Abstract
Background
Systemic inflammation, the most representative tumour–host interaction, plays a crucial role in disease progression and prognosis in patients with non‐small cell lung cancer (NSCLC). Few studies have compared the performance of existing haematological systemic inflammation biomarkers in predicting the prognosis of NSCLC patients. The purpose of this study was to compare the prognostic value of existing systemic inflammation biomarkers and determine the optimal systemic inflammation biomarker in patients with NSCLC through a multicentre prospective study.
Methods
The predictive accuracy of systemic inflammation biomarkers for prognostic assessment in NSCLC was assessed using C‐statistics. Inter‐group differences in survival were assessed using the log‐rank test and visualized using the Kaplan–Meier method. A restricted cubic spline (RCS) curve was used to explore the association between the biomarkers and survival. Independent prognostic biomarkers for overall survival were determined using multivariable Cox proportional hazards regression analysis. Logistic regression analysis was used to determine independent predictors of 90‐day outcomes, length of hospitalization, hospitalization expenses and cachexia.
Results
The inflammatory burden index (IBI) had the highest C‐statistic for predicting the prognosis of patients with NSCLC, reaching 0.640 (0.617, 0.663). Patients with a high IBI had significantly worse outcomes than those with a low IBI (35.46% vs. 57.22%; log‐rank P < 0.001). The IBI was also able to differentiate the prognosis of patients with NSCLC with the same pathological stage. The RCS curve showed an inverted L‐shaped dose–response relationship between the IBI and survival of patients with NSCLC. Multivariable Cox proportional hazards regression analysis showed that a high IBI was an independent risk factor for death of patients with NSCLC (hazard ratio = 1.229, 95% confidence interval [CI]: 1.131–1.335, P < 0.001). A high IBI was an independent predictor of 90‐day outcomes (odds ratio [OR] = 1.789, 95% CI: 1.489–2.151, P < 0.001), prolonged hospital stays (OR = 1.560, 95% CI: 1.256–1.938, P < 0.001), high hospitalization expenses (OR = 1.476, 95% CI: 1.195–1.822, P < 0.001) and cachexia (OR = 1.741, 95%CI = 1.374–2.207, P < 0.001) in patients with NSCLC.
Conclusions
The IBI was independently associated with overall survival, 90‐day outcomes, length of hospitalization, hospitalization expenses and cachexia in NSCLC patients. As an optimal systemic inflammation biomarker, the IBI has broad clinical application prospects in predicting the prognosis of patients with NSCLC.
Keywords: Systemic inflammation, Biomarker, Prognosis, Cachexia, Expenses, Non‐small cell lung cancer
Introduction
Lung cancer causes high morbidity and mortality worldwide and therefore seriously affects human health. There were 2.26 million new cases of lung cancer and 2.04 million deaths from lung cancer worldwide in 2019. 1 In China, the burden of lung cancer is very high. Lung cancer has the highest cancer‐associated morbidity and mortality in China, and both show an increasing annual trend. 2 Non‐small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Despite recent significant advances in the treatment of NSCLC, the long‐term survival of patients with NSCLC remains unsatisfactory. Therefore, there is an urgent need to find simple, inexpensive, effective biomarkers for predicting the prognosis of NSCLC to help improve survival assessment.
Tumour‐related pathological factors, such as pathological stage and histological subtype, have been widely used to assess survival of patients with NSCLC. 3 , 4 However, some patients with NSCLC have the same pathological stage or histological subtype but different outcomes. Although the detection of genetic biomarkers to assess prognosis is gaining popularity, it is limited by the cost and inconvenience of testing. In addition to tumour‐related pathological factors, the interaction between the tumour and host is also an important factor affecting patient prognosis. If cancer is a wound that never heals, then systemic inflammation, the most representative tumour–host interaction, is the likely cause of non‐healing. 5 , 6 Systemic inflammation, an important feature of the tumour microenvironment, plays a crucial role in disease progression and prognosis in patients with cancer. 7 , 8
Haematological inflammatory parameters, such as neutrophils, lymphocytes, platelets and C‐reactive protein (CRP), can effectively reflect the systemic inflammatory state of cancer. 9 , 10 Many studies have evaluated several systemic inflammation biomarkers composed of these inflammatory parameters and demonstrated that these biomarkers have important prognostic predictive value in different cancers, including NSCLC. 11 , 12 , 13 , 14 , 15 , 16 However, it remains unclear which combination of inflammatory parameters is the optimal systemic inflammation biomarker to assess prognosis in patients with NSCLC. Therefore, this study aimed to compare the prognostic value of existing systemic inflammation biomarkers and determine the optimal systemic inflammation biomarker in patients with NSCLC through a multicentre prospective setting. Particular attention was paid to the potential feasibility of our newly developed inflammatory burden index (IBI) as a prognostic biomarker for patients with NSCLC. We focused on the relationship between IBI and overall survival (OS), 90‐day outcomes, length of hospitalization, hospitalization expenses and cachexia to illustrate the potential of IBI in predicting the prognosis in patients with NSCLC.
Materials and methods
Study population
This prospective cohort study was conducted at multiple centres in China. Patients were recruited from a national multicentre project, the Investigation on Nutrition Status and its Clinical Outcome of Common Cancers (INSCOC). The inclusion criteria were pathologically proven NSCLC, administration of anticancer therapy and age older than 18 years. Patients with severe infection or immunodeficiency, other tumours, incomplete serological data, severe cardiopulmonary co‐morbidities or loss to follow‐up were excluded. If patients were hospitalized two or more times during the investigation, only data from the first survey were included. Written informed consent was obtained from all participating patients. This study was approved by the ethics committees of all participating institutions.
Data acquisition
Patient baseline characteristics were assessed by experienced physicians and included the following: sex, age, height, weight, body mass index, co‐morbidities (hypertension and diabetes), lifestyle (smoking and alcohol consumption), family history, tumour‐node‐metastasis (TNM) stage, treatment modality (surgery, radiotherapy or chemotherapy), length of hospitalization, hospitalization cost, Karnofsky Performance Scale score, Patient‐Generated Subjective Global Assessment (PG‐SGA) score, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (Version 3.0) and cachexia. Fasting venous blood was collected from patients at their respective central laboratories within 1 week prior to receiving anticancer therapy for the detection of serological parameters. Haematological parameters included whole white blood cells, neutrophils, lymphocytes, platelets, red blood cells, haemoglobin, CRP and albumin.
In this study, the survival status of patients with NSCLC was determined through regular follow‐up. Follow‐up began on October 2013 and lasted until October 2020 or until patient death. The primary outcome was OS, defined as the interval from cancer diagnosis to death from any cause or the last follow‐up. The secondary outcomes included 90‐day outcomes, length of hospitalization (≥14 days), hospitalization expenses (≥20 000 RMB) and cachexia. The 90‐day outcomes were defined as the 90‐day outcomes after anticancer treatment. Cachexia was defined according to international standards 17 : (i) unintentional weight loss of more than 5% in the past 6 months; (ii) BMI < 20 kg/m2 and any degree of weight loss >2%; and (iii) skeletal muscle mass (sarcopenia) and any degree of weight loss >2%. The skeletal muscle depletion was assessed as follows: mid‐upper‐arm muscle area by anthropometry (men <32 cm2, women <18 cm2).
Statistical analysis
Categorical variables are expressed as frequencies and percentages and were compared using the chi‐squared test or Fisher's exact test. Continuous non‐normally distributed variables are expressed as medians and interquartile ranges and were compared using the log‐rank test, whereas continuous normally distributed variables are expressed as means ± standard deviations and were compared using Student's t‐test. The predictive accuracy of systemic inflammation biomarkers for the prognostic assessment of patients with NSCLC was assessed using C‐statistics. Maximally selected log‐rank statistics were used to determine the optimal cut‐off value of the IBI for predicting prognosis in NSCLC patients. A restricted cubic spline (RCS) curve was used to explore the association between the IBI and survival in patients with NSCLC. Inter‐group differences in survival were assessed using the log‐rank test and visualized using the Kaplan–Meier method. Independent prognostic factors for OS were determined using multivariable Cox proportional hazards regression analysis and assessed using Wald's test. Logistic regression analysis was used to determine factors influencing the secondary outcomes. Statistical analysis and plotting were performed using R version 4.0.5 (http://www.r‐project.org), and a two‐sided P < 0.05 was considered statistically significant.
Results
Demographic and clinicopathological features of NSCLC patients
Initially, 2428 patients with NSCLC were screened from the INSCOC study. After excluding patients with missing serological data, a total of 1843 patients with NSCLC were included in the study, including 1197 (64.9%) men and 646 (35.1%) women. The mean age was 60.71 (±9.81) years. There were 133 (7.2%) patients with stage I disease, 277 (15.0%) with stage II disease, 410 (22.2%) with stage III disease and 1023 (55.5%) with stage IV disease. The demographic and clinicopathological features of the patients are summarized in Table S1 .
Comparison of haematological systemic inflammation biomarkers
Systemic inflammation is characterized by an increased proportion of pro‐inflammatory parameters (neutrophils, platelets and CRP) and a decreased proportion of anti‐inflammatory parameters (lymphocytes and albumin). We combined these five serum pro‐inflammatory and anti‐inflammatory parameters separately for assessment. Ultimately, we identified 16 systemic inflammation biomarkers (Figure 1 ). Table 1 summarizes the formulas used to calculate these biomarkers. These included previously reported existing and unevaluated systemic inflammation biomarkers. 11 , 18 , 19 , 20
Figure 1.
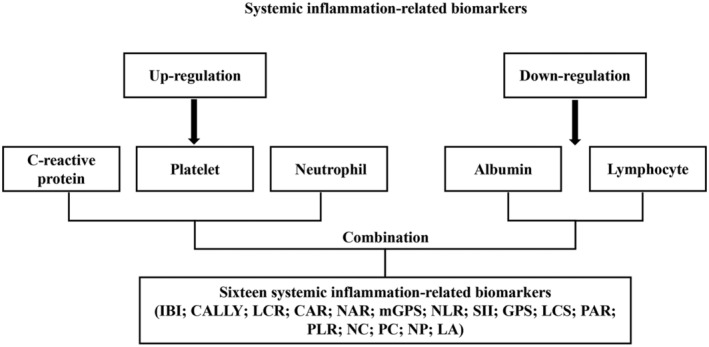
Study design.
Table 1.
Sixteen systemic inflammation biomarkers evaluated in this study
| Biomarkers | Biomarker formulas |
|---|---|
| C‐reactive protein‐to‐albumin ratio (CAR) | C‐reactive protein (mg/dL)/albumin (g/dL) |
| C‐reactive protein‐albumin‐lymphocyte index (CALLY) | Albumin = (g/dL) × Lymphocyte(/uL) /CRP (mg/dL) |
| Platelet‐to‐albumin ratio (PAR) | Platelet (/uL)/albumin (g/dL) |
| Neutrophil‐to‐albumin ratio (NAR) | Neutrophil (/uL)/albumin (g/dL) |
| Lymphocyte‐to‐C‐reactive protein ratio (LCR) | Lymphocyte (/uL)/C‐reactive protein (mg/L) |
| Platelet‐to‐lymphocyte ratio (PLR) | Platelet (/uL)/lymphocyte (/uL) |
| Neutrophil‐to‐lymphocyte ratio (NLR) | Neutrophil (/uL)/lymphocyte (/uL) |
| Systemic‐immune‐inflammation index (SII) | Platelet (/uL) × neutrophil (/uL)/lymphocyte (/uL) |
| C‐reactive protein–neutrophil–lymphocyte ratio (inflammatory burden index, IBI) | C‐reactive protein (mg/L) × neutrophil (/uL)/lymphocyte (/uL) |
| Glasgow Prognostic Score (GPS) |
C‐reactive protein ≤ 10 mg/L and albumin ≥35 g/L: 0 score C‐reactive protein ≤ 10 mg/L or albumin < 35 g/L: 1 score C‐reactive protein > 10 mg/L and albumin < 35 g/L: 2 score |
| Modified Glasgow Prognostic Score (mGPS) |
C‐reactive protein ≤ 10 mg/L and albumin ≥ 35 g/L: 0 score C‐reactive protein ≤ 10 mg/L and albumin < 35 g/L: 0 score C‐reactive protein > 10 mg/L: 1 score C‐reactive protein > 10 mg/L and albumin < 35 g/L: 2 score |
| Lymphocyte C‐reactive protein score (LCS) |
Lymphocyte ≥ 1 × 10^9/L and C‐reactive protein ≤ 3 mg/L: 0 score; Lymphocyte ≤ 1 × 10^9/L or C‐reactive protein ≥ 3 mg/L: 1 score; Lymphocyte < 1 × 10^9/L and C‐reactive protein > 3 mg/L: 2 score |
| Neutrophil‐C‐reactive protein score (NC) | Neutrophil (/uL) × C‐reactive protein score (mg/L) |
| Platelet‐C‐reactive protein score (PC) | Platelet (/uL) × C‐reactive protein score (mg/L) |
| Neutrophil–platelet score (NP) | Neutrophil (/uL) × platelet score (/uL) |
| Lymphocyte–albumin score (LA) | Lymphocyte (/uL) × albumin (g/dL) score |
These systemic inflammation biomarkers were independently associated with OS in this cohort, except for lymphocyte‐to‐CRP ratio and platelet‐to‐lymphocyte ratio (Table S2 ). We found that, among these systemic inflammation biomarkers, the IBI had the highest C‐statistic for predicting the prognosis of patients with NSCLC, reaching 0.640 (0.617, 0.663). None of the remaining systemic inflammation biomarkers were able to improve on this value (Table 2 ). These results suggest that the IBI is the optimal systemic inflammation biomarker for assessing the prognosis of patients with NSCLC. Therefore, we further evaluated the potential of the IBI as a predictive prognostic biomarker in patients with NSCLC.
Table 2.
Comparative analysis of the discrimination of each systemic inflammation‐related biomarkers for all‐cause mortality in non‐small cell lung cancer
| Discrimination ability | C‐statistic | ||
|---|---|---|---|
| Difference | Difference | P value | |
| IBI | 0.640(0.617,0.663) | Ref | |
| NLR | 0.633(0.610,0.656) | −0.007(−0.025, 0.013) | 0.470 |
| CALLY | 0.631(0.608,0.655) | −0.008(−0.015, −0.001) | 0.018 |
| LCR | 0.629(0.606,0.653) | −0.011(−0.018, −0.004) | 0.003 |
| NC | 0.629(0.606,0.652) | −0.011(−0.017, −0.005) | <0.001 |
| CAR | 0.623(0.599,0.646) | −0.017(−0.025, −0.009) | <0.001 |
| SII | 0.614(0.591,0.638) | −0.026(−0.044, −0.007) | 0.007 |
| GPS | 0.610(0.588,0.631) | −0.030(−0.044, −0.017) | <0.001 |
| NAR | 0.614(0.590,0.638) | −0.026(−0.046, −0.005) | 0.013 |
| PC | 0.611(0.588,0.635) | −0.029(−0.038, −0.019) | <0.001 |
| mGPS | 0.605(0.584,0.626) | −0.035(−0.048, −0.022) | <0.001 |
| PLR | 0.580(0.556,0.604) | −0.060(−0.082, −0.038) | <0.001 |
| NP | 0.579(0.555,0.603) | −0.061(−0.085, −0.038) | <0.001 |
| LA | 0.581(0.557,0.604) | −0.059(−0.084, −0.035) | <0.001 |
| LCS | 0.567(0.547,0.588) | −0.072(−0.089, −0.054) | <0.001 |
| PAR | 0.550(0.526,0.574) | −0.090(−0.114, −0.065) | <0.001 |
CALLY, C‐reactive protein–albumin–lymphocyte index; CAR, C‐reactive protein‐to‐albumin ratio; GPS, Glasgow Prognostic Score; IBI, inflammatory burden index; LA, lymphocyte–albumin score; LCR, lymphocyte‐to‐C‐reactive protein ratio; LCS, lymphocyte C‐reactive protein score; mGPS, Modified Glasgow Prognostic Score; NAR, neutrophil‐to‐albumin ratio; NC, neutrophil‐C‐reactive protein score; NLR, neutrophil‐to‐lymphocyte ratio; NP, neutrophil–platelet score; PAR, platelet‐to‐albumin ratio; PC, platelet‐C‐reactive protein score; PLR, platelet‐to‐Lymphocyte ratio; SII, systemic‐immune‐inflammation index.
Relationship between the IBI and disease development
Maximally selected log‐rank statistics determined that the optimal threshold of the IBI of patients with NSCLC was 17 (Figure S1 ). Based on this threshold, 644 (34.9%) patients were identified as having a high IBI. Compared with patients with a low IBI, those with a high IBI had higher hospitalization costs, longer hospital stays and advanced pathological stages. A high IBI was significantly associated with high inflammatory status (high white blood cell count, high neutrophil count, low lymphocyte count, high platelet count and high CRP), malnutrition (low red blood cell count, low haemoglobin, low albumin, high PG‐SGA score and cachexia), poor quality of life and poor physical function (Table S3 ).
Kaplan–Meier survival analysis
Patients with a high IBI had significantly worse outcomes than those with a low IBI (35.46% vs. 57.22%; log‐rank P < 0.001) (Figure 2 ). Notably, we found that the IBI could differentiate the prognosis of patients with NSCLC with the same pathological stage. A high IBI was associated with significantly worse prognosis than a low IBI at all stages (Figure S2 ). Further, we found that the IBI could effectively differentiate the prognosis of patients receiving radiotherapy, chemotherapy or surgery (Figure S3 ).
Figure 2.
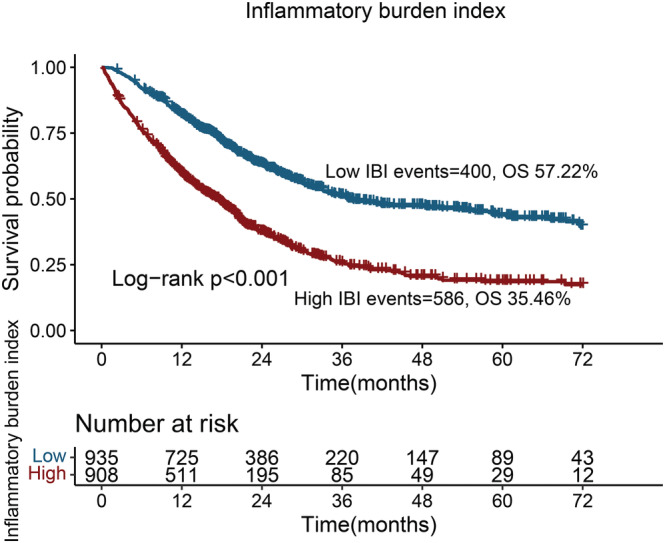
Kaplan–Meier curve of inflammatory burden index in patients with non‐small cell lung cancer.
Univariate and multivariable survival analyses
Univariate and multivariable RCS curves showed an inverted L‐shaped dose–response relationship between the IBI as a continuous variable and survival of patients with NSCLC (Figure 3 ). Multivariable Cox proportional hazards regression analysis showed that the IBI as a continuous variable was an independent factor for the prognosis of patients with NSCLC (hazard ratio [HR] = 1.229, 95% confidence interval [CI]: 1.131–1.335, P < 0.001). When assessing the IBI as a categorical variable (high vs. low), it was also an independent factor affecting patients with NSCLC (HR = 1.592, 95% CI: 1.393–1.819, P < 0.001). Using the Q1 group as a reference, the risks of adverse prognosis in the Q2, Q3 and Q4 groups gradually increased, with HRs of 1.078, 1.472 and 1.926, respectively (Table 3 ). Subsequent multivariable subgroup analysis revealed that a high IBI was an independent risk factor affecting most of the subgroups (Figure S4 ).
Figure 3.
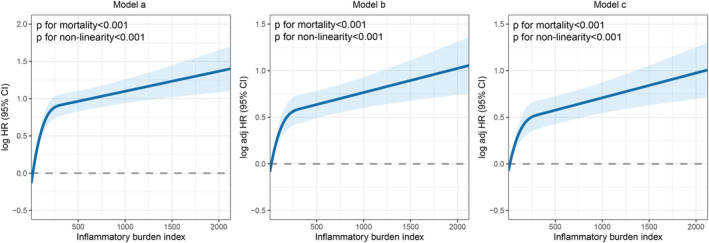
The association between inflammatory burden index and overall survival in patients with non‐small cell lung cancer. Notes: Model a: Not adjusted. Model b: Adjusted for age, sex, BMI and TNM stage. Model c: Adjusted for age, sex, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoking, drinking and family history.
Table 3.
Association between inflammatory burden index and overall survival of patients with non‐small cell lung cancer
| IBI | Model a | P value | Model b | P value | Model c | P value |
|---|---|---|---|---|---|---|
| Continuous (per SD) | 1.376 (1.275,1.486) | <0.001 | 1.255 (1.158,1.36) | <0.001 | 1.229 (1.131,1.335) | <0.001 |
| Cut‐off value | <0.001 | <0.001 | <0.001 | |||
| C1 (<17) | ref | ref | ref | |||
| C2 (≥17) | 2.192 (1.929,2.492) | 1.704 (1.495,1.944) | 1.592 (1.393,1.819) | |||
| Quartiles | ||||||
| Q1 (<5.23) | ref | ref | ref | |||
| Q2 (5.23–16.38) | 1.227 (1.006,1.497) | 0.044 | 1.105 (0.905,1.349) | 0.326 | 1.078 (0.883,1.317) | 0.459 |
| Q3 (16.38–80.63) | 2.025 (1.677,2.445) | <0.001 | 1.611 (1.332,1.949) | <0.001 | 1.472 (1.214,1.785) | <0.001 |
| Q4 (80.63) | 3.022 (2.516,3.63) | <0.001 | 2.083 (1.724,2.516) | <0.001 | 1.926 (1.591,2.331) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 |
Model a: No adjusted. Model b: Adjusted for age, sex, BMI, TNM stage. Model c: Adjusted for age, sex, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoking, drinking, family history.
Logistic regression analysis of the IBI and secondary outcomes
In this study, 146 patients (7.9%) experienced 90‐day outcomes. Multivariable‐adjusted logistic regression analysis revealed that a high IBI was an independent risk factor for 90‐day outcomes in patients with NSCLC (odds ratio [OR] = 1.789, 95% CI: 1.489–2.151; P < 0.001). In total, 594 (32.2%) patients with NSCLC were hospitalized for more than 14 days. With an increase in the IBI, the risk of prolonged hospitalization gradually increased. After excluding patients with infectious complication (61 cases), the IBI was still an independent factor affecting length of hospitalization (OR = 1.560, 95% CI: 1.256–1.938, P < 0.001). With the Q1 group as the reference, the ORs in the Q2, Q3 and Q4 groups were 1.146, 1.430 and 1.993, respectively. There were 628 (34.1%) patients whose hospitalization expenses exceeded 20,000 yuan. A high IBI was an adverse factor affecting hospitalization expenses in patients with NSCLC (OR = 1.476, 95% CI: 1.195–1.822, P < 0.001). Patients with an extremely high IBI had a >2‐fold higher risk of high hospitalization expenses than those with a low IBI (Q4 vs. Q1). Further, IBI was also an independent factor affecting cachexia (OR = 1.741, 95%CI = 1.374–2.207, P < 0.001). Compared with the Q1 group, the ORs of the Q2, Q3 and Q4 groups were 1.124, 1.529 and 2.318, respectively (Table 4 ).
Table 4.
Logistic regression analysis of inflammatory burden index associated with secondary outcomes
| 90‐day outcomes | ||||||
|---|---|---|---|---|---|---|
| IBI | Model a | P value | Model b | P value | Model c | P value |
| Continuous (per SD) | 2.049 (1.727,2.431) | <0.001 | 1.839 (1.541,2.196) | <0.001 | 1.789 (1.489,2.151) | <0.001 |
| Cut‐off value | <0.001 | <0.001 | <0.001 | |||
| C1 (<17) | ref | ref | ref | |||
| C2 (≥17) | 6.564 (4.13,10.432) | 4.551 (2.834,7.309) | 4.037 (2.497,6.527) | <0.001 | ||
| Quartiles | ||||||
| Q1 (<5.23) | ref | ref | ref | |||
| Q2 (5.23–16.38) | 0.564 (0.234,1.357) | 0.2011 | 0.453 (0.186,1.101) | 0.080 | 0.410 (0.168,1.004) | 0.051 |
| Q3 (16.38–80.63) | 2.069 (1.075,3.984) | 0.0295 | 1.439 (0.738,2.805) | 0.286 | 1.152 (0.584,2.274) | 0.683 |
| Q4 (≥80.63) | 8.398 (4.714,14.961) | <0.001 | 4.733 (2.604,8.602) | <0.001 | 3.876 (2.114,7.105) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Length of hospitalization (≥14 days), excluding patients with infectious complication (61 cases) | ||||||
|---|---|---|---|---|---|---|
| IBI | Model a | P value | Model b | P value | Model c | P value |
| Continuous (per SD) | 1.103 (0.957,1.271) | 0.175 | 1.101 (0.954,1.27) | 0.187 | 1.099 (0.95,1.271) | 0.204 |
| Cut‐off value | <0.001 | <0.001 | <0.001 | |||
| C1 (<17) | ref | ref | ref | |||
| C2 (≥17) | 1.563 (1.277,1.911) | 1.604 (1.299,1.981) | 1.560 (1.256,1.938) | |||
| Quartiles | ||||||
| Q1 (<5.23) | ref | ref | ref | |||
| Q2 (5.23–16.38) | 1.165 (0.865,1.569) | 0.269 | 1.168 (0.865,1.577) | 0.311 | 1.146 (0.846,1.554) | 0.379 |
| Q3 (16.38–80.63) | 1.471 (1.099,1.968) | 0.010 | 1.497 (1.111,2.016) | 0.008 | 1.430 (1.053,1.941) | 0.022 |
| Q4 (≥80.63) | 1.944 (1.46,2.588) | <0.001 | 2.065 (1.527,2.793) | <0.001 | 1.993 (1.462,2.717) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Hospitalization expenses (≥20 000 RMB) | ||||||
|---|---|---|---|---|---|---|
| IBI | Model a | P value | Model b | P value | Model c | P value |
| Continuous (per SD) | 1.212 (1.056,1.392) | 0.006 | 1.206 (1.049,1.386) | 0.009 | 1.198 (1.04,1.381) | 0.012 |
| Cut‐off value | <0.001 | <0.001 | <0.001 | |||
| C1 (<17) | ref | ref | ref | |||
| C2 (≥17) | 1.468 (1.21,1.782) | 1.486 (1.214,1.820) | 1.476 (1.195,1.822) | |||
| Quartiles | ||||||
| Q1 (<5.23) | ref | ref | ref | |||
| Q2 (5.23–16.38) | 1.410 (1.061,1.872) | 0.018 | 1.434 (1.077,1.909) | 0.014 | 1.443 (1.077,1.934) | 0.014 |
| Q3 (16.38–80.63) | 1.470 (1.108,1.951) | 0.008 | 1.514 (1.134,2.023) | 0.005 | 1.498 (1.110,2.023) | 0.008 |
| Q4 (≥80.63) | 2.060 (1.560,2.720) | <0.001 | 2.159 (1.611,2.895) | <0.001 | 2.146 (1.582,2.910) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| Cachexia | ||||||
|---|---|---|---|---|---|---|
| IBI | Model a | P value | Model b | P value | Model c | P value |
| Continuous (per SD) | 1.388 (1.200,1.606) | <0.001 | 1.237 (1.063,1.439) | 0.006 | 1.223 (1.047,1.427) | 0.011 |
| Cut‐off value | <0.001 | <0.001 | <0.001 | |||
| C1 (<17) | ref | ref | ref | |||
| C2 (≥17) | 2.263 (1.831,2.795) | 1.796 (1.423,2.267) | 1.741 (1.374,2.207) | |||
| Quartiles | ||||||
| Q1 (<5.23) | ref | ref | ref | |||
| Q2 (5.23–16.38) | 1.184 (0.852,1.645) | 0.3149 | 1.138 (0.801,1.616) | 0.470 | 1.124 (0.790,1.599) | 0.517 |
| Q3 (16.38–80.63) | 1.84 (1.346,2.517) | <0.001 | 1.593 (1.138,2.232) | 0.007 | 1.529 (1.086,2.154) | 0.015 |
| Q4 (≥80.63) | 3.418 (2.528,4.623) | <0.001 | 2.403 (1.724,3.351) | <0.001 | 2.318 (1.654,3.248) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
Model a: No adjusted. Model b: Adjusted for age, sex, BMI, TNM stage. Model c: Adjusted for age, sex, BMI, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoking, drinking, family history.
Internal cohort validation
To further validate the relationship between the IBI and survival in NSCLC patients, we randomly divided the total population into validation cohorts A (1291 patients) and B (552 patients) at a ratio of 7:3. A comparison of clinicopathological features between the cohorts indicated good independence (Table S4 ). The IBI still provided good prognostic differentiation in the two validation cohorts. NSCLC patients with a high IBI had a significantly worse prognosis than those with a low IBI (validation cohort A, 37.00% vs. 57.52%; validation cohort B, 31.68% vs. 56.55%) (Figure S5 ). Multivariable Cox proportional hazards regression analysis showed that the IBI was an independent factor affecting the prognosis of NSCLC patients in validation cohort A (HR = 1.268, 95% CI: 1.132–1.421, P < 0.001) and validation cohort B (HR = 1.280, 95% CI: 1.132–1.448, P < 0.001) (Table S5 ).
Discussion
It is widely believed that tumour‐associated inflammation is the response of the body's immune system to tumour cells. Non‐controllable inflammation is closely related to tumour occurrence, development, invasion and metastasis. 21 , 22 As the most representative tumour–host interaction, tumour‐associated inflammation is considered a hallmark of cancer. 23 It can alter the tumour microenvironment by altering the rate of stromal cell turnover and polarizing the immunosuppressive capacity of immune cells, thereby promoting tumour growth. 24 The haematological products of the inflammatory process can be considered as potential biomarkers. However, few studies have compared the performances of existing haematological systemic inflammation biomarkers for predicting the prognosis of NSCLC patients. In the current study, we systematically and comprehensively compared the prognostic value of 16 systemic inflammation biomarkers composed of peripheral blood features in patients with NSCLC. We found that the majority of systemic inflammation biomarkers were independently associated with the prognosis of patients with NSCLC. Notably, the IBI had the best accuracy in predicting the prognosis of patients with NSCLC.
Utility of the IBI as a biomarker
The IBI is a haematological biomarker newly developed by our team to evaluate the inflammatory status and survival of patients with cancer. 11 At present, the prognostic value and scope of application of the IBI in the prognostic assessment of patients with NSCLC are still unknown. We determined the optimal threshold for the IBI for predicting prognosis in patients with NSCLC to be 17. A high IBI was significantly associated with adverse outcomes, including poor nutritional status, high inflammation and advanced pathological stage. A high IBI also seriously affected the quality of life and physical function of patients with NSCLC. In addition, it significantly increased the cost of medical care, including higher hospitalization costs and longer hospitalization. These findings suggest that IBI monitoring can serve as a reference for monitoring disease progression and treatment efficacy. A high IBI was significantly associated with poor prognosis and was an independent prognostic factor for OS in patients with NSCLC. In addition, the IBI effectively differentiated the prognosis of NSCLC patients with the same pathological stage; therefore, it can provide additional prognostic guidance for patients with NSCLC beyond the pathological stage. Subsequent internal validation cohorts demonstrated that the IBI is a simple, effective, reproducible biomarker for the prognostic assessment of patients with NSCLC. Weight loss is an important factor in the assessment of cachexia and prognostic evaluation of patients with cancer. 25 However, the clinical assessment of weight loss depends mainly on self‐reporting of cancer patients, and weight loss data are often lacking clinically. In this study, we found that IBI was independently associated with 90‐day outcomes and cachexia in patients with NSCLC, which indicated that IBI can be used as an objective and effective tool for cancer cachexia in addition to weight loss.
The IBI combines multiple systemic markers to improve its effectiveness
The advantage of the IBI in the prognostic assessment of patients with NSCLC may be that it measures the balance between acute and immune inflammation by combining CRP, neutrophils and lymphocytes. CRP is the most widely used biomarker for clinical measurement of inflammation. CRP is an acute‐phase protein synthesized by hepatocytes in response to inflammatory stimuli. 22 Persistently elevated CRP levels often predict poor prognosis and indicate metastasis in cancer patients. 23 , 24 CRP is not only a marker of inflammation but also has many key pro‐inflammatory properties. CRP can induce the initiation of endothelial and smooth muscle cells and promote the expression of adhesion molecules, chemoattractants and vascular endothelial growth factor, which are essential for tumour invasion. 25 , 26 In addition, some studies have found that CRP can directly affect the function of immune active cells, thereby affecting the body's cytotoxic immune response and promoting tumour immune escape. 27 , 28 Neutrophils and lymphocytes are important components of humoral immunity, playing key roles in chemotaxis, phagocytosis, intracellular killing and adaptive immune regulation. 29 , 30 , 31 As a systemic inflammation biomarker, the IBI has broad clinical application prospects for predicting the prognosis of patients with NSCLC.
Study strengths and limitation
To our knowledge, this is the first study to comprehensively assess the prognostic value of haematological systemic inflammation biomarkers in patients with NSCLC. We found for the first time that the IBI was the best systemic inflammation biomarker for predicting the prognosis of patients with NSCLC. We subsequently determined that the IBI was independently associated with OS, 90‐day outcomes, length of hospitalization, hospitalization expenses and cachexia in NSCLC patients. In addition, we determined that the optimal threshold of the IBI for predicting prognosis of patients with NSCLC was 17. Our findings may contribute to a deeper understanding of the nature of the relationship between systemic inflammation and survival in NSCLC patients, thereby providing a valuable reference for the selection of systemic inflammation biomarkers for prognostic assessment, prediction of treatment efficacy and follow‐up monitoring of NSCLC patients.
In previous exploration, 16 nutritional and systemic inflammation indicators are often mixed and compared in the prediction of tumour prognosis, which is obviously inappropriate. Systemic inflammation may change earlier than nutritional status and may be a more sensitive symbol of host‐tumour related effects. Changes in nutritional indicators (BMI and weight loss) are more likely to be a late manifestation of cancer patients. As a result, the weight of nutritional indicators is usually too large in comparison. Different from the previous research, this study comprehensively evaluated the prognostic value of various combinations of common, clinically used serum pro‐inflammatory parameters and anti‐inflammatory parameters in patients with NSCLC. This study was purely performed to evaluate the relationship between serum systemic inflammation markers and NSCLC, and it provides a direction for the comprehensive evaluation of systemic inflammation in the macroscopic phenotype of NSCLC. It may be a potential direction of serological research to shift from the composite study of nutritional/systemic inflammation indicators to the study of pure systemic inflammation indicators.
However, this study has a few limitations. First, the patients included in this study were all Chinese, and it is unclear whether these findings are applicable to other ethnic groups. Second, this study was a national multicentre study. Although the selection bias found in single‐centre studies was not a concern, there may have been a bias due to differences in the treatment level and testing equipment of each participating centre. The institutions were all tertiary hospitals in China, and the staff were uniformly trained before the patients were enrolled. These measures can reduce the bias caused by differences between different centres to a certain extent. In addition, although the results of this study were internally validated, further external validation is needed through stricter inclusion criteria, larger sample sizes and prospective studies. Finally, due to the lack of additional survival data, including cancer‐specific survival and recurrence‐free survival, this study cannot explore the relationship between IBI and other survival outcomes in NSCLC patients, which needs to be further explored in the future.
Conclusion
Our study demonstrates that the IBI is the optimal systemic inflammation biomarker for predicting the prognosis of NSCLC. The IBI was independently associated with OS, 90‐day outcomes, length of hospitalization, hospitalization expenses and cachexia in NSCLC patients.
Conflict of interest
The authors declare that there is no conflict of interest.
Supporting information
Figure S1. Cut‐off of inflammatory burden index in patients with non‐small cell lung cancer.
Figure S2. Subgroup survival analysis of inflammatory burden index based on pathological stage.
Figure S3. Subgroup survival analysis of inflammatory burden index based on anti‐tumour therapy method.
Figure S4. The association between inflammatory burden index and hazard risk of overall survival in various subgroups.
Figure S5. Kaplan–Meier curve of inflammatory burden index in patients with non‐small cell lung cancer at internal validation cohorts.
Table S1. The clinicopathological features in patients with non‐small cell lung cancer.
Table S2. The prognostic value of systemic inflammation‐related biomarkers in the prognosis assessment in patients with non‐small cell lung cancer.
Table S3. Characteristics by level of inflammatory burden index in patients with non‐small cell lung cancer.
Table S4. Demographics of patients with non‐small cell lung cancer between validation cohort A and validation cohort B.
Table S5. Association between inflammatory burden index and overall survival of patients with non‐small cell lung cancer at validation cohorts.
Acknowledgements
We all appreciate the support from the National Key Research and Development Program to Dr Hanping Shi (2022YFC2009600) and the Beijing Municipal Science and Technology Commission (SCW2018‐06).
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 32 This study followed the tenets of the Helsinki Declaration. All participants signed an informed consent form, and this study was approved by the Institutional Review Board of each hospital (registration number: ChiCTR1800020329).
Xie H., Ruan G., Wei L., Deng L., Zhang Q., Ge Y., Song M., Zhang X., Lin S., Liu X., Yang M., Song C., Zhang X., and Shi H. (2023) The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non‐small cell lung cancer, Journal of Cachexia, Sarcopenia and Muscle, 14, 869–878, 10.1002/jcsm.13199
Hailun Xie, Guotian Ruan, Lishuang Wei and Li Deng contributed equally to this work.
References
- 1. Global Burden of Disease 2019 Cancer Collaboration , Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol 2022;8:420–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent 2022;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami‐Porta R, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706–714. [DOI] [PubMed] [Google Scholar]
- 4. Noma D, Inamura K, Matsuura Y, Hirata Y, Nakajima T, Yamazaki H, et al. Prognostic effect of lymphovascular invasion on TNM staging in stage I non‐small‐cell lung cancer. Clin Lung Cancer 2018;19:e109–e122. [DOI] [PubMed] [Google Scholar]
- 5. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–545. [DOI] [PubMed] [Google Scholar]
- 6. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- 7. Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation‐mediated promotion of invasion and metastasis. Cancer Metastasis Rev 2010;29:243–248. [DOI] [PubMed] [Google Scholar]
- 8. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019;51:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga‐Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta‐analyses of observational studies. BMC Med 2020;18:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer 2020;20:485–503. [DOI] [PubMed] [Google Scholar]
- 11. Xie H, Ruan G, Ge Y, Zhang Q, Zhang H, Lin S, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr 2022;41:1236–1243. [DOI] [PubMed] [Google Scholar]
- 12. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐Lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176–181. [DOI] [PubMed] [Google Scholar]
- 13. Matsubara T, Takamori S, Haratake N, Fujishita T, Toyozawa R, Ito K, et al. Identification of the best prognostic marker among immunonutritional parameters using serum C‐reactive protein and albumin in non‐small cell lung cancer. Ann Surg Oncol 2021;28:3046–3054. [DOI] [PubMed] [Google Scholar]
- 14. Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: a meta‐analysis. Medicine (Baltimore) 2020;99:e23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie H, Yuan G, Huang S, Kuang J, Yan L, Ruan G, et al. The prognostic value of combined tumor markers and systemic immune‐inflammation index in colorectal cancer patients. Langenbeck's Arch Surg 2020;405:1119–1130. [DOI] [PubMed] [Google Scholar]
- 16. Song M, Zhang Q, Song C, Liu T, Zhang X, Ruan G, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle 2022;13:2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 18. Jiang Y, Xu D, Song H, Qiu B, Tian D, Li Z, et al. Inflammation and nutrition‐based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta‐analysis. BMJ Open 2021;11:e048324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, et al. Lymphocyte‐C‐reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg 2020;272:342–351. [DOI] [PubMed] [Google Scholar]
- 20. Müller L, Hahn F, Mähringer‐Kunz A, Stoehr F, Gairing SJ, Michel M, et al. Immunonutritive scoring for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: evaluation of the CALLY index. Cancers (Basel) 2021;13:5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anastasiou D. Tumour microenvironment factors shaping the cancer metabolism landscape. Br J Cancer 2017;116:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 24. Zhu H, Cao X. NLR members in inflammation‐associated carcinogenesis. Cell Mol Immunol 2017;14:403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oswalt C, Liu Y, Pang H, Le‐Rademacher J, Wang X, Crawford J. Associations between body mass index, weight loss and overall survival in patients with advanced lung cancer. J Cachexia Sarcopenia Muscle 2022;13:2650–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223–226. [DOI] [PubMed] [Google Scholar]
- 27. Riedl JM, Barth DA, Brueckl WM, Zeitler G, Foris V, Mollnar S, et al. C‐reactive protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non‐small cell lung cancer: a bi‐center study. Cancers (Basel) 2020;12:2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin Y, Sun Y, Shi X, Zhao J, Shi L, Yu X. Prognostic value of circulating C‐reactive protein levels in patients with non‐small cell lung cancer: a systematic review with meta‐analysis. J Cancer Res Ther 2014;10:C160–C166. [DOI] [PubMed] [Google Scholar]
- 29. Kemik O, Kemik AS, Begenik H, Erdur FM, Emre H, Sumer A, et al. The relationship among acute‐phase response proteins, cytokines, and hormones in various gastrointestinal cancer types patients with cachectic. Hum Exp Toxicol 2012;31:117–125. [DOI] [PubMed] [Google Scholar]
- 30. Hattori Y, Matsumura M, Kasai K. Vascular smooth muscle cell activation by C‐reactive protein. Cardiovasc Res 2003;58:186–195. [DOI] [PubMed] [Google Scholar]
- 31. Van Vré EA, Bult H, Hoymans VY, Van Tendeloo VF, Vrints CJ, Bosmans JM. Human C‐reactive protein activates monocyte‐derived dendritic cells and induces dendritic cell‐mediated T‐cell activation. Arterioscler Thromb Vasc Biol 2008;28:511–518. [DOI] [PubMed] [Google Scholar]
- 32. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cut‐off of inflammatory burden index in patients with non‐small cell lung cancer.
Figure S2. Subgroup survival analysis of inflammatory burden index based on pathological stage.
Figure S3. Subgroup survival analysis of inflammatory burden index based on anti‐tumour therapy method.
Figure S4. The association between inflammatory burden index and hazard risk of overall survival in various subgroups.
Figure S5. Kaplan–Meier curve of inflammatory burden index in patients with non‐small cell lung cancer at internal validation cohorts.
Table S1. The clinicopathological features in patients with non‐small cell lung cancer.
Table S2. The prognostic value of systemic inflammation‐related biomarkers in the prognosis assessment in patients with non‐small cell lung cancer.
Table S3. Characteristics by level of inflammatory burden index in patients with non‐small cell lung cancer.
Table S4. Demographics of patients with non‐small cell lung cancer between validation cohort A and validation cohort B.
Table S5. Association between inflammatory burden index and overall survival of patients with non‐small cell lung cancer at validation cohorts.


