Abstract
Background
Maximum muscle power (Pmax) is a biomarker of physical performance in all ages. No longitudinal studies have assessed the effects of aging on Pmax obtained from the torque‐velocity (T‐V) relationship, which should be considered the ‘gold standard’. This study evaluated the longitudinal changes in the T‐V relationship and Pmax of the knee‐extensor muscles in young, middle‐aged, and older adults after 10 years of follow‐up.
Methods
Four hundred eighty‐nine subjects (311 men and 178 women; aged 19–68 years) were tested at baseline and after a 10‐year follow‐up. Anthropometric data, daily protein intake, physical activity level (PAL), and knee‐extension muscle function (isometric, isokinetic, and isotonic) were evaluated. A novel hybrid equation combining a linear and a hyperbolic (Hill‐type) region was used to obtain the T‐V relationship and Pmax of the participants, who were grouped by sex and age (young: 20–40 years; middle‐aged: 40–60 years; and old: ≥60 years). Linear mixed‐effect models were used to assess effects of time, sex, and age on T‐V parameters, Pmax, and body mass index (BMI). Additional analyses were performed to adjust for changes in daily protein intake and PAL.
Results
Pmax decreased in young men (−0.6% per year; P < 0.001), middle‐aged men and women (−1.1% to −1.4% per year; P < 0.001), and older men and women (−2.2% to −2.4% per year; P ≤ 0.053). These changes were mainly related to decrements in torque at Pmax at early age and to decrements in both torque and velocity at Pmax at older age. BMI increased among young and middle‐aged adults (0.2% to 0.5% per year; P < 0.001), which led to greater declines in relative Pmax in those groups. S/T0, that is, the linear slope of the T‐V relationship relative to maximal torque, exhibited a significant decline over time (−0.10%T0·rad·s−1 per year; P < 0.001), which was significant among middle‐aged men and old men and women (all P < 0.05). Annual changes in PAL index were significantly associated to annual changes in Pmax (P = 0.017), so the overall decline in Pmax was slightly attenuated in the adjusted model (−5.26 vs. −5.05 W per year; both P < 0.001).
Conclusions
Pmax decreased in young, middle‐aged, and older adults after a 10‐year follow‐up. The early declines in Pmax seemed to coincide with declines in force, whereas the progressive decline at later age was associated with declines in both force and velocity. A progressively blunted ability to produce force, especially at moderate to high movement velocities, should be considered a specific hallmark of aging.
Keywords: Torque‐velocity, Force‐velocity, Sarcopenia, Torque, Knee extension, Aging
Introduction
Muscle power is defined as the rate at which mechanical work is performed and is one of the main biomarkers of physical performance in humans during actions that can be determining for success in sport activities in the case of athletes 1 or in activities of daily living in older individuals. 2 In the latter case, muscle power is negatively and independently associated with the risk of cognitive decline, 3 mobility limitations and disability, 4 , 5 hospitalization, 6 and mortality in older adults. 6 , 7 The clinical relevance of low muscle power seems to exceed that shown by sarcopenia (i.e., low muscle mass and strength). 8 , 9 Previous studies have shown that the decline in muscle power initiates after the age of 40 years in both women and men, although between‐sex differences can be observed during certain stages of life, 10 , 11 which makes it necessary to investigate men and women separately. Evidence from studies conducted in master athletes even suggests that, despite their greater muscle power levels throughout the lifespan when compared with their sedentary counterparts, the age‐related decline in power is inevitable. 12
The amount of muscle power that an individual produces varies as a function of movement velocity, showing lower values under either relatively slow or fast contractions (i.e., heavy and light loading conditions, respectively) and higher values (maximum muscle power (Pmax)) during moderate contraction velocities (i.e., moderate loading conditions). 13 Therefore, Pmax (i.e., apex of the power‐velocity (P‐V) curve) can occur at different absolute and relative loading conditions among study participants. Unfortunately, age‐related declines in muscle power are usually derived from studies in which muscle power was assessed under a single loading condition, 10 , 11 , 14 so it is possible that differences in muscle power among participants and over time are at least partially due to the chosen loading conditions. A ‘gold standard’ approach to determine the effect of aging on Pmax would be the assessment of the force‐velocity (F‐V) or torque‐velocity (T‐V) relationship and recording of derived muscle power values across contraction velocities. This method can also help discern whether decrements in Pmax are related to changes in force, velocity, or both. 15 Results derived from a cross‐sectional study suggest that the force component is already relevant in mid‐life, whereas the velocity component becomes relevant at an older age. 16 Importantly, these changes are driven by different physiological mechanisms (e.g., decrease in muscle size 17 vs. slower single muscle fibre phenotype 18 ), having important implications for designing effective countermeasures. Nevertheless, no previous longitudinal studies using the above‐mentioned approach have been found in the literature. 19 The evidence about changes in muscle power with aging is limited to either cross‐sectional studies, 10 , 12 longitudinal studies that assessed upper‐limb, but not lower‐limb, muscle power, 11 or studies that did not measure Pmax obtained from the F‐V or T‐V relationship. 19
Therefore, the main goal of the present investigation was to assess the longitudinal changes in the T‐V and P‐V relationships, and in Pmax of the knee extensors in community‐dwelling young, middle‐aged, and older men and women. Our main hypotheses were that Pmax would decrease with aging in young, middle‐aged, and older participants, whereas these decrements would be motivated mainly by torque‐related factors in young individuals and by both torque‐ and velocity‐related factors in middle‐aged and older participants.
Methods
Study design
This is a longitudinal study in which data were collected in the framework of the first (2002–2004; baseline) and third (2012–2015; follow‐up) waves of the Flemish Policy Research Center on Sport. The study sample was randomly selected among community‐dwelling people aged 18 to 80 years living in the region of Flanders (Belgium), as previously described in detail. 20 , 21
Participants
A total of 1569 subjects (923 men and 646 women) were tested at baseline, of which a total of 652 subjects (420 men and 232 women) were tested again at follow‐up. Exclusion criteria, reasons for dropping out, and differences between the drop‐out and follow‐up participants have been previously reported. 21 , 22 Briefly, subjects were excluded in the case of cardiovascular disease or acute thrombosis, recent surgery, neuromuscular disease, infection or fever, diabetes, and/or pregnancy. In the current study, only those participants that presented the full set of data from the anthropometric and T‐V assessments were included. Thus, a total 489 subjects (178 women and 311 men; aged from 19 to 68 years at baseline) participated in this longitudinal study (Table 1). Median follow‐up time and inter‐quartile range was 9.6 [9.3–10.4] years. All the subjects gave their written informed consent; all the procedures were performed in accordance with the Declaration of Helsinki, and the study was approved by the Ethics Committee Research UZ/KU Leuven.
Table 1.
Main characteristics of the study participants.
| Age groups | |||
|---|---|---|---|
| Young (20.0–39.9 years) | Middle‐aged (40.0–59.9 years) | Old (≥60.0 years) | |
| Women, n | 44 | 124 | 10 |
| Age, years | 33.2 ± 5.8 | 46.9 ± 5.2*, a | 62.9 ± 2.8ab |
| Height, m | 1.65 ± 0.05* | 1.65 ± 0.06* | 1.61 ± 0.10* |
| Body mass, kg | 61.4 ± 8.1* | 63.9 ± 8.9* | 59.5 ± 6.6* |
| BMI, kg/m2 | 22.4 ± 2.4* | 23.3 ± 3.0* | 22.9 ± 3.4* |
| Protein, g·kg−1 | 1.24 ± 0.35 | 1.29 ± 0.38 | 1.37 ± 0.32 |
| PAL index | 1.73 ± 0.25 | 1.71 ± 0.16* | 1.59 ± 0.13 |
| EEsports, MET·h·week−1 | 26.0 ± 37.4 | 24.7 ± 30.1 | 14.8 ± 14.3 |
| Men, n | 85 | 202 | 24 |
| Age, years | 33.1 ± 5.9 | 47.9 ± 4.9*, a | 63.9 ± 2.5ab |
| Height, m | 1.78 ± 0.06* | 1.77 ± 0.06* | 1.73 ± 0.06* ab |
| Body mass, kg | 76.2 ± 10.6* | 79.8 ± 10.0*, a | 78.6 ± 8.3* |
| BMI, kg/m2 | 23.9 ± 2.8* | 25.4 ± 2.7*, a | 26.2 ± 2.8*, a |
| Protein, g·kg−1 | 1.25 ± 0.35 | 1.23 ± 0.32 | 1.31 ± 0.30 |
| PAL index | 1.80 ± 0.21 | 1.79 ± 0.22* | 1.70 ± 0.20 |
| EEsports, MET·h·week−1 | 22.2 ± 23.8 | 23.6 ± 23.7 | 20.3 ± 20.7 |
Note: Baseline differences were assessed using linear mixed‐effect models with two fixed factors (sex and age group) and one random factor (subject ID).
Abbreviations: BMI, body mass index. PAL, physical activity level. EEsports, energy expenditure in sports.
Significant differences between men and women (P < 0.05).
Significant differences compared with young adults (P < 0.05).
Significant differences compared with middle‐aged adults (P < 0.05).
Outcomes
Anthropometrics
The participants were barefoot and with minimal clothing for the anthropometric measurements. Height was measured to the nearest 0.01 m using a stadiometer (Holtain, Crymych, UK), and body mass was measured to the nearest 0.1 kg with a digital scale (Seca 841, Seca GmbH, Hamburg, Germany). Body mass index (BMI) was calculated as the ratio between body mass and height squared (kg·m−2).
Protein intake
Protein intake was assessed by asking the participants to register all food and drinks consumed in a validated 3‐day diet record (two weekdays and one weekend day). 23 The food diary was sent to the participants prior to the test session in the laboratory, so that they could fill it in beforehand. The participants had to weigh the amount of food and drinks or otherwise estimate the amounts by using standard household measures (e.g., spoon and cup). Then, daily protein intake relative to body mass (g·kg−1) was calculated using Becel Nutrition software (Unilever CO., Rotterdam, the Netherlands).
Physical activity
Physical activity and participation in sports was assessed by the Flemish Physical Activity Computerized Questionnaire. 24 Briefly, this questionnaire registers information on the participants' engagement in leisure activities and sports participation, household and garden activities, transportation, occupation, sleeping, and sedentary behaviour in a normal week. From these data, energy expenditure due to sport activities (EEsports) was estimated based on the Compendium of Physical Activity as the average metabolic equivalents of energy expenditure hours per week (MET·h·week−1). 25 Physical activity level (PAL) was assessed as the ratio between daily total energy expenditure and basal metabolic rate. 24
Knee extension torque‐velocity relationship and muscle power
Unilateral knee extension muscle function of the right leg (left leg in case of history of injury) was assessed using an isokinetic dynamometer (Biodex System Pro 3, Biodex Medical Systems, USA). Torque and angular velocity signals were sampled at 100 Hz and processed offline through a commercial software package (Matlab R2015b; The MathWorks Inc., USA). Subjects were seated upright on the dynamometer chair with the axis of the dynamometer corresponding to the knee joint axis. The participants' shoulder, waist, thigh, and lower leg were secured with straps, and arms were kept crossed over the chest during all the trials. After an adequate familiarization and warm‐up, several isometric, isotonic, and isokinetic trials were carried out. Briefly, peak torque values were recorded during maximal isometric contractions (3–4 s) at knee joint angles of 30°, 60°, and 90° (full extension = 0°). Two trials were performed for each isometric condition and the highest peak torque (Nm) registered among the six trials was defined as the maximum isometric torque. In addition, peak torque and corresponding angular velocity values were recorded during maximal isotonic contractions against loads corresponding to 40%, 20%, 0%, and 60% of maximum isometric torque (three trials per each loading condition), and during maximal isokinetic contractions performed at 1.05 rad·s−1 (four trials) and 4.19 rad·s−1 (six trials) (i.e., 60° and 240°·s−1, respectively). Torque (Nm) and angular velocity (rad·s−1) at peak power registered against each load/angular velocity was used for further analysis. All the participants received the instruction to perform every repetition as fast and strong as possible through the whole range of movement (90° to full extension). Strong verbal encouragement was provided during each trial, and adequate resting periods (20–120 s) were allowed between trials and loading conditions.
Torque‐velocity equation
In recent years, the linear F‐V relationship has been suggested as a featured property of only multi‐joint muscle actions. However, the linear F‐V relationship is a characteristic also shared by single‐joint muscle actions. 18 , 26 In both multi‐joint and single‐joint muscle actions, force decreases linearly as a function of velocity in a specific limited region (from high to moderate forces), whereas force decreases as velocity increases in a curvilinear (convex) fashion in other region (from moderate to null forces). 27 Therefore, a hybrid equation 28 (Equation 1 ) that combines a linear and a curvilinear (Hill‐type) region was used in the current study. Briefly, this equation includes the classical linear 29 and hyperbolic 30 equations, as well as an associated coefficient to each of the two [c 1 (Equation 2 ) and c 2 (Equation 3 ), respectively] that provides different albeit complementary weights (c 1 + c 2 = 1) to each of them as a function of relative intensity (i.e., in this case torque relative to maximum isometric torque). 28
| (1) |
| (2) |
| (3) |
where V is velocity (angular velocity in this case), c 1 is the coefficient associated to the linear equation, c 2 is the coefficient associated to the hyperbolic equation, T is torque, T 0 is estimated maximum isometric torque, S is the slope of the linear region, a and b are Hill‐type constants, k is a constant that determines the point of transition from the linear to the hyperbolic equation (i.e., when c 1 and c 2 are both 0.5), and s is a constant that determines how smooth the model runs from the linear to the hyperbolic region. Estimated maximal unloaded velocity (V0) was calculated as the intercept of the velocity axis, and S/T0 and a/T0 were calculated to describe the decrease in torque as a function of contraction velocity in the linear (i.e., slope) and hyperbolic (i.e., curvature) regions, respectively, of the T‐V relationship. Power was calculated as the product of torque and angular velocity; maximum muscle power (Pmax) was identified at the apex of the P‐V relationship, and optimal torque (Topt) and optimal velocity (Vopt) as those produced at Pmax. With the torque and angular velocity values measured in the current experiments, and k = 0.45 based on previous evidence, 31 a commercial software package (Solver VBA, Microsoft Excel, USA) was used to calculate the rest of the constants of the T‐V equation (non‐linear least squares method). To ensure an optimal assessment of the T‐V relationship, and based on basic physiological principles, the following automatized instructions were implemented in the software for the selection of suitable torque and angular velocity data (Figure 1): Maximum isometric torque values lower than any of the registered dynamic torque values were discarded; dynamic trials showing a lower torque value for a corresponding angular velocity compared with a faster contraction were discarded; and dynamic trials showing a lower power for a corresponding angular velocity compared with both a slower and a faster contraction were discarded. In addition, those isometric or dynamic trials that by visual inspection showed diminished torque compared with the one that would be expected according to the rest of the trials were discarded only if after excluding those trials the difference between measured and estimated values reached ≥10%. Participants that did not have at least three suitable trials (at least one trial below 45% and two trials above 45% of T0) were excluded from further analysis. The average number of T‐V data points included was 5.4 ± 1.1 (excluded: 1.6 ± 1.0), coefficient of determination (R2) was 0.99 ± 0.02, and standard error of the estimate (SEE) was 0.44 ± 0.32. A total of 50 participants (baseline and follow‐up; i.e., 100 T‐V tests) were randomly selected and assessed independently by two different evaluators to assess the inter‐rater reliability of this procedure. Reliability values (intraclass correlation coefficient (ICC2,1) for absolute agreement [95% confidence interval]) for the main outcomes derived from the T‐V relationship were T0: 0.99 [0.98–0.99]; V0: 0.85 [0.77–0.90]; and Pmax: 0.99 [0.99–0.99].
Figure 1.
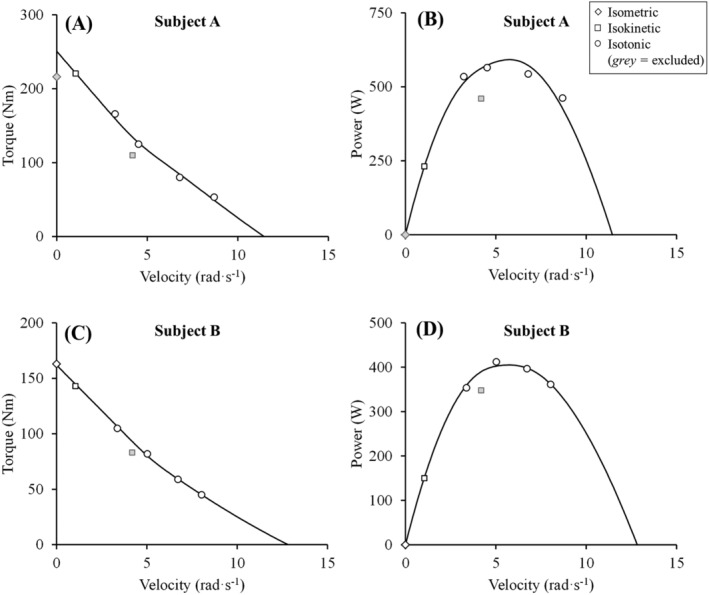
Analysis of the torque‐velocity (A and C) and power velocity (B and D) relationships in two participants (subject 1: A and B; subject 2: C and D). A hybrid equation 28 was applied to measured data after excluding those data that fulfilled the following criteria: Maximum isometric torque values lower than any of the registered dynamic torque values (in A); dynamic trials showing a lower torque value for a corresponding velocity compared with a faster contraction (in A); and dynamic trials showing a lower power for a corresponding velocity compared with both a slower and a faster contraction (in B and D).
Data analyses
Next to the analyses of absolute values, torque and power outcomes were normalized to body mass (i.e., relative T0 and relative Pmax). Relative values have a greater functional relevance compared with absolute values. 2 , 32 Longitudinal yearly changes (i.e., ∆·year−1) in daily protein intake, physical activity behaviour, and outcomes derived from the T‐V and P‐V relationships (both absolute and relative to body mass) were calculated as the difference between follow‐up and baseline values divided by follow‐up time in years; then, percentage changes (i.e., %∆·year−1) were calculated relative to baseline levels.
Statistical analyses
Data were presented as mean ± standard deviation or 95% confidence interval unless otherwise stated. Cross‐sectional baseline or follow‐up differences regarding anthropometry, daily protein intake, physical activity behaviour, and outcomes derived from T‐V and P‐V relationship between men and women and between age groups were assessed using linear mixed‐effect models with two fixed factors (sex and age group) and one random factor (subject ID). Then, longitudinal changes (i.e., absolute and relative change scores as dependent variable) were assessed with linear mixed‐effect models with two fixed factors (sex and age group) and one random factor (subject ID). Maximum likelihood estimation and the best‐fitting covariance structure were applied. Pairwise comparisons were corrected by the Bonferroni method. For reasons of clarity and readability, the results reported in the text refer to longitudinal absolute changes and are reported as overall changes over time (i.e., all participants merged), main effect of sex (i.e., all age groups merged), main effect of age (i.e., men and women merged), sex‐by‐age interaction and subgroup comparisons (i.e., classified by sex and age). Tables 2, 3, 4 also include longitudinal percentage changes and subgroup comparisons. In addition, to assess the influence of changes in protein intake and physical activity behaviour on changes in the outcomes derived from the T‐V and P‐V relationships, the above‐mentioned analyses were adjusted by changes in protein intake and physical activity behaviour. Finally, regression analyses (2nd order polynomials) were used to represent the continuum of longitudinal percentage changes as a function of baseline age. All statistical analyses were carried out with SPSS, version 24 (SPSS Inc., USA), and the level of significance was set at α = 0.05.
Table 2.
Changes in parameters obtained from the torque‐velocity relationship.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Young | Middle‐aged | Old | Young | Middle‐aged | Old | |
| T0 (Nm) | ||||||
| Pre | 159.5 ± 27.9* | 153.4 ± 27.7* | 115.6 ± 23.7* ab | 242.7 ± 47.8* | 234.0 ± 47.3* | 203.6 ± 41.2* ab |
| Post | 154.6 ± 32.0* | 138.3 ± 23.8* | 103.8 ± 28.6* ab | 234.1 ± 41.8* | 219.3 ± 39.9* | 178.8 ± 35.6* ab |
| ∆ (year−1) | −0.45 ± 3.12 | −1.57 ± 2.27 | −1.26 ± 1.79* | −0.82 ± 3.88 | −1.52 ± 3.75 | −2.58 ± 2.60 |
| ∆% (year−1) | −0.28 ± 1.96 | −1.02 ± 1.48 * , a | −1.09 ± 1.55 | −0.34 ± 1.60 | −0.65 ± 1.60 * | −1.27 ± 1.28 a |
| P‐value | 0.368 | <0.001 | 0.256 | 0.022 | <0.001 | <0.001 |
| V0 (rad·s−1) | ||||||
| Pre | 11.6 ± 2.1* | 10.6 ± 1.7* | 10.2 ± 2.2 | 12.9 ± 2.8* | 12.1 ± 2.9* | 10.8 ± 2.0ab |
| Post | 11.8 ± 3.6* | 10.5 ± 1.9*, a | 9.4 ± 1.2 a | 13.3 ± 3.4* | 11.1 ± 2.4*, a | 9.8 ± 1.7 a |
| ∆ (year−1) | 0.03 ± 0.37 | −0.01 ± 0.24* | −0.10 ± 0.25 | 0.03 ± 0.37 | −0.11 ± 0.33 * , a | −0.10 ± 0.28 |
| ∆% (year−1) | 0.26 ± 3.19 | −0.09 ± 2.26* | −0.98 ± 2.45 | 0.23 ± 2.87 | −0.91 ± 2.73 * , a | −0.93 ± 2.59 |
| P‐value | 0.609 | 0.740 | 0.365 | 0.388 | <0.001 | 0.105 |
| S/T0 (%T0·rad·s−1) | ||||||
| Pre | −11.5 ± 1.8 | −12.5 ± 1.8*, a | −13.7 ± 2.8 a | −10.9 ± 1.9 | −11.6 ± 2.1*, a | −12.5 ± 2.2 a |
| Post | −12.0 ± 2.2 | −12.7 ± 2.4 | −15.3 ± 2.9ab | −11.4 ± 1.9 | −12.8 ± 2.6 a | −14.0 ± 2.8ab |
| ∆ (year−1) | −0.05 ± 0.23 | −0.03 ± 0.24* | −0.19 ± 0.44 | −0.05 ± 0.23 | −0.13 ± 0.26 * | −0.16 ± 0.39 |
| ∆% (year−1) | −0.43 ± 2.00 | −0.24 ± 1.92 | −1.39 ± 3.21 | −0.46 ± 2.11 | −1.12 ± 2.24 | −1.28 ± 3.12 |
| P‐value | 0.240 | 0.200 | 0.028 | 0.052 | <0.001 | 0.002 |
| a/T0 | ||||||
| Pre | 2.45 ± 1.35 | 2.48 ± 1.10 | 1.94 ± 0.69 | 2.35 ± 1.45 | 2.43 ± 1.45 | 2.49 ± 1.30 |
| Post | 2.06 ± 1.10 | 2.46 ± 0.99 | 2.00 ± 1.15 | 2.04 ± 1.30 | 2.49 ± 1.28 | 2.32 ± 1.25 |
| ∆ (year−1) | −0.04 ± 0.18 | <0.01 ± 0.15 | 0.01 ± 0.07 | −0.03 ± 0.17 | 0.01 ± 0.19 | −0.02 ± 0.19 |
| ∆% (year−1) | −1.63 ± 7.35 | <0.01 ± 6.05 | 0.52 ± 3.61 | −1.28 ± 7.23 | 0.41 ± 7.82 a | −0.80 ± 7.63 |
| P‐value | 0.115 | 0.944 | 0.904 | 0.110 | 0.659 | 0.583 |
Note: Linear mixed‐effect models with two fixed factors (sex and age group) and one random factor (subject ID) were performed and reported. The P‐value in the table represents the time effect within each subgroup for absolute changes. Bold values indicate a significant change over time (P < 0.05).
Abbreviations: T0, estimated maximum isometrid torque; V0, estimated maximum unloaded velocity; S/T0, linear slope of the torque‐velocity relationship; a/T0, curvature of the torque‐velocity relationship.
Significant differences between men and women (P < 0.05).
Significant differences compared with young adults (P < 0.05).
Significant differences compared with middle‐aged adults (P < 0.05).
Table 3.
Changes in maximum muscle power, optimal force, and optimal velocity.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Young | Middle‐aged | Old | Young | Middle‐aged | Old | |
| Pmax (W) | ||||||
| Pre | 358.4 ± 68.2* | 319.4 ± 59.9* | 228.8 ± 70.6* ab | 591.1 ± 131.1* | 536.4 ± 122.9*, a | 429.9 ± 96.7* ab |
| Post | 338.5 ± 71.5* | 286.1 ± 58.1* | 178.4 ± 33.8* ab | 557.8 ± 114.2* | 464.5 ± 102.9*, a | 340.6 ± 78.4* ab |
| ∆ (year−1) | −2.02 ± 5.76 | −3.48 ± 5.00 * | −5.50 ± 4.90 | −3.54 ± 11.38 | −7.56 ± 9.56 * , a | −9.47 ± 8.62 a |
| ∆% (year−1) | −0.56 ± 1.61 | −1.09 ± 1.57 | −2.40 ± 2.14 a | −0.60 ± 1.93 | −1.41 ± 1.78 a | −2.20 ± 2.01 a |
| P‐value | 0.117 | <0.001 | 0.053 | <0.001 | <0.001 | <0.001 |
| Topt (Nm) | ||||||
| Pre | 69.8 ± 12.5* | 67.2 ± 11.7* | 49.2 ± 11.1* ab | 102.3 ± 19.5* | 98.9 ± 20.6* | 87.8 ± 18.4* ab |
| Post | 66.0 ± 15.7* | 60.3 ± 10.7* | 42.6 ± 11.3* ab | 95.3 ± 20.0* | 92.2 ± 18.9* | 75.6 ± 14.7* ab |
| ∆ (year−1) | −0.38 ± 1.40 | −0.72 ± 1.18 | −0.71 ± 0.73 | −0.68 ± 2.09 | −0.70 ± 2.04 | −1.28 ± 1.38 |
| ∆% (year−1) | −0.54 ± 2.01 | −1.07 ± 1.76 * | −1.44 ± 1.48 | −0.66 ± 2.04 | −0.71 ± 2.06 * | −1.46 ± 1.57 |
| P‐value | 0.157 | <0.001 | 0.229 | <0.001 | <0.001 | <0.001 |
| Vopt (rad·s−1) | ||||||
| Pre | 5.20 ± 0.87* | 4.79 ± 0.72* | 4.63 ± 0.99 | 5.84 ± 1.27* | 5.51 ± 1.13* | 4.96 ± 0.89ab |
| Post | 5.30 ± 1.41* | 4.78 ± 0.81*, a | 4.29 ± 0.53 a | 5.99 ± 1.34* | 5.12 ± 1.01*, a | 4.54 ± 0.79ab |
| ∆ (year−1) | 0.01 ± 0.15 | <0.01 ± 0.10* | −0.04 ± 0.11 | 0.01 ± 0.15 | −0.04 ± 0.13 * , a | −0.05 ± 0.12 |
| ∆% (year−1) | 0.19 ± 2.88 | <0.01 ± 2.09* | −0.86 ± 2.38 | 0.17 ± 2.57 | −0.73 ± 2.36 * , a | −1.01 ± 2.42 |
| P‐value | 0.646 | 0.909 | 0.367 | 0.367 | <0.001 | 0.082 |
Note: Linear mixed‐effect models with two fixed factors (sex and age group) and one random factor (subject ID) were performed and reported. The P‐value in the table represents the time effect within each subgroup for absolute changes. Bold values indicate a significant change over time (P < 0.05).
Abbreviations: Pmax, maximum muscle power; Topt, optimal torque; Vopt, optimal velocity.
Significant differences between men and women (P < 0.05).
Significant differences compared with young adults (P < 0.05).
Significant differences compared with middle‐aged adults (P < 0.05).
Table 4.
Changes in body mass index, relative T0 and relative Pmax.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Young | Middle‐aged | Old | Young | Middle‐aged | Old | |
| BMI (kg·m−2) | ||||||
| Pre | 22.4 ± 2.4* | 23.3 ± 3.0* | 23.1 ± 2.9* | 23.9 ± 2.8* | 25.4 ± 2.7*, a | 26.2 ± 2.8*, a |
| Post | 23.4 ± 2.9* | 23.9 ± 3.4* | 23.2 ± 3.5* | 24.7 ± 3.0* | 25.8 ± 3.1*, a | 25.9 ± 3.3* |
| ∆ (year−1) | 0.10 ± 0.19 | 0.06 ± 0.16 | 0.01 ± 0.18 | 0.08 ± 0.17 | 0.04 ± 0.15 | −0.03 ± 0.13 a |
| ∆% (year−1) | 0.45 ± 0.85 | 0.26 ± 0.69 | 0.04 ± 0.78 | 0.33 ± 0.71 | 0.16 ± 0.59 | −0.11 ± 0.50 a |
| P‐value | <0.001 | <0.001 | 0.878 | <0.001 | <0.001 | 0.396 |
| Relative T0 (Nm·kg−1) | ||||||
| Pre | 2.62 ± 0.48* | 2.43 ± 0.44* | 1.94 ± 0.37* ab | 3.20 ± 0.58* | 2.94 ± 0.53*, a | 2.60 ± 0.47* ab |
| Post | 2.41 ± 0.47* | 2.14 ± 0.39*, a | 1.74 ± 0.39*, a | 2.99 ± 0.56* | 2.73 ± 0.45*, a | 2.32 ± 0.39* ab |
| ∆ (year−1) | −0.02 ± 0.05 | −0.03 ± 0.04 | −0.02 ± 0.02 | −0.02 ± 0.05 | −0.02 ± 0.05 | −0.03 ± 0.04 |
| ∆% (year−1) | −0.76 ± 1.91 | −1.23 ± 1.65 * | −1.03 ± 1.03 | −0.63 ± 1.56 | −0.68 ± 1.70 * | −1.15 ± 1.54 |
| P‐value | 0.002 | <0.001 | 0.135 | <0.001 | <0.001 | 0.001 |
| Relative Pmax (W·kg−1) | ||||||
| Pre | 5.84 ± 0.84* | 5.04 ± 0.93*, a | 3.85 ± 1.24* ab | 7.79 ± 1.54* | 6.72 ± 1.24*, a | 5.49 ± 1.17* ab |
| Post | 5.28 ± 1.04* | 4.43 ± 0.92*, a | 3.03 ± 0.65* ab | 7.11 ± 1.41* | 5.77 ± 1.17*, a | 4.44 ± 1.03* ab |
| ∆ (year−1) | −0.06 ± 0.10 | −0.06 ± 0.07 * | −0.09 ± 0.08 | −0.07 ± 0.13 | −0.10 ± 0.11 * | −0.11 ± 0.11 |
| ∆% (year−1) | −1.03 ± 1.71 | −1.19 ± 1.39 | −2.34 ± 2.08 | −0.90 ± 1.67 | −1.49 ± 1.64 a | −2.00 ± 2.00 a |
| P‐value | <0.001 | <0.001 | 0.009 | <0.001 | <0.001 | <0.001 |
Note: Linear mixed‐effect models with two fixed factors (sex and age group) and one random factor (subject ID) were performed and reported. The P‐value in the table represents the time effect within each subgroup for absolute changes. Bold values indicate a significant change over time (P < 0.05).
Abbreviations: BMI, body mass index; T0, estimated maximum isometric torque; Pmax, maximum muscle power.
Significant differences between men and women (P < 0.05).
Significant differences compared with young adults (P < 0.05).
Significant differences compared with middle‐aged adults (P < 0.05).
Results
Table 1 gives an overview of the main characteristics and subgroup comparisons at baseline.
Longitudinal changes in lifestyle factors
Daily protein intake decreased significantly from baseline to follow‐up (mean [95% CI] = −0.01 [−0.016 to −0.004] g·kg−1 per year; P = 0.001), but no effects of sex (P = 0.998), age group (P = 0.508), or sex‐by‐age interaction (P = 0.513) were noted. However, post hoc within‐subgroup analyses showed significant changes only in middle‐aged women (mean [95% CI] = −0.01 [−0.018 to −0.003] g·kg−1 per year; P = 0.007) and old men (mean [95% CI] = −0.02 [−0.035 to −0.003] g·kg−1 per year; P = 0.017). There were no significant differences between subgroups (all P > 0.05). No significant changes were noted in PAL index (mean [95% CI] = −0.002 [−0.006 to 0.001] per year; P = 0.176) or EEsports (mean [95% CI] = −0.25 [−0.65 to 0.16] MET·h·week−1 per year; P = 0.234), and no main effects of sex (P = 0.911 and 0.331, respectively), age group (P = 0.477 and 0.586, respectively), or sex‐by‐age interaction (P = 0.384 and 0.673, respectively) were found. However, post hoc within‐subgroup analyses showed that PAL index diminished significantly in middle‐aged men (mean [95% CI] = −0.004 [−0.007 to −0.001] per year; P = 0.004), whereas EEsports did in middle‐aged women (mean [95% CI] = −0.52 [−0.98 to −0.07] MET·h·week−1 per year; P = 0.023). There were no significant differences between subgroups (all P > 0.05). In general, changes in protein intake (all P ≥ 0.133), PAL index (P ≥ 0.100) or EEsports (all P ≥ 0.372) did not contribute significantly to changes in the T‐V and P‐V relationships over the follow‐up, with a few exceptions that are reported below.
Longitudinal changes in the torque‐velocity and power‐velocity relationships
Individual changes and regression lines observed in the main T‐V and P‐V outcomes after the 10‐year follow‐up in men and women as a function of age are shown in Figure 2.
Figure 2.
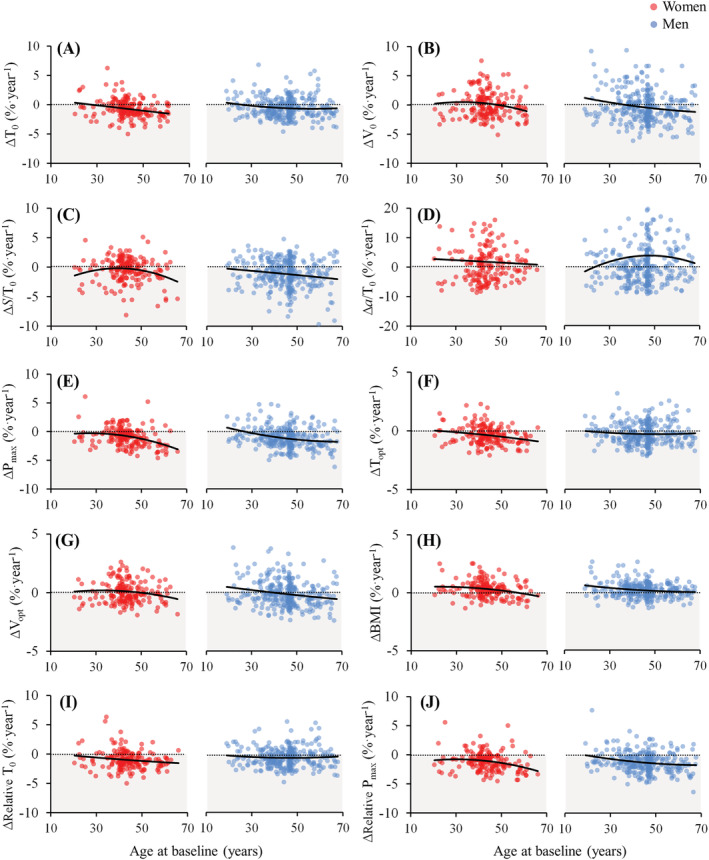
Individual longitudinal annual percentage changes in the study outcomes as a function of baseline age in women and men. Longitudinal changes were calculated as the difference between follow‐up and baseline values divided by follow‐up time in years, and then relative to baseline levels. The dotted lines represent no change (i.e., zero) whereas the solid lines were calculated using regression analyses (2nd order polynomials) to represent the continuum of longitudinal percentage changes as a function of baseline age. ∆, change in a respective outcome; T0, estimated maximum isometric torque; V0, estimated maximum unloaded contraction velocity; S/T0, slope of the linear part of the torque‐velocity relationship; a/T0, curvature of the hyperbolic part of the torque‐velocity relationship; Pmax, maximum muscle power; Topt, optimal torque; Vopt, optimal velocity; BMI, body mass index.
Knee extension torque‐velocity relationship
The results from each study subgroup and multiple comparisons regarding T0, V0, S/T0, and a/T0 are provided in Table 2. T0 decreased significantly over time (mean [95% CI] = −1.37 [−1.85 to −0.88] Nm per year; P < 0.001). There was no main effect of sex (P = 0.268), but there was a main effect of age group (P = 0.027). Specifically, T0 decreased significantly in middle‐aged women, and in young, middle‐aged, and old men (all P < 0.05). Also, the T0 decrement was significantly higher in middle‐aged compared with young participants (P = 0.036). No sex‐by‐age interaction was observed (P = 0.541).
Changes in V0 over time did not reach statistical significance (mean [95% CI] = −0.05 [−0.091 to 0.001] %T0 rad·s−1 per year; P = 0.057), and no effect of sex was detected (P = 0.452), whereas a significant main effect of age group was observed (P = 0.022). V0 diminished significantly in middle‐aged men only (P < 0.001). Moreover, the middle‐aged group exhibited a significantly greater decrease in V0 than the young group (P = 0.030). No sex‐by‐age interaction was observed (P = 0.258).
S/T0 exhibited a significant decline over time (mean [95% CI] = −0.10 [−0.14 to −0.06] %T0·rad·s−1 per year; P < 0.001), but no significant main effects of sex (P = 0.501) or age group (P = 0.080) were detected. The decline in S/T0 was statistically significant among old women, middle‐aged men and old men (all P < 0.05). No sex‐by‐age interaction was observed (P = 0.190).
No significant changes for a/T0 were found (mean [95% CI] = −0.01 [−0.04 to 0.01] per year; P = 0.312) nor main effects of sex (P = 0.901) or age group (P = 0.872). No sex‐by‐age interaction was observed (P = 0.872). Figure 3 shows the changes observed in the T‐V relationship for each subset of participants.
Figure 3.
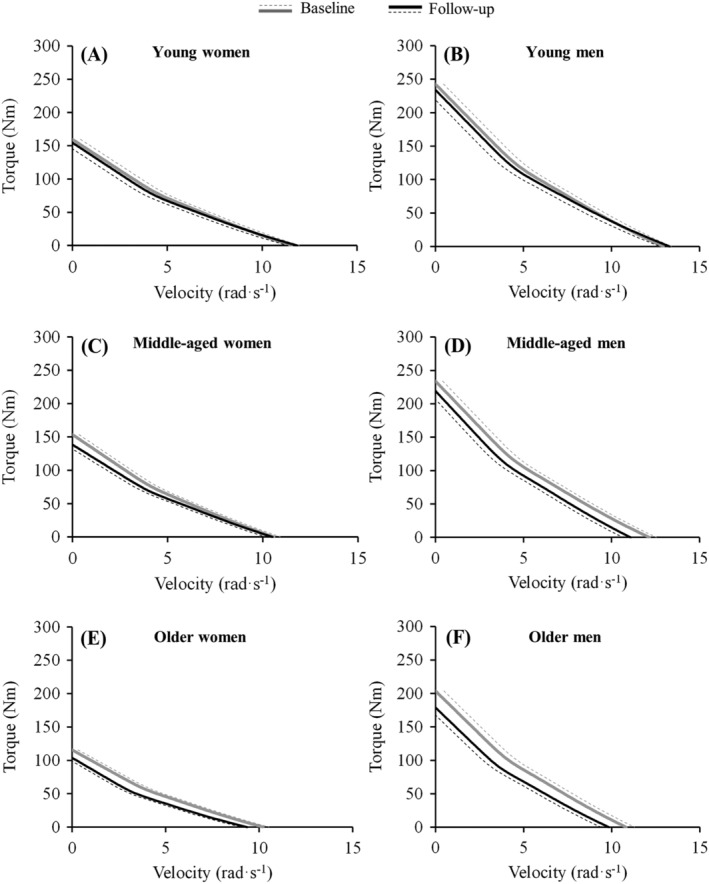
Longitudinal changes observed after the 10‐year follow‐up in the torque‐velocity relationship in young women and men, middle‐aged women and men, and older women and men. The dotted lines represent standard error of the estimate values, and the solid lines represent the hybrid equation used for modelling the torque‐velocity relationship.
Knee extension maximum muscle power
The results from each study subgroup and multiple comparisons regarding Pmax, Topt, and Vopt are provided in Table 3. Pmax was found to decrease significantly over time (mean [95% CI] = −5.26 [−6.52 to −4.00] W per year; P < 0.001), whereas significant main effects of sex (P = 0.013) and age group (P = 0.004) were also noted. Pmax decreased significantly in middle‐aged women, and in young, middle‐aged, and old men (all P < 0.001). Also, Pmax declined more in men compared with women (P = 0.013), and more in middle‐aged and old adults compared with young participants (P = 0.010 and 0.034, respectively). No sex‐by‐age interaction was observed (P = 0.385). Annual changes in PAL index were significantly associated to annual changes in Pmax (P = 0.017), so the overall decline in Pmax was slightly attenuated in the adjusted model (mean [95% CI] = −5.05 [−6.39 to −3.72] W per year; P < 0.001), but it did not influence comparisons between sex or age groups (Table S1 ).
In addition, absolute Topt declined significantly over time (mean [95% CI] = −0.74 [−1.00 to −0.49] Nm per year; P < 0.001), but no main effects of sex (P = 0.273) or age group (P = 0.416) were observed. Specifically, it decreased significantly in middle‐aged women, and in young, middle‐aged and old men (all P < 0.001). No sex‐by‐age interaction was observed (P = 0.540). In terms of Topt relative to T0, there was a significant decrement (from 43.0 ± 4.2 to 42.3 ± 4.2% of T0; mean [95% CI] = −0.11 [−0.21 to −0.02] % of T0 per year, P < 0.017) with no main effects of sex (P = 0.914) or age group (P = 0.115). No sex‐by‐age interaction was observed (P = 0.883).
Changes in absolute Vopt did not reach statistical significance (mean [95% CI] = −0.02 [−0.036 to 0.001] rad·s−1 per year; P = 0.064), although there was a significant main effect of age group (P = 0.035), but not sex (P = 0.449). Vopt decreased significantly in middle‐aged men only (P < 0.001). Despite the higher Vopt decrements observed in middle‐aged compared with young adults, the difference was not statistically significant (P = 0.059). No sex‐by‐age interaction was observed (P = 0.265). Furthermore, Vopt as a percentage of V0 increased significantly over time (from 45.5 ± 1.6 to 45.9 ± 1.8% of V0; mean [95% CI] = 0.04 [0.001 to 0.070] % of V0 per year; P = 0.043), although no significant main effects of sex (P = 0.825) or age group (P = 0.129) were reported. Figure 4 shows the changes observed in the P‐V relationship for each subset of participants. No sex‐by‐age interaction was observed (P = 0.522).
Figure 4.
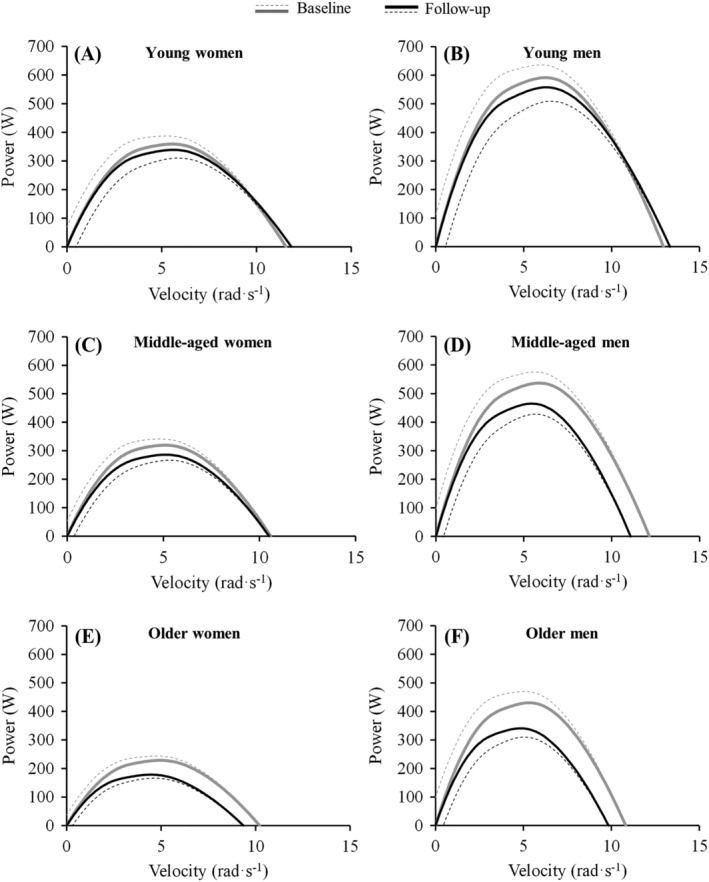
Longitudinal changes observed after the 10‐year follow‐up in the power‐velocity relationship in young women and men, middle‐aged women and men, and older women and men. The dotted lines represent standard error of the estimate values, and the solid lines represent the hybrid equation used for modelling the torque‐velocity relationship that resulted in the power‐velocity relationship.
Longitudinal changes in body mass index and muscle function measures relative to body mass
The results from each study subgroup and multiple comparisons regarding BMI, relative T0, and relative Pmax are provided in Table 4. There was a significant increase in BMI (mean [95% CI] = 0.04 [0.02 to 0.07] kg·m−2 per year; P < 0.001), and a significant main effect of age group (P = 0.010), but not sex (P = 0.263). BMI increased significantly in young and middle‐aged women and men (all P < 0.001). The young group experienced a significant increase in BMI compared with a non‐significant decline in the old group (P = 0.016). Annual changes in protein intake were significantly associated to annual changes in BMI (P < 0.001), although with no apparent variation in the overall increase in BMI in the adjusted model (mean [95% CI] = 0.04 [0.01 to 0.06] kg·m−2 per year; P < 0.001) nor in comparisons between sex or age groups (Table S2 ).
Both relative T0 and relative Pmax decreased significantly (mean [95% CI] = −0.02 [−0.03 to −0.02] Nm·kg−1 per year, and −0.08 [−0.10 to −0.07] W·kg−1 per year; both P < 0.001), but no effects of sex (P = 0.972 and 0.133, respectively) or age group (P = 0.492 and 0.147, respectively) were noted. Finally, the declines were statistically significant for relative T0 in young and middle‐aged women, and in young, middle‐aged, and old men; whereas regarding relative Pmax, the declines were statistically significant for all the subgroups (all P < 0.01). No sex‐by‐age interaction was observed (P = 0.597). Annual changes in protein intake were significantly associated to annual changes in relative Pmax (P = 0.010), but the overall decline in relative Pmax remained the same in the adjusted model (mean [95% CI] = −0.08 [−0.10 to −0.07] W·kg−1 per year; P < 0.001) and did not influence comparisons by sex or age groups (Table S2 ).
Discussion
The main findings of the present longitudinal investigation were that after 10 years, Pmax decreased at different rates in young (−0.6%·year−1) compared with middle‐aged (−1.1% to −1.4%·year−1) and older (−2.2% to −2.4%·year−1) adults. These changes were in general accompanied by decreases in Topt and Vopt. Due to concomitant changes in BMI, relative Pmax was found to decline in young (−0.9% to −1.0%·year−1), middle‐aged (−1.2% to −1.5%·year−1), and older (−2.0% to −2.3%·year−1) adults. Of note, an overall longitudinal decline in protein intake was detected, whereas physical activity and sports participation remained unchanged in the whole cohort of participants. Changes in physical activity were associated to changes in absolute Pmax, whereas changes in protein intake were associated to changes in BMI and relative Pmax.
Early cross‐sectional studies demonstrated that older people show a downward shift in the F‐V relationship (i.e., lower force production at given movement velocities, lower maximal isometric force, and lower maximal unloaded contraction velocity) 33 , 34 , 35 when compared with their younger counterparts. Of note, muscle power is the product of force and velocity, so changes in the F‐V relationship impact muscle power output severely. In addition, age‐related percentage decrements in the F‐V relationship seem to be greater at higher versus lower contraction velocities, 6 , 36 , 37 , 38 , 39 and so dynamic muscle function is more affected by aging than isometric muscle function. 37 , 40 By analysing changes in the slope of the T‐V relationship relative to maximum isometric torque (i.e., S/T0), we were able to examine how torque decreases when movement velocity increases. Our results show that (i) torque decreased as a function of velocity more abruptly in middle‐aged and older adults compared with young adults and (ii) significant longitudinal decreases were observed in older women and in middle‐aged and older men. Basically, these results confirm that middle‐aged and especially older people exhibit a more compromised muscle function with aging as the required movement velocity increases. Another study has also shown that older people exhibit a lower resistance to fatigue compared with young people during fast muscle actions, 38 which would impair even more their ability to conduct repeated fast dynamic actions. Therefore, as previously noted, 41 the inclusion of explosive exercises within resistance training programmes might be paramount for the prevention and treatment of this hallmark of aging.
Regarding the potential mechanisms leading to these changes, one strong candidate might be the loss of skeletal muscle. Nevertheless, muscle force is less dependent on muscle size as muscle contraction velocity increases, 38 , 42 and no significant longitudinal changes in whole‐body skeletal muscle mass assessed by bioelectrical impedance analysis have been previously reported for the present cohort of participants. 22 Although skeletal muscle mass was diminished by ~3% after the 10‐year follow‐up in the older participants (i.e., ~0.3% per year), other factors should be considered to explain the observed 21–23% decrease in Pmax. For example, lower overall single muscle fibre ATPase activity 43 or a fast‐to‐slow shift in the single muscle fibre phenotype 44 with aging would fit with the observed changes. In addition, an age‐related decrease in tendon stiffness and skeletal muscle fascicle length would cause a decrease in torque at given velocities, especially during fast contractions. 45,46 Also, considering the time constraints inherent to fast contractions, a slower ability to maximally evoke voluntary contractions, convey action potentials through motor nerves and translate the electrical signal into a mechanical signal (excitation‐contraction coupling) might explain a greater decline in high‐ versus low‐velocity actions. A possible disproportionate coactivation of the antagonist muscles during fast contractions, or impaired muscle gearing, may also account for the greater decrements observed during fast versus slow muscle actions. Among these hypotheses, specific atrophy and loss of type II fibres, 47 reduced shortening velocity within fibre types due to slowed rates of myosin head detachment, 48 muscle fascicles operating in a faster region of the F‐V relationship for a given external velocity, 49 reduced muscle fibre conduction velocity, 50 decreased tendon stiffness 51 and muscle fascicle length, 52 and lower rate of EMG rise 53 and decreased neural drive during the early phase of the muscle contraction 54 have been found to be associated with aging in previous studies. The assessment of the potential role of the other proposed factors and the finding of molecular markers 55,56 might be of relevance for future studies.
Apart from the changes in absolute Pmax values, we noted a significant decline in relative Pmax at all age and sex groups. These declines in relative Pmax values apparently exceeded the decline in absolute Pmax values in younger individuals, whereas they were slightly lower in older adults. This results from concomitant changes in BMI occurring during aging. Of note, BMI was found to augment in the young and middle‐aged participants (0.3–0.5% and 0.2–0.3%·year−1, respectively), whereas no significant changes were reported in our 60‐ to 70‐year‐old adults. This matches with a previous cross‐sectional study showing an increase in BMI up to the age of 65 years in men and 75 years in women, after which it starts to decline. 10 Likewise, sex differences in changes in BMI have been confirmed in a previous 10‐year longitudinal follow‐up study, 57 and the current data show that older women and men in their 60s increased (0.04% year−1, not significant) and decreased (−0.1% year−1, not significant) in BMI, respectively. Important to note is that the potential loss of BMI at advancing age cannot compensate the progressively larger loss of muscle power as people age, which can reach up to 4–9%·year−1 after the eighth decade of life. 10 , 58 As low relative muscle power is more strongly associated with several negative outcomes occurring at older age (e.g., mobility limitations, hospitalization, or mortality) than sarcopenia, low handgrip strength, or absolute muscle power per se, 6 , 8 , 9 , 14 , 59–61 it should be evaluated and diagnosed in older people according to a proposed operational algorithm and cut‐off points. 62
The temporal sequence of the declines in the different parameters can be viewed in Figure 2, that is, by investigating when the regression lines crossed the zero line and changes became negative. Maximum isometric torque (i.e., T0) started to decline after the age of 27 years in men and 28 years in women, whereas Pmax declined after the age of 27 years in men and 20 years in women. Although longitudinal changes in power were greater than those found in maximum isometric torque, both started approximately in the same period in life. In addition, torque produced at Pmax (i.e., Topt) started to decline after the age of 20 years in men and 22 years in women, whereas velocity at Pmax (i.e., Vopt) decreased after the age of 39 years in men and 49 years in women. This denotes that early decrements in Pmax are associated with mechanisms directly related to torque production, whereas in middle‐aged and older adults, both torque‐ and velocity‐related mechanisms are associated with the age‐related loss of Pmax. A previous cross‐sectional study also reported that the main determinant of the lower maximum power observed in older versus young women was a decreased optimal force, whereas optimal velocity was reduced to a lesser extent. 63 In any case, the great interindividual variability observed in our study indicates that evaluation and individualization should be the preferred choice to establish a correct diagnosis and treatment for each individual.
Finally, there are several considerations and limitations that deserve to be mentioned to interpret adequately the current findings. Firstly, the present study assessed knee extension muscle function, so the findings can only apply for this muscle action. Secondly, in some subgroups, there were changes in physical activity level, sports energy expenditure, and protein intake over the 10‐year follow‐up period, which can influence the changes in muscle function. Although we have performed additional analyses to adjust for those variables, we should be aware that these measurements only address two specific points in the 10‐year period (i.e., baseline and follow‐up) and might not represent what occurred during follow‐up period. In addition, there might be other covariates that may have influenced our results, such as co‐morbidity. Although we registered some disease‐related data for the exclusion criteria and risk stratification for a maximal graded exercise test within the overall study protocol, these were not collected in sufficient detail to include co‐morbidity as a covariate in the study. Thirdly, the results derived from older women are based on a relatively small group of participants, which may explain the lack of statistical significance in some outcomes. Finally, longitudinal studies are limited by a potential survival bias, and thus, our finding may underestimate the actual changes occurring during aging in the whole set of participants evaluated at baseline.
Conclusions
Maximum muscle power of the knee extensors declined significantly after a period of 10 years in young men, middle‐aged women and men, and older women and men. The early declines in muscle power seemed to coincide with declines in force production, whereas the progressive decline in muscle power was associated with declines in both force and velocity production. The analysis of the torque‐velocity relationship also showed a progressively blunted ability to produce force at moderate‐to‐high movement velocities (i.e., maximum muscle power) as a specific hallmark of aging.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This study was conducted under the authority of the First and Third waves of the Flemish Policy Research Center on Sport and was funded by the Flemish Government. E. Van Roie was supported by the Research Foundation Flanders, Belgium (senior postdoctoral fellowship 12Z5720N). J. Alcazar was supported by the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and FEDER funds from the European Union (CB16/10/00477). C. Rodriguez‐Lopez was supported by the Ministerio de Economía y Competitividad of the Government of Spain (DEP2015‐69386‐R and BES‐2016‐077199).
Supporting information
Data S1. Supporting Information
Table S1. Changes in maximum muscle power adjusted by changes in PAL index.
Table S2. Changes in body mass index and relative Pmax adjusted by changes in daily protein intake.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 64
Alcazar J., Rodriguez‐Lopez C., Delecluse C., Thomis M., and Van Roie E. (2023) Ten‐year longitudinal changes in muscle power, force, and velocity in young, middle‐aged, and older adults, Journal of Cachexia, Sarcopenia and Muscle, 14, 1019–1032, 10.1002/jcsm.13184
References
- 1. Valenzuela PL, McGuigan M, Sánchez‐Martínez G, Torrontegi E, Vázquez‐Carrión J, Montalvo Z, et al. Reference power values for the jump squat exercise in elite athletes: A multicenter study. J Sports Sci 2020;38:2273–2278. [DOI] [PubMed] [Google Scholar]
- 2. Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, et al. Association of muscle power with functional status in community‐dwelling elderly women. J Gerontol A Biol Sci Med Sci 2000;55:M192–M199. [DOI] [PubMed] [Google Scholar]
- 3. Steves CJ, Mehta MM, Jackson SH, Spector TD. Kicking Back Cognitive Ageing: Leg Power Predicts Cognitive Ageing after Ten Years in Older Female Twins. Gerontology 2016;62:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alcazar J, Alegre LM, Suetta C, Júdice PB, Van Roie E, González‐Gross M, et al. Threshold of Relative Muscle Power Required to Rise from a Chair and Mobility Limitations and Disability in Older Adults. Med Sci Sports Exerc 2021;53:2217–2224. [DOI] [PubMed] [Google Scholar]
- 5. Kuo HK, Leveille SG, Yen CJ, Chai HM, Chang CH, Yeh YC, et al. Exploring how peak leg power and usual gait speed are linked to late‐life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999‐2002. Am J Phys Med Rehabil 2006;85:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Losa‐Reyna J, Alcazar J, Carnicero J, Alfaro‐Acha A, Castillo‐Gallego C, Rosado‐Artalejo C, et al. Impact of relative muscle power on hospitalization and all‐cause mortality in older adults, Vol. 77. Biological sciences and medical sciences: The journals of gerontology Series A; 2021. p 781–789. [DOI] [PubMed] [Google Scholar]
- 7. Alcazar J, Navarrete‐Villanueva D, Mañas A, Gómez‐Cabello A, Pedrero‐Chamizo R, Alegre LM, et al. 'Fat but powerful' paradox: association of muscle power and adiposity markers with all‐cause mortality in older adults from the EXERNET multicentre study. Br J Sports Med 2021;55:1204–1211. [DOI] [PubMed] [Google Scholar]
- 8. Losa‐Reyna J, Alcazar J, Rodríguez‐Gómez I, Alfaro‐Acha A, Alegre LM, Rodríguez‐Mañas L, et al. Low relative mechanical power in older adults: An operational definition and algorithm for its application in the clinical setting. Exp Gerontol 2020;142:111141. [DOI] [PubMed] [Google Scholar]
- 9. Bahat G, Kilic C, Eris S, Karan MA. Power Versus Sarcopenia: Associations with Functionality and Physical Performance Measures. J Nutr Health Aging 2021;25:13–17. [DOI] [PubMed] [Google Scholar]
- 10. Alcazar J, Aagaard P, Haddock B, Kamper RS, Hansen SK, Prescott E, et al. Age‐ and Sex‐Specific Changes in Lower‐Limb Muscle Power Throughout the Lifespan. J Gerontol A Biol Sci Med Sci 2020;75:1369–1378. [DOI] [PubMed] [Google Scholar]
- 11. Metter EJ, Conwit R, Tobin J, Fozard JL. Age‐associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 1997;52:B267–B276. [DOI] [PubMed] [Google Scholar]
- 12. Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M, et al. Muscle function in elite master weightlifters. Med Sci Sports Exerc 2002;34:1199–1206. [DOI] [PubMed] [Google Scholar]
- 13. Alcazar J, Guadalupe‐Grau A, Garcia‐Garcia FJ, Ara I, Alegre LM. Skeletal Muscle Power Measurement in Older People: A Systematic Review of Testing Protocols and Adverse Events. J Gerontol A Biol Sci Med Sci 2018;73:914–924. [DOI] [PubMed] [Google Scholar]
- 14. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of applied physiology (Bethesda, Md: 1985) 2003;1851–1860, 10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- 15. Alcazar J, Rodriguez‐Lopez C, Ara I, Alfaro‐Acha A, Rodriguez‐Gomez I, Navarro‐Cruz R, et al. Force‐velocity profiling in older adults: An adequate tool for the management of functional trajectories with aging. Exp Gerontol 2018;108:1–6. [DOI] [PubMed] [Google Scholar]
- 16. Pojednic RM, Clark DJ, Patten C, Reid K, Phillips EM, Fielding RA. The specific contributions of force and velocity to muscle power in older adults. Exp Gerontol 2012;47:608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blazevich AJ, Coleman DR, Horne S, Cannavan D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol 2009;105:869–878. [DOI] [PubMed] [Google Scholar]
- 18. MacIntosh BR, Herzog W, Suter E, Wiley JP, Sokolosky J. Human skeletal muscle fibre types and force: velocity properties. Eur J Appl Physiol Occup Physiol 1993;67:499–506. [DOI] [PubMed] [Google Scholar]
- 19. Raj IS, Bird SR, Shield AJ. Aging and the force‐velocity relationship of muscles. Exp Gerontol 2010;45:81–90. [DOI] [PubMed] [Google Scholar]
- 20. Matton L, Beunen G, Duvigneaud N, Wijndaele K, Philippaerts R, Claessens A, et al. Methodological issues associated with longitudinal research: findings from the Leuven Longitudinal Study on Lifestyle, Fitness and Health (1969‐2004). J Sports Sci 2007;25:1011–1024. [DOI] [PubMed] [Google Scholar]
- 21. Wijndaele K, Duvigneaud N, Matton L, Duquet W, Thomis M, Beunen G, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc 2007;39:233–240. [DOI] [PubMed] [Google Scholar]
- 22. Charlier R, Knaeps S, Mertens E, Van Roie E, Delecluse C, Lefevre J, et al. Age‐related decline in muscle mass and muscle function in Flemish Caucasians: a 10‐year follow‐up. Age (Dordr) 2016;38:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deriemaeker P, Aerenhouts D, Hebbelinck M, Clarys P. Validation of a 3‐day diet diary: comparison with a 7‐day diet diary and a FFQ. Med Sci Sports Exerc 2006;38:S328. [Google Scholar]
- 24. Matton L, Wijndaele K, Duvigneaud N, Duquet W, Philippaerts R, Thomis M, et al. Reliability and validity of the Flemish Physical Activity Computerized Questionnaire in adults. Res Q Exerc Sport 2007;78:293–306. [DOI] [PubMed] [Google Scholar]
- 25. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor‐Locke C, et al. Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- 26. Fontana Hde B, Roesler H, Herzog W. In vivo vastus lateralis force‐velocity relationship at the fascicle and muscle tendon unit level. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology 2014;24:934–940. [DOI] [PubMed] [Google Scholar]
- 27. Alcazar J, Csapo R, Ara I, Alegre LM. On the Shape of the Force‐Velocity Relationship in Skeletal Muscles: The Linear, the Hyperbolic, and the Double‐Hyperbolic. Front Physiol 2019;10:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alcazar J, Pareja‐Blanco F, Rodriguez‐Lopez C, Gutierrez‐Reguero H, Sanchez‐Valdepeñas J, Cornejo‐Daza PJ, et al. A novel equation that incorporates the linear and hyperbolic nature of the force‐velocity relationship in lower and upper limb exercises. Eur J Appl Physiol 2022;122:2305–2313. [DOI] [PubMed] [Google Scholar]
- 29. Jaric S. Two‐Load Method for Distinguishing Between Muscle Force, Velocity, and Power‐Producing Capacities. Sports medicine (Auckland, NZ) 2016;46:1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hill AV. The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society of London Series B 1938;126:136–195. [DOI] [PubMed] [Google Scholar]
- 31. Alcazar J, Pareja‐Blanco F, Rodriguez‐Lopez C, Navarro‐Cruz R, Cornejo‐Daza PJ, Ara I, et al. Comparison of linear, hyperbolic and double‐hyperbolic models to assess the force‐velocity relationship in multi‐joint exercises. Eur J Sport Sci 2021;21:359–369. [DOI] [PubMed] [Google Scholar]
- 32. Alcazar J, Aagaard P, Haddock B, Kamper RS, Hansen SK, Prescott E, et al. Assessment of functional sit‐to‐stand muscle power: Cross‐sectional trajectories across the lifespan. Exp Gerontol 2021;152:111448. [DOI] [PubMed] [Google Scholar]
- 33. Harries UJ, Bassey EJ. Torque‐velocity relationships for the knee extensors in women in their 3rd and 7th decades. Eur J Appl Physiol Occup Physiol 1990;60:187–190. [DOI] [PubMed] [Google Scholar]
- 34. Aniansson A, Grimby G, Hedberg M. Muscle function in old age. Scand J Rehabil Med 1978;10:43–49. [PubMed] [Google Scholar]
- 35. Harridge SD, White MJ, Carrington CA, Goodman M, Cummins P. Electrically evoked torque‐velocity characteristics and isomyosin composition of the triceps surae in young and elderly men. Acta Physiol Scand 1995;154:469–477. [DOI] [PubMed] [Google Scholar]
- 36. Gerstner GR, Giuliani HK, Mota JA, Ryan ED. Age‐related reductions in muscle quality influence the relative differences in strength and power. Exp Gerontol 2017;99:27–34. [DOI] [PubMed] [Google Scholar]
- 37. Charlier R, Mertens E, Lefevre J, Thomis M. Muscle mass and muscle function over the adult life span: a cross‐sectional study in Flemish adults. Arch Gerontol Geriatr 2015;61:161–167. [DOI] [PubMed] [Google Scholar]
- 38. Callahan DM, Kent‐Braun JA. Effect of old age on human skeletal muscle force‐velocity and fatigue properties. J Appl Physiol 2011;111:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kennis E, Verschueren S, Van Roie E, Thomis M, Lefevre J, Delecluse C. Longitudinal impact of aging on muscle quality in middle‐aged men. Age (Dordr) 2014;36:9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allison SJ, Brooke‐Wavell K, Folland JP. Multiple joint muscle function with ageing: the force‐velocity and power‐velocity relationships in young and older men. Aging Clin Exp Res 2013;25:159–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Table S1. Changes in maximum muscle power adjusted by changes in PAL index.
Table S2. Changes in body mass index and relative Pmax adjusted by changes in daily protein intake.


