Abstract
Objective
Developing and assessing a risk prediction model of postoperative atrial fibrillation (POAF) after coronary artery bypass grafting (CABG), and aims to provide a reference for the prediction and prevention.
Design
A retrospective case-control study.
Setting
Three major urban teaching and university hospitals and tertiary referral centers.
Participants
consecutive patients undergoing CABG.
Interventions
The study was retrospective and no interventions were administered to patients.
Measurements and main results
In the study, the overall new-onset POAF prevalence was approximately 28%. A prediction model for POAF with nine significant indicators was developed, and identified new predictors of POAF: left ventricular end diastolic diameter (LVEDD), intraoperative defibrillation, and intraoperative temporary pacing lead implantation. The model had good discrimination in both the derivation and validation cohorts, with the area under the receiver operating characteristic curves (AUCs) of 0.621 (95% CI = 0.602–0.640) and 0.616 (95% CI = 0.579–0.651), respectively, and showed good calibration. Compared with CHA2DS2-VASc, HATCH score, and the prediction model of POAF after CABG developed based on a small sample of clinical data from a single center in China, the model in this study had better discrimination.
Conclusion
We have developed and validated a new prediction model of POAF after CABG using multicenter data that can be used in the clinic for early identification of high-risk patients of POAF, and to help effectively prevent POAF in postoperative patients.
Keywords: Coronary artery bypass grafting, Atrial fibrillation, Prediction
1. Introduction
Atrial fibrillation (AF) is one of the common complications after coronary artery bypass grafting (CABG), with an incidence of about 21%–33%, and the onset is usually 2–3 days after surgery [[1], [2], [3], [4], [5]]. Postoperative AF (POAF) after CABG affects the patient's cardiac function, which in severe cases can lead to risks such as hemodynamic impairment, cerebrovascular accident, acute limb ischemia, renal failure, ventricular arrhythmias [6,7], which in turn affects the patient's postoperative recovery, resulting in a longer intensive care unit (ICU) stays, longer hospital stays, increased medical costs, intra-aortic balloon pump (IABP) assistance requirements, and even death [4,[8], [9], [10]]. Therefore, the prediction and prevention of POAF after CABG is particularly important.
In a cost-effectiveness study of intravenous amiodarone for the prevention of POAF in patients undergoing cardiac surgery, Mahoney et al. suggested that the cost-effectiveness of prophylactic intravenous amiodarone varied according to the predicted risk of AF [11]. Therefore, for patients at high risk of POAF, early identification and targeted use of pharmacological or non-pharmacological interventions to prevent POAF are expected to shorten hospital stays, reduce hospital costs, and reduce the incidence of cerebrovascular accidents [12]. In patients at low risk for POAF, early identification may prevent antiarrhythmic drug abuse and increased hospitalization costs. At present, there is still no effective prediction method for CABG patients with preoperative sinus rhythm in China. Although there are various prediction models for POAF after CABG [3,4,[13], [14], [15]], the predictive value of POAF in the Chinese CABG population, especially the predictive value of new-onset POAF, remains to be studied.
In summary, this study aimed to establish and validate a reliable and accurate risk prediction model by analyzing clinical data from three medical centers of different sizes in China, and to present the model visualized to improve the practical usability of the prediction model and provide clues for early identification of patients at high risk of POAF after CABG, to provide timely individualized preventive guidance for perioperative clinical management strategies.
2. Methods
2.1. Study population
Derivation cohort data were from consecutive patients undergoing CABG at TEDA International Cardiovascular Hospital from June 2020 to December 2021; Validation cohort data were from consecutive patients undergoing CABG at the First Affiliated Hospital of Zhengzhou University from January 2021 to December 2021, and consecutive patients undergoing CABG at Nanyang Central Hospital from January 2020 to December 2021. All data from national triple A, first-class medical center in China with extensive experience in CABG. And all operations are performed by cardiac surgeons with more than 10 years of experience in CABG.
The inclusion criteria were as follows: (1) patients with no previous history of cardiac surgery who were admitted for the first time and underwent CABG; (2) bypass vascular material was internal mammary artery, radial artery, and/or great saphenous vein; (3) Preoperative admission routine ECG was sinus rhythm and no antiarrhythmic drugs (except β-blockers) were used before undergoing CABG. The exclusion criteria were as follows: (1) patients with preoperative combined aortic coarctation; (2) patients with small incision, thoracoscopic or robotic-assisted CABG; (3) patients with permanent cardiac pacemaker implantation. (4) previous history of atrial fibrillation (5) Perioperative prophylactic use of amiodarone. (i.e., preoperative, intraoperative or postoperative prophylactic use of amiodarone).
2.2. Potential risk factors
Based on the analysis of previous studies and expert consensus, and based on the principle of easy clinical access and refinement, the following potential risk factors were selected for inclusion in this study.
-
(1)
Preoperative characteristics: gender, age, body mass index (BMI), smoking history, history of diabetes mellitus, history of hypertension (Class 1: systolic blood pressure 140–159 mmHg or diastolic blood pressure 90–99 mmHg; Class 2: systolic blood pressure 160–179 mmHg or diastolic blood pressure 100–109 mmHg; Class 3: systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥110 mmHg), history of percutaneous coronary intervention (PCI) treatment.
-
(2)
Preoperative medication status: beta-blockers, statins, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB).
-
(3)
Preoperative examination and laboratory indicators: last preoperative arterial blood gas (including partial pressure of oxygen, partial pressure of carbon dioxide, pH, lactate); last preoperative routine blood count/blood biochemistry (including white blood cell count, red blood cell count, neutrophil to lymphocyte ratio (NLR), hemoglobin, platelet count, albumin-to-globulin ratio (A/G), low-density lipoprotein to high-density lipoprotein ratio (LDL/HDL), aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT), creatinine, glucose); Coronary angiography and cardiac ultrasound (including left main coronary artery condition of lesion, left ventricular ejection fraction (LVEF), condition of ventricular aneurysm, left ventricular end-diastolic diameter (LVEDD), left atrial diameter (LAD))
-
(4)
Intraoperative conditions: whether cardiopulmonary bypass (CPB), duration of CPB, intraoperative defibrillation, and temporary pacing lead implantation.
-
(5)
Postoperative care: The fluid balance in the first 24 h after surgery (the first 24 h after the patient leaves the OR), i.e. the actual net 24-h balance, is the difference between the total 24-h in and total 24-h out volume.
2.3. Definition of new-onset POAF
New-onset AF after CABG was the outcome variable of the prediction model, defined as paroxysmal and permanent AF excluded by both preoperative clinical diagnosis and ECG, and the disappearance of p waves indicated by ECG monitoring or ECG after surgery, replaced by f waves of variable size and the duration of the episode was more than 10 min.
2.4. Ethics and study quality control
The Ethics Review Committee of TEDA International Cardiovascular Hospital approved the study and exempted informed consent. The study followed the TRIPOD guidelines and completed all the items in the guidelines. The study uses Epidata 3.1 for all data entry, and after completing the data entry, a comprehensive systematic review is performed and the original data is carefully checked.
2.5. Statistical methods
Data were analyzed by SPSS (ver 26.0, USA) and R software (ver 4.0.5, USA). Continuous variables that did not conform to a normal distribution were expressed as median and interquartile spacing, and the Mann-Whitney U test was used for comparison between groups. Continuous variables that conformed to a normal distribution were expressed as mean ± standard deviation, and two independent samples t-test was used for comparison between groups. The χ2 test was used for categorical variables, which were statistically described as percentages or frequencies (%). The Box-Tidwell method was used to test the existence of a linear relationship between continuous variables and logit transformed values of dependent variables. Continuous variables that did not conform to a linear relation were transformed into categorical variables according to the Youden index of the receiver operator characteristic curve (ROC) or clinically common cut-off value. Factors relevant to new-onset AF preoperatively, intraoperatively and postoperatively were analyzed by univariate logistic regression, with P < 0.2 as the screening criterion, and observed indicators that met the criteria and indicators with clear clinical significance were added to the multivariate logistic regression analysis. Forward stepwise regression analysis based on maximum likelihood estimation was used to select variables and construct a prediction model. The discrimination of the model is evaluated by the ROC curve and calculating the area under the curve (AUC). The calibration of the model was evaluated by the Hosmer-Lemeshow goodness-of-fit test.
3. Results
3.1. Comparison among groups
The study included a total of 3259 patients from three medical centers, including 2531 patients in the derivation cohort and 728 patients in the validation cohort. The incidence of new-onset AF after CABG was 28.45% and 28.98%, respectively (P = 0.778). The comparison between the POAF group and the no-POAF group in the derivation cohort is shown in Table 1. Patients in the POAF group had greater age, LVEDD, LAD, and LVEF than those in the no-POAF group (P < 0.05), and a higher proportion of patients in the POAF group had intraoperative temporary pacing leads implanted (P < 0.05).
Table 1.
Intergroup comparison between the postoperative new-onset AF group and the normal group in the derivation cohort.
| Variables | Non-AF (n = 1811) | AF (n = 720) | Global cohort (n = 2531) | P |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| gender | 0.591 | |||
| male | 1253 (69.19%) | 506 (70.28%) | 1759 (69.50%) | |
| female | 558 (30.81%) | 214 (29.72%) | 772 (30.50%) | |
| age (years) | 64.00 (57.00, 69.00) | 66.00 (60.00, 70.00) | 63.48 ± 8.37 | <0.001 |
| BMI(a) (kg/m2) | 25.67 (23.51, 27.82) | 25.72 (23.73, 27.93) | 25.83 ± 3.24 | 0.262 |
| smoking history | 0.013 | |||
| no | 1116 (61.62%) | 405 (56.25%) | 1521 (60.10%) | |
| yes | 695 (38.38%) | 315 (43.75%) | 1010 (39.90%) | |
| diabetes | 0.173 | |||
| no | 1122 (61.96%) | 425 (59.03%) | 1547 (61.12%) | |
| yes | 689 (38.05%) | 295 (40.97%) | 984 (38.88%) | |
| hypertension | 0.259 | |||
| no | 548 (30.26%) | 220 (30.56%) | 768 (30.34%) | |
| class 1 | 101 (5.58%) | 31 (4.31%) | 132 (5.22%) | |
| class 2 | 382 (21.09%) | 136 (18.89%) | 518 (20.47%) | |
| class 3 | 780 (43.07%) | 333 (46.25%) | 1113 (43.98%) | |
| PCI(b) | 0.237 | |||
| no | 1486 (82.05%) | 605 (84.03%) | 2091 (82.62%) | |
| yes | 325 (17.95%) | 115 (15.97%) | 440 (17.38%) | |
| Preoperative medications | ||||
| β-blockers | 0.549 | |||
| no | 420 (23.19%) | 159 (22.08%) | 579 (22.88%) | |
| yes | 1391 (76.81%) | 561 (77.92%) | 1952 (77.12%) | |
| statins | 0.773 | |||
| no | 120 (6.63%) | 50 (6.94%) | 170 (6.72%) | |
| yes | 1691 (93.37%) | 670 (93.06%) | 2361 (93.28%) | |
| ACEI(c)/ARB(d) | 0.019 | |||
| no | 1240 (68.47%) | 458 (63.61%) | 1698 (67.09%) | |
| yes | 571 (31.53%) | 262 (36.39%) | 833 (32.91%) | |
| Preoperative examination and testing | ||||
| PaO2 (mmHg) | 88.00 (79.00, 100.00) | 86.00 (78.00, 98.00) | 96.58 ± 35.51 | 0.021 |
| PaCO2 (mmHg) | 35.00 (32.00, 38.00) | 35.00 (33.00, 38.00) | 35.09 ± 4.12 | 0.145 |
| PH | 7.42 (7.40, 7.43) | 7.42 (7.40, 7.43) | 7.42 ± 0.03 | 0.913 |
| Lactate (mmol/L) | 0.80 (0.70, 1.10) | 0.80 (0.70, 1.10) | 0.93 ± 0.42 | 0.638 |
| white blood cell count ( × 109/L) | 6.40 (5.40, 7.50) | 6.25 (5.30, 7.30) | 6.49 ± 1.62 | 0.151 |
| red blood cell count ( × 1012/L) | 4.40 (4.00, 4.70) | 4.40 (4.10, 4.70) | 4.41 ± 0.93 | 0.491 |
| NLR(e) | 2.09 (1.58, 2.78) | 2.04 (1.54, 2.75) | 2.38 ± 1.42 | 0.160 |
| Hemoglobin (g/L) | 135.00 (123.00, 146.00) | 136.00 (125.00, 146.00) | 134.89 ± 19.06 | 0.182 |
| platelet count ( × 109/L) | 216.00 (182.00, 257.00) | 214.50 (179.00, 250.00) | 221.57 ± 64.21 | 0.153 |
| A/G(f) | 1.61 (1.44, 1.81) | 1.63 (1.46, 1.79) | 1.63 ± 0.29 | 0.336 |
| LDL(g)/HDL(h) | 2.66 (2.07, 3.34) | 2.53 (1.95, 3.25) | 2.74 ± 1.04 | 0.004 |
| AST(i)/ALT(j) | 0.91 (0.70, 1.17) | 0.90 (0.72, 1.16) | 1.04 ± 0.77 | 0.924 |
| Creatinine (μmol/L) | 69.00 (59.00, 81.00) | 68.00 (58.00, 79.00) | 72.19 ± 31.80 | 0.179 |
| Glucose (mmol/L) | 5.80 (5.10, 6.80) | 5.60 (5.10, 6.80) | 6.25 ± 2.34 | 0.228 |
| left main coronary artery lesion | 0.159 | |||
| no | 1336 (74.18%) | 510 (71.43%) | 1846 (73.40%) | |
| yes | 465 (25.82%) | 204 (28.57%) | 669 (26.60%) | |
| LVEF(k) (%) | 61.00 (55.00, 65.00) | 62.00 (57.00, 66.00) | 59.67 ± 9.30 | 0.001 |
| ventricular aneurysm | 0.009 | |||
| no | 1750 (96.79%) | 681 (94.58%) | 2431 (96.16%) | |
| yes | 58 (3.21%) | 39 (5.42%) | 97 (3.84%) | |
| LVEDD(l) (mm) | 48.00 (45.00, 51.00) | 48.00 (46.00, 52.00) | 48.75 ± 5.41 | <0.001 |
| LAD(m) (mm) | 37.00 (35.00, 40.00) | 38.00 (35.00, 41.00) | 38.10 ± 8.29 | <0.001 |
| Intraoperative conditions | ||||
| CPB(n) | 0.820 | |||
| no | 535 (29.54%) | 216 (30.00%) | 751 (29.67%) | |
| yes | 1276 (70.46%) | 504 (70.00%) | 1780 (70.33%) | |
| duration of CPB (min) | 81.50 (0.00, 107.00) | 82.00 (0.00, 109.00) | 74.13 ± 61.68 | 0.664 |
| intraoperative defibrillation | 0.050 | |||
| no | 1070 (59.12%) | 395 (54.86%) | 1465 (57.91%) | |
| yes | 740 (40.88%) | 325 (45.14%) | 1065 (42.10%) | |
| temporary pacing lead implantation | <0.001 | |||
| no | 1544 (85.30%) | 564 (78.33%) | 2108 (83.32%) | |
| yes | 266 (14.70%) | 156 (21.67%) | 422 (16.68%) | |
| Postoperative Care | ||||
| actual 24 h net balance | 0.321 | |||
| total volume in < total volume out | 1264 (69.80%) | 488 (67.78%) | 1811 (71.55%) | |
| total volume in ≥ total volume out | 547 (30.20%) | 232 (32.22%) | 720 (28.45%) |
Values are mean ± SD, n (%), or median (interquartile range). BMI(a): body mass index; PCI(b): percutaneous coronary intervention; ACEI(c): angiotensin-converting enzyme inhibitor; ARB(d): angiotensin receptor blocker; NLR(e): neutrophil to lymphocyte ratio; A/G(f): albumin/globulin; LDL(g): low-density lipoprotein; HDL(h): high-density lipoprotein; AST(i): aspartate aminotransferase; ALT(j): alanine aminotransferase; LVEF(k): left ventricular ejection fraction; LVEDD(l): left ventricular end-diastolic diameter; LAD(m): left atrial diameter; CPB(n): cardiopulmonary bypass.
3.2. Logistic regression analysis and prediction model development
In this study, 43 items were included in the linearity test, and the significance level should be α = 0.00116 corrected by the Bonferroni method (). According to this level of significance, the p-values of all interaction terms in this study are above 0.00116, so there is a linear relationship between all continuous independent variables and dependent logit transformation values. An univariate logistic regression analysis of potential risk factors for new-onset AF after CABG in the derivation cohort showed that age, BMI, smoking history, history of diabetes mellitus, use of ACEI/ARB, last preoperative arterial partial pressure of oxygen, partial pressure of carbon dioxide, lactate, white blood cell count, hemoglobin, platelet count, LDL/HDL, AST/ALT, presence of left main coronary artery lesion, LVEF, presence of ventricular aneurysm, LVEDD, intraoperative defibrillation, and intraoperative temporary pacing lead implantation may be statistically significant (P < 0.2) in association with new-onset AF after CABG (Table 2).
Table 2.
univariate and multivariate logistic regression analysis.
| Variables | Univariate Logistic |
Multivariate Logistic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR(a) | 95%CI of OR |
P | β | OR | 95%CI of OR |
P | |||
| Lower | Upper | Lower | Upper | ||||||
| gender | 1.05 | 0.87 | 1.27 | 0.591 | |||||
| age | 1.03 | 1.02 | 1.04 | 0.000 | 0.034 | 1.03 | 1.02 | 1.05 | 0.000 |
| BMI(b) | 1.02 | 0.99 | 1.05 | 0.151 | 0.033 | 1.03 | 1.01 | 1.06 | 0.022 |
| smoking history | 1.25 | 1.05 | 1.49 | 0.013 | 0.306 | 1.36 | 1.13 | 1.63 | 0.001 |
| diabetes | 1.13 | 0.95 | 1.35 | 0.173 | |||||
| hypertension | 1.06 | 0.87 | 1.30 | 0.551 | |||||
| PCI(c) | 0.87 | 0.69 | 1.10 | 0.237 | |||||
| β-blockers | 1.07 | 0.87 | 1.31 | 0.549 | |||||
| statins | 0.95 | 0.68 | 1.34 | 0.773 | |||||
| ACEI(d)/ARB(e) | 1.24 | 1.04 | 1.49 | 0.019 | 0.250 | 1.29 | 1.07 | 1.55 | 0.008 |
| PaO2 | 1.00 | 1.00 | 1.00 | 0.179 | |||||
| PaCO2 | 1.02 | 1.00 | 1.04 | 0.134 | |||||
| PH | 0.85 | 0.04 | 18.40 | 0.920 | |||||
| lactate | 1.17 | 0.96 | 1.44 | 0.127 | |||||
| white blood cell count | 0.95 | 0.90 | 1.01 | 0.084 | |||||
| red blood cell count | 0.99 | 0.90 | 1.09 | 0.838 | |||||
| NLR(f) | 0.97 | 0.91 | 1.03 | 0.343 | |||||
| hemoglobin | 1.00 | 1.00 | 1.01 | 0.100 | |||||
| platelet count | 1.00 | 1.00 | 1.00 | 0.159 | |||||
| A/G(g) | 1.05 | 0.78 | 1.41 | 0.754 | |||||
| LDL(h)/HDL(i) | 0.89 | 0.82 | 0.98 | 0.011 | −0.102 | 0.90 | 0.83 | 0.99 | 0.024 |
| AST(j)/ALT(k) | 0.91 | 0.79 | 1.04 | 0.175 | |||||
| creatinine | 1.00 | 1.00 | 1.00 | 0.853 | |||||
| glucose | 0.98 | 0.94 | 1.03 | 0.436 | |||||
| left main coronary artery lesion | 1.15 | 0.95 | 1.40 | 0.159 | |||||
| LVEF(l) | 1.01 | 1.00 | 1.02 | 0.005 | 0.014 | 1.01 | 1.00 | 1.02 | 0.006 |
| ventricular aneurysm | 1.73 | 1.14 | 2.62 | 0.010 | |||||
| LVEDD(m) | 1.03 | 1.02 | 1.05 | 0.000 | 0.024 | 1.02 | 1.01 | 1.04 | 0.004 |
| LAD(n) | 1.01 | 1.00 | 1.02 | 0.211 | |||||
| CPB(o) | 0.98 | 0.81 | 1.18 | 0.820 | |||||
| duration of CPB | 1.00 | 1.00 | 1.00 | 0.307 | |||||
| intraoperative defibrillation | 1.20 | 1.00 | 1.42 | 0.051 | 0.187 | 1.21 | 1.01 | 1.44 | 0.040 |
| temporary pacing lead implantation | 1.61 | 1.29 | 2.00 | 0.000 | 0.398 | 1.49 | 1.18 | 1.88 | 0.001 |
| actual 24 h net balance | 1.10 | 0.91 | 1.32 | 0.321 | |||||
β is the regression coefficient, and the regression coefficient of “constant” in the multivariate logistic regression analysis is −6.015. OR(a): odds ratio; BMI(b): body mass index; PCI(c): percutaneous coronary intervention; ACEI(d): angiotensin-converting enzyme inhibitor; ARB(e): angiotensin receptor blocker; NLR(f): neutrophil to lymphocyte ratio; A/G(g): albumin/globulin; LDL(h): low-density lipoprotein; HDL(i): high-density lipoprotein; AST(j): aspartate aminotransferase; ALT(k): alanine aminotransferase; LVEF(l): left ventricular ejection fraction; LVEDD(m): left ventricular end-diastolic diameter; LAD(n): left atrial diameter; CPB(o): cardiopulmonary bypass.
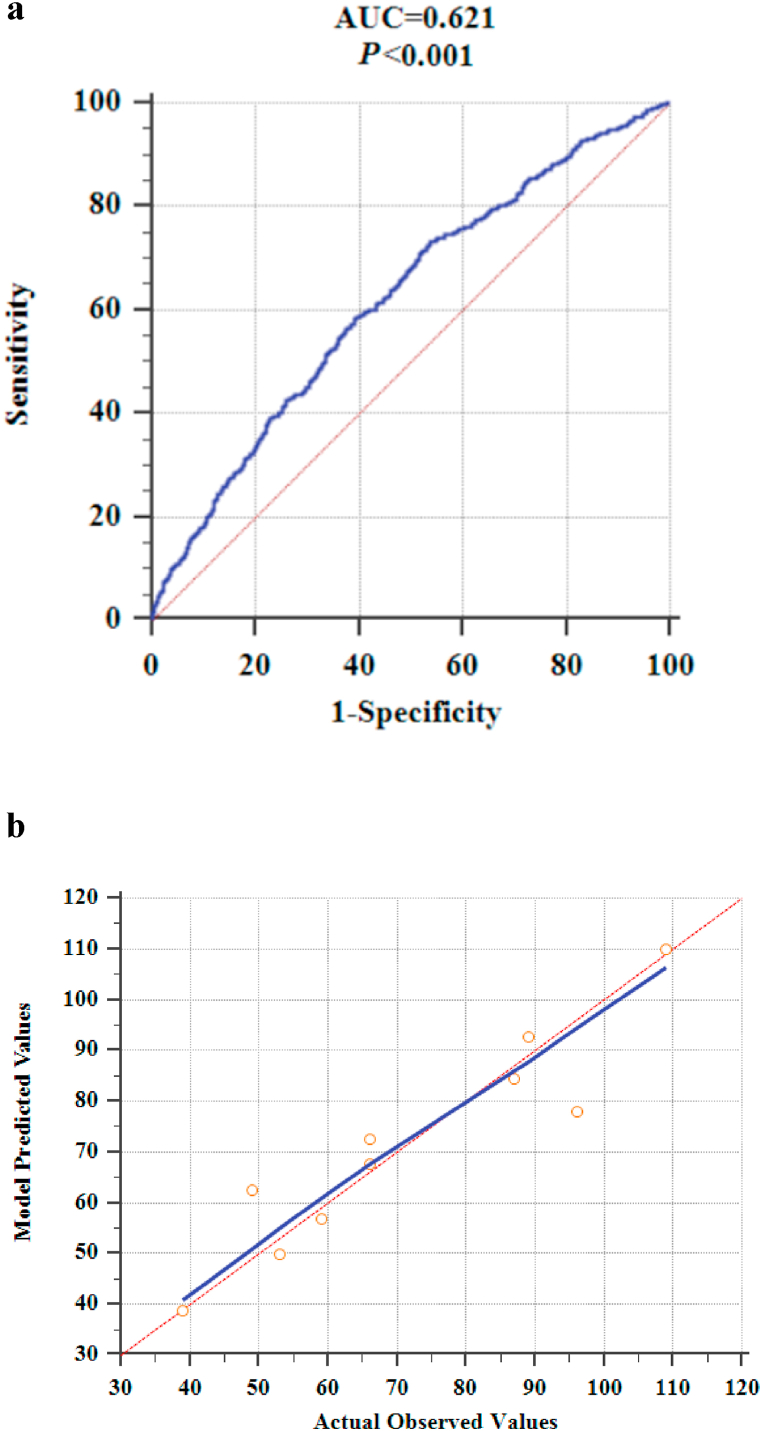
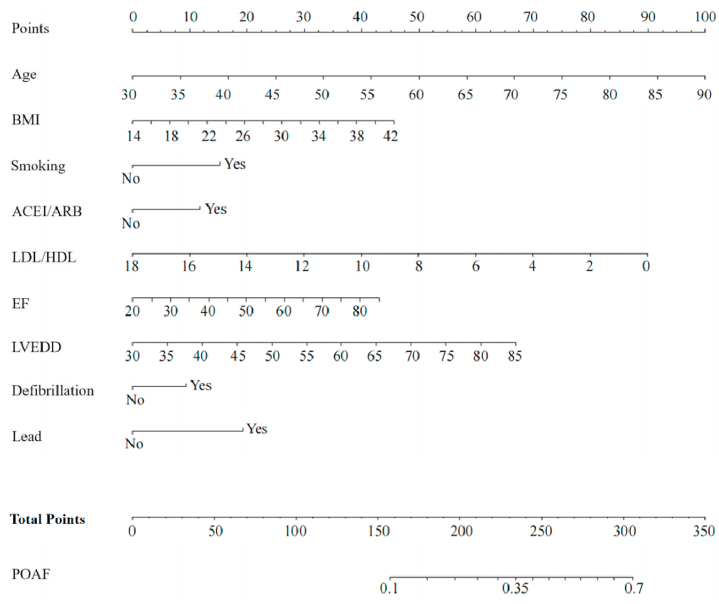
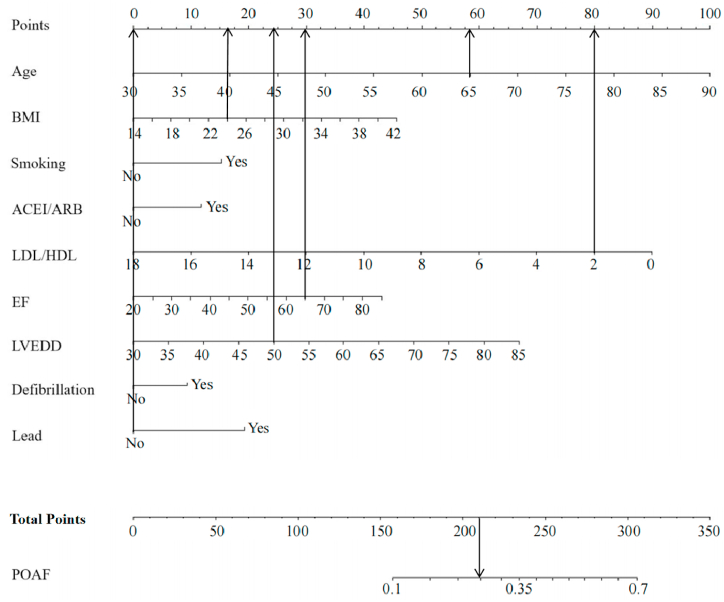
The above factors were included in a multivariate logistic regression analysis along with gender, and results showed that age, BMI, smoking history, use of ACEI/ARB, LDL/HDL, LVEF, LVEDD, intraoperative defibrillation, and intraoperative temporary pacing lead implantation were independent predictors of POAF (Table 2). The logistic regression model is as follows: . P is the probability of new-onset AF after CABG. The model was statistically significant (χ2 = 91.886, P = 0.000) and its percent correct classification (i.e., agreement rate in diagnostic tests) was 71.8%. ROC curve analysis showed that the area under the curve was 0.621 (95% CI = 0.602–0.640). The sensitivity and specificity at the optimal cut-point (i.e., at POAF probability >26.22%) were 73.3% (95% CI = 0.699–0.765) and 46.2% (95% CI = 0.438–0.485), respectively (Fig. 1a). The Hosmer-Lemeshow goodness-of-fit test statistic was 11.448, p = 0.178 > 0.05, and the calibration curve is shown in Fig. 1b. The nomogram of the model is shown in Fig. 2.
Fig 1.
a. ROC curve of the prediction model.
b. The calibration curve of the prediction model (the blue line is the calibration curve; the red line is the standard curve, which means that the prediction risk of the model is the same as the actual observed risk. The closer the calibration curve and the standard curve are, the better the calibration capability of the prediction model is).
Fig. 2.
The prediction model is presented as nomogram (BMI: body mass index; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; LDL: low-density lipoprotein; HDL: high-density lipoprotein; EF (LVEF): left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter).
3.3. External validation
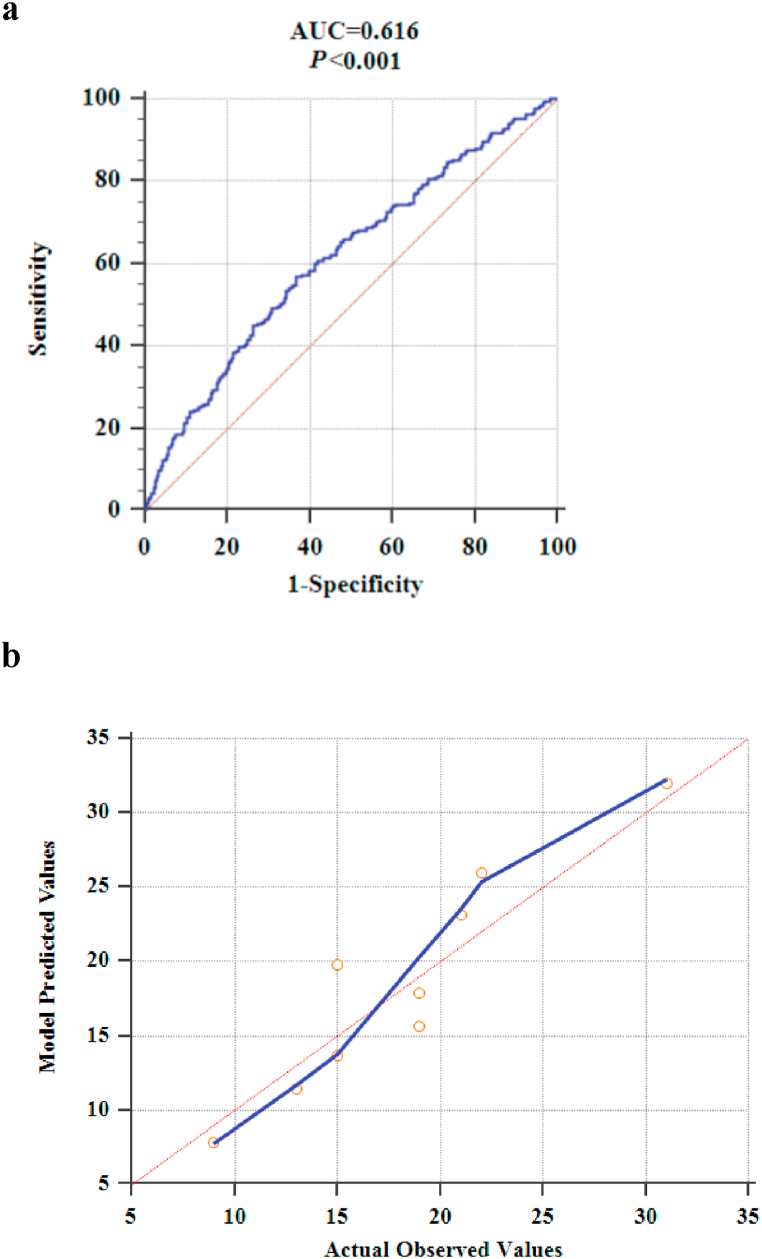
The validation cohort evaluated the model developed in the study in terms of both discrimination and calibration, and the results showed that the area under the ROC curve was 0.616 (95% CI = 0.579 to 0.651). The sensitivity and specificity at the optimal cut-point (i.e., POAF probability >24.87%) were 56.9% (95% CI = 0.499–0.637) and 63.3% (95% CI = 0.589–0.674) (Fig. 3a). The difference between the area under the ROC curve of the derivation cohort and the validation cohort is 0.005. The Z statistic of the Delong test was 0.187, P = 0.852 > 0.05, so the difference in AUC between the two cohorts was not statistically significant. The Hosmer-Lemeshow goodness-of-fit test statistic was 5.204, p = 0.736 > 0.05, and the calibration curve is shown in Fig. 3b.
Fig 3.
a. Discrimination assessment of external validation of prediction model.
b. Calibration assessment for external validation of prediction model (the blue line is the calibration curve; the red line is the standard curve, which means that the prediction risk of the model is the same as the actual observed risk. The closer the calibration curve and the standard curve are, the better the calibration capability of the prediction model is).
3.4. Comparison of different models
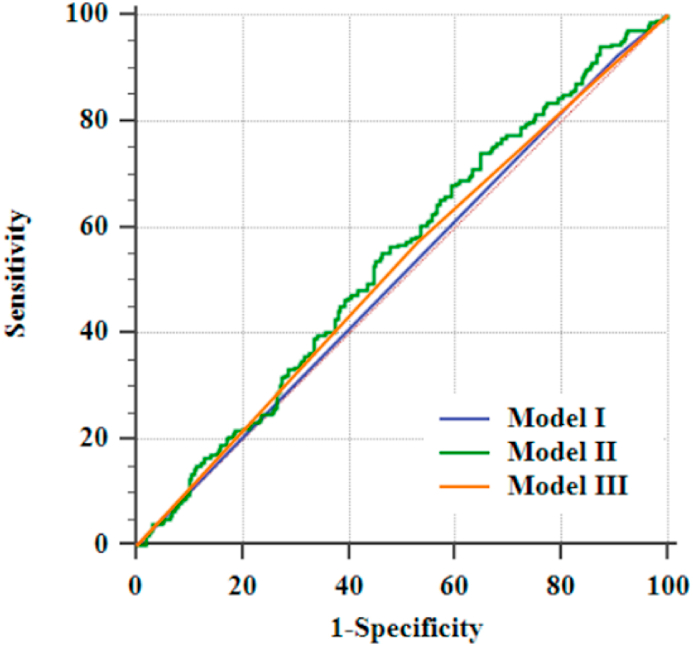
The discrimination was assessed in the validation cohort on CHA2DS2-VASc [16], HATCH score [17], and the prediction model developed based on a small sample of clinical data from a single center in China [18], and the results are shown in Fig. 4.
Fig. 4.
Assessment of the discrimination of the validation group against other models (Model Ⅰ is the HATCH score, AUC = 0.509, 95%CI = 0.472–0.646, P = 0.446; Model Ⅱ is the prediction model developed by Li et al. AUC = 0.541, 95%CI = 0.504–0.577, P = 0.083; Model Ⅲ is CHA2DS2-VASc, AUC = 0.521, 95%CI = 0.484–0.558, P = 0.313).
3.5. Model visualization
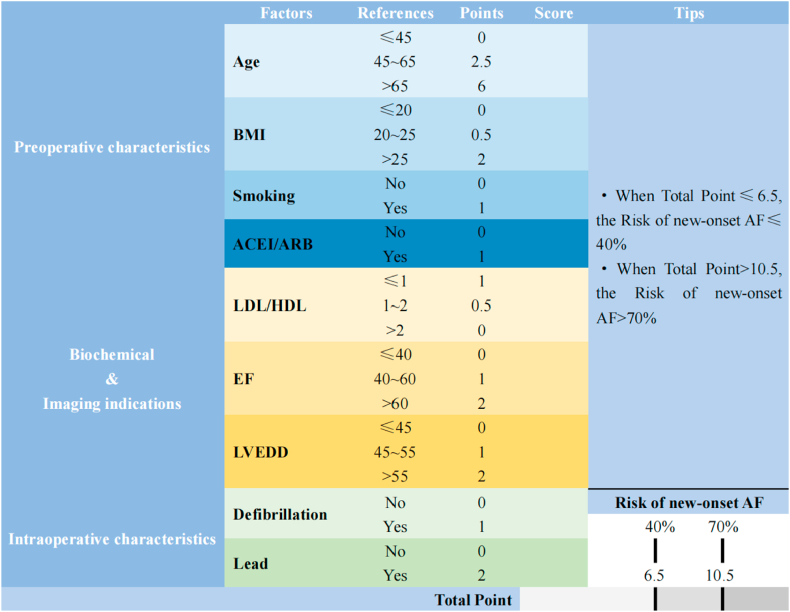
Predictors in the prediction model are classified according to their clinical significance or common cut-off values. Based on logistic regression, appropriate values in each subgroup were selected as reference values to construct the scoring tool (Fig. 5).
Fig. 5.
Scoring Scale (BMI: body mass index; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; LDL: low-density lipoprotein; HDL: high-density lipoprotein; EF (LVEF): left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter).
4. Discussion
The majority of patients at TEDA are from northern China, while patients at the other two medical centers are mainly from the mid-China region, covering a large part of China, so the data could better represent the CABG population in China. The study population was derived from 3 medical centers of different sizes (with different annual surgical volumes) in China, and the data were patients consecutively receiving CABG alone in the last 2 years, with an overall incidence of new-onset POAF of about 28%, generally consistent with the results of previous studies [[1], [2], [3], [4]]. As one of the common complications after CABG, POAF is associated with several adverse complications and affects the patient's postoperative recovery, even leading to death [[6], [7], [8], [9], [10]]. Therefore, effective identification of patients at high risk for POAF can guide targeted prevention strategies during the perioperative period. In the study, a prediction model for POAF after CABG was developed by logistic regression based on general demographic characteristics, preoperative biological markers, intraoperative conditions, and postoperative fluid balance in CABG patients, and the model was evaluated and externally validated in terms of both discrimination and calibration. Multivariate logistic regression analysis showed that age, BMI, smoking history, use of ACEI/ARB, LDL/HDL, LVEF, LVEDD, intraoperative defibrillation, and intraoperative temporary pacing lead implantation were independent predictors of POAF. Among them, age, BMI, smoking history, use of ACEI/ARB, and LDL/HDL were consistent with the results of previous studies [14,19,20]. However, the predictive role of LVEDD, intraoperative defibrillation, and intraoperative temporary pacing lead implantation for new-onset AF after CABG has rarely been reported.
Age is a common risk factor or predictor of POAF after cardiac surgery, and it is generally accepted that the risk of POAF increases with age [3,4,13,14,[19], [20], [21], [22], [23], [24], [25], [26]]. The results of this study were similar to most previous studies. Multivariate logistic regression analysis revealed that age was a predictor of new-onset POAF after CABG (P < 0.05), and the risk of new-onset POAF after CABG increased with age (OR>1). A possible explanation may be that age-related atrial fibrosis [27] and degeneration and loss of atrial myocytes [28] may increase conduction heterogeneity through discrete regions of slow conduction, thereby promoting and maintaining AF by favoring intra-atrial reentry [29,30], giving rise to an electrophysiological substrate that predisposes to AF in elderly patients. And the observed negative correlation of increasing age with hypoxic signals (hypoxia-inducible factor-1α, vascular endothelial growth factor, etc.) in atrial tissue may reduce adaptation to hypoxia in old age [27], and contribute to age-related atrial fibrosis.
In addition to age, overweight and obesity are also considered to be associated with AF [19,[31], [32], [33]]. Multivariate logistic regression analysis showed that BMI was a predictor of new-onset POAF after CABG (OR>1, P < 0.05). The results of Zacharias et al. are the same as our study, which concluded that obesity is an important determinant of new-onset POAF after cardiac surgery and that POAF risk models should incorporate BMI or obesity levels [34]. The results of this study suggest that the risk of POAF after CABG increases with increasing BMI. Possible reasons may be increased BMI or obesity affecting the patient's epicardial adipose volume. Epicardial adipose tissue (EAT) is an adipose depot with specific endocrine functions, producing a large number of adipokines such as adiponectin, leptin, resistin, visfatin, omentin, zinc-alpha 2-glycoprotein, dextran-4, etc [35,36]. Since there is no tissue like fascia separating EAT and myocardial tissue, adipokines and/or inflammatory mediators secreted by EAT can act directly on myocardial tissue and interact with surrounding atrial myocytes, extracellular matrix and vascular endothelial cells to induce or participate in the process of AF development [37,38].
A meta-analysis shows that smoking is associated with an increased risk of AF [39]. The results of this study also confirmed that smoking was associated with an increased risk of new-onset AF after CABG (OR>1, P < 0.05). Nicotine increases oxidative stress by stimulating sympathetic neurotransmission, thereby altering serum catecholamine concentrations and ion channel status, activating the inflammatory system, and triggering atrial remodeling through pro-fibrotic effects [[40], [41], [42]]. In contrast, carbon monoxide in tobacco induces structural remodeling that may facilitate arrhythmias by causing tissue hypoxia and inducing myocardial fibrosis [41].
The effect of ACEI on AF is currently controversial. This study found that preoperative use of ACEI increased the risk of POAF after CABG (OR>1, P < 0.05). Magee et al. found in their prediction model that the use of ACEI was associated with an increased risk of POAF after CABG [14], contrary to Mathew et al.'s study in the Multicenter Risk Index, in which they stated that pre- and post-operatively initiated ACEI therapy was associated with a reduced risk of AF, while withdrawal from therapy was associated with an increased risk [4]. ACEI has been shown to reduce the incidence of atrial fibrillation in patients with left ventricular dysfunction after acute myocardial infarction [43]. This may be related to ACEI blocking the myocardial remodeling process associated with the renin-angiotensin system, and atrial remodeling appears to be the central mechanism promoting AF recurrence and maintenance [44]. Therefore, in patients with a high probability of myocardial remodeling, such as heart failure and myocardial infarction, the anti-AF effect of ACEI was demonstrated and reported. However, the effect of ACEI on POAF in patients without preoperative heart failure is unclear and controversial, and further studies are needed.
LDL/HDL has become one of the predictive indicators of atherosclerosis severity [45], bypass stenosis after CABG [46,47], and carotid intima-media thickness [48]. And few reports are available on the relationship between LDL/HDL and AF. In the study, multivariate logistic regression analysis showed that LDL/HDL was a protective factor for POAF after CABG (OR<1, P < 0.05). This result is consistent with previous research suggesting a “cholesterol paradox”, in which higher levels of lipids, although an important risk factor for cardiovascular disease, are negatively associated with the risk of AF [48].
Echocardiographic indices are increasingly being used in the evaluation and prediction of POAF [49]. The previous study results suggested that low LVEF is a predictor of POAF or AF recurrence [3,49,50]. In Stefàno et al. [51], LVEF was found to be independent of AF after cardiac surgery in patients without previous AF. No association between POAF and preoperative LVEF was also found in a study of preoperative sinus rhythm patients who underwent CABG under CPB [52]. However, high preoperative LVEF is a predictor of POAF after CABG by multivariate logistic regression analysis in our study (OR>1, P < 0.05). The reasons may be the study population and the definitions of outcomes were different; the population in this study was patients with preoperative sinus rhythm before CABG and the outcome was new-onset AF, whereas the population in previous studies was mostly all cardiac surgery patients and the outcome included new-onset AF and recurrent AF. However, it is not clear why this association was seen, and the relationship between LVEF and new-onset POAF needs further investigation. In addition to LVEF, LVEDD is also considered to be associated with POAF or AF recurrence [49,53]. The same results were observed in our study, i.e., the risk of POAF after CABG increased with preoperative LVEDD (OR>1, P < 0.05). According to Frank-Starling mechanism, LVEDD reflects left ventricular end-diastolic pressure (LVEDP) to some extent. Animal experiments revealed that in a mouse model of ventricular pressure overload, the extent of atrial remodeling was directly correlated with LVEDP and atrial gene expression related to fibrosis was increased [54]. As mentioned previously, atrial remodeling and atrial fibrosis are involved in the development and progression of AF.
Intraoperative defibrillation and temporary pacing lead implantation were also predictors of POAF after CABG in this study (P < 0.05). Patients undergoing intraoperative defibrillation and/or implantation of temporary pacing leads were more likely to develop POAF postoperatively (OR<1). Arrigo et al. [55]demonstrated that in patients with POAF, external electrical cardioversion restored sinus rhythm immediately, but recurrence was common within 24 h. A multicenter study showed the possibility of arrhythmic complications remaining after AF electrical resuscitation [56]. In the CABG population in this study, the potential causes of new-onset POAF in intraoperative defibrillation and temporary pacing of lead implantation may be as follows: (1) Intraoperative electrical cardioversion of the heart surface, although low in energy, where defibrillation electrodes directly interface with the myocardium of the atria and ventricles, may lead to dysfunction of sinus nodes, and thus become a potential risk for POAF. (2) Patients defibrillated intraoperatively for any reason commonly have transient sinus bradycardia and frequently require temporary pacemaker implantation, the common type of implantation is atrial pacing or atrioventricular sequential pacing, regardless of postoperative use or not, the pacemaker electrodes may stimulate the atria and thus produce AF. At present, there are few studies on the mechanism of electrical cardioversion associated with POAF, and the specific causes and potential mechanisms need to be further investigated.
Using the above predictors, the study developed prediction models through logistic regression and presented them visually through nomogram and scoring scale. The model had fair discrimination (AUC = 0.621) and had a high ability to identify patients (sensitivity of 73.3%) but could be misdiagnosed (specificity of 46.2%). The incidence of POAF after CABG predicted by this model was in good consistency with the actual incidence by the Hosmer-Lemeshow goodness-of-fit test.
CHA2DS2-VASc is a clinical scoring tool to assess stroke risk in patients with AF and has also been used in recent years to predict AF or POAF [16]. The HATCH score is primarily used to assess the progression of persistent AF [17], but it has also been demonstrated as a predictor of POAF after CABG [57,58]. Li et al. [18], on the other hand, developed a prediction model for POAF after non-extracorporeal CABG using a small sample of data from a single center in China. In contrast, this study has a large sample size and the model was externally validated. External validation results showed that the discrimination of the model developed in this study (AUC = 0.616) > Li et al.'s model (AUC = 0.541) > CHA2DS2-VASc (AUC = 0.521) > HATCH score (AUC = 0.509).
Nomogram or scoring scale simplifies the cumbersome logistic regression model for clinical use. For example, a 65-year-old male patient with CABG in preoperative sinus rhythm, preoperative BMI of 24 kg/m2, no smoking history, without the use of ACEI or ARB, LDL/HDL = 2.08, LVEF = 65%, LVEDD = 50 mm, and no intraoperative defibrillation or temporary pacing lead implantation. The total score obtained by using the nomogram for this patient was approximately 208 = 57 + 16+0 + 0+80 + 30+25 + 0+0, which corresponds to a risk probability of POAF after CABG <35% (Fig. 6). The total score obtained from the scoring scale was 6 = 2.5 + 0.5+0 + 0+0 + 0+2 + 1+0 + 0, which corresponds to a risk probability of POAF after CABG <40%.
Fig. 6.
Examples of nomogram applications (BMI: body mass index; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; LDL: low-density lipoprotein; HDL: high-density lipoprotein; EF (LVEF): left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter).
The study is retrospective and has some limitations: First, some of the preoperative biochemical markers and/or coronary angiography specifics were not included because of severe missing, which may lead to biased study results and also limit the validation and comparison of this study cohort to other more prediction models. Second, the study only recorded AF occurred during the inpatient period, and no long-term follow-up of patients was recorded, which may lead to an underestimation of the incidence of postoperative AF in this study. In future studies, it is highly necessary to conduct prospective multicenter studies, standardize patient follow-up, collect patient survival data, and construct models with COX regression to improve the models.
5. Conclusion
Age, preoperative BMI, smoking history, use of ACEI/ARB, LDL/HDL, LVEF, LVEDD, intraoperative defibrillation, and temporary pacing lead implantation were independent predictors of POAF after CABG. This prediction model can be used for the early identification of patients at high risk of POAF and helps to differentiate patients in the postoperative period. Nomogram and scoring scale visualization models are more convenient for the assessment of POAF and have certain advantages for clinical use. In future studies, it is highly necessary to conduct prospective multicenter studies, standardize patient follow-up, collect patient survival data, and construct models with COX regression to improve the models.
Author contribution statement
Renjianzhi zhang; Xin-Yi Yu; Jing Wang; Jian Lv: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yan Zheng: Analyzed and interpreted the data.
Ming-Huan Yu; Yi-Rui Zang; Jian-Wei Shi; Jia-Hui Wang: Contributed reagents, materials, analysis tools or data.
Li Wang; Zhi-Gang Liu: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research is funded by Tianjin Key Medical Discipline (Specialty) Construction Project.
Data availability statement
The authors do not have permission to share data.
Additional information
Supplementary content related to this article has been published online at [URL].
Declaration of interest's statement
The authors declare no conflict of interest.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- AF
atrial fibrillation
- A/G
albumin/globulin
- ALT
alanine aminotransferase
- ARB
angiotensin receptor blocker
- AST
aspartate aminotransferase
- AUC
area under the curve
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CPB
cardiopulmonary bypass
- EAT
epicardial adipose tissue
- HDL
high density lipoprotein
- IABP
intra-aortic balloon pump
- ICU
intensive care unit
- LAD
left atrial diameter
- LDL
low density lipoprotein
- LVEDD
left ventricular end-diastolic diameter
- LVEDP
left ventricular end-diastolic pressure
- LVEF
left ventricular ejection fraction
- NLR
neutrophil to lymphocyte ratio
- OR
odds ratio
- PCI
percutaneous coronary intervention
- POAF
postoperative atrial fibrillation
- ROC
receiver operator characteristic curve
References
- 1.Perrier S., et al. Predictors of atrial fibrillation after coronary artery bypass grafting: a bayesian analysis. Ann. Thorac. Surg. 2017;103(1):92–97. doi: 10.1016/j.athoracsur.2016.05.115. [DOI] [PubMed] [Google Scholar]
- 2.Filardo G., et al. Epidemiology of new-onset atrial fibrillation following coronary artery bypass graft surgery. Heart. 2018;104(12):985–992. doi: 10.1136/heartjnl-2017-312150. [DOI] [PubMed] [Google Scholar]
- 3.Mariscalco G., et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J. Am. Heart Assoc. 2014;3(2):e000752. doi: 10.1161/JAHA.113.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew J.P., et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 5.Filardo G., et al. Underestimation of the incidence of new-onset post-coronary artery bypass grafting atrial fibrillation and its impact on 30-day mortality. J. Thorac. Cardiovasc. Surg. 2017;154(4):1260–1266. doi: 10.1016/j.jtcvs.2017.05.104. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg B.A., et al. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the Society of Thoracic Surgeons CAPS-Care Atrial Fibrillation Registry. Clin. Cardiol. 2014;37(1):7–13. doi: 10.1002/clc.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedetto U., et al. Postoperative atrial fibrillation and long-term risk of stroke after isolated coronary artery bypass graft surgery. Circulation. 2020;142(14):1320–1329. doi: 10.1161/CIRCULATIONAHA.120.046940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra P., et al. Postoperative atrial fibrillation in coronary artery bypass grafting herald poor outcome. Ann. Card Anaesth. 2021;24(4):464–469. doi: 10.4103/aca.ACA_30_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eikelboom R., et al. Postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Ann. Thorac. Surg. 2021;111(2):544–554. doi: 10.1016/j.athoracsur.2020.05.104. [DOI] [PubMed] [Google Scholar]
- 10.Kerwin M., et al. New-onset atrial fibrillation and outcomes following isolated coronary artery bypass surgery: a systematic review and meta-analysis. Clin. Cardiol. 2020;43(9):928–934. doi: 10.1002/clc.23414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahoney E.M., et al. Cost-effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J. Am. Coll. Cardiol. 2002;40(4):737–745. doi: 10.1016/s0735-1097(02)02003-x. [DOI] [PubMed] [Google Scholar]
- 12.Arsenault K.A., et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst. Rev. 2013;2013(1):Cd003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amar D., et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J. Am. Coll. Cardiol. 2004;44(6):1248–1253. doi: 10.1016/j.jacc.2004.05.078. [DOI] [PubMed] [Google Scholar]
- 14.Magee M.J., et al. Atrial fibrillation after coronary artery bypass grafting surgery: development of a predictive risk algorithm. Ann. Thorac. Surg. 2007;83(5):1707–1712. doi: 10.1016/j.athoracsur.2006.12.032. ; discussion 1712. [DOI] [PubMed] [Google Scholar]
- 15.Hung L.T., et al. Predicting atrial fibrillation after cardiac surgery using a simplified risk index. J. Electrocardiol. 2021;67:45–49. doi: 10.1016/j.jelectrocard.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Borde D., et al. Prediction of postoperative atrial fibrillation after coronary artery bypass grafting surgery: is CHA 2 DS 2 -VASc score useful? Ann. Card Anaesth. 2014;17(3):182–187. doi: 10.4103/0971-9784.135841. [DOI] [PubMed] [Google Scholar]
- 17.de Vos C.B., et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010;55(8):725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Li F.L., Feng Z.B. Establishment of a nomogram to predict the risk of atrial fibrillation after coronary artery bypass grafting. J. Clin. Cardiovasc. Dis. 2020;36(4):335–340. [Google Scholar]
- 19.El-Chami M.F., et al. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. Am. J. Cardiol. 2012;110(5):649–654. doi: 10.1016/j.amjcard.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 20.Thorén E., et al. Prediction of postoperative atrial fibrillation in a large coronary artery bypass grafting cohort. Interact. Cardiovasc. Thorac. Surg. 2012;14(5):588–593. doi: 10.1093/icvts/ivr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolek M.J., et al. Genetic and clinical risk prediction model for postoperative atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8(1):25–31. doi: 10.1161/CIRCEP.114.002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan K., et al. Predicting new-onset postoperative atrial fibrillation following isolated coronary artery bypass grafting: development and validation of a novel nomogram. Int. J. Gen. Med. 2022;15:937–948. doi: 10.2147/IJGM.S346339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaman A.G., et al. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation. 2000;101(12):1403–1408. doi: 10.1161/01.cir.101.12.1403. [DOI] [PubMed] [Google Scholar]
- 24.Rizvi F., et al. Noninvasive biomarker-based risk stratification for development of new onset atrial fibrillation after coronary artery bypass surgery. Int. J. Cardiol. 2020;307:55–62. doi: 10.1016/j.ijcard.2019.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgos L.M., et al. Development and validation of A simple clinical risk prediction model for new-onset postoperative atrial fibrillation after cardiac surgery: nopaf score. J. Atr. Fibrillation. 2020;13(2):2249. doi: 10.4022/jafib.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banach M., et al. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ. J. 2006;70(4):438–441. doi: 10.1253/circj.70.438. [DOI] [PubMed] [Google Scholar]
- 27.Gramley F., et al. Age-related atrial fibrosis. Age (Dordr) 2009;31(1):27–38. doi: 10.1007/s11357-008-9077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gramley F., et al. Decreased plasminogen activator inhibitor and tissue metalloproteinase inhibitor expression may promote increased metalloproteinase activity with increasing duration of human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007;18(10):1076–1082. doi: 10.1111/j.1540-8167.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 29.Li D., et al. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100(1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 30.Shinagawa K., et al. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105(22):2672–2678. doi: 10.1161/01.cir.0000016826.62813.f5. [DOI] [PubMed] [Google Scholar]
- 31.Frost L., Hune L.J., Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am. J. Med. 2005;118(5):489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Korantzopoulos P., Kolettis T.M. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2005;293(16):1974. doi: 10.1001/jama.293.16.1974-a. ; author reply 1975. [DOI] [PubMed] [Google Scholar]
- 33.Goudis C.A., et al. Obesity and atrial fibrillation: a comprehensive review of the pathophysiological mechanisms and links. J. Cardiol. 2015;66(5):361–369. doi: 10.1016/j.jjcc.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Zacharias A., et al. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. 2005;112(21):3247–3255. doi: 10.1161/CIRCULATIONAHA.105.553743. [DOI] [PubMed] [Google Scholar]
- 35.Thanassoulis G., et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3(4):345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong C.X., et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J. Am. Coll. Cardiol. 2011;57(17):1745–1751. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 37.Liberale L., et al. The role of adipocytokines in coronary atherosclerosis. Curr. Atherosclerosis Rep. 2017;19(2):10. doi: 10.1007/s11883-017-0644-3. [DOI] [PubMed] [Google Scholar]
- 38.Toczylowski K., et al. Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J. Transl. Med. 2019;17(1):310. doi: 10.1186/s12967-019-2060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aune D., et al. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta-analysis of prospective studies. Eur. J. Prev. Cardiol. 2018;25(13):1437–1451. doi: 10.1177/2047487318780435. [DOI] [PubMed] [Google Scholar]
- 40.Haass M., Kübler W. Nicotine and sympathetic neurotransmission. Cardiovasc. Drugs Ther. 1997;10(6):657–665. doi: 10.1007/BF00053022. [DOI] [PubMed] [Google Scholar]
- 41.D'Alessandro A., et al. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol. 2012;19(3):297–305. doi: 10.1177/1741826711411738. [DOI] [PubMed] [Google Scholar]
- 42.Goette A., et al. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart. 2007;93(9):1056–1063. doi: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen O.D., et al. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999;100(4):376–380. doi: 10.1161/01.cir.100.4.376. [DOI] [PubMed] [Google Scholar]
- 44.Allessie M.A. Atrial electrophysiologic remodeling: another vicious circle? J. Cardiovasc. Electrophysiol. 1998;9(12):1378–1393. doi: 10.1111/j.1540-8167.1998.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 45.Paramsothy P., et al. Age-modification of lipoprotein, lipid, and lipoprotein ratio-associated risk for coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am. J. Cardiol. 2010;105(3):352–358. doi: 10.1016/j.amjcard.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y.Y., et al. Effect of lipid exposure on graft patency and clinical outcomes: arteries and veins are different. Eur. J. Cardio. Thorac. Surg. 2014;45(2):323–328. doi: 10.1093/ejcts/ezt261. [DOI] [PubMed] [Google Scholar]
- 47.Song Y., et al. The apoB100/apoAI ratio is independently associated with the severity of coronary heart disease: a cross sectional study in patients undergoing coronary angiography. Lipids Health Dis. 2015;14:150. doi: 10.1186/s12944-015-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah A.S., et al. Lipids and lipoprotein ratios: contribution to carotid intima media thickness in adolescents and young adults with type 2 diabetes mellitus. J Clin Lipidol. 2013;7(5):441–445. doi: 10.1016/j.jacl.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magne J., et al. Echocardiography is useful to predict postoperative atrial fibrillation in patients undergoing isolated coronary bypass surgery: a prospective study. Eur Heart J Acute Cardiovasc Care. 2019;8(2):104–113. doi: 10.1177/2048872616688419. [DOI] [PubMed] [Google Scholar]
- 50.Li Y., et al. Value of echocardiography in evaluating efficacy of radiofrequency catheter ablation in patients with atrial fibrillation. Am J Transl Res. 2022;14(3):1778–1787. [PMC free article] [PubMed] [Google Scholar]
- 51.Stefàno P.L., et al. Overweight and aging increase the risk of atrial fibrillation after cardiac surgery independently of left atrial size and left ventricular ejection fraction. J. Cardiothorac. Surg. 2020;15(1):316. doi: 10.1186/s13019-020-01366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Massih T.E., et al. Myocardial extraction of intracellular magnesium and atrial fibrillation after coronary surgery. Int. J. Cardiol. 2012;160(2):114–118. doi: 10.1016/j.ijcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Houltz B., et al. Left ventricular diastolic function and right atrial size are important rhythm outcome predictors after intraoperative ablation for atrial fibrillation. Echocardiography. 2010;27(8):961–968. doi: 10.1111/j.1540-8175.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- 54.De Jong A.M., et al. Atrial remodeling is directly related to end-diastolic left ventricular pressure in a mouse model of ventricular pressure overload. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0072651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrigo M., et al. Disappointing success of electrical cardioversion for new-onset atrial fibrillation in cardiosurgical ICU patients. Crit. Care Med. 2015;43(11):2354–2359. doi: 10.1097/CCM.0000000000001257. [DOI] [PubMed] [Google Scholar]
- 56.Grönberg T., et al. Arrhythmic complications after electrical cardioversion of acute atrial fibrillation: the FinCV study. Europace. 2013;15(10):1432–1435. doi: 10.1093/europace/eut106. [DOI] [PubMed] [Google Scholar]
- 57.Emren V., et al. Usefulness of HATCH score as a predictor of atrial fibrillation after coronary artery bypass graft. Kardiol. Pol. 2016;74(8):749–753. doi: 10.5603/KP.a2016.0045. [DOI] [PubMed] [Google Scholar]
- 58.Selvi M., et al. A new predictor of atrial fibrillation after coronary artery bypass graft surgery: HATCH score. J. Invest. Med. 2018;66(3):648–652. doi: 10.1136/jim-2017-000525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.