Abstract
Objective:
To identify associations between occupational settings and self-reported occupational exposures on amyotrophic lateral sclerosis (ALS) survival and phenotypes.
Methods:
All patients seen in the University of Michigan Pranger ALS Clinic were invited to complete an exposure assessment querying past occupations and exposures. Standard occupational classification (SOC) codes for each job and the severity of various exposure types were derived. Cox proportional hazards models associated all-cause mortality with occupational settings and the self-reported exposures after adjusting for sex, diagnosis age, revised El Escorial criteria, onset segment, revised ALS Functional Rating Scale (ALSFRS-R), and time from symptom onset to diagnosis. Multinomial logistic regression models with three categories, adjusted for age, assessed the association between occupational settings and exposures to onset segment.
Results:
Among the 378 ALS participants (median age, 64.7 years; 54.4% male), poorer survival was associated with work in SOC code “Production Occupations” and marginally with work in “Military Occupation”; poor survival associated with self-reported occupational pesticide exposure in adjusted models. Among onset segments: cervical onset was associated with ALS participants having ever worked in “Buildings and Grounds Cleaning and Maintenance Occupations”, “Construction and Extraction Occupations”, and “Production Occupations”; bulbar onset with self-reported occupational exposure to radiation; and cervical onset with exposure to particulate matter, volatile organic compounds, metals, combustion and diesel exhaust, electromagnetic radiation, and radiation.
Conclusion:
Occupational settings and self-reported exposures influence ALS survival and onset segment. Further studies are needed to explore and understand these relationships, most advantageously using prospective cohorts and detailed ALS registries.
Keywords: amyotrophic lateral sclerosis, occupation, survival, phenotype
Introduction
Amyotrophic lateral sclerosis (ALS) is a complex and fatal neurodegenerative disease caused by genetic and non-genetic factors. Of the non-genetic factors, exposures in occupational, residential, and avocational settings linked to ALS risk include pesticides and metals exposure.1 Identifying and confirming risk factors is critical to pinpoint modifiable ALS risks. Recently we reported that occupational exposure to particulate matter, volatile organic compounds, combustion and diesel exhaust, and especially metals, along with a history of working in production occupations, correlated with increased ALS risk.2 We have found that persistent organic pollutant exposure associates with ALS risk3 and survival.4 Therefore, identifying links between occupational factors and ALS survival and onset segment could have important implications on understanding how exposures influence the progression and presentation of disease. This study uses a prospective ALS cohort to explore the association of ALS survival and phenotype with occupational histories and self-reported exposures.
Methods
Participants
Full cohort details were previously published.3–6 Briefly, all patients attending the University of Michigan Pranger ALS Clinic were invited to join this study provided they were older than 18 years. Participants were required to carry a diagnosis of ALS and to provide written informed consent in English. Participants were enrolled between June 2010 and March 2020. The study received Institutional Review Board (IRB) approval.
Data Collection and Processing
Participants completed a paper survey requesting information on four previous occupations: the current or most recent job, the job prior to the current/most recent, and the other two longest held jobs. For each job, participants provided the job title and description, and answered detailed prompts about occupational exposures using instruments modified from the Agency for Toxic Substances and Disease Registry.2 All responses were assessed for completeness and accuracy, and follow up phone calls for clarifications were made as needed. Responses were entered into an electronic database and checked at random to ensure correct data entry. Jobs and job years following ALS symptom onset were removed. Standardized Occupation Classification (SOC) Coding was completed and exposure scores were derived for each job.2 Briefly, SOCs were initially generated using both National Institutes of Health (NIH) (SOCcer model version 2.0,7 available via National Cancer Institute, https://soccer.nci.nih.gov/soccer/) and Centers for Disease Control (CDC) (NIOCCS; 2010 coding scheme available from https://www.cdc.gov/niosh/topics/coding/code.html) systems. SOCs that were discordant, had low confidence scores, could not be assigned using the two coding systems, occurred in a military setting, or had high exposure risk were reviewed by two exposure scientists (C.G., S.A.B.) for accuracy. Coding was blinded to clinical outcome data. Next, survey questions were assigned to nine exposure categories (particulate matter, volatile organic compounds, pesticides, metals, biologicals, combustion and diesel exhaust, electromagnetic radiation, and corrosives) and combined to generate composite exposure scores, as detailed previously.2
Participant demographics (age, sex, race), date of symptom onset, date of diagnosis, and onset segment were abstracted from the survey and medical records. Participants were followed prospectively and last contact (death or censoring) was recorded. Surveys were returned by March 2020 and prospective survival data were collected through July 2021.
Statistical Analysis
Demographic, clinical, and occupational characteristics for the study population were calculated. For each of the nine occupational exposure scores,2 Kaplan-Meier curves were generated and log-rank tests ascertained differences in survival for individuals with or without the respective exposures. To understand associations between occupational history and ALS survival, Cox models regressed job-years worked in each individual SOC code against survival post-diagnosis adjusted for sex, diagnosis age (quartiles), log-transformed time between symptom onset and diagnosis, revised El Escorial criteria (definite or not), onset segment (bulbar or not), and revised ALS functional rating scale (ALSFRS-R) at first visit (quartiles). The Benjamini-Hochberg procedure with a false discovery rate threshold of 0.2 determined significance after correcting for multiple testing.8 An adaptive lasso penalized Cox regression model with the same adjustment variables above fit job-years worked in all SOC codes simultaneously against survival. Because the distribution of job-years worked was right-skewed, Cox models were re-run using binary indicators of ever having worked in each SOC code as a sensitivity analysis. To estimate the mixture effect corresponding to all two-digit SOC codes simultaneously conditional on adjustment covariates, we use a Cox proportional hazards model with the framework of quantile g-computation.9 Non-parametric bootstrap is used to obtain standard error estimates, and are subsequently used to construct confidence intervals and p-values. Quantile g-computation is implemented for Cox Proportional Hazards models in the qgcomp package in R. For occupational exposure scores, Cox models were fit for each exposure score with survival as the clinical endpoint adjusted for the same covariates as the SOC survival models. After the single exposure models, an unpenalized multivariable Cox model and an adaptive lasso penalized Cox model simultaneously modeled all exposures with survival, again adjusted for covariates. Because the occupational exposure scores were right-skewed, we also considered an unpenalized multivariable Cox regression model where exposure scores were treated as a binary variable (zero exposure vs non-zero exposure), as a sensitivity check.
Also, as a small subset of participants survived much longer than average, survival analyses were repeated after restricting to those less than 5 years post-diagnosis to ensure that long survivors were not overly influential.
Next, occupational association with onset segment (bulbar, cervical, lumbar) was explored. Job-years and number of unique jobs worked grouped by onset segment were tabulated. Age-adjusted univariate multinomial logistic regression models associated job-years worked in each SOC code, and binary indicators of ever working in a job code, against onset segment. Age-adjusted multinomial logistic regression associated continuous and binarized occupational exposure scores with onset segment. The Benjamini-Hochberg procedure with a false discovery rate threshold of 0.2 determined significance after correcting for multiple testing.
Analyses were performed in R version 4.1.1. Key packages used were glmnet version 4.1-2, nnet version 7.3-16, survival version 3.2-13, and survminer version 0.4-9.
Results
Participants
Of the 378 ALS participants with demographic, ALS phenotyping, and occupational exposure data, 96.3% completed all occupational exposure data from the questionnaire (Table 1).
Table 1.
Participant demographics and results of univariable unadjusted Cox proportional hazards models.
| Covariate | ALS Cases (N = 378) | HR | 95% CI | P-Value |
|---|---|---|---|---|
| Age at diagnosis (years)* | 64.7 (57.5-71.2) | 1.03 | (1.01, 1.04) | <0.001 |
| Status | NA | |||
| Censored | 92 (24.3) | |||
| Observed Death | 286 (75.7) | |||
| Race | NA | |||
| American Indian and Alaska Native | 1 (0.3) | |||
| Black or African American | 3 (0.8) | |||
| White or Caucasian | 374 (98.9) | |||
| Sex | ||||
| Female | 172 (45.5) | 0.65 | (0.51, 0.82) | <0.001 |
| Male | 206 (54.5) | Ref | ||
| Military Service | ||||
| Enlisted | 60 (15.9) | 0.96 | (0.70, 1.32) | 0.807 |
| Neither | 318 (84.1) | Ref | ||
| Education | ||||
| High-school or Less | 106 (28.0) | Ref | ||
| Some College, Associate’s Degree | 122 (32.3) | 0.68 | (0.51, 0.91) | 0.010 |
| Bachelor’s Degree | 85 (22.5) | 0.55 | (0.39, 0.76) | <0.001 |
| Graduate Degree | 61 (16.1) | 0.52 | (0.36, 0.75) | 0.001 |
| Missing | 4 (1.1) | |||
| Smoking Status | ||||
| Non-smoker | 172 (45.8) | Ref | ||
| Current Smoker | 29 (7.7) | 0.57 | (0.35, 0.92) | 0.022 |
| Former Smoker | 173 (45.8) | 1.01 | (0.79, 1.28) | 0.965 |
| Missing | 4 (1.1) | |||
| Revised El Escorial Criteria | ||||
| Possible | 41 (10.8) | 0.43 | <0.001 | |
| Probable, LS | 104 (27.5) | 0.52 | <0.001 | |
| Probable | 127 (33.6) | 0.67 | 0.008 | |
| Definite | 94 (24.9) | Ref | ||
| Suspected | 12 (3.2) | 0.28 | <0.001 | |
| Onset Segment | ||||
| Bulbar | 110 (29.1) | Ref | ||
| Cervical | 130 (34.4) | 0.44 | (0.33, 0.59) | <0.001 |
| Lumbar | 138 (36.5) | 0.54 | (0.40, 0.72) | <0.001 |
| Time between symptom onset and diagnosis (years)** | 1.04 (0.67-1.76) | 0.75 | (0.65, 0.87) | <0.001 |
| Initial ALSFRS-R*** | 37 (33-41) | 0.97 | (0.95, 0.98) | <0.001 |
Table of descriptive statistics for the overall ALS participant study population. For continuous variables, median (25th – 75th percentile), and for categorical variables, N (%). Hazard ratios, 95% confidence intervals, and p-values correspond to univariable unadjusted Cox proportional hazards models.
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, Revised ALS Functional Rating Scale; CI, confidence interval; HR, hazard ratio; LS, lab supported; Ref, reference category;
Interpretation of hazard ratio is for one year change in age at diagnosis.
Interpretation of hazard ratio is for one log-year change in time between symptom onset and diagnosis.
369 out of 378 have observed ALSFRS-R. Interpretation of ratio is for one point change in ALSFRS-R.
Occupational settings and exposures
Of the 23 reported 2-digit SOC codes, “Education, Training, and Library Occupations,” “Sales and Related Occupations,” “Management Occupations,” “Production Occupations,” and “Office and Administrative Support Occupations” accounted for the most job-years. “Construction and Extraction Occupations,” “Healthcare Practitioners and Technical Occupations,” “Education, Training, and Library Occupations,” “Farming, Fishing, and Forestry Occupations,” and “Legal Occupations” had the highest average job-years per participant (Supplemental Table 1). Exposure to particulate matter, volatile organic compounds, and metals were most frequent (Supplemental Table 2).
Survival associations
We first evaluated associations between occupation and ALS survival. In adjusted models, an additional 5 years worked corresponded to a hazard ratio (HR) of 1.06 (95% CI 1.00-1.12, p-value = 0.040, q-value = 0.416) in “Production Occupations” (Table 2). No SOC codes were significant after multiple comparisons correction. Further, no occupations were selected by adaptive lasso regression. The estimated hazard ratio corresponding to a per quartile increase in the joint exposure to all two-digit SOC codes conditional on adjustment covariates is 0.99 (95% CI: 0.76, 1.29; p = 0.948), suggesting that there is no evidence of an occupational mixture effect on ALS survival. Although not statistically significant, perhaps owing to only 8 participants, “Military Occupation” correlated with a marginally significant shorter survival in unadjusted models (HR = 2.04, 95% CI 0.87-4.81, p-value = 0.103, q-value = 0.572; adjusted HR = 2.22, 95% CI 0.97-5.07, p-value = 0.058, q-value = 0.416). Excluding long surviving participants did not significantly alter the results (Supplemental Table 3). In models further adjusted for education and smoking status, the HR for “Production Occupations” was no longer significant (HR = 1.04, 95% CI 0.98-1.11, p-value = 0.207), however, occupation and education were correlated. (Supplemental Table 4).
Table 2. Job-years worked with two-digit SOC codes associated with survival: single SOC code models.
Single standard occupational classification (SOC) Cox models where outcome is survival post-diagnosis in years for the number of job-years worked within two-digit SOC codes. Covariates in adjusted models are sex, age at diagnosis (quartiles), log-transformed time between symptom onset and diagnosis, El Escorial criteria (definite or not), onset segment (bulbar or not), and ALSFRS-R at first visit (quartiles). Interpretation of hazard ratios (HR) correspond to five additional years worked within the SOC code. BH is the Benjamini-Hochberg P-Value. Statistically significant or marginally significant values in bold.
| Unadjusted Model | Adjusted Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Two-digit SOC Code | Description | HR | 95% CI | P-Value | Q-Value (BH) | HR | 95% CI | P-Value | Q-Value (BH) |
| 11-0000 | Management Occupations | 1.00 | (0.93, 1.08) | 0.977 | 0.981 | 0.99 | (0.92, 1.08) | 0.894 | 0.987 |
| 13-0000 | Business and Financial Operations Occupations | 1.05 | (0.96, 1.13) | 0.277 | 0.647 | 1.02 | (0.94, 1.11) | 0.690 | 0.987 |
| 15-0000 | Computer and Mathematical Occupations | 0.92 | (0.76, 1.13) | 0.446 | 0.855 | 0.87 | (0.69, 1.09) | 0.214 | 0.614 |
| 17-0000 | Architecture and Engineering Occupations | 0.92 | (0.84, 1.01) | 0.087 | 0.572 | 0.96 | (0.87, 1.06) | 0.442 | 0.923 |
| 19-0000 | Life, Physical, and Social Science Occupations | 0.82 | (0.61, 1.09) | 0.169 | 0.602 | 0.77 | (0.57, 1.04) | 0.086 | 0.416 |
| 21-0000 | Community and Social Services Occupations | 1.06 | (0.9, 1.25) | 0.501 | 0.871 | 1.09 | (0.92, 1.3) | 0.332 | 0.764 |
| 23-0000 | Legal Occupations | 0.85 | (0.67, 1.08) | 0.183 | 0.602 | 0.83 | (0.65, 1.06) | 0.141 | 0.539 |
| 25-0000 | Education, Training, and Library Occupations | 1.02 | (0.94, 1.1) | 0.651 | 0.871 | 1.02 | (0.94, 1.1) | 0.608 | 0.987 |
| 27-0000 | Arts, Design, Entertainment, Sports, and Media Occupations | 0.92 | (0.78, 1.08) | 0.309 | 0.647 | 0.89 | (0.76, 1.05) | 0.180 | 0.590 |
| 29-0000 | Healthcare Practitioners and Technical Occupations | 0.98 | (0.9, 1.07) | 0.706 | 0.871 | 1.00 | (0.92, 1.09) | 0.987 | 0.987 |
| 31-0000 | Healthcare Support Occupations | 1.02 | (0.83, 1.26) | 0.844 | 0.925 | 0.89 | (0.71, 1.12) | 0.321 | 0.764 |
| 33-0000 | Protective Service Occupations | 1.05 | (0.88, 1.25) | 0.574 | 0.871 | 1.04 | (0.87, 1.25) | 0.638 | 0.987 |
| 35-0000 | Food Preparation and Serving Related Occupations | 1.21 | (1.06, 1.39) | 0.006 | 0.146 | 1.15 | (0.98, 1.34) | 0.081 | 0.416 |
| 37-0000 | Building and Grounds Cleaning and Maintenance Occupations | 0.97 | (0.83, 1.13) | 0.730 | 0.871 | 1.00 | (0.86, 1.17) | 0.967 | 0.987 |
| 39-0000 | Personal Care and Service Occupations | 0.96 | (0.83, 1.12) | 0.611 | 0.871 | 0.89 | (0.77, 1.02) | 0.091 | 0.416 |
| 41-0000 | Sales and Related Occupations | 0.98 | (0.9, 1.06) | 0.627 | 0.871 | 1.00 | (0.92, 1.08) | 0.959 | 0.987 |
| 43-0000 | Office and Administrative Support Occupations | 1.04 | (0.99, 1.09) | 0.124 | 0.572 | 1.00 | (0.95, 1.06) | 0.934 | 0.987 |
| 45-0000 | Farming, Fishing, and Forestry Occupations | 1.10 | (0.95, 1.28) | 0.211 | 0.608 | 1.01 | (0.87, 1.17) | 0.896 | 0.987 |
| 47-0000 | Construction and Extraction Occupations | 0.95 | (0.86, 1.04) | 0.294 | 0.647 | 0.98 | (0.89, 1.08) | 0.651 | 0.987 |
| 49-0000 | Installation, Maintenance, and Repair Occupations | 1.02 | (0.92, 1.13) | 0.757 | 0.871 | 1.03 | (0.93, 1.15) | 0.567 | 0.987 |
| 51-0000 | Production Occupations | 1.06 | (1, 1.12) | 0.042 | 0.484 | 1.06 | (1.00, 1.12) | 0.040 | 0.416 |
| 53-0000 | Transportation and Material Moving Occupations | 1.00 | (0.9, 1.12) | 0.981 | 0.981 | 1.01 | (0.9, 1.13) | 0.918 | 0.987 |
| 55-0000 | Military Occupations | 2.04 | (0.87, 4.81) | 0.103 | 0.572 | 2.22 | (0.97, 5.07) | 0.058 | 0.416 |
We next investigated associations between self-reported occupational exposure and survival (Table 3). Kaplan-Meier plots for each occupational exposure category differed significantly only for pesticides, which had a median survival of 1.49 years (IQR 1.14-2.43) versus 2.32 years (IQR 1.39-3.92; p-value = 0.046) for those without this exposure (Figure 1, Supplemental Figure 1). In multivariable models and adaptive lasso, mortality rate increased for exposures to pesticides (HR = 1.25, 95% CI 1.09-1.44, p-value = 0.002 and HR = 1.246, respectively). Results were similar after adjusting for education and smoking status (HR = 1.26, 95% CI 1.09-1.46, p-value = 0.002) (Supplemental Table 5). The ever-exposed (binary model, occupational exposure score > 0) sensitivity analysis showed similar results (Supplemental Table 6). Across all models, occupational exposure to pesticides consistently correlated with poorer ALS survival. Multivariable models excluding long surviving participants also yielded similar results (Supplemental Table 7).
Table 3. Occupational exposure scores associated with survival.
Results of three types of Cox models for survival post-diagnosis in years: univariate models with occupational exposure scores as a continuous variable; multivariable models with occupational exposure scores as a continuous variable, and adaptive lasso penalized models with occupational exposure scores as a continuous variable. Covariates in all models include sex, age at diagnosis (quartiles), log-transformed time between symptom onset and diagnosis, El Escorial criteria (definite or not), onset segment (bulbar or not), and initial ALSFRS-R (quartiles). CI, confidence interval; HR, hazard ratio.
| Univariate Model with Exposure Scores as Continuous Variable | Multivariable Model with Exposure Scores as Continuous Variable | Adaptive lasso | ||||||
|---|---|---|---|---|---|---|---|---|
| Exposure Score | n | HR | 95% CI | P-Value | HR | 95% CI | P-Value | HR |
| Particulate Matter | 378 | 1.02 | (0.9, 1.16) | 0.771 | 0.97 | (0.74, 1.27) | 0.835 | 1.000 |
| Volatile Organic Compounds | 378 | 1.14 | (1.01, 1.29) | 0.038 | 1.07 | (0.89, 1.29) | 0.451 | 1.000 |
| Pesticides | 370 | 1.29 | (1.15, 1.46) | 0.000 | 1.25 | (1.09, 1.44) | 0.002 | 1.246 |
| Metals | 378 | 1.11 | (0.97, 1.26) | 0.134 | 1.08 | (0.85, 1.38) | 0.541 | 1.000 |
| Biologicals | 373 | 1.01 | (0.88, 1.14) | 0.934 | 0.98 | (0.86, 1.11) | 0.750 | 1.000 |
| Combustion/Diesel Exhaust | 376 | 0.95 | (0.84, 1.08) | 0.426 | 0.97 | (0.84, 1.12) | 0.674 | 1.000 |
| Electromagnetic radiation | 378 | 1.06 | (0.94, 1.2) | 0.340 | 1.08 | (0.93, 1.25) | 0.315 | 1.000 |
| Radiation | 378 | 0.98 | (0.87, 1.1) | 0.725 | 0.93 | (0.81, 1.06) | 0.271 | 1.000 |
| Corrosives | 375 | 0.93 | (0.81, 1.07) | 0.298 | 0.88 | (0.74, 1.04) | 0.140 | 1.000 |
Figure 1. Kaplan-Meier survival plot by occupational pesticide exposure.
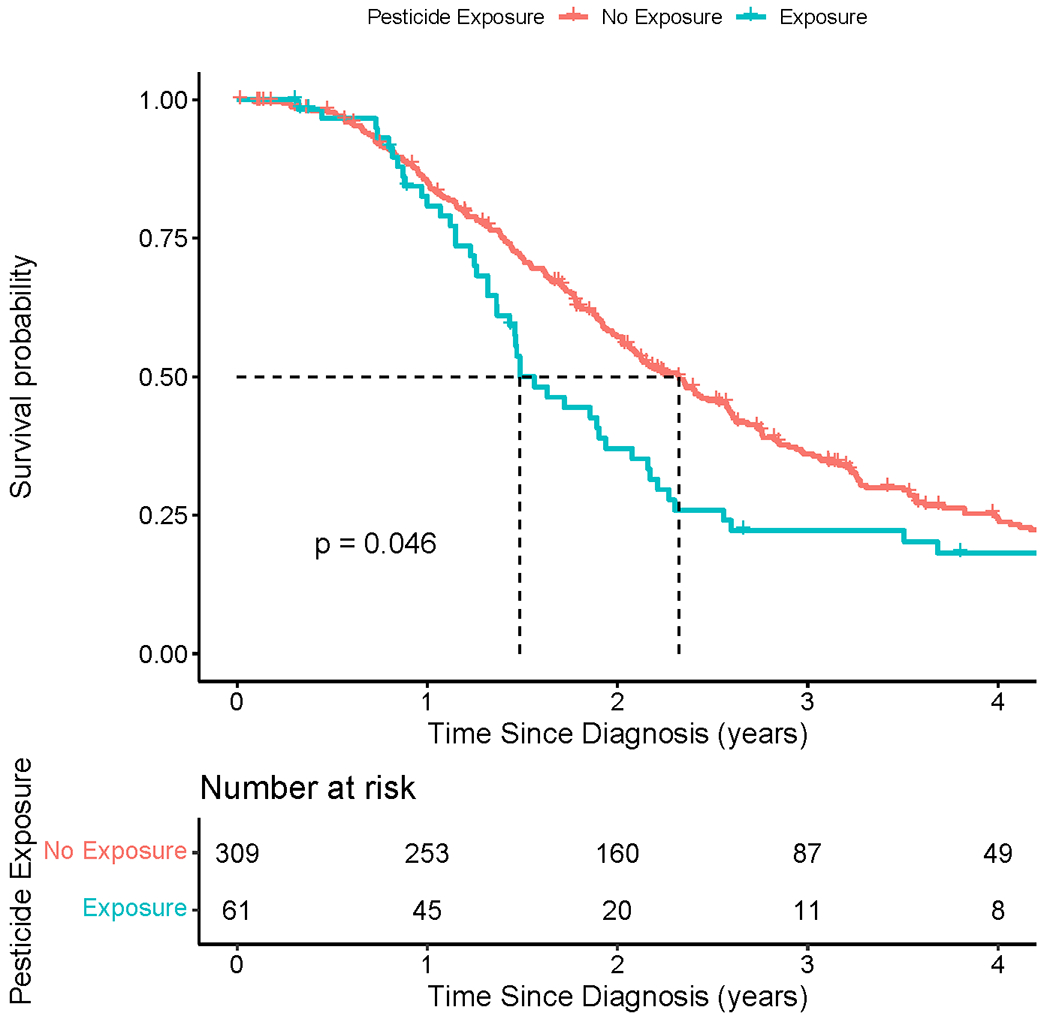
Kaplan-Meier survival plot comparing ALS participants who report occupational exposure to pesticides (blue line; median survival = 1.49 years) versus those who do not report occupational pesticide exposure (red line; median survival = 2.32 years; p-value = 0.046).
Onset segment associations
The association of onset segment with occupational settings and exposures was investigated using multinomial logistic regression models. Initial models examined duration of each occupation by SOC code (Table 4, Supplemental Figure 2). For every 5 years of work in “Construction and Extraction Occupations,” the odds of cervical compared with lumbar onset increased 31% (OR 1.31, 95% CI = 1.02-1.68, p-value = 0.031, q-value = 0.846) after adjusting for age at diagnosis. This same association was seen when considering whether a participant “ever-worked” in an occupation (Supplemental Table 8).
Table 4. SOC codes associated with onset segment.
Results of multinomial logic regression models associating standard occupational classification (SOC) codes with onset segment. Shows unadjusted models with SOC codes treated as total years of exposure and adjusted models with SOC codes treated as total years of exposure Interpretation of odds ratios correspond to five additional years worked within the respective SOC code. Adjusted multinomial logistic regression models are adjusted with age at diagnosis (quartiles). BH, Benjamini-Hochberg; CI, confidence interval; OR, odds ratio. Statistically significant results are bolded.
| Unadjusted model with SOC as continuous variable | Adjusted model with SOC as continuous variable | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulbar vs lumbar | Cervical vs lumbar | Bulbar vs lumbar | Cervical vs lumbar | ||||||||||||||
| Two-digit SOC Code | Description | OR | 95% CI | P-Value | Q-Value (BH) | OR | 95% CI | P-Value | Q-Value (BH) | OR | 95% CI | P-Value | Q-Value (BH) | OR | 95% CI | P-Value | Q-Value (BH) |
| 11-0000 | Management Occupations | 1.02 | (0.87, 1.2) | 0.786 | 0.974 | 1.04 | (0.89, 1.21) | 0.656 | 0.974 | 1.00 | (0.85, 1.18) | 0.954 | 0.979 | 1.05 | (0.9, 1.23) | 0.533 | 0.846 |
| 13-0000 | Business and Financial Operations Occupations | 0.98 | (0.82, 1.19) | 0.867 | 0.974 | 0.93 | (0.76, 1.13) | 0.460 | 0.961 | 0.96 | (0.8, 1.16) | 0.705 | 0.979 | 0.93 | (0.75, 1.14) | 0.460 | 0.846 |
| 15-0000 | Computer and Mathematical Occupations | 1.03 | (0.7, 1.51) | 0.875 | 0.974 | 1.03 | (0.71, 1.48) | 0.888 | 0.974 | 0.99 | (0.68, 1.46) | 0.972 | 0.979 | 1.04 | (0.72, 1.51) | 0.836 | 0.979 |
| 17-0000 | Architecture and Engineering Occupations | 1.01 | (0.84, 1.2) | 0.951 | 0.974 | 0.94 | (0.78, 1.13) | 0.506 | 0.966 | 1.01 | (0.84, 1.21) | 0.922 | 0.979 | 0.93 | (0.77, 1.12) | 0.436 | 0.846 |
| 19-0000 | Life, Physical, and Social Science Occupations | 0.47 | (0.15, 1.47) | 0.196 | 0.855 | 0.90 | (0.59, 1.36) | 0.609 | 0.966 | 0.45 | (0.14, 1.51) | 0.198 | 0.846 | 0.87 | (0.57, 1.31) | 0.500 | 0.846 |
| 21-0000 | Community and Social Services Occupations | 0.74 | (0.39, 1.41) | 0.364 | 0.855 | 1.05 | (0.76, 1.45) | 0.776 | 0.974 | 0.70 | (0.36, 1.34) | 0.279 | 0.846 | 1.04 | (0.75, 1.44) | 0.836 | 0.979 |
| 23-0000 | Legal Occupations | 1.00 | (0.74, 1.34) | 0.974 | 0.974 | 0.77 | (0.45, 1.34) | 0.358 | 0.855 | 0.99 | (0.73, 1.33) | 0.941 | 0.979 | 0.77 | (0.44, 1.36) | 0.371 | 0.846 |
| 25-0000 | Education, Training, and Library Occupations | 0.97 | (0.83, 1.13) | 0.669 | 0.974 | 0.93 | (0.79, 1.09) | 0.372 | 0.855 | 0.94 | (0.81, 1.1) | 0.469 | 0.846 | 0.94 | (0.8, 1.1) | 0.424 | 0.846 |
| 27-0000 | Arts, Design, Entertainment, Sports, and Media Occupations | 1.00 | (0.77, 1.32) | 0.972 | 0.974 | 0.83 | (0.56, 1.22) | 0.335 | 0.855 | 1.02 | (0.77, 1.35) | 0.884 | 0.979 | 0.83 | (0.56, 1.23) | 0.352 | 0.846 |
| 29-0000 | Healthcare Practitioners and Technical Occupations | 1.10 | (0.89, 1.36) | 0.361 | 0.855 | 1.10 | (0.9, 1.35) | 0.355 | 0.855 | 1.11 | (0.9, 1.37) | 0.317 | 0.846 | 1.10 | (0.9, 1.35) | 0.345 | 0.846 |
| 31-0000 | Healthcare Support Occupations | 1.16 | (0.85, 1.57) | 0.343 | 0.855 | 0.65 | (0.33, 1.27) | 0.207 | 0.855 | 1.15 | (0.85, 1.57) | 0.362 | 0.846 | 0.66 | (0.33, 1.3) | 0.229 | 0.846 |
| 33-0000 | Protective Service Occupations | 0.68 | (0.27, 1.69) | 0.404 | 0.885 | 1.06 | (0.72, 1.55) | 0.777 | 0.974 | 0.68 | (0.28, 1.64) | 0.387 | 0.846 | 1.09 | (0.74, 1.6) | 0.662 | 0.979 |
| 35-0000 | Food Preparation and Serving Related Occupations | 1.01 | (0.76, 1.34) | 0.957 | 0.974 | 0.83 | (0.58, 1.19) | 0.306 | 0.855 | 1.01 | (0.76, 1.34) | 0.961 | 0.979 | 0.82 | (0.56, 1.2) | 0.298 | 0.846 |
| 37-0000 | Building and Grounds Cleaning and Maintenance Occupations | 0.96 | (0.66, 1.38) | 0.806 | 0.974 | 1.09 | (0.81, 1.47) | 0.578 | 0.966 | 1.02 | (0.7, 1.47) | 0.930 | 0.979 | 1.07 | (0.79, 1.45) | 0.671 | 0.979 |
| 39-0000 | Personal Care and Service Occupations | 0.77 | (0.52, 1.15) | 0.209 | 0.855 | 0.85 | (0.63, 1.15) | 0.303 | 0.855 | 0.78 | (0.52, 1.15) | 0.209 | 0.846 | 0.86 | (0.63, 1.17) | 0.325 | 0.846 |
| 41-0000 | Sales and Related Occupations | 1.05 | (0.88, 1.26) | 0.565 | 0.966 | 1.02 | (0.85, 1.22) | 0.807 | 0.974 | 1.06 | (0.88, 1.27) | 0.522 | 0.846 | 1.02 | (0.85, 1.22) | 0.821 | 0.979 |
| 43-0000 | Office and Administrative Support Occupations | 1.03 | (0.92, 1.15) | 0.601 | 0.966 | 0.99 | (0.89, 1.11) | 0.865 | 0.974 | 1.01 | (0.9, 1.13) | 0.882 | 0.979 | 1.00 | (0.89, 1.12) | 0.979 | 0.979 |
| 45-0000 | Farming, Fishing, and Forestry Occupations | 0.99 | (0.7, 1.41) | 0.965 | 0.974 | 0.92 | (0.62, 1.37) | 0.685 | 0.974 | 0.96 | (0.67, 1.36) | 0.810 | 0.979 | 0.92 | (0.62, 1.37) | 0.682 | 0.979 |
| 47-0000 | Construction and Extraction Occupations | 1.21 | (0.93, 1.57) | 0.158 | 0.855 | 1.32 | (1.03, 1.68) | 0.026 | 0.855 | 1.22 | (0.94, 1.6) | 0.136 | 0.846 | 1.31 | (1.02, 1.68) | 0.031 | 0.846 |
| 49-0000 | Installation, Maintenance, and Repair Occupations | 0.86 | (0.63, 1.18) | 0.351 | 0.855 | 1.13 | (0.94, 1.37) | 0.197 | 0.855 | 0.85 | (0.63, 1.15) | 0.281 | 0.846 | 1.15 | (0.94, 1.39) | 0.173 | 0.846 |
| 51-0000 | Production Occupations | 1.11 | (0.97, 1.27) | 0.113 | 0.855 | 1.09 | (0.96, 1.25) | 0.187 | 0.855 | 1.11 | (0.97, 1.26) | 0.144 | 0.846 | 1.10 | (0.96, 1.26) | 0.165 | 0.846 |
| 53-0000 | Transportation and Material Moving Occupations | 0.94 | (0.75, 1.18) | 0.604 | 0.966 | 0.89 | (0.7, 1.13) | 0.328 | 0.855 | 0.92 | (0.73, 1.16) | 0.500 | 0.846 | 0.89 | (0.69, 1.13) | 0.340 | 0.846 |
| 55-0000 | Military Occupations | 4.64 | (0.36, 59.7) | 0.239 | 0.855 | 2.51 | (0.17, 36.84) | 0.502 | 0.966 | 3.93 | (0.29, 53.92) | 0.306 | 0.846 | 2.79 | (0.18, 43.39) | 0.463 | 0.846 |
Similar analyses correlated several occupational exposure scores with onset segment (Table 5, Figure 2). Using the continuous self-reported occupational exposure scores adjusted for age at diagnosis, for every 5 years of work: the odds of bulbar onset disease relative to lumbar onset was 1.38 (95% CI 1.01-1.88, p-value = 0.040, q-value = 0.103) for radiation exposure; the odds of cervical onset relative to lumbar onset was higher for exposures to particulate matter (OR = 1.49, 95% CI 1.15-1.92, p-value = 0.002, q-value = 0.043), volatile organic compounds (OR = 1.32, 95% CI 1.03-1.69, p-value = 0.027, q-value = 0.103), metals (OR = 1.31, 95% CI 1.02-1.68, p-value = 0.033, q-value = 0.103), combustion and diesel exhaust (OR = 1.30, 95% CI 1.01-1.67, p-value = 0.040, q-value = 0.103), electromagnetic radiation (OR = 1.39, 95% CI 1.06-1.83, p-value = 0.016, q-value = 0.103), and radiation (OR = 1.43, 95% CI 1.06-1.92, p-value = 0.018, q-value = 0.103). Pesticides (OR = 1.25, 95% CI 1.02-1.68, p-value = 0.077, q-value = 0.173) and biological exposures (OR = 1.31, 95% CI 0.96-1.80, p-value = 0.089, q-value = 0.177) also favored cervical onset after FDR correction. Overall, findings were similar for logistic regression models that considered exposure scores as binary, ever-exposed variables (Supplemental Table 9).
Table 5. Occupational exposure scores associated with Onset Segment.
Interpretation of odds ratios correspond to a standard deviation increase in the respective occupational exposure score. Adjusted multinomial logistic regression models are adjusted for age at diagnosis (quartiles). BH, Benjamini-Hochberg; CI, confidence interval; OR, odds ratio. Statistically significant or near significant results are bolded.
| Exposure score as a continuous variable | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bulbar vs lumbar | Cervical vs lumbar | |||||||
| Exposure Score | OR | 95% CI | P-Value | Q-Value (BH) | OR | 95% CI | P-Value | Q-Value (BH) |
| Particulate Matter | 1.11 | (0.83, 1.49) | 0.476 | 0.659 | 1.49 | (1.15, 1.92) | 0.002 | 0.043 |
| VOCs | 1.04 | (0.79, 1.38) | 0.770 | 0.866 | 1.32 | (1.03, 1.69) | 0.027 | 0.103 |
| Pesticides | 0.97 | (0.72, 1.3) | 0.828 | 0.877 | 1.25 | (0.98, 1.59) | 0.077 | 0.173 |
| Metals | 1.06 | (0.8, 1.4) | 0.687 | 0.825 | 1.31 | (1.02, 1.68) | 0.033 | 0.103 |
| Biological Exposures | 1.01 | (0.68, 1.51) | 0.959 | 0.959 | 1.31 | (0.96, 1.8) | 0.089 | 0.177 |
| Combustion (Diesel) | 1.15 | (0.87, 1.52) | 0.335 | 0.502 | 1.30 | (1.01, 1.67) | 0.040 | 0.103 |
| Electromagnetic | 1.24 | (0.93, 1.67) | 0.145 | 0.260 | 1.39 | (1.06, 1.83) | 0.016 | 0.103 |
| Radiation | 1.38 | (1.01, 1.88) | 0.040 | 0.103 | 1.43 | (1.06, 1.92) | 0.018 | 0.103 |
| Corrosives | 1.09 | (0.82, 1.45) | 0.538 | 0.692 | 1.19 | (0.92, 1.53) | 0.178 | 0.292 |
Figure 2. Onset Segment Multinomial Logistic Regression Model and Distribution.
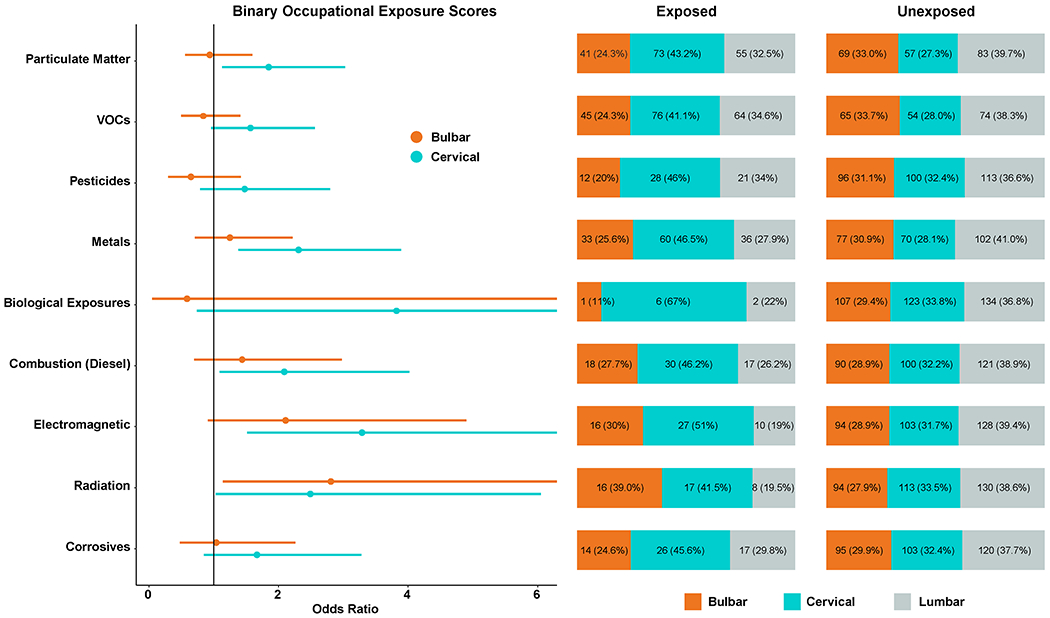
On the left, forest plot of the adjusted multinomial logistic regression models from Table 5 showing the odds ratio for bulbar and cervical onset with lumbar onset as the reference. On the right, the distribution of each onset segment by exposed and unexposed.
Discussion
Previously, we have shown that plasma persistent organic pollutant levels correlate with ALS survival4 and self-reported occupational exposures associate with ALS risk.2 Here, we investigated whether occupational settings, via SOC codes, and self-reported exposures are linked to survival and onset segment and found several associations in this Michigan ALS cohort. First, regarding survival, we identified that work in “Production Occupations” correlated with a decrease in ALS survival. Lack of significance following multiple comparison testing could be related to small sample size rather than from a lack of an effect. Additionally, work in “Military Occupation” associated with a large reduction in survival, although the effect was only marginally significant. Military service is a known ALS risk10 and raises the possibility of additional or cumulative exposures that may impact disease progression. Future research with larger sample sizes is encouraged to determine if these marginal associations, including the joint effect of military and other exposure types. Larger samples might also reveal interaction effects, such as between education and occupation, which would better identify culpable exposures and potentially lead to interventions to reduce ALS risk.
Few studies have explored the role of occupation on ALS survival. In the Cancer Prevention Study cohort, occupations associated with increased ALS mortality included programmers and laboratory technicians in males and machine assemblers in females.11 A mortality study showed an excess number of ALS deaths among persons working in the computer and mathematical, architecture and engineering, legal, and education, training, and library fields.12 However, few studies have explored the survival duration by occupational exposures.
The “Production Occupations” SOC code encompasses a wide range of occupational settings and tasks, e.g., assemblers, fabricators, and operators in food, metal, plastic, printing, textile, wood, and other industries. These workers may experience exposures to metals, fumes, and industrial chemicals and may perform repetitive and strenuous tasks. Regarding “Military Occupations,” veterans are exposed to a range of hazards, including herbicides, pesticides, heavy metals, combustion products, jet fuels, and chemicals released from burn pits,10 many already linked to ALS risk.13,14 Thus, workers in such specific SOCs have increased likelihood of exposure to certain hazards, e.g., metals, fumes, and other toxics. While 2-digit SOC job codes are very broad and only a subset of workers in a particular code are likely to receive a specific exposure, we still found that several SOCs were correlated with ALS survival.
We next examined self-reported exposure and survival. Our analysis showed that self-reported pesticide exposure associated with poorer survival, a finding consistent across several different analytical models. This aligned with our previous reports, which identified persistent organic pollutants including pesticides in blood as both ALS risk factors3 and determinants of disease progression and survival.4 Moreover, our methods showed consistency with studies utilizing self-reported questionnaires to identify occupational exposures.15,16 Pesticides in current use, e.g., organophosphates, can cause widespread occupational exposure to farm workers, building and grounds maintenance staff, and production and distribution workers in the agrichemical industry, which can be assessed by self-reported questionnaires. Recently, a study of 94 ALS participants recruited from the United States National ALS Registry reported no survival association with occupational exposure to agricultural chemicals based on exposure classification by a single industrial hygienist.17 Our study classified exposures based on assessments from two trained assessors using self-reported data, methods established in the literature.15,16 However, since we did not classify exposures with an industrial hygienist, we could not confirm or account for the intensity of particular exposures, e.g., frequency, duration and level of exposure events, and recall bias is always a concern with retrospective studies.
Regarding our second study goal, the linkage of SOC or self-reported exposure with onset segment, we identified several important associations in this cohort. Specifically, cervical onset disease correlated with “Construction and Extraction Occupations” when job duration was considered, and with “ever-working” in “Buildings and Grounds Cleaning and Maintenance Occupations”, “Construction and Extraction Occupations”, and “Production Occupations”. Workers in these occupations typically perform strenuous and repetitive physical movements, especially in the upper body. Whether this leads to injury in the cervical motor neurons or reflects the physical activity risk with ALS is unknown. Strenuous physical activity associates with ALS risk, most recently shown via genetic risk18 and a survey of vigorous leisure-time physical activity in the National ALS Registry.19 Professions characterized by strenuous physical activity linked to ALS include manual laborers,20–24 professional athletes,25–28 and military veterans.29,30 With respect to correlations with onset segment, in contrast to our findings, a recent Maltese study found that workers in construction occupations31 and agricultural workers in Brittany, France24 tended to have bulbar onset. In professional Italian soccer players, bulbar onset was predominant, although the numbers were small.25 In Israeli triathletes, bulbar onset was more frequent.32 This paucity of studies warrants further research, especially given the possible cumulative impact of physical activity with other exposure types.
When we examined the relationship of self-reported exposures instead of SOC codes to segment onset, radiation exposure was linked to bulbar and cervical onset disease as opposed to lumbar onset disease. Radiation can cause injury to the brainstem and spinal cord; the cervical spine may be more susceptible to damage compared to the thoracic spine due to lower dispersion of radiation across the tissue,33 which might explain the pattern we observed. This exposure only occurred in a subset of workers in the “Healthcare Practitioners and Technical Occupations” and “Healthcare Support Occupations”. In contrast, most other occupational scores correlated with cervical onset disease, which may reflect a cumulative effect in individuals exposed to pollutants in occupational settings with strenuous upper body physical activity. An Australian study did find that workers exposed to metals tended to have limb-onset disease.16
Causes of phenotypic heterogeneity in ALS remain largely unknown. Some genetic mutations are associated with predisposition to certain onset segments, but these are largely in rare genes.13,14 Therefore, the finding that some occupations lead to a higher odds of cervical onset disease is of interest in terms of our appreciation for why disease starts in certain areas of the nervous system.
This study has strengths. The use of expert assessment of occupation with SOC codes and self-reported occupational exposures improves the accuracy of assigning exposures.34,35 Moreover, the dual approach of leveraging self-reported exposures and exposure scores in addition to SOC codes overcomes the weaknesses of using SOC codes alone. Occupational exposure scores yield insight beyond the occupational SOC codes, which tend to be broad and encompass diverse workplace activities and exposure types. Thus, sole reliance on SOC codes can lead to exposure misclassification, including both false positives and false negatives. For example, workers in managerial roles in job codes usually associated with specific exposures, e.g., volatile organic compounds in the petrochemical industry, may not actually be exposed if not working at the production site. Conversely, some workers in “Management Occupations” or “Architectural and Engineering Operations” working in production facilities or at construction sites may potentially be exposed to hazards. Thus, we accounted for such situations by considering both SOC codes and self-reported exposures. Moreover, this allowed us to investigate environmental effects in ALS, which is a rare disease, making large prospective cohorts difficult. Further, our analysis included many ALS participants recruited from a single center, which captured a large fraction of ALS cases in Michigan. Next, the consistency of results across multiple analyses improved the confidence of our results.
This study also has limitations. Although all patients seen in our Pranger ALS Clinic are invited to participate, ALS is not a reportable disease in the State of Michigan and therefore we were unable to recruit from all incident cases in the State. Further, we could not account for bias that exists if people in certain occupations preferentially seek care at another center or choose not to receive care at all. Further, we are unable to account for bias if certain occupations or occupational exposures are differentially associated with a rapid disease progression, if individuals do not survive to diagnosis, or if individuals are so overwhelmed at the time of diagnosis that they choose not to enroll in observational research, i.e. recruitment bias. Additionally, we could neither isolate nor confirm the exposures associated with a specific job from the SOC code. For example, physical activity is linked to ALS through polygenic risk;18 however, the association we identified of survival with “Production Occupations” may be linked to the level of regular physical activity required to perform the job as opposed to the job setting. This might be addressed by adjusting our analyses for activity level, but we did not collect data on occupational physical activity levels. Models that adjusted for education and smoking did not show the same association with “Production Occupations.” Workers in this occupational category typically report lower educational attainment, which could suggest confounding in prior studies that associated educational attainment with increased ALS risk. In this case, the true driver may be workplace exposures to chemicals, particles and other physical stressors. While we show associations with pesticides, we did not capture specific pesticide formulations, which have changed considerably over time. In our study, many workers had long job tenures and often transitioned to similar jobs, which could involve the same exposure types. Thus, older workers may have had occupational exposure to both “legacy” pesticides, like organochlorines, as well as currently used pesticides. Also, it is extremely challenging to quantify occupational exposure over the life course, although a combination of job history, self-reported exposures, and biological monitoring can help address this important gap. Further unknown is whether exposures are only relevant in certain critical exposure windows. Leveraging prospective cohorts of workers in specific occupations provides a possible alternative, but very large sample sizes would be needed given the low incidence of ALS. In addition, creative approaches are also needed to collect the necessary data given the fragmented reporting of this disease.
Conclusion
Few prior studies have linked occupational job or exposures to ALS survival. Working in “Production Occupations” and self-reported occupational exposure to pesticides worsened ALS survival, as did “Military Occupations” although this occupation was only marginally significant. In addition, several occupations associated with typically strenuous physical activity were associated with cervical onset disease. Occupational settings and self-reported occupational exposures have important associations with ALS phenotypes. These intriguing findings regarding the impact of occupations and occupational exposures on ALS survival and phenotypes help build the case for prospective cohorts and registries to better assess disease risk.
Supplementary Material
Acknowledgements
We are indebted to the study participants that provided information. We thank Blake Swihart, Adam Patterson, Jayna Duell, RN, Daniel Berger, Amanda Williams, and Scott Dent for study support. We thank Stacey Sakowski, PhD for assistance with figures.
Funding
National ALS Registry/CDC/ATSDR (1R01TS000289); National ALS Registry/CDC/ATSDR CDCP-DHHS-US (CDC/ATSDR 200-2013-56856); NIEHS K23ES027221; NIEHS R01ES030049; NIEHS P30ES017885
Footnotes
Competing interests
SAG served on a DSMB. The other authors declare they have no competing interests.
Data availability
Sharing of non-identifiable data will be considered at the reasonable request of a qualified investigator.
References
- 1.Wang MD, Little J, Gomes J, Cashman NR & Krewski D Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 61, 101–130, doi: 10.1016/j.neuro.2016.06.015 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Goutman SA et al. Associations of self-reported occupational exposures and settings to ALS: a case–control study. International Archives of Occupational and Environmental Health, doi: 10.1007/s00420-022-01874-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su FC et al. Association of Environmental Toxins With Amyotrophic Lateral Sclerosis. JAMA neurology 73, 803–811, doi: 10.1001/jamaneurol.2016.0594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goutman SA et al. High plasma concentrations of organic pollutants negatively impact survival in amyotrophic lateral sclerosis. Journal of neurology, neurosurgery, and psychiatry 90, 907–912, doi: 10.1136/jnnp-2018-319785 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goutman SA et al. Untargeted metabolomics yields insight into ALS disease mechanisms. Journal of neurology, neurosurgery, and psychiatry, doi: 10.1136/jnnp-2020-323611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y et al. Environmental risk factors and amyotrophic lateral sclerosis (ALS): a case-control study of ALS in Michigan. PloS one 9, e101186, doi: 10.1371/journal.pone.0101186 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russ DE et al. Computer-based coding of free-text job descriptions to efficiently identify occupations in epidemiological studies. Occup Environ Med 73, 417–424, doi: 10.1136/oemed-2015-103152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamini Y & Hochberg Y Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300 (1995). [Google Scholar]
- 9.Keil AP et al. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environmental Health Perspectives 128, 047004, doi:doi: 10.1289/EHP5838 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geretto M et al. Occupational Exposures and Environmental Health Hazards of Military Personnel. Int J Environ Res Public Health 18, doi: 10.3390/ijerph18105395 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisskopf MG et al. Prospective Study of Occupation and Amyotrophic Lateral Sclerosis Mortality. American Journal of Epidemiology 162, 1146–1152, doi: 10.1093/aje/kwi343 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Beard JD et al. Mortality from Amyotrophic Lateral Sclerosis and Parkinson’s Disease Among Different Occupation Groups - United States, 1985–2011. MMWR Morb Mortal Wkly Rep 66, 718–722, doi: 10.15585/mmwr.mm6627a2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goutman SA et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. The Lancet. Neurology, doi: 10.1016/s1474-4422(21)00465-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goutman SA et al. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. The Lancet. Neurology, doi: 10.1016/s1474-4422(21)00414-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malek AM et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neuro-degenerative diseases 14, 31–38, doi: 10.1159/000355344 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Pamphlett R Exposure to environmental toxins and the risk of sporadic motor neuron disease: an expanded Australian case-control study. European journal of neurology : the official journal of the European Federation of Neurological Societies 19, 1343–1348, doi: 10.1111/j.1468-1331.2012.03769.x (2012). [DOI] [PubMed] [Google Scholar]
- 17.Mitsumoto H et al. Case-control study in ALS using the National ALS Registry: lead and agricultural chemicals are potential risk factors. Amyotrophic lateral sclerosis & frontotemporal degeneration 23, 190–202, doi: 10.1080/21678421.2021.1936556 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Julian TH et al. Physical exercise is a risk factor for amyotrophic lateral sclerosis: Convergent evidence from Mendelian randomisation, transcriptomics and risk genotypes. EBioMedicine 68, 103397, doi: 10.1016/j.ebiom.2021.103397 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond J et al. History of vigorous leisure-time physical activity and early onset amyotrophic lateral sclerosis (ALS), data from the national ALS registry: 2010–2018. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 1–10, doi: 10.1080/21678421.2021.1910308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrew AS et al. Risk factors for amyotrophic lateral sclerosis: A regional United States case-control study. Muscle Nerve 63, 52–59, doi: 10.1002/mus.27085 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippini T et al. Environmental and Occupational Risk Factors of Amyotrophic Lateral Sclerosis: A Population-Based Case-Control Study. Int J Environ Res Public Health 17, doi: 10.3390/ijerph17082882 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerson AS et al. Study of occupation and amyotrophic lateral sclerosis in a Danish cohort. Occup Environ Med 75, 630–638, doi: 10.1136/oemed-2018-105110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govoni V, Granieri E, Fallica E & Casetta I Amyotrophic lateral sclerosis, rural environment and agricultural work in the Local Health District of Ferrara, Italy, in the years 1964–1998. Journal of neurology 252, 1322–1327, doi: 10.1007/s00415-005-0859-z (2005). [DOI] [PubMed] [Google Scholar]
- 24.Furby A, Beauvais K, Kolev I, Rivain JG & Sébille V Rural environment and risk factors of amyotrophic lateral sclerosis: a case-control study. Journal of neurology 257, 792–798, doi: 10.1007/s00415-009-5419-5 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Chiò A et al. ALS in Italian professional soccer players: The risk is still present and could be soccer-specific. Amyotrophic Lateral Sclerosis 10, 205–209, doi: 10.1080/17482960902721634 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Chiò A, Benzi G, Dossena M, Mutani R & Mora G Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain : a journal of neurology 128, 472–476, doi: 10.1093/brain/awh373 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Lehman EJ, Hein MJ, Baron SL & Gersic CM Neurodegenerative causes of death among retired National Football League players. Neurology 79, 1970–1974, doi: 10.1212/WNL.0b013e31826daf50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pupillo E et al. Increased risk and early onset of ALS in professional players from Italian Soccer Teams. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 21, 403–409, doi: 10.1080/21678421.2020.1752250 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Seals RM, Kioumourtzoglou MA, Hansen J, Gredal O & Weisskopf MG Amyotrophic Lateral Sclerosis and the Military: A Population-based Study in the Danish Registries. Epidemiology 27, 188–193, doi: 10.1097/ede.0000000000000417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKay KA et al. Military service and related risk factors for amyotrophic lateral sclerosis. Acta neurologica Scandinavica 143, 39–50, doi: 10.1111/ane.13345 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrugia Wismayer M et al. Occupation and amyotrophic lateral sclerosis risk: a case-control study in the isolated island population of Malta. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 1–7, doi: 10.1080/21678421.2021.1905847 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Gotkine M, Friedlander Y & Hochner H Triathletes are over-represented in a population of patients with ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 15, 534–536, doi: 10.3109/21678421.2014.932383 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Kirkpatrick JP, van der Kogel AJ & Schultheiss TE Radiation dose-volume effects in the spinal cord. International journal of radiation oncology, biology, physics 76, S42–49, doi: 10.1016/j.ijrobp.2009.04.095 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Teschke K et al. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med 59, 575–593; discussion 594, doi: 10.1136/oem.59.9.575 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge CB et al. Use and Reliability of Exposure Assessment Methods in Occupational Case-Control Studies in the General Population: Past, Present, and Future. Annals of work exposures and health 62, 1047–1063, doi: 10.1093/annweh/wxy080 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sharing of non-identifiable data will be considered at the reasonable request of a qualified investigator.


