Abstract
Background
Myosteatosis and systemic inflammation are well‐known prognostic factors in patients with colorectal cancer (CRC). The serum albumin level is a reflection of malnutrition and systemic inflammation, which in turn plays a key role in the development of myosteatosis. However, few studies have been conducted on these synergistic effects. This study aimed to examine the individual and synergistic effects of different prognostic markers related to skeletal muscle quality and serum albumin levels in patients with CRC.
Methods
This study enrolled patients with stage I–III CRC who underwent surgical resection between July 2006 and February 2014. Skeletal muscle index (SMI) and skeletal muscle radiodensity (SMD) were calculated using computed tomography at the L3 level obtained within 2 months prior to surgery. The albumin‐myosteatosis gauge (AMG) was defined as SMD × albumin. Patients were divided into sex‐specific quartiles (G1 to G4) according to the AMG, and analysis of variance for continuous variables and chi‐square test for categorical variables were used to compare variables among quartiles. Cox proportional hazard models were constructed and integrated receiver operating characteristic curve (iAUC) analysis was used to compare the prognostic performance of SMD, albumin and AMG.
Results
Among the 906 participants, the median (interquartile) age was 64 (55–72) years, and 365 (40.3%) were female. AMG was significantly correlated with the occurrence of complications, albumin level, SMI and SMD (all P < 0.001). Overall survival (OS) differed significantly according to the AMG group, with 5‐year OS for G1–G4 being 73.4%, 86.2%, 91.1% and 95.5%, respectively (P < 0.0001). Although SMI, SMD, albumin and AMG were all significant individual prognostic markers of OS in the univariable analysis, AMG remained the only independent prognostic factor in the multivariable analysis (G1 vs. G2, P = 0.045, G1 vs. G3, P = 0.005, G1 vs. G4, P < 0.001, respectively). The iAUC value of AMG [0.681, 95% confidence interval (CI) = 0.638–0.723] was superior to that of SMD (0.610, 95% CI = 0.566–0.654) (bootstrap iAUC mean difference = 0.071, 95% CI = 0.034–0.106), SMI (0.551, 95% CI = 0.511–0.594) (bootstrap iAUC mean difference = 0.129, 95% CI = 0.076–0.181) and albumin (0.627, 95% CI = 0.585–0.668) (bootstrap iAUC mean difference = 0.053, 95% CI = 0.010–0.098).
Conclusions
In patients with stage I–III CRC, AMG is a meaningful predictor of survival, with superior prognostic value compared to SMI, SMD or albumin alone. Further studies are needed to determine their significance in different ethnic groups.
Keywords: myosteatosis, albumin, albumin‐myosteatosis gauge, colorectal cancer, sarcopenia, inflammation
Introduction
Colorectal cancer (CRC) is the third most common malignancy and one of the leading causes of cancer‐related death worldwide. Despite much progress in the surgical and medical treatment of CRC, 20–30% of patients with stage I–III disease still develop recurrence, and it is the third highest cause of cancer‐related mortality in South Korea. 1 , 2 Many studies have focused on identifying clinical and treatment‐related factors associated with prognosis in patients with CRC. 3
Cancer cachexia, a syndrome characterized by multiple factors including reduced nutritional status and a chronic inflammatory response, is associated with poor survival. 4 Skeletal muscle wasting is an important factor in cancer cachexia. The definition of sarcopenia used by the European Working Group on Sarcopenia in Older People (EWGSOP) has recently been changed to highlight that both muscle mass and muscle quality, reflected in part by intermuscular or intramyocelluar fat deposition (myosteatosis), are important. 5 A meta‐analysis showed that myosteatosis was a major prognostic factor for survival in a variety of cancer types, reporting 73% higher mortality in cancer patients with myosteatosis than in those without myosteatosis [hazard ratio (HR) 1.73, 95% confidence interval (CI) 1.58–1.90, P < 0.0001]. 6
Systemic inflammation is also a promising factor for predicting the outcome of CRC, as well as a key component in the pathogenesis of muscle wasting by pro‐inflammatory cytokines, such as interleukin 1 (IL‐1), interleukin 6 (IL‐6) and tumour necrosis factor (TNF)‐α, which influence muscle progenitor cells and muscle turnover. 7 , 8 These cytokines also affect the production of albumin in the liver, which is an indicator of malnutrition, as well as a factor for inflammation. 9 Taking this into consideration, muscle quality and albumin share a common pathway; however, the synergistic effects of both in patients with cancer have not been thoroughly investigated.
Therefore, this study aimed to examine and compare different prognostic markers for overall survival (OS) related to skeletal muscle quality and serum albumin in patients with CRC.
Methods
Study population
This retrospective study enrolled patients diagnosed with CRC who were surgically treated between July 2006 and February 2014 at Gangnam Severance Hospital, Yonsei University College of Medicine. This study was approved by the Institutional Review Board of our hospital. The requirement for informed consent was waived owing to the retrospective nature of the study.
The following patients were eligible for study inclusion: (i) diagnosed with stage I–III CRC, (ii) underwent blood testing within 1 month prior to surgery, including serum albumin measurements, and (iii) underwent routine abdominal‐pelvic computed tomography (CT) within 2 months prior to surgery. The exclusion criteria were as follows: (i) neuroendocrine or gastrointestinal stromal tumour, (ii) appendiceal or anal cancer, (iii) double primary cancers, (iv) preoperative chemoradiotherapy or radiotherapy, (v) emergency operations and (vi) hereditary non‐polyposis syndrome, familial adenomatous polyposis, ulcerative colitis and Crohn's disease. Details of the inclusion and exclusion criteria are shown in Figure S1.
Clinical variables
Patient source data were obtained from a review of electronic medical records (EMR) and included information on disease stage, tumour characteristics such as tumour location, size, histological grade, lymphovascular invasion (LVI), total lymph nodes, receipt of chemotherapy and demographic characteristics, including sex, age, American Society of Anesthesiologists (ASA) grade and body mass index (BMI). Height and weight measured at the clinical visit closest to the diagnostic CT scan were used to calculate BMI, expressed as weight in kilograms (kg) divided by height in meters squared (m2). Other relevant covariates, such as carcinoembryonic antigen (CEA) and serum albumin levels, and complications, were retrieved from the EMR.
Measurement of skeletal muscle index and skeletal muscle radiodensity
Abdominopelvic CT images were obtained within 2 months prior to surgery. The CT protocol is described in Supplementary Paragraph 1. CT images taken at the level of the third lumbar vertebra (L3) were used to measure skeletal muscle area (SMA) and skeletal muscle density (SMD). To measure SMA, we used in‐house open‐source software ‘BMI_CT’ available at https://sourceforge.net/projects/muscle‐fat‐area‐measurement. 10 The SMD was measured using 3DSlicer, which is also available online at https://www.slicer.org. 11 The SMA was segmented using a threshold of −29 to 150 Hounsfield units (HU). SMA normalized to height (cm2/m2) was defined as the skeletal muscle index (SMI). The SMD was assessed by estimating the mean HU value of the SMA. The intra‐class correlation coefficients pre‐determined via this software by two investigators of SMD and SMI were reported to be 0.99 (range, 0.97–0.99) and 0.97 (range, 0.95–0.99), respectively, in our previous report. 12
Albumin‐myosteatosis gauge
The albumin‐myosteatosis gauge (AMG) was calculated using the following formula: serum albumin (g/dL) × SMD (HU). For simplicity, an arbitrary unit was used in the study instead of g/dL × HU as the AMG unit.
Defining low and high level of SMD and albumin
In this study, SMD was divided into low and high according to the criteria suggested by Martin et al., which was 41 in patients with BMI < 25 kg/m2 and 33 in patients with BMI ≥ 25 kg/m2. 13 Albumin was classified into low and high groups based on the median value.
Patient follow‐up
Patient follow‐up was performed regularly every 3 months at outpatient clinics for the first 3 years post‐operatively and then every 3–6 months for the next 2 years. Routine chemistry and complete blood counts, including serum CEA levels, were recorded during follow‐up. Chest and abdominopelvic CT images were obtained every 6 or 12 months, considering the patient's pathological stage, for 5 years. Procedures, such as colonoscopy, pelvic magnetic resonance imaging and other imaging studies, were performed according to the judgement of the physician.
Statistical analysis
Patients were divided into sex‐specific quartiles based on their AMG levels at the time of enrolment. Clinicopathological characteristics between the groups were compared using analysis of variance for continuous variables and chi‐square test for categorical variables. Bonferroni's post hoc test was performed to assess the magnitude of the differences.
The primary outcome of the study was OS, which was defined as the time from the date of surgery to the date of death from any cause or the date of the last follow‐up. Patients with OS periods longer than 5 years were censored. OS was estimated using the Kaplan–Meier method and compared between groups using the log‐rank test. Cox proportional hazard models were used to test the relationship of between AMG and OS. Variables that were significant (P < 0.05) in the univariable analyses were entered into a multivariable analysis using backward selection. The results were reported using HR with corresponding 95% CIs.
To compare the prognostic capabilities of AMG, albumin and SMD, the integrated areas under the time‐dependent receiver operating characteristic (ROC) curves (iAUC) were calculated. The time‐dependent ROC curve is the weighted average of the AUC in a specific time period and is used as a continuous marker to evaluate the discriminatory power for time‐dependent disease outcomes. The time‐dependent ROC curve was used to measure the predictive value of the model during a certain period, mostly during follow‐up. We used bootstrapping to assess between‐group risk differences. All analyses were repeated according to sex, as shown in the Supporting Information .
All statistical analyses were performed using R version 4.1.0 (R‐project, Institute for Statistics and Mathematics, Vienna, Austria). Statistical significance was set at P < 0.05.
Results
Distribution of AMG according to the sex
The study sample consisted of 906 patients with stage I–III CRC. The median values of albumin, SMD and AMG differed considerably according to sex (Figure S2). With respect to AMG, each quartile was divided into 158.66, 189.95 and 219.52 in male patients and 138.43, 174.06 and 200.54 in female patients, respectively (Table S1). We divided AMG into four subgroups according to sex quartile methods: G1, G2, G3 and G4. The numbers of male patients in groups G1, G2, G3 and G4 were 136, 135, 135 and 135, respectively. The number of female patients in groups G1, G2, G3 and G4 was 96, 95, 95 and 95, respectively.
Clinicopathological characteristics according to the quartile of AMG
Analysis of variance for continuous variables and chi‐square test for categorical variables were used to detect correlations between quartile AMG groups and clinicopathological characteristics, such as sex, age, BMI, ASA grade, CEA level, tumour location, tumour size, histologic grade, LVI, total lymph nodes, stage, complications, chemotherapy, albumin level, SMI and SMD. Patients in the lowest quartile AMG group were older (age ≥70 years; 64.5%, 38.5%, 21.2% and 5.3%, P < 0.001), had higher ASA grade (grades III and IV, 17.5%, 10.6%, 8.8% and 4%, P < 0.001; G1 vs. G4, P < 0.05; G2 vs. G3, P < 0.05), higher CEA (≥5 ng/mL, 36%, 27%, 21.7% and 21.2%, P < 0.001; G1 vs. G3, P < 0.05; G1 vs. G4, P < 0.05), larger tumour size (≥5 cm, 53.5%, 42%, 27.4% and 28.3%, P < 0.001; G1 vs. G3, P < 0.05; G1 vs. G4, P < 0.05; G2 vs. G3, P < 0.05; G2 vs. G4, P < 0.05), more complications (32%, 19.9%, 17.3% and 16.8%, P < 0.001; G1 vs. G2, P < 0.05; G1 vs. G3, P < 0.05; G1 vs. G4, P < 0.05), lower mean serum albumin levels (g/dL) (3.8, 4.2, 4.4 and 4.6, P < 0.001; G1 vs. G2, P < 0.05; G1 vs. G3, P < 0.05; G1 vs. G4, P < 0.05; G2 vs. G3, P < 0.05; G2 vs. G4, P < 0.05; G3 vs. G4, P < 0.05), lower mean SMI (cm2/m2) (47.1, 47.9, 49.3 and 49.8, P < 0.001; G1 vs. G3, P < 0.05; G1 vs. G4, P < 0.05) and lower mean SMD (HU) (32.2, 40.3, 45.6 and 50.9, P < 0.001; G1 vs. G2, P < 0.05; G1 vs. G3, P < 0.05; G1 vs. G4, P < 0.05; G2 vs. G3, P < 0.05; G2 vs. G4, P < 0.05; G3 vs. G4, P < 0.05) than those in the other groups (Table 1).
Table 1.
Patient characteristics according to the quartile of albumin‐myosteatosis gauge
| G1 group(n = 228) | G2 group(n = 226) | G3 group(n = 226) | G4 group(n = 226) | |||
|---|---|---|---|---|---|---|
| Variables | Categorization | n (%) | n (%) | n (%) | n (%) | P |
| Sex | Female | 92 (40.4) | 91 (40.3) | 91 (40.3) | 91 (40.3) | |
| Male | 136 (59.6) | 135 (59.7) | 135 (59.7) | 135 (59.7) | >0.999 | |
| Age (years) | <70 | 81 (35.5) † , ‡ , § | 139 (61.5) * , ‡ , § | 178 (78.8) * , † , § | 214 (94.7) * , † , ‡ | |
| ≥70 | 147 (64.5) | 87 (38.5) | 48 (21.2) | 12 (5.3) | <0.001 | |
| BMI (kg/m2) | Mean (SD) | 23.7 (3.5) | 23.6 (2.9) | 23.5 (2.8) | 23.0 (2.9) | 0.051 |
| ASA grade | I | 84 (36.8) § | 95 (42) ‡ | 108 (47.8) † | 135 (59.7) * | |
| II | 86 (37.7) | 96 (42.5) | 87 (38.5) | 73 (32.3) | ||
| III and IV | 40 (17.5) | 24 (10.6) | 20 (8.8) | 9 (4) | ||
| Unknown | 18 (7.9) | 11 (4.9) | 11 (4.9) | 9 (4) | <0.001 | |
| CEA (ng/mL) | <5 | 141 (61.8) ‡ , § | 158 (69.9) | 158 (69.9) * | 164 (72.6) * | |
| ≥5 | 82 (36) | 61 (27) | 49 (21.7) | 48 (21.2) | ||
| Unknown | 5 (2.2) | 7 (3.1) | 19 (8.4) | 14 (6.2) | <0.001 | |
| Tumour location | Colon | 174 (76.3) § | 163 (72.1) | 156 (69) | 140 (61.9) * | |
| Rectum | 54 (23.7) | 63 (27.9) | 70 (31) | 86 (38.1) | 0.008 | |
| Tumour size (cm) | <5 | 106 (46.5) ‡ , § | 131 (58.0) ‡ , § | 164 (72.6) * , † | 162 (71.7) * , † | |
| ≥5 | 122 (53.5) | 95 (42.0) | 62 (27.4) | 64 (28.3) | <0.001 | |
| Histologic grade | G1 and G2 | 204 (89.5) | 208 (92.0) | 215 (95.1) | 211 (93.4) | |
| G3 and MC and SRC | 24 (10.5) | 18 (8.0) | 11 (4.9) | 15 (6.6) | 0.134 | |
| LVI | Absent | 162 (71.1) | 164 (72.6) | 184 (81.4) | 174 (77.0) | |
| Present | 60 (26.3) | 56 (24.8) | 36 (15.9) | 40 (17.7) | ||
| Unknown | 6 (2.6) | 6 (2.7) | 6 (2.7) | 12 (5.3) | 0.029 | |
| Total lymph nodes | <12 | 36 (15.8) | 28 (12.4) | 34 (15.0) | 32 (14.2) | |
| ≥12 | 192 (84.2) | 198 (87.6) | 192 (85.0) | 194 (85.8) | 0.756 | |
| Stage | I and II | 128 (56.1) | 132 (58.4) | 138 (61.1) | 124 (54.9) | |
| III | 100 (43.9) | 94 (41.6) | 88 (38.9) | 102 (45.1) | 0.560 | |
| Complications | No | 155 (68.0) † , ‡ , § | 181 (80.1) * | 187 (82.7) * | 188 (83.2) * | |
| Yes | 73 (32.0) | 45 (19.9) | 39 (17.3) | 38 (16.8) | <0.001 | |
| Chemotherapy | No | 105 (46.1) | 94 (41.6) | 93 (41.2) | 87 (38.5) | |
| Yes | 123 (53.9) | 132 (58.4) | 133 (58.8) | 139 (61.5) | 0.432 | |
| Albumin (g/dL) | Mean (SD) | 3.8 (0.5) † , ‡ , § | 4.2 (0.3) * , ‡ , § | 4.4 (0.3) * , † , § | 4.6 (0.3) * , † , ‡ | <0.001 |
| SMI (cm2/m2) | Mean (SD) | 47.1 (8.8) ‡ , § | 47.9 (7.6) | 49.3 (8.6) * | 49.8 (9.2) * | <0.001 |
| SMD (HU) | Mean (SD) | 32.2 (7.1) † , ‡ , § | 40.3 (4.2) * , ‡ , § | 45.6 (3.7) * , † , § | 50.9 (4.1) * , † , ‡ | <0.001 |
ASA, American Society of Anesthesiologists; BMI, body mass index; CEA, carcinoembryonic antigen; HU, Hounsfield unit; LVI, lymphovascular invasion; MC, mucinous adenocarcinoma; SD, standard deviation; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; SRC, signet‐ring cell.
P < 0.05 vs. G1 group.
P < 0.05 vs. G2 group.
P < 0.05 vs. G3 group.
P < 0.05 vs. G4 group.
We compared SMD and albumin levels among the AMG groups in men and women. There were significant differences in the median SMD values according to the AMG group in both men and women (all P < 0.001). In addition, there were significant differences in the median albumin level according to the AMG group in the men and women (all P < 0.001) (Figure S3).
Kaplan–Meier survival curve according to the quartiles of AMG
Kaplan–Meier analysis showed significant differences in 5‐year OS among the AMG groups (G1–73.4%, G2–86.2%, G3–91.1% and G4–95.5%), respectively (P < 0.0001; G1 vs. G2, P = 0.005; G1 vs. G3, P < 0.001; G1 vs. G4, P < 0.001; G2 vs. G3, P = 0.592; G2 vs. G4, P = 0.051; G3 vs. G4, P = 0.400) (Figure 1). In subgroup analysis of stage II and III patients, the 5‐year OS rates for G1, G2, G3 and G4 were 69.6%, 83.2%, 87.9% and 94.7%, respectively (P < 0.0001) (Figure S4).
Figure 1.
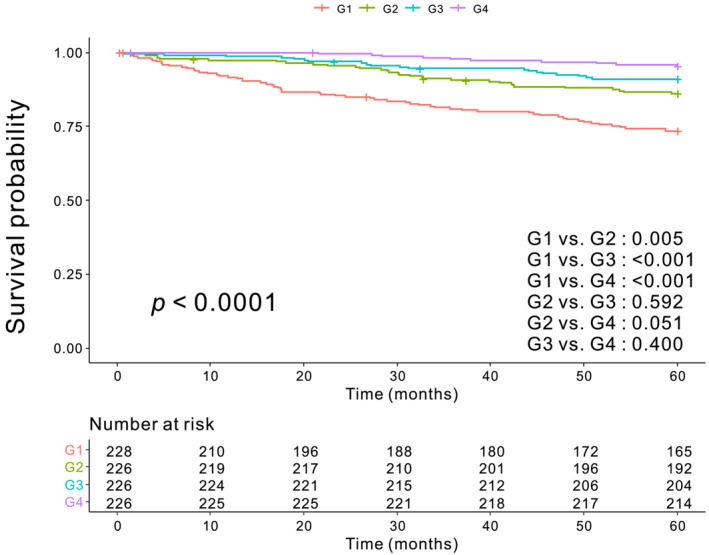
Kaplan–Meier survival curve. The 5‐year overall survival for G1, G2, G3 and G4 were 73.4%, 86.2%, 91.1% and 95.5%, respectively (P < 0.0001).
Univariable and multivariable analyses of factors associated with OS
Univariable analysis showed that age, BMI, CEA level, tumour size, complications, histologic grade, total lymph nodes, LVI, stage, SMI, SMD, albumin level and AMG were all significantly associated with OS (Table 2). In a multivariable analysis adjusted for age, sex, BMI, CEA level, tumour size, histologic grade, complications, total lymph nodes, LVI, stage, SMI, SMD and albumin level, AMG was identified as an independent prognostic factor for OS (G1 vs. G2, P = 0.045; G1 vs. G3, P = 0.005; G1 vs. G4, P < 0.001) (Table 3).
Table 2.
Univariable analysis of factors associated with overall survival
| Univariable analysis | |||
|---|---|---|---|
| Variables | Categorization | HR (95% CI) | P |
| Sex | Female | 1 | |
| Male | 1.343 (0.921–1.957) | 0.125 | |
| Age (years) | <70 | 1 | |
| ≥70 | 2.757 (1.927–3.943) | <0.001 | |
| BMI (kg/m2) | <25 | 1 | |
| ≥25 | 0.524 (0.330–0.832) | 0.006 | |
| ASA grade | I | 1 | |
| II | 1.181 (0.798–1.748) | 0.405 | |
| III and IV | 1.006 (0.524–1.931) | 0.985 | |
| Unknown | 1.721 (0.873–3.389) | 0.117 | |
| CEA (ng/mL) | <5 | 1 | |
| ≥5 | 1.940 (1.340–2.807) | <0.001 | |
| Unknown | 1.031 (0.415–2.558) | 0.946 | |
| Tumour location | Colon | 1 | |
| Rectum | 0.944 (0.637–1.4) | 0.777 | |
| Tumour size (cm) | <5 | 1 | |
| ≥5 | 2.184 (1.526–3.126) | <0.001 | |
| Complications | No | 1 | |
| Yes | 2.043 (1.402–2.977) | <0.001 | |
| Histologic grade | G1 and G2 | 1 | |
| G3 and MC and SRC | 1.862 (1.084–3.197) | 0.024 | |
| Total lymph nodes | <12 | 1 | |
| ≥12 | 0.636 (0.409–0.987) | 0.043 | |
| LVI | Absent | 1 | |
| Present | 2.456 (1.698–3.552) | <0.001 | |
| Unknown | 0.605 (0.148–2.468) | 0.484 | |
| Stage | I and II | 1 | |
| III | 2.644 (1.822–3.837) | <0.001 | |
| Chemotherapy | No | 1 | |
| Yes | 1.044 (0.726–1.501) | 0.815 | |
| SMI (cm2/m2) | Low | 1 | |
| High | 0.607 (0.417–0.885) | 0.009 | |
| SMD (HU) | Low | 1 | |
| High | 0.387 (0.271–0.552) | <0.001 | |
| Albumin | Low | 1 | |
| High | 0.351 (0.239–0.514) | <0.001 | |
| AMG | G1 | 1 | |
| G2 | 0.472 (0.306–0.729) | <0.001 | |
| G3 | 0.296 (0.178–0.491) | <0.001 | |
| G4 | 0.144 (0.073–0.281) | <0.001 | |
AMG, albumin‐myosteatosis gauge; ASA, American Society of Anesthesiologists; BMI, body mass index; CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; HU, Hounsfield unit; LVI, lymphovascular invasion; MC, mucinous adenocarcinoma; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; SRC, signet‐ring cell.
Table 3.
Multivariable analysis of factors associated with overall survival
| Variables | Categorization | HR (95% CI) | P |
|---|---|---|---|
| AMG | G1 | 1 | |
| G2 | 0.627 (0.397–0.989) | 0.045 | |
| G3 | 0.457 (0.263–0.794) | 0.005 | |
| G4 | 0.223 (0.107–0.464) | <0.001 |
AMG, albumin‐myosteatosis gauge; CI, confidence interval; HR, hazard ratio.
Multivariable analysis was adjusted for age, sex, body mass index, carcinoembryonic antigen, tumour size, histologic grade, complications, lymph node numbers, lymphovascular invasion, stage, skeletal muscle index, skeletal muscle radiodensity and albumin.
Comparison between AMG and albumin, SMI and SMD
We compared iAUC values to evaluate the predictive power of AMG as a prognostic factor during the follow‐up period. The integrated AUC value of AMG (0.681, 95% CI = 0.638–0.723) was superior to that of SMD (0.610, 95% CI = 0.566–0.654) (bootstrap iAUC mean difference = 0.071, 95% CI = 0.034–0.106), SMI (0.551, 95% CI = 0.511–0.594) (bootstrap iAUC mean difference = 0.129, 95% CI = 0.076–0.181) and albumin (0.627, 95% CI = 0.585–0.668) (bootstrap iAUC mean difference = 0.053, 95% CI = 0.010–0.098) (Table S2).
Clinical significance of AMG according to sex
We have added statistical analyses according to sex to the supplementary file (Tables S3–S8). In male patients, AMG was identified as a significant prognosticator, whereas albumin level and SMD were not selected in the final multivariable model with backward selection (Tables S3 and S4). Based on the integrated AUC comparison (Table S5), the discriminatory ability of AMG may be better than that of albumin level, SMD and SMI in male patients.
In female patients, AMG was also identified as an independent indicator of survival in multivariable analysis (Tables S6 and S7). The discriminatory ability of AMG was better than that of SMD and SMI but similar to that of albumin level (Table S8).
Clinical significance of category‐based combination of albumin and SMD
In addition, the patients were divided into four groups as follows: low SMD and low albumin level (LL), low SMD but high albumin level (LH), high SMD but low albumin level (HL) and high SMD and high albumin level (HH). Five‐year OS was significantly different among the four groups (72.1%, 86.1%, 84.9% and 93.7%, respectively (P < .0001); LL vs. LH, P = 0.047; LL vs. HL, P = 0.010; LL vs. HH, P < 0.001; LH vs. HL, P > 0.05; LH vs. HH, P = 0.084; HL vs. HH, P = 0.003) (Figure S5). Univariable analysis according to categorical classification showed statistical significance (LL vs. LH, P = 0.005; LL vs. HL, P < 0.001; LL vs. HH, P < 0.001), whereas multivariable analysis showed significance only for LL versus HH (P < 0.001) (Table S9).
Discussion
This study demonstrated the superior predictive capability of AMG as a predictor of OS compared with serum albumin, SMI and SMD in patients with stage I–III CRC. AMG can be used as a novel prognostic biomarker that reflects the risk of cachexia and nutritional status of patients with CRC.
Sarcopenia was initially suggested to represent muscle loss observed in older people. 14 The EWGSOP currently defines sarcopenia according to muscle mass, strength and physical performance. When CT is performed, sarcopenia can be estimated using the SMI obtained from a cross‐sectional CT image at the L3 level. 5 Myosteatosis is the infiltration of adipose tissue into the skeletal muscle and is associated with muscle strength per size. 15 Due to CT examination being routinely performed in most patients with CRC, the assessment of muscle quality has also been extensively studied. 6 , 16 , 17 , 18 Although the exact pathophysiology of myosteatosis is yet to be discovered, some clinical data suggest that sarcopenia and myosteatosis may partially share systemic inflammation as a common mechanism underscored by IL‐6‐mediated catabolic activity. 7 , 8 The prognostic value of sarcopenia and myosteatosis in patients with cancer is well known. 19 , 20 , 21
The prognostic impacts of SMI and SMD are often not directly compared, and studies to date have reported contradictory results. Cortellini et al. found that, whereas SMI was significantly associated with progression‐free survival in patients with CRC (HR, 0.54; 95% CI: 0.31–0.93), low SMD was not (HR, 0.67; 95% CI: 0.36–1.24). 22 Malietzis et al. reported that, in patients who received surgical treatment of CRC, SMI was a prognostic factor for OS and disease‐free survival (DFS) (P < 0.001 and P = 0.011, respectively), whereas SMD was not (P = 0.069 and P = 0.622, respectively). 7 In contrast, other studies have demonstrated that SMD is a better prognostic factor than SMI. 23 , 24 Maurits et al. reported that higher SMD was associated with better OS, although no significant association with SMI was found in patients with renal cell carcinoma. 23 Another study that enrolled patients with gastric cancer treated with radical gastrectomy showed similar findings, with SMD being significantly associated with OS and DFS and SMI was excluded in a multivariate analysis using forward stepwise selection. 24 Although it is very difficult to determine the reason underscoring the contradictory associations of SMI and SMD evident from prior studies, the absence of definite criteria to diagnose sarcopenic status using SMI or SMD may be one fundamental reason. A recent review observed diverse SMI cut‐off values for the 156 included studies with 39 (25%), 47 (30.1%) and 70 (44.9%) using the criteria put forth by Martin et al., Prado et al. and their own, respectively. 25 Also, a review of 73 studies described the use of 32 different cut‐off values when determining the prognostic value of myosteatosis. 17 These situations may hinder the reliable determination of the prevalence of sarcopenia using SMI and/or SMD and the incorporation of these biomarkers in the process of clinical decision in patients with cancer. Therefore, future investigations are required to identify additional universal biomarkers of CRC prognosis.
Serum albumin, which is produced in the liver and is abundant in the blood, is a well‐known marker of systemic inflammation and nutritional status. 26 Gastrointestinal tumours, including CRC, can influence serum albumin levels in two ways. Malnutrition due to impaired food absorption is associated with decreased survival in patients with cancer. 27 , 28 Systemic inflammation secondary to cancer also lowers serum albumin levels, 29 , 30 and this anti‐tumour response even promotes cancer growth and progression, 31 resulting in a worse prognosis. 26 A meta‐analysis of 29 studies showed that serum albumin measured before cancer treatment was a meaningful prognostic factor for better survival, supporting this hypothesis. 32 In addition, Haskins et al. showed that serum albumin was independently associated with higher 30‐day mortality post‐operatively in patients with CRC. 33 Although albumin acts as an independent prognostic marker in CRC, non‐tumour factors such as diet and hydration state influence albumin levels, which can hinder the clinical applications of this marker for risk stratification in patients with CRC.
Post‐operative complications are associated with poor survival and disease recurrence in patients with CRC. 34 , 35 Similarly, complications were identified as independent prognostic factors for OS in our multivariable analysis (data not shown). The percentage of patients with complications was highest in the G1 group, at 32.0%, 19.9%, 17.3% and 16.8% for G1–G4, respectively (P < 0.001). With regard to age and tumour size, all variables were identified as independent prognostic factors in the multivariable analysis. Our study showed that the sex–quartile AMG was significantly associated with age (P < 0.001) and tumour size (P < 0.001). Thus, we believe that the relationship between AMG groups and these clinical situations may explain, in part, the survival discrimination among the AMG groups.
Myosteatosis and serum albumin levels are associated with tumour‐induced inflammation and cancer cachexia. Recent studies have suggested that muscle proteolysis and inhibition of hepatic albumin production are caused by increased production of pro‐inflammatory mediators such as IL‐6. 7 , 8 , 29 , 30 The tumour microenvironment produces pro‐inflammatory cytokines such as TNF‐α and IL‐6. 36 These cytokines may mediate the redistribution of adipose tissue and intramuscular fat infiltration by inducing the differentiation of muscle progenitor cells to an adipocyte‐like phenotype. 37 , 38 Based on these observations, myosteatosis and albumin may reflect cachexic status in different ways; thus, integrating these two factors may have a synergistic effect on the stratification of prognosis. In our study, although albumin, SMI, SMD and AMG had a significant prognostic impact at the univariate level, only AMG remained an independent prognostic risk factor in the multivariable analysis. Furthermore, the discriminatory ability of AMG outperformed that of serum albumin, SMI and SMD.
Our study has several limitations. Fat infiltration of the skeletal muscle increases with obesity, 39 the prevalence of which differs between ethnicities. Whether our results are applicable to ethnicities other than those of self‐reported Asian ancestry requires further investigation. This was a retrospective cross‐sectional study; therefore, we could not examine the causal relationship between AMG and risk factors for poor OS, such as post‐operative complications. Since each patient received different post‐operative treatments depending on their pathological stage, we could not determine how this affected the prognosis of each patient. However, this could have been corrected to some extent in our multivariable analysis. Our study included the receipt of post‐operative chemotherapy as a covariant. Based on the Kaplan–Meier survival curves according to patients with stage II and III CRC, which are potential candidates for adjuvant chemotherapy, survival also significantly differed according to AMG. Weight loss might be associated with prognosis in patients with various types of cancer. 13 Thus, consideration of this parameter might be necessary when evaluating the effect of nutritional status or body composition. However, we did not include weight loss as a clinical parameter in this study. Further research is needed to overcome these limitations.
In conclusion, AMG, which is calculated using serum albumin levels and SMD, is a meaningful and novel prognostic risk factor for patients with stage I–III CRC. AMG can be used as a more reliable prognostic marker than SMI and SMD alone. Because albumin levels and SMD are both readily accessible, the potential role of AMG as an easily accessible and affordable measure for predicting patient outcomes is promising. Further evaluation of AMG in different ethnicities is required.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1F1A1074811).
Supporting information
Figure S1. Inclusion criteria
Supplementary paragraph 1. CT protocol
Figure S2. Comparison of skeletal muscle radiodensity, albumin, and albumin‐myosteatosis gauge according to the sex
Table S1. Quartile distribution of AMG according to the sex
Figure S3. Comparison of skeletal muscle radiodensity and albumin according to the albumin‐myosteatosis gauge in men and women respectively
Figure S4. Kaplan–Meier survival curve of AMG groups in stage II and III patients
Table S2. Comparison of integrated AUC between AMG and other variables in all patients (n = 906)
Table S3. Univariable analysis of factors associated with overall survival in male patients (n = 541)
Table S4. Multivariable analysis of factors associated with overall survival in male patients (n = 541)
Table S5. Comparison of integrated AUC between AMG and other variables in male patients (n = 541)
Table S6. Univariable analysis of factors associated with overall survival in the female patients (n = 365)
Table S7. Multivariable analysis of factors associated with overall survival in the female patients (n = 365)
Table S8. Comparison of integrated AUC between AMG and other variables in female patients (n = 365)
Figure S5. Kaplan–Meier survival curve of new categorical classifications using SMD and albumin.
Table S9. Univariable and multivariable analysis producing hazard ratios of new categorical classifications using SMD and albumin among all patients (n = 906)
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle. 40
Kim Y., Lee J.‐H., Cho E.‐S., Lee H. S., Shin S.‐J., Park E. J., Baik S. H., Lee K. Y., and Kang J. (2023) Albumin‐myosteatosis gauge as a novel prognostic risk factor in patients with non‐metastatic colorectal cancer, Journal of Cachexia, Sarcopenia and Muscle, 14, 860–868, 10.1002/jcsm.13183
References
- 1. Khil H, Kim SM, Hong S, Gil HM, Cheon E, Lee DH, et al. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci Rep 2021;11:2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Stok EP, Spaander MCW, Grünhagen DJ, Verhoef C, Kuipers EJ. Surveillance after curative treatment for colorectal cancer. Nat Rev Clin Oncol 2017;14:297–315. [DOI] [PubMed] [Google Scholar]
- 3. Marks KM, West NP, Morris E, Quirke P. Clinicopathological, genomic and immunological factors in colorectal cancer prognosis. Br J Surg 2018;105:e99–e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peixoto da Silva S, Santos JMO, Costa ESMP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 2020;11:619–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: systematic review and meta‐analysis. Crit Rev Oncol Hematol 2020;145:102839. [DOI] [PubMed] [Google Scholar]
- 7. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne‐Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 2016;103:572–580. [DOI] [PubMed] [Google Scholar]
- 8. Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005;41:2645–2654. [DOI] [PubMed] [Google Scholar]
- 9. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005;39:S143–S146. [DOI] [PubMed] [Google Scholar]
- 10. Kim SS, Kim JH, Jeong WK, Lee J, Kim YK, Choi D, et al. Semiautomatic software for measurement of abdominal muscle and adipose areas using computed tomography: a STROBE‐compliant article. Medicine (Baltimore) 2019;98:e15867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedorov A, Beichel R, Kalpathy‐Cramer J, Finet J, Fillion‐Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 2012;30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung E, Lee HS, Cho ES, Park EJ, Baik SH, Lee KY, et al. Changes in body composition during adjuvant FOLFOX chemotherapy and overall survival in non‐metastatic colon cancer. Cancers (Basel) 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 14. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 15. Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care 2010;13:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsieh YC, Joo SK, Koo BK, Lin HC, Lee DH, Chang MS, et al. Myosteatosis, but not sarcopenia, predisposes NAFLD subjects to early steatohepatitis and fibrosis progression. Clin Gastroenterol Hepatol 2022. 10.1016/j.cgh.2022.01.020 [DOI] [PubMed] [Google Scholar]
- 17. Ahn H, Kim DW, Ko Y, Ha J, Shin YB, Lee J, et al. Updated systematic review and meta‐analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev 2021;70:101398. [DOI] [PubMed] [Google Scholar]
- 18. Lee CM, Kang J. Prognostic impact of myosteatosis in patients with colorectal cancer: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2020;11:1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamashita M, Kamiya K, Matsunaga A, Kitamura T, Hamazaki N, Nozaki K, et al. Low skeletal muscle density combined with muscle dysfunction predicts adverse events after adult cardiovascular surgery. Nutr Metab Cardiovasc Dis 2021;31:1782–1790. [DOI] [PubMed] [Google Scholar]
- 20. Ubachs J, Ziemons J, Minis‐Rutten IJG, Kruitwagen R, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2019;10:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 22. Cortellini A, Palumbo P, Porzio G, Verna L, Giordano AV, Masciocchi C, et al. Single‐institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non‐small cell lung cancer patients treated with first‐line chemotherapy. Thorac Cancer 2018;9:1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maurits JSF, Sedelaar JPM, Mulders PFA, Aben KKH, Kiemeney L, Vrieling A. Skeletal muscle radiodensity and visceral adipose tissue index are associated with survival in renal cell cancer ‐ a multicenter population‐based cohort study. Clin Nutr 2022;41:131–143. [DOI] [PubMed] [Google Scholar]
- 24. Dong QT, Cai HY, Zhang Z, Zou HB, Dong WX, Wang WB, et al. Influence of body composition, muscle strength, and physical performance on the postoperative complications and survival after radical gastrectomy for gastric cancer: a comprehensive analysis from a large‐scale prospective study. Clin Nutr 2021;40:3360–3369. [DOI] [PubMed] [Google Scholar]
- 25. McGovern J, Dolan RD, Horgan PG, Laird BJ, McMillan DC. Computed tomography‐defined low skeletal muscle index and density in cancer patients: observations from a systematic review. J Cachexia Sarcopenia Muscle 2021;12:1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med 2020;133:713–722.e717. [DOI] [PubMed] [Google Scholar]
- 27. Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol 2012;19:2128–2134. [DOI] [PubMed] [Google Scholar]
- 28. von Meyenfeldt M. Cancer‐associated malnutrition: an introduction. Eur J Oncol Nurs 2005;9:S35–S38. [DOI] [PubMed] [Google Scholar]
- 29. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev 2011;25:2559–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shinko D, Diakos CI, Clarke SJ, Charles KA. Cancer‐related systemic inflammation: the challenges and therapeutic opportunities for personalized medicine. Clin Pharmacol Ther 2017;102:599–610. [DOI] [PubMed] [Google Scholar]
- 32. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haskins IN, Baginsky M, Amdur RL, Agarwal S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr 2017;36:1333–1338. [DOI] [PubMed] [Google Scholar]
- 34. Salvans S, Mayol X, Alonso S, Messeguer R, Pascual M, Mojal S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg 2014;260:939–943, discussion 943–934. [DOI] [PubMed] [Google Scholar]
- 35. Kang J, Choi GS, Oh JH, Kim NK, Park JS, Kim MJ, et al. Multicenter analysis of long‐term oncologic impact of anastomotic leakage after laparoscopic total mesorectal excision: the Korean laparoscopic colorectal surgery study group. Medicine (Baltimore) 2015;94:e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kasprzak A. The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci 2021;22:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li CW, Yu K, Shyh‐Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle 2022;13:781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kob R, Bollheimer LC, Bertsch T, Fellner C, Djukic M, Sieber CC, et al. Sarcopenic obesity: molecular clues to a better understanding of its pathogenesis? Biogerontology 2015;16:15–29. [DOI] [PubMed] [Google Scholar]
- 39. Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000;49:467–472. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Inclusion criteria
Supplementary paragraph 1. CT protocol
Figure S2. Comparison of skeletal muscle radiodensity, albumin, and albumin‐myosteatosis gauge according to the sex
Table S1. Quartile distribution of AMG according to the sex
Figure S3. Comparison of skeletal muscle radiodensity and albumin according to the albumin‐myosteatosis gauge in men and women respectively
Figure S4. Kaplan–Meier survival curve of AMG groups in stage II and III patients
Table S2. Comparison of integrated AUC between AMG and other variables in all patients (n = 906)
Table S3. Univariable analysis of factors associated with overall survival in male patients (n = 541)
Table S4. Multivariable analysis of factors associated with overall survival in male patients (n = 541)
Table S5. Comparison of integrated AUC between AMG and other variables in male patients (n = 541)
Table S6. Univariable analysis of factors associated with overall survival in the female patients (n = 365)
Table S7. Multivariable analysis of factors associated with overall survival in the female patients (n = 365)
Table S8. Comparison of integrated AUC between AMG and other variables in female patients (n = 365)
Figure S5. Kaplan–Meier survival curve of new categorical classifications using SMD and albumin.
Table S9. Univariable and multivariable analysis producing hazard ratios of new categorical classifications using SMD and albumin among all patients (n = 906)


