Abstract
Background
Changes in body composition and systemic inflammation are important characteristics of cancer cachexia. This multi‐centre retrospective study aimed to explore the prognostic value of the combination of body composition and systemic inflammation in patients with cancer cachexia.
Methods
The modified advanced lung cancer inflammation index (mALI), which combines body composition and systemic inflammation, was defined as appendicular skeletal muscle index (ASMI) × serum albumin/neutrophil‐lymphocyte ratio. The ASMI was estimated according to a previously validated anthropometric equation. Restricted cubic splines were used to evaluate the relationship between mALI and all‐cause mortality in patients with cancer cachexia. Kaplan–Meier analysis and Cox proportional hazard regression analysis were used to evaluate the prognostic value of mALI in cancer cachexia. A receiver operator characteristic curve was used to compare the effectiveness of mALI and nutritional inflammatory indicators in predicting all‐cause mortality in patients with cancer cachexia.
Results
A total of 2438 patients with cancer cachexia were enrolled, including 1431 males and 1007 females. The sex‐specific optimal cut‐off values of mALI for males and females were 7.12 and 6.52, respectively. There was a non‐linear relationship between mALI and all‐cause mortality in patients with cancer cachexia. Low mALI was significantly associated with poor nutritional status, high tumour burden, and high inflammation. Patients with low mALI had significantly lower overall survival (OS) than those with high mALI (39.5% vs. 65.5%, P < 0.001). In the male population, OS was significantly lower in the low mALI group than in the high group (34.3% vs. 59.2%, P < 0.001). Similar results were also observed in the female population (46.3% vs. 75.0%, P < 0.001). mALI was an independent prognostic factor for patients with cancer cachexia (hazard ratio [HR] = 0.974, 95% confidence interval [CI] = 0.959–0.990, P = 0.001). For every standard deviation [SD] increase in mALI, the risk of poor prognosis for patients with cancer cachexia was reduced by 2.9% (HR = 0.971, 95%CI = 0.943–0.964, P < 0.001) in males and 8.9% (HR = 0.911, 95%CI = 0.893–0.930, P < 0.001) in females. mALI is an effective complement to the traditional Tumour, Lymph Nodes, Metastasis (TNM) staging system for prognosis evaluation and a promising nutritional inflammatory indicator with a better prognostic effect than the most commonly used clinical nutritional inflammatory indicators.
Conclusions
Low mALI is associated with poor survival in both male and female patients with cancer cachexia and is a practical and valuable prognostic assessment tool.
Keywords: Body composition, Systemic inflammation, Cancer cachexia, Prognostic
1. Introduction
According to the Global Cancer Statistics 2020, 1 cancer is one of the major causes affecting patients' lives and health, and its morbidity and mortality are rising rapidly. It is estimated that there are approximately 19.3 million new cancer cases and ten million related deaths worldwide, with China leading the world in both the number of new cases (approximately 4.57 million) and deaths (approximately three million). China has an average of approximately 125 000 people diagnosed with cancer per day, with 8.7 people diagnosed with cancer per minute, indicating a very heavy tumour burden. Cachexia is considered the leading cause of death in patients with cancer. It is estimated that more than 30% of cancer patients die from cachexia, and more than 50% of patients suffer from cachexia of varying degrees. 2 , 3
Despite considerable growth in our understanding of cancer cachexia in recent years, it remains an unmet medical need due to a number of confounding factors, such as cachexia‐induced complications of treatment and sarcopenic obesity. 4 Moreover, effective prognostic factors for cancer cachexia are still lacking, especially simple and economical biomarkers. Therefore, there is an urgent need to identify potential prognostic markers to stratify the prognosis of patients with cancer cachexia in order to reduce the mortality associated with it. Cancer cachexia is defined as a multifactorial syndrome characterised by continuous loss of skeletal muscle mass. Quantisation of skeletal muscle mass is an important component of cancer cachexia and serves as a significant prognostic indicator for cancer. Some studies have shown that skeletal muscle mass is associated with dysfunction, an increased risk of chemotherapy‐related toxicity, and reduced survival. 5 , 6 , 7 , 8 , 9 Recently, a newly developed nutritional inflammatory indicator, the advanced lung cancer inflammation index (ALI), combining body mass index (BMI), albumin, and neutrophil‐lymphocyte ratio (NLR), has been reported to assess the prognosis of various malignancies. 10 , 11 , 12 Currently, there are still relatively few studies on the relationship between the combination of body composition and systemic inflammation biomarkers and the prognosis of cancer cachexia. Thus, we intend to develop a prognostic marker related to skeletal muscle mass on the basis of existing ALI to predict the prognosis of patients in the cancer cachexia population.
At present, the commonly used methods to assess the quality of skeletal muscle mass include computed tomography (CT) or magnetic resonance imaging (MRI), dual energy X‐ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA). 13 However, these methods may be restricted owing to high instrument requirements and high cost. Wen et al. 14 developed a simple, non‐invasive, and effective anthropometric equation of appendicular skeletal muscle mass (ASM) based on the Chinese population and verified its accuracy in multiple studies. 15 , 16 ALI, defined as BMI × albumin/NLR, was originally designed for patients with lung cancer. It is still unknown whether it can be applied to patients with cancer cachexia. We attempted to modify ALI by replacing BMI with ASM in the original anthropometric equation to make it more suitable for application in patients with cancer cachexia. Therefore, this study aimed to evaluate the prognostic value of modified ALI (mALI) in patients with cancer cachexia.
2. Materials and methods
2.1. Population
We abstracted data from the Investigation on Nutrition Status and its Clinical Outcome of Common Cancers (INSCOC) project of China for cancer patients, which had more than 40 clinical centres in China between June 2012 and December 2019. The inclusion criteria were as follows: (1) patients who were histopathologically diagnosed with cancer and (2) patients who met the diagnostic criteria for cachexia. The exclusion criteria were as follows: (1) patients with missing or incomplete clinicopathological parameters; (2) patients with granulocytopenia (neutrophils <0.5 × 109/L) or significant infection (white blood cells [WBCs] ≥ 15 × 109/L); and (3) patients lost to follow‐up.
2.2. Measurements of body composition
ASM was estimated according to a previously validated anthropometric equation, which was described and validated in the Chinese population: ASM = 0.193 × weight (kg) + 0.107 × height (cm) − 4.157 × sex (male = 1, female = 2) − 0.037 × age (year) − 2.631. The ASM equation model is in good agreement with the DXA (adjusted R 2 = 0.90, standard error of estimate = 1.63 kg). 14 , 15 , 16 The ASM index (ASMI) was generated in terms of standardising height: ASMI = ASM/height. 2
2.3. Cancer cachexia definition
In this study, the diagnosis of cancer cachexia was based on Fearon's criteria 17 as follows: (1) loss of >5% in body weight within 6 months without dieting; (2) BMI < 20 kg/m2 accompanied by weight loss >2%; and (3) the skeletal muscle depletion met the criteria for sarcopenia and weight loss >2%. Patients who met one or more of the above criteria were diagnosed with cancer cachexia. Skeletal muscle depletion was assessed using mid‐upper arm muscle area (MAMA) anthropometry (male <32 cm2, female <18 cm2).
2.4. Data collection and definition
The descriptive data collected included the following aspects: population characteristics including sex, age, height, weight, weight loss, hypertension, diabetes, smoking, drinking, and family history; clinical characteristics including types of tumours, tumour‐node‐metastasis (TNM) stage, surgery, radiotherapy, and chemotherapy; and serological tests including albumin, WBC, neutrophil, lymphocyte, platelet, red blood cell (RBC), and haemoglobin counts. All serological tests were performed within 1 week of admission. Nutritional inflammatory indicators included NLR, patient‐generated subjective nutrition assessment (PG‐SGA), hand‐grip strength (HGS), Karnofsky performance status (KPS), mid‐arm circumference (MAC), triceps fold thickness (TSF), MAMA, and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30). TNM staging was based on the American Joint Committee on Cancer staging standard. BMI was defined as weight (kg)/height squared (m2). NLR was defined as neutrophil (109/L)/lymphocyte count (109/L). mALI was defined as ASMI × albumin/NLR. The patients were followed up every 3 months for 2 years, and every 6 months thereafter. The last follow‐up was September 2019. Follow‐up included serological tests, plain radiographs, CT, or invasive examinations, such as oesophagus and gastrointestinal endoscopy. Overall survival (OS) was defined as the time interval between the date of cancer diagnosis and death from any cause or last follow‐up.
2.5. Statistical analysis
Based on patient survival, the optimal stratification method was used to determine the sex‐specific optimal cut‐off value for mALI. The chi‐square test, t‐test, or non‐parametric test was used to analyse the relationship between mALI and clinical characteristics. The mean ± standard deviation (SD) was used to describe normally distributed data, and the median (interquartile range) or frequency (percentage) was used to describe non‐normally distributed data. Restricted cubic splines (RCS) were used to evaluate the relationship between mALI and OS in patients with cancer cachexia. The Kaplan–Meier method and log‐rank test were used to compare the survival rates of patients with high and low mALI. Univariate and multivariate Cox proportional hazard regression analyses were used to assess independent risk factors for OS in patients with cancer cachexia. Receiver operator characteristic (ROC) curve analysis was used to compare the effectiveness of mALI and nutritional inflammatory indicators in predicting OS in patients with cancer cachexia. Stratified analysis was used to assess the relationship between mALI and all‐cause mortality in the different subgroups. In addition, we also examined the tendency of mALI to affect all‐cause mortality in patients with cancer cachexia using the multiequal method. In this study, a P value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 24.0; IBM Corporation, Armonk, NY) and R software (3.5.3; http://www.r‐Project.org).
3. Results
3.1. Population
The study initially included 12 792 patients from multiple centres in China. Of these, 1732 were excluded due to incomplete parameters, 8084 were not compatible with the diagnostic criteria for cancer cachexia, and 538 were diagnosed with demonstrable infections or granulocytopenia. A total of 2438 patients with cancer cachexia were enrolled (Figure S1). The sex‐specific optimal cut‐off values for male and female patients with cancer cachexia were 7.12 and 6.52, respectively (Figure S2). There were 958 patients with low mALI, comprising 543 males and 415 females. There were 1480 patients with high mALI, comprising 888 males and 592 females.
3.2. The relationship between mALI and clinicopathologic characteristics
We compared the clinicopathological characteristics of patients with high and low mALI and found that low mALI was significantly associated with male sex, advanced age, low BMI, hypertension, tumours, high TNM stage, low albumin, high WBC, high neutrophil, low lymphocyte, high platelet, low haemoglobin, and low RBC count. In terms of other nutritional inflammatory indicators, low mALI was associated with low BMI, KPS, MAC, TSF, MAMA, and HGS, and high PG‐SGA scores (Table 1). mALI was found to have a weak negative correlation with age and a strong negative correlation with PG‐SGA. mALI was positively correlated with BMI, HGS, and KPS, but weakly and positively correlated with MAC, TSF, and MAMA (Figure S3). These results were consistent for both males and females. We further analysed the differences in clinicopathological characteristics between the cachectic and non‐cachectic populations. Cachexia was associated with sex, age, BMI, smoking, alcohol, tumours, TNM stage, radiotherapy, chemotherapy, albumin, WBC, neutrophils, lymphocytes, platelets, RBCs, haemoglobin, mALI, KPS, MAC, TSF, MAMA, HGS, weight loss, PG‐SGA, and EORTC QLQ‐C30 (Table S1). In addition, we compared the mALI values of cachexia and non‐cachexia patients with different cancer types. The results showed that the mALI values in the cachexia population were generally lower than those in non‐cachexia patients in both the general population and sex‐specific populations, especially in gastrointestinal cancer prone to malnutrition, such as hepatobiliary cancer and pancreatic cancer. Among patients with different stages, patients with cancer cachexia with advanced stages had lower mALI values than those with early stages (Figure S4).
Table 1.
Characteristic of patients with cancer cachexia stratified by mALI.
| Characteristic | Overall (n = 2438) | Low‐mALI (n = 958) | High‐mALI (n = 1480) | P value |
|---|---|---|---|---|
| Population characteristic | ||||
| Sex, male, n (%) | 1431 (58.7%) | 543 (56.7%) | 888 (60.0%) | <0.001 |
| Age, years, mean (SD) | 58.73 (11.73) | 60.51 (12.03) | 57.57 (11.40) | <0.001 |
| BMI, kg/m2, mean (SD) | 20.88 (3.26) | 20.15 (3.18) | 21.36 (3.22) | <0.001 |
| Hypertension, yes, n (%) | 394 (16.2%) | 175 (18.3%) | 219 (14.8%) | 0.023 |
| Diabetes, yes, n (%) | 201 (8.2%) | 92 (9.6%) | 109 (7.4%) | 0.050 |
| Smoke, yes, n (%) | 1111 (45.6%) | 425 (44.4%) | 686 (46.4%) | 0.336 |
| Alcohol, yes, n (%) | 567 (23.3%) | 219 (22.9%) | 348 (23.5%) | 0.709 |
| Family history, yes, n (%) | 357 (14.6%) | 146 (15.2%) | 211 (14.3%) | 0.502 |
| Clinical characteristic | ||||
| Tumours, yes, n (%) | <0.001 | |||
| Lung cancer | 466 (18.4%) | 213 (21.2%) | 253 (16.7%) | |
| Gastric cancer | 528 (20.9%) | 203 (20.2%) | 325 (21.4%) | |
| Oesophagus cancer | 237 (9.4%) | 77 (7.6%) | 160 (10.5%) | |
| Hepatic‐biliary cancer | 121 (4.8%) | 71 (7.1%) | 50 (3.3%) | |
| Colorectal cancer | 627 (24.8%) | 219 (21.7%) | 408 (26.9%) | |
| Pancreatic cancer | 77 (3.0%) | 37 (3.7%) | 40 (2.6%) | |
| Breast cancer | 131 (5.2%) | 36 (3.6%) | 95 (6.3%) | |
| Cervical cancer | 108 (4.3%) | 56 (5.6%) | 52 (3.4%) | |
| Ovarian cancer | 68 (2.7%) | 30 (3.0%) | 38 (2.5%) | |
| Urologic cancer | 21 (0.8%) | 12 (1.2%) | 9 (0.6%) | |
| Nasopharynx cancer | 93 (3.7%) | 34 (3.4%) | 59 (3.9%) | |
| Other cancer | 49 (1.9%) | 19 (1.9%) | 30 (2.0%) | |
| TNM stage, n (%) | <0.001 | |||
| I | 210 (8.6%) | 75 (7.8%) | 135 (9.1%) | |
| II | 527 (21.6%) | 185 (19.3%) | 342 (23.1%) | |
| III | 656 (26.9%) | 204 (21.3%) | 452 (30.5%) | |
| IV | 1045 (42.9%) | 494 (51.6%) | 551 (37.2%) | |
| Surgery | 1033 (42.4%) | 370 (38.6%) | 663 (44.8%) | 0.003 |
| Radiotherapy, yes, n (%) | 179 (7.3%) | 69 (7.2%) | 110 (7.4%) | 0.832 |
| Chemotherapy, yes, n (%) | 1396 (57.3%) | 586 (61.2%) | 810 (54.7%) | 0.002 |
| Serological tests | ||||
| Albumin, g/L, mean (SD) | 37.57 (5.36) | 34.86 (5.44) | 39.32 (4.52) | <0.001 |
| WBC, 109/L, mean (SD) | 6.62 (2.54) | 7.91 (2.80) | 5.78 (1.95) | <0.001 |
| Neutrophil, 109/L, mean (SD) | 4.41 (2.39) | 6.03 (2.54) | 3.36 (1.56) | <0.001 |
| Lymphocyte, 109/L, mean (SD) | 1.46 (0.81) | 1.03 (0.49) | 1.74 (0.85) | <0.001 |
| Platelet, 109/L, mean (SD) | 238.82 (99.83) | 246.06 (110.41) | 234.13 (92.08) | 0.004 |
| RBC, 1012/L, median (IQR) | 4.10 (0.85) | 3.86 (0.94) | 4.21 (0.76) | <0.001 |
| Haemoglobin, g/L, median (IQR) | 120.00 (27.00) | 112.00 (28.00) | 124.00 (24.00) | <0.001 |
| KPS, mean (SD) | 83.17 (15.06) | 77.67 (18.08) | 86.73 (11.40) | <0.001 |
| MAC, cm, mean (SD) | 24.98 (3.77) | 24.26 (3.92) | 25.45 (3.60) | <0.001 |
| TSF, cm, mean (SD) | 14.33 (7.90) | 13.26 (7.49) | 15.03 (8.08) | <0.001 |
| MAMA, cm, mean (SD) | 33.75 (11.05) | 32.53 (10.90) | 34.53 (11.08) | <0.001 |
| HGS, kg, mean (SD) | 23.40 (10.41) | 21.03 (9.68) | 24.97 (10.59) | <0.001 |
| Weight loss, mean (SD) | 5.81 (3.81) | 5.97 (3.95) | 5.71 (3.72) | 0.102 |
| PG‐SGA, mean (SD) | 9.38 (4.52) | 10.98 (4.84) | 8.35 (3.97) | <0.001 |
| EORTC QLQ‐C30, median (IQR) | 50.00 (13.00) | 53.00 (16.25) | 48.00 (11.00) | <0.001 |
| Recurrence and progression | 666 (27.3) | 323 (33.7) | 343 (23.2) | <0.001 |
| Death | 1090 (44.7) | 580 (60.5) | 510 (34.5) | <0.001 |
| Length of stay, mean (SD) | 15.76 (12.49) | 16.54 (12.00) | 15.26 (12.78) | 0.013 |
Note: Data are represented as mean (standard deviation, SD), median (interquartile range, IQR) or number (%).
Abbreviations: BMI, body mass index; EORTC QLQ‐C30EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HGS, hand grip strength; KPS, Karnofsky Performance Status; MAC, mid‐arm circumference; mALI, modified advanced lung cancer inflammation index; MAMA, mid‐upper arm muscle area; PG‐SGA, patient‐generated subjective nutrition assessment; TSF, triceps fold thickness. For male cut‐off as 7.12 or female cut‐off as 6.52.
3.3. Kaplan–Meier curves of mALI in patients with cancer cachexia
In this study, the median follow‐up time was 18.86 months. A total of 1090 patients (44.71%) died, including 580 patients with low mALI (53.21% of the total low mALI group) and 510 patients with high mALI (34.46% of the total high mALI group). In the male population, OS was significantly lower in the low mALI group than in the high mALI group (34.3% vs. 59.2%, P < 0.001) (Figure 1A). Similar results were also observed in the female population (46.3% vs. 75.0%, P < 0.001) (Figure 1B). In all population, patients with low mALI had significantly lower OS than those with high mALI (39.5% vs. 65.5%, P < 0.001) (Figure 1C). We also performed a stratified survival analysis based on the TNM stage. For stage I–II, OS in the low mALI group (all patients: 59.3% vs. 74.1%, P < 0.001; male: 59.3% vs. 74.1%, P < 0.001; female: 59.3% vs. 74.1%, P < 0.001) was significantly lower than in the high mALI group. For stage III, patients with low mALI also had significantly lower OS (all patients: 46.1% vs. 72.8%, P < 0.001; male: 35.9% vs. 70.3%, P < 0.001; female: 59.8% vs. 77.2%, P < 0.001) than those with high mALI. Similarly, a significant survival difference was also observed in stage IV patients (all patients: 19.0% vs. 41.7%, P < 0.001; male: 15.4% vs. 31.9%, P < 0.001; female: 23.9% vs. 56.9%, P < 0.001). In the stratified survival analysis of various tumours, low mALI was still associated with poor survival of patients with cancer cachexia (Figure S5).
Figure 1.
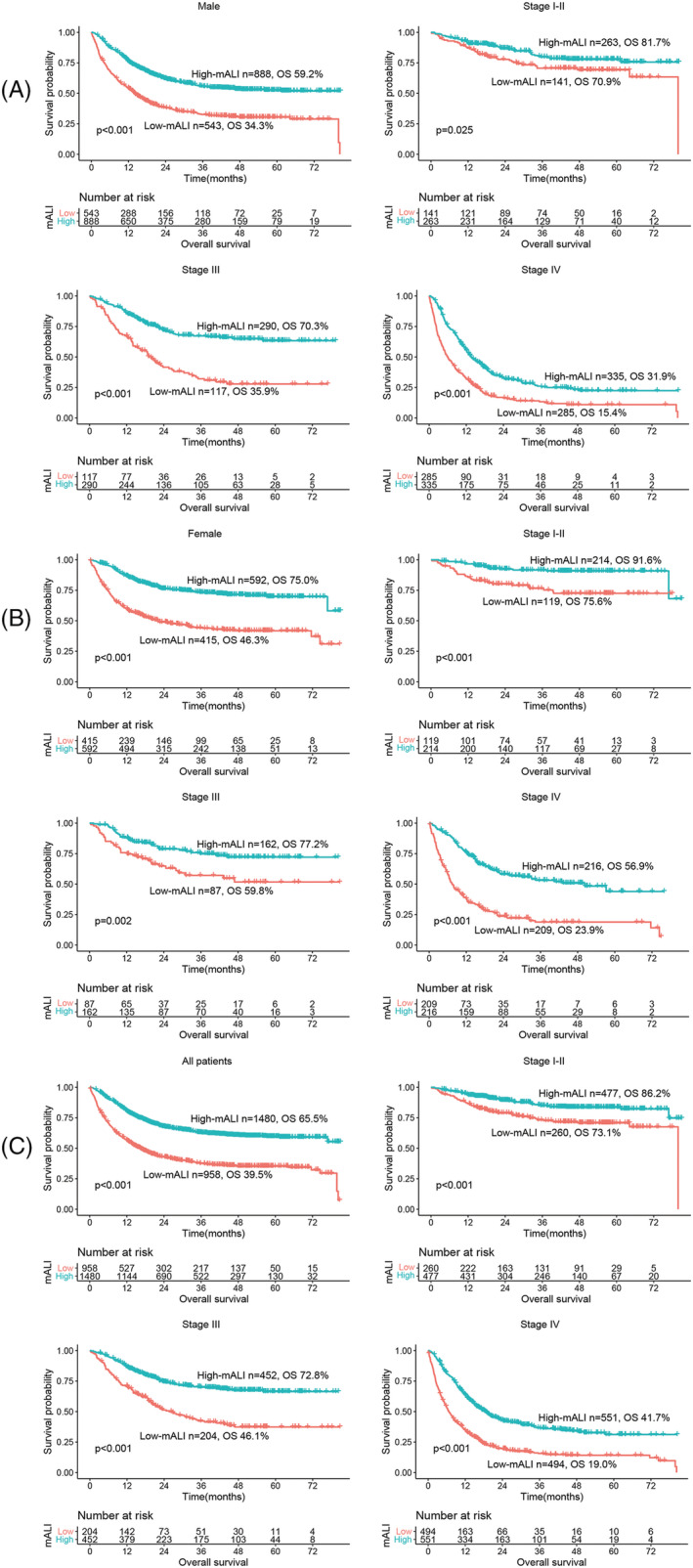
Survival and stratified analysis of cachexia patients with high and low ALI. Notes: (A) K‐M survival curve of male patients, (B) K‐M survival curve of female patients, (C) K‐M survival curve of all patients. Abbreviations: mALI, modified advanced lung cancer inflammation index; OS, overall survival.
3.4. Survival analysis of mALI in patients with cancer cachexia
After multivariate adjustment, there was still a non‐linear relationship between mALI and all‐cause mortality in patients with cancer cachexia (Figure 2). With the increase in mALI, the all‐cause mortality of patients gradually decreased and became flat after mALI > 15. Notably, low mALI in female patients had a stronger correlation with all‐cause mortality compared with male patients. In the univariate analysis, it was found that a variety of clinical characteristics, including mALI, were associated with patient's prognosis in those with cancer cachexia. After adjusting for confounding factors, multivariate analysis revealed that mALI was an independent prognostic factor for cancer cachexia (HR = 0.974, 95%CI = 0.959–0.990, P = 0.001) (Table 2). In the stratified analysis, we divided the patients into 31 clinicopathological characteristic subgroups. After adjusting for confounding factors, the results were similar, indicating that a low mALI was an independent risk factor for the poor prognosis of cancer cachexia (all P < 0.05) (Figure 3). Because mALI had an interactive effect with sex and tumours, we conducted a covariate interaction analysis. In terms of sex, mALI had a greater effect on the prognosis of female patients (Figure S6A, sex). For tumours, the most severe effects were among other and lower digestive cancer, followed by upper digestive cancer, and then lung cancer (Figure S6A, tumours). The combination of interactive factors for survival analysis can effectively stratify the prognosis of patients. Males with low mALI had the worst prognosis, whereas female patients with high mALI had a relatively better prognosis (Figure S6B, sex). The low mALI and lung cancer subgroups had the lowest prognoses, whereas the low mALI and other cancer subgroups had the best prognoses (Figure S6B, tumours).
Figure 2.
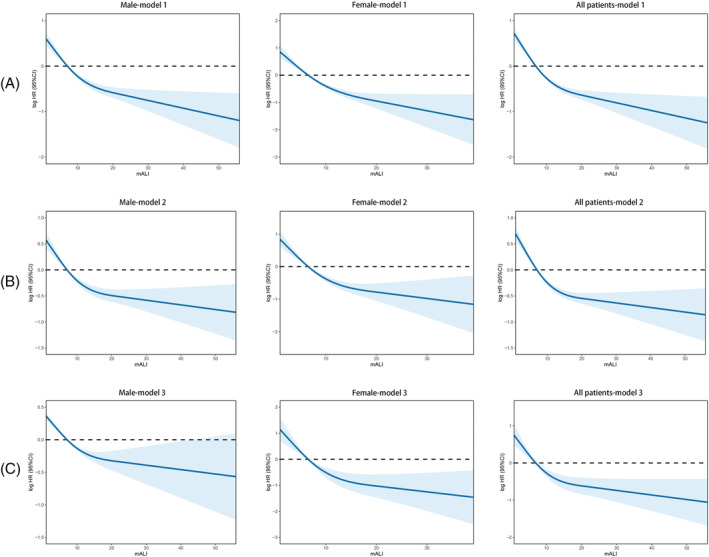
The association between ALI (continuous) and hazard risk of overall survival in cachexia patients by cut‐off of ALI. Notes: Model 1: No adjusted. Model 2: Adjusted for age, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoke, alcohol, family history, weight loss. Model 3: Adjusted for age, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoke, alcohol, family history, weight loss, KPS, MAC, TSF, MAMA, HGS, PG‐SGA, EORTC QLQ‐C30, albumin, white blood cell, neutrophil, lymphocyte, platelet, red blood cell, haemoglobin. Abbreviation: HR, hazard ratio.
Table 2.
Univariate and multivariate cox regression analysis of factors associated with overall survival.
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR, 95% CI | P value | HR, 95% CI | P value | |
| Sex, female | 0.669 (0.590, 0.759) | <0.001 | 0.654 (0.540, 0.792) | <0.001 |
| Age, per SD | 1.019 (1.014, 1.025) | <0.001 | 1.007 (1.001, 1.012) | 0.022 |
| BMI, per SD | 0.940 (0.922, 0.958) | <0.001 | 1.001 (0.975, 1.027) | 0.945 |
| Family history, yes | 1.022 (0.865, 1.208) | 0.797 | ||
| Hypertension, yes | 1.137 (0.972, 1.330) | 0.109 | ||
| Diabetes, yes | 1.171 (0.952, 1.441) | 0.136 | ||
| Smoke, yes | 1.367 (1.213, 1.539) | <0.001 | 1.015 (0.866, 1.190) | 0.851 |
| Alcohol, yes | 1.118 (0.974, 1.282) | 0.112 | ||
| Weight loss, per SD | 1.032 (1.020, 1.045) | <0.001 | 1.017 (1.001, 1.033) | 0.036 |
| Tumour stage | <0.001 | |||
| I | Ref. | Ref. | ||
| II | 1.798 (1.180, 2.740) | 0.006 | 1.562 (1.021, 2.391) | 0.04 |
| III | 3.460 (2.322, 5.155) | <0.001 | 2.926 (1.955, 4.379) | <0.001 |
| IV | 10.273 (6.989, 15.102) | <0.001 | 6.932 (4.659, 10.315) | <0.001 |
| Surgery, yes | 0.535 (0.471, 0.607) | <0.001 | 0.695 (0.605, 0.798) | <0.001 |
| Radiotherapy, yes | 1.168 (0.937, 1.455) | 0.774 | ||
| Chemotherapy, yes | 1.215 (1.079, 1.369) | 0.001 | 1.170 (1.020, 1.341) | 0.024 |
| Albumin, per SD | 0.929 (0.919, 0.939) | <0.001 | 0.984 (0.970, 0.998) | 0.024 |
| White blood cell, per SD | 1.099 (1.076, 1.124) | <0.001 | 1.026 (0.966, 1.091) | 0.403 |
| Neutrophil, per SD | 1.131 (1.106, 1.156) | <0.001 | 0.994 (0.929, 1.063) | 0.858 |
| Lymphocyte, per SD | 0.777 (0.710, 0.850) | <0.001 | 1.033 (0.910, 1.173) | 0.615 |
| Platelet, per SD | 1.001 (1.001, 1.002) | <0.001 | 1.001 (1.000, 1.001) | 0.028 |
| mALI, per SD | 0.434 (0.385, 0.489) | <0.001 | 0.974 (0.959, 0.990) | 0.001 |
| Red blood cell, per SD | 0.652 (0.599, 0.709) | <0.001 | 0.802 (0.697, 0.924) | 0.002 |
| Haemoglobin, per SD | 0.989 (0.987, 0.992) | <0.001 | 1.002 (0.997, 1.007) | 0.452 |
| KPS, per SD | 0.978 (0.975, 0.982) | <0.001 | 0.997 (0.992, 1.002) | 0.259 |
| MAC, per SD | 0.951 (0.936, 0.966) | <0.001 | 1.003 (0.980, 1.026) | 0.807 |
| TSF, per SD | 0.957 (0.948, 0.965) | <0.001 | 0.977 (0.966, 0.988) | <0.001 |
| MAMA, per SD | 1.001 (0.996, 1.006) | 0.718 | ||
| HGS, per SD | 0.978 (0.972, 0.985) | <0.001 | 0.989 (0.981, 0.997) | 0.005 |
| PG‐SGA, per SD | 1.082 (1.069, 1.095) | <0.001 | 1.009 (0.992, 1.026) | 0.285 |
| EORTC QLQ‐C30, per SD | 0.960 (0.954, 0.967) | <0.001 | 0.990 (0.982, 0.998) | 0.019 |
Abbreviations: BMI, body mass index; EORTC QLQ‐C30EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HGS, hand grip strength; KPS, Karnofsky performance status; MAC, mid‐arm circumference; mALI, Modified advanced lung cancer inflammation index; MAMA, mid‐upper arm muscle area; PG‐SGA, patient‐generated subjective nutrition assessment; SD, standard deviation; TSF, triceps fold thickness.
Figure 3.
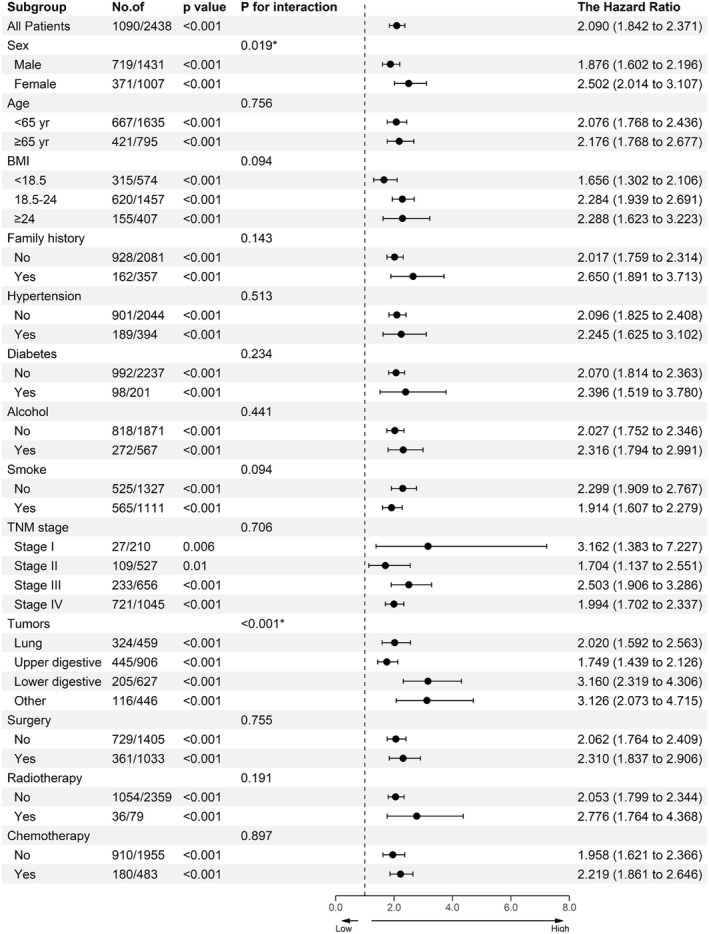
The association between mALI (stratified by male cut‐off as 7.12 or female cut‐off as 6.52) and hazard risk of overall survival in various subgroups. Notes: The model adjusted for age, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoke, alcohol, family history, weight loss. Abbreviation: BMI, body mass index.
3.5. The relationship between mALI and the all‐cause mortality of patients with cancer cachexia
We explored the relationship between mALI and all‐cause mortality in patients with cancer cachexia based on sex stratification. All‐cause mortality had a significant positive association with mALI in male patients (Table 3). When mALI was divided into quintiles, the lowest quintile Q1 (~4.43) was used as a reference. Q2 (4.43–7.34), Q3 (7.34–11.12), Q4 (11.12–16.48), and Q5 (16.48~) were all positively associated with better prognosis (P < 0.001). After adjusting for confounding factors, the HRs for all‐cause mortality were 0.751 (0.574, 0.982), 0.567 (0.410, 0.785), 0.461 (0.314, 0.677), and 0.302 (0.184, 0.494), respectively. Similarly, in female patients (Table 4), both continuous and multi‐categorical variables of mALI could effectively stratify the prognosis of patients without being affected by confounding factors. With a decrease in mALI, the all‐cause mortality of cancer cachexia patients gradually decreased. We also excluded patients dying within 3 months and those with haematological disease or abnormal liver function for sensitivity analysis to assess the impact of potential factors on the overall results (Table S2 and Table S3). The results showed that mALI was an independent prognostic factor in both male and female patients with cancer cachexia.
Table 3.
The association between mALI and hazard ratio of male patients with cachexia.
| mALI | Male | |||||
|---|---|---|---|---|---|---|
| Model 1, HR (95% CI) | P value | Model 2, HR (95% CI) | P value | Model 3, HR (95% CI) | p | |
| As continuous (per SD) | 0.954 (0.943, 0.964) | <0.001 | 0.965 (0.954, 0.976) | <0.001 | 0.613 (0.489, 0.769) | <0.001 |
| Binaries | ||||||
| B1 (~7.12) | Ref | Ref | Ref | |||
| B2 (7.12~) | 0.475 (0.410, 0.550) | <0.001 | 0.530 (0.453, 0.620) | <0.001 | 0.735 (0.624, 0.866) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Tertiles | ||||||
| T1 (~6.45) | Ref | Ref | Ref | |||
| T2 (6.45–12.51) | 0.625 (0.528, 0.741) | <0.001 | 0.663 (0.556, 0.792) | <0.001 | 0.730 (0.581, 0.918) | 0.007 |
| T3 (12.51~) | 0.397 (0.330, 0.479) | <0.001 | 0.473 (0.388, 0.576) | <0.001 | 0.541 (0.390, 0.751) | <0.001 |
| P for trend | <0.001 | <0.001 | 0.001 | |||
| Quartiles | ||||||
| Q1 (~5.17) | Ref | Ref | Ref | |||
| Q2 (5.17–8.97) | 0.708 (0.587, 0.856) | <0.001 | 0.669 (0.550, 0.814) | <0.001 | 0.693 (0.541, 0.887) | 0.004 |
| Q3 (8.97–14.85) | 0.527 (0.432, 0.643) | <0.001 | 0.544 (0.442, 0.671) | <0.001 | 0.566 (0.417, 0.769) | <0.001 |
| Q4 (14.85~) | 0.334 (0.268, 0.417) | <0.001 | 0.396 (0.313, 0.502) | <0.001 | 0.383 (0.254, 0.578) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Quintile | ||||||
| Q1 (~4.43) | Ref | Ref | Ref | |||
| Q2 (4.43–7.34) | 0.824 (0.672, 1.010) | <0.001 | 0.743 (0.602, 0.917) | 0.006 | 0.751 (0.574, 0.982) | 0.036 |
| Q3 (7.34–11.12) | 0.572 (0.459, 0.712) | <0.001 | 0.586 (0.466, 0.738) | <0.001 | 0.567 (0.410, 0.785) | 0.001 |
| Q4 (11.12–16.48) | 0.481 (0.383, 0.602) | <0.001 | 0.480 (0.378, 0.610) | <0.001 | 0.461 (0.314, 0.677) | <0.001 |
| Q5 (16.48~) | 0.299 (0.232, 0.385) | <0.001 | 0.358 (0.273, 0.470) | <0.001 | 0.302 (0.184, 0.494) | <0.001 |
| P for trend | <0.001 | <0.001 | ||||
Note: Model 1: No adjusted. Model 2: Adjusted for age, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoke, alcohol, family history, weight loss. Model 3: Adjusted for age, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoke, alcohol, family history, weight loss, KPS, MAC, TSF, MAMA, HGS, PG‐SGA, EORTC QLQ‐C30, albumin, white blood cell, neutrophil, lymphocyte, platelet, red blood cell, haemoglobin.
Abbreviations: BMI, body mass index; EORTC QLQ‐C30EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HGS, hand grip strength; KPS, Karnofsky Performance Status; MAC, mid‐arm circumference; mALI, Modified advanced lung cancer inflammation index; MAMA, mid‐upper arm muscle area; PG‐SGA, patient‐generated subjective nutrition assessment; TSF, triceps fold thickness.
Table 4.
The association between mALI and hazard ratio of female patients with cachexia.
| mALI | Female | |||||
|---|---|---|---|---|---|---|
| Model 1, HR (95% CI) | P value | Model 2, HR (95% CI) | P value | Model 3, HR (95% CI) | P value | |
| As continuous (per SD) | 0.911 (0.893, 0.930) | <0.001 | 0.924 (0.904, 0.944) | <0.001 | 0.945 (0.911, 0.982) | 0.003 |
| Binaries | ||||||
| B1 (~6.52) | Ref | Ref | Ref | |||
| B2 (6.52~) | 0.352 (0.286, 0.434) | <0.001 | 0.400 (0.322, 0.497) | <0.001 | 0.492 (0.358, 0.676) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Tertiles | ||||||
| T1 (~5.57) | Ref | Ref | Ref | |||
| T2 (5.57–11.20) | 0.566 (0.451, 0.710) | <0.001 | 0.566 (0.448, 0.715) | <0.001 | 0.671 (0.485, 0.927) | <0.001 |
| T3 (11.20~) | 0.252 (0.189, 0.336) | <0.001 | 0.323 (0.239, 0.435) | <0.001 | 0.448 (0.275, 0.732) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Quartiles | ||||||
| Q1 (~4.40) | Ref | Ref | Ref | |||
| Q2 (4.40–7.78) | 0.619 (0.482, 0.795) | <0.001 | 0.565 (0.439, 0.729) | <0.001 | 0.611 (0.432, 0.866) | 0.006 |
| Q3 (7.78–13.41) | 0.432 (0.329, 0.568) | <0.001 | 0.451 (0.341, 0.596) | <0.001 | 0.496 (0.315, 0.781) | 0.002 |
| Q4 (13.41~) | 0.197 (0.139, 0.280) | <0.001 | 0.241 (0.168, 0.346) | <0.001 | 0.276 (0.148, 0.517) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Quintile | ||||||
| Q1 (~3.60) | Ref | Ref | Ref | |||
| Q2 (3.60–6.37) | 0.775 (0.593, 1.013) | 0.062 | 0.728 (0.555, 0.955) | 0.022 | 0.731 (0.511, 1.048) | 0.088 |
| Q3 (6.37–9.76) | 0.425 (0.315, 0.573) | <0.001 | 0.389 (0.286, 0.530) | <0.001 | 0.370 (0.233, 0.589) | <0.001 |
| Q4 (9.76–14.77) | 0.335 (0.242, 0.464) | <0.001 | 0.381 (0.273, 0.531) | <0.001 | 0.373 (0.213, 0.654) | 0.001 |
| Q5 (14.77~) | 0.191 (0.130, 0.281) | <0.001 | 0.232 (0.155, 0.348) | <0.001 | 0.219 (0.106, 0.453) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
Note: Model 1: No adjusted. Model 2: Adjusted for age, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoke, alcohol, family history, weight loss. Model 3: Adjusted for age, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoke, alcohol, family history, weight loss, KPS, MAC, TSF, MAMA, HGS, PG‐SGA, EORTC QLQ‐C30, albumin, white blood cell, neutrophil, lymphocyte, platelet, red blood cell, haemoglobin.
Abbreviations: BMI, body mass index; EORTC QLQ‐C30EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HGS, hand grip strength; KPS, Karnofsky Performance Status; MAC, mid‐arm circumference; mALI, Modified advanced lung cancer inflammation index; MAMA, mid‐upper arm muscle area; PG‐SGA, patient‐generated subjective nutrition assessment; TSF, triceps fold thickness.
3.6. Comparison of mALI and other nutritional inflammatory indicators
First, we compared the effectiveness of mALI and its component factors in predicting the clinical outcome of patients with cancer cachexia at 1‐, 3‐, and 5‐year time points by using time‐dependent ROC curves. Compared with the component factors, mALI as a combined indicator can effectively improve prognosis prediction performance. In addition, we also compared the efficacy of the commonly used nutritional inflammatory indicators in predicting the clinical outcome of cancer cachexia, from which we could see that the efficacy of mALI in predicting the prognosis was better than most nutritional inflammatory indicators. The same results were observed at the 1‐, 3‐, and 5‐year time points (Figure S7). Furthermore, we compared the prognostic benefits of these indicators for OS in patients with cancer cachexia. We calculated the C‐statistic, continuous net reclassification improvement (cNRI), and integrated discrimination improvement (IDI) (Table S4 and Table S5). Compared with mALI, the other indicators failed to bring significant gains (where all increments were negative) in both male and female patients. In the analysis of the combined TNM stage, most indicators provided significant incremental prognostic value for the TNM stage. The incremental value of mALI was considerable and statistically significant in both male and female patients (all P < 0.05). We also compared the prognostic efficacy of mALI and the original ALI using BMI. In male patients, although the C‐statistic was positive, no statistical difference was observed, whereas the results of cNRI and IDI both showed that the prognostic gain of mALI was superior to that of ALI (BMI). It also demonstrated that the benefits of mALI in the combined TNM stage were significantly higher than those in ALI (BMI). In female patients, the prognostic gain due to mALI was more obvious than that of ALI (BMI). These results indicate that mALI has a natural advantage over ALI (BMI).
3.7. Correlation analysis of mALI with recurrence, metastasis, and quality of life
Among the enrolled patients, 666 patients experienced recurrence and progression, including 323 (33.7%) patients with low mALI and 343 (23.2%) patients with high mALI (Table 1). Correlation analysis showed that patients with low mALI were more likely to relapse and progress. In the univariate logistic regression analysis, mALI was associated with recurrence and progression in patients with cancer cachexia. Multivariate analysis showed that mALI was not an independent factor affecting recurrence and progression (Table S6). In this study, we found that low mALI was significantly associated with low EORTC QLQ‐C30 scores (Table S7).
4. Discussion
The tumour inflammatory microenvironment plays an important role in cancer progression. 18 , 19 Virchow 20 first detected the presence of tumour‐infiltrating lymphocytes in 1881 and speculated that the occurrence of tumours may be related to inflammation. Hanahan et al. 21 further found that immune and inflammatory cells are an important part of the tumour inflammatory microenvironment, which can affect tumour growth through the production of cytokines and chemokines in autocrine and paracrine pathways. In addition, due to the vigorous metabolism and abnormally accelerated proliferation of tumour cells, cancer patients are more prone to malnutrition, resulting in loss of muscle, fat, and body weight, which in turn leads to adverse clinical outcomes for patients. Malnutrition further damages the immune system, leading to an imbalance between immunosuppression and tumour proliferation; thus, the body's immune system cannot eliminate cancer cells, which further increases the risk of tumour‐related death. The increase in certain clinical inflammatory factors can be used to predict the prognosis of cancer patients. The combination of a variety of inflammatory factors and nutrition‐related indicators can further improve the efficacy of prognostic prediction for cancer patients. Recently, many prognostic indicators based on cancer‐related inflammation and nutrition have been developed, including the Glasgow prognostic score, geriatric nutritional risk index, and controlling nutritional status score, which have been reported as effective prognostic predictors in cancer patients. 22 , 23 , 24 Cancer cachexia is considered the clinical outcome of the interaction of tumours, host metabolism, and pro‐inflammatory cytokines. 25 Jafri et al. 7 proposed a BMI‐based nutritional inflammatory indicator, ALI, to predict the poor prognosis of cancer patients in 2013. However, with the increase in number of obese cancer patients, sarcopenic obesity has received increasing attention and has been proven to be an important factor affecting the prognosis. The controversy of the “obesity paradox” among cancer patients has also been raised. In addition, confounding factors, such as dystrophic oedema, cancerous pleural effusion, and visceral fat increase, have an influence on BMI. The emergence of these problems makes BMI increasingly limited in prognosis assessment, which suggests that a more accurate and individualised measurement of body composition is necessary. In this study, we developed mALI by replacing BMI with ASMI based on previous studies to generate a novel method for prognosis assessment of patients with cancer cachexia.
We established a sex‐specific mALI cut‐off value for patients with cancer cachexia and found that low mALI was associated with poor survival in both male and female patients. Correlation analysis showed that low mALI was significantly correlated with poor nutritional status, degree of malignancy, and high inflammation. In addition, low mALI was more likely to be associated with low BMI, KPS, MAC, and HGS, but with high PG‐SGA, consistent with conventional nutritional assessment tools. This indicates that mALI can be used as a routine nutritional assessment tool for cancer patients. In the TNM stratification analysis, mALI can significantly stratify the prognosis of cachectic patients with all stages in cancer. This indicates that mALI can be used as an effective complement to the traditional TNM staging system for prognosis assessment. Whether as a continuous or a categorical variable, mALI was an independent prognostic factor for patients with cancer cachexia. In addition, after adjusting for confounding factors, it was found that low mALI was an independent risk factor for all subgroups. Overall, low mALI was associated with a poor prognosis in different tumours. The stratification effect of mALI is better in gastrointestinal tumours, but is relatively insignificant in breast cancer. In RCS, the all‐cause mortality of patients gradually decreased with an increase in mALI, but gradually levelled off after mALI > 15. This may be explained by the fact that cancer is a multifactorial disease. Nutrition‐related factors may be another important prognostic factor in addition to TNM stage. In the nutritional deficiency period, nutrition‐related factors have a greater prognostic impact. When the nutritional state is in a relatively abundant state, other factors, such as tumour infiltration and metastasis, gradually play a leading role. It can also be noted that mALI had a greater impact on females than males. This may be due to the fact that males have more skeletal muscle mass than females; under the same skeletal muscle reduction, female patients are more likely to experience reduced activity, increased chemotherapy toxicity, increased complications, and more difficulty in coping with the shock of cancer and treatment, thus affecting the prognosis. Low mALI was associated with the recurrence and progression of cancer patients and prolonged hospital stay, but it is not an independent risk factor that affects recurrence and progression. In addition, patients with low mALI are more likely to have a worse quality of life.
We found that the ability of mALI to predict the prognosis of patients was better than that of its constituent factors. Furthermore, mALI is a promising nutritional inflammatory indicator with a better prognostic effect than the most commonly used clinical indicators. This may be due to the combination of three important indicators of ASMI, albumin, and NLR, which comprehensively reflect changes in muscle mass, nutrition, and inflammation. In the evaluation of additional prognostic benefits, mALI combined with TNM stage provided significantly more benefits than other nutritional inflammatory indicators. The previous research of Kim et al. 26 reported that mALI had no additional prognostic value beyond the original ALI using BMI. However, in our study, the results showed that mALI was superior to ALI (BMI) in predicting prognosis, and the additional prognostic benefit of mALI to TNM stage was much higher than that of ALI (BMI). mALI assesses a more accurate body composition (skeletal muscle mass) based on the original ALI (BMI), which is a powerful and attractive prognostic assessment tool. It is worth noting that mALI has shown a good prognostic ability for cancer cachexia patients with all BMI classes, suggesting that it can be used to assess occult sarcopenia, thereby further screening out easily overlooked patients with poor prognosis at normal or high BMI. This greatly improves the accuracy of the prognosis assessment of patients with cancer cachexia. In summary, mALI, a reliable, objective, reproducible, and inexpensive prognostic indicator for patients with cancer cachexia, can provide more prognostic benefits than the original ALI (BMI), which can be considered as a routine clinical application.
This study has some limitations. This study is essentially a multi‐centre, retrospective study which needs to be further verified in a prospective cohort. Due to data limitations, this study only included patients with cancer in Chinese hospitals. In the future, we hope to verify our results in multiple countries and ethnic groups. In addition, due to the lack of data on skeletal muscle mass measured by other methods (such as CT, MRI, DXA, BIA, etc.), it is regrettable that the prognostic ability of different methods of mALI has not been compared and requires further exploration in subsequent studies.
5. Conclusion
This study demonstrated that low mALI is associated with poor survival in both male and female patients with cancer cachexia and is a practical and valuable tool for evaluating the prognosis of such patients.
Funding
This work was supported by the National Key Research and Development Program to Dr. Hanping Shi (No. 2017YFC1309200 and No. 2022YFC2009600).
Conflict of interest
The authors declare no conflict of interest. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 27
Supporting information
Figure S1. Study design
Figure S2. Cut‐off of mALI in patients with cancer cachexia. (A, cut‐off of mALI in male; B, cut‐off of mALI in female)
Abbreviations: mALI, modified advanced lung cancer inflammation index.
Figure S3. Correlation analysis between mALI and other parameters.
Abbreviations: mALI, modified advanced lung cancer inflammation index; BMI, body mass index; KPS, Karnofsky performance status; PG‐SGA, patient‐generated subjective nutrition assessment; HGS, hand‐grip strength; MAC, mid‐arm circumference; TSF, triceps fold thickness; MAMA, mid‐upper arm muscle area.
Figure S4. mALI in different cancer types different TNM stage of patients with cachexia.
Notes: A. Stratified by whether patients with cachexia ALI, B, Stratified by TNM stage. ns p‐value >0.05, * p‐value<0.05, ** p‐value<0.01, *** p‐value<0.001.
Abbreviations: mALI, modified advanced lung cancer inflammation index.
Figure S5. Kaplan–Meier curves of OS for cachexia patients stratified by low‐ and high‐AGR for multiple cancer types in training cohort (including lung cancer, gastric cancer, oesophagus cancer, colorectal cancer, hepatic‐biliary cancer, pancreatic cancer, cervical ‐ endometrial cancer, ovarian cancer, urologic cancer, nasopharynx cancer, breast cancer, and other cancer).
Abbreviations: mALI, modified advanced lung cancer inflammation index; OS, overall survival.
Figure S6. Combined survival analysis of mALI and covariates interaction.
Abbreviations: mALI, modified advanced lung cancer inflammation index.
Figure S7. Comparison of the ability of prognostic tools in predicting prognosis of cachexia patients using ROC curves. (A, male cachexia patients, B, female cachexia patients)
Abbreviations: mALI, modified advanced lung cancer inflammation index; ASMI, appendicular skeletal muscle index; NLR, neutrophil‐lymphocyte ratio; ALB, albumin; BMI, body mass index; PG‐SGA, patient‐generated subjective nutrition assessment; HGS, hand‐grip strength; KPS, Karnofsky performance status; MAC, mid‐arm circumference; TSF, triceps fold thickness; MAMA, mid‐upper arm muscle area.
Xie H.‐L., Ruan G.‐T., Wei L., Zhang Q., Ge Y.‐Z., Song M.‐M., et al (2023) The prognostic value of the combination of body composition and systemic inflammation in patients with cancer cachexia, Journal of Cachexia, Sarcopenia and Muscle, 14, 879–890, 10.1002/jcsm.13205
Hai‐Lun Xie, Guo‐Tian Ruan and Lishuang Wei contributed equally to this work.
Contributor Information
Wei Li, Email: drweili@yahoo.com.
Kun‐Hua Wang, Email: wkh@ydyy.cn.
Han‐Ping Shi, Email: shihp@ccmu.edu.cn.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Hu C, Zhang Q, Wang K, Li W, Xu H. Cancer cachexia statistics in China. Precis Nutr 2022;1:e00008. [Google Scholar]
- 4. Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer Cachexia: Beyond Weight Loss. J Oncol Pract 2016;12:1163–1171. [DOI] [PubMed] [Google Scholar]
- 5. Cherin P, Voronska E, Fraoucene N, de Jaeger C. Prevalence of sarcopenia among healthy ambulatory subjects: the sarcopenia begins from 45 years. Aging Clin Exp Res 2014;26:137–146. [DOI] [PubMed] [Google Scholar]
- 6. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 7. Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou‐Rouquette P, et al. Sarcopenia and body mass index predict sunitinib‐induced early dose‐limiting toxicities in renal cancer patients. Br J Cancer 2013;108:1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne‐Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 2016;103:572–580. [DOI] [PubMed] [Google Scholar]
- 9. Xie H, Gong Y, Kuang J, Yan L, Ruan G, Tang S, et al. Computed Tomography‐Determined Sarcopenia Is a Useful Imaging Biomarker for Predicting Postoperative Outcomes in Elderly Colorectal Cancer Patients. Cancer Res Treat 2020;52:957–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie H, Huang S, Yuan G, Kuang J, Yan L, Wei L, et al. The advanced lung cancer inflammation index predicts short and long‐term outcomes in patients with colorectal cancer following surgical resection: a retrospective study. PeerJ 2020;8:e10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin C, Toiyama Y, Okugawa Y, Omura Y, Kusunoki Y, Kusunoki K, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin Nutr 2021;40:1130–1136. [DOI] [PubMed] [Google Scholar]
- 12. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non‐small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper C, Fielding R, Visser M, van Loon LJ , Rolland Y, Orwoll E, et al. Tools in the assessment of sarcopenia. Calcif Tissue Int 2013;93:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen X, Wang M, Jiang CM, Zhang YM. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr 2011;20:551–556. [PubMed] [Google Scholar]
- 15. Yang M, Hu X, Wang H, Zhang L, Hao Q, Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle 2017;8:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu X, Zhang L, Wang H, Hao Q, Dong B, Yang M. Malnutrition‐sarcopenia syndrome predicts mortality in hospitalized older patients. Sci Rep 2017;7:3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 18. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 19. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–e503. [DOI] [PubMed] [Google Scholar]
- 20. Virchow R. An Address on the Value of Pathological Experiments. Br Med J 1881;2:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 22. Lu X, Guo W, Xu W, Zhang X, Shi Z, Zheng L, et al. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta‐analysis of 9,839 patients. Cancer Manag Res 2019;11:229–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie H, Tang S, Wei L, Gan J. Geriatric nutritional risk index as a predictor of complications and long‐term outcomes in patients with gastrointestinal malignancy: a systematic review and meta‐analysis. Cancer Cell Int 2020;20:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie H, Nong C, Yuan G, Huang S, Kuang J, Yan L, et al. The value of preoperative controlling nutritional status score in evaluating short‐term and long‐term outcomes of patients with colorectal cancer following surgical resection. J Cancer 2020;11:7045–7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 26. Kim EY, Kim N, Kim YS, Seo JY, Park I, Ahn HK, et al. Prognostic Significance of Modified Advanced Lung Cancer Inflammation Index (ALI) in Patients with Small Cell Lung Cancer_ Comparison with Original ALI. PLoS ONE 2016;11:e0164056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study design
Figure S2. Cut‐off of mALI in patients with cancer cachexia. (A, cut‐off of mALI in male; B, cut‐off of mALI in female)
Abbreviations: mALI, modified advanced lung cancer inflammation index.
Figure S3. Correlation analysis between mALI and other parameters.
Abbreviations: mALI, modified advanced lung cancer inflammation index; BMI, body mass index; KPS, Karnofsky performance status; PG‐SGA, patient‐generated subjective nutrition assessment; HGS, hand‐grip strength; MAC, mid‐arm circumference; TSF, triceps fold thickness; MAMA, mid‐upper arm muscle area.
Figure S4. mALI in different cancer types different TNM stage of patients with cachexia.
Notes: A. Stratified by whether patients with cachexia ALI, B, Stratified by TNM stage. ns p‐value >0.05, * p‐value<0.05, ** p‐value<0.01, *** p‐value<0.001.
Abbreviations: mALI, modified advanced lung cancer inflammation index.
Figure S5. Kaplan–Meier curves of OS for cachexia patients stratified by low‐ and high‐AGR for multiple cancer types in training cohort (including lung cancer, gastric cancer, oesophagus cancer, colorectal cancer, hepatic‐biliary cancer, pancreatic cancer, cervical ‐ endometrial cancer, ovarian cancer, urologic cancer, nasopharynx cancer, breast cancer, and other cancer).
Abbreviations: mALI, modified advanced lung cancer inflammation index; OS, overall survival.
Figure S6. Combined survival analysis of mALI and covariates interaction.
Abbreviations: mALI, modified advanced lung cancer inflammation index.
Figure S7. Comparison of the ability of prognostic tools in predicting prognosis of cachexia patients using ROC curves. (A, male cachexia patients, B, female cachexia patients)
Abbreviations: mALI, modified advanced lung cancer inflammation index; ASMI, appendicular skeletal muscle index; NLR, neutrophil‐lymphocyte ratio; ALB, albumin; BMI, body mass index; PG‐SGA, patient‐generated subjective nutrition assessment; HGS, hand‐grip strength; KPS, Karnofsky performance status; MAC, mid‐arm circumference; TSF, triceps fold thickness; MAMA, mid‐upper arm muscle area.


