Abstract
Inflammation is a complicated physiological process that results in a variety of disorders. Several inflammatory mediators are produced during this process, which is responsible for long-term inflammatory conditions like osteoarthritis, rheumatoid arthritis, asthma, cancer, and neurological disorders. Inflammatory mediators are produced by an arachidonic acid pathway that gives us several anti-inflammatory targets. The most commonly used medications are NSAIDs to treat inflammation by inhibiting cyclooxygenase (COX) and lipoxygenase enzymes (5-LOX). However, this therapy is associated with adverse events like gastrointestinal disorders, renal failure, etc., limiting its use. Therefore, novel, efficacious, and safer anti-inflammatory agents are prerequisites for inhibiting both cyclooxygenase and lipoxygenase pathways. Though several synthetic analogs are under development, natural products may act as a potential source to identify novel molecules and herbal remedies. Valuable contributions have been made in this direction by the scientific communities. This review article briefly discusses the implications of phytochemicals and bioactive fractions in the development of dual COX-LOX inhibitors while highlighting different classes of phytoconstituents such as tannins, steroids, flavonoids, alkaloids, terpenoids, among others, that showed significant dual COX-LOX inhibition.
Keywords: Inflammation, Natural products, Cyclooxygenase, 5-Lipoxygenase, Dual COX-LOX inhibitors
1. Introduction
Inflammation is a critical defense mechanism that keeps us healthy. Our immune system plays a vital role in countering harmful stimuli like poisonous substances, damaged cells, and pathogens which removes harmful stimuli and commences the healing process. The cellular and molecular connections that result from acute inflammatory reactions can reduce the risk of infection or injury. This process also aids in tissue homeostasis and stops acute inflammation. On the other hand, uncontrolled inflammation results in chronic inflammatory disorders like obesity (OB), coronary heart disease (CHD), Parkinson's disease (PD), Alzheimer's disease, (AD) diabetes mellitus, osteoarthritis (OA), and cancer [1]. Additionally, lipid and protease mediators are among the significant components that must be produced in a complex and coordinated manner for the widespread phenomena of inflammation. Inflammation initiates and progresses due to the overexpression of arachidonic acid (AA) cascade mediators, specifically those arising from 5-lipoxygenase (5-LOX) and cyclooxygenase (COX) pathways [2]. The Cyclooxygenase pathway is one of the most widely studied inflammatory pathways in mammals. The chemical mediators include prostaglandins (PG) and leukotrienes (LT) that induce specific inflammatory reactions [3].
Corticosteroids (glucocorticoids) and Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly utilized for treating inflammation, as well as to relieve pain and fever [4]. Corticosteroids block the phospholipase-A2 enzyme, preventing the production of AAs and their downstream products, including COX and LOX, whereas NSAIDs block the COX pathway. Two different COX isoforms are COX-1 and COX-2 which were implicated in different functions and have other gene codes [5,6]. COX-2 majorly accounts for the harmful inflammatory responses whereas COX-1 is linked to hemostatic functions. Thereby, selective COX-2 inhibitors were developed [7]. With NSAIDs inhibiting the COX-2 enzyme route, substrate diversion of AA metabolism occurs to the other major LOX pathway, resulting in increased LT production and causing an inflammatory reaction [8].
1.1. COX and LOX pathways in inflammation
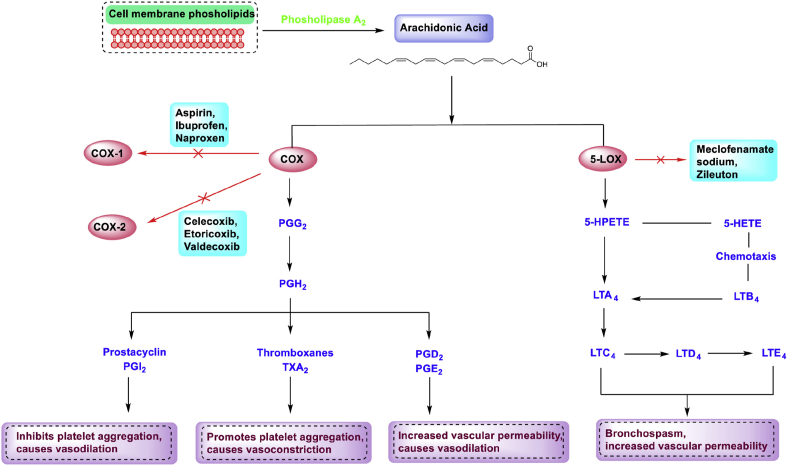
Arachidonic acid (AA) is the most crucial precursor of inflammatory pathways. There is external and internal factor which activates the phospholipase-A2. This activation cleaves membrane-bound AA and makes it available for the COX and LOX pathways (Fig. 1) [9]. COX and LOX are well-known pro-inflammatory enzymes, such as prostaglandins and leukotrienes, required for eicosanoids' physiological production. COX oxidizes arachidonate to form PGG2, and the released arachidonate is also available for oxidation to PGH2 precursor and other PGs, including thromboxane production [10,11]. Leukotrienes are another family of AA derivatives synthesized by LOX and play a significant role in the inflammation process [12,13]. There are different isoforms of LOX enzymes like 5-LOX, 8-LOX, 12-LOX, and 15-LOX, which catalyze the formation of 4 hydroperoxy eicosatetraenoic acids (HPETEs; 5-, 8-, 12-, and 15-HPETE). These HPETEs are further converted into Leukotrienes (LTs), Hepoxilins, and Lipoxins [13]. 5-LOX, a well-overviewed isoenzyme of LOX, triggers the crucial biosynthetic pathway to liberate 5-HPETE and further LTA4 (Precursor of the LTs like LTC4, LTD4, LTB4, and LTE4) [14,15]. The 5-LOX pathway ceases with the release of a by-product, Leukotriene B4 (LTB4), which mediates numerous inflammatory and allergic illnesses, such as atherosclerosis, cancer, and cardiovascular conditions [16,17]. Other isoforms like 12-LOX and 15-LOX also play an important role in generating leukotrienes. Subsequently, COX and LOX are rate-limiting enzymatic steps in producing eicosanoids and are frequently used for testing and evaluating anti-inflammatory drugs [18,19]. Since inhibition of COX enzymes (COX-1, COX-2) stimulates the LOX pathway and increases leukotriene production, parallel inhibition of LOX may assist in reducing the risk of cardiovascular and gastrointestinal diseases while preserving the anti-inflammatory effects of COX-2 inhibition [20,21].
Fig. 1.
Arachidonic acid pathway and targets of anti-inflammatory drugs. (Reprinted from M. A. Meshram, U. O. Bhise, P.N. Makhal, V. R. Kaki, Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR, European Journal of Medicinal Chemistry, 225 (2021) 113804. (Copyright with permission from Elsevier).
2. Rationale of developing dual COX-LOX inhibitors
A thorough literature search found that the drugs commonly used (Ex. NSAIDs) for treating inflammation showed several adverse effects like gastrointestinal disorders, cardiovascular diseases, kidney problems, etc. For achieving greater therapeutic efficacy, the adverse effects need to be addressed, and for this purpose “multi-target approach” was utilized. Dual COX-LOX inhibitors come under this approach, a viable alternative to curtail the adverse effects caused by NSAIDs. By inhibiting 5-LOX and COX-2 enzymes, dual COX-LOX inhibitors simultaneously inhibited leukotrienes and prostaglandins, the significant mediators released during inflammation [9]. Thus the concurrent inhibition of both pathways helps to develop novel anti-inflammatory agents with a broader anti-inflammatory efficacy and fewer side effects [22,23]. This novel approach also substantiates a better gastrointestinal pharmacological safety profile and has a protective impact on the GI mucosa. For designing dual COX-2-5-LOX inhibitors the information regarding the binding sites of Arachidonic acid in these enzymes is very much useful. This inspired K. K. Reddy et al. to do a comparative study on the characteristics of the binding sites of Arachidonic acid metabolizing enzymes and to highlight the important amino acids that could be targeted for drug design. This study also provides the basic approach to developing multitargeted drugs. The common characteristics of the binding sites of COX-2 with 5-LOX, 15-LOX, and 12-LOX were identified and important interactions were observed. A total of 15 physicochemical features were found to be similar in the case of COX-2 and 5-LOX enzymes and the interactions observed were: hydrogen bond acceptor, aromatic and aliphatic pi contacts, and mixed hydrogen bond donor and acceptor. In the Arachidonic acid binding site of both enzymes, three amino acids named Leu, Phe and Tyr were observed in common. Aromatic pi contact interactions were more in comparison to aliphatic pi contact interactions. Serine (Ser 530) of COX-2 and glutamine (Gln 363) of 5-LOX possess hydrogen bond acceptor interactions, and tyrosine (Tyr 355) and histidine (His 372) possesses mixed hydrogen bond donor and acceptor interactions. In the case of COX-2-15-LOX and COX-2-12-LOX enzymes, total of 12 and 10 physicochemical features were found to be similar. Seven aliphatic and one aromatic pi contact interactions were observed. Other interactions like mixed hydrogen bond donor and acceptor and hydrogen bond acceptor interactions were also observed [24].
3. Materials and methods
This review work was carried out by thoroughly exploring different research articles collected from online databases like CAS SciFinder, Google Scholar, Pubmed, ScienceDirect, Wiley Online Library, and SpringerLink. A total of 65 publications were initially collected from different online journals, which were published between 2015 and 2022. After screening thoroughly, 20 research publications were reviewed, which highlighted not only the importance of natural product-driven dual COX-LOX inhibition but also the potential bioactive compounds obtained from different medicinal plants for preparing this manuscript. The terminologies utilized in the search were “Inflammation”, “Pathophysiology of inflammation”, “COX and LOX pathway”, “Natural products”, “Medicinal Plants”, “Anti-inflammatory activity”, “Inhibition of COX and LOX”, and “Dual COX-LOX inhibition.”
4. Natural products used as COX and LOX inhibitors
Several pieces of research highlighted that cyclooxygenase and lipoxygenase enzymes present a significant aspect in the pathophysiology of inflammation. By inhibiting different isoforms of both enzymes, desired anti-inflammatory activity can be achieved. The anti-inflammatory activity of herbal drug extracts, phytochemicals, and natural product-derived compounds has been investigated for many years.
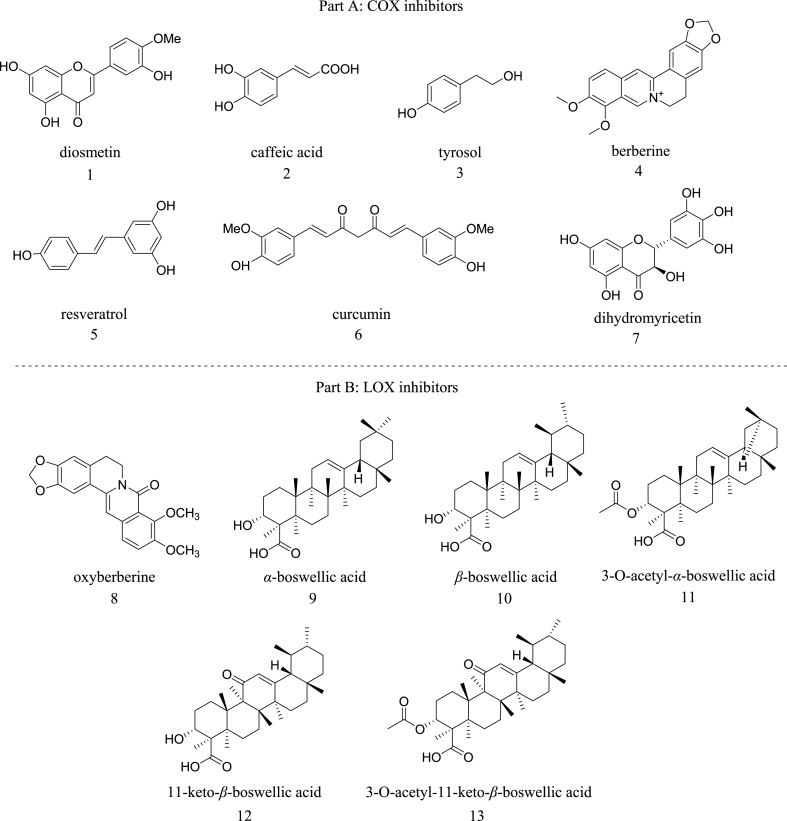
A. Attiq et al. stated that different classes of plant-based natural products, such as alkaloids, terpenoids, flavonoids, saponins, fatty acids, etc., as potential COX-2 inhibitors (Fig. 2, Part A) [25]. A recent review by G. Ambati G et al. also highlighted the importance of utilizing natural products obtained from plant and marine sources as COX-2 inhibitors [26]. Similar reviews have also been done on natural product-based 5-LOX inhibitors, which may lead to the discovery of novel anti-inflammatory agents. S. Sinha et al. stated that different classes of plant-based natural products, like coumarin derivatives, benzoquinones, phenolics, triazole caffeic acid analogs, etc., could be helpful as potential 5-LOX inhibitors (Fig. 2, Part B) [27].
Fig. 2.
Part A: Structures of well known natural products used as an COX inhibitors. Part B: Structures of well known natural products used as an LOX inhibitors.
5. Natural products used as dual COX-LOX inhibitors
Over the years, several researchers mentioned the importance of dual COX-LOX inhibitors and their role in designing novel anti-inflammatory agents. Natural products can be utilized as potential alternatives for developing novel anti-inflammatory agents, and several types of research proved the importance of utilizing natural products in developing dual COX-LOX inhibitors. A book chapter entitled “Generating and screening a natural product library for cyclooxygenase and lipoxygenase dual inhibitors” highlighted the importance of developing novel dual COX-LOX inhibitors [28]. This chapter highlighted different classes of natural products like alkaloids, flavonoids, terpenoids, iridoids, coumarins, etc., which were utilized as dual COX-LOX inhibitors and made a library of them. Later in 2021, M. A. Meshram et al. gave a detailed review of synthetically developed and natural product-based dual inhibitors. This review mentioned the advantages of isolating natural products and plant extracts as dual inhibitors [9].
5.1. Tannins
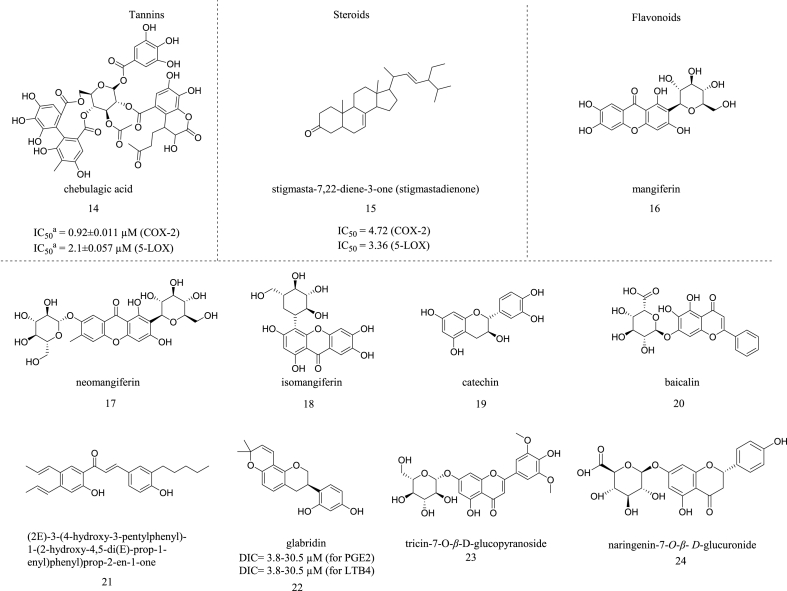
Tannins are an important category of natural products widely employed as digestive, antidiabetic, antiasthmatic, etc. Though some preliminary research stated that tannins could be used as an anti-inflammatory agent, no specific research has been reported on tannins' dual COX-LOX inhibition potential. This inspired D. B. Reddy et al. to evaluate the dual inhibitory activity of chebulagic acid (14) (Fig. 3), a benzopyran tannin class of compound derived from the Terminalia chebula fruits.
Fig. 3.
Structures of tannins, steroids, and flavonoids with dual COX-LOX inhibition, aIC50 value calculated for the fraction.
In this research work, the dried fruits were extracted with ethanol, and the ethanolic extract was evaluated based on its inhibitory potential against COX and LOX enzymes. It was observed that the prepared extract showed excellent inhibition against 5-LOX, COX-1 and COX-2 with IC50 values of 20, 90 and 3.75 μg/ml, respectively. So, it was evident that the ethanolic extract showed more affinity towards COX-2 (25-fold selectivity). Further, fractionation of the ethanolic extract has been done by using RP-HPLC; overall, eight individual peaks were eluted and collected as fractions and evaluated for the same. It was found that out of eight fractions, only the fifth fraction (identified as chebulagic acid, (14)) showed inhibition against 5-LOX, COX-1 and COX-2 with IC50 values of 2.1 ± 0.057 μM, 15 ± 0.288 μM, and 0.92 ± 0.011 μM respectively. Thus, the fifth fraction displayed ∼16-fold COX-2 selectivity along with dual inhibition of COX and LOX. The structural elucidation of this fraction using IR spectroscopy, NMR, and LC-MS identified the chemical component as chebulagic acid. The reported activity was comparable to celecoxib [29]. The authors concluded that chebulagic acid showed significant dual COX-LOX inhibition and can be utilized further for developing novel anti-inflammatory agents.
5.2. Steroids
Steroids have their contribution in development of novel anti-inflammatory agents. Several research works proved the anti-inflammatory potential of steroidal components (ex. β-sitosterol) by inhibiting inflammatory mediators like pro-inflammatory cytokines (IL-6, TNF-α, etc.). But, specific research work on dual COX-LOX inhibitory activity of the steroidal components has been seldom reported. In the year 2021, Y. S. Alqahtani took a research study to determine the dual COX-LOX inhibitory activity of a steroidal component isolated from Isodon rugosus and named as stigmasta-7,22-diene-3-one (stigmastadienone) (15) (Fig. 3).
The phytoconstituent was tested in cyclooxygenase and lipoxygenase assays, and their inhibition potential was determined. It showed significant inhibition of both 5-LOX and COX-2 enzymes with the IC50 values of 3.36 and 4.72 μg/ml, respectively, in comparison with the IC50 value of 3.81 μg/ml of standard drug celecoxib (for COX-2) and 2.74 μg/ml of standard drug montelukast (for 5-LOX) [30]. This research highlighted the importance of steroidal compounds as an effective dual COX-LOX inhibitor with anti-inflammatory indication.
5.3. Flavonoids
Flavonoids are widely explored natural products in anti-inflammatory therapy or for developing novel anti-inflammatory agents. Several research works highlighted the importance of utilizing flavonoids as a dual COX-LOX inhibitor to develop novel anti-inflammatory agents further. Q. Jia collected and summarized the information regarding the bioactive natural products against dual COX/LOX enzymes [28].
In this review, it was stated that different types of flavonoids, like flavones - ex. Crysin, hypocretin, morusin, etc.; flavonols - ex. kaempferol, fisetin, morin, quercetin, etc.; flavanones - ex. naringenin, naringin, etc.; chalcones - ex. 3,4-dihydroxy chalcone etc. showed dual COX-LOX inhibitory potential [28]. To further append to the work on flavonoids; Xiaotong Cao et al. isolated three flavonoids from the dried rhizome of Anemarrhena asphodeloides named mangiferin (16), neomangiferin (17), and iso-mangiferin (18) (Fig. 3). These flavonoids inhibited the production of COX-2 and 5-LOX simultaneously, thereby, exerting an anti-inflammatory response. For determining the anti-inflammatory activity of the flavonoids, the rat BPH inflammation model was utilized. In this experiment, Testosterone propionate (TP) at the dose of 10 mg/kg was injected subcutaneously into the male rats for inducing prostate hyperplasia. Further, the rats were treated with the isolated flavonoids (550 mg/kg), standard drugs zileuton (18 mg/kg), and Celecoxib (18 mg/kg). The prostate tissues were collected and the total RNA was extracted. Thereafter, the western blotting technique was performed and it was observed that the flavonoids showed potential downregulation of COX-2 and 5-LOX at both mRNA and protein levels (P < 0.01) [31]. W. Kong et al. evaluated the dual COX-LOX inhibitory potential of flavocoxoid, a purified fraction consisting of a mixture of catechin flavans, (19) and baicalin flavonoids, (20) (Fig. 3) isolated from Acacia catechu and Scutellaria baicalensis, respectively. In this study, C57BL/6 mice were taken and experimental autoimmune encephalomyelitis was induced. Thereafter the mice were treated with 100 mg/kg flavocoxoid. The spleens and spinal cords of the flavocoxoid treated mice were collected. By using the qRT-PCR technique the levels of the expression of 5-LOX and COX-2 were checked and it was found that flavocoxoid exhibited potent inhibition of the expression of both enzymes. The dual inhibition potential of the same was also tested on peritoneal macrophages which were stimulated with lipopolysaccharide (LPS) and a significant reduction of the levels of the expression of COX-2 and 5-LOX was observed [32]. R S. Sudheesh et al. isolated a bioactive flavonoid named (2E)-3-(4-hydroxy-3-pentylphenyl)-1-(2-hydroxy-4,5-di((E)-prop-1-enyl)phenyl)prop-2-en-1-one, (21) (Fig. 3) from the plant Punica granatum which showed significant dual COX/LOX enzyme inhibition. The isolated flavonoid showed 78.48% inhibition of the LOX enzyme against 89.87% inhibition by the standard compound vanillin. It also showed significant COX enzyme inhibition with the activity of 25 μmol/min compared with the activity of 38 and 29 μmol/min of the standard drugs - Nimesulide and Aspirin. This activity might be observed due to the polyphenolic nature of this compound [33].
C.V. Chandrasekharan et al. evaluated the dual COX/5-LOX inhibition potential of “Gutguard™,” a standardized extract prepared from Glycyrrhiza glabra roots. Further HPLC analysis was done to identify the potential bioactive compounds present in the extract, and it was found that the extract contains two important constituents named glabridin (4.5% w/w) and isoliquiritigenin (0.1% w/w). After that, this extract was tested to determine its dual inhibition potential with the help of TXB2, LTB4, and PGE2 inhibition assay. From these assays, it was observed that glabridin exhibited potent inhibition of the levels of TXB2, PGE2, and LTB4 at the concentrations of 1.9–30.5 μM, 3.8–30.5 μM, and 3.8–30.5 μM respectively. The reference standards utilized for these assays were dexamethasone, acetylsalicylic acid, and captopril. This research work stated that glabridin, (22) (Fig. 3) could be used as a lead molecule to develop novel dual COX-LOX inhibitors and help achieve better therapeutic efficacy [34].
A.K.M. Jacinto et al. prepared methanolic extracts from the aerial shoots of Eleusine indica and this extract was further fractionated by using the following solvents: Water, Dichloromethane, Ethyl acetate, and hexane. The crude extract and the prepared fractions were evaluated for dual COX-LOX inhibitory activity. From initial screening, it was observed that the crude extract showed significant inhibition of COX-2, COX-1, and 5-LOX at the concentration of 100 and 50 μg mL−1 (The percentage of inhibition obtained was as follows: 84.33 ± 0.89% and 77.26 ± 3.13%, 81.40 ± 1.46% and 74.72 ± 0.85%, 85.49 ± 9.74% and 73.84 ± 1.07%). The prepared fractions were also evaluated at the same concentrations (100 and 50 μg mL−1) and the results showed that the ethyl acetate and dichloromethane fractions showed higher dual inhibition potential than the methanolic extract. (P < 0.05) Further, the ethyl acetate fraction was undergone sub fractionation by using the solvent system Ethyl acetate- Acetic acid- Formic acid- Water (100:11:11:27) for isolation of bioactive phytocompounds. These subfractions (at the concentration of 50 μg mL−1) were again pharmacologically screened for dual inhibitory activity and it was found that the subfractions 8, 6, and 9 showed significant inhibition against COX-2 and 5-LOX enzymes. Subfraction 6 exhibited maximum dual inhibition. (The percentage of inhibition obtained for COX-2, 5-LOX, and COX-1 was as follows: 66.23 ± 5.81%, 80.17 ± 2.60%, and 65.24 ± 8.47%, respectively). Finally, two flavonoids were isolated from this subfraction named Tricin-7-O-β-d-glucopyranoside, (23) and naringenin-7-O-β-D-glucuronide, (24) (Fig. 3). Diclofenac and indomethacin were utilized as standard drug in this study (at the concentration of 50 μg mL−1) [35].
5.4. Alkaloids
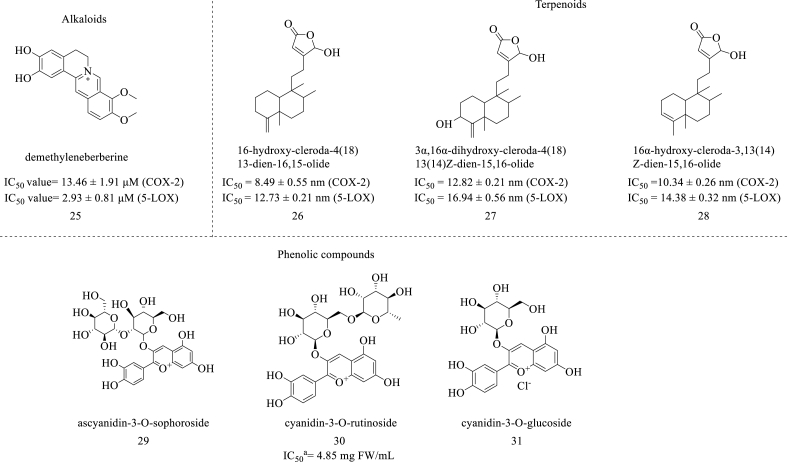
Alkaloids are another category of natural products that have been widely utilized in the field of phytochemistry. Different research works explained the utilization of alkaloids in a variety of disorders. Some research works stated that this phytoconstituent could be used for preparing effective anti-inflammatory agents. But not much research was reported on its dual COX-LOX inhibition potential. So J. Jenny et al. prepared a methanolic fraction from Tinospora cordifolia and evaluated its inhibition against COX and LOX. In this study, the bioactive fraction was tested for COX and LOX inhibition assay, and it was found that it showed significant inhibition on 5-LOX and COX-2 with the IC50 values of 0.041 ± 0.0003 ng/μl and 59.46 ± 0.42 ng/μl respectively [36]. S. Wang et al. isolated seven alkaloids from the alkaloidal extract of Cortex phellodendri and determined their inhibitory activity against COX-2 and 5-LOX enzymes. In this study, the BPH rat model has been utilized and the rats were treated with alkaloidal extract at the concentration of 400 mg/kg and with the standard drugs celecoxib (18 mg/kg) and Zileuton (100 mg/kg), respectively. The prostate tissues were collected from the rats and the Western Blotting technique was performed. It was observed that the alkaloidal extract showed a potent reduction in the levels of expression of 5-LOX and COX-2 (P < 0.01). three compounds named demethyleneberberine, palmatine, and berberine downregulated the protein expressions of COX-2 and 5-LOX (P < 0.01) and thus could be helpful as a potent dual COX-LOX antagonist. These alkaloids were also tested against lipoxygenase and cyclooxygenase enzymes by using inhibitory assays. It was found that demethyleneberberine, (25) (Fig. 4) showed potent inhibition against COX-2 and 5-LOX enzymes with IC50 values of 13.46 ± 1.91 μM and 2.93 ± 0.81 μM, respectively (Fig. 3). It was evident that demethyleneberberine, (25) may evolve as a potent dual COX-LOX inhibitor. The obtained IC50 values for Celecoxib and Zileuton were 0.07 ± 0.02 and 0.25 ± 0.06 μM, respectively [37].
Fig. 4.
Structure of alkaloids, terpenoids and phenolic compounds with dual COX-LOX inhibition, aIC50 value calculated for the fraction.
5.5. Terpenoids
The anti-inflammatory activity of different classes of terpenoids was reported in several research studies. Out of these, some research works highlighted the dual COX-LOX inhibition potential of the same. In a review work, Q. Jia reported the classes of terpenoids that showed potent inhibition against COX and LOX (Sesquiterpenes - ex. Buddledin A; Diterpenes-ex. Teucrin A, Lagascatrieol, etc.; Triterpenoids - ex. Ursolic acid, etc.) [28]. Further research has been carried out to develop terpenoid-based anti-inflammatory agents.
H. T. Nguyen et al. isolated and identified five different clerodane diterpenes from the methanolic extract prepared from the seeds of Polyalthia longifolia. These compounds were named as 16-oxo-cleroda-3,13(14)E-dien-15-oic acid, 16-hydroxy-cleroda-3,13-dien-15-oic acid, 16-hydroxy-cleroda-4(18), 13-dien-16,15-olide, 3α,16α-dihydroxy-cleroda-4(18),13(14)Z-dien-15,16-olide, and 16α-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide and further investigated for their anti-inflammatory activities and tested for COX and LOX assays. From these assays, it was observed the following compounds 16-hydroxy-cleroda-4(18), 13-dien-16,15-olide (26), 3α,16α-dihydroxy-cleroda-4(18),13(14)Z-dien-15,16-olide (27), and 16α-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide (28) (Fig. 4), showed potent dual COX/LOX inhibition with the IC50 values calculated for COX-2 were 8.49 ± 0.55, 12.82 ± 0.21, and 10.34 ± 0.26 nm, respectively, and for 5-LOX, the calculated IC50 values were 12.73 ± 0.21, 16.94 ± 0.56 and 14.38 ± 0.32 nm, respectively. Diclofenac and indomethacin were used as standard drugs with IC50 values of 23.28 ± 0.31 nm and 12.84 ± 0.32 nm, respectively [38].
5.6. Phenolic compounds
Until now, varied research has been done on the anti-inflammatory activity of phenolic compounds and substantial phenolic compounds were identified for developing novel anti-inflammatory agents with dual COX-LOX inhibition. It was found that the following important phenolic compounds, named phenethyl ferulate, bornyl ferulate, gossypol, etc., showed significant inhibition against both COX and LOX enzymes. Z. Rabiu et al. collected the palm kernel shells and converted them into pyroligneous acid by using a pyrolysis process. Further, this pyroligneous acid was fractionated by using different organic solvents like hexane, ethyl acetate, and methanol. The collected fractions were tested for cyclooxygenase and lipooxygenase assays. It was found that the ninth fraction (coded as IX in the study) and another five fractions (coded as XXI-XXV) - showed significant COX-2 inhibition with an IC50 value of 17.04–18.56 μg/ml. The series of Fractions coded as XXI-XXV and XXVI-XXX in the study showed significant 5-LOX inhibition with an IC50 value of 5.25–10.07 μg/mL [39]. By taking cues from this outcome, the phenolic compounds can be isolated from the same or different plants for developing dual COX-LOX inhibitors. U. Szymanowska et al. isolated phenolic compounds from raspberry pomace, and the purified anthocyanin fractions were evaluated for anti-inflammatory activity. This study found that the anthocyanin fraction showed good dual COX/LOX inhibition with an average IC50 value of 4.85 mg FW/mL. Using HPLC analysis, three anthocyanin compounds were identified and named ascyanidin-3-O-sophoroside (29), cyanidin-3-O-rutinoside (30), and cyanidin-3-O-glucoside (31) (Fig. 4) [40].
5.7. Resins
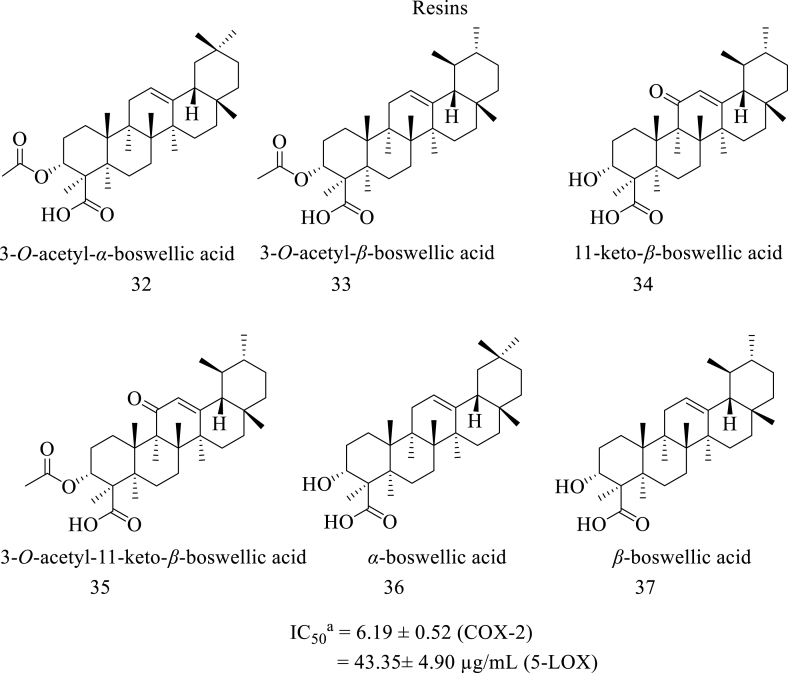
A research study done by V. K. Alluri et al. reported the dual COX-LOX inhibition activity of a novel composition named Serratrin, which contains the nonacidic and acidic fractions prepared from the aqueous ethanolic extract of boswellia oleo-gum-resin. This composition is composed of six boswellic acids called 3-O-acetyl-α-boswellic acid (α-ABA) (32), 3-O-acetyl-β-boswellic acid (β-ABA) (33), 11-keto-β-boswellic acid (KBA) (34), 3-O-acetyl-11-keto-β-boswellic acid (AKBA) (35), α-boswellic acid (α-BA) (36), and β-boswellic acid (β-BA) (37) (Fig. 5, Fig. 6). This study found that this composition showed significant inhibition of 5-LOX with an IC50 value of 43.35 ± 4.90 μg/mL and inhibition of PGE2, a potent inflammatory mediator in the COX pathway with an IC50 value of 6.19 ± 0.52 μg/ml. This research has highlighted an essential aspect of the dual inhibitory potentials of boswellic acids, which could be a new approach for developing novel anti-inflammatory agents as boswellic acids were known as effective LOX inhibitors [41].
Fig. 5.
Structure of resins with dual COX-LOX inhibition, aIC50 value calculated for the composition named serratrin comprising the above mentioned boswellic acids.
Fig. 6.
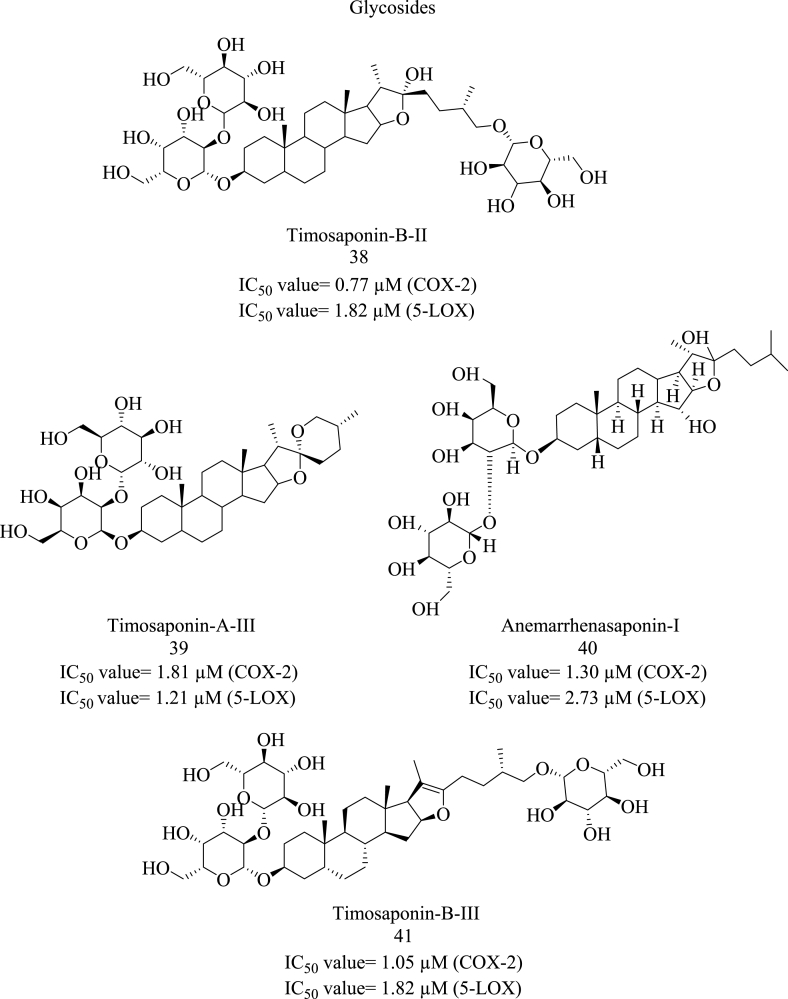
Structure of glycosides with dual COX-LOX inhibition.
5.8. Glycosides
L. Xie et al. coupled HPLC-MS and affinity ultrafiltration (AUF) to screen and characterize COX/LOX dual-targeted inhibitors from the extracts of Anemarrhenae rhizome. This research study specifically focussed on two classes of glycosides: spirostanol and furostanol glycosides. By means of affinity ultrafiltration, a total of four spirostanol glycosides named sarsapogenin, timosaponin I-III and four furostanol glycosides named timosaponin D, anemarrhenasaponin I, and timosaponin B II-III were identified (Fig. 5). Thereafter, AUF-LC-MS was used to determine ligand-receptor binding properties and calculate binding values. According to Munigunti et al. , for compounds with a specific binding value less than 1.5 are considered non-ligand. On the other hand, if the values of the compounds were between 1.5 and 2.0, 2.0–3.0, and more than 3.0, then they were considered weak ligands, moderate ligands, and strong ligands, respectively [42]. The ultrafiltration proved that these glycosidic compounds could bind with 5-LOX. It was observed that timosaponin A-II (specific binding value 2.817) showed more binding affinity to 5-LOX. Other glycosides, timosaponin B-II (38) (Specific binding value 2.297), sarsapogenin (2.134), timosaponin A-1 (2.119), timosaponin B-III (41) (1.792), timosaponin A-III (39) (2.379), showed good binding affinity to 5-LOX. Only timosaponin D (1.395) showed moderate binding to 5-LOX. AUF-LC-MS was done to screen the potential COX-2 inhibitors. The results suggested that timosaponin B-III (6.931) showed more binding affinity to COX-2 as compared to other glycosides, timosaponin A-II (3.930), timosaposin A-III (5.293), sarsapogenin (2.484), timosaponin A-I (3.182), timosaponin B-II (5.8460) and anemarrhenasaponin I (40) (6.586). Therefore, the authors concluded that a total of seven bioactive compounds identified in this extract bind explicitly with 5-LOX and COX-2. Further, the COX/LOX inhibitory potential of these bioactive compounds was evaluated by using in-vitro COX-LOX inhibition assays. These assays depicted that timosaponin A-II and timosaponin B-III showed maximum inhibition against COX-2 and 5-LOX with IC50 values of 1.05 μM and 0.48 μM, respectively. The compounds which showed weak inhibition against COX-2 were timosaponin A-I, timosaponin A-II, timosaponin B-II, timosaponin A-III, sarsapogenin, and anemarrhenasaponin I. (The obtained IC50 values were as follows: 36.43 μM, 6.07 μM, 0.77 μM, 1.81 μM, 19.27 μM, and 1.30 μM, respectively). In contrast, the compounds (other than timosaponin A-II) showed better inhibition against 5-LOX. (The obtained IC50 values for timosaponin A-III, timosaponin A-I, timosaponin B-III, timosaponin B-II, anemarrhenasaponin I and sarsapogenin were as follows: 1.21, 3.29, 1.82, 1.57, 2.73 and 2.21 μM, respectively.) Zileuton and celecoxib were used as standard drugs for this assay. (The obtained IC50 values were as follows: 0.24 μM and 0.06 μM respectively) The outcomes suggested that glycosides could present potential dual COX-LOX inhibitors and further aid the development of safe, effective anti-inflammatory agents [43].
5.9. Miscellaneous
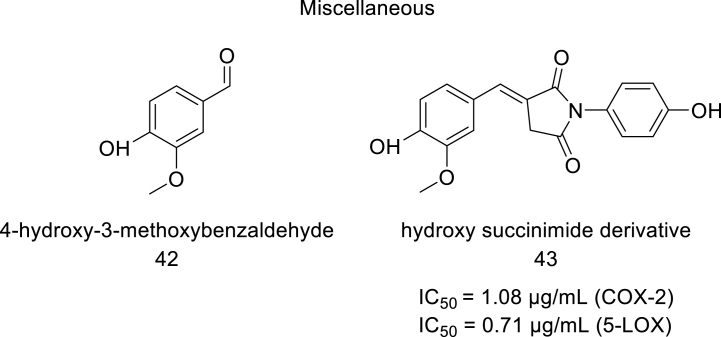
L. Langhansova et al. prepared ethanolic extracts from the plant Myrica Rubra. The crude ethanolic extract fractionation was done using organic solvents like butanol, ethyl acetate, hexane, and water. All bioactive fractions were collected and tested for anti-inflammatory activity based on dual COX-LOX antagonism. It was observed that ethyl acetate fraction showed potential resistance against isoforms of COX and LOX enzymes with IC50 values obtained for COX-2, 5-LOX and COX-1 were 2.54 μg mL-1, 8.30 μg/mL and 3.29 μg/mL, respectively [44]. M.H. Mahnashi et al . tried to validate the ethnopharmacological claims of utilizing two plants named Habenaria digitata and Habenaria plantaginea for the treatment of inflammation and analgesia. For this purpose, methanolic extracts were prepared and further fractionated by using different organic solvents like hexane, chloroform, ethyl acetate, and n-butanol. The crude extracts and the prepared fractions were evaluated for COX-LOX inhibition assays. It was observed that the ethyl acetate and chloroform fractions prepared from H. digitata showed more significant inhibition against COX-2 enzyme (at 1000 μg/ml concentration, ethyl acetate and chloroform fractions showed 83.81% and 84.69% inhibition with the IC50 values of 32.39 μg/ml and 21.30 μg/ml respectively). Similarly, the ethyl acetate and chloroform fractions prepared from H. plantaginea showed potent inhibition against COX-2 enzyme (at 1000 μg/ml concentration, ethyl acetate and chloroform fractions showed 76.38 ± 0.76% and 77.40 ± 0.25% inhibition with the IC50 values of 87.56 μg/ml, and 33.81 μg/ml respectively). Celecoxib was used as a standard drug for comparing the inhibition potential of the fractions prepared from both plants and it showed 96.00% and 84.51 ± 0.30% inhibition of COX-2 enzyme at 1000 μg/ml concentration with the IC50 values of 15.13 μg/ml and 23.30 μg/ml respectively. The ethyl acetate and chloroform fractions prepared from H. digitata showed potent inhibition of 5-LOX enzyme (at 1000 μg/ml concentration ethyl acetate and chloroform fractions showed 84.37% and 87.30% inhibition with IC50 values of 16.40 μg/ml and 14.42 μg/ml respectively. Similarly, the ethyl acetate and chloroform fractions prepared from H. plantaginea also showed potent inhibition against 5-LOX enzyme (at 1000 μg/ml concentration ethyl acetate and chloroform fractions showed 80.47 ± 0.70% and 81.73 ± 0.37% inhibition with IC50 values of 67.51 μg/ml and 26.74 μg/ml respectively). Montelukast and linoleic acid (1000 μg/ml) were used as standard drugs for comparing the inhibition potential of the fractions prepared from both plants and showed 87.30% and 87.66 ± 0.45% inhibition with the IC50 values of 9.45 μg/ml and 17.47 μg/ml respectively [45,46]. Later, they isolated and characterized six phytocompounds from Polygonum aviculare, on the basis of its chemical nature 4-hydroxy-3-methoxybenzaldehyde, (42) was taken for derivatization and a hydroxy succinimide derivative (43) (Fig. 7) was prepared and further evaluated for COX-LOX inhibition assays. This compound displayed significant inhibition against COX-2. COX-1 and 5-LOX enzymes with IC50 values of 1.08 μg/ml, 13.91 μg/ml, and 0.71 μg/ml, respectively, reflecting COX-2 selectivity (selectivity index-12.9). Zileuton and Diclofenac were used as standard drugs in this study and the IC50 values for COX-2, COX-1, and 5-LOX were found to be 10.80 μg/mL, 4:48 μg/mL, and 5.29 μg/mL, respectively [47].
Fig. 7.
Structure of hydroxy succinimide derivative.
6. Conclusion
Dual COX-LOX inhibition has evolved as an excellent strategy to develop novel anti-inflammatory medications because of the inhibition of both cyclooxygenase and lipooxygenase enzymes. The dual COX-LOX inhibitors are known to improve the pharmacological safety profile and prevent gastrointestinal damage. With significant contribution of natural products in the development of anti-inflammatory therapy, they can be utilized for developing dual COX-LOX inhibitors. Different research studies have been done for exploring natural product-based dual COX-LOX inhibitors. From the cumulative research, it was observed that the various classes of natural products, like flavonoids, terpenoids, alkaloids, glycosides, phenolics, resins, etc., could show potent inhibition against both LOX and COX isoforms. Our present review emphasized the importance of natural product-derived dual COX-LOX inhibitors and summarized the bioactive natural products isolated from different medicinal plants which showed potent dual COX-LOX inhibition. This review also highlighted the utilization of potent bioactive fractions which were prepared from the crude extract of the medicinal plants and showed significant inhibition of both COX and LOX enzymes. This work may help the researchers to find a direction for the development of safe, efficacious dual COX-LOX inhibitors by further isolation of similar classes of phytochemicals from the unexplored bioactive fractions and by investigating structurally similar phytocompounds. Further, the semisynthetic modification of the biologically active scaffolds discussed in this review may help to develop more potent dual COX-LOX inhibitors.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Acknowledgements
This manuscript bears the NIPER-HYDERABAD communication number NIPERHYD/2022/118. The authors are acknowledged to National Institute of Pharmaceutical Education and Research Hyderabad for providing the necessary facilities to carry out this review work.
References
- 1.Das K., Buchholz N. Benign prostate hyperplasia and nutrition. Clinical Nutrition ESPEN. 2019;33:5–11. doi: 10.1016/j.clnesp.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Jacob P J., Manju S.L., Ethiraj K.R., Elias G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: a structure-based approach. Eur. J. Pharmaceut. Sci. 2018;121:356–381. doi: 10.1016/j.ejps.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Yoon J.H., Baek S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005;46(5):585–596. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuzick J., Sasieni P., Myles J., Tyrer J. Estimating the effect of treatment in a proportional hazards model in the presence of non-compliance and contamination. J. Roy. Stat. Soc. B Stat. Methodol. 2007;69:565–588. [Google Scholar]
- 6.Smith W.L., Garavito M.R., DeWitt D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 7.Yunus M.H.M., Nordin A., Kamal H. Pathophysiological perspective of osteoarthritis. Medicina. 2020;56:1–13. doi: 10.3390/medicina56110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottani A., Bertolini M.Sandrini. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks. Curr. Med. Chem. 2005;9:1033–1043. doi: 10.2174/0929867024606650. [DOI] [PubMed] [Google Scholar]
- 9.Meshram M.A., Bhise U.O., Makhal P.N., Kaki V.R. Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: structural aspects and SAR. Eur. J. Med. Chem. 2021;225 doi: 10.1016/j.ejmech.2021.113804. [DOI] [PubMed] [Google Scholar]
- 10.Steinmeyer J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. 2000;2:379–385. doi: 10.1186/ar116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antman E.M., Demets D., Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- 12.Fiorucci S., Meli R., Bucci M., Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem. Pharmacol. 2001;62:1433–1438. doi: 10.1016/s0006-2952(01)00747-x. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Golden M., Canetti C., Mancuso P., Coffey M.J. Leukotrienes: underappreciated mediators of innate immune responses. J. Immunol. 2005;174:589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- 14.Maxis K., Delalandre A., Martel-Pelletier J., Pelletier J.P., Duval N., Lajeunesse D. Arthritis Research & Therapy; 2006. The Shunt from the Cyclooxygenase to Lipoxygenase Pathway in Human Osteoarthritic Subchondral Osteoblasts Is Linked with a Variable Expression of the 5 Lipoxygenase-Activating Protein; p. R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutil Z., Temml V., Maghradze D., Pribylova M., Dvorakova M., Schuster D., Vanek T., Landa P. Mediators of Inflammation; 2014. Impact of Wines and Wine Constituents on Cyclooxygenase-1, Cyclooxygenase-2, and 5-lipoxygenase Catalytic Activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen H.T., Vu T.Y., Chandi V., Polimati H., Tatipamula V.B. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur G., Silakari O. Multiple target-centric strategy to tame inflammation. Future Med. Chem. 2017;9:1361–1376. doi: 10.4155/fmc-2017-0050. [DOI] [PubMed] [Google Scholar]
- 18.Hyde C.A.C., Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int. Immunopharm. 2009;9:701–715. doi: 10.1016/j.intimp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Prasher P., Mudila H., Sharma M., Khati B. Developmental perspectives of the drugs targeting enzyme-instigated inflammation: a mini review. Med. Chem. Res. 2019;28:417–449. [Google Scholar]
- 20.Nikalje P.G., Tiwari S.V., Sangshetti J.N., et al. Ultrasound-mediated synthesis, biological evaluation, docking and in- vivo acute oral toxicity study of novel indolin-2-one coupled pyrimidine derivatives. Res. Chem. Intermed. 2018;44:3031–3059. [Google Scholar]
- 21.Alasmary F.A.S., Awaad A.S., Alafeefy A.M., El-Meligy R.M., Alqasoumi S.I. Novel quinazoline and acetamide derivatives as safe anti-ulcerogenic agent and anti-ulcerative colitis activity. Saudi Pharmaceut. J. 2018;26:138–143. doi: 10.1016/j.jsps.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carty T.J., Marfat A., Rasamune J. In: Annual Reports in Medicinal Chemistry. Allen R.C., editor. Academic Press; New York: 1988. Modulation of AA metabolites in the treatment of rheumatoid arthritis; pp. 181–189. [Google Scholar]
- 23.Horizoe T., Nagakura N., Chiba K., Shirota H., Shinoda M., Numata H., Kobayashi S., Abe C. Effect of ER-34122, a novel dual 5-lipoxygenase/cyclooxygenase inhibitor, on indices of early articular lesion in MRL/MpJ-lpr/lpr mice. Inflamm. Res. 1999;48:432–436. doi: 10.1007/s000110050483. [DOI] [PubMed] [Google Scholar]
- 24.Reddy K.K., Rajan V.K.V., Gupta A., Aparoy P., Reddanna P. Exploration of binding site pattern in arachidonic acid metabolizing enzymes, Cyclooxygenases and Lipoxygenases. BMC Res. Notes. 2015;8:1–10. doi: 10.1186/s13104-015-1101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attiq J.Jalil, Husain K., Ahmad W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018;9:976. doi: 10.3389/fphar.2018.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambati G G., Jachak S.M. Natural product inhibitors of cyclooxygenase (COX) enzyme: a review on current status and future perspective. Curr. Med. Chem. 2020;27:1–28. doi: 10.2174/0929867327666200602131100. [DOI] [PubMed] [Google Scholar]
- 27.Sinha S., Doble M., Manju S.L. 5-Lipoxygenase as a drug target: a review on trends in inhibitors structural design, SAR and mechanism-based approach. Bioorg. Med. Chem. 2019;27:3745–3759. doi: 10.1016/j.bmc.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Jia Q. Generating and screening a natural product library for cyclooxygenase and lipoxygenase dual inhibitors, Bioactive Natural products. Stud. Nat. Prod. Chem. 2003:643–718. [Google Scholar]
- 29.Reddy D.B., Reddy T.C.M., Jyotsna G., Sharan S., Priya Nalini, Lakshmipathi V., Reddanna P. Chebulagic acid, a COX–LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J. Ethnopharmacol. 2009;124:506–512. doi: 10.1016/j.jep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Alqahtani Y.S. Bioactive stigmastadienone from Isodon rugosusas potential anticholinesterase, α-glucosidase and COX/LOX inhibitor: in-vitro and molecular docking studies. Steroids. 2021;172 doi: 10.1016/j.steroids.2021.108857. [DOI] [PubMed] [Google Scholar]
- 31.Cao X., Shang Y., Kong W., Jiang S., Liao J., Dai R. Flavonoids derived from Anemarrhenae Rhizoma ameliorate inflammation of benign prostatic hyperplasia via modulating COX/LOX pathways. J. Ethnopharmacol. 2022;284 doi: 10.1016/j.jep.2021.114740. [DOI] [PubMed] [Google Scholar]
- 32.Kong W., Hooper K.M., Ganea D. The natural dual cyclooxygenase and 5-lipoxygenase inhibitor Flavocoxid is protective in EAE through effects on Th1/Th17 differentiation and macrophage/Microglia activation. Brain Behav. Immun. 2015;53:59–71. doi: 10.1016/j.bbi.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Sudheesh S., Soumya K., James J. A novel chalcone derivative from Punica granatum peel inhibits LOX/COX enzyme activity. Beni-Suef Univ. J. Basic and Appl. Sci. 2018;7:593–597. [Google Scholar]
- 34.Chandrasekaran C.V., Deepak H.B., Thiyagarajan P., Kathiresan S., Sangli G.K., Deepak M., Agarwal Amit. Dual inhibitory effect of Glycyrrhiza glabra (GutGardTM) on COX and LOX products. Phytomedicine. 2011;18:278–284. doi: 10.1016/j.phymed.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Jacinto K.M., Heralde F.M., Moreno P.G.G., Santos J.H., Toralba J.V., Quiming N.S. Isolates from Eleusine indica (poaceae) aerial shoot fraction dually inhibits 5-LOX and COX enzyme systems. Philipp. J. Sci. 2022;151(6B):2365–2384. [Google Scholar]
- 36.Jenny J., Kumar P.B. Dihydroxy berberine from Tinospora cordifolia: in silico evidences for the mechanism of anti-inflammatory action through dual inhibition of Lipoxygenase and Cyclooxygenase. Indian J. Biochem. Biophys. 2021;58:244–252. [Google Scholar]
- 37.Wang S., Lee D.Y., Shang Y., Liao J., Cao X., Xie L., et al. The bioactive alkaloids identified from Cortex phellodendri ameliorate benign prostatic hyperplasia via LOX-5/COX-2 pathways. Phytomedicine. 2021;93 doi: 10.1016/j.phymed.2021.153813. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen H.T., Vu T.Y., Chandi V., Polimati H., Tatipamula V.B. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabiu Z., Hasham R., Zakaria Z.A. Dual COX/LOX inhibition: screening and evaluation of the effect of pyroligneous acid fractions of palm kernel shell as an anti-inflammatory agents. Jurnal Kejuruteraan. 2019;2:51–57. [Google Scholar]
- 40.Szymanowska U., Baraniak B. Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants. 2019;8:299. doi: 10.3390/antiox8080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alluri V.K., Kundimi S., Sengupta K., Golakoti T., Kilari E.K. An anti-inflammatory composition of Boswellia serrata resin extracts alleviates pain and protects cartilage in monoiodoacetate-induced osteoarthritis in rats. Evid. base Compl. Alternative Med. 2020:1–11. doi: 10.1155/2020/7381625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munigunti R., Mulabagal V., Calderon A.I. Screening of natural compounds for ligands to PfTrxR by ultrafiltration and LC-MS based binding assay. J. Pharmaceut. Biomed. Anal. 2011;55:265–271. doi: 10.1016/j.jpba.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Xie L., Lee D.Y.W., Shang Y., Cao X., Wang S., Liao J., Zhang T., Dai R. Characterization of spirostanol glycosides and furostanol glycosides from Anemarrhenae rhizoma as dual targeted inhibitors of 5-lipoxygenase and Cyclooxygenase-2 by employing a combination of affinity ultrafiltration and HPLC/MS. Phytomedicine. 2020;77 doi: 10.1016/j.phymed.2020.153284. [DOI] [PubMed] [Google Scholar]
- 44.Langhansova L., Landa P., Kutil Z., Tauchen J., Marsik P., Rezek J., Dong Lou J., Li Yun Z., Vanek T. Myrica rubra leaves as a potential source of a dual 5-LOX/COX inhibitor. Food Agric. Immunol. 2017;28:343–353. [Google Scholar]
- 45.Mahnashi M.H., Alyami B.A., Alqahtani Y.S., Jan M.S., Rashid U., Sadiq A., Alqarni A.O. Phytochemical profiling of bioactive compounds, anti-inflammatory and analgesic potentials of Habenaria digitata Lindl.: molecular docking based synergistic effect of the identified compounds. J. Ethnopharmacol. 2021;273 doi: 10.1016/j.jep.2021.113976. [DOI] [PubMed] [Google Scholar]
- 46.Mahnashi M.H., Alqahtani Y.S., Alyami B.A., Alqarni A.O., Alshrahili M.A., Salim M.A.A., Alqahtani M.N., Mushtaq S., Sadiq A., Jan M.S. GC-MS analysis and various in vitro and in vivo pharmacological potential of Habenaria plantaginea lindl. Evid. base Compl. Alternative Med. 2022 doi: 10.1155/2022/7921408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahnashi M.H., Alyami B.A., Alqahtani Y.S., Alqarni A.O., Jan M.S., Hussain F., Zafar R., Rashid U., Abbas M., Tariq M., Sadiq A. Antioxidant molecules isolated from edible prostrate knotweed: rational derivatization to produce more potent molecules. Oxid. Med. Cell. Longev. 2022 doi: 10.1155/2022/3127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.