Abstract
Sarcopenia is a syndrome characterized by progressive loss of skeletal muscle mass, strength and function, which is one of the most important clinical features of cancer malnutrition, representing a poor prognostic indicator in oncology. Sarcopenia is commonly assessed by measuring the skeletal muscle index (SMI) of the third lumbar spine (L3) using computed tomography (CT). The primary aim of this meta‐analysis was to study the association between low SMI and comprehensive clinicopathological characteristics as well as prognosis in patients with ovarian cancer. Data were searched in PubMed, EMBASE and Cochrane databases from inception to 10 June 2022. Studies evaluating the prognostic effect of SMI on ovarian cancer survival or chemotherapy‐related side effects were included. The risk of bias and study quality were assessed via the Newcastle–Ottawa Scale (NOS). The search strategy yielded 1286 hits in all three databases combined. Thirteen studies were included for qualitative and quantitative analysis, comprising 1814 patients. Our meta‐analysis revealed the significant association between low SMI and progression‐free survival (PFS) [P = 0.02; hazard ratio (HR): 1.40, 95% confidence interval (CI): 1.06–1.85], as well as 5‐year overall survival (OS) [P = 0.02; odds ratio (OR): 1.35, 95% CI: 1.05–1.74]. Low SMI was also obviously associated with body mass index (BMI) < 25 (P < 0.00001; OR: 5.08, 95% CI: 3.54–7.30), FIGO stage (P = 0.02; OR: 1.62, 95% CI: 1.06–2.45) and R0 cytoreduction (P = 0.04;OR: 1.34, 95% CI: 1.01–1.79). There was no correlation between low SMI and histological types, pathological grades and chemotherapy‐related toxicity. The quality of the evidence was relatively high according to NOS. Our meta‐analysis indicated that sarcopenia assessed by SMI was associated with poor clinical characteristics and adverse prognosis in patients with ovarian cancer. Consensus should be reached on standardized cut‐off values for defining sarcopenia in patients with ovarian cancer.
Keywords: skeletal muscle index, ovarian cancer, clinical characteristic, prognosis, meta‐analysis
Introduction
Among gynaecological malignancies, ovarian cancer is one of the most prominent and leading causes of female mortality worldwide. Over the past 25 years, only few improvements have been made in long‐term survival rates. 1 Owing to the lack of specific symptoms and effective screening modalities, the majority of patients are diagnosed at advanced stage with large tumours and ascites. Therefore, patients with ovarian cancer often appear malnourished at the presentation time. 2
Sarcopenia is one of the most important clinical features of cancer malnutrition, which is a complex syndrome characterized by progressive decreases in skeletal muscle mass, strength and function. 3 Computed tomography (CT) has been used to image tumours and respond to treatment of patients with cancer and could be used to assess skeletal muscle mass. CT scan assessment of skeletal muscle index (SMI) is significantly correlated with whole‐body muscle mass and is the most commonly used method for defining sarcopenia. 3 , 4 A systematic review that included 35 articles involving 6894 patients showed that the prevalence of pre‐therapeutic sarcopenia was 38.6% in cancer patients. Meanwhile, pre‐therapeutic sarcopenia was significantly and independently associated with postoperative complications, chemotherapy‐induced toxicity and poor survival. 5
There was limited information on impact of SMI on ovarian cancer. One systematic review with meta‐analysis published in 2019 revealed a significant association between SMI and overall survival (OS), 1 and another meta‐analysis published in 2020 indicated that there was no correlation, 6 which means there was no definitive conclusions of the impact of SMI on the prognosis of ovarian cancer can be drawn. The current study aimed to perform a systematic review and meta‐analysis of the association between low L3 SMI and clinicopathological features as well as adverse outcomes in patients with ovarian cancer. To our knowledge, this is the first meta‐analysis to comprehensively assess the relationship between low SMI and clinicopathological features, chemotherapy sensitivity and prognosis in ovarian cancer.
Methods
Search strategy
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐analyses and registered with the PROSPERO International Prospective Register of Systematic Reviews (CRD). 7 A systematic literature search was conducted using PubMed, EMBASE and Cochrane databases on 10 June 2022. The following terms were used in the search strategy: ‘sarcopenia’, ‘skeletal muscle’, ‘cachexia’, ‘ovarian cancer’, ‘ovarian tumour’ and ‘ovarian malignancy’.
Eligibility criteria
Studies were included based on predefined inclusion criteria and were limited to English language studies describing human subjects with ovarian cancer undergoing treatment. Studies must have assessed body composition with CT, described sarcopenia in the context of SMI, with PFS or OS or chemotherapy‐related toxicity as the reported outcomes, and the association between clinicopathologic variables and SMI should be described. Studies were limited to those with SMI measurements of total skeletal muscle area, including the psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques and rectus abdominis muscles. Studies using CT analysis of psoas muscle only were excluded. Studies were excluded if they were conference abstracts, review articles or case reports or reported on cohorts of less than five patients or where data were unavailable or not interpretable.
Data extraction
Data extraction was performed by one author (N. Zhang), and the data were independently checked by two authors (Y. Jin and X. Ma). The following data were extracted from included articles: author, year, study/cohort, country/region, demographical information [sample size, age (mean/median)], body composition assessment methods and definitions and survival outcomes.
Methodological quality assessment
Two researchers (X. Ma and Z. Yang) independently assessed the methodological quality of the included studies via the Newcastle–Ottawa Scale (NOS). We assigned tailored quality‐related criteria to the domains: patient selection, study comparability and study endpoints, resulting in a maximum score of nine. The high quality was defined as a score greater than 7, whereas moderate quality was described as a score between 5 and 7. Studies that earned scores of less than 5 were considered low quality. 8
Data synthesis and analysis
We estimated the odds ratio (OR) or hazard ratio (HR) for clinicopathologic variables (FIGO III–IV vs. FIGO I–II; Grade 3 or Grade 2 vs. Grade 1), chemotherapy‐related toxicity, PFS and 5‐year OS. Statistical heterogeneity assumption among studies was checked using the χ 2‐based Q‐test. When I 2 was less than 50%, pooled odds ratios, relative risk and 95% confidence intervals (CIs) were calculated using Mantel–Haenszel method with fixed effects models. Where significant heterogeneity among the studies was detected (I 2 > 50%), a random‐effect model was adopted. If necessary, a sensitivity analysis was also performed to evaluate the influence of individual studies on the final effect. All P values were two sided. A P value <0.05 was considered significant. All the statistical analyses were performed using RevMan 5.0 software (The Cochrane Collaboration, Oxford, UK).
Results
Search results and included studies
A comprehensive search of three databases resulted in the identification of 1286 studies. After the automatic removal of 876 duplicates, 410 titles and abstracts were screened by two independent researchers (N. Zhang and X. Ma). Abstracts and titles were screened, and consensus was reached. Total 81 full‐text articles were considered for inclusion. After full‐text review of these 81 articles, 13 studies met the inclusion criteria and were included in the meta‐analysis. 2 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 The selection process and reasons for exclusion were illustrated in Figure 1 . The studies identified were all retrospective studies published between 2015 and 2022. The main characteristics of the included studies were summarized in Table 1 .
Figure 1.
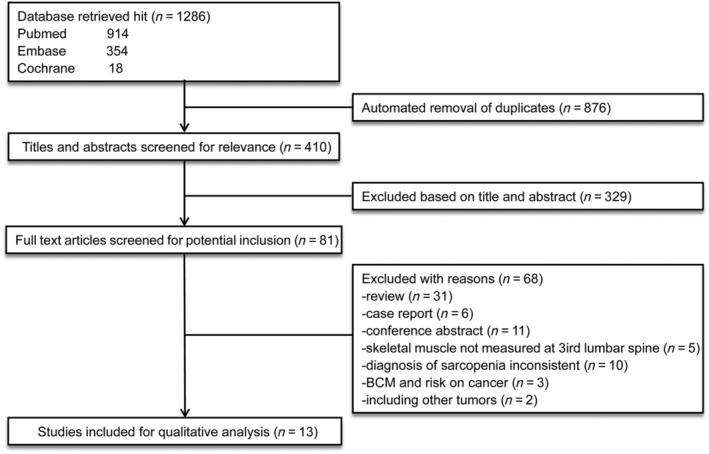
Flowchart of study selection. Thirteen independent studies were included in the final review. BCM, body composition measurement.
Table 1.
Characteristics of studies included in this meta‐analysis
| Author | Year | Country | Study design | Study period | n | Mean age | Median Age | Mean BMI(kg/m2) | Median BMI(kg/m2) | Disease stage(FIGO) | Time of assessment | SMI cut‐point (cm2/m2) | Prevalence of sarcopenia (%) (based on SMI) | Outcome | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Su Hyun Chae 13 | 2021 | Korean | RS | 2002–2017 | 82 | n.a. | 52 | n.a. | 22.6 | I–II | Pre‐operation | ≤38.73 | 20.7 | PFS, OS | 8 |
| Angiolo Gadducci 15 | 2022 | Roma | RS | 2010–2020 | 134 | n.a. | 59 | n.a. | 22.5 | I–IV | Pre‐operation | <40.1 | 50.0 | PFS, OS | 8 |
| Beyan Ataseven 9 | 2018 | Germany | RS | 2011–2016 | 323 | n.a. | 67 | n.a. | n.a. | IIIB–IVB | Pre‐operation |
<38.5 <39 <41 |
29.4 33.7 47.1 |
OS | 9 |
| Holger Bronger 11 | 2017 | Germany | RS | 2003–2013 | 105 | n.a. | 65 | 25.0 ± 5.4 | n.a. | III–IV | At diagnosis | ≤38.5 | 11.4 | PFS, OS | 9 |
| S. Allison Staley 20 | 2020 | America | RS | 2000–2017 | 201 | n.a. | 63.6 | n.a. | 26.9 | I–IV | Pre‐operation | ≤41 | 63.7 | PFS, OS, chemo toxicity | 9 |
| Amanika Kumar 17 | 2016 | America | RS | 2006–2012 | 296 | 64.6 ± 10.6 | n.a. | n.a. | n.a. | IIIC–IV | Pre‐operation | <39.0 | 44.6 | PFS, OS | 9 |
| I.J.G. Rutten 18 | 2017 | Netherlands | RS | 2000–2015 | 216 | 63.1 ± 0.8 | n.a. | 24.9 ± 0.3 | n.a. | III–IV | Not specified | ≤38.73 | 32.4 | OS | 8 |
| I.J.G. Rutten 19 | 2016 | Netherlands | RS | 2000–2014 | 123 | 66.5 ± 0.8 | n.a. | 25.9 ± 0.5 | n.a. | II–IV | At diagnosis | <41.5 | 50.4 | Chemo toxicity, OS | 9 |
| Stefanie Aust 10 | 2015 | Austria | RS | 2004–2012 | 140 | 60 ± 13 | n.a. | 24.9 ± 4.8 | n.a. | I–IV | Pre‐operation | <41 | 28.9 | PFS, OS | 9 |
| Chueh‐Yi Huang 2 | 2020 | Taiwan | RS | 2008–2017 | 147 | 54.5 ± 10.5 | n.a. | 22.3 ± 3.3 | n.a. | IIIA–IIIB | Pre‐operation | <39.1 | 34.1 | PFS, OS | 7 |
| Se Ik Kim 16 | 2020 | Korean | RS | 2010–2017 | 179 | 57.5 ± 10.6 | n.a. | 23.6 ± 3.2 | n.a. | IIIA–IVB | Pre‐treatment | <39 | 42.5 | PFS, OS | 8 |
| Maria Del Grande 14 | 2021 | Switzerland | RS | 2011–2020 | 69 | 65 ± 11.45 | n.a. | 24.9 ± 6.1 | n.a. | IB–IV | At diagnosis | <41 | 29.0 | Chemo toxicity, OS | 9 |
| Karine de Aguiar Bruno 12 | 2021 | Brazil | RS | 2008–2017 | 239 | 56.3 ± 11.4 | n.a. | n.a. | n.a. | I–IV | Pre‐treatment | <38.9 | 35.1 | Chemo toxicity, OS | 9 |
BMI, body mass index; FIGO, International Federation for Gynecologic Oncology; n.a., not applicable; NOS, Newcastle–Ottawa Scale; OS, overall survival; PFS, progression−free survival; RS, retrospective; SMI, skeletal muscle index.
Patient characteristics
The sample size of included studies ranged from 69 to 323 women, comprising a total of 1814 patients. All studies used SMI in L3 to assess sarcopenia, and the cut‐off used to define sarcopenia ranged from 38.5 to 41.5 cm2/m2. Owing to the different SMI cut‐offs for assessing sarcopenia, sarcopenia prevalence estimated by SMI in the included studies of our meta‐analysis of ovarian cancer cases had a wide range from 11.4% to 63.7%. The patient's mean age ranged from 54.5 ± 10.5 to 66.5 ± 0.8 years, whereas median age ranged from 52 to 67 years. The mean BMI ranged from 22.3 ± 3.3 to 25.9 ± 0.5 kg/m2, and the median BMI ranged from 22.5 to 26.9 kg/m2. All patients underwent primary or interval debulking surgery combined with adjuvant or neoadjuvant chemotherapy. FIGO (International Federation for Gynecologic Oncology) stage across all studies ranged from I to IV. The distribution of patients according to the FIGO stage was as follows: I–II, n = 216(12%); III–IV, n = 1598 (88%). The European Working Group on Sarcopenia in Older People (EWGSOP) made a major change to the definition of sarcopenia in 2018; it added muscle function to former definitions based only on detection of low muscle mass. 3 Because muscle strength was not measured in the articles included in our meta‐analysis, we defined the patients in our analysis as ovarian cancer patients with low SMI and analysed the association between low SMI and clinicopathological features of patients with ovarian cancer.
Low SMI and BMI
Five studies were included in the meta‐analysis to assess the effect of SMI‐assessed sarcopenia on BMI, 11 , 15 , 16 , 17 , 20 depicted in Figure 2 . According to the data from the studies, patients with low SMI and those with high SMI were divided into two groups, respectively, BMI less than 25 kg/m2 and BMI more than 25 kg/m2. The correlation between low SMI and BMI was significant (P < 0.00001; OR:5.08, 95% CI:3.54–7.30), which means that patients with low SMI usually had lower BMI. Again, the fixed effects model was chosen. A χ 2‐test P value of 1.00 and an I 2 of 0% indicated methodological homogeneity between studies.
Figure 2.
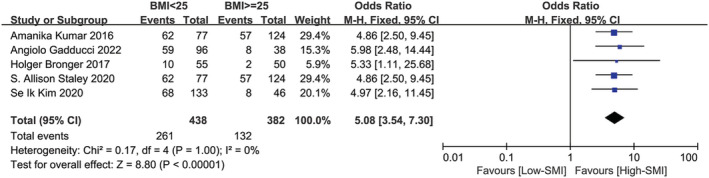
Forest plot assessing the correlation between low SMI and BMI. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.
Low SMI and Clinicopathological features
The meta‐analysis of the association between SMI‐assessed sarcopenia and clinicopathological features, including FIGO stage, pathological types, grades of pathology and intraoperative R0 cytoreduction, which were shown in Figure 3 . There were 127 patients in stages I–II and 638 patients in stages III–IV in the four studies included in the meta‐analysis. 12 , 15 , 18 , 20 The correlation between low SMI and FIGO stage was significant (P = 0.02; OR: 1.62, 95% CI:1.06–2.45). A χ 2‐test P‐value of 0.38 and an I 2 of 4% indicated methodological homogeneity between studies. Six studies were included to analyse the relationship between low SMI and R0 cytoreduction. 2 , 11 , 13 , 15 , 17 , 18 A total of 421 patients achieved R0 cytoreduction, including 174 patients with low SMI. A total of 457 patients did not achieve R0 cytoreduction, including 174 patients with low SMI, indicating a significant correlation between low SMI and R0 cytoreduction (P = 0.04; OR: 1.34, 95% CI:1.01–1.79), a χ 2‐test P value of 0.95 and an I 2 of 0%. These findings confirmed that low SMI might be associated with advanced stage, and it was more challenging to achieve R0 cytoreduction in ovarian cancer patients with low SMI. Six 2 , 11 , 13 , 17 , 18 , 20 and five 2 , 12 , 13 , 17 , 20 articles were included respectively to analyse the correlation between low SMI and pathological grades as well as pathological types of ovarian cancer patients, but the results were negative, which were depicted in Figures S1 and S2 , respectively.
Figure 3.
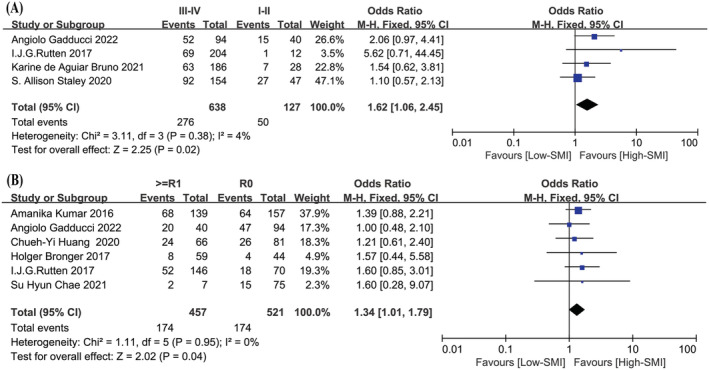
The correlation between low SMI and clinicopathological features. (A) Forest plot assessing the correlation between low SMI and FIGO stage. (B) Forest plot assessing the correlation between low SMI and R0 cytoreduction. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.
Low SMI and chemotherapy‐related toxicity
In terms of chemotherapy‐related toxicity, according to the analysis of the three included studies, 12 , 14 , 20 there was no association between low SMI and delays of chemotherapy and reduction of chemotherapy drugs. Two studies were included in the meta‐analysis about the effect of low SMI on platinum sensitivity. 12 , 16 Obviously, no association was found between low SMI and platinum sensitivity, as depicted in Figure 4 .
Figure 4.
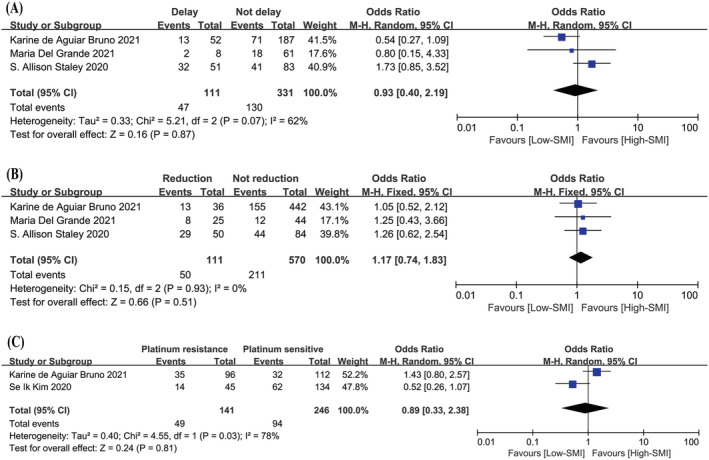
The correlation between low SMI and chemotherapy‐related toxicity. (A) Forest plot assessing the correlation between low SMI and delays of chemotherapy. (B) Forest plot assessing the correlation between low SMI and reduction of chemotherapy. (C) Forest plot assessing the correlation between low SMI and platinum sensitivity. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.
Low SMI and PFS
Three studies involving 424 patients were included into the meta‐analysis of the multivariate data on the influence of sarcopenia assessed by SMI on PFS, 10 , 11 , 16 which was depicted in Figure 5 . The overall effect of low SMI on PFS was significant (P = 0.02; HR: 1.40, 95% CI:1.06–1.85). Statistical consistency between the compared HRs and 95% CIs was evaluated with the χ 2 and I 2 tests, which returned a χ 2‐test P‐value of 0.34 and an I 2 of 9%, indicating methodological homogeneity between studies. The fixed effects model was deliberately chosen because the methodologies for measuring SMI and its association with PFS used in the studies were homogeneous.
Figure 5.
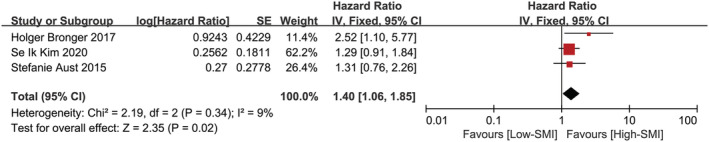
Forest plot assessing the correlation between low SMI and PFS. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Low SMI and OS
Eight studies involving 1356 patients were included in the meta‐analysis for 5‐year survival rate, 2 , 9 , 11 , 14 , 15 , 16 , 18 , 19 which was depicted in Figure 6 . The result confirmed that low SMI might have an appreciable impact on poor 5‐year survival rate (P = 0.02; OR: 1.35, 95% CI:1.05–1.74). A χ 2‐test P‐value of 0.08 and an I 2 of 45% indicated consistency between studies for this association.
Figure 6.
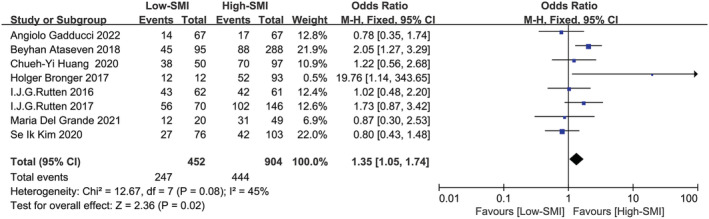
Forest plot assessing the correlation between low SMI and 5‐year overall survival rate. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.
Discussion
The purpose of this meta‐analysis was to summarize the relationship between CT‐defined SMI at L3 and the clinical characteristics as well as prognosis of the patients with ovarian cancer. We found evidence from 13 studies that low SMI‐assessed sarcopenia might be significantly associated with poorer PFS and 5‐year OS in patients with ovarian cancer, as well as with BMI, FIGO stage and R0 cytoreduction, which means ovarian cancer patients with low SMI assessed sarcopenia had lower BMI and advanced FIGO stage and were less likely to achieve R0 cytoreduction at the time of surgery. However, low SMI assessed sarcopenia was not associated with histological types, pathological grades and chemotherapy‐related toxicity.
SMI assessed by CT at the L3 vertebra level is significantly correlated with total body muscle measurements and an important indicator of the diagnosis of sarcopenia. 3 Although 38.5 and 41 cm2/m2 were the most widely used SMI cut‐offs for the diagnosis of sarcopenia for women with respiratory and gastrointestinal cancers, 21 there were no uniform SMI cut‐offs used in ovarian cancer studies. The SMI cut‐off used to define sarcopenia will directly influence the outcomes of associations made between SMI and the clinical characteristics or prognosis, and significant heterogeneity in cut‐offs for sarcopenia may lead to difficulty in interpreting the data. In the current cohort, the cut‐off used for defining sarcopenia in ovarian cancer patients ranged from 38.5 to 41 cm2/m2. Ubachs et al. observed that studies with cut‐offs between 38.5 and 38.73 cm2/m2 were more likely to report a prognostic effect of sarcopenia and proposed the use of lower cut‐off (<38.5 cm2/m2) to distinguish sarcopenic from non‐sarcopenic patients. 1 However, among the 13 studies included in our analysis, four studies demonstrated that sarcopenia assessed by low SMI was associated with poorer outcomes in patients with ovarian cancer and the SMI cut‐offs used to define sarcopenia were 38.5, 38.73, 39 and 39.1 cm2/m2, respectively. The lower cut‐off (<38.5 cm2/m2) might miss some patients (38.5–39.1 cm2/m2) with poor prognosis associated with low SMI. Furthermore, none of the studies included in our analysis with SMI cut‐off greater than 39.1 cm2/m2 drew the conclusion that sarcopenia assessed by low SMI was related to OS. 2 , 11 , 13 , 17 Therefore, we believed that the SMI cut‐off value less than 39.1 cm2/m2 could strike a balance between drawing meaningful conclusions and diagnosing more ovarian cancer patients with sarcopenia.
Our meta‐analysis demonstrated a few noteworthy aspects. Our study first found that low SMI was significantly associated with BMI, FIGO stage and R0 cytoreduction. This could be explained to an extent by the fact that women with low BMI, advanced FIGO stage and no R0 cytoreduction were usually characterized by extensive disease, poor nutritional and performance status, which were also correlated with sarcopenia. Nonetheless, in light of the high heterogeneity observed, these findings should be interpreted with caution. Body composition has been an increasingly important prognostic factor in malignant tumours. 4 Previous publications mainly focused on the correlation between sarcopenia and overall survival in patients with ovarian cancer. One systematic review with meta‐analysis published in 2019 revealed a significant association between the SMI and OS no matter univariate analysis included six studies involving 1203 patients (P = 0.007, HR: 1.11, 95% CI:1.03–1.20) or multivariate Cox regression analysis included three studies involving 461 patients (P = 0.02, HR: 1.17, 95% CI: 1.03–1.33), 1 which were consistent with our research. However, another meta‐analysis published in 2020 indicated that there was no correlation between sarcopenia and 5‐year OS (P = 0.07, OR:1.8, 95% CI 1.0–3.2) in patients with epithelial ovarian cancer (EOC), which included four studies involving 790 patients published before 2018. 6 Our meta‐analysis included eight studies involving 1356 patients suggested that low SMI was significantly associated with 5‐year survival rate; four studies were published between 2020 and 2022, which might contribute to the different results. The direct causality of low SMI on survival remains unclear. Sarcopenia assessed by SMI represents one of the hallmarks of cancer cachexia. 22 The catabolic and inflammatory changes associated with cachexia could partially explain the shorter OS. 3 It is noteworthy that low SMI also had a significant effect on PFS. Hence, it is possible that low SMI has an adverse impact not only on overall health and ability to tolerate treatment but also on disease course.
The present meta‐analysis reported a quantitative assessment of low SMI and its correlation with clinicopathological features and clinical outcomes of ovarian cancer patients. The strength of this meta‐analysis was that only ovarian cancer patients whose SMI was assessed with total skeletal muscle in the third lumbar evaluated by CT scan were included, and this condition created a homogeneous cohort study. The limitations of this meta‐analysis were that the studies included in this analysis were all retrospective. In addition, the reported prevalence of sarcopenia in ovarian cancer is heterogeneous due to the different SMI cut‐off points that defined sarcopenia. A clear understanding of the correlation between sarcopenia and clinicopathology might be helpful for precise treatment, such as whether to choose primary debulking surgery or neoadjuvant chemotherapy. Variations in diagnostic criteria of sarcopenia represent a barrier to develop guideline to improve clinical practice. Of note, the EWSOP recommend incorporating both low muscle mass and low muscle function into the definition of sarcopenia. 3 The wide use of CT scanning provides investigators useful tool to further explore the causes and definitions of sarcopenia and its association with treatment outcomes. Increased understanding of sarcopenia in patients with ovarian cancer is necessary to better develop interventions and treatment strategy. Larger prospective studies on the interventions to reverse sarcopenia and how such interventions might improve outcomes in patients with ovarian cancer are needed.
Conclusions
Our findings suggested that the SMI assessed sarcopenia evaluated by CT at L3 was seemingly prevalent in patients with ovarian cancer, especially among patients with advanced‐stage disease. Low SMI in ovarian cancer patients might be not only associated with poor PFS and 5‐year OS but also associated with low BMI and advanced FIGO stage. It is worth noting that ovarian cancer patients with low SMI were more difficult to achieve R0 cytoreduction, and complete cytoreduction was one of the most important prognostic factors for patients with ovarian cancer. The correlation between low SMI and pathological grade and pathological type, as well as chemotherapy toxicity in ovarian cancer patients cannot be clarified. Although sarcopenia appears to be associated with poorer outcome in patients with ovarian cancer, the lack of standardized SMI cut‐offs for assessing the prevalence of sarcopenia hampers the interpretation of this association and its strength. A consensus on standardized cut‐off values to define sarcopenia in patients with ovarian cancer needs to be found. Information on muscle strength and nutritional assessments should be incorporated in the future studies.
Conflict of interest
The authors declare that they have no conflicts of interest relevant to the content of this review. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 23
Funding
This study was supported by the Shanghai Sailing Program (Grant No. 22YF1424400) and the National Natural Science Foundation of China (Grant No. 82102479).
Supporting information
Figure S1. The correlation between low SMI and grade stage. (A) Forest plot assessing the prevalence of low SMI in Grade 3 patients versus that in Grade 1 patients. (B) Forest plot assessing the prevalence of low SMI in Grade 3 patients versus that in Grade 2 patients. (C) Forest plot assessing the prevalence of low SMI in Grade 2 patients versus that in Grade 1 patients. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.
Figure S2. The correlation between low SMI and pathological types. (A) Forest plot assessing the prevalence of low SMI in epithelial ovarian cancer groups versus that in other type groups. (B) Forest plot assessing the prevalence of low SMI in serous ovarian cancer groups versus that in other type groups. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.
Jin Y., Ma X., Yang Z., and Zhang N. (2023) Low L3 skeletal muscle index associated with the clinicopathological characteristics and prognosis of ovarian cancer: a meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 14, 697–705, 10.1002/jcsm.13175
Yue Jin and Xiaowei Ma contributed equally to this work.
References
- 1. Ubachs J, Ziemons J, Minis‐Rutten IJG, Kruitwagen RFPM, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2019;10:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang CY, Sun FJ, Lee J. Prognostic value of muscle measurement using the standardized phase of computed tomography in patients with advanced ovarian cancer. Nutrition 2020;72:110642. [DOI] [PubMed] [Google Scholar]
- 3. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 5. Pamoukdjian F, Bouillet T, Levy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre‐therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 2018;37:1101–1113. [DOI] [PubMed] [Google Scholar]
- 6. McSharry V, Mullee A, McCann L, Rogers AC, McKiernan M, Brennan DJ. The impact of sarcopenia and low muscle attenuation on overall survival in epithelial ovarian cancer: a systematic review and meta‐analysis. Ann Surg Oncol 2020;27:3553–3564. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 8. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 9. Ataseven B, Luengo TG, du Bois A, Waltering KU, Traut A, Heitz F, et al. Skeletal muscle attenuation (sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol 2018;25:3372–3379. [DOI] [PubMed] [Google Scholar]
- 10. Aust S, Knogler T, Pils D, Obermayr E, Reinthaller A, Zahn L, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS One 2015;10:e0140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bronger H, Hederich P, Hapfelmeier A, Metz S, Noël PB, Kiechle M, et al. Sarcopenia in advanced serous ovarian cancer. Int J Gynecol Cancer 2017;27:223–232. [DOI] [PubMed] [Google Scholar]
- 12. Bruno KA, Sobreira da Silva MJ, Chaves GV. Association of body composition with toxicity to first‐line chemotherapy and three‐year survival in women with ovarian adenocarcinoma. Acta Oncol 2021;60:1611–1620. [DOI] [PubMed] [Google Scholar]
- 13. Chae SH, Lee C, Yoon SH, Shim SH, Lee SJ, Kim SN, et al. Sarcopenia as a predictor of prognosis in early stage ovarian cancer. J Korean Med Sci 2021;36:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Grande M, Rizzo S, Nicolino GM, Colombo I, Rossi L, Manganaro L, et al. Computed tomography‐based body composition in patients with ovarian cancer: association with chemotoxicity and prognosis. Front Oncol 2021;11:718815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gadducci A, Simonetti E, Mezzapesa F, Cosio S, Miccoli M, Frey J, et al. Computed tomography‐assessed skeletal muscle index and skeletal muscle radiation attenuation in patients with ovarian cancer treated with primary surgery followed by platinum‐based chemotherapy: a single‐center Italian study. Anticancer Res 2022;42:947–954. [DOI] [PubMed] [Google Scholar]
- 16. Kim SI, Kim TM, Lee M, Kim HS, Chung HH, Cho JY, et al. Impact of CT‐determined sarcopenia and body composition on survival outcome in patients with advanced‐stage high‐grade serous ovarian carcinoma. Cancers (Basel) 2020;12:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol 2016;142:311–316. [DOI] [PubMed] [Google Scholar]
- 18. Rutten IJ, Ubachs J, Kruitwagen RF, van Dijk DP, Beets‐Tan RG, Massuger LF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol 2017;43:717–724. [DOI] [PubMed] [Google Scholar]
- 19. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets‐Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle 2016;7:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Staley SA, Tucker K, Newton M, Ertel M, Oldan J, Doherty I, et al. Sarcopenia as a predictor of survival and chemotoxicity in patients with epithelial ovarian cancer receiving platinum and taxane‐based chemotherapy. Gynecol Oncol 2020;156:695–700. [DOI] [PubMed] [Google Scholar]
- 21. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 22. Bozzetti F. Chemotherapy‐induced sarcopenia. Curr Treat Options Oncol 2020;21:7. [DOI] [PubMed] [Google Scholar]
- 23. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The correlation between low SMI and grade stage. (A) Forest plot assessing the prevalence of low SMI in Grade 3 patients versus that in Grade 1 patients. (B) Forest plot assessing the prevalence of low SMI in Grade 3 patients versus that in Grade 2 patients. (C) Forest plot assessing the prevalence of low SMI in Grade 2 patients versus that in Grade 1 patients. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.
Figure S2. The correlation between low SMI and pathological types. (A) Forest plot assessing the prevalence of low SMI in epithelial ovarian cancer groups versus that in other type groups. (B) Forest plot assessing the prevalence of low SMI in serous ovarian cancer groups versus that in other type groups. CI, confidence interval; df, degrees of freedom; M‐H, Mantel–Haenszel; SE, standard error.


