Abstract
Background
The associations of multimorbidity patterns with transitions between frailty states remain unclear in older individuals.
Methods
We used data from the National Health and Aging Trends Study 2011–2019. Frailty was measured annually using the Fried frailty phenotype. Multimorbidity patterns at baseline were identified using latent class analysis based on 14 chronic conditions. We used the semi‐Markov multi‐state model to investigate the influences of multimorbidity characterized by condition counts and patterns on subsequent frailty transitions over follow‐ups.
Results
Among 9450 participants aged ≥65 years at baseline, 34.8% were non‐frail, 48.1% were pre‐frail and 17.0% were frail. Over a median follow‐up of 4.0 years, 16 880 frailty transitions were observed, with 10 527 worsening and 6353 improving. For 7675 participants with multimorbidity, four multimorbidity patterns were identified: osteoarticular pattern (62.4%), neuropsychiatric–sensory pattern (17.2%), cardiometabolic pattern (10.3%) and complex multimorbidity pattern (10.1%). Compared with no disease, multimorbidity was significantly associated with an increased risk of worsening transitions, including from non‐frail to pre‐frail (hazard ratio [HR] = 1.35; 95% confidence interval [CI] = 1.21–1.52), from non‐frail to frail (HR = 1.68; 95% CI = 1.04–2.73), from pre‐frail to frail (HR = 2.19; 95% CI = 1.66–2.90) and from pre‐frail to death (HR = 1.64; 95% CI = 1.11–2.41). Compared with the osteoarticular pattern, neuropsychiatric–sensory, cardiometabolic and complex multimorbidity patterns had a significantly higher risk of worsening frailty (all P < 0.05).
Conclusions
Multimorbidity was associated with dynamic transitions between frailty states and death among older American adults, and the associations varied across multimorbidity patterns. The findings could offer significant implications for public health policymakers in planning interventions and healthcare resources. They also might inform clinicians regarding providing targeted clinical treatment and health management based on multimorbidity patterns of older people.
Keywords: frailty, multimorbidity pattern, older adults, transition
Introduction
Multimorbidity and frailty have received increasing attention as clinical and public health challenges with the rapid growth of the older adult population. 1 , 2 Multimorbidity is commonly defined as the coexistence of two or more chronic diseases in an individual, 2 whereas frailty is a geriatric syndrome characterized by increased vulnerability to stressors due to the declined reserve and function in the multisystem. 1 , 3 Previous studies have demonstrated that phenotypic frailty (or physical frailty) can present independently of any specific chronic disease but is aggravated by the coexistence of multiple diseases. 4 , 5 Uncovering relationships between multimorbidity and frailty can advance the understanding of mechanisms underlying the ageing process and facilitate targeted interventions for multimorbid patients with a greater risk of adverse outcomes. 5 The importance of identifying frailty status when managing older multimorbid adults has been emphasized by the National Institute for Health and Care Excellence (NICE) and the British Geriatrics Society. 6 , 7
Although multimorbidity has been associated with frailty, a meta‐analysis showed that one fourth of patients with multimorbidity do not have frailty, suggesting the need to recognize specific multimorbidity status at risk for frailty. 5 Previous studies have reported that increased unweighted and weighted disease counts were associated with a higher risk of frailty. 5 , 8 , 9 However, fully understanding how multimorbidity relates to frailty requires consideration of potential interactions between particular groups of conditions that commonly exist and may have synergistic effects on health‐related outcomes. 9 , 10 , 11 , 12
Furthermore, frailty denotes a gradual change in losing capacities to handle stressors and has been considered as a dynamic changeable state. 1 Previous studies mostly define frailty at one time point when investigating the associations between multimorbidity patterns and frailty; it is still unclear how multimorbidity patterns predict the change of frailty states. 9 , 10 Evaluating the effect of different multimorbidity patterns on the dynamics of frailty over time is essential for developing interventions to prevent, ameliorate and potentially reverse frailty among older adults with multimorbidity. 13 , 14 This study aimed to explore baseline multimorbidity patterns and examine their associations with the subsequent transitions between frailty states and death among older American adults.
Methods
Study design
Data came from the National Health and Aging Trends Study (NHATS). The NHATS was a nationally representative longitudinal study of Medicare beneficiaries aged ≥65 years that aims to evaluate national trends in late‐life functioning and examine the effect of late life on individuals, families and society. 15 The NHATS participants were initially enrolled in 2011 and replenished in 2015. Data were collected annually via in‐person or proxy interviews (if participants could not self‐report). All‐cause mortality was ascertained through an annual proxy respondent interview. More details about the study design were provided elsewhere. 15 The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved the NHATS protocol. Signed informed consent was obtained from the study participants or their proxy respondents.
Our sample derived from the NHATS 2011–2019 cycles was restricted to participants completing sample person interviews and dwelling in community or non‐nursing home residential care settings at baseline. To ensure a sufficient sample size, we combined participants recruited in 2011 (N = 7609) and 2015 (N = 3949). 16 The exclusion criteria were as follows: (1) missing ≥3 frailty components (i.e., exhaustion, low physical activity, shrinking, weakness and slowness) at baseline (N = 93) 17 and (2) missing any annual follow‐ups (N = 2015). A total of 9450 participants were finally included in the analyses. The flow chart and comparison of baseline characteristics between included and excluded participants are shown in Figure S1 and Table S1 .
Chronic conditions and multimorbidity
Fourteen chronic conditions at baseline were included in this study, covering somatic diseases and mental disorders frequently used in defining multimorbidity. 2 Nine chronic conditions, including heart attack, heart disease (angina or congestive heart failure), hypertension, arthritis (osteoarthritis and rheumatoid arthritis), osteoporosis, diabetes, lung disease, stroke and cancer, were ascertained through self‐reported physicians' diagnoses. Vision impairment was defined as blindness or inability to see well enough even with glasses or contacts to recognize someone across the street, to watch television or to read newspaper print. 18 , 19 Participants were identified as having hearing impairment if they were deaf or unable to hear well enough even with hearing aids to use the telephone and to carry on a conversation in a room with a radio or TV playing or in a quiet room. 18 , 19 Anxiety symptoms and depressive symptoms were assessed via the Generalized Anxiety Disorder‐2 (GAD‐2) and Patient Health Questionnaire‐2 (PHQ‐2) scales, both ranging from 0 to 6. Anxiety was defined as a GAD‐2 score ≥ 3, whereas depression was defined as a PHQ‐2 score ≥ 3, as previously validated. 20 Cognitive tests, including orientation, memory and executive functioning domains, were used to evaluate the cognitive capacity of participants. 21 Additionally, the AD8 Dementia Screening Interview was administered to proxy respondents. 21 Participants were classified as having dementia if they had a self‐reported physician diagnosis of dementia or Alzheimer's disease, an AD8 score ≥ 2 or the cognitive test performance ≤ 1.5 standard deviations (SDs) below the mean for at least one of the three domains. 21 Multimorbidity was defined as the coexistence of two or more chronic conditions within one person.
Frailty
Frailty was assessed each year by the Fried frailty phenotype. 3 The criteria of five phenotypes (exhaustion, low physical activity, shrinking, weakness and slowness) followed the approach of Bandeen‐Roche et al. 17 Exhaustion was defined as having low energy or being easily exhausted to the point of limiting activities in the last month. Participants met the criteria for low physical activity if they never walked for exercise or engaged in vigorous activities in the last month. Shrinking was defined as body mass index (BMI) < 18.5 kg/m2, based on self‐reported weight and height, or unintentional weight loss ≥ 10 lb in the last year. Weakness was measured by the best of two dominant handgrip strength measurements (measured by the Jamar Plus+ Digital Hand Dynamometer). Participants with handgrip strength ≤ 20th percentile of the population distribution stratified by sex and BMI groups (<18.5, 18.5–24.9, 25.0–29.9 and ≥30.0 kg/m2) were defined as having weakness. Slowness was assessed by gait speed from the first of two 3 m walking trails. Gait speed ≤ 20th percentile of the population distribution stratified by sex and height (≤175 or >175 cm for men; ≤159 or >159 cm for women) indicated slowness. Following recommended practice, if participants were not eligible due to surgery or pain, or did not attempt to test physical performance because of safety concerns, or attempted but failed to complete a test, their grip strength and gait speed were scored as ‘0’. 17 , 22 Detailed definitions are described in Table S2 . Participants were categorized into ‘frail’, ‘pre‐frail’ and ‘non‐frail’ for those meeting ≥3, 1–2 criteria and none of the criteria, respectively.
Confounding variables
Age (65–74, 75–84 or ≥85 years), sex (male or female), race/ethnicity (non‐Hispanic White, non‐Hispanic Black, Hispanic or other), residence (community or non‐nursing home residential care settings), marital status (married/partnered or unmarried, including separated, divorced, widowed or never married), educational levels (<high school, high school, some college or ≥college graduate), smoking status (never, former or current) and chronic pain (no pain, bothersome pain or activity‐limiting pain) were collected at baseline.
Statistical analyses
Baseline characteristics were summarized using frequencies (percentages) for categorical variables and medians (inter‐quartile ranges [IQRs]) for continuous variables. Chi‐squared tests for categorical variables and Kruskal–Wallis tests for nonnormally distributed continuous variables were used to compare baseline characteristics between groups with different condition counts or multimorbidity patterns.
Multimorbidity patterns were explored using latent class analysis (LCA) (poLCA package) among multimorbid participants at baseline. 23 LCA modelling does not need to define cluster distance, or select cluster algorithms, but rather allows statistical testing of model fit and classifies objects according to membership probabilities, which performs more objectively and rigorously than traditional hierarchical clustering methods. 24 We examined 3 to 10 latent classes (i.e., multimorbidity patterns) in the LCA models and determined the optimal number of the classes based on the Bayesian information criterion (BIC), Pearson χ 2 goodness of fit and clinical interpretability. Participants were assigned to the class for which they had the highest probability of membership.
We used the semi‐Markov multi‐state model (mstate package) to determine the covariate‐adjusted association of multimorbidity with the transitions between frailty states (i.e., non‐frail, pre‐frail and frail) and death. 25 Participants with any frailty states at baseline could remain in the entry state or transition to another state (Figure S2 ). It is impossible to emerge further transitions for participants once death occurs. Transition intensities were modelled using the ‘clock reset’ approach, where time was measured since entering each state (Figure S3 ). Participants were censored if they were lost to follow‐up or their frailty state did not change during the follow‐up period. The ‘clock reset’ modelling approach of the semi‐Markov multi‐state model relaxed the Markov assumption based on the ‘clock forward’ approach by considering both the current frailty state and the time spent in that state when predicting the future state, thus improving the biological plausibility of models. 25 , 26
We developed Cox proportional hazards models stratified by transitions to estimate transition‐specific hazard ratios (HRs) with 95% confidence intervals (CIs), which represent the effect of multimorbidity on each transition in the multi‐state model. We first included condition counts as the independent variable in the multi‐state model to investigate the influence of multimorbidity on transitions in frailty states among all participants. We then examined the effects of different multimorbidity patterns on transitions among multimorbid participants. To assess the independent association of multimorbidity patterns with frailty transitions, we further added condition counts as a continuous variable in the models. Because the associations between multimorbidity patterns and incident frailty have been suggested to vary by age, we performed all analyses stratified by age (65–74, 75–84 and ≥85 years). 9
To test the robustness of our results, we performed sensitivity analyses after excluding the participants living in the non‐nursing home residential care settings (N = 444). Additionally, to address missing data on frailty assessments, we employed multiple imputation by chained equations, incorporating all variables in this study, and conducted primary analyses using imputed datasets. Final estimates were generated by pooling the results of five imputed datasets. Because the analytic weights were not available for the combined samples in the current study, our statistical analyses did not incorporate the sample weights, which has been considered acceptable for modelling the relationship between the independent and dependent variables. 27 All statistical analyses were conducted using R software Version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). A two‐sided P < 0.05 was considered statistically significant.
Results
Table 1 shows the characteristics of the study population at baseline. Of the 9450 participants, 42.2% were aged 65–74 years and 42.9% were male. The majority enrolled were non‐Hispanic Whites (68.6%). The proportions of non‐frail, pre‐frail and frail participants were 34.8%, 48.1% and 17.0%, respectively. Significant differences in baseline characteristics were observed between participants with no disease, only one disease and multimorbidity (Table S3 ).
Table 1.
Baseline characteristics of all participants (N = 9450)
| Characteristics | N (%) |
|---|---|
| Age group (years) | |
| 65–74 | 3986 (42.2) |
| 75–84 | 3608 (38.2) |
| ≥85 | 1856 (19.6) |
| Male | 4051 (42.9) |
| Race/ethnicity | |
| Non‐Hispanic White | 6479 (68.6) |
| Non‐Hispanic Black | 2005 (21.2) |
| Hispanic | 574 (6.1) |
| Other a | 392 (4.1) |
| Community | 9006 (95.3) |
| Married or living with a partner | 4765 (50.4) |
| Educational levels | |
| <High school | 2294 (24.3) |
| High school | 2558 (27.1) |
| Some college | 2463 (26.1) |
| ≥College graduate | 2135 (22.6) |
| Smoking status | |
| Never | 4688 (49.6) |
| Former | 4039 (42.7) |
| Current | 723 (7.7) |
| Chronic pain | |
| No pain | 4310 (45.6) |
| Bothersome pain | 2348 (24.8) |
| Activity‐limiting pain | 2792 (29.5) |
| Frailty states | |
| Non‐frail | 3292 (34.8) |
| Pre‐frail | 4548 (48.1) |
| Frail | 1610 (17.0) |
Other race/ethnicity includes American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander or other.
Among the LCA models with 3 to 10 classes, the four‐class model with the optimal fit and the most reasonable clinical interpretability was finally selected (Table S5 ). Four classes were labelled according to chronic conditions having excess prevalence in each class than the prevalence in all multimorbid participants (Figure 1 ): osteoarticular pattern (N = 4786, 62.4%), neuropsychiatric–sensory pattern (N = 1322, 17.2%), cardiometabolic pattern (N = 788, 10.3%) and complex multimorbidity pattern (N = 779, 10.1%). The baseline characteristics by patterns are reported in Table S4 .
Figure 1.
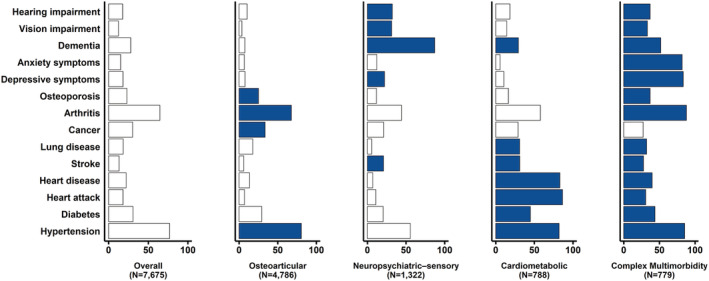
Prevalence (%) of having chronic conditions for each latent class. The dark blue‐coloured condition indicates that its prevalence exceeds the population prevalence.
The observed transitions among all participants and those with multimorbidity at baseline are summarized in Figure 2 . Over the 41 519 person‐years of follow‐up, with a median (IQR) follow‐up of 4.0 (2.2–7.5) years, we observed 16 880 transitions among all participants. Of those, 10 527 (62.4%) were worsening transitions and 6353 (37.6%) were improving transitions. The percentage of worsening transitions among participants with multimorbidity (62.9%) was higher than that among those with no disease (58.2%) or only one disease (60.2%). For older multimorbid patients, worsening transitions accounted for 62.9% of all 14 091 transitions. Older people in the neuropsychiatric–sensory pattern showed the highest percentage of worsening transitions (68.3%).
Figure 2.
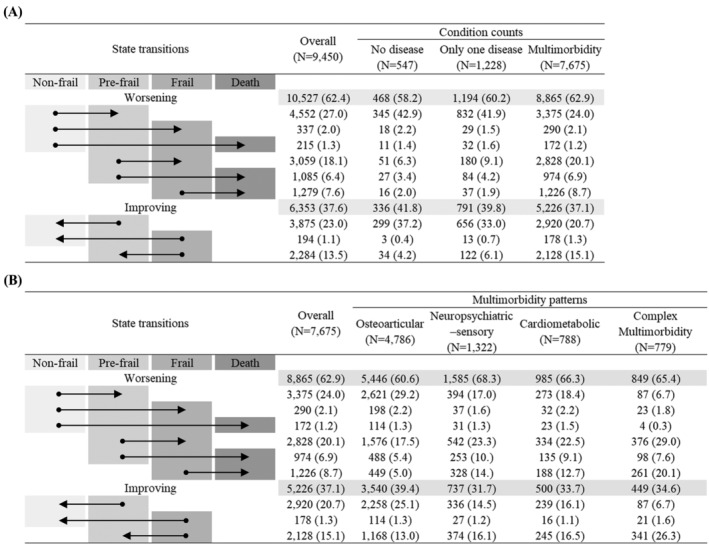
Number (%) of observed transitions between frailty states and death by (A) condition counts and (B) multimorbidity patterns. Data are presented as numbers (percentages). The arrow displays the direction of the transition between frailty states and death.
Figure 3 shows the associations between multimorbidity and the transitions between frailty states and death. After adjusting for covariates, multimorbidity was significantly associated with an increased risk of worsening transitions from non‐frail to pre‐frail (HR = 1.35; 95% CI = 1.21–1.52), from non‐frail to frail (HR = 1.68; 95% CI = 1.04–2.73), from pre‐frail to frail (HR = 2.19; 95% CI = 1.66–2.90) and from pre‐frail to death (HR = 1.64; 95% CI = 1.11–2.41), compared with no disease. Multimorbidity also reduced the chance of improving transitions from pre‐frail to non‐frail (HR = 0.60; 95% CI = 0.53–0.68). Regarding analyses stratified by age categories, adjusted HRs among participants aged 65–74 years were similar to our primary results, whereas the effect of multimorbidity on most transitions was not statistically significant among those aged 75–84 and ≥85 years (Figure S4 ).
Figure 3.
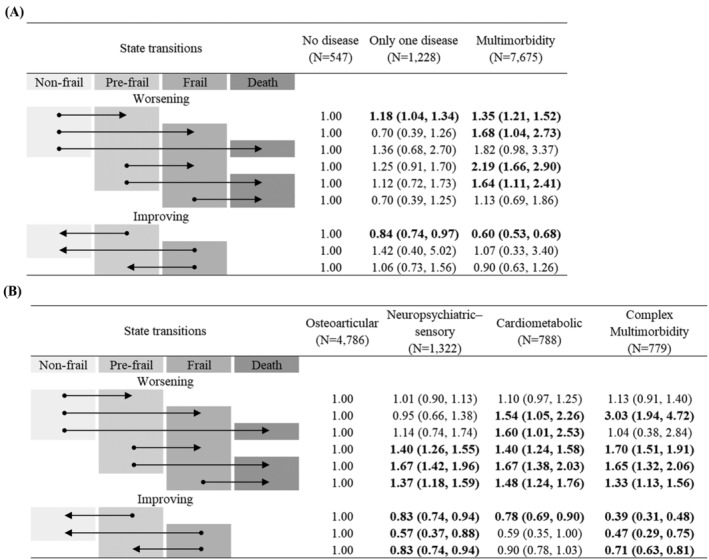
Adjusted hazard ratios (95% confidence intervals) for (A) condition counts and (B) multimorbidity patterns. All models were adjusted for age, sex, race/ethnicity, residence, marital status, educational levels, smoking status and chronic pain. The arrow displays the direction of the transition between frailty states and death. Boldface indicates statistical significance (P < 0.05).
The complex multimorbidity pattern was associated with a higher risk than the osteoarticular pattern in all worsening transitions except that from non‐frail to pre‐frail or death (all P < 0.05). Additionally, individuals in the complex multimorbidity pattern were less likely to recover from pre‐frail to non‐frail (HR = 0.39; 95% CI = 0.31–0.48), from frail to non‐frail (HR = 0.47; 95% CI = 0.29–0.75) and from frail to pre‐frail (HR = 0.71; 95% CI = 0.63–0.81), compared with those in the osteoarticular pattern. Similarly, compared with the osteoarticular pattern, the increased risk of worsening transitions and reduced likelihood of improving transitions were observed in neuropsychiatric–sensory and cardiometabolic patterns (all P < 0.05). Multimorbidity patterns were independently associated with frailty transitions after adjusting for condition counts (Figure S6 ). It was observed that the associations between multimorbidity patterns and frailty transitions did not vary by age (Figure S5 ).
Multiple imputations of missing frailty criteria did not alter the results (Figure S7 ). There was little difference in the results derived from the model with and without excluding participants living in the non‐nursing home residential care settings (Figure S8 ).
Discussion
This is the first study to investigate the role of multimorbidity characterized by both condition counts and patterns on frailty transitions. Our findings showed that multimorbidity was associated with worsening transitions in frailty states among older American adults, and there were variations in the relationships between four multimorbidity patterns and frailty transitions. Compared with the osteoarticular pattern, neuropsychiatric–sensory, cardiometabolic and complex multimorbidity patterns had a significantly higher risk of worsening frailty.
In line with previous studies, we found that older people with multimorbidity were more likely to experience worsened frailty states over time. 5 , 8 , 9 Disease‐associated declines in several physiological systems have been reported as a major risk factor for worsening frailty status. 5 , 28 In addition, our study showed that multimorbidity had no significant effect on transitions between frailty states and death among adults aged ≥85 years, which is similar to results from a German study. 29 This finding may support the idea previously proposed that ageing‐related decreases in gait speed and grip strength, two domains of frailty, were greater than those related to chronic diseases. 30
Four identified multimorbidity patterns reported in this study, including cardiometabolic, osteoarticular/musculoskeletal, neuropsychiatric–sensory multimorbidity and complex multimorbidity patterns, were concordant with prior studies. 23 , 31 , 32 In the current study, although the prevalence of hypertension and arthritis was relatively high in most multimorbidity patterns, we could observe the differences in major chronic diseases of each pattern. 23
Our study found that multimorbidity patterns were significantly associated with frailty transitions even after adjusting for condition counts. Prior research also documented different risks of health‐related outcomes in distinct multimorbidity patterns with similar condition counts, which suggests the importance of identifying specific patterns in the health management of older multimorbid adults. 9 , 11 However, due to variations in multimorbidity definitions and data‐driven methods, multimorbidity patterns in these studies might not be directly comparable, limiting the understanding in the mechanism of different associations between multimorbidity patterns and adverse outcomes. 31
Our study showed a lower risk of worsening frailty in older patients with osteoarticular diseases than the other three multimorbidity patterns, consistent with previous studies. 12 , 33 One possible reason is that osteoarticular multimorbidity pattern, such as coexisting arthritis and osteoporosis, may receive effective management through lifestyle intervention (e.g., physical activities) and specific medications (e.g., angiotensin‐converting enzyme [ACE] inhibitors), following the current clinical guidance. 34 We observed that complex multimorbidity pattern, as well as patterns characterized by cardiometabolic diseases and neuropsychiatric–sensory disorders, had a positive association with worsening frailty status, in line with prior findings. 9 , 35 A plausible explanation is that disease combinations affecting multiple organ systems may be more detrimental than combinations sharing common etiologic or pathophysiologic mechanisms. 35 Moreover, low awareness of mild cognitive impairment, difficulty to recognize sensory impairment in patients with dementia and reluctance to seek healthcare services for neuropsychiatric–sensory diseases could also possibly lead to subsequent progression of existing diseases, form a vicious circle and thus accelerate the functional decline among older individuals. 16 , 36 , 37
The main strength of our study is the use of a large sample with an extensive annual follow‐up that allowed us to capture the dynamic nature of frailty among older American adults. Furthermore, we not only compared the risk of worsening frailty between participants with and without multimorbidity but also investigated the influence of multimorbidity patterns identified by LCA on transitions between frailty states and death within patients with multimorbidity. In addition, our study used multi‐state models based on the semi‐Markov assumption to enhance the biological plausibility of models. Nonetheless, several limitations should be noted. First, most of the chronic conditions were assessed by self‐reported questionnaires rather than clinical records, which might have led to recall bias and information bias. Second, we did not have information on the severity, duration, treatment of each condition or biomarkers, and their influence on frailty needs to be considered in further research. Finally, applying these data‐driven multimorbidity patterns directly to the clinical classification of individual patients is challenging. 38 However, the identified patterns, similar to those in previous studies, and their different associations with frailty transition may provide insights into possible common underlying causes or shared risk pathways for multiple coexisting chronic conditions and help optimize the prevention and treatment of multimorbidity to improve frailty status among older adults. 38 , 39
Conclusions
Multimorbidity was associated with dynamic transitions between frailty states and death among older American adults, and the associations varied across multimorbidity patterns. Our findings may guide public health policymakers to plan preventative interventions and healthcare resources for older adults with multimorbidity in community settings. Understanding the relationship between multimorbidity and frailty can also inform clinicians to provide targeted clinical treatment and health management to prevent or delay the progression of frailty according to individual multimorbidity status. Further research is needed to investigate the underlying mechanisms through which different multimorbidity patterns affect frailty.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Numbers 81973130 and 81703304). The sponsors did not play a role in the study design, methods, subject recruitment, data collection, analysis and preparation of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Table S1. Baseline characteristics of included and excluded participants.
Table S2. Definitions of the Fried frailty phenotype.
Table S3. Baseline characteristics of all participants by conditions counts.
Table S4. Baseline characteristics of multimorbid participants by multimorbidity patterns.
Table S5. Statistics of latent class analysis models.
Figure S1. Flow chart of participant selection.
Figure S2. Frailty‐death multi‐state model with allowed transitions.
Figure S3. Follow‐up time of one participant (A) and time scale for the “clock forward” and “clock reset” approach (B).
Figure S4. Adjusted hazard ratios (95% confidence interval) for condition counts and transitions between frailty states and death among participants aged 65–74 (A), 75–84 (B), and ≥85 years (C).
Figure S5. Adjusted hazard ratios (95% confidence interval) for multimorbidity patterns and transitions between frailty states and death among participants aged 65–74 (A), 75–84 (B), and ≥85 years (C).
Figure S6. Condition counts‐adjusted hazard ratios (95% confidence interval) for multimorbidity patterns and transitions between frailty states and death.
Figure S7. Adjusted hazard ratios (95% confidence interval) for condition counts (A) and multimorbidity patterns (B) using imputed datasets.
Figure S8. Adjusted hazard ratios (95% confidence interval) for condition counts (A) and multimorbidity patterns (B) after excluding participants living in the non‐nursing home residential care settings
Acknowledgements
We thank the NHATS research and field teams for collecting the data. The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved the NHATS protocol. Signed informed consent was obtained from the study participants or their proxy respondents. 40
Luo Y., Chen Y., Wang K., De Fries C. M., Huang Z., Xu H., Yang Z., Hu Y., and Xu B. (2023) Associations between multimorbidity and frailty transitions among older Americans, Journal of Cachexia, Sarcopenia and Muscle, 14, 1075–1082, 10.1002/jcsm.13197
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Academy of Medical Sciences . Multimorbidity: a priority for global health research. Available from: https://acmedsci.ac.uk/policy/policy‐projects/multimorbidity. Accessed 12 Feb 2022.
- 3. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Cohen AA, Xue QL, Walston J, Bandeen‐Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nature Aging 2021;1:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller‐Wirnsberger R, et al. Frailty and multimorbidity: a systematic review and meta‐analysis. J Gerontol A Biol Sci Med Sci 2019;74:659–666. [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence . Multimorbidity: clinical assessment and management. Available from: https://www.nice.org.uk/guidance/ng56. Accessed 6 Nov 2021.
- 7. Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014;43:744–747. [DOI] [PubMed] [Google Scholar]
- 8. Mendonça N, Kingston A, Yadegarfar M, Hanson H, Duncan R, Jagger C, et al. Transitions between frailty states in the very old: the influence of socioeconomic status and multi‐morbidity in the Newcastle 85+ cohort study. Age Ageing 2020;49:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tazzeo C, Rizzuto D, Calderon‐Larranaga A, Roso‐Llorach A, Marengoni A, Welmer AK, et al. Multimorbidity patterns and risk of frailty in older community‐dwelling adults: a population‐based cohort study. Age Ageing 2021;50:2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho HE, Yeh CJ, Wei JC, Chu WM, Lee MC. Multimorbidity patterns and their relationships with incident disability and frailty among older adults in Taiwan: a 16‐year, population‐based cohort study. Arch Gerontol Geriatr 2022;101:104688. [DOI] [PubMed] [Google Scholar]
- 11. Zheng DD, Loewenstein DA, Christ SL, Feaster DJ, Lam BL, McCollister KE, et al. Multimorbidity patterns and their relationship to mortality in the US older adult population. PLoS One 2021;16:e0245053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson CA, Jones M, Tooth L, Mishra GD, Byles J, Dobson A. Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age Ageing 2015;44:810–816. [DOI] [PubMed] [Google Scholar]
- 13. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019;394:1365–1375. [DOI] [PubMed] [Google Scholar]
- 14. Dent E, Martin FC, Bergman H, Woo J, Romero‐Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet 2019;394:1376–1386. [DOI] [PubMed] [Google Scholar]
- 15. Freedman VA, Kasper JD. Cohort profile: the National Health and Aging Trends Study (NHATS). Int J Epidemiol 2019;48:1044–5g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen SP, Azad AD, Pershing S. Bidirectional association between visual impairment and dementia among older adults in the United States over time. Ophthalmology 2021;128:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bandeen‐Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015;70:1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chi WC, Wolff J, Greer R, Dy S. Multimorbidity and decision‐making preferences among older adults. Ann Fam Med 2017;15:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuo PL, Huang AR, Ehrlich JR, Kasper J, Lin FR, McKee MM, et al. Prevalence of concurrent functional vision and hearing impairment and association with dementia in community‐dwelling Medicare beneficiaries. JAMA Netw Open 2021;4:e211558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris D, McNicoll L, Epstein‐Lubow G, Thomas KS. Association between anxious symptoms and sleeping medication use among US older adults. Int J Geriatr Psychiatry 2018;33:e307–e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasper JD, Freedman VA, Spillman B. Classification of persons by dementia status in the National Health and Aging Trends Study. NHATS Technical Paper #5. Baltimore: Johns Hopkins University School of Public Health; 2013.
- 22. Kasper JD, Freedman VA, Niefeld MR. Construction of performance‐based summary measures of physical capacity in the National Health and Aging Trends Study. NHATS Technical Paper #4. Baltimore: Johns Hopkins University School of Public Health; 2012.
- 23. Nguyen QD, Wu C, Odden MC, Kim DH. Multimorbidity patterns, frailty, and survival in community‐dwelling older adults. J Gerontol A Biol Sci Med Sci 2019;74:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chidumwa G, Maposa I, Corso B, Minicuci N, Kowal P, Micklesfield LK, et al. Identifying co‐occurrence and clustering of chronic diseases using latent class analysis: cross‐sectional findings from SAGE South Africa Wave 2. BMJ Open 2021;11:e041604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non‐ and semi‐parametric multi‐state and competing risks models. Comput Methods Programs Biomed 2010;99:261–274. [DOI] [PubMed] [Google Scholar]
- 26. Meira‐Machado L, de Uña‐Alvarez J, Cadarso‐Suárez C, Andersen PK. Multi‐state models for the analysis of time‐to‐event data. Stat Methods Med Res 2009;18:195–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bollen KA, Biemer PP, Karr AF, Tueller S, Berzofsky ME. Are survey weights needed? A review of diagnostic tests in regression analysis. Annu Rev Stat Appl 2016;3:375–392. [Google Scholar]
- 28. Ho LYW, Cheung DSK, Kwan RYC, Wong ASW, Lai CKY. Factors associated with frailty transition at different follow‐up intervals: a scoping review. Geriatr Nurs 2021;42:555–565. [DOI] [PubMed] [Google Scholar]
- 29. Hajek A, Brettschneider C, Posselt T, Lange C, Mamone S, Wiese B, et al. Predictors of frailty in old age—results of a longitudinal study. J Nutr Health Aging 2016;20:952–957. [DOI] [PubMed] [Google Scholar]
- 30. Newman AB, Sanders JL, Kizer JR, Boudreau RM, Odden MC, Zeki Al Hazzouri A, et al. Trajectories of function and biomarkers with age: the CHS All Stars Study. Int J Epidemiol 2016;45:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prados‐Torres A, Calderón‐Larrañaga A, Hancco‐Saavedra J, Poblador‐Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014;67:254–266. [DOI] [PubMed] [Google Scholar]
- 32. Juul‐Larsen HG, Christensen LD, Bandholm T, Andersen O, Kallemose T, Jørgensen LM, et al. Patterns of multimorbidity and differences in healthcare utilization and complexity among acutely hospitalized medical patients (≥65 years)—a latent class approach. Clin Epidemiol 2020;12:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Peng W, Li M, Li X, Yang T, Li C, et al. Association between multimorbidity patterns and disability among older people covered by long‐term care insurance in Shanghai, China. BMC Public Health 2021;21:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raterman HG, Lems WF. Pharmacological management of osteoporosis in rheumatoid arthritis patients: a review of the literature and practical guide. Drugs Aging 2019;36:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marengoni A, Akugizibwe R, Vetrano DL, Roso‐Llorach A, Onder G, Welmer AK, et al. Patterns of multimorbidity and risk of disability in community‐dwelling older persons. Aging Clin Exp Res 2021;33:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alzheimer's Association . 2022 Alzheimer's disease facts and figures. Alzheimers Dement 2022;18:700–789. [DOI] [PubMed] [Google Scholar]
- 37. Vetrano DL, Rizzuto D, Calderon‐Larranaga A, Onder G, Welmer AK, Bernabei R, et al. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med 2018;15:e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitson HE, Johnson KS, Sloane R, Cigolle CT, Pieper CF, Landerman L, et al. Identifying patterns of multimorbidity in older Americans: application of latent class analysis. J Am Geriatr Soc 2016;64:1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Busija L, Lim K, Szoeke C, Sanders KM, McCabe MP. Do replicable profiles of multimorbidity exist? Systematic review and synthesis. Eur J Epidemiol 2019;34:1025–1053. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of included and excluded participants.
Table S2. Definitions of the Fried frailty phenotype.
Table S3. Baseline characteristics of all participants by conditions counts.
Table S4. Baseline characteristics of multimorbid participants by multimorbidity patterns.
Table S5. Statistics of latent class analysis models.
Figure S1. Flow chart of participant selection.
Figure S2. Frailty‐death multi‐state model with allowed transitions.
Figure S3. Follow‐up time of one participant (A) and time scale for the “clock forward” and “clock reset” approach (B).
Figure S4. Adjusted hazard ratios (95% confidence interval) for condition counts and transitions between frailty states and death among participants aged 65–74 (A), 75–84 (B), and ≥85 years (C).
Figure S5. Adjusted hazard ratios (95% confidence interval) for multimorbidity patterns and transitions between frailty states and death among participants aged 65–74 (A), 75–84 (B), and ≥85 years (C).
Figure S6. Condition counts‐adjusted hazard ratios (95% confidence interval) for multimorbidity patterns and transitions between frailty states and death.
Figure S7. Adjusted hazard ratios (95% confidence interval) for condition counts (A) and multimorbidity patterns (B) using imputed datasets.
Figure S8. Adjusted hazard ratios (95% confidence interval) for condition counts (A) and multimorbidity patterns (B) after excluding participants living in the non‐nursing home residential care settings


