Abstract
Aim
To determine the presence of Streptococcus mutans (S. mutans) and Streptococcus sobrinus (S. sobrinus) and their association with extrinsic and intrinsic variables in 6–18-month-old infants.
Methods
This was an analytical, cross-sectional study of 65 6–18-month-old infants who visited the Centers for Early Childhood in Buenos Aires City. Three groups were established according to the presence of teeth—group I (GI)—edentulous infants, group II (GII)—infants with 1–8 teeth, and group III (GIII)—infants with 9–16 teeth. Data on the variables, diet, use of artificial teats, and oral hygiene were gathered using a self-administered questionnaire. An oral examination was performed according to the International Caries Detection and Assessment System (ICDAS II) criterion. A saliva sample was taken by aspiration with a sterile plastic syringe. Cariogenic Streptococci (CS) were counted using the adherence test in modified gold broth (AT-MGB). Molecular detection and quantification were performed by quantitative polymerase chain reaction (qPCR) (gtfB, gtfT, and tuf).
Results
A total of 12% of infants received oral hygiene, 38% used bottles, 30% used pacifiers, and 55% had sugar intake. S. sobrinus and S. mutans were detected in 57.1 and 28.6% of the children with caries, respectively. Groups I, II, and III had CS counts of log 2, 3.4, and 3.7, respectively. S. sobrinus was detected in 26.7% of GI, 52.9% of GII, and 85.7% of GIII, while S. mutans was detected in 13.3%, 35.3%, and 57.7%, respectively.
Conclusion
The prevalence of S. sobrinus was higher than S. mutans in all groups. The presence of CS was significantly associated with sugar intake. No association was found between S. mutans and S. sobrinus and the presence of caries, hygiene habits, or use of artificial teats.
Clinical Significance
This study supports the role of diet in developing a cariogenic biofilm in children under 2 years of age.
How to cite this article
Cornejo CF, Soken LJ, Salgado PA, et al. Detection of Streptococcus mutans and Streptococcus sobrinus and Their Association with Oral Microbiome Stressors in 6–18-month-old Infants. Int J Clin Pediatr Dent 2023;16(1):68-73.
Keywords: Colony forming units, Early childhood caries, Quantitative polymerase chain reaction, Streptococcus mutans, Streptococcus sobrinus, Sugar intake
Introduction
Understanding the relationship between the oral microbiome and oral health has evolved. Biomarkers that participate in the states of eubiosis and dysbiosis have been identified, establishing a predictive association between microbial communities and oral diseases such as dental caries.
From birth to adulthood, the oral microbiome is modulated by different factors. These factors include intrinsic factors (biological characteristics inherent to the host) and extrinsic factors (external stimuli with the potential to influence host characteristics), which can be divided into those modulated by the host (lifestyle and behavior) and those not intentionally modulated by the host (environmental factors).1
Within the host-modulated extrinsic factors, diet and oral hygiene habits have significant effects on the oral microbiome and may or may not favor an imbalance of the oral medium.
Frequent intake of fermentable carbohydrates is a necessary, though not sufficient, condition for developing dental caries. Fermentation of carbohydrates in the diet by microorganisms present in the microbiota produces organic acids that lower oral pH. When acid production exceeds salivary buffer capacity, the pH descends, favoring an increase in acid-producing and acid-tolerant bacterial species. When this condition persists over time, the oral microbiome reaches a state of dysbiosis.
From a social standpoint, extrinsic environmental factors such as socioeconomic situation and access to dental care also contribute to the development and composition of the oral microbiome.2,3 Like other countries in Latin America, Argentina uses a direct multidimensional method to assess poverty, which measures economic poverty and the living conditions of groups and communities known as the Unmet Basic Needs (UBN) method; it seeks to measure deficiencies in a series of needs considered essential and generally discriminated into two main dimensions. The first is associated with minimum requirements related to private household consumption—food, housing, and clothing. The second is associated with public services provided to the community—potable water, sanitation, public transport, healthcare, education, and cultural facilities. Based on a standardized definition, a given condition can be compared, need by need, or satisfier by satisfier, thereby providing direct observation of unmet needs.4
The process of tooth eruption is one of the intrinsic factors that modulate the oral microbiome because it creates a new habitat in the ecosystem. The formation of the gingival sulcus at the beginning of the eruptive period develops a new ecosystem that favors colonization by anaerobic microorganisms. At the same time, the emergence of teeth provides surfaces to which biofilm-forming microorganisms such as viridans group Streptococcus, in particular S. mutans, can adhere.5
Some authors consider that colonization by CS (S. mutans-S. sobrinus) occurs only after the dental eruption, while others have detected the presence of microorganisms in predentate infants. It has been reported that at least 20% of 2–3-year-old have been colonized by S. mutans before tooth eruption, with colonization increasing to 80% after tooth eruption.6,7
Recent studies on caries at early ages show that it is associated with various cariogenic microorganisms. However, in agreement with classical studies, new research based on molecular techniques continues to highlight the presence of S. mutans (serotypes c, e, f, and k) and S. sobrinus (serotypes d and g) as cariogenic microorganisms causing dental caries where the coexistence of S. mutans and S. sobrinus would have a synergetic effect.8,9 Some pathogenicity factors that could explain the cariogenic activity of these microorganisms are related to the synthesis and use of different glycosyltransferase-type enzymes and the presence of glucan-binding proteins, which are essential during the process of colonization of the enamel surface. Rupf et al.10 reported the presence and acidogenic capacity of S. sobrinus, relating it to a higher probability of developing dental caries on smooth surfaces even though it is present in a lower proportion than S. mutans.
From birth to 2–3 years of age, children may be fed (exclusive or mixed feeding) using artificial teats and bottles and maybe given pacifiers to calm anxiety or pain and regulate feeding or sleeping schedules. Studies have analyzed how the use of artificial teats and pacifiers, the quality of their components, the frequency with which they are used, and the addition of sugar may impact the development of dental caries in infants.11,12
Aim
The aim of this study was to determine the presence of S. mutans and S. sobrinus and their association with intrinsic and extrinsic factors in the establishment of dental caries in 6–18-month-old infants.
Methods
This was an analytical, observational, and cross-sectional study conducted at two early childhood centers [Certificate of Parental Improvement (CPI), according to the acronym in Spanish], which are facilities that aim to ensure the healthy growth and development of underprivileged children from 45 days to 3 years of age. Both these CPIs are located in District 4, which has one of the highest percentages of households with UBN in Buenos Aires City, and where people's only access to healthcare is via the government-managed system.13
The eligible population for this study comprised 6–18-month-old infants who attended the CPIs. Inclusion criteria for the sample were 6–18 months of age, attending the selected early childhood centers, and signed informed consent from legal guardians (approved by CUPAD Ethics Committee—EXP-UBA—0072332/201 7 Nº O12/2018 CETICA FOUBA). Exclusion criteria were preexisting systemic diseases that could affect the oral microbiome or having received antibiotic therapy within the month prior to being included in the study. Elimination criteria were any infant not accompanied by parents or legal guardians at the time of sampling and infants who were edentulous at 12 months of age.
The study sample consisted of 65 infants who met the legal and ethical principles and the inclusion and exclusion criteria.
The presence of teeth was the criterion used to form three study groups—GI n = 30 edentulous infants, GII n = 20 infants with 1–8 teeth, and GIII n = 15 infants with 9–16 teeth. Demographics, diet, use of artificial teats, and oral hygiene of each participating infant were surveyed through a questionnaire containing closed questions (yes/no), self-administered by infants’ parents and/or legal guardians. The item “diet” or sugar intake was considered as the intake of sucrose in the form of refined sugars added to the infant's usual diet. “Artificial teats” included the use of bottles and/or pacifiers. “Oral hygiene” was recorded as the presence or absence of hygiene practices at some time of the day.
Dental clinical diagnosis was performed by a calibrated professional (κ > 0.7), following ICDAS II criteria.14
For the microbiological and molecular study, saliva samples were taken from the floor of the mouth by aspiration with a sterile plastic syringe. Samples were sent to the laboratory (Microbiological and Molecular Diagnosis Laboratory of the School of Dentistry, Buenos Aires University, Buenos Aires, Argentina), where they were vortexed (”Velp Scientifica” ZX-3, Analogical 3000 rpm, Italy) and divided into equal portions, of which one portion was preserved at −20°C for molecular processing and another used for microbiological culture.
Cariogenic Streptococci (CS) were counted using the AT-MGB.15 Cultures were incubated under anaerobic conditions (Jarra 2,5 L, AnaeroJar OXOID™, AnaeroGen™ AN0035 kit, OXOID™) for 48 hours at 36° ± 1°C, after which they were assessed by calibrated personnel (κ > 0.7) under a stereoscopic microscope (binocular stereomicroscope “Arcano” ST30-L, China) at 50× magnification.
Molecular detection of S. mutans and S. sobrinus was done by qPCR (real-time PCR). The genomic material was obtained using a commercial kit (PrestoTM Mini gDNA Bacteria Kit, Geneaid Biotecj Ltd., New Taipei city, Taiwan), following the manufacturer's instructions.
Deoxyribonucleic acid (DNA) integrity was quantified and evaluated by spectrometry (CYTATION 3 Cell Imaging reader, Biotek, Winooski, Vermont, United States of America). The original extracts were normalized at 20 ng/mL for use in the qPCR reactions.
Presence and quantification of S. mutans and S. sobrinus were performed using the qPCR technique in a CFX96TM Real-Time System thermocycler (Bio-Rad Laboratories, Inc., Hercules, California, United States of America). Species-specific primers were used, and the target was the gene encoding the glycosyltransferase enzymes gtfB for S. mutans and gtfT for S. sobrinus.16 Total Streptococci were quantified by detection of the gene tuf.17 The reactions were performed in duplicate, using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, California, United States of America) in a final volume of 10 mL, following the cycling conditions recommended by the manufacturer.
The calibration curves for the genes gtfB, gtfT, and tuf were based on a series of dilutions of DNA from strains of S. mutans (ATCC 2517) and S. sobrinus (Ss001—culture collection of the Department of Microbiology at the School of Dentistry, Universidad de Buenos Aires, Buenos Aires, Argentina) and by sequencing two gtfT genes) (ABI 3130xl Genetic Analyzer, Applied Biosystems, Waltham, Massachusetts, United States of America). The number of copies per dilution was calculated considering the genome size for S. mutans as 2.6 Mb18 and for S. sobrinus as 2.20 Mb.19 The targets employed correspond to single-copy genes. The quantifiable range for both species was 1 × 101–1 × 108 c/µL (copies/µL). The curves employed were accepted with an efficiency of 84.3% for S. sobrinus and 93.6% for S. mutans.
Statistical Treatment
Qualitative variables were described by calculating the frequency distribution, percentages, and 95% confidence intervals (CIs). For quantitative variables, median, minimum, and maximum were calculated. To compare the association between qualitative variables, the independence test (Chi-square) was applied, and for expected frequencies lower than five, exact tests were used. To compare proportions between groups, independent proportion comparison tests with Bonferroni correction were used, and 95% CIs were calculated for the difference in proportions. To evaluate risk, the OR with 95% CIs was applied.
Statistical tests for independent samples were applied in all cases, with a significance level lower than 5%, to reject the null hypothesis.
Data were processed using the software Statistical Package for the Social Sciences (version 27), Statistics and data 14, MS Excel 2019, MedCalc; Epidat 4.2.
Results
Demographics are shown in Table 1, with distribution according to sex and age for each study group.
Table 1.
Demographic characteristics of the infants
Three infants from G2 and one from G3 were not included in the microbiological/molecular analysis because it was not possible to obtain a large enough sample. The presence of mutans group Streptococcus per study group was as follows—GI, S. sobrinus in 26.7% (95% CI, 13.5–44.1) and S. mutans in 6.7% (95% CI, 1.4–19.7); GII, S. sobrinus in 52.9% (95% CI, 30.3–74.6) and S. mutans in 35.3% (95% CI, 16.3–58.9); and GIII, S. sobrinus in 85.7% (95% CI, 61.5–96.9) and S. mutans in 57.1% (95% CI, 31.9–79.7) (Fig. 1).
Fig. 1.
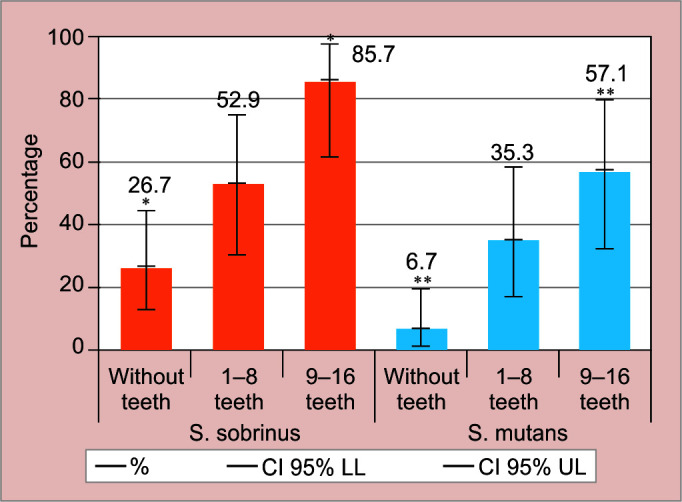
Distribution of cariogenic Streptococci by groups
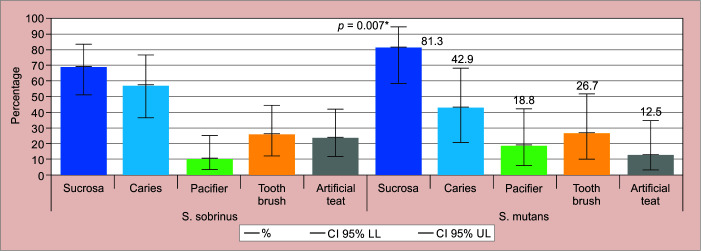
The data from the self-administered questionnaire showed that 22.2% of the infants received oral hygiene, 38.5% used a bottle, 30.8% used a pacifier, and 55.4% had refined sugar intake. Figure 2 shows the detection of S. mutans and S. sobrinus and their association with the extrinsic factors studied.
Fig. 2.
Association of S. sobrinus and S. mutans with extrinsic variables
Clinical diagnosis showed that infants in G2 had a 55.0% prevalence of dental caries, with a maximum of four surfaces affected by incipient lesions (code 1-2-3 ICDAS II) and two cavitated surfaces (code 4-5-6 ICDAS II). Infants in GIII had a 66.7% prevalence of dental caries, with a maximum of three surfaces affected by incipient lesions and eight cavitated surfaces. In samples corresponding to patients with carious lesions in GII, the presence of CS was detected in 18.20% of S. mutans and 36.4% of S. sobrinus, while in GIII, it was 40% for S. mutans and 80% for S. sobrinus.
Cariogenic Streptococci (CS) counts by AT-MGB expressed in colony-forming units per mL of saliva (CFU/mL) were 1 × 102 (Log10 2) in G1; 2.5 × 103 (Log10 3.4) in G2 and 5.01 × 103 (Log10 3.7) in GIII, with significant difference between GI and GIII, p < 0.01. Molecular detection showed the presence of CS in edentulous infants, and significant increases were observed in groups with teeth (p < 0.01).
The relative abundance of Streptococcus spp (total Streptococci—gene tuf) was 0.04% ± 0.048 for S. mutans and 0.017% ± 0.018 for S. sobrinus, resulting in a mean relative abundance of 0.03%.
The extrinsic variable “sugar intake” was significantly associated with the presence of both S. mutans and S. sobrinus, with p = 0.007 [odds ratio (OR) 5.93 (95% CI, 1.48–23.750] and p = 0.014 [OR = 3.70 (95% CI, 1.27–10.72)], respectively.
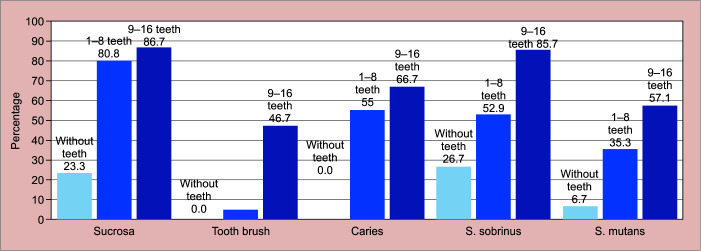
Detection of S. mutans and S. sobrinus with relation to the extrinsic and intrinsic variables studied in each group showed greater exponential growth of S. sobrinus than S. mutans (Fig. 3).
Fig. 3.
Distribution of intrinsic and extrinsic variables according to the quantity of teeth
Discussion
The results of our study showed that the detection of CS increased in direct relation to infant age and the number of teeth, in agreement with data published by Avasare et al.,20 who consider the presence of more than five teeth to be a risk factor for acquiring CS. This linear increase in detection in relation to the number of teeth may result from the dynamism in microbial communities associated with tooth eruption.21
S. mutans and S. sobrinus were detected in the current study from the group of 6-month-old infants. This finding agrees with the data reported by Plonka et al.22 for a study on S. mutans acquisition time and age of caries onset in a cohort of children from birth to 36 months of age, which established that 6% of the children who had developed caries at 30 months of age had been colonized by S. mutans at the age of 6 months. Thus, early detection of these cariogenic species could have a predictive effect and enable preventive interventions to modulate oral microbiota.
In contrast to other studies that report only the presence of S. mutans, the techniques used in our study found a more significant presence of S. sobrinus among total participating infants as well as within each study group. These results are similar to those reported by Gizani et al.23 Recent studies have reported the high acidogenic capacity of S. sobrinus and established that when it is detected, there is a greater probability of dental caries on smooth surfaces even if it is present in a lower proportion.24
Through the data obtained from clinical diagnosis, the current study showed the presence of incipient and cavitated carious lesions but was unable to establish a significant association with the presence of CS. Our data are consistent with those reported by Li et al.,5 establishing the relationship between the presence of incipient lesions and bacterial species in children with and without lesions.
The use of saliva samples may be insufficient for establishing an association between the presence of carious lesions and CS in this age group. Thus, future research should use alternative sampling techniques and new sequencing techniques for sample processing.
The presence of carious lesions in infants in our study had values similar to those reported in Ecuador by Valarezo-Bravo and Marino-Solis,25 who used the same diagnostic criterion and reported a prevalence of 49.3%. The systematic review by Ganesh et al.26 on early childhood caries in India reported a prevalence higher than 40%. However, studies by other authors such as Un Lam et al.27 in Singapore and He et al.28 in China found values lower than 35% for caries prevalence in infants up to 2 years old. These differences may respond to socioeconomic and cultural differences between countries. The role of CS as primary pathogens in dental caries may depend on the study population, with their weight being lower in populations with access to prevention programs and higher in populations lacking access to prevention strategies and treatment of caries.29
The CFU/mL count is another method used to establish the relationship between cariogenic microorganisms and the presence or progression of carious lesions. Various longitudinal studies have demonstrated the predictive value of the microbial count and established that high CS counts are associated with the presence and progression of dental caries.30,31 The results of the counts in our study are similar to those reported by Edelstein et al. 2016.32 However, we were unable to establish an association between CS count and carious lesions. Fontana et al.33 found that the S. mutans count and its association with total Streptococci did not behave as significant predictors for caries progression in 2–5-year-olds.
Streptococcus mutans (S. mutans) and S. sobrinus relative abundance found by qPCR in the current study was similar to that reported by Teng et al.34 and Dzidic et al.35 for 6–24-month-old infants. Those studies also found no significant association between the levels of these microbial species and the development of carious lesions. However, other authors36,37 had found significant associations when the relative abundance of CS (S. mutans-S. sobrinus) reached 0.73%, establishing that values higher than this may be good estimators for future caries development. It is worth highlighting that even though the frequency of detection of S. sobrinus in our study was higher than the detection of S. mutans, the quantification and relative abundance for S. mutans compared to Streptococcus spp. were higher than for S. sobrinus. It would therefore be interesting to increase the number of samples and perform a longitudinal study to evaluate the behavior of the intrinsic and extrinsic variables with regard to the onset of carious lesions and how they are associated with the proportions of CS in infants up to the age of 24 months.
Our study found that more than half the population had refined sugar intake, which acted as a stress factor and favored the presence of CS, as reported by Wan et al.38 Studies of infant nutritional transition from birth to 5 years of age have claimed that this is the period when infants learn what, when, and how much to eat, based on the transmission of beliefs, attitudes, and cultural practices regarding nutrition. It has been reported that some of the influencing factors involved in an early introduction to sugary foods and beverages are related to affordability of and greater access to foods rich in refined sugars, low socioeconomic level, and parents who study or work outside the home for long periods.39,40
In the current study, no association was found between the use of artificial teats and the presence of carious lesions or CS levels. Other studies claim that the use of artificial teats is associated with a higher frequency of dental caries in infants and the early acquisition of CS, and a lower level of dental hygiene habits,11,12 although these findings were also linked to the addition of refined sugars to those artificial teats.
Conclusion
It is relevant to highlight that S. mutans and S. sobrinus presented low relative abundance in relation to total Streptococci detected in the saliva samples analyzed. However, there was a significant predominance of S. sobrinus to S. mutans in all three study groups. Both species were observed to increase in direct relation to infant age and the number of teeth according to dental chronology. Analysis of the relationship between microbiology and the different extrinsic factors showed a significant association with refined carbohydrate intake.
Clinical Significance
This study supports the role of diet in developing a cariogenic biofilm in children under 2 years of age. This study supports the role of diet in developing a cariogenic biofilm in children under 2 years of age and the need to implement interventions to educate parents or caregivers on feeding practices that may avoid dysbiosis.
Acknowledgment
This study was supported by the University of Buenos Aires Grant–UBACYT 20720190100007BA.
Footnotes
Source of support: Nil
Conflict of interest: None
Patient consent statement: The author(s) have obtained written informed consent from the patient's parents/legal guardians for publication of the case report details and related images.
References
- 1.Cornejo Ulloa P, van der Veen MH, Krom BP. Review: modulation of the oral microbiome by the host to promote ecological balance. Odontology. 2019;107(4):437–448. doi: 10.1007/s10266-019-00413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce A, Singh S, Lee J, et al. The burden of early childhood caries in Canadian children and associated risk factors. Front Public Health. 2019;7:328. doi: 10.3389/fpubh.2019.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh A, Peres MA, Watt RG. The relationship between income and oral health: a critical review. J Dent Res. 2019;98(8):853–860. doi: 10.1177/0022034519849557. [DOI] [PubMed] [Google Scholar]
- 4.Lépore E. Hacia una definición de la pobreza centrada en los derechos humanos: Aproximaciones conceptuales Acta Académica 2007. https://www.aacademica.org/000-028/129 https://www.aacademica.org/000-028/129 Available from:
- 5.Li F, Tao D, Feng X, et al. Establishment and development of oral microflora in 12-24 month-old toddlers monitored by high-throughput sequencing. Front Cell Infect Microbiol. 2018;8:422. doi: 10.3389/fcimb.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson S, Albert JM, Soderling E, et al. Increased number of teeth predict acquisition of mutans Streptococci in infants. Eur J Oral Sci. 2014;122(5):346–352. doi: 10.1111/eos.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seow WK. Early childhood caries. Pediatr Clin North Am. 2018;65(5):941–954. doi: 10.1016/j.pcl.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis E, Parsaei Y, Klein MI, et al. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol Oral Microbiol. 2017;32(1):24–34. doi: 10.1111/omi.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faustova MO, Ananieva MM, Basarab YO, et al. Bacterial factors of cariogenicity (literature review). Wiad Lek. 2018;71(2 pt 2):378–382. [PubMed] [Google Scholar]
- 10.Rupf S, Merte K, Eschrich K, et al. Streptococcus sobrinus in children and its influence on caries activity. Eur Arch Paediatric Dent. 2006;7(1):17–22. doi: 10.1007/BF03320810. [DOI] [PubMed] [Google Scholar]
- 11.Colombo S, Gallus S, Beretta M, et al. Prevalence and determinants of early childhood caries in Italy. Eur J Paediatr Dent. 2019;20(4):267–273. doi: 10.23804/ejpd.2019.20.04.02. [DOI] [PubMed] [Google Scholar]
- 12.Dahas ZA, Khormi HA, Vishwanathaiah S, et al. Correlation of feeding practices and dental caries among preschool children of Jazan, KSA: a cross-sectional study. Int J Clin Pediatr Dent. 2020;13(4):327–331. doi: 10.5005/jp-journals-10005-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argentina GOdE-SdPS-MdS. Análisis de Situación de Salud de la Ciudad Autónoma de Buenos Aires – Año 2016 2017. https://www.buenosaires.gob.ar/sites/gcaba/files/asis_caba_2016_dic17_vf_1.pdf https://www.buenosaires.gob.ar/sites/gcaba/files/asis_caba_2016_dic17_vf_1.pdf Available from:
- 14.Pitts N. ”ICDAS”—an international system for caries detection and assessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health. 2004;21(3):193–198. [PubMed] [Google Scholar]
- 15.Gliosca LA, Stoppani N, Lamas NS, et al. Validation of an adherence assay to detect group mutans Streptococci in saliva samples. Acta Odontol Latinoam. 2019;32(2):97–102. [PubMed] [Google Scholar]
- 16.Yoshida A, Suzuki N, Nakano Y, et al. Development of a 5’ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J Clin Microbiol. 2003;41(9):4438–4441. doi: 10.1128/JCM.41.9.4438-4441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard FJ, Ke D, Boudreau DK, et al. Use of tuf sequences for genus-specific PCR detection and phylogenetic analysis of 28 streptococcal species. J Clin Microbiol. 2004;42(8):3686–3695. doi: 10.1128/JCM.42.8.3686-3695.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajdić D, McShan WM, McLaughlin RE, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99(22):14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sales MJ, Herbert WG, Du Y, et al. Complete genome sequences of Streptococcus sobrinus SL1 (ATCC 33478 = DSM 20742), NIDR 6715-7 (ATCC 27351), NIDR 6715-15 (ATCC 27352), and NCTC 10919 (ATCC 33402). Microbiol Resour Announc. 2018;7(3):e00804–e00818. doi: 10.1128/MRA.00804-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avasare T, Warren J, Qian F, et al. Longitudinal study assessing factors associated with mutans streptococci acquisition in infants and toddlers. Oral Health Prev Dent. 2017;15(6):543–548. doi: 10.3290/j.ohpd.a39226. [DOI] [PubMed] [Google Scholar]
- 21.Caufield PW, Dasanayake AP, Li Y, et al. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infection And Immunity. 2000;68(7):4018–4023. doi: 10.1128/IAI.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plonka KA, Pukallus ML, Barnett AG, et al. A longitudinal case-control study of caries development from birth to 36 months. Caries Res. 2013;47(2):117–127. doi: 10.1159/000345073. [DOI] [PubMed] [Google Scholar]
- 23.Gizani S, Papaioannou W, Haffajee AD, et al. Distribution of selected cariogenic bacteria in five different intra-oral habitats in young children. Int J Paediatr Dent. 2009;19(3):193–200. doi: 10.1111/j.1365-263X.2008.00956.x. [DOI] [PubMed] [Google Scholar]
- 24.Dye BA, Hsu KL, Afful J. Prevalence and measurement of dental caries in young children. Pediatric Dent. 2015;37(3):200–216. [PubMed] [Google Scholar]
- 25.Valarezo-Bravo TL, Mariño-Solis SM. Prevalencia de caries temprana de la infancia en cuatro guarderías del norte de Quito-Ecuador. Dominio de las Ciencias. 2017;3(1):278–297. [Google Scholar]
- 26.Ganesh A, Muthu MS, Mohan A, et al. Prevalence of early childhood caries in india—a systematic review. Indian J Pediatr. 2019;86(3):276–286. doi: 10.1007/s12098-018-2793-y. [DOI] [PubMed] [Google Scholar]
- 27.Un Lam C, Khin LW, Kalhan AC, et al. Identification of caries risk determinants in toddlers: results of the GUSTO birth cohort study. Caries Res. 2017;51(4):271–282. doi: 10.1159/000471811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He XL, Chen D, Yan ZL, et al. Investigation on the prevalence of dental caries and family oral health behaviors in young children aged 1 to 3 years in Chengdu city. Shanghai Kou Qiang Yi Xue. 2020;29(1):80–84. [PubMed] [Google Scholar]
- 29.Johansson I, Witkowska E, Kaveh B, et al. The microbiome in populations with a low and high prevalence of caries. J Dent Res. 2016;95(1):80–86. doi: 10.1177/0022034515609554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piva F, Pereira JT, Luz PB, et al. A longitudinal study of early childhood caries and associated factors in Brazilian children. Braz Dent J. 2017;28(2):241–248. doi: 10.1590/0103-6440201701237. [DOI] [PubMed] [Google Scholar]
- 31.Xiao J, Fiscella KA, Gill SR. Oral microbiome: possible harbinger for children's health. Int J Oral Sci. 2020;12(1):12. doi: 10.1038/s41368-020-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edelstein BL, Ureles SD, Smaldone A. Very high salivary Streptococcus mutans predicts caries progression in young children. Pediatr Dent. 2016;38(4):325–330. [PubMed] [Google Scholar]
- 33.Fontana M, Jackson R, Eckert G, et al. Identification of caries risk factors in toddlers. J Dent Res. 2011;90(2):209–214. doi: 10.1177/0022034510385458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng F, Yang F, Huang S, et al. Prediction of early childhood caries via spatial-temporal variations of oral microbiota. Cell Host Microbe. 2015;18(3):296–306. doi: 10.1016/j.chom.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Dzidic M, Collado MC, Abrahamsson T, et al. Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018;12(9):2292–2306. doi: 10.1038/s41396-018-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dashper SG, Mitchell HL, Lê Cao KA, et al. Temporal development of the oral microbiome and prediction of early childhood caries. Sci Rep. 2019;9(1):19732. doi: 10.1038/s41598-019-56233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agnello M, Marques J, Cen L, et al. Microbiome associated with severe caries in Canadian first nations children. J Dent Res. 2017;96(12):1378–1385. doi: 10.1177/0022034517718819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan AK, Seow WK, Purdie DM, et al. A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J Dent Res. 2003;82(7):504–508. doi: 10.1177/154405910308200703. [DOI] [PubMed] [Google Scholar]
- 39.Levin A, Sokal-Gutierrez K, Hargrave A, et al. Maintaining traditions: a qualitative study of early childhood caries risk and protective factors in an indigenous community. Int J Environ Res Public Health. 2017;14(8):907. doi: 10.3390/ijerph14080907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ha DH, Do LG, Spencer AJ, et al. Factors influencing early feeding of foods and drinks containing free sugars—a birth cohort study. Int J Environ Res Public Health. 2017;14(10):1270. doi: 10.3390/ijerph14101270. [DOI] [PMC free article] [PubMed] [Google Scholar]