetically associate with the metal ions transfer a positive charge that promote an electrostatic interaction force between these two opposite charges [24]. The metal ions are perhaps reduced by the enzymes or polysaccharides within the cell wall, which promote the accumulation of metal ions and the development of NPs [108].
5. Antimicrobial peptide as an alternative agent
Nature provides a defence system as a part of innate immunity, which is produced as antimicrobial substances by animals, plants, insects, and bacteria in the form of hydrogen peroxide, fatty acids, organic acids, ethanol, antibiotics, and antimicrobial peptides (AMPs). AMPs possess broad spectrum effectiveness against multidrug-resistant pathogens, which make them very interesting compounds for the search of new drug molecules [112]. AMPs are small-molecular-weight proteins/peptides generally produced by different multicellular organisms. Most of the AMPs possess net positive charge because of the presence of multiple lysine and arginine residues along with ≥ 30 % hydrophobic residues. Till date, hundreds of such AMPs have been searched with activity against various pathogenic microorganisms, fungi, yeast, viruses and others [113], [114].
5.1. Bacteriocins: Antimicrobial peptide from bacteria
The antimicrobial peptides (AMPs) secreted by the bacterial cells are known as bacteriocins. Limited nutrients in the atmosphere cause production of diverse bacteriocins for survival. Bacteriocins are ribosomally synthesized peptides which display narrow spectrum potential (kills relatively close species) or broad-spectrum activity (kills non-related microbial group). Most of the bacteria have the capability of producing at least one bacteriocin, many of them, are still not identified [115]. Although the antibiotics and bacteriocins have a bacterial origin and pose similar function but they differ. Antibiotics are secondary metabolites of bacterial cells while bacteriocins are synthesized by ribosomes [116].
5.2. Sources of bacteriocin
In the current scenario, bacteriocins have been anticipated as a replacement for antibiotics to which the pathogenic bacteria are developing resistance. In previous studies, bacteriocin was isolated from various genera including Bifidobacteria, LAB, and yeast (Table 2 ). The bacteriocin production is not limited to any specific bacteria as it can be produced by both Gram-positive and Gram-negative bacteria [117]. The first bacteriocin named colicin was isolated and purified from Gram-negative bacteria E. coli [118]. Klebicins, marcescins, alveicins, cloacin and pyocin are the other known bacteriocins from Gram-negative bacteria secreted by K. pneumoniae, Serratia marcescens, Hafnia alvei, Enterobacter cloacae and Pseudomonads, respectively [119]. They are classified into two groups, 30–80 kDa molecular mass bacteriocins secreted by Gram-negative bacteria are known as colicins and 1–10 kDa were known are microcin. Most of the bacteriocins of Gram-negative bacteria belonging to colicin are heat labile peptides which cannot survive in extreme conditions [118].
Table 2.
List of bacteriocin producing microorganisms.
| S. No. | Bacteria | Bacteriocins | Antimicrobial Activity | Reference |
|---|---|---|---|---|
| Lactic acid bacteria | ||||
| 1 | L. lactis strain CH3 | Nisin | C. albicans, A. fumigatus, S. flexneri, K. pneumoniae, S. aureus, and S. pyogenes | [120] |
| 2 | L. lactis subsp. lactis B14 | Bozacin B14 | LAB and food borne pathogens | [121] |
| 3 | L. lactis subsp. lactis Q1-2 | Lactococcin 972 | L. sakei, L. lactis subsp. lactis and L. cremoris | [122] |
| 4 | L. lactis subsp. cremoris 2A27 | Lactococcin G | L. sakei, L. lactis subsp. lactis and L. cremoris | [122] |
| 5 | L. lactis subsp. lactis | Nisin A |
S. aureus, Listeria innocua, L. sakei, and L. plantarum |
[123] |
| 6 | L. lactis subsp. lactis 1AA17 and 2BB9 | Nisin Z | S. aureus, L. innocua, L. sakei, and L. plantarum | [123] |
| 7 | Lactiplantibacillus plantarum KLDS1.0391 | Plantaricin MG | L. monocytogenes, S. aureus, S. typhimurium and E. coli | [124] |
| 8 | Lactococcus garvieae BCC 43578 | Garvieacin Q | L. garvieae, Enterococcus faecium, and L. monocytogenes | [125] |
| 9 | Lactobacillus animalis TSU4 | Bacteriocin TSU4 | A. hydrophila, P. aeruginosa, S. flexneri, S. typhimurium, S. aureus, S. paratyphi, and E. coli | [126] |
| 10 | Lactobacillus coryniformis MXJ 32 | Lactocin MXJ 32A |
E. coli, S.aureus, Salmonella sp., L. monocytogenes, and C. sakazakii |
[127] |
| 11 | L. plantarum JLA-9 | Pantaricin JLA-9 | and M. Luteus, S. aureus, C. sporogenes, C. perfringens, C. difficile, P. polymyxa, A. acidoterrestris, G. stearothermophilus, B. subtilis, B. coagulans, and B. cereus, | [128] |
| 12 | B.animals BB04 | Bifidocin A | E.coli, Listeria monocytogenes, S.aureus | [129] |
| Yeast | ||||
| 13 | S. boulardii CNCM I-745 | leucocin C | Antilisterial activity (L. monocytogenes) | [130] |
| Bacillus | ||||
| 14 | Bacillus licheniformis | Bacteriocin BL8 | B. circulans, S. aureus, B. coagulans, B. cereus, C. perfringens and B. pumilis | [131] |
| 15 | Bacillus subtilis | Bacteriocin | diabetic foot ulcer patients to Pseudomonas sp., S. aureus sp., Klebsiella sp. and Proteus sp. | [132] |
| Streptococcus | ||||
| 17 | S. thermophilus ST109 | Thermophilin 109 | E. faecalis and S. pyogenes | [133] |
| 18 | S.thermophilus SBT1277 | Thermophilin 1277 | C. butylicum, C. sprogenes and B. cereus | [134] |
| 19 | Streptococcus mutans N | Mutacin N | S. pyogenes and Oral Streptococci | [135] |
| Enterococcus | ||||
| 20 | E. faecalis 478 | Enterocin 478 | multidrug-resistant enterococci and vancomycin-resistant enterococci |
[136] |
| 21 | E. faecalis NKR-4–1 | Enterocin W | B. coagulans, B. circulans, B. subtilis, and Listeria innocua | [137] |
| 22 | Enterococcus durans L28-1 | Durancin L28-1A | LAB and food borne pathogens | [138] |
| 23 | Enterococcus mundtii QU 2 | Mundticin KS | E. faecalis, E. hirae, L. plantarum, L. sakei ssp. sakei, Leuconostoc mesenteroides, and P. acidilactici | [139] |
5.3. Classification of bacteriocin
The classification of bacteriocin is based on their structure, size, and sensitivity towards enzymes, thermo stability and post translational modifications (Table 3 ).
Table 3.
Types of bacteriocin and their characteristics.
| Class | Subgroups | Characteristics feature of bacteriocin | Examples | Reference |
|---|---|---|---|---|
| Class I | Lantibiotics, small size peptides, presence of unusual amino acids | |||
| Type A | Amphipathic peptides, known for voltage-dependent pore formation | Nisin | [140] | |
| Type B | Small anionic or neutral peptides | Mutacin II | [141] | |
| Class II | Non-lantibiotics Absence of unusual amino acids, heat stable |
|||
| IIa | Bacteriocin possessing anti-listeral activity | Leucocin A | [142] | |
| IIb | Composed of two complementary peptides whose mechanism depend upon both peptides | Lactococcin Q | [143] | |
| IIc | Cyclic bacteriocins formed by the linkage of C and N terminals of the peptide | Lactocyclicin Q | [144] | |
| IId | Non pediocin like linear peptide | Lacticin Q | [145] | |
5.4. Mechanism of action
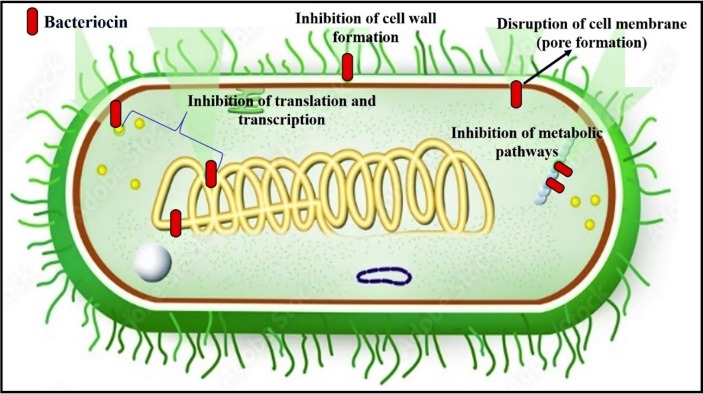
The detailed study of physical and chemical parameters of bacteriocin is very important for targeting the pathogenic bacteria [146]. The bacteriocin differs in their molecular structure, amino acid composition, molecular weight, thermo stability, pH, antimicrobial activity and mode of action. The in-vitro and in-vivo investigations reveal the promising compound to become a therapeutic drug molecule [147]. Many of the bacteriocins are bactericidal in action in which lysis of the cell takes place. In few cases, the bacteriocin activity is bacteriostatic in nature. Many of the bacteriocins (e.g. colicins) are acting on sensitive cells by pore formation and weakens the integrity of the membrane thus results in leakage of cellular constituents [148]. The gram-positive bacteria are made up of peptidoglycan whereas the gram-negative bacteria are made up of lipopolysaccharides. This feature greatly influences the mode of action of bacteriocin on pathogenic bacteria. In recent study, it was reported that the inhibitory activity determination is difficult if the producer strain produces several bacteriocins, organic acids and hydrogen peroxide [149]. Various mode of bacteriocin action on microorganisms was represented in Fig. 6 . The class I peptide bacteriocins attacks on microorganisms by attaching to the cell wall membrane, inserted into the membrane followed by pore formation results in cellular constituents leakage [150], [151]. The class II bacteriocins integrate into the cell wall membrane causes depolarization of the membrane and membrane potential disrupts that leads to death of the cell [152]. In a recent study by Colombo et al. (2018) reported that the bacteriocins act on cytoplasmic membrane and dissipate the electric potential of the membrane [153]. Krishnamoorthi et al., (2022) reported that bacteriocin significant antimicrobial effects against C. albicans, A. fumigatus, S. flexneri, K. pneumoniae, S. aureus and S. pyogens. Moreover, the bacteriocin mode of action against human pathogens was revealed that cell lysis and rupture the cell wall as reported in SEM studies [120]. The bacteriocin mode of action results from its binding to the receptors present on the surface of bacterial membrane of the target. In another mechanism the interaction of charged ions between bacteriocins and membrane lipids results in the generation of electrostatic forces followed by bacteriocin entry into the bacterial cell [154].
Fig. 6.
Mechanism of antibacterial action of bacteriocin.
Members of class I bacteriocins target the lipid II, an intermediate in the peptidoglycan biosynthesis [155]. Some of the bacteriocins have been reported to form pore by their capability to directly insert themselves into bacterial cells after binding to the lipid II. Bacteriocins from class I have also been reported for depletion of membrane potential which causes efflux of compounds. Type A subgroup of lantibiotics of class I which includes nisin, epidermin, and galidermin, targets the bacterial cells by two mechanisms, one membrane permeabilization and another inhibition of cell wall [156]. Mersacidin, a member of type B has been revealed to inhibit peptidoglycan synthesis by reacting with lipid II without interacting with transglycosylation enzyme [157]. Plantaricin C, the member of type B lantibiotic, targets bacterial cell by direct lipid II-mediated action [155].
Class II bacteriocins have been reported for the disintegration of plasma membrane of the bacterial cell [158]. Class II bacteriocins are a diverse class of short thermostable peptides (10 kDa) having an amphiphilic helical shape that enables their entry into the cytoplasmic membrane of the target cell, leading to membrane depolarization and cell death. They induce the leakage of internal substances such as monovalent cations, phosphate and ATP. The members of class II, PA-1, leucocin A, and mesentericin Y105 are known for binding with specific receptors present on the bacterial cell surface. Mannose phospho transferase system (PTS) and EIIt Man permease act as a receptor for members of class II, which directs its activity against L. monocytogenes and Enterococcus faecalis [159].
Similar to most bacteriocins, class IIc cyclic bacteriocins permeabilize the membrane of susceptible cells, resulting in the leakage of ions, dissipation of the membrane potential, and cell apoptosis [160]. Class III bacteriocins are bigger in size having molecular weight>30KDa. The bacteriocins are hydrophilic and heat labile in nature. Their action mode differs compared to other bacteriocins in that they encourage the destruction of the target microorganism's cell envelope. Their C-terminal region is in charge of recognising the target cell, while their N-terminal region is similar to an endopeptidase responsible for the synthesis of cell walls. The bacteriocins interfere with different cellular functions such as cell wall synthesis, transcription, translation and replication [152]. The peptides possessing good antimicrobial activity have been discovered recently and some of them are under clinical trials [161], [162], [163].
6. Bio-functional potential of metal NPs
Nanomaterials reveals antimicrobial property by promoting antibiotics administration effectiveness and safety [86], [93], [164]. The antimicrobial property of NPs cannot pose direct and severe consequence, while promising toxicity upon long-term exposure. The development of antimicrobial NPs could be non-toxic, cost-effective when compared with antibiotics fabrication, and they are relatively stable enough for long-term storage with an extended shelf-life [86]. Besides, the synthesized metal NPs can tolerate hard state of affairs, such as high-temperature sterilization, under which conventional antibiotics are usually inactivated. Antibiotics distribution using metal NPs offer various superiority: i) reduced side effects, ii) sustained and controlled release, iii) controllable and relatively uniform distribution in the target tissue, iv) enhanced cellular internalization, v) improved patient-compliance, and vi) improved solubility [32], [86], [93].
Metal NPs make reactive oxygen species (ROS) under UV light and find their increasing applications in antimicrobial formulations and dressings. In particular, silver, gold, zinc, and their compounds fruitfully inactivate many pathogenic microorganisms. Currently, tetracycline hydrochloride development in polymeric NPs has demonstrated increased antimicrobial properties and anti-methicillin resistant Staphylococcus aureus (MRSA) properties with non-polymerized forms of penicillin and N-methylthio β-lactams [165], [166], [167]. Vancomycin-capped Au NPs have also revealed increasing antimicrobial properties against vancomycin-resistant enterococci (VRE), and E. coli strains [168]. Metal NPs based bionanocomposite with bacteriocin could enhance their antimicrobial potential against MDR pathogenic microorganisms.
6.1. Antibacterial potential of Ag NPs and Au NPs
In the last few years, bacterial infection is common in healthcare settings, especially in hospital-acquired infections, contamination of water, food, and agriculture sectors worldwide. Hospital patients mostly pneumonia, bacteraemia, meningitis, urinary tract infections, as well as skin and soft-tissue infections caused by Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter spp. Microbial pollutions are key for the most common clinical infections around worldwide, conveying considerable dangers to the general well-being of humans. Presently, familiar market obtainable antimicrobial agents are antibiotics, metal salt arrangements, and ammonium salt [165]. Unfortunately, most antimicrobial agents are poor, and misapplication has led to MDR of pathogenic bacteria, yeast, and fungal microorganisms. The notable pollution that have happened because of MDR microbes of Pseudomonas sp, Klebsiella sp., and Acinetobacter baumannii are rise at an alarming signal [61]. In this way, it is critical to detect novel antimicrobial agents with less toxic qualities and high potency to combat this catastrophe. Therefore, it is necessary to create innovative antimicrobial treatments based on metal NPs that have high antibacterial effectiveness, low toxicity, and good compatibility. Metal NPs have been considered as possible substitute to commercial antibiotics.
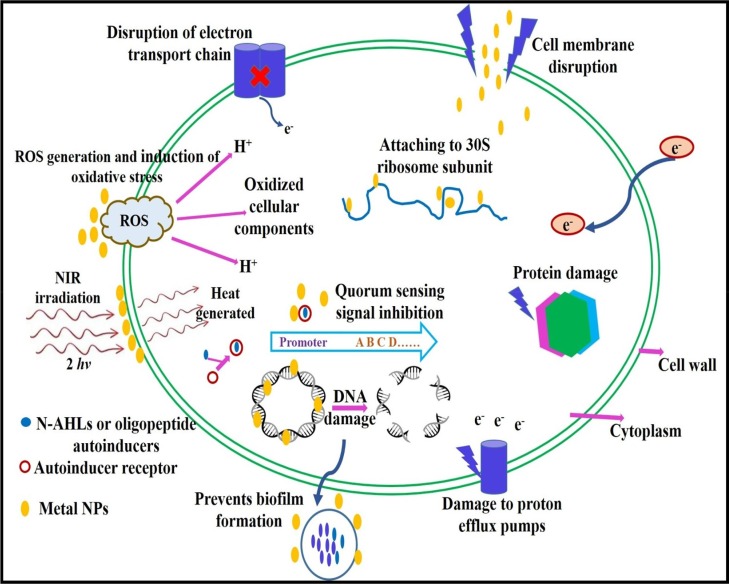
The therapeutic properties of many antibiotics originate from their capability to “inhibit the cell wall synthesis, interfering with essential proteins expression and disrupting the DNA replication machinery”. However, bacteria have developed the capability to resist each of these mechanisms of action. One of the fundamental principle of bacterial resistance is the change of the antibiotics target [169]. Over the years, the use of nanomaterials in photo thermal therapy has received considerable attention [170], [171]. The light-responsive technique specifically has been used in the development of drugs. It has also found application in the design of drug carrier systems and as an antibacterial agent. The light-responsive structure can be easily regulated and it has low invasiveness. Its mechanism of activity is based on the alteration of the light sensitive molecules when stimulated by light, thus enabling the release of the encapsulated or conjugated drug [172]. Metal NPs can overcome the drug resistance principle owing to their distinctive physicochemical activity, enabling nanomaterials to carry out various new bactericidal pathways (Fig. 7 and Table 4 ) to attain antimicrobial properties such as i) photocatalytic generation of ROS that damage cellular constituents, ii) inhibition of enzyme property and DNA synthesis, iii) interruption of energy transduction, and iv) compromising the bacterial cell membrane [173], [174], [175]. The antibacterial mechanisms of metal NPs is naturally relative their core material, shape, size, and surface functionalization. Besides their broad-spectrum antibacterial activity, metal NPs have been used as a vectors to deliver antimicrobial component that outstandingly enhance their biocidal activities [176]. Metal NPs shows entirely new or enhanced activities based on particular characteristics such as size, shape, and NPs distribution. Some of the benefits of using metal NPs as vectors are their protective action against enzymes that would otherwise kills antimicrobial agents; their capability to deliver antibiotics actively, and their capability to combine many therapeutic modalities onto an one individual nanomaterials [165], [176].
Fig. 7.
Mechanisms of antibacterial action of metal NPs.
Table 4.
Mechanism of antibacterial action of Ag/Au NPs against target bacteria.
| NPs type | Targeted bacteria | Concentration of NPs | Mechanisms of antibacterial action | Reference |
|---|---|---|---|---|
| Ag | Streptococcus pneumoniae | 20 µL | ROS generation, cellular uptake of silver ions, cascade of intracellular reaction. | [183] |
| Ag | Streptococcus pyogenes, S. aureu, K. pneumoniae and Shigella flexneri | 100 µg/ ml | Disruption of the bacterial cell wall, DNA damage, and ROS generation. | [86] |
| Ag | Staphylococcus aureus | 100 µg/ ml | Cell surface damage and loss of the chain integrity. | [31] |
| Ag | Pseudomonas aeruginosa and E. coli | 1 µg/disc | Evade multidrug efflux pumps. | [30] |
| Ag | Enterobacter cloacae and Streptococcus mutans | 180 µg/ ml | ROS production and membrane disruption. | [184] |
| Ag |
S. epidermidis and S. aureus |
80 µg/ ml | Penetration in the bacterial biofilm using an external magnetic field. | [185] |
| Ag | Escherichia coli and P. aeruginosa | 50 mM | Inhibition of cell wall synthesis, protein synthesis and nucleic acid synthesis. | [15] |
| Ag | S. aureus and E. coli | 80–160 µg/ ml |
Physical adhesion to the bacterial cell. | [186] |
| Ag | Acinetobacter baumannii |
3.06 µg/ ml |
Attach to the cell wall leading to structural changes in the permeability of the cell membrane. | [187] |
| Ag | A. calcoaceticus | 50 µg/ ml | Not revealed. | [188] |
| Ag | S. aureus and E. coli | 100 µg/ ml | Upregulation of the expression of antioxidant genes and ATP pumps. | [189] |
| Au | Klebsiella pneumoniae and E. coli | 1.009 mg/L | Disruption of the bacterial cell wall, DNA damage. | [190] |
| Au | Streptococcus pyogenes, S. aureu, K. pneumoniae and Shigella flexneri | 100 µg/ ml | Disruption of the bacterial cell wall, DNA damage. | [93] |
| Au | Streptococcus bovis and Streptococcus epidermidis | 50 to 512 µg/ml | Disruption of the bacterial cell wall. | [191] |
| Au | Acinetobacter baumannii | 40 µg/ml | Disturb of osmotic balance and disrupt the integrity of cell bacterial cell wall. | [192] |
| Au | E. coli | 4 mg/mL | Interaction between lysozyme microbubbles and cell wall. | [193] |
| Au | P. aeruginosa | >78 ppm | Interaction with cell surface. | [194] |
| Au | S. aureus | 70 µg/mL | Laser excitation of the near IR LSPR lead to an efficient photothermal response with efficient killing of bacteria biofilms. | [195] |
| Au | E. coli | 0.005 % v/v | Penetration through biofilm layers and interaction with cellular components. | [196] |
| Au | Proteus species | 2 μM | Interaction between proteins and NPs. | [197] |
The Au-Ag NPs, which are positively charged, aggregate on negatively charged bacterial cell walls. They release Ag NPs and generate ROS, which are antibacterial agents. Strong emission under near-infrared (NIR) irradiation allows these bacteria to be easily damaged. The NIR irradiation also increases the antibacterial effect of the NPs through the photo-thermal effect (heat generation using energy converted from the absorbed photons), it brings about a considerable conformational change in the cell wall. It eventually loses permeability control, which generate to cell death [172]. Once metal NPs enter the bacterial cell, they would affect with the bacterial multiplication signalling pathway by modulating tyrosine phosphorylation of putative peptides substrates important for cell division and cell viability [86], [177]. The other mechanism is the development of free radicals, which subsequently promote membrane damage leading to an effective antimicrobial activity of metal NPs against S. flexneri, K. pneumoniae, S. aureus, and S. pyogene at 100 µg/ml. Further, the metal ions interfere with the bacterial cell wall and electron transport at the same time causing DNA damage [86]. Strong antibacterial action by Ag NPs against human bacterial pathogens that are resistant to many drugs has been established.
In previously so many studies was reported that Ag and Au NPs as a broad spectrum of antimicrobial effects. For example, S. aureus and Edwardesiella tarda bacteria were resistant to Ag NPs inspired with citrus lemon juice as a reducing agent, while Oscillatoria species were resistant to the cyanobacterial effects of the latter [178]. Researchers have looked into using Au NPs as an effective antibacterial to inhibit the growth of common waterborne infections like Salmonella typhi and E. coli that are developing resistance to conventional bactericides [179]. Carrizales et al. documented that AgNPs can reduce bacterial adherence in the early stages of biofilm development and enhance the impact of antibiotics against bacteria that are resistant to them [180]. According to Fernando et al. (2019), Ag NPs containing garcinol from G. quaesita Pierre on top effectively suppressed the development of a methicillin-resistant strain of Staphylococcus aureus with a 21.7 mm zone of inhibition [181].
Priyadharshni et al., (2022) reported that, the synergistic antimicrobial effects of Ag NPs and mushroom extract showed efficient antibacterial effects against human pathogens and Ag NPs with mushroom extract may be attributed by their tiny size and large surface area to contact the microbial cells and thus makes them interact effectively with the cell wall of the disease causing microbes [164]. The mushroom based Au NPs may bind with the cytoplasmic membrane and destroys the bacterial cell due to the electrostatic affinity between positively charged Au NPs and negatively charged cell membrane of the pathogenic microbes [111]. Krishnamoorthi et al., (2022) clearly reported that mushroom based Ag NPs may induce the release of reactive oxygen species, which leads to the destruction of proteins and DNA of bacteria cells, ultimately cause cell death [86].
Also, metal NPs could produce ROS that interfere the amino acid synthesis and DNA in bacterial cells. On the other hand, metal oxide-based nanomaterials cause cell wall damage and produce ROS to destroy bacteria [86], [167]. Metallic nanomaterial can offer a wide range of antibacterial mechanisms (Fig. 7) could combat drug-resistant superbugs [182]. The Ag NPs and Au NPs reveals efficient antimicrobial activity due to their extensive surface area, which provides superior contact with human pathogens at 100 µg/ml [90], [93]. Nanomaterials surface chemistry is important to modulate their interaction with bacteria, enhancing their broad-spectrum property at the same time decreasing their toxicity against mammalian cells [86], [165].
6.2. Antifungal potential of Ag NPs and Au NPs
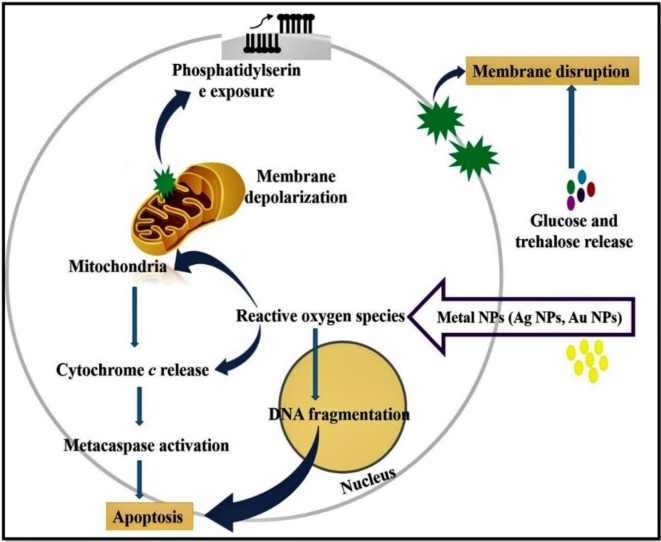
Around the world, fungus infections are the most prevalent infectious disease. Fungal diseases appear in individuals when an invasive fungus invades a body organ and multiplies beyond the capacity of the immune system to manage it. The most frequent fungal pathogens are Candida albicans, Aspergillus spp., Pneumocystis spp., and Cryptococcus spp. C. albicans is an opportunistic pathogen cause sickness to humans like bloodstream infections and oral thrush [198]. After occasionally it will become acute to die. The Indian public is dealing with a brand-new issue as reports of recent COVID-19 patients who have died with mucormycosis, it is sometimes known as “black fungus,” emerge. Sinus fungus infections are challenging to cure and frequently lethal. Of late, the resistance of fungal pathogens to antifungal drugs has received worldwide recognition. Thus, there is a growing demand for novel antifungal agents. To overcome this issue, metallic NPs as powerful nano weapon to fight fungal pathogens. The Ag/Au NPs are the most frequently studied NPs that demonstrate a potent antifungal effects through various mechanisms viz., induction of cell wall interfere, cell wall depolarization, cell cycle arrest, promotion of bacteria cell apoptosis via metacaspase activation, and cytochrome c release [199]. The action of metal NPs and their particular antifungal mechanisms are displayed in Fig. 8 .
Fig. 8.
Mechanism of antifungal action of metal NPs.
The interaction of Au NPs with fungal pathogens and effective enzymes may change their regular conformations superior to the activity loss [200]. Thus, the fungal cell wall is impotent of controlling the H+ transport across the cell wall, leading to the cell growth's retardation, which ultimately causes cell death. The antifungal property of metal NPs is size-dependent and the extent of inhibition enhanced with the particle size decreased. Since, the decreased particle size of Au NPs has an increased surface area that enhances its interaction with the binding sites of the plasma membrane proteins.
A small sized Au NPs may diffuse rapidly through the cell wall to the cells inside. Au (weak acid) has a better tendency to behave with the sulphur and phosphorus-containing soft bases [201]. Therefore, Au NPs may interact with phosphorus-containing bases in DNA or sulfur-containing proteins in cell wall proteins to prevent cells from operating normally, such as replicating, synthesising, repairing, and ultimately cell death. Krishnamoorthi et al. 2021 reported that Au NPs obtained from A. bisporus, which have great antifungal potential in C. albicans and A. fumigatus at 100 µg/ml [111]. The findings of Priyadarshni et al. 2022, green synthesis of Ag NPs with mushroom extract have antifungal efficacy in A. fumigatus and C. albicans [164] . Various fungal pathogens are more susceptible for Ag/Au NPs and their particular antifungal mechanism are displayed in Table 5 .
Table 5.
Mechanism of antifungal action of Ag/Au NPs against target fungi.
| NPs type | Targeted fungi | Concentration of NPs | Mechanisms of antifungal action | Reference |
|---|---|---|---|---|
| Ag | Aspergillus niger, and Candida albicans | 25–50 µg/ ml | DNA replication, cellular proteins and enzyme essential for ATP production is inactivated of living cells. | [202] |
| Ag | C. albicans and A. fumigatus | 100 µg/ ml | Disruption of cell membrane and cell lysis | [86] |
| Ag | Candida albicans | 2 µg /ml | Disrupted the cell membrane and inhibited the normal budding process. | [200] |
| Ag | Sclerotinia homoeocarpa | 200 μg/ml | Oxidative damage. | [203] |
| Ag | C. albicans and A. fumigatus | 100 μg/ml | cell membrane disruption and cell lysis | [204] |
| Ag |
Saccharomyces boulardii and C. tropicals |
25 μg/mL | Damage to cell death. Cell wall disruption and osmotic imbalance. | [205] |
| Ag | Aspergillus niger | 200 μL | Pitting of the cell membranes and finally cell death. | [206] |
| Au | Aspergillus nigerandFusarium oxysporum | 100 μL | Inactivation of sulfhydryl groups in the fungal cell wall and disruption of membrane bound enzymes and lipids which causes cell lysis. Disruption of cell membrane and cell lysis | [201] |
| Au | C. albicans | >166 ppm | Interaction with cell surface. | [194] |
| Au | C. albicans and A. fumigatus | 100 µg/ ml | Disruption of cell membrane and cell lysis | [93] |
6.3. Antiviral potential of Ag NPs and Au NPs
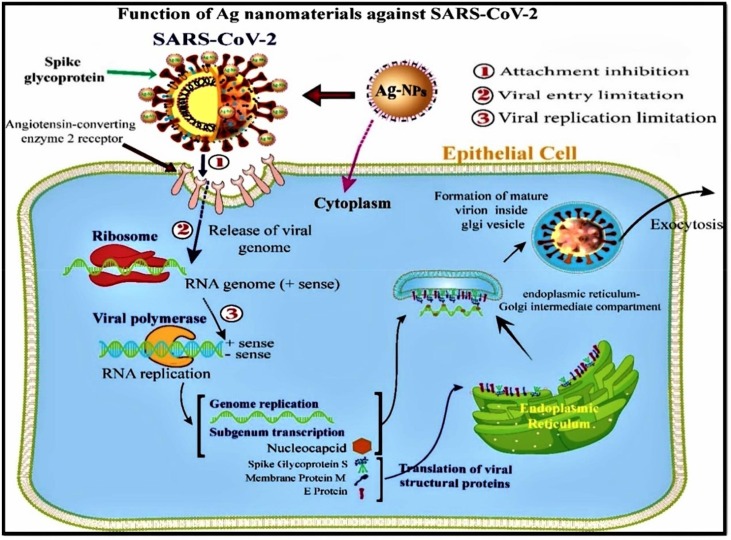
Viral infectious diseases like Human Immunodeficiency viruses (HIV) and influenza including Ebola [207], Zika [208] and Avian Influenza as well as corona virus (CoV) are common throughout the world. COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are responsible for huge mortality globally [8], [9], [10]. Fruitful remedy of viral infections are limited by enhanced drug resistance, mostly related to HIV [209] and influenza [210]. Viruses are rigidly intracellular pathogenic microbes composing of DNA or RNA as genetic factor and a protein coat (capsid). The genetic code for enzymes requires in replication and for many structural proteins which confers infection inducing virulence factor of viruses. All the DNA and RNA viruses enter into the host cell through a particular receptor on the cell membrane of host cells by connection glycoprotein implant in the viral envelope [211], [212]. The CoV enters the host cell membrane by binding on the specific receptor of the angiotensin-converting enzyme 2 (ACE2) protein site. Followed by entry into the host cell membrane, the RNA viruses undergo reverse transcription (viral mRNA synthesis) [36]. Utmost severe lung contagions were caused by CoV, such as SARS-Cov, MERS and SARS-CoV-2 [176]. Currently, appropriate, safe, biocompatible, and alternative antiviral materials to avert pandemic contagions is required. Generally, metal NPs possess remarkable antimicrobial and antiviral properties [55]. Therefore, it is crucial to highlight the significance of particular metal NPs to be used as antiviral and drug delivery agents.
Fabrication of metal nanomaterial drugs having various antiviral mechanisms viz., “inhibition of virus fusion with the host cell membrane, anatomization of cell surface receptors, inhibition of uncoating, inhibition of transcription of viral RNA or DNA, inhibition of enzymes involved in virus replication, inhibition of protease as well as neuraminidase production” as displayed in Fig. 9 [213]. The working of metal NPs and their particular antiviral mechanisms are shown in Table 6 .
Fig. 9.
Mechanisms of antiviral action of metal NPs against SARS-CoV-2 (Source. [213]).
Table 6.
Mechanism of antiviral action of Ag/Au NPs against target viruses.
| NPs type | Targeted virus | Concentration of NPs | Mechanisms of antiviral action | Reference |
|---|---|---|---|---|
| PVP-coated Ag | Human immunodeficiency virus type 1 (HIV-1) |
50 mM |
Interaction with gp120 | [214] |
| Ag | HIV-1 | 0.44 mg/mL | Interaction with gp120 | [215] |
| Ag | Herpes simplex virus type 1 (HSV-1) |
200 μg/ml |
Competition for the binding of the virus to the cell | [216] |
| Au | Herpes simplex virus type 1 (HSV-1) |
400 μg/ml |
Competition for the binding of the virus to the cell | [217] |
| Ag | Tacaribe virus (TCRV) |
10 or 50 μg/ ml |
Inactivation of virus particles prior to entry | [218] |
| Ag | Hepatitis B virus (HBV) |
5–50 μM |
Interaction with double-stranded DNA and/or binding with viral particles | [219] |
| Ag | HIV-1 | 100 μg/ ml | surface transformation | [220] |
| Ag | Corona viruses |
10 and 100 ppm |
viral entry limitation, attachment inhibition, and viral replication limitation | [213] |
| Ag | SARS-CoV-2 |
2 ppm |
inhibited viral entry step via disrupting viral integrity | [221] |
| Ag | monkeypox virus and Respiratory syncytial virus | 0.3 & 0.5 ml | inhibiting the viral adherence to host cell | [222] |
6.3.1. Therapeutic approaches for COVID-19
Nowadays, the epidemic of the tremendous contagion novel COVID-19 associated pneumonia is a worldwide pandemic. Various therapeutic approaches for COVID-19 treatment viz., renal replacement/transplant, rescue therapy, immunotherapy, mechanical ventilation, oxygen therapy, antiviral therapy, blood purification and circulatory support and plasma therapy were reported by sheanhan et al., (2012), which was not only to treat the symptoms of COVID-19, and also capability to reduce the viral load [223]. Most of the anti-viral drugs can be used for preliminary treatment of COVID-19 infections likewise Arbidol, Chloroquine phosphate, Ribavirin, Lopinavir/ritonavir, IFN-α, remdesiver and hydroxychloroquine along with azithromycin [224], [225], [226]. Due to the global challenge of finding a long-term solution to the issue, various vaccinations, medications, and immunotherapeutic candidates started entering clinical trials concurrently. As per report of WHO and COVID-19 vaccine tracker, 74 vaccine are being completed or in-completed globally. Amid these limited number of vaccines have done 3rd phase trials successfully and now those are in use and the reports were displayed in Table 7 . Additionally, the only option to discover efficient and secure COVID-19 remedies is through simultaneous and rapid ambulatory and medical care combined with randomized clinical trials [227], [228].
Table 7.
List of currently available COVID-19 vaccines and their efficacy.
| S.No | Trail ID & Vaccine name | Developed countries | Efficiency (%) |
Ag Type | Study evolution | Reference |
|---|---|---|---|---|---|---|
| 1 | CanSino:Convidecia (Ad5-nCoV) NCT04526990 |
China | 65 | Viral vector | Tolerability and immunogenicity from different strain |
[229] |
| 2 |
Sinopharm [Vero Cell] BBIBP -CorV NCT04560881 |
China | 79.3 | Inactivated | Variant neutralization | [230] |
| 3 | Pfizer-BioNTech (BNT162b2) NCT04368728 |
USA | 95 | mRNA | Tolerability and immunogenicity from different strain |
[231] |
| 4 | EpiVacCorona NCT04527575 |
Russia | 90 | Peptide | Increased immune response |
[232] |
| 5 | Sinovac-Corona Vac NCT04456595 |
China | 50.4 | Inactivated | Effective against UK, South African variants |
[233] |
| 6 | Janssen (Johnson & Johnson) Ad26. CoV2.S NCT04505722 |
USA | 66 | Viral vector | Effective against severe condition |
[234] |
| 7 | Sputnik V NCT04530396 |
Russian | 91 | Viral vector | Immune system |
[235] |
| 8 | CovishieldAZD1222 NCT04516746 |
UK | 65 | Viral vector | Neutralization against B.1.1.7 variant |
[236] |
| 9 | Covaxin (BBV152) NCT04471519 |
India | 50 | Inactivated | Neutralization against UK variant strain |
[237] |
| 10 | Moderna (mRNA-1273) NCT04470427 |
USA | 94 | mRNA | Neutralization against UK variant strain B.1.351varient |
[238] |
In the regards, the metal NPs in relation to CoV, is that they are capable to suppress viral binding to the host cell- specific surface receptor [239]. Metal NPs perform as superior antiviral drug transporter because of their large surface-to-volume ratios, tiny, modifiable surfaces, and controllable hydrophobicity, which enable the drug-loaded NPs to target particular biological sites [240]. Dendrimers, quantum dot and micelles are the most often used NPs against viruses [241]. In recent times, many researchers dealing with metal NPs against COVID-19, but they have more limitations like NPs effective against CoV was 20 nm size with 50–70 ppm dose concentration which may cause cytotoxicity [221]. Bacteriocin obstruct with viruses entry of host cell by blocking the surface receptor and simultaneously decrease the virus load into the host cells [242]. Bacteriocin have a potential anti-viral effects against SARS-CoV-2 infections, and not only to treat the symptoms of SARS-CoV-2, while act as an immunomodulatory for humans. Balemh et al., (2021) was also reported that bacteriocin have promising anti-viral effects against COVID-19 and prophylaxis of SARS-CoV-2 [243].
Kim and colleagues found that the affinity of Au NPs for the thiolated domain of hemagglutinin, a highly conserved fusion protein of influenza virus, is a factor in the antiviral action of Au NPs. The findings demonstrate that after receiving Au NPs therapy, the vitality of virus-infected MDCK cells improved to 96.8 % compared to 33.9 % in the control group. The greater affinity of AuNPs for the disulfide bond with HA, which inhibits the fusion of the viral membrane and is supported by real-time RT-PCR, is thought to be the probable mechanism of the anti-influenza effect of these particles. Because the SARS-CoV-2 virus also includes HA, like the influenza virus, these careful arguments lead researchers to consider using AuNPs as a supplement to the Covid-19 therapy [244], [245]. Alghrair et al. examine the anti-influenza effect of Flu-Pep modified Ag and Au NPs in canine MDCK cells. The results suggest that both functionalized nano-systems have high antiviral efficacy, with an IC50 value of 2.1 nM, although they are lower effective than Flu-Pep itself, which has an IC50 value of 140 pM [246]. While the immunomodulatory action of AgNPs is connected to the down-regulation of pro-inflammatory cytokines including IL-6, IFN, and CCL5, the virucidal efficacy of produced AgNPs may be attributed to their capacity to bind with surface glycoprotein of virus over respiratory epithelium. The authors also stated that A549 epithelial cells are not cytotoxic to when treated to AgNPs at a dosage of 50 µg/ml. These findings inspire researchers to employ AgNPs as a supplement to COVID-19 therapy [247]. Bacteriocin like pediocin PA-1 significantly act against delta variant (L452R-T478K double mutant) SARS-CoV-2 and to enhance the immune systems [248]. Based on the above facts, novel development of bacteriocin composite with metal NPs as a less toxic compared to other nanomaterials, long-term stability and high efficiency with low volume of drug concentration is developed.
6.4. Cytotoxicity effects of Ag NPs and Au NPs
Human revelation to metal NPs is unavoidable as they become generally used for antimicrobial purposes. This has led to cytotoxicity in humans (Table 8 ). Because these metal NPs can interact with cells, it is essential to confirm that these activities do not generate any harmful impact on the human body. The harmful impact of metal-based nanoparticles varied with various kinds of nanomaterials. For metal NPs, intracellular aggregate can cause less cytotoxicity to humans because of their small size. Ag NPs can induce dose-dependent toxicity in mammalian cells, as well as oxidative stress and genetic material damage, finally occur in cell death [86], [165].
Table 8.
Cytotoxicity of Ag/Au NPs on human cell lines.
| NPs Type | Cell line | Cytotoxicity effects | Reference |
|---|---|---|---|
| Ag | HeLa and U937 | Induce cytotoxicity in both HeLa and U937 | [252] |
| Ag | HepG2 cell | Induce size-dependent toxicity through autophagy of lysosomal system and inflammasome activation | [254] |
| Ag, CuO, ZnO | Mammalian cell | Order of cytotoxicity on mammalian cell. Ag > CuO > ZnO | [255] |
| Ag | MCF-7 (Human breast cancer cell | IC50 value of 42.19 mg/mL along with cell shrinkage, blebbing and restricted cell spreading patterns compared to control cells | [256] |
| Ag | NHDF cell | Less toxicity while using 100 µg/mL | [86] |
| Ag & Au | Cancerous cells (MCF-7 and RAW 264.7) | ROS generation and induce cytotoxicity | [257] |
| Au | LoVo cancer cell | Endocytosis | [258] |
| Au | MCF-7 human breast cancer cell line and NCI-N87 human stomach cancer cell line | Induce size-dependent toxicity and cell membrane disturb and finally cell death | [259] |
| Au | MCF-7 cell line | ROS generation | [260] |
| Chitosan coated Ag/ZnO NPs | RAW 264.7 murine macrophages | Inhibition of biofilm through ROS generation | [250] |
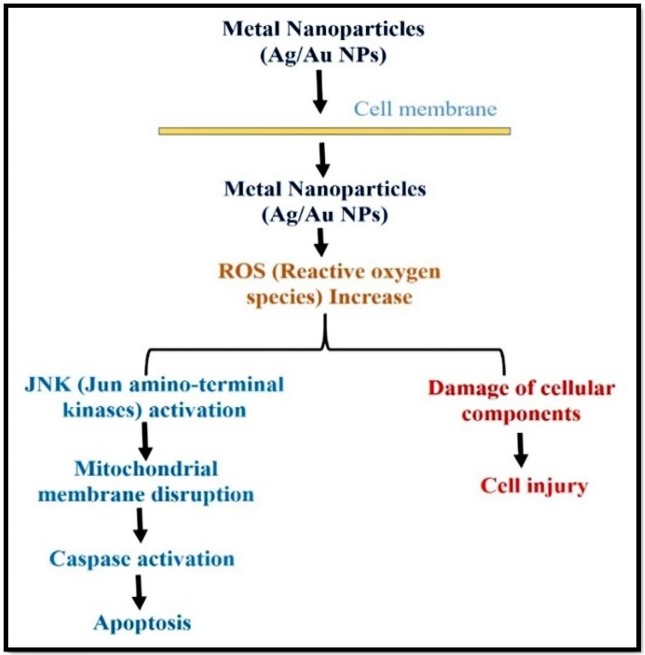
The interaction of metal NPs with cell membrane proteins stimulates signalling pathways that generate ROS, which in turn damages proteins and genetic material due to the strong link between gold and sulphur and ultimately induces death and inhibits cell growth [249]. Thus, metal NPs can cause apoptosis via mitochondria and caspase dependent pathway mediated by Jun amino-terminal kinase (JNK) (Fig. 10 ). Most of the studies have pointed to the forgoing research in cytotoxicological pathways of metal NPs.
Fig. 10.
Antimicrobial mechanism of metal NPs inducing cell death.
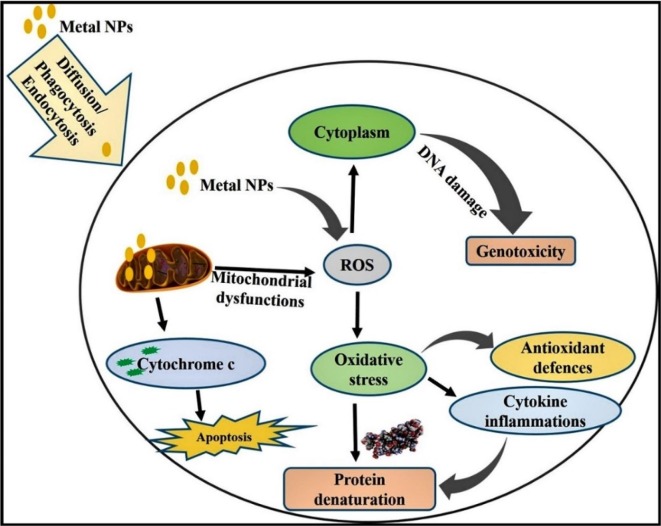
Two main mechanisms underlying the cytotoxicity of metal NPs have been reported: i) production of ROS and ii) causing of apoptosis. In chitosan coated Ag NPs, nanocomposite with zinc oxide NPs are less toxic and have immense potential for cancerous cells [250]. Nano size zerovalent ion of zinc oxide causes toxicity via mixture of oxidative stress to disruption of cell membrane, hypoxia, and chlorosis [251]. Metal NPs can enter into the cell through the action of diffusion, endocytosis or phagocytosis. Inside the cell, metal NPs itself or ionized (Ag+ or Au3+) produce ROS and induce oxidative stress (Fig. 11 ). Over the generation of ROS, it can denature various antiapoptotic proteins and start proapoptotic proteins expression. Thus, expression of apoptotic proteins starts apoptosis signalling pathway (p53, AKT, MAPK activation to inhibit ROS production by metal NPs).
Fig. 11.
Mechanisms of cytotoxicity of metal NPs in different cell lines.
In general, very tiny metal NPs are more cytotoxic than the highlighted entities, with Ag NPs below 40 nm, commonly induce abundant toxicity [252]. The cytotoxicity of metal-based nanomaterial was correlated with their clinical applications. Various efforts are devoted to decrease the cytotoxicity of metal NPs. Researchers have suggested nanomaterial immobilization in polymeric matrices such as polyethylene glycol, chitosan, silica, proteins, bacteriocins, titania, alginate beads, and dextran to reduce the cytotoxicity of metal NPs [165], [253]. Another approach for decreasing the toxicity of metal NPs is to use green chemistry fabrication technique. For example, mushroom-mediated fabrication of metal NPs formed fewer toxic by-products that demonstrate antimicrobial properties on Gram-positive and Gram-negative bacteria as well as fungi, but with little cytotoxicity on mammalian cells.
6.5. Development process of adverse outcome pathway (AOP) of relevance to metal NPs composite bacteriocin
In the last decades, the toxicity pathways were recommended by the US “Environmental Protection Agency” and the “National Research Council” as the favored toxicity analyzing approach [261]. The novel research review evaluates the bioanalytical examination approach and techniques for the safety assessment of metal NPs composite peptides. Thus, bioanalysis comes from the findings of the bio-responses of metal NPs at the cell, tissue, and organ levels after exposure. Disruption of cellular properties committed to the toxicity outcomes in biological systems (i.e., fibrosis, DNA damage, necrosis, inflammation, apoptosis, carcinogenesis, and hypertrophy). On keep viewing the mind, toxicity analysis on in vitro cellular models focusing on adverse outcome pathways (AOPs) is the favored approach. Various researchers on the effective toxicity of metal NPs in different animal systems, such as Daphnia, zebrafish, mice, guinea pigs, human skin, etc., have been documented (Table 8) [262], [263]. Chemical initiators (Metal NPs-Bacterocin nanocomposite) are used in the development of the AOP process and will be linked to proteins, receptors, and DNA to change the signalling pathway in a cascade. The molecular initiating event (MIE) is the first interaction with the biological system; it then spurs the formation of key events (KEs), which ultimately result in apical unfavourable effects (AO). Key event relationships (KER) are used to describe the connection between the two major events, and Fig. 12 illustrates this. Nano-toxicological research requires filling the gap of combined toxicity in the complex system. Fabricated metal NPs must have a maximum level of biocompatibility compared with other agents and have low adverse effects on human health (Table 9 ). It is an important to understand the biological interactions of combined metal NPs with organisms and design safe nanoproducts for human use. Based on the literature survey, in our knowledge, metal-based NPs composite bacteriocin of in-vitro studies, predictive computational tools, and in-vivo models is efficacious and potentially be used to explore environmental and human hazard assessment in the future.
Fig. 12.
A schematic representation of the adverse outcome pathway (AOP) framework. The risk assessment for developing occupational exposure limits for metal NPs- bacteriocin nanocomposite.
Table 9.
Adverse outcome pathway of metal NPs and their toxicity risk assessment.
| NPs type | Test type models | Adverse Outcome | Report of the study | Reference |
|---|---|---|---|---|
| Ag, Zn O, iron oxide, and TiO2 | In-vitro | Compromised phagocytosis | NPs interact with membrane/ biomolecules | [264] |
| Ag, CeO2, and ZnO | In-vitro | Progression of cancer and death | ROS formation | [265] |
| PVP capped Ag NPs | In-vitro & In-vivo | Brain and liver damage | Dopamine receptor antagonism, ROS formation | [266] |
| CeO2, ZnO, TiO2, Ag and silica NPs | In-vitro | Cell death | Lysosomal acidification | [267] |
| Au, Ag, ZnO, CNTs and TiO2 | In-vitro | Impaired cytoskeleton | DNA methylation | [268] |
| Ag NPs (PVP Coated and uncoated) | In-vitro | Increased mortality and decreased reproduction | apoptosis stimulation, ROS formation and DNA damage | [269] |
| Ag NPs, ZnO, and TiO2, | In-vitro | Dysregulation of Immune system | Activation of intracellular pattern recognition receptors | [270] |
| Ag NPs & Selenium NPs | In-vitro & In-vivo | Destroy tumor cell | micronuclei formation & chromosomal aberrations, and DNA damage | [271] |
| Inorganic nanomaterial | In-vivo | Proinflammatory cytokines | ROS and angiogenesis | [272] |
| Metal NPs | In-vitro & In-vivo | Wound healing | angiogenesis pathway, and ATP synthase enzyme inhibiting | [273] |
| Au NPs | In-vivo | Eye defect | Oxidative stress | [262] |
| Au/ manganese dioxide nanocomposite | In-vitro & In-vivo | Destroy malignant cell and melanoma tumor | ROS geenration and oxidative stress | [274] |
| Metal NPs | In-vivo | Liver damage and lung damage | Cell damage, ROS formed | [275] |
| Ag/80S bioactive nanocomposites | In-vitro & In-vivo | Cell death | ROS production, and Cell membrane damage | [276] |
| Chitosan- Ag NPs composite | In-vitro & In-vivo | Cell lysis and promote dissemination | Cell membrane damage, ROS generation | [277] |
| Au NPs/ gelation fibers nanocomposite | In-vitro & In-vivo | Cell proliferation | NADPH oxidase generating a maximum level of ROS in the lysosome | [278] |
7. Bionanocomposites strategies for metal NPs with biomolecules
The synthesis of nanomaterials with well-defined surface chemistry, controlled size/shape and unique optoelectronic properties is the basis of their wide range of applications [279]. The large surface to volume ratio and high reactivity with biological systems provide a platform for surface modifications. The modification of NPs provides enhancement in the interaction between NPs and biomolecules. The advancements in the field of nanotechnology have opened a new era of environment friendly and bio-inspired synthesis of NPs. The surface modified NPs plays a key role in the synthesis of non-toxic and biocompatible formulations. They can be conjugated to variety of antibiotic drugs, biomolecules such as peptides, proteins, antibodies, nucleotides and enzymes. These nanocomposite based products can be used in the treatment of various bacterial infections by enhancing the therapeutic efficacy of various antibiotic drugs or biomolecules [280].
In general, the nanocomposite of biomolecules on the surface of metal NPs can be achieved by using four various strategies: (i) Direct attachment, (ii) electrostatic interaction, (iii) covalent attachment, and (iv) encapsulation [281] (Fig. 13 ).
Fig. 13.
Methods of formation of metal NPs and bacteriocin nanocomposite.
Direct attachment approaches involves the conjugation of biomolecules to NPs that can attach directly through the high affinity interactions depending on the surface exposure of the NPs to the surrounding environment as shown in Fig. 13 a [282]. The thiol groups are the most commonly used for this type of interactions, especially biomolecules have thiol groups with directly attachment on Au and Ag NPs. Furthermore, the high affinity strength of the solution, which ultimately prevents NPs aggregation owing to the screening of direct attachment between Au-TMA and the oligo anions. Electrostatic interactions have a vital significance in the formation of various self-assembled nanostructures. Although, a positively/negatively charged metal NPs and oppositely charged biomolecules by simply mixing the solution of the NPs and biomolecules [283] . In this method, it is assumed that all the biomolecules would fit on the surface of NPs (Fig. 13 b). However, it is difficult to control the final orientation of the bio-conjugate because of the possibility of non-specific interactions. This method has been applied successfully to conjugate citrate-stabilized Au NPs to variety of positively charged biomolecules such as peptides, proteins, polymers etc., [284]. They provide stable and robust interaction among various enzymes, peptides, and proteins. Covalent attachment is the one of the most commonly employed method for the coupling of biomolecules to the metal NPs. In this method primary amines, thiols and carboxyl groups have been used for the conjugation of NPs as these groups are generally present on biomolecules (Fig. 13 c) [282]. This method includes 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC. HCl) and N-hydroxysuccinimide (NHS) as coupling reagents which catalyze the formation of amide bonds [285]. The potential advantage of this method is their bio-orthogonality, as the coupling of two groups takes place in a specific manner without any manipulation of the other groups. Covalent approach has some disadvantages, such as poor scoring functions, and limitations in speed and accuracy. Encapsulation interaction is generally used to encapsulate a variety of antibiotics, targeted molecules etc. on NPs for drug delivery [286]. It includes the encapsulation of biomolecules using organic NPs such as liposomes polymeric NPs, solid lipid NPs etc. Fig. 13 d revealed that metal precursor strongly encapsulates with biomolecules with the help of ligands. Advantages of encapsulation approach such as protection of API from degradation, targeted drug delivery with surface coating or conjugation, PEGylation for extended circulation time, modification to surface charge can promote cell entry, surface function for cell entry and fluorescent labelling for imaging. Disadvantages of encapsulation approach such as control of the particle size is difficult, special handling and storage conditions can be required, moderate yields for small batches, and can degraded highly temperature sensitive compounds.
8. Synergistic effects of metal NPs with bacteriocin for increased antimicrobial efficacy
The integration of nanotechnology and biotechnology may be considered as the most recent example of this hurdle technology. One of the possible methods to target MDR pathogens is a combination of one or more antimicrobial agents. In combinatorial studies, two or more compounds target the bacterial cell at the same time with their own mode of action which shows the enhanced effect on the antimicrobial potential than the individual compounds. The reason for the enhancement could be the multipronged strategy to target the bacterial cell growth. The combinatorial studies can result in; synergistic, antagonism and indifferent or zero interaction [287]. In the synergistic activity of oil compounds with antibiotics exhibited an enhanced activity at lower concentration and lower down the cytotoxicity against cancerous cells as compared to the individual compound. Currently, several research suggest that metal NPs can be combined with biomolecules to increase their effectiveness.
Ag NPs with peptide molecules has been emerging as a promising candidate for biological applications due to their bioavailability, higher specificity and lower toxicity [288], [289]. The antimicrobial activity of individual components was enhanced by the cationic peptide Odorranain-A-OA1 (OA1) conjugated Ag NPs, which had no significant IC50 value compared to Ag NPs, which had an IC50 value of 96 μg mL−1, implying non-cytotoxicity of the conjugate against a mammalian cell line [290]. Recently, Ag NPs were conjugated with an antimicrobial peptide nisin [291]. The Ag peptide conjugate showed that the antimicrobial activity of peptide nisin was enhanced after bioconjugation with Ag NPs. Bacteriocin capped Ag NPs have a broad spectrum of antimicrobial property against food spoiling bacteria [292]. In another study, the antibacterial effects of peptide capped Ag NPs on gram-negative bacteria was studied, where, casein peptide stabilized Ag NPs strongly inhibited the biofilm formation of P. aeruginosa, E. coli, and Serratia proteamaculans at concentration of 10 μg mL−1, 4–5 μg mL−1 and 10–20 μg mL−1, respectively [293].
In recent years, peptide functionalized Au NPs have been used in wide number of therapeutic applications. Recently, glucagon like peptide-1 (GLP-1) was modified at the C-terminal by the addition of cysteine to promote the conjugation with Au NPs [294]. The intraperitoneal delivery of the GLP-1 gold conjugate to normoglycemic rats showed a decrease in the level of blood glucose similar to that of a native GLP-1 and demonstrated the stability of the conjugate which maintains the activity of this incretin. In another report, Au NPs were functionalized with therapeutic peptide (NH2-TSFAEYWNLLSP-NH2) and a targeted peptide (CRGDK) to achieve the selective binding with NRP-1 receptor over expressed on the cancer cells which regulates the process of membrane receptor mediated internalization. It has been found that CRGDK peptide facilitates the intracellular uptake of AuNPs which exhibited stronger in-vitro anti-cancer activity compared to other conjugates due to strong binding interaction between peptide CRGDK and targeted Nrp-1 receptor [295]. Peng et al. (2016) reported that the integration of antimicrobial peptides with Au NPs had great transfection efficacy and synergetic effects on human pathogens [296]. Bacteriocins produced by
L. plantarum ATM11 and commercial nisin were conjugated with Au NPs having enhance antimicrobial potential against food spoilage microorganisms [297]. The disadvantages of using single drug such as high dose, toxicity, alter cellular metabolisms, and generation of drug resistance with a minimum period of time. The interaction between the NPs and bacteriocins thus holds the potential to lead to a reduction in the requirement of high bacteriocin dosage, and an extension in the shelf life of food. Nano-encapsulations of bacteriocins when used in food preservation, protect them from degradation by gastrointestinal enzymes, and can also increase their commercial yield and stability. However, the advantages of combination of bacteriocins with NPs is more effective than bacteriocins alone owing to their increased antibacterial efficacy with possibly lower doses, synergistic effects, decrease the probability of resistance evaluation, broad spectrum of action, and multiple targets. Hence, metallic NPs can be used to conjugate bacteriocins to increase the antimicrobial spectrum of the former, which in turn can prove to be an efficient weapon in the fight against MDR pathogens.
9. Future prospects
Despite the advantages that NPs offer, such as a broad therapeutic index, controlled drug release, less prone to bacterial resistance, and fewer side effects than chemical antimicrobials [298], to treat infections caused by the ESKAPE pathogens, there are still challenges remaining to be tackled such as improvement of physicochemical properties, better pharmacokinetic profiles, and comprehensive studies on long-term exposure to humans. Metal NPs-based compounds (alone or in combination with other antimicrobial agents) provide promising alternatives to combat the development of antibacterial resistance (Shaikh et al., 2019). The strategies to reduce antibiotic resistance include the limited use of antibiotics and the application of more effective antimicrobial therapies. Because the time of exposure to antibiotics correlates with the development of resistance, it is necessary to use drugs with a broad spectrum of action and pharmacokinetic properties that facilitate their rapid access to the target site. However, most of the available treatments do not have all these characteristics, so an alternative option is the use of combination therapies, which can lead to a synergistic and more effective response. The combination of drugs leads to a considerably more potent effect, compared to the individual drug. Therefore, it is imperative to develop a comprehensive understanding of the mechanisms of action responsible for the bactericidal properties as well as the identification of the most promising antimicrobial agents for future clinical translation [299], [300], [301]. For an example, metal NPs will made up wound-dressing materials, catheters, bone cement, cardiovascular implants, and dental restorative materials that are available for clinical uses. Nevertheless, antimicrobial metal NPs are more effective for fighting human contagion diseases involving the entire body and its associated organs were displayed in Fig. 14 . Ag NPs is a well-known antimicrobial drugs which has a wealth of experience of topical use versus domestic contagion [302]. Still now, there is a need for fabrication of more effective drugs and vaccines for treating pandemic contagion diseases [303] or as antimicrobial drug delivery systems [304]. However, there is evidence for many clinical experiments on metal NPs based antimicrobials materials.
Fig. 14.
Antimicrobial efficacy of nanomaterials in fighting infections in various parts of the human body ().
Source: [305]
Apart from the approach stated above, mushroom as a bio factory, which have wide number of mycomolecules (alkaloids, proteins, polysaccharides, terpenoids, aminoacids, vitamins and polyphenols) could have wide range of food and biomedical applications. Based on the above facts mushroom could be uses in a green chemistry synthesis and is more helpful for increasing biocompatible capabilities of metal NPs. The use of metal NPs is not only narrow to the biomedical field. It can be generally applicable in water treatment, textiles, food packaging, cosmetics industry, and agriculture system (nano fertilizer and nano pesticides). The mycosynthesized metal NPs will be a novel field and finds the right balance between the production cost, scalability, and applicability. Hence, further studies will be required to focus on economical ways of mycosynthesized metal NPs fabricated, producing them readily available for all kinds of future prospects. Metal NPs and nanocomposites with biomolecules play a crucial role in the production of non-toxic and biocompatible formulations, based on the aforementioned valuable discussion points. However, a novel system with distinctive antimicrobial potential of Ag and Au NPs bionanocomposite with bacteriocin obtain many benefits without the use of hazardous chemicals. Bio-nanotechnology-based monoclonal antibodies and vaccines precisely deliver the active agents to targeted tissues and provide very rapid detection of these viruses. Green synthesized nanomaterials could also play a crucial role in developing a vaccine. Nowadays, vaccines are delivered through slow-release implants, single-dose followed by the second dose, and plant viral nanoparticles for drug delivery. That day seems far off in the race to develop a novel SARS CoV-2 vaccine that works, but it remains to be seen whether SARS CoV-2 will be more like flu, requiring an unknown shot every year. The COVID-19 focus is on the solution, and nanomedicine is the part of the solution that enables delivery of bacteriocin composite metal NPs. This paradigm shift from “what are the unknown risks of nanomedicines?” to “what clinical solutions can we solve with nanomedicine” has opened the door to translational nanomedicine applications beyond SARS-CoV-2 vaccines. Finally, the biggest challenge will always remain the possibility of these nanocomposite metal NPs as a potential therapeutic agent for SARS CoV-2. The broad range of antimicrobial properties of metal NPs against MDR pathogens (bacteria, fungi, and viruses), as well as the sustainability of mass manufacturing. Considerations of the bacteriocin composite metal NPs drug delivery mechanisms and effective in vivo safety should be paved to ensure enhanced treatment of COVID-19 and MDR infectious diseases with maximum biosafety.
10. Conclusion
Microbial resistance has emerged as a serious threat to public health due to indiscriminate and abusive use of antibiotics thereby causing a need to search for novel antibiotics. In recent years, metal NPs and nanocomposites with antimicrobial effective biomolecules appear as a promising candidate for various biological applications. However, most of the work in this direction has been performed with antimicrobial macromolecules. Keeping the focus on cost effective synthesis for design of novel nano-antibiotics, mushroom has been found as a suitable green source for nanomaterials synthesis with biomedical applications due their non-toxic, and biocompatible nature. The size, shape, and morphology of metal NPs were well controlled by mushroom-based synthesis. This review focused on evaluating the possible mechanisms of antibacterial, antifungal, antiviral and cytotoxicity of metal NPs. Bacteriocin as a nature source of antimicrobial peptides from probiotic bacteria has broad spectrum of antimicrobial effects on MDR pathogens and COVID-19. Synergistic effects of metal NPs nanocomposite with various biomolecules to enhance the antimicrobial effects and non-toxic property are reported. Based on the above facts, we focus on metal NPs nanocomposite with bacteriocin as a perfect augmentation to enhance their antimicrobial effects and increasing their long-term stability. We believe that bacteriocin nanocomposite metal NPs would become a more effective approach to combat the ongoing global health emergency and future pandemics.
CRediT authorship contribution statement
Moovendran Srinivash: Writing – original draft. Raman Krishnamoorthi: Conceptualization, Writing - original draft, Writing - review & editing. Pambayan Ulagan Mahalingam: Validation, Supervision. Balasubramanian Malaikozhundan: Writing – review & editing. Subramanian Bharathakumar: Resources, Visualization. Krishnamoorthy Gurushankar: Formal analysis, Data curation. Kasi Karuppa Samy: Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We express our sincere gratitude to the Head of the Department of Biology, The Gandhigram Rural Institute – (Deemed to be University), Gandhigram, Dindigul, for the research support and encouragement.
Biographies

Moovendran Srinivash, pursuing Ph.D Researcher in the Gandhigram Rural Institute (Deemed to be University), Dindigul, Tamil Nadu, India. He has published 3 research articles in reputed journals and participated various international conferences and workshop. He has total citations: 11, and h-index: 2, Research interests are microbiology, probiotics, Exopolysaccharide, and cancer biology. E-mail: nivashsri896@@gmail.com

Raman Krishnamoorthi is a Spices Research Trainee at Indian Cardamom Research Institute, Spices Board (Govt. of. India), Kerala, India. He received his Ph.D in Microbiology January-2023 from The Gandhigram Rural Institute (Deemed to be University), Dindigul, Tamil Nadu, India. He had research intern to the Molecular Microbiology Division, ICMR-CRME, India. His research work focused on microbiology, bionanotechnology, Nanomaterials, wound dressing materials, cancer biology, food and medical microbiology. He has authored 11 peer reviewed publications with a total citations of over 68, with h-index of 5. E-mail: krishmicro3@gmail.com

Pambayan Ulagan Mahalingam received his Ph. D in Microbiology from The Gandhigram Rural Institute (Deemed to be University), Dindigul, Tamil Nadu, and India. He have 25 years research and Teaching experience. He has passed SELT (1997 & 1998) examinations. Currently, he is Professor at Department of Biology, The Gandhigram Rural Institute (Deemed to be University), India. He received research project funding from DBT . He had research exposure visits to various countries such as Malaysia, and Srilanka. He guided 9 Ph. D candidates and 8 candidate in pipeline. His research work focused on Microbial consortium technology, Bionanotechnology, Biofuel technology, and Bio fertilizer technology. He has published more than 70 peer reviewed publications and numerous book chapters with a total citations of over 285, with h-index of 9.

Balasubramanian Malaikozhundan done his Ph. D in Zoology at Alagappa University, India. He have 16 years research and Teaching experience. He has passed SET examinations in 2008. Currently, he is Guest Lecture at Department of Biology, The Gandhigram Rural Institute (Deemed to be University), India. He received research project funding from DST-SERB, and UGC. He had research exposure visits to Bangkok, Thailand. He is a potential reviewer on various standard scientific journals. He received best researcher and best paper awards. He guided 25 M.Sc candidates and 6 candidate in pipeline. His research work focused on Agricultural entomology, Insect Biocontrol, Nanopesticides, Nanotherapeutics, and Aquaculture. He has published more than 54 peer reviewed publications and numerous book chapters with a total citations of over 1998, h-index of 25, with i10-index of 35. E-mail: kozhundan.malai@gmail.com

Balasubramanian Bharathakumar is a highly accomplished and talented individual with an impressive track record of success in the field of biotechnology. . He completed his B.Tech and M.Tech in Biotechnology at PSR Engineering College, India. He has significant research experience and has made significant contributions to the field in various organization ICMR-CRME, Indian Institute of Information Technology Materials and Device, CSIR-Institute of Microbial Technology, CSIR-Central Electrochemical Research Institute. He received best researcher and best paper awards. Moreover, his current role as CEO of Ereky Labs Private Limited and his involvement in various other organizations indicate his leadership qualities and his commitment to innovation and environmental technology.

Krishnamoorthy Gurushankar, (b. 1987) done his Ph.D at Annamalai University, Tamilnadu in 2015, majoring in Ph.D. Currently he works as the Senior Researcher, Laboratory of Computational Modeling of Drugs, South Ural State University, Chelyabinsk, Russia. He has published more than 30 research articles in reputed journals including Thomson Reuters (SCI & Web of Science) and conferences and it’s also available online. He has total citations: 361, h-index: 11, i-10 index: 12. Research interests are molecular modeling of phytopreparations, nanotechnology and DFT study for drug design. E-mail: gurushankar01051987@gmail.com

K. Dhanapal done his Ph. D in Botany & Plant Pathology at Bharathiyar University, India. He have 33 years of research experience. Currently, he is worked as a Scientist- C and Director in-charge of the Indian Cardamom Research Institute, Spices Board (Govt. of. India), Kerala, India. He received research project funding from ICRI and various external projects. He had research exposure visits to Germany, Columbia, South Africa, and Nepal. He received best researcher and Dr. Ambedkar fellowship award. He guided 10 M.Sc candidates and 4 candidate in pipeline. His research work focused on Agriculture, plant pathology, Spices, Insect Biocontrol, Biocontrol agents, and Cardamom. He has published more than 55 peer reviewed publications and numerous book chapters.

Kasi Karuppa Samy, done his Ph. D in Botany at The Gandhigram Rural Institute,Tamil Nadu in 2023. Currently he works as the Spices Research Trainee at Indian Cardamom Research Board, Spices Board of India. He has published 8 research articles in reputed journals including Acta Ecologica Sinica, Asia Pacific Biodiversity, Phytotaxa. His Research area of interests are Plant Reproductive Biology, Biodiversity Conservation, Plant Pollinators interaction, Plant frugivore interaction, Ethnobotany, phytomedicine and Natural Dye extraction from plants.

Anand Babu Perumal is a Post-Doctoral Research Fellow at the Zhejiang University, College of Biosystems Engineering and Food Science. He received his PhD in 2018 from SRM University, Chennai, India. He worked in DST-NRF Indo-South Africa Bilateral Project during his doctoral research. He had research exchange visits to the Department of Chemical, Metallurgical and Materials Engineering, Polymer Division, Tshwane University of Technology, South Africa. His research work focused on packaging and postharvest preservation of fresh produce, Raman Spectroscopy, Agricultural Biotechnology, Microbiology, Biochemistry, Nanotechnology, Biocomposite Film, Nanomaterials. He has authored more than 44 peer reviewed publications and numerous book chapters with a total citations of over 770, with h-index of 15. More information can be found at: https://person.zju.edu.cn/en/anandbabu
Data availability
Data will be made available on request.
References
- 1.Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century, Perspectives. Med. Chem. 2014:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies J. Where have all the raises gone?, Canadian Journal of Infectious Diseases and Medical. Microbiology. 2006;17:287–290. doi: 10.1155/2006/707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alavi M., Rai M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. Anti Infect. Ther. 2019;17:419–428. doi: 10.1080/14787210.2019.1614914. [DOI] [PubMed] [Google Scholar]
- 4.Nepal K., Pant N.D., Neupane B., Belbase A., Baidhya R., Shrestha R.K., Lekhak B., Bhatta D.R., Jha B. Extended spectrum beta-lactamase and metallo beta-lactamase production among Escherichia coli and Klebsiella pneumoniae isolated from different clinical samples in a tertiary care hospital in Kathmandu, Nepal. Ann. Clin. Microbiol. Antimicrob. 2017;16:1–7. doi: 10.1186/s12941-017-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford P.A. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saravanan M., Ramachandran B., Barabadi H. The prevalence and drug resistance pattern of extended spectrum β–lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb. Pathog. 2018;114:180–192. doi: 10.1016/j.micpath.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Alves P.M., Al-Badi E., Withycombe C., Jones P.M., Purdy K.J., Maddocks S.E. Interaction between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathogens Disease. 2018;76 doi: 10.1093/femspd/fty003. [DOI] [PubMed] [Google Scholar]
- 8.Baharoon S., Memish Z.A. MERS-CoV as an emerging respiratory illness: A review of prevention methods. Travel Med. Infect. Dis. 2019;32 doi: 10.1016/j.tmaid.2019.101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aydemir D., Ulusu N.N. Correspondence: Angiotensin-converting enzyme 2 coated nanoparticles containing respiratory masks, chewing gums and nasal filters may be used for protection against COVID-19 infection. Travel Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of Co-infection between SARS-CoV-2 and Other Respiratory Pathogens. JAMA – J. Am Med. Assoc. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kailasa S.K., Mehta V.N., Koduru J.R., Basu H., Singhal R.K., Murthy Z.V.P., Park T.J. An overview of molecular biology and nanotechnology based analytical methods for the detection of SARS-CoV-2: Promising biotools for the rapid diagnosis of COVID-19. Analyst. 2021;146:1489–1513. doi: 10.1039/d0an01528h. [DOI] [PubMed] [Google Scholar]
- 12.Ramakrishnan S.G., Robert B., Salim A., Ananthan P., Sivaramakrishnan M., Subramaniam S., Natesan S., Suresh R., Rajeshkumar G., Maran J.P., Al-Dhabi N.A., Karuppiah P., M. Valan Arasu, Nanotechnology based solutions to combat zoonotic viruses with special attention to SARS, MERS, and COVID 19: Detection, protection and medication. Microb. Pathog. 2021;159 doi: 10.1016/j.micpath.2021.105133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek S.H., Park C.Y., Nguyen T.P., Kim M.W., Park J.P., Choi C., Kim S.Y., Kailasa S.K., Park T.J. Novel peptides functionalized gold nanoparticles decorated tungsten disulfide nanoflowers as the electrochemical sensing platforms for the norovirus in an oyster. Food Control. 2020;114 doi: 10.1016/j.foodcont.2020.107225. [DOI] [Google Scholar]
- 14.Nakonechny F., Nisnevitch M., Nitzan Y., Firer M.A. New techniques in antimicrobial photodynamic therapy: scope of application and overcoming drug resistance in nosocomial infections. Sci. Again. Microb. Pathog. 2011:684–691. http://www.formatex.info/microbiology3/book/684-691.pdf [Google Scholar]
- 15.Lara H.H., Ayala-Núñez N.V., del Turrent L.C.I., Padilla C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria, World. J. Microbiol. Biotechnol. 2010;26:615–621. doi: 10.1007/s11274-009-0211-3. [DOI] [Google Scholar]
- 16.Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Sun S.S., Chen Y., Yang J., Tian T.F., Deng H.X., Li W.H., Du H., Alici G. The development of an adaptive tuned magnetorheological elastomer absorber working in squeeze mode. Smart Mater. Struct. 2014;23 doi: 10.1088/0964-1726/23/7/075009. [DOI] [Google Scholar]
- 18.Nath A., Bhattacharjee R., Nandi A., Sinha A., Kar S., Manoharan N., Mitra S., Mojumdar A., Panda P.K., Patro S., Dutt A., Ahuja R., Verma S.K., Suar M. Phage delivered CRISPR-Cas system to combat multidrug-resistant pathogens in gut microbiome. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113122. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjee R., Jana A., Nandi A., Sinha A., Bhattacharjee A., Mitra S., Kar S., Dey A., Singh S.K., Varma R.S., Panda P.K., Suar M., Verma S.K. Synergy of nanocarriers with CRISPR-Cas9 in an emerging technology platform for biomedical appliances: Current insights and perspectives. Mater. Des. 2022;224 doi: 10.1016/j.matdes.2022.111415. [DOI] [Google Scholar]
- 20.Willems N.I.T. Roadmap Report on Nanoparticles. Methodology. 2005;1–57 [Google Scholar]
- 21.Philip D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract, Spectrochim. Acta - Part A: Mol. Biomol.Spectros. 2009;73:374–381. doi: 10.1016/j.saa.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Sreelakshmi C., Datta K.K.R., Yadav J.S., Subba Reddy B.V. Honey derivatized Au and Ag nanoparticles and evaluation of its antimicrobial activity. J. Nanosci. Nanotechnol. 2011;11:6995–7000. doi: 10.1166/jnn.2011.4240. [DOI] [PubMed] [Google Scholar]
- 23.Venu R., Ramulu T.S., Anandakumar S., Rani V.S., Kim C.G. Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf A Physicochem Eng Asp. 2011;384:733–738. doi: 10.1016/j.colsurfa.2011.05.045. [DOI] [Google Scholar]
- 24.Khan M., Naqvi A.H., Ahmad M. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol. Rep. 2015;2:765–774. doi: 10.1016/j.toxrep.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramyadevi J., Jeyasubramanian K., Marikani A., Rajakumar G., Rahuman A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012;71:114–116. doi: 10.1016/j.matlet.2011.12.055. [DOI] [Google Scholar]
- 26.P.J.P. Espitia, N. de F.F. Soares, J.S. dos R. Coimbra, N.J. de Andrade, R.S. Cruz, E.A.A. Medeiros, Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications, Food and Bioprocess Technology. 5 (2012) 1447–1464 10.1007/s11947-012-0797-6.
- 27.Saravanan M., Nanda A. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf. B Biointerfaces. 2010;77:214–218. doi: 10.1016/j.colsurfb.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Merk A., Bartesaghi A., Banerjee S., Falconieri V., Rao P., Davis M.I., Pragani R., Boxer M.B., Earl L.A., Milne J.L.S., Subramaniam S. Breaking Cryo-EM Resolution Barriers to Facilitate Drug Discovery. Cell. 2016;165:1698–1707. doi: 10.1016/j.cell.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ethiraj A.S., Jayanthi S., Ramalingam C., Banerjee C. Control of size and antimicrobial activity of green synthesized silver nanoparticles. Mater. Lett. 2016;185:526–529. doi: 10.1016/j.matlet.2016.07.114. [DOI] [Google Scholar]
- 30.Arya G., Kumari R.M., Sharma N., Gupta N., Kumar A., Chatterjee S., Nimesh S. Catalytic, antibacterial and antibiofilm efficacy of biosynthesised silver nanoparticles using Prosopis juliflora leaf extract along with their wound healing potential. J. Photochem. Photobiol. B Biol. 2019;190:50–58. doi: 10.1016/j.jphotobiol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Otari S.V., Patil R.M., Nadaf N.H., Ghosh S.J., Pawar S.H. Green synthesis of silver nanoparticles by microorganism using organic pollutant: Its antimicrobial and catalytic application, Environ. Sci. Pollut.Res. 2014;21:1503–1513. doi: 10.1007/s11356-013-1764-0. [DOI] [PubMed] [Google Scholar]
- 32.Saravanan M., Barik S.K., MubarakAli D., Prakash P., Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018;116:221–226. doi: 10.1016/j.micpath.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Balakumaran M.D., Ramachandran R., Balashanmugam P., Mukeshkumar D.J., Kalaichelvan P.T. Mycosynthesis of silver and gold nanoparticles: Optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 2016;182:8–20. doi: 10.1016/j.micres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Saravanan M., Arokiyaraj S., Lakshmi T., Pugazhendhi A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018;117:68–72. doi: 10.1016/j.micpath.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Qu Y., Shen W., Wang J., Li H., Zhang Z., Li S., Zhou J. Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surf A Physicochem Eng Asp. 2016;497:280–285. doi: 10.1016/j.colsurfa.2016.02.033. [DOI] [Google Scholar]
- 36.Shu M., He F., Li Z., Zhu X., Ma Y., Zhou Z., Yang Z., Gao F., Zeng M. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res. Lett. 2020;15 doi: 10.1186/s11671-019-3244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa L.H., Hemmer J.V., Wanderlind E.H., Gerlach O.M.S., Santos A.L.H., Tamanaha M.S., Bella-Cruz A., Corrêa R., Bazani H.A.G., Radetski C.M., Almerindo G.I. Green Synthesis of Gold Nanoparticles Obtained from Algae Sargassum cymosum: OptimizationCharacterization and Stability. BioNanoScience. 2020;10:1049–1062. doi: 10.1007/s12668-020-00776-4. [DOI] [Google Scholar]
- 38.Ulagesan S., Nam T.J., Choi Y.H. Biogenic preparation and characterization of Pyropia yezoensis silver nanoparticles (P.y AgNPs) and their antibacterial activity against Pseudomonas aeruginosa. Bioprocess Biosyst. Eng. 2021;44:443–452. doi: 10.1007/s00449-020-02454-x. [DOI] [PubMed] [Google Scholar]
- 39.Narayanan K.B., Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Salem S.S., El-Belely E.F., Niedbała G., Alnoman M.M., Hassan S.E.D., Eid A.M., Shaheen T.I., Elkelish A., Fouda A. Bactericidal and in-vitro cytotoxic efficacy of silver nanoparticles (Ag-NPs) fabricated by endophytic actinomycetes and their use as coating for the textile fabrics. Nanomaterials. 2020;10:1–20. doi: 10.3390/nano10102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koduru J.R., Kailasa S.K., Bhamore J.R., Kim K.H., Dutta T., Vellingiri K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018;256:326–339. doi: 10.1016/j.cis.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Panda P.K., Kumari P., Patel P., Samal S.K., Mishra S., Tambuwala M.M., Dutt A., Hilscherová K., Mishra Y.K., Varma R.S., Suar M., Ahuja R., Verma S.K. Molecular nanoinformatics approach assessing the biocompatibility of biogenic silver nanoparticles with channelized intrinsic steatosis and apoptosis. Green Chem. 2022;24:1190–1210. doi: 10.1039/d1gc04103g. [DOI] [Google Scholar]
- 43.Kasithevar M., Saravanan M., Prakash P., Kumar H., Ovais M., Barabadi H., Shinwari Z.K. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients, Journal of Interdisciplinary. Nanomedicine. 2017;2:131–141. doi: 10.1002/jin2.26. [DOI] [Google Scholar]
- 44.Sunkar S., Nachiyar C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012;2:953–959. doi: 10.1016/S2221-1691(13)60006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owaid M.N., Ibraheem I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017;9:5–23. doi: 10.1515/ejnm-2016-0016. [DOI] [Google Scholar]
- 46.Bernardshaw S., Johnson E., Hetland G. An extract of the mushroom Agaricus blazei murill administered orally protects against systemic Streptococcus pneumoniae infection in mice. Scand. J. Immunol. 2005;62:393–398. doi: 10.1111/j.1365-3083.2005.01667.x. [DOI] [PubMed] [Google Scholar]
- 47.Velusamy P., Kumar G.V., Jeyanthi V., Das J., Pachaiappan R. Bio-inspired green nanoparticles: Synthesis, mechanism, and antibacterial application, Toxicol. Res. 2016;32:95–102. doi: 10.5487/TR.2016.32.2.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balaji D.S., Basavaraja S., Deshpande R., Mahesh D.B., Prabhakar B.K., Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B Biointerfaces. 2009;68:88–92. doi: 10.1016/j.colsurfb.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Netala V.R., Bethu M.S., Pushpalatha B., Baki V.B., Aishwarya S., Rao J.V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016;11:5683–5696. doi: 10.2147/IJN.S112857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cotter P.D., Hill C., Ross R.P. Food microbiology: Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005;3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 51.Bowdish D., Davidson D., Hancock R. A Re-evaluation of the Role of Host Defence Peptides in Mammalian Immunity. Curr. Protein Pept. Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 52.Hegarty J.W., Guinane C.M., Ross R.P., Hill C., Cotter P.D. Bacteriocin production: A relatively unharnessed probiotic trait? F1000. Research. 2016;5 doi: 10.12688/f1000research.9615.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diop M.B., Dubois-Dauphin R., Tine E., Ngom A., Destain J., Thonart P. Bacteriocin producers from traditional food products. Biotechnol., Agronom, Soc Environ. 2007;11:275–281. [Google Scholar]
- 54.Seil J.T., Webster T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erkoc P., Ulucan-Karnak F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis. 2021;3:25–52. doi: 10.3390/prosthesis3010005. [DOI] [Google Scholar]
- 56.Panda P.K., Arul M.N., Patel P., Verma S.K., Luo W., Rubahn H.G., Mishra Y.K., Suar M., Ahuja R. Structure-based drug designing and immunoinformatics approach for SARS-CoV-2. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shang Y., Min C., Hu J., Wang T., Liu H., Hu Y. Synthesis of gold nanoparticles by reduction of HAuCl4 under UV irradiation. Solid State Sci. 2013;15:17–23. doi: 10.1016/j.solidstatesciences.2012.09.002. [DOI] [Google Scholar]
- 58.Herizchi R., Abbasi E., Milani M., Akbarzadeh A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016;44:596–602. doi: 10.3109/21691401.2014.971807. [DOI] [PubMed] [Google Scholar]
- 59.Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci.. 2014;9:385–406. [PMC free article] [PubMed] [Google Scholar]
- 60.H.A. Salam, P. Rajiv, M. Kamaraj, P. Jagadeeswaran, S. Gunalan, R. Sivaraj, Plants : Green Route for Nanoparticle Synthesis, 1 (2012) 85–90.
- 61.Dhanaraj S., Thirunavukkarasu S., Allen John H., Pandian S., Salmen S.H., Chinnathambi A., Alharbi S.A. Novel marine Nocardiopsis dassonvillei-DS013 mediated silver nanoparticles characterization and its bactericidal potential against clinical isolates. Saudi J. Biol. Sci. 2020;27:991–995. doi: 10.1016/j.sjbs.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agnihotri M., Joshi S., Kumar A.R., Zinjarde S., Kulkarni S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater. Lett. 2009;63:1231–1234. doi: 10.1016/j.matlet.2009.02.042. [DOI] [Google Scholar]
- 63.Annamalai J., Nallamuthu T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. (Switzerland). 2015;5:603–607. doi: 10.1007/s13204-014-0353-y. [DOI] [Google Scholar]
- 64.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. doi: 10.1039/c1gc15386b. [DOI] [Google Scholar]
- 65.Alomar T.S., AlMasoud N., Awad M.A., El-Tohamy M.F., Soliman D.A. An eco-friendly plant-mediated synthesis of silver nanoparticles: Characterization, pharmaceutical and biomedical applications. Mater. Chem. Phys. 2020;249 doi: 10.1016/j.matchemphys.2020.123007. [DOI] [Google Scholar]
- 66.Syed B., Karthik N., Bhat P., Bisht N., Prasad A., Satish S., Prasad M.N.N. Phyto-biologic bimetallic nanoparticles bearing antibacterial activity against human pathogens. J. King Saud Univ. - Sci. 2019;31:798–803. doi: 10.1016/j.jksus.2018.01.008. [DOI] [Google Scholar]
- 67.Ahmed T.A., Aljaeid B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aljabali A.A.A., Akkam Y., Al Zoubi M.S., Al-Batayneh K.M., Al-Trad B., Alrob O.A., Alkilany A.M., Benamara M., Evans D.J. Synthesis of gold nanoparticles using leaf extract of ziziphus zizyphus and their antimicrobial activity. Nanomaterials. 2018;8:1–15. doi: 10.3390/nano8030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klekotko M., Brach K., Olesiak-Banska J., Samoc M., Matczyszyn K. Popcorn-shaped gold nanoparticles: Plant extract-mediated synthesis, characterization and multiphoton-excited luminescence properties. Mater. Chem. Phys. 2019;229:56–60. doi: 10.1016/j.matchemphys.2019.02.066. [DOI] [Google Scholar]
- 70.Mariychuk R., Smolková R., Bartošová V., Eliašová A., Grishchenko L.M., Diyuk V.E., Lisnyak V.V. The regularities of the Mentha piperita L. extract mediated synthesis of gold nanoparticles with a response in the infrared range. Appl. Nanosci. (Switzerland) 2022;12:1071–1083. doi: 10.1007/s13204-021-01740-8. [DOI] [Google Scholar]
- 71.Kanmani P., Lim S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem. 2013;48:1099–1106. doi: 10.1016/j.procbio.2013.05.011. [DOI] [Google Scholar]
- 72.Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces. 2003;28:313–318. doi: 10.1016/S0927-7765(02)00174-1. [DOI] [Google Scholar]
- 73.Vetchinkina E., Loshchinina E., Kupryashina M., Burov A., Pylaev T., Nikitina V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ. 2018;2018 doi: 10.7717/peerj.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srivastava S., Bhargava A., Pathak N., Srivastava P. Production, characterization and antibacterial activity of silver nanoparticles produced by Fusarium oxysporum and monitoring of protein-ligand interaction through in-silico approaches. Microb. Pathog. 2019;129:136–145. doi: 10.1016/j.micpath.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Sudheer S., Bai R.G., Muthoosamy K., Tuvikene R., Gupta V.K., Manickam S. Biosustainable production of nanoparticles via mycogenesis for biotechnological applications: A critical review. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.111963. [DOI] [PubMed] [Google Scholar]
- 76.El-Sonbaty S.M. Fungus-mediated synthesis of silver nanoparticles and evaluation of antitumor activity. Cancer Nanotechnol. 2013;4:73–79. doi: 10.1007/s12645-013-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Sheekh M.M., Shabaan M.T., Hassan L., Morsi H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020;00:1–12. doi: 10.1080/09603123.2020.1789946. [DOI] [PubMed] [Google Scholar]
- 78.Panicker S., Ahmady I.M., Han C., Chehimi M., Mohamed A.A. On demand release of ionic silver from gold-silver alloy nanoparticles: fundamental antibacterial mechanisms study. Mater. Today Chem. 2020;16 doi: 10.1016/j.mtchem.2019.100237. [DOI] [Google Scholar]
- 79.M. Shah, D. Fawcett, S. Sharma, S.K. Tripathy, G.E.J. Poinern, Green synthesis of metallic nanoparticles via biological entities, 2015 10.3390/ma8115377. [DOI] [PMC free article] [PubMed]
- 80.Lindequist U., Niedermeyer T.H.J., Jülich W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Alternat. Med. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manoharachary C., Sridhar K., Singh R., Adholeya A., Suryanarayanan T.S., Rawat S., Johri B.N. Fungal biodiversity: Distribution, conservation and prospecting of fungi from India. Curr. Sci. 2005;89:58–71. [Google Scholar]
- 82.Barros L., Cruz T., Baptista P., Estevinho L.M., Ferreira I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008;46:2742–2747. doi: 10.1016/j.fct.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 83.Owaid M.N., Raman J., Lakshmanan H., Al-Saeedi S.S.S., Sabaratnam V., Ali Abed I. Mycosynthesis of silver nanoparticles by Pleurotus cornucopiae var. citrinopileatus and its inhibitory effects against Candida sp. Mater. Lett. 2015;153:186–190. doi: 10.1016/j.matlet.2015.04.023. [DOI] [Google Scholar]
- 84.Krishnamoorthi R., Srinivash M., Mahalingam P.U., Malaikozhundan B. Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochem. 2022;121:10–17. doi: 10.1016/j.procbio.2022.06.021. [DOI] [Google Scholar]
- 85.Priyadarshni K.C., Mahalingam P.U. Antimicrobial and anticancer activity of silver nanoparticles from edible mushroom: A review, Asian J. Pharm. Clin. Res. 2017;10:37–40. doi: 10.22159/ajpcr.2017.v10i3.16027. [DOI] [Google Scholar]
- 86.Krishnamoorthi R., Ulagan Mahalingam P., Malaikozhundan B. Edible mushroom extract engineered Ag NPs as safe antimicrobial and antioxidant agents with no significant cytotoxicity on human dermal fibroblast cell. Inorg. Chem. Commun. 2022;139 doi: 10.1016/j.inoche.2022.109362. [DOI] [Google Scholar]
- 87.N. Prabhu, T. Nadu, Biogenic synthesis of myconanoparticles from mushroom extracts and its medical applications: a review. Int. J. Pharm. Sci. Res. 10 (2019) 2108–2118. 10.13040/IJPSR.0975-8232.1 0(5). 2108-18.
- 88.Oladipo O.G., Awotoye O.O., Olayinka A., Bezuidenhout C.C., Maboeta M.S. Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Braz. J. Microbiol. 2018;49:29–37. doi: 10.1016/j.bjm.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saglam N., Yesilada O., Cabuk A., Sam M., Saglam S., Ilk S., Emul E., Celik P.A., Gurel E. Innovation of Strategies and Challenges for Fungal. NanoBiotechnology. 2016:25–46. doi: 10.1007/978-3-319-42990-8_2. [DOI] [Google Scholar]
- 90.Priyadarshni K.C., Mahalingam P.U. Biofunctionalization of mycosynthesized silver nanoparticles on selected drug resistant human pathogens. Mater. Res. Express. 2019;6 doi: 10.1088/2053-1591/ab1d8c. [DOI] [Google Scholar]
- 91.Bhat R., Sharanabasava V.G., Deshpande R., Shetti U., Sanjeev G., Venkataraman A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus florida and its anticancer evaluation. J. Photochem. Photobiol. B Biol. 2013;125:63–69. doi: 10.1016/j.jphotobiol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Sen I.K., Maity K., Islam S.S. Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Carbohydr. Polym. 2013;91:518–528. doi: 10.1016/j.carbpol.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 93.Krishnamoorthi R., Bharathakumar S., Malaikozhundan B., Mahalingam P.U. Mycofabrication of gold nanoparticles : Optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatal. Agric. Biotechnol. 2021;35:102107. doi: 10.1016/j.bcab.2021.102107. [DOI] [Google Scholar]
- 94.Politi J., De Stefano L., Rea I., Gravagnuolo A.M., Giardina P., Methivier C., Casale S., Spadavecchia J. One-pot synthesis of a gold nanoparticle-Vmh2 hydrophobin nanobiocomplex for glucose monitoring. Nanotechnology. 2016;27 doi: 10.1088/0957-4484/27/19/195701. [DOI] [PubMed] [Google Scholar]
- 95.Owaid M.N., Al-Saeedi S.S.S., Abed I.A. Biosynthesis of gold nanoparticles using yellow oyster mushroom Pleurotus cornucopiae var. citrinopileatus, Environmental Nanotechnology. Monitor Manage. 2017;8:157–162. doi: 10.1016/j.enmm.2017.07.004. [DOI] [Google Scholar]
- 96.Owaid M.N., Rabeea M.A., Abdul Aziz A., Jameel M.S., Dheyab M.A. Mushroom-assisted synthesis of triangle gold nanoparticles using the aqueous extract of fresh Lentinula edodes (shiitake) Omphalotaceae. Environ. Nanotechnol., Monitor. Manage. 2019;12 doi: 10.1016/j.enmm.2019.100270. [DOI] [Google Scholar]
- 97.Narayanan K.B., Park H.H., Han S.S. Synthesis and characterization of biomatrixed-gold nanoparticles by the mushroom Flammulina velutipes and its heterogeneous catalytic potential. Chemosphere. 2015;141:169–175. doi: 10.1016/j.chemosphere.2015.06.101. [DOI] [PubMed] [Google Scholar]
- 98.V.R. Manzoor-ul-Haq, D. Singh, A.K. Singh, S. Ninganagouda, J. Hiremath, Dried Mushroom Agaricus bisporus mediated synthesis of silver nanoparticles from Bandipora District (Jammu and Kashmir) and their efficacy against Methicillin Resistant Staphylococcus aureus (MRSA) strains, 5 (2014) 1–8.
- 99.Dhanasekaran D., Latha S., Saha S., Thajuddin N., Panneerselvam A. Extracellular biosynthesis, characterisation and in-vitro antibacterial potential of silver nanoparticles using Agaricus bisporus. J. Exp. Nanosci. 2013;8:579–588. doi: 10.1080/17458080.2011.577099. [DOI] [Google Scholar]
- 100.Sujatha S., Tamilselvi S., Subha K., Panneerselvam A. Studies on biosynthesis of silver nanoparticles using mushroom and its antibacterial activities. Int. J. Curr. Microbiol. App. Sci. 2013;2:605–614. [Google Scholar]
- 101.P. Balashanmugam, S. Santhosh, H. Giyaullah, M.D. Balakumaran, Mycosynthesis , Characterization and Antibacterial Activity of Silver Nanoparticles From Microporus Xanthopus : a Macro Mushroom, 2 (2013) 6262–6270.
- 102.Maurya S., Bhardwaj A.K., Gupta K.K., Agarwal S., Kushwaha A., Chaturvedi V., Pathak R.K., Gopal R., Uttam K.N., Singh A.K., Verma V. Green Synthesis of Silver Nanoparticles using Pluerotus and its Bactericidal Activity. Cell. Mol. Biol. 2016;62 doi: 10.4172/1165-158X.1000131. [DOI] [Google Scholar]
- 103.Bhat R., Deshpande R., Ganachari S.V., Huh D.S., Venkataraman A. Photo-irradiated biosynthesis of silver nanoparticles using edible mushroom Pleurotus florida and their antibacterial activity studies. Bioinorg. Chem. Appl. 2011;2011 doi: 10.1155/2011/650979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anthony K.J.P., Murugan M., Jeyaraj M., Rathinam N.K., Sangiliyandi G. Synthesis of silver nanoparticles using pine mushroom extract: A potential antimicrobial agent against E. coli and B. subtilis. J. Ind. Eng. Chem. 2014;20:2325–2331. doi: 10.1016/j.jiec.2013.10.008. [DOI] [Google Scholar]
- 105.Arun G., Eyini M., Gunasekaran P. Green synthesis of silver nanoparticles using the mushroom fungus Schizophyllum commune and its biomedical applications, Biotechnology and Bioprocess Engineering. 2014;19:1083–1090. doi: 10.1007/s12257-014-0071-z. [DOI] [Google Scholar]
- 106.Mirunalini S., Arulmozhi V., Deepalakshmi K., Krishnaveni M. Intracellular Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Edible Mushrooms. Notulae Scientia Biologicae. 2012;4:55–61. doi: 10.15835/nsb448051. [DOI] [Google Scholar]
- 107.Sanghi R., Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009;100:501–504. doi: 10.1016/j.biortech.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 108.Alghuthaymi M.A., Almoammar H., Rai M., Said-Galiev E., Abd-Elsalam K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015;29:221–236. doi: 10.1080/13102818.2015.1008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rai M., Ingle A., Gupta I., Birla S., Yadav A., Abd-Elsalam K. Potential Role of Biological Systems in Formation of Nanoparticles: Mechanism of Synthesis and Biomedical Applications. Curr. Nanosci. 2013;9:576–587. doi: 10.2174/15734137113099990092. [DOI] [Google Scholar]
- 110.Mandal D., Bolander M.E., Mukhopadhyay D., Sarkar G., Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006;69:485–492. doi: 10.1007/s00253-005-0179-3. [DOI] [PubMed] [Google Scholar]
- 111.Krishnamoorthi R., Bharathakumar S., Malaikozhundan B., Mahalingam P.U. Mycofabrication of gold nanoparticles: Optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatal. Agric. Biotechnol. 2021;35 doi: 10.1016/j.bcab.2021.102107. [DOI] [Google Scholar]
- 112.Martin E., Ganz T., Lehrer R.I. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 1995;58:128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- 113.Pinheiro Da Silva F., MacHado M.C.C. Antimicrobial peptides: Clinical relevance and therapeutic implications. Peptides. 2012;36:308–314. doi: 10.1016/j.peptides.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 114.Rahnamaeian M. Antimicrobial peptides: Modes of mechanism, modulation of defense responses. Plant Signal. Behav. 2011;6:1325–1332. doi: 10.4161/psb.6.9.16319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Riley M.A., Wertz J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 116.Devi Avaiyarasi N., David Ravindran A., Venkatesh P., Arul V. In vitro selection, characterization and cytotoxic effect of bacteriocin of Lactobacillus sakei GM3 isolated from goat milk. Food Control. 2016;69:124–133. doi: 10.1016/j.foodcont.2016.04.036. [DOI] [Google Scholar]
- 117.Cotter P.D., Ross R.P., Hill C. Bacteriocins-a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 118.O. Sharma, S.D. Zakharov, M.V. Zhalnina, E. Yamashita, W.A. Cramer, Colicins, Second Ed., Elsevier Inc., 2013 10.1016/B978-0-12-385095-9.00017-8.
- 119.Chavan M.A., Riley M.A. Molecular Evolution of Bacteriocins in Gram-Negative Bacteria. Bacteriocins. 2007:19–43. doi: 10.1007/978-3-540-36604-1_3. [DOI] [Google Scholar]
- 120.Krishnamoorthi R., Srinivash M., Ulagan P., Malaikozhundan B., Suganya P., Gurushankar K. Antimicrobial, anti-biofilm, antioxidant and cytotoxic effects of bacteriocin by Lactococcus lactis strain CH3 isolated from fermented dairy products — An in vitro and in silico approach. Int. J. Biol. Macromol. 2022;220:291–306. doi: 10.1016/j.ijbiomac.2022.08.087. [DOI] [PubMed] [Google Scholar]
- 121.Ivanova I., Kabadjova P., Pantev A., Danova S., Dousset X. Detection, purification and partial characterization of a novel bacteriocin substance produced by Lactococcus lactis subsp. lactis B14 isolated from boza-Bulgarian traditional cereal beverage. Biocatalysis. 2000;41:47–53. [Google Scholar]
- 122.Todorov S.D., Danova S.T., Van Reenen C.A., Meincken M., Dinkova G., Ivanova I.V., Dicks L.M.T. Characterization of bacteriocin HV219, produced by Lactococcus lactis subsp. lactis HV219 isolated from human vaginal secretions. J. Basic Microbiol. 2006;46:226–238. doi: 10.1002/jobm.200510037. [DOI] [PubMed] [Google Scholar]
- 123.Alegría Á., Delgado S., Roces C., López B., Mayo B. Bacteriocins produced by wild Lactococcus lactis strains isolated from traditional, starter-free cheeses made of raw milk. Int. J. Food Microbiol. 2010;143:61–66. doi: 10.1016/j.ijfoodmicro.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 124.Gong H.S., Meng X.C., Wang H. Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1.0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control. 2010;21:89–96. doi: 10.1016/j.foodcont.2009.04.005. [DOI] [Google Scholar]
- 125.Tosukhowong A., Zendo T., Visessanguan W., Roytrakul S., Pumpuang L., Jaresitthikunchai J., Sonomoto K. Garvieacin Q, a novel class II bacteriocin from Lactococcus garvieae BCC 43578. Appl. Environ. Microbiol. 2012;78:1619–1623. doi: 10.1128/AEM.06891-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sahoo T.K., Jena P.K., Patel A.K., Seshadri S. Purification and Molecular Characterization of the Novel Highly Potent Bacteriocin TSU4 Produced by Lactobacillus animalis TSU4. Appl. Biochem. Biotechnol. 2015;177:90–104. doi: 10.1007/s12010-015-1730-z. [DOI] [PubMed] [Google Scholar]
- 127.Lü X., Yi L., Dang J., Dang Y., Liu B. Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant microorganisms. Food Control. 2014;46:264–271. doi: 10.1016/j.foodcont.2014.05.028. [DOI] [Google Scholar]
- 128.Zhao S., Han J., Bie X., Lu Z., Zhang C., Lv F. Purification and Characterization of Plantaricin JLA-9: A Novel Bacteriocin against Bacillus spp. Produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a Traditional Chinese Fermented Cabbage. J. Agric. Food Chem. 2016;64:2754–2764. doi: 10.1021/acs.jafc.5b05717. [DOI] [PubMed] [Google Scholar]
- 129.Liu G., Song Z., Yang X., Gao Y., Wang C., Sun B. Antibacterial mechanism of bifidocin A, a novel broad-spectrum bacteriocin produced by Bifidobacterium animalis BB04. Food Control. 2016;62:309–316. doi: 10.1016/j.foodcont.2015.10.033. [DOI] [Google Scholar]
- 130.Li R., Yassami S., Kiviniemi E.A., Qiao W., Takala T.M., Saris P.E.J. Listeria decontamination of chicken meat with beer brewed with bacteriocin producing Saccharomyces boulardii. Lwt. 2021;152 doi: 10.1016/j.lwt.2021.112323. [DOI] [Google Scholar]
- 131.Smitha S., Bhat S.G. Thermostable Bacteriocin BL8 from Bacillus licheniformis isolated from marine sediment. J. Appl. Microbiol. 2013;114:688–694. doi: 10.1111/jam.12097. [DOI] [PubMed] [Google Scholar]
- 132.Joseph B., Dhas B., Hena V., Raj J. Bacteriocin from Bacillus subtilis as a novel drug against diabetic foot ulcer bacterial pathogens. Asian Pacific J. Trop. Biomed. 2013;3:942–946. doi: 10.1016/S2221-1691(13)60183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Renye J.A., Somkuti G.A., Steinberg D.H. Thermophilin 109 is a naturally produced broad spectrum bacteriocin encoded within the blp gene cluster of Streptococcus thermophilus. Biotechnol. Lett. 2019;41:283–292. doi: 10.1007/s10529-018-02637-3. [DOI] [PubMed] [Google Scholar]
- 134.Kabuki T., Uenishi H., Watanabe M., Seto Y., Nakajima H. Characterization of a bacteriocin, Thermophilin 1277, produced by Streptococcus thermophilus SBT1277. J. Appl. Microbiol. 2007;102:971–980. doi: 10.1111/j.1365-2672.2006.03159.x. [DOI] [PubMed] [Google Scholar]
- 135.Balakrishnan M., Simmonds R.S., Carne A., Tagg J.R. Streptococcus mutans strain N produces a novel low molecular mass non- lantibiotic bacteriocin. FEMS Microbiol. Lett. 2000;183:165–169. doi: 10.1016/S0378-1097(99)00661-8. [DOI] [PubMed] [Google Scholar]
- 136.Phumisantiphong U., Siripanichgon K., Reamtong O., Diraphat P. A novel bacteriocin from Enterococcus faecalis 478 exhibits a potent activity against vancomycin-resistant enterococci. PLoS One. 2017;12:1–22. doi: 10.1371/journal.pone.0186415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sawa N., Wilaipun P., Kinoshita S., Zendo T., Leelawatcharamas V., Nakayama J., Sonomoto K. Isolation and characterization of enterocin W, a novel two-peptide lantibiotic produced by Enterococcus faecalis NKR-4-1. Appl. Environ. Microbiol. 2012;78:900–903. doi: 10.1128/AEM.06497-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yanagida F., Chen Y., Onda T., Shinohara T. Durancin L28–1A, a new bacteriocin from Enterococcus durans L28–1, isolated from soil. Lett. Appl. Microbiol. 2005;40:430–435. doi: 10.1111/j.1472-765X.2005.01693.x. [DOI] [PubMed] [Google Scholar]
- 139.Zendo T., Eungruttanagorn N., Fujioka S., Tashiro Y., Nomura K., Sera Y., Kobayashi G., Nakayama J., Ishizaki A., Sonomoto K. Identification and production of a bacteriocin from Enterococcus mundtii QU 2 isolated from soybean. J. Appl. Microbiol. 2005;99:1181–1190. doi: 10.1111/j.1365-2672.2005.02704.x. [DOI] [PubMed] [Google Scholar]
- 140.Matsusaki H., Endo N., Sonomoto K., Ishizaki A. Lantibiotic nisin Z fermentative production by Lactococcus lactis 10–1: Relationship between production of the lantibiotic and lactate and cell growth. Appl. Microbiol. Biotechnol. 1996;45:36–40. doi: 10.1007/s002530050645. [DOI] [PubMed] [Google Scholar]
- 141.Nissen-Meyer J., Rogne P., Oppegard C., Haugen H., Kristiansen P. Structure-Function Relationships of the Non-Lanthionine-Containing Peptide (class II) Bacteriocins Produced by Gram-Positive Bacteria. Curr. Pharm. Biotechnol. 2009;10:19–37. doi: 10.2174/138920109787048661. [DOI] [PubMed] [Google Scholar]
- 142.Sawa N., Okamura K., Zendo T., Himeno K., Nakayama J., Sonomoto K. Identification and characterization of novel multiple bacteriocins produced by Leuconostoc pseudomesenteroides QU 15. J. Appl. Microbiol. 2010;109:282–291. doi: 10.1111/j.1365-2672.2009.04653.x. [DOI] [PubMed] [Google Scholar]
- 143.Zendo T., Koga S., Shigeri Y., Nakayama J., Sonomoto K. Lactococcin Q, a novel two-peptide bacteriocin produced by Lactococcus lactis QU 4. Appl. Environ. Microbiol. 2006;72:3383–3389. doi: 10.1128/AEM.72.5.3383-3389.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sawa N., Zendo T., Kiyofuji J., Fujita K., Himeno K., Nakayama J., Sonomoto K. Identification and characterization of lactocyclicin Q, a novel cyclic bacteriocin produced by lactococcus sp. strain QU 12. Appl. Environ. Microbiol. 2009;75:1552–1558. doi: 10.1128/AEM.02299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fujita K., Ichimasa S., Zendo T., Koga S., Yoneyama F., Nakayama J., Sonomoto K. Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Appl. Environ. Microbiol. 2007;73:2871–2877. doi: 10.1128/AEM.02286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Srinivash M., Krishnamoorthi R., Ulagan P. Probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from homemade fermented food products. J. Agric. Food Res. 2023;11 doi: 10.1016/j.jafr.2023.100517. [DOI] [Google Scholar]
- 147.Mokoena M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules. 2017;22 doi: 10.3390/molecules22081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang S.C., Lin C.H., Sung C.T., Fang J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014;5:1–10. doi: 10.3389/fmicb.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Silva C.C.G., Silva S.P.M., Ribeiro S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.N. Malanovic, K. Lohner, Antimicrobial peptides targeting Gram-positive bacteria, 2016 10.3390/ph9030059. [DOI] [PMC free article] [PubMed]
- 151.Perez R.H., Zendo T., Sonomoto K. Circular and leaderless bacteriocins: Biosynthesis, mode of action, applications, and prospects. Front. Microbiol. 2018;9:1–18. doi: 10.3389/fmicb.2018.02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Strompfová V., Kubašová I., Ščerbová J., Maďari A., Gancarčíková S., Mudroňová D., Miltko R., Belzecki G., Lauková A. Oral administration of bacteriocin-producing and non-producing strains of Enterococcus faecium in dogs. Appl. Microbiol. Biotechnol. 2019;103:4953–4965. doi: 10.1007/s00253-019-09847-3. [DOI] [PubMed] [Google Scholar]
- 153.Ríos Colombo N.S., Chalón M.C., Navarro S.A., Bellomio A. Pediocin-like bacteriocins: new perspectives on mechanism of action and immunity. Curr. Genet. 2018;64:345–351. doi: 10.1007/s00294-017-0757-9. [DOI] [PubMed] [Google Scholar]
- 154.Abdulhussain Kareem R., Razavi S.H. Plantaricin bacteriocins: As safe alternative antimicrobial peptides in food preservation—A review. J. Food Saf. 2020;40:1–12. doi: 10.1111/jfs.12735. [DOI] [Google Scholar]
- 155.Martin N.I., Breukink E. Expanding role of lipid II as a target for lantibiotics. Future Microbiol. 2007;2:513–525. doi: 10.2217/17460913.2.5.513. [DOI] [PubMed] [Google Scholar]
- 156.H. Hashim, Bacteriocin: the avenues of innovation towards applied microbiology, Pure Appl. Biol. 7 (2018) 460–478. 10.19045/bspab.2018.700205.
- 157.Brötz H., Bierbaum G., Reynolds P.E., Sahl H.G. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur. J. Biochem. 1997;246:193–199. doi: 10.1111/j.1432-1033.1997.t01-1-00193.x. [DOI] [PubMed] [Google Scholar]
- 158.Paiva A.D., Breukink E., Mantovani H.C. Role of lipid II and membrane thickness in the mechanism of action of the lantibiotic bovicin HC5. Antimicrob. Agents Chemother. 2011;55:5284–5293. doi: 10.1128/AAC.00638-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gravesen A., Ramnath M., Rechinger K.B., Andersen N., Jänsch L., Héchard Y., Hastings J.W., Knøchel S. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology. 2002;148:2361–2369. doi: 10.1099/00221287-148-8-2361. [DOI] [PubMed] [Google Scholar]
- 160.Van Belkum M.J., Martin-Visscher L.A., Vederas J.C. Structure and genetics of circular bacteriocins. Trends Microbiol. 2011;19:411–418. doi: 10.1016/j.tim.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 161.Dawson M.J., Scott R.W. New horizons for host defense peptides and lantibiotics. Curr. Opin. Pharmacol. 2012;12:545–550. doi: 10.1016/j.coph.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koo H.B., Seo J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019;111 doi: 10.1002/pep2.24122. [DOI] [Google Scholar]
- 163.Gradisteanu Pircalabioru G., Popa L.I., Marutescu L., Gheorghe I., Popa M., Czobor Barbu I., Cristescu R., Chifiriuc M.C. Bacteriocins in the era of antibiotic resistance: rising to the challenge. Pharmaceutics. 2021;13:1–15. doi: 10.3390/pharmaceutics13020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chandran Priyadarshni K., Krishnamoorthi R., Mumtha C., Ulagan Mahalingam P. Biochemical analysis of cultivated mushroom, Pleurotus florida and synthesis of silver nanoparticles for enhanced antimicrobial effects on clinically important human pathogens. Inorg. Chem. Commun. 2022;142 doi: 10.1016/j.inoche.2022.109673. [DOI] [Google Scholar]
- 165.Vijayakumar S., Malaikozhundan B., Parthasarathy A., Saravanakumar K., Wang M.H., Vaseeharan B. Nano Biomedical Potential of Biopolymer Chitosan-Capped Silver Nanoparticles with Special Reference to Antibacterial, Antibiofilm, Anticoagulant and Wound Dressing Material. J. Clust. Sci. 2020;31:355–366. doi: 10.1007/s10876-019-01649-x. [DOI] [Google Scholar]
- 166.Malaikozhundan B., Krishnamoorthi R., Vinodhini J., Nambi K.S.N., Palanisamy S. Multifunctional iron oxide nanoparticles using Carica papaya fruit extract as antibacterial, antioxidant and photocatalytic agent to remove industrial dyes. Inorg. Chem. Commun. 2022;144 doi: 10.1016/j.inoche.2022.109843. [DOI] [Google Scholar]
- 167.Malaikozhundan B., Lakshmi V.N., Krishnamoorthi R. Copper oxide nanoparticles using Mentha spicata leaves as antibacterial, antibiofilm, free radical scavenging agent and efficient photocatalyst to degrade methylene blue dyes. Mater. Today Commun. 2022;33 doi: 10.1016/j.mtcomm.2022.104348. [DOI] [Google Scholar]
- 168.Gu H., Ho P.L., Tong E., Wang L., Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003;3:1261–1263. doi: 10.1021/nl034396z. [DOI] [Google Scholar]
- 169.Neu H.C. The crisis in antibiotic resistance [See comments] Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 170.C.D.P. Roberto Canaparo, Federica Foglietta, Francesca Giuntini, F.D. and L. Serpe, Recent Developments in Antibacterial Therapy : and Therapeutic Nanoparticles, 2019. [DOI] [PMC free article] [PubMed]
- 171.Ramezani M., Parissa F., Parsi K., Hossein G., Mehdi R., Ardestani S. PEGylation of graphene / iron oxide nanocomposite : assessment of release of doxorubicin, magnetically targeted drug delivery and photothermal therapy. Appl. Nanosci. 2020 doi: 10.1007/s13204-020-01255-8. [DOI] [Google Scholar]
- 172.P. Zhao, Z. Jin, Q. Chen, T. Yang, D. Chen, J. Meng, X. Lu, Z. Gu, Q. He, photothermal therapy, (n.d.) 1–12. 10.1038/s41467-018-06630-2.
- 173.Weir E., Lawlor A., Whelan A., Regan F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst. 2008;133:835–845. doi: 10.1039/b715532h. [DOI] [PubMed] [Google Scholar]
- 174.Rabea E.I., Badawy M.E.T., Stevens C.V., Smagghe G., Steurbaut W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 175.Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effects of silver nanoparticles, Nanomed: Nanotechnol. Biol., Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 176.Hadiya S., Liu X., Abd El-Hammed W., Elsabahy M., Aly S.A. Levofloxacin-loaded nanoparticles decrease emergence of fluoroquinolone resistance in Escherichia coli. Microb. Drug Resist. 2018;24:1098–1107. doi: 10.1089/mdr.2017.0304. [DOI] [PubMed] [Google Scholar]
- 177.Slavin Y.N., Asnis J., Häfeli U.O., Bach H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017;15:1–20. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Swain P., Nayak S.K., Sasmal A., Behera T., Barik S.K., Swain S.K., Mishra S.S., Sen A.K., Das J.K., Jayasankar P. Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture, World. J. Microbiol. Biotechnol. 2014;30:2491–2502. doi: 10.1007/s11274-014-1674-4. [DOI] [PubMed] [Google Scholar]
- 179.Lima E., Guerra R., Lara V., Guzmán A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem. Cent. J. 2013;7:1–7. doi: 10.1186/1752-153X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lopez-Carrizales M., Velasco K.I., Castillo C., Flores A., Magaña M., Martinez-Castanon G.A., Martinez-Gutierrez F. In vitro synergism of silver nanoparticles with antibiotics as an alternative treatment in multiresistant uropathogens. Antibiotics. 2018;7:1–13. doi: 10.3390/antibiotics7020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fernando H.N., Kumarasinghe K.G.U.R., Gunasekara T.D.C.P., Wijekoon H.P.S.K., Ekanayaka E.M.A.K., Rajapaksha S.P., Fernando S.S.N., Jayaweera P.M. Synthesis, characterization and antimicrobial activity of garcinol capped silver nanoparticles. J. Microbiol. Biotechnol. 2019;29:1841–1851. doi: 10.4014/jmb.1904.04032. [DOI] [PubMed] [Google Scholar]
- 182.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 183.Thapa R., Bhagat C., Shrestha P., Awal S., Dudhagara P. Enzyme-mediated formulation of stable elliptical silver nanoparticles tested against clinical pathogens and MDR bacteria and development of antimicrobial surgical thread. Ann. Clin. Microbiol. Antimicrob. 2017;16:1–10. doi: 10.1186/s12941-017-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kulshrestha S., Qayyum S., Khan A.U. Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: A comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 2017;103:167–177. doi: 10.1016/j.micpath.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 185.Mahmoudi M., Serpooshan V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano. 2012;6:2656–2664. doi: 10.1021/nn300042m. [DOI] [PubMed] [Google Scholar]
- 186.lin Su D., jun Li P., Ning M., yang Li G., Shan Y. Microwave assisted green synthesis of pectin based silver nanoparticles and their antibacterial and antifungal activities. Mater Letters. 2019;244:35–38. doi: 10.1016/j.matlet.2019.02.059. [DOI] [Google Scholar]
- 187.T.Y. Chang, C.C. Chen, K.M. Cheng, C.Y. Chin, Y.H. Chen, X.A. Chen, J.R. Sun, J.J. Young, T.S. Chiueh, Trimethyl chitosan-capped silver nanoparticles with positive surface charge: Their catalytic activity and antibacterial spectrum including multidrug-resistant strains of Acinetobacter baumannii, 2017, Elsevier B.V 10.1016/j.colsurfb.2017.03.054. [DOI] [PubMed]
- 188.Gaidhani S., Singh R., Singh D., Patel U., Shevade K., Yeshvekar R., Ananda Chopade B. Biofilm disruption activity of silver nanoparticles synthesized by Acinetobacter calcoaceticus PUCM 1005. Mater. Lett. 2013;108:324–327. doi: 10.1016/j.matlet.2013.07.023. [DOI] [Google Scholar]
- 189.Nagy A., Harrison A., Sabbani S., Munson R.S., Dutta P.K., Waldman W.J. Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011;6:1833–1852. doi: 10.2147/ijn.s24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shaikh S., Rizvi S.M.D., Shakil S., Hussain T., Alshammari T.M., Ahmad W., Tabrez S., Al-Qahtani M.H., Abuzenadah A.M. Synthesis and Characterization of Cefotaxime Conjugated Gold Nanoparticles and Their Use to Target Drug-Resistant CTX-M-Producing Bacterial Pathogens. J. Cell. Biochem. 2017;118:2802–2808. doi: 10.1002/jcb.25929. [DOI] [PubMed] [Google Scholar]
- 191.Payne J.N., Waghwani H.K., Connor M.G., Hamilton W., Tockstein S., Moolani H., Chavda F., Badwaik V., Lawrenz M.B., Dakshinamurthy R. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front. Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shaker M.A., Shaaban M.I. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: In vitro antibacterial study. Int. J. Pharm. 2017;525:71–84. doi: 10.1016/j.ijpharm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 193.Cui Y., Zhao Y., Tian Y., Zhang W., Lü X., Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 194.Yu Q., Li J., Zhang Y., Wang Y., Liu L., Li M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016;6:26667. doi: 10.1038/srep26667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Okkeh M., Bloise N., Restivo E., De Vita L., Pallavicini P., Visai L. Gold nanoparticles: Can they be the next magic bullet for multidrug-resistant bacteria? Nanomaterials. 2021;11:1–30. doi: 10.3390/nano11020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mohankandhasamy R., Lee J.H., Lee J. Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomed. 2017;12:2813–2828. doi: 10.2147/IJN.S132784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vinoj G., Pati R., Sonawane A., Vaseeharan B. In vitro cytotoxic effects of gold nanoparticles coated with functional acyl homoserine lactone lactonase protein from Bacillus licheniformis and their antibiofilm activity against proteus species. Antimicrob. Agents Chemother. 2015;59:763–771. doi: 10.1128/AAC.03047-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rodríguez-Cerdeira C., Arenas R., Moreno-Coutiño G., Vásquez E., Fernández R., Chang P. Systemic fungal infections in patients with human inmunodeficiency virus. Actas Dermo-Sifiliograficas. 2014;105:5–17. doi: 10.1016/j.adengl.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 199.Hwang I.S., Lee J., Hwang J.H., Kim K.J., Lee D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012;279:1327–1338. doi: 10.1111/j.1742-4658.2012.08527.x. [DOI] [PubMed] [Google Scholar]
- 200.Kim K.J., Sung W.S., Suh B.K., Moon S.K., Choi J.S., Kim J.G., Lee D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals. 2009;22:235–242. doi: 10.1007/s10534-008-9159-2. [DOI] [PubMed] [Google Scholar]
- 201.Smitha S.L., Gopchandran K.G. Surface enhanced Raman scattering, antibacterial and antifungal active triangular gold nanoparticles. Spectrochim. Acta - Part A: Mol. Biomol. Spectrosc. 2013;102:114–119. doi: 10.1016/j.saa.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 202.Das J., Velusamy P. Biogenic synthesis of antifungal silver nanoparticles using aqueous stem extract of banana. Nano Biomed. Eng. 2013;5:34–38. doi: 10.5101/nbe.v5i1. [DOI] [Google Scholar]
- 203.Li J., Sang H., Guo H., Popko J.T., He L., White J.C., Parkash Dhankher O., Jung G., B. Xing, Antifungal mechanisms of ZnO and Ag nanoparticles to Sclerotinia homoeocarpa. Nanotechnology. 2017;28 doi: 10.1088/1361-6528/aa61f3. [DOI] [PubMed] [Google Scholar]
- 204.Chinnaiah K., Krishnamoorthi R., Kannan K., Sivaganesh D., Saravanakumar S., Theivasanthi T., Palko N., Grishina M., Maik V., Gurushankar K. Ag nanoparticles synthesized by Datura metel L Leaf extract and their charge density distribution, electrochemical and biological performance. Chem. Phys. Lett. 2022;807 doi: 10.1016/j.cplett.2022.140083. [DOI] [Google Scholar]
- 205.Guerra J.D., Sandoval G., Avalos-Borja M., Pestryakov A., Garibo D., Susarrey-Arce A., Bogdanchikova N. Selective antifungal activity of silver nanoparticles: A comparative study between Candida tropicalis and Saccharomyces boulardii. Colloid Interface Sci. Commun. 2020;37 doi: 10.1016/j.colcom.2020.100280. [DOI] [Google Scholar]
- 206.Jaidev L.R., Narasimha G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Colloids Surf. B Biointerfaces. 2010;81:430–433. doi: 10.1016/j.colsurfb.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 207.Spengler J.R., Ervin E.D., Towner J.S., Rollin P.E., Nichol S.T. Perspectives on West Africa Ebola Virus Disease Outbreak, 2013–2016. Emerg. Infect. Dis. 2016;22:956–963. doi: 10.3201/eid2206.160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.P. Brasil, G.A. Calvet, A.M. Siqueira, M. Wakimoto, P.C. de Sequeira, A. Nobre, M. de S.B. Quintana, M.C.L. de Mendonça, O. Lupi, R.V. de Souza, C. Romero, H. Zogbi, C. da S. Bressan, S.S. Alves, R. Lourenço-de-Oliveira, R.M.R. Nogueira, M.S. Carvalho, A.M.B. de Filippis, T. Jaenisch, Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects, PLoS Neglected Tropical Diseases. 10 (2016) 1–13. 10.1371/journal.pntd.0004636. [DOI] [PMC free article] [PubMed]
- 209.Maseko S.B., Natarajan S., Sharma V., Bhattacharyya N., Govender T., Sayed Y., Maguire G.E.M., Lin J., Kruger H.G. Purification and characterization of naturally occurring HIV-1 (South African subtype C) protease mutants from inclusion bodies. Protein Expr. Purif. 2016;122:90–96. doi: 10.1016/j.pep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 210.Hayden F. Developing new antiviral agents for influenza treatment: What does the future hold? Clin. Infect. Dis. 2009;48:3–13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- 211.Chen L., Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C. 2020;112 doi: 10.1016/j.msec.2020.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang M., Sunderland K., Mao C. Virus-Derived Peptides for Clinical Applications. Chem. Rev. 2017;117:10377–10402. doi: 10.1021/acs.chemrev.7b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Khoshnevisan K., Maleki H., Baharifar H. Nanobiocide Based-Silver Nanomaterials Upon Coronaviruses: Approaches for Preventing Viral Infections. Nanoscale Res. Lett. 2021;16 doi: 10.1186/s11671-021-03558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sun R.W.Y., Chen R., Chung N.P.Y., Ho C.M., Lin C.L.S., Che C.M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. 2005:5059–5061. doi: 10.1039/b510984a. [DOI] [PubMed] [Google Scholar]
- 215.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010;8:1–10. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Baram-Pinto D., Shukla S., Perkas N., Gedanken A., Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 2009;20:1497–1502. doi: 10.1021/bc900215b. [DOI] [PubMed] [Google Scholar]
- 217.Baram-Pinto D., Shukla S., Gedanken A., Sarid R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small. 2010;6:1044–1050. doi: 10.1002/smll.200902384. [DOI] [PubMed] [Google Scholar]
- 218.Speshock J.L., Murdock R.C., Braydich-Stolle L.K., Schrand A.M., Hussain S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010;8:1–9. doi: 10.1186/1477-3155-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lu L., Sun R.W.Y., Chen R., Hui C.K., Ho C.M., Luk J.M., Lau G.K.K., Che C.M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008;13:252–262. [PubMed] [Google Scholar]
- 220.Elechiguerra J.L., Burt J.L., Morones J.R., Camacho-Bragado A., Gao X., Lara H.H., Yacaman M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005;3:1–10. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jeremiah S.S., Miyakawa K., Morita T., Yamaoka Y., Ryo A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020;533:195–200. doi: 10.1016/j.bbrc.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Avilala J., Golla N. Antibacterial and Antiviral Properties of Silver Nanoparticles Synthesized By Marine Actinomycetes. Int. J. Pharm. Sci. Res. 2019;10:1223–1228. doi: 10.13040/IJPSR.0975-8232.10(3).1223-28. [DOI] [Google Scholar]
- 223.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2012 doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.L. Dong, S. Hu, J. Gao, Discovering drugs to treat coronavirus disease 2019 (COVID-19), 14 (2020) 58–60. 10.5582/ddt.2020.01012. [DOI] [PubMed]
- 225.Mckee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gautret P., Lagier J., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Esteves V., Tissot H., Honoré S., Colson P., Chabrière E., La B., Rolain J., Brouqui P., Raoult D. International Journal of Antimicrobial Agents Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial ✩. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 227.A.C. Kalil, Treating COVID-19 — Off-Label Drug Use , Compassionate Use , and Randomized Clinical Trials During Pandemics, 68135 (2020). 10.1056/NEJMoa1604330. [DOI] [PubMed]
- 228.Shree P., Mishra P., Selvaraj C., Singh S.K., Chaube R., Garg N., Tripathi Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants – Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) – a molecular docking study. J. Biomol. Struct. Dyn. 2022;40:190–203. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhu F., Li Y., Guan X., Hou L., Wang W., Li J., Wu S., Wang B., Wang Z., Wang L., Jia S. Articles Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.H. Wang, Y. Zhang, B. Huang, G.F. Gao, W. Tan, X. Yang, Article Development of an Inactivated Vaccine Candidate , BBIBP-CorV , with Potent Protection against SARS- ll Article Development of an Inactivated Vaccine Candidate , BBIBP-CorV , with Potent Protection against SARS-CoV-2, (2020) 713–721. 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed]
- 231.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.B. Doroftei, A. Ciobica, O. Ilie, R. Maftei, C. Ilea, Mini-Review Discussing the Reliability and Efficiency of COVID-19 Vaccines, (2021) 1–11. [DOI] [PMC free article] [PubMed]
- 233.Shen X., Tang H., Pajon R., Smith G., Glenn G.M., Shi W., Korber B., Montefiori D.C. Neutralization of SARS-CoV-2 Variants B.1.429 and B.1.351. N. Engl. J. Med. 2021;384:2352–2354. doi: 10.1056/nejmc2103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K.L., Robb M.L., Treanor J., Barouch D.H., Stoddard J., Ryser M.F., Marovich M.A., Neuzil K.M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guiñazú J., Le Gars M., Schuitemaker H., Van Hoof J., Struyf F., Douoguih M. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/nejmoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jones I., Roy P. Comment Sputnik V COVID-19 vaccine candidate appears safe and effective Next-generation COVID-19 vaccines : here come the proteins. Lancet. 2021;397:642–643. doi: 10.1016/S0140-6736(21)00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.M. Voysey, S. Ann, C. Clemens, S.A. Madhi, L.Y. Weckx, P.M. Folegatti, P.K. Aley, B. Angus, V.L. Baillie, S.L. Barnabas, Q.E. Bhorat, S. Bibi, C. Briner, P. Cicconi, A.M. Collins, R. Colin-jones, C.L. Cutland, D.M. Ferreira, A. Finn, A.L. Goodman, C.M. Green, C.A. Green, P.T. Heath, C. Hill, H. Hill, I. Hirsch, S.H.C. Hodgson, A. Izu, S. Jackson, D. Jenkin, C.C.D. Joe, S. Kerridge, A. Koen, G. Kwatra, R. Lazarus, A. Mcgregor, H. Morrison, Y.F. Mujadidi, A. Nana, P.J.O. Reilly, S.D. Padayachee, A. Pittella, E. Plested, E. Sprinz, R.K. Sutherland, R. Tarrant, E.C. Thomson, M.E. Török, M. Toshner, D.P.J. Turner, J. Vekemans, T.L. Villafana, M.E.E. Watson, C.J. Williams, A.D. Douglas, A.V.S. Hill, T. Lambe, S.C. Gilbert, Articles Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2 : an interim analysis of four randomised controlled trials in Brazil , South Africa , and the UK, (n.d.) 1–13. 10.1016/S0140-6736(20)32661-1.
- 237.Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., Ganneru B., Sapkal G. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., Cai H., Sarkar R., Chen W., Cutler M., Cooper D., Weaver S.C., Muik A., Sahin U., Jansen K.U., Xie X., Dormitzer P.R., Shi P.-Y. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021;384:1466–1468. doi: 10.1056/nejmc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li W., Hulswit R.J.G., Kenney S.P., Widjaja I., Jung K., Alhamo M.A., van Dieren B., van Kuppeveld F.J.M., Saif L.J., Bosch B.J. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. PNAS. 2018;115:E5135–E5143. doi: 10.1073/pnas.1802879115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tarallo R., Carberry T.P., Falanga A., Vitiello M., Galdiero S., Galdiero M., Weck M. Dendrimers functionalized with membrane-interacting peptides for viral inhibition. Int. J. Nanomed. 2013;8:521–534. doi: 10.2147/IJN.S37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Saravanan M., Asmalash T., Gebrekidan A., Gebreegziabiher D., Araya T., Hilekiros H., Barabadi H., Ramanathan K. Nano-medicine as a newly emerging approach to combat Human Immunodeficiency Virus (HIV), Pharmaceutical. Nanotechnology. 2018;06:17–27. doi: 10.2174/2211738506666180209095710. [DOI] [PubMed] [Google Scholar]
- 242.Maiti B.K. Potential Role of Peptide-Based Antiviral Therapy against SARS-CoV-2 Infection. ACS Pharmacol. Translat. Sci. 2020;3:783–785. doi: 10.1021/acsptsci.0c00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Balmeh N., Mahmoudi S., Allahyari N. Informatics in Medicine Unlocked Manipulated bio antimicrobial peptides from probiotic bacteria as proposed drugs for COVID-19 disease. Inf. Med. Unlocked. 2021;23 doi: 10.1016/j.imu.2021.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kim J., Yeom M., Lee T., Kim H.O., Na W., Kang A., Lim J.W., Park G., Park C., Song D., Haam S. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020;18:1–11. doi: 10.1186/s12951-020-00611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.B. Kar, D. Pradhan, P. Mishra, S.K. Bhuyan, G. Ghosh, G. Rath, Exploring the Potential of Metal Nanoparticles as a Possible Therapeutic Adjunct for Covid-19 Infection, Proceedings of the National Academy of Sciences India Section B - Biological Sciences. 92 (2022) 511–521. 10.1007/s40011-022-01371-1. [DOI] [PMC free article] [PubMed]
- 246.Alghrair Z.K., Fernig D.G., Ebrahimi B. Enhanced inhibition of influenza virus infection by peptide-noble-metal nanoparticle conjugates, Beilstein. J. Nanotechnol. 2019;10:1038–1047. doi: 10.3762/BJNANO.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Morris D., Ansar M., Speshock J., Ivanciuc T., Qu Y., Casola A., Garofalo R. Antiviral and immunomodulatory activity of silver nanoparticles in experimental rsv infection. Viruses. 2019;11 doi: 10.3390/v11080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Erol I., Enes S., Ozkan K., Ahmet F., Serdar E.Y., Fatih D. In Silico Analysis of Bacteriocins from Lactic Acid Bacteria Against SARS - CoV - 2. Probiotics Antimicrob. Proteins. 2021 doi: 10.1007/s12602-021-09879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Haase A., Rott S., Mantion A., Graf P., Plendl J., Thünemann A.F., Meier W.P., Taubert A., Luch A., Reiser G. Effects of silver nanoparticles on primary mixed neural cell cultures: Uptake, oxidative stress and acute calcium responses. Toxicol. Sci. 2012;126:457–468. doi: 10.1093/toxsci/kfs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Thaya R., Malaikozhundan B., Vijayakumar S., Sivakamavalli J., Jeyasekar R., Shanthi S., Vaseeharan B., Ramasamy P., Sonawane A. Chitosan coated Ag/ZnO nanocomposite and their antibiofilm, antifungal and cytotoxic effects on murine macrophages. Microb. Pathog. 2016;100:124–132. doi: 10.1016/j.micpath.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 251.Yoon H., Pangging M., Jang M.H., Hwang Y.S., Chang Y.S. Impact of surface modification on the toxicity of zerovalent iron nanoparticles in aquatic and terrestrial organisms. Ecotoxicol. Environ. Saf. 2018;163:436–443. doi: 10.1016/j.ecoenv.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 252.Wang X., Ji Z., Chang C.H., Zhang H., Wang M., Liao Y.P., Lin S., Meng H., Li R., Sun B., Van Winkle L., Pinkerton K.E., Zink J.I., Xia T., Nel A.E. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small. 2014;10:385–398. doi: 10.1002/smll.201301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chia S.L., Leong D.T. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mishra A.R., Zheng J., Tang X., Goering P.L. Silver nanoparticle-induced autophagic-Lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol. Sci. 2016;150:473–487. doi: 10.1093/toxsci/kfw011. [DOI] [PubMed] [Google Scholar]
- 255.Bondarenko O., Juganson K., Ivask A., Kasemets K., Mortimer M., Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013;87:1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.AlSalhi M.S., Elangovan K., Ranjitsingh A.J.A., Murali P., Devanesan S. Synthesis of silver nanoparticles using plant derived 4-N-methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci. 2019;26:970–978. doi: 10.1016/j.sjbs.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Huo Y., Singh P., Kim Y.J., Soshnikova V., Kang J., Markus J., Ahn S., Castro-Aceituno V., Mathiyalagan R., Chokkalingam M., Bae K.S., Yang D.C. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif. Cells Nanomed. Biotechnol. 2018;46:303–312. doi: 10.1080/21691401.2017.1307213. [DOI] [PubMed] [Google Scholar]
- 258.Maliszewska I. Microbial mediated synthesis of gold nanoparticles: Preparation, characterization and cytotoxicity studies. Dig. J. Nanomater. Biostruct. 2013;8:1123–1131. [Google Scholar]
- 259.Lee K.D., Nagajyothi P.C., Sreekanth T.V.M., Park S. Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J. Ind. Eng. Chem. 2015;26:67–72. doi: 10.1016/j.jiec.2014.11.016. [DOI] [Google Scholar]
- 260.Sunderam V., Thiyagarajan D., Lawrence A.V., Mohammed S.S.S., Selvaraj A. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J Biol. Sci. 2019;26:455–459. doi: 10.1016/j.sjbs.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chen R., Qiao J., Bai R., Zhao Y., Chen C. Intelligent testing strategy and analytical techniques for the safety assessment of nanomaterials. Anal. Bioanal. Chem. 2018;410:6051–6066. doi: 10.1007/s00216-018-0940-y. [DOI] [PubMed] [Google Scholar]
- 262.Kim K.T., Zaikova T., Hutchison J.E., Tanguay R.L. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol. Sci. 2013;133:275–288. doi: 10.1093/toxsci/kft081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jia H.R., Zhu Y.X., Duan Q.Y., Chen Z., Wu F.G. Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. J. Control. Release. 2019;311–312:301–318. doi: 10.1016/j.jconrel.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 264.J. Zhang, S. Wang, M. Gao, R. Li, S. Liu, Multihierarchically Profiling the Biological Effects of Various Metal-Based Nanoparticles in Macrophages under Low Exposure Doses, 2018. 10.1021/acssuschemeng.8b01744 10.1021/acssuschemeng.8b01744.
- 265.Dekkers S., Williams T.D., Zhang J., Zhou J., Vandebriel R.J., De La Fonteyne L.J.J., Gremmer E.R., He S., Guggenheim E.J., Lynch I., Cassee F.R., De Jong W.H., Viant M.R. Multi-omics approaches confirm metal ions mediate the main toxicological pathways of metal-bearing nanoparticles in lung epithelial A549 cells, Environmental Science. Nano. 2018;5:1506–1517. doi: 10.1039/c8en00071a. [DOI] [Google Scholar]
- 266.Garcia-Reyero N., Kennedy A.J., Escalon B.L., Habib T., Laird J.G., Rawat A., Wiseman S., Hecker M., Denslow N., Steevens J.A., Perkins E.J. Differential effects and potential adverse outcomes of ionic silver and silver nanoparticles in vivo and in vitro. Environ. Sci. Tech. 2014;48:4546–4555. doi: 10.1021/es4042258. [DOI] [PubMed] [Google Scholar]
- 267.Hansjosten I., Rapp J., Reiner L., Vatter R., Fritsch-Decker S., Peravali R., Palosaari T., Joossens E., Gerloff K., Macko P., Whelan M., Gilliland D., Ojea-Jimenez I., Monopoli M.P., Rocks L., Garry D., Dawson K., Röttgermann P.J.F., Murschhauser A., Rädler J.O., Tang S.V.Y., Gooden P., Belinga-Desaunay M.F.A., Khan A.O., Briffa S., Guggenheim E., Papadiamantis A., Lynch I., Valsami-Jones E., Diabaté S., Weiss C. Microscopy-based high-throughput assays enable multi-parametric analysis to assess adverse effects of nanomaterials in various cell lines. Arch. Toxicol. 2018;92:633–649. doi: 10.1007/s00204-017-2106-7. [DOI] [PubMed] [Google Scholar]
- 268.Chen Y., Xu M., Zhang J., Ma J., Gao M., Zhang Z., Xu Y., Liu S. Genome-Wide DNA Methylation Variations upon Exposure to Engineered Nanomaterials and Their Implications in Nanosafety Assessment. Adv. Mater. 2017;29 doi: 10.1002/adma.201604580. [DOI] [PubMed] [Google Scholar]
- 269.Gomes S.I.L., Roca C.P., Scott-Fordsmand J.J., Amorim M.J.B. High-throughput transcriptomics reveals uniquely affected pathways: AgNPs, PVP-coated AgNPs and Ag NM300K case studies. Environmental Science Nano. 2017;4:929–937. doi: 10.1039/C6EN00652C. [DOI] [Google Scholar]
- 270.Poon W.L., Alenius H., Ndika J., Fortino V., Kolhinen V., Meščeriakovas A., Wang M., Greco D., Lähde A., Jokiniemi J., Lee J.C.Y., El-Nezami H., Karisola P. Nano-sized zinc oxide and silver, but not titanium dioxide, induce innate and adaptive immunity and antiviral response in differentiated THP-1 cells. Nanotoxicology. 2017;11:936–951. doi: 10.1080/17435390.2017.1382600. [DOI] [PubMed] [Google Scholar]
- 271.Mittal A.K., Banerjee U.C. In vivo safety, toxicity, biocompatibility and anti-tumour efficacy of bioinspired silver and selenium nanoparticles. Mater. Today Commun. 2021;26 doi: 10.1016/j.mtcomm.2020.102001. [DOI] [Google Scholar]
- 272.Nethi S.K., Das S., Patra C.R., Mukherjee S. Recent advances in inorganic nanomaterials for wound-healing applications, Biomaterials. Science. 2019;7:2652–2674. doi: 10.1039/c9bm00423h. [DOI] [PubMed] [Google Scholar]
- 273.Boomi P., Ganesan R., Prabu Poorani G., Jegatheeswaran S., Balakumar C., Gurumallesh Prabu H., Anand K., Marimuthu Prabhu N., Jeyakanthan J., Saravanan M. Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in vitro and in vivo Conditions. Int. J. Nanomed. 2020;15:7553–7568. doi: 10.2147/IJN.S257499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Soratijahromi E., Mohammadi S., Dehdari Vais R., Azarpira N., Sattarahmady N. Photothermal/sonodynamic therapy of melanoma tumor by a gold/manganese dioxide nanocomposite: In vitro and in vivo studies. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101846. [DOI] [PubMed] [Google Scholar]
- 275.Oberdörster G., Kuhlbusch T.A.J. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact. 2018;10:38–60. doi: 10.1016/j.impact.2017.10.007. [DOI] [Google Scholar]
- 276.Yang T.Y., Hsieh Y.J., Lu P.L., Lin L., Wang L.C., Wang H.Y., Tsai T.H., Shih C.J., Tseng S.P. In vitro and in vivo assessments of inspired Ag/80S bioactive nanocomposites against carbapenem-resistant Klebsiella pneumoniae. Mater. Sci. Eng. C. 2021;125 doi: 10.1016/j.msec.2021.112093. [DOI] [PubMed] [Google Scholar]
- 277.Hassanen E.I., Ragab E. In Vivo and In Vitro Assessments of the Antibacterial Potential of Chitosan-Silver Nanocomposite Against Methicillin-Resistant Staphylococcus aureus–Induced Infection in Rats. Biol. Trace Elem. Res. 2021;199:244–257. doi: 10.1007/s12011-020-02143-6. [DOI] [PubMed] [Google Scholar]
- 278.Samadian H., Khastar H., Ehterami A., Salehi M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: in vitro and in vivo study. Sci. Rep. 2021;11:13877. doi: 10.1038/s41598-021-93367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.G.A. Somorjai, Y. Li, Impact of surface chemistry, Proceedings of the National Academy of Sciences of the United States of America. 108 (2011) 917–924. 10.1073/pnas.1006669107. [DOI] [PMC free article] [PubMed]
- 280.Wang A.Z., Gu F., Zhang L., Chan J.M., Radovic-Moreno A., Shaikh M.R., Farokhzad O.C. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin. Biol. Ther. 2008;8:1063–1070. doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mu Q., Jiang G., Chen L., Zhou H., Fourches D., Tropsha A., Yan B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014;114:7740–7781. doi: 10.1021/cr400295a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pensa E., Cortés E., Corthey G., Carro P., Vericat C., Fonticelli M.H., Benítez G., Rubert A.A., Salvarezza R.C. The chemistry of the sulfur-gold interface: In search of a unified model. Acc. Chem. Res. 2012;45:1183–1192. doi: 10.1021/ar200260p. [DOI] [PubMed] [Google Scholar]
- 283.Sperling R.A., Parak W.J. Surface modification, functionalization and bioconjugation of colloidal Inorganic nanoparticles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010;368:1333–1383. doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- 284.DeLong R.K., Reynolds C.M., Malcolm Y., Schaeffer A., Severs T., Wanekaya A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010;3:53–63. doi: 10.2147/NSA.S8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Totaro K.A., Liao X., Bhattacharya K., Finneman J.I., Sperry J.B., Massa M.A., Thorn J., Ho S.V., Pentelute B.L. Systematic Investigation of EDC/sNHS-Mediated Bioconjugation Reactions for Carboxylated Peptide Substrates. Bioconjug. Chem. 2016;27:994–1004. doi: 10.1021/acs.bioconjchem.6b00043. [DOI] [PubMed] [Google Scholar]
- 286.Ismail N.S., Gopinath S.C.B. Enhanced antibacterial effect by antibiotic loaded starch nanoparticle. J. Assoc. Arab Univ. Basic Appl. Sci. 2017;24:136–140. doi: 10.1016/j.jaubas.2016.10.005. [DOI] [Google Scholar]
- 287.Langeveld W.T., Veldhuizen E.J.A., Burt S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014;40:76–94. doi: 10.3109/1040841X.2013.763219. [DOI] [PubMed] [Google Scholar]
- 288.De Leon Rodriguez L.M., Kaur H., Brimble M.A. Synthesis and bioactivity of antitubercular peptides and peptidomimetics: An update. Org. Biomol. Chem. 2016;14:1177–1187. doi: 10.1039/c5ob02298c. [DOI] [PubMed] [Google Scholar]
- 289.Anbazhagan S., Azeez S., Morukattu G., Rajan R., Venkatesan K., Thangavelu K.P. Synthesis, characterization and biological applications of mycosynthesized silver nanoparticles. 3 Biotech. 2017;7:1–9. doi: 10.1007/s13205-017-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Pal I., Brahmkhatri V.P., Bera S., Bhattacharyya D., Quirishi Y., Bhunia A., Atreya H.S. Enhanced stability and activity of an antimicrobial peptide in conjugation with silver nanoparticle. J. Colloid Interface Sci. 2016;483:385–393. doi: 10.1016/j.jcis.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 291.Pandit R., Rai M., Santos C.A. Enhanced antimicrobial activity of the food-protecting nisin peptide by bioconjugation with silver nanoparticles. Environ. Chem. Lett. 2017;15:443–452. doi: 10.1007/s10311-017-0626-2. [DOI] [Google Scholar]
- 292.Sidhu P.K., Nehra K. Bacteriocin-capped silver nanoparticles for enhanced antimicrobial efficacy against food pathogens. IET Nanobiotechnol. 2020;14:245–252. doi: 10.1049/iet-nbt.2019.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Radzig M.A., Nadtochenko V.A., Koksharova O.A., Kiwi J., Lipasova V.A., Khmel I.A. Colloids and Surfaces B : Biointerfaces Antibacterial effects of silver nanoparticles on gram-negative bacteria : Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces. 2013;102:300–306. doi: 10.1016/j.colsurfb.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 294.Pérez-Ortiz M., Zapata-Urzúa C., Acosta G.A., Álvarez-Lueje A., Albericio F., Kogan M.J. Gold nanoparticles as an efficient drug delivery system for GLP-1 peptides. Colloids Surf. B Biointerfaces. 2017;158:25–32. doi: 10.1016/j.colsurfb.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 295.Bartczak D., Kanaras A.G. Preparation of peptide-functionalized gold nanoparticles using one pot EDC/Sulfo-NHS coupling. Langmuir. 2011;27:10119–10123. doi: 10.1021/la2022177. [DOI] [PubMed] [Google Scholar]
- 296.Peng L.H., Huang Y.F., Zhang C.Z., Niu J., Chen Y., Chu Y., Jiang Z.H., Gao J.Q., Mao Z.W. Integration of antimicrobial peptides with gold nanoparticles as unique non-viral vectors for gene delivery to mesenchymal stem cells with antibacterial activity. Biomaterials. 2016;103:137–149. doi: 10.1016/j.biomaterials.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 297.Thirumurugan A., Ramachandran S., Shiamala Gowri A. Combined effect of bacteriocin with gold nanoparticles against food spoiling bacteria - an approach for food packaging material preparation. Int. Food Res. J. 2013;20:1909–1912. [Google Scholar]
- 298.Lee B., Lee D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J. Appl. Microbiol. 2019;127:701–712. doi: 10.1111/jam.14357. [DOI] [PubMed] [Google Scholar]
- 299.Jamil B., Syed M.A. Nano-antimicrobials, A viable approach to tackle multidrug-resistant pathogens. Nanotechnol. Appl. Pharm. Technol. 2017:31–54. doi: 10.1007/978-3-319-70299-5_2. [DOI] [Google Scholar]
- 300.Sinha A., Simnani F.Z., Singh D., Nandi A., Choudhury A., Patel P., Jha E., chouhan R.S., Kaushik N.K., Mishra Y.K., Panda P.K., Suar M., Verma S.K. The translational paradigm of nanobiomaterials: Biological chemistry to modern applications. Materials Today Bio. 2022;17 doi: 10.1016/j.mtbio.2022.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Husain S., Nandi A., Simnani F.Z., Saha U., Ghosh A., Sinha A., Sahay A., Samal S.K., Panda P.K., Verma S.K. Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology. J. Funct. Biomater. 2023;14:47. doi: 10.3390/jfb14010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ge L., Li Q., Wang M., Ouyang J., Li X., Xing M.M.Q. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014;9:2399–2407. doi: 10.2147/IJN.S55015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Graves J.L., Thomas M., Ewunkem J.A. Antimicrobial nanomaterials: Why evolution matters. Nanomaterials. 2017;7:1–10. doi: 10.3390/nano7100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Gao W., Chen Y., Zhang Y., Zhang Q., Zhang L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018;127:46–57. doi: 10.1016/j.addr.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Makvandi P., yu Wang C., Zare E.N., Borzacchiello A., na Niu L., Tay F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201910021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.