Abstract
Purpose of Review
Disparities in prostate cancer care and outcomes have been well recognized for decades. The purpose of this review is to methodically highlight known racial disparities in the care of prostate cancer patients, and in doing so, recognize potential strategies for overcoming these disparities moving forward.
Recent Findings
Over the past few years, there has been a growing recognition and push towards addressing disparities in cancer care. This has led to improvements in care delivery trends and a narrowing of racial outcome disparities, but as we highlight in the following review, there is more to be addressed before we can fully close the gap in prostate cancer care delivery.
Summary
While disparities in prostate cancer care are well recognized in the literature, they are not insurmountable, and progress has been made in identifying areas for improvement and potential strategies for closing the care gap.
Keywords: Prostate cancer, Disparities, Equity, Care delivery, Review
Introduction and General Outcome Data
Prostate cancer is the most common cancer type diagnosed in men in the USA [1]. Despite advancements in screening and treatment regimens, several factors have resulted in racial disparities for many races and ethnicities, but particularly for Black men living with prostate cancer in quality of care and survival. Our hope is that by writing this review article and surveying the existing body of literature, we will be able to supply providers with the knowledge they need to improve the standard of care for marginalized communities.
To contextualize, it has been well established that Black men experience greater risk for many types of cancer. The American Cancer Society was one of the first to highlight the disparate incidence and mortality experienced by Black men compared to White men in 1993, with 52% of Black men and 26% of White men presenting with stage 4 prostate cancer at diagnosis [2]. Subsequently, the survival rates for Black men were lower than White men. After controlling for grade and stage, however, survival was similar for both groups. One common explanation is that White men had better access to medical care, but the study was restricted to patients who were initially diagnosed in the same VA medical center. Therefore, factors such as differences in access (e.g., transportation) or medical care usage rates, stemming from the social determinants of medicine, were given as possible explanations.
Following that discovery, one of the largest and highest impact early outcome studies examining racial disparities in prostate cancer outcomes showed that traditional socioeconomic, clinical, and pathologic factors accounted for the increased relative risk for individuals diagnosed with advanced stage prostate cancer in Hispanic men but not Black men [3]. These studies highlight the existence of other racial structural factors beyond those stated above that contribute to the inequities in treatment and late stage diagnosis of prostate cancer for Black men.
While other review papers focus mainly on either primary, or advanced cancer treatment or screening, we aim to provide a comprehensive picture of the entire disease continuum from diagnosis to disease outcomes. This is because social determinants of health (SDOH) consistently appear to impact race/ethnic disparities across every stage of care. SDOH are defined as the social “conditions in the environments in which people are born, live, work” [4]. Differences in SDOH are believed to result from systemic discrimination and policies that have existed at the societal level (e.g., redlining/housing policies/implicit biases). SDOH are often explained in terms of 5 main domains: economic stability (e.g., employment, poverty), education (e.g., high school graduation), social and community context (e.g., social support, perceived discrimination, mistrust), health and healthcare access (e.g., insurance status, health literacy), and neighborhood and built environment (e.g., neighborhood socioeconomic status, quality of housing) [4]. The lines between these determinants are blurred, with multiple buckets often applying to patients.
The majority of SDOH studies in prostate cancer evaluate the associations between incidence and mortality outcomes with the five SDOH domains. However, not all domains are consistently evaluated across the continuum. Here, we summarize and provide some examples of studies investigating SDOH and racial disparities across several key turning points in the disease course. However, we recognize that there are gaps in the literature that will need to be filled to understand how SDOH (same or different domains/factors) impact prostate cancer care from screening and diagnosis to treatment, barriers, and clinical trial enrollment, in primary and advanced prostate cancer. We feel it is imperative to fill in the gaps so that providers are best able to identify the root of the problem and direct future efforts to implement solutions. Following this framework, we will present findings on screening and diagnosis, treatment, and barriers to trial enrollment in primary and advanced prostate cancer.
Screening and Diagnosis Disparities
With groundbreaking improvements in prostate cancer screening technologies, one would expect that participation in screening programs would increase. However, overall PSA screening rates declined from 2012 to 2018, and screening rates among non-Hispanic Black men declined at a significantly higher rate, driven primarily by decreased screenings in the 40–54-year-old cohort [5•]. This could possibly be an effect of the recommendation for PSA screenings being updated in 2018 [6]. However, still among all groups, PSA screening is lowest among Hispanic, Native American Indian/Alaska Natives, and Native Asian/Pacific Islander men [5•]. Also, in one cohort of privately insured men, there was little effect on PSA screening from changes to the USPSTF recommendation [7]. There was a lower utilization in Black men compared to other races and higher utilization for the higher income and privately insured population for mpMRI diagnostic imaging [8]. In addition, non-White males were more likely to get imaging that was not in concordance with NCCN guidelines [9]. From a performance standpoint, there was mixed data on mpMRI performance, with one study showing similar performance for Black patients compared to White patients and another with worse performance for Asian American patients [10, 11]. Detecting prostate cancer early permits fuller discussion of options between patient and provider: whether to pursue treatment vs active surveillance. This issue is multifaceted but has many downstream implications. The incidence rate for prostate cancer has grown by 3% every year from 2014 to 2019, driven by advanced cases [12]. A higher proportion of Black men classified as low risk and intermediate risk had a higher Decipher score than White men [13]. Basourkas et al. estimated that the number needed to diagnose to prevent one death was 11 to 14 for men of all races, compared to 5 to 9 for Black men [14]. In general, screening benefits Black men, which warrants a focus on improving screening rates in Black men.
Holmes et al. ask the question of whether an increased distance to a urologist, a surrogate for economic stability and health and healthcare access, is associated with a delayed diagnosis of prostate cancer among Black and White patients, as manifested by higher risk disease at diagnosis. They found that high-risk cancer rate increased with distance to a urologist and low-risk cancer rate decreased with longer distance [15]. On the race stratified multivariate analysis, longer distance was associated with higher risk prostate cancer for White and Black patients (P = 0.04 and < 0.01, respectively), but the effect was larger for Black men [15]. The authors concluded that longer distance to a urologist might disproportionately impact Black patients. Education has been shown to be positively associated with prostate cancer screening rates, specifically with men who have higher educational attainment [16, 17]. Using data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial, Barocas et al. found that non-Hispanic Black men aged < 65 years had 45% lower odds of undergoing a repeat PSA test or prostate biopsy compared with non-Hispanic White men [18•]. There was no racial difference in the follow-up among older men. Limitations in access to care among Black men below Medicare eligibility age in the USA may underlie the low rates of follow-up diagnostic care among Black men. Hispanic and Black cohorts tend to be diagnosed at a later stage than non-Hispanic White cohorts [19]. Individuals who were uninsured were more likely to be diagnosed with prostate cancer at a more advanced stage (OR = 1.47, P = 0.02) [19]. The interaction between the Hispanic ethnicity term and being uninsured in the multivariate logistic regression model was statistically significant. From a Switzerland-based population study, insurance characteristics like low deductibles, supplementary health insurance, and enrollment in a managed care plan were associated with higher screening utilization [20]. However, a gap in knowledge exists in the association between insurance status and specifically prostate cancer screening utilization in the USA. Table 1 summarizes the recent findings on the disparities in screening and diagnosis in prostate cancer.
Table 1.
Select data published on screening and diagnostic disparities in prostate cancer between various racial groups
| Study | Question | Findings |
|---|---|---|
| Effects of Health Insurance and Race on Early Detection of Cancer (1999) | Asks the question of whether differences in type of health insurance helps explain the racial disparities we see at time of cancer diagnosis |
• Found that high-risk cancer rate increased with distance to a urologist and low-risk cancer rate decreased with longer distance • On race stratified multivariate analysis, longer distance was associated with higher risk prostate cancer for White and Black patients (P = 0.04 and < 0.01, respectively), but the effect was larger in the latter group |
| Impact of Distance to a Urologist on Early Diagnosis of Prostate Cancer Among Black and White Patients (2012) | Asks the question of whether an increased distance to a urologist is associated with a delayed diagnosis of prostate cancer among Black and White patients, as manifested by higher risk disease at diagnosis |
• Persons who were uninsured were more likely diagnosed at a late stage (prostate cancer OR = 1.47, P = 0.02) • Non-Hispanic Black patients were more likely diagnosed with late stage breast and prostate cancers than were non-Hispanic White men |
| Association between race and follow-up diagnostic care after a positive prostate cancer screening test in the Prostate, Lung, Colorectal, and Ovarian cancer screening trial (2013) | Used data from the PLCO screening study to see if there was racial variation in how long it took to follow up on a high PSA and whether participants received either a repeat PSA, a prostate biopsy, or both within 9 months |
• Non-Hispanic Black men aged < 65 years had 45% lower odds of undergoing a repeat PSA test or prostate biopsy compared with non-Hispanic White men • There was no racial difference in follow-through among older men |
| Trends in Prostate Cancer Incidence Rates and Prevalence of Prostate Specific Antigen Screening by Socioeconomic Status and Regions in the United States, 2004 to 2013 (2018) | Investigators looked at census level data to examine trends in PSA testing by race and socioeconomic groups between the years of 2003 and 2013, leading up to revised USPSTF recommendations against routine PSA screening |
• Higher incidence in AA men and in counties with low poverty rate • Overall incidence decreased; decreased earlier in White men vs other race/ethnicities |
| Health disparities and inequities in the utilization of diagnostic imaging for prostate cancer (2020) | Systematic review examining the utilization of imaging in detecting prostate cancer |
• Non-White males tended to get more imaging not in concordance with NCCN guidelines • Higher income level more associated with appropriate imaging use, mpMRI use, but also imaging overuse |
| Racial and Ethnic Variation in PSA Testing and Prostate Cancer Incidence Following the 2012 USPSTF Recommendation (2021) | Investigators looked at the impact of USPSTF recommendations against routine prostate cancer screening after 2012 |
• PSA screening overall declined • PSA screening in non-Hispanic Black men declined at a significantly higher rate, driven by decreased screening in the 40–54-year-old cohort • PSA screening is lowest among Hispanic, NH American Indian/Alaska Native, and NH Asian/Pacifica Islander men |
Disparities in Care Delivery and Access
Multiple studies have described clear evidence of delivery and outcome disparities for both localized and advanced prostate cancer treatments. Historical data has consistently suggested higher mortality rates for Black men when compared to White patients [21–24]. However, in recent years, research suggests that overall survival and prostate cancer specific mortality outcomes are similar between White and Black men once they receive treatment [25] and may even be slightly favorable for Black men with localized prostate cancer [26]. Overall, this hopeful finding suggests that bridging the gap in care delivery and access to standard of care therapies may be an effective way to alleviate disparities in vulnerable communities.
Regardless, there remains evidence of clear differences in access, delivery, and even response to standard of care therapies between racial groups. The mechanism underlying these disparities is unclear, and there is limited evidence to suggest whether these observations are driven by factors on the provider side (e.g., implicit biases, access, and availability among underserved communities) or the patient side (e.g., distrust of the medical system, socialization of medical knowledge). However, there is evidence to suggest that the driving force is likely a combination of both. This section attempts to describe some of these mechanisms driving disparities in prostate cancer treatment outcomes to highlight key opportunities for growth in these areas.
Standard of Care Delivery
Various studies have described racial group differences in the time from diagnosis to delivery of standard of care may be one driving mechanisms behind the outcome differences observed. One study attempted to identify differences in time from diagnosis to definitive treatment in Black versus White localized prostate cancer patients. The study found that the time from diagnosis to definitive treatment and risk groups (with either prostatectomy or radiation) was longer for Black patients across all risk groups and was most pronounced in the high-risk cancer group (96 versus 105 days, P < 0.001) [27••]. These data are further corroborated by a study examining racial variations in the use of ADT and time to receive ADT among patients with metastatic prostate cancer [28]. Investigators found that White men were significantly more likely to receive ADT after a diagnosis of prostate cancer than Black men (P < 0.001). Differences even exist in fundamental treatment patterns among racial groups with advanced prostate cancer. One study that queried the SEER database to examine treatment trends among racial groups found that Black patients were more likely to go without treatment than White patients even after accounting for early mortality and TNM stage [29•]. Even in the adjuvant setting, investigators have observed disparities in delivery of ADT even in its most appropriate settings where Black men and men of other races are less likely to receive ADT compared to their White counterparts [30].
Within localized prostate cancer treatment, evidence suggests that compared to White men, Black men are significantly less likely to receive treatment across all Gleason scores and all D’Amico risk classifications [31]. Hispanic men were also less likely to receive treatment for Gleason score ≥ 7 disease and for intermediate or high-risk disease. Furthermore, a survey of the NCDB database from 2004 to 2012 revealed that non-White men were more likely to present with high-risk disease and were less likely to receive radical prostatectomies [32]. A subsequent study also using the NCDB database examined individual facility level variations in care delivery among men with nonmetastatic Gleason score ≥ 7 disease. Black men had significantly higher median PSA, were less likely to receive definitive treatment, and were more likely to be Gleason grade 8 or higher when compared to White men [33].
Access to Standard of Care
Disparities in access to standard of care options between different racial groups have been a longstanding and well-defined phenomenon for decades. The mechanisms behind these variations in access are multifactorial. Early studies all showed clear differences in availability [34] and willingness to access healthcare [35]. More recent studies have examined these access issues on a deeper level.
Tangible barriers such as lack of transportation or health insurance have been well described in the literature [36]. Medical distrust among vulnerable populations is a very well described (and unfortunately well founded [37]) phenomenon that serves as an access barrier to healthcare in general [38, 39•]. The evidence also suggests that endemic socioeconomic disparities likely play a key role in the access disparities that we observe between different racial groups [40, 41]. These disparities carry over in the post treatment cohort of prostate cancer survivors, with data suggesting that Black Americans may be at greater risk for disease recurrence after treatment [42, 43]. On the other hand, associations between education and especially the built environment with standard of care access have gone largely unstudied.
Variability in Standard of Care Efficacy Among Groups
However, even upon receipt of standard of care, there are response differences to standard of care along different racial groups. For example, a study of older men treated for locoregional prostate cancer examined survival differences between patients that received ADT [44]. Investigators found that the receipt of ADT was significantly lower in Black men (24%) relative to White (27%), Asian (34%), and Hispanic men (28.7%) (P < 0.05). Investigators also found that Black men had a statistically significant increase in mortality that remains significant even after adjusting for receipt of ADT. Notably, this mortality difference decreased after controlling for primary therapies such as prostatectomy, radiation, and surveillance and was no longer statistically significant after controlling for baseline comorbidities.
Interestingly, Asian men have been observed to receive ADT at higher rates than other racial and ethnic groups [44] and also respond more favorably to hormonal therapy [45]. This in turn may translate into more favorable survival outcomes in the Asian community [25]. One Japanese study attempted to examine this phenomenon more mechanistically to explain why Asian men responded more favorably to ADT than other racial and ethnic groups [46]. Investigators found higher rates of the active androgen transport genotypes of SLCO2B1 (GG allele) among Black and White racial populations than in Japanese and Han Chinese populations. This active genotype in turn exhibited a median time to progression that was 7 months shorter than that of patients with impaired androgen transporting activity polymorphisms. The literature discussed in this section reveals that care delivery and access to care vary along racial lines (Table 2).
Table 2.
Select data published on treatment and outcome disparities in prostate cancer between various racial groups
| Study | Question | Finding |
|---|---|---|
| Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men (2006) | Asked if there was a difference in the effectiveness of hormonal therapy in patients with prostate cancer among Japanese and White Americans |
• Japanese American Men who had received hormonal treatment had a better outcome than White men for overall and cause-specific survival rate • Race was one of the significant prognostic factors in the multivariate analysis |
| Impact of Androgen Deprivation Therapy on Racial/Ethnic Disparities in the Survival of Older Men Treated for Locoregional Prostate Cancer (2009) | Asked if there were survival differences between racial groups that received ADT | • Authors found that the receipt of ADT was significantly lower in Black men (24%) relative to White (27%), Asian (34%), and Hispanic men (28.7%) (P < 0.05) |
| Trends and Racial Differences in the Use of Androgen Deprivation Therapy for Metastatic Prostate Cancer (2010) | Authors wanted to see whether there were racial variations to use of ADT and time to receipt of ADT in metastatic prostate cancer | • Authors concluded that Black men with metastatic prostate cancer were significantly less likely to receive ADT and, when treated, had a slightly longer time to receipt than White men |
| Racial differences in time from prostate cancer diagnosis to treatment initiation (2013) | Asked if there was a difference between time from diagnosis to treatment in Black and White prostate cancer patients | • Authors concluded that AA patients with prostate cancer experienced longer time from diagnosis to treatment than White patients with prostate cancer, including with advanced systemic care |
| Racial/Ethnic Disparity in Treatment for Prostate Cancer: Does Cancer Severity Matter? (2016) | Authors attempted to determine whether there was variation in the receipt of treatment based on race and disease severity |
• Compared to White men, Black men sig less likely to receive treatment across all Gleason scores and all D’Amico risk classifications • Hispanic men less likely to receive treatment for G7 + disease and for int/high-risk disease • Asian men are no different from reference |
| Racial Disparity in Delivering Definitive Therapy for Intermediate/High-risk Localized Prostate Cancer: The Impact of Facility Features and Socioeconomic Characteristics (2018) | Authors reviewed the NCDB database between 2004 and 2013 to examine facility level variations in the use of definitive therapy for localized prostate cancer between different racial groups | • After adjusting for sociodemographic and clinical factors, investigators found that most facilities favored definitive therapy in White prostate cancer patients compared to their Black counterparts |
| Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: A SEER-Medicare investigation (2019) | Investigators queried SEER to examine treatment patterns for metastatic and CR prostate cancer and stratified by race determine whether there is a difference in adherence to standard of care treatment in advanced prostate cancer |
• Non-Hispanic Black (NHB) patients were more likely to go without treatment (P < 0.001) compared to non-Hispanic white (NHW) patients, even after accounting for early mortality and TNM stage • The frequency of nearly all forms of treatment was lower among NHB with the exception of orchiectomy, which was significantly higher (10.1% vs 6.1%, P < 0.001) |
| Racial differences in the treatment and outcomes for prostate cancer in Massachusetts (2021) | Investigators examined whether prostate cancer outcomes were different in Massachusetts, which was the earliest US state to mandate universal insurance coverage (in 2006) | • Despite lower odds of definitive treatment, Black men with prostate cancer experience reduced cancer-specific mortality in comparison with White men in Massachusetts |
Disparities in Trial Enrollment and Possible Barriers
Given the complex and often fraught relationship between various marginalized groups and the medical establishment that ranges back to before the now infamous Tuskegee syphilis experiments [47] and eventually culminating in the World Medical Association’s Declaration of Helsinki, it is no wonder that trust in the medical establishment is divided along racial lines, the degree of which has been unmasked even further in the recent COVID-19 pandemic [48]. Our current social and community context is a consequence of this complex history. Within the realm of prostate cancer, this can manifest in multiple ways, in addition to acting as a potential barrier to clinical trial enrollment. This is evidenced by a history of clear racial disparities in trial enrollment, even in classic trials like the PLCO (Prostate, Lung, Colorectal, and Ovarian) study [49]. Recent studies have identified possible barriers to trial enrollment that will need to be unpacked.
Ensuring that a sample is representative of the larger population is an important principle in experimental design. Despite this, many studies fail to present data on the diversity of their study population. One review found that in prostate, kidney, and bladder/urothelial cancer interventional phase II and III trials, only 169 of 341 (49.7%) reported race and ethnicity data [50•]. Black and Asian patients were poorly represented across all cancer types. Among prostate cancer patients enrolled on registrational trials, only 2.9% are self-reported Black patients, and other minority race patients were enrolled at ≤ 0.5% [51]. One reason for this is that many of the studies included in the Lythgoe et al. paper are multinational clinical trials that partner with small countries with small percentages of minorities. However, RTOG/NRG Oncology consistently enrolls Black men in prostate cancer trials at a rate of ~ 15% [52••]. More of these studies need to be replicated so that data reflects our country’s diversity. Considering the increased risk of presenting with advanced stage cancers and treatment outcomes for Black and other minority patients, research must focus on eliminating these disparities and including more representative samples. Whether because of distrust in the medical community, issues with accessibility to medical services or hidden financial costs, several SDOH drivers contribute to racial disparities in prostate cancer trial enrollment. Per the VOICES study which looked at community-based interviews in New York City in regard to research conducted in emergency situations, minority patients tend to have higher levels of perceived discrimination and deception in accordance with social and community context [39•]. Mistrust ultimately results in discordance of treatment recommendations and lack of trial participation. Galsky et al. evaluated metastatic prostate cancer clinical trials and found that 50.2% of trials would require a one-way drive of > 60 min for patients to access clinical trial sites [52••]. In addition, as trials become increasingly complex, it is common for frequent study visits to get billed as follow-up visits and charged to the patient rather than the study, unbeknownst to the patient [52••]. It is important to note that well-documented barriers related to SDOH (e.g., socioeconomic status, insurance state, poverty, education) generally are underrepresented and underreported in clinical trials.
On the policy side, Congress enacted the NIH Revitalization Act that addressed representation of women and minority patients in NIH-sponsored research through the creation of Minority Community Clinical Oncology Programs, collaboration in strategic initiatives with the CDC and teaching hospitals, and specialized trials for the elderly [53]. In a paper by the current surgeon general, Dr. Murthy quantified disparities in enrollment between minority groups as “enrollment fraction” which is defined as the number of trial enrollees divided by the estimated US cancer cases in each race and age subgroup. Compared with a 1.8% enrollment fraction among White patients, lower enrollment fractions were noted in Hispanic (1.3%; odds ratio (OR) vs White, 0.72; 95% confidence interval (CI), 0.68–0.77; P < 0.001) and Black (1.3%; OR, 0.71; 95% CI, 0.68–0.74; P < 0.001) patients [53]. Although both the National Cancer Institute (NCI) budget doubled from 1993 to 2002 and the number of trial participants increased, the proportion of trial participants who are Black decreased [53]. Given the ongoing lack of minority representation, however, previous mandates created by the government have fallen short.
Discussion and Opportunities for Improvement
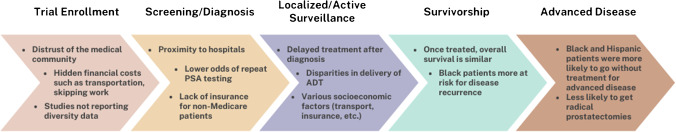
While there is clear documentation of disparities experienced by prostate cancer patients at every step of the continuum, most prominently among disadvantaged and marginalized groups, there also exists evidence that conveys a more hopeful message: that normalization of these disparities helps to improve differences in outcomes (Fig. 1). Here, we highlight and discuss a variety of data in the literature that portends to this space, with an emphasis on screening, access and care delivery, and trial enrollment.
Fig. 1.
Challenges for care delivery in the prostate cancer continuum. Well-described barriers and disparities in care evident in the literature, as stratified by discrete phases along the prostate cancer care continuum
Screening
Several groups have deployed solutions in various settings and populations to help combat the issue of low screening rates by deploying information and education about prostate cancer tailored for Black men in the barbershop. The barbershop is an advantageous environment because it is often regarded as a trusted space specific to Black culture to discuss vulnerable topics, thereby moving the prostate cancer discussion to a safe social and community context [54]. In a survey of 64 barbershops in the Richmond area, not only did 100% of proprietors agree that more promotional activities and programs should be directed to the Black community, but also 100% of proprietors reported that they would consider allowing their barbershops to be used to help Black men learn about prostate cancer [54]. By navigating historically safe spaces for minority communities, providers can help establish patient-provider trust and reach vulnerable populations. In another study conducted in rural Georgia, an area which has limited access to education, there was both high receptivity from Black men about the topic and an increase in the average score on a 17-item prostate cancer knowledge assessment from 72% pretest to 89% posttest (P = 0.03) [55]. A similar approach, the Detroit Education and Early Detection (DEED) study recruited participants from Black churches and had promising results. Compared with the population presenting to the urological clinic in which 35% of Black men were diagnosed with pathologically organ-confined prostate cancer, the men who underwent radical prostatectomy in the DEED project were diagnosed at a statistically significant higher rate of 11 of 17 men or 65%. They also recurred at a smaller rate of 1 of 15 (7%) in the DEED group and 39 of 157 (25%) in the clinic population [56••]. Another example of this method, Project HEAL (Health through Early Awareness and Learning), two peer community health advisors each were trained to host educational seminars for fifteen churches through traditional- and technology-based methods with an overall adoption rate of 41% for 375 total participants [57]. The common theme in all these implementations is tackling gaps in awareness through educational programs that transplant the discussion from doctor’s offices to settings that empower and uplift Black communities.
Access and Care Delivery
As aforementioned, evidence of disparities in access to care both in the localized and advanced settings of prostate cancer is abundant in the literature. Reassuringly however, there is a preponderance of evidence suggesting that once access disparities are eliminated, long-term survival outcomes between different groups begin to normalize. One classic study evaluated the long-term survival of Black and White prostate cancer patients among US Department of Defense active duty and retired service members [58]. The authors believed that these patients represented a population with equal access to a medical care system with relatively uniform screening and care delivery practices. The results of the study found no survival differences along racial lines. These data have been reproduced on multiple occasions in the modern era with studies finding that Black men in the VA system have similar or even better survival outcomes when compared to their White counterparts [26, 59].
Outside of the VA system, improved access to medical infrastructure consistently translates to better outcomes in vulnerable populations. One study examined differences in prostate cancer treatment outcomes between Black and White men in Massachusetts, which holds the distinction of being the earliest US state to mandate universal health insurance back in 2006 [60]. Black prostate cancer patients in Massachusetts experienced reduced prostate cancer-related mortality in comparison with White prostate cancer patients. These data as a whole support the hypothesis that improvements and access to quality care and screening help mitigate disparities among racial groups.
Interestingly, there is evidence that improved access to care may also translate into improved care delivery among racial groups. In a 2013 study of 777 North Carolinian men with newly diagnosed prostate cancer, 83.5% of men received guideline-concordant care within 1 year of diagnosis which did not differ by race [61]. What was most remarkable, however, was that patients’ perceived access to care had a statistically significant association with the receipt of guideline-concordant care, and those who had the lowest levels of perceived access to care were among the least likely to receive guideline-concordant care for prostate cancer.
Trial Enrollment
A diverse study cohort could potentially help identify any differences in treatment outcomes between different ethnic groups, which will allow patients and physician to make more informed care decisions. Including a representative study population is essential for conducting high-quality, reliable, and generalizable research. However, there is a fine line between inclusivity and exploitation. It might be important to ask the question if participating in research is actually beneficial for minority racial groups and ethnicities. From the perspective of equality and fairness, it is important to remove barriers to participate in clinical trials, but also not increase representation of minorities strictly for research purposes [62]. The ultimate objective should always be to reduce health disparities in disease outcomes. Financial incentives and outreach to community centers such as churches and schools are effective ways of bolstering access to clinical trials, but great care must be taken not to violate core ethical principles.
Given a history of unethical scientific research practices as well as a well-documented distrust in medicine, one might be led to believe that underrepresented people might be more unwilling to participate in clinical trials than White people. A 2006 study by Wendler et al. found that there were very small differences between the willingness of Hispanic, Black, and White men to participate in clinical trial [62]. Rather, the issue lied with Hispanic and Black patients not being asked to participate in studies [62]. As mentioned above for screening, outreach to cultural enclaves such as churches, restaurants, and barbershops can be an effective way of increasing awareness for people who live far away from healthcare infrastructure. In reaching out to populations with a lack of access to healthcare, the financial strain of enrolling in a clinical trial is more daunting than for high-income patients. After the implementation of a novel fee-assistance cancer care equity program (CCEP), Nipp et al. discovered that cancer clinical trial enrollment increased compared with enrollment from previous years [63]. A vital corollary to this is developing an accurate and efficient way of quantifying a patient’s level of financial burden and comprehensively presenting all risks to avoid taking advantage of low-income individuals.
Final Conclusions
Although there continues to be more progress in the literature in understanding how the SDOH are impacting patient diagnosis, treatment, and outcomes in prostate cancer, a lack of standardized, widespread data across health systems is limiting our capacity to draw generalizable conclusions from studies. Incorporating SDOH measures in EMR records to allow for more analytical assessment of what factors are contributing to prostate cancer care, although requiring some investment upfront by providers, would allow for more comprehensive investigation into the often overlooked SDOH domains and help the care team identify and address any potential barriers to care. The National Association of Community Health Centers and Centers for Medicare & Medicaid Services both have protocols and tools for how to address the SDOH for patients, and some clinicians are advocating for their use in clinical practices [64, 65•].
While disparities in prostate cancer care still exist, it is encouraging to see that progress is being made. A recent analysis of cancer statistics from the American Cancer Society found that Black men had an approximately twofold higher mortality from prostate cancer than White men, but it also found that the overall cancer mortality disparity is narrowing, particularly because of a steeper drop in lung and prostate cancers [66]. From the data, we also see a clear pathway forward, and encouraging evidence that improvements in screening, access, care delivery, and trial enrollment may translate out into meaningful outcome improvements for all prostate cancer patients. It is the sincere hope of the authors that this progress that has been made in remedying known inequities in our healthcare system continues into the future.
Acknowledgements
This work was supported by the National Cancer Institute (5P30CA006927) and the TUFCCC/HC Regional Comprehensive Cancer Health Disparity Partnership, Award Number U54 CA221704 from the National Cancer Institute of National Institutes of Health (NCI/NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI/NIH. This work was also supported in part by funding from the American Cancer Society (ACS) [MRSG-18-098-01-CPHPS to SML] and the Department of Defense [E01 W81XWH2210368 to SML].
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jerry Fu and Chen Fu equally contributed to this manuscript.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.National Institutes of Health. Cancer stat facts: prostate cancer In: Cancer.gov. 2020. https://seer.cancer.gov/statfacts/html/prost.html. Accessed 22 Aug 2022.
- 2.Brawn N, et al. Stage at presentation and survival of white and black patients with prostate carcinoma. Cancer. 1993;71(8):2569–2573. doi: 10.1002/1097-0142(19930415)71:8<2569::aid-cncr2820710822>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman RM, et al. Racial and ethnic differences in advanced-stage prostate cancer: the prostate cancer outcomes study. JNCI: J Nat Cancer Inst. 2001;93(5):388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Social determinants of health at CDC. In: CDC official website. 2022. https://www.cdc.gov/about/sdoh/index.html. Accessed 8 Dec 2022.
- 5.Kensler KH, et al. Racial and ethnic variation in PSA testing and prostate cancer incidence following the 2012 USPSTF recommendation. J Natl Cancer Inst. 2021;113(6):719–726. doi: 10.1093/jnci/djaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force. Prostate cancer: screening. In: USPSTF official website. 2018. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/prostate-cancer-screening. Accessed 22 Aug 2022.
- 7.Kim SP, et al. Contemporary national trends of prostate cancer screening among privately insured men in the United States. Urology. 2016;97:111–117. doi: 10.1016/j.urology.2016.06.067. [DOI] [PubMed] [Google Scholar]
- 8.El Khoury CJ, Ros R. A systematic review for health disparities and inequities in multiparametric magnetic resonance imaging for prostate cancer diagnosis. Acad Radiol. 2021;28(7):953–962. doi: 10.1016/j.acra.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Washington C, Deville C., Jr Health disparities and inequities in the utilization of diagnostic imaging for prostate cancer. Abdom Radiol (NY) 2020;45(12):4090–4096. doi: 10.1007/s00261-020-02657-6. [DOI] [PubMed] [Google Scholar]
- 10.Gross MD, et al. Variation in magnetic resonance imaging-ultrasound fusion targeted biopsy outcomes in Asian American men: a multicenter study. J Urol. 2020;203(3):530–536. doi: 10.1097/JU.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 11.Falagario UG, et al. Staging accuracy of multiparametric magnetic resonance imaging in Caucasian and African American men undergoing radical prostatectomy. J Urol. 2020;204(1):82–90. doi: 10.1097/JU.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, et al. Cancer statistics 2023. CA: A Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 13.Awasthi S, et al. Genomic testing in localized prostate cancer can identify subsets of African Americans with aggressive disease. J Natl Cancer Inst. 2022;114(12):1656–1664. doi: 10.1093/jnci/djac162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basourakos SP, et al. Harm-to-benefit of three decades of prostate cancer screening in Black men. NEJM Evidence. 2022;1(6):EVIDoa2200031. doi: 10.1056/evidoa2200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes JA, et al. Impact of distance to a urologist on early diagnosis of prostate cancer among Black and White patients. J Urol. 2012;187(3):883–888. doi: 10.1016/j.juro.2011.10.156. [DOI] [PubMed] [Google Scholar]
- 16.Steenland K, et al. Prostate cancer incidence and survival in relation to education (United States) Cancer Causes Control. 2004;15(9):939–945. doi: 10.1007/s10552-004-2231-5. [DOI] [PubMed] [Google Scholar]
- 17.Rapiti E, et al. Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer. 2009;115(23):5556–5565. doi: 10.1002/cncr.24607. [DOI] [PubMed] [Google Scholar]
- 18.Barocas DA, et al. Association between race and follow-up diagnostic care after a positive prostate cancer screening test in the Prostate, Lung, Colorectal, and Ovarian cancer screening trial. Cancer. 2013;119(12):2223–2229. doi: 10.1002/cncr.28042. [DOI] [PubMed] [Google Scholar]
- 19.Roetzheim RG, et al. Effects of health insurance and race on early detection of cancer. JNCI: J Natl Cancer Inst. 1999;91(16):1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 20.Ulyte A, et al. Variation of colorectal, breast and prostate cancer screening activity in Switzerland: Influence of insurance, policy and guidelines. PLoS ONE. 2020;15(4):e0231409. doi: 10.1371/journal.pone.0231409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang KM, et al. Evaluation of recent trends in cancer mortality and incidence among blacks. Cancer. 1988;61(6):1255–1261. doi: 10.1002/1097-0142(19880315)61:6<1255::aid-cncr2820610634>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Austin JP, et al. Diminished survival of young blacks with adenocarcinoma of the prostate. Am J Clin Oncol. 1990;13(6):465–469. doi: 10.1097/00000421-199012000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Austin JP, Convery K. Age-race interaction in prostatic adenocarcinoma treated with external beam irradiation. Am J Clin Oncol. 1993;16(2):140–145. doi: 10.1097/00000421-199304000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Aziz H, et al. Radiation-treated carcinoma of prostate. Comparison of survival of black and white patients by Gleason's grading system. Am J Clin Oncol. 1988;11(2):166–71. doi: 10.1097/00000421-198804000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Bernard B, et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer. 2017;123(9):1536–1544. doi: 10.1002/cncr.30503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKay RR, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403–411. doi: 10.1002/cncr.33224. [DOI] [PubMed] [Google Scholar]
- 27.Stokes WA, et al. Racial differences in time from prostate cancer diagnosis to treatment initiation. Cancer. 2013;119(13):2486–2493. doi: 10.1002/cncr.27975. [DOI] [PubMed] [Google Scholar]
- 28.Carson AP, et al. Trends and racial differences in the use of androgen deprivation therapy for metastatic prostate cancer. J Pain Symptom Manage. 2010;39(5):872–881. doi: 10.1016/j.jpainsymman.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beebe-Dimmer JL, et al. Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: A SEER-Medicare investigation. Cancer Medicine. 2019;8(6):3325–3335. doi: 10.1002/cam4.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen C, et al. Racial, socioeconomic, and geographic disparities in the receipt, timing to initiation, and duration of adjuvant androgen deprivation therapy in men with prostate cancer. J Racial Ethn Health Disparities. 2019;6(1):133–142. doi: 10.1007/s40615-018-0508-8. [DOI] [PubMed] [Google Scholar]
- 31.Moses KA, et al. Racial/ethnic disparity in treatment for prostate cancer: does cancer severity matter? Urology. 2017;99:76–83. doi: 10.1016/j.urology.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray J, et al. Temporal trends and the impact of race, insurance, and socioeconomic status in the management of localized prostate cancer. Eur Urol. 2017;71(5):729–737. doi: 10.1016/j.eururo.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 33.Friedlander DF, et al. Racial disparity in delivering definitive therapy for intermediate/high-risk localized prostate cancer: the impact of facility features and socioeconomic characteristics. Eur Urol. 2018;73(3):445–451. doi: 10.1016/j.eururo.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Blendon RJ, et al. Access to medical care for Black and White Americans: a matter of continuing concern. jAMA. 1989;261(2):278–281. [PubMed] [Google Scholar]
- 35.Loehrer J, Sr, et al. Knowledge and beliefs about cancer in a socioeconomically disadvantaged population. Cancer. 1991;68(7):1665–1671. doi: 10.1002/1097-0142(19911001)68:7<1665::aid-cncr2820680734>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Connell CL, et al. Barriers to healthcare seeking and provision among African American adults in the rural Mississippi delta region: community and provider perspectives. J Community Health. 2019;44(4):636–645. doi: 10.1007/s10900-019-00620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Washington HA. Medical apartheid : the dark history of medical experimentation on black americans from colonial times to the present. 1. New York: Doubleday; 2006. [Google Scholar]
- 38.Richardson A, et al. Effects of race/ethnicity and socioeconomic status on health information-seeking, confidence, and trust. J Health Care Poor Underserved. 2012;23(4):1477–1493. doi: 10.1353/hpu.2012.0181. [DOI] [PubMed] [Google Scholar]
- 39.Smirnoff M, et al. A paradigm for understanding trust and mistrust in medical research: the Community VOICES study. AJOB Empir Bioeth. 2018;9(1):39–47. doi: 10.1080/23294515.2018.1432718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pamies RJ, Woodard LJ. Cancer in socioeconomically disadvantaged populations. Primary Care: Clin Office Pract. 1992;19(3):443–450. [PubMed] [Google Scholar]
- 41.Yin D, et al. Does socioeconomic disparity in cancer incidence vary across racial/ethnic groups? Cancer Causes Control. 2010;21(10):1721–1730. doi: 10.1007/s10552-010-9601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen JH, et al. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States) Cancer Causes Control. 2006;17(6):803–811. doi: 10.1007/s10552-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 43.Barocas DA, Penson DF. Racial variation in the pattern and quality of care for prostate cancer in the USA: mind the gap. BJU Int. 2010;106(3):322–328. doi: 10.1111/j.1464-410X.2010.09467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes L, et al. Impact of androgen deprivation therapy on racial/ethnic disparities in the survival of older men treated for locoregional prostate cancer. Cancer control. 2009;16(2):176–185. doi: 10.1177/107327480901600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukagai T, et al. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU International. 2006;97(6):1190–1193. doi: 10.1111/j.1464-410X.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto N, et al. Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis. 2013;16(4):336–340. doi: 10.1038/pcan.2013.23. [DOI] [PubMed] [Google Scholar]
- 47.Clinton B. Remarks by the president in apology for study done in Tuskegee. Washington D.C.: The East Room of The White House; 1997.
- 48.Funk C, Tyson A. Intent to get a COVID-19 vaccine rises to 60% as confidence in research and development process increases. In: Pew Research Foundation. 2020. https://www.pewresearch.org/science/2020/12/03/intent-to-get-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-process-increases/. Accessed 22 Aug 2022.
- 49.Pinsky F, et al. Enrollment of racial and ethnic minorities in the prostate, lung, colorectal and ovarian cancer screening trial. J Natl Med Assoc. 2008;100(3):291–298. doi: 10.1016/s0027-9684(15)31241-4. [DOI] [PubMed] [Google Scholar]
- 50.Owens-Walton J, et al. Minority enrollment in phase II and III clinical trials in urologic oncology. Journal of Clinical Oncology. 2022;40(14):1583–1589. doi: 10.1200/JCO.21.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lythgoe MP, et al. Race reporting and diversity in US food and drug administration (FDA) registration trials for prostate cancer; 2006–2020. Prostate Cancer Prostatic Dis. 2021;24(4):1208–1211. doi: 10.1038/s41391-021-00361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vince R, Spratt DE. Drivers of racial disparities in prostate cancer trial enrollment. Prostate Cancer Prostatic Dis. 2021;24(4):946–947. doi: 10.1038/s41391-021-00427-z. [DOI] [PubMed] [Google Scholar]
- 53.Murthy VH, Krumholz HM, Gross CP. participation in cancer clinical trialsrace-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 54.Hart A, et al. Recruiting African-American barbershops for prostate cancer education. J Natl Med Assoc. 2008;100(9):1012–1020. doi: 10.1016/s0027-9684(15)31437-1. [DOI] [PubMed] [Google Scholar]
- 55.Luque JS, et al. Feasibility study of engaging barbershops for prostate cancer education in rural African-American communities. J Cancer Educ. 2015;30(4):623–628. doi: 10.1007/s13187-014-0739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell Isaac J, et al. Outcome of African American men screened for prostate cancer: the Detroit education and early detection study. J Urol. 1997;158(1):146–149. doi: 10.1097/00005392-199707000-00045. [DOI] [PubMed] [Google Scholar]
- 57.Santos SL, et al. Adoption, reach, and implementation of a cancer education intervention in African American churches. Implement Sci. 2017;12(1):36. doi: 10.1186/s13012-017-0566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Optenberg SA, et al. Race, Treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995;274(20):1599–1605. [PubMed] [Google Scholar]
- 59.Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer. 2020. 10.1002/cncr.32666 [DOI] [PubMed]
- 60.Cole AP, et al. Racial differences in the treatment and outcomes for prostate cancer in Massachusetts. Cancer. 2021;127(15):2714–2723. doi: 10.1002/cncr.33564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellis SD, et al. Receipt of National Comprehensive Cancer Network guideline-concordant prostate cancer care among African American and Caucasian American men in North Carolina. Cancer. 2013;119(12):2282–2290. doi: 10.1002/cncr.28004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wendler D, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nipp RD, et al. Financial burden of cancer clinical trial participation and the impact of a cancer care equity program. Oncologist. 2016;21(4):467–474. doi: 10.1634/theoncologist.2015-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Association of Community Health Centers. PRAPARE (version date September 20, 2016). National Association of Community Health Centers website. 2017. http://www.nachc.org/research-and-data/prapare/. Accessed 22 Aug 2022.
- 65.• Billioux, A., Verlander K, Anthony S, and Alley D. Standardized screening for health-related social needs in clinical settings: the accountable health communities screening tool. NAM Perspectives. 2017. 10.31478/201705b. This perspective piece discusses the use of the CMS screening tool for social determinants of health.
- 66.Giaquinto AN, et al. Cancer statistics for African American/Black people 2022. CA: A Cancer J Clin. 2022;72(3):202–229. doi: 10.3322/caac.21718. [DOI] [PubMed] [Google Scholar]