Abstract
Background
Ageing is accompanied by a progressive loss of skeletal muscle mass and strength, potentially determining the insurgence of sarcopenia. Evidence suggests that motoneuron and neuromuscular junction (NMJ) degeneration contribute to sarcopenia pathogenesis. Seeking for strategies able to slow down sarcopenia insurgence and progression, we investigated whether a 2‐year mixed‐model training involving aerobic, strength and balance exercises would be effective for improving or preserving motoneuronal health and NMJ stability, together with muscle mass, strength and functionality in an old, sarcopenic population.
Methods
Forty‐five sarcopenic elderly (34 females; 11 males) with low dual‐energy X‐ray absorptiometry (DXA) lean mass and Short Physical Performance Battery (SPPB) score <9 were randomly assigned to either a control group [Healthy Aging Lifestyle Education (HALE), n = 21] or an intervention group [MultiComponent Intervention (MCI), n = 24]. MCI trained three times per week for 2 years with a mix of aerobic, strength and balance exercises matched with nutritional advice. Before and after the intervention, ultrasound scans of the vastus lateralis (VL), SPPB and a blood sample were obtained. VL architecture [pennation angle (PA) and fascicle length (Lf)] and cross‐sectional area (CSA) were measured. As biomarkers of neuronal health and NMJ stability status, neurofilament light chain (NfL) and C‐terminal agrin fragment (CAF) concentrations were measured in serum. Differences in ultrasound parameters, NfL and CAF concentration and physical performance between baseline and follow‐up were tested with mixed ANOVA or Wilcoxon test. The relationship between changes in physical performance and NfL or CAF concentration was assessed through correlation analyses.
Results
At follow‐up, MCI showed preserved VL architecture (PA, Lf) despite a reduced CSA (−8.4%, P < 0.001), accompanied by maintained CAF concentration and ameliorated overall SPPB performance (P = 0.007). Conversely, HALE showed 12.7% decrease in muscle CSA (P < 0.001), together with 5.1% and 5.5% reduction in PA and Lf (P < 0.001 and P = 0.001, respectively), and a 6.2% increase in CAF (P = 0.009) but improved SPPB balance score (P = 0.007). NfL concentration did not change in either group. In the population, negative correlations between changes in CAF concentration and SPPB total score were found (P = 0.047), whereas no correlation between NfL and SPPB variations was observed.
Conclusions
The present findings suggest that our 2‐year mixed aerobic, strength and balance training seemed effective for preventing the age and sarcopenia‐related increases in CAF concentration, preserving NMJ stability as well as muscle structure (PA and Lf) and improving physical performance in sarcopenic older individuals.
Keywords: C‐terminal agrin fragment (CAF), sarcopenia, neurofilament light chain (NfL), exercise intervention, skeletal muscle, elderly
Introduction
Ageing is accompanied by a progressive decline in muscle mass and functionality, associated with an increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality, possibly leading to a clinical syndrome known as sarcopenia. 1 This condition affects the performance of daily life activities, reducing quality of life and ultimately leading to loss of independence in advanced age. For these reasons, sarcopenia represents a heavy burden for healthcare systems. 1 Hence, countermeasures aiming at combatting this condition are warranted within the ageing modern society.
Among the causes of sarcopenia, motoneuron and neuromuscular junction (NMJ) degeneration have been proposed as key determinants. 2 , 3 , 4 , 5 Neuronal degeneration and NMJ instability may be assessed in vivo in humans by measuring the blood concentration of neurofilaments and C‐terminal agrin fragment (CAF), respectively.
Neurofilaments constitute the most abundant structural scaffolding proteins of neuronal axons; they are exclusively expressed in neurons and are released in the cerebrospinal fluid and consequently in blood upon neuronal cell damage and death. 6 Among the four subunits constituting neurofilaments, neurofilament light chain (NfL) is that with the lowest molecular weight and, thus, with putatively enhanced diffusive capacity. 7 These features highlight the pertinence of NfL as a blood‐based biomarker. Neurofilament abundance is particularly high in several pathologies involving neuronal degeneration, such as amyotrophic lateral sclerosis, Alzheimer's disease, multiple sclerosis and frontotemporal dementia (see previous works 6 , 8 ). Other reports showed an increase in NfL with age 9 , 10 and negative correlations of this biomarker with physical performance, 11 suggesting a link between this phenomenon and the ongoing neuromuscular degeneration that is known to accompany ageing. 2 , 6 A very recent study reported higher NfL concentrations in sarcopenic than in pre‐sarcopenic and non‐sarcopenic older individuals, suggesting that the neuronal degeneration underlying sarcopenia development may be reflected in higher NfL serum concentrations. 12
The stability of NMJs can be investigated by measuring the serum concentration of CAF, the 22‐KDa C‐terminal fragment of the protein agrin, which is a heparan‐sulphate proteoglycan that stabilizes synaptic structures by aggregating acetylcholine receptors 13 and is irreversibly inactivated by the enzyme neurotrypsin. Upon agrin cleavage at the synaptic cleft, CAF is released in the circulation, with a consequent destabilization of the NMJ through degeneration of its endplate. 14 Elevated CAF levels have been associated with sarcopenia in community‐dwelling elderly, 15 , 16 and this marker has been reported to be an easy‐accessible and reliable indicator of NMJ instability. 3 , 17
Exercise training has been consistently shown to be highly effective in slowing down and possibly preventing the insurgence of sarcopenia in older people, 18 counteracting loss of muscle mass, strength and increases in intramuscular fat infiltrations. 19 While stressing the importance of a personalized approach, general physical activity guidelines suggest that a combination of moderate‐intensity aerobic exercise, progressive resistance and balance training might be the ideal intervention for a sarcopenic population. 18 Interestingly, a very recent cross‐sectional study conducted on healthy older adults reported a negative relationship between physical activity levels and NfL concentration, showing individuals performing at least 90 MET‐min/week of physical activity to have lower probability of having high NfL concentration. 20
However, despite the recognized importance of physical activity for slowing down sarcopenia, no previous investigations assessed NfL concentration in response to longitudinal training protocols, and only two studies performed this analysis on NMJ stability, through CAF evaluation, in a mobility‐limited population. 21 , 22 In both cases, the authors showed that either aerobic or strength training or a combination of aerobic, strength and balance training induced no changes in CAF serum concentration over 6 or 12 months, potentially preserving NMJ stability and preventing its deterioration. In addition, a neuroprotective effect of regular training has been observed in seniors with a long history of high‐level recreational sporting activities who display fewer denervated fibres 23 and superior reinnervation capacity. 24 These studies support the concept that exercise may be an effective tool for slowing down sarcopenia progression by reducing NMJ degeneration and promoting fibre reinnervation.
Hence, the aim of the present work was to investigate the effects of a 2‐year multimodal training intervention involving aerobic, strength and balance exercises on muscle mass and function, motoneuronal and NMJ health in a population of older individuals classified as sarcopenic following EWSGSOP 2010 guidelines. 25 We hypothesized that a better preservation of the neuromuscular system would be the main outcome of this interventional study.
Materials and methods
Ethical approval and participants
The elderly volunteers enrolled in the present investigation were participants of MUS‐C‐1 study, a project that aimed to assess the relationship between muscle ultrasound and estimates of muscle mass [obtained through dual‐energy X‐ray absorptiometry (DXA) scan and bioimpedance analysis] in a group of outpatients with suspected sarcopenia. All patients enrolled underwent a comprehensive geriatric assessment at the Cognitive and Motoric Disorders Clinic of the University‐Hospital of Parma, Medical Geriatric Rehabilitation Department.
Among the cohort of the MUS‐C‐1 study, in 2017, some participants who were diagnosed for low muscle mass and low muscle function were recruited to participate in the SPRINTT project. 26 A detailed description of the inclusion and exclusion criteria and of the SPRINTT clinical trial (Sarcopenia and Physical fRailty IN older people: multi‐componenT Treatment strategies) is provided in Data S1. At the end of the 2‐year duration of the SPRINTT intervention, participants were finally enrolled in the follow‐up study (MUS‐C‐2) and underwent the same geriatric assessment performed in 2017.
The protocol of the MUS‐C‐1, MUS‐C‐2 and SPRINTT studies were approved by the Ethics Committee of Parma province (ID 164/2018/OSS/AOUPR, ID 912/2018/OSS/AOUPR and ID 82/2016/SPER/AOUPR, respectively).
SPRINTT intervention protocol
For the present investigation, 45 participants from MUS‐C‐1 study were selected to participate in the SPRINTT project (see ‘Statistical analysis’ section). At baseline, BL (MUS‐C‐1) and follow‐up (MUS‐C‐2), volunteers underwent a visit to assess their appendicular lean mass (DXA) and performance [Short Physical Performance Battery (SPPB)], upper limb strength (handgrip), lower limb muscle morphology and architecture (ultrasound) and a blood sampling to assess motoneurons and NMJ damage via serum NfL and CAF concentration detection. Once enrolled, participants were randomly assigned to two different groups: MultiComponent Intervention (MCI), performing two‐center and one home‐based session of mixed aerobic, strength and balance training per week and receiving nutritional counselling, n = 24; and Healthy Aging Lifestyle Education (HALE), n = 21. SPRINTT physical activity intervention had a duration of 2 years; details can be found elsewhere 26 and in the Data S1.
DXA
DXA exams were performed with a Hologic™ QDR 4500 A densitometer, US (Hologic, Inc., USA) device to quantify appendicular lean mass (aLM). Low muscle mass was defined according to the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project criteria. 27
SPPB and handgrip strength
Participants were evaluated in terms of physical performance by means of SPPB, according to Guralnik et al., 28 and maximal handgrip strength, measured as the average of three trials per arm (90° flexion angle) by using a dynamometer (Baseline Hydraulic Hand Dynamometer, Irvington, USA). Further details can be found in the Data S1.
Muscle morphology assessment
Each participant underwent an ultrasound scan to assess vastus lateralis (VL) muscle morphology. Longitudinal images of the right VL muscle were collected using B‐mode ultrasonography (Mylab70, Esaote, Genoa, Italy) while participants were resting in a supine position. VL architecture [fascicle length (Lf) and pennation angle (PA)] was measured according to the method described by Narici et al. 29 Additionally, VL CSA was assessed through the extended‐field‐of‐view (EFOV) ultrasonography technique (Mylab70, Esaote, Genoa, Italy) at the 35% of the femur length. For a detailed description concerning how ultrasound measures were collected and analysed, see Data S1.
Determination of serum biomarkers of neurodegeneration
All participants underwent a blood sampling of 5 mL from the median cubital vein, and serum was obtained. NfL concentration was determined at the facility ‘Centro Piattaforme Tecnologiche’ of the University of Verona (8 Le Grazie St., 37134, Verona VR, Italy) using the SIMOA Bead Technology (Quanterix Corporation 900 Middlesex Turnpike, Billerica, MA 10821) on Quanterix SR‐x (#1913QP0444) platform with Simoa® Nf‐light Advantage Kit (SR‐x). Samples were diluted 1:4 and run in duplicate. The measurement was performed following the manufacturer's instructions.
CAF serum concentration was determined using a commercially available enzyme‐linked immunosorbent assay (ELISA) kit (Human Agrin SimpleStep ELISA, Ab216945, Abcam, Cambridge, UK) following the manufacturer's instructions. Samples were diluted 1:4 and run in duplicate.
Statistical analysis
Definition of sample size was not estimated according to a statistical method; the participants considered for the present study were selected basing on MUS‐C‐1 and SPRINTT projects and on availability of biological samples.
Data are presented as mean ± SD or median [interquartile range], as appropriate. Normality and shape of data were assessed through Q‐Q plot and Shapiro–Wilk tests; when the dataset was not Gaussian, a logarithmic transformation was applied; hence, parametric or non‐parametric statistics was performed, as appropriate. The specific test used for each analysis is reported in the figure captions and in the Data S1. All statistical decisions were made at P ≤ 0.05. Statistical analyses were performed through SPSS Statistics 25 (IBM, Inc., Chicago, IL, USA), whereas GraphPad Prism software (version 8.0; GraphPad software Inc., San Diego, CA) was used to prepare graphs.
Results
Baseline characteristics of subjects enrolled in the study
In the present study, we included 45 sarcopenic participants (34 females and 11 males), whose features are reported in Table 1. Mean age was 78.7 ± 5.9 years; mean BMI was in the range of overweight (28 ± 4.4 kg/m2). Height and body mass were 153.6 ± 15.6 cm and 70.1 ± 17.7 kg, respectively. The median score of Mini‐Mental State Examination Test was 28 (suggestive of a normal cognitive status of participants); the median DXA aLMcrude and aLM/BMI were 14.9 kg and 0.564, respectively, reflecting low muscle mass; the median score of SPPB test showed an impairment of physical performance (median score 7, interquartile range 7–8).
Table 1.
Baseline characteristics of the volunteers enrolled in the study (n = 45, 75.6% females and 24.4% males)
| HALE(n = 21) | MCI(n = 24) | MCI + HALE pooled(n = 45) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median[Q1–Q3] | Mean ± SD | Median[Q1–Q3] | Mean ± SD | Median[Q1–Q3] | |
| Age (years) | 79.6 ± 5.8 | 78.0 ± 6.1 | 78.7 ± 5.9 | |||
| BMI (kg/m2) | 26.9 ± 2.9 | 29.0 ± 5.2 | 28 ± 4.4 | |||
| MMSE (score) | 29[25–30] | 28[27–29] | 28[26.5–30] | |||
| aLMcrude (kg) | 14.78[14.78–18.68] | 15.00[14.44–16.75] | 14.90[14.48–18.05] | |||
| aLM/BMI | 0.596[0.539–0.723] | 0.541[0.494–0.607] | 0.564[0.506–0.685] | |||
| CAF (pg/mL) | 5871.1 ± 1317.7 | 6360.7 ± 1441.2 | 6133.0 ± 1390.9 | |||
| NfL (pg/mL) | 24.1 ± 13.2 | 19.9 ± 11.0 | 21.8 ± 12.1 | |||
| PA (°) | 13.5 ± 1.5 | 13.1 ± 2.1 | 13.3 ± 1.8 | |||
| Lf (mm) | 70.9 ± 8.5 | 76.3 ± 14.7 | 73.8 ± 12.4 | |||
| CSA (cm2) | 9.9 ± 2.0 | 10.5 ± 3.1 | 10.2 ± 2.7 | |||
| SPPB Balance (score) | 3[2–3] | 3[2–4] | 3[2–3] | |||
| SPPB Gait (score) | 3[2.5–4] | 3[3–4] | 3[3–4] | |||
| SPPB Chair stand (score) | 1[1–2] | 1[1–2] | 1[1–2] | |||
| SPPB Total (score) | 7[7–7.5] | 7[7–9] | 7[7–8] | |||
| Handgrip strength (kg) | 22[20.75–27] | 21.5[19.5–28.5] | 22[20–27] | |||
aLM/BMI, BMI‐adjusted appendicular lean mass; aLMcrude, raw value of appendicular lean mass; BMI, body mass index; CSA, cross‐sectional area obtained at the 35% of the femur length; Lf, length of fascicles; MMSE, Mini‐Mental Status Examination; PA, pennation angle; SPPB, Short Physical Performance Battery.
Physical performance: SPPB tests output
The physical performance measured by SPPB and the strength changes observed in response to the intervention are reported in Table 2. Within HALE, a significant amelioration in SPPB balance and total score was observed (P = 0.007, P = 0.019, respectively), whereas MCI improved SPPB for balance, chair stand and total score (P = 0.034, P = 0.006, P = 0.007, respectively). Force was preserved in both groups. Such results were in line with those observed in the global population participating to the SPRINTT programme. 30
Table 2.
Differences of physical performance [measured by Short Physical Performance Battery (SPPB)] endpoints between baseline (BL) and follow‐up in control (HALE) and intervention (MCI) groups determined by Wilcoxon rank test
| HALE (n = 21) | MCI (n = 24) | |||||
|---|---|---|---|---|---|---|
| BL | Follow‐up | P value | BL | Follow‐up | P value | |
| SPPB Balance (score) | 3[2–3] | 3[2–4] | 0.007 | 3[2–4] | 4[3–4] | 0.034 |
| SPPB Gait (score) | 3[2.5–4] | 4[3–4] | 0.197 | 3[3–4] | 4[3.25–4] | 0.134 |
| SPPB Chair stand (score) | 1[1–2] | 1[1–3] | 0.468 | 1[1–2] | 2[1.25–3] | 0.006 |
| SPPB Total (score) | 7[7–7.5] | 9[6.5–10.5] | 0.019 | 7[7–9] | 10[7.25–11] | 0.007 |
| Handgrip strength (kg) | 22[20.75–27] | 20[18.5–25] | 0.229 | 22[19–28] | 21.5[19.5–28.5] | 0.512 |
In bold significant P values.
Muscle morphological adaptations
Muscle morphological adaptations to the 2‐year intervention in terms of muscular architecture—PA and Lf—and VL CSA are reported in Figure 1. The mixed ANOVA revealed a significant time and time × group interaction effect for PA (P = 0.003 and P = 0.007, respectively) and a significant time effect (P = 0.001) and a strong trend time × group interaction effect (P = 0.056) for Lf. In HALE, both PA and Lf decreased significantly at follow‐up if compared with BL, from 13.5° to 12.8° (−5.1%, P < 0.001) and from 70.9 to 67.0 mm (−5.5%, P = 0.012), although this drop was not present in MCI. In contrast, mixed ANOVA revealed only a time effect for VL CSA, which decreased significantly in both groups, from 9.9 to 8.5 cm2 in HALE (−12.7%, P < 0.001) and from 10.5 to 9.6 cm2 in MCI (−8.4%, P < 0.001). Although unpaired t‐test between HALE and MCI percentage decrements in VL CSA revealed no significant differences (P = 0.14), a 35.8% lower decrement in this parameter in the MCI group, if compared with HALE, was observed.
Figure 1.
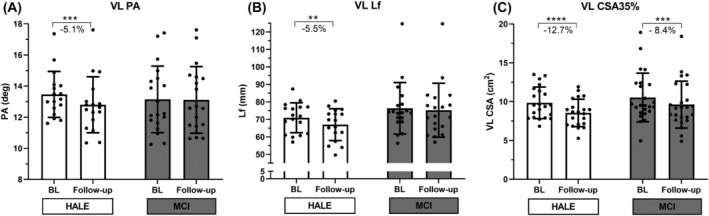
Muscle size adaptations to 2‐year intervention: vastus lateralis (VL), pennation angle (PA) (A), length of fascicles (Lf) (B) and cross‐sectional area (CSA) obtained at the 35% of the femur length (C) at baseline (BL) and follow‐up for control (HALE) and intervention (MCI) groups, determined by mixed ANOVA. **P < 0.01 follow‐up versus BL. ***P < 0.001 follow‐up versus BL. ****P < 0.0001 follow‐up versus BL.
Motoneuron and NMJ health status
Motoneuron health was inferred by measuring NfL concentration in blood (Figure 2). HALE group increased by 16.6% (from 24.1 ± 13.2 pg/mL to 27.7 ± 20.1 pg/mL), whereas MCI experienced a slight 4.1% increment (from 19.9 ± 11.0 pg/mL to 20.6 ± 11.8 pg/mL). However, mixed ANOVA reported no significant effect, neither on time nor on group or their interaction.
Figure 2.
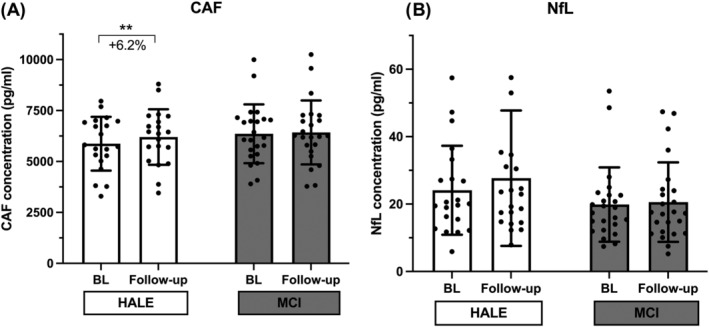
C‐terminal agrin fragment (CAF) (A) and neurofilament light chain (NfL) (B) serum concentration variations in response to the 2‐year intervention: CAF and NfL values at baseline (BL) and follow‐up for the control (HALE) and intervention (MCI) groups, determined by mixed ANOVA. NfL values did not pass the normality test; thus, log10 transformation was applied; thereafter, normality test was passed. **P < 0.01 follow‐up versus BL.
The NMJ health status was inferred through the dosage of CAF (Figure 2). Mixed ANOVA revealed time effect (P = 0.012) and a trend for time × group interaction effect (P = 0.082), and the analysis showed that CAF values were significantly increased in HALE (from 5871.1 ± 1317.7 pg/mL to 6202.3 ± 1362.9 pg/mL, +6.2%, P = 0.009), whereas they were unchanged in MCI (from 6360.7 ± 1441.2 pg/mL to 6424.5 ± 1569.4 pg/mL, n.s.). According to the trend for time × group interaction, the difference in ΔCAF between the two groups showed a trend for significance (P = 0.082).
Correlation between changes in serum biomarkers and parameters of muscle function
The relationship between variations in biomarkers of NMJ stability and neuronal damage and changes in functional outputs within the 2‐year intervention was assessed through correlation analyses (Tables 3 and 4). Considering all participants, ΔCAF was significantly and negatively correlated to ΔSPPB total score (r = −0.305, P = 0.047). Performing the analysis by group, we did not observe the same effect. However, an inverse and significant correlation was observed between ΔCAF and Δhandgrip strength in HALE group (r = −0.578 P = 0.015). Changes in NfL were not correlated to ΔSPPB performance, neither considering single subtask nor ΔSPPB total score, in the entire population and in the two groups.
Table 3.
Spearman correlations between changes in physical performance outcomes measured through Short Physical Performance Battery (SPPB) and handgrip strength and changes of C‐terminal Agrin fragment (CAF) concentration within 2‐year intervention
| ΔCAF | ||||||
|---|---|---|---|---|---|---|
| HALE | MCI | HALE + MCI pooled | ||||
| r | P value | r | P value | r | P value | |
| ΔSPPB Balance | −0.235 | 0.32 | −0.122 | 0.58 | −0.164 | 0.29 |
| ΔSPPB Gait | 0.076 | 0.75 | −0.41 | 0.852 | 0.06 | 0.69 |
| ΔSPPB Chair stand | −0.200 | 0.40 | −0.029 | 0.90 | −0.127 | 0.42 |
| ΔSPPB Total | −0.392 | 0.09 | −0.214 | 0.33 | −0.305 | 0.047 |
| ΔHandgrip strength | −0.578 | 0.015 | −0.05 | 0.72 | −0.229 | 0.10 |
Δ: change between BL and follow‐up.
In bold significant P values.
Table 4.
Spearman correlations between changes in physical performance outcomes measured through Short Physical Performance Battery (SPPB) and handgrip strength and changes of neurofilament light chain (NfL) concentration within 2‐year intervention
| ΔNfL | ||||||
|---|---|---|---|---|---|---|
| HALE | MCI | HALE + MCI pooled | ||||
| r | P value | r | P value | r | P value | |
| ΔSPPB Balance | −0.081 | 0.73 | −0.176 | 0.41 | −0.109 | 0.48 |
| ΔSPPB Gait | 0.396 | 0.08 | 0.025 | 0.91 | 0.231 | 0.13 |
| ΔSPPB Chair stand | −0.359 | 0.11 | 0.284 | 0.18 | −0.041 | 0.79 |
| ΔSPPB Total | −0.299 | 0.19 | 0.178 | 0.41 | −0.048 | 0.76 |
| Δ Handgrip strength | 0.049 | 0.85 | 0.417 | 0.06 | 0.266 | 0.10 |
Δ: change between BL and follow‐up.
Discussion
The present study investigated the effects of a 2‐year multimodal training intervention on muscle mass and function, motoneuron health and NMJ stability in an elderly sarcopenic population.
In line with our hypothesis, our mixed aerobic, strength and balance training seemingly preserved NMJ stability, preventing serum CAF concentration raise in the MCI group, although this biomarker increased significantly only in the HALE group (+6.2% at follow‐up). Conversely, NfL concentration did not change in either group. In addition, we observed a significant effect of the exercise protocol on muscle pennation angle, with a strong trend also on fascicle length, which were both maintained in the MCI group but decreased in the HALE group. Surprisingly, an increase in SPPB balance and total score was found in both groups; on the other hand, only MCI showed improvements in SPPB chair stand score. Finally, we found that serum CAF concentration variation over the 2‐year observation was negatively correlated with SPPB total score in the population considered and to handgrip strength changes only in HALE.
Effects of a 2‐year multi‐modal training intervention on serum NfL and CAF concentration
NfL and CAF are well‐established biomarkers of either neuronal cell damage and death 6 and NMJ stability. 3 , 17 At baseline, NfL and CAF concentrations were not different between HALE and MCI.
However, both NfL and CAF concentrations were markedly higher than those of a young reference population analysed in our laboratory (Figure 3), highlighting that, in older individuals, sarcopenia is associated with NMJ instability 5 and axonal damage. 9 , 10
Figure 3.
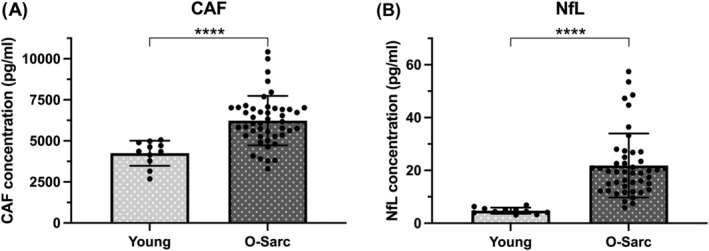
C‐terminal agrin fragment (CAF) (A) and neurofilament light chain (NfL) (B) serum concentration differences between a young (Young) reference population measured in our laboratory and the pooled old sarcopenic (O‐Sarc) participants of the present study at baseline. Differences were assessed via unpaired t‐test. NfL values did not pass the normality test; thus, log10 transformation was applied; thereafter, normality test was passed. ****P < 0.0001 versus Young.
Previous research showed that 1 month of aerobic exercise was not able to prevent motoneuron degeneration in aged animals but rescued their NMJs and muscle fibre innervation. 31
Based on these findings, we hypothesized that following the 2‐year intervention serum concentrations of NfL would raise in both groups, whereas CAF would be maintained in MCI. We also expected ageing and sarcopenia to exacerbate the increments of CAF in HALE. However, this was only partially confirmed.
Indeed, whereas CAF concentration at follow‐up was found to be unaltered in MCI but increased in HALE, NfL did not change in either group. Although this result may seem surprising, some important considerations should be made. On the one hand, we observed a 16.6% and 4.1% NfL concentration increment in HALE and MCI, respectively. Although these changes were scored as not significant by the mixed ANOVA, potentially due to the high individual variability in response to training, our sample size was quite small. Hence, we cannot exclude the possibility that, by testing a larger sample, NfL concentration in sarcopenic elderly would increase significantly over time. In fact, on larger cohorts, NfL blood concentration has been shown to augment with age in neurological disease‐free individuals 9 , 10 to different rates and up to 4.3% per year, potentially due to motoneuronal loss (especially fast‐twitch fibre innervating motoneurons). 5 Thus, up to about 10% of the total NfL increment observed in HALE might be explained by the pure ageing process. In addition, it cannot be excluded that sarcopenia, which is associated with higher NfL concentrations, 12 could have induced a faster neurodegenerative decay, reflected by further NfL increments (total of about 17% in HALE). Importantly, this phenomenon seemed to be attenuated in MCI, in which a much lower increase was observed. Therefore, and considering that the variability of NfL concentration has been reported to increase with ageing, 9 it would be interesting to longitudinally assess the variations of this parameter on a lager exercising cohort, and potentially also over a longer time course, to understand whether physical exercise may have a protective effect on motoneurons in humans.
From a different point of view, one should also acknowledge that neurofilaments are scaffolding proteins constituting neuronal axons 6 ; hence, this marker is not selectively specific for motoneurons, but rather represents the whole neuronal pool health. Our participants were free from neurological disease (known to be associated with consistent neuronal wasting and NfL increments; see comprehensive works 6 , 8 ) and did not experience any trauma, which could induce an acute and important neuronal death. Hence, the lack of NfL increment might suggest that no substantial loss of neuronal cells massively occurred in our participants over the course of 2 years. Considering that the number of motoneurons is much lower than that of neurons, the highly variable response observed in our participants may indicate that NfL is a less specific marker than CAF when assessing the neuromuscular system responses to training in humans.
Conversely, CAF significantly increased only in HALE, suggestive of an increased NMJ instability, although its concentration was maintained in MCI. Furthermore, the interaction time × group (mixed ANOVA) showed a trend for statistical significance, suggesting a potential neuroprotective effect of the exercise programme. This may have possibly delayed further increases in NMJ instability and consequently reduced CAF concentration increments. Our hypothesis is supported by previous studies, reporting decreased or unchanged CAF in both non‐sarcopenic and sarcopenic elderly populations following different typologies of intervention. 14 , 21 , 32 , 33 In contrast, two studies reported a trend for increased CAF following 6–12 weeks of training. 33 , 34 Interestingly, one of these studies found a differential effect of the same exercise protocol on peri‐menopausal and post‐menopausal women: CAF was found to be higher and lower after the intervention, respectively, in the first and second groups, thus stressing the potential importance of age and hormonal status on this biomarker. 33
Cross‐sectionally, both ageing and sarcopenia have been shown to be linked to higher NfL and CAF concentration, 9 , 12 , 15 , 16 possibly reflecting a differently preserved neuronal/motoneuronal and NMJ health. Longitudinally, our results confirm that ageing and, potentially, sarcopenia contribute to the natural increase in these biomarker concentrations. As NfL increments were not significant, no inference about the effect of our exercise protocol on axonal integrity may be made. On the other hand, oxidative stress, due to mitochondrial dysfunction, 35 muscle fibre denervation and decreased PGC‐1α expression 36 are considered major age‐related neuromuscular alterations triggering NMJ damage, also implicated in the biogenesis of sarcopenia. 18 These mechanisms might be responsible also for the increase in CAF observed in the HALE group. Noteworthy, in the MCI group, CAF concentration was unchanged with a trend in favour of the efficacy of exercise intervention in preserving NMJ stability.
In support of this concept, animal studies have shown that training has a protective effect on the NMJ 37 ; for example, aerobic exercise induced a partial reversion of the NMJ alterations that had already occurred in 22‐month‐old mice after 1 month of running protocol. 31
One may speculate that our mixed aerobic, strength and balance training induced beneficial effects at the muscle and NMJ level (including mitochondrial increased biogenesis and turnover, 38 increased muscle protein synthesis 39 and neurotrophin production and release 37 ), thus better promoting NMJ stability maintenance and CAF concentrations, together with preventing muscle architecture declines and inducing ameliorations in muscle performance.
Effects of a 2‐year multimodal training intervention on muscle size, architecture and function
Another important finding of this study was the unchanged muscle architecture (PA and Lf), associated with an improved physical performance in MCI, as opposed to the decrease in PA and Lf despite increased SPPB balance and total scores in HALE. The significant time × group interaction effect described for PA and the strong trend for Lf (P = 0.056) suggest that the mixed aerobic, strength and balance training was able to preserve muscle architecture in MCI. This result is relevant especially considering the very old (80 years old), sarcopenic population involved in this study. Indeed, low weights were used within the strength training, likely reducing the potential for hypertrophy. Accordingly, MCI VL CSA decreased by about 8%. This strongly suggests that the ageing process acts as an important determinant of the loss of muscle mass but also that training volume, intensity or a combination of both was not sufficient to contrast the muscle mass decrement. In support of this view, a 1‐year study by Goodpaster et al. 19 on elderly people demonstrated that, after a 12‐month intervention, both controls and trained volunteers experienced a loss of muscle mass, although muscle force was preserved in the exercised group only. In addition, it is worth noting that a trend for a greater reduction in muscle CSA was observed in HALE (−13%) than in MCI (−8.4%), highlighting the protective effect of the training intervention on muscle mass and architecture, as opposed to the significant decrease of all muscle morphological parameters (PA, Lf and CSA) in HALE, reflecting a worsening of the sarcopenic condition.
In this study, muscle function and performance measured through SPPB was surprisingly ameliorated both in MCI and HALE groups, although MCI improved balance, chair‐stand and total SPPB scores, while in the HALE group only balance and total SPPB changed. Whereas the balance test may reflect a preserved or improved balance capacity, which is necessary to everyday life activity performance and might explain the observed output, the chair stand is considered a measure of lower limb muscle power. Hence, MCI selective chair‐stand score improvement may be reflective of a beneficial effect of the strength component in the proposed training.
Interestingly, SPPB total score was negatively correlated with changes in CAF concentration in the whole population considered. Longitudinally, no previous work investigated this relationship; it could be speculated that CAF concentration increments, reflective of NMJ destabilization processes, would negatively affect the performance of motor tasks. In support of this, we also observed CAF variation to be inversely correlated to handgrip strength only in HALE, who experienced a higher (although not significant) loss of muscle strength associated with increased CAF. Similar correlations were reported in COPD patients undergoing pulmonary rehabilitation 40 and patients increasing their lean mass during a post‐stroke rehabilitation protocol. 41
Methodological considerations
The observed baseline pooled MCI and HALE NfL concentration was 21.8 pg/mL, while CAF was 6133 pg/mL. Whereas NfL concentration values are in line with previous literature 8 and have been assessed with the gold‐standard technique SIMOA, the observed CAF concentration in our sample deserves a brief critical discussion.
In fact, the baseline CAF concentration observed in the present cohort (6133 pg/mL) was significantly greater than that found in non‐sarcopenic sedentary (5161.6 pg/mL) and active (4388.6 pg/mL) elderly involved in a recent study of our laboratory. 42 Noteworthy, very similar results were also reported by previous work comparing non‐sarcopenic to sarcopenic elderly. 14 , 15 , 16 Interestingly, in the first study conducted on circulating CAF in healthy and sarcopenic elderly, this peptide was dosed in serum using western blot (WB), 14 , 15 and its concentration was reported to be around 3 ± 0.86 ng/mL (3000 ± 860 pg/mL) in healthy elderly and to range between 4.5 ± 2.2 and 5.4 ± 2.9 ng/mL (4500 ± 2200 to 5400 ± 2900 pg/mL) in a sarcopenic population. In our study, as in many more recent others, CAF serum concentrations were dosed using an ELISA kit, which seems to slightly, but systematically, overestimate values obtained by WB analyses (around 4400 for healthy active elderly and 6100 for sarcopenic ones). Such systematic overestimation might be due to the different techniques used (WB vs ELISA); however, it is noteworthy that the proportions between CAF concentrations in healthy and sarcopenic old people are in line with those reported by the abovementioned studies from Drey and Hettwer groups. 14 , 15
Limitations
An important limitation of our study is that the present research included a small number of participants; enlarging the sample size would be appropriate to confirm the relationships we have described.
A second limitation is represented by the absence of muscle biopsies. Indeed, the observation of a better maintained NMJ stability would have been strengthened by analysis of muscle fibre cross‐sectional area, grouping and denervation or reinnervation biomarkers. The hypothesis, open for being tested in future studies, is that exercise would result in a lower amount of denervated fibres or an increased abundance of regenerating, reinnervated fibres associated with more stable and innervated NMJs.
Conclusions
In conclusion, our 2‐year multimodal training intervention including aerobic, strength and balance exercises seemed effective for preventing CAF concentration increments, suggesting a positive effect on NMJ stability. Importantly, also architectural alterations of pennation angle and, with a strong trend, of fascicle length were prevented in the trained group, whereas the control group experienced a decrease in both parameters. Finally, improvements of physical performance were correlated with changes of serum biomarkers of NMJ stability, suggesting a potential role of CAF (but not of NfL) as proxy of muscle function.
Conflict of interest
Elena Monti, Sara Tagliaferri, Sandra Zampieri, Fabio Sarto, Giuseppe Sirago, Martino V. Franchi, Andrea Ticinesi, Yari Longobucco, Elisa Adorni, Fulvio Lauretani, Riccardo Calvani and Marco V. Narici declare that they have no conflict of interests. Stephan Von Haehling, Emanuele Marzetti, Roberto Bernabei, Matteo Cesari and Marcello Maggio have received research grants from the Innovative Medicines Initiative – Joint Undertaking (IMI‐JU 115621).
Funding
The present work was funded by a grant from the Innovative Medicines Initiative – Joint Undertaking (IMI‐JU 115621) and by PRIN project ‘NeuAge’ (2017CBF8NJ_001, Narici).
Ethics statement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 43
As reported in the ‘Methods’ section, this study has been approved by the ethics committee, being performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
All the participants gave their informed consent prior to their inclusion in the study.
Supporting information
Data S1. Supporting Information
Acknowledgements
The authors would like to thank all the participants taking part to the study for their priceless effort and Dr Marta Canato for helping during experiment performance.
Monti E., Tagliaferri S., Zampieri S., Sarto F., Sirago G., Franchi M. V., Ticinesi A., Longobucco Y., Adorni E., Lauretani F., Von Haehling S., Marzetti E., Calvani R., Bernabei R., Cesari M., Maggio M., and Narici M. V. (2023) Effects of a 2‐year exercise training on neuromuscular system health in older individuals with low muscle function, Journal of Cachexia, Sarcopenia and Muscle, 14, 794–804, 10.1002/jcsm.13173
Elena Monti and Sara Tagliaferri have contributed to the work equally and share first co‐authorship.
References
- 1. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gonzalez‐Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: Aging at the crossroad between nerves and muscle. Front Aging Neurosci 2014;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalinkovich A, Livshits G. Sarcopenia ‐ The search for emerging biomarkers. Ageing Res Rev 2015;22:58–71. [DOI] [PubMed] [Google Scholar]
- 4. Bütikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J 2011;25:4378–4393. [DOI] [PubMed] [Google Scholar]
- 5. Moreira‐Pais A, Ferreira R, Oliveira PA, Duarte JA. A neuromuscular perspective of sarcopenia pathogenesis: Deciphering the signaling pathways involved. GeroScience 2022;44:1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–589. [DOI] [PubMed] [Google Scholar]
- 7. Kušnierová P, Zeman D, Hradílek P, Čábal M, Zapletalová O. Neurofilament levels in patients with neurological diseases: A comparison of neurofilament light and heavy chain levels. J Clin Lab Anal 2019;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bornhorst JA, Figdore D, Campbell MR, Pazdernik VK, Mielke MM, Petersen RC, et al. Plasma neurofilament light chain (NfL) reference interval determination in an age‐stratified cognitively unimpaired cohort. Clin Chim Acta 2022;535:153–156. [DOI] [PubMed] [Google Scholar]
- 11. O'Bryant SE, Petersen M, Hall J, Large S, Johnson LA. Plasma biomarkers of Alzheimer's disease are associated with physical functioning outcomes among cognitively normal adults in the multi‐ethnic HABS‐HD cohort. J Gerontol A Biol Sci Med Sci 2022;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pratt J, De Vito G, Segurado R, Pessanha L, Dolan J, Narici M, et al. Plasma neurofilament light levels associate with muscle mass and strength in middle‐aged and older adults: Findings from GenoFit. J Cachexia Sarcopenia Muscle 2022;13:1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stephan A, Mateos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, et al. Neurotrypsin cleaves agrin locally at the synapse. FASEB J 2008;22:1861–1873. [DOI] [PubMed] [Google Scholar]
- 14. Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, et al. C‐terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol 2013;48:76–80. [DOI] [PubMed] [Google Scholar]
- 15. Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, et al. Elevated levels of a C‐terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol 2013;48:69–75. [DOI] [PubMed] [Google Scholar]
- 16. Landi F, Calvani R, Lorenzi M, Martone AM, Tosato M, Drey M, et al. Serum levels of C‐terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community‐dwellers: Results from the ilSIRENTE study. Exp Gerontol 2016;79:31–36. [DOI] [PubMed] [Google Scholar]
- 17. Liguori I, Russo G, Aran L, Bulli G, Curcio F, Della‐Morte D, et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin Interv Aging 2018;13:913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phu S, Boersma D, Duque G. Exercise and sarcopenia. J Clin Densitom 2015;18:488–492. [DOI] [PubMed] [Google Scholar]
- 19. Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: A randomized controlled trial. J Appl Physiol 2008;105:1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raffin J, Rolland Y, Aggarwal G, Nguyen AD, Morely JE, Li Y, et al. Associations between physical activity, blood‐based biomarkers of neurodegeneration, and cognition in healthy older adults: The MAPT study. J Gerontol A Biol Sci Med Sci 2021;76:1382–1390. [DOI] [PubMed] [Google Scholar]
- 21. Bondoc I, Cochrane SK, Church TS, Dahinden P, Hettwer S, Hsu FC, et al. Effects of a one‐year physical activity program on serum C‐terminal agrin fragment (CAF) concentrations among mobility‐limited older adults. J Nutr Health Aging 2015;19:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento‐Villareal R, Sun Z, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite calorie restriction. Cell Metab 2020;176:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, et al. Long‐term high‐level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 2014;73:284–294. [DOI] [PubMed] [Google Scholar]
- 24. Sonjak V, Jacob K, Morais JA, Rivera‐Zengotita M, Spendiff S, Spake C, et al. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol 2019;597:5009–5023. [DOI] [PubMed] [Google Scholar]
- 25. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landi F, Cesari M, Calvani R, Cherubini A, Di Bari M, Bejuit R, et al. The “Sarcopenia and Physical fRailty IN older people: multi‐componenT Treatment strategies” (SPRINTT) randomized controlled trial: Design and methods. Aging Clin Exp Res 2017;29:89–100. [DOI] [PubMed] [Google Scholar]
- 27. McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The Foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol ‐ Ser A Biol Sci Med Sci 2014;69A:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Narici M, McPhee J, Conte M, Franchi MV, Mitchell K, Tagliaferri S, et al. Age‐related alterations in muscle architecture are a signature of sarcopenia: The ultrasound sarcopenia index. J Cachexia Sarcopenia Muscle 2021;12:973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bernabei R, Landi F, Calvani R, Cesari M, Del Signore S, Anker SD, et al. Multicomponent intervention to prevent mobility disability in frail older adults: Randomised controlled trial (SPRINTT project). BMJ 2022;377:e068788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW, et al. Attenuation of age‐related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A 2010;107:14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bigdeli S, Dehghaniyan MH, Amani‐Shalamzari S, Rajabi H, Gahreman DE. Functional training with blood occlusion influences muscle quality indices in older adults. Arch Gerontol Geriatr 2020;90:104110. [DOI] [PubMed] [Google Scholar]
- 33. Willoughby DS, Beretich KN, Chen M, Funderburk LLK. Decreased serum levels of C‐terminal agrin in postmenopausal women following resistance training. J Aging Phys Act 2020;28:73–80. [DOI] [PubMed] [Google Scholar]
- 34. Fragala MS, Jajtner AR, Beyer KS, Townsend JR, Emerson NS, Scanlon TC, et al. Biomarkers of muscle quality: N‐terminal propeptide of type III procollagen and C‐terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle 2014;5:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rygiel KA, Picard M, Turnbull DM. The ageing neuromuscular system and sarcopenia: A mitochondrial perspective. J Physiol 2016;594:4499–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging‐associated mitochondrial dysfunction in rat skeletal muscle: Role of PGC‐1α. Exp Gerontol 2013;48:1343–1350. [DOI] [PubMed] [Google Scholar]
- 37. Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve 2014;49:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hood DA, Memme JM, Oliveira AN, Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol 2019;81:19–41. [DOI] [PubMed] [Google Scholar]
- 39. Lavin KM, Roberts BM, Fry CS, Moro T, Rasmussen BB, Bamman MM. The importance of resistance exercise training to combat neuromuscular aging. Phys Ther 2019;34:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karim A, Muhammad T, Qaisar R. Prediction of sarcopenia using multiple biomarkers of neuromuscular junction degeneration in chronic obstructive pulmonary disease. J Pers Med 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scherbakov N, Knops M, Ebner N, Valentova M, Sandek A, Grittner U, et al. Evaluation of C‐terminal agrin fragment as a marker of muscle wasting in patients after acute stroke during early rehabilitation. J Cachexia Sarcopenia Muscle 2016;7:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marcolin G, Franchi MV, Monti E, Pizzichemi M, Sarto F, Sirago G, et al. Active older dancers have lower C‐terminal agrin fragment concentration and better balance and gait performance than sedentary peers. Exp Gerontol 2021;153:111469. [DOI] [PubMed] [Google Scholar]
- 43. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: Update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


