Abstract
Ageing is accompanied by an inexorable loss of muscle mass and functionality and represents a major risk factor for numerous diseases such as cancer, diabetes and cardiovascular and pulmonary diseases. This progressive loss of muscle mass and function may also result in the insurgence of a clinical syndrome termed sarcopenia, exacerbated by inactivity and disease. Sarcopenia and muscle weakness yield the risk of falls and injuries, heavily impacting on health and social costs. Thus, screening, monitoring and prevention of conditions inducing muscle wasting and weakness are essential to improve life quality in the ageing modern society. To this aim, the reliability of easily accessible and non‐invasive blood‐derived biomarkers is being evaluated. C‐terminal agrin fragment (CAF) has been widely investigated as a neuromuscular junction (NMJ)‐related biomarker of muscle dysfunction. This narrative review summarizes and critically discusses, for the first time, the studies measuring CAF concentration in young and older, healthy and diseased individuals, cross‐sectionally and in response to inactivity and physical exercise, providing possible explanations behind the discrepancies observed in the literature. To identify the studies investigating CAF in the above‐mentioned conditions, all the publications found in PubMed, written in English and measuring this biomarker in blood from 2013 (when CAF was firstly measured in human serum) to 2022 were included in this review. CAF increases with age and in sarcopenic individuals when compared with age‐matched, non‐sarcopenic peers. In addition, CAF was found to be higher than controls in other muscle wasting conditions, such as diabetes, COPD, chronic heart failure and stroke, and in pancreatic and colorectal cancer cachectic patients. As agrin is also expressed in kidney glomeruli, chronic kidney disease and transplantation were shown to have a profound impact on CAF independently from muscle wasting. CAF concentration raises following inactivity and seems to be lowered or maintained by exercise training. Finally, CAF was reported to be cross‐sectionally correlated to appendicular lean mass, handgrip and gait speed; whether longitudinal changes in CAF are associated with those in muscle mass or performance following physical exercise is still controversial. CAF seems a reliable marker to assess muscle wasting in ageing and disease, also correlating with measurements of appendicular lean mass and muscle function. Future research should aim at enlarging sample size and accurately reporting the medical history of each patient, to normalize for any condition, including chronic kidney disease, that may influence the circulating concentration of this biomarker.
Keywords: C‐terminal agrin fragment, Ageing, Sarcopenia, Cancer cachexia, Muscle wasting, Muscle weakness
Introduction
Ageing and age‐associated diseases inducing muscle wasting and weakness
In the last decades, Western countries faced a profound demographic change, reflected in the average expectancy of life progressively increasing up to more than 75 years. 1 The number of people over 60 has been estimated to rise from 600 million (in 2000) to about 2 billion by 2050. 2 Hence, one of the challenges of the modern society is to increase the expectancy of healthy life.
Ageing is accompanied by a progressive decline of muscle mass and performance, yielding increased incidence of falls, fractures and hospitalization, consequently reducing the quality of life and increasing the healthcare expenditures. The clinical manifestation of this phenomenon is termed sarcopenia. 3 According to a recent meta‐analysis, sarcopenia prevalence ranges between 10% and 27% in people aged >60 years old, widely depending on the definition used for diagnosis. 4 Even with a conservative estimate, sarcopenia affects >50 million people today and will affect >200 million people in the next 40 years. 2
This impressive prevalence is due to the fact that sarcopenia is not only caused by ageing (primary sarcopenia) but may be linked to the concurrent presence of other modifying conditions (secondary sarcopenia), such as inactivity, advanced organ failure (disease‐related sarcopenia) or inadequate intake of energy/proteins. 3 , 5 , 6 Importantly, ageing is accompanied by increased inactivity 7 , 8 and represents a major risk factor for disease such as diabetes, 9 cardiovascular, pulmonary diseases, cancer and cancer‐related cachexia. 10 Cancer cachexia is a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass not reversible by nutritional support. 11 All these conditions in turn determine an increased likelihood of secondary sarcopenia development, 10 , 12 , 13 implementing a vicious cycle.
To achieve an early diagnosis and assessment of sarcopenia or cachexia, screening procedures in clinical settings are needed. 10 , 11 , 14 , 15 , 16 , 17 Several blood biomarkers have been investigated as they represent easy‐accessible and non‐invasive potential hallmarks to discriminate between individuals at high and low risk to develop muscle wasting conditions.
Denervation and NMJ degradation have been recently proposed as key determinants of age‐related muscle wasting diseases. 15 , 18 , 19 , 20 , 21 NMJ dismantling may be detected by measuring the serum concentration of the C‐terminal agrin fragment (CAF). 22 , 23 This is a 22‐kDa peptide, deriving from the cleaved protein agrin, that has been proposed as a possible biomarker for assessing NMJ‐related muscle dysfunction. 24
Ever since the first reports by Drey et al., 24 an increasing number of studies have investigated CAF concentration in different populations at muscle dysfunction risk, ranging from sarcopenic, 25 , 26 to cachectic, 27 to diseased patients. 28 , 29 , 30 , 31 , 32 , 33
The focus of the most updated guidelines shifted from the ‘single‐biomarker explains all’ to the search for a battery of circulating biomarkers able to address and discriminate the pathogenesis of different muscle wasting conditions. 17 , 30 However, we believe that a narrative review summarizing and discussing the findings concerning CAF as a biomarker of NMJ instability and possibly of muscle dysfunction and wasting is still lacking. This may be a useful tool for those who intend to include CAF in the list of biomarkers assessed both in research studies and clinical practice.
Thus, we provide, for the first time, an overview of all the studies measuring cross‐sectional and longitudinal changes in circulating CAF levels in ageing, sarcopenia, muscle wasting conditions such as diabetes, COPD, chronic heart failure, stroke and cancer cachexia as well as in response to disuse and physical activity. In addition, some methodological aspects concerning CAF assessments and the directions that may be pursued by future research in this regard are highlighted.
CAF: where does it come from? The agrin pathway and its relevance in NMJ maintenance
Agrin (from the Greek ‘agrein’, meaning ‘to assemble’) was firstly described in 1987 by Nitkin and colleagues, who purified it from the basal laminae of the electric organ of Torpedo californica , a giant homologue of the NMJ. 34 In 1990, McMahan postulated the so‐called 'agrin hypothesis', 35 stating that agrin is a nerve‐derived trophic factor, responsible for the assembly of the post‐synaptic apparatus in vivo. Few years later, the main predictions of the ‘agrin hypothesis’ were proved by experimental evidence: agrin‐deficient mice died because of a lack of NMJ formation, and forced agrin expression or injection in non‐synaptic regions of innervated muscles established the formation of an ectopic and fully differentiated post‐ synaptic apparatus. 36 , 37 , 38
To date, we have gained several insights into the structure and roles of agrin, whose core protein is known to have a molecular mass of about 225 kDa. However, it is extensively glycosylated at its NH2‐terminal half; thus, it migrates around 400–600 kDa on SDS‐PAGE. 39 Agrin can undergo differential splicing leading to the formation of many isoforms. 40 Essentially, two main different amino‐terminus can be formed: (i) one encoding for a cleaved signal sequence (SS) and an amino (N)‐terminal agrin domain (SS‐NtA agrin), which allows binding to laminins; (ii) the other encoding for a shorter amino‐acid terminus that converts the protein into a type II transmembrane protein (TM agrin), unable to bind to laminins. 41 , 42 SS‐NtA agrin is expressed in those tissues containing basal lamina, such as the NMJ and the muscle, whereas TM agrin is present in many cells of the central nervous system, where basal lamina is absent. 40 Additionally, in the carboxy‐terminal laminin‐globular domains 2 (LG2) and 3 (LG3), two other differential splicing sites are present, named A/y and B/z. 43 A/y site can contain 0 or a 4‐amino acid insert; B/z site can contain 0, 8, 11 or 19 (8 + 11) amino acid inserts. 43 Importantly, the B/z site inserts are crucial for agrin‐induced AChR aggregation capacity, as only the isoforms that contain an insert are able to induce AChR clustering. 44 These isoforms are mainly expressed by neurons and motor neurons (neural agrin), while being absent in skeletal muscles that only contain agrin isoforms without B/z inserts (muscular agrin). 40
At the NMJ level, SS‐NtA neural agrin is bound to the NMJ basal‐lamina laminins; it activates the single transmembrane receptor tyrosine kinase MuSK (muscle‐specific kinase), via its binding to MuSK co‐receptor low‐density lipoprotein receptor‐related protein 4 (Lrp4). 39 Neural agrin carboxy‐terminal LG3 domain has been shown to bind the YWTD repeat‐containing β‐propeller of Lrp4, inducing MuSK phosphorylation and activation. 45 From the cytoplasmic side, the protein downstream of tyrosine kinases‐7 (Dok‐7) also binds to MuSK to allow for its compete activation. 46 MuSK activation is responsible for the formation of the postsynaptic apparatus, inducing AChR clustering and anchoring to the NMJ postsynaptic membrane (see Figure 1). 39 , 47
Figure 1.
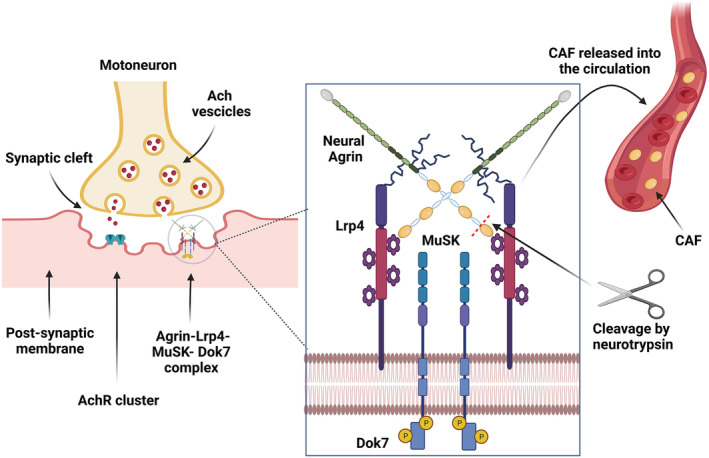
The agrin pathway. Agrin complex (Agrin, Lrp4, MuSK, Dok7) localization within the neuromuscular junction structure (right panel); agrin complex detailed structure and site of proteolytic cleavage of C‐terminal agrin fragment (CAF) by neurotrypsin (middle panel); schematic representation of agrin cleavage by neurotrypsin and CAF release within the blood circulation (middle and right panels).
Neural agrin presence at the synaptic cleft is regulated by its proteolytic cleavage. 22 Stephan et al. showed that the pre‐synaptic held enzyme neurotrypsin, whose activity is regulated by pH and calcium concentration, 48 when released was able to cleave agrin locally at the nervous system synapses. The authors further reported that agrin cleavage by neurotrypsin induced the release of a 90‐kDa and a 22‐kDa fragment from the C‐terminal end 22 (Figure 1). Two years later, Bolliger et al. demonstrated that agrin cleavage at the NMJ determined its maturation: Overexpression of neurotrypsin, leading to an increased agrin cleavage, caused precocious maturation of NMJs followed by their disassembling within few days. 23
Importantly, muscular agrin co‐localizes with AChRs at the post‐synaptic site of the NMJ and is also cleaved by neurotrypsin. Its expression is regulated by the interactions with neurons and collaborates with neural agrin to organize NMJ formation. 49
In 2011, Bütikofer et al. 50 demonstrated in vivo that neurotrypsin overexpression, leading to excessive agrin cleavage, resulted in a muscle phenotype typically observed in advanced ageing (reduced number of muscle fibres, increased heterogeneity of fibre thickness, more centralized nuclei, fibre‐type grouping and an increased proportion of type I fibres) as well as NMJ fragmentation. Such muscle phenotype is referred as ‘sarcopenic’. The authors also observed that the absence of post‐synaptic AChR aggregates in neurotrypsin overexpressing mice was always linked with the absence of C terminal agrin‐22 fragment (i.e. CAF) and that loss of CAF at the NMJ preceded AChR dispersal. However, in neurotrypsin‐null mice, the age‐dependent sarcopenic phenotype was still developed, thus highlighting that both ageing per se and NMJ dismantling could be two phenomena contributing to muscle wasting and weakness. Three years later, Hettwer et al. 51 treated neurotrypsin‐overexpressing mice (presenting the sarcopenic phenotype) with a neurotrypsin‐resistant compound (NT‐1654) derived from murine agrin. The authors showed treated animals to display an almost full rescue of muscle weight and fibre number, strength and NMJ morphology.
From this body of literature, the European Working Group on Sarcopenia in older people (EWGSOP) proposed, in 2012, that the investigation of a biomarker of NMJ stability (CAF) might be useful when assessing sarcopenia. 52
The following year, Drey et al. proved CAF to be released and detectable within the blood circulation. The authors measured CAF concentration in old sarcopenic and non‐sarcopenic patients by using western blot, reporting this biomarker to be higher in the first than in the second group and to increase with age. 24 , 26
From 2013, many studies investigated CAF concentration in serum and plasma from patients belonging to several populations at risk of developing muscle wasting and weakness by using ELISA techniques.
CAF assessment: which conditions, and which results?
CAF in ageing and sarcopenia
The main findings concerning circulating CAF measurement in ageing and sarcopenia are summarized in Table 1.
Table 1.
CAF and ageing, sarcopenia or frailty. Total number of participants, number of sarcopenic (S), non sarcopenic (NS), healthy controls (HC) or physically frail and sarcopenic (PF&S) and non‐physically frail and sarcopenic (NPF&S) participants involved in the studies
| Population | Finding | Reference | |||
|---|---|---|---|---|---|
| N (sex) | Age (yrs) | Definition of sarcopenia | CAF concentration | Correction applied | |
| Ageing | |||||
| 169 all‐age individuals (86 F, 83 M) | 19–74 | N/A | Increase with increasing age, more marked in S | None | Hettwer et al., 2013 26 |
| 200 healthy adults (103 F, 97 M) | 18 to over 65 | N/A | Increase with increasing age, significant in females | Age | Yu et al., 2017 53 |
|
15 young M 15 old M |
21 (young) 72 (old) |
N/A | No differences | None | Hester et al., 2022 54 |
| Sarcopenia | |||||
|
133 elderlies 73 S; 60 HC (62 F; 71 M) |
71 | EWGSOP(Cruz‐Jentoft et al., 2010) | S > HC* | Age, sex, disease, confounding effects | Hettwer et al., 2013 26 |
|
42 hip fractured elderlies 7 S; 35 NS (32 F, 10 M) |
83 | EWGSOP(Cruz‐Jentoft et al., 2010) | S > NS* | Variables showing a significant difference between S and NS at the univariate analysis | Marzetti et al., 2014 32 |
|
332 elderlies 101 S; 231 NS (225 F, 107 M) |
85–88 | EWGSOP(Cruz‐Jentoft et al., 2010) | S > NS* | Age‐cognitive capacity, daily physical activity, disease | Landi et al., 2016 25 |
| 150 very old hip‐fractured 140 S; 9 NS (118 F, 32M) | 87 |
EWGSOP2(Cruz‐Jentoft et al., 2019) |
S > NS |
None | Sanchez‐Castellano et al., 2020 59 |
| 87 COPD and chronic heart failure free elderlies (87 M) | 63 | Low SPPB score or SARC‐F questionnaire | S > NS* | None | Qaisar et al., 2021 30 |
|
300 elderlies 31 S; 269 NS (150 F; 150 M) |
64 | EWGSOP2(Cruz‐Jentoft et al., 2019) | S > NS* | Age and BMI | Pratt et al., 2021 60 |
|
200 elderlies 100 PF&S; 100 NPF&S (125 F, 75 M) |
75–78 | EWGSOP(Cruz‐Jentoft et al., 2010) | PF&S males > NPF&S males | Analysed through a multi‐block method called SO‐CovSel | Calvani et al., 2021 17 |
SPPB, Short Physical Performance Battery; yrs, years.
SARC‐F questionnaire: tool developed to assess sarcopenia. It comprises various measures of functional independence such as strength, assistance in walking, rising from a chair, climbing stairs and falls.
Statistically significant.
Hettwer et al. 26 showed that ageing per se resulted in a significant increase in CAF concentration from young (19–29 years) to middle (30–59 years) and old‐age (60–74 years) with no significant gender differences. Similar results were observed in a Chinese population, 53 although the age‐related CAF differences were significant only among females. A recent study reported lower, but non‐significant, CAF values in younger than healthy older people 54 ; significance may have not been achieved due to the small sample size (15 young and 15 elderly).
Interestingly, in longitudinal studies, Bondoc et al. 55 reported a higher increase in CAF concentration within the oldest participants over a period of 12 months, and Gagliano‐Jucá et al. 56 observed CAF increments in aged individuals with low muscle mass and function in a 6‐month study.
Taken together, these findings suggest that ageing is linked to increased circulating CAF concentration, likely because of NMJ degeneration and increased denervation known to accompany the ageing process. 19 , 57 , 58
Higher circulating CAF levels have been documented also in sarcopenia. The first paper investigating this topic, by Hettwer et al., 26 stratified sarcopenic patients in high‐CAF and low‐CAF holders. The latter group presented CAF concentration very similar to those of the age‐matched healthy counterpart involved in the same study. The authors concluded that CAF was a biomarker able to distinguish between who developed a ‘neurogenic sarcopenia’ (high CAF) from those developing a ‘natural muscle aging related sarcopenia’ (low CAF). 26 However, all the following studies pooled together sarcopenic participants, without considering low‐CAF versus high‐CAF individuals, and this aspect was no longer investigated.
Marzetti et al. 32 and Sanchez‐Castellano et al. 59 reported higher CAF serum levels in old to very old sarcopenic hip‐fractured patients than in non‐sarcopenic ones. Similarly, sarcopenic individuals with chronic heart failure or COPD were shown to have higher CAF than disease‐matched, non‐sarcopenic ones. 29 , 30
Landi et al., 25 in 2016, observed higher CAF concentrations in sarcopenic versus non‐sarcopenic people within a prospective cohort of 332 participants, also when adjusting the values for age, sex and different pathological conditions and confounding factors, including congestive heart failure, lung disease, diabetes and renal failure. Following studies confirmed these findings. 30 , 60
Interestingly, a well‐designed, recent study searching for a battery of sarcopenia‐associated biomarkers reported that, based on the data collected and the mathematical model employed, CAF might be a reliable sarcopenia‐associated biomarker only in males. 17
From the above‐mentioned studies, a trend for higher serum CAF concentration in sarcopenic versus non‐sarcopenic individuals emerges, although not always significant. Likely, this is due to the fact that (i) the studies evaluating CAF in sarcopenic patients do not report whether this was likely primary or secondary. 6 Participants enrolled in these studies were neurological, inflammation and cardiovascular‐disease free (unless otherwise stated), or a correction for these conditions was applied. Hence, disease‐related secondary sarcopenia is not expected to significantly influence the results reported. On the other side, it is well known that ageing is accompanied by a decreased physical activity 7 a recent survey showed that in 16 European countries, the overall prevalence of inactivity among individuals aged 55 or older is 12.5%, ranging from 4.9% (Sweden) to 29% (Portugal). 8 Because inactivity seems to enhance CAF concentration (see next section), this should be considered as an important factor to be used for CAF concentration corrections. (ii) It is important to emphasize that the definition of sarcopenia has been changing over time, shifting the focus from the sole ‘loss of muscle mass’ 61 to similar importance of loss of muscle mass and strength or functionality, 62 to finally emphasize more the loss of muscle force than that of muscle mass or quality. 3 Recently, sarcopenia has also been recognized as a disease. 63 Coherently, the criteria to diagnose sarcopenia have been modified to meet its updated definition. 3 As such, the studies reported over time defined ‘sarcopenic’ participants with different features, thus potentially explaining the partial discrepancies between the reported results.
Nonetheless, it seems worth pointing out that most reports suggest that CAF might be a good candidate, if included in a clinical routine together with other biomarkers, to distinguish between non‐sarcopenic and sarcopenic people. This finding supports the concept of an important role played by increased NMJ dismantling and muscle denervation as co‐factors for sarcopenia. 15 , 18 , 19 , 21
CAF in cancer cachexia and other muscle wasting diseases
A recent study investigating the possible mechanisms of cancer‐induced muscle wasting (i.e. cachexia) in colorectal and pancreatic patients reported for the first time fibre‐type grouping and increased CAF concentration in pre‐cachectic and cachectic patients when compared with age‐matched controls. These findings suggest that early instability of the NMJ precedes the marked atrophy and the higher amounts of denervated fibres present in cachectic patients. 27 Accordingly, in a preclinical murine model of cancer cachexia, the authors observed that denervation and NMJ morphological alterations preceded the onset of muscle atrophy. Moreover, NMJ functional alterations were observed in the muscles of cachectic mice. 27 These results, supporting and further developing the denervation issue described in a previous report, 64 propose the concept of denervation and NMJ impairment as factors potentially involved in the pathogenesis of muscle wasting in cancer cachexia, even if only longitudinal studies in humans will confirm a causality link. On the other hand, Boehm et al. 65 showed NMJs to be morphologically stable among 10 oesophageal patients with cancer cachexia; however, CAF concentration was not measured in this study.
Hence, cachexia‐induced muscle wasting might derive, at least in some cancer types, also from denervation and NMJ instability, thus highlighting CAF as a potential biomarker to assess disease progression towards cachexia in some cancer patients.
CAF was shown to be higher than controls also in other diseases where mild‐to‐severe muscle wasting or dysfunction is developed such as chronic heart failure 29 , 30 and acute stroke 31 and patients with COPD or other pulmonary diseases. 28 , 30 , 66 , 67 A recent work reported CAF to be higher in patients affected by type 2 diabetes compared with pre‐diabetic and control volunteers; in this context, CAF also positively correlated with the concentration of glycated haemoglobin, a marker of diabetes progression. 68 The authors reported diabetic patients to present lower muscle strength and quality, also correlating with higher CAF concentration. Thus, CAF might be a useful marker also when assessing muscle dysfunction in different muscle‐wasting‐inducing diseases.
CAF and inactivity
Given the increased attention that CAF has gained as a biomarker of muscle dysfunction, recent studies have considered the sole inactivity‐related changes of CAF in young, healthy populations undergoing unloading protocols.
In a recent short‐term (10 days) bed rest study, we found that CAF concentration raised by about 19% in healthy young males and that this was accompanied by initial and partial signs of denervation in their muscle biopsies. 69 Conversely, a study by Ganse et al. assessed CAF variations through a longer bed rest (60 days) with or without 30 min/day of continuous or intermittent permanence in human centrifuges generating gravity forces similar to those experienced on Earth. CAF was unaltered at the end of the 60‐day bed rest independently from the experimental condition. 70 Accordingly, the authors observed no changes in muscle wasting biomarkers. Importantly, 60 days of bed rest represent a very long‐term unloading, and the acute CAF increments observed after 10 days in our study may have been blunted by the end of the 2‐month observation time. Indeed, CAF cleavage could have been stabilized because the NMJ remodelling due to unloading reached a steady state. In the study by Ganse et al., 70 CAF concentrations were much lower (about 10 times) than those reported in all the other papers investigating this biomarker and had a high variability among the three groups (average mean of controls, continuous or intermittent centrifugation: 129, 344 and 65 pg/mL, respectively) and the time course considered, thus the comparison results to be difficult. Another recent work from Narici's group showed a 5.5% increase in CAF after 10 days of unilateral lower limb suspension. 71 This seems reasonable as unilateral lower limb suspension is considered a milder unloading model compared with bed rest, due to the smaller amount of muscle mass subjected to inactivity.
In conclusion, it seems likely that whole‐body unloading induces acute increase in CAF concentration also in healthy, young people, which we observed to rapidly decrease already after 2 days of reloading (unpublished data). This concept would support the evidence of an early‐induced morphological NMJ remodelling with unloading, although whether such phenomenon would precede (and cause) or accompany muscle atrophy is currently unknown. Further, such observed CAF raising induced by inactivity may corroborate the findings showing CAF to be higher in the more prone‐to‐inactivity sarcopenic population. As no longitudinal studies have determined the effects of inactivity‐related CAF variations in elderly, this aspect remains to be investigated.
CAF and physical exercise
As one of the most effective strategies to counteract muscle wasting and weakness is physical exercise, which is also well known to have positive effects on NMJ 72 and reinnervation, 73 it is not surprising that many research groups focused their attention on the effects of different training modalities on circulating CAF levels, especially in the ageing population. The results of the studies conducted so far, together with the training mode and duration, are summarized in Table 2.
Table 2.
CAF and physical activity. Sample size, average age and sex of the different population undergoing exercise training protocols and the effects of training intervention of CAF concentration
| Population | Exercise type | Duration | CAF changes | Reference | ||
|---|---|---|---|---|---|---|
| N | Age(yrs) | Sex | ||||
| 69 pre‐frail (according to previous study 74 ) elderlies | 76–79 | M + F |
‐ Power training ‐ Strength training ‐ Control |
12 wks |
|
Drey et al., 2013 24 |
| 23 elderlies | 70–71 | M + F |
‐ Resistance training ‐ Control |
6 wks | = (trend for increase) in intervention group | Fragala et al., 2014 76 |
| 26 elderlies | 70–72 | M + F |
‐ Skiing ‐ Control |
12 wks | = | Narici et al., 2015 80 |
| 333 elderlies with SPPB <9 | 77 | M + F |
‐ Mixed aerobic, strength and balance training ‐Control (health education) |
12 mo. | = | Bondoc et al., 2015 55 |
|
12 peri‐menopause 23 post‐menopause |
48 57 |
F | ‐ Resistance training | 10 wks |
|
Willoughby et al., 2020 77 |
| 30 elderlies | 60 | M |
‐ Functional training ‐ Functional training with blood flow restriction ‐ Control |
6 wks | ↓ in intervention groups | Bigdeli et al., 2020 75 |
| 46 obese elderlies mild‐to‐moderately frail | 71 | M + F |
‐ Aerobic training ‐ Aerobic and resistance training ‐ Resistance training ‐ Control |
6 mo. | = | Colloluori et al., 2020 78 |
| 37 healthy elderlies | 71 | M + F |
‐ Dance ‐ General fitness training |
24 mo. |
|
Franchi et al., 2022 79 |
| 24 healthy elderlies | 63 | M + F |
‐ Dual task (treadmill and cognitive) ‐ Dual task and blood‐flow restriction ‐ Control |
8 wks |
|
Kargaran et al., 2021 82 |
| 123 patients after stroke | 70 | M + F | ‐ Rehabilitation programme | 4 wks |
|
Scherbakov et al., 2016 31 |
| 84 patients with COPD | 70 | M | ‐ Pulmonary rehabilitation | 6 mo. |
|
Karim et al., 2021 67 |
=, non changes; ↑, increase; ↓, decrease; mo., months; wks, weeks.
Overall, these studies report less coherent results than the ones focusing on CAF and sarcopenia. Here, we provide a comprehensive overview of those studies and the specifics of each training regime employed in order to contribute new tools for the interpretation of the data reported in the literature.
The majority of the longitudinal studies investigating CAF serum levels in response to exercise were focused on non‐sarcopenic or pre‐frail (according to previous study 74 ) elderly 24 , 75 , 76 , 78 , 79 , 80 ; only few works were aimed at assessing longitudinally training effects in populations of elderlies with low muscle mass and function. 55 In two cross‐sectional studies, the levels of CAF were assessed within active and inactive non‐sarcopenic and healthy elderly populations. 79 , 81
Some authors reported decreased CAF concentrations following training interventions 31 , 67 , 75 , 82 ; others observed no changes 55 , 78 , 79 , 80 or even a trend for increase in CAF. 76 In addition, in two studies, CAF varied differentially in response to the same exercise protocol between groups with low versus high baseline CAF concentration 24 or pre‐menopausal versus post‐menopausal women. 77 Therefore, there are apparent discrepancies within the reported results. However, when critically looking at these studies, some interesting elements emerge. (i) The mode, duration, intensity and volume of exercise were very different; (ii) the sex and hormonal status of the involved participants were unequal; (iii) the healthy or sarcopenic condition was thoroughly stratified only in few studies.
Exercise and decreased CAF
Drey et al.24 reported a trend for a better effect of power training compared with strength training in decreasing CAF. 24 Bigdeli et al. employed a functional‐type, balance‐based training, 75 which was effective in reducing CAF, whereas Kargaran et al. 82 reported CAF to, respectively, decrease and trend to decrease after a combined aerobic and cognitive training with and without blood‐flow restriction. 82 The blood‐flow restriction applied to the dual aerobic and cognitive training by Karagan et al.was the determinant for a significant versus a not significant decrement in CAF, as blood‐flow restriction is known to increase the exercise intensity. Also two studies by Narici's group 79 , 81 showed that active individuals, practicing dance, presented lower CAF values than sedentary peers. When training for 6 months two groups of elderly, the one practising dance presented decreased CAF values, whereas in the group practising general fitness CAF was unchanged. 79 These results suggest that the intensity and the type of physical activity might play a very important role in inducing changes in circulating CAF. Importantly, activities involving fine coordination and cognition (such as dance and balance based or cognitive training) were able to reduce the circulating levels of this biomarker, potentially acting on mechanisms involved in NMJ integrity to a higher extent than other training modalities (such as general fitness training). 75 , 79 , 81 In a context of rehabilitation, two studies investigated circulating CAF on patients affected by stroke 31 or COPD 67 and found them to be decreased after the rehabilitation physical intervention.
Exercise and unchanged CAF
It is interesting to note that the majority of the studies in which no differences in the trained groups were detected had long duration (i.e. at least 6 months). 55 , 78 Bondoc et al. 55 reported no differences in the control, non‐exercising group after 1‐year follow‐up observation in low muscle function individuals 55 ; the comparison with the intervention group performing physical exercise showed no difference, although the authors stated that the adherence to the training protocol in the last months was lower. Colleluori et al. 78 trained for 6 months obese elderlies with either only aerobic, only resistance training or a combination of both, also prescribing a diet to their participants. 78 The authors observed no variations in CAF after the three training interventions and suggested that in the context of obesity, exercise training was able to preserve but not improve NMJ health over 6 months although diet‐induced body weight loss was experienced. 78 In these contexts, the training intervention might have only helped maintaining CAF concentration, preventing its raise, instead of resulting in a lowering of this parameter.
Exercise and increased CAF
Only in a short‐term interventional study (6 weeks) conducted by Fragala et al. 76 a resistance training in elderlies 76 was not able to induce any change (or, even more, seemed to tend to an opposite result) in CAF concentration. From the above‐mentioned studies reporting no changes in circulating CAF serum levels, it could be speculated that resistance training, not involving fine motricity and coordinative tasks, may produce less beneficial effects on the NMJ.
On the contrary, Gagliano‐Jucá et al. 56 observed a raise in CAF concentration over 6 months in frail, old individuals; interestingly, testosterone administration was not able to prevent such raise, despite increasing muscle strength. The authors suggested that this hormone exploits pathways other than a restore in NMJ stability to induce force ameliorations. 56
Exercise and differential effects of the same protocol on CAF
Lastly, Willoughby et al. 77 showed that resistance training resulted in increased CAF in peri‐menopausal women, although it was able to reduce CAF in post‐menopausal ones. These results stress the importance of sex and hormonal status in regulating CAF concentration in the bloodstream.
Hence, contrarily to the conditions of ageing, sarcopenia or inactivity, it is difficult to draw definite conclusions on the effects of physical exercise on CAF circulating concentrations. Indeed, only some speculation may be done, as studies directly comparing different training modalities (e.g. rehabilitation programmes, resistance training, functional training or dance and aerobic training) in different populations (males, females, healthy or sarcopenic) are lacking or involve small sample size or confounding effects (such as weight loss). Thus, so far, what seems important is to consider sarcopenia, sex and hormonal status, together with the intensity, duration and type of the intervention, when planning and interpreting the results of studies assessing training‐induced CAF variations.
CAF: a marker of muscle wasting or of muscle weakness?
As discussed in the previous paragraphs, CAF has been investigated over the years in different young or aged and healthy or diseased populations, also before and after training or unloading protocols, and the associations of this biomarker with indexes of muscle mass and function have been reported (summarized in Table 3).
Table 3.
CAF concentration and its association to parameters of muscle wasting and weakness. Muscle mass and function parameters and their correlation with CAF cross‐sectionally or longitudinally
| Type of variable | Studies finding associations with CAF | ||
|---|---|---|---|
| Reference | Sex | Type of association, type of study | |
| Appendicular lean mass | Drey et al., 2013 24 ; Qaisar et al., 2021 30 | M | Negative, cross‐sectional |
| Landi et al., 2016 25 | F+M | Negative (trend in M), cross‐sectional | |
| Pratt et al., 2021 60 | M + F | Negative, cross‐sectional | |
| Racha et al., 2022 68 | Not specified | Negative, cross‐sectional | |
| Hester et al., 2022 54 | M | No association, cross‐sectional | |
| Vastus lateralis cross‐sectional area | Fragala et al., 2014 76 | M + F | Positive, longitudinal |
| Vastus lateralis + rectus femoris cross‐sectional area | Hester et al., 2022 54 | M | No association, cross‐sectional |
| Handgrip strength | Landi et al., 2016 25 | M + F | Negative, cross‐sectional |
| Qaisar et al., 2020a 66 and 2020b 28 ; Pratt et al., 2021 60 | M | Negative, cross‐sectional | |
| Steinbeck et al., 2015 29 | M + F (patients with chronic heart failure) | Negative, cross‐sectional | |
| Karim et al., 2021 67 | M | Negative, longitudinal | |
| Scherbakov et al., 2016 31 | M + F (only stroke patients increasing ALM) | Negative, longitudinal | |
| Gait speed | Bondoc et al., 2015 55 ; Landi et al., 2016 25 | M + F | Negative, cross‐sectional |
| Steinbeck et al., 2015 29 | M + F (patients with chronic heart failure) | Negative, cross‐sectional | |
| SPPB total score | Bondoc et al., 2015 55 | M + F | No association, cross‐sectional |
| 1RM strength (knee‐extension and chest press) | Bigdeli et al., 2020 75 | M | Negative, cross‐sectional |
| Fragala et al., 2014 76 | M + F | No association, longitudinal | |
| 1 RM strength (leg press and chest press) | Gagliano‐Juca et al., 2018 56 | M | No association, longitudinal |
| Isometric specific peak torque | Hester et al., 2022 54 | M | No association, cross‐sectional |
| Static/dynamic balance | Bigdeli et al., 2020 75 | M | Negative (static); positive (dynamic), cross‐sectional |
| Marcolin et al., 2021 81 | M + F | Negative, cross‐sectional | |
| Muscle quality based on measurement of maximum isometric strength (60°) and maximum isokinetic strength | Racha et al., 2022 68 | Not specified | Negative, cross‐sectional |
| Electromyographic activity during neuromuscular fatigue test | Stout et al., 2015 88 | M | Negative, cross‐sectional |
F, females; M, males; SPPB, Short Physical Performance Battery; RM, repetition maximum
Two cross‐sectional studies reported CAF to be negatively correlated with appendicular lean mass (ALM), only in male participants. 24 , 30 Landi et al. 25 and Pratt et al. 60 found an inverse correlation between CAF and ALM, but the first study observed this association only in females (and a trend in males), whereas the second observed this finding in both genders. 60 On the contrary, Hester et al. 54 reported no correlation between muscle cross‐sectional area or ALM and this biomarker. 54
In longitudinal studies, only Fragala et al. 76 reported that the increase in muscle mass or morphology were positively correlated with changes in CAF, 76 whereas other reports suggest that higher CAF is associated with lower muscle/lean body mass or increased muscle wasting in patients affected by type 2 diabetes, chronic heart failure or acute stroke. 29 , 31 , 68
Physiologically, the rationale for proposing CAF as a biomarker of muscle wasting is that increased NMJ destabilization (resulting in higher agrin cleavage at the synapses and thus increased CAF blood concentration) would determine atrophy of the muscle fibres whose NMJs are dismantled. Such NMJ disarrangement could be influenced by both motor neuronal degeneration and failure of reinnervation (from the nerve side), 83 alterations of autophagy, 84 mitochondrial function, 85 protein synthesis inhibition 86 and increased ROS accumulation 87 in muscle fibres (muscle side).
So far, some reports (cited above) seem to suggest that CAF may be quite sensitive to these neuromuscular changes; however, deeper investigation should clarify their underlying mechanism.
On the other hand, some studies linked CAF concentration to functional parameters such as handgrip strength, gait speed, short physical performance battery (SPPB) or frailty scales (summarized in Table 3), reporting conflicting results: some found different degrees of negative correlation between CAF and handgrip or gait speed in cross‐sectional studies 25 , 28 , 30 , 55 , 60 , 66 or between CAF and handgrip in longitudinal studies. 31 , 67 Further, some studies found negative associations between CAF and 1RM strength, 75 static/dynamic balance, 75 , 81 muscle quality based on measurements of maximum isometric or isokinetic strength 68 or neuromuscular activity during fatiguing tests. 88 Interestingly, some of these correlations were observed to be gender specific. 60 , 88 Conversely, other studies reported no correlation between parameters of muscle function and CAF. 54 , 55 , 56 , 76
The rationale behind a link between increased CAF and a decline in muscle function would be explained by two elements: (i) NMJ disruption, which causes disconnection from muscle fibres that become denervated, atrophic and unfunctional; (ii) NMJ remodelling, which might affect the transmission of action potentials to the muscle fibre. As for the first point, many studies have shown ageing to be accompanied by a reduction of motor unit number. 83 , 89 The reduced reinnervation capacity of denervated fibres, together with the increased instability of the dismantled NMJs, 90 , 91 would contribute to the decline in muscle function. On the other hand, surviving NMJs with an altered morphology (potentially contributing to CAF elevation) might be less efficient in transmitting action potentials, although this event has been suggested to happen very late during the lifespan. 92 , 93 Thus, it might be possible that in very old subjects, the correlation between CAF and functional parameters could be stronger than in younger ones, helping to explain some discrepancies observed among the presented results.
Overall, most of the literature seems to suggest that CAF could be quite sensitive in detecting changes in muscle mass or function; however, more investigation concerning age, sex and other confounding factors dependent on such relationship must be addressed before a definitive conclusion is drawn.
Methodological considerations
A last but very important aspect that deserves attention concerns the different CAF concentrations reported over the studies published in the literature.
Indeed, when trying to establish cut‐offs of a biomarker, age, sex, race and co‐morbidities should be considered; furthermore, also the technique used for its measurement may be relevant. Both aspects are essential to correctly interpreting the results and compare studies from different laboratories.
Concerning age, sex and race, we have listed several studies showing that these parameters need to be considered. 25 , 26 , 53 , 60 Co‐morbidities assessment may also be crucial, as demonstrated by the higher circulating CAF levels observed in the muscle dysfunction‐inducing chronic heart failure, 29 , 30 stroke, 31 pulmonary diseases, 28 diabetes 68 and cancer cachexia. 27
Importantly, also renal function has been linked to raised circulating CAF, because this biomarker has been reported to be higher in patients developing acute kidney injury 33 and undergoing kidney transplantation. 53 , 94 Two days after transplantation, CAF concentration was observed to significantly decrease and reach the controls levels, remaining stable until at least 6 months after surgery. 53 An extremely important note is that agrin has been reported to be expressed in kidney, contributing to the formation of the glomerular basement membrane (GBM). 94 Thus, increased CAF concentration in these patients has been hypothesized to be due either to reduced glomerular filtration/tubular secretion or to increased degradation of the GBM causing a decline in glomerular function or their loss. 94 As animal studies investigating glomerular formation in agrin‐deficient mice demonstrated that no differences in the structure of glomeruli and renal function were observable, 95 a decrement in renal clearance [measurable as a reduced glomerular filtration rate (GRF)] was proposed as the predominant determinant of the higher CAF concentration observed in patients with kidney dysfunction. 53 Supporting this concept, several studies reported strong positive correlation between CAF concentration and creatinine (a well‐established marker of kidney functionality) and negative correlation between CAF and estimated GFR in patients with chronic kidney disease 96 and undergoing kidney transplantation. 53 , 94 In a 57‐week study, CAF was reported to have a high predictive power in determining rapid kidney function decline in patients affected by chronic kidney disease, independently from estimated GFR. 97 Hence, although the authors acknowledged that NMJ‐derived CAF may play a partial role also in the elevated concentrations observed among kidney‐dysfunctional patients, the presence of kidney‐related diseases seems an independent predictor of elevated CAF. In this context, particular attention should be paid when investigating CAF in diabetic patients, as this pathology is the leading cause of chronic kidney disease. 98 Devetzis et al. 98 measured serum CAF concentration, estimated GFR and proteinuria in spot urine in type 2 elderly patients with diabetic nephropathy and observed a negative and a positive correlation between CAF and estimated GFR and CAF and proteinuria, respectively, at baseline and after 12 months of follow‐up. 98 Similar results were reported in a separate cohort on one abstract published by Roos et al. 99 Additionally, higher CAF concentration in diabetic nephropathic patients was also associated with progression to end‐stage renal disease within 24 months of follow‐up. 98 These results strongly suggest that, both in the general population and specifically in diabetic individuals, a detailed medical history of the patient and a follow‐up to exclude that chronic kidney disease is the cause of the higher CAF observed are essential for a correct interpretation of the elevated concentration of this biomarker.
Concerning the different detection methodologies, CAF was originally measured by using the western blot technique, 24 , 26 which was quickly replaced by ELISA immunosorbent assays. ELISA kits produced by different brands have been used in various studies, and the concentrations reported vary from 3.6 88 to 18–340 70 , 77 , 100 to about 20 300 53 pg/mL. The last value was measured in patients before kidney transplantation and might lead to misleading interpretation due to the concomitant kidney disease. However, the majority of the studies on healthy subjects reported ranges between 1700 and 5000 pg/mL on average, 25 , 26 , 29 , 31 , 32 , 53 , 60 , 66 , 69 , 71 , 78 , 79 , 81 with increasing concentration as age increases. In general, using ELISA kits produced by different companies led to different concentrations estimation, but most of the studies using the commercially available kits provided results that remained within the above‐indicated range. Only four studies 70 , 77 , 88 , 100 reported values in a very different scale (more than 10‐fold lower) compared with the remaining body of literature.
Overall, in sarcopenic volunteers or participants with low muscle function, CAF was reported to range from 2100 to 6400 pg/mL, 24 , 25 , 26 , 56 and in patients with different co‐morbidities from 2300 to 20 300 pg/mL. 28 , 29 , 30 , 31 , 32 , 53 , 59 , 66 , 67 Although there is still quite a high variability, it is noteworthy that, within the same study, CAF values proportions were maintained (sarcopenic participants presented values significantly higher than their healthy counterparts, elderly people displayed higher values than young, and diseased patients had higher CAF than controls).
Importantly, by using WB, Hettwer et al. 26 reported that they were able to purify muscular B/z negative (i.e. possessing no aminoacidic insertion at the B/z splicing site) and neural B/z positive (possessing 8‐aminoacidic insertion at the B/z splicing site) isoforms of agrin. However, they used the B/z negative isoform as reference standard for the calculation of serum CAF concentration in their sample. 26 Similarly, the two most widespread ELISA kits for CAF measurement (Neurotune and Abcam) measure the B/z negative isoform of C‐terminal cleaved agrin (from the reference of Sherbakov et al. 31 and our internal analysis). Because this isoform is the one mostly expressed in muscles, and 10–20 times more concentrated than neural agrin, these authors concluded that observing a consistent increase in the cleaved muscular agrin isoform would be reflective of a higher activity of the enzyme neurotrypsin, also determining higher amounts of neural agrin cleavage. 31
However, this point should be clarified by future studies aiming to quantify circulating levels of both neural and muscular agrin also optimizing specific ELISA assays to fully establish the robustness of CAF as a marker of muscle wasting or weakness caused by NMJ degeneration.
Conclusions and future directions
In this review, we summarize for the first time the studies investigating CAF concentration in ageing, sarcopenia, disease and physical activity or inactivity (Figure 2). Overall, CAF seems a good candidate to distinguish between sarcopenic volunteers and their age‐matched, healthy peers. Similarly, CAF concentration is higher in other muscle‐wasting‐inducing diseases, such as diabetes, COPD, chronic heart failure and stroke, as well as in pancreatic and colorectal cancer cachectic patients. CAF concentrations also seem to raise following muscle unloading and to be lowered or maintained throughout time in different populations undergoing various types of exercise training and rehabilitation protocols. Hence, overall, CAF seems to be a good candidate when assessing muscle dysfunction or ‘NMJ‐related skeletal muscle status’ 14 in ageing and disease, although its reliability in monitoring the effects of exercise should be further methodically investigated.
Figure 2.
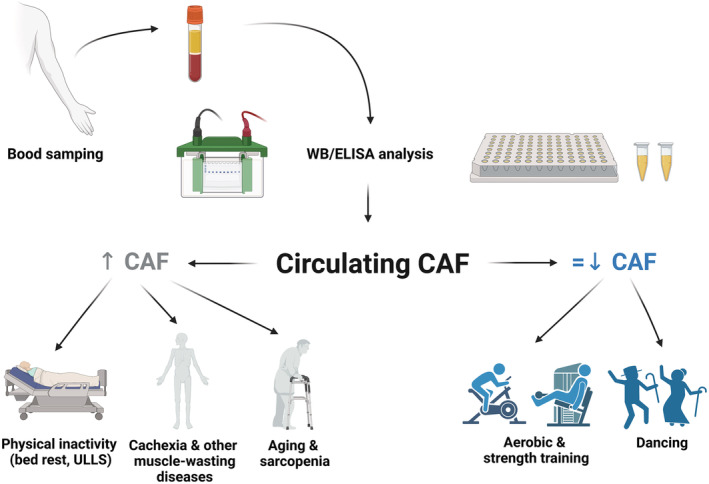
CAF and ageing, sarcopenia, disease and physical activity and inactivity. Schematic representation of CAF concentration measurement and the main findings provided by the literature. On the left side of the picture: Physical inactivity, ageing and sarcopenia, muscle‐wasting disease (in grey), as the conditions in which CAF has been reported to increase or be higher than controls. On the right side of the picture: Physical activity and dance (in blue), as the conditions in which CAF has been mainly reported to be either maintained or reduced. WB, western blot; ELISA, enzyme‐linked immunosorbent assay; ULLS, unilateral lower limb suspension; CAF, C‐terminal agrin fragment.
The direction of the future studies should be therefore aimed at (i) assessing CAF in the context of age‐adjusted, sex‐adjusted, race‐adjusted and disease‐adjusted models in combination with other biomarkers for muscle wasting assessment (this road has been explored very recently 17 ); (ii) determining which type of exercise or rehabilitation intervention is more effective to reduce CAF concentration; (iii) addressing whether CAF might better detect muscle wasting, muscle weakness or both; and (iv) extending the findings to larger cohorts in order to strengthen the results obtained.
Finally, future works should aim to an increased reproducibility of the results within different laboratories to allow for ‘standard reference cut‐offs setting’ adjusted for age, sex, race, disease and other confounding factors. This would finally result in the real possibility to insert CAF in clinical routine practice, when pertinent.
Conflict of interest
Elena Monti, Fabio Sarto, Roberta Sartori, Gianpietro Zanchettin, Stefan Löfler, Marco V. Narici and Sandra Zampieri declare they have no conflict of interests.
Funding
The present work was funded by Ludwig Boltzmann Institute for Rehabilitation Research (Vienna, Austria); European Regional Development Fund‐Cross Border Cooperation Program SLOVAKIA‐AUSTRIA (Interreg Va) project ‘Center of Active Aging’ (CAA, Sankt Poelten, Austria); Italian Space Agency (ASI, MARS‐PRE Project, n. DC‐VUM‐2017‐006, Narici); and PRIN (Progetti di Ricerca di Interesse Nazionale) project ‘NeuAge’ (2017CBF8NJ_001, Narici).
Acknowledgements
The authors would like to thank Dr Sara Tagliaferri for the discussion concerning CAF concentration data.
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 101
Monti E., Sarto F., Sartori R., Zanchettin G., Löfler S., Kern H., Narici M. V., and Zampieri S. (2023) C‐terminal agrin fragment as a biomarker of muscle wasting and weakness: a narrative review, Journal of Cachexia, Sarcopenia and Muscle, 14, 730–744, 10.1002/jcsm.13189
References
- 1. World Health Organization . Monitoring health for the SDGs annex 2. 2020.
- 2. Dhillon RJS, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med 2018;176:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petermann‐Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2022;13:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz‐Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle 2019;10:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiedmer P, Jung T, José J, Castro P, Pomatto LCD, Sun PY, et al. Sarcopenia‐molecular mechanisms and open questions. Ageing Res Rev 2020;65:101200. [DOI] [PubMed] [Google Scholar]
- 7. McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology 2016;17:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gomes M, Figueiredo D, Teixeira L, Poveda V, Paúl C, Santos‐Silva A, et al. Physical inactivity among older adults across Europe based on the SHARE database. Age Ageing 2017;46:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol 2021;17:534–548. [DOI] [PubMed] [Google Scholar]
- 10. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Prim 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 11. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 12. Sepúlveda‐Loyola W, Osadnik C, Phu S, Morita AA, Duque G, Probst VS. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2020;11:1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013;34:512–519. [DOI] [PubMed] [Google Scholar]
- 14. Kalinkovich A, Livshits G. Sarcopenia ‐ the search for emerging biomarkers. Ageing Res Rev 2015;22:58–71. [DOI] [PubMed] [Google Scholar]
- 15. Curcio F, Ferro G, Basile C, Liguori I, Parrella P, Pirozzi F, et al. Biomarkers in sarcopenia: a multifactorial approach. Exp Gerontol 2016;85:1–8. [DOI] [PubMed] [Google Scholar]
- 16. Calvani R, Marini F, Cesari M, Tosato M, Picca A, Anker SD, et al. Biomarkers for physical frailty and sarcopenia. Aging Clin Exp Res 2017;29:29–34. [DOI] [PubMed] [Google Scholar]
- 17. Calvani R, Picca A, Marini F, Biancolillo A, Gervasoni J, Persichilli S, et al. Identification of biomarkers for physical frailty and sarcopenia through a new multi‐marker approach: results from the BIOSPHERE study. GeroScience 2021;43:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casati M, Costa AS, Capitanio D, Ponzoni L, Ferri E, Agostini S, et al. The biological foundations of sarcopenia: established and promising markers. Front Med 2019;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwan P. Sarcopenia, a neurogenic syndrome? J Aging Res 2013;2013:791679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreira‐Pais A, Ferreira R, Oliveira PA, Duarte JA. A neuromuscular perspective of sarcopenia pathogenesis: deciphering the signaling pathways involved. GeroScience 2022;44:1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalez‐Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci 2014;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stephan A, Mateos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, et al. Neurotrypsin cleaves agrin locally at the synapse. FASEB J 2008;22:1861–1873. [DOI] [PubMed] [Google Scholar]
- 23. Bolliger MF, Zurlinden A, Lüscher D, Bütikofer L, Shakhova O, Francolini M, et al. Specific proteolytic cleavage of agrin regulates maturation of the neuromuscular junction. J Cell Sci 2010;123:3944–3955. [DOI] [PubMed] [Google Scholar]
- 24. Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, et al. C‐terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol 2013;48:76–80. [DOI] [PubMed] [Google Scholar]
- 25. Landi F, Calvani R, Lorenzi M, Martone AM, Tosato M, Drey M, et al. Serum levels of C‐terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community‐dwellers: results from the ilSIRENTE study. Exp Gerontol 2016;79:31–36. [DOI] [PubMed] [Google Scholar]
- 26. Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, et al. Elevated levels of a C‐terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol 2013;48:69–75. [DOI] [PubMed] [Google Scholar]
- 27. Sartori R, Hagg A, Zampieri S, Armani A, Winbanks CE, Viana LR, et al. Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci Transl Med 2021; In press. [DOI] [PubMed] [Google Scholar]
- 28. Qaisar R, Karim A, Muhammad T, Shah I. Circulating biomarkers of accelerated sarcopenia in respiratory diseases. Biology (Basel) 2020;9:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steinbeck L, Ebner N, Valentova M, Bekfani T, Elsner S, Dahinden P, et al. Detection of muscle wasting in patients with chronic heart failure using C‐terminal agrin fragment: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur J Heart Fail 2015;17:1283–1293. [DOI] [PubMed] [Google Scholar]
- 30. Qaisar R, Karim A, Muhammad T, Shah I, Khan J. Prediction of sarcopenia using a battery of circulating biomarkers. Sci Rep 2021;11:8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scherbakov N, Knops M, Ebner N, Valentova M, Sandek A, Grittner U, et al. Evaluation of C‐terminal agrin fragment as a marker of muscle wasting in patients after acute stroke during early rehabilitation. J Cachexia Sarcopenia Muscle 2016;7:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marzetti E, Calvani R, Lorenzi M, Marini F, D'Angelo E, Martone AM, et al. Serum levels of C‐terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol 2014;60:79–82. [DOI] [PubMed] [Google Scholar]
- 33. Arampatzis S, Chalikias G, Devetzis V, Konstantinides S, Huynh‐Do U, Tziakas D. C‐terminal fragment of agrin (CAF) levels predict acute kidney injury after acute myocardial infarction. BMC Nephrol 2017;18:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nitkin R, Smith M, Magill C, Fallon J, Yao Y‐M, Wallace B, et al. Identification of agrin, a synaptic organizing protein from torpedo electric organ. J Cell Biol 1987;105:2471–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McMahan UJ. The agrin hypothesis. Cold Spring Harb Symp Quant Biol 1990;55:407–418. [DOI] [PubMed] [Google Scholar]
- 36. Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, et al. Defective neuromuscular synaptogenesis in agrin‐deficient mutant mice. Cell 1996;85:525–535. [DOI] [PubMed] [Google Scholar]
- 37. Jones G, Meier T, Lichtsteiner M, Witzemann V, Sakmann B, Brenner HR. Induction by agrin of ectopic and functional postsynaptic‐like membrane in innervated muscle. Proc Natl Acad Sci U S A 1997;94:2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bezakova G, Rabben I, Sefland I, Fumagalli G, Lømo T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proc Natl Acad Sci U S A 2001;98:9924–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tintignac LA, Brenner HR, Rüegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev 2015;95:809–852. [DOI] [PubMed] [Google Scholar]
- 40. Bezakova G, Ruegg MA. New insights into the roles of agrin. Nat Rev Mol Cell Biol 2003;4:295–308. [DOI] [PubMed] [Google Scholar]
- 41. Neumann FR, Bittcher G, Annies M, Schumacher B, Kröger S, Ruegg MA. An alternative amino‐terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol Cell Neurosci 2001;17:208–225. [DOI] [PubMed] [Google Scholar]
- 42. Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J Cell Biol 2000;151:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruegg MA, Bixby JL. Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci 1998;21:22–27. [DOI] [PubMed] [Google Scholar]
- 44. Burgess RW, Nguyen QT, Young‐Jin S, Lichtman JW, Sanes JR. Alternatively spliced isoforms of nerve‐ and muscle‐derived agrin: their roles at the neuromuscular junction. Neuron 1999;23:33–44. [DOI] [PubMed] [Google Scholar]
- 45. Zong Y, Zhang B, Gu S, Lee K, Zhou J, Yao G, et al. Structural basis of agrin–LRP4–MuSK signaling. Genes Dev 2012;1:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, et al. The muscle protein Dok‐7 is essential for neuromuscular synaptogenesis. Science 2006;312:1802–1805. [DOI] [PubMed] [Google Scholar]
- 47. DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996;85:501–512. [DOI] [PubMed] [Google Scholar]
- 48. Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wölfel J, et al. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J 2007;21:3468–3478. [DOI] [PubMed] [Google Scholar]
- 49. Lieth E, Fallon JR. Muscle agrin: neural regulation and localization at nerve‐induced acetylcholine receptor clusters. J Neurosci 1993;13:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bütikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J 2011;25:4378–4393. [DOI] [PubMed] [Google Scholar]
- 51. Hettwer S, Lin S, Kucsera S, Haubitz M, Oliveri F, Fariello RG, et al. Injection of a soluble fragment of neural agrin (NT‐1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS One 2014;9:e88739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, van Kan GA, et al. Biomarkers of sarcopenia in clinical trials‐recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle 2012;3:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu D, Li HX, Liu Y, Ying ZW, Guo JJ, Cao CY, et al. The reference intervals for serum C‐terminal agrin fragment in healthy individuals and as a biomarker for renal function in kidney transplant recipients. J Clin Lab Anal 2017;31:e22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hester GM, Vandusseldorp TA, Ha PL, Kiani K, Olmos AA, Jabbari M, et al. Microbiopsy sampling for examining age‐related differences in skeletal muscle fiber morphology and composition. Front Physiol 2022;12:756626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bondoc I, Cochrane SK, Church TS, Dahinden P, Hettwer S, Hsu F‐C, et al. Effects of a one‐year physical activity program on serum C‐terminal agrin fragment (CAF) concentrations among mobility‐limited older adults. J Nutr Heal Aging 2015;19:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gagliano‐Jucá T, Storer TW, Pencina KM, Travison TG, Li Z, Huang G, et al. Testosterone does not affect agrin cleavage in mobility‐limited older men despite improvement in physical function. Andrology 2018;6:29–36. [DOI] [PubMed] [Google Scholar]
- 57. Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjær M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sport 2010;20:49–64. [DOI] [PubMed] [Google Scholar]
- 58. Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW, et al. Attenuation of age‐related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A 2010;107:14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sánchez‐Castellano C, Martín‐Aragón S, Bermejo‐Bescós P, Vaquero‐Pinto N, Miret‐Corchado C, Merello de Miguel A, et al. Biomarkers of sarcopenia in very old patients with hip fracture. J Cachexia Sarcopenia Muscle 2020;11:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pratt J, De Vito G, Narici M, Segurado R, Pessanha L, Dolan J, et al. Plasma C‐terminal agrin fragment as an early biomarker for sarcopenia: results from the Genofit study. Biomedgerontology 2021;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rosenberg IH. Summary comments. Am J Clin Nutr 1989;50:1231–1233. [Google Scholar]
- 62. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Daou N, Hassani M, Matos E, De Castro GS, Costa RGF, Seelaender M, et al. Displaced myonuclei in cancer cachexia suggest altered innervation. Int J Mol Sci 2020;21:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boehm I, Miller J, Wishart TM, Wigmore SJ, Skipworth RJE, Jones RA, et al. Neuromuscular junctions are stable in patients with cancer cachexia. J Clin Invest 2020;130:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qaisar R, Karim A, Muhammad T. Plasma CAF22 levels as a useful predictor of muscle health in patients with chronic obstructive pulmonary disease. Biology (Basel) 2020;9:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Karim A, Muhammad T, Qaisar R. Prediction of sarcopenia using multiple biomarkers of neuromuscular junction degeneration in chronic obstructive pulmonary disease. J Pers Med 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Racha P, Selvam S, Bose B, Bantwal G, Sambashivaiah S. Circulating C‐terminal agrin fragment: a potential marker for sarcopenia among type 2 diabetes. Indian J Endocrinol Metab 2022. 10.4103/ijem.ijem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Monti E, Reggiani C, Franchi MV, Toniolo L, Sandri M, Armani A, et al. Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans. J Physiol 2021;599:3037–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ganse B, Bosutti A, Drey M, Degens H. Sixty days of head‐down tilt bed rest with or without artificial gravity do not affect the neuromuscular secretome. Exp Cell Res 2021;399:112463. [DOI] [PubMed] [Google Scholar]
- 71. Sarto F, Stashuk D, Franchi MV, Monti E, Zampieri S, Valli G, et al. Effects of short‐term unloading and active recovery on human motor unit properties, neuromuscular junction transmission and transcriptomic profile. J Physiol 2022;600:4731–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle and Nerve 2014;49:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, et al. Long‐term high‐level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 2014;73:284–294. [DOI] [PubMed] [Google Scholar]
- 74. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. Journals Gerontol ‐ Ser A Biol Sci Med Sci 2001;56:146–157. [DOI] [PubMed] [Google Scholar]
- 75. Bigdeli S, Dehghaniyan MH, Amani‐Shalamzari S, Rajabi H, Gahreman DE. Functional training with blood occlusion influences muscle quality indices in older adults. Arch Gerontol Geriatr 2020;90:104110. [DOI] [PubMed] [Google Scholar]
- 76. Fragala MS, Jajtner AR, Beyer KS, Townsend JR, Emerson NS, Scanlon TC, et al. Biomarkers of muscle quality: N‐terminal propeptide of type III procollagen and C‐terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle 2014;5:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Willoughby DS, Beretich KN, Chen M, Funderburk LLK. Decreased serum levels of C‐terminal agrin in postmenopausal women following resistance training. J Aging Phys Act 2020;28:73–80. [DOI] [PubMed] [Google Scholar]
- 78. Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento‐Villareal R, Sun Z, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite calorie restriction. Cell Metab 2020;176:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Franchi MV, Badiali F, Sarto F, Müller P, Müller NG, Rehfeld K, et al. Neuromuscular aging: a case for the neuroprotective effects of dancing. Gerontology 2022;69:1–9. [DOI] [PubMed] [Google Scholar]
- 80. Narici M, Conte M, Salvioli S, Franceschi C, Selby A, Dela F, et al. Alpine skiing with total knee arthroplasty (ASWAP): impact on molecular and architectural features of musculo‐skeletal ageing. Scand J Med Sci Sport 2015;25:33–39. [DOI] [PubMed] [Google Scholar]
- 81. Marcolin G, Franchi MV, Monti E, Pizzichemi M, Sarto F, Sirago G, et al. Active older dancers have lower C‐terminal agrin fragment concentration and better balance and gait performance than sedentary peers. Exp Gerontol 2021;153:111469. [DOI] [PubMed] [Google Scholar]
- 82. Kargaran A, Abedinpour A, Saadatmehr Z, Yaali R, Amani‐Shalamzari S, Gahreman D. Effects of dual‐task training with blood flow restriction on cognitive functions, muscle quality, and circulatory biomarkers in elderly women. Physiol Behav 2021;239:113500. [DOI] [PubMed] [Google Scholar]
- 83. Deschenes MR. Effects of aging on muscle fibre type and size. Sport Med 2004;34:809–824. [DOI] [PubMed] [Google Scholar]
- 84. Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 2014;8:1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tezze C, Romanello V, Desbats MA, Fadini GP, Albiero M, Favaro G, et al. Age‐associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab 2017;25:1374–1389.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McCann CM, Nguyen QT, Neto HS, Lichtman JW. Rapid synapse elimination after postsynaptic protein synthesis inhibition in vivo. J Neurosci 2007;27:6064–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, et al. Increased superoxide in vivo accelerates age‐associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 2010;24:1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stout JR, Fragala MS, Hoffman JR, Robinson EH, Mccormack WP, Townsend JR, et al. C‐terminal agrin fragment is inversely related to neuromuscular fatigue in older men. Muscle and Nerve 2015;51:132–133. [DOI] [PubMed] [Google Scholar]
- 89. Piasecki M, Ireland A, Jones DA, McPhee JS. Age‐dependent motor unit remodelling in human limb muscles. Biogerontology 2016;17:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pratt J, De Vito G, Narici MV, Boreham C. Neuromuscular junction aging: a role for biomarkers and exercise. Gerontology 2020;26:979–993. [DOI] [PubMed] [Google Scholar]
- 91. Piasecki M, Ireland A, Stashuk D, Hamilton‐Wright A, Jones DA, McPhee JS. Age‐related neuromuscular changes affecting human vastus lateralis. J Physiol 2016;594:4525–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chugh D, Iyer CC, Wang X, Bobbili P, Rich MM, Arnold WD. Neuromuscular junction transmission failure is a late phenotype in aging mice. Neurobiol Aging 2020;86:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Padilla CJ, Harrigan ME, Harris H, Schwab JM, Rutkove SB, Rich MM, et al. Profiling age‐related muscle weakness and wasting: neuromuscular junction transmission as a driver of age‐related physical decline. GeroScience 2021;43:1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dominik S, Stefan H, Wim V, Petra W, Peter L, Carsten W, et al. C‐terminal agrin fragment ‐ a new fast biomarker for kidney function in renal transplant recipients C‐terminal agrin fragment (CAF) – a new biomarker for evaluating kidney function. Abteilung für Nephrologie, Klinikum rechts der Isar, München, Germany 2013;38:501–508. [Google Scholar]
- 95. Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan‐heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant 2009;24:2044–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Steubl D, Roos M, Hettwer S, Satanovskij R, Tholen S, Wen M, et al. Plasma total C‐terminal agrin fragment (tCAF) as a marker for kidney function in patients with chronic kidney disease. Clin Chem Lab Med 2016;54:1487–1495. [DOI] [PubMed] [Google Scholar]
- 97. Lorenz G, Hettwer S, McCallum W, Angermann S, Wen M, Schmaderer C, et al. Plasma C‐terminal agrin fragment and rapid kidney function decline in chronic kidney disease patients. Medicine (Baltimore) 2019;98:e15597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Devetzis V, Daryadel A, Roumeliotis S, Theodoridis M, Wagner CA, Hettwer S, et al. C‐terminal fragment of agrin (CAF): a novel marker for progression of kidney disease in type 2 diabetics. PLoS One 2015;10:e0143524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Roos M, Kopf S, Hettwer S, Oikonomou D, Von Eynatten M, Heemann U et al. C‐terminal agrin fragment (CAF) – a potential new biomarker for prediction of diabetic kidney disease. 2015. https://www.thieme‐connect.com/products/ejournals/abstract/101055/s‐0035‐1549587 10.1055/s-0035-1549587 [DOI]
- 100. de Souza Ramos JTG, Ferrari FS, Andrade MF, de Melo CS, Boas PJFV, Costa NA, et al. Association between frailty and C‐terminal agrin fragment with 3‐month mortality following ST‐elevation myocardial infarction. Exp Gerontol 2021;158:111658. [DOI] [PubMed] [Google Scholar]
- 101. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]


