Abstract
Background
Cancer cachexia is prevalent in digestive tract cancer patients and has significant impacts on prognosis; it is vital to identify individuals who are at risk of cancer cachexia to allow for appropriate evaluation and treatment. This study evaluated whether digestive tract cancer patients with a risk of cancer cachexia and who had a risk of adverse survival could be identified before abdominal surgery.
Methods
This large‐scale cohort study involved patients who underwent abdominal surgery between January 2015 and December 2020 to treat digestive tract cancer. Participants were allocated to the development cohort, the validation cohort, or the application cohort. Univariate and multivariate analyses of the development cohort were performed to detect distinct risk variables for cancer cachexia to create a cancer cachexia risk score. The performance of the risk score across all the three cohorts was assessed through calculating the area under the receiver operating characteristic curve (AUC), as well as calibration and decision curves. We tested how well the score predicted survival outcomes in the application cohort.
Results
A total of 16 264 patients (median 64 years of age; 65.9% male) were included, with 8743 in the development cohort, 5828 in the validation cohort, and 1693 in the application cohort. Seven variables were identified as independent predictive factors and were included in the cancer cachexia risk score: cancer site, cancer stage, time from symptom onset to hospitalization, appetite loss, body mass index, skeletal muscle index, and neutrophil‐lymphocyte ratio. The risk score predicting cancer cachexia owns a good discrimination, with the mean AUC of 0.760 (P < 0.001) in the development cohort, 0.743 (P < 0.001) in the validation cohort, and 0.751 (P < 0.001) in the application cohort, respectively, and had an excellent calibration (all P > 0.05). The decision curve analysis revealed net benefits of the risk score across a range of risk thresholds in the three cohorts. In the application cohort, compared with the high‐risk group, the low‐risk group experienced significantly longer overall survival [hazard ratio (HR) 2.887, P < 0.001] as well as relapse‐free survival (HR 1.482, P = 0.01).
Conclusions
The cancer cachexia risk score constructed and validated demonstrated good performance in identifying those digestive tract cancer patients before abdominal surgery at a higher risk of cancer cachexia and unfavourable survival. This risk score can help clinicians to enhance their capabilities to screen for cancer cachexia, assess patient prognosis, and strengthen early decision‐making on targeted approaches to attune cancer cachexia for digestive tract cancer patients before abdominal surgery.
Keywords: Cancer cachexia, Prediction, Digestive tract cancer, Surgery, Prognosis
1. Introduction
Cachexia is a recognized multifactorial illness in which there is a continuing loss of skeletal muscle mass with or without fat wasting. 1 Conventional nutritional support cannot fully reverse the condition. 2 Cachexia frequently occurs in patients with cancer, with approximately half of advanced cancer patients, particularly digestive tract cancer, experiencing cancer cachexia. 3 , 4 , 5 Cancer cachexia is related to fatigue, functional impairment, an increase in toxicity related to treatment, poor life quality, and reduced survival, all of which greatly impact patients' lives and place a heavy burden on the health care system. 6 , 7 , 8 Cancer cachexia is a direct cause of death for more than 20% of cancer patients but is often not recognized in clinical practice, and therapies to stop or even reverse cancer cachexia are lacking. 9 Thus, cancer cachexia is an important unmet medical need and of great research interest. 10
Since cancer cachexia is potentially a determinant of the cancer patient's prognosis and there are no effective therapies, identifying high‐risk patients is crucial for the treatment and assessment of cancer cachexia. 11 Nevertheless, there are major barriers to identifying patients who are at risk of cancer cachexia. First, cancer cachexia is a form of malnutrition, 12 and there are many tools that identify the nutritional risk, but few are developed especially to assess the risk of cancer cachexia. The Cachexia score (CASCO) is the only validated cachexia screening tool for cancer patients, 13 but it is imperfect. It includes numerous questions and metrics but no disease‐state questions; 14 therefore, its potential to predict patient prognosis is unknown. Second, many studies, including the international consensus on definition and classification of cancer cachexia, recommend that patients can be screened for cancer cachexia using body mass index (BMI), weight loss, or direct measure of muscularity, 1 but these commonly used measures also have limitations. For example, weight loss is a diagnostic and risk factor for cancer cachexia, but in general, many patients cannot accurately provide information regarding when their weight loss began and how much it is, which limits the possibility of using weight loss to early identify patients at risk of cancer cachexia. In addition, these commonly used measures are often used alone in clinical practice, showing relatively low accuracy. In fact, cancer cachexia is characterized by a wide spectrum, ranging from non‐symptomatic inflammatory alterations with minimal muscle and weight loss in an early stage to severe muscle wasting and low‐performance status in more advanced stages; 11 therefore, a screening tool based on as many clinical, nutritional, and oncological variables as possible would provide better screening results. Nevertheless, to our knowledge, there is no such screening tool available. 2 , 9 , 11 , 15 Finally, when some nutritional screening tools are used to identify cancer cachexia, they are applied to only a subset of patients with most digestive tract cancer patients who had a high risk of developing cancer cachexia did not have their cancer cachexia assessed at all.
In the present study, due to the high volume of abdominal surgery for digestive tract cancer in China, we conducted a large‐scale cohort study of digestive tract cancer patients before abdominal surgery to develop and validate a cancer cachexia risk score to identify patients at risk of cancer cachexia and adverse survival.
2. Methods
2.1. Study design and participants
This study was performed in the Department of General Surgery/Shanghai Clinical Nutrition Research Center, Zhongshan Hospital, Fudan University, China, was approved by our institutional review board (B2020‐296R), and was conducted in compliance with the Declaration of Helsinki and its later amendments on ethical standards. Individuals aged ≥18 years in our clinical database who underwent abdominal surgery for digestive tract cancer (liver, gallbladder, pancreatic, gastric, or colorectal cancer) in our institution were retrospectively recruited from 1 January 2015 to 31 December 2020. Patients were excluded if they had no complete clinical data for the diagnosis of cachexia, underwent emergency, or had a previous cancer history. Participants who were eligible for the study from 1 January 2015 to 31 December 2019, were allocated randomly into development and validation cohorts in a ratio of 6:4. Participants who were eligible for the study between 1 January 2020 and 31 December 2020 formed the application cohort. Based on the international consensus, 1 the diagnostic criterion for cancer cachexia was weight loss greater than 5% over past 6 months, or any degree of weight loss greater than 2% in individuals who had a BMI lower than 20 kg/m2 or skeletal muscle depletion consistent with sarcopenia. Skeletal muscle depletion was defined as skeletal muscle index (SMI) based on the diagnostic cut‐off values (37.81 cm2/m2 for women and 43.13 cm2/m2 for men) according to our recent research. 16 , 17 , 18 , 19
2.2. Data collection
In development and validation cohorts, data about the candidate predictor variables, including demographic, clinical, nutritional, and oncologic variables, were collected before surgery from medical records and patient interviews. Candidate predictors for cancer cachexia were selected based on knowledge of the subject and a literature review. 3 , 11 , 20 The demographic data included age, gender, height, smoking and drinking status. The clinical data included time from symptom onset to hospitalization, co‐morbidity, especially respiratory co‐morbidity, cardiovascular co‐morbidity and diabetes, lymphocyte count, leukocyte count, and platelet count. Nutritional data included weight, weight loss, appetite loss, BMI measured as weight (kg)/height (m2), albumin, haemoglobin, and SMI calculated by dividing the skeletal muscle area (SMA) by the height [SMA (cm2)/height (m2)]. SMA was determined by calculating the average area on two contiguous computed tomography images at the third lumbar vertebra between the range of −29 to +150 Hounsfield units. 21 Oncologic data included cancer site, histologic type including differentiated and undifferentiated status, abnormal tumour biomarker, and the cancer stage grouped I, II, III, or IV based on the 8th edition of the American Joint Committee on Cancer staging system.
In the application cohort, in addition to the collected data in development and validation cohorts, long‐term postoperative outcomes, including overall survival and relapse‐free survival, were collected. The follow‐up data on survival status were updated in January 2022.
2.3. Statistical analysis
The data are presented as median [interquartile range (IQR)] for continuous variables and frequencies and proportions for categorical variables. Comparisons between continuous and categorical variables were conducted using the Mann–Whitney U test and χ 2 test, respectively. Cancer cachexia risk variables were evaluated in the development cohort by multivariate analysis on variables with P < 0.05 in the univariate analysis using a binary logistic backward stepwise regression. The univariate risk variables with P < 0.05 were incorporated as independent factors in a binary logistic backward stepwise regression analysis to assess cancer cachexia. Using predictive parameters, a nomogram for cancer cachexia risk was created and each patient's cancer cachexia prediction was used in a receiver‐operating characteristic (ROC) study to determine the best cutoff value. If a patient's probability was over the cutoff, the risk of cancer cachexia was identified. Using the area under the receiver operating characteristic curve (AUC), calibration curve, and decision curve analysis, 22 the performance of the risk score was evaluated in the three cohorts. Kaplan–Meier curves were constructed to examine the risk score's capacity to stratify the overall and relapse‐free survival among the two risk groups in the application cohort. Multivariate analysis on variables with P < 0.05 in the univariate analysis was performed using Cox proportional backward stepwise procedure to test the prognostic value of the risk score for overall survival and relapse‐free survival. If P < 0.05, differences were statistically significant. All statistical analyses used software SPSS (26.0), R (4.1.2), and Stata (15.0) for Windows.
3. Results
In total, 16 264 participants were included in this study (Figure 1). The development cohort included 8743 patients with a median (IQR) age of 64 (15) years, and 5731 (65.5%) were men, and the validation cohort included 5828 patients with a median (IQR) age of 64 (16) years, and 3903 (67.0%) were men, while the application cohort included 1693 patients with a median (IQR) age of 64 (14) years, and 1081 (63.9%) were men. In the three cohorts, gastric, colorectal and liver cancers accounted for most cancer types, and more than half of patients were in cancer stage I and II. Cancer cachexia was observed in 2699 individuals (1377, 911, and 411 from development, validation, and application cohorts, respectively). Descriptive patient characteristics of study populations in the three cohorts are shown in Table 1.
Figure 1.
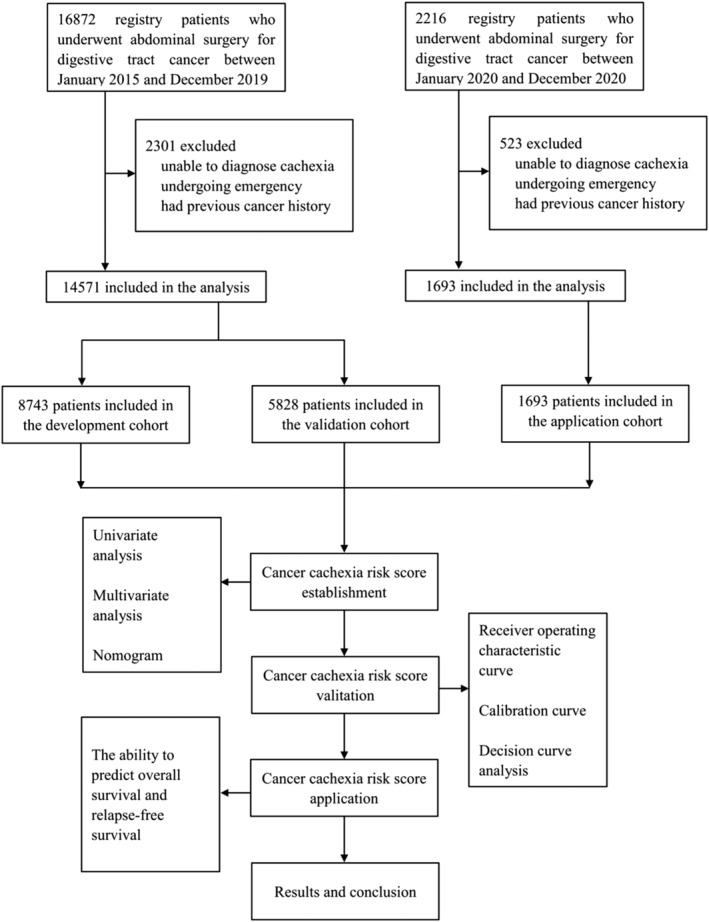
Study profile.
Table 1.
Participant characteristics of three cohorts.
| Development cohort (n = 8743) | Validation cohort (n = 5828) | Application cohort (n = 1693) | |
|---|---|---|---|
| Age, years | 64 (15) | 64 (16) | 64 (14) |
| Sex | |||
| Male | 5731 (65.5%) | 3903 (67.0%) | 1081 (63.9%) |
| Female | 3012 (34.5%) | 1925 (33.0%) | 612 (36.1%) |
| Smoking status | |||
| Never | 7310 (83.6%) | 4926 (84.5%) | 1401 (82.8%) |
| Former/current | 1433 (16.4%) | 902 (15.5%) | 292 (17.2%) |
| Drinking status | |||
| Never | 7589 (86.8%) | 5066 (86.9%) | 1470 (86.8%) |
| Former/current | 1154 (13.2%) | 762 (13.1%) | 223 (13.2%) |
| Co‐morbidity | 2563 (29.3%) | 1735 (29.8%) | 513 (30.3%) |
| Respiratory co‐morbidity | 82 (0.9%) | 50 (0.9%) | 23 (1.4%) |
| Cardiovascular co‐morbidity | 2207 (25.2%) | 1484 (25.5%) | 446 (26.3%) |
| Diabetes | 763 (8.7%) | 538 (9.2%) | 156 (9.2%) |
| Time from symptom onset to hospitalization, month | 1 (2.5) | 1(2.5) | 1 (3.5) |
| Neutrophil‐lymphocyte ratio | 2.02 (1.3) | 2.03 (1.31) | 2.2 (1.46) |
| Platelet‐lymphocyte ratio | 134.17 (79.31) | 134.05 (81.07) | 136.81 (85.15) |
| Albumin, g/L | 41.1 (5.4) | 41.2 (5.5) | 41.0 (5.8) |
| Haemoglobin, g/L | 128 (28) | 128 (28) | 126 (26) |
| Weight, kg | 64 (15) | 63 (19) | 64 (15) |
| BMI, kg/m2 | 23.24 (4.26) | 23.01 (4.99) | 23.18 (4.09) |
| SMI, cm2/m2 | 46.07 (11.22) | 45.93 (11.04) | 45.78 (12.57) |
| Appetite loss | 1828 (20.9%) | 1250 (21.4%) | 366 (21.6%) |
| Cancer site | |||
| Liver | 1385 (15.8%) | 989 (17.0%) | 216 (12.8%) |
| Gallbladder | 532 (6.1%) | 361 (6.2%) | 74 (4.4%) |
| Pancreas | 361 (4.1%) | 242 (4.2%) | 78 (4.6%) |
| Stomach | 3043 (34.8%) | 2006 (34.4%) | 566 (33.4%) |
| Colorectum | 3422 (39.1%) | 2230 (38.3%) | 759 (44.8%) |
| Cancer stage | |||
| I | 2551 (29.2%) | 1690 (29.0%) | 494 (29.2%) |
| II | 3056 (35.0%) | 2068 (35.5%) | 551 (32.5%) |
| III | 2659 (30.4%) | 1750 (30.0%) | 464 (27.4%) |
| IV | 477 (5.5%) | 320 (5.5%) | 184 (10.9%) |
| Histologic type | |||
| Differentiated | 4346 (49.7%) | 2880 (49.4%) | 836 (49.4%) |
| Undifferentiated | 4397 (50.3%) | 2948 (50.6%) | 857 (50.6%) |
| Abnormal tumour biomarker | 7912 (90.5%) | 5276 (90.5%) | 1541 (91.0%) |
| Cachexia | 1377 (15.7%) | 911 (15.6%) | 411 (24.3%) |
Note: Data are median (IQR) or n (%).
Abbreviations: BMI, body mass index; SMI, skeletal muscle index.
Univariate analysis identified 12 factors linked to cancer cachexia (P < 0.05) in the development cohort, and they were subsequently subjected to multivariate analysis. Cachexia risk was independently and significantly (all P < 0.05) linked to cancer site, cancer stage, time from symptom onset to hospitalization, appetite loss, BMI, SMI, and neutrophil‐lymphocyte ratio (NLR) (Table 2). Figure 2 shows the nomogram for the cancer cachexia risk score. ROC analysis was used to determine the appropriate cutoff value of 0.18 based on the predictive probability of cancer cachexia for each patient.
Table 2.
Univariate and multivariate analyses for cancer cachexia in the development cohort.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, years | 1.014 (1.009–1.020) | 0.000 | ||
| Sex, male | 1.103 (0.976–1.247) | 0.117 | ||
| Smoke, former/current | 1.119 (0.961–1.302) | 0.147 | ||
| Drink, former/current | 1.017 (0.859–1.205) | 0.845 | ||
| Co‐morbidity, yes | 0.964 (0.849–1.095) | 0.576 | ||
| Respiratory co‐morbidity, yes | 1.511 (0.893–2.557) | 0.124 | ||
| Cardiovascular co‐morbidity, yes | 0.997 (0.873–1.139) | 0.968 | ||
| Diabetes, yes | 0.903 (0.732–1.114) | 0.340 | ||
| Time from symptom onset to hospitalization, month | 1.220 (1.188–1.254) | 0.000 | 1.143 (1.109–1.178) | 0.000 |
| Neutrophil‐lymphocyte ratio | 1.105 (1.081–1.128) | 0.000 | 1.094 (1.069–1.119) | 0.000 |
| Platelet‐lymphocyte ratio | 1.003 (1.002–1.003) | 0.000 | ||
| Albumin, g/L | 0.956 (0.942–0.969) | 0.000 | ||
| Haemoglobin, g/L | 0.987 (0.985–0.989) | 0.000 | ||
| Weight, kg | 0.953 (0.948–0.959) | 0.000 | ||
| BMI, kg/m2 | 0.831 (0.815–0.848) | 0.000 | 0.874 (0.854–0.895) | 0.000 |
| SMI, cm2/m2 | 0.941 (0.934–0.948) | 0.000 | 0.977 (0.968–0.986) | 0.000 |
| Appetite loss, yes | 1.988 (1.750–2.259) | 0.000 | 1.589 (1.384–1.825) | 0.000 |
| Cancer site | Reference | Reference | ||
| Liver | 4.739 (3.369–6.668) | 0.000 | 3.925 (2.755–5.592) | 0.000 |
| Gallbladder | 8.229 (5.813–11.648) | 0.000 | 7.788(5.418–11.193) | 0.000 |
| Pancreas | 6.176 (4.702–8.111) | 0.000 | 4.984 (3.756–6.615) | 0.000 |
| Stomach | 3.430 (2.601–4.523) | 0.000 | 2.679 (2.012–3.568) | 0.000 |
| Colorectum | ||||
| Cancer stage, n (%) | ||||
| I | Reference | Reference | ||
| II | 1.342 (1.138–1.583) | 0.000 | 1.311 (1.100–1.563) | 0.002 |
| III | 2.448 (2.091–2.866) | 0.000 | 1.959 (1.654–2.320) | 0.000 |
| IV | 3.136 (2.466–3.988) | 0.000 | 2.496 (1.915–3.253) | 0.000 |
| Histologic type, differentiated | 1.084 (0.966–1.217) | 0.168 | ||
| Abnormal tumour biomarker, yes | 1.182 (0.962–1.452) | 0.112 | ||
Abbreviations: BMI, body mass index; SMI, skeletal muscle index; OR, odds ratio; CI, confidence interval.
Figure 2.
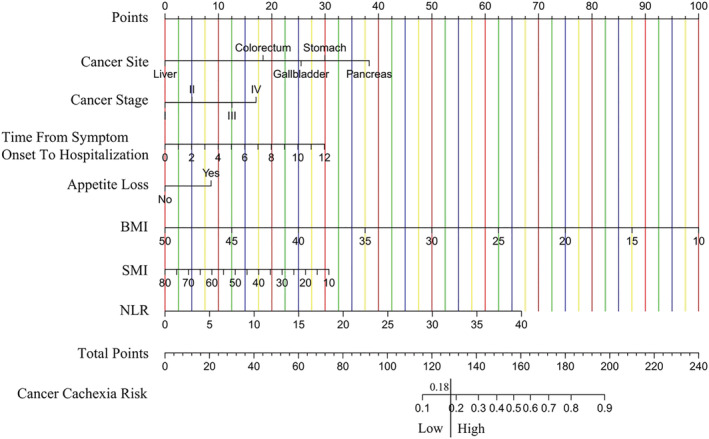
The risk score to identify cancer cachexia in digestive tract cancer before abdominal surgery.
ROC curves were drawn, and AUC was generated to analyse the risk score's capacity to discriminate (Figure 3), demonstrating that the risk score had an AUC value of 0.760 (95% CI 0.747–0.774, P < 0.001), 0.743 (95% CI 0.726–0.761, P < 0.001), and 0.751 (95% CI 0.725–0.777, P < 0.001) in development, validation, and application cohorts, respectively. This indicates that the risk score can distinguish between individuals at risk of cancer cachexia and those who are not.
Figure 3.
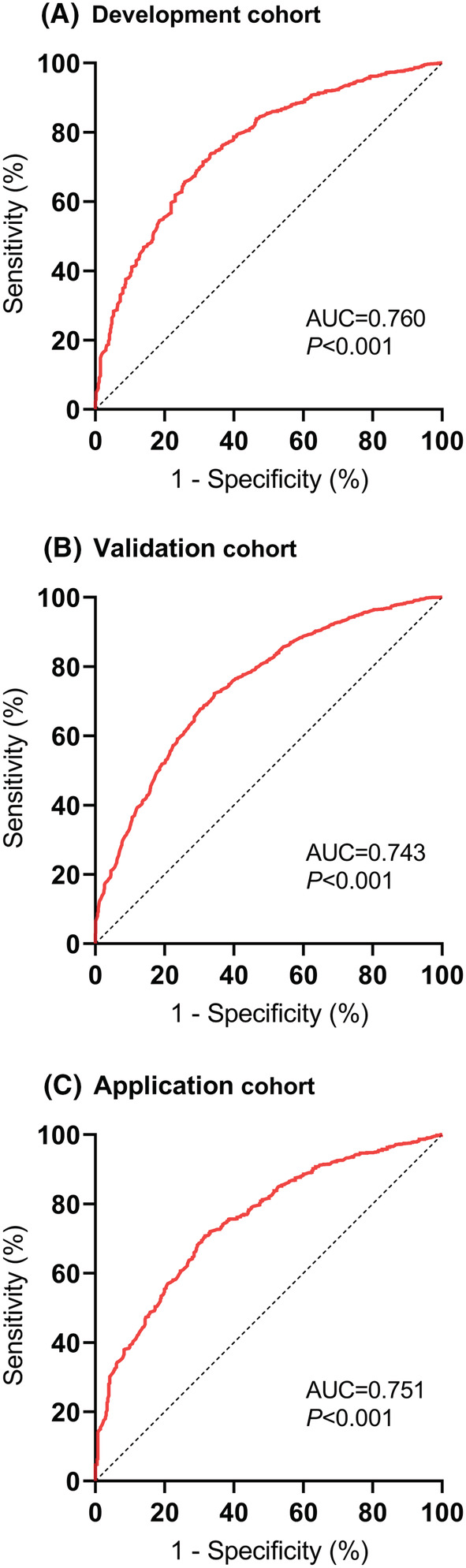
Receiver‐operating characteristic (ROC) curves of the risk score to identify cancer cachexia in the development cohort (A), validation cohort (B), and application cohort (C).
The calibration of the risk score was assessed through the calibration curve using Hosmer–Lemeshow test. The calibration curve demonstrated agreement between the prediction and the observation (Figure 4), and Hosmer–Lemeshow test indicated insignificant statistics (all P > 0.05) in the three cohorts, showing that there was no deviation from the perfect fit and the risk score had an excellent calibration. The decision curve analysis reported the net benefits of the risk score across a range of risk thresholds in the three cohorts (Figure 5).
Figure 4.
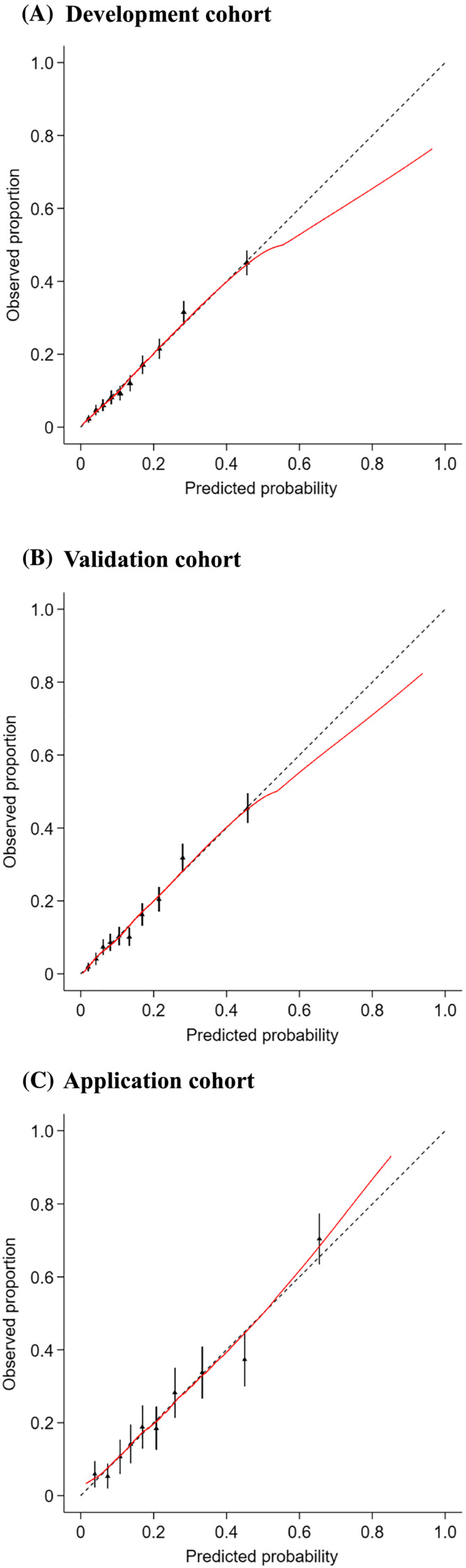
Calibration plots for the risk score to identify cancer cachexia in the development cohort (A), validation cohort (B), and application cohort (C).
Figure 5.
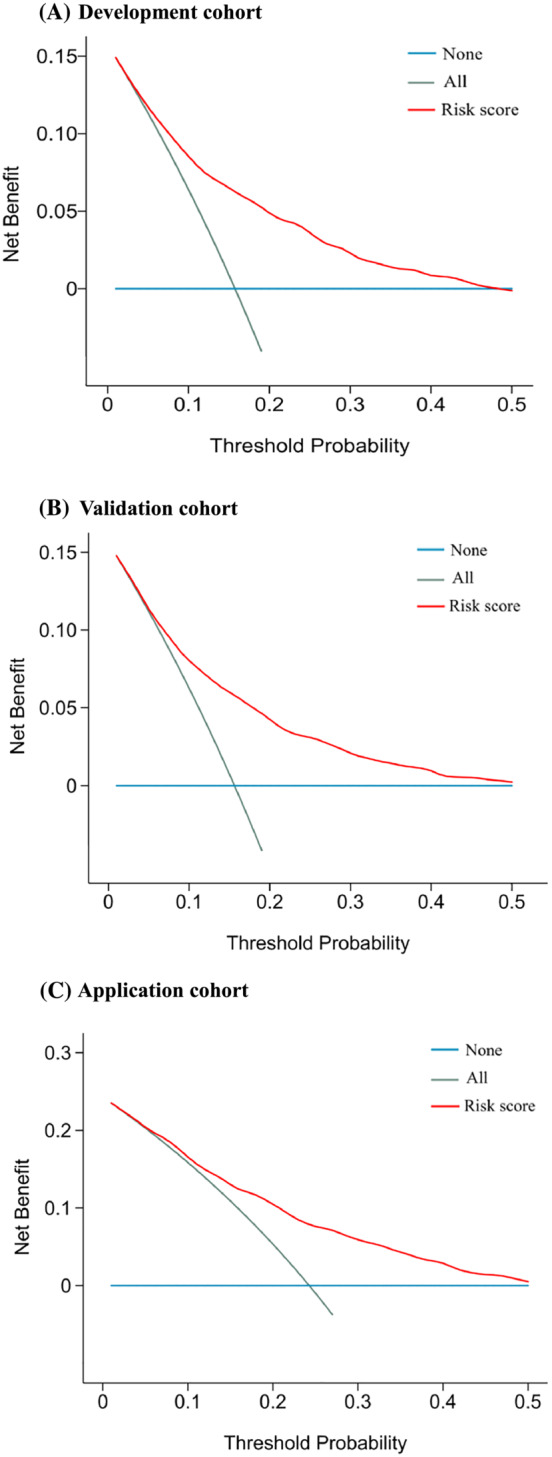
Decision curve analysis plots for the risk score to identify cancer cachexia in the development cohort (A), validation cohort (B), and application cohort (C).
Low‐ and high‐risk groups were defined in the application cohort according to the cutoff value of predictive probability (0.18), and the observed prevalence rates of cancer cachexia for these two groups were 12.5% and 42.3%, respectively (P < 0.001). The low‐risk group, compared to the high‐risk group, had significantly longer overall survival [hazard ratio (HR) 2.887, 95% CI 1.513–5.508, P < 0.001], as well as relapse‐free survival (HR 1.482, 95% CI 1.087–2.020, P = 0.01) (Figure 6). In multivariate Cox regression analyses (Table 3A, 3B), the risk score was found to be an independent prognostic factor for overall survival (HR 7.797, 95% CI 1.431–42.477, P = 0.018) and relapse‐free survival (HR 4.793, 95% CI 1.798–12.779, P = 0.002).
Figure 6.
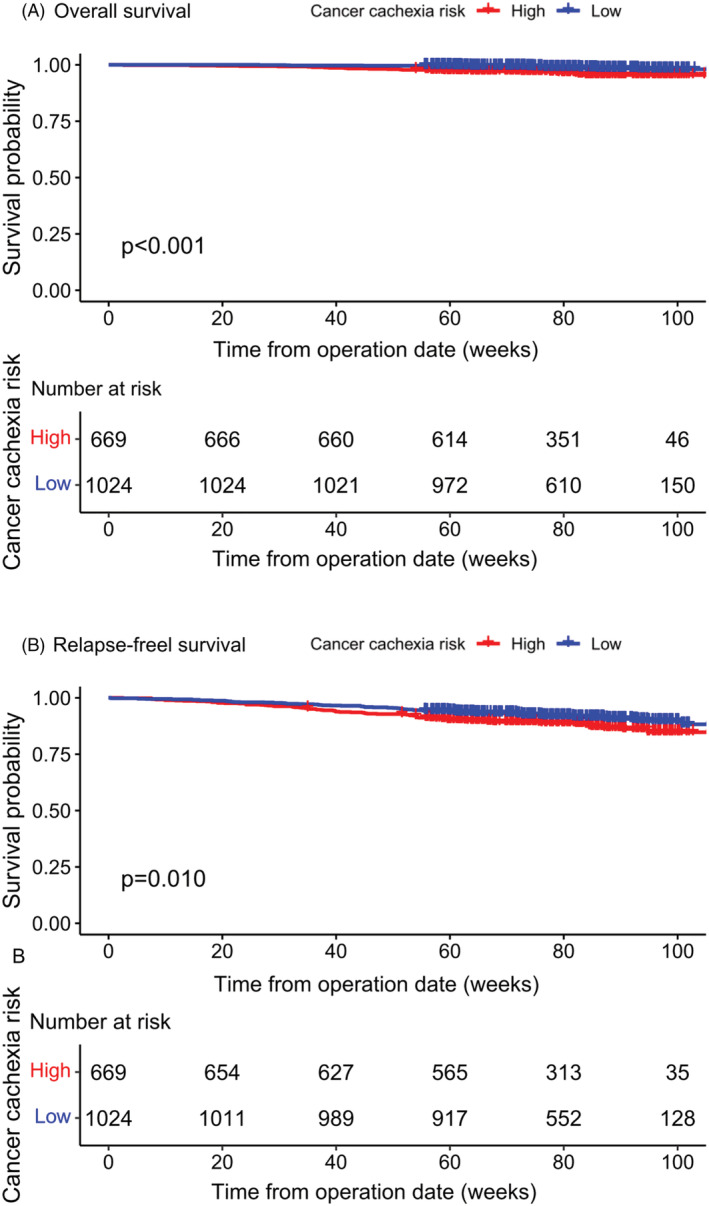
Kaplan–Meier curves for overall survival (A) and relapse‐free survival (B), stratified by risk of cancer cachexia in the application cohort.
Table 3A.
Univariate and multivariate analyses for overall survival in the application cohort.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | 1.034 (1.005–1.063) | 0.022 | 1.031 (1.003–1.060) | 0.027 |
| Sex, male | 1.283 (0.590–2.786) | 0.529 | ||
| Smoke, former/current | 1.087 (0.485–2.439) | 0.839 | ||
| Drink, former/current | 2.093 (0.648–6.760) | 0.217 | ||
| Co‐morbidity, yes | 0.685 (0.338–1.386) | 0.293 | ||
| Respiratory co‐morbidity, yes | 1.104 (0.955–1.278) | 0.182 | ||
| Cardiovascular co‐morbidity, yes | 0.721 (0.346–1.500) | 0.381 | ||
| Diabetes, yes | 0.730 (0.226–2.358) | 0.599 | ||
| Time from symptom onset to hospitalization, month | 1.116 (0.986–1.263) | 0.083 | ||
| Neutrophil‐lymphocyte ratio | 1.099 (1.007–1.201) | 0.035 | ||
| Platelet‐lymphocyte ratio | 1.001 (0.999–1.003) | 0.459 | ||
| Albumin, g/L | 0.919 (0.860–0.981) | 0.012 | 0.931 (0.872–0.994) | 0.032 |
| Haemoglobin, g/L | 0.985 (0.972–0.998) | 0.026 | ||
| Weight, kg | 0.973 (0.945–1.002) | 0.064 | ||
| BMI, kg/m2 | 0.921 (0.856–0.992) | 0.030 | ||
| SMI, cm2/m2 | 0.957 (0.923–0.992) | 0.017 | 0.962 (0.927–0.997) | 0.034 |
| Appetite loss, yes | 1.142 (0.289–4.512) | 0.850 | ||
| Cancer site | ||||
| Liver | Reference | Reference | ||
| Gallbladder | 6.910 (2.128–22.438) | 0.001 | 5.202 (1.560–17.351) | 0.007 |
| Pancreas | 4.544 (1.280–16.131) | 0.019 | 5.250(1.460–18.876) | 0.011 |
| Stomach | 1.147 (0.370–3.556) | 0.812 | 0.882 (0.268–2.907) | 0.836 |
| Colorectum | 0.910 (0.297–2.791) | 0.869 | 0.585 (0.185–1.851) | 0.362 |
| Cancer stage, n (%) | ||||
| I | Reference | Reference | ||
| II | 2.365 (0.627–8.915) | 0.204 | 3.348 (0.878–12.763) | 0.077 |
| III | 6.041 (1.770–20.613) | 0.004 | 9.093 (2.551–32.406) | 0.001 |
| IV | 14.828 (4.320–50.889) | 0.000 | 18.670 (5.397–64.580) | 0.000 |
| Histologic type, differentiated | 0.621 (0.338–1.139) | 0.124 | ||
| Abnormal tumour biomarker, yes | 1.989 (0.481–8.219) | 0.342 | ||
| Cancer cachexia risk score | 22.533 (4.886–103.930) | 0.000 | 7.797 (1.431–42.477) | 0.018 |
Abbreviations: BMI, body mass index; SMI, skeletal muscle index; HR, hazard ratio; CI, confidence interval.
Table 3B.
Univariate and multivariate analyses for relapse‐free survival in the application cohort.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | 1.032 (0.983–1.084) | 0.204 | ||
| Sex, male | 1.025 (0.976–1.076) | 0.327 | ||
| Smoke, former/current | 1.183 (0.827–1.693) | 0.358 | ||
| Drink, former/current | 1.048 (0.663–1.655) | 0.841 | ||
| Co‐morbidity, yes | 0.818 (0.580–1.153) | 0.251 | ||
| Respiratory co‐morbidity, yes | 1.379 (0.440–4.320) | 0.581 | ||
| Cardiovascular co‐morbidity, yes | 0.814 (0.567–1.168) | 0.264 | ||
| Diabetes, yes | 0.820 (0.465–1.443) | 0.490 | ||
| Time from symptom onset to hospitalization, month | 1.001 (0.954–1.050) | 0.975 | ||
| Neutrophil‐lymphocyte ratio | 1.478 (1.061–2.060) | 0.021 | ||
| Platelet‐lymphocyte ratio | 1.000 (0.999–1.002) | 0.656 | ||
| Albumin, g/L | 0.989 (0.981–0.998) | 0.021 | ||
| Haemoglobin, g/L | 0.996 (0.989–1.002) | 0.193 | ||
| Weight, kg | 0.990 (0.976–1.004) | 0.154 | ||
| BMI, kg/m2 | 0.809 (0.596–1.098) | 0.173 | ||
| SMI, cm2/m2 | 0.977 (0.959–0.995) | 0.011 | 0.978 (0.960–0.997) | 0.025 |
| Appetite loss, yes | 1.564 (1.097–2.230) | 0.013 | ||
| Cancer site | ||||
| Liver | Reference | Reference | ||
| Gallbladder | 2.025 (1.326–3.094) | 0.001 | 2.927 (1.870–4.584) | 0.000 |
| Pancreas | 2.406 (1.322–4.380) | 0.004 | 2.372(1.296–4.341) | 0.005 |
| Stomach | 1.756 (0.899–3.429) | 0.099 | 2.128 (1.077–4.203) | 0.030 |
| Colorectum | 1.220 (0.844–1.763) | 0.290 | 1.324 (0.911–1.925) | 0.142 |
| Cancer stage, n (%) | ||||
| I | Reference | Reference | ||
| II | 1.262 (0.741–2.149) | 0.392 | 1.386 (0.811–2.370) | 0.233 |
| III | 3.113 (1.937–5.004) | 0.000 | 4.155 (2.528–6.829) | 0.000 |
| IV | 6.297 (3.830–10.352) | 0.000 | 6.659 (4.035–10.989) | 0.000 |
| Histologic type, differentiated | 0.984 (0.970–0.999) | 0.037 | ||
| Abnormal tumour biomarker, yes | 1.237 (0.915–1.674) | 0.167 | ||
| Cancer cachexia risk score | 4.576 (1.843–11.360) | 0.001 | 4.793 (1.798–12.779) | 0.002 |
Abbreviations: BMI, body mass index; SMI, skeletal muscle index; HR, hazard ratio; CI, confidence interval.
4. Discussion
In the present study with 16 264 patients, a cancer cachexia risk score, comprising the variables cancer site, cancer stage, time from symptom onset to hospitalization, appetite loss, BMI, SMI, and NLR, was developed and validated to identify patients with cancer cachexia risk and who are at risk of adverse survival in digestive tract cancer patients before abdominal surgery. The cancer cachexia risk score demonstrated reasonably good discrimination and calibration, had net benefits across a range of risk thresholds, and predicted adverse postoperative overall survival and relapse‐free survival. To our knowledge, this is the largest study of cancer cachexia involving most types of digestive tract cancer before abdominal surgery, and the first risk score for identifying patients with risk of cancer cachexia and who are at risk of adverse survival using readily available clinical data in digestive tract cancer patients before surgery.
Malnutrition is a global public health concern due to its adverse impact on patients' treatment and clinical outcomes, and a variety of methods such as Malnutrition Screening Tool, Malnutrition Universal Screening Tool, and Nutritional Risk Screening‐2002 have been established to screen and assess the risk of malnutrition to guide treatment. 23 Nutritional alterations are common in patients with cancer, especially those with digestive system cancer.
Due to the complexities of the catabolic pathway activation and the predominance of muscle loss in the pathophysiology of cancer‐related nutritional abnormalities, the term ‘cachexia’ is currently preferred to the previous term ‘malnutrition’ for patients with cancer. 12 , 24 , 25 , 26 Patients with digestive tract cancer are more likely to develop cancer cachexia, which is associated with poor outcomes, although little research has specifically addressed cancer cachexia. Few methods have been constructed specifically to determine the risk of cancer cachexia, despite cancer cachexia being a form of malnutrition. Therefore, identifying individuals at high risk of cancer cachexia and poor survival is critical for treatment planning and improving the prognosis of those with digestive tract cancer.
There is a wide spectrum of symptoms associated with cancer cachexia, ranging from non‐symptomatic inflammatory alterations with minimal weight and muscle loss in the early stages to severe muscle wasting and low‐performance status in the later stages; Consequently, a screening tool for cancer cachexia based on the maximum number of factors is required rather than one subset of these factors for better screening results. This large‐scale cohort study involving 16 264 patients in a high‐volume surgery centre for digestive tract cancer in China was conducted to uncover independent characteristics that are strongly linked with cancer cachexia.
Seven risk factors associated with cancer cachexia were identified, including cancer site, cancer stage, time from symptom onset to hospitalization, appetite loss, BMI, SMI, and NLR. Notably, these variables data can be collected from the medical records of patients with digestive tract cancer before abdominal surgery in the hospital. It is of particular significance because clinicians can use such routine data to assess these risk factors to identify patients at risk of cancer cachexia.
Nutritional variables such as weight, BMI, and serum albumin are commonly used to assess nutritional status and identify cancer cachexia risk in clinical practice, but each parameter has different merits. Some reports have demonstrated that some variables such as low BMI and low serum albumin were risk factors for cancer cachexia and were associated with more complications and poor survival after surgery. 7 , 11 , 27 Patients with cancer who have a low haemoglobin level are more likely to die from their disease. In patients with cancer cachexia, low haemoglobin levels have been linked to death, 28 and haemoglobin can be used as a predictive indicator for prognosis. However, some reports stated negative results regarding the assessment of nutritional status, including cancer cachexia using these nutritional variables. 29 , 30 In this study, we found that variables of weight, serum albumin, and haemoglobin were unrelated to cancer cachexia, while a low BMI was an independent risk factor for cancer cachexia, similar to earlier research. 31 Patients with cancer cachexia often have a loss of appetite and reduced food intake, which affects their nutritional status and quality of life. 32 , 33 , 34 In this study, we found that appetite loss was another independent risk factor for cancer cachexia. Notably, in clinical practice, most patients could inaccurately describe the range of appetite changes; therefore, in this study, we used the loss of appetite or not as an assessment indicator, confirming that this indicator can be used as a cancer cachexia risk predictor, which greatly improves the practicability of the clinical application. Cancer cachexia is also characterized by the decrease of skeletal muscle mass, which can be used to identify cancer cachexia risk. In this study, we also found that low skeletal muscle mass is an independent risk factor for cancer cachexia; consequently, in clinical practice, if low skeletal muscle mass is observed, clinicians should be alerted to cancer cachexia.
Cancer is the major cause of cachexia; accordingly, it is important to investigate cancer‐related factors to evaluate the risk of cachexia in cancer patients. 5 , 26 , 29 For example, many studies have revealed that the incidence of cancer cachexia varies in different cancer sites and stages, and cachexia was associated with clinical outcomes independently of these factors. 3 , 11 , 20 In this study, we found that cancer stage and site are significantly correlated with cancer cachexia, in line with other studies. 3 , 11 , 20 Cancer patients often present with abnormal tumour biomarkers, especially those with advanced cancer; therefore, detecting tumour biomarkers is routine in cancer management. During the study design, we raised the question of whether abnormal tumour biomarkers can be used as a predictor of cachexia, but there was no correlation between abnormal tumour biomarkers and cachexia. In addition, the results demonstrated that tumour histologic type, differentiated or not, has no significant impact on cancer cachexia. It is hypothesized that this may be because the histologic type is not independently associated with the patient's metabolism status, thus having no significant impact on weight loss and cachexia. Considering these factors, these cancer‐related indicators such as cancer stage and site can be used to assess the cancer cachexia risk in patients with digestive tract cancer before abdominal surgery.
Systemic inflammation is another important feature of cancer, which can promote catabolism and is one of the causes of body consumption. 32 , 35 , 36 Previous studies also assessed inflammation when assessing the cachexia stage in cancer patients. 37 , 38 The commonly used inflammation indicators are C‐reactive protein, interleukin, and so on, but these indicators are uncommonly used in the clinic, which will limit their clinical use. NLR has recently been considered a new indicator for inflammation assessment and can be obtained simply from a blood routine test. A previous study reported that NLR was a significant negative prognostic biomarker for patients with cachexia. 35 In this study, NLR was an independent risk factor for cancer cachexia; therefore, it is convenient to assess inflammation in clinical practice, especially for cachexia risk assessment.
Notably, the variable time from symptom onset to hospitalization for cancer was an important factor in the cancer cachexia risk. Due to economic, cultural, and social factors, when patients suffer from cancer, the time from symptom onset to hospitalization varies between patients, which may lead to varying degrees of cancer progression, different nutritional status at hospital admission, as well as mood and other factors, which will significantly affect cachexia. Thus, assessing the time from symptom onset to hospitalization is an important factor in identifying cancer cachexia in the present study, although it has not been investigated previously. As expected, the time from symptom onset to hospitalization is a risk factor for cancer cachexia, suggesting that patients should visit their doctors as soon as possible if they are unwell, and clinicians should be highly vigilant for cachexia in those patients with advanced cancer.
The seven risk factors were then used to construct a nomogram of the cancer cachexia risk score, which was validated in the three cohorts displaying good discrimination and excellent calibration. The decision curve analysis also reported the net benefits of the risk score across a range of risk thresholds in the three cohorts. To further assess its application value, low‐ and high‐risk groups were defined in the application cohort according to the cutoff value of predictive probability to test the ability of the score to stratify overall survival and relapse‐free survival in these two risk groups. The results demonstrated that the observed prevalence rates of cancer cachexia were significantly different between the two groups (high‐ and low‐risk). In addition, the low‐risk group's overall survival and relapse‐free survival were much longer than those of the high‐risk group. In multivariate Cox regression analyses, the risk score itself provided independent prognostic value. Therefore, the established and validated score may be used in the clinic to identify patients with cancer cachexia who are at risk of death.
There are several limitations to this study. First, because this research was retrospective, it is prone to bias. For example, functional measures such as grip strength is associated with prognosis in cancer patients, and it may be a potential factor to identify patients with risk of cancer cachexia and who are at risk of adverse survival. However, functional measures such as grip strength is not a routine test in the clinical practice, and this study was retrospective and the number of patients having data on functional measures such as grip strength was small. Therefore, it is regretted that functional measures such as grip strength was not included in the candidate predictor selection. Prospective studies are required to optimize the risk score. Second, this was a single‐centre study, and although the performance of the risk score has been assessed by three different independent cohorts, additional validation at other centers is required to confirm the reliability of this score. Third, this study included a large number of Chinese patients, and further studies on other Asian and non‐Asian populations should be conducted. Consequently, in future studies, this risk score will need to be tested in different clinical settings and countries to confirm whether cancer cachexia can be predicted in digestive tract cancer patients before abdominal surgery.
In conclusion, a cancer cachexia risk score was constructed and validated to identify digestive tract cancer patients who have a risk of cancer cachexia and adverse postoperative survival. Accordingly, this risk score could help clinicians better screen for cancer cachexia, take targeted approaches to better understand the prognosis of cancer cachexia patients, and strengthen early decisions for digestive tract cancer patients before undergoing abdominal surgery.
Conflict of interest
The authors declare that there are no conflicts of interest.
Funding
This study was funded by Shanghai ‘Rising Stars of Medical Talent’ Youth Development Program ([2022]65), Excellent Young Program of Zhongshan Hospital, Fudan University (2021ZSYQ14), and Clinical Research Special Fund of Zhongshan Hospital, Fudan University (2020ZSLC17 and 2020ZSLC53).
Acknowledgements
The first author Dr. Shanjun Tan expresses his deepest love, sincerest gratitude and endless miss to his father, Mr. Weilong Tan, for his cultivating parenting and always encouraging during his life and work. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 39
Tan S., Xu J., Wang J., Zhang Z., Li S., Yan M., et al (2023) Development and validation of a cancer cachexia risk score for digestive tract cancer patients before abdominal surgery, Journal of Cachexia, Sarcopenia and Muscle, 14, 891–902, 10.1002/jcsm.13207
References
- 1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Siff T, Parajuli P, Razzaque MS, Atfi A. Cancer‐Mediated Muscle Cachexia: Etiology and Clinical Management. Trends Endocrinol Metab 2021;32:382–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poisson J, Martinez‐Tapia C, Heitz D, Geiss R, Albrand G, Falandry C, et al. Prevalence and prognostic impact of cachexia among older patients with cancer: a nationwide cross‐sectional survey (NutriAgeCancer). J Cachexia Sarcopenia Muscle 2021;12:1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rich NE, Phen S, Desai N, Mittal S, Yopp AC, Yang JD, et al. Cachexia is Prevalent in Patients With Hepatocellular Carcinoma and Associated With Worse Prognosis. Clin Gastroenterol Hepatol 2022;20:e1157–e1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akezaki Y, Kikuuchi M, Hamada K, Ookura M. Incidence of cachexia in patients with advanced gastrointestinal cancer at the beginning of rehabilitation intervention. J Phys Ther Sci 2020;32:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer Cachexia: Its Mechanism and Clinical Significance. Int J Mol Sci 2021;22:8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wallengren O, Lundholm K, Bosaeus I. Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer 2013;21:1569–1577. [DOI] [PubMed] [Google Scholar]
- 8. da Rocha IMG, Marcadenti A, de Medeiros GOC, Bezerra RA, Rego JFM, Gonzalez MC, et al. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J Cachexia Sarcopenia Muscle 2019;10:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, et al. Management of Cancer Cachexia: ASCO Guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 10. Garcia JM, Dunne RF, Santiago K, Martin L, Birnbaum MJ, Crawford J, et al. Addressing unmet needs for people with cancer cachexia: recommendations from a multistakeholder workshop. J Cachexia Sarcopenia Muscle 2022;13:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines(☆). ESMO Open 2021;6:100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition ‐ An ESPEN Consensus Statement. Clin Nutr 2015;34:335–340. [DOI] [PubMed] [Google Scholar]
- 13. Argilés JM, Betancourt A, Guàrdia‐Olmos J, Peró‐Cebollero M, López‐Soriano FJ, Madeddu C, et al. Validation of the CAchexia SCOre (CASCO). Staging Cancer Patients: The Use of miniCASCO as a Simplified Tool. Front Physiol 2017;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller J, Wells L, Nwulu U, Currow D, Johnson MJ, Skipworth RJE. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: a systematic review. Am J Clin Nutr 2018;108:1196–1208. [DOI] [PubMed] [Google Scholar]
- 15. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr 2021;40:2898–2913. [DOI] [PubMed] [Google Scholar]
- 16. Tan S, Wang J, Zhou F, Tang M, Xu J, Zhang Y, et al. Validation of GLIM malnutrition criteria in cancer patients undergoing major abdominal surgery: a large‐scale prospective study. Clin Nutr 2022;41:599–609. [DOI] [PubMed] [Google Scholar]
- 17. Tan S, Meng Q, Jiang Y, Zhuang Q, Xi Q, Xu J, et al. Impact of oral nutritional supplements in post‐discharge patients at nutritional risk following colorectal cancer surgery: A randomised clinical trial. Clin Nutr 2021;40:47–53. [DOI] [PubMed] [Google Scholar]
- 18. Meng Q, Tan S, Jiang Y, Han J, Xi Q, Zhuang Q, et al. Post‐discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin Nutr 2021;40:40–46. [DOI] [PubMed] [Google Scholar]
- 19. Zhang S, Tan S, Jiang Y, Xi Q, Meng Q, Zhuang Q, et al. Sarcopenia as a predictor of poor surgical and oncologic outcomes after abdominal surgery for digestive tract cancer: A prospective cohort study. Clin Nutr 2019;38:2881–2888. [DOI] [PubMed] [Google Scholar]
- 20. Vagnildhaug OM, Brunelli C, Hjermstad MJ, Strasser F, Baracos V, Wilcock A, et al. A prospective study examining cachexia predictors in patients with incurable cancer. BMC Palliat Care 2019;18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han J, Tang M, Lu C, Shen L, She J, Wu G. Subcutaneous, but not visceral, adipose tissue as a marker for prognosis in gastric cancer patients with cachexia. Clin Nutr 2021;40:5156–5161. [DOI] [PubMed] [Google Scholar]
- 22. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition ‐ A consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Gaafer OU, Zimmers TA. Nutrition challenges of cancer cachexia. JPEN J Parenter Enteral Nutr 2021;45:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stubbins R, Bernicker EH, Quigley EMM. Cancer cachexia: a multifactoral disease that needs a multimodal approach. Curr Opin Gastroenterol 2020;36:141–146. [DOI] [PubMed] [Google Scholar]
- 26. McGovern J, Dolan RD, Skipworth RJ, Laird BJ, McMillan DC. Cancer cachexia: a nutritional or a systemic inflammatory syndrome? Br J Cancer 2022;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sadeghi M, Keshavarz‐Fathi M, Baracos V, Arends J, Mahmoudi M, Rezaei N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit Rev Oncol Hematol 2018;127:91–104. [DOI] [PubMed] [Google Scholar]
- 28. Zhang XW, Zhang Q, Song MM, Zhang KP, Zhang X, Ruan GT, et al. The prognostic effect of hemoglobin on patients with cancer cachexia: a multicenter retrospective cohort study. Support Care Cancer 2022;30:875–885. [DOI] [PubMed] [Google Scholar]
- 29. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 30. Bouillanne O, Hay P, Liabaud B, Duché C, Cynober L, Aussel C. Evidence that albumin is not a suitable marker of body composition‐related nutritional status in elderly patients. Nutrition 2011;27:165–169. [DOI] [PubMed] [Google Scholar]
- 31. Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the Consensus‐Definition for Cancer Cachexia and evaluation of a classification model—a study based on data from an international multicentre project (EPCRC‐CSA). Ann Oncol 2014;25:1635–1642. [DOI] [PubMed] [Google Scholar]
- 32. Martin L, Muscaritoli M, Bourdel‐Marchasson I, Kubrak C, Laird B, Gagnon B, et al. Diagnostic criteria for cancer cachexia: reduced food intake and inflammation predict weight loss and survival in an international, multi‐cohort analysis. J Cachexia Sarcopenia Muscle 2021;12:1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang G, Zhang H, Lyden D. Tumour‐regulated anorexia preceding cachexia. Nat Cell Biol 2021;23:111–1113. [DOI] [PubMed] [Google Scholar]
- 34. Peixoto da Silva S, Santos JMO, Costa ESMP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 2020;11:619–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Q, Song MM, Zhang X, Ding JS, Ruan GT, Zhang XW, et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle 2021;12:1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345–1350. [DOI] [PubMed] [Google Scholar]
- 37. Argilés JM, López‐Soriano FJ, Toledo M, Betancourt A, Serpe R, Busquets S. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle 2011;2:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prokopchuk O, Hermann CD, Schoeps B, Nitsche U, Prokopchuk OL, Knolle P, et al. A novel tissue inhibitor of metalloproteinases‐1/liver/cachexia score predicts prognosis of gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2021;12:378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


