Summary
Branched-chain amino acids (BCAAs) are effectors of metabolic diseases, but their impact on mortality is largely unknown. We investigated the association of BCAA with risk factors and mortality in 2,236 participants of the Ludwigshafen Risk and Cardiovascular Health (LURIC) study using linear and Cox regression. Adiponectin, hemoglobin, C-peptide, hemoglobin A1c, and homoarginine showed the strongest association with BCAA concentration (all p < 0.001). During a median follow-up of 10.5 years, 715 participants died, including 450 cardiovascular-related deaths. BCAA concentrations were inversely associated with the risk of all-cause and cardiovascular mortality (HR [95% CI] per 1-SD increase in log-BCAA: 0.75 [0.69–0.82] and 0.72 [0.65–0.80], respectively) after adjustment for potential confounders. BCAAs are directly associated with metabolic risk but inversely with mortality in persons with intermediate-to-high cardiovascular risk. Further studies are warranted to evaluate the diagnostic and therapeutic utility of BCAA in the context of cardiovascular diseases.
Subject areas: Health sciences, Cardiovascular medicine, Association analysis
Graphical abstract
Highlights
-
•
Direct associations of serum BCAA concentrations and metabolic risk factors were found
-
•
Adiponectin and BCAA were inversely correlated in diabetic-/nondiabetic patients
-
•
BCAAs were inversely related to mortality risk irrespective of obesity or diabetes
-
•
Considering BCAA concentrations substantially improved risk prediction models
Health sciences; Cardiovascular medicine; Association analysis;
Introduction
Branched-chain amino acids (BCAAs; leucine, isoleucine, and valine) are essential amino acids, which account for up to 40% of the preformed amino acids required by humans.1,2 Increasing intake of proteins in general or specifically, BCAAs is associated with both elevated circulating BCAA levels and changes in body composition in mice and humans.3 BCAA serum concentrations therefore partly reflect total protein intake whose association with mortality has been studied extensively. For the intake of plant proteins, most studies report inverse associations with mortality and no or direct associations for the intake of animal proteins.4,5,6,7 Mechanistic studies in animals have provided evidence that BCAAs play an integral role in glucose homeostasis and cardiac physiology with partly differing effects of the individual three amino acids.3,8,9
It has been shown that under a high-fat diet, leucine mediates beneficial effects on adiposity and insulin sensitivity in mice while valine feeding leads to reduced glucose tolerance/insulin sensitivity.10 Another study demonstrated that reducing isoleucine or valine but not leucine rapidly restores metabolic health in obese mice by increasing hepatic insulin sensitivity and ketogenesis and increasing energy expenditure.11 Mice with incomplete catabolism of BCAAs due to a genetic defect showed arrhythmias as well as cardiac conduction and repolarization disturbances.12 A study in a murine model of myocardial infarction (MI) found that myocardial BCAA catabolism was significantly impaired in response to permanent MI leading to elevated myocardial BCAA abundance, with oral BCAA administration further increasing cardiac BCAA concentrations, which lead to the activation of mammalian target of rapamycin (mTOR) signaling and exacerbated cardiac dysfunction and remodeling.13
In human heart failure (HF), impaired cardiac BCAA catabolism and insulin signaling were demonstrated, and it was shown that enhancing BCAA oxidation can improve cardiac function in the failing mouse heart.14
Furthermore, restricting dietary BCAA intake increases health span and longevity in mice.15 Metabolomic profiling in large community-based cohorts indicated that elevated BCAA concentrations are related to facets of the metabolic syndrome, incident hyperglycemia, and diabetes mellitus.16,17,18 Shah et al. observed higher blood concentrations of BCAAs in patients with coronary artery disease (CAD) and a positive association between BCAAs and prevalent, but not incident MI as compared to persons without CAD.19 In a large study of patients undergoing coronary angiography, higher BCAAs in conjunction with related metabolites emerged as independent predictors of the risk of death and MI.20 A mendelian randomized study provided evidence for causal impacts of genetically predicted circulating BCAA levels on blood pressure (BP).21
While BCAAs appear to augment cardiometabolic risk and reflect prevalent CAD, the association between BCAAs and the risk of incident cardiovascular mortality irrespective of related metabolites remains obscure. In a large-scale metabolomics study opposite associations with mortality were observed for the individual BCAAs with leucine and valine being associated with reduced risk and isoleucine with increased risk.22
Understanding the impact of BCAAs on cardiovascular risk may afford additional insight into the complex mechanisms involved in the development of and/or progression of cardiovascular diseases. We used ten-year longitudinal data from the Ludwigshafen Risk and Cardiovascular Health (LURIC) study, a prospective cohort study in patients routinely referred to coronary angiography, to examine the associations between serum BCAA concentrations and cardiometabolic risk factors while further evaluating the association between circulating BCAA concentrations with the risk of overall and cardiovascular death.
Results
Distribution of BCAA and baseline characteristics
Among the 2,236 Caucasian participants with a mean age of 62.5 ± 10.8 years, 29.9% were women. Overall, 1,566 (68.7%) had angiographically proven CAD (1, 2, and 3 vessels disease: 17.7%, 19.8%, and 31.2%, respectively). Of the 1,566 individuals with CAD, 934 (59.6%) presented with stable CAD, while the remaining patients were admitted with unstable angina pectoris (n = 357), non-ST-elevation myocardial infarction (NSTEMI) (troponin-T > 0.1 mg/L, n = 74), or ST-elevation MI (STEMI) (troponin-T > 0.1 mg/L, n = 191). The prevalence of diabetes mellitus and arterial hypertension was 37.7% (women: 38.8%; men 37.2%; p = 0.495) and 73.1% (women: 76.5%; men: 71.6%; p = 0.017), respectively. The mean estimated glomerular filtration rate, eGFR was 85.5 ± 23.6 mL/min/1.73 m2.
Baseline characteristics of the 2,236 patients according to quartiles of serum BCAA concentration are shown in Table 1. Compared with participants in the lowest BCAA quartile, those with higher concentrations were more frequently women and had a higher prevalence of diabetes mellitus and insulin resistance. Also, individuals with higher serum BCAA concentrations had higher left ventricular ejection fraction (LVEF) and homoarginine concentrations as well as lower concentrations of N-Terminal pro B-type natriuretic peptide-1 (NT-pro-BNP) and galectin-3.
Table 1.
Baseline characteristics of participants of the Ludwigshafen Risk and Cardiovascular Health (LURIC) study stratified by baseline serum branched-chain amino acid (BCAA) concentrations (N = 2236)
| Variable | Quartiles of serum BCAA sum |
p Value | |||
|---|---|---|---|---|---|
| 1st quartile (n = 559) < 398.7 μmol/L | 2nd quartile (n = 559) 398.7–462.5 μmol/L | 3rd quartile (n = 559) 462.5–528.1 μmol/L | 4thquartile (n = 559) > 528.1 μmol/L | ||
| BCAA sum, μmol/L | 346 ± 42.4 | 432 ± 18.2 | 494 ± 19.0 | 597 ± 58.7 | – |
| Leucine, μmol/L | 102 ± 14.2 | 129 ± 9.2 | 147 ± 12.2 | 179 ± 20.8 | <0.001 |
| Isoleucine, μmol/L | 53 ± 9.1 | 66 ± 7.7 | 75 ± 9.0 | 91 ± 12.4 | <0.001 |
| Valine, μmol/L | 191 ± 26.4 | 238 ± 14.6 | 272 ± 15.1 | 326 ± 34.7 | <0.001 |
| Age (years) | 62.4 ± 11.1 | 62.0 ± 11.3 | 63.0 ± 10.6 | 62.6 ± 10.2 | 0.294 |
| Sex (male), % | 53.1 | 71.0 | 74.4 | 81.9 | <0.001 |
| Diabetes mellitus, % | 30.6 | 27.9 | 37.9 | 54.4 | <0.001 |
| Hemoglobin A1c, mmol/mol | 38.8 (35.5–44.3) | 39.9 (36.6–45.4) | 42.1 (36.6–48.6) | 44.3 (38.8–54.1) | <0.001 |
| Fasting glucose, mmol/L | 4.9 (4.4–5.4) | 4.9 (4.5–5.7) | 5.1 (4.6–5.8) | 5.4 (4.8–6.9) | <0.001 |
| Fasting C-peptide, mg/dL | 2.8 ± 2.1 | 2.8 ± 1.5 | 2.8 ± 1.6 | 2.9 ± 1.8 | 0.599 |
| HOMA-IR | 1.8 (1.1–2.9) | 1.9 (1.2–3.0) | 2.2 (1.4–3.6) | 2.9 (1.8–4.7) | <0.001 |
| Oral antidiabetic treatment, % | 4.1 | 5.5 | 8.9 | 14.7 | <0.001 |
| Insulin therapy, % | 3.9 | 4.7 | 5.4 | 7.0 | 0.020 |
| BMI, kg/m2 | 26.2 ± 4.0 | 26.8 ± 3.8 | 27.7 ± 3.8 | 28.3 ± 3.8 | 0.761 |
| LDL-cholesterol, mmol/L | 3.0 (2.4–3.6) | 2.9 (2.5–3.5) | 2.9 (2.4–3.5) | 2.9 (2.3–3.5) | 0.894 |
| HDL-cholesterol, mmol/L | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.139 |
| Triglycerides, mmol/L | 1.4 (1.1–2.0) | 1.6 (1.2–2.1) | 1.7 (1.3–2.3) | 1.9 (1.4–2.6) | <0.001 |
| Lipid-lowering therapy, % | 38.6 | 46.0 | 49.6 | 51.9 | <0.001 |
| Adiponectin, μM | 10.9 (7.7–16.9) | 8.6 (6.1–12.9) | 7.8 (5.4–11.3) | 6.7 (4.9–9.4) | <0.001 |
| Hypertension, % | 72.3 | 70.1 | 74.4 | 75.5 | 0.096 |
| Antihypertensive medication, % | 83.5 | 84.6 | 88.0 | 90.0 | <0.001 |
| Systolic blood pressure, mm Hg | 140 ± 24 | 140 ± 24 | 143 ± 23 | 144 ± 24 | 0.610 |
| Diastolic blood pressure, mm Hg | 80 ± 12 | 80 ± 11 | 81 ± 11 | 82 ± 12 | 0.893 |
| Mean heart rate, bpm | 70 ± 13 | 68 ± 12 | 67 ± 11 | 69 ± 12 | 0.006 |
| Physical activity: inactive, % | 9.5 | 7.2 | 9.7 | 6.1 | |
| Current smokers, % | 21.3 | 19.5 | 16.5 | 17.4 | 0.044 |
| Coronary artery disease, % | 62.7 | 68.1 | 74.4 | 73.3 | <0.001 |
| Previous myocardial infarction, % | 37.6 | 40.4 | 44.9 | 46.0 | 0.001 |
| Acute coronary syndrome, % | 24.3 | 27.5 | 32.4 | 35.2 | <0.001 |
| NYHA classification | 0.213 | ||||
| NYHA I | 45.1 | 53.8 | 49.9 | 48.5 | |
| NYHA II | 31.3 | 26.3 | 29.3 | 32.4 | |
| NYHA III | 19.5 | 16.1 | 18.1 | 15.2 | |
| NYHA IV | 4.1 | 3.8 | 2.7 | 3.9 | |
| Left ventricular ejection fraction, % | 55.2 ± 18.8 | 57.3 ± 17.2 | 58.7 ± 17.4 | 58.5 ± 16.6 | 0.026 |
| NT-pro-BNP, pg/mL | 394 (130.0–1499.0) | 303 (104.0–949.5) | 270 (108.0–629.0) | 264 (93.0–664.0) | <0.001 |
| Galectin-3, ng/mL | 14.6 (11.5–19.0) | 13.8 (11.0–17.7) | 13.7 (11.3–17.0) | 13.5 (11.0–17.1) | 0.001 |
| Homoarginine, μmol/L | 2.3 ± 1.0 | 2.6 ± 1.1 | 2.7 ± 1.1 | 2.8 ± 1.0 | <0.001 |
| High-sensitivity-CRP, mg/L | 3.5 (1.2–8.9) | 3.1 (1.1–8.4) | 3.5 (1.3–8.5) | 3.6 (1.5–8.8) | 0.196 |
| eGFR, mL/min per 1.73 m2 | 82.5 ± 21.8 | 84.3 ± 19.9 | 82.6 ± 20.4 | 82.0 ± 19.2 | 0.848 |
| Hemoglobin, g/dL | 13.2 ± 1.6 | 13.7 ± 1.3 | 13.9 ± 1.4 | 14.3 ± 1.4 | 0.175 |
| De Ritis ratio | 0.87 (0.70–1.13) | 0.80 (0.63–1.00) | 0.75 (0.60–1.00) | 0.71 (0.60–0.89) | <0.001 |
Values are given as median (25th, 75th percentile), as mean with SD and as percentage for categorical data. ANOVA with P for trend was used for continuous, χ2 test for categorical variables.
Abbreviations: BCAA, branched-chain amino acids; eGFR estimated glomerular filtration rate.
Diabetic patients (481.2 ± 4.0 vs. 442.1 ± 1.0 μmol/L; p < 0.001) and patients with CAD (461.0 ± 1.2 vs. 440.7 ± 1.3 μmol/L; p < 0.001) had higher BCAA values compared with nondiabetic patients and those without CAD, respectively. In contrast, patients with New York Heart Association stage (NYHA) III/IV had lower concentrations of BCAA compared with those with NYHA I/II (458.7 ± 1.2 vs. 448.1 ± 1.2 μmol/L; p = 0.041).
Predictors of serum BCAA concentrations
In the overall cohort, adiponectin (β = −0.27), hemoglobin (β = 0.25), and C-peptide (β = −0.17; p < 0.001 for all) emerged as the strongest predictors of serum BCAA concentrations, whereas age, sex, BMI, ongoing antidiabetic, antihypertensive and/or lipid-lowering treatment, CAD, and NT-pro-BNP were not predictors (Table 2).
Table 2.
Predictors of serum BCAA concentrations determined by linear regression
| Overall (n = 2236) |
β-coefficient | No DM (n = 1392) |
β-coefficient | DM (n = 844) |
β-coefficient |
|---|---|---|---|---|---|
| (R2: 0.31) | (R2: 0.24) | (R2: 0.35) | |||
| Adiponectin (missing n = 67) | −0.27∗∗ | Adiponectin (missing n = 42) | −0.29∗∗ | Hemoglobin | 0.28∗∗ |
| Hemoglobin | 0.25∗∗ | Hemoglobin | 0.27∗∗ | Adiponectin (missing n = 25) | −0.23∗∗ |
| C-peptide (missing n = 188) | −0.17∗∗ | eGFR (missing n = 6) | −0.19∗∗ | Hemoglobin A1c | 0.17∗∗ |
| Hemoglobin A1c (missing n = 3) | 0.16∗∗ | HOMA-IR (missing n = 49) | 0.15∗∗ | Homoarginine (missing n = 4) | 0.15∗∗ |
| HOMA-IR (missing n = 74) | 0.16∗∗ | C-peptide (missing n = 118) | −0.14∗∗ | C-peptide (missing n = 70) | −0.13∗∗ |
| eGFR (missing n = 9) | −0.15∗∗ | Galectin-3 (missing n = 442) | −0.11∗∗ | Triglycerides | 0.13∗ |
| Galectin-3 (missing n = 728) | −0.10∗∗ | hsCRP (missing n = 4) | 0.10∗∗ | Mean heart rate | −0.08∗∗ |
| Triglycerides | 0.10∗∗ | Current smokers | −0.06 | HOMA-IR (missing n = 25) | 0.08∗∗ |
| hsCRP (missing n = 6) | 0.10∗∗ | Homoarginine (missing n = 6) | 0.04 | hsCRP (missing n = 2) | 0.07∗∗ |
| Homoarginine (missing n = 10) | 0.09∗∗ | ||||
| Current smokers | −0.06∗∗ | ||||
| Mean heart rate | −0.05∗ |
Excluded variables: age, sex, BMI, fasting glucose, oral antidiabetic treatment, insulin therapy, HDL-cholesterol, LDL-cholesterol, lipid-lowering treatment, De Ritis ratio, physical activity, arterial hypertension, antihypertensive treatment, acute coronary syndrome, coronary artery disease, and NT-pro-BNP. Abbreviations: BMI, body-mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoproteins; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity-CRP; LDL, low-density lipoproteins; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide-1; significance levels: ∗∗ (0.01), ∗ (0.05).
Repeating the analysis in the subgroup of participants with LVEF measurement at baseline, yielded similar results but without showing an association between LVEF and serum BCAA concentrations.
Associations between baseline serum BCAA concentration and outcomes
During a median follow-up of 10.5 years (25th–75th percentile: 8.3–11.0), corresponding to 22,648 person-years, 450 (20.1%) patients experienced fatal cardiovascular and 265 (11.9%) had fatal non-cardiovascular events, respectively. Among specific cardiovascular events, we recorded 174 (7.8%) SCDs, 79 (3.5%) fatal myocardial infarctions, 108 (4.8%) deaths due to HF, and 89 deaths due to other cardiovascular causes. Fatal non-cardiovascular events comprised fatal infection 54 (2.4%), fatal cancer 104 (4.7%), and other causes of death 93 (4.2%).
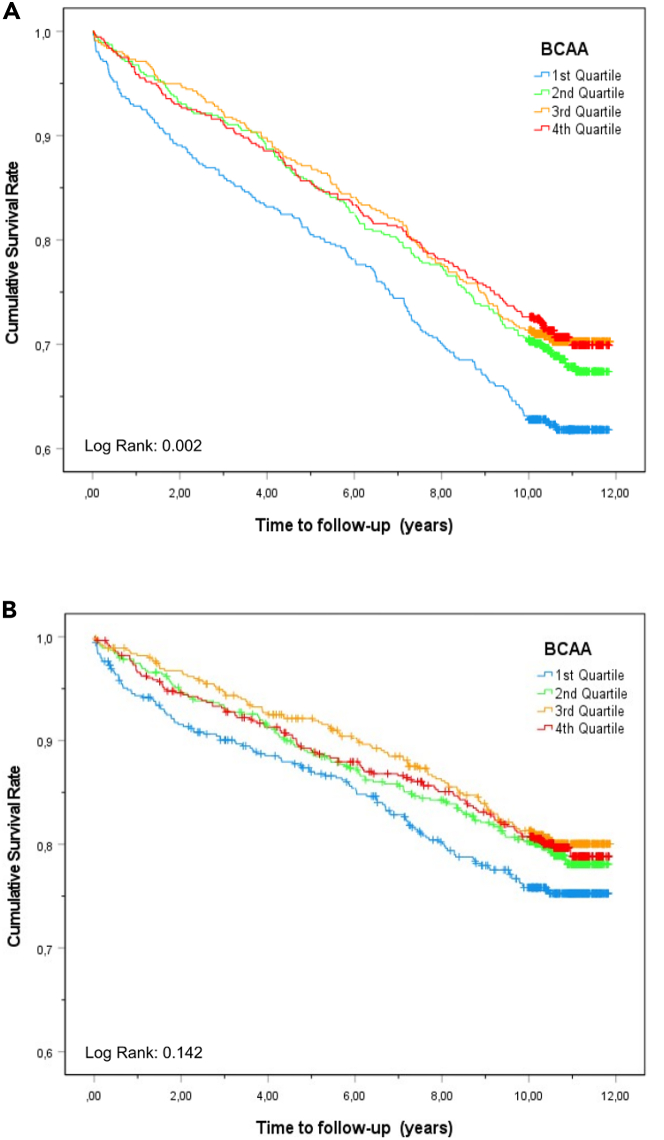
Kaplan-Meier curves for overall and cardiovascular mortality according to quartiles of serum BCAA concentrations are shown in Figures 1A and 1B. Decreasing quartiles of baseline serum BCAA concentrations were strongly associated with the risk of subsequent fatal cardiovascular and fatal non-cardiovascular events (Table 3), all three BCAAs (leucine, isoleucine, and valine) are independently associated with mortality (Figures S1–S3). Participants in the lowest quartile of serum BCAA concentrations had a 2.2 times higher risk of overall death and a 2.1 times higher risk of death from cardiovascular causes in comparison with the highest quartile, after adjusting for demographics and established cardiovascular risk factors. Additional consideration of ongoing antihypertensive medication, eGFR, hemoglobin, and liver function as potential confounders did not materially attenuate these associations (i.e., fourth vs. first BCAA quartile for all-cause mortality: Hazard ratio (HR) 1.98 [95% confidence intervals (CI), 1.58–2.49] and cardiovascular mortality: HR 1.95 [95% CI 1.46–2.60]).
Figure 1.
Kaplan-Meier curve for the time to all-cause and cardiovascular mortality according to serum branched-chain amino acid (BCAA) concentrations in quartiles at baseline
(A) Kaplan-Meier curve for the time to all-cause mortality according to serum BCAA concentrations in quartiles at baseline.
(B) Kaplan-Meier curve for the time to cardiovascular mortality according to serum BCAA concentrations in quartiles at baseline.
Table 3.
Hazard ratios (95% CI) for ten-year all-cause and cardiovascular mortality and cardiovascular death
| Quartiles (n) | BCAA Quartiles |
Linear Model |
||||
|---|---|---|---|---|---|---|
| 1st quartile (n = 559) | 2nd quartile (n = 559) | 3rd quartile (n = 559) | 4th quartile (n = 559) | Per SD BCAA | p Value | |
| Events (cardiovascular events) | 212 (127) | 176 (112) | 165 (103) | 162 (108) | ||
| Person-years | 5796 | 5843 | 5156 | 5853 | ||
| Incidence Rate for death/cardiovascular death per 100 person-years | 3.66 (2.19) | 3.01 (1.92) | 3.20 (2.00) | 2.77 (1.85) | ||
| All-cause mortality | ||||||
| Event rate (%) | 37.9 (212/559) | 31.5 (176/559) | 29.5 (165/559) | 29 (162/559) | – | – |
| Crude | 1.40 (1.14–1.72) | 1.09 (0.88–1.35) | 1.01 (0.82–1.26) | 1.00 (ref) | 0.87 (0.80–0.93) | <0.001 |
| Model 1 | 1.60 (1.30–1.97) | 1.18 (0.95–1.46) | 1.00 (0.80–1.23) | 1.00 (ref) | 0.82 (0.76–0.89) | <0.001 |
| Model 2 | 1.67 (1.34–2.08) | 1.28 (1.02–1.60) | 1.00 (0.80–126) | 1.00 (ref) | 0.82 (0.75–0.89) | <0.001 |
| Model 3 | 1.98 (1.58–2.49) | 1.43 (1.14–1.80) | 1.13 (0.91–1.42) | 1.00 (ref) | 0.76 (0.69–0.82) | <0.001 |
| Cardiovascular mortality | ||||||
| Event rate (%) | 23.0 (127/551) | 20.2 (112/554) | 18.5 (103/556) | 19.4 (108/558) | – | – |
| Crude | 1.26 (0.98–1.63) | 1.04 (0.80–1.36) | 0.95 (0.73–1.24) | 1.00 (ref) | 0.91 (0.82–1.00) | 0.04 |
| Model 1 | 1.43 (1.10–1.86) | 1.12 (0.86–1.47) | 0.93 (0.71–1.22) | 1.00 (ref) | 0.86 (0.78–0.95) | <0.001 |
| Model 2 | 1.49 (1.12–1.97) | 1.24 (0.94–1.65) | 0.90 (0.68–1.20) | 1.00 (ref) | 0.73 (0.66–0.82) | <0.001 |
| Model 3 | 1.95 (1.46–2.60) | 1.45 (1.09–1.91) | 1.08 (0.82–1.43) | 1.00 (ref) | 0.76 (0.69–0.85) | <0.001 |
| Quartiles (n) | Isoleucine Quartiles |
Linear Model |
||||||
|---|---|---|---|---|---|---|---|---|
| 1st quartile (n = 559) | 2nd quartile (n = 559) | 3rd quartile (n = 559) | 4th quartile (n = 559) | Per SD Isoleucine | p Value | |||
| Events (cardiovascular events) | 196 (118) | 175 (108) | 181 (113) | 163 (111) | ||||
| Person-years | 5796 | 5843 | 5156 | 5853 | – | – | ||
| Incidence Rate for death/cardio-vascular death per 100 person-years | 3.38 (2.04) | 3.00 (1.85) | 3.51 (2.19) | 2.78 (1.90) | – | – | ||
| All-cause mortality | ||||||||
| Event rate (%) | 35.1 (196/559) | 31.3 (175/559) | 32.4 (181/559) | 29.2 (163/559) | – | – | ||
| Crude | 1.24 (1.00–1.52) | 1.07 (0.86–1.32) | 1.11 (0.90–1.38) | 1.00 (ref) | 0.93 (0.87–1.01) | 0.08 | ||
| Model 1 | 1.36 (1.10–1.68) | 1.14 (0.92–1.42) | 1.16 (0.94–1.43) | 1.00 (ref) | 0.90 (0.83–0.98) | 0.01 | ||
| Model 2 | 1.79 (1.42–2.26) | 1.40 (1.12–1.76) | 1.28 (1.03–1.60) | 1.00 (ref) | 0.80 (0.73–0.87) | <0.001 | ||
| Model 3 | 1.80 (1.42–2.27) | 1.47 (1.17–1.83) | 1.30 (1.05–1.62) | 1.00 (ref) | 0.80 (0.73–0.87) | <0.001 | ||
| Cardiovascular mortality | ||||||||
| Event rate (%) | 21.4 (118/559) | 19.6 (108/559) | 20.5 (113/559) | 20.1 (111/559) | – | – | ||
| Crude | 1.10 (0.85–1.42) | 0.97 (0.75–1.27) | 1.02 (0.79–1.33) | 1.00 (ref) | 0.98 (0.89–1.08) | 0.688 | ||
| Model 1 | 1.19 (0.91–1.55) | 1.03 (0.79–1.35) | 1.06 (0.81–1.38) | 1.00 (ref) | 0.95 (0.87–1.05) | 0.338 | ||
| Model 2 | 1.71 (1.28–2.28) | 1.32 (1.00–1.75) | 1.19 (0.91–1.56) | 1.00 (ref) | 0.83 (0.74–0.92) | <0.001 | ||
| Model 3 | 1.71 (1.28–2.28) | 1.39 (1.06–1.83) | 1.22 (0.93–1.59) | 1.00 (ref) | 0.82 (0.73–0.91) | <0.001 | ||
| Quartiles (n) | Valine Quartiles |
Linear Model |
||||
|---|---|---|---|---|---|---|
| 1st quartile (n = 559) | 2nd quartile (n = 559) | 3rd quartile (n = 559) | 4th quartile (n = 559) | Per SD Valine | p Value | |
| Events (cardiovascular events) | 219 (135) | 174 (107) | 159 (100) | 163 (108) | – | – |
| Person-years | 5796 | 5843 | 5156 | 5853 | – | – |
| Incidence Rate for death/cardiovascular death per 100 person-years | 3.78 (2.33) | 2.98 (1.83) | 3.08 (1.94) | 2.78 (1.85) | – | – |
| All-cause mortality | ||||||
| Event rate (%) | 39.2 (219/559) | 31.1 (174/559) | 28.4 (159/559) | 29.2 (163/559) | – | – |
| Crude | 1.45 (1.18–1,78) | 1.07 (0.86–1.33) | 0.97 (0.78–1.21) | 1.00 (ref) | 0.87 (0.80–0.94) | <0.001 |
| Model 1 | 1.71 (1.39–2.30) | 1.17 (0.94–1.45) | 1.02 (0.82–1.27) | 1.00 (ref) | 0.81 (0.75–0.88) | <0.001 |
| Model 2 | 2.22 (1.78–2.77) | 1.48 (1.18–1.85) | 1.14 (1.18–1.85) | 1.00 (ref) | 0.73 (0.67–0.79) | <0.001 |
| Model 3 | 2.01 (1.61–2.52) | 1.40 (1.14–1.78) | 1.11 (0.89–1.40) | 1.00 (ref) | 0.76 (0.70–0.83) | <0.001 |
| Cardiovascular mortality | ||||||
| Event rate (%) | 24.2 (135/559) | 19.1 (107/559) | 17.9 (100/559) | 19.3 (108/559) | – | – |
| Crude | 1.35 (1.05–1.74) | 1.00 (0.76–1.30) | 0.92 (0.70–1.21) | 1.00 (ref) | 0.90 (0.82–0.99) | 0.029 |
| Model 1 | 1.59 (1.23–2.05) | 1.09 (0.83–1.43) | 0.97 (0.74–1.27) | 1.00 (ref) | 0.85 (0.77–0.93) | <0.001 |
| Model 2 | 2.24 (1.70–2.95) | 1.42 (1.07–1.87) | 1.09 (0.83–1.44) | 1.00 (ref) | 0.73 (0.66–0.81) | <0.001 |
| Model 3 | 2.02 (1.53–2.68) | 1.37 (1.03–1.82) | 1.07 (0.81–1.41) | 1.00 (ref) | 0.77 (0.69–0.85) | <0.001 |
| Quartiles (n) | Leucine Quartiles |
Linear Model |
||||||
|---|---|---|---|---|---|---|---|---|
| 1st quartile (n = 559) | 2nd quartile (n = 559) | 3rd quartile (n = 559) | 4th quartile (n = 559) | Per SD Leucine | p Value | |||
| Events (cardiovascular events) | 218 (133) | 175 (111) | 167 (104) | 155 (102) | ||||
| Person-years | 5796 | 5843 | 5156 | 5853 | ||||
| Incidence Rate for death/cardiovascular death per 100 person-years | 3.76 (2.29) | 3.01 (1.90) | 3.2 (2.02) | 2.77 (1.74) | ||||
| All-cause mortality | ||||||||
| Event rate (%) | 39.0 (218/559) | 31.3 (175/559) | 29.9 (167/559) | 27.7 (155/559) | – | – | ||
| Crude | 1.53 (1.24–1.88) | 1.15 (0.93–1.43) | 1.09 (0.88–1.36) | 1.00 (ref) | 0.85 (0.79–0.92) | <0.001 | ||
| Model 1 | 1.66 (1.34–2.05) | 1.19 (0.96–1.47) | 1.05 (0.84–1.31) | 1.00 (ref) | 0.83 (0.76–0.89) | <0.001 | ||
| Model 2 | 2.09 (1.67–2.62) | 1.49 (1.19–1.87) | 1.18 (0.95–1.48) | 1.00 (ref) | 0.74 (0.68–0.81) | <0.001 | ||
| Model 3 | 1.98 (1.57–2.49) | 1.45 (1.16–1.82) | 1.19 (0.95–1.49) | 1.00 (ref) | 0.76 (0.70–0.83) | <0.001 | ||
| Cardiovascular mortality | ||||||||
| Event rate (%) | 23.8 (133/559) | 20.0 (111/559) | 18.7 (104/559) | 18.3 (102/559) | – | – | ||
| Crude | 1.42 (1.10–1.84) | 1.11 (0.85–1.45) | 1.03 (0.79–1.36) | 1.00 (ref) | 0.89 (0.81–0.98) | 0.016 | ||
| Model 1 | 1.52 (1.17–1.99) | 1.14 (0.87–1.49) | 0.99 (0.75–1.30) | 1.00 (ref) | 0.87 (0.79–0.96) | 0.006 | ||
| Model 2 | 2.10 (1.58–2.79) | 1.50 (1.13–1.99) | 1.12 (0.84–1.48) | 1.00 (ref) | 0.75 (0.68–0.84) | <0.001 | ||
| Model 3 | 1.99 (1.49–2.65) | 1.48 (1.12–1.96) | 1.14 (0.86–1.51) | 1.00 (ref) | 0.77 (0.69–0.86) | <0.001 | ||
Abbreviations: BMI, body-mass index; eGFR, estimated glomerular filtration rate; hemoglobin A1c, HbA1c; LDL, low-density lipoproteins.
Model 1: adjusted for age and sex.
Model 2: additionally adjusted for BMI, LDL-cholesterol, triglycerides, active smoking status, systolic blood pressure, daily physical activity, coronary artery disease, and HbA1c.
Model 3: additionally adjusted for intake of antihypertensive medication, eGFR, hemoglobin, and De Ritis ratio.
Analyzing specific causes of cardiovascular death, persons in the lowest quartile of BCAA had a 2.5-, 2.0-, and 2.1-fold higher risk for death due to MI, sudden cardiac death (SCD), and HF, respectively, compared with persons in the highest BCAA quartile. Analyses performed using BCAA as a continuous variable (per SD) showed a 25% decreased risk of all-cause and a 28% decreased risk of cardiovascular mortality for each increment per one SD BCAA in the fully adjusted model. Serum BCAA concentrations were associated with a decreased risk of incident fatal MI, death due to HF, and SCD (HR, 0.68; 95% CI, 0.52–0.89; HR, 0.76; 95% CI, 0.61–0.94; and HR, 0.75; 95% CI, 0.63–0.89 per one SD increment in BCAA, respectively) (Table 3).
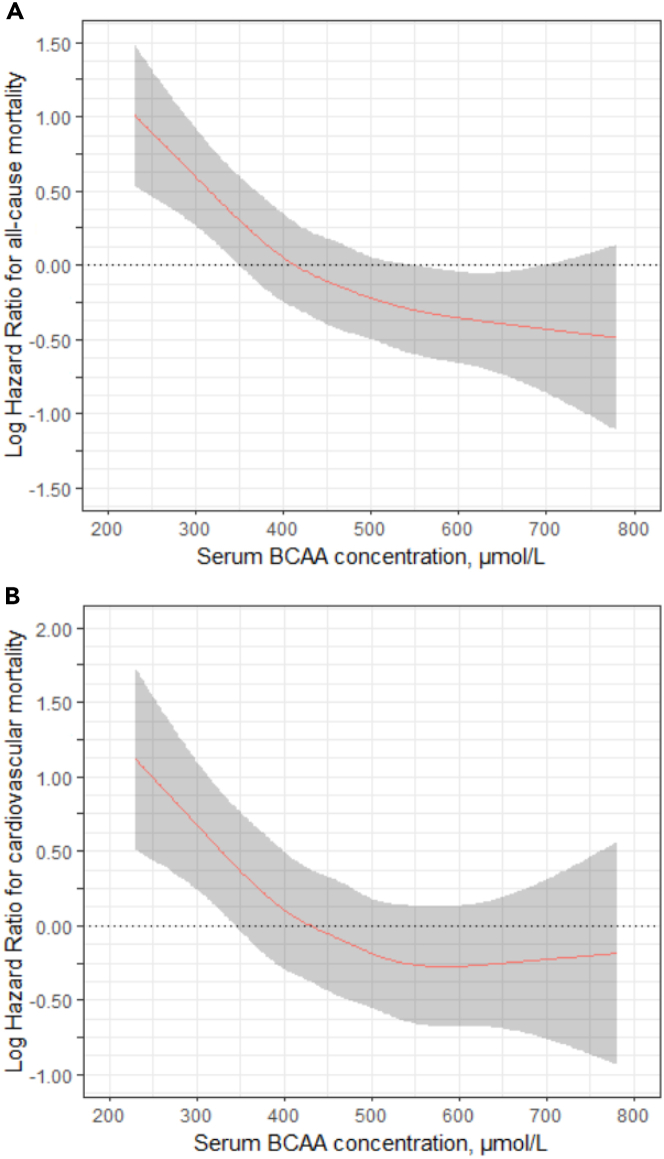
When serum BCAA concentration was considered as a continuous variable by calculating restricted cubic splines, a non-linear association of BCAA with all-cause (p = 0.005) and cardiovascular mortality risk (p = 0.01) was revealed (Figures 2A and 2B).
Figure 2.
Cubic spline graph depicting the association of continuous serum concentrations of branched-chain amino acids (BCAAs) with all-cause and cardiovascular mortality
(A) Cubic spline graph depicting the association of continuous serum concentrations of BCAAs with all-cause mortality. The model is adjusted for age, sex, BMI, LDL-cholesterol, triglycerides, active smoking status, systolic blood pressure, daily physical activity, coronary artery disease, hemoglobin A1c, intake of antihypertensive medication, eGFR, hemoglobin, and De Ritis ratio.
(B) Cubic spline graph depicting the association of continuous serum BCAA concentrations of BCAAs with cardiovascular mortality. The model is adjusted as in (A).
C-statistic, discrimination and reclassification
The additional consideration of BCAA to the fully adjusted model increased the area under the receiver operating characteristic curve (C-statistic) for the prediction of all-cause mortality and cardiovascular mortality from 0.716 to 0.724 and 0.711 to 0.718, respectively (Table 4).
Table 4.
Performance of risk prediction models with and without inclusion of BCAA
| C-statistics | AUC | NRI (95% CI) | |
|---|---|---|---|
| All-cause death | |||
| All univariate prediction | 0.716 | 0.748 (0.726–0.770) | Ref. |
| All univariate prediction. + BCAA | 0.724 | 0.756 (0.734–0.778) p =0.038 | 18.7 (9.65–31.3) |
| Cardiovascular death | |||
| All univariate prediction | 0.711 | 0.747 (0.722–0.773) | Ref. |
| All univariate prediction + BCAA | 0.718 | 0.752 (0.727–0.777) p = 0.237 | 18.1 (6.46–30.6) |
Abbreviations: BCAA, branched-chain amino acids; Harrell’s C (C statistics); AUC (Area under the curve and NRI (95% CI), net reclassification improvement Comparisons are adjusted to a baseline model according to Model 3 (see Table 3).
Reclassification as assessed by area under the curve (AUC) and net reclassification improvement metrics (NRI) was significantly improved with the addition of serum BCAA concentrations to the fully adjusted model for all-cause mortality, cardiovascular mortality, SCD, and death due to HF (Table 4).
BCAA concentration-associated cardiovascular mortality in various subgroups
To determine whether the association between serum BCAA concentrations and cardiovascular mortality was homogeneous across the cohort, we conducted subgroup analyses with formal testing for interactions (Table S1). A decrement per one SD decrease in BCAAs for cardiovascular mortality was stronger in participants with lower age, male sex, higher BMI, lower serum triglyceride concentrations, higher LDL-cholesterol, increasing severity of HF, higher NT-pro-BNP concentrations, and lower homoarginine concentrations. The associations between BCAA concentrations and risk of mortality persisted in patients with and without diabetes, respectively.
Discussion
We found strong associations between serum BCAA concentrations and clinical correlates of metabolic risk. Paradoxically, increased circulating concentrations of BCAAs were related to lower long-term risk of all-cause death and death from cardiovascular causes. The association between serum BCAA concentrations and risk of death persisted irrespective of prevailing obesity and/or diabetes. Furthermore, serum BCAA concentrations substantially improved the risk prediction model for cardiovascular mortality based on traditional cardiovascular risk factors. Our findings offer potentially new insights regarding the role of serum BCAA concentrations in the pathogenesis of cardiovascular diseases.
BCAA and metabolic risk factors
BCAAs contribute to the maintenance of β-cell mass and stimulate insulin release in pancreatic β-cells.23,24,25 The effect of BCAA and particularly of leucine on insulin secretion is elusive, as mTOR, which mediates leucine-induced insulin signaling by triggering auto-phosphorylation of insulin substrate 1 (IRS-1), creates negative feedback loops by inhibiting IRS-1 and subsequently insulin secretion26 This notion is supported by Wang et al., who reported that BCAA concentrations predicted incident diabetes independent of stimulatory effects on insulin secretion.17
Elevated concentrations of circulating BCAA concentrations induce skeletal muscle insulin resistance by inhibition of glucose transport and phosphorylation.27 Large-scale metabolomic profiling studies documented that high circulating BCAA concentrations are related to the development of obesity,16,17,28,29,30,31 impaired glucose tolerance, insulin resistance, and incident diabetes. Our study highlights the associations between increasing BCAA concentrations and aggravated insulin resistance resulting in worsened glycemic controls in participants with and without diabetes.
High adiponectin concentrations predict increased risk of HF and cardiovascular mortality in the general population and in patients at cardiovascular risk.32,33,34 Studies in rodents indicate modulatory effects of leucine on adiponectin concentrations.35,36 The current study revealed a strong inverse relationship between serum adiponectin and BCAA concentrations in diabetic and nondiabetic participants. Adiponectin resistance at the level of the skeletal muscle is frequently found in the setting of chronic HF.37 Hence, impaired energy formation due to lower fatty acid oxidation might hypothetically favor BCAAs as an alternative energy substrate but may result in decreased protein synthesis contributing to a worse cardiovascular outcome observed in patients with lower BCAA concentrations.
BCAAs and risk of mortality
Increased BCAA serum concentration is associated with a lower risk of overall and cardiovascular mortality independent of related metabolites. While the risk of all-cause mortality steadily declines with increasing serum concentration of BCAAs, this is not the case for cardiovascular mortality, where the risk only declines up to a concentration of approximately 500 μmol/L (Figure 2). Besides cardiovascular death, the main causes of death were cancer and fatal infections. When looking at specific causes of death in study participants with BCAA concentrations below or above 500 μmol/L, we noticed slightly lower percentages of patients that died from stroke (2.4% vs. 1.9%), fatal infection (2.6% vs. 2.2%), cancer (4.8% vs. 4.5%), and other causes of death (4.5% vs. 3.6%), while the percentages for cardiac death were comparable (Table S2). A role of the dysregulated BCAA metabolism in cancer has been described.38 This highlights the potential role of BCAA as an eminent factor of overall and cardiovascular prognosis. In our analysis, each of the individual BCAAs was inversely associated with mortality. That differs from the results of Deelen et al. who reported in their meta-analysis a reduced risk only for leucine and valine but an increased risk for higher levels of isoleucine.22
There are several mechanisms by which BCAA may be related to the development of cardiovascular diseases, in particular HF and arrhythmia. The myocardium is a known site for multiple BCAA-mediated effects. Although inappropriately elevated concentrations of BCAAs contribute to the development of dilated and hypertrophic cardiomyopathy, insufficient concentrations of BCAAs may also play an important role in the development and progression of cardiovascular diseases.39
The catabolism of BCAAs is tightly regulated via oxidative decarboxylation by a mitochondrial branched-chain α-ketoacid dehydrogenase complex (BCKDC), which is highly expressed in the heart.27,28 The down-regulation of BCKDC and the subsequent branched-chain a-keto-acid accumulation in hypertrophic and failing hearts supports a higher demand of BCAAs in the setting of myocardial ischemia and dysfunction.40 However, restoring BCAA catabolic flux in hearts through the application of BCKDC inhibitors are a promising new therapeutic target for the treatment of HF.41
In contrast, a combined marked increase in BCAA catabolism (e.g., due to inflammation) might play a pivotal role in promoting myocardial muscle wasting and subsequent HF development.42 In fact, BCAA infusion in postabsorptive patients with CAD resulted in increased protein synthesis and BCAA uptake in the myocardium independent of prevailing cardiac output, and myocardial oxygen consumption.43 During a median follow-up of 3.1 years Shah et al. revealed in CAD risk patients a decreasing risk for fatal MI with increasing concentrations of combined BCAA and related catabolites.20 Notably, we extend these findings by documenting that BCAA concentrations without conjointly considering related metabolites predict long-term mortality even under consideration of established cardiovascular risk factors. A restricted cubic spline regression analysis, however, indicates a U-shaped association of serum BCAA concentrations with mortality.
Cardiac mTOR activity is strongly involved in myocardial hypertrophy.42,44,45,46 Notably, we found inverse correlations between serum BCAA concentrations and galectin-3, an indicator of cardiac fibrosis, particularly in diabetic patients. Besides fibrosis, bioenergen defects are established critical contributors to the disease progression of HF. Hence, circulating BCAAs might serve as a potential alternative energy substrate, particularly in the setting of myocardial dysfunction and ischemia.47 We observed a strong positive correlation between homoarginine, an indicator of myocardial energy metabolism, and serum BCAA concentrations and a stronger impact on BCAA-related mortality risk in patients with lower homoarginine concentrations.48,49
Finally, animal studies identified cigarette smoke as a potential factor that decreases BCAA concentrations both in plasma and skeletal muscle.50
This is in line with both the inverse relationship between active smoking status and serum BCAA concentrations and the significant interaction between smoking and BCAA-related risk of mortality, respectively, documented in the present study.
Lower hemoglobin concentrations are an independent predictor of incident HF and adverse mortality.51 In elite rugby players, supplementation of an amino acid mixture containing BCAAs resulted in significantly increased hemoglobin concentrations.52 With this in line, we observed a strong positive correlation between hemoglobin and serum BCAA concentrations and revealed a strong interaction between BCAA-related cardiovascular mortality risk and hemoglobin. Whether BCAA supplementation reduces cardiovascular risk by increasing hemoglobin concentrations remains to be determined. However, the effect is unlikely to be related to the erythropoiesis alone, as previous clinical trials testing the hypothesis of increasing hemoglobin in the absence of severe chronic kidney diseases were unable to show any clinical benefit.53
Limitations of the study
Several limitations of the present investigation merit consideration. Our cohort was primarily composed of elderly of European ancestry referred to for coronary angiography; thus, generalizations to other ethnicities and younger individuals cannot be made. Furthermore, despite extensive adjustments of the statistical models, we cannot exclude residual confounding. The observational design of our study precludes conclusions with regard to causal relationships.
Measurements of muscle and fat-free mass were not available. As increased leucine intake prevented excessive protein waste in conditions of large weight loss, we cannot exclude the possibility, that the association between serum BCAA concentrations and mortality is significantly related to wasting.54 However, the additional consideration of hemoglobin and BMI did not significantly attenuate the relationship between serum BCAA concentrations and mortality risk.
In view of the shared anabolic and catabolic pathways of BCAA metabolism, we conducted the present study by using the sum of leucine, isoleucine, and valine. It has been suggested that the simultaneous supplementation of all three BCAAs is necessary to reveal the potential of leucine-mediated effects.55 Dietary pattern might not have strongly impacted our findings as it was previously indicated that the association between BCAAs and insulin resistance and risk for diabetes was not materially influenced by dietary protein intake.17,56 A serious limitation of this study is that dietary data are not available.
An important strength of the current investigation is the high in-depth clinical and biochemical characterization of the patients. Moreover, multiple established risk factors representing key pathways implicated in the pathogenesis of cardiovascular diseases were available for conjoint and comparative multivariate analyses. Finally, our results are strengthened by the high number of participants eligible for mortality analyses and a considerable number of deaths within a long-term follow-up of almost ten years.
Ethical statement
Due to the articles of Ludwigshafen Risk and Cardiovascular Health (LURIC) Study GmbH, which needs to acknowledge the German Data Protection Act and the consent given by the study participants, data cannot be released to the public domain. The exploitation of the LURIC Study database is governed by the articles of the LURIC Study GmbH (non-profit LLC), registered under number HRB 7668 at the commercial registry of Freiburg in Breisgau, Germany. According to the articles of the organization, data may be made available to researchers upon request and approval. This procedure implies that data cannot be released to the public without a formal agreement and makes sure that rules of good scientific practice are followed and that credit is given to the people who have been in charge of the design and the organization of the study. Interested researchers are invited to address their request or proposal to Kai Grunwald (Kai.Grunwald@weitnauer.net) or to the Principal Investigator of the LURIC Study, Winfried März (winfried.maerz@luric-online.de). Finally, the authors confirm that they accessed these data upon approval by LURIC and that all other researchers can access the data in the same manner the authors did. The ethics approval number is (LURIC, #837.255.97(1394)).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| NT-pro-BNP | electro-chemiluminescence on an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim, Germany) | N/A |
| hsCRP | N Latex CRP mono/Behring nephelometer II (Dade Behring, Marburg, Germany) | N/A |
| Triglycerides | WAKO (Neuss, Germany) | N/A |
| LDL-cholesterol | WAKO (Neuss, Germany) | N/A |
| HDL-cholesterol | WAKO (Neuss, Germany) | N/A |
| Fasting plasma glucose | GLU/Hitachi 717 (Roche, Mannheim, Germany) | N/A |
| Glycated hemoglobin A1c (HbA1c) | Hemoglobin A1c UNI-MATE 5 (Hoffmann-La Roche, Grenzach-Whylen, Germany) | N/A |
| C-peptide | AIA-Pack C-Peptide/AIA 1200 (Eurogenetics Germany, Eschborn, Germany) | N/A |
| Serum aspartate transaminase (AST) | AST / Hitachi 717 (Roche, Mannheim, Germany) | N/A |
| Alanine transaminase (ALT) | ALT / Hitachi 717 (Roche, Mannheim, Germany) | N/A |
| Adiponectin | ELISA (Biovendor Laboratory Medicine, Brno, Czech Republic) | N/A |
| Homoarginine | Reversed phase high-performance liquid chromatography, Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria | N/A |
| Galectin-3 | ARCHITECT analyzer (Abbott Diagnostics, Abbott Park, IL, USA) | N/A |
| Hemoglobin | Technicon H-1 (Technicon, Bad Vilbel, Germany) and Advia 120 (Bayer Diagnostics (Tarrytown,LA, USA) | N/A |
| Software and algorithms | ||
| SPSS Statistics Version 27.0.0.0 | IBM, Chicago, IL, USA | https://www.ibm.com/products/spss-statistics |
| R version 4.1.1 | R Foundation for Statistical Computing, Vienna, Austria | https://www.r-project.org |
| Deposited data | ||
| code availability for BCAA Figures only ISCIENCE-D-22-03679R2.R | http://dx.doi.org/10.17632/rjnc3x6yyr.1 | |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, MD Prof. Dr. Winfried März (winfried.maerz@synlab.com)
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Study population
The LURIC study is an ongoing prospective cohort study designed to investigate environmental and endogenous risk factors for cardiovascular diseases. Recruitment of patients was performed consecutively on regular working days. Inclusion criteria were availability of a coronary angiogram, Caucasians of German ancestry to limit genetic heterogeneity, and clinical stability, with the exception of acute coronary syndromes (ACS). Coronary angiography was performed as part of the clinical routine and indications were mainly chest pain or non-invasive tests consistent with myocardial ischemia. Participants with a history of malignancy within the past five years, or any predominant non-cardiac disease were excluded from the study. The LURIC study was approved by the ethics committee at the Landesärztekammer Rheinland-Pfalz (Mainz, Germany). Informed written consent was obtained from all participants.
Samples of 3316 Caucasian patients hospitalized for coronary angiography were collected from June 1997 to May 2001.57 Concentrations of leucine, isoleucine and valine were determined in a random sample of 2,236 participants (males, n = 1568 (mean age 62.6 years); females, n = 668 (mean age 65.1 years), who did not significantly differ from participants without BCAA measurement at baseline regarding to age (p = 0.147), sex (p = 0.404), and prevalence of ACS (p = 0.127). CAD was defined as the occurrence of 1, 2 or 3 vessel disease. Angiograms were analyzed as described previously.57 In addition, in 700 participants, LVEF, which showed a high correlation with semiquantitative left ventricular function assessment (Spearman’s correlation coefficient −0.834, p < 0.001), was calculated from the right anterior oblique view during catheterization.
Method details
Blood sampling and biomarker measurement
Blood samples were drawn (in the supine position) by venous puncture in the morning before cardiac catheterization, after participants had fasted overnight. The blood was allowed to clot at room temperature, and serum was obtained by centrifugation at 3220 × g for 15 min. Remaining blood samples were shock frozen in liquid nitrogen and stored at −80°C for later use. Routine laboratory parameters were immediately measured on a daily basis as previously described.57
Concentrations of leucine, isoleucine and valine were measured in serum by ion-exchange chromatography followed by post-column continuous reaction with ninhydrin after about one year of storage at −80° and had not been thawed before.58 Within-day and between-day coefficients of variation at different concentrations were below 9% throughout.
The measurements of selected biomarkers, representing key pathways implicated in the pathogenesis of CAD, HF and diabetes mellitus [N-terminal pro-B-type natriuretic peptide-1 (NT-pro-BNP), high-sensitivity C-reactive protein (hsCRP), triglycerides, LDL-cholesterol, HDL-cholesterol, fasting plasma glucose, glycated hemoglobin A1c (HbA1c), C-peptide, hemoglobin, serum aspartate transaminase (AST) and alanine transaminase (ALT)] have been described.57 All variables have finally been converted into SI units.
Adiponectin serum concentrations were measured by ELISA (Biovendor Laboratory Medicine, Brno, Czech Republic). Homoarginine was measured using reverse phase high-performance liquid chromatography. Galectin-3 concentrations were measured in plasma samples taken at baseline and stored at −80°C until analysis on an ARCHITECT analyzer (Abbott Diagnostics, Abbott Park, IL, USA). The De Ritis ratio was calculated by dividing AST through ALT and used as an index of liver damage.
Clinical definitions
A detailed questionnaire was used to collect a range of demographic characteristics from the study participants, including lifestyle factors such as smoking and physical activity. Daily physical activity was recorded using an eleven-point scale (i.e., key points were as follows: 1, bed rest, 2, mostly supine, 3, not very active, 6, usual office work, 9, heavy work or sports, and 11, extremely sportive) and was categorized into three groups according to “below average” (not very active, ranged from daily physical activity 1 to 3), “average” (usual office work, ranged from daily physical activity 4 to 8) and “above average” (heavy work or sports, ranged from daily physical activity ≥9).
Diabetes mellitus was diagnosed according to the revised recommendations of the American Diabetes Association.59 The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.
BP was measured with an automated oscillometric device (Omron MX4, Omron Healthcare, Hamburg, Germany) while in the supine position for at least 10 min. Five consecutive measures of systolic and diastolic BP were taken 30 s apart, with the average obtained from the last two. Hypertension was diagnosed (i) if the systolic and/or diastolic BP was 140 and/or 90 mm Hg or higher or (ii) if individuals were on antihypertensive medication (e.g., angiotensin-converting enzyme (ACE)-inhibitors, angiotensin-II type-1 (AT1) receptor antagonists, beta-blockers, calcium channel blockers, and/or diuretics).
The eGFR was calculated using the Chronic Kidney Disease Epidemiologic Collaboration formula.60
Severity of HF was assessed using the NYHA classification.
Follow-up on mortality
No patient was lost to follow-up. Information on vital status was obtained from local community registries. During a median follow-up of 10.5 (8.3–11.0) years, 715 (32.0%) patients died. Death certificates were reviewed to classify the deceased into those who died from cardiovascular and non-cardiovascular events. Death from cardiovascular causes included SCD, fatal MI, death due to HF, death after intervention to treat CAD, stroke, and other deaths due to heart disease. The cause of death of 23 participants was unknown. Two experienced physicians that were masked to any data of the study participants except for information regarding death certificates independently classified the causes of death. In the case of disagreement concerning classification, it was discussed, and the final decision was made by one of the principal investigators of LURIC (W.M.), who was also masked to any data except for death certificates. 450 participants (20.1%) died from cardiovascular causes.
Quantification and statistical analysis
Participants were divided into quartiles according to their baseline serum BCAA (sum of leucine, isoleucine and valine) concentrations. We also conducted analyses for the individual amino acids leucine, valine and isoleucine.
Baseline characteristics were compared across BCAA quartiles using analysis of variance (ANOVA) for continuous variables and the χ2 trend test for categorical variables. Normally distributed continuous variables were reported as means (±SD), while variables with nonnormal distribution were reported as medians with interquartile ranges.
Categorical variables were presented as proportions. For parametric procedures, all non-normally distributed variables were logarithmically (log-10) transformed, when appropriate.
Multivariate linear regression models were constructed to assess the relation between BCAA and cardiometabolic risk factors in the overall cohort and in patients with and without diabetes, respectively.
The models were adjusted for age, sex, body-mass index (BMI), HbA1c, fasting C-peptide, fasting glucose, HOMA-IR, oral antidiabetic treatment, insulin therapy, HDL-cholesterol, LDL-cholesterol, serum triglycerides, lipid-lowering treatment, adiponectin, hemoglobin, De Ritis ratio, hsCRP, smoking status, eGFR, physical activity, arterial hypertension, antihypertensive treatment, ACS, CAD, galectin-3, NT-pro-BNP, homoarginine and mean heart rate.
A Kaplan-Meier curve was employed to calculate the cumulative event rates according to quartiles of BCAA, which were compared by use of a trend test.
The association between serum BCAA concentrations and outcomes was examined using Cox proportional-hazard regression models reporting hazard ratios (HR) with 95% CI. The final step derived from backward procedures is presented. Besides a crude model, the additional models were adjusted for the following clinical risk factors: age and sex (model 1), age, sex, BMI, LDL-cholesterol, triglycerides, active smoking, systolic BP, daily physical activity, CAD, HbA1c (model 2), intake of antihypertensive drugs, eGFR, hemoglobin and De Ritis ratio (model 3).
To increase statistical power, we additionally modeled BCAA concentrations continuously, estimating changes in mortality per 1 SD. The proportional-hazard assumption was estimated by using Schoenfeld's tests and by visual inspection.
Restricted cubic splines were used in fully adjusted Cox models to evaluate whether there is a dose response or non-linear association of BCAA as a continuous variable with all-cause and cardiovascular mortality risk. Five knots were pre-specified located at the minimum, 25th, 50th, 75th percentile and maximum.
To test for effect modification, fully adjusted Cox proportional-hazard analyses (according to model 3) were created that included interaction terms between BCAA and age, sex, body-mass index (BMI), HOMA-IR, diabetes mellitus, HbA1c, CAD, arterial hypertension, triglycerides, LDL-cholesterol, NYHA, NT-pro-BNP, adiponectin, galectin-3, smoking status, homoarginine, hemoglobin and De Ritis ratio.
Subcategorization was done by stratifications according to (i) the median of age, HOMA-IR, HbA1c, serum triglycerides, LDL-cholesterol, NT-pro-BNP, adiponectin, galectin-3, homoarginine, hemoglobin and De Ritis ratio; (ii) by BMI </> 25 kg/m2; and (iii) by sex, presence of diabetes, presence of CAD, NYHA classification I/II and III/IV, history of arterial hypertension and no/active smoking status.
The incremental utility of BCAAs was also evaluated by calculating changes in the C-statistic, AUC, and by NRI.
All P-values were two-sided and values of p < 0.05 were considered statistically significant. Data were analyzed using SPSS Statistics Version 27.0.0.0 (IBM, Chicago, IL, USA) and R version 4.1.1 (http://www.r-project.org).
Acknowledgments
We thank the LURIC team involved in patient recruitment and sample and data handling and the laboratory staffs at the Ludwigshafen General Hospital, the Universities of Freiburg, Ulm, Heidelberg, and Graz.
Sources of funding: This work was supported by the 6th Framework Program (integrated project Bloodomics, grant LSHM-CT-2004-503485) and the 7th Framework Program (integrated project Atheroremo, grant agreement number 201668, RiskyCAD, grant agreement number 305739) of the European Union, by the INTERREG IV Oberrhein Program (Project A28, Genetic mechanisms of cardiovascular diseases) with support from the European Regional Development Fund (ERDF) and the Wissenschaftsoffensive TMO, and by the German Ministry for Education and Research, project e:AtheroSysMed (Systems medicine of coronary heart disease and stroke), grant number 01ZX1313A-K. The work of SL, APM, MEK, and WM was also supported by the German Ministry for Education and Research as part of the Competence Cluster for Nutrition and Cardiovascular Health (nutriCARD) Halle-Jena-Leipzig (grant numbers 01EA1411A and 01EA1808A).
Author contributions
Conceptualization, Methodology, Software: A.T., W.M., S.L., and A.P.M.; Data curation: G.E.D. and W.M.; Formal analysis: A.P.M. and M.E.K.; Writing- Original draft: A.P.M., W.M., A.T., and SL; Critical review of the manuscript: S.L., A.M., S.P., H.S., G.E.D., M.E.K., B. K. K., B.P., M.R. G., H.B., D. v. L., B.M., H.T., A.F-H., W.M., and A.T.; Resources: W.M., G.E.D., M.E.K., and H.S.; Visualization, Investigation: A.P.M., S.L., W.M., and A.T.; Supervision: S.L. and W.M.; Software, Validation: A.P.M. and M.E.K.
Declaration of interests
Marcus E. Kleber and Winfried März are employed with SYNLAB Germany.
The other authors do not report any conflict of interest.
Published: March 21, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106459.
Supplemental information
Data and code availability
-
•
The data reported in this study cannot be deposited in a public repository due to the articles of the LURIC Study GmbH, which needs to acknowledge the German Data Protection Act and the consent given by the study participants. The exploitation of the (LURIC) Study database is governed by the articles of the LURIC Study GmbH (non-profit LLC), registered under number HRB 7668 at the commercial registry of Freiburg in Breisgau, Germany. To request access, contact Kai Grunwald (Kai.Grunwald@weitnauer.net) or the Principal Investigator of the LURIC Study, Winfried März (winfried.maerz@luric-online.de).
-
•
This paper does only report original code for figures. Raw data R code from figure 1 and 2 were deposited on Mendeley at http://dx.doi.org/10.17632/rjnc3x6yyr.1.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Proud C.G. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y., Zhou M., Sun H., Wang Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc. Res. 2011;90:220–223. doi: 10.1093/cvr/cvr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro R.V., Solon-Biet S.M., Pulpitel T., Senior A.M., Cogger V.C., Clark X., O'Sullivan J., Koay Y.C., Hirani V., Blyth F.M., et al. Of older mice and men: branched-chain amino acids and body composition. Nutrients. 2019;11 doi: 10.3390/nu11081882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z., Glisic M., Song M., Aliahmad H.A., Zhang X., Moumdjian A.C., Gonzalez-Jaramillo V., van der Schaft N., Bramer W.M., Ikram M.A., Voortman T. Dietary protein intake and all-cause and cause-specific mortality: results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020;35:411–429. doi: 10.1007/s10654-020-00607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J., Liao L.M., Weinstein S.J., Sinha R., Graubard B.I., Albanes D. Association between plant and animal protein intake and overall and cause-specific mortality. JAMA Intern. Med. 2020;180:1173–1184. doi: 10.1001/jamainternmed.2020.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song M., Fung T.T., Hu F.B., Willett W.C., Longo V.D., Chan A.T., Giovannucci E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016;176:1453–1463. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virtanen H.E.K., Voutilainen S., Koskinen T.T., Mursu J., Kokko P., Ylilauri M.P.T., Tuomainen T.-P., Salonen J.T., Virtanen J.K. Dietary proteins and protein sources and risk of death: the kuopio ischaemic heart disease risk factor study. Am. J. Clin. Nutr. 2019;109:1462–1471. doi: 10.1093/ajcn/nqz025. [DOI] [PubMed] [Google Scholar]
- 8.Nishitani S., Takehana K., Fujitani S., Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1292–G1300. doi: 10.1152/ajpgi.00510.2003. [DOI] [PubMed] [Google Scholar]
- 9.Shin A.C., Fasshauer M., Filatova N., Grundell L.A., Zielinski E., Zhou J.-Y., Scherer T., Lindtner C., White P.J., Lapworth A.L., et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014;20:898–909. doi: 10.1016/j.cmet.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop C.A., Machate T., Henning T., Henkel J., Püschel G., Weber D., Grune T., Klaus S., Weitkunat K. Detrimental effects of branched-chain amino acids in glucose tolerance can be attributed to valine induced glucotoxicity in skeletal muscle. Nutr. Diabetes. 2022;12:20. doi: 10.1038/s41387-022-00200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D., Richardson N.E., Green C.L., Spicer A.B., Murphy M.E., Flores V., Jang C., Kasza I., Nikodemova M., Wakai M.H., et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021;33:905–922.e6. doi: 10.1016/j.cmet.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portero V., Nicol T., Podliesna S., Marchal G.A., Baartscheer A., Casini S., Tadros R., Treur J.L., Tanck M.W.T., Cox I.J., et al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc. Res. 2022;118:1742–1757. doi: 10.1093/cvr/cvab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Zhang F., Xia Y., Zhao S., Yan W., Wang H., Lee Y., Li C., Zhang L., Lian K., et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H1160–H1169. doi: 10.1152/ajpheart.00114.2016. [DOI] [PubMed] [Google Scholar]
- 14.Uddin G.M., Zhang L., Shah S., Fukushima A., Wagg C.S., Gopal K., Al Batran R., Pherwani S., Ho K.L., Boisvenue J., et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc. Diabetol. 2019;18:86. doi: 10.1186/s12933-019-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson N.E., Konon E.N., Schuster H.S., Mitchell A.T., Boyle C., Rodgers A.C., Finke M., Haider L.R., Yu D., Flores V., et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat. Aging. 2021;1:73–86. doi: 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng S., Rhee E.P., Larson M.G., Lewis G.D., McCabe E.L., Shen D., Palma M.J., Roberts L.D., Dejam A., Souza A.L., et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125:2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E., Lewis G.D., Fox C.S., Jacques P.F., Fernandez C., et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Würtz P., Soininen P., Kangas A.J., Rönnemaa T., Lehtimäki T., Kähönen M., Viikari J.S., Raitakari O.T., Ala-Korpela M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–655. doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah S.H., Bain J.R., Muehlbauer M.J., Stevens R.D., Crosslin D.R., Haynes C., Dungan J., Newby L.K., Hauser E.R., Ginsburg G.S., et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 20.Shah S.H., Sun J.-L., Stevens R.D., Bain J.R., Muehlbauer M.J., Pieper K.S., Haynes C., Hauser E.R., Kraus W.E., Granger C.B., et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012;163:844–850.e1. doi: 10.1016/j.ahj.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Lin C., Sun Z., Mei Z., Zeng H., Zhao M., Hu J., Xia M., Huang T., Wang C., Gao X., Zheng Y. The causal associations of circulating amino acids with blood pressure: a Mendelian randomization study. BMC Med. 2022;20:414. doi: 10.1186/s12916-022-02612-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deelen J., Kettunen J., Fischer K., van der Spek A., Trompet S., Kastenmüller G., Boyd A., Zierer J., van den Akker E.B., Ala-Korpela M., et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 2019;10:3346. doi: 10.1038/s41467-019-11311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NEWSHOLME P., BRENNAN L., RUBI B., MAECHLER P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin. Sci. 2005;108:185–194. doi: 10.1042/CS20040290. [DOI] [PubMed] [Google Scholar]
- 24.Miyake T., Abe M., Furukawa S., Tokumoto Y., Toshimitsu K., Ueda T., Yamamoto S., Hirooka M., Kumagi T., Hiasa Y., et al. Long-term branched-chain amino acid supplementation improves glucose tolerance in patients with nonalcoholic steatohepatitis-related cirrhosis. Intern. Med. 2012;51:2151–2155. doi: 10.2169/internalmedicine.51.7578. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Guo K., LeBlanc R.E., Loh D., Schwartz G.J., Yu Y.-H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 26.Dugan C.E., Fernandez M.L. Effects of dairy on metabolic syndrome parameters: a review. Yale J. Biol. Med. 2014;87:135–147. [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs M., Krssak M., Bernroider E., Anderwald C., Brehm A., Meyerspeer M., Nowotny P., Roth E., Waldhäusl W., Roden M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 28.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., Haqq A.M., Shah S.H., Arlotto M., Slentz C.A., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaham O., Wei R., Wang T.J., Ricciardi C., Lewis G.D., Vasan R.S., Carr S.A., Thadhani R., Gerszten R.E., Mootha V.K. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol. Syst. Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wopereis S., Rubingh C.M., van Erk M.J., Verheij E.R., van Vliet T., Cnubben N.H.P., Smilde A.K., van der Greef J., van Ommen B., Hendriks H.F.J. Metabolic profiling of the response to an oral glucose tolerance test detects subtle metabolic changes. PLoS One. 2009;4:e4525. doi: 10.1371/journal.pone.0004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah S.H., Crosslin D.R., Haynes C.S., Nelson S., Turer C.B., Stevens R.D., Muehlbauer M.J., Wenner B.R., Bain J.R., Laferrère B., et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321–330. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldasseroni S., Antenore A., Di Serio C., Orso F., Lonetto G., Bartoli N., Foschini A., Marella A., Pratesi A., Scarantino S., et al. Adiponectin, diabetes and ischemic heart failure: a challenging relationship. Cardiovasc. Diabetol. 2012;11:151. doi: 10.1186/1475-2840-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekker J.M., Funahashi T., Nijpels G., Pilz S., Stehouwer C.D.A., Snijder M.B., Bouter L.M., Matsuzawa Y., Shimomura I., Heine R.J. Prognostic value of adiponectin for cardiovascular disease and mortality. J. Clin. Endocrinol. Metab. 2008;93:1489–1496. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 34.Pilz S., Mangge H., Wellnitz B., Seelhorst U., Winkelmann B.R., Tiran B., Boehm B.O., März W. Adiponectin and mortality in patients undergoing coronary angiography. J. Clin. Endocrinol. Metab. 2006;91:4277–4286. doi: 10.1210/jc.2006-0836. [DOI] [PubMed] [Google Scholar]
- 35.Torres-Leal F.L., Fonseca-Alaniz M.H., Teodoro G.F., de Capitani M.D., Vianna D., Pantaleão L.C., Matos-Neto E.M., Rogero M.M., Donato J., Tirapegui J. Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutr. Metab. 2011;8:62. doi: 10.1186/1743-7075-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruckbauer A., Zemel M.B., Thorpe T., Akula M.R., Stuckey A.C., Osborne D., Martin E.B., Kennel S., Wall J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012;9:77. doi: 10.1186/1743-7075-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Berendoncks A.M., Garnier A., Beckers P., Hoymans V.Y., Possemiers N., Fortin D., Martinet W., van Hoof V., Vrints C.J., Ventura-Clapier R., Conraads V.M. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ. Heart Fail. 2010;3:185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- 38.Sivanand S., Vander Heiden M.G. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell. 2020;37:147–156. doi: 10.1016/j.ccell.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harper A.E., Miller R.H., Block K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 40.Lu G., Ren S., Korge P., Choi J., Dong Y., Weiss J., Koehler C., Chen J.n., Wang Y. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–796. doi: 10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen M., Gao C., Yu J., Ren S., Wang M., Wynn R.M., Chuang D.T., Wang Y., Sun H. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J. Am. Heart Assoc. 2019;8:e011625. doi: 10.1161/JAHA.118.011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura J., Masaki T., Arakawa M., Seike M., Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARα and uncoupling protein in diet-induced obese mice. J. Nutr. 2010;140:496–500. doi: 10.3945/jn.109.108977. [DOI] [PubMed] [Google Scholar]
- 43.Young L.H., McNulty P.H., Morgan C., Deckelbaum L.I., Zaret B.L., Barrett E.J. Myocardial protein turnover in patients with coronary artery disease. Effect of branched chain amino acid infusion. J. Clin. Invest. 1991;87:554–560. doi: 10.1172/JCI115030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katta A., Kundla S., Kakarla S.K., Wu M., Fannin J., Paturi S., Liu H., Addagarla H.S., Blough E.R. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1666–R1675. doi: 10.1152/ajpregu.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang D., Contu R., Latronico M.V.G., Zhang J., Rizzi R., Catalucci D., Miyamoto S., Huang K., Ceci M., Gu Y., et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marazzi G., Rosanio S., Caminiti G., Dioguardi F.S., Mercuro G. The role of amino acids in the modulation of cardiac metabolism during ischemia and heart failure. Curr. Pharm. Des. 2008;14:2592–2604. doi: 10.2174/138161208786071227. [DOI] [PubMed] [Google Scholar]
- 48.Pilz S., Meinitzer A., Tomaschitz A., Drechsler C., Ritz E., Krane V., Wanner C., Boehm B.O., März W. Low homoarginine concentration is a novel risk factor for heart disease. Heart. 2011;97:1222–1227. doi: 10.1136/hrt.2010.220731. [DOI] [PubMed] [Google Scholar]
- 49.Tomaschitz A., Meinitzer A., Pilz S., Rus-Machan J., Genser B., Drechsler C., Grammer T., Krane V., Ritz E., Kleber M.E., et al. Homoarginine, kidney function and cardiovascular mortality risk. Nephrol. Dial. Transplant. 2014;29:663–671. doi: 10.1093/ndt/gft512. [DOI] [PubMed] [Google Scholar]
- 50.Tomoda K., Kubo K., Hino K., Kondoh Y., Nishii Y., Koyama N., Yamamoto Y., Yoshikawa M., Kimura H. Branched-chain amino acid-rich diet improves skeletal muscle wasting caused by cigarette smoke in rats. J. Toxicol. Sci. 2014;39:331–337. doi: 10.2131/jts.39.331. [DOI] [PubMed] [Google Scholar]
- 51.Anand I.S., Kuskowski M.A., Rector T.S., Florea V.G., Glazer R.D., Hester A., Chiang Y.T., Aknay N., Maggioni A.P., Opasich C., et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation. 2005;112:1121–1127. doi: 10.1161/CIRCULATIONAHA.104.512988. [DOI] [PubMed] [Google Scholar]
- 52.Ohtani M., Maruyama K., Sugita M., Kobayashi K. Amino acid supplementation affects hematological and biochemical parameters in elite rugby players. Biosci. Biotechnol. Biochem. 2001;65:1970–1976. doi: 10.1271/bbb.65.1970. [DOI] [PubMed] [Google Scholar]
- 53.Swedberg K., Young J.B., Anand I.S., Cheng S., Desai A.S., Diaz R., Maggioni A.P., McMurray J.J.V., O'Connor C., Pfeffer M.A., et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N. Engl. J. Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 54.Pedroso J.A.B., Nishimura L.S., de Matos-Neto E.M., Donato J., Tirapegui J. Leucine improves protein nutritional status and regulates hepatic lipid metabolism in calorie-restricted rats. Cell Biochem. Funct. 2014;32:326–332. doi: 10.1002/cbf.3017. [DOI] [PubMed] [Google Scholar]
- 55.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 56.Tai E.S., Tan M.L.S., Stevens R.D., Low Y.L., Muehlbauer M.J., Goh D.L.M., Ilkayeva O.R., Wenner B.R., Bain J.R., Lee J.J.M., et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkelmann B.R., März W., Boehm B.O., Zotz R., Hager J., Hellstern P., Senges J., LURIC Study Group LUdwigshafen RIsk and Cardiovascular Health Rationale and design of the LURIC study--a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics. 2001;2:S1–S73. doi: 10.1517/14622416.2.1.S1. [DOI] [PubMed] [Google Scholar]
- 58.Sourij H., Meinitzer A., Pilz S., Grammer T.B., Winkelmann B.R., Boehm B.O., März W. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis. 2011;218:220–225. doi: 10.1016/j.atherosclerosis.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 59.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inker L.A., Schmid C.H., Tighiouart H., Eckfeldt J.H., Feldman H.I., Greene T., Kusek J.W., Manzi J., van Lente F., Zhang Y.L., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The data reported in this study cannot be deposited in a public repository due to the articles of the LURIC Study GmbH, which needs to acknowledge the German Data Protection Act and the consent given by the study participants. The exploitation of the (LURIC) Study database is governed by the articles of the LURIC Study GmbH (non-profit LLC), registered under number HRB 7668 at the commercial registry of Freiburg in Breisgau, Germany. To request access, contact Kai Grunwald (Kai.Grunwald@weitnauer.net) or the Principal Investigator of the LURIC Study, Winfried März (winfried.maerz@luric-online.de).
-
•
This paper does only report original code for figures. Raw data R code from figure 1 and 2 were deposited on Mendeley at http://dx.doi.org/10.17632/rjnc3x6yyr.1.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.