Abstract
Anorexia/appetite loss in older subjects is frequently underrecognized in clinical practice, which may reflect deficient understanding of clinical sequelae. Therefore, we performed a systematic literature review to assess the morbidity and mortality burden of anorexia/appetite loss in older populations. Following PRISMA guidelines, searches were run (1 January 2011 to 31 July 2021) in PubMed, Embase® and Cochrane databases to identify English language studies of adults aged ≥ 65 years with anorexia/appetite loss. Two independent reviewers screened titles, abstracts and full text of identified records against pre‐defined inclusion/exclusion criteria. Population demographics were extracted alongside risk of malnutrition, mortality and other outcomes of interest. Of 146 studies that underwent full‐text review, 58 met eligibility criteria. Most studies were from Europe (n = 34; 58.6%) or Asia (n = 16; 27.6%), with few (n = 3; 5.2%) from the United States. Most were conducted in a community setting (n = 35; 60.3%), 12 (20.7%) were inpatient based (hospital/rehabilitation ward), 5 (8.6%) were in institutional care (nursing/care homes) and 7 (12.1%) were in other (mixed or outpatient) settings. One study reported results separately for community and institutional settings and is counted in both settings. Simplified Nutritional Appetite Questionnaire (SNAQ Simplified, n = 14) and subject‐reported appetite questions (n = 11) were the most common methods used to assess anorexia/appetite loss, but substantial variability in assessment tools was observed across studies. The most commonly reported outcomes were malnutrition and mortality. Malnutrition was assessed in 15 studies, with all reporting a significantly higher risk of malnutrition in older individuals with anorexia/appetite loss (vs. without) regardless of country or healthcare setting (community n = 9, inpatient n = 2, institutional n = 3, other n = 2). Of 18 longitudinal studies that assessed mortality risk, 17 (94%) reported a significant association between anorexia/appetite loss and mortality regardless of either healthcare setting (community n = 9, inpatient n = 6, institutional n = 2) or method used to assess anorexia/appetite loss. This association between anorexia/appetite loss and mortality was observed in cohorts with cancer (as expected) but was also observed in older populations with a range of comorbid conditions other than cancer. Overall, our findings demonstrate that, among individuals aged ≥ 65 years, anorexia/appetite loss is associated with increased risk of malnutrition, mortality and other negative outcomes across community, care home and hospital settings. Such associations warrant efforts to improve and standardize screening, detection, assessment and management of anorexia/appetite loss in older adults.
Keywords: anorexia, appetite loss, malnutrition, mortality, prevalence, systematic literature review
Introduction
Appetite loss is common among older adults and may occur as a symptom of underlying disease and/or as a side effect of medication use. 1 Appetite loss may also occur in the absence of any evident medical cause and be attributed to the aging process; this has been termed ‘anorexia of aging’. 1 , 2
Clinical and societal recognition of the burden of appetite loss in older adults is likely deficient for a variety of reasons. For example, there is no standardized approach to appetite assessment. 3 Similarly, there is no standardized terminology or definition for the condition, with terms such as appetite loss, anorexia of aging, unintentional weight loss, undernutrition and malnutrition used interchangeably across studies. 4 There is also a common assumption that appetite loss is a normal part of aging. These factors underpin variation in the reported prevalence of appetite loss, which ranges from 5% to 25% in community‐dwelling older adults. 5 , 6 , 7 , 8 Prevalence is increased among nursing home residents and older hospitalized subjects, but the exact prevalence in these care settings is uncertain as appetite loss may go undetected, particularly if associated weight loss is minimal or absent. 3 , 9 , 10 , 11
As such, there is a need to improve both the recognition and management of appetite loss among the growing population aged 65 years or older. To achieve these goals, however, a better understanding of the effects of appetite loss on morbidity, mortality and healthcare burden in older adults is required. The objective of this systematic literature review (SLR), therefore, was to characterize the relationship between appetite loss and negative outcomes in populations aged ≥ 65 years.
Methods
Study design and data sources
This SLR was conducted across PubMed, Embase®, Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials (CENTRAL) using a standardized methodical approach following guidance presented in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 statement. 12 The review is registered on PROSPERO under the identifier CRD42021270876. For the purposes of this SLR, the term ‘anorexia/appetite loss’ incorporated the following terms: anorexia (but not anorexia nervosa), loss or lack of appetite, appetite loss, decreased or reduced appetite, poor appetite or hyporexia (defined as a pathologically decreased appetite).
Search strategy: Inclusion/exclusion criteria
The full search strategies for each of the databases is given in Table S1 . Inclusion criteria targeted studies in adults aged ≥ 65 years old with anorexia/appetite loss or at risk of anorexia, with a sample size of ≥ 20. Observational and real‐world studies, economic models and interventional studies published in English language from 1 January 2011 to 31 July 2021 were eligible for inclusion. Studies in populations aged < 65 years, in a mixed age population that did not report separate results for those aged ≥ 65 years or in subjects with anorexia nervosa were excluded. Similarly, preclinical studies, clinical trials in healthy populations, case studies, notes/commentaries/editorials/opinions, congress abstracts or meta‐analyses/reviews were not eligible for inclusion.
A rigorous, two‐stage screening process was carried out independently by two reviewers (C. R. and K. S.). Stage 1 involved screening titles and abstracts based on inclusion/exclusion criteria; Stage 2 involved full‐text screening of publications included at Stage 1 (title and abstract) review, including any for which there was a discrepancy between the two reviewers. Reference lists were also screened to identify any additional literature for further review. Reasons for exclusion were cross‐checked between the two reviewers. The inclusion/exclusion agreement rate between the two reviewers was 90.5% for Stage 1 and 79% for Stage 2. Discrepancies were resolved by discussion to achieve agreement. Identification of papers and reasons for exclusion are presented in Figure 1 .
Figure 1.
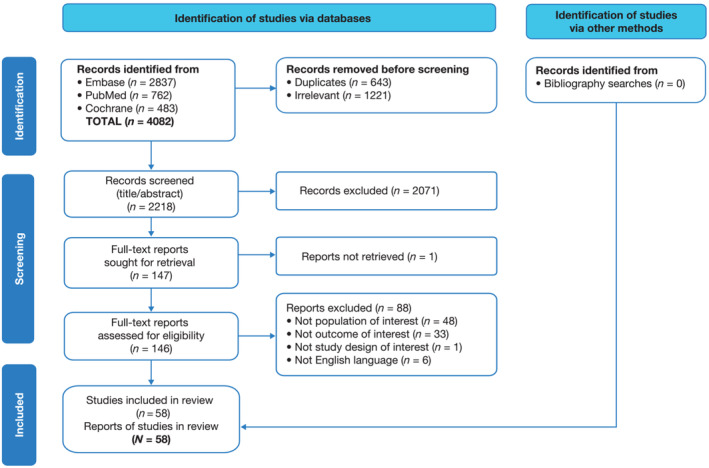
PRISMA flow diagram. Publications were identified from searches of Embase, PubMed and Cochrane databases. Duplicate entries or irrelevant types of publications (e.g., editorials, letters, reviews, congress abstracts, case reports, animal studies and paediatric studies) were removed initially before screening.
Outcomes of interest included, but were not limited to, mortality, malnutrition, disability, health‐related quality of life and healthcare resource utilization (Table 1 ). For the purpose of this paper, subjects were categorized into four healthcare setting cohorts: community‐dwelling (independent living, free living in the community); inpatient (hospitalized, staying in a hospital or rehabilitation ward at the time of evaluation); institutional care (nursing care facility or geriatric day home); or other (mixed settings or settings not covered in previous categories).
Table 1.
Outcomes and healthcare setting cohorts assessed in the systematic literature review
| Outcomes | Healthcare setting cohorts |
|---|---|
| Mortality, malnutrition, sarcopenia, functional status, increased care, hospitalization, falls, health‐related quality of life, cognition, depression, disability, frailty, general health, infection, major adverse cardiovascular or cerebrovascular events, sleep quality, stroke, treatment (chemotherapy) interruption, weight loss |
|
Data extraction and quality assessment
Full publications of retained records were examined in detail for information relating to study size and location, subject characteristics and demographics of interest, healthcare setting, and outcomes of interest, according to population, interventions, comparator, outcomes, and study design (PICOS) criteria. Information on prevalence of anorexia and presence of key comorbidities including cognitive impairment, cancer or heart failure was also captured. Details of the instrument used to assess or screen for anorexia/appetite loss were recorded.
For observational longitudinal or prospective studies, quality/risk of bias was assessed using the Newcastle‐Ottawa Scale (NOS). 13 The quality of observational cross‐sectional studies was assessed using the modified NOS. 14 All studies except one 15 had either low or medium risk of bias, as detailed in Table S2 .
Results
Study selection
Overall, 4082 unique citations were identified from the systematic searches of the three literature databases; 2218 passed through first screen (title/abstract), and 146 articles were retained for full‐text review. In total, 58 publications meeting pre‐defined criteria were included in the SLR (Figure 1 ).
Study characteristics
All 58 studies were observational studies, of which 60% (n = 35) were longitudinal and 40% (n = 23) were cross‐sectional in design (Table 2 ). Sample sizes ranged widely, from ≤ 100 subjects to 1 466 598 for longitudinal 16 and to 13 151 for cross‐sectional 55 studies. Most studies were conducted in Europe (n = 34; 58.6%) or Asia (n = 16; 27.6%) and only a few were carried out in the United States (n = 3; 5.2%), Australia (n = 2; 3.4%), the Middle East (n = 2; 3.4%) or Canada (n = 1; 1.7%). More than half of all studies were carried out in the community setting (n = 35; 60.3%), with the remainder carried out in inpatient (n = 12; 20.7%), institutional care (n = 5; 8.6%) or other (n = 7; 12.1% [outpatient n = 4; mixed n = 3]) settings. One study was conducted in community and institutional settings and reported results separately for each. 48 This study, therefore, is counted in both the community and institutional settings throughout the manuscript.
Table 2.
Design and subject characteristics of studies identified during the systematic literature review (n = 58)
|
Author; year; country |
Study design; sample size; study period |
Study setting; age; percentage female |
BMI, kg/m2 | Anorexia or appetite assessment method; definition of anorexia/loss of appetite | Prevalence of anorexia or poor appetite | Outcomes assessed |
|---|---|---|---|---|---|---|
| Longitudinal studies | ||||||
|
Hippisley‐Cox and Coupland; 2017; UK (England) 16 |
Prospective; N = 1 466 598; 1 Jan 2012 to 30 Sep 2016 |
Not reported but appears to be community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age = 75.3 (8.0) years; 54.9% female |
Mean (SD) BMI = 27.3 (4.9) | In healthcare record; defined as the subject visiting a general practitioner for loss of appetite in the previous 12 months. | 0.20% | Mortality |
|
Cai et al.; 2020; UK (England) 17 |
Retrospective; N = 4243; 1 Jan 2008 to 31 Mar 2017 |
Community‐dwelling adults with depression; aged ≥ 65 years, mean (SD) age = 77.0 (7.9) years; 61.2% female |
BMI not reported | In healthcare record; anorexia/loss of appetite criteria not defined. | 54.8% | Mortality |
|
Landi et al.; 2012; Italy 18 |
Prospective; N = 2787; 1998 to 2000 |
Community‐dwelling and receiving in‐home care; all subjects aged ≥ 65 years, mean (SD) age = 80.4 (7.5) years; 60% female |
BMI not reported |
Food consumption (staff assessed); defined as staff responding yes to the question, ‘In at least 4 of the last 7 days, the client ate one or fewer meals a day,’ or ‘In the last 3 days, noticeable decrease in the amount of food client usually eats.’ |
26.7% | Mortality |
|
Huang et al.; 2014; Taiwan 19 |
Retrospective; N = 1856; 1999 to 2000 with survivorship ascertained from the death registry until 31 Dec 2008 |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SE) age = 74.3 (0.3) years; 47.7% female |
Mean (SE) BMI = 23.6 (0.2) Category: < 18.5 = 7.1% 18.5–23.9 = 48.2% 24.0–26.9 = 27.7% ≥ 27.0 = 17.0% |
Appetite question (subject‐reported); defined as subject responding ‘poor’ to the question, ‘How is your current appetite?’ Other possible responses included ‘good’ or ‘fair’. |
7.6% | Mortality |
|
Huang et al.; 2018; Taiwan 20 |
Retrospective; Male n = 970, female n = 967; 1999 to 2000 with survivorship ascertained from the death registry until 31 Dec 2008 |
Community‐dwelling; age not reported; 49.9% female |
Category: Male: < 18.5 = 4.4% 18.5–23.9 = 65.4% 24.0–26.9 = 20.9% ≥ 27.0 = 9.2% Female: < 18.5: 4.3% 18.5–23.9 = 49.1% 24.0–26.9 = 31.4% ≥ 27.0 = 15.2% |
MNA‐SF Q1 (food intake question); defined as subject responding ‘poor’ to the question, ‘How is your current appetite?’ Other possible responses included ‘good’ or ‘fair’. |
Male = 7.5% Female = 12.4% |
Mortality |
|
St John and Montgomery; 2014; Canada 21 |
Prospective; N = 1751; baseline: 1991 to 1992, follow‐up: 1996 to 1997 |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age = 77.5 (7.1) years; 58% female |
BMI not reported | CES‐D; defined as subject responding ‘some or little of the time (1–2 days)’, ‘occasionally or moderate amount of time (3–4 days)’ or ‘most of time (5–6 days)’ to the question, ‘I did not feel like eating, my appetite was poor,’ in the past week. Other possible responses included ‘rarely or none of time (< 1 day)’. | 18% | Mortality |
|
Wijnhoven et al.; 2012; the Netherlands 22 |
Retrospective; N = 1687; LASA 1: 1992 to 2007 |
Community‐dwelling; LASA cohort, mean (SD) age = 74.5 (5.8) years; 50.4% female |
Mean (SD) BMI = 26.7 (4.1) | CES‐D; defined as subject responding ‘some or little of the time (1–2 days)’, ‘occasionally or moderate amount of time (3–4 days)’ or ‘most of time (5–6 days)’ to the question, ‘I did not feel like eating, my appetite was poor,’ in the past week. Other possible responses included ‘rarely or none of time (< 1 day)’. | 13.1% | Mortality |
|
Fournier et al.; 2016; France 23 |
Prospective; N = 156 (152 with appetite loss data); Mar 2003 to Sep 2006 |
Not reported but appears to be community‐dwelling; age range, n (%): 65–74 years = 83 (53.2) ≥ 75 years = 73 (46.8); 48.7% female |
BMI not reported | EORTC QLQ‐C30; defined as a deterioration (from baseline) of > 10 points in appetite score on a scale from 0 to 100. |
3 months = 16.7% 6 months = 13.7% |
Mortality |
|
Engelheart et al.; 2021; Sweden 24 |
Prospective; N = 69; 2014 to 2017 |
Community‐dwelling; all subjects aged ≥ 65 years, mean (range) age = 81.9 (65–97) years; 64% female |
Mean [range] BMI = 26.8 [16–50], n = 61 | Appetite question (subject‐reported) with a visual analogue scale from 0 (much reduced appetite) to 10 (very good appetite); criteria for anorexia/loss of appetite not defined. | Prevalence of anorexia or appetite loss not reported | Mortality |
|
Cox et al.; 2020; UK (England) 25 |
Retrospective; N = 296; 2014 and 2017 |
Inpatient; all subjects aged ≥ 70 years, mean (SD) age = 82.7 (6.9) years; 43% female |
Median (IQR) BMI = 25 (21, 28) | SNAQ Simplified; defined as score < 14. | 41% | Mortality |
|
Pilgrim et al.; 2016; UK (England) 26 |
Prospective; N = 178; dates not reported |
Inpatient (geriatric ward); all subjects described as ‘older’, mean (SD) age = 86.6 (4.7) years; 100% female |
Mean (SD) BMI = 24.8 (1.3), n = 172 | SNAQ Simplified; defined as score < 14. |
Admission = 42%, 6‐month follow‐up = 38% |
Mortality Infection Increased care Weight loss Sarcopenia Hospitalization |
|
Hofer et al.; 2018; Austria 27 |
Prospective; N = 149; Jan 2009 to Apr 2016 |
Inpatient; all subjects aged > 67 years, mean [range] age = 77.8 [67.1–95.3] years; 46% female |
BMI not reported | EORTC QLQ‐C30; defined as no appetite loss (score of 0), mild loss (score of 1–66) and moderate to severe loss (score of 67–100) on a 100‐point numeric rating scale for appetite question. |
Mild appetite loss = 30% Moderate to severe appetite loss = 13% |
Mortality |
|
Taniguchi et al.; 2019; Japan 28 |
Retrospective; N = 139; TAVI received Jul 2014 and Oct 2018, follow‐up: max of 2 years |
Inpatient followed by community or institution upon discharge, all subjects underwent TAVI; mean (SD) age = 83.6 (5.2) years; 63.3% female |
Mean (SD) BMI = 22.4 (4.0) |
Food consumption (staff‐assessed); assessed averaged intake rate for breakfast, lunch and dinner on the day just before discharge. The good appetite group was defined as having a dietary intake rate > 90% just before discharge, whereas the less appetite group was defined as a dietary intake rate ≥ 90%. | 24.5% |
Mortality MACCE Stroke Hospitalization |
|
Rawle et al.; 2020; UK 29 |
Retrospective; N = 134: Community‐dwelling n = 70, care (nursing) home n = 64; 10 Mar to 8 Apr 2020 |
Inpatients hospitalized with COVID‐19 (with subgroups of subjects previously residing in community or care [nursing] home); all subjects aged ≥ 80 years, median (IQR) age, years: Total = 86 (7.6), community = 85 (6), care home = 88.5 (7), P < 0.001; % female: Total = 45.5%, community = 44.3%, care home = 46.9% |
BMI not reported | Mentioned in health record; defined as being present in the health record. |
Total = 21.2% Community = 15.7% Care (nursing) home = 28.1% |
Mortality |
|
Schmidt et al.; 2014; Germany 30 |
Prospective; N = 126; Jun 2008 to Jul 2010 |
Inpatients scheduled for surgery for gastrointestinal, genitourinary or gynaecological cancer; all subjects aged ≥ 65 years, mean (SD) age = 72 (5.6) years; 55.6% female |
Mean (SD) BMI = 26.7 (4.9) | EORTC QLQ‐C30; anorexia/appetite loss criteria not defined. | Prevalence not reported | Mortality |
|
Landi et al.; 2013; Italy 8 |
Prospective; N = 1904; Enrolment: Feb to Dec 2005, follow‐up: 12 months (Dec 2006) |
Institution (nursing home); all subjects aged ≥ 65 years, mean (SD) age = 83.5 (8.1) years; 71.6% female |
BMI not reported | Food consumption (staff‐assessed); defined as staff answering ‘yes’ to the question, ‘In at least 4 of the last 7 days, the client ate one or fewer meals a day.’ | 12.6% | Mortality |
|
Mikami et al.; 2019; Japan 31 |
Prospective; N = 316; Jan 2014 to Jan 2015 |
Institution (nursing home); all subjects described as ‘elderly’, mean (SD) age = 84.9 (8.3) years; 81% female |
Mean (SD) BMI = 21 (3.5) | CNAQ, SNAQ Simplified; defined as CNAQ total scores ≤ 28 (poor appetite) and > 28 (good appetite). SNAQ criteria not defined. | Prevalence of anorexia or poor appetite not reported | Mortality |
|
Kanamori et al.; 2012; Japan 32 |
Prospective; N = 72; 2000 to 2003 |
Other (outpatients receiving haemodialysis, ≥ 65 years subset); mean (SD) age = 71.8 (5.6) years; 41.7% female |
BMI not reported | Appetite question (subject‐reported) based on a 100‐mm visual analogue scale; appears to be defined as scores ≤ 74 (less appetite) and ≥ 75 (more appetite). | Prevalence of anorexia or poor appetite not reported | Mortality |
|
de Almeida Mello et al.; 2020; Belgium 33 |
Retrospective; N = 6334; dates not reported |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age = 80.6 (6.9) years; 70.6% female |
BMI not reported | Appetite assessment method not reported. | Prevalence of anorexia or poor appetite not reported | Malnutrition |
|
Schilp et al.; 2011; the Netherlands 34 |
Prospective; N = 1120; 1992 to 1993 |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age = 74.1 (5.7) years; 51.5% female |
Mean (SD) BMI = 27.2 (3.9) | CES‐D; defined as subject responding ‘some or little of the time (1–2 days)’, ‘occasionally or moderate amount of time (3–4 days)’ or ‘most of time (5–6 days)’ to the question, ‘I did not feel like eating, my appetite was poor,’ in the past week. Other possible responses included ‘rarely or none of time (< 1 day)’. | 10.9% | Malnutrition |
|
Lambert et al.; 2017; Germany 15 |
Prospective; N = 317; Enrolment: June 2014 to June 2015, follow‐up: Length not reported |
Inpatient (followed by community or nursing home on discharge); subset of subjects aged ≥ 70 years hospitalized ≥ 2 nights; % female not reported |
BMI not reported for the ≥ 70‐year‐old subset | Appetite assessment method not reported; anorexia/loss of appetite criteria not defined. | Prevalence of anorexia or poor appetite not reported | Malnutrition |
|
Cox et al.; 2021; UK (England) 35 |
Prospective; N = 204; SNAQ measure: 5 Mar to 30 Apr 2019, sarcopenia measures: 2006 to 2018 |
Community‐dwelling: Case (SNAQ < 14) n = 102, control (SNAQ > 14) n = 102; all subjects aged ≥ 65 years, mean (SD) age, years: Case = 68.0 (5.7), control = 67.6 (5.4); % female: Case = 95.1%, control = 95.1% |
Mean (SD) BMI: Case = 25.6 (5.1) Control = 25.8 (4.1) |
SNAQ Simplified; defined as score < 14. | Prevalence of anorexia or poor appetite not reported | Sarcopenia |
|
Senoo et al.; 2020; Japan 36 |
Prospective; N = 173; baseline: 2015, follow‐up: 2016 to 2018 |
Community‐dwelling; all subjects aged ≥ 75 years, median (IQR) age = 80 (78–83) years; 64.7% female |
BMI category, n (%) < 18.5 = 7 (4.1) |
SNAQ Simplified; defined as score ≤ 14. | 32.9% | Sarcopenia |
|
Tsutsumimoto et al.; 2018; Japan 7 |
Prospective; N = 4393; dates not reported |
Community‐dwelling; all subjects aged ≥ 70 years, mean (SD) age = 75.8 (4.3) years; 52.6% female |
Mean (SD) BMI = 22.9 (3.0) | SNAQ Simplified; defined as score ≤ 13. | 10.7% | Disability |
|
Martinez‐Reig et al.; 2014; Spain 37 |
Prospective; N = 678; 2007 to 2009 |
Community‐dwelling; all subjects aged ≥ 70 years, mean (SD) age = 78.0 (5.7) years; 57.8% female |
Mean (SD) BMI = 29.3 (4.8) | MNA‐SF Q1 (food intake question); criteria for anorexia/loss of appetite not defined. |
Severe decrease in food intake = 0.4% Moderate decrease = 15.2% |
Disability |
|
Hsu et al.; 2019; Taiwan 38 |
Retrospective; N = 1986; 1999 to 2003 |
Community‐dwelling; all subjects aged ≥ 70 years, mean (SD) age = 75.8 (4.9) years; 44.5% female |
Mean (SD) BMI = 22.9 (3.4) | CES‐D; authors combined the two ‘no and seldom’ responses of the CES‐D into ‘no’, poor appetite loss and the two ‘often and usually’ responses into ‘yes’ poor appetite. | Prevalence of anorexia or appetite loss not reported | Increased care |
|
Salminen et al.; 2018; Finland 39 |
Prospective; N = 1032; 1920 birth cohort, data collected Jan 1991 |
Community‐dwelling; all subjects aged ≥ 70 years, mean age = 70 years; 64% female |
BMI category, n (%): < 25 = 297 (38) 25.0–29.9 = 354 (45) ≥ 30 = 134 (17) |
Appetite question (subject‐reported); defined as subject responding ‘sometimes or daily’ to lack of appetite question. Other possible responses included ‘never’. | 10% | Increased care |
|
Sheppard et al.; 2013; USA 40 |
Prospective; admission to nursing home: Yes n = 75, no n = 924; baseline: Nov 1999 to Feb 2001, follow‐up: 8.5 years |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age, years: Yes nursing home admission = 79.4 (7.2), no nursing home admission = 74.9 (6.6), P < 0.001; % female: Yes nursing home admission = 56%, no nursing home admission = 49.3% |
BMI not reported | PROMIS; defined as subjects responding ‘fair’ or ‘poor’ to the question, ‘Would you say your appetite is usually very good, good, fair or poor?’ |
Yes nursing home = 29.3% No nursing home = 16.2% |
Increased care |
|
Salanitro et al.; 2012; USA 41 |
Prospective; N = 980; Dec 1999 to Feb 2001 |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age = 75.3 (6.7) years; 49.5% female |
BMI category, n (%): < 18.5 = 21 (2.1) 18.5–25 = 288 (29.4) 25–30 = 369 (37.7) 30–35 = 199 (20.3) 35 + = 103 (10.5) |
Appetite question (subject‐reported); defined as subject responding ‘fair’ or ‘poor’ to the question, ‘Would you say your appetite is usually very good, good, fair or poor?’ | 16.9% | Hospitalization |
|
Van Dronkelaar et al.; 2019; the Netherlands 42 |
Prospective; N = 400; Oct 2015 to Feb 2017 |
Inpatient (acute inpatients); all subjects aged ≥ 70 years, mean (SD) age = 80.1 (6.7) years; 48.5% female |
Median (IQR) BMI = 24.5 (21.9–28.6) | SNAQ Short AL question; defined as subject responding ‘yes’ to the question, ‘Have you experienced a decreased appetite over the last month?’ at hospital admission or to the question, ‘Have you experienced a decreased appetite since hospital admission?’ for discharge and post‐discharge assessments. |
50.5% at hospital admission 34.0% at discharge 27.8% at 1‐month post‐discharge 17.0% at 3‐month post‐discharge |
Sarcopenia |
|
Dent et al.; 2015; Australia 43 |
Prospective; N = 172; 22 Oct 2010 to 23 Dec 2011 |
Inpatient; all subjects aged ≥ 70 years, mean (SD) = 85.2 (6.4) years; 72% female |
BMI category, n (%): < 22 = 58 (34) 22–30 = 75 (44) > 30 = 39 (23) |
SNAQ Simplified; defined as score ≤ 14. | 63% |
Functional status Increased care Hospitalization |
|
Mendelson et al.; 2018; Israel 44 |
Retrospective; N = 107: FIM > 70 n = 56, FIM ≥ 90 n = 37; Feb to Jul 2014 |
Inpatient (rehab centre); all subjects with hip fracture, FIM > 70: mean (SD) age = 78.7 (7.0) years; 71.4% female |
BMI not reported | SNAQ Short AL question; defined as subject responding ‘yes’ to the question, ‘Have you experienced a decreased appetite over the last month?’ |
FIM ≥ 90 = 40.5% FIM < 90 (and > 70) = 68.4% P = 0.048 |
Functional status |
|
Van Grootven et al.; 2020; Belgium 45 |
Prospective; N = 189; Sep 2016 to Jun 2017 |
Inpatient; all subjects aged ≥ 75 years, mean (SD) age = 84 (5) years; 47% female |
BMI not reported | Appetite question (subject‐reported); defined as self‐reported loss of appetite in the past 3 months. | 40% | Functional status |
|
Kirkhus et al.; 2019; Norway 46 |
Prospective; N = 288; Jan 2013 to Apr 2015 |
Other (outpatient with cancer aged ≥ 70 years); mean (SD) age = 76.9 (5.1) years; 44% female |
BMI not reported | EORTC QLQ‐C30; anorexia/appetite loss criteria not defined. | Prevalence of anorexia or appetite loss not reported |
Functional status HRQoL |
|
Won et al.; 2019; Korea 47 |
Prospective; N = 75; Apr 2012 to Nov 2013 |
Other (outpatient); mean (SD) = 72.7 (5.0) years; 36% female |
BMI category, n (%): < 23 = 43 (57.3) ≥ 23 = 32 (42.7) |
EORTC QLQ‐C30; anorexia/appetite loss criteria not defined. | Prevalence of anorexia or appetite loss not reported | Treatment interruption |
| Cross‐sectional | ||||||
|
Kiesswetter et al.; 2020; Germany 48 |
Cross‐sectional; community‐dwelling: N = 1073 2009 Geriatric day hospital: N = 180 2012 Home care: N = 335 2010 Nursing home: N = 197 2007 |
Multiple settings, but reports reported separately for each: Community‐dwelling, geriatric day hospital, home care and nursing home with results reported separately for each; mean (SD) age: Community = 76 (6.6) Geriatric day hospital = 79.3 (6.2) Home care = 80.9 (7.7) Nursing home = 85.5 (7.9); % female: Community = 50.2% Geriatric day hospital = 71.7% Home care = 63.6% Nursing home = 73.6% |
BMI not reported | Appetite question (subject‐reported); anorexia/appetite loss criteria not defined. |
Community = 9.5% Geriatric day hospital = 25.6% Home care = 37.3% Nursing home = 35.4% |
Malnutrition |
|
Hirose et al.; 2014; Japan 49 |
Cross‐sectional; N = 1098 Malnourished n = 235 Risk of malnutrition n = 596 Well‐nourished n = 267; 1 May 2009 to 30 Nov 2009 |
Other (mixed community‐dwelling or nursing home); mean (SD) age, years: Malnourished = 84.6 (8.2) Risk of malnutrition = 83.8 (8.1) Well‐nourished = 81.0 (7.5); % female: Malnourished = 74.5% Risk of malnutrition = 72.7% Well‐nourished = 62.2% |
BMI not reported | MNA‐SF Q1 (food intake question); defined as subject self‐reported appetite loss or reduced food intake not attributable to specific reasons. | 26.6% | Malnutrition |
|
Pohlhausen et al.; 2016; Germany 50 |
Cross‐sectional; N = 353: Female n = 225, male n = 128; 2010 |
Community‐dwelling (receiving home care); all subjects aged > 64 years, mean (SD) age: Female = 82.0 (7.5) years, male = 79.1 (7.8) years; 63.7% female |
Mean (SD) BMI: Female = 28.2 (6.5) Male = 28.3 (5.7) |
Appetite question (subject‐reported); defined as subjects responding ‘poor’ to the question asking them to rank their appetite. Other possible responses included ‘very good’, ‘good’ or ‘moderate’. |
Female = 3.1% Male = 5.5% |
Malnutrition |
|
van der Pols‐Vijlbrief et al.; 2016; the Netherlands 51 |
Cross‐sectional; N = 300; dates not reported |
Community‐dwelling, (receiving home care); all subjects aged ≥ 65 years, mean (SD) age = 81.7 (7.6); 68.3% female |
Mean (SD) BMI = 25.8 (5.2) | SNAQ65+ Simplified 4‐items; anorexia/loss of appetite criteria not defined. | 21.3% | Malnutrition |
|
Nambooze et al.; 2014; Laos 52 |
Cross‐sectional; 3 ethnic groups (N = 144): Oy n = 53, Brau n = 41, Lao n = 50; 2012 and 2013 |
Community‐dwelling; all subjects aged ≥ 65 years, age range by ethnic group, n (%): Oy: 65–74 = 25 (47.2) 75–84 = 19 (35.8) > 85 = 9 (17.0) Brau: 65–74 = 28 (68.3) 75–84 = 8 (19.5) > 85 = 5 (12.2) Lao: 65–74 = 30 (60.0) 75–84 = 13 (26.0) > 85 = 7 (14.0); % female: Oy = 54.7%, Brau = 61%, Lao = 70% |
BMI category: n (%): Oy < 21 = 49 (92.5) ≥ 21 = 4 (7.5) Brau < 21 = 37 (90.2) ≥ 21 = 4 (9.8) Lao < 21 = 29 (58.0) ≥ 21 = 21 (42.0) |
MNA Q1 (food intake question); anorexia/loss of appetite criteria not defined. | Prevalence of anorexia or appetite loss not reported | Malnutrition |
|
Buhl et al.; 2021; Denmark 53 |
Cross‐sectional; N = 126: Normal protein intake ≥ 1.0 g/kg/day (n = 58), low protein intake ≤ 1.0 g/kg/day (n = 68); Jan 2017 to Aug 2018 |
Community‐dwelling; all subjects aged ≥ 80 years, mean (SD) age = 86 (3.6) years; 63.5% female |
Median [95% CI] BMI: Normal protein intake = 25.1 [24.1, 26.1] Low protein intake = 27.4 [26.5, 28.4] |
SNAQ Simplified; score ≤ 14. |
Normal protein intake = 3.4% (0.8, 13.1) Low protein intake = 10.3% (4.9, 20.3) |
Malnutrition |
|
Berggren et al.; 2020; Sweden 54 |
Cross‐sectional; N = 121; 2011 and 2012 |
Community‐dwelling, receiving home care; mean [95% CI] age = 83.5 [82.3, 84.7] years; 67.8% female |
Median [95% CI] BMI = 23.3 [22.6, 24.7] | ESAS Q7 and FAACT Q1; anorexia/appetite loss criteria not defined. | Prevalence of anorexia or appetite loss not reported | Malnutrition |
|
Arkkukangas et al.; 2020; Sweden 55 |
Cross‐sectional; N = 13 151: 12 290 with fall data, 3385 with fall and appetite data (all female); 2017 |
Other (mixed community‐dwelling or nursing home [4%]); all subjects aged ≥ 70 years, age range, n (%): 70–74 = 4858 (36.9) 75–79 = 3296 (25.1) 80–84 = 1942 (14.8) 85–89 = 2041 (15.5) 90 + = 1014 (7.7); 48.6% female |
BMI not reported | Appetite question (subject‐reported); anorexia/loss of appetite criteria not defined. | Women with falls in the past 12 months = 6.6% | Falls |
|
Tsutsumimoto et al.; 2020; Japan 56 |
Cross‐sectional; N = 9496; Nagoya: Jul 2013 to Dec 2013, Obu: Feb 2015 to Aug 2016 |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age = 74.1 (5.4) years; 53% female |
BMI not reported | SNAQ Simplified; defined as score ≤ 13. | 9.8% | Sarcopenia |
|
Tsutsumimoto et al.; 2017; Japan 57 |
Cross‐sectional; N = 4417; dates not reported |
Community‐dwelling; all subjects aged ≥ 70 years, mean (SD) age = 75.8 (4.3) years; 52.7% female |
Mean (SD) BMI = 22.9 (3.0) | SNAQ Simplified; defined as score ≤ 13. |
Total = 10.6% Non‐frail = 7.9% Pre‐frail: 14.8% Frail: 21.2%, P < 0.001 |
Frailty |
|
Pisu et al.; 2018; USA 58 |
Cross‐sectional; N = 1457; Nov 2013 to Jun 2015 |
Community‐dwelling with cancer; all subjects aged ≥ 65 years, mean (SD) [range] age = 74.2 (5.8) [65–99] years; 59.8% female |
Mean (SD) BMI = 27.1 (5.1) | MDASI; anorexia/loss of appetite criteria not defined. | Prevalence of anorexia or appetite loss not reported | HRQoL |
|
Yamamoto et al.; 2020; Japan 59 |
Cross‐sectional; N = 1042; Oct to Dec 2016 |
Community‐dwelling; mean (SD) age = 77.5 (4.9) years; 56.1% female |
Mean (SD) BMI = 23.2 (10.7) | CNAQ; anorexia/loss of appetite criteria not defined. | Prevalence of anorexia or appetite loss not reported | Sleep quality |
|
Kim et al.; 2019; Japan 60 |
Cross‐sectional; normal n = 822: Physical frailty n = 30, mild cognitive impairment n = 314, cognitive frailty n = 25; 2016 |
Community‐dwelling; mean (SD) age, years: Normal = 76.6 (4.5), physical frailty = 80.9 (5.3), mild cognitive impairment = 77.8 (4.7), cognitive frailty = 82.3 (6.6); % female: Normal = 61.5%, physical frailty = 46.7%, mild cognitive impairment = 56.2%, cognitive frailty = 64% |
Mean (SD) BMI: Normal = 22.9 (3.2) Physical frailty = 22.8 (4.0) Mild cognitive impairment = 23.4 (3.1) Cognitive frailty = 21.5 (3.8) |
CNAQ; anorexia/loss of appetite criteria not defined. | Prevalence of anorexia or appetite loss not reported |
Cognition Falls |
|
Donini et al.; 2011; Italy 61 |
Cross‐sectional; N = 527: With anorexia n = 112, without anorexia n = 415; Apr 2006 to Jun 2007 |
Other (mixed community‐dwelling or nursing home, or rehabilitation/acute wards ≥ 65 years); mean (SD) age, years: With anorexia = 83.0 (7.0), without anorexia = 76.6 (8.0); % female: With anorexia = 63.4%, without anorexia = 59.8% |
Mean (SD) BMI: With anorexia = 22.6 (5.0) Without anorexia = 26.7 (4.0) P < 0.05 |
Food consumption (staff‐assessed); defined as a ≥ 50% reduction in food intake versus a standard meal (over 3 days), in absence of oral disorders preventing mastication. | 21.3% |
Cognition Depression Functional status General health Increased care |
|
Fonad et al.; 2015; Sweden 62 |
Cross‐sectional; Fallers n = 434, non‐fallers n = 759; Oct 2008 to Mar 2009 |
Community‐dwelling; Age range: Fallers aged ≥ 75 years 75–84 years = 59.9% ≥ 85 years = 40.1% Non‐fallers aged ≥ 75 years 75–84 years = 66.7% ≥ 85 years = 33.3%; % female: Fallers aged ≥ 75 years = 64%, non‐fallers aged ≥ 75 years = 61% |
BMI not reported | Appetite question (subject‐reported); defined as answering ‘yes’ to the questions, ‘Has your appetite decreased in the previous 6‐month period?’ or ‘Has your food intake declined in the previous 6‐month period?’ |
Fallers, n (%): Appetite decrease = 94 (22) Food intake decline = 108 (25) Non‐fallers, n (%): Appetite decrease = 84 (11) Food intake decline = 90 (12) |
Falls |
|
Acar Tek and Karaçil‐Ermumcu; 2018; Turkey 63 |
Cross‐sectional; N = 407; dates not reported |
Community‐dwelling; all subjects described as ‘elders’, mean (SD) age, years: Men = 72.8 (6.7), women = 71.1 (6.4); 65% female |
Mean (SD) BMI: Men = 27.5 (4.3) Women = 29.8 (5.8) |
SNAQ Simplified; defined as score ≤ 14. | 28.7% | HRQoL |
|
Landi et al.; 2013; Italy 64 |
Cross‐sectional; N = 354; dates not reported |
Community‐dwelling; mean (SD) age = 85.8 (4.9) years; 66.7% female |
Mean (SD) BMI = 25.6 (4.5) | Food consumption and appetite questions (subject‐reported); defined as answering ‘yes’ to the question, ‘Is the amount of food intake decreased in the last year?’ or ‘poor’ to the question, ‘How is your appetite in general?’ Other possible responses included ‘normal’ or ‘good’. | 20.6% | Sarcopenia |
|
Reijnierse et al.; 2015; the Netherlands 65 |
Cross‐sectional; N = 185; Mar 2011 to Jan 2012 |
Community‐dwelling (but all pts seen in geriatric outpatient clinic); mean (SD) age = 82.0 (7.3) years; 60% female |
Mean (SD) BMI = 25.7 (4.4) | SNAQ Short AL question; anorexia/loss of appetite criteria not defined. | 27.6% | Sarcopenia |
|
Nakatsu et al.; 2015; Japan 66 |
Cross‐sectional; N = 84; Aug 2013 to Sep 2013 |
Community‐dwelling; all subjects aged ≥ 65 years, mean (SD) age = 76.4 (9.3) years; 64% female |
Mean (SD) BMI = 23.5 (3.2) | SNAQ Simplified; anorexia/loss of appetite criteria not defined. | Prevalence of anorexia or appetite loss not reported |
Depression Functional status |
|
Dent et al.; 2018; Australia 67 |
Cross‐sectional a ; N = 172; Oct 2010 to Dec 2011 |
Inpatient; all subjects aged ≥ 70 years, mean (SD) age = 85.2 (6.4) years; 72% female |
Mean (SD) BMI = 25.3 (6.5) | SNAQ Simplified; defined as score ≤ 14. | 63% | Malnutrition |
|
Madeira et al.; 2018; Portugal 68 |
Cross‐sectional; N = 1186; Oct 2015 to Apr 2016 |
Institution (nursing home); all subjects aged ≥ 65 years, mean [95% CI] age = 83.4 [82.8, 83.7] years; 72.8% female |
Mean [95% CI] BMI = 27.4 [27.0, 27.8] | Appetite question (subject‐reported); anorexia/loss of appetite criteria not defined. |
% [95% CI] on a scale from 1 = no appetite to 5 = lots of appetite: 1 = 5.8 [3.9, 8.6] 2 = 18.4 [15.9, 21.2] 3 = 42.7 [38.5, 46.9] 4 = 22.6 [19.0, 26.6] 5 = 10.5 [8.3, 13.3] |
Malnutrition |
|
Donini et al.; 2013; Italy 9 |
Cross‐sectional; N = 100; Jan to Jun 2005 |
Institution (nursing home); mean (SD) age = 80.2 (10) years; 71% female |
BMI category, %: Male: < 18.5 = 0% 18.5–24.9 = 50% 25–29.9 = 25% ≥ 30 = 25% Female: < 18.5 = 6.7 18.5–24.9 = 30 25–29.9 = 26.7 ≥ 30 = 36.6 Mean (SD) BMI by MNA nutritional status: Normal = 32.6 (6) At risk = 26.9 (5) Malnutrition = 19.3 (1) P = 0.00 |
Food consumption (staff‐assessed); anorexia/loss of appetite criteria not defined. |
Subjects (%) with decreased, scarce or absent appetite: Normal nutrition status = 16.7% At‐risk status = 54.4% Malnutrition status = 61% |
Malnutrition |
|
van Steijn et al.; 2014; the Netherlands 69 |
Cross‐sectional; N = 102; Nov 2010 to Feb 2011 |
Other (outpatients with Parkinson's disease); mean (SD) age = 76.4 (6.3) years; 47.1% female |
Mean (SD) BMI = 25.2 (3.6) | CNAQ; anorexia/loss of appetite criteria not defined. | Prevalence of anorexia or appetite loss not reported | Malnutrition |
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; CES‐D, Center for Epidemiological Studies Depression Scale; CI, confidence interval; CNAQ, Council on Nutrition Appetite Questionnaire; COPD, chronic obstructive pulmonary disease; EORTC QLQ‐C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire; ESAS, Edmonton Symptom Assessment System; FAACT, Functional Assessment of Anorexia/Cachexia Treatment; FIM, Functional Independence Measure; FRAIL, Fatigue, Resistance, Ambulation, Illnesses, and Loss of Weight; GDS, Geriatric Depression Scale; IQR, interquartile range; LASA, Longitudinal Aging Study Amsterdam; MDASI, MD Anderson Symptom Inventory; MNA, Mini Nutritional Assessment; MNA‐SF, Mini Nutritional Assessment‐Short Form; PROMIS, Patient‐Reported Outcomes Measurement Information System; SD, standard deviation; SE, standard error; SNAQ Short, Short Nutritional Assessment Questionnaire; SNAQ Simplified, Simplified Nutritional Appetite Questionnaire; TAVI, transcatheter aortic valve implantation.
Dent (2018) is the same study population as Dent (2015). Although both were prospective longitudinal studies, Dent (2018) is classified here as cross‐sectional because the outcome of interest for the systematic literature review (malnutrition) was assessed at the same time point as appetite (at hospital admission), whereas outcomes in Dent (2015) were assessed at discharge.
By design, all subjects were aged ≥ 65 years. Twenty‐three studies reported a mean or median age > 80 years (Table 2 ). Cohorts were predominantly female. Mean body mass index (BMI) at study entry ranged between 21.0 and 29.3 kg/m2. 31 , 37 Six studies were in subjects with active cancer or a history of cancer 23 , 27 , 30 , 46 , 47 , 58 ; two studies were in subjects hospitalized for a fracture 44 or other trauma 15 ; and one study each was in subjects with Parkinson's disease, 69 subjects receiving haemodialysis 32 or subjects hospitalized for COVID‐19. 29 The remaining studies were not specific to a given health condition (Table 2 ).
Assessment of anorexia/appetite loss
Methods used to assess anorexia/appetite loss were reported in nearly all (n = 57; 98.3%) studies (Tables 2 and S3 ). The most commonly used assessment tool for appetite was the self‐reported Simplified Nutritional Appetite Questionnaire (SNAQ Simplified, 14 studies) 70 ; most of these studies employed a SNAQ Simplified score of ≤ 14 or ≤ 13 (in some studies) out of 20 as the threshold for defining anorexia/appetite loss. Four studies used the Mini Nutritional Assessment (MNA) or the Mini Nutritional Assessment Short Form (MNA‐SF), three studies used the Short Nutritional Assessment Questionnaire (SNAQ Short), 71 three studies used the Council on Nutrition Appetite Questionnaire (CNAQ) 70 and one study used the Functional Assessment of Anorexia/Cachexia Treatment (FAACT). 72 The remaining studies used assessments of quality of life that included specific questions on appetite, subject‐ or staff‐reported assessments of food intake and/or appetite, or history of loss of appetite taken from subject medical records (Tables 2 and S3 ).
Prevalence of anorexia/appetite loss
Prevalence of anorexia/appetite loss was reported in 41 (70.7%) studies and ranged widely, from 0.20% in a study of 1 466 598 older UK residents (anorexia/appetite loss was derived from medical records and was defined as the patient visiting a general practitioner for loss of appetite in the previous 12 months) 16 to 63% in a study of hospitalized older subjects in South Australia (anorexia/appetite loss was derived from the SNAQ Simplified and defined as a score ≤ 14) 43 (Table 2 ). When estimated by residential setting, prevalence ranged from 0.20% 16 to 55% 17 in community‐dwelling subjects, 5.8% 68 to 61% 9 among subjects in institutional care, 13% 27 to 63% 43 in inpatient settings and 6.6% 55 to 26.6% 65 in other settings (mixed and outpatient). The wide variation in reported prevalence across studies is explained, in part, by heterogeneity with respect to populations assessed, study design and the methods used to evaluate or define anorexia/appetite loss.
Eleven studies with a reported prevalence defined anorexia/appetite loss as a SNAQ Simplified score of ≤ 14 or ≤ 13 (Table 2 ). 7 , 25 , 26 , 36 , 43 , 51 , 53 , 56 , 57 , 63 , 67 Among these 11 studies with a consistent definition, the prevalence of anorexia/appetite loss ranged from 3.4% 53 to 33% 36 for community‐dwelling older adults (n = 7 studies) and from 38% 26 to 63% 43 in the inpatient care setting (n = 4 studies).
Relationship between anorexia/appetite loss and malnutrition
All 15 studies (3 longitudinal and 12 cross‐sectional) that assessed the association of anorexia/appetite loss and malnutrition confirmed a higher risk of malnutrition in populations with anorexia/appetite loss (vs. without), regardless of country of origin, healthcare setting or measure of malnutrition (Figure 2 and Table S4 ). Ten of the 15 studies reported odds ratios (ORs) (Figure 3 ). Measures of malnutrition included mostly the MNA (full and short forms) (Table S4 ) as well as measures of unintentional weight loss, BMI, upper arm and calf circumferences, and protein intake (Table S4 ).
Figure 2.
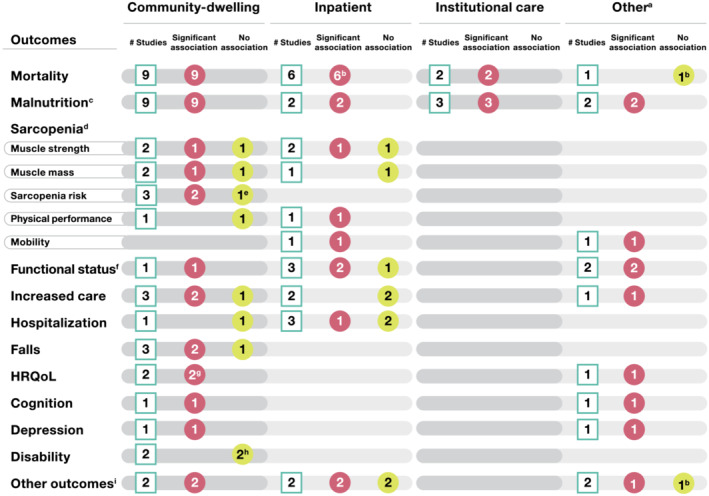
Summary of association between anorexia/appetite loss and outcomes in studies identified (n = 58 articles), shown by healthcare setting. White squares indicate the number of studies assessing the outcome; red circles indicate the number of studies where anorexia/appetite loss was associated with poor outcomes; and yellow circles indicate the number of studies where anorexia/appetite loss was not associated with the outcome. HRQoL, health‐related quality of life. a‘Other’ includes studies with mixed settings (no separate results by setting) and studies where care was provided in an outpatient setting. bBased on findings from a univariate analysis, a significant association was found between anorexia/appetite loss and mortality in reference 27 but not in reference. 32 An association between anorexia/appetite loss and treatment interruption was found in univariate, but not multivariate, analysis. 47 cIn Kiesswetter et al. (2020), 48 an association of appetite loss with malnutrition was reported separately for both community‐dwelling and institutional care cohorts; therefore, this study has been counted in both the categories. dSarcopenia was reported in seven studies. ePoor appetite alone was not significantly associated with sarcopenia development in participants with poor appetite and without low masticatory function; however, a significantly higher risk of sarcopenia was observed in those who had both poor appetite and low masticatory function. 36 fIn Nakatsu et al. (2015), 66 the authors measured walking speed, chair stand time, hand‐grip strength and timed ‘Up and Go’ test and characterized these outcomes as measures of physical performance and not sarcopenia; however, other studies also used these measures to assess sarcopenia. A significant correlation between walking speed, chair stand time and timed ‘Up and Go’ test and better appetite was noted, whereas the correlation between hand‐grip strength and appetite was not statistically significant. gLack of appetite was found to be associated with HRQoL as assessed by the Short Form‐12 mental component summary score only, but not with the Short Form‐12 physical component summary score. 58 hAnorexia was found to be associated with a significantly higher risk of disability in both studies in unadjusted or non‐fully adjusted models, but this association was no longer significant in fully adjusted models. 7 , 37 i‘Other outcomes’ includes frailty, general health, infection, major adverse cardiovascular or cerebrovascular events, sleep quality, stroke, treatment (chemotherapy) interruption and weight loss, which are reported in one study each. The two inpatient studies both had some “other outcomes” that were associated with anorexia/appetite loss and some that were not. Thus, they are shown in both categories in the figure.
Figure 3.
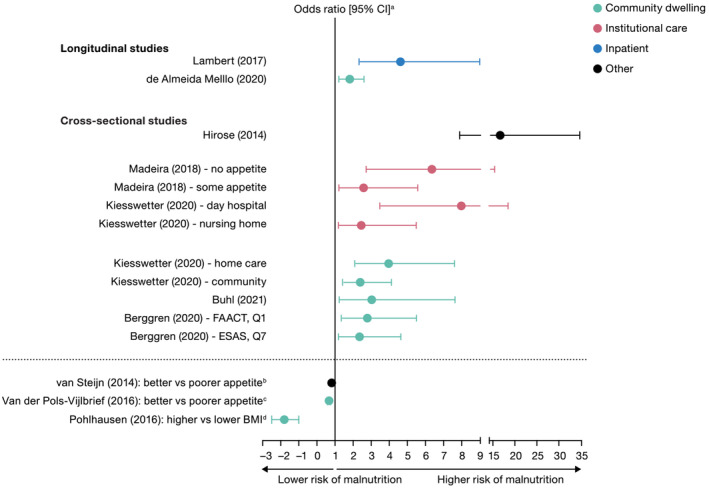
Relationship between anorexia/appetite loss and malnutrition in studies reporting odds ratios. Community‐dwelling represents independent living or free living in the community. Institutional care represents living in a nursing care facility or care home. Inpatient represents being hospitalized or staying in a hospital ward. Other represents living in healthcare setting not covered (e.g., outpatient or conducted in mixed healthcare setting). Studies above the dashed line used better appetite as the reference group (positive odds ratio = more risk of malnutrition), whereas studies below the dashed line used worse appetite as the reference group (negative odds ratio = less risk of malnutrition). BMI, body mass index; CI, confidence interval; ESAS, Edmonton Symptom Assessment System; FAACT, Functional Assessment of Anorexia/Cachexia Treatment; Q, question. aFifteen of the 58 studies identified investigated and reported a significant relationship between anorexia/poor appetite and malnutrition, with 10 studies presenting data as an odds ratio. bOdds ratio [95% CI] = 0.82 [0.70, 0.95]. cUnadjusted odds for better appetite associated with a lower risk of malnutrition, other studies were adjusted for baseline variables. dUnivariate odds ratio for relationship between BMI and malnutrition (i.e., higher BMI is associated with a lower risk of malnutrition).
Of the three longitudinal studies, two community‐based studies concluded that anorexia/appetite loss was associated with a 76% increased risk 33 of unintentional weight loss and a 63% increased risk 34 of malnutrition (vs. those without anorexia/appetite loss) over a period of 1 and 9 years, respectively (Figure 3 and Table S4 ). A third study of 317 orthopaedic subjects hospitalized for at least two nights reported that the likelihood of malnutrition was 4.5 times higher among those with anorexia/appetite loss than those without anorexia/appetite loss (OR [95% confidence interval, CI] = 4.54 [2.31, 8.90], P < 0.001) 15 (Figure 3 ).
The 12 cross‐sectional studies assessed populations with different comorbidities and included a range of care settings (Table S4 ). Regardless of setting, a higher risk of malnutrition was reported in those with anorexia/poor appetite than in those without anorexia/appetite loss. The highest risk of malnutrition was reported from a multivariate analysis of 1098 Japanese individuals with anorexia/appetite loss living either in institutional care (nursing home) or within the community (OR [95% CI] = 16.45 [7.84, 34.54], P < 0.001) 49 (Figure 3 ). A study in the Netherlands reported that the odds of an unfavourable nutritional status increased by 18% for every point decrease of the CNAQ score (OR [95% CI] = 0.82 [0.70, 0.95], P = 0.008) in 102 older outpatients with Parkinson's disease. 69 In a separate study of 300 community‐dwelling individuals receiving home care in the Netherlands, better appetite was significantly associated with lower risk of malnutrition (OR [95% CI] = 0.66 [0.58, 0.76], P < 0.05), for each point increase in SNAQ Simplified score. 51 Finally, in a community‐dwelling population from Germany receiving home care (n = 353), a significantly negative association (P < 0.001) was found between risk of malnutrition and physiological measures of nutritional body status (e.g., BMI, mid‐upper arm circumference and calf circumference). 50
One study specifically investigated an association between anorexia/appetite loss and malnutrition among older adults in Germany across different care settings: community (cohorts with and without home care) and institutional (geriatric day care and nursing home cohorts). 48 The association between appetite and malnutrition was significant in all cohorts regardless of residential setting in univariate analyses, and the odds of malnutrition were more than double in community‐dwelling older adults without home care (OR [95% CI] = 2.42 [1.43, 4.10]), nearly four‐fold greater for those receiving home care (OR [95% CI] = 3.99 [2.10, 7.58]) and eight‐fold higher for geriatric day care subjects (OR [95% CI] = 8.01 [3.48, 18.44]), 48 reporting poor appetite after adjusting for covariates in a stepwise logistic regression analysis.
Relationship between anorexia/appetite loss and mortality
Eighteen of 35 (51.4%) longitudinal studies assessed the relationship between anorexia/appetite loss and mortality (Table S5 ). Mortality was assessed for up to 15 years of follow‐up, 22 but studies most commonly reported associations with 1‐year mortality (n = 6). 16 , 18 , 28 , 30 , 31 Anorexia/appetite loss was associated with increased risk of mortality in 17 of the 18 studies (94%; Figure 4 ), regardless of method used to assess anorexia/appetite loss (Figure S1 ). A positive association with mortality was noted across a variety of settings, including nine in community‐dwelling older adults, six in an inpatient setting and two in an institutional setting (Figure 4 ). In 16 of the studies, the association between anorexia/appetite loss and mortality was maintained in multivariate analyses controlling for known confounders. Appetite loss increased the risk of death by 30–35% in the largest study of > 1.4 million community‐dwelling older people (hazard ratio [HR] [95% CI]: women = 1.30 [1.21, 1.39]; men = 1.35 [1.24, 1.48]). 16
Figure 4.
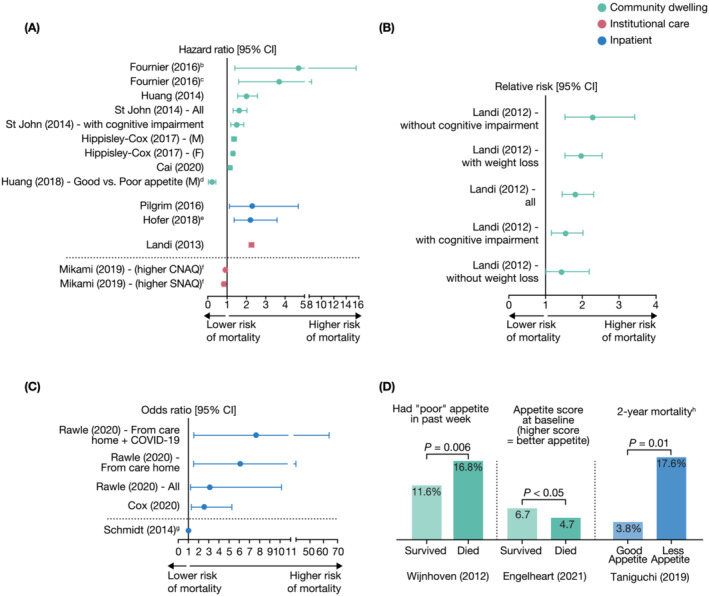
Relationship between anorexia/appetite loss and mortalitya. (A) Data as hazard ratio. (B) Data as relative risk. (C) Data as odds ratio. (D) Other mortality data. CI, confidence interval; CNAQ, Council on Nutrition Appetite Questionnaire; COVID‐19, coronavirus disease 2019; F, female; M, male; SNAQ, Simplified Nutritional Appetite Questionnaire. aOf the 58 studies identified, 18 longitudinal studies investigated the relationship between anorexia/poor appetite and mortality. Of these, 17 studies showed a significant association (P < 0.05) and are presented. bFive‐year survival based on decline in appetite from baseline to 3 months. cFive‐year survival based on decline in appetite from baseline to 6 months. dAnalysis in female was non‐significant. eUnadjusted hazard ratio, other studies adjusted for baseline variables as detailed in Table S5 . fData show that better appetite (i.e., higher CNAQ/SNAQ scores) is associated with lower risk of mortality. Findings agree with other studies showing that loss of appetite is associated with higher risk of mortality. gOdds ratio [95% CI] = 1.02 [1.00, 1.03], P = 0.01, with higher scores indicating better appetite (score inversely related to mortality). hOne‐year mortality was not significant for good appetite (3.8%) versus less appetite (5.9%), P = 0.46.
In a study of 296 older individuals admitted to hospital, a SNAQ Simplified score < 14 was associated with 2.62‐fold increased odds of mortality at 6 months (OR [95% CI] = 2.62 [1.30, 5.27], P = 0.007). 25 In this study, every 1‐point decrease in SNAQ Simplified score predicted a 22% increase in odds of 6‐month mortality (OR [95% CI] = 1.22 [1.07, 1.39], P = 0.002) after adjustment for length of stay, Charlson comorbidity index and gender. 25 Similarly, another study reported that a SNAQ Simplified score < 14 was associated with more than two‐fold higher risk of mortality (adjusted HR [95% CI] = 2.29 [1.12, 4.68], P = 0.023) in older women during acute hospitalization (n = 178). 26
Anorexia/appetite loss was associated with a significantly higher risk of mortality in individuals with a range of comorbidities, including community‐dwelling individuals with depressive disorder, 17 cognitive impairment 8 , 21 and weight loss 8 (Table S5 ). Three studies demonstrated that anorexia/appetite loss is associated with worse survival among subjects with cancer. 23 , 27 , 30
The lone study that did not report a significant relationship between anorexia/appetite loss and mortality was a longitudinal Japanese study in haemodialysis outpatients that assessed the impact of quality of life on 3‐year mortality. 32 Univariate Kaplan–Meier analysis demonstrated no significant difference in mortality for subjects aged ≥ 65 years with appetite scores > 75 (on 100 mm of visual analogue scale) versus those with scores ≤ 74 (P = 0.6832). 32 In a multivariate analysis (adjusted for age, gender, duration of haemodialysis, clinical data and comorbidities), a small but statistically significant increased (rather than decreased) risk of mortality was observed in subjects aged ≥ 65 years with a better self‐reported appetite (adjusted relative risk [95% CI] = 1.048 [1.006, 1.103], P = 0.0247). 32 The authors noted that the lack of correlation between appetite (and other measures) and mortality among subjects aged ≥ 65 years may be due to the low subject number (n = 72) because significant associations were seen in the larger cohort aged < 65 years. 32
Relationship between anorexia/appetite loss and additional outcomes of interest
The relationship between anorexia/appetite loss and outcomes of interest other than malnutrition or mortality was examined in several studies, and results are summarized below, arranged by the number of studies (greatest to lowest number) for each outcome.
Sarcopenia indicators
Seven studies (four longitudinal and three cross‐sectional) assessed the association of anorexia/appetite loss with sarcopenia indicators (Figure 2 and Table S6 ). 26 , 35 , 36 , 42 , 56 , 64 , 65 A variety of methods were used to assess sarcopenia including risk of sarcopenia, sarcopenia incidence (3‐year), grip strength, muscle strength, chair stand time, reduced muscle mass, absolute muscle mass, skeletal muscle mass, mobility and physical performance. In all three cross‐sectional studies, the presence of anorexia/appetite loss was significantly associated with a higher risk of most, but not all, sarcopenia‐related outcomes in older subjects. 56 , 64 , 65 The association between anorexia/appetite loss and the risk of sarcopenia was mixed in the four longitudinal studies. 26 , 35 , 36 , 42
Decreased physical function
The association of anorexia/appetite loss with decreased physical function was evaluated in five longitudinal studies and one cross‐sectional study (Figure 2 and Table S7 ). 43 , 44 , 45 , 46 , 61 , 66 Assessments included subject‐reported physical function, hospitalization‐associated functional decline (Katz Index of Activities of Daily Living [ADL]), Functional Independence Measure ≥ 90, physical performance and functional change (Barthel Index or Instrumental ADL score). All but one 43 study reported a significant association between anorexia/appetite loss and decreased physical function.
Increased care
Six studies (five longitudinal and one cross‐sectional) reported the association of anorexia/appetite loss and increased care requirements (Figure 2 and Table S8 ). 26 , 38 , 39 , 40 , 43 , 61 Increased care was measured using a variety of definitions including receipt of formal or informal long‐term care, entry/moved to nursing home or sheltered housing, discharged to higher level of care (includes death) and by residential accommodation type (habilitation/acute geriatric ward vs. nursing home vs. free living). Three of the studies noted an association between anorexia/appetite loss and the need for increased care. 38 , 40 , 61
Hospitalization‐related outcomes
The association between anorexia/appetite loss and hospitalization‐related outcomes was assessed in four longitudinal studies (Figure 2 and Table S9 ). 26 , 28 , 41 , 43 Outcomes included hospital utilization, time to first hospital or emergency room admission, hospital length of stay, readmission to hospital and hospitalization due to acute decompensated heart failure. Only one study found an association between anorexia/appetite loss and hospitalization‐related outcomes. 28
Falls
The association between anorexia/appetite loss and falls was assessed in three cross‐sectional studies (Figure 2 and Table S10 ). 55 , 60 , 62 Falls were measured as falls/fall‐related injuries in the last 12 months or cognitive frailty‐related falls. Anorexia/appetite loss was associated with a significantly increased risk of falls in two of the three studies. 55 , 60
Health‐related quality of life
Three studies (one longitudinal and two cross‐sectional) reported the association between anorexia/appetite loss and health‐related quality of life (HRQoL) (Figure 2 and Table S11 ). 46 , 58 , 63 Methods to assess HRQoL included the Short Form‐12 or ‐36 Health Surveys and global quality of life scores using the EORTC QLQ‐C30. In two studies, good appetite was associated with better HRQoL. 46 , 63 In the remaining study, lack of appetite was associated with HRQoL, as assessed by the Short Form‐12 mental, but not physical, component score. 58
Cognition, depression and disability
Two studies (both cross‐sectional) reported that anorexia/appetite loss was associated with poor cognition (Figure 2 and Table S12 ). 60 , 61 Likewise, two cross‐sectional studies reported that the likelihood of depression was higher in subjects with anorexia than in subjects without anorexia (Figure 2 and Table S12 ). 61 , 66 Two longitudinal studies reported that anorexia/appetite loss was associated with a significantly higher risk of disability in unadjusted or non‐fully adjusted models, but this association was not significant in fully adjusted models (Figure 2 and Table S12 ). 7 , 37
Other outcomes
The association between anorexia/appetite loss and other outcomes (e.g., hospital‐acquired infection, treatment interruption, major adverse cardiac and cerebrovascular events [MACCE], life‐threatening stroke, comorbidity burden, sleep quality and physical frailty) was assessed in six studies (three longitudinal and three cross‐sectional) (Figure 2 and Table S13 ). 26 , 28 , 47 , 57 , 59 , 61 Anorexia/appetite loss was significantly associated with hospital‐acquired infection, 26 MACCE, 28 comorbidity burden, 61 frailty 57 and sleep efficiency. 59 In contrast, anorexia/appetite loss was not significantly associated with life‐threatening stroke. 28 Anorexia/appetite loss was significantly associated with treatment interruption in a univariate, but not multivariate, analysis. 47
Discussion
Although anorexia/appetite loss is common among older populations and represents a significant health‐related burden, it is under‐evaluated, under‐diagnosed and under‐treated in clinical practice. 73 Therefore, we sought to characterize the association of anorexia/appetite loss with negative outcomes using a comprehensive systematic search of published literature. Overall, 58 studies (both longitudinal and cross‐sectional) in a variety of healthcare settings (community‐dwelling, institutional care, inpatient and other) were identified that assessed the relationship of anorexia/appetite loss with mortality, morbidity and other healthcare outcomes in populations aged ≥ 65 years.
Key findings
A majority of studies (70.7%) reported on the prevalence of anorexia/appetite loss, although results varied greatly (from 0.20% to 63%). 16 , 43 , 67 This variation is explained, in part, by heterogeneity with respect to populations assessed, study design and the methods used to define and assess anorexia/appetite loss. A total of 15 studies examined the relationship between anorexia/appetite loss and malnutrition. As expected, each of these studies reported a statistically significant relationship between anorexia/appetite loss and malnutrition, including when adjusting for known confounders. Whereas the odds of malnutrition increased with anorexia/appetite loss regardless of healthcare setting, odds were increased in institutional‐based studies versus community‐based studies. One study, for example, demonstrated that the risk of malnutrition among those with anorexia/appetite loss was more than two‐fold higher in a community setting and eight‐fold higher in an institutional setting compared with those without anorexia/appetite loss. 48 A total of 18 studies examined the relationship between anorexia/appetite loss and mortality, with 17 (94.4%) showing a significant relationship, regardless of treatment setting. In 16 of the 17 studies, the association between anorexia/appetite loss and mortality remained significant after adjusting for known confounders. Whereas this association with mortality was expected among persons with cancer, it was also observed in other older populations with a range of comorbid conditions other than cancer. Other negative outcomes were studied less frequently in the literature, but associations between anorexia/poor appetite and sarcopenia, functional status, increased care, hospitalization, falls, HRQoL, cognition, depression, disability and other outcomes were also observed.
Associations of anorexia/appetite loss with these adverse outcomes are likely mediated via both direct and indirect mechanisms. Appetite loss will result in reduced food intake and precipitate or exacerbate weight loss, malnutrition, sarcopenia, declining strength and frailty, which in turn can impair functional status and HRQoL and increase falls, care requirements, hospitalizations, disability and mortality. 3 Specific micronutrient deficiencies may also mediate poor health outcomes associated with anorexia/appetite loss. 64 Relationships between anorexia/appetite loss may also be bi‐directional. For example, whereas anorexia/appetite loss may contribute to the aetiology of multimorbidity, severe and chronic diseases may, in turn, drive anorexia/appetite loss. 18 Finally, associations of anorexia/appetite loss with adverse outcomes may not always represent a causal relationship. For example, diminished appetite may occur as a side effect of polypharmacy required for management of comorbidities, and these comorbidities may be the primary drivers of poor outcomes. 74
The study of appetite loss and its health‐related impact is complicated by a lack of standardized terminology describing the condition. In addition to appetite loss, terms such as poor appetite, reduced appetite, decreased appetite, lack of appetite, risk of malnutrition, undernutrition, malnourishment, weight loss, risk of weight loss, unexplained or abnormal weight loss, anorexia and anorexia of aging were used interchangeably across publications, geographies and settings. These terms, however, are not always equivalent. Clearer consensus definitions for, and more careful application of, these terms would be helpful for the field. For example, there is often a lack of distinction between anorexia (specific appetite loss) and malnutrition (lack of intake or uptake of nutrients). 75 , 76 Whereas malnutrition may be a secondary cause or symptom of anorexia, malnutrition can be caused by a variety of other physical or socioeconomic factors. 76
The field is also complicated by the lack of a standardized and reliable method to define and assess anorexia/appetite loss. A wide range of methods were used in the 57 studies that described how anorexia/appetite loss was assessed (see Tables 2 and S3 ). These included appetite‐specific tools (e.g., SNAQ Simplified and CNAQ), nutritional assessment tools (e.g., SNAQ Short and MNA/MNA‐SF), appetite‐related questions from broader health‐related tools (e.g., CES‐D, EORTC QLQ‐C30 and Edmonton Symptom Assessment System [ESAS]) and a variety of subject‐ or staff‐reported assessments. As such, these tools vary in the breadth of what is actually assessed, ranging from a single appetite‐specific question to a range of questions across various domains that include appetite. Even within more appetite‐specific tools, such as the SNAQ Simplified, some questions extend beyond appetite to surrogates such as taste and meal frequency. Furthermore, these assessment tools vary greatly in terms of format (e.g., Likert, numeric rating scale or categorical), validation methodology, recall period (e.g., past day, past week, past month or not specified) and administration (e.g., subject‐reported or staff‐reported). This undoubtedly contributed to the great variation seen in prevalence estimates and complicates comparisons across different studies. Although the SNAQ Simplified was the most frequently used tool, it was only utilized in approximately one quarter (n = 14) of the studies identified. Further, only 11 of these 14 studies used a consistent definition (SNAQ Simplified score of ≤ 14 or ≤ 13 out of 20) to define and report the prevalence of anorexia/appetite loss.
Gaps in the literature
There was also a notable paucity of information on the burden of anorexia/appetite loss in the United States (only 3 studies, 5.2%), with a majority of studies based in Europe (59%) or Asia (28%). This geographical discrepancy may reflect a heightened awareness of the role of nutrition in health and a holistic approach to patient management among geriatricians in Europe and Asia. Further, none of the US‐based studies identified assessed the association of anorexia/appetite loss with mortality, malnutrition, sarcopenia, functional status, falls, cognition, depression or disability.
Whereas several studies examined the association of anorexia/appetite loss with malnutrition (n = 15) and mortality (n = 18), fewer studies examined the association with other outcomes (e.g., sarcopenia, functional status and HRQoL; see Figure 2 ), and no studies examined the association with healthcare costs, healthcare resource utilization (apart from basic information on hospitalization/length of stay or nursing home admission) and caregiver burden.
Strengths and limitations
The strength of this review is based on the systematic approach (following PRISMA guidelines) to study identification and the quality assessments performed on included studies (all studies except one 15 had low or medium risk of bias).
Despite the comprehensive, systematic approach, however, there are some limitations to our study. For example, it is not possible to rule out selection and publication bias as only English language studies were included. Additionally, although most studies utilized a multivariate analysis, it is difficult to separate out the effects of anorexia/loss of appetite from other comorbidities because not all studies adjusted for the same confounding variables. Likewise, there was substantial heterogeneity across studies in terms of country, population, appetite assessment, outcomes measured, hospital systems, medical technologies and healthcare settings, which may affect interpretation of our results. Despite these limitations, this comprehensive SLR provides robust evidence that anorexia/appetite loss increases the risk of a number of adverse outcomes, particularly mortality and malnutrition, in populations aged ≥ 65 years.
Conclusions
This SLR demonstrates that, among persons aged ≥ 65 years, anorexia/appetite loss is associated with increased risk of adverse outcomes (particularly malnutrition and mortality) across community living, nursing/care homes and inpatient settings and across disease states. These associations warrant efforts to improve the detection, evaluation and management of anorexia/appetite loss in older adults. Such efforts to improve management of anorexia/appetite loss are ongoing. The Society on Sarcopenia, Cachexia and Muscle Wasting Disorders, for example, will be publishing international guidelines on Anorexia of Aging shortly. 77 However, this SLR demonstrates that efforts are hindered by the lack of a standardized approach to appetite assessment and the lack of distinction between anorexia/appetite loss and malnutrition. Consensus guidance on the optimal appetite assessment tool in older populations, on the definition for anorexia/appetite loss in older adults and on standard of care would provide a much needed framework for intervention trials of candidate therapeutics for this target indication. In addition, there remains a need for well‐designed longitudinal cohort studies (particularly in the United States) with clearly defined/standardized measures of anorexia/appetite loss and suitable control for confounding factors, including the interplay of factors such as malnutrition, cachexia, sarcopenia, cognitive decline, loss of mobility, social isolation and socioeconomic disadvantage.
Conflict of interest
Roger A. Fielding reports grants, personal fees and other from Axcella Health, other from Juvicell, other from Inside Tracker, grants and personal fees from Biophytis, personal fees from Amazentis, personal fees from Nestle and personal fees from Pfizer, outside the submitted work. Roger A. Fielding is also partially supported by the US Department of Agriculture (USDA), under Agreement No. 58‐8050‐9‐004 and by NIH Boston Claude D. Pepper Center (OAIC; 1P30AG031679). Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. Francesco Landi reports invited lectures for Abbott and Nutricia. Karen E. Smoyer is an employee of Curo, part of Envision Pharma group who were paid consultants to Pfizer in relation to this project. Lisa Tarasenko and John Groarke are employees of Pfizer and may hold stock or stock options.
Supporting information
Table S1. Search strategy for (A) Embase, (B) Pubmed, and (C) Cochrane central register of controlled trials or of systematic reviews. Searches conducted on July 31, 2021
Table S2. Analysis of risk of bias for (A) longitudinal cohort studies (Newcastle‐Ottawa Scale) and for (B) longitudinal cross‐sectional sectional studies (modified Newcastle‐Ottawa Scale) identified in the systematic literature review
Table S3. Measures used to define anorexia/appetite loss
Table S4. Relationship between anorexia/appetite loss and malnutrition, as reported in 15 identified studies
Table S5. Relationship between anorexia/appetite loss and mortality as reported in 18 identified studies
Table S6. Relationship between anorexia/appetite loss and sarcopenia indicators as reported in 7 identified studies
Table S7. Relationship between anorexia/appetite loss and functional status as reported in 6 identified studies
Table S8. Relationship between anorexia/appetite loss and increased care as reported in 6 identified studies
Table S9. Relationship between anorexia/appetite loss and hospitalization as reported in 4 identified studies
Table S10. Relationship between anorexia/appetite loss and falls as reported in 3 identified studies
Table S11. Relationship between anorexia/appetite loss and health‐related quality of life as reported in 3 identified studies
Table S12. Relationship between anorexia/appetite loss and cognition, depression, or disability as reported in 5 identified studies
Table S13. Relationship between anorexia/appetite loss and other outcomes as reported in 6 identified studies
Figure S1. Summary of association between anorexia/appetite loss and scale used to assess appetite in studies identified (n = 58)
Acknowledgements
Medical writing support was provided by Karen Burrows, MPhil, CMPP, and Matt Soulsby, PhD, CMPP, of Engage Scientific Solutions and was funded by Pfizer. Database searches were conducted by Catherine Rolland, whereas screening, data extraction and quality assessment were carried out by Catherine Rolland and Karen E. Smoyer of CURO (part of the Envision Pharma group), funded by Pfizer. The authors also wish to thank Jatin Gupta for assistance with developing the SLR report. This study was funded by Pfizer. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 78
Fielding R. A., Landi F., Smoyer K. E., Tarasenko L., and Groarke J. (2023) Association of anorexia/appetite loss with malnutrition and mortality in older populations: A systematic literature review, Journal of Cachexia, Sarcopenia and Muscle, 14, 706–729, 10.1002/jcsm.13186
References
- 1. Pilgrim AL, Robinson SM, Sayer AA, Roberts HC. An overview of appetite decline in older people. Nurs Older People 2015;27:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morley JE, Silver AJ. Anorexia in the elderly. Neurobiol Aging 1988;9:9–16. [DOI] [PubMed] [Google Scholar]
- 3. Cox NJ, Morrison L, Ibrahim K, Robinson SM, Sayer AA, Roberts HC. New horizons in appetite and the anorexia of ageing. Age Ageing 2020;49:526–534. [DOI] [PubMed] [Google Scholar]
- 4. Cox NJ, Ibrahim K, Sayer AA, Robinson SM, Roberts HC. Assessment and treatment of the anorexia of aging: a systematic review. Nutrients 2019;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Meij BS, Wijnhoven HAH, Lee JS, Houston DK, Hue T, Harris TB, et al. Poor appetite and dietary intake in community‐dwelling older adults. J Am Geriatr Soc 2017;65:2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres SJ, McCabe M, Nowson CA. Depression, nutritional risk and eating behaviour in older caregivers. J Nutr Health Aging 2010;14:442–448. [DOI] [PubMed] [Google Scholar]
- 7. Tsutsumimoto K, Doi T, Makizako H, Hotta R, Nakakubo S, Makino K, et al. Aging‐related anorexia and its association with disability and frailty. J Cachexia Sarcopenia Muscle 2018;9:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landi F, Lattanzio F, Dell'Aquila G, Eusebi P, Gasperini B, Liperoti R, et al. Prevalence and potentially reversible factors associated with anorexia among older nursing home residents: results from the ULISSE project. J Am Med Dir Assoc 2013;14:119–124. [DOI] [PubMed] [Google Scholar]
- 9. Donini LM, Neri B, De Chiara S, Poggiogalle E, Muscaritoli M. Nutritional care in a nursing home in Italy. PLoS One. 2013;8:e55804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mion LC, McDowell JA, Heaney LK. Nutritional assessment of the elderly in the ambulatory care setting. Nurse Pract Forum 1994;5:46–51. [PubMed] [Google Scholar]
- 11. Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti‐inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007;128:92–105. [DOI] [PubMed] [Google Scholar]
- 12. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol 2020. https://osf.io/preprints/metaarxiv/v7gm2/ Accessed 18 Aug 2022. [DOI] [PubMed] [Google Scholar]
- 13. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 18 Aug 2022. [Google Scholar]
- 14. Herzog R, Álvarez‐Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review BMC Public Health 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lambert C, Nüssler A, Biesalski HK, Freude T, Bahrs C, Ochs G, et al. Age‐dependent risk factors for malnutrition in traumatology and orthopedic patients. Nutrition 2017;37:60–67. [DOI] [PubMed] [Google Scholar]
- 16. Hippisley‐Cox J, Coupland C. Development and validation of QMortality risk prediction algorithm to estimate short term risk of death and assess frailty: cohort study. BMJ 2017;358:j4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai W, Mueller C, Shetty H, Perera G, Stewart R. Predictors of mortality in people with late‐life depression: a retrospective cohort study. J Affect Disord 2020;266:695–701. [DOI] [PubMed] [Google Scholar]
- 18. Landi F, Liperoti R, Lattanzio F, Russo A, Tosato M, Barillaro C, et al. Effects of anorexia on mortality among older adults receiving home care: an observation study. J Nutr Health Aging 2012;16:79–83. [DOI] [PubMed] [Google Scholar]
- 19. Huang YC, Wahlqvist ML, Lee MS. Appetite predicts mortality in free‐living older adults in association with dietary diversity. A NAHSIT cohort study Appetite 2014;83:89–96. [DOI] [PubMed] [Google Scholar]
- 20. Huang YC, Wahlqvist ML, Lo YTC, Lin C, Chang HY, Lee MS. A non‐invasive modifiable Healthy Ageing Nutrition Index (HANI) predicts longevity in free‐living older Taiwanese. Sci Rep 2018;8:7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St John PD, Montgomery PR. Utility of Hippocrates' prognostic aphorism to predict death in the modern era: prospective cohort study. BMJ 2014;349:g7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wijnhoven HAH, Schilp J, van Bokhorst‐de van der Schueren MAE, de Vet HCW, Kruizenga HM, Deeg DJH, et al. Development and validation of criteria for determining undernutrition in community‐dwelling older men and women: the Short Nutritional Assessment Questionnaire 65+. Clin Nutr 2012;31:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fournier E, Jooste V, Woronoff AS, Quipourt V, Bouvier AM, Mercier M. Health‐related quality of life is a prognostic factor for survival in older patients after colorectal cancer diagnosis: a population‐based study. Dig Liver Dis 2016;48:87–93. [DOI] [PubMed] [Google Scholar]
- 24. Engelheart S, Forslund HB, Brummer RJ, Ljungqvist O. Dehydration and loss of appetite: key nutrition features in older people receiving home health care. Nutrition 2021;91‐92:111385. [DOI] [PubMed] [Google Scholar]
- 25. Cox NJ, Lim SE, Howson F, Moyses H, Ibrahim K, Sayer AA, et al. Poor appetite is associated with six month mortality in hospitalised older men and women. J Nutr Health Aging 2020;24:1107–1110. [DOI] [PubMed] [Google Scholar]
- 26. Pilgrim AL, Baylis D, Jameson KA, Cooper C, Sayer AA, Robinson SM, et al. Measuring appetite with the Simplified Nutritional Appetite Questionnaire identifies hospitalised older people at risk of worse health outcomes. J Nutr Health Aging 2016;20:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hofer F, Koinig KA, Nagl L, Borjan B, Stauder R. Fatigue at baseline is associated with geriatric impairments and represents an adverse prognostic factor in older patients with a hematological malignancy. Ann Hematol 2018;97:2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taniguchi Y, Sakakura K, Yuri K, Nomura Y, Tamanaha Y, Akashi N, et al. Appetite predicts clinical outcomes in high risk patients undergoing trans‐femoral TAVI. Int Heart J 2019;60:1350–1357. [DOI] [PubMed] [Google Scholar]
- 29. Rawle MJ, Bertfield DL, Brill SE. Atypical presentations of COVID‐19 in care home residents presenting to secondary care: a UK single centre study. Aging Med 2020;3:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt M, Neuner B, Kindler A, Scholtz K, Eckardt R, Neuhaus P, et al. Prediction of long‐term mortality by preoperative health‐related quality‐of‐life in elderly onco‐surgical patients. PLoS One 2014;9:e85456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mikami Y, Watanabe Y, Edahiro A, Motokawa K, Shirobe M, Yasuda J, et al. Relationship between mortality and Council of Nutrition Appetite Questionnaire scores in Japanese nursing home residents. Nutrition 2019;57:40–45. [DOI] [PubMed] [Google Scholar]
- 32. Kanamori H, Nagai K, Matsubara T, Mima A, Yanagita M, Iehara N, et al. Comparison of the psychosocial quality of life in hemodialysis patients between the elderly and non‐elderly using a visual analogue scale: the importance of appetite and depressive mood. Geriatr Gerontol Int 2012;12:65–71. [DOI] [PubMed] [Google Scholar]
- 33. de Almeida MJ, Favaro‐Moreira NC, Krausch‐Hofmann S, Vanneste D, Matthys C, Declercq A, et al. Can the interRAI home care instrument be applied to the definition criteria of the Global Leadership Initiative on Malnutrition (GLIM)? A longitudinal study Clin Nutr 2020;39:3477–3482. [DOI] [PubMed] [Google Scholar]
- 34. Schilp J, Wijnhoven HAH, Deeg DJH, Visser M. Early determinants for the development of undernutrition in an older general population: Longitudinal Aging Study Amsterdam. Br J Nutr 2011;106:708–717. [DOI] [PubMed] [Google Scholar]
- 35. Cox NJ, Bowyer RCE, Ni Lochlainn M, Wells PM, Roberts HC, Steves CJ. The composition of the gut microbiome differs among community dwelling older people with good and poor appetite. J Cachexia Sarcopenia Muscle 2021;12:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Senoo S, Iwasaki M, Kimura Y, Kakuta S, Masaki C, Wada T, et al. Combined effect of poor appetite and low masticatory function on sarcopenia in community‐dwelling Japanese adults aged ≥ 75 years: a 3‐year cohort study. J Oral Rehabil 2020;47:643–650. [DOI] [PubMed] [Google Scholar]
- 37. Martinez‐Reig M, Gomez‐Arnedo L, Alfonso‐Silguero SA, Juncos‐Martinez G, Romero L, Abizanda SP. Nutritional risk, nutritional status and incident disability in older adults. The FRADEA study. J Nutr Health Aging 2014;18:270–276. [DOI] [PubMed] [Google Scholar]
- 38. Hsu WC, Wang JY, Tsai AC. Predictors of developing a new need for long‐term care of older adults aged ≥70 years: results from a population‐based cohort study in Taiwan. Geriatr Gerontol Int 2019;19:641–646. [DOI] [PubMed] [Google Scholar]
- 39. Salminen M, Eloranta S, Vire J, Viikari P, Viikari L, Vahlberg T, et al. Prediction of the future need for institutional care in Finnish older people: a comparison of two birth cohorts. Gerontology 2017;64:19–27. [DOI] [PubMed] [Google Scholar]
- 40. Sheppard KD, Brown CJ, Hearld KR, Roth DL, Sawyer P, Locher JL, et al. Symptom burden predicts nursing home admissions among older adults. J Pain Symptom Manage 2013;46:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salanitro AH, Hovater M, Hearld KR, Roth DL, Sawyer P, Locher JL, et al. Symptom burden predicts hospitalization independent of comorbidity in community‐dwelling older adults. J Am Geriatr Soc 2012;60:1632–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Dronkelaar C, Tieland M, Aarden JJ, Reichardt LA, Van Seben R, Van Der Schaaf M, et al. Decreased appetite is associated with sarcopenia‐related outcomes in acute hospitalized older adults. Nutrients 2019;11:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dent E, Chapman I, Piantadosi C, Visvanathan R. Nutritional screening tools and anthropometric measures associate with hospital discharge outcomes in older people. Australas J Ageing 2015;34:E1–E6. [DOI] [PubMed] [Google Scholar]
- 44. Mendelson G, Katz Y, Shahar DR, Bar O, Lehman Y, Spiegel D, et al. Nutritional status and osteoporotic fracture rehabilitation outcomes in older adults. J Nutr Gerontol Geriatr 2018;37:231–240. [DOI] [PubMed] [Google Scholar]
- 45. Van Grootven B, Jeuris A, Jonckers M, Devriendt E, Dierckx de Casterlé B, Dubois C, et al. Predicting hospitalisation‐associated functional decline in older patients admitted to a cardiac care unit with cardiovascular disease: a prospective cohort study. BMC Geriatr 2020;20:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kirkhus L, Harneshaug M, Saltyte Benth J, Gronberg BH, Rostoft S, Bergh S, et al. Modifiable factors affecting older patients' quality of life and physical function during cancer treatment. J Geriatr Oncol 2019;10:904–912. [DOI] [PubMed] [Google Scholar]
- 47. Won HS, Sun DS, Choi JY, An HJ, Ko YH. Factors associated with treatment interruption in elderly patients with cancer. Korean J Intern Med 2019;34:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kiesswetter E, Colombo MG, Meisinger C, Peters A, Thorand B, Holle R, et al. Malnutrition and related risk factors in older adults from different health‐care settings: an enable study. Public Health Nutr 2020;23:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hirose T, Hasegawa J, Izawa S, Enoki H, Suzuki Y, Kuzuya M. Accumulation of geriatric conditions is associated with poor nutritional status in dependent older people living in the community and in nursing homes. Geriatr Gerontol Int 2014;14:198–205. [DOI] [PubMed] [Google Scholar]
- 50. Pohlhausen S, Uhlig K, Kiesswetter E, Diekmann R, Heseker H, Volkert D, et al. Energy and protein intake, anthropometrics, and disease burden in elderly home‐care receivers—a cross‐sectional study in Germany (ErnSIPP study). J Nutr Health Aging 2016;20:361–368. [DOI] [PubMed] [Google Scholar]
- 51. van der Pols‐Vijlbrief R, Wijnhoven HA, Molenaar H, Visser M. Factors associated with (risk of) undernutrition in community‐dwelling older adults receiving home care: a cross‐sectional study in the Netherlands. Public Health Nutr 2016;19:2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nambooze J, Fujimura M, Inaoka T. Nutritional status and functional capacity of community‐dwelling elderly in Southern Laos. Environ Health Prev Med 2014;19:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buhl SF, Beck AM, Christensen B, Kock G, Boyle E, Caserotti P. Prevalence of low protein intake in 80+ year‐old community‐dwelling adults and association with dietary patterns and modifiable risk factors: a cross‐sectional study. Br J Nutr 2021;127:266–277. [DOI] [PubMed] [Google Scholar]
- 54. Berggren E, Strang P, Orrevall Y, Odlund Olin A, Tornkvist L. Symptom burden in patients with home care who are at risk for malnutrition: a cross‐sectional study. J Palliat Care 2020;35:103–109. [DOI] [PubMed] [Google Scholar]
- 55. Arkkukangas M, Eriksson HG, Dension E. Risk factors for fall‐related injuries among community‐dwelling men and women over 70 years of age, based on social cognitive theory: results from a population study. Eur J Physiother 2020;23:221–226. [Google Scholar]
- 56. Tsutsumimoto K, Doi T, Nakakubo S, Kim M, Kurita S, Ishii H, et al. Association between anorexia of ageing and sarcopenia among Japanese older adults. J Cachexia Sarcopenia Muscle 2020;11:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsutsumimoto K, Doi T, Makizako H, Hotta R, Nakakubo S, Makino K, et al. The association between anorexia of aging and physical frailty: results from the National Center for Geriatrics and Gerontology's study of geriatric syndromes. Maturitas 2017;97:32–37. [DOI] [PubMed] [Google Scholar]
- 58. Pisu M, Azuero A, Halilova KI, Williams CP, Kenzik KM, Kvale EA, et al. Most impactful factors on the health‐related quality of life of a geriatric population with cancer. Cancer 2018;124:596–605. [DOI] [PubMed] [Google Scholar]
- 59. Yamamoto K, Motokawa K, Yoshizaki T, Yano T, Hirano H, Ohara Y, et al. Association of dietary variety and appetite with sleep quality in urban‐dwelling older Japanese adults. J Nutr Health Aging 2020;24:152–159. [DOI] [PubMed] [Google Scholar]
- 60. Kim H, Awata S, Watanabe Y, Kojima N, Osuka Y, Motokawa K, et al. Cognitive frailty in community‐dwelling older Japanese people: prevalence and its association with falls. Geriatr Gerontol Int 2019;19:647–653. [DOI] [PubMed] [Google Scholar]
- 61. Donini LM, Dominguez LJ, Barbagallo M, Savina C, Castellaneta E, Cucinotta D, et al. Senile anorexia in different geriatric settings in Italy. J Nutr Health Aging 2011;15:775–781. [DOI] [PubMed] [Google Scholar]
- 62. Fonad E, Robins Wahlin TB, Rydholm Hedman AM. Associations between falls and general health, nutrition, dental health and medication use in Swedish home‐dwelling people aged 75 years and over. Health Soc Care Community 2015;23:594–604. [DOI] [PubMed] [Google Scholar]
- 63. Acar Tek N, Karaçil‐Ermumcu M. Determinants of health related quality of life in home dwelling elderly population: appetite and nutritional status. J Nutr Health Aging 2018;22:996–1002. [DOI] [PubMed] [Google Scholar]
- 64. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Barillaro C, et al. Association of anorexia with sarcopenia in a community‐dwelling elderly population: results from the ilSIRENTE study. Eur J Nutr 2013;52:1261–1268. [DOI] [PubMed] [Google Scholar]
- 65. Reijnierse EM, Trappenburg MC, Leter MJ, Blauw GJ, De Van Der Schueren MAE, Meskers CGM, et al. The association between parameters of malnutrition and diagnostic measures of sarcopenia in geriatric outpatients. PLoS One 2015;10:e0135933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakatsu N, Sawa R, Misu S, Ueda Y, Ono R. Reliability and validity of the Japanese version of the simplified nutritional appetite questionnaire in community‐dwelling older adults. Geriatr Gerontol Int 2015;15:1264–1269. [DOI] [PubMed] [Google Scholar]
- 67. Dent E, Wright O, Hoogendijk EO, Hubbard RE. Nutritional screening and dietitian consultation rates in a geriatric evaluation and management unit. Nutr Diet 2018;75:11–16. [DOI] [PubMed] [Google Scholar]
- 68. Madeira T, Peixoto‐Placido C, Sousa‐Santos N, Santos O, Alarcao V, Goulao B, et al. Malnutrition among older adults living in Portuguese nursing homes: the PEN‐3S study. Public Health Nutr 2018;1‐12:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van Steijn J, van Harten B, Flapper E, Droogsma E, van Walderveen P, Blaauw M, et al. The nutritional status of Dutch elderly patients with Parkinson's disease. J Nutr Health Aging 2014;18:601–607. [DOI] [PubMed] [Google Scholar]
- 70. Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community‐dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074–1081. [DOI] [PubMed] [Google Scholar]
- 71. Kruizenga HM, Seidell JC, de Vet HC, Wierdsma NJ, van Bokhorst‐de van der Schueren MA. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin Nutr 2005;24:75–82. [DOI] [PubMed] [Google Scholar]
- 72. Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, et al. Re‐validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 2000;9:1137–1146. [DOI] [PubMed] [Google Scholar]
- 73. de Souto BP, Cesari M, Morley JE, Roberts S, Landi F, Cederholm T, et al. Appetite loss and anorexia of aging in clinical care: an ICFSR task force report. J Frailty Aging 2022;11:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nakamura T, Itoh T, Yabe A, Imai S, Nakamura Y, Mizokami Y, et al. Polypharmacy is associated with malnutrition and activities of daily living disability among daycare facility users: a cross‐sectional study. Medicine (Baltimore) 2021;100:e27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Frostad S, Bentz M. Anorexia nervosa: outpatient treatment and medical management. World J Psychiatry 2022;12:558–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Serón‐Arbeloa C, Labarta‐Monzón L, Puzo‐Foncillas J, Mallor‐Bonet T, Lafita‐López A, Bueno‐Vidales N, et al. Malnutrition screening and assessment. Nutrients 2022;14:2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aprahamian I. Appetite is key in geriatric syndromes: a pre‐release of SCWD International Guidelines on Anorexia of Aging. In Presentation at the 15th International Conference on Cachexia, Sarcopenia, and Muscle Wasting. June 24–26, 2002. Lisbon, Portugal; 2022. [Google Scholar]
- 78. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Measur 1977;1:385–401. [Google Scholar]
- 80. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 81. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 82. Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000;89:1634–1646. [DOI] [PubMed] [Google Scholar]
- 83. Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short‐form mini‐nutritional assessment (MNA‐SF). J Gerontol A Biol Sci Med Sci 2001;56:M366–M372. [DOI] [PubMed] [Google Scholar]
- 84. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999;15:116–122. [DOI] [PubMed] [Google Scholar]
- 85. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient‐Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy for (A) Embase, (B) Pubmed, and (C) Cochrane central register of controlled trials or of systematic reviews. Searches conducted on July 31, 2021
Table S2. Analysis of risk of bias for (A) longitudinal cohort studies (Newcastle‐Ottawa Scale) and for (B) longitudinal cross‐sectional sectional studies (modified Newcastle‐Ottawa Scale) identified in the systematic literature review
Table S3. Measures used to define anorexia/appetite loss
Table S4. Relationship between anorexia/appetite loss and malnutrition, as reported in 15 identified studies
Table S5. Relationship between anorexia/appetite loss and mortality as reported in 18 identified studies
Table S6. Relationship between anorexia/appetite loss and sarcopenia indicators as reported in 7 identified studies
Table S7. Relationship between anorexia/appetite loss and functional status as reported in 6 identified studies
Table S8. Relationship between anorexia/appetite loss and increased care as reported in 6 identified studies
Table S9. Relationship between anorexia/appetite loss and hospitalization as reported in 4 identified studies
Table S10. Relationship between anorexia/appetite loss and falls as reported in 3 identified studies
Table S11. Relationship between anorexia/appetite loss and health‐related quality of life as reported in 3 identified studies
Table S12. Relationship between anorexia/appetite loss and cognition, depression, or disability as reported in 5 identified studies
Table S13. Relationship between anorexia/appetite loss and other outcomes as reported in 6 identified studies
Figure S1. Summary of association between anorexia/appetite loss and scale used to assess appetite in studies identified (n = 58)


