Abstract
Background
Sarcopenia is defined by the progressive and generalized loss of muscle mass and function associated with aging. We have previously proposed that aging‐related hyperphosphataemia is linked with the appearance of sarcopenia signs. Because there are not effective treatments to prevent sarcopenia, except for resistance exercise, we propose here to analyse whether the dietary restriction of phosphate could be a useful strategy to improve muscle function and structure in an animal model of aging.
Methods
Five‐month‐old (young), 24‐month‐old (old) and 28‐month‐old (geriatric) male C57BL6 mice were used. Old and geriatric mice were divided into two groups, one fed with a standard diet (0.6% phosphate) and the other fed with a low‐phosphate (low‐P) diet (0.2% phosphate) for 3 or 7 months, respectively. A phosphate binder, Velphoro®, was also supplemented in a group of old mice, mixed with a standard milled diet for 3 months. Muscle mass was measured by the weight of gastrocnemius and tibial muscles, and quality by nuclear magnetic resonance imaging (NMRI) and histological staining assays. Muscle strength was measured by grip test and contractile properties of the tibialis muscle by electrical stimulation of the common peroneal nerve. Gait parameters were analysed during the spontaneous locomotion of the mice with footprinting. Orientation and motor coordination were evaluated using a static rod test.
Results
Old mice fed with low‐P diet showed reduced serum phosphate concentration (16.46 ± 0.77 mg/dL young; 21.24 ± 0.95 mg/dL old; 17.46 ± 0.82 mg/dL low‐P diet). Old mice fed with low‐P diet displayed 44% more mass in gastrocnemius muscles with respect to old mice (P = 0.004). NMRI revealed a significant reduction in T2 relaxation time (P = 0.014) and increased magnetization transfer (P = 0.045) and mean diffusivity (P = 0.045) in low‐P diet‐treated mice compared with their coetaneous. The hypophosphataemic diet increased the fibre size and reduced the fibrotic area by 52% in gastrocnemius muscle with respect to old mice (P = 0.002). Twitch force and tetanic force were significantly increased in old mice fed with the hypophosphataemic diet (P = 0.004 and P = 0.014, respectively). Physical performance was also improved, increasing gait speed by 30% (P = 0.032) and reducing transition time in the static rod by 55% (P = 0.012). Similar results were found when diet was supplemented with Velphoro®.
Conclusions
The dietary restriction of phosphate in old mice improves muscle quantity and quality, muscle strength and physical performance. Similar results were found using the phosphate binder Velphoro®, supporting the role of phosphate in the impairment of muscle structure and function that occurs during aging.
Keywords: aging, hyperphosphataemia, phosphate binders, sarcopenia, skeletal muscle
Introduction
Aging is an irreversible and universal process that usually leads to the appearance of chronic diseases and conditions associated with loss of functional reserve, frailty, dependence and, eventually, death. Sarcopenia, defined by the European Working Group on Sarcopenia in Older People (EWGSOP), as the progressive and generalized skeletal muscle disorder associated with aging, 1 profoundly affects the quality of life and overall health status of the elderly. Different grades of sarcopenia can be diagnosed when one, two or three of the most important variables of sarcopenia are present: low muscle strength, low muscle mass quantity or quality and low physical performance, 1 which can be detected by using different validated tests and tools. 2
The sarcopenic muscle is characterized by numerous conditions such as type II fibre atrophy, fibre size reduction, infiltration of adipose and fibrotic tissue, decline in mitochondrial function, increased inflammation, 3 , 4 reduction in the number and function of satellite cells and, consequently, an impaired capacity to regenerate after muscle damage. 5
Causes of sarcopenia are not fully understood, but several factors have been proposed, notably a decrease in the levels of sexual hormones, reduced physical activity levels, low dietary protein intake or even chronic inflammation. 1 In this sense, we have previously proposed hyperphosphataemia as a potential important factor in the development of sarcopenia. Serum phosphate concentration rises with aging due to the disturbance of the Klotho/FGF23 axis. 6 We have demonstrated a link between hyperphosphataemia and some of the signs characterizing sarcopenia, because we demonstrated that hyperphosphataemia induces myoblast senescence and diminishes its myogenic differentiation, avoiding the correct regeneration process of skeletal muscle and favouring the appearance of fibrosis. 7 , 8 In this way, hyperphosphataemia might contribute to the loss of muscle strength and mass in old mice. However, a direct link between sarcopenia and hyperphosphataemia remains to be clarified.
Strategies improving sarcopenia signs include the combination of resistance‐based training and some nutritional interventions, like the increase in protein and caloric intake, but evidence is still not very consistent due to the heterogeneity of the performed studies. 9 The present work proposes different strategies to reduce serum phosphate levels to improve signs of sarcopenia in old mice.
The aim of this work was to evaluate whether the reduction of serum phosphate levels, by means of dietary phosphate restriction or through supplementation with phosphate binders, improves sarcopenia indicators (skeletal muscle mass and quality, muscle strength and physical performance) in an animal model of aged mice.
Methods
Animal studies
We describe the study design following the ARRIVE guideline. 10 Male C57BL6 mice, purchased from Janvier Laboratories, were used. To analyse the effect of low‐phosphate diet on sarcopenic signs, we compared three experimental conditions: young mice (5 months) fed with a pelleted standard diet (Tekland global 14% protein, provided for INVIGO, IN, USA, containing 0.6% of phosphate), old mice (24 months) fed with the same pelleted standard diet and old mice fed with a pelleted hypophosphataemic diet (EF Low Phosphate S9723‐E020 provided for Sniff, Spezialdiäten GmbH, containing 0.2% of phosphate) for the last 3 months of life. Diets composition is described in Table S1 . This experimental design was replicated in three cohorts with sample sizes of 16, 31 and 22 animals, respectively.
To analyse the effect of low‐phosphate diet in geriatric mice (28 months), we compared animals fed with a pelleted standard diet to mice fed with a pelleted low‐phosphate diet for the last 7 months of life. This experiment was made in only one cohort of 12 animals.
To analyse the effect of a phosphate binder (sucroferric oxyhydroxide powder: Velphoro®, Vifor Pharma, Zurich, Switzerland), we created four experimental conditions: young mice (5 months) fed with a standard milled diet, old mice (24 months) fed with the standard milled diet, old mice fed with milled low‐phosphate diet and old mice fed with a standard milled diet supplemented with 5% Velphoro® during the last 3 months of life. This experiment was performed in only one cohort of 34 mice.
Animals were randomly housed in cages with an average number of animals per cage of three (range one to five). Cages contained environmental enrichment items, such as nesting material (cotton string and cardboard shelters) and wood chips. Cages were placed in pathogen‐free rooms with controlled temperature (24°C) and lighting (12:12‐h light–dark cycle) and receiving food and water ad libitum.
After treatment, arterial pressure was measured as described in the Supplementary Methods section of the supporting information and muscle strength and physical performance were analysed as described below. Blood glucose was measured via tail bleeding using a glucometer (Accu‐Check Aviva; Roche). No fasting was performed in any case. At the time of euthanasia, mice were anaesthetized, blood samples were obtained by cardiac puncture and serum was collected after centrifugation (2200 g, 10 min). Gastrocnemius and tibialis muscles were collected, flash‐frozen in liquid nitrogen‐cooled isopentane and stored at −80°C for histological studies. Serum phosphate levels were measured using a commercial kit (quantiChrom Phosphate Assay Kit [DIPI‐500]) according to the manufacturer's instructions.
The study design and the experimental protocols were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85‐23, revised 1996) and with the European Union and national regulations (EU Directive 2010/63/EU, the Spanish State law 3/2007 on animal care and the Royal Decrees RD1201/2005 and RD53/2013 on the protection of experimental animals and other scientific purposes). The study was revised and accepted by the Ethics Committee from the University of Alcalá for mice (Madrid, Spain) and by the Community of Madrid (PROEX 210/17).
Nuclear magnetic resonance imaging
Skeletal muscle quality was assessed by nuclear magnetic resonance imaging (NMRI). Experiments were performed on a Bruker Pharmascan 7.0‐T horizontal‐bore system (Bruker Medical GmbH, Ettlingen, Germany) equipped with a 1H selective birdcage resonator of 23 mm and a Bruker gradient insert with 90 mm of diameter (maximum intensity of 30 G/cm), at the Institute of Biomedical Research Alberto Sols CSIC‐UAM (Madrid, Spain). All data were acquired using a Hewlett‐Packard console running Paravision software (Bruker Medical GmbH) operating on a Linux platform. The entire procedure was performed under general anaesthesia by inhalation of 2% isoflurane in O2. Mice temperature was maintained at 37°C with a heated probe and the respiratory rate of the animals was monitored using a Biotrig physiological monitor (Bruker). For the specific procedure of data acquisition, see the supporting information.
Quantitative RT‐PCR
Total RNA from quadriceps muscles were isolated using TRIzol reagent according to the manufacturer's protocol. cDNA was synthesized using a High‐Capacity cDNA reverse transcription kit. The expression of atrogin‐1 and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was measured by quantitative PCR using Taqman genes: atrogin‐1 (Mm00499523_m1) and the endogenous control GAPDH (Mm99999915_g1). The ABI Prism 7500 Fast Real‐Time PCR System was used and analysed with 7500 Fast sequence detection software v1.3.1 (Applied Biosystems Inc., Foster City, CA, USA) with the double delta Ct method.
Histological staining
For histological analysis, 10‐μm serial transverse sections of frozen gastrocnemii were cut at −20°C by cryostat (Leica CM3000 Cryostat, Leica Biosystems, Nussloch, Germany), mounted on glass slides and stored at −80°C until assayed. Samples were stained with haematoxylin and eosin for measuring the fibre cross‐sectional area (FCSA), with a staining solution containing succinate for calculating the percentage of oxidative fibres, with fast or slow myosin heavy chain (MHC) antibodies for detecting the muscle fibre type or with the Picro‐Sirius Red Stain Kit (Abcam, Cambridge, UK) for analysing the amount of connective tissue. For the specific procedure of the staining assays, see the supporting information.
Western blot assays
Protein content was obtained from quadriceps muscles as previously described. 8 In brief, blots were incubated with different primary antibodies O/N at 4°C in Tween Tris‐buffered saline (TTBS) (20‐mM Tris–HCl pH 7.5, 0.9% NaCl, 0.05% Tween 20 with 3% bovine serum albumin [BSA]), using 1:1000 dilution. Finally, blots were reblotted with a mouse anti‐GAPDH (Merck Life Science, Madrid, Spain) antibody in order to normalize protein levels. Primary antibodies (myomesin‐2, vimentin, MyoD and HDAC‐1) were obtained from Santa Cruz Biotechnology, TX, USA.
Neuromuscular electrical stimulation of tibialis anterior
Contractile properties of tibialis anterior muscle were assessed by electrical stimulation of common peroneal nerve with two needle electrodes. First, mice were anaesthetized by inhalation of O2 containing 2% isoflurane, and placed in a supine position on a circulating heat‐water blanket to maintain core body temperature. The tendon of the tibialis muscle was tied to a force transducer (TRI201, Letica Scientific Instruments, Spain) using suture thread. The optimal length of the muscle (tension that enables the strongest contraction in response to an electrical twitch) was established and kept steady during all the measurements for each mouse. Strength measures were obtained with a 16‐bit analogue‐to‐digital converter (PowerLab/16SP; AD Instruments, UK) using the Power Lab Chart 5 Software (AD Instruments). The following parameters were evaluated: twitch force evoked by a single electrical stimulus (pulse width 0.3 ms, voltage 20 V); maximal tetanic force evoked through a train of high‐frequency stimuli (pulse width 0.3 ms, voltage 20 V, frequency 100 Hz, 30 pulses); and fatigue resistance, measured as the time until which force decreased below 50% of the maximal force during a long‐duration tetanic contraction (pulse width 0.3 ms, voltage 20 V, frequency 60 Hz, 1200 pulses). After the procedure, tibialis muscles were removed, and mice were sacrificed.
Gait test
Gait parameters were analysed during the spontaneous locomotion of the mice along a catwalk 60 cm long and 3 cm wide covered with absorbent paper. Non‐toxic ink was used to stain the mouse footprint. After familiarization, each animal was tested in four free walks interspersed by 5‐min intervals. Gait speed was estimated from time and distance, and stride length and hind paw base width were measured analysing the spatial pattern of the ink footprints.
Static rod test
Orientation and motor coordination were evaluated using a wooden rod 60 cm long and 28 mm in diameter fixed to a laboratory bench 60 cm above a padded surface, with a mark 10 cm from the supported end to indicate the finish line. Mice were placed on the ‘open’ end of the cantilever rod facing away from the bench. After familiarization, animals were tested three times interspersed by 5‐min intervals. Two parameters were measured: orientation time (time taken to rotate 180° from the initial position and face the supported end) and transition time (time taken to reach the finish line). If a mouse fell twice during the orientation or transition times, a score of 25 s was assigned.
Forelimb grip strength test
The Grip Strength Meter (UGO BASILE) purchased from PSYMTEC (Madrid, Spain) was used for measuring forelimb grip strength. For the procedure, the animal was placed over the device in front of the grasping bar attached to a force transducer that is connected to an amplifier for monitoring data. Then, it is allowed to grasp the bar and pulled by its tail backwards in the horizontal plane. Each animal was tested in three trials of five consecutive attempts interspersed by 1‐min intervals. Grip strength of each mouse was the result of dividing the mean of all its peak forces data per its body weight.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 software (GraphPad Prism Software Inc., San Diego, CA, USA) and Stata (StataCorp. 2021, Stata Statistical Software: Release 16, College Station, TX, USA). Results are expressed as mean ± standard error of the mean in tables or as box plot graphs.
To account for cage and cohort effects, we analysed data using multilevel linear mixed effects models with cohort and experimental groups as fixed effects and cage as random intercepts. Between‐group comparisons for NMRI were made using the analysis of variance (ANOVA) test followed by the Bonferroni post hoc tests for multiple comparisons. Correlations were performed using the Pearson correlation test. Differences were considered statistically significant at P < 0.05. No adjustments were made for multiple comparisons.
The cage effect was summarized as intraclass correlation coefficients (ICCs), which ranges between 0 and 1. The higher the ICC, the greater the correlation between experimental units within a cage. Because the number of animals per cage varied between one and five, in some instances, it was not possible to estimate this cage effect.
Results
Dietary phosphate restriction reduced serum phosphate concentration in old mice
Twenty‐four‐month‐old mice showed higher serum phosphate concentration than young mice (Table 1 ). To assess whether the restriction of phosphate intake could reduce serum phosphate levels, a group of 24‐month‐old mice was fed with a diet containing less phosphate (0.2%) than the standard diet (0.6%) during the 3 months before sacrifice. The total intake was similar in both groups (3.72 ± 0.35 g/mouse/day for mice fed with standard diet and 3.65 ± 0.48 g/animal/day for mice fed with hypophosphataemic diet). Low‐phosphate diet significantly reduced the serum phosphate concentration in old mice with respect to old mice fed with the standard diet, reaching similar values to young mice (Table 1 ). No changes were found in the body weight, body mass index (BMI) or blood glucose levels between the different groups of mice. Arterial pressure was reduced in both groups of old mice with respect to young mice (Table 1 ). Tibia lengths were measured from six animals per group and no differences were found (young mice: 1.82 ± 0.03 cm; old mice: 1.82 ± 0.03 cm; and old mice fed with the low‐phosphate diet: 1.87 ± 0.02 cm).
Table 1.
(A) Biochemical and physiological parameters of the three experimental groups: Young mice, old mice (Old‐24m) and old mice fed with the hypophosphataemic diet (Old‐LowP); and (B) the Pearson correlation coefficients (r), and its P value (P), between serum concentration of phosphate and gastrocnemius mass, tibialis mass, mean fibre cross‐sectional area (FCSA), Sirius red intensity, tetanic force, gait speed, orientation time and transition time, obtained from all animals fed with the pellet diet.
| (A) Biochemical and physiological parameters | |||
|---|---|---|---|
| Young | Old‐24m | Old‐LowP | |
| Serum P levels (mg/dL) | 16.46 ± 0.77 (n = 15) | 21.24 ± 0.95* (n = 21) | 17.46 ± 0.82 # (n = 22) |
| Body weight (g) | 33.64 ± 0.72 (n = 19) | 34.19 ± 1.15 (n = 21) | 33.10 ± 1.05 (n = 24) |
| Body mass index (g/cm2) | 3.51 ± 0.07 (n = 19) | 3.38 ± 0.11 (n = 21) | 3.27 ± 0.08 (n = 24) |
| Blood glucose (mg/dL) | 173.00 ± 10.43 (n = 5) | 159.00 ± 7.33 (n = 8) | 160.00 ± 5.73 (n = 9) |
| Beats per minute | 654.50 ± 7.79 (n = 14) | 659.00 ± 10.13 (n = 16) | 663.70 ± 4.76 (n = 9) |
| Systolic blood pressure (mmHg) | 115.00 ± 0.939 (n = 14) | 101.00 ± 2.15* (n = 16) | 101.50 ± 3.55* (n = 9) |
| Diastolic blood pressure (mmHg) | 76.50 ± 1.67 (n = 14) | 69.43 ± 1.58* (n = 16) | 71.87 ± 1.97 (n = 9) |
| (B) Correlation between serum phosphate levels and sarcopenia signs | |||
|---|---|---|---|
| Serum phosphate levels | |||
| r | P | n | |
| Gastrocnemius mass | −0.390 | 0.001 | 68 |
| Tibialis mass | −0.322 | 0.006 | 68 |
| Mean FCSA | −0.390 | 0.019 | 36 |
| Sirius red intensity | 0.398 | 0.020 | 34 |
| Tetanic force | −0.682 | >0.001 | 36 |
| Gait speed | −0.534 | >0.001 | 65 |
| Transition time | 0.491 | >0.001 | 58 |
Note: Results are expressed as mean ± standard error.
P < 0.05 versus Young.
P < 0.05 versus Old‐24m.
Dietary phosphate restriction improved skeletal muscle mass and quality in old mice
To analyse the effect of the low‐phosphate diet on the mass and the quality of skeletal muscle, several methods of diagnosis were used. First, the gastrocnemius and tibialis muscles were weighed just after the isolation and the weight corrected by body weight. We found that muscle from old mice fed with a low‐phosphate diet had significantly higher mass than their coetaneous fed with the standard diet and quite similar to the young mice one (Figure 1 A,B ).
Figure 1.
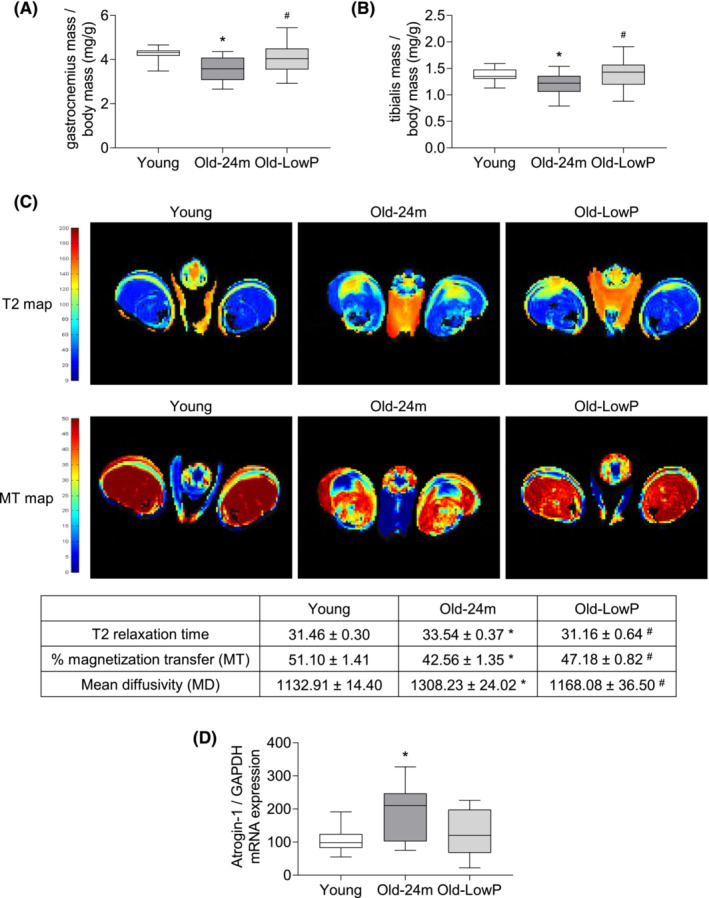
Dietary restriction of phosphate decreases the loss of mass and quality of skeletal muscle in old mice. Five‐month‐old mice (Young), 24‐month‐old mice fed with a standard diet (Old‐24m) and 24‐month‐old mice fed with a low‐phosphate diet (Old‐LowP) were evaluated. (A, B) Muscle mass was estimated by analysing the dry weight of gastrocnemius and tibialis muscles and corrected by body weight. The graphs represent the box plot from values obtained from 19 young mice, 21 old mice and 24 old mice fed with the hypophosphataemic diet. (C) Nuclear magnetic resonance image of the hind legs was performed. T2 map and magnetization transfer (MT) map images of each group are shown. The table shows the mean ± standard error from values obtained from three young mice, five old mice and five old mice fed with the hypophosphataemic diet. (D) Atrogin‐1 mRNA expression was analysed by qPCR in quadriceps samples. The graph represents the box plot from values obtained from 10 young mice, 9 old mice and 9 old mice fed with the hypophosphataemic diet. *P < 0.05 versus Young, # P < 0.05 versus Old‐24m.
Second, muscle quality was analysed by performing a study of NMRI in the hind legs of the mice. Old mice displayed a significant increase in T2 relaxation time and mean diffusivity (MD) and a reduction in the percentage of magnetization transfer (MT) compared with young mice, showing an important deterioration in the skeletal muscle. Dietary phosphate restriction prevented the alteration of the three parameters (Figure 1 C ). In addition, the expression of atrogin‐1 was analysed by RT‐PCR as a marker of muscle damage, finding a significant increase in the quadriceps of old animals fed with a standard diet, whereas old animals fed with the low‐phosphate diet have a lower expression (Figure 1 D ).
Third, histological studies were performed in gastrocnemius muscles to analyse the FCSA, the percentage of oxidative fibres and the percentage of type I and type II muscle fibres. Muscle from old mice fed with the standard diet showed a higher number of small fibres (<2000 μm2) with respect to young mice and a lower number of larger ones (between 2001 and 3000 μm2). These changes were prevented by the low‐phosphate diet (Figure 2 A,B ). Succinate dehydrogenase (SDH) staining (Figure 2 C ) revealed an increase in the oxidative capacity of muscle fibres in gastrocnemius isolated from old mice that was also prevented with the low‐phosphate diet (Figure 2 D ). Moreover, we analysed the expression of myomesin‐2 by western blot, as a component of the M‐line of sarcomere, which is predominant in type II fibres, and found a decreased expression in the quadriceps isolated from old mice fed with a standard diet as compared with the quadriceps from young and old mice fed with low‐phosphate diet (Figure 2 E ).
Figure 2.
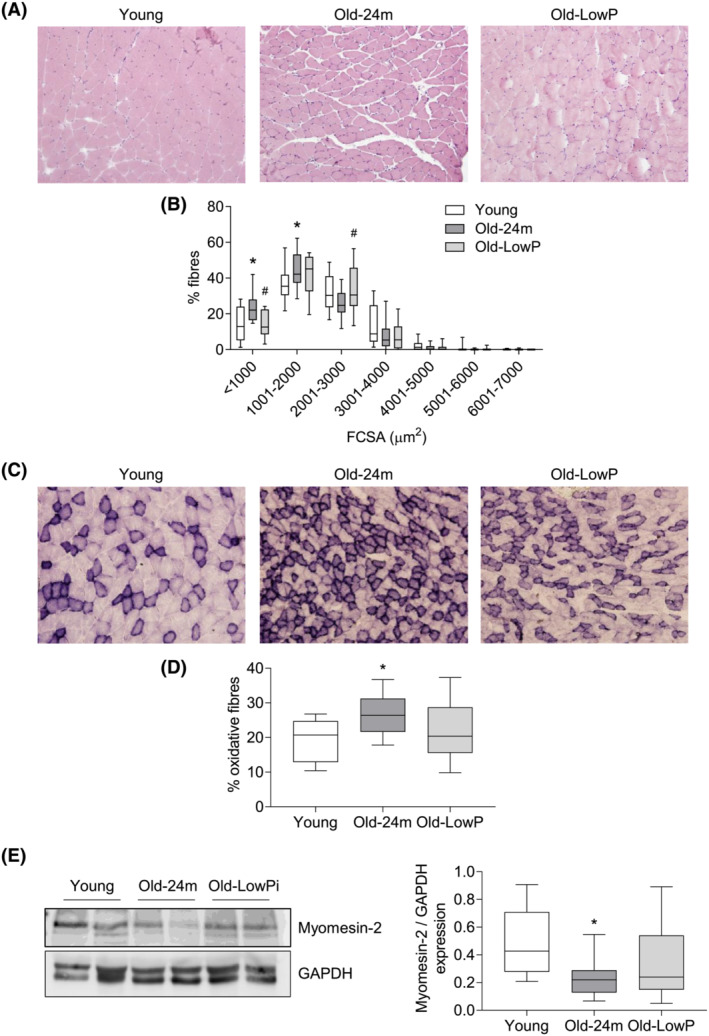
Dietary restriction of phosphate prevents the fibre cross‐sectional area (FCSA) reduction observed in old mice. Five‐month‐old mice (Young), 24‐month‐old mice fed with a standard diet (Old‐24m) and 24‐month‐old mice fed with a low‐phosphate diet (Old‐LowP) were evaluated. (A) Haematoxylin and eosin staining was performed in gastrocnemius muscle to measure the FCSAs (B). (C) Succinate dehydrogenase staining was also performed in gastrocnemius muscle to calculate the percentage of oxidative fibres (D). The graphs represent the box plot from values obtained from 15 young mice, 13 old mice and 13 old mice fed with the hypophosphataemic diet. (E) Myomesin‐2 expression was analysed by western blot in quadriceps samples. A represent blot is shown in the left panel. The graph represents the box plot from values obtained from 15 young mice, 16 old mice and 16 old mice fed with the hypophosphataemic diet. *P < 0.05 versus Young, # P < 0.05 versus Old‐24m.
To analyse changes in the percentage of fast and slow fibres, we performed an MHC staining (Figure 3 A ). The study revealed that muscle from old mice showed a greater percentage of type I oxidative fibres (slow‐twitch fibres) and a lower percentage of type II glycolytic fibres (fast‐twitch fibres) with respect to young mice (Figure 3 B ). These changes were partially reverted with the low‐phosphate diet without reaching statistically significant differences between both old mice groups (Figure 3 B ).
Figure 3.
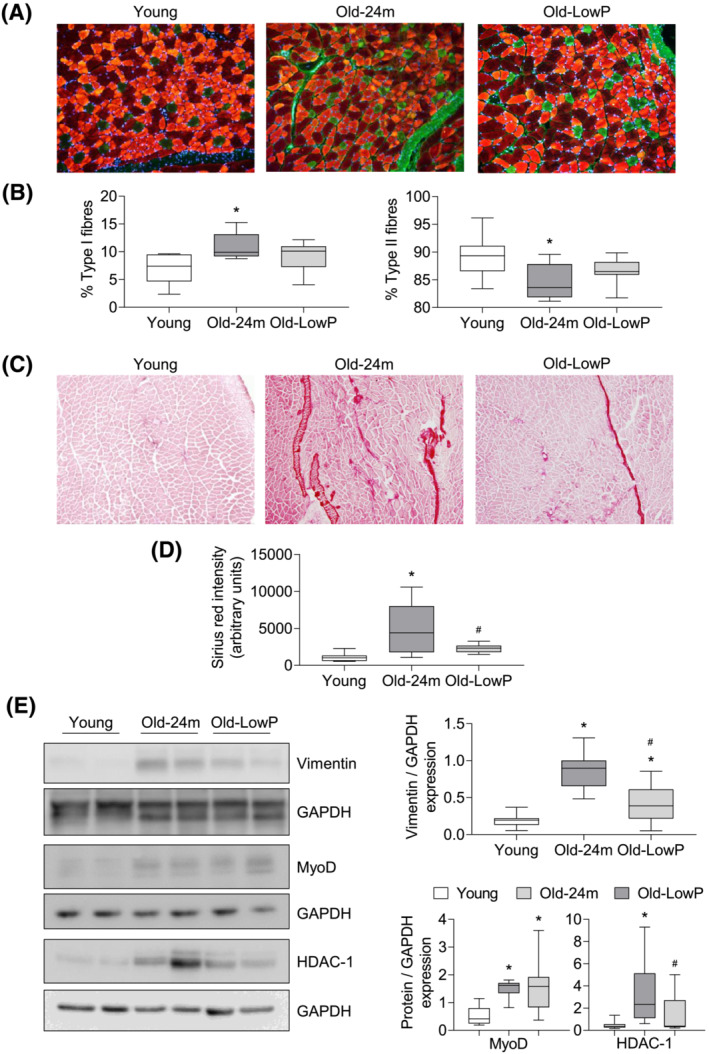
Dietary restriction of phosphate prevents the aging‐related fibre‐type switching and muscle fibrosis observed in old mice. (A) Slow‐twitch (type I) and fast‐twitch (type II) fibres staining was performed in all groups. (B) The graphs represent the percentage of type I fibres (green) (left panel) or type II fibres (red) (right panel) and are expressed as the box plot from values obtained from eight young mice, eight old mice and nine old mice fed with the hypophosphataemic diet. (C) Collagen content in gastrocnemius muscle was measured by Sirius red staining. (D) The graph represents the densitometric analysis of the staining and the results are expressed as the box plot from 14 young mice, 12 old mice and 10 old mice fed with the hypophosphataemic diet. (E) Vimentin, MyoD and HDAC‐1 expressions were analysed by western blot in quadriceps samples. A represent blot of each protein is shown in the left panel. The graphs represent the box plot from values obtained from 15 young mice, 16 old mice and 16 old mice fed with the hypophosphataemic diet. *P < 0.05 versus Young, # P < 0.05 versus Old‐24m.
Finally, the presence of fibrosis in gastrocnemius muscle was evaluated by Sirius red staining (Figure 3 C,D ) and by vimentin expression in quadriceps, a marker of fibrogenic differentiation, assessed by western blot (Figure 3 E ). Results showed that muscles from old mice presented significantly more fibrotic tissue and more vimentin expression than young mice, but these differences were prevented in old mice fed with the low‐phosphate diet (Figure 3 D,E ).
Additionally, we analyse in isolated quadriceps, the expression of MyoD and its epigenetic negative regulator HDAC‐1, by western blot, as markers of myogenic differentiation (Figure 3 E ) and found a light increased expression of MyoD in old mice comparing with young mice, whereas HDAC‐1 expression was significantly higher in old mice fed with standard diet than in young animals and old mice fed with low‐phosphate diet (Figure 3 E ). These results suggest that muscle from old animals shows increased expression of fibrogenic differentiation markers and decreased expression of myogenic differentiation marker, which can be prevented partially with a low‐phosphate diet.
Dietary phosphate restriction improved the muscle strength in old mice
Muscle strength, assessed by the grip test, diminishes progressively from 18 months of age in old mice (Figure S1 ). We had previously published that old mice fed with the standard diet have a significant loss of muscle strength, as expected, which was improved when old mice were fed with low‐phosphate diet. 8 Now, we analysed muscle contraction of the tibialis muscle after electrical stimulation of peroneal nerve. Old mice showed a lower twitch and tetanic force than young mice. Hypophosphataemic diet increased twitch force and tetanic force in old mice compared with old mice fed with a standard diet (Figure 4 A,B ). Finally, old mice displayed a lower fatigue resistance in response to a long‐duration contraction than young mice, whereas these differences were not observed in old mice fed with the low‐phosphate diet (Figure 4 C ).
Figure 4.
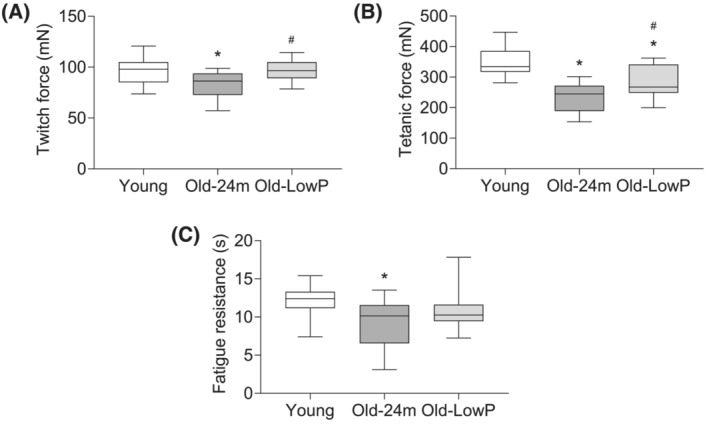
Dietary restriction of phosphate prevents the loss of muscle strength in old mice. Five‐month‐old mice (Young), 24‐month‐old mice fed with a standard diet (Old‐24m) and 24‐month‐old mice fed with a low‐phosphate diet (Old‐LowP) were evaluated. Electrical stimulation of the peroneal nerve was performed in all groups. Twitch force (A), tetanic force (B) and fatigue resistance (C) were measured after nerve stimulation. The graphs represent the box plot from values obtained from 13 young mice, 13 old mice and 14 old mice fed with the hypophosphataemic diet. *P < 0.05 versus Young, # P < 0.05 versus Old‐24m.
Dietary phosphate restriction improved physical performance in old mice
Physical performance was assessed through the measurement of gait speed, the stride length and the hind paw base width by footprint test. A significantly slower gait speed was observed in old mice with respect to young ones (Figure 5 A ), as well as a shorter stride length (Figure 5 B ) and a greater hind paw base width (Figure 5 C ). The restriction of phosphorus in the diet improved gait speed and increased the stride length in old mice compared with the mice fed with the standard diet, although no differences were observed in the hind paw base width (Figure 5 A–C ).
Figure 5.
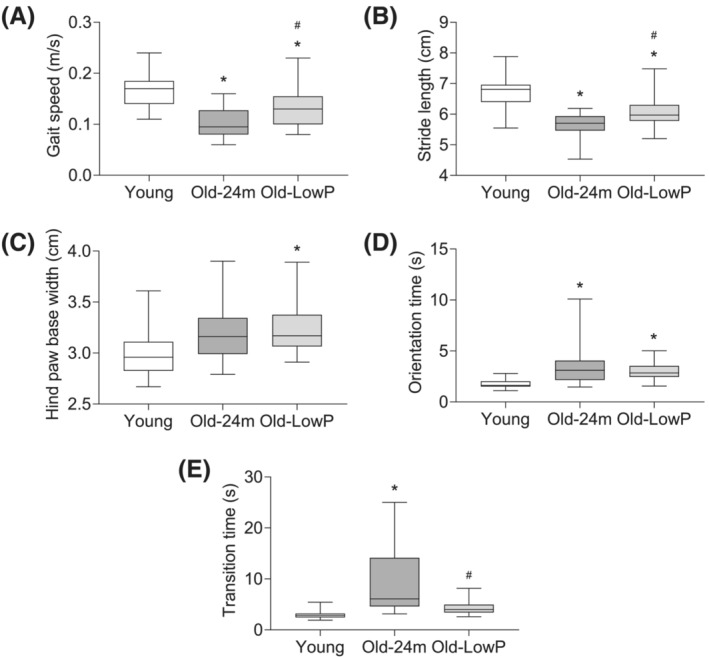
Dietary restriction of phosphate prevents the loss of physical performance in old mice. Five‐month‐old mice (Young), 24‐month‐old mice fed with a standard diet (Old‐24m) and 24‐month‐old mice fed with a low‐phosphate diet (Old‐LowP) were evaluated. Gait test (A–C) and static bar test (D, E) were performed in all groups. Gait speed (A), stride length (B), hind paw base width (C), orientation time in static bar (D) and transition time in static bar (E) were measured. The graphs represent the box plot from values obtained from 19 young mice, 21 old mice and 24 old mice fed with the hypophosphataemic diet (Panels A–C) or 15 young mice, 18 old mice and 20 old mice fed with the hypophosphataemic diet (Panels D and E). *P < 0.05 versus Young, # P < 0.05 versus Old‐24m.
In the same way, the analysis of orientation and motor coordination in the static bar test showed a significant higher orientation and transition time in old mice with respect to the young mice group (Figure 5 D,E ). Once again, low‐phosphate diet significantly managed to reduce the transition time in those old mice fed with this diet (Figure 5 E ); however, no changes were observed in orientation time (Figure 5 D ).
Dietary phosphate restriction improved muscle strength and physical performance in geriatric mice
A group of 21‐month‐old mice was fed with either standard diet or low‐phosphate diet for the next 7 months, and they were sacrificed at 28 months, as geriatric mice groups. Low‐phosphate diet reduced significantly phosphate levels in this group of geriatric mice compared with those fed with a standard diet (Figure 6 A ). No differences were found in the gastrocnemius and tibialis mass between both groups (Figure 6 B ). By contrast, the low‐phosphate diet improved the muscle strength (Figure 6 C ) and the orientation and motor coordination of geriatric mice, resulting in a lower orientation and transition times in the static bar test compared with the group of geriatric mice fed with standard diet (Figure 6 D ). Finally, the low‐phosphate diet also significantly improved the physical performance, as reflected by a higher gait speed without affecting the stride length and the hind paw base width (Figure 6 E,F ).
Figure 6.
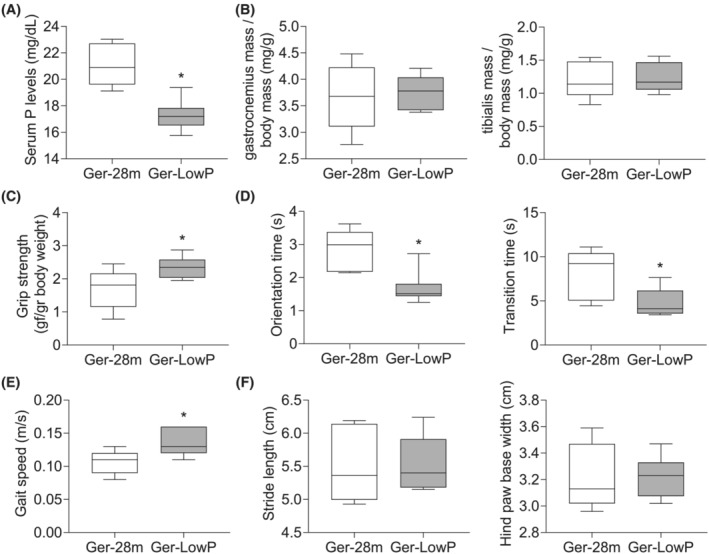
Dietary restriction of phosphate prevents the loss of muscle strength and physical performance in geriatric mice. Twenty‐eight‐month‐old mice fed with a standard diet (Ger‐28m) and 28‐month‐old mice fed with a low‐phosphate diet (Ger‐LowP) were evaluated. (A) Serum phosphate levels were measured in all groups. (B) Muscle mass was estimated analysing the dry weight of gastrocnemius (left panel) and tibial (right panel) muscles and corrected by body weight. Muscle strength was estimated by the grip test (C) and physical performance by the static bar test (D) and the gait test (E, F). Orientation and transition times (D), gait speed (E) and gait parameters (stride length and hind paw base width) (F) were measured. The graphs represent the box plot from values obtained from five geriatric mice and seven geriatric mice fed with the standard diet. *P < 0.05 versus Ger‐28m.
Phosphate binder, sucroferric oxyhydroxide, reduced the sarcopenia signs in old mice
A group of 21‐month‐old mice was fed during the next 3 months with a millet standard diet supplemented with 5% sucroferric oxyhydroxide powder (Velphoro®). The old mice Velphoro® group was compared with young mice and old mice, which were fed with a millet standard diet, and with old mice fed with a hypophosphataemic millet diet. Results showed that Velphoro® reduced significantly the serum phosphate concentration in old mice, even more efficiently than the hypophosphataemic diet (Figure 7 A ). Regarding the effect of Velphoro® supplementation on sarcopenia signs, we did not observe differences in gastrocnemius mass with respect to the old mice group (Figure 7 B ). By contrast, old mice treated with Velphoro® showed an improvement in muscle strength with respect to old mice (Figure 7 C ) and some parameters of physical performance, such as gait speed (Figure 7 D ), and the transition time (Figure 7 F ) in the static bar without changes in the orientation time (Figure 7 E ).
Figure 7.
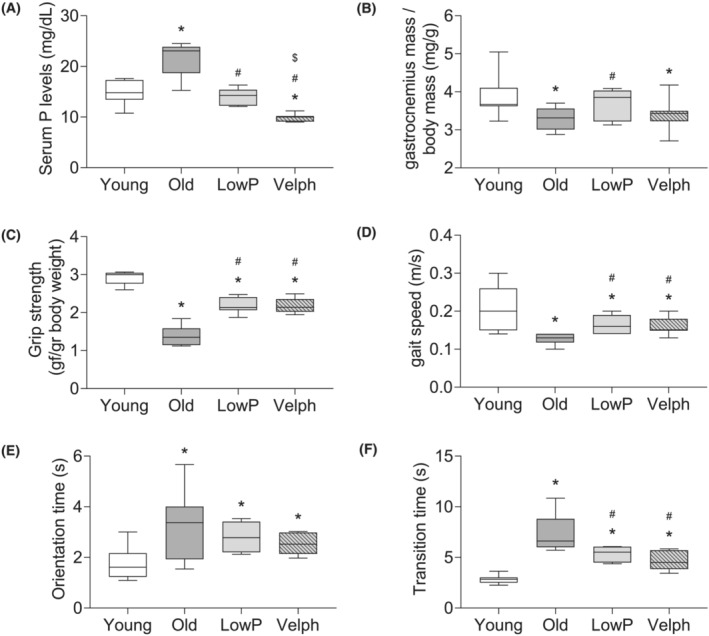
Velphoro® (Velph) administration reduces the development of sarcopenia signs in old mice. Four groups of mice were evaluated: 5‐month‐old mice (Young), 24‐month‐old mice fed with a millet standard diet (Old), 24‐month‐old mice fed with a millet hypophosphataemic diet (LowP) and 24‐month‐old mice fed with a millet standard diet supplemented with 5% Velph. (A) Serum phosphate levels were measured in all groups. (B) Muscle mass was estimated analysing the dry weight of gastrocnemius muscles and corrected by body weight. Muscle strength was estimated by the grip test (C) and physical performance by the gait test (D) and the static bar test (E, F). Gait speed (D) and orientation (E) and transition (F) times in static bar were measured. The graphs represent the box plot from values obtained from 9 young mice, 10 old mice, 7 old mice fed with the hypophosphataemic diet and 8 old mice fed with the Velph supplemented diet. *P < 0.05 versus Young, # P < 0.05 versus Old, $ P < 0.05 versus LowP.
Serum phosphate concentration correlated with sarcopenia signs in old mice
Finally, we performed a correlation analysis with all data obtained in this study with animals fed with the pelleted diet in order to test whether serum phosphate concentration was correlated with specific signs of sarcopenia. The results presented in Table 1 show that serum phosphate concentration was positively correlated with Sirius red intensity and transition time but negatively correlated with gastrocnemius and tibialis mass, mean FCSA, tetanic force and gait speed.
As per the ARRIVE recommendations, because animals were housed in cages containing from one to five animals, we estimated the cage effect using ICC, which is presented in Table S2 . These estimations indicate a low cage effect for all analyses except for only one parameter.
Discussion
In the present work, we show the relevant role of hyperphosphataemia in the development of sarcopenia signs in an animal model of aging. We found that serum phosphate concentration increases progressively with age in mice in parallel with the loss of muscle mass, strength and physical performance. In line with these findings, we had previously shown that hyperphosphataemia induces myoblasts senescence, 7 reducing myogenic differentiation and promoting fibrogenic differentiation in cultured myoblasts. 8 Now, we demonstrate that when phosphorus serum levels are reduced in old mice by means of dietary phosphate restriction, the muscle strength, the muscle mass and the physical performance from those animals improve significantly. To reduce serum phosphate, old mice were fed with a hypophosphataemic diet containing only 0.2% phosphorus instead of 0.6% from a standard diet. Although the standard diet and the low‐phosphate diet do not have identical composition, the results obtained using a phosphate binder mixed with the standard diet showed that the differences found in muscle are due to phosphorus intake.
Age‐related loss of muscle function involves qualitative and quantitative changes in skeletal muscle structure and function that results in sarcopenia. 11 In the present study, the effects of hypophosphataemic diet on sarcopenic signs were evaluated following the criteria defined by the EWGSOP group for humans, that is, through the assessment of muscle mass, muscle strength and physical performance. 1 Our analysis reveals an age‐related decrease in the relative mass of the tibialis and gastrocnemius muscles that was prevented by the restriction of phosphate intake, suggesting a relationship between hyperphosphataemia and loss of muscle mass. To further analyse the quality of the muscle, NMRI tests were performed in some animals. NMRI is considered one of the best research methods for estimating muscle mass 1 because it not only allows the quantification of skeletal mass but also provides information about muscle quality in relation to the presence of oedema, inflammation, fibrotic or fat infiltration and atrophy. 12 , 13 Old mice had significantly higher MD values than young mice. MD refers to the mobility of water molecules in tissue and provides information about the microstructure and arrangement of muscle fibres. 14 An increase in the value of MD has been related to frailty in older people and is associated with an increased atrophy of muscle fibres, as well as with a greater presence of intramuscular connective tissue. 15 Another parameter, MT, represents the ratio of the immobile protons bound to macromolecules and the protons that are part of free water. 16 Old mice presented lower MT percentages than those found in young mice, indicating a lower presence of macromolecules and protein content in skeletal muscle. 17 Finally, T2 relaxation time offers information about the histological organization of water molecules in muscle tissue. 18 An increase in T2 relaxation time has been related to aging, 15 , 19 as well as with greater presence of inflammation, 20 oedema 21 or atrophy of type II muscle fibres. 22 Consistent with these findings, old mice showed higher levels of T2 relaxation time relative to young mice. In turn, dietary phosphate restriction prevented the modification of the three aforementioned parameters, reflecting a better muscle quality. Atrophy and muscle wasting were also examined by atrogin‐1 expression, 23 corroborating the results obtained with the NMRI studies.
Some of the changes evidenced by NMRI were confirmed with the histological analysis of the isolated muscles. Old animals presented a rise in the number of small fibres and a decline in the largest ones, suggesting aging‐related muscle atrophy, even though the total number of fibres is increased. 24 The low‐phosphate diet had a positive effect on muscle atrophy from old mice, as it was able to increase the FCSA. Another parameter to assess muscle integrity is the activity of SDH, which reflects the oxidative metabolism of muscle fibres. 25 It has been previously described that the number of positive SDH fibres increases in relation to age, from 6% at 49 years of age to 31% at 92 years. 26 Our data confirmed a significant increase in the number of positive SDH fibres in old mice compared with young mice, which were reduced with dietary phosphate restriction.
During aging, the decrease in muscle mass has been mainly attributed to the atrophy of type II fibres, 27 which results in a reduction in the proportion of fast‐twitch fibres. 25 We found a greater number of type I fibres and less type II fibres in our aged‐mice model than in young mice, thus confirming a typical fibre profile of aging. Also, the expression of myomesin‐2, a component of the M‐line predominant in type II fibres, was reduced in old mice. 28 Again, mice fed with a low‐phosphate diet presented more percentage of type II fibres than control old mice, although no statistical significance was found, and higher expression of myomesin‐2. Finally, it was analysed whether there was fibrosis in the skeletal muscle of the old animals. An increase in the amount of muscle collagen is associated with muscle aging. 11 , 29 , 30 A higher Sirius red intensity was found in the muscle of old mice when compared with young mice, correlating positively with serum phosphate concentration. In addition, fibrotic areas from skeletal muscle of old mice fed with a low‐phosphate diet were significantly lower. This would indicate that the low‐phosphate diet could delay the appearance of age‐related fibrosis. We have previously published that hyperphosphataemia induced fibrogenic differentiation and reduced myogenic differentiation by reducing myogenin and MEF2c expression in muscle isolated from old mice. 8 Now, we demonstrate that muscles from old mice also have a higher expression of vimentin, a marker of fibrogenic differentiation, and HDAC‐1, which is an epigenetic inhibitor of the transcriptional activity of MyoD. 31 MyoD, one of the principal directors of myogenic differentiation, activates the transcription of myogenin and MEF2c, which are essential for the formation of new myotubes. 32
Regarding the loss of muscle function, several studies have shown that muscle force can decrease in humans by up to 60%, between the ages of 30 and 80. 33 In mice, a diminished capacity to generate muscle force is not only due to a loss of muscle mass but also due to a reduction in the capacity to generate force per cross‐sectional area of the muscle, that is, of specific force. 24 , 34 It has been described that the loss of this specific force could occur in the initial stages of sarcopenia 24 ; for this reason, we studied the contractile activity of the tibialis muscle. 35 , 36 The 24‐month‐old mice had a decreased twitch and tetanic strength and fatigue resistance compared with the 5‐month‐old mice. In addition, mice fed with the low‐phosphate diet prevented these changes.
Finally, physical performance was also evaluated by gait analysis, a widely used system for studying locomotion. 37 Age‐related gait disturbances and balance disorders are a major cause of falls and injuries in the elderly population. 38 The association of gait speed with aging has been consistently found in humans, although not yet in mice. 39 Age‐related changes in gait parameters in rodents are not well defined, although they are used as the primary preclinical model for many disease states associated with motor disturbances. 39 Our 24‐month‐old mice showed a decrease in gait speed when compared with the 5‐month‐old mice that correlated negatively with serum phosphorus, which suggests that a restriction in phosphate intake might improve the motor function from old mice. On the other hand, when analysing various gait parameters, we found impairments in stride length associated with aging. This parameter is also modified in the elderly and in people with diseases that affect their mobility and is directly associated with walking speed. 40 Finally, motor coordination and orientation capacity were assessed by means of the static bar test. 41 Old mice showed an increase in the times of orientation and transition on the balance beam when compared with the young mice, which again indicates a decline in the physical performance of the aged animals. Nevertheless, animals fed with a low‐phosphorus diet showed an improvement in transition and orientation times when compared with mice of the same age fed with the standard diet.
Next, a group of 21‐month‐old mice was maintained for 7 months with the hypophosphataemic diet and compared with 28‐month‐old mice fed with the standard diet to know whether the effect on dietary restriction remains beneficial in older animals. The results showed that whereas muscle strength and physical performance were improved with phosphate dietary restriction, there was no improvement in the muscle mass in geriatric mice.
The reduction of phosphate in the diet could involve nutritional deficiency because one of the main sources of phosphate is proteins. In this sense, the restriction of the phosphate intake by using different treatments with phosphate binders has been done with satisfactory results in chronic kidney disease (CKD) patients to reduce mortality. 42 For this reason, we treated our old animals with sucroferric oxyhydroxide in powder as a phosphate binder for 3 months to assess the effect on muscle. No changes were found in muscle mass, but muscle strength and gait speed were significantly improved, indicating a potential therapeutic tool to avoid loss of muscle function with age.
In summary, the structural and functional evaluation of the skeletal muscle of old mice revealed a close relationship between serum phosphate levels and the appearance of signs of sarcopenia, such as loss of muscle mass, changes in muscle fibres and the appearance of fibrosis, as well as age‐dependent changes in muscle and motor function. These results support the importance of restricting phosphorus intake for improving muscle structure and function during aging in mice. In humans, a study has correlated dietary phosphate intake with some accelerated aging signs. 43 However, clinical studies will be necessary to analyse the effect of phosphorus intake in the diet or the use of phosphate binders in sarcopenia in order to translate these results into clinical practice.
Funding
This work was supported by grants from the Fondo de Investigaciones Sanitarias from Instituto de Salud Carlos III and FEDER funds (grants PI19/01339, PI19/00502, PI16/02082, PI16/01619), a grant from the Principado de Asturias FICYT (IDI/2021/0000080), the Networks Program REDinREN from Instituto de Salud Carlos III and FEDER funds (grant RETIC REDinREN: RD016/0009/0018, RD16/0009/0017), IRYCIS group 3.07 and FRIAT. E. Alcalde‐Estévez holds a project‐associated contract from Instituto de Salud Carlos III and FEDER funds (RD016/0009/0018) and held a predoctoral contract from the Ministerio de Educación, Cultura y Deportes (FPU16/01450). A. Asenjo‐Bueno held a contract from the Programa de Garantía Juvenil of CAM as a research assistant (PEJ‐2020‐AI/BMD‐18125), held a project‐associated contract from the Fondo de Investigaciones Sanitarias from Instituto de Salud Carlos III (PI16/01619) and FEDER funds and holds a predoctoral contract from the Universidad de Alcalá (FPI‐UAH). P. Plaza held a contract from the Programa de Garantía Juvenil of CAM as a technician (PEJ‐2017‐TL/BMD‐5956), and P. Sosa held a predoctoral contract from the Universidad de Alcalá (FPI16‐UAH) and holds a post‐doctoral contract from the Instituto de Salud Carlos III (Sara Borrell). S. López‐Ongil holds a contract from the Research Stabilization Program from Instituto de Salud Carlos III (CES07/032). P.L. Valenzuela holds a post‐doctoral contract from the Instituto de Salud Carlos III (Sara Borrell; CD21/00138).
Conflict of interest
All authors declare that they have no conflict of interest.
Supporting information
Table S1. Diets composition.
Table S2. Intraclass correlation coefficients (ICC) values of the results depicted in box plots.
Figure S1. Muscle strength measured by a grip test from fourteen 5‐month‐old animals (5m), ten 9‐month‐old animals (9m), ten 12‐month‐old animals (12m), fourteen 18‐month‐old animals (18m), and thirteen 24‐month‐old animals (24m). *p < 0.05 vs 5m.
Acknowledgements
Velphoro® was supplied by the company Vifor Pharma, Zurich, Switzerland. We acknowledge Prof. Raymond Stallings for English spelling and grammar revision. We thank Prof. Javier Zamora (Institute Ramon y Cajal of Biomedical Research‐IRYCIS) for his statistical advice and for performing statistical analysis. We also thank two anonymous reviewers for providing valuable suggestions to the manuscript. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 44
Alcalde‐Estévez E., Sosa P., Asenjo‐Bueno A., Plaza P., Valenzuela P. L., Naves‐Díaz M., Olmos G., López‐Ongil S., and Ruiz‐Torres M. P. (2023) Dietary phosphate restriction prevents the appearance of sarcopenia signs in old mice, Journal of Cachexia, Sarcopenia and Muscle, 14, 1060–1074, 10.1002/jcsm.13194
Elena Alcalde‐Estévez, Patricia Sosa, Susana López‐Ongil and María P. Ruiz‐Torres contributed equally to the manuscript.
References
- 1. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 3. Verdijk LB, Snijders T, Beelen M, Savelberg HH, Meijer K, Kuipers H, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc 2010;58:2069–2075. [DOI] [PubMed] [Google Scholar]
- 4. Nair KS. Aging muscle. Am J Clin Nutr 2005;81:953–963. [DOI] [PubMed] [Google Scholar]
- 5. Sousa‐Victor P, Munoz‐Canoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med 2016;50:109–117. [DOI] [PubMed] [Google Scholar]
- 6. Troyano N, Nogal MD, Mora I, Diaz‐Naves M, Lopez‐Carrillo N, Sosa P, et al. Hyperphosphatemia induces cellular senescence in human aorta smooth muscle cells through integrin linked kinase (ILK) up‐regulation. Mech Ageing Dev 2015;152:43–55. [DOI] [PubMed] [Google Scholar]
- 7. Sosa P, Alcalde‐Estevez E, Plaza P, Troyano N, Alonso C, Martínez‐Arias L, et al. Hyperphosphatemia promotes senescence of myoblasts by impairing autophagy through Ilk overexpression, a possible mechanism involved in sarcopenia. Aging Dis 2018;9:769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sosa P, Alcalde‐Estévez E, Asenjo‐Bueno A, Plaza P, Carrillo‐López N, Olmos G, et al. Aging‐related hyperphosphatemia impairs myogenic differentiation and enhances fibrosis in skeletal muscle. J Cachexia Sarcopenia Muscle 2021;12:1266–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sayer AA, Cruz‐Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing 2022;51:afac220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging‐related loss of muscle mass and function. Physiol Rev 2019;99:427–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: old evidence and new insights. Eur Radiol 2020;30:2199–2208. [DOI] [PubMed] [Google Scholar]
- 13. Lee K, Shin Y, Huh J, Sung YS, Lee IS, Yoon KH, et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol 2019;20:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li K, Dortch RD, Welch EB, Bryant ND, Buck AK, Towse TF, et al. Multi‐parametric MRI characterization of healthy human thigh muscles at 3.0 T—relaxation, magnetization transfer, fat/water, and diffusion tensor imaging. NMR Biomed 2014;27:1070–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farrow M, Biglands J, Tanner SF, Clegg A, Brown L, Hensor EMA, et al. The effect of ageing on skeletal muscle as assessed by quantitative MR imaging: an association with frailty and muscle strength. Aging Clin Exp Res 2021;33:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louie EA, Gochberg DF, Does MD, Damon BM. Transverse relaxation and magnetization transfer in skeletal muscle: effect of pH. Magn Reson Med 2009;61:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Power GA, Allen MD, Booth WJ, Thompson RT, Marsh GD, Rice CL. The influence on sarcopenia of muscle quality and quantity derived from magnetic resonance imaging and neuromuscular properties. Age (Dordr) 2014;36:9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damon BM, Li K, Bryant ND. Magnetic resonance imaging of skeletal muscle disease. Handb Clin Neurol 2016;136:827–842. [DOI] [PubMed] [Google Scholar]
- 19. Azzabou N, Hogrel JY, Carlier PG. NMR based biomarkers to study age‐related changes in the human quadriceps. Exp Gerontol 2015;70:54–60. [DOI] [PubMed] [Google Scholar]
- 20. Maillard SM, Jones R, Owens C, Pilkington C, Woo P, Wedderburn LR, et al. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology (Oxford) 2004;43:603–608. [DOI] [PubMed] [Google Scholar]
- 21. Hiba B, Richard N, Hébert LJ, Coté C, Nejjari M, Vial C, et al. Quantitative assessment of skeletal muscle degeneration in patients with myotonic dystrophy type 1 using MRI. J Magn Reson Imaging 2012;35:678–685. [DOI] [PubMed] [Google Scholar]
- 22. Hatakenaka M, Ueda M, Ishigami K, Otsuka M, Masuda K. Effects of aging on muscle T2 relaxation time: difference between fast‐ and slow‐twitch muscles. Invest Radiol 2001;36:692–698. [DOI] [PubMed] [Google Scholar]
- 23. Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin‐1, a muscle‐specific F‐box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 2001;98:14440–14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballak SB, Degens H, Buse‐Pot T, de Haan A, Jaspers RT. Plantaris muscle weakness in old mice: relative contributions of changes in specific force, muscle mass, myofiber cross‐sectional area, and number. Age (Dordr) 2014;36:9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 2011;91:1447–1531. [DOI] [PubMed] [Google Scholar]
- 26. Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 2013;45:2288–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 2013;48:492–498. [DOI] [PubMed] [Google Scholar]
- 28. Murgia M, Nogara L, Baraldo M, Reggiani C, Mann M, Schiaffino S. Protein profile of fiber types in human skeletal muscle: a single‐fiber proteomics study. Skelet Muscle 2021;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldspink G, Fernandes K, Williams PE, Wells DJ. Age‐related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 1994;4:183–191. [DOI] [PubMed] [Google Scholar]
- 30. Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 2010;11:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mal A, Sturniolo M, Schiltz RL, Ghosh MK, Harter ML. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program. EMBO J 2001;20:1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 2005;132:2685–2695. [DOI] [PubMed] [Google Scholar]
- 33. Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age‐related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 2007;34:1091–1096. [DOI] [PubMed] [Google Scholar]
- 34. Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve 2003;27:339–347. [DOI] [PubMed] [Google Scholar]
- 35. Lovering RM, Roche JA, Goodall MH, Clark BB, McMillan A. An in vivo rodent model of contraction‐induced injury and non‐invasive monitoring of recovery. J Vis Exp 2011;11:2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hakim CH, Wasala NB, Duan D. Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterior muscle in situ in mice. J Vis Exp 2013;9:50183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Batka RJ, Brown TJ, Mcmillan KP, Meadows RM, Jones KJ, Haulcomb MM. The need for speed in rodent locomotion analyses. Anat Rec (Hoboken) 2014;297:1839–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarantini S, Yabluchanskiy A, Fülöp GA, Kiss T, Perz A, O'Connor D, et al. Age‐related alterations in gait function in freely moving male C57BL/6 mice: translational relevance of decreased cadence and increased gait variability. J Gerontol A Biol Sci Med Sci 2019;74:1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mock JT, Knight SG, Vann PH, Wong JM, Davis DL, Forster MJ, et al. Gait analyses in mice: effects of age and glutathione deficiency. Aging Dis 2018;9:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parvathy SS, Masocha W. Gait analysis of C57BL/6 mice with complete Freund's adjuvant‐induced arthritis using the CatWalk system. BMC Musculoskelet Disord 2013;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deacon RM. Measuring motor coordination in mice. J Vis Exp 2013;29:e2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barreto FC, Barreto DV, Massy ZA, Drueke TB. Strategies for phosphate control in patients with CKD. Kidney Int Rep 2019;4:1043–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McClelland R, Christensen K, Mohammed S, McGuinness D, Cooney J, Bakshi A, et al. Accelerated ageing and renal dysfunction links lower socioeconomic status and dietary phosphate intake. Aging (Albany NY) 2016;8:1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Diets composition.
Table S2. Intraclass correlation coefficients (ICC) values of the results depicted in box plots.
Figure S1. Muscle strength measured by a grip test from fourteen 5‐month‐old animals (5m), ten 9‐month‐old animals (9m), ten 12‐month‐old animals (12m), fourteen 18‐month‐old animals (18m), and thirteen 24‐month‐old animals (24m). *p < 0.05 vs 5m.


