Abstract
Background
Scutellaria baicalensis Georgi is a famous traditional Chinese medicine, which is widely used in treating fever, upper respiratory tract infection and other diseases. Pharmacology study showed it can exhibit anti-bacterial, anti-inflammation and analgesic effects. In this study, we investigated the effect of baicalin on the odonto/osteogenic differentiation of inflammatory dental pulp stem cells (iDPSCs).
Methods and results
iDPSCs were isolated from the inflamed pulps collected from pulpitis. The proliferation of iDPSCs was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2,5-tetrazolium bromide (MTT) assay and flow cytometry. Alkaline phosphatase (ALP) activity assay, alizarin red staining, Real-time reverse transcription-polymerase chain reaction (RT-PCR) and Western blot assay were conducted to examine the differentiation potency along with the involvement of nuclear factor kappa B(NF-κB) and β-catenin/Wnt signaling pathway. MTT assay and cell-cycle analysis demonstrated that baicalin had no influence on the proliferation of iDPSCs. ALP activity assay and alizarin red staining demonstrated that baicalin could obviously enhance ALP activity and calcified nodules formed in iDPSCs. RT-PCR and Western blot showed that the odonto/osteogenic markers were upregulated in baicalin-treated iDPSCs. Moreover, expression of cytoplastic phosphor-P65, nuclear P65, and β-catenin in iDPSCs was significantly increased compared with DPSCs, but the expression in baicalin-treated iDPSCs was inhibited. In addition, 20 µM Baicalin could accelerate odonto/osteogenic differentiation of iDPSCs via inhibition of NF-κB and β-catenin/Wnt signaling pathways.
Conclusion
Baicalin can promote odonto/osteogenic differentiation of iDPSCs through inhibition of NF-κB and β-catenin/Wnt pathways, thus providing direct evidence that baicalin may be effective in repairing pulp with early irreversible pulpitis.
Keywords: Baicalin, Dental pulp stem cells, Nuclear factor kappa B, β-catenin/Wnt signaling pathway, Odonto/osteogenic differentiation
Introduction
Scutellaria baicalensis, Chinese traditional Herbal medicine, is the dried root of Scutellaria baicalensis Georgi. Its use first appeared in the Han Dynasty classic ShenNongBenCaoJing, which recorded its efficacy in treating lung and liver diseases [1]. Baicalin, one of the flavonoids purified from S. baicalensis, can exhibit anti-bacterial [2],anti-inflammation [3], analgesic effects [4]. In the field of stomatology related research, baicalin has been shown to significantly enhance type I collagen and protein synthesis in gingival fibroblasts [5]. Baicalin inhibits the inflammatory root resorption induced by orthodontic treatment by reducing inflammatory factors and enhancing the expression of osteogenic factors [6]. Scutellaria baicalensis extract including baicalin has certain value in the treatment of oral squamous cell carcinoma due to its tumor inhibition and angiogenesis [7].
The dental pulp consists of fibroblasts, preodontoblasts, odontoblasts, immune cells, ectomesenchymal cells and dental pulp stem cells (DPSCs) [8]. DPSCs, a type of stem cells that exhibits multipotent differentiation ability and has been isolated from dental pulp for the first time in 2000 [9], are a predictable source of cells that can be used in various fields of medical regeneration. Some evidence has suggested that inflammation can reduce the differentiation potency of DPSCs [10]. Recent years, some studies have also displayed the phenomenon that the inherent anti-inflammatory potential and high dentin regeneration capacity of the pulp stimulated by inflammation can be expressed at various dental positions at all ages [11]. Moreover, studies have found that in addition to controlling bacterial and inflammatory reactions, stem cells are found in the pulp of both permanent and deciduous teeth, the former can generate regenerative signals such as growth factors to induce proliferation, migration, and regeneration of dentin-pulp tissue after traumatic injury or pulp infection [12, 13] In other words, these DPSCs can migrate to the damaged dental pulp tissue for differentiation, such as odontoblast-like cells that respond to adverse external stimuli [8].
Previous studies have shown that Gram-negative bacteria's lipopolysaccharide (LPS), causing severe inflammatory damage so far as to necrosis of tissues [14, 15]. LPS-induced inflammatory damage involves the TLR4/NF-κB P65 inflammatory signaling pathway. TLR4, the receptor of LPS, activates the MyD88-dependent signaling pathway upon LPS, triggering the transcription program of NF-κB P65 and inducing secreta of pro-inflammatory cytokines from some epithelial cells, macrophages and dendritic cells [16]. Therefore, many anti-inflammatory drugs, including some TCM components, target the TLR4/NF-κB P65 inflammatory signaling pathway.
The transcription factors NF-κB play a significant role in multiple cellular processes, including development, immune system, inflammatory, proliferation, and cancer [17–19]. NF-κB can be activated by various extracellular signaling factors, such as cytokines and hormones in immune cells and other cell types [20, 21]. In iDPSCs, the NF-κB pathway is activated and osteogenic differentiation marker (ALP/RUNX2/OCN/COL-I) is down-regulated [22]. Also, baicalin can inhibit the NF-κB signaling pathway to prevent the expression of tumor necrosis factor-α(TNF-α) and interleukin-1β(IL-1β) related to human periodontal ligament stem cells(PDLSCs) [23].
Wnt are a family of secreted glycoproteins that may regulate developmental processes. Wnt signal is significant in the growth and development of tooth embryo and the biological behavior after eruption [24]. Wnt family can be divided into canonical and non-canonical (Wnt/Ca2+ and Wnt polarity pathways), including 19 proteins. Inflammatory could affect the differentiation of stem cells through cytokines and mediator by activating the canonical Wnt signaling pathway [25]. Under inflammatory conditions, canonical Wnt signaling showed significant difference in osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and PDLSCs [26, 27]. The β-catenin/Wnt pathway inhibited odontoblast-like differentiation in DPSCs, resulting in low expression of ALP activity and mineralization [28]. Moreover, it has been found that baicalin induces osteoblastic differentiation via β-catenin/Wnt pathway [29], while its mode of action is not clear yet.
In this research, iDPSCs were extracted from patients clinically diagnosed with irreversible pulpitis [30], the impact of baicalin on the cell multiplication and odonto/osteogenic differentiation of iDPSCs was explored, the role of NF-κB and β-catenin/Wnt signaling pathway and their intrinsic mechanisms in this process was also investigated.
Materials and methods
Study samples and cell culture
Baicalin (Lot.Number: Z28S11X125952; CAS: 21967-41-9, Shanghai yuanye Bio-Technology Co., Ltd, China), with a purity of 99.3%, was dissolved in dimethylsulfoxide (DMSO) and stored in the dark at -20℃. Different concentrations of baicalin (10 µM, 20 µM, 50 µM, 100 µM) were used for the cell experiment. Cells passaged 2–4 times were used for in vitro experiments.
Normal and inflammatory dental pulp tissues were isolated from teeth without caries or other dental tissue diseases and from clinically diagnosed irreversible pulpitis. The pulp samples were then chopped and digested for 30 min in a solution containing 3 mg/mL collagenase type I (Sigma, USA) and 4 mg/mL dispase (Sigma, USA) at 37℃. Afterwards, the single-cell suspensions were obtained and incubated in alpha minimum essential medium (α-MEM, Gibco, NY) mixed with 10% fetal bovine serum (FBS, Gibco), 100 mg/mL streptomycin and 100U/mL penicillin in 5% CO2 at 37 °C.
This research was approved by the consent of the patients and was given permission by the Ethical Committee of the Stomatological School of Nanjing Medical University (Reference #200900128).
Immunofluorescence
nDPSCs (normal dental pulp stem cells) and iDPSCs were incubated in different petri dishes. On day 3, the cells were cleaned, fixed, perforated and sealed at 37 °C for 0.5 h. The cells were subsequently incubated with primary antibodies at 4 °C for one night, and then with fluorescence-labeled secondary antibody for 1 h at 25 °C. Nuclei was stained by 4,6-diamidino-2-phenylindole (DAPI) and the treated cells mentioned above were visualized using a microscope (LEICA DM4000B) for their immunofluorescence.
MTT assay
The cells proliferation of nDPSCs, iDPSCs, and iDPSCs treated by baicalin was investigated by MTT assay (Sigma, USA). The general process is to incubate the cells in complete culture media for 24 h, and then in serum-free α-MEM for another 24 h. Cells were then treated by baicalin at different concentrations (10, 20, 50, 100 µM) for 0, 1, 3, 5, 7, and 9 days. After each time point, MTT solution (Sigma-Aldrich) prepared on the spot was added and incubated at 37 °C for 4 h. After removing the solution, dimethyl sulfoxide (DMSO, Sigma) was employed to dissolve the formazan. Finally, the absorbance at 490 nm was measured by an automatic enzyme-linked immunosorbent assay reader (ELx800, Grand Island, NY, USA).
Flow cytometry
nDPSCs along with iDPSCs were seeded and cultured in complete culture media for 24 h. After 24 h of serum starvation, cells were cultured in complete culture media containing 20 µM balcalin or not. After reaching 70%-80% confluence, harvested the cells and then fixed them with 75% ice-cold ethanol for 0.5 h without light. Cell cycle fractions were subsequently measured by flow cytometry.
Alkaline phosphatase activity and alizarin red staining
nDPSCs and iDPSCs treated or untreated by baicalin were seeded in six-well plates at a density of 2 × 105 cells/well for 3, 5, and 7 days, 4% paraformaldehyde was used for fixation for 30 min at room temperature. Cells were stained with the ALP staining kit in the dark according to manufacturer’s protocols. Images were taken under the scanner (EPSON printer, Japan). Similarly, cells were cultured under these groups, and the lysate protein concentration in each group was measured. Then, ALP activity assay was assessed by ALP activity kit, measuring at 520 nm optical density, which was performed as formerly studies [31]. To evaluate the ability of mineralized nodule formation, the cells were seeded in complete culture media or mineralization-inducing media (MM) with or without 20 μM baicalin. At day 14, Alizarin red staining was conducted as formerly mentioned [32]. After acquiring the images, nodule staining was discolored by 1mL 10% cetylpyridinium chloride (CPC) per well to quantify the calcified nodules.
Real-time reverse transcriptase-polymerase chain reaction (Real-time RT-PCR)
After 7 days of culture, nDPSCs and iDPSCs treated with or without baicalin, along with cells cultured in the existence or non-existence of BMS345541 or DKK1, were gained using TRIzol reagent (Invitrogen, USA). On the foundation of the instructions, total cell RNA was extracted and the concentration of it was measured. Only after being reversely transcribed into cDNA via a Prime Script RT Master Mix kit (TaKaRa Biotechnology, China) then can the mRNA be performed for Real-time RT-PCR using SYBR1 Premix Ex Taq™ kit (TaKaRa, Otsu, Japan) and ABI 7300 real-time PCR system. The primers used for the genes are listed in Table 1. GAPDH was applied as an internal control.
Table 1.
Sense and antisense primers for Real-time RT-PCR
| Genes | Sequences (5'-3') | Length of product |
|---|---|---|
| RUNX2 | F: TGGAACATCTCCATCAAGGCAG | 89 bp |
| R: TCAGGATATTCGGGACGTTGGA | ||
| OSX | F: CCTCCTCAGCTCACCTTCTC | 148 bp |
| R: GTTGGGAGCCCAAATAGAAA | ||
| OCN | F: AGCAAAGGTGCAGCCTTTGT | 63 bp |
| R: GCGCCTGGGTCTCTTCACT | ||
| DSPP | F: ATATTGAGGGCTGGAATGGGGA | 136 bp |
| R: TTTGTGGCTCCAGCATTGTCA | ||
| GAPDH | F: GAAGGTGAAGGTCGGAGTC | 225 bp |
| R: GAGATGGTGATGGGATTTC | ||
| IL-1β | F: CCAGGGACAGGATATGGAGCA | 129 bp |
| R: TTCAACACGCAGGACAGGTACAG | ||
| TNF-α | F: CTCAGCAAGGACAGCAGAGG | 83 bp |
| R: ATGTGGCGTCTGAGGGTTGTT |
Western blot
For evaluating the expression of odonto/osteognic proteins, nDPSCs and iDPSCs treated or untreated with baicalin or BMS345541 or DKK1 were harvested, washed and lysed. To analyze the expressions of NF-κB and β-catenin/Wnt pathway related proteins, the cytoplasm protein as well as nucleoprotein were extracted from iDPSCs treated with 20 µM baicalin for 0, 15, 30, and 60 min. After loading the protein onto a 10% SDS-PAGE gel for electrophoresis, electroblotted (Bio-Rad, USA) the protein onto 0.22 μm polyvinylidene fluoride (PVDF) membrane (Millipore, USA) at 300 mA for 1 h. After blocking membranes with solution at 25℃, incubated them with primary antibodies (DSP, Santa Cruz; RUNX2, Abcam; OSX, Abcam; OCN, Abcam; phosphor-P65; Cell Signaling, Boston, MA, USA, P65, Cell Signaling, Boston, MA, USA; phosphor IκBα, Cell Signaling; IκBα, Cell Signaling; β-catenin, Abcam; GSK-3β, Cell Signaling; NLK, Cell Signaling; CaMKII, Cell Signaling; β-ACTIN, Bioworld, Minneapolis, MN, USA; Histone, Bioworld) at 4 °C all night long. Membranes were then washed for 1 h with PBST blended with secondary antibodies (Boster, China) at 37 °C before visualized with ImageQuant LAS4000 system (GE Healthcare, USA). β-Actin and H3 were set as the internal parameters, respectively.
Statistical analysis
Each experiment was repeated three times at each time point. All the results were expressed as mean ± standard deviation, and statistical analysis was carried out using one-way analysis of variance (ANOVA) or Student's t-test on SPSS 17.0. P < 0.05 was considered statistically significant.
Results
Cell morphology and characterization
nDPSCs and iDPSCs were used for all experiments. The cells presented spindle- or classical fibroblast-like morphology, and there was no difference between nDPSCs and iDPSCs (Fig. 1A-D). The expression of TNF-α and interleukin-1β(IL-1β)in iDPSCs was vastly up-regulated in nDPSCs (P < 0.05) (Fig. 1E). Additionally, cells showed positive expression of vimentin (Fig. 1F) and negative expression of cytokeratin, conforming to be of mesenchymal origin. Negative immunofluorescence was not shown.
Fig. 1.
Characterization and Colony of nDPSCs and iDPSCs. (A, B 40 × , scale bar = 80 μm) Observation of colony-forming of nDPSCs and iDPSCs cultured in vitro; (C, D 100 × , scale bar = 200 μm) Observation of nDPSCs and iDPSCs cultured in vitro. E The expression of TNF-αand IL-1βin iDPSCs was significantly up-regulated in nDPSCs (P < 0.05). F Representative immunofluorescence staining of cultured nDPSCs and iDPSCs for vimentin. (200 × , Scale bar = 100 μm)
Low concentrations of baicalin have no effect on the proliferation of iDPSCs
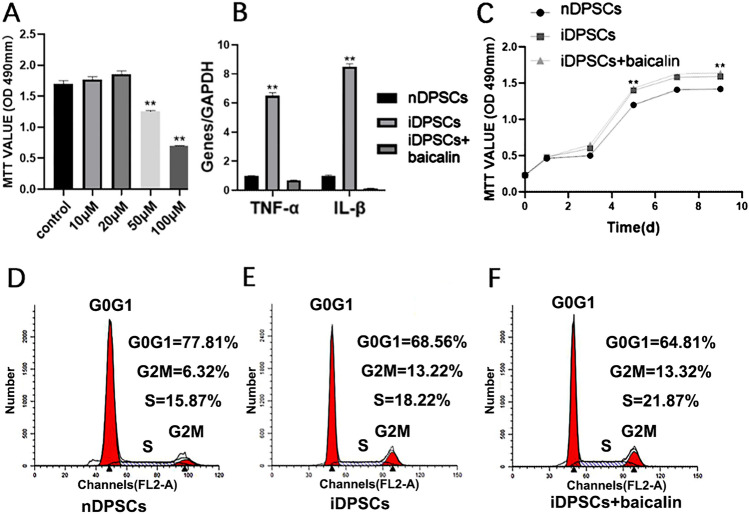
MTT assay was implemented to select the appropriate concentration and investigate the effects of baicalin on iDPSCs proliferation. 10–20 µM baicalin exposure did not impact the proliferation of iDPSCs (P > 0.05). Yet, the decrease in iDPSCs growth was seen after treatment with 50–100 µM baicalin (Fig. 2A). Thus, 20 µM was chosen as the first-rank concentration of baicalin for succedent experiments.
Fig. 2.
Construction of an inflammatory environment and the optium concentration of baicalin. A Dose-dependent effects of baicalin on iDPSCs. MTT detected cell proliferation, baicalin under the concentration of 20 µM had no inhibiting effect (P > 0.05). B Real-time RT-PCR analysed inflammatory indicators in iDPSCs compared with nDPSCs group. C Effects of 20 µM baicalin on proliferation rates of iDPSCs. D–F Flow cytometry (FCM) assay examed proliferation of each group. **P < 0.01, *P < 0.05, n = 3
Furthermore, MTT assay (Fig. 2C) and flow cytometry analysis (Fig. 2D-F) showed that the proliferation index was analogous in baicalin-treated iDPSCs and untreated cells, and iDPSCs increased as compared with nDPSCs. The above results revealed that the proliferation of iDPSCs was higher than that of nDPSCs, and low concentrations of baicalin (≤ 20 µM) had no effect on the proliferation of iDPSCs.
Baicalin downregulates cytokines in iDPSCs
Real-time RT-PCR demonstrated that the expressions of cytokines in iDPSCs were vastly higher than those in nDPSCs (P < 0.05), and baicalin could down-regulate the expression of them (Fig. 2B) in iDPSCs (P < 0.01).
Baicalin promotes odonto/osteogenic differentiation in iDPSCs
After detecting the ALP activity of nDPSCs, iDPSCs, and iDPSCs treated with 20 µM baicalin at day 3, 5, and 7 (P < 0.01), we found that nDPSCs showed higher ALP activity than iDPSCs. Moreover, iDPSCs treated with baicalin presented higher ALP activity compared with iDPSCs (Fig. 3A). Alizarin red staining along with quantitative calcium measurement further indicated that nDPSCs had higher mineralization ability compared with iDPSCs, and baicalin could enhance the ability of mineralization in iDPSCs (P < 0.01) (Fig. 3B, C). Moreover, real-time RT-PCR assessment of odonto/osteogenic genes in iDPSCs was decreased than that in nDPSCs, while it was significantly up-regulated in response to 20 µM baicalin (Fig. 4A). Consistently, the assessment in the protein levels showed a similar trend where inflammatory decreased the odonto/osteogenic differentiation marker (RUNX2, DSP, OSX, OPN, OCN), and baicalin enhanced the expression level of proteins in iDPSCs (Fig. 4B, C). These outcomes revealed that baicalin heightens the odonto/osteogenic differentiation of iDPSCs.
Fig. 3.

Baicalin promoted odonto/osteogenic differentiation of iDPSCs. A The ALP activity of each group for 3,5,7. ALP activity of nDPSCs was higher than iDPSCs, while iDPSCs treated by baicalin exhibited the significantly higher ALP activity than iDPSCs. B Alizarin red staining revealed the mineralized nodules in each group. C CPC assay revealed that baicalin-treated group had significantly higher calcium content. **P < 0.01, *P < 0.05, n = 3
Fig. 4.
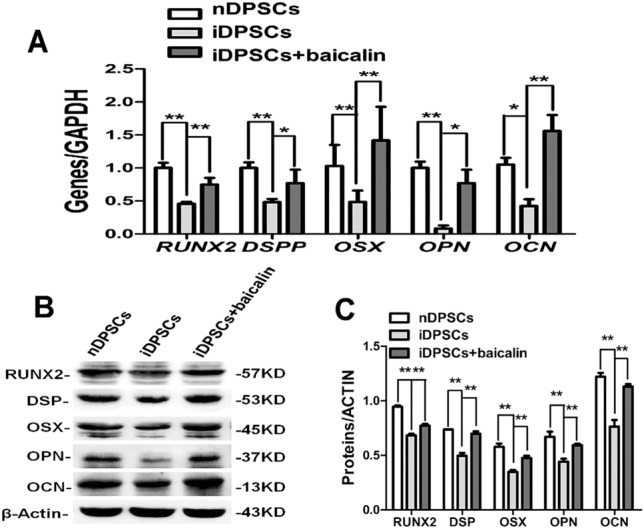
Baicalin contributed to odonto/osteogenic differentiation of iDPSCs. A Real-time RT-PCR analysed odonto/osteogenic genes in nDPSCs, iDPSCs and baicalin-treated iDPSCs on day 7. (**2-∆∆Ct.32, P < 0.01; *1 < 2-∆∆Ct < 2, P < 0.01), n = 3. B, C Western blot assessments of odonto/osteogenic proteins were vastly increased under the stimulation of baicalin on day 7
Baicalin inhibits NF-κB and β-catenin/Wnt pathway in iDPSCs
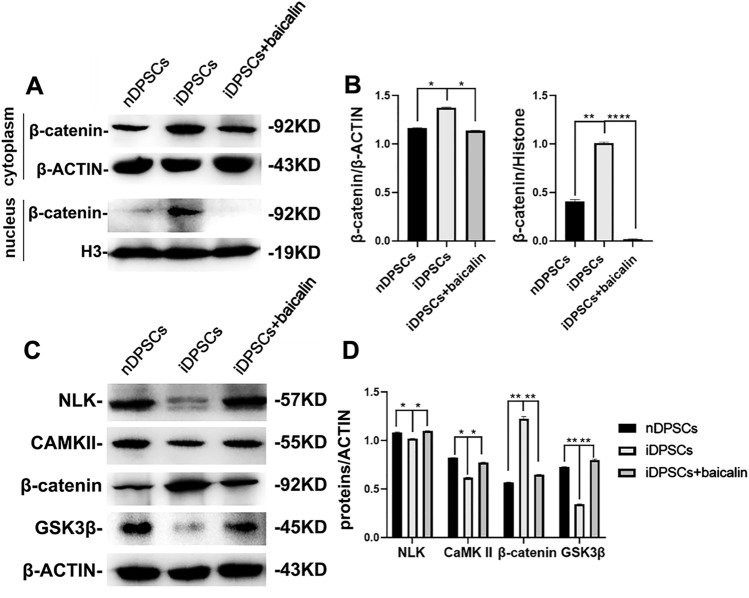
To explore whether baicalin inhibits NF-κB and β-catenin/Wnt Pathway in iDPSCs, we collected cytoplasmic and nuclear protein of iDPSCs treated with baicalin at 0,15, 30, 60 min, and 48 h, and measured the levels of related proteins using Western blot. The expression of NF-κB pathway proteins (Fig. 5A–D) and Wnt pathway-related protein in iDPSCs were significantly enhanced as compared with those in nDPSCs. However, the proteins in the baicalin-treated group were lower than iDPSCs (Fig. 6A, B); the expression of P65, IκBα and CaMKII, NLK in iDPSCs were decreased as compared with those in nDPSCs, and the proteins in the baicalin-treated group were enhanced (Fig. 6C, D). In short, these findings indicated that baicalin can inhibit the NF-κB and β-catenin/Wnt signaling pathways activated by inflammation in iDPSCs.
Fig. 5.
Baicalin inhibited the NF-κB pathway in iDPSCs. A, B Protein expressions of IκBα, p-IκBα, P65, and p-P65 in baicalin-treated iDPSCs. C, D Protein expressions of P65 in nuclear in baicalin -treated iDPSCs
Fig. 6.
Baicalin inhibited the β-catenin/Wnt signaling pathway. A, B Protein expressions of β-catenin in baicalin-treated iDPSCs at each time point. C, D The NLK, CaMKII,β-catenin and GSK3β expression of baicalin-treated iDPSCs were investigated by western blot at indicated time points
Inhibition of the NF-κB and β-catenin/Wnt pathway upregulated odonto/osteogenic differentiation of baicalin-treated iDPSCs
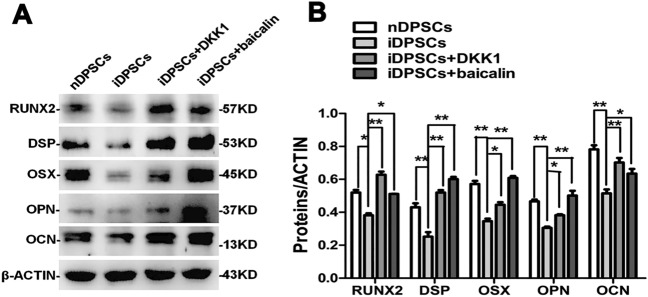
To detect whether the suppression of NF-κB and β-catenin/Wnt signaling pathway was crucial to enhance odonto/osteogenic differentiation of iDPSCs for baicalin, BMS345541 and DKK1 were used to block the NF-κB and β-catenin/Wnt signaling pathway, respectively. The gene and protein levels both showed a semblable trend where the expression of odonto/osteogenic markers was upregulated by BMS345541 (Fig. 7) and DKK1 (Fig. 8) in iDPSCs; these results were consistent with iDPSCs treated with baicalin. These data demonstrated that baicalin accelerated the differentiation of iDPSCs by inhibiting NF-κB and β-catenin/Wnt pathways.
Fig. 7.
Impacts of baicalin and BMS345541 on the odonto/osteogenic differentiation. Real-time RT-PCR analysed odonto/osteogenic genes at day 7. (**2-∆∆Ct32, P < 0.01; *1 < 2-∆∆Ct < 2, P < 0.01), n = 3. B, C Western blot demonstrated a similar trend
Fig. 8.
Impacts of baicalin and DKK1 on the β-catenin/Wnt pathway and odonto/osteogenic differentiation in iDPSCs. A, B Western blot assessments of odonto/osteogenic markers were vastly upregulated (P < 0.01) by baicalin and DKK1 at day 7, as comparison with iDPSCs
Discussion
Under severe dental pulp damage, DPSCs can be activated, after which they enter odontogenesis to produce tertiary dentin [33]. There have been studies reported that dental tissue-derived stem cells can also be found under inflammation [34–36]. It has also been shown that DPSCs isolated from inflammatory dental pulp have stem cell-like properties [30]. Based on the above discussion, we successfully extracted and cultured stem cells from healthy and inflamed dental pulp tissue for subsequent research.
Lipopolysaccharide (LPS) is the major causative factor of pulpitis that can cause severe dental pulp inflammation. LPS can induce varieties of cells to produce cytokines, including IL, TNF and interferon (IFN), subsequently leading to pulp tissue damage and inflammation. Baicalin can repress IL-6 and TNF-α levels to improve LPS-stimulated cell viability [37]. Baicalin protects against tissue damage in ligature-induced periodontitis by down-regulating the MMP-1 induced by IL-1β and promoting the production of COL-I [38]. Based on relevant studies, IL-1 β and TNF-α were selected from many inflammatory factors to observe the effect of baicalin on the inflammatory pulp.
In the current study, we successfully isolated iDPSCs from inflammatory dental pulp. nDPSCs and iDPSCs showed similar morphology, and both stained positively for vimentin, which confirmed their mesenchymal origin. A previous study showed that the expression quantity of inflammatory cytokines was up-regulated in inflammatory dental pulp [39]. In our study, the gene levels indicated that the expressions of TNF-α and IL-1β in iDPSCs were upregulated, while those in iDPSCs treated with baicalin were down-regulated. iDPSCs have higher proliferation and lower differentiation compared to nDPSCs. Baicalin at concentration of 20 µM showed to facilitate the odonto/osteogenic differentiation of iDPSCs, but made no difference in the proliferation of cells. Therefore, the phenomenon shown in our study is that the specific concentration of baicalin can safely reduce some inflammatory indicators in the inflamed pulp, in other words, it has some anti-inflammatory function.
RUNX2, as the first transcription factor, determines the lineage of osteoblasts [40, 41], whereas OSX is the downstream gene [42]. DSP protein, Dspp mRNA and OPN, have multifaceted roles in tooth mineralization, dental biofilm formation and osteogenic differentiation [43–46]. In the late phase of osteogenesis, OCN is secreted as the major non-collagenous protein in the bone cells [47]. Our experiment chose the public recognition mineralized indicators, to study in addition to being able to reduce inflammation factors as mentioned above narration, what kind of the biological role baicalin played in the mineralization. And on this basis, the mineralization mechanism of baicalin after inflammatory pulp reaction was further discussed, and the characteristics of baicalin were studied from surface to interior. These results revealed that inflammation increased the proliferation and decreased the differentiation of DPSCs, while baicalin at the low concentration made no difference in the proliferation, but enhanced the differentiation capacity of iDPSCs.
Under unstimulated conditions, NF-κB is retained in the cytoplasm by the inhibitory protein IκBα and presents in an inactive form. LPS, TNF, and IL-1 can activate NF-κB in human DPSCs [48] and IKK complex plays an essential role in this program. BMS345541 is a selective inhibitor of IKK [49]. Activation of NF-κB can inhibit osteogenesis of stem cells from dental apical papilla and PDLSCs in inflammatory microenvironments [23, 50]. In this paper, NF-κB pathway-related protein expressions in iDPSCs were highly enhanced, while it was lower in the baicalin-treated group. In order to illustrate that the inhibition of the NF-κB signaling pathway is pivotal to enhance odonto/osteogenic differentiation of iDPSCs for baicalin, we used IKK inhibitors to block IDPSCs, which led to increased odonto/osteogenic differentiation markers in iDPSCs.
By increasing Wnt/β-catenin signaling pathway, baicalin can accelerate hair follicle development [51]. Bone homeostasis is proverbial for being essentially directed by Wnt-mediated signals [52]. By binding to low-density lipoprotein receptor-related protein 5/6 (LRP5/6), this cascade can be blocked by antagonists such as Dickkopf-1 (DKK-1) [52]. β-catenin/Wnt pathway can inhibit DPSCs differentiation [53]. In this study, β-catenin in iDPSCs was significantly enhanced as compared with in nDPSCs; however, the proteins in baicalin-treated group were lower than iDPSCs. Moreover, CaMKII, and NLK in iDPSCs were decreased compared with in nDPSCs, and the proteins in baicalin-treated group that were enhanced using DKK1 to block IDPSCs led to significant increase in odonto/osteogenic differentiation markers in iDPSCs. These discoveries revealed that baicalin heightened odonto/osteogenic differentiation of iDPSCs via inhibiting β-catenin/Wnt pathway.
Baicalin has been demonstrated to have multiple pharmacological activities but low solubility. Various baicalin hydrogels or scaffolds have been used to break its limitation in clinical applications, for example, a dynamic covalent hydrogel consisting of baicalin and inorganic borate exhibited significant antibacterial activities, which is considered as a potential drug delivery system for biomedical applications[54]. In recent years, polylactic acid materials and graphene hybrid scaffolds have been increasingly studied in bone regeneration [55, 56]. Studies revealed that load baicalin into polylactic-co-glycolic acid based fibrous scaffolds could regulate inflammation and osteoclast differentiation, favor neovascularization and bone formation [57]. According to some researchers, a kind of biomimetic baicalin incorporating graphene oxide-demineralized bone matrix hybrid scaffold could enhance beneficial cross-talk among bone cells and inflammatory cells, which may be utilized as an effective strategy for bone regeneration [58]. These findings provide a viable option for effective use of baicalin in pulpitis to enhance odonto/osteogenic differentiation.
Our study researched the impacts of baicalin on dental pulp from aspects of anti-inflammatory and mineralization promotion, and discussed the mechanism of mineralization in depth, but this is far from enough for long-term clinical application. In recent years, TCM has gradually received attention in the medical field, especially after the outbreak of COVID-19, TCM has played a vital role in preventing and treating diseases effectively. Most TCM containing natural ingredients, which are highly safe and have little adverse effects even after long-term use, are best when treating chronic diseases. To treat stomatology diseases, even now TCM has the long-term application, there is still a challenge for western colleagues to understand and accept this traditional medicine because of accumulated differences in traditional culture, values and treatment concepts, and it is also our great responsibility as healthcare workers to bring excellent medicines to the world. In order to narrow the chasm between TCM and evidence-based medicine, clinical trials owing clear evaluation criteria are also needed.
Conclusion
Our data suggested that 20 µM baicalin effectively promote odonto/osteogenic differentiation of iDPSCs through inhibiting NF-κB and β-catenin/Wnt pathways, thus providing direct evidence that baicalin may be effective in repairing pulp with early irreversible pulpitis.
Acknowledgements
This research was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant number PAPD_2018-87) and Natural Science Foundation of Jiangsu Province (grant number BK_20191347).
Abbreviations
- ALP
Alkaline phosphatase
- BMSCs
Bone marrow mesenchymal stem cells
- COL-I
Type I collagen I
- CPC
Cetylpyridinium chloride
- DAPI
4,6-Diamidino-2-phenylindole
- DKK-1
Dickkopf-1
- DMSO
Dimethyl sulfoxide
- DPSCs
Dental pulp stem cells
- DSP
Dentin sialoprotein
- DSPP
Dentin sialophosphoprotein
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- iDPSCs
Inflammatory dental pulp stem cells
- IFN
Interferon
- IL
Interleukin
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IκBα
Inhibitor of nuclear factor kappa-B kinase
- LPS
Lipopolysaccharide
- LRP5/6
Lipoprotein receptor-related protein 5/6
- MM
Mineralization-inducing media
- MMP-1
Matrix metalloproteinase-1
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2,5-tetrazolium bromide
- nDPSCs
Normal dental pulp stem cells
- NF-κB
Nuclear factor kappa B
- OCN
Osteocalcin
- OPN
Osteopontin
- OSX
Osterix
- PAMP
Pathogen-associated molecular pattern
- PBS
Phosphate buffered saline
- PDLSCs
Periodontal ligament stem cells
- PMSF
Phenylmethylsulphonyl fluoride
- PVDF
Polyvinylidene fluoride
- RT-PCR
Real-time reverse transcription-polymerase chain reaction
- RUNX2
Runt-related transcription factor 2
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TCM
Traditional Chinese medicine
- TNF
Tumor necrosis factor
- α-MEM
Alpha minimum essential medium
Author contributions
ML, YW: conducted the vast majority of the experiments, wrote the manuscript, made equal contribution to this research as co-first authors. JX, QX, YZ, JL, HX, ZG, CB: Provided reagents and materials to assist experiments. YY: Conceptualization, Project administration, Supervision. GZ: Helped to analyze data, design experiment, suggest manuscript outline and guide manuscript writing.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
This research was approved by the consent of the patients and was given permission by the Ethical Committee of the Stomatological School of Nanjing Medical University (Reference #200900128).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability statements
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mengyuan Li and Yumeng Wang are co-first authors.
Contributor Information
Mengyuan Li, Email: 644927169@qq.com.
Yumeng Wang, Email: 1875154596@qq.com.
Jing Xue, Email: xuej0521@163.com.
Qingqing Xu, Email: laporteduciel@163.com.
Yuerong Zhang, Email: 812124327@qq.com.
Jie Liu, Email: 2834999474@qq.com.
Hai Xu, Email: 598904131@qq.com.
Zhuo Guan, Email: 3272313221@qq.com.
Chengyue Bian, Email: 1032180986@qq.com.
Guangdong Zhang, Email: egd_zhang@njmu.edu.cn.
Yan Yu, Email: yuyan0102@163.com.
References
- 1.Zhao T, Tang H, Xie L, Zheng Y, Ma Z, Sun Q, Li X (2019) Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol 71(9):1353–1369 [DOI] [PubMed]
- 2.Cheng X, Cao Z, Luo J, Hu R, Cao H, Guo X, Xing C, Yang F, Zhuang Y, Hu G. Baicalin ameliorates APEC-induced intestinal injury in chicks by inhibiting the PI3K/AKT-mediated NF-κB signaling pathway. Poult Sci. 2022;101(1):101572. doi: 10.1016/j.psj.2021.101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An HJ, Lee JY, Park W (2022) Baicalin modulates inflammatory response of macrophages activated by LPS via calcium-CHOP pathway. Cells 11(19):3076. 10.3390/cells11193076 [DOI] [PMC free article] [PubMed]
- 4.Kim E, Ham S, Jung BK, Park JW, Kim J, Lee JH (2022) Effect of baicalin on wound healing in a mouse model of pressure ulcers. Int J Mol Sci 24(1):329. 10.3390/ijms24010329 [DOI] [PMC free article] [PubMed]
- 5.Chung CP, Park JB, Bae KH. Pharmacological effects of methanolic extract from the root of Scutellaria baicalensis and its flavonoids on human gingival fibroblast. Planta Med. 1995;61(2):150–153. doi: 10.1055/s-2006-958036. [DOI] [PubMed] [Google Scholar]
- 6.Lin P, Guo XX, Wang YL, Wei ZL, Xin HY, Liu TB. Inhibitory effect of baicalin on orthodontically induced inflammatory root resorption in rats. J Int Med Res. 2020;48(9):300060520955070. doi: 10.1177/0300060520955070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato D, Kondo S, Yazawa K, Mukudai Y, Li C, Kamatani T, Katsuta H, Yoshihama Y, Shirota T, Shintani S. The potential anticancer activity of extracts derived from the roots of Scutellaria baicalensis on human oral squamous cell carcinoma cells. Mol Clin Oncol. 2013;1(1):105–111. doi: 10.3892/mco.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrelli M, Codispoti B, Shelton RM, Scheven BA, Cooper PR, Tatullo M, Paduano F. Dental pulp stem cell mechanoresponsiveness: effects of mechanical stimuli on dental pulp stem cell behavior. Front Physiol. 2018;9:1685. doi: 10.3389/fphys.2018.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothermund K, Calabrese TC, Syed-Picard FN. Differential effects of Escherichia coli-versus Porphyromonas gingivalis-derived Lipopolysaccharides on dental pulp stem cell differentiation in scaffold-free engineered tissues. J Endod. 2022;48(11):1378–1386.e1372. doi: 10.1016/j.joen.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraud T, Jeanneau C, Rombouts C, Bakhtiar H, Laurent P, About I. Pulp capping materials modulate the balance between inflammation and regeneration. Dent Mater. 2019;35(1):24–35. doi: 10.1016/j.dental.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Inostroza C, Vega-Letter AM, Brizuela C, Castrillón L, Saint Jean N, Duran CM, Carrión F. Mesenchymal stem cells derived from human inflamed dental pulp exhibit impaired immunomodulatory capacity in vitro. J Endod. 2020;46(8):1091–1098.e1092. doi: 10.1016/j.joen.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Xia K, Chen Z, Chen J, Xu H, Xu Y, Yang T, Zhang Q. RGD- and VEGF-mimetic peptide epitope-functionalized self-assembling peptide hydrogels promote dentin-pulp complex regeneration. Int J Nanomed. 2020;15:6631–6647. doi: 10.2147/IJN.S253576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu YJ, Xu B, Huang SW, Luo X, Deng XL, Luo S, Liu C, Wang Q, Chen JY, Zhou L. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol Sin. 2021;42(1):88–96. doi: 10.1038/s41401-020-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Meng L, Qiao W, Yang R, Gao Q, Peng Y, Bian Z. Vascular endothelial growth factor A/Vascular endothelial growth factor receptor 2 axis promotes human dental pulp stem cell migration via the FAK/PI3K/Akt and p38 MAPK signalling pathways. Int Endod J. 2019;52(12):1691–1703. doi: 10.1111/iej.13179. [DOI] [PubMed] [Google Scholar]
- 16.Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78(4):1233–1261. doi: 10.1007/s00018-020-03656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang J, Alafate W, Wu W, Wang Y, Li X, Xie W, Bai X, Li R, Wang M, Wang J. NEK2 enhances malignancies of glioblastoma via NIK/NF-κB pathway. Cell Death Dis. 2022;13(1):58. doi: 10.1038/s41419-022-04512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Songkiatisak P, Rahman SMT, Aqdas M, Sung MH. NF-κB, a culprit of both inflamm-ageing and declining immunity? Immun Ageing. 2022;19(1):20. doi: 10.1186/s12979-022-00277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 2022;43(9):757–775. doi: 10.1016/j.it.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 20.De Luca F. Regulatory role of NF-κB in growth plate chondrogenesis and its functional interaction with growth hormone. Mol Cell Endocrinol. 2020;514:110916. doi: 10.1016/j.mce.2020.110916. [DOI] [PubMed] [Google Scholar]
- 21.Burns VE, Kerppola TK. Virus infection induces Keap1 binding to cytokine genes, which recruits NF-κB p50 and G9a-GLP and represses cytokine transcription. J Immunol. 2021;207(5):1437–1447. doi: 10.4049/jimmunol.2100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie X, Hu L, Mi B, Panayi AC, Xue H, Hu Y, Liu G, Chen L, Yan C, Zha K, et al. SHIP1 activator AQX-1125 regulates osteogenesis and osteoclastogenesis through PI3K/Akt and NF-κb signaling. Front Cell Dev Biol. 2022;10:826023. doi: 10.3389/fcell.2022.826023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan X, He Y, Li Y, Shi C, Wei Z, Zhao R, Han Y, Pan L, Yang J, Hou T. Gremlin aggravates periodontitis via activation of the nuclear factor-kappa B signaling pathway. J Periodontol. 2022;93(10):1589–1602. doi: 10.1002/JPER.21-0474. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. doi: 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astudillo P, Larraín J. Wnt signaling and cell-matrix adhesion. Curr Mol Med. 2014;14(2):209–220. doi: 10.2174/1566524014666140128105352. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Liang T, Zhang Z, Chen J, Xue J, Zhan X, Ren L. Transfer of microRNA-22-3p by M2 macrophage-derived extracellular vesicles facilitates the development of ankylosing spondylitis through the PER2-mediated Wnt/β-catenin axis. Cell Death Discov. 2022;8(1):269. doi: 10.1038/s41420-022-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu Y, Bai Y (2023) Osteogenic effect of crocin in human periodontal ligament stem cells via Wnt/β-catenin signaling. Oral Dis. 10.1111/odi.14523 [DOI] [PubMed]
- 28.Liu Y, Liu N, Na J, Li C, Yue G, Fan Y, Zheng L. Wnt/β-catenin plays a dual function in calcium hydroxide induced proliferation, migration, osteogenic differentiation and mineralization in vitro human dental pulp stem cells. Int Endod J. 2023;56(1):92–102. doi: 10.1111/iej.13843. [DOI] [PubMed] [Google Scholar]
- 29.Guo AJ, Choi RC, Cheung AW, Chen VP, Xu SL, Dong TT, Chen JJ, Tsim KW. Baicalin, a flavone, induces the differentiation of cultured osteoblasts: an action via the Wnt/beta-catenin signaling pathway. J Biol Chem. 2011;286(32):27882–27893. doi: 10.1074/jbc.M111.236281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Pan J, Wright JT, Bencharit S, Zhang S, Everett ET, Teixeira FB, Preisser JS. Putative stem cells in human dental pulp with irreversible pulpitis: an exploratory study. J Endod. 2010;36(5):820–825. doi: 10.1016/j.joen.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Mu J, Fan Z, Yu Y, Yan M, Lei G, Tang C, Wang Z, Zheng Y, Yu J, et al. Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res. 2012;8(3):346–356. doi: 10.1016/j.scr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, He H, Tang C, Zhang G, Li Y, Wang R, Shi J, Jin Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010;11:32. doi: 10.1186/1471-2121-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandomme J, Touil Y, Ostyn P, Olejnik C, Flamenco P, El Machhour R, Segard P, Masselot B, Bailliez Y, Formstecher P, et al. Insulin-like growth factor 1 receptor and p38 mitogen-activated protein kinase signals inversely regulate signal transducer and activator of transcription 3 activity to control human dental pulp stem cell quiescence, propagation, and differentiation. Stem Cells Dev. 2014;23(8):839–851. doi: 10.1089/scd.2013.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira LO, Rubini MR, Silva JR, Oliveira DM, Silva IC, Pocas-Fonseca MJ, Azevedo RB. Comparison of stem cell properties of cells isolated from normal and inflamed dental pulps. Int Endod J. 2012;45(12):1080–1090. doi: 10.1111/j.1365-2591.2012.02068.x. [DOI] [PubMed] [Google Scholar]
- 35.Liao J, Al Shahrani M, Al-Habib M, Tanaka T, Huang GT. Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J Endod. 2011;37(9):1217–1224. doi: 10.1016/j.joen.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JC, Kim JM, Jung IH, Kim JC, Choi SH, Cho KS, Kim CS. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: in vitro and in vivo evaluations. J Clin Periodontol. 2011;38(8):721–731. doi: 10.1111/j.1600-051X.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Zhang R, Wang J, Yu P, Liu Q, Zeng D, Song H, Kuang Z. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can J Physiol Pharmacol. 2015;93(4):233–237. doi: 10.1139/cjpp-2014-0262. [DOI] [PubMed] [Google Scholar]
- 38.Shen B, Zhang H, Zhu Z, Ling Z, Zeng F, Wang Y, Wang J (2023) Baicalin relieves LPS-induced lung inflammation via the NF-κB and MAPK pathways. Molecules 28(4):1873. 10.3390/molecules28041873 [DOI] [PMC free article] [PubMed]
- 39.Arora S, Cooper PR, Friedlander LT, Rizwan S, Seo B, Rich AM, Hussaini HM. Potential application of immunotherapy for modulation of pulp inflammation: opportunities for vital pulp treatment. Int Endod J. 2021;54(8):1263–1274. doi: 10.1111/iej.13524. [DOI] [PubMed] [Google Scholar]
- 40.Wade-Gueye NM, Boudiffa M, Vanden-Bossche A, Laroche N, Aubin JE, Vico L, Lafage-Proust MH, Malaval L. Absence of bone sialoprotein (BSP) impairs primary bone formation and resorption: the marrow ablation model under PTH challenge. Bone. 2012;50(5):1064–1073. doi: 10.1016/j.bone.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Komori T. Whole aspect of Runx2 functions in skeletal development. Int J Mol Sci. 2022;23(10):5776. doi: 10.3390/ijms23105776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gopinathan G, Luan X, Diekwisch TGH. Epigenetic repression of RUNX2 and OSX promoters controls the nonmineralized state of the periodontal ligament. Genes (Basel) 2023;14(1):201. doi: 10.3390/genes14010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E, Lee NP, Wu GH, Luk JM. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol. 2012;18(30):3923–3930. doi: 10.3748/wjg.v18.i30.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi C, Ma N, Zhang W, Ye J, Shi H, Xiang D, Wu C, Song L, Zhang N, Liu Q. Haploinsufficiency of Dspp gene causes dentin dysplasia Type II in Mice. Front Physiol. 2020;11:593626. doi: 10.3389/fphys.2020.593626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang MC, Chang KW, Lin SC, Hung PS. Biodentine but not MTA induce DSPP expression of dental pulp cells with different severity of LPS-induced inflammation. Clin Oral Investig. 2023;27(3):1207–1214. doi: 10.1007/s00784-022-04734-0. [DOI] [PubMed] [Google Scholar]
- 46.Wu Q, Li L, Miao C, Hasnat M, Sun L, Jiang Z, Zhang L. Osteopontin promotes hepatocellular carcinoma progression through inducing JAK2/STAT3/NOX1-mediated ROS production. Cell Death Dis. 2022;13(4):341. doi: 10.1038/s41419-022-04806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang JS, Mazur CM, Wein MN. Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol (Lausanne) 2021;12:584147. doi: 10.3389/fendo.2021.584147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang J, Zhang C, Tani-Ishii N, Shi S, Wang C-Y. NF-kappaB activation in human dental pulp stem cells by TNF and LPS. J Dent Res. 2005;84(11):994–998. doi: 10.1177/154405910508401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Zhang J, Sun H, Brasier AR. Crosstalk of the IkappaB kinase with spliced X-Box binding Protein 1 couples inflammation with glucose metabolic reprogramming in epithelial-mesenchymal transition. J Proteome Res. 2021;20(7):3475–3488. doi: 10.1021/acs.jproteome.1c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo L, Zhang Y, Liu H, Cheng Q, Yang S, Yang D. All-trans retinoic acid inhibits the osteogenesis of periodontal ligament stem cells by promoting IL-1β production via NF-κB signaling. Int Immunopharmacol. 2022;108:108757. doi: 10.1016/j.intimp.2022.108757. [DOI] [PubMed] [Google Scholar]
- 51.Xing F, Yi WJ, Miao F, Su MY, Lei TC. Baicalin increases hair follicle development by increasing canonical Wnt/betacatenin signaling and activating dermal papillar cells in mice. Int J Mol Med. 2018;41(4):2079–2085. doi: 10.3892/ijmm.2018.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda K, Takahashi N, Kobayashi Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J Mol Med (Berl) 2013;91(1):15–23. doi: 10.1007/s00109-012-0974-0. [DOI] [PubMed] [Google Scholar]
- 53.Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87(2):126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang ZZ, Jia Y, Wang G, He H, Cao L, Shi Y, Miao M, Li XM. Dynamic covalent hydrogel of natural product baicalin with antibacterial activities. RSC Adv. 2022;12(14):8737–8742. doi: 10.1039/D1RA07553E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian G, Zhang L, Shuai Y, Wu X, Zeng Z, Peng S, Shuai C. 3D-printed CuFe2O4-MXene/PLLA antibacterial tracheal scaffold against implantation-associated infection. Appl Surf Sci. 2023;614:156108. doi: 10.1016/j.apsusc.2022.156108. [DOI] [Google Scholar]
- 56.Shuai C, Guo W, Wu P, Yang W, Hu S, Xia Y, Feng P. A graphene oxide-Ag co-dispersing nanosystem: dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem Eng J. 2018;347:322–333. doi: 10.1016/j.cej.2018.04.092. [DOI] [Google Scholar]
- 57.Jin S, Gao J, Yang R, Yuan C, Wang R, Zou Q, Zuo Y, Zhu M, Li Y, Man Y, et al. A baicalin-loaded coaxial nanofiber scaffold regulated inflammation and osteoclast differentiation for vascularized bone regeneration. Bioact Mater. 2022;8:559–572. doi: 10.1016/j.bioactmat.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo B, Feng X, Wang Y, Wang X, He Y. Biomimetic and immunomodulatory baicalin-loaded graphene oxide-demineralized bone matrix scaffold for in vivo bone regeneration. J Mater Chem B. 2021;9(47):9720–9733. doi: 10.1039/D1TB00618E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.