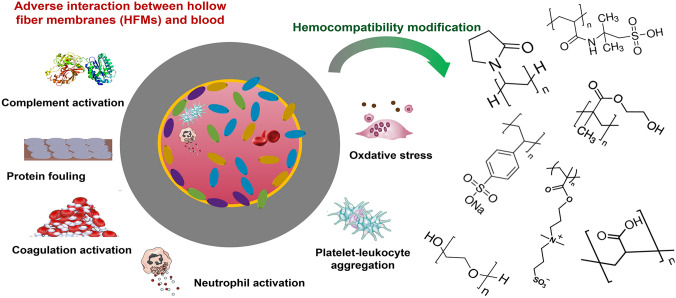
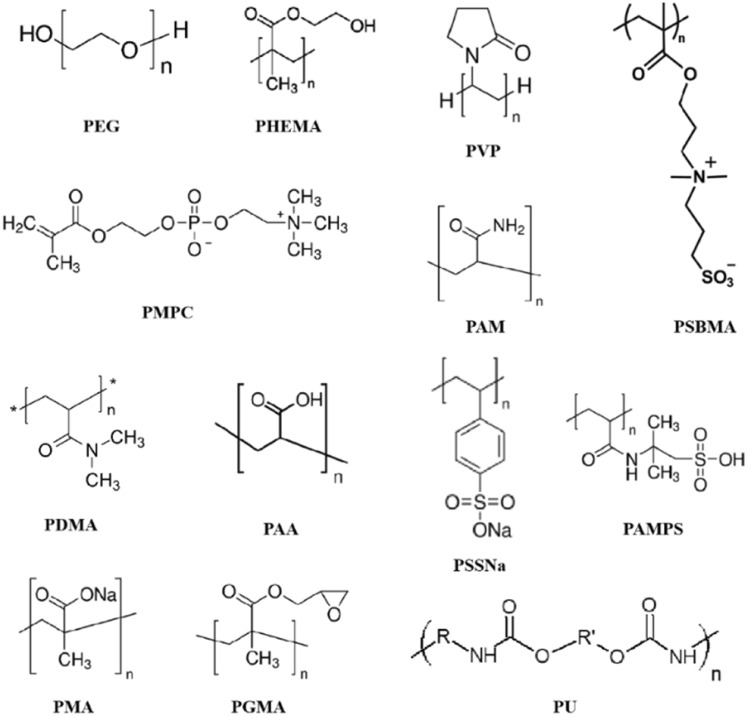
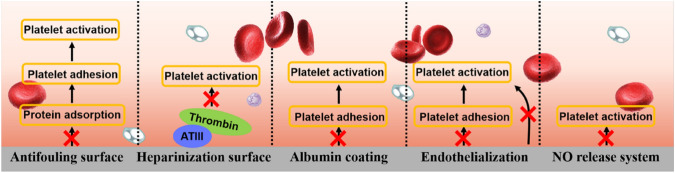
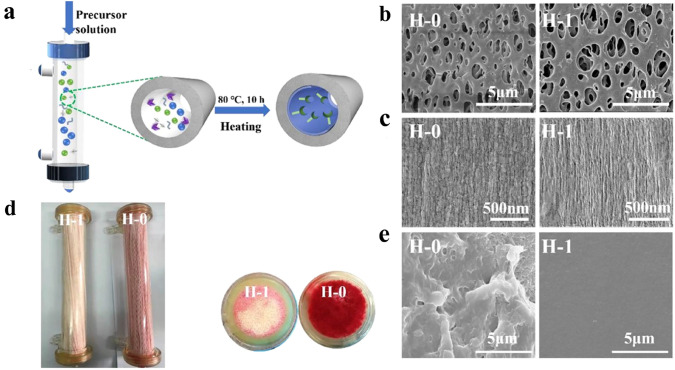
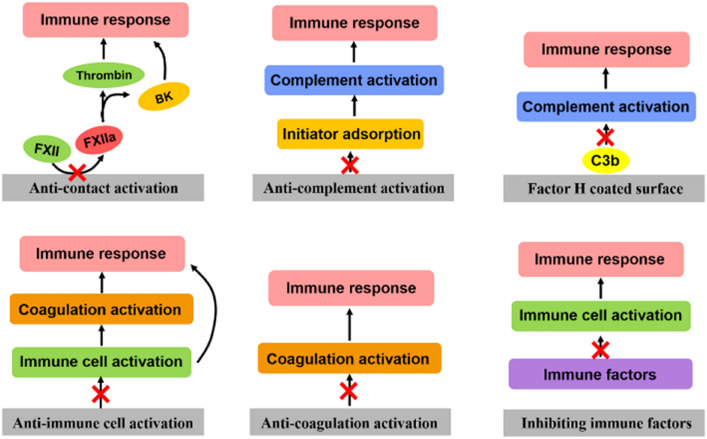
Abstract
Hemodialysis, the most common modality of renal replacement therapy, is critically required to remove uremic toxins from the blood of patients with end-stage kidney disease. However, the chronic inflammation, oxidative stress as well as thrombosis induced by the long-term contact of hemoincompatible hollow-fiber membranes (HFMs) contribute to the increase in cardiovascular diseases and mortality in this patient population. This review first retrospectively analyzes the current clinical and laboratory research progress in improving the hemocompatibility of HFMs. Details on different HFMs currently in clinical use and their design are described. Subsequently, we elaborate on the adverse interactions between blood and HFMs, involving protein adsorption, platelet adhesion and activation, and the activation of immune and coagulation systems, and the focus is on how to improve the hemocompatibility of HFMs in these aspects. Finally, challenges and future perspectives for improving the hemocompatibility of HFMs are also discussed to promote the development and clinical application of new hemocompatible HFMs.
Graphical Abstract
Keywords: Hemodialysis, Hollow fiber membranes, Hemocompatibility, Surface modification, Hemocompatibility characterization
Introduction
The prevalence of end-stage renal disease (ESKD) has been increasing over the past two decades and is associated with increased medical costs, decreased quality of life, and severe adverse health outcomes such as cardiovascular diseases, infection, depression, and mortality [1–4]. Renal replacement therapy (RRT), including kidney transplantation, peritoneal dialysis and hemodialysis, is essential to maintain homeostasis by removing metabolic waste accumulated in the body of ESKD patients [5, 6]. By 2030, the worldwide use of RRT is projected to be more than 5.4 million and remains a public health challenge [5]. Hemodialysis, the most prevalent modality of RRT, removes solutes and water utilizing diverse mass separation mechanisms involving diffusion, convection, and ultrafiltration [1, 2, 6]. Currently, approximately 300 million hollow-fiber hemodialyzers are used annually to administer chronic hemodialysis to 2.6 million ESKD patients worldwide [6].
Conventionally, HFMs are classified into cellulose (natural) and synthetic membrane groups based on their material composition [7, 8]. Unmodified cellulose membranes, the first generation of hollow-fiber hemodialyzers, were used extensively in the last century. However, their use has gradually dropped during the past two decades because of their poor hemocompatibility and limited permeability [8–10]. Synthetic membranes, such as polymethylmethacrylate (PMMA), polyacrylonitrile (PAN), polyester polymers, polysulfone (PSF), and polyethersulfone (PES) have been developed for dialysis applications to address the limitations of cellulose-based membranes for over 40 years [7]. Among them, the HFMs based on PSF and PES show lower mortality and better hemocompatibility than other membranes and have gradually become the dominant choice for clinical dialysis [11]. However, adverse blood-membrane interactions (including protein fouling, leukocyte activation, complement activation and surface-induced thrombosis) still occur when these synthetic membranes come in contact with blood [12–14]. For instance, chronic inflammation and oxidative stress induced by synthetic membranes further contribute to an increased risk of cardiovascular events, anemia, malnutrition, and mortality in patients receiving long-term dialysis [15, 16]. Although heparin administration to prevent blood clotting in extracorporeal circuits and dialyzers is still of great importance, systemic heparization during hemodialysis sessions inevitably increases the risk of bleeding in ESKD patients [17, 18].
Modifications of HFMs by bioactive substances such as heparin and vitamin E were thus initiated to enhance the hemocompatibility of conventional HFMs [15]. Representative commercial products such as AN69ST membrane and Excerbrane® membrane (vitamin E-coated cellulose membrane) came into the market based on these improvements. There have been several attempts to improve the hemocompatibility of HFMs in the last two decades, including antithrombotic, antifouling, and antiadhesion properties [19]. Diverse modification and characterization methods have been developed, and significant improvements in our understanding of blood-membrane interactions have been made [19–22]. However, the performance of commercially available hemocompatible HFM has not been systematically reviewed, which makes it challenging to propose the gap between current clinical requirements and laboratory development. In addition, although there have been a few excellent review papers on blood-material interactions as well as hemocompatibility enhancement of dialysis membranes [19, 21, 23], the practical significance of these publications decreases partially because of missing discussions on either translational value or in-depth exploration of underlying mechanisms.
In this review, we first retrospectively analyzed the current clinical research progress in improving the hemocompatibility of HFMs. Then, we elaborate on the adverse blood-membrane interactions, involving protein adsorption, platelet adhesion and activation, and the activation of immune and coagulation systems, and focus our effort on how to improve the hemocompatibility of HFMs regarding these aspects. Finally, we highlight the challenges in this field and perspectives to promote the development and clinical application of hemocompatible HFMs.
Rationale, History, Basic Characteristics, and Clinical Evaluation of Contemporary HFMs
Rationale of Hemodialysis with HFMs
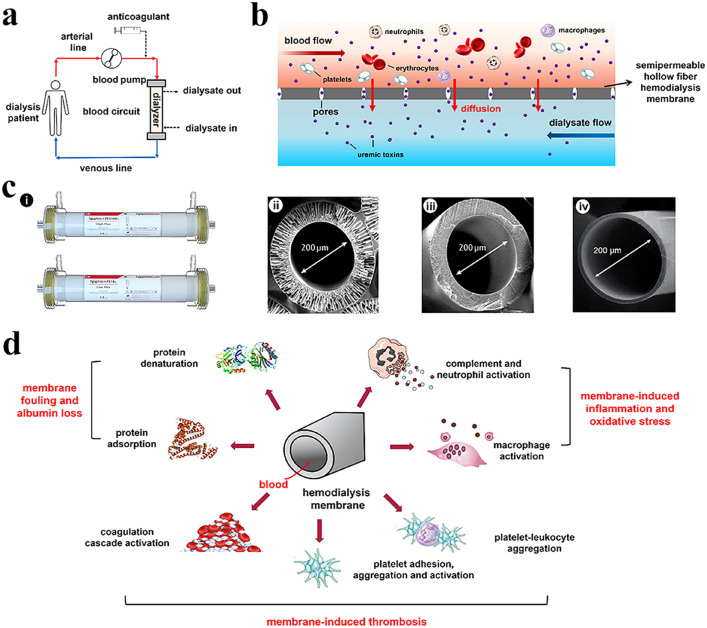
As shown in Fig. 1a, b, hemodialysis leverages the extracorporeal exchange process between blood and dialysate through semi-permeable HFMs which is driven by a diffusion- and/or convection mechanism [24]. Modern dialyzers vary greatly not only in membrane microstructure, including pore radius and wall thickness (see Fig. 1c), but also in solute permeability, surface area, and hemocompatibility in particular. HFMs per se can also induce adverse protein adsorption and denaturation and the activation of other blood components, such as blood cells, complement proteins and coagulation factors when they come in contact with blood as illustrated in Fig. 1d. It has been well established that semi-permeable HFMs play a pivotal role in routine hemodialysis because their solute removal efficacy and hemocompatibility can remarkably affect the clinical outcomes of ESKD patients.
Fig. 1.
A brief introduction to hemodialysis procedures and adverse membrane-blood interactions. a Schematic diagram for hemodialysis procedures. Hemodialysis is performed by an extracorporeal method where blood is pumped through a semi-permeable hollow hemodialysis membrane (also termed a dialyzer or hemodialyzer) and then instilled into the intracorporeal blood circulation. Anticoagulants are usually administered to preserve the patency of the dialyzer and the blood flow. b Classical mechanism of solute exchange by diffusion during hemodialysis. Uremic toxins are transported across the dialyzer under the driving force of their concentration gradients between blood and dialysate. c Representative digital photographs of commercial polyethersulfone high-flux dialyzer (the upper) and polysulfone low-flux dialyzer (the lower) manufactured by NephroCan Inc. (i) and the cross-section scanning electron microscope (SEM) morphologies of polysulfone (ii), polyethersulfone (iii), and cellulose triacetate (iv) hollow-fiber hemodialysis membranes. d Adverse membrane-blood interactions when HFMs come into contact with blood. HFMs per se can induce protein adsorption and denaturation, as well as the activation of diverse blood cells, complement and coagulation systems, which finally result in unexpectedly decreased membrane diffusive permeability, increased serum albumin/protein loss, inflammatory cytokine release and oxidative stress and promote thrombus formation in the extracorporeal blood circuit, occlusion of hollow fibers and early termination of dialysis treatment in the worst situation
History, Basic Characteristics, and Clinical Evaluation of Contemporary HFMs
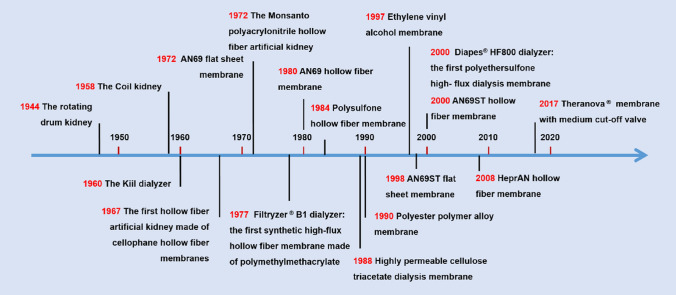
Since the 1960s, progress in both material technologies and polymer chemistry has enabled the development of HFMs with higher permeability, hydrophilicity and better hemocompatibility, which significantly contributed to the significant improvement in the welfare and quality of life of chronic dialysis patients [6, 24]. A brief history of the evolution of modern hemodialyzers is shown in Fig. 2. Traditionally, dialysis membranes have been divided into cellulosic and synthetic groups based on their material composition.
Fig. 2.
A brief history of the evolution of modern hemodialyzers
Cellulosic Membranes
In general, cellulose membranes have symmetric structures and favorable properties for removing small solutes. Application of Cuprophane® membranes made of cellulose resulted in poor outcomes for hemodialysis patients, triggering further investigations intended to optimize their biocompatibility. The poor hemocompatibility (e.g., complement activation and leukopenia) of unmodified Cuprophane® membranes is due to the presence of free hydrophilic hydroxyl groups in the regular linear chain structure of cellulose [24]. Since the1980s, improved performance of cellulose membranes has been achieved through strategic chemical masking of hydroxyl groups that contribute to the complement activation through the alternative pathway [15]. The conversion of hydroxyl groups to acetates is correlated with an increase in membrane porosity, with the most pronounced effect on the permeability of water and small solute molecules [7]. Among them, cellulose triacetate membranes demonstrate the lowest complement activation and are the most biocompatible of all existing cellulose membranes [25]. Cellulose triacetate membranes are also manufactured with a variety of permeability properties, from low to ultra-high-flux performance (the standard definition of high-flux hemodialyzers is not readily available. However, based on various high throughput dialyzers from different commercial vendors, the hemodialyzers with ultrafiltration coefficients higher than 40 mL/mmHg per hour are defined as high-flux hemodialyzers) and can thus be used for the clearance of medium-molecular-weight solutes, including β2-microglobulin [25].
Unmodified cellulose membranes have been widely used over the last century. Nevertheless, their use has declined dramatically in recent decades and has practically disappeared from the market. The primary manufacturers of cellulose dialysis membranes (Wuppertal, Germany, the manufacturer of Cuprophan® and Hemophan®, Membrana GmbH) even halted production in 2006. Although some modern (modified) cellulose membranes have performance and functional attributes similar to those of synthetic membranes, their use is still declining. This steep reduction in the clinical use of cellulose membranes may be primarily attributed to an improved understanding of uremic toxicity, hemocompatibility challenges, and the need to further enhance the survival rate of dialysis patients.
Synthetic Membranes
Dialyzers made of synthetic membranes are the essential part of modern hemodialysis [26]. Synthetic membranes were initially developed more than 40 years ago for use in dialysis, to solve the problems of poor hemocompatibility and limited permeability of unmodified cellulose membranes [6]. The early synthetic membranes had a wall thickness of 100 μm, which was not permeable enough to allow diffusion-based separation, and thus limited in use in hemofiltration (purely convection-based). However, subsequent modifications, particularly the significant reduction in wall thickness, enable the synthetic membrane to be suitable for high-flux hemodialysis and hemofiltration [6]. As shown in Table 1, modern synthetic membranes typically have 20–50 μm wall thickness.
Table 1.
Basic membrane structure characteristics, main strengths and limitations of commercially available hemodialysis hollow-fiber membranes
| Category | Membrane name | Basic membrane structure characteristics | Main strengths and limitations | References | ||||
|---|---|---|---|---|---|---|---|---|
| Composition | Structure | Pore radius (Å) | Wwall thickness (μm) | Ultrafiltration coefficient (ml/hr/mmHg) | ||||
| Cellulosic membrane | (Unmodified) cuprophane membrane | Cellulose | Symmetric | 21 | 5–17 | Low-flux (3.8 -8.4) | Negligible middle molecule removal, significant complement activation and leukopenia, only for standard hemodialysis | [56–58] |
| Cellulose triacetate membrane | Cellulose triacetate | Symmetric | 58 ~ 78 | 15 | Low- to high-flux (9.5–85.6) | Minimal protein adsorption, attenuated complement activation, high antithrombotic performance, high phosphorus clearance, improved lipid metabolism | [25, 59–62] | |
| Cellulose (di) acetate membrane | Cellulose diacetate | Symmetric | NA | 14–30 | Low- to high-flux | Improved biocompatibility and hydrophobicity vs cuprophane membrane, low middle molecule removal | [24, 63] | |
| Hemophan membrane | Cellulose and diethylaminoethyl | Symmetric | 21 | 5–20 | Low-flux | Negligible middle molecule removal, improved biocompatibility and hydrophobicity vs cuprophane membrane, higher pro-inflammatory cytokine production than polyamide membrane, only for standard hemodialysis | [24] | |
| Cuprammonium rayon membrane | Polyethylene and cellulose | Asymmetric | NA | 9–26 | Low- to high-flux (9–19) | Improved biocompatibility and hydrophobicity vs cuprophane membrane, less platelet activation and higher albumin loss vs polysulfone membrane | [15, 24, 64, 65] | |
| Synthetic membrane | AN69 membrane | Copolymer of acrylonitrile and sodium methallyl sulfonate | Symmetric | 25–55 | 30 | High-flux (19.42) | With a specific hydrogel structure on its surface; bulk adsorption of low-molecular-weight proteins such as cytokines; decreased complement activation, platelet adhesion and activation; increased activation of kallikrein-kinase system, production of bradykinin, available for high-flux hemodialysis, hemodiafiltration, and hemofiltration | [24, 66] |
| PAN membrane | Copolymer of acrylonitrile, methacrylate and acrylic acid | Asymmetric | NA | 19–55 | High-flux (45–54) | Yield of bradykinin, risk of allergic reactions higher than other synthesis membranes, sustained mild complement activation | [15, 67] | |
| PSF membrane | Polysulfone, blending with polyvinylpyrrolidone or polyethylene glycol in most occasions | Asymmetric | NA | 30–104 | Low- to high-flux | Meets solute and fluid removal needs in all therapy modalities (low and high throughput dialysis, in-line hemofiltration and hemofiltration), efficient endotoxin retaining capacity, significant intrinsic biocompatibility, low cytotoxicity, the most widely used dialysis membrane in routine hemodialysis, Optimal chemical and thermal stability for steam sterilization, Increased β2 macroglobulin clearance through advanced PSF-based Helixone® without any albumin loss | [6, 24, 26] | |
| PMMA membrane | Isotactic and syndiotactic polymethylmethacrylate | Asymmetric | NA | 20–40 | Low- to high-flux | Reduced complement activation compared to cuprophane membranes with increased removal of β2 microglobulin by adsorption, sustained mild complement activation, and unexpected slight leukopenia | [10, 15, 24, 28] | |
| PES membrane | Polyethersulfone, blending with polyvinylpyrrolidone in most occasions | Asymmetric | NA | 30–104 | Low- to high-flux | Meets solute and fluid removal needs in all therapy modalities (low and high throughput dialysis, in-line hemofiltration and hemofiltration), provides significant intrinsic biocompatibility, favorable chemical and thermal stability, allows for steam sterilization, and delivers dialysate uniformly through Diapes® | [24, 30] | |
| EVOH membrane | Copolymer of ethylene and vinyl alcohol | Symmetric | NA | 25–32 | Low-flux (5.8–11.1) | Smooth inner surface; few plasma protein adsorption; weak interactions with blood cells | [24, 68] | |
| MCO membrane | Polyethersulfone, blending with polyvinylpyrrolidone | Asymmetric | 50 | 35 | High-flux (48–59) | Medium apertures, uniform distribution of pores, steep sieve curve and internal filtration–backfiltration mechanism, greater removal of middle-molecule toxins than conventional high-flux dialysis, insufficient removal of protein-bound and large-molecule uremic toxins and unwanted albumin loss | [31, 33] | |
| Bioactive membrane | AN69ST membrane | Copolymer of acrylonitrile and methallylsulfonate (AN69) coated with polyethyleneimine | Symmetric | NA | 45 | High-flux | Lower surface charge, kallikrein-kinase system activation and high-molecular weight protein adsorption than AN69 membrane due to surface treatment technique with cationic polyethyleneimine, specific antithrombin III adsorption by binding to heparin coating, unique self-anticoagulant membrane for heparin-free dialysis | [27, 69, 70] |
| HeprAN membrane | Copolymer of acrylonitrile and methallylsulfonate (AN69) coated with polyethyleneimine and heparin | Symmetric | NA | 45 | High-flux | Further superficial treatment by cationic polyethyleneimine polymer and grafting of heparin on the inner surface, specific antithrombin III adsorption by binding to heparin coating, unique self-anticoagulant membrane for heparin-free dialysis, effective endotoxin retention capacity | [27, 69] | |
| Vitamin-E coated membrane | Cellulose-based or polysulfone-based membrane coated with vitamin-E | NA | NA | NA | Low- to high-flux | Decreased oxidative stress, improved inflammation status and anemia, persistence of complement activation | [15, 52, 55, 71, 72] | |
Although some synthetic membranes, such as sulfonated polyacrylonitrile (AN69) and PMMA membranes, are structurally symmetric with a homogeneous composition throughout, the most contemporary synthetic membranes for hemodialysis have an asymmetrical structure, with a thin inner 'skin' layer at the membrane-blood interface (approximately ≤ 1 µm in width) being the main size differentiating factor for solute removal [6, 27, 28]. The remainder of the wall (the 'matrix') provides a large surface area as a support structure for removing molecules by adsorption as well. The structure of the matrix is more macroporous than that of the ‘skin’ layer, as the ‘matrix’ has a spongy or finger-shaped structure [6].
In 1969, Rhône-Poulenc SA, a French chemical and pharmaceutical manufacturer, started the development of AN69 membranes for hemodialysis. The AN69 membrane is symmetric and homogeneous with a high density of medium-sized pores, making it highly permeable not only to large solutes but also to the fluid. Uniquely, the AN69 membrane is able to remove low/medium-molecular-weight β2-microglobulin and inflammatory cytokines by membrane adsorption through electrostatic binding with negative sulfonic groups as well as by transmembrane transport [29]. Owing to these unique membrane characteristics, the latest surface-treated AN69 membrane, namely the oXiris membrane, has been widely used for RRT of critically ill patients with sepsis or COVID-19 [29].
Among all dialysis membranes made from synthetic polymers, 93% are derived from the parent polyarylsulfone family, including 71% from PSF and 22% from PES. PSF and PES are amorphous polymers that contain a sulfone group in their structure and exhibit excellent chemical and thermal stability. Compared to all synthetic polymers available for dialysis, PSF and PES are unique in that they can be sterilized by all the main modes of sterilization (steam, ethylene oxide and gamma radiation). This characteristic is critical, as steam sterilization of medical devices is becoming of increasing importance given the clinically significant hypersensitivity reactions associated with ethylene oxide and the adverse effects of irradiation on polymer and membrane structures [24]. PSF and PES dialyzers also fulfill the solute and fluid removal requirements of all therapeutic modalities (low- and high-flux dialysis, online hemodiafiltration, and hemofiltration), as well as other demands of contemporary dialysis therapies, such as effective endotoxin retention capacity, intrinsic biocompatibility and low cytotoxicity [26, 30]. As per the literature, PSF and PES membranes have demonstrated superior performance over other synthetic types in terms of solute removal and biocompatibility [26].
Most recently, advances in the membrane manufacturing have led to the development of a novel class of dialysis membranes with a molecular weight cut-off close to the molecular weight of albumin called medium cut-off (MCO) membrane (Theranova hemodialyzer) [31]. The MCO membrane has medium apertures, uniform distribution of pores, a steep sieve curve close to the native kidney, and unique internal filtration–backfiltration mechanism [32]. Growing clinical evidence collectively suggests that expanded hemodialysis with the MCO membrane shows greater capacity to remove middle-molecular weight toxins than conventional high-flux dialysis, and the MCO membrane may alleviate the status of micro-inflammation and oxidative stress, and may partly improve quality of life in patients receiving chronic hemodialysis [33–35]. However, the removal of protein-bound and large-molecule uremic toxins is not solved by expanded hemodialysis with the MCO membrane [36].
Bioactive Membranes
Despite the technological improvements in membrane manufacturing over the past decades, the most biocompatible membranes cannot avoid unwanted complement activation, release of pro-inflammatory cytokines and reactive oxygen species (ROS) during prolonged contact with blood during dialysis. The increased inflammatory status can greatly contribute to the long-term comorbidities of ESRD patients [37]. Thus, innovative approaches for improving the biocompatibility and bioactivity of HFMs are needed. The main focus is to endow the current HFMs with antithrombotic and antioxidant properties using bioactive heparin- or vitamin E-coatings, which can significantly reduce the bleeding risk of systemic heparization and oxidative stress in traditional dialysis sessions, respectively.
Chronic hemodialysis usually requires systemic anticoagulation to prevent thrombosis in the dialyzers and extracorporeal circuits [17, 38, 39]. However, the use of heparin can increase the risk of bleeding [38] and is thus generally contraindicated in dialysis patients with fatal active bleeding, such as severe gastrointestinal bleeding and cerebral hemorrhage [40]. An alternative dialysis option in patients at high risk of bleeding is heparin-free dialysis with heparin-grafted HFMs to prevent thrombosis. However, the efficacy and safety of heparin-free hemodialysis with heparin-grafted HFMs in such patients remains contradictory. Clinical trials of heparin-coated PSF dialyzers showed that these dialyzers reduced heparin requirements in patients on continuous RRT [41]. Nevertheless, Wright et al. found that heparin-grafted cellulose membranes did not alleviate heparin requirements in dialysis patients in contrast to conventional Cuprophan® membranes [42]. These early explorations have greatly fueled the development and application of heparin-grafted AN69 membranes (including AN69ST and HeprAN membranes) during the last two decades [27]. AN69ST is a modified AN69 membrane whose surface electronegativity has been neutralized by layering polyethyleneimine [43]. Compared with native AN69, PSF, and triacetate membranes, the AN69 ST membrane binds significant amounts of heparin with an equilibrated adsorption capacity of 1000 ± 200 IU/m2 within 3–5 min [43]. HeprAN (the membrane of Evodial® dialyzer) uses improved AN69ST technology where heparin is grafted to the membrane during the manufacturing process, thereby eliminating the need for heparin priming at the dialysis center [27].
In 2014, the HepZero study showed that dialysis sessions with Evodial® membranes had significantly higher success rate than those with current standard-of-care such as intermittent saline flushes and predilution in patients receiving heparin-free dialysis (68.5 vs 50.4%) [44]. However, the higher success rate in the Evodial® group did not reach the prespecified superiority threshold. Similar results were obtained in a later study in which Evodial® membrane was used with a modified anticoagulation protocol with combined heparin/albumin priming with citrate-containing dialysate, and showed much better performance than heparin saline priming in PSF membrane [45]. In another prospective randomized study, the anticoagulation performance of the heparin-grafted membrane was found to be inferior to that of routine regional citrate anticoagulation [46]. The success rate of dialysis sessions with AN69ST membrane and heparin saline priming was significantly lower than those with PSF membranes and regional citrate anticoagulation (61% vs 87%). Most recently, the EvoCit study evaluated the performance of the Evodial® membrane during hemodialysis with and without systemic anticoagulation. The results suggest that hemodialysis using the Evodial® membrane combined with a 1.0 mmol/L citrate-enriched dialysate is non-inferior to that combined with systemic heparinization with respect to solute clearance. However, this protocol results in a greater number of shortened treatments, more membrane clotting, and greater thrombin generation [47]. These findings suggest that the advantage of heparin-grafted membranes in lowering heparin dose in high-bleeding risk patients is at the cost of increasing unexpected clotting events in extracorporeal circuits, making it challenging to utilize these heparin-grafted membranes in conventional dialysis sessions.
Oxidative stress and chronic inflammation are also prevalent in dialysis patients and have been implicated in increased cardiovascular diseases, anemia, and malnutrition [16, 48]. As early as 1984, Giardini et al. first described evidence for increased oxidative stress in hemodialysis patients [49]. During the process of oxidative stress, red blood cells are subject to membrane lipid peroxidation and are susceptible to destruction [50]. Vitamin E is a lipophilic antioxidant and can be coated on HFMs to reduce the generation of ROS [48]. In 2006, Yang et al. showed that a vitamin E-coated Excerbrane EE18 membrane (Terumo Co., Shibuyaku, Japan) per se is effective in reducing lipid peroxidation and protecting RBCs from oxidative stress in dialysis patients [51]. The use of the Excerbrane membrane also resulted in significant (50%) decrease in ROS formation in whole blood. Similar results were found in another prospective, controlled, observational cohort study, in which the vitamin E-coated Clirans® E hollow-fiber dialyzer (Terumo Co., Shibuyaku, Japan) resulted in a significant increase in total antioxidant status (TAS) and a decrease in thiobarbituric acid reactive substances (TBARS, a sensitive marker of oxidative stress) and oxidant low-density lipoprotein (ox-LDL, a sensitive marker of oxidative stress) [52]. After the use of the Clirans® E dialyzer for 6 months, pro-inflammatory cytokines such as C-reactive protein (CRP) and interleukin-6 (IL-6) markedly decreased compared to baseline, suggesting the anti-inflammatory effect of the vitamin E-coated membrane. Another study investigated the protective effect of vitamin E-coated PSF membranes on the levels of genomic and DNA oxidative damage [53]. After 6 months of follow-up, 29 patients treated with vitamin E-coated membranes showed lower levels of oxidative DNA damage (assessed by lower levels of oxidized DNA bases) than those treated with conventional PSF membranes. In 2022, Zhang et al. demonstrated that hemodialysis with vitamin E-coated Excerbrane EE15 membrane effectively restored the imbalance of redox metabolism, and improved the oxidative phosphorylation [54]. A recent meta-analysis in which 15 studies were included also showed that vitamin E-coated HFMs were capable of decreasing oxidative stress biomarkers such as TBARS and ox-LDL and pro-inflammatory cytokines such as CRP and IL-6 [55]. However, it should be noted that there is no solid clinical evidence showing the protective effect of vitamin E-coated HFMs on patient-centered outcomes including mortality and major cardiovascular events and therefore. Accordingly, clinical studies with larger sample size and longer follow-up period are still required to determine whether the use of vitamin E-coated HFMs is associated with the long-term protection of dialysis patients. Table 1 further summarizes detailed information on the basic membrane structural characteristics, major advantages, and limitations of diverse contemporary HFMs.
Concept and Principles of Hemocompatibility of HFMs
Blood-contacting devices are widely used in clinical practice, and hemocompatibility is recognized as one of the most crucial properties of blood-contacting devices such as HFMs. The developmental history of HFMs was also linked to the process of enhancing their hemocompatibility. However, currently used synthetic polymer membranes (e.g., made of PSF and PES) still have insufficient hemocompatibility and show activation of complement, protein adsorption and denaturation, immune cell activation, inflammation and so forth [27]. Although there has been some progress, such as the development of vitamin E-coated and heparin-modified membranes (see “Rationale, History, Basic Characteristics, and Clinical Evaluation of Contemporary HFMs”), these advances only partly addressed the challenges associated with the hemocompatibility of membranes. In addition, new advances in hemodialysis and the emergence of new hemodialysis technologies, such as microfluidic devices, wearable dialyzers and human nephron filters, advocate for higher hemocompatibility profiles [73–76].
Protein Adsorption
Protein adsorption is widely recognized as the initial event occurring when blood comes into contact with foreign surfaces such as HFMs [77]. The knowledge and research on protein adsorption on material surfaces have been extensively investigated, and there are many excellent reviews reported to summarize this aspect [21, 78]. In the case of hemodialysis, the membrane matrices used in current clinical practice are poorly resistant to protein adhesion and denaturation. Thus, low protein fouling is particularly important and needs to be resolved urgently. Protein adsorption on HFM surfaces is known to affect their toxin clearance [79, 80], promote cell adhesion and activation, induce inflammation and thrombogenesis [81, 82]. In this section, the progress in protein interactions on HFM surfaces and subsequent downstream reactions are introduced.
Driving Forces for Protein Adsorption on Membrane Surfaces
When HFMs come in contact with blood, non-specific plasma protein adsorption on the surface is inevitable and can occur within a few seconds [83, 84]. Plasma consists of thousands of proteins with different molecular sizes and surface characteristics, including charge and hydrophobicity. Due to the surface structure of HFMs and proteins, many interactions of proteins with the HFM surface are possible. Non-specific protein adsorption occurs mainly via van der Waals forces, hydrogen bonding, electrostatic interactions, and hydrophobic dehydration on the HFM surface [15, 78]. The initial adsorption can be reversible or irreversible depending on the surface characteristics. In most cases, smaller proteins with higher concentrations and low surface affinity in blood quickly build a layer on the interface; the composition of this initially attached layer, however, may change over time as these proteins are substituted by large proteins with high surface affinity.
Most proteins show extremely rapid and essentially irreversible adsorption on HFM surfaces, which remain attached even after extensive rinsing with buffers. Irreversible protein adsorption is typically modeled in a series of multi-steps that follow a path to a lower Gibbs free energy (G), ΔG = ΔH-TΔS. The minimum value of G can be obtained by decreasing the enthalpy (H) and/or increasing the entropy (S) at a fixed temperature [85]. Blood proteins are stabilized through interactions between amino acid residues, including hydrogen bonds, ionic bonds and hydrophobic-hydrophobic interactions. Their surfaces exposed to water are usually hydrophilic, with bound water molecules and associated small counter ions. When proteins come into contact with HFM surfaces, they tend to liberate these water molecules and the bound small counterions, leading to an entropy increase. Subsequent conformational changes with weakened internal stabilizing interactions result in the exposure of hydrophobic regions to the aqueous environment, leading to the adsorption of proteins to the HFM surface. The formation of new bonds/interactions on the surface with net positive enthalpy change is due to the adsorption and denaturation of proteins on the HFM surface. In this process, the increase in entropy due to the release of water molecules and counterions is sufficient to offset the detrimental enthalpy of adsorption, giving rise to a net reduction in Gibbs free energy [21].
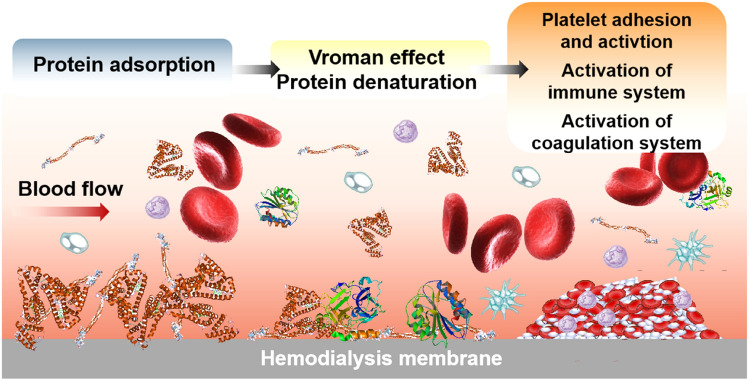
Vroman Effect and Protein Denaturation
Reversible protein adsorption from blood can occur through interactions between HFM membrane surfaces and proteins [86] and can be explained by the “Vroman effect” [87]. The conformational change of many proteins occurs upon non-specific adsorption to the interface [88, 89] (Fig. 3). Surface protein contact areas induce an increase in free energy, so proteins tend to maximize their footprint through conformational recombination, as shown in numerous experimental reports [90]. Structural reorganization of proteins on a surface is driven by conducive protein-surface interactions, resulting in an increase in entropy due to loss in ordered protein structures and the release of water molecules or counter ions [91]. As a consequence, conformational changes during the adsorption process lead to macroscopically measurable effects, such as reduction in the amount of adsorbed proteins, changes in secondary structure and increased elution resistance [92, 93].
Fig. 3.
Protein adsorption process on the HFM surface. Small proteins that are present at higher concentrations and low surface affinity in blood, such as albumin, are rapidly and reversibly adsorbed and may subsequently be replaced by larger proteins with a higher surface affinity. The adsorbed proteins tend to maximize their footprint through conformational reorganization since surface-protein contact induces a gain in free energy. The adsorbed and denatured proteins trigger the activation of coagulation, immune and inflammatory responses at the HFM surface during dialysis
Significant conformational changes in proteins are usually a slow process. Many proteins bind loosely on the surface initially and then increase their surface affinity with time by structural reorganization due to the unfolding of proteins. As a result, the desorption kinetics of the protein layers on HFM strongly depended on the elapsed time due to the adsorption event. This means that the freshly established protein layer is easily eluted from the HFM membrane surface; however, the protein layers that are already adsorbed early in the event (ranging from a few minutes to several hours) might be difficult to remove by elution. Of note, conformational change after adsorption also affects the biological function of absorbed proteins and can further trigger cellular responses. For example, fibrinogen or vWF is known to expose more binding domains to platelets and leukocytes after conformational changes [94–97]. The adsorbed proteins on HFM can also lose their biological activity due to the change in their native conformation [98]. In addition, it is worth noting that the HFM surface properties also closely affect protein denaturation, an important topic for further studies [99, 100].
Protein Adsorption and Its Consequences
The antifouling properties of HFMs are critical for several reasons. The protein contaminants adsorbed on the HFM surface not only reduce the efficacy of clearance of solutes but also trigger a series of subsequent contact reactions, possibly due to the conformational changes of adsorbed proteins [77, 94]. It is widely recognized that adsorbed and denatured proteins can initiate coagulation activation and immune-inflammatory responses [101, 102]. Therefore, avoiding and controlling protein adsorption may greatly improve the hemocompatibility of current HFMs. The adverse contact reactions induced by plasma protein adsorption from blood on HFM are briefly introduced here, and details on how protein adsorption induces these reactions will be explained in the subsequent sections.
The adsorption of fibrinogen and vWF can trigger further conformational changes exposing binding domains for interacting blood cells [94–97]. For instance, platelets bind to adsorbed vWF via the GPIb-V-IX complex and to fibrinogen via the αIIbβ3 receptor [95, 103]. Neutrophils are known to bind to fibrinogen by the CD11b/CD18 receptor, and erythrocytes can be aggregated with platelets by adhesive molecules (fibrinogen with aIIbβ3 and ICAM-4) [96, 104]. Such events play important roles in inflammatory and immune responses to HFM, and the coagulation activation results in thrombogenesis [105, 106]. For instance, FXII binding to a fiber surface gives rise to a conformational change, which initiates the intrinsic coagulation pathway [107]; βFXIIa cleaved from FXII can initiate the formation of kallikrein (KK) cleaved from prekallikrein, and KK cleaves high-molecular weight kininogen (HK) and releases bradykinin (BK), a non-peptide with pro-inflammatory and angio-active properties [108].
In another example, the binding of ficolin-2 and C3b to the membrane surfaces, which can trigger complement activation through the lectin pathway (LP) and the alternative pathway (AP), could result in inflammation activation [109]. These cascades of events, the activation of hemocytes, the immune system and the coagulation system, are very closely related to providing a potent amplification loop of further activation [110]. Thus, studying these events as standalone, although informative, may not result in proper understanding of the influence of membrane properties on hemocompatibility and our ability to innovate membrane surface chemistry.
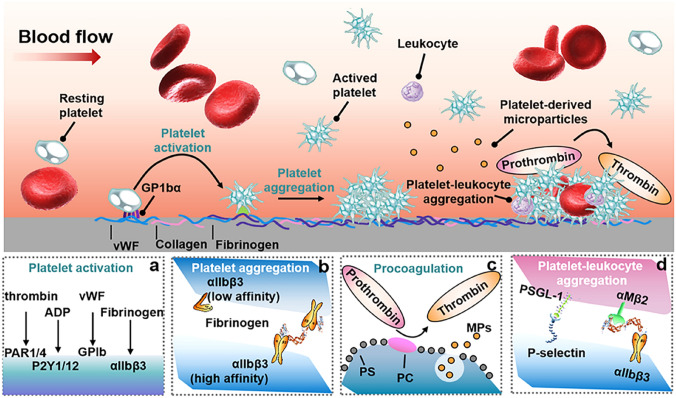
Platelet Adhesion, Aggregation and Activation
Platelet adhesion and activation are important contributing factors on the performance and the hemocompatibility of HFMs. A plethora of biomolecules and cytokines can be released by the activated platelets, which can trigger the intrinsic and extrinsic coagulation pathways, inflammation and immune responses [111, 112]. These activated platelets can also form aggregates with erythrocytes via fibrinogen [95, 104]. There are approximately 250,000 platelets per microliter of blood in circulation. Platelet quiescence is maintained in part by the action of signals in the blood including prostaglandin I2 (PGI2), nitric oxide (NO), prostaglandin E2 (PGE2) and ecto-nucleoside triphosphate diphosphohydrolase (CD39) [113, 114]. In the quiescence state, platelets are about 1 to 2 μm in size [115]. The platelet surface has a fuzzy proteo-glycan layer composed of membrane glycoproteins, glycolipids and mucopolysaccharides [116]. The platelet surface is negatively charged, which can prevent the resting platelets from adhering to other platelets or the vascular endothelial surface through electrostatic repulsion. In addition, platelets have ion channels, and studies have shown that these channels are continuous with the depression of the platelet membrane surface. These channels can provide pathways for both inside-out and outside-in signaling. After platelet activation, these channels can also be modified as surface membranes [117, 118].
When blood comes into contact with HFMs, the adsorbed proteins facilitate the adhesion and activation of platelets. In this case, platelets are preprogrammed to respond quickly to surface-bound and soluble protein signals, initiating a sequence of events essential to blood clotting and inflammation [119]. Platelet activation can occur in fractions of a second, leading to the formation of a clot in less than five minutes. Compared with quiescence conditions, the hemodialysis process is often accompanied by higher shear stress [120], which will not only affect the adsorption and denaturation of proteins, but also induce platelets to undergo deformation and thus increase their binding domains with the adsorbed proteins [121, 122]. It is clearly documented that shear and flow have an influence on platelet adhesion and activation [123, 124]. Thus, in this section, we will elaborate on how platelets adhere to the HFM surface, how they are activated, and their interactions between the coagulation and immune systems.
HFM-Induced Platelet Adhesion and Aggregation
Initial binding of platelets to the surface can be intermediated by cell surface glycoproteins [119]. Fibrinogen, vWF, collagen, etc., play important roles in platelet adhesion [125–127], as illustrated in Fig. 4. vWF is a large, multimeric adhesive glycoprotein synthesized by endothelial cells and megakaryocytes. Adsorption and denaturation of vWF on the HFM surface revealed the vWF-A1 structural domain with a positive charge, which binds to the ligand binding domain on GP1bα with a negative charge [128]. GPIbα is the only receptor on a nonactivated platelet with a significant affinity for surface-bound vWF. In normal conditions, no interaction between native vWF and GPIbα is present as GPIbα binding sites are hidden on the vWF-A1 domain. Nevertheless, the immobilization of vWF and/or high shear forces (e.g., under hemodialysis conditions) eliminate the shielding effect of other vWF domains, permitting platelets to bind and activate [129].
Fig. 4.
Illustration of HFM-induced platelet adhesion and activation. a-b Platelet binding to a surface can be mediated by cell surface glycoproteins, and platelets can be activated via different signal transduction pathways, including tyrosine kinase activation and G-protein coupled receptors. c When platelets are activated by strong agonists, the negatively charged phospholipids are redistributed to the outer leaflets of platelet membranes, and platelet activation also leads to the formation of microparticles and the release of highly anionic biomolecules such as polyP, thereby promoting the activation of the coagulation pathway. d In addition, the platelet-leukocyte interaction is mainly initiated by P-selectin expressed on the surface of activated platelets and P-selectin glycoprotein ligand-1 on the surface of neutrophils and monocytes. Abbreviations: vWF von Willebrand factor, ADP adenosine diphosphate, PS phosphatidylserine, PC choline phospholipids, PSGL-1 P-selectin glycoprotein ligand-1, PAR1/4 protease activated receptor-1 or -4
After platelet activation, platelet adhesion mediated by fibrinogen plays a major role, and αIIbβ3 is recognized as the most abundant receptor on the platelet surface [21]. Under resting conditions, the low affinity of integrin αIIbβ3 for its ligands, fibrinogen and vWF, increases dramatically in response to platelet activation [126], as illustrated in Fig. 4. Lower affinity conformation is sustained by interactions between the proximal cellular regions of the α and β subunits of the platelet membrane, stabilized by a salt bridge between Arg995 of αIIb integrin and Asp723 of β3 integrin as well as the N-terminal hydrophobic residues immediately adjacent to the arginine and aspartate residues [130]. It is worth noting that platelet adhesion on a membrane surface is mediated by factors related to adsorption-induced conformational changes in fibrinogen rather than adsorption concentration.
In addition, platelet adhesion and aggregation on HFM surfaces are also related to the shear rate, which is also an important hemodynamic parameter in hemodialysis sessions [95]. In-depth knowledge of the platelet aggregation process has resulted in three distinct routes. Under low shear rates (below 1000 s−1), the aggregation of platelets is mainly regulated by αIIbβ3-fibrinogen interactions [131]. In contrast, when the shear rate is between 1000 and 10,000 s−1, platelet aggregation occurs in 2 steps [132]. A preliminary step relies on the adhesive function of GPIbα and αIIbβ3, and is regulated by reversible platelet aggregate development. In the second step, the irreversible activation of αIIbβ3 and generation of platelet agonists result in the development of stable platelet aggregates. Under shear rates greater than 10,000 s−1, platelet aggregation is fully regulated by GPIbα-vWF interactions and can arise in the absence of platelet or αIIbβ3 activation [133].
HFM-Induced Platelet Activation
Platelets can be activated by different signal transduction pathways, including tyrosine kinase activation and G-protein coupled receptors, as illustrated in Fig. 4a, b. For activation of the tyrosine kinase cascade, some evidence suggests that such signals are generated in response to vWF occupying GPIb [134], the antibody complexes occupying FcRγII receptor, the GAS6 occupying receptors Axl and Mer [135], the collagen occupying GPVI-FcRγ [136] and podoplanin occupying CLEC-2, which equally result in platelet aggregation [137]. For the activation of a cascade of transmembrane G-protein coupled receptors, evidence is available that such signals are generated upon the occupancy of thromboxane receptor (TP) by TXA2 [138], of protease activated receptor-1 (PAR-1) and protease activated receptor-4 (PAR-4) by thrombin [139], of P2Y1 and P2Y12 by adenosine diphosphate (ADP) [114, 140], and of αIIbβ3 by fibrinogen.
Once platelets are activated, they can rapidly change their shapes through cytoskeletal rearrangement [95]. The activation of platelets is distinguished by a shift in morphology from a disk shape (inactivated) to a spherical shape and the appearance of appendages, called pseudopods, promoting both platelet contact and adhesion [21]. Partially activated platelets exhibit short or long dendrites, whereas fully activated platelets are flat and spread over the surface of HFMs. Activated platelets can defect their granules to the external membrane, vacating the contents to enrich the local context with a large number of bioactive molecules [141]. The paracrine or/and autocrine properties allow the initial signal to feedback rapidly into the process, increasing the activation of nearby platelets in number and extent, thus inducing secondary secretion and leading to a dramatic amplification of platelet activation.
Platelets and Coagulation
In the resting state, negatively charged phospholipids containing phosphatidylserine (PS) and phosphatidylethanolamine (PE) are mostly stored in the inner leaflets of cell membranes, while neutral phosphocholine dominates the outer leaflets (Fig. 4c) [142]. When platelets are activated by strong agonists, the negatively charged phospholipids are redistributed to the outer leaflets of platelet membranes, thereby promoting the activation of the intrinsic coagulation pathway [142]. This negatively charged surface also acts as a template to assemble coagulation factors toward further generation of factor Xa and downstream generation of thrombin, leading to clot formation. Platelet activation can also release negatively charged polyphosphate (polyP), an accelerator of blood coagulation, from dense granules, leading to further thrombin generation and clot formation [141, 143]. PolyP is a linear polymer of inorganic phosphate units that can aggregate as nanoparticles on the activated platelet surface, further activating the coagulation system. PolyP affects the coagulation process in multiple stages, and it can accelerate the activation of FXII, FXI, and FV and change the structure of the fibrin clot [143].
The rise in the concentration of intracellular calcium ions, the activation of calpain, the rearrangement of the cytoskeleton, the phosphorylation of proteins and the translocation of phospholipids are all related to the formation of platelet-derived procoagulant microparticles. In vitro, the microparticles released by activated platelets can be realized by stimulation of collagen, thrombin, tissue factor (TF), complement C5b-9 or a high shear rate [144]. The microparticles can induce contact activation, bind FVIII, FVa and FXa, and finally form FXa and prothrombin complexes. They can also bind protein S to promote the inactivation of FVa and FVIIIa and thus inhibit the coagulation cascade [95, 145]. In addition, the microparticles can activate platelets by supplying arachidonic acid.
In summary, there is irrefutable evidence that platelet activation on HFM surfaces can accelerate thrombin generation: (1) activated platelets can induce contact activation, (2) platelets accelerate FX activation through FIXa and VIIIa and prothrombin activation through FXa and Va, (3) the binding of activated coagulation factors to the platelet membrane surface can protect them from inactivation caused by inhibitors from plasma, (4) platelets can help the reaction between fibrinogen and thrombin, enhance the positioning of coagulation factors through vWF, accelerate the activation of FXI by thrombin, and release FXIII to promote the cross-linking of fibrinogen [95, 146], and (5) activated platelets can release polyP, which accelerates thrombin generation, resulting in the amplification of clot generation [143, 147].
Platelets and Leukocytes
The participation of platelets in the inflammatory response can be achieved through the synergistic effect on leukocytes [148, 149]. The platelet-leukocyte interaction is mainly initiated by P-selectin expressed on the activated platelet surface and P-selectin glycoprotein ligand-1 on the leukocyte surface, as illustrated in Fig. 4d. In addition to P-selectin glycoprotein ligand-1, CD24 on the surface of leukocytes can also bind P-selectin [148]. These instantaneous interactions mediated by P-selectin can be stabilized by subsequent contact mediation mainly by activated leukocyte β2 integrin [150]. In addition, platelets can synthesize and release platelet active factors to activate αMβ2 of leukocytes [151], which interacts with platelet GPIbα and platelet-bound fibrinogen.
The tight complementary relationship between leukocytes and platelets leads to the subsequent inflammatory response, including the release of leukocyte chemokines [152], the release of platelet-derived growth factor that can affect fibroblasts and smooth muscle cells [153], the release of transforming growth factor that can stimulate and inhibit cell growth [154], the release of platelet factor 4 (PF4) that can activate neutrophils and have anti-angiogenic activity [155], and the release of cytokine IL-1B that irritates lymphocytes [156]. Platelets also contain FcγIIA receptors that can bind immunoglobulin G (IgG) and immune complexes [157], which in turn affects complement activation. In addition, platelets express CD40 ligands on their membrane surfaces after being activated, which can bind to the CD40 factor located on leukocytes [158]. This combination leads to the activation of leukocytes and the synthesis of many inflammatory molecules. Meanwhile, the platelet-leukocyte interaction can promote the generation of ROS, leading to oxidative stress [159]. Thus, activated platelets on the HFM surface play a key role in inflammatory and immune responses associated with hemodialysis.
HFM-Induced Inflammation
The life expectancy of uremic patients is significantly decreased as a result of compounding factors such as arteriosclerosis and partly due to the prevailing chronic systemic inflammatory condition triggered by the underlying renal disease, comorbidities, and the bioincompatibility of HFMs [160, 161]. Based on these considerations, the inflammatory response during hemodialysis sessions has always been the focus of medical staff. For example, cellulose-based membranes which are known to cause complement activation, result in the activation of mononuclear leukocytes and the release of pro-inflammatory cytokines. Activated leukocytes are more prone to undergo apoptosis, which may lead to leukopenia in this patient group [15]. Therefore, cellulose-based membranes were replaced by synthetic polymer membranes such as PSF and PES in clinical use, which significantly reduced the inflammatory response. However, these membranes still do not have satisfactory anti-inflammatory properties, possibly due to their poor hemocompatibility [79, 80]. In this section, HFM-induced inflammation activation and inflammation-associated side effects are discussed.
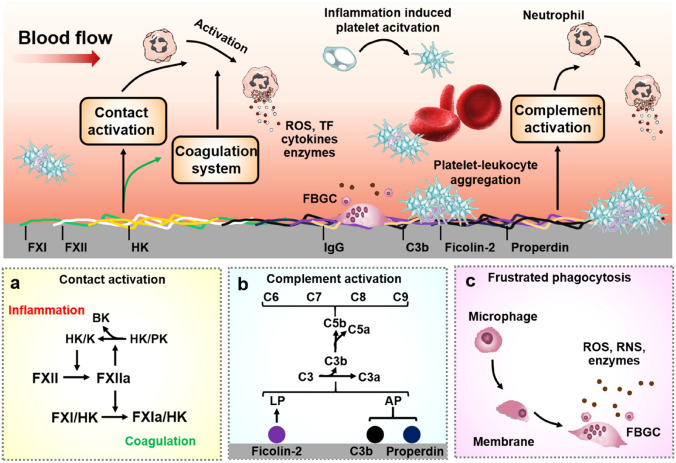
The Contact/Kallikrein System
The proteins in the contact activation system that circulate as zymogens are FXII, FXI, and prekallikrein (PK), while HK acts as a cofactor in the cleavage of PK and FXI [81]. The conformational change of FXII is induced by the binding or contact of FXII with HFMs, triggering the unprotected autoactivation of the moiety into α-FXIIa, which cleaves FXII to activate β-FXIIa enzymatically.
β-FXIIa can be initiated by two pathways. In the first pathway, PK cleaves to KK, with HK as a cofactor. Then, KK activates FXII to β-FXIIa, which in turn produces more KK, offering an efficient amplification cycle. Additionally, HK can be cleaved by KK and release BK, a potent vasodilator, vascular permeability inducer and pro-inflammatory peptide. In the second pathway, FXI is activated to FXIa, which again uses HK as a cofactor, initiating the intrinsic coagulation pathway and ultimately leading to thrombin generation [162], as illustrated in Fig. 5a. The generated proteases FXIIa, FXIa, and KK can be suppressed by the C1 inhibitor (serine protease inhibitor) and antithrombin (AT), which are ubiquitous in plasma [21]. A direct relationship was reported between the electronegativity of the membrane assessed by its zeta potential and the activation of the contact system during the first five minutes of plasma circulation [163].
Fig. 5.
Illustration of HFM-induced inflammation. The membrane first induces autoactivation of FXII to FXIIa. FXIIa activates HK-bound PK to K, which cleaves HK to BK and induces an inflammatory response. a The contact activation of coagulation and complement activation can lead to leukocyte activation, and the activated leukocytes can secrete ROS, TF, pro-inflammatory cytokines, and enzymes. b The principal mechanism leading to complement activation on HFM is the binding of ficolin-2 and MBL to the membrane, resulting in LP activation. Simultaneously, properdin and/or C3b bind to the membrane, resulting in AP activation. Surface functional groups on HFM, such as hydroxyl group, can also contribute to AP activation. c) Frustrated phagocytosis is thought to be the primary initiator of macrophage fusion, leading to the formation of multinucleated FBGCs, which are characteristic of the foreign body reaction. Abbreviations: PK prekallikrein, K kallikrein, HK high-molecular-weight kininogen, BK bradykinin, FBGC foreign body giant cells, TF tissue factor, IgG immunoglobulin G, ROS reactive oxygen species, RNS reactive nitrogen species, LP lectin pathway, AP alternative pathway
Complement Activation
Before diving into HFM-induced complement activation, the pathways leading to complement activation should be first retrospectively reviewed. Activation of complement can occur via three different pathways, including the classical the classical, lectin and alternative pathway [164]. Irrespective of the pathways, complement activation leads to the formation of C3 convertase, which catalyzes the cleavage of C3 into split products C3a and C3b. C3b plays an important role in forming a C3 convertase when bound to Factor B cleaved by Factor D (C3bBb complex), which magnifies C3b generation. After the local concentration of C3b attains a cut-off threshold, the C3 convertase shifts the substrate specificity to C5.
C5 convertase cleave C5 into C5a and C5b [165]. C5a is released as an anaphylatoxin, and C5b activates the terminal pathway of the complement system. C5b rapidly conjugates to C6 and forms the transferable C5b,6 complex. In rapid sequence, the C5b,6 complex binds rapidly and tightly to C7 (C5b-7), C8 (C5b-8) and multiple copies of C9 (C5b-9) that form a membrane attack complex (C5b-9), causing osmotic lysis of the cell [166]. The number of complement-derived mediation molecules shows the important role of complement in acute inflammation [164].
The principal mechanism leading to complement activation on HFMs is the binding of ficolin-2 and MBL to these membranes, resulting in the activation of the Lectin pathway [109]. It is also found that properdin and/or C3b bind to the surfaces of HFMs and thus activate the alternative pathway [167], as illustrated in Fig. 5b. Surface functionalities such as hydroxyl groups and primary amine groups are also known to activate the alternative pathway [168]. A mechanism that modulates complement activation during hemodialysis is the adsorption of complement inhibitors onto HFMs. In hemodialysis, PSF or PES membranes are shown to absorb factor H (the main regulator of alternative pathway) and clusterin (an inhibitor of terminal pathway activation), thereby stopping the formation of C5a and C5b-9 [169, 170].
Immune Cells
Growing evidence suggests that adhesion of neutrophils to HFM may be facilitated by the adsorbed fibrinogen [96]. Following surface adhesion, neutrophils alter the context by releasing proteases, ROS and TF [171], as illustrated in Fig. 5, contributing significantly to thrombogenesis and other complications. Neutrophils also release pro-inflammatory cytokines including macrophage chemoattractants and inflammatory proteins, as well as interleukin (IL) IL-8 [172]. Notably, activated neutrophils also release neutrophil extracellular traps (NETs), which promote thrombus formation and immune activation, as reviewed recently [173, 174].
Monocytes can be recruited to sites of inflammation through chemoattractant signals [175]. Some of their activity on the surface of HFMs is associated with the chronic phase. Monocytes differentiate into macrophages; they may promote inflammation or contribute to resolution, depending on local microenvironmental cues [176]. The phagocytic action of macrophages on HFMs leads to a state called “frustrated phagocytosis” [177], as illustrated in Fig. 5c. Frustrated phagocytosis is believed to be the primary initiator of macrophage fusion, leading to the formation of multinucleated foreign body giant cells (FBGCs), a feature of the foreign body response. Such cells subsequently produce ROS reactive nitrogen species (RNS) and enzymes that can alter the surface chemistry of HFMs [178].
Surface-Induced Thrombosis
The development of antithrombotic surface characteristics of HFMs is recognized as an important research focus. Hemodialysis sessions are often accompanied by the use of anticoagulants, which effectively inhibit coagulation activation induced by HFMs. To avoid thrombosis during hemodialysis, Nephrologists tend to use high-dose anticoagulants to ensure the progress of these sessions [17, 179]. However, the excessive use of anticoagulants can lead to bleeding risk in patients [180]. In addition, it is difficult for medical staff to determine the accurate dose of anticoagulants for each patient because of their individual disease status and clearance [181]. HFMs with sufficient antithrombotic properties can potentially reduce the use of anticoagulants [182]. Recent investigations have indicated that new hemodialysis technologies, such as wearable artificial kidneys, could greatly improve the quality of life of dialysis patients. However, these new technologies require an urgent need for HFMs with good hemocompatibility. This will not only reduce the weight of the entire device by eliminating the need for heparin pumps but also significantly diminish the risk of bleeding [74].
As discussed in previous sections, reducing the adsorption of proteins may effectively inhibit the activation of HFM-induced coagulation. However, evidence suggests that antifouling fouling surfaces alone may not be sufficient, as such surfaces can still activate the intrinsic coagulation pathway. To date, there are no successful studies that show anticoagulant-free hemodialysis using HFM membranes with only antifouling properties. In recent years, the heparin-grafted AN69 membrane [183] and the recent discovery of a blood-thinning device (pseudohemophilia model) without the need for anticoagulants from our group may lead to anticoagulant-free hemodialysis in the future [18]. In this section, the coagulation pathways altered by HFMs are discussed.
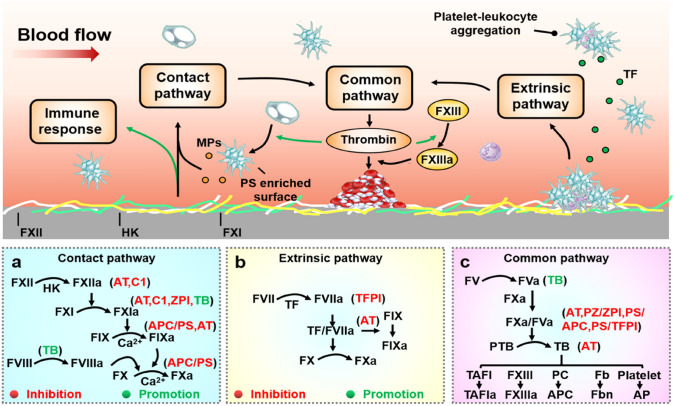
The blood coagulation system consists of a series of proenzyme molecules [184]. The coagulation cascade can be activated through either the “extrinsic pathway” (Fig. 6a) or the “intrinsic pathway” (Fig. 6b) [185]. These two pathways converge upon FX activation. The activated FX (FXa) and the subsequent steps constitute the “common pathway”. In this pathway, prothrombin is cleaved to thrombin, which in turn cleaves fibrinogen into fibrin. Fibrin polymerizes into a cross-linked network of fibrin fibers forming the primary structure of blood clots, as illustrated in Fig. 6c. The fibrin network can promote platelet aggregation by providing adhesive links. Besides, thrombin can provide positive feedback to both pathways.
Fig. 6.
Illustration of HFM-induced thrombosis. Coagulation can be activated through either the “extrinsic pathway” or the “intrinsic pathway”. a In the intrinsic pathway, FXII adsorbs to a surface and becomes activated. b In the extrinsic pathway, activated cells express TF, which forms a complex on the surfaces of cells with activated FVII. These two pathways converge upon FX activation. c The activated FX (FXa) and the subsequent steps constitute the “common pathway”. In this pathway, prothrombin is cleaved to thrombin which in turn cleaves fibrinogen into fibrin. Abbreviations: MPs microparticles, TF tissue factor, TFPI tissue factor pathway inhibitor, HK high molecular weight kininogen, PS phosphatidylserine, AT antithrombin, TB thrombin, C1 serine protease inhibitor C1, PC protein C, APC activated protein C, APC/PS complex of activated protein C and protein S, ZPI Z-dependent protease inhibitor, TAFI thrombin-activatable fibrinolysis inhibitorase inhibitor, TAFIa activated thrombin-activatable fibrinolysis inhibitor, Fb fibrinogen, Fbn fibrin, AP activated platelets
In addition, there are other proteins in blood that can regulate coagulation and avoid excessive coagulation [186, 187]: (1) FIXa, FXa and thrombin can be inhibited by AT, which acts as a natural inhibitor of these activated factors, and the presence of heparin will greatly catalyze the action of AT [188]; (2) thrombomodulin can bind with thrombin, and the bound thrombin loses its ability to cleave fibrinogen; and (3) thrombin can also activate protein C, thereby inactivating FVa and FVIIIa. Activated protein C can also form a complex with protein S, inactivating FVa and FVIIIa; (4) the complex of serine protease inhibitor and protein Z will inactivate FXIa and FXa; the serine protease inhibitor C1 is also the main factor of FXIIa inhibitors; (5) the TF pathway inhibitor can inhibit the TF/FVIIa complex and inhibit the hydrolysis ability of FXa [189]; and (6) subsequent to the blood clot formation, the fibrinolytic system can dissolve thrombus. Fibrin is finally degraded by plasmin to produce soluble fibrin degradation products [190]. The amount of thrombin generated during the clotting process also dictates the clot structure, which can alter the ability of the fibrinolytic system to degrade the clot [191].
During the hemodialysis process, the activation of the intrinsic coagulation (contact activation) pathway may be the main reason inducing the initial clotting process. Important considerations are (1) membrane-induced thrombosis is mainly triggered by the contact activation pathway; (2) the use of FXIIa antagonists can effectively avoid thrombosis during extracorporeal blood therapy [192], and the potential of FXIa inhibitors should also be investigated; and (3) the concentration of TF during hemodialysis shows limited change [193]. However, in the case of anticoagulant-free hemodialysis, this argument needs to be reconsidered.
Fabrication and Modification Approaches of HFMs
Fabrication Approaches of HFMs
The process of converting polymers into fibers is recognized as spinning. Spinning technology governs the structure of the membrane. HFM spinning involves consecutive fiber production by extrusion through a spinneret and then reverting it to the solid state. Depending on the parameters and processes used, fibers are produced with different properties.
The early HFMs were manufactured by melt extrusion, a solvent-free technique in which the polymer is heated above the melting point and extruded into a thin sheet, which is then stretched to make it porous [194]. This technique is suitable for highly crystalline polymers. Typically, stretching is conducted in two steps, first cold stretching and then hot stretching. In this process, the physical properties (such as crystallinity, melting point, tensile strength, etc.) and the applied processing parameters control the final porous structure and properties of the membranes. The HFMs fabricated by this approach are symmetrical, and the flux is low. In addition, only a specific substrate can be applied in this way, resulting in the recent discontinuation of this method for the fabrication of HFM.
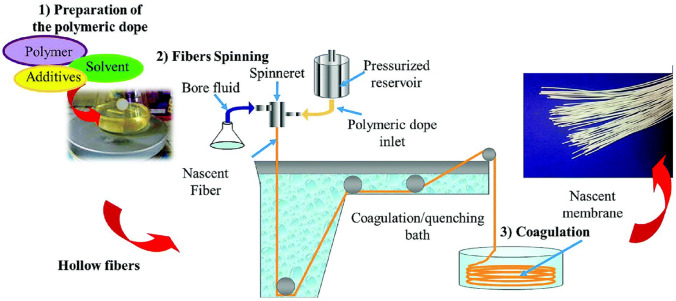
For solution spinning, a professional apparatus containing at least a spinneret and a take-up unit are needed, as shown in Fig. 7. HFMs are prepared by a dry‒wet spinning technique (the wet-spinning technique is seldom used) based on phase inversion. In this process, the polymer solution is extruded through the spinneret and then immersed in a coagulation bath containing a nonsolvent, where an exchange of solvent and nonsolvent takes place, and finally the asymmetric HFM is formed [195]. To date, various approaches, such as the selection of polymers, solvents and non-solvents, additives, precipitation time, bath temperature and other parameters during immersion precipitation, can be applied to control the pore structure of HFMs. Different casting conditions and post-treatments were proposed to improve the water flux and salt rejection of the cellulose membranes. Currently, solution spinning dominates the fabrication of HFMs.
Fig. 7.
Flat sheet and hollow fiber membrane preparation via phase inversion. Represented with permission from ref. [195]. Copyright (2014) Royal Society of Chemistry
Modification Approaches for HFMs
Based on the characteristics and fabrication process of these polymeric membranes, three main strategies are commonly used: bulk modification, blend modification, and surface modification.
Bulk Modifications
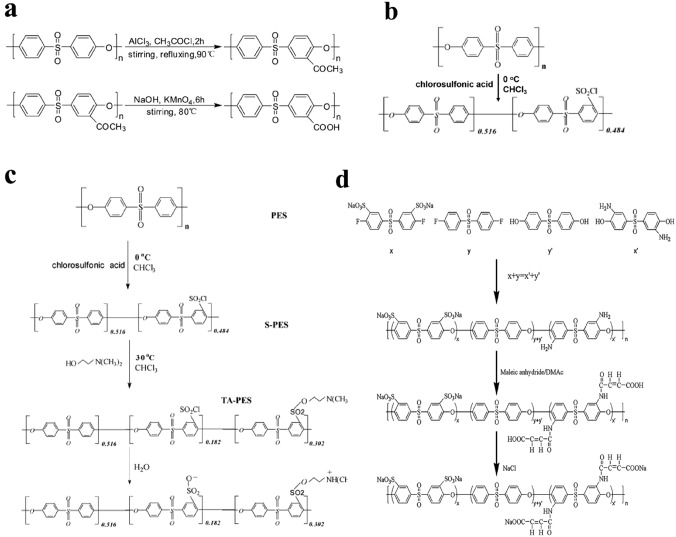
The bulk modification directly modifies the polymer substrate itself through chemical means, and the modified membranes can be directly prepared and shaped by the modified polymer substrate via phase inversion. This method can endow the polymer matrix with functional groups to improve hemocompatibility. For example, carboxylic PES can be prepared by controlled acetylating and oxidizing reactions using acetyl chloride as the acetylating reagent and KMnO4 as the oxidant [196], as illustrated in Fig. 8a. Tang et al. prepared sulfonated PES using chlorosulfonic acid [197] as shown in Fig. 8b. Additionally, PES can be directly modified with zwitterions via bulk modification using chlorosulfonic acid and dimethylaminoethanol [198] as shown in Fig. 8c. In addition, PES membranes modified with both carboxyl and sulfonic acid groups via bulk modification have also been reported [199] as shown in Fig. 8d.
Fig. 8.
Bulk modification for HFMs. a The process for the fabrication of carboxylic PES. b The process for the fabrication of sulfonic PES. c The process for the fabrication of zwitterionic PES. d The process for the fabrication of heparin-mimicking PES. a is represented with permission from ref. [196]. Copyright (2011) Elsevier, b and d are represented with permission from ref. [199]. Copyright (2013) Elsevier, c is represented with permission from ref. [198]. Copyright (2008) Elsevier
Moreover, amino acids can also be used to modify HFMs via bulk modification. Shi et al. used a 1-ethyl-(3–3-dimethylaminopropyl)-carbodiimide hydrochloride EDC and NHS coupling reaction to modify PAN substrates with amino acids (glycine, serine, and lysine) [200]. In fact, in addition to directly improving the antifouling performance, bulk modification can also endow the polymer substrate with functional groups for further modification. Tripathi et al. first prepared sulfonated PES and then modified the sulfonated PES with aminated PEG using 1,1′-carbonyldiimidazole. Xiang et al. initially prepared an azido-PSF membrane by chloromethylation and azide conversion reactions and then modified the membranes with zwitterions via a click chemistry-enabled layer-by-layer assembly [201]. The advantage of bulk modification is that the modification effect is relatively stable, but complex and rigorous modification conditions are usually needed. Meanwhile, chain breakage of the polymer substrates is easily induced during the modification process, which may lead to a decrease in the mechanical properties of the modified membranes.
Blending Modifications
Compared to bulk modification, blending modification is much easier and more suitable for industrial production. By blending polymer substrates with functional modifiers, the functionalization of the substrates can be easily improved. However, there are two critical aspects of blending modification that need to be addressed. The first point is that the hydrophilic nature of blends will lead to the “elution” (the hydrophilic modifiers may be dissolved out by water) of the blend components during HFM preparation, storage and use, thereby reducing the modification performance. The second aspect is that many hydrophilic blends do not have good miscibility with HFM matrices, leading to poor integrity or insufficient mechanical properties of the blending-modified HFMs.
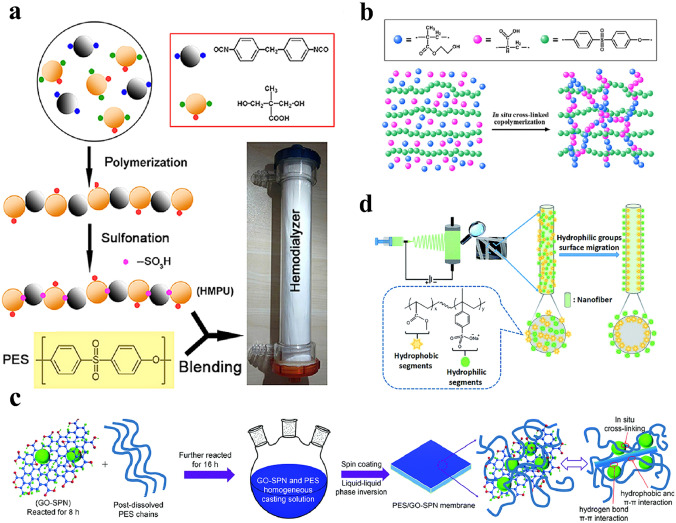
There are many reports on improving the hydrophilicity of HFMs by blending hydrophilic polymers such as PVP [202], PAA [203], PHEMA [204], polyurethane (PU, as shown in Fig. 9a) and so forth, but the immiscibility of hydrophilic polymers with hydrophobic membrane materials and elution of hydrophilic polymers limits the modification effect. Due to the poor performance of such an approach, an in-situ cross-linking strategy was proposed. By directly initiating the cross-linking polymerization of the hydrophilic monomers in the polymer substrate solution, a semi-interpenetrating network structure is formed, which can effectively reduce the elution of hydrophilic polymeric components (as shown in Fig. 9b). Although the in-situ cross-linking method can effectively prevent the elution of blend components, its modification effect is still not satisfactory due to the poor miscibility between blends and substrates.
Fig. 9.
Blending modification methods for HFMs. a Preparation of modified PES membranes by blending heparin-mimicking PU. b Preparation of modified PES membranes by the in-situ cross-linking method. c Preparation of modified PES membranes by blending functionalized nanogels. d Preparation of modified PES membranes by blending amphiphilic polymers. a is represented with permission from ref. [208]. Copyright (2014) Elsevier, b is represented with permission from ref. [209]. Copyright (2014) Elsevier, c is represented with permission from ref. [210]. Copyright (2016) Royal Society of Chemistry, d is represented with permission from ref. [211]. Copyright (2018) Royal Society of Chemistry
In this decade, large 3D or 2D modifiers that were shown to have good miscibility with the membrane substrates were investigated (Fig. 9c). For example, Wang et al. blended carbon nanotubes (CNTs) modified with sulfonic groups into a membrane matrix [23]. In addition, hydrophilic microgels have been used to modify HFMs. Xia et al. prepared polyethylene glycol methacrylate microgels and blended them into a membrane substrate [205]. However, the elution of microgels cannot be prevented even though the size of microgels is larger than that of the pores under hydrostatic pressures relevant to filtration [206]. Therefore, networked submicrogels were prepared and blended into a membrane matrix. As a result, the submicrogels linked by PES chains effectively prevented them from eluting from the membrane matrix [207]. However, one of the challenges associated with the blending method is controlling the balance between the surface segregation and the elution of hydrophilic components of the blends. The high entanglement between the blend components and substrates will lead to insufficient surface enrichment of functional groups. Conversely, the problem of elution is likely to occur. The use of amphiphilic polymers may be a strategy to solve this problem (see Fig. 9d. In addition, blending methods can endow the membrane matrix with active functional groups for further chemical modification [204]. Figure 9 summarizes the different blending methods for the modification of HFMs.
Surface Modifications
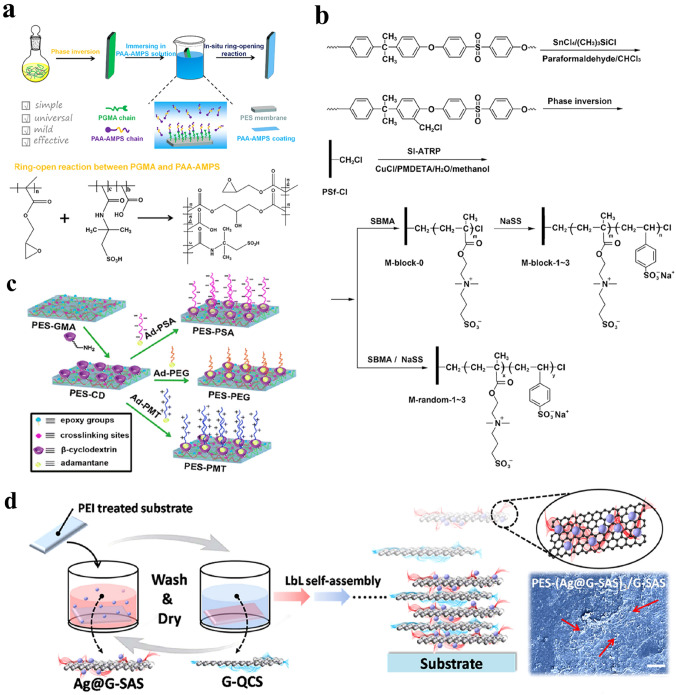
Compared with blending modification, the effect of surface modification is generally better because it can enrich more functional groups on the membrane surface. In addition, a large number of strategies can be applied to realize surface modification, such as hydrogen bonding, surface self-assembly, host–guest interactions, hydrophilic-hydrophobic interactions, click chemistry, mussel-inspired coatings, surface-initiated atom-transfer radical polymerization (SI-ATRP), surface click chemistry, and other surface grafting methods. The effect of surface modifications is more controllable and diversified due to the abundant modification methods. For traditional surface modification methods, membranes can be modified with reaction sites to attach diverse functional groups over the membrane surface. Then, different biocompatible materials are cross-linked or immobilized on the membrane surface. For example, polyglycidyl methacrylate (PGMA) was first prepared in PES solution by in situ cross-linking polymerization, after which PAA-AMPS was grafted onto the HFM surface via an in situ ring-opening reaction (Fig. 10a) [79]. Additionally, SI-ATRP is also a good approach to control the effect of surface modification. Xiang et al. first prepared chloromethylated PSF membranes, after which block zwitterionic copolymers of P(SBMA-b-SSNa) were synthesized via consecutive SI-ATRP (Fig. 10b) [212].
Fig. 10.
Surface modification methods for HFMs. a Illustration of the fabrication of PAA-AMPS-modified PES membranes via a surface ring-opening reaction. b Illustration of the fabrication of PSBMA-NaSS-modified PSF membranes via SI-ATRP. c Illustration of the fabrication of PSA-, PEG- and PMT-modified PSF membranes via host–guest interactions. d Construction of a sodium alginate sulfate coating through the immersed LbL self-assembly method and SEM image of the modified membrane surface. a is represented with permission from ref. [79]. Copyright (2019) Elsevier, b is represented with permission from ref. [212]. Copyright (2014) Elsevier, c is represented with permission from ref. [213]. Copyright (2015) American Chemical Society, d is represented with permission from ref. [215], Copyright (2018) American Chemical Society
Host–guest interactions are another method for surface modification. Deng et al. first prepared PES-cyclodextrin host membranes and an adamantane-PEG guest modifier, and then the post-functionalization of the membranes was realized (Fig. 10c) [213]. In addition, surface self-assembly is also reported to realize the surface modification of the membrane (as shown in Fig. 10d). Xia et al. first prepared positively charged membranes by immersing the membranes in a positively charged polyethyleneimine solution, after which negatively charged nanogels and PSSNa were assembled onto the membrane surface [214]. Figure 10 summarizes the commonly used surface modification methods for HFMs.
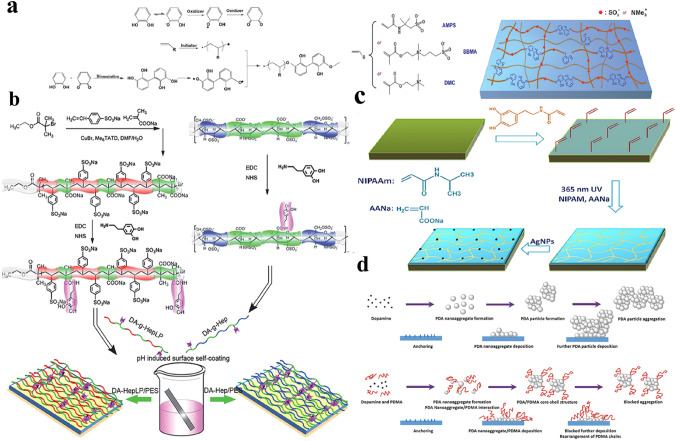
A mussel-inspired strategy is also used to modify the hemodialyzer. Although many studies are based on flat membranes, a mussel-inspired strategy can be applied in HFMs theoretically; thus, a summary is provided here. As illustrated in Fig. 11. Shao et al. prepared PAMPS-g-catechol, PSBMA-g-catechol, and poly(dimethylaminoethyl methacrylate methyl chloride)-g-catechol and then coated them onto PES membranes (Fig. 11a) [23]. Although the above methods are relatively simple in the membrane modification process, complex synthesis is required to prepare modifiers. In recent years, many simple dopamine-based modification methods have been developed, such as free-radical polymerization initiated by dopamine self-polymerization (Fig. 11b) or grafting to polydopamine-provided sites (Fig. 11c). In addition, co-immobilization of polydopamine and the designed polymer is also a convenient method to enhance the antifouling property of the membranes (Fig. 11d).
Fig. 11.
Metal-inspired modification of HFMs. a Modification of PES membranes via dopamine-triggered surface free-radical polymerization. b Schematic procedure to modify the PES membrane with heparin or heparin-mimicking polymer via mussel-inspired self-coating. c Process of chemically attaching the hydrogel thin layer onto the PES membrane with the assistance of N-(3,4-dihydroxyphenethyl) acrylamide. d The one-pot method for preparing PDOPA coatings utilizes a precursor solution containing a mixture of dopamine and molecules to be co-immobilized with PDOPA. a is represented with permission from ref. [23]. Copyright (2018) Wiley, b is represented with permission from ref. [216]. Copyright (2014) Royal Society of Chemistry, c is represented with permission from ref. [217]. Copyright (2017) American Chemical Society and d is represented with permission from ref. [218]. Copyright (2018) American Chemical Society
Hemocompatibility Modification Strategies for HFMs
Antifouling Modification Strategies
Endowing HFMs with antifouling properties can be achieved by a series of physical or chemical methods to control their surface wettability, charge, and topology. Of all the parameters available, hydrophobicity and hydrochemical forces mediated by water are regarded as the main contributors to protein adsorption. A surface is considered hydrophilic if its water contact angle 'θ' is lower than a critical value of approximately 65°, where proteins may not displace water on the surface and be adsorbed. Whereas for hydrophobic surfaces, the opposite behavior is expected. However, the observation of strong protein adsorption on many types of hydrophilic surfaces is a clear contrast to the abovementioned rules. Four types of molecular-level characteristics of protein-resistant monolayers have been proposed, and are now recognized as the “Whitesides rules”: 1) the presence of polar functional groups, 2) the presence of hydrogen bond acceptor groups, 3) the absence of hydrogen bond donor groups, and 4) the absence of net charge [219, 220]. Membranes that fulfill all of these rules could provide an additional hydration layer that prevents protein adsorption. Recently, with the development of bionics, it has also been found that bioinspired superhydrophobic surfaces (self-cleaning coatings) or lubricant surfaces can effectively create an energy barrier to prevent liquid from penetrating into the pores, thereby blocking contamination from the proteins present in the blood and endowing the surface with antifouling properties [221]. However, these general principles cannot be fully applied to HFMs. It is currently unrealistic to ensure the superhydrophobicity and flux of the membrane simultaneously. Therefore, hydrophilic modification of the membranes to achieve antifouling properties is mainly discussed here.
To date, several modifiers that can be used to achieve the antifouling properties in membranes have been investigated. These include polyethylene glycol (PEG) or polyethylene oxide (PEO), zwitterionic polysaccharides, polymers such as poly(N,N-dimethylacrylamide) (PDMA), poly(hydroxyethylmethacrylate) (PHEMA), and PVP [222]. In addition, negatively charged polymers (e.g., poly(acrylic acid) (PAA)) are also commonly used for enhancing the antifouling property of membranes [79]. These modifiers can be directly used to modify HFMs (e.g., blending) or they can also be grafted onto HFMs [23, 207]. Figure 12 summarizes the chemical structures of polymeric modifiers used to achieve the antifouling characteristics of HFMs.
Fig. 12.
Polymers used for HFM modification. Chemical structures of common polymers used to enhance the antifouling property of HFMs. Abbreviations: PEG Poly(ethylene glycol), PHEMA Poly(hydroxyethylmethacrylate), PVP Poly(vinylpyrrolidone), PSBMA Poly(sulfobetaine methacrylate), PMPC Poly(2-methacryloyloxyethyl phosphorylcholine), PAM Poly(acrylamide), PDMA Poly(N,N-dimethylacrylamide), PAA Poly(acrylic acid), PSSNa Poly(sodium 4-styrenesulfonate), PAMPS Poly(2-acrylamido-2-methyl-1-propanesulfonic acid), PMA Poly(methyl acrylate), PGMA Poly(glycidyl methacrylate), PU Poly(urethane)
Currently, most of the experiments to determine the antifouling performance of HFMs are mostly conducted in static environments. However, the use of HFMs is often accompanied by high shear force [223] which may lead to the denaturation of the protein. This process can expose more binding sites on proteins to facilitate tighter binding to HFMs. Recent studies from our group using proteomic studies on hydrophilic surfaces under dynamic conditions in blood plasma and whole blood demonstrated that the protein composition on surfaces that underwent a high shear rate was different from those adsorbed under static conditions [123]. Therefore, it is highly relevant to investigate protein binding in relevant shear conditions especially in the case of HFMs.
Most common examples of protein binding of HFMs are performed using fibrinogen (the most important protein that affects the coagulation system and hemocyte adhesion) and albumin (the most abundant protein in the blood). However, in real world conditions, these high binding proteins could be replaced by other proteins in blood due to competitive binding over time (Vroman effect) on the HFM surface. Therefore, new research should include other relevant proteins, such as HK, vWF, and complement proteins in addition to fibrinogen and albumin.
Exploring the protein adsorption process on HFMs is still quite difficult at present, mainly because the surface of HFMs is much rougher and more porous than other substrates (e.g., silicon wafers or controlled substrates used in most studies). Therefore, achieving more precise modifications on the membrane surface such as controllable surface topology and surface charge density, may provide a powerful approach to alter the protein adsorption process on the membrane surface. As mentioned previously, unbiased proteomics approaches are also highly relevant here.
Improving the protein resistance of the surface is now recognized as one of the important parameters to improve hemocompatibility. However, increasing evidence also shows that protein adsorption is not the only condition for the protein-induced activation of downstream pathological events. This evidence suggests that we should reconsider protein contamination on the material surface. Defining protein adsorption as a process that leads to protein restriction within an interfacial region (soft protein corona) separating bulk solution from the adsorbed surface should also be taken into consideration.
Prevention of Platelet Adhesion and Activation on HFMs
The adhesion and activation of platelets on the membrane surface is inseparable from the participation of proteins such as fibrinogen and vWF. It has been widely reported that improving the antifouling performance of HFMs can also improve their performance against platelet adhesion and activation. Therefore, improving the antifouling property of HFMs is recognized as a convenient and effective strategy to prevent platelet adhesion on HFM surfaces [224, 225]. Heparinization and heparin-mimicking modification on the membrane surface are also shown to effectively improve the platelet adhesion resistance of HFMs. For example, Wang et al. prepared a blood-compatible PES membrane by blending heparin-mimicking polyurethanes (containing carboxyl and sulfonic acid groups) with PES. It was shown that the number of adhered platelets on the modified surface was reduced by nearly 96% [226].
Albumin coating on the membrane surface can also inhibit platelet adhesion and activation as platelets do not have receptors that bind to albumin. Xie et al. decorated PSF membranes with bovine serum albumin. The platelet adhesion resistance of modified membrane was significantly improved by this modification [227]. As described previously, in a complex environment such as in blood, the adsorbed albumin can be replaced by other proteins with high affinity. Thus, the long-term effect of this modification needs to be further evaluated. Inspired by the phenomenon that platelets maintain a quiescent condition in blood vessels, endothelialization of membranes was also tested to inhibit platelet adhesion and activation [228, 229]. However, the current sterilization process could significantly restrict the application of endothelialized HFMs.
In blood vessels, the platelet activation is inhibited by NO, a molecule released by the endothelium. Thus, the incorporation of NO release modification was considered as another strategy to improve the platelet adhesion resistance on material surfaces. Modifying blood contact materials with NO-donating groups or catalytic systems were used to inhibit platelet activation [230, 231]. The strategies that might be used to inhibit platelet adhesion and activation on HFM surface are illustrated in Fig. 13.
Fig. 13.
Schematic illustration of different strategies for enhancing the platelet adhesion resistance and activation on HFMs
Most of the platelet activation and adhesion studies on HFM surface were performed by incubating the membranes in platelet-rich plasma (PRP) containing anticoagulants such as citrate or heparin. After a period of incubation, platelets were fixed on HFM surfaces and scanning electron microscopy was used to observe platelet adhesion and activation on the surface. To a certain extent, this method can reflect the platelet adhesion resistance of HFMs. However, there are other factors that need to be considered for studying platelet adhesion resistance by HFMs. Studies under relevant shear conditions of HFM operation should be included in these studies to generate realistic performance of platelet adhesion resistance of the membranes. As explained previously shear force can induce the denaturation of the adsorbed proteins and the shape change of the platelets. Such factors can lead to tighter binding between platelets and proteins [96]. It is also known that shear conditions can expose more binding sites on adsorbed vWF which can increase the adhesion and activation of platelets.
In addition to platelet activation and adhesion, the HFM surfaces can also induce immune response as well as thrombin generation. These can lead to further activation of platelets and other immune cells in circulating blood [101, 109]. Although these activated platelets may not be bound to the membrane surface, they can still release platelet microparticles or other molecules to enhance the amplification loop.
Material scientists tend to consider platelet adhesion first and then platelet activation. However, when platelets are activated, their ability to adhere to the surface is magnified several times due to the more exposed binding sites, as discussed in Sect. 3.2. Platelets have a large number of receptors that can be activated by various signals, especially those derived from immune activation or coagulation activation. It is worth noting that uremic patients who require hemodialysis are often in a state of hyperinflammation as described. Therefore, including studies using blood from these patients may be more effective in obtaining relevant results.
For platelet adhesion resistance analysis, PRP is used with blood-thinning agents (e.g., sodium citrate), thus the coagulation pathways are largely blocked. The removal of calcium from the blood by citrate can also inhibit many calcium-dependent enzymatic reactions in the blood. Thus, studies that mimic relevant conditions, such as recalcified PRP or recalcified PRP with minimal amount of heparin or other anticoagulants (enable experiments without unwanted clotting), should be considered to better understand platelet adhesion or activation resistance of HFMs.
Antithrombotic Modification Strategies for HFMs
The current strategies used for enhancing the antithrombotic property of blood-contacting materials can be divided into the following categories: (1) modifying the surfaces with heparin, heparin-mimicking polymers, and anticoagulants that inhibit specific coagulation activators such argatroban or hirudin (argatroban and hirudin can inhibit thrombin activity); (2) complexing calcium ions in blood to stop coagulation activation by the key enzymes that require calcium as a cofactor; (3) combining or inactivating blood factors to stop the coagulation pathway; and (4) modifying the surfaces with active proteins that can inhibit coagulation activation or enhance clot lysis [232].
Heparin coatings to improve the hemocompatibility of surfaces have been in clinical application for over 30 years. Heparin binds ATIII through an active pentasaccharide sequence and inhibits FXIa, FIXa, FXa and FIIa, thereby inhibiting blood clot formation [233]. According to clinical reports, the success rate of heparin-grafted membranes is far from the requirements for safe blood purification without systemic anticoagulation [44]. To circumvent these issues, many approaches are provided based on the knowledge that the anticoagulant function of heparin is due to its functional groups, such as sulfonic groups and carboxyl groups. For example, heparin-mimicking polymers with sulfonic acid and carboxyl groups were proposed [18, 79]. However, the anticoagulation mechanism of heparin-mimicking polymers with only sulfonic acid groups and carboxyl groups is not similar to that of heparin. These polymers cannot effectively bind ATIII to inhibit thrombin (FIIa) activity, thus reducing the potency.
Another strategy is endowing the membranes with the ability to sequester calcium ions from blood to achieve anticoagulant function. Calcium ions play indispensable roles in blood coagulation pathways. In clinical practice, regional citrate anticoagulation has been widely used and is effective in reducing the bleeding risk in patients [234]. Inspired by this, the introduction of negatively charged groups on HFMs to bind calcium ions has also become an option. Liu et al. modified PSA-AA hydrogel onto PSF membranes, and the modified membrane delayed the coagulation time in platelet-poor plasma (PPP). More importantly, successful heparin-free hemodialysis in animals was achieved using self-anticoagulation HFMs, although the duration was only one hour [20] (Fig. 14). However, this strategy has an important challenge, as the dialysis process continuously replenishes the calcium ions, and the modified membranes will reach adsorption saturation and lose their anticoagulation function with time resulting in clot formation. In addition, if the surface realizes its anticoagulant function only by complexing calcium ions, the coagulation time of the co-incubated plasma may not change significantly because the calcium ions will be continuously replenished during the run. Therefore, approaches that improve and provide long-acting calcium sequestration to achieve anticoagulant function still need to be explored.
Fig. 14.
Representation of a dialysis session using a self-anticoagulant dialyzer. a Schematic illustration of the anti-coagulant dialyzer via hydrogel skin modification. SEM micrographs of b inner surfaces and c outer surfaces of pristine dialyzer H-0 and self-anticoagulant dialyzer H-1. d Digital photos of the H-1 and H-0 shell sides and ports after a 1 h dialysis session. e SEM micrographs of hollow fiber inner surfaces after dialysis. Represented with permission from ref. [20]. Copyright (2020) Elsevier
Inhibiting the activity of blood factors is another strategy for endowing HFMs with anticoagulant properties. It has been reported that the half-life of blood factors in our body is short; thus, the consumed blood factors can be quickly replenished, and therefore, the patient can recover the balance of coagulation and anticoagulation [235]. A large number of studies have demonstrated that targeting blood FXII seems to be a safer option. Animal experiments have shown that it is possible to effectively avoid clot formation without the additional risk of bleeding by inhibiting FXII activity [192, 236]. For membrane-induced coagulation activation, FXII can be directly activated to trigger the intrinsic coagulation pathway. However, the TF released by HFM induced activation of immune cells first triggers the extrinsic coagulation pathway to generate thrombin, and thrombin can bypass FXII and directly activate FXI, thereby triggering the intrinsic coagulation pathway. Thus, the combination of inhibitors of these factors and HFMs is expected to reduce the bleeding risk in hemodialysis patients.
Some proteins in blood, such as protein C, TF pathway inhibitor, thrombomodulin, and tissue plasminogen activator (tPA), can prevent excessive coagulation reactions. Therefore, researchers have attempted to bind these proteins onto material surfaces to endow them with antithrombotic properties. Among these proteins, using tPA to activate the fibrinolysis system to dissolve thrombi is the most studied strategy [237, 238]. However, this strategy cannot effectively inhibit the generation of thrombin, which can still induce a series of side effects, such as immune activation, coagulation factor consumption, and platelet activation. Other surface modification strategies using anticoagulant proteins have also been investigated [239]; however, these proteins often cannot withstand routine sterilization methods used for HFMs. Therefore, this may not be an ideal strategy to impart antithrombotic properties to HFMs.
In hemodialysis, administered anticoagulants such as heparin remain in the patient’s body and continue to exert a blood-thinning effect even after treatment. This may increase the risk of bleeding in patients. Compared with systemic administration of anticoagulants, the use of self-antithrombotic HFMs can effectively prevent blood clotting on the membrane surface and avoid the use of anticoagulants. Although many studies on HFMs with self-anticoagulation function have been reported [208, 240], most of them are still far from clinical application.
To date, there is no clear definition of standard parameters that need to be achieved for the self-antithrombotic properties of HFMs. Many studies have reported on membrane preparations with self-anticoagulant/antithrombotic properties as these membranes can delay the coagulation time in plasma or in whole blood [241, 242]. However, blood coagulation is a complex process and is inseparable from the activation of the immune system and different blood cells. In addition, blood fluid dynamics, changes in blood cell composition, etc., could have a significant impact. Therefore, the conclusions drawn from performing only coagulation time measurements are sometimes unreliable. Thus, there is an urgent need to reach a consensus on how to evaluate the self-anticoagulation function of membranes in clinically relevant conditions.
In theory, the use of HFMs with self-anticoagulation properties can effectively reduce the bleeding risk in patients. Thus, to ensure that HFM can effectively achieve its self-anticoagulation function, many researchers hypothesized that membranes that can significantly extend the blood clotting time can function as self-anticoagulant membranes. For instance, some reports have shown that modified membranes can extend the APTT by more than 600 s (greater than the detection limit of this assay) [203]. Although, such a delay in clotting may be beneficial, it also poses a hidden danger of increasing the bleeding risk.
Negatively charged polymers have been widely used to increase the surface negative charge to improve the self-anticoagulant performance of HFMs. However, it is recognized that negatively charged surfaces can promote the contact activation of FXII [243]. For example, glass can activate coagulation [244]. Negatively charged heparin can also promote the activation of FXII [245]. Despite its ability to activate FXII, heparin functions as an anticoagulant by its ability to enhance the activity of ATIII. However, most negatively charged polymers cannot perform in this fashion [18]. PolyP and nucleic acids with multiple negative charges are also known activators of coagulation [243]. Therefore, it is necessary to explore the anticoagulant mechanism of HFM modified with negatively charged polymers, as it might pose a thrombotic risk in patients.
Modifications which Suppress the Immune Activation by HFMs
The pathways in which the membrane induces immune activation can be divided into the following categories. The immune response triggered by complement activation and immune cell activation and the immune activation triggered by blood coagulation (e.g., contact activation) pathways. Uremic patients themselves are in a state of immune activation due to the membrane-induced activation during the hemodialysis. The enhancement of the anti-inflammatory property of HFMs can also be realized via multiple approaches as illustrated in Fig. 15.
Fig. 15.
Schematic illustration of different strategies for endowing HFMs with resistance to immune activation
The immune activation induced by contact activation can be inhibited by preventing the activation of FXII on membrane surface. Negatively charged surfaces are more likely to activate FXII. Hydrophilic surfaces are known to inhibit the contact activation of FXII. However, recent evidence has shown that hydrophilic surfaces can activate FXII by collisional activation [246]. In contrast, some hydrophobic surfaces can also inhibit the contact activation on material surface due to the competitive effect. The influence of surface properties of HFMs on contact activation needs to be explored further. Similarly, reducing the coagulation activation induced by the membrane, in general, can also reduce immune activation. For example, grafting heparin onto the membrane surface can effectively reduce the immune activation triggered by HFMs [101].
To reduce complement activation induced by the HFM surface, the following principles are recommended for designing HFMs. First, the surface of the membrane should have only a limited number of hydroxyl or primary amine groups as these groups can form covalent bonds with complement proteins (e.g., C3b), which could lead to complement activation by the alternate pathway. Second, surfaces with good resistance to protein fouling are recommended; these surfaces can effectively reduce the adhesion of complement proteins thereby preventing the activation of the complement system. However, this principle may not be generalizable. It is worth noting that highly protein-resistant hydrophilic surfaces can trigger the activation of complement proteins [247]. In contrast, some relatively hydrophobic surfaces have less complement activation. This is mainly due to the protein adsorption competition and the adsorption of complement inhibitory proteins. In addition, proactively recruiting inhibitory proteins such as factor H and C1INH on material surfaces can also inhibit complement activation [248]. By conjugating the 5C6 peptide, a molecule that has strong binding affinity toward factor H without modifying its inhibitory activity, is shown to be effective [249].
By inhibiting the immune factors generated by the uremic patients themselves or due to hemodialysis treatment, immune activation can be prevented from being aggravated. For example, vitamin E-coated membranes are currently the most mature HFM that can suppress immune activation [250]. The use of vitamin E-coated membranes can decrease oxidative stress and improve inflammatory status and anemia but cannot suppress complement activation [251]. In addition, immobilization of the superoxide dismutase/catalase enzyme combination, tannic acid, resveratrol, alpha-tocopherol and alpha-lipoic acid are used to generate antioxidative surfaces [252–257].
In the process of pursuing HFMs with self-anticoagulant properties, the immune activation is particularly worthy of attention. Since the anticoagulant function of HFMs may not be as strong as that of systemic anticoagulants, there is a greater risk of coagulation during hemodialysis due to immune activation, which can result in thrombosis.
Many studies have explored the phenomenon of immune activation on the surface of nanoparticles [168]. Due to the difficulty in characterization, the investigation of immune activation by HFMs has not been systematically studied but we can still learn from the experience of nanoparticles. In terms of complement activation, simply by improving the material’s protein fouling resistance, we may not be able to lower complement activation by HFMs. In contrast, there is evidence that the graft density, molecular weight, and even molecular weight pairing of the same modifier can have large influences on complement activation levels [247, 258]. Similarly, in terms of contact activation, the curvature of the material can even force the FXII to appear in two forms: lying down or standing [259, 260]. It can be argued that modification with different polymers and the architecture of the polymer on the HFM surface, can lead to greater differences in immune activation. These thoughts need to be systematically explored in the future. The rapid development of computer simulations may provide convenient avenues for future exploration.
Although many studies have explored the adsorption and denaturing of immune-related proteins on material surfaces, it is worth noting that studying single proteins may lead to incorrect interpretation. This is mainly because many immune-related proteins exist in the form of complexes or act in a complex manner. For the material surface, the adsorption of immune-related proteins may lead to the activation or adsorption of other proteins [168]. For example, the adsorption of immunoglobulins can determine the complement opsonization efficiency of materials [261]. These complex situations also pose challenges for future mechanism exploration.
Current State of the Art on Laboratory-based Hemocompatibility Modifications on HFMs
The reported hemocompatibility modification methods for HFMs are summarized in Table 2. As shown in Table 2 (in order of publication date), a large number of surface modification methods are applied on diverse substrates and membranes. These modified membranes can minimize protein fouling and inhibit coagulation, complement activation, and platelet adhesion and activation to a certain extent. As shown in Table 2, in addition to exploring the antifouling property of the membrane, the most explored aspect is the effect of membrane on coagulation which indicates that improving the anticoagulant property of membrane is one of the current research focuses [20, 23]. There are only a handful of reports on the influence of HFMs on the activation of immune system including the activation of the immune cells [262]. Although this information is very important in the design of improved hemocompatible HFMs, it is clearly overlooked. Further research is needed in this direction to improve the performance of HFMs. Another important information gained from the summary is that a large number of HFM modification methods reported are not suitable for HFMs or large-scale production. This points to the challenges in the adoption of laboratory research into industrial manufacturing and clinical practice. Finally, it is worth noting that the interaction of HFMs with blood is multifaceted but the currently used methods to characterize such interactions are relatively incomplete. This can affect the quality of the data generated and the assessment of the hemocompatibility of HFMs. Therefore, it is necessary to adopt more comprehensive, holistic and specific characterization methods to assess the hemocompatibility of HFMs.
Table 2.
State of the art on laboratory hemodialysis membranes
| Substrate | Modification method | Functional modifier | Protein Adhesion | Platelet adhesion/activation | Immune activation | Clotting time | Reference |
|---|---|---|---|---|---|---|---|
| PES | Bulk modification | Ionic liquid | BSA adsorption: 52% decreased | Platelet adhesion:95% decrease calculated from SEM picture | No significant change | [263] | |
| PES | Surface modification | PEtO | BSA adsorption: 56% decreased | Low platelet adhesion as indicated by SEM | No significant change | [254] | |
| PES | Surface modification | Tannic acid | Reduced BSA adsorption | Low platelet adhesion as indicated by SEM | Increased radical scavenging capacity | Prolonged clotting times and reduced blood coagulation index | [253] |
| PSF | Blending modification | Resveratrol | Reduced BSA adsorption | Low platelet adhesion as indicated by SEM | Increased radical scavenging capacity, and reduced C3a and C5a generation | – | [256] |
| PES | Surface modification | Chitosan and heparin | Reduced BSA adsorption as indicated by fluorescence microscope | Reduced PF4 concentration | Reduced C3a and C5a generation | Reduced TAT generation | [264] |
| PES | Blending modification | Ticagrelor | Reduced platelet adhesion and P-selectin expression | Reduced IL-6, THF-α, IL-10 generation | No significant change | [265] | |
| PSF | Surface modification | Sodium alginate, acrylic acid | Low protein adhesion as indicated by SEM |
Low platelet adhesion as indicated by SEM, PF4: 70% decrease |
C3a: 70% decreased, C5a: 75% decreased |
APTT: 775% prolonged, TT: 94.4% prolonged, TAT: 50% decreased |
[20] |
| PAN | Surface modification | Heparin-poly(vinyl alcohol) hydrogel |
Bovine serum albumin (BSA) adsorption: 98% decreased, Flux recovery: 21% increased |
– | – |
APTT: 225% prolonged, PT: 233% prolonged, TT: 200% prolonged |
[22] |
| PES | Blending/surface modification | PEGMA |
BSA adsorption: 35% decreased, Flux recovery: 20% increased |
Platelet adhesion: 50% decrease calculated from SEM picture | – | – | [266] |
| PAN | Surface modification | Heparin functionalized MWCNTs/PVA | BSA adsorption: 55% decreased | Platelet adhesion: 80% decrease calculated from SEM picture |
C3a: 15% decreased, C5a: 20% decreased |
Plasma recalcification time: 123% prolonged | [267] |
| PES | Bulk/blending modification | Amphiphilic zwitterionic polymer |
BSA adsorption: 85% decreased, Bovine fibrinogen (BFG) adsorption: 87.5% decreased, Flux recovery: 16% increased |
Low platelet adhesion as indicated by SEM |
C3a: 5% decreased, C5a: 5% decreased |
Plasma recalcification time: 36% prolonged | [268] |
| PES | Blending/surface modification | PAA-AMPS |
BSA adsorption: 55% decreased, BFG adsorption: 70% decreased, whole blood protein adsorption: 50% decreased, Flux recovery: 7% increased |
Platelet adhesion: 80% decrease calculated from SEM picture |
C3a: 10% decreased, C5a: 5% decreased, BK: 10% decreased |
APTT: 157% prolonged, TT: 584% prolonged |
[79] |
| Polyvinylidene Fluoride (PVDF) | Blending modification | Poly(ethylene glycol)-multi-walled carbon nanotubes |
BSA adsorption: 80% decreased, Flux recovery: 20% increased |
– | – | – | [269] |
| PSF | Surface modification | Poly AA-HEMA | Flux recovery: 116% increased | – | – | APTT: 33% prolonged | [241] |
| PSF | Blending modification | P(VP-VTES)-b-P(SSNa-VTES-AA) | – | Low platelet adhesion as indicated by SEM |
C3a: 25% decreased, C5a: 50% decreased |
APTT: 1900% prolonged, PT: 100% prolonged, TT: 80% prolonged |
[203] |
| PES | Surface modification | Sulfonated sodium alginate |
BSA adsorption: 33% decreased, BFG adsorption: 50% decreased, |
Platelet adhesion: 90% decrease calculated from SEM picture | – |
APTT: 30% prolonged, PT: 10% prolonged, TT: 20% prolonged |
[270] |
| PES | Bulk/surface modification | Sulphated carboxymethylcellulose |
BSA adsorption: 70% decreased, Flux recovery: 150% increased |
Low platelet adhesion as indicated by SEM | – | – | [271] |
| PAN | Surface modification | Branched polyethylenimine | – | Platelet adhesion: 45% decrease calculated from SEM picture | – | Plasma recalcification time: 60% prolonged | [272] |
| PES | Blending modification | GO | Flux recovery: 30% increased | Low platelet adhesion as indicated by SEM | – |
APTT: 25% prolonged, PT: 10% prolonged, TT: 10% prolonged |
[273] |
| PES | Blending/surface modification | P(AA-co-HEMA)/PSSNa |
BSA adsorption: 66% decreased, BFG adsorption: 50% decreased, Flux recovery: 20% increased |
Platelet adhesion: 98% decrease calculated from SEM picture, PF4: 20% decrease |
C3a: 45% decreased, C5a: 10% decreased |
APTT: 125% prolonged, TT: 66% prolonged |
[274] |
| PES | Bulk modification | Heparin-like PES |
BSA adsorption: 60% increased, BFG adsorption: 65% decreased |
PF4: 10% decrease |
C3a: 16% decreased, C5a: 33% decreased |
APTT: 225% prolonged, TT: 40% prolonged |
[100] |
| PSF | Surface modification | Sulfonated hydroxypropyl chitosan | BSA adsorption: 85% decreased | Low platelet adhesion as indicated by SEM | – |
Whole blood clotting time: 190% prolonged, APTT: 10% prolonged, TT: 100% prolonged |
[275] |
| PES | Blending modification | Heparin-mimicking polyurethanes |
BSA adsorption: 50% increased, BFG adsorption: 40% decreased |
Platelet adhesion: 95% decrease calculated from SEM picture |
C3a: 45% decreased, C5a: 15% decreased |
APTT: 162% prolonged, TT: No change |
[226] |
| PSF | Blending modification | Poly(vinyl pyrrolidone-co-vinyltriethoxysilane |
BSA adsorption: 60% decreased, Flux recovery: 50% increased |
Low platelet adhesion as indicated by SEM | – |
APTT: 30% prolonged, PT: 20% prolonged |
[276] |
| PAN | Surface modification | Polyvinyl alcohol | BSA adsorption: 60% decreased | Low platelet adhesion as indicated by SEM |
C3a: 5% decreased, C5a: 20% decreased |
– | [277] |
Conclusions and Outlook
Great progress has been made in altering the surface properties of HFMs to improve their hemocompatibility. HFMs have evolved from the original cellulose-based membranes to the current high-flux synthetic polymer-based membranes. HFMs have been further modified to improve their hemocompatibility (e.g., heparin-coated and vitamin E-coated HFMs) and are currently used in clinics. Many modification methods have also emerged to improve the hemocompatibility of HFMs, and some of these methods are simple and efficient for HFM modification. These emerging new methods undoubtedly provide convenient ways to further improve the hemocompatibility of HFM.
Recent developments in surface modification approaches and self-anticoagulant materials have demonstrated significant improvements in the antifouling and antithrombotic performances of HFMs. These modified membranes are expected to play an important role in heparin-free hemodialysis in the future, which may reduce the bleeding risk. Importantly, the demonstration of such heparin-free hemodialysis using self-anticoagulation HFMs has been reported recently using animal models. Although the duration of such experiments is only one hour, it undoubtedly demonstrates the outlook of such technologies and the future heparin-free hemodialysis model.
Although great progress has been achieved in improving the antifouling and antithrombotic properties of HFMs, these evaluations also need to be further combined with assays that mimic clinical conditions. For instance, the inclusion of flow and shear conditions in the analysis should be important. In addition, the analysis and approaches to prevent immune activation by HFMs are also important, as uremic patients are in a state of hyperinflammation, which is possibly caused by exposure to HFMs. Many of the developed modification techniques for HFMs are demonstrated on flat sheet membranes, however, these methods may not be suitable for hollow fibrous membranes due to the complexity of dialyzer manufacturing. Thus, new methods that are suitable for use with hollow fibrous membrane dialyzers should be developed.
In addition, the mechanistic understanding of blood interactions with the surface of HFMs is not deep enough currently. This has further limited our ability to develop novel modification strategies to improve the hemocompatibility of HFMs. Many modification strategies are available; however, due to the lack of deeper understanding of the blood interaction with HFMs, it is difficult to adopt such methods to improve the performance of HFMs. For example, a large number of convenient chemical tools have been developed for surface modification of HFMs; however, there are not enough data available currently on how to control the adsorption behavior of proteins on HFM surfaces and how the protein adsorption behavior relates to the surface properties.
To obtain these advanced guidelines, interdisciplinary collaboration will be essential. For example, developing techniques of gene editing and recombinant protein technology are time-consuming for material-based groups, but these techniques are essential for exploring the function of proteins on the material's surface; thus, collaboration is mandatory. The exploration of the development of guidelines on hemocompatibility is challenging for material-based groups, including the requirement for significant R&D funds, low near-term rewards, the risk of disclosing research results, and the difficulty of establishing links with expert groups. Thus, materials scientists must step out of their comfort zones to make this happen. Other future perspectives include collaboration with the hematology group to develop practical methods for blood interaction characterization methods and investigating the impact of materials on the blood system. Work with immunology experts to develop practical strategies for immune-response characterization and investigation of the impact of materials on the immune system. Further collaboration with pathology experts to develop practical animal models of renal failure, etc.
To gain a deeper understanding of the interaction between blood and HFMs, more comprehensive and professional characterization methods that mimic clinical conditions are necessary. The common methods currently used to characterize the hemocompatibility of HFMs are not sufficient to allow us to conduct a targeted analysis of the interaction between the blood and the HFMs. Therefore, a systematic hemocompatibility evaluation process and holistic process urgently need to be established.
In addition, HFMs can also be used in the treatment of other diseases, but these applications put forward higher requirements for the hemocompatibility of HFMs. Taking the treatment of sepsis as an example, HFMs can bind endotoxins through electrostatic interactions, but such HFMs tend to have severe protein adsorption and platelet activation. It is also worth noting that some advanced treatment models have emerged for efficient and convenient blood purification, such as microfluidic hemodialysis, artificial nephron filters, and active dialyzers. In addition, the application of other adjunctive blood contact materials, such as self-anticoagulant materials instead of direct administration of heparin, can potentially reduce the bleeding risk suffered by patients and will be a future investigational area of interest.
Acknowledgements
This work was financially sponsored by the National Natural Science Foundation of China (No. 51773127, 51873115 and 52073190). HF acknowledges the financial support of the China Scholarship Council (CSC) for the visiting study at the University of British Columbia. JNK acknowledges the funding from Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council (NSERC) of Canada and Canada Foundation for Innovation for funding. JNK was a receipt of a Michael Smith Foundation for Health Research Scholar award. JNK is a Tier 1 Canada Research Chair. JNK also acknowledges previous trainees who contributed to blood materials research at the University of British Columbia.
Biographies
Haifeng Ji
completed his bachelor’s and doctoral degrees from Sichuan University in 2016 and 2022, respectively, and now serves as a postdoctoral fellow at the University of British Columbia. Dr. Ji’s research is primarily concerned with exploring changes in protein formation induced by surfaces, alongside the creation of associated detection tools, and guiding innovative advancements in hemocompatible materials.
Dr. Yupei Li
is currently a resident physician in the Department of Nephrology, at West China Hospital of Sichuan University. He received his Master’s degree in Nephrology and PhD degree in Emergency Medicine from Sichuan University in 2019 and 2022, respectively. His research interests mainly include the R&D evaluation of blood-contacting biomaterials, anticoagulation strategies for extracorporeal circuits, and dialysis membrane innovation.
Baihai Su
is currently a full professor in the Department of Nephrology, at West China Hospital of Sichuan University. He received his MBBS and MMed degrees from West China School of Medicine, Sichuan University in 1998 and 2005, respectively. Then, he obtained his PhD degree in biomedical engineering from the College of Polymer Science and Engineering, Sichuan University in 2012. His research interests encompass the R&D of blood-contacting biomaterials, acute kidney injury and chronic hemodialysis.
Weifeng Zhao
received his BS degree in Polymer Science & Engineering in 2009 and then obtained his PhD degree in Biomedical Engineering in 2014 from Sichuan University. He did another PhD study at KTH Royal Institute of Technology from 2012 to 2015 and received that degree in 2015. Subsequently, Dr Zhao joined the College of Polymer Science and Engineering, Sichuan University as an Associate Professor in 2016, and was promoted to Full Professor in 2020. Dr Zhao’s research interests are mostly focused on the development of bio- and blood-compatible polymers and functional hydrogels and he is also dedicated to the design of hemoperfusion adsorbents.
Dr. Kizhakkedathu
is a Professor and Tier 1 Canada Research Chair in the Department of Pathology and Laboratory Medicine and a Principal Investigator at the Centre for Blood Research (CBR) at the University of British Columbia (UBC), Canada. Dr. Kizhakkedathu completed PhD in 2000 from Indian Institute of Chemical Technology, India, and completed a postdoctoral fellowship from 2000–2005. Dr. Kizhakkedathu joined UBC as an Assistant Professor in 2005 and rose through the ranks. He is leading a cutting-edge translational research program at CBR to innovate materials and therapeutics that can treat and prevent immunothrombosis and infection, immunological rejection of organs and cells, protection of tissues and organs in inflammatory conditions and blood-compatible surfaces.
Changsheng Zhao
received his BS degree in Polymer Materials from Sichuan University in 1991 and then obtained his PhD degree in Biomedical Engineering from Sichuan University in 1998. From 2001 to 2003, he did research work at Hokkaido University (Japan) as a postdoctoral research fellow. Subsequently, he joined Sichuan University and became a professor in 2004. Prof. Zhao’s research interests are mostly focused on the development of hemodialysis materials with bioactive and anticoagulant properties, he is also dedicated to the design of heparin-inspired hydrogels and composite biomaterials for tissue engineering and regeneration medicine.
Declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haifeng Ji and Yupei Li are contributed equally to this work.
Contributor Information
Weifeng Zhao, Email: zhaoscukth@163.com.
Jayachandran N. Kizhakkedathu, Email: jay@pathology.ubc.ca
Changsheng Zhao, Email: zhaochsh70@163.com.
References
- 1.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen XY, Gipson D, Gu HY, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li YT, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin MG, Zhang XS, Shahinian V. Us renal data system annual data report: epidemiology of kidney disease in the united states. Am J Kidney Dis. 2020 doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Yang C, Yang Z, Wang J, Wang H-Y, Su Z, Chen R, Sun X, Gao B, Wang F, Zhang L, Jiang B, Zhao M-H. China kidney disease network work g estimation of prevalence of kidney disease treated with dialysis in china: a study of insurance claims data. Am J Kidney Dis. 2021;77:889. doi: 10.1053/j.ajkd.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, Jindal K, Salako BL, Rateb A, Osman MA, Qarni B, Saad S, Lunney M, Wiebe N, Johnson DW. Assessment of global kidney health care status. JAMA-J Am Med Assoc. 1864;2017:317. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo KT, Choong HL, Wong KS, Tan HB, Chan CM. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2012;81:1044. doi: 10.1038/ki.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv JC, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 1975;2015:385. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 6.Ronco C, Clark WR. Haemodialysis membranes. Nat Rev Nephrol. 2018;14:394. doi: 10.1038/s41581-018-0002-x. [DOI] [PubMed] [Google Scholar]
- 7.Clark WR, Hamburger RJ, Lysaght MJ. Effect of membrane composition and structure on solute removal and biocompatibility in hemodialysis. Kidney Int. 2005;1999:56. doi: 10.1046/j.1523-1755.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- 8.Lysaght MJ. Evolution of hemodialysis membranes. In: Bonomini V, Berland Y, editors. Dialysis membranes: Structure and predictions Contributions to nephrology. Basel: S Karger AG; 1995. p. 1. [DOI] [PubMed] [Google Scholar]
- 9.Craddock PR, Fehr J, Dalmasso AP, Brigham KL, Jacob HS. Hemodialysis leukopenia-pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977;59:879. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakim RM, Fearon DT, Lazarus JM. Biocompatibility of dialysis membranes-effects of chronic complement activation. Kidney Int. 1984;26:194. doi: 10.1038/ki.1984.155. [DOI] [PubMed] [Google Scholar]
- 11.Kreusser W, Reiermann S, Vogelbusch G, Bartual J, Schulze-Lohoff E. Effect of different synthetic membranes on laboratory parameters and survival in chronic haemodialysis patients. NDT Plus. 2010;3:i12. doi: 10.1093/ndtplus/sfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irfan M, Idris A. Overview of pes biocompatible/hemodialysis membranes: Pes-blood interactions and modification techniques. Mater Sci Eng C-Mater Biol Appl. 2015;56:574. doi: 10.1016/j.msec.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Ward RA, Ouseph R, McLeish KR. Effects of high-flux hemodialysis on oxidant stress. Kidney Int. 2003;63:353. doi: 10.1046/j.1523-1755.2003.00741.x. [DOI] [PubMed] [Google Scholar]
- 14.Tielemans C, Madhoun P, Lenaers M, Schandene L, Goldman M, Vanherweghem JL. Anaphylactoid reactions during hemodialysis on an69 membranes in patients receiving ace inhibitors. Kidney Int. 1990;38:982. doi: 10.1038/ki.1990.301. [DOI] [PubMed] [Google Scholar]
- 15.Kohlova M, Amorim CG, Araujo A, Santos-Silva A, Solich P, Montenegro M. The biocompatibility and bioactivity of hemodialysis membranes: Their impact in end-stage renal disease. J Artif Organs. 2019;22:14. doi: 10.1007/s10047-018-1059-9. [DOI] [PubMed] [Google Scholar]
- 16.Liakopoulos V, Roumeliotis S, Zarogiannis S, Eleftheriadis T, Mertens PR. Oxidative stress in hemodialysis: causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial. 2019;32:58. doi: 10.1111/sdi.12745. [DOI] [PubMed] [Google Scholar]
- 17.Claudel SE, Miles LA, Murea M. Anticoagulation in hemodialysis: a narrative review. Semin Dial. 2020;34:103. doi: 10.1111/sdi.12932. [DOI] [PubMed] [Google Scholar]
- 18.Song X, Ji HF, Li YP, Xiong YQ, Qiu L, Zhong R, Tian M, Kizhakkedathu JN, Su BH, Wei Q, Zhao WF, Zhao CS. Transient blood thinning during extracorporeal blood purification via the inactivation of coagulation factors by hydrogel microspheres. Nat Biomed Eng. 2021;5:1143. doi: 10.1038/s41551-020-00673-x. [DOI] [PubMed] [Google Scholar]
- 19.Mollahosseini A, Abdelrasoul A, Shoker A. A critical review of recent advances in hemodialysis membranes hemocompatibility and guidelines for future development. Mater Chem Phys. 2020;248:122911. doi: 10.1016/j.matchemphys.2020.122911. [DOI] [Google Scholar]
- 20.Liu Y, Li GL, Han Q, Lin HB, Li Q, Hua J, Liu F. Anticoagulant dialyzer with enhanced ca2+ chelation and hydrophilicity for heparin free hemodialysis. J Membr Sci. 2020;604:118082. doi: 10.1016/j.memsci.2020.118082. [DOI] [Google Scholar]
- 21.Hedayati M, Neufeld MJ, Reynolds MM, Kipper MJ. The quest for blood-compatible materials: Recent advances and future technologies. Mater Sci Eng R-Rep. 2019;138:118. doi: 10.1016/j.mser.2019.06.002. [DOI] [Google Scholar]
- 22.Yu XF, Zhu YD, Zhang TH, Deng L, Li PY, Wang XF, Hsiao BS. Heparinized thin-film composite membranes with sub-micron ridge structure for efficient hemodialysis. J Membr Sci. 2020;599:117706. doi: 10.1016/j.memsci.2019.117706. [DOI] [Google Scholar]
- 23.Jaffer IH, Weitz JI. The blood compatibility challenge. Part 1: Blood-contacting medical devices: the scope of the problem. Acta Biomater. 2019;94:2. doi: 10.1016/j.actbio.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Ronco C, Ghezzi PM, Bowry SK. Membranes for hemodialysis. In: Hörl WH, Koch KM, Lindsay RM, Ronco C, Winchester JF, editors. Replacement of renal function by dialysis. Netherlands, Dordrecht: Springer; 2004. p. 301. [Google Scholar]
- 25.Sunohara T, Masuda T. Cellulose triacetate as a high-performance membrane. In: Saito A, Kawanishi H, Yamashita AC, Mineshima M, editors. High-performance membrane dialyzers. Karger, Basel: Contributions to nephrology; 2011. p. 156. [DOI] [PubMed] [Google Scholar]
- 26.Bowry SK, Gatti E, Vienken J. Contribution of polysulfone membranes to the success of convective dialysis therapies. In: Saito A, Kawanishi H, Yamashita AC, Mineshima M, editors. High-performance membrane dialyzers. Karger, Basel: Contributions to nephrology; 2011. p. 110. [DOI] [PubMed] [Google Scholar]
- 27.Thomas M, Moriyama K, Ledebo I. An69: Evolution of the world's first high permeability membrane. In: Saito A, Kawanishi H, Yamashita AC, Mineshima M, editors. High-performance membrane dialyzers. Karger, Basel: Contributions to nephrology; 2011. p. 119. [DOI] [PubMed] [Google Scholar]
- 28.Sakai Y. Polymethylmethacrylate membrane with a series of serendipity. 2011 In: Saito A, Kawanishi H, Yamashita AC, Mineshima M, (eds). High-performance membrane dialyzers. Contributions to nephrology,. pp. 137. [DOI] [PubMed]
- 29.Li Y, Sun P, Chang K, Yang M, Deng N, Chen S, Su B. Effect of continuous renal replacement therapy with the oxiris hemofilter on critically ill patients: a narrative review. J Clin Med. 2022;11:6719. doi: 10.3390/jcm11226719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieter DH, Lemke HD. Polyethersulfone as a high-performance membrane. In: Saito A, Kawanishi H, Yamashita AC, Mineshima M, editors. High-performance membrane dialyzers. Karger, Basel: Contributions to nephrology; 2011. p. 130. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Yang T, Li Y, Li J, Yang Q, Wang L, Jiang L, Su B. Effects of expanded hemodialysis with medium cut-off membranes on maintenance hemodialysis patients: a review. Membranes. 2022;12:253. doi: 10.3390/membranes12030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzin A, Golino G, de Cal M, Pajarin G, Savastano S, Lupi A, Sandini A, Fiorin F, Ronco C. Flow dynamic analysis by contrast-enhanced imaging techniques of medium cutoff membrane hemodialyzer. Blood Purif. 2022;51:138. doi: 10.1159/000516411. [DOI] [PubMed] [Google Scholar]
- 33.Jonny J, Teressa M. Expanded hemodialysis: a new concept of renal replacement therapy. J invest med. 2023;71:38. doi: 10.1136/jim-2022-002431. [DOI] [PubMed] [Google Scholar]
- 34.Hung YH, Lai TS, Belmouaz M, Tu YC, Lai CF, Lin SL, Chen YM. Effects of medium cut-off polyarylethersulfone and polyvinylpyrrolidone blend membrane dialyzers in hemodialysis patients: a systematic review and meta-analysis of randomized controlled trials. Membranes. 2022;12:443. doi: 10.3390/membranes12050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner DE, Falzon L, Skoufos L, Bernardo A, Beck W, Xiao M, Tran H. Efficacy and safety of expanded hemodialysis with the theranova 400 dialyzer: a randomized controlled trial. Clin J Am Soc Nephrol: CJASN. 2020;15:1310. doi: 10.2215/CJN.01210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YG, Lee SH, Jung SW, Jung GT, Lim HJ, Kim KP, Jo YI, Jin K, Moon JY. The medium cut-off membrane does not lower protein-bound uremic toxins. Toxins. 2022;14:779. doi: 10.3390/toxins14110779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Renal Nutr. 2012;22:149. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davenport A. What are the anticoagulation options for intermittent hemodialysis? Nat Rev Nephrol. 2011;7:499. doi: 10.1038/nrneph.2011.88. [DOI] [PubMed] [Google Scholar]
- 39.Ouseph R, Ward RA. Anticoagulation for intermittent hemodialysis. Semin Dial. 2000;13:181. doi: 10.1046/j.1525-139X.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 40.Fischer KG. Essentials of anticoagulation in hemodialysis. Hemodial Int. 2007;11:178. doi: 10.1111/j.1542-4758.2007.00166.x. [DOI] [PubMed] [Google Scholar]
- 41.Davenport A. Anticoagulation for continuous renal replacement therapy. In: Ronco C, Bellomo R, Brendolan A, editors. Sepsis, kidney and multiple organ dysfunction. Karger, Basel: Contributions to nephrology; 2004. p. 228. [Google Scholar]
- 42.Wright MJ, Woodrow G, Umpleby S, Hull S, Brownjohn AM, Turney JH. Low thrombogenicity of polyethylene glycol-grafted cellulose membranes does-not influence heparin requirements in hemodialysis. Am J Kidney Dis. 1999;34:36. doi: 10.1016/S0272-6386(99)70105-8. [DOI] [PubMed] [Google Scholar]
- 43.Chanard J, Lavaud S, Paris B, Toure F, Rieu P, Renaux JL, Thomas M. Assessment of heparin binding to the an69 st hemodialysis membrane: I. Preclin stud Asaio J. 2005;51:342. doi: 10.1097/01.mat.0000169119.06419.ed. [DOI] [PubMed] [Google Scholar]
- 44.Laville M, Dorval M, Ros JF, Fay R, Cridlig J, Nortier JL, Juillard L, Debska-Slizien A, Lorente LF, Thibaudin D, Franssen C, Schulz M, Moureau F, Loughraieb N, Rossignol P. Results of the hepzero study comparing heparin-grafted membrane and standard care show that heparin-grafted dialyzer is safe and easy to use for heparin-free dialysis. Kidney Int. 2014;86:1260. doi: 10.1038/ki.2014.225. [DOI] [PubMed] [Google Scholar]
- 45.Skagerlind MSE, Stegmayr BG. An evaluation of four modes of low-dose anticoagulation during intermittent haemodialysis. Eur J Clin Pharmacol. 2018;74:267. doi: 10.1007/s00228-017-2389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evenepoel P, Dejagere T, Verhamme P, Claes K, Kuypers D, Bammens B, Vanrenterghem Y. Heparin-coated polyacrylonitrile membrane versus regional citrate anticoagulation: a prospective randomized study of 2 anticoagulation strategies in patients at risk of bleeding. Am J Kidney Dis. 2007;49:642. doi: 10.1053/j.ajkd.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 47.François K, De Clerck D, Tonnelier A, Cambier ML, Orlando C, Jochmans K, Cools W, Wissing KM. Dialyzer performance during hemodialysis without systemic anticoagulation using a heparin-grafted dialyzer combined with a citrate-enriched dialysate: results of the randomized crossover noninferiority evocit study. Am J Kidney Dis. 2022;79:79. doi: 10.1053/j.ajkd.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Sosa MA, Balk EM, Lau J, Liangos O, Balakrishnan VS, Madias NE, Pereira BJG, Jaber BL. A systematic review of the effect of the excebrane dialyser on biomarkers of lipid peroxidation. Nephrol Dial Transplant. 2006;21:2825. doi: 10.1093/ndt/gfl376. [DOI] [PubMed] [Google Scholar]
- 49.Giardini O, Tacconegallucci M, Lubrano R, Ricciarditenore G, Bandino D, Silvi I, Ruberto U, Casciani CU. Evidence of red-blood-cell membrane lipid-peroxidation in hemodialysis-patients. Nephron. 1984;36:235. doi: 10.1159/000183160. [DOI] [PubMed] [Google Scholar]
- 50.Claster S, Chiu DTY, Quintanilha A, Lubin B. Neutrophils mediate lipid-peroxidation in human red-cells. Blood. 1984;64:1079. doi: 10.1182/blood.V64.5.1079.1079. [DOI] [PubMed] [Google Scholar]
- 51.Yang CC, Hsu SP, Wu MS, Hsu SM, Chien CT. Effects of vitamin c infusion and vitamin e-coated membrane on hemodialysis-induced oxidative stress. Kidney Int. 2006;69:706. doi: 10.1038/sj.ki.5000109. [DOI] [PubMed] [Google Scholar]
- 52.Kirmizis D, Papagianni A, Belechri AM, Memmos D. Effects of vitamin e-coated membrane dialyser on markers of oxidative stress and inflammation in patients on chronic haemodialysis. Nephrol Dial Transplant. 2011;26:2296. doi: 10.1093/ndt/gfq715. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Ribera L, Corredor Z, Silva I, Diaz JM, Ballarin J, Marcos R, Pastor S, Coll E. Vitamin e-coated dialysis membranes reduce the levels of oxidative genetic damage in hemodialysis patients. Mutat Res Genet Toxicol Environ Mutagen. 2017;815:16. doi: 10.1016/j.mrgentox.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Gao W, Li X. Vitamin e-coated dialyzer alleviates erythrocyte deformability dysfunction in patients with end-stage renal disease undergoing hemodialysis. Exp Ther Med. 2022;24:480. doi: 10.3892/etm.2022.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang SK, Xiao L, Xu B, Xu XX, Liu FY, Sun L. Effects of vitamin e-coated dialyzer on oxidative stress and inflammation status in hemodialysis patients: A systematic review and meta-analysis. Ren Fail. 2014;36:722. doi: 10.3109/0886022X.2014.890858. [DOI] [PubMed] [Google Scholar]
- 56.Hakim RM, Breillatt J, Lazarus JM, Port FK. Complement activation and hypersensitivity reactions to dialysis membranes. N Engl J Med. 1984;311:878. doi: 10.1056/NEJM198410043111403. [DOI] [PubMed] [Google Scholar]
- 57.Kabanda A, Jadoul M, Pochet JM, Lauwerys R, Destrihou CV, Bernard A. Determinants of the serum concentrations of low-molecular-weight proteins in patients on maintenance hemodialysis. Kidney Int. 1994;45:1689. doi: 10.1038/ki.1994.221. [DOI] [PubMed] [Google Scholar]
- 58.Vanholder RC, Ringoir SM. Intradialytic body-weight changes and dialyzer pore-size as main contributing factors to the evolution of beta-2-microglobulin in dialysis. Blood Purif. 1990;8:32. doi: 10.1159/000169922. [DOI] [PubMed] [Google Scholar]
- 59.Kalocheretis P, Vlamis I, Belesi C, Makriniotou I, Zerbala S, Savidou E, Zorbas S, Arvanitis N, Iatrou C. Residual blood loss in single use dialyzers: Effect of different membranes and flux. Int J Artif Organs. 2006;29:286. doi: 10.1177/039139880602900306. [DOI] [PubMed] [Google Scholar]
- 60.Liu S, Shi W, Liang X, Su Y, Liang Y, Zhang B, Ye Z, Xu L, Xia Y, He C, Ma J, Tao Y, Ye H, Li H. Cellulose triacetate dialyzer reduces platelet loss during continuous veno-venous hemofiltration. Blood Purif. 2010;29:375. doi: 10.1159/000314649. [DOI] [PubMed] [Google Scholar]
- 61.Grooteman MP, Nube MJ, van Limbeek J, van Houte AJ, Daha MR, van Geelen JA. Biocompatibility and performance of a modified cellulosic and a synthetic high flux dialyzer a randomized crossover comparison between cellulose triacetate and polysulphon. ASAIO J. 1995;41:215. doi: 10.1097/00002480-199541020-00018. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita AC. Commercially available high-performance membrane dialyzers and several special kinds on the japanese market. In: Saito A, Kawanishi H, Yamashita AC, Mineshima M, editors. high-performance membrane dialyzers. KARGER, Basel: Contributions to nephrology; 2011. p. 191. [DOI] [PubMed] [Google Scholar]
- 63.Ingram AJ, Parbtani A, Churchill DN. Effects of two low-flux cellulose acetate dialysers on plasma lipids and lipoproteins - a cross-over trial. Nephrol Dial Transplant. 1998;13:1452. doi: 10.1093/ndt/13.6.1452. [DOI] [PubMed] [Google Scholar]
- 64.Thijs A, Grooteman MPC, Zweegman S, Nube MJ, Huijgens PC, Stehouwer CDA. Platelet activation during haemodialysis: comparison of cuprammonium rayon and polysulfone membranes. Blood Purif. 2007;25:389. doi: 10.1159/000108628. [DOI] [PubMed] [Google Scholar]
- 65.Opatrny K, Sulkova S, Lopot F, Vit L, Valek A. Clinical-study of high-flux cuprammonium rayon hemodialysis membranes. Artif Organs. 1993;17:971. doi: 10.1111/j.1525-1594.1993.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 66.Chanard J, Brunois JP, Melin JP, Lavaud S, Toupance O. Long-term results of dialysis therapy with a highly permeable membrane. Artif Organs. 1982;6:261. doi: 10.1111/j.1525-1594.1982.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 67.Jindal KK, McDougall J, Woods B, Nowakowski L, Goldstein MB. A study of the basic principles determining the performance of several high-flux dialyzers. Am J Kidney Dis. 1989;14:507. doi: 10.1016/S0272-6386(89)80151-9. [DOI] [PubMed] [Google Scholar]
- 68.Nakano A. Ethylene vinyl alcohol co-polymer as a high-performance membrane: An evoh membrane with excellent biocompatibility. In: Saito A, Kawanishi H, Yamashita AC, Mineshima M, editors. High-performance membrane dialyzers. Basel, Karger: Contributions to nephrology; 2011. p. 164. [DOI] [PubMed] [Google Scholar]
- 69.Malard B, Florens N, Lambert C, Juillard L. Adsorption as a contributor for inflammatory mediators removal by different hemofiltration membranes: a pilot study. Artif Organs. 2017;41:545. doi: 10.1111/aor.12785. [DOI] [PubMed] [Google Scholar]
- 70.Brunet P, Frances J, Vacher-Coponat H, Jaubert D, Lebrun G, Gondouin B, Duval A, Berland Y. Hemodialysis without heparin: a randomized, controlled, crossover study of two dialysis membranes (an69st and polysulfone f60) Int J Artif Organs. 2011;34:1165. doi: 10.5301/ijao.5000033. [DOI] [PubMed] [Google Scholar]
- 71.Huang J, Yi B, Li AM, Zhang H. Effects of vitamin e-coated dialysis membranes on anemia, nutrition and dyslipidemia status in hemodialysis patients: a meta-analysis. Ren Fail. 2015;37:398. doi: 10.3109/0886022X.2014.1001281. [DOI] [PubMed] [Google Scholar]
- 72.Panichi V, Rosati A, Paoletti S, Ferrandello P, Migliori M, Beati S, Bernabini G, Daini R, Casani A, Angelini D, Parrini M, Rossi A, Petrone I, Barsotti G, Donadio C, Donati G, Grazi G, Rizza GM, Garosi G, Sansoni E, Braccagni B, Sidoti A, Boracelli D, Biagioli M, Moriconi L, Finato V, Mannarino A, Grimaldi C, Pansa F, Imperiali P, Mura C, Bianchi S, Bigazzi R. A vitamin e-coated polysulfone membrane reduces serum levels of inflammatory markers and resistance to erythropoietin-stimulating agents in hemodialysis patients: Results of a randomized cross-over multicenter trial. Blood Purif. 2011;32:7. doi: 10.1159/000321369. [DOI] [PubMed] [Google Scholar]
- 73.Gura V, Rivara MB, Bieber S, Munshi R, Smith NC, Linke L, Kundzins J, Beizai M, Ezon C, Kessler L, Himmelfarb J. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016;1:86397. doi: 10.1172/jci.insight.86397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo J, Fan JB, Wang ST. Recent progress of microfluidic devices for hemodialysis. Small. 2020;16:1904076. doi: 10.1002/smll.201904076. [DOI] [PubMed] [Google Scholar]
- 75.Fissell WH, Roy S, Davenport A. Achieving more frequent and longer dialysis for the majority: Wearable dialysis and implantable artificial kidney devices. Kidney Int. 2013;84:256. doi: 10.1038/ki.2012.466. [DOI] [PubMed] [Google Scholar]
- 76.Nicolau DV. A model of protein adsorption to solid surfaces from solution. In: Bornhop DJ, Dunn DA, Mariella RP, Murphy CJ, Nicolau DV, Nie S et al., editors. Biomedical nanotechnology architectures and applications. Proceedings of spie. Bellingham: Spie-Int Soc Optical Engineering; 2002. pp. 1.
- 77.Brash JL, Horbett TA, Latour RA, Tengvall P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019;94:11. doi: 10.1016/j.actbio.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei Q, Becherer T, Angioletti-Uberti S, Dzubiella J, Wischke C, Neffe AT, Lendlein A, Ballauff M, Haag R. Protein interactions with polymer coatings and biomaterials. Angew Chem-Int Edit. 2014;53:8004. doi: 10.1002/anie.201400546. [DOI] [PubMed] [Google Scholar]
- 79.Ji HF, Xu H, Jin LQ, Song X, He C, Liu XL, Xiong L, Zhao WF, Zhao CS. Surface engineering of low-fouling and hemocompatible polyethersulfone membranes via in-situ ring-opening reaction. J Membr Sci. 2019;581:373. doi: 10.1016/j.memsci.2019.03.082. [DOI] [Google Scholar]
- 80.Zhao CS, Xue JM, Ran F, Sun SD. Modification of polyethersulfone membranes - a review of methods. Prog Mater Sci. 2013;58:76. doi: 10.1016/j.pmatsci.2012.07.002. [DOI] [Google Scholar]
- 81.Schmaier AH. The contact activation and kallikrein/kinin systems: Pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14:28. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 82.Arvidsson S, Askendal A, Tengvall P. Blood plasma contact activation on silicon, titanium and aluminium. Biomaterials. 2007;28:1346. doi: 10.1016/j.biomaterials.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Ahmad AL, Abdulkarim AA, Ooi BS, Ismail S. Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem Eng J. 2013;223:246. doi: 10.1016/j.cej.2013.02.130. [DOI] [Google Scholar]
- 84.Horbett TA. Fibrinogen adsorption to biomaterials. J Biomed Mater Res Part A. 2018;106:2777. doi: 10.1002/jbm.a.36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rabe M, Verdes D, Seeger S. Understanding protein adsorption phenomena at solid surfaces. Adv Colloid Interface Sci. 2011;162:87. doi: 10.1016/j.cis.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 86.Fagerstam LG, Frostellkarlsson A, Karlsson R, Persson B, Ronnberg I. Biospecific interaction analysis using surface-plasmon resonance detection applied to kinetic binding-site and concentration analysis. J Chromatogr. 1992;597:397. doi: 10.1016/0021-9673(92)80137-J. [DOI] [PubMed] [Google Scholar]
- 87.Elwing H, Askendal A, Lundstrom I. Competition between adsorbed fibrinogen and high-molecular-weight kininogen on solid-surfaces incubated in human-plasma (the vroman effect)-influence of solid-surface wettability. J Biomed Mater Res. 1987;21:1023. doi: 10.1002/jbm.820210808. [DOI] [PubMed] [Google Scholar]
- 88.Lindon JN, McManama G, Kushner L, Merrill EW, Salzman EW. Does the conformation of adsorbed fibrinogen dictate platelet interactions with artificial surfaces. Blood. 1986;68:355. doi: 10.1182/blood.V68.2.355.355. [DOI] [PubMed] [Google Scholar]
- 89.Yang LW, Han LL, Liu Q, Xu YG, Jia LY. Galloyl groups-regulated fibrinogen conformation: understanding antiplatelet adhesion on tannic acid coating. Acta Biomater. 2017;64:187. doi: 10.1016/j.actbio.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto M. Spread monolayers of a water-soluble protein (ferritin) and its 2-dimensional and 3-dimensional arrays. Langmuir. 1994;10:3922. doi: 10.1021/la00023a002. [DOI] [Google Scholar]
- 91.Norde W. Driving forces for protein adsorption at solid surfaces. Macromol Symp. 1996;103:5. doi: 10.1002/masy.19961030104. [DOI] [Google Scholar]
- 92.Engel MFM, Visser A, van Mierlo CPM. Conformation and orientation of a protein folding intermediate trapped by adsorption. Proc Natl Acad Sci U S A. 2004;101:11316. doi: 10.1073/pnas.0401603101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu JR, Zhao XB, Yaseen M. Biomimetic amphiphiles: biosurfactants. Curr Opin Colloid Interface Sci. 2007;12:60. doi: 10.1016/j.cocis.2007.05.004. [DOI] [Google Scholar]
- 94.Gorbet M, Sperling C, Maitz MF, Siedlecki CA, Werner C, Sefton MV. The blood compatibility challenge. Part 3: material associated activation of blood cascades and cells. Acta Biomater. 2019;94:25. doi: 10.1016/j.actbio.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 95.Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128:753. doi: 10.1182/blood-2016-05-718114. [DOI] [PubMed] [Google Scholar]
- 97.Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2017;37:E13. doi: 10.1161/ATVBAHA.117.308564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fredenburgh JC, Gross PL, Weitz JI. Emerging anticoagulant strategies. Blood. 2017;129:147. doi: 10.1182/blood-2016-09-692996. [DOI] [PubMed] [Google Scholar]
- 99.Ji HF, Xiong L, Shi ZQ, He M, Zhao WF, Zhao CS. Engineering of hemocompatible and antifouling polyethersulfone membranes by blending with heparin-mimicking microgels. Biomater Sci. 2017;5:1112. doi: 10.1039/C7BM00196G. [DOI] [PubMed] [Google Scholar]
- 100.Wang LR, He M, Gong T, Zhang X, Zhang LC, Liu T, Ye W, Pan CJ, Zhao CS. Introducing multiple bio-functional groups on the poly(ether sulfone) membrane substrate to fabricate an effective antithrombotic bio-interface. Biomater Sci. 2017;5:2416. doi: 10.1039/C7BM00673J. [DOI] [PubMed] [Google Scholar]
- 101.Ekdahl KN, Huang S, Nilsson B, Teramura Y. Complement inhibition in biomaterial- and biosurface-induced thromboinflammation. Semin Immunol. 2016;28:268. doi: 10.1016/j.smim.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemostasis. 2015;13:S72. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 103.Xu LC, Bauer JW, Siedlecki CA. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloid Surf B-Biointerfaces. 2014;124:49. doi: 10.1016/j.colsurfb.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17:271. doi: 10.1111/jth.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joseph L, Fink LM, Hauer-Jensen M. Cytokines in coagulation and thrombosis: a preclinical and clinical review. Blood Coagul Fibrinolysis. 2002;13:105. doi: 10.1097/00001721-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 106.van der Poll T, de Jonge E, Levi M. Regulatory role of cytokines in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27:639. doi: 10.1055/s-2001-18868. [DOI] [PubMed] [Google Scholar]
- 107.Yin J, Xu L, Ahmed A. Batch preparation and characterization of electrospun porous polylactic acid-based nanofiber membranes for antibacterial wound dressing. Adv Fiber Mater. 2022;4:832. doi: 10.1007/s42765-022-00141-y. [DOI] [Google Scholar]
- 108.Vanderkamp K, Vanoeveren W. Factor-xii fragment and kallikrein generation in plasma during incubation with biomaterials. J Biomed Mater Res. 1994;28:349. doi: 10.1002/jbm.820280309. [DOI] [PubMed] [Google Scholar]
- 109.Poppelaars F, Faria B, da Costa MG, Franssen CFM, van Son WJ, Berger SP, Daha MR, Seelen MA. The complement system in dialysis: a forgotten story? Front Immunol. 2018 doi: 10.3389/fimmu.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kizhakkedathu JN, Conway EM. Biomaterial and cellular implants: Foreign surfaces where immunity and coagulation meet. Blood. 1987;2022:139. doi: 10.1182/blood.2020007209. [DOI] [PubMed] [Google Scholar]
- 111.Swirski FK. Platelets have a hold over immune cells. Nature. 2020;577:323. doi: 10.1038/d41586-019-03732-9. [DOI] [PubMed] [Google Scholar]
- 112.Rayes J, Bourne JH, Brill A, Watson SP. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res Pract Thromb Haemost. 2020;4:23. doi: 10.1002/rth2.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ross R, Nist C, Kariya B, Rivest MJ, Raines E, Callis J. Physiological quiescence in plasma-derived serun-influence of platelet-derived growth-factor on cell-growth in culture. J Cell Physiol. 1978;97:497. doi: 10.1002/jcp.1040970325. [DOI] [PubMed] [Google Scholar]
- 114.Gayle RB, Maliszewski CR, Gimpel SD, Schoenborn MA, Caspary RG, Richards C, Brasel K, Price V, Drosopoulos JHF, Islam N, Alyonycheva TN, Broekman MJ, Marcus AJ. Inhibition of platelet function by recombinant soluble ecto-adpase/cd39. J Clin Invest. 1851;1998:101. doi: 10.1172/JCI1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holme S, Heaton A, Konchuba A, Hartman P. Light scatter and total protein signal distribution of platelets by flow-cytometry as parameters of size. J Lab Clin Med. 1988;112:223. [PubMed] [Google Scholar]
- 116.Almazov VA, Gurevich VS, Shatilina LV, Bykova NO, Bershadskii BG. Role of lipid hyperperoxidation in disturbance of the structural organization of platelet membranes. Bull Exp Biol Med. 1992;114:1276. doi: 10.1007/BF00809555. [DOI] [PubMed] [Google Scholar]
- 117.Bodin S, Tronchere H, Payrastre B. Lipid rafts are critical membrane domains in blood platelet activation processes. Biochim Biophys Acta-Biomembr. 2003;1610:247. doi: 10.1016/S0005-2736(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 118.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42:423. doi: 10.1016/S0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 119.Rao GHR, Chandy T. Role of platelets in blood-biomaterial interactions. Bull Mat Sci. 1999;22:633. doi: 10.1007/BF02749979. [DOI] [Google Scholar]
- 120.Fontana F, Ballestri M, Makomi C, Morandi R, Cappelli G. Hemorheologic alterations in peritoneal dialysis. Clin Hemorheol Microcirc. 2017;65:175. doi: 10.3233/CH-16152. [DOI] [PubMed] [Google Scholar]
- 121.Chen ZS, Li TL, Kareem K, Tran D, Griffith BP, Wu ZJJ. The role of pi3k/akt signaling pathway in non-physiological shear stress-induced platelet activation. Artif Organs. 2019;43:897. doi: 10.1111/aor.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Majumdar S, Patil CN, Ladner-Threadgill T, Randolph E, Burgreen GW, Kermode JC. Platelet activation and erythrocyte lysis during brief exposure of blood to pathophysiological shear stress in vitro. Clin Hemorheol Microcirc. 2017;67:159. doi: 10.3233/CH-170256. [DOI] [PubMed] [Google Scholar]
- 123.Yu K, Andruschak P, Yeh HH, Grecov D, Kizhakkedathu JN. Influence of dynamic flow conditions on adsorbed plasma protein corona and surface-induced thrombus generation on antifouling brushes. Biomaterials. 2018;166:79. doi: 10.1016/j.biomaterials.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 124.Chen ZS, Mondal NK, Zheng SR, Koenig SC, Slaughter MS, Griffith BP, Wu ZJJ. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets. 2019;30:112. doi: 10.1080/09537104.2017.1384542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vanhoorelbeke K, Deckmyn H. The role of vwf-collagen interaction in acute platelet thrombus formation. Drug Future. 2003;28:61. doi: 10.1358/dof.2003.028.01.856926. [DOI] [Google Scholar]
- 126.Hou Y, Carrim N, Wang YM, Gallant RC, Marshall A, Ni HY. Platelets in hemostasis and thrombosis: novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J Biomed Res. 2015;29:437. doi: 10.7555/JBR.29.20150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peerschke EIB. Reversible and irreversible binding of fibrinogen to platelets. Platelets. 1997;8:311. doi: 10.1080/09537109777177. [DOI] [PubMed] [Google Scholar]
- 128.Nurden AT. Platelet membrane glycoproteins: a historical review. Semin Thromb Hemost. 2014;40:577. doi: 10.1055/s-0034-1383826. [DOI] [PubMed] [Google Scholar]
- 129.Fredrickson BJ, Dong JF, McIntire LV, Lopez JA. Shear-dependent rolling on von willebrand factor of mammalian cells expressing the platelet glycoprotein ib-ix-v complex. Blood. 1998;92:3684. doi: 10.1182/blood.V92.10.3684. [DOI] [PubMed] [Google Scholar]
- 130.Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas TA, Plow EF, Qin J. A structural mechanism of integrin alpha(iib)beta(3) "inside-out" activation as regulated by its cytoplasmic face. Cell. 2002;110:587. doi: 10.1016/S0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 131.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von willebrand factor. Cell. 1996;84:289. doi: 10.1016/S0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 132.Dopheide SM, Maxwell MJ, Jackson SP. Shear-dependent tether formation during platelet translocation on von willebrand factor. Blood. 2002;99:159. doi: 10.1182/blood.V99.1.159. [DOI] [PubMed] [Google Scholar]
- 133.Bluestein D, Niu LJ, Schoephoerster RT, Dewanjee MK. Fluid mechanics of arterial stenosis: relationship to the development of mural thrombus. Ann Biomed Eng. 1997;25:344. doi: 10.1007/BF02648048. [DOI] [PubMed] [Google Scholar]
- 134.Du XP. Signaling and regulation of the platelet glycoprotein ib-ix-v complex. Curr Opin Hematol. 2007;14:262. doi: 10.1097/MOH.0b013e3280dce51a. [DOI] [PubMed] [Google Scholar]
- 135.Cosemans J, Van Kruchten R, Olieslagers S, Schurgers LJ, Verheyen FK, Munnix ICA, Waltenberger J, Angelillo-Scherrer A, Hoylaerts MF, Carmeliet P, Heemskerk JWM. Potentiating role of gas6 and tyro3, axl and mer (tam) receptors in human and murine platelet activation and thrombus stabilization. J Thromb Haemost. 2010;8:1797. doi: 10.1111/j.1538-7836.2010.03935.x. [DOI] [PubMed] [Google Scholar]
- 136.Watson SP, Herbert JMJ, Pollitt AY. Gpvi and clec-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8:1457. doi: 10.1111/j.1538-7836.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 137.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor clec-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 138.Zhang S, Zhang Y, Shen J, Chen L, Mruk JM, Zhu L, Kunapuli SP, Ding Z. Tumor vascular disrupting agent dmxaa inhibits platelet activation and thrombosis via inhibition of txa2-tp signaling and pde. J Thromb Haemost. 2013;11:443. doi: 10.1111/jth.12362. [DOI] [PubMed] [Google Scholar]
- 139.Centellas DS, Gudlur S, Vicente-Carrillo A, Ramstrom S, Lindahl TL. A cluster of aspartic residues in the extracellular loop ii of par 4 is important for thrombin interaction and activation of platelets. Thromb Res. 2017;154:84. doi: 10.1016/j.thromres.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 140.Rabani V, Montange D, Meneveau N, Davani S. Impact of ticagrelor on p2y1 and p2y12 localization and on cholesterol levels in platelet plasma membrane. Platelets. 2018;29:709. doi: 10.1080/09537104.2017.1356453. [DOI] [PubMed] [Google Scholar]
- 141.Morel O, Morel N, Freyssinet JM, Toti F. Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses. Platelets. 2008;19:9. doi: 10.1080/09537100701817232. [DOI] [PubMed] [Google Scholar]
- 142.Heemskerk JWM, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186. doi: 10.1055/s-0037-1613209. [DOI] [PubMed] [Google Scholar]
- 143.Morrissey JH, Choi SH, Smith SA. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012;119:5972. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Elia E, Montecucco F, Portincasa P, Sahebkar A, Mollazadeh H, Carbone F. Update on pathological platelet activation in coronary thrombosis. J Cell Physiol. 2019;234:2121. doi: 10.1002/jcp.27575. [DOI] [PubMed] [Google Scholar]
- 145.Navas-Carrillo D, Marin F, Valdes M, Orenes-Pinero E. Deciphering acute coronary syndrome biomarkers: High-resolution proteomics in platelets, thrombi and microparticles. Crit Rev Clin Lab Sci. 2017;54:49. doi: 10.1080/10408363.2016.1241214. [DOI] [PubMed] [Google Scholar]
- 146.Greer JP, Arber DA, Glader BE, List AF, Means RM, Rodgers GM. Wintrobe's clinical hematology. Lippincott Williams & Wilkins; 2018.
- 147.Zhu S, Travers RJ, Morrissey JH, Diamond SL. Fxia and platelet polyphosphate as therapeutic targets during human blood clotting on collagen/tissue factor surfaces under flow. Blood. 2015;126:1494. doi: 10.1182/blood-2015-04-641472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rossaint J, Margraf A, Zarbock A. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9:2712. doi: 10.3389/fimmu.2018.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dziedzic A, Bijaka M. Interactions between platelets and leukocytes in pathogenesis of multiple sclerosis. Adv Clin Exp Med. 2019;28:277. doi: 10.17219/acem/83588. [DOI] [PubMed] [Google Scholar]
- 150.Bednarczyk M, Stege H, Grabbe S, Bros M. Beta 2 integrins-multi-functional leukocyte receptors in health and disease. Int J Mol Sci. 2020 doi: 10.3390/ijms21041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang L. The alpha(m)beta(2) integrin and its role in neutrophil function. Cell Res. 1999;9:171. doi: 10.1038/sj.cr.7290015. [DOI] [PubMed] [Google Scholar]
- 152.David BA, Kubes P. Exploring the complex role of chemokines and chemoattractants in vivo on leukocyte dynamics. Immunol Rev. 2019;289:9. doi: 10.1111/imr.12757. [DOI] [PubMed] [Google Scholar]
- 153.Wang CB, Liu YB, He DN. Diverse effects of platelet-derived growth factor-bb on cell signaling pathways. Cytokine. 2019;113:13. doi: 10.1016/j.cyto.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 154.Hartley J, Abken H. Chimeric antigen receptors designed to overcome transforming growth factor-beta-mediated repression in the adoptive t-cell therapy of solid tumors. Clin Transl Immunol. 2019;8:1064. doi: 10.1002/cti2.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schiemann F, Grimm TA, Hoch J, Gross R, Lindner B, Petersen F, Bulfone-Paus S, Brandt E. Mast cells and neutrophils proteolytically activate chemokine precursor ctap-iii and are subject to counterregulation by pf-4 through inhibition of chymase and cathepsin g. Blood. 2006;107:2234. doi: 10.1182/blood-2005-06-2424. [DOI] [PubMed] [Google Scholar]
- 156.Yang M, Li SY, Zhang X, Cao R, Li CF, Chong BH. Pro-inflammatory cytokine il-1b on megakaryopoiesis: a possible explanation of thrombocytosis in inflammation. Blood. 2017;130:4851. [Google Scholar]
- 157.Sun B, Li J, Kambayashi J. Interaction between gpib alpha and fc gamma iia receptor in human platelets. Biochem Biophys Res Commun. 1999;266:24. doi: 10.1006/bbrc.1999.1761. [DOI] [PubMed] [Google Scholar]
- 158.Biagi E, Dotti G, Yvon E, Lee E, Pule M, Vigouroux S, Gottschalk S, Popat U, Rousseau R, Brenner M. Molecular transfer of cd40 and ox40 ligands to leukemic human b cells induces expansion of autologous tumor-reactive cytotoxic t lymphocytes. Blood. 2005;105:2436. doi: 10.1182/blood-2004-07-2556. [DOI] [PubMed] [Google Scholar]
- 159.Del Principe D, Frega G, Savini I, Catani MV, Rossi A, Avigliano L. The plasma membrane redox system in human platelet functions and platelet-leukocyte interactions. Thromb Haemost. 2009;101:284. doi: 10.1160/TH08-05-0300. [DOI] [PubMed] [Google Scholar]
- 160.Jing YJ, Ni JW, Ding FH, Fang YH, Wang XQ, Wang HB, Chen XN, Chen N, Zhan WW, Lu L, Zhang RY. P-cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoe -/- mice. Kidney Int. 2016;89:439. doi: 10.1038/ki.2015.287. [DOI] [PubMed] [Google Scholar]
- 161.Seyrek N, Balal M, Karayaylali I, Paydas S, Aikimbaev K, Cetiner S, Seydaoglu G. Which parameter is more influential on the development of arteriosclerosis in hemodialysis patients? Ren Fail. 2003;25:1011. doi: 10.1081/JDI-120026036. [DOI] [PubMed] [Google Scholar]
- 162.Schmaier AH, McCrae KR. The plasma kallikrein-kinin system: Its evolution from contact activation. J Thromb Haemost. 2007;5:2323. doi: 10.1111/j.1538-7836.2007.02770.x. [DOI] [PubMed] [Google Scholar]
- 163.Renaux JL, Thomas M, Crost T, Loughraieb N, Vantard G. Activation of the kallikrein-kinin system in hemodialysis: role of membrane electronegativity, blood dilution, and ph. Kidney Int. 1999;55:1097. doi: 10.1046/j.1523-1755.1999.0550031097.x. [DOI] [PubMed] [Google Scholar]
- 164.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part i - molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liszewski MK, Atkinson JP. Complement regulator cd46: genetic variants and disease associations. Hum Genomics. 2015;9:13. doi: 10.1186/s40246-015-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Podack ER, Mullereberhard HJ. Limited proteolysis of c5b-6-functional stability of the degraded complex. J Immunol. 1980;124:332. doi: 10.4049/jimmunol.124.1.332. [DOI] [PubMed] [Google Scholar]
- 167.Poppelaars F, da Costa MG, Faria B, Berger SP, Assa S, Daha MR, Pestana JOM, van Son WJ, Franssen CFM, Seelen MA. Intradialytic complement activation precedes the development of cardiovascular events in hemodialysis patients. Front Immunol. 2018;9:2070. doi: 10.3389/fimmu.2018.02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen FF, Wang GK, Griffin JI, Brenneman B, Banda NK, Holers VM, Backos DS, Wu LP, Moghimi SM, Simberg D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12:387. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mares J, Thongboonkerd V, Tuma Z, Moravec J, Matejovic M. Specific adsorption of some complement activation proteins to polysulfone dialysis membranes during hemodialysis. Kidney Int. 2009;76:404. doi: 10.1038/ki.2009.138. [DOI] [PubMed] [Google Scholar]
- 170.Mares J, Richtrova P, Hricinova A, Tuma Z, Moravec J, Lysak D, Matejovic M. Proteomic profiling of blood-dialyzer interactome reveals involvement of lectin complement pathway in hemodialysis-induced inflammatory response. Proteom Clin Appl. 2010;4:829. doi: 10.1002/prca.201000031. [DOI] [PubMed] [Google Scholar]
- 171.Siedlecki C. Hemocompatibility of biomaterials for clinical applications: Blood-biomaterials interactions. Woodhead Publishing; 2018.
- 172.Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J. Biomed Mate. Res. 2017;105:927. doi: 10.1002/jbm.a.35958. [DOI] [PubMed] [Google Scholar]
- 173.Castanheira FVS, Kubes P. Neutrophils and nets in modulating acute and chronic inflammation. Blood. 2019;133:2178. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 174.Noubouossie DF, Reeves BN, Strahl BD, Key NS. Neutrophils: back in the thrombosis spotlight. Blood. 2019;133:2186. doi: 10.1182/blood-2018-10-862243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Korabecna M, Tesar V. Netosis provides the link between activation of neutrophils on hemodialysis membrane and comorbidities in dialyzed patients. Inflamm Res. 2017;66:369. doi: 10.1007/s00011-016-1010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 177.Anderson JM. Biocompatibility and bioresponse to biomaterials. Principles of regenerative medicine: Elsevier, Amsterdam; 2019. p. 675. [Google Scholar]
- 178.Weber M, Steinle H, Golombek S, Hann L, Schlensak C, Wendel HP, Avci-Adali M. Blood-contacting biomaterials: in vitro evaluation of the hemocompatibility. Front Bioeng Biotechnol. 2018;6:99. doi: 10.3389/fbioe.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mahieu E, Claes K, Jacquemin M, Evenepoel P, De Beek KO, Bogaert AM, Kuypers D, Verhamme P, Meijers B. Anticoagulation with fondaparinux for hemodiafiltration in patients with heparin-induced thrombocytopenia: Dose-finding study and safety evaluation. Artif Organs. 2013;37:482. doi: 10.1111/aor.12002. [DOI] [PubMed] [Google Scholar]
- 180.Schmaier AH. Extracorporeal circulation without bleeding. Sci Trans Med. 2014;6:222fs7. doi: 10.1126/scitranslmed.3008497. [DOI] [PubMed] [Google Scholar]
- 181.Breuer G, Weiss DR, Ringwald J. 'New' direct oral anticoagulants in the perioperative setting. Curr Opin Anesthesiol. 2014;27:409. doi: 10.1097/ACO.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 182.Fransson F, Kyrk T, Skagerlind M, Stegmayr B. Rinsing the extra corporeal circuit with a heparin and albumin solution reduces the need for systemic anticoagulant in hemodialysis. Int J Artif Organs. 2013;36:725. doi: 10.5301/ijao.5000253. [DOI] [PubMed] [Google Scholar]
- 183.Lavaud S, Paris B, Maheut H, Randoux C, Renaux JL, Rieu P, Chanard J. Assessment of the heparin-binding an69 st hemodialysis membrane: Ii Clinical studies without heparin administration. Asaio J. 2005;51:348. doi: 10.1097/01.mat.0000169121.09075.53. [DOI] [PubMed] [Google Scholar]
- 184.Yang YT, Du YZ, Zhang J, Zhang HL, Guo BL. Structural and functional design of electrospun nanofibers for hemostasis and wound healing. Adv Fiber Mater. 2022;4:1027. doi: 10.1007/s42765-022-00178-z. [DOI] [Google Scholar]
- 185.Furie B, Furie BC. Mechanisms of disease: mechanisms of thrombus formation. N Engl J Med. 2008;359:938. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 186.Sachetto ATA, Mackman N. Modulation of the mammalian coagulation system by venoms and other proteins from snakes, arthropods, nematodes and insects. Thromb Res. 2019;178:145. doi: 10.1016/j.thromres.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 187.Morrissey JH, Smith SA. Polyphosphate as modulator of hemostasis, thrombosis, and inflammation. J Thromb Haemost. 2015;13:S92. doi: 10.1111/jth.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chuang YJ, Swanson R, Raja SM, Olson ST. Heparin enhances the specificity of antithrombin for thrombin and factor xa independent of the reactive center loop sequence - evidence for an exosite determinant of factor xa specificity in heparin-activated antithrombin. J Biol Chem. 2001;276:14961. doi: 10.1074/jbc.M011550200. [DOI] [PubMed] [Google Scholar]
- 189.Girolami A, Cosi E, Ferrari S, Girolami B. Heparin, coumarin, protein c, antithrombin, fibrinolysis and other clotting related resistances: old and new concepts in blood coagulation. J Thromb. 2018;45:135. doi: 10.1007/s11239-017-1559-0. [DOI] [PubMed] [Google Scholar]
- 190.Opneja A, Kapoor S, Stavrou EX. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb Res. 2019;179:56. doi: 10.1016/j.thromres.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29:17. doi: 10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Larsson M, Rayzman V, Nolte MW, Nickel KF, Bjorkqvist J, Jamsa A, Hardy MP, Fries M, Schmidbauer S, Hedenqvist P, Broome M, Pragst I, Dickneite G, Wilson MJ, Nash AD, Panousis C, Renne T. A factor xiia inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- 193.Li JM, Sun S, Han M, Wang LY, Liao RX, Xiong YQ, Li YP, Jiang H, Qin Z, Maharjan A, Cozzolino M, Zarbock A, Su BH. The effects of citrate dialysate in hemodialysis on polymorphonuclear elastase interaction with tissue factor and its inhibitor. Ann Transl Med. 2019;7:11. doi: 10.1186/1479-5876-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lalia BS, Kochkodan V, Hashaikeh R, Hilal N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination. 2013;326:77. doi: 10.1016/j.desal.2013.06.016. [DOI] [Google Scholar]
- 195.Figoli A, Marino T, Simone S, Di Nicolo E, Li XM, He T, Tornaghi S, Drioli E. Towards non-toxic solvents for membrane preparation: a review. Green Chem. 2014;16:4034. doi: 10.1039/C4GC00613E. [DOI] [Google Scholar]
- 196.Wang DS, Zou W, Li LL, Wei Q, Sun SD, Zhao CS. Preparation and characterization of functional carboxylic polyethersulfone membrane. J Membr Sci. 2011;374:93. doi: 10.1016/j.memsci.2011.03.021. [DOI] [Google Scholar]
- 197.Tang M, Xue JM, Yan KL, Xiang T, Sun SD, Zhao CS. Heparin-like surface modification of polyethersulfone membrane and its biocompatibility. J Colloid Interface Sci. 2012;386:428. doi: 10.1016/j.jcis.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 198.Shi Q, Su YL, Zhao W, Li C, Hu YH, Jiang ZY, Zhu SP. Zwitterionic polyethersulfone ultrafiltration membrane with superior antifouling property. J Membr Sci. 2008;319:271. doi: 10.1016/j.memsci.2008.03.047. [DOI] [Google Scholar]
- 199.Wang LR, Qin H, Nie SQ, Sun SD, Ran F, Zhao CS. Direct synthesis of heparin-like poly(ether sulfone) polymer and its blood compatibility. Acta Biomater. 2013;9:8851. doi: 10.1016/j.actbio.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 200.Shi Q, Su YL, Chen WJ, Peng JM, Nie LY, Zhang L, Jiang ZY. Grafting short-chain amino acids onto membrane surfaces to resist protein fouling. J Membr Sci. 2011;366:398. doi: 10.1016/j.memsci.2010.10.032. [DOI] [Google Scholar]
- 201.Xiang T, Wang R, Zhao WF, Sun SD, Zhao CS. Covalent deposition of zwitterionic polymer and citric acid by click chemistry-enabled layer-by-layer assembly for improving the blood compatibility of polysulfone membrane. Langmuir. 2014;30:5115. doi: 10.1021/la5001705. [DOI] [PubMed] [Google Scholar]
- 202.Daugirdas JT, Bernardo AA. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 2012;82:147. doi: 10.1038/ki.2012.130. [DOI] [PubMed] [Google Scholar]
- 203.Wang H, Hou WJ, Liu F, Han Q, Li TT, Lin HB, Deng G, He JD. Preparation and evaluation of a self-anticlotting dialyzer via an interface crosslinking approach. J Membr Sci. 2018;563:115. doi: 10.1016/j.memsci.2018.05.056. [DOI] [Google Scholar]
- 204.Sakai K. Determination of pore-size and pore-size distribution 2Dialysis membranes. J Membr Sci. 1994;96:91. doi: 10.1016/0376-7388(94)00127-8. [DOI] [Google Scholar]
- 205.Xia Y, Cheng C, Wang R, Nie CX, Deng J, Zhao CS. Ag-nanogel blended polymeric membranes with antifouling, hemocompatible and bactericidal capabilities. J Mat Chem B. 2015;3:9295. doi: 10.1039/C5TB01523E. [DOI] [PubMed] [Google Scholar]
- 206.Hendrickson GR, Lyon LA. Microgel translocation through pores under confinement. Angew Chem-Int Edit. 2010;49:2193. doi: 10.1002/anie.200906606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ji HF, He C, Wang R, Fan X, Xiong L, Zhao WF, Zhao CS. Multifunctionalized polyethersulfone membranes with networked submicrogels to improve antifouling property, antibacterial adhesion and blood compatibility. Mater Sci Eng C-Mater Biol Appl. 2019;96:402. doi: 10.1016/j.msec.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 208.Ma L, Su BH, Cheng C, Yin ZH, Qin H, Zhao JM, Sun SD, Zhao CS. Toward highly blood compatible hemodialysis membranes via blending with heparin-mimicking polyurethane: study in vitro and in vivo. J Membr Sci. 2014;470:90. doi: 10.1016/j.memsci.2014.07.030. [DOI] [Google Scholar]
- 209.Qin H, Sun CC, He C, Wang DS, Cheng C, Nie SQ, Sun SD, Zhao CS. High efficient protocol for the modification of polyethersulfone membranes with anticoagulant and antifouling properties via in situ cross-linked copolymerization. J Membr Sci. 2014;468:172. doi: 10.1016/j.memsci.2014.06.006. [DOI] [Google Scholar]
- 210.He C, Cheng C, Nie SQ, Wang LR, Nie CX, Sun SD, Zhao CS. Graphene oxide linked sulfonate-based polyanionic nanogels as biocompatible, robust and versatile modifiers of ultrafiltration membranes. J Mater Chem B. 2016;4:6143. doi: 10.1039/C6TB01855F. [DOI] [PubMed] [Google Scholar]
- 211.Xu YT, Yuan DD, Bao JX, Xie Y, He M, Shi ZQ, Chen SQ, He C, Zhao WF, Zhao CS. Nanofibrous membranes with surface migration of functional groups for ultrafast wastewater remediation. J Mater Chem A. 2018;6:13359. doi: 10.1039/C8TA04005B. [DOI] [Google Scholar]
- 212.Xiang T, Zhang LS, Wang R, Xia Y, Su BH, Zhao CS. Blood compatibility comparison for polysulfone membranes modified by grafting block and random zwitterionic copolymers via surface-initiated atrp. J Colloid Interface Sci. 2014;432:47. doi: 10.1016/j.jcis.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 213.Deng J, Liu XY, Zhang SQ, Cheng C, Nie CX, Zhao CS. Versatile and rapid postfunctionalization from cyclodextrin modified host polymeric membrane substrate. Langmuir. 2015;31:9665. doi: 10.1021/acs.langmuir.5b02038. [DOI] [PubMed] [Google Scholar]
- 214.Xia Y, Cheng C, Wang R, Qin H, Zhang Y, Ma L, Tan H, Gu ZW, Zhao CS. Surface-engineered nanogel assemblies with integrated blood compatibility, cell proliferation and antibacterial property: towards multifunctional biomedical membranes. Polym Chem. 2014;5:5906. doi: 10.1039/C4PY00870G. [DOI] [Google Scholar]
- 215.Fan X, Yang F, Nie CX, Yang Y, Ji HF, He C, Cheng C, Zhao CS. Mussel-inspired synthesis of nir-responsive and biocompatible ag-graphene 2d nanoagents for versatile bacterial disinfections. ACS Appl Mater Interfaces. 2018;10:296. doi: 10.1021/acsami.7b16283. [DOI] [PubMed] [Google Scholar]
- 216.Ma L, Qin H, Cheng C, Xia Y, He C, Nie CX, Wang LR, Zhao CS. Mussel-inspired self-coating at macro-interface with improved biocompatibility and bioactivity via dopamine grafted heparin-like polymers and heparin. J Mat Chem B. 2014;2:363. doi: 10.1039/C3TB21388A. [DOI] [PubMed] [Google Scholar]
- 217.He M, Wang Q, Zhang J, Zhao WF, Zhao CS. Substrate-independent ag-nanoparticle-loaded hydrogel coating with regenerable bactericidal and thermoresponsive antibacterial properties. ACS Appl Mater Interfaces. 2017;9:44782. doi: 10.1021/acsami.7b13238. [DOI] [PubMed] [Google Scholar]
- 218.Mei Y, Yu K, Lo JCY, Takeuchi LE, Hadjesfandiari N, Yazdani-Ahmadabadi H, Brooks DE, Lange D, Kizhakkedathu JN. Polymer-nanoparticle interaction as a design principle in the development of a durable ultrathin universal binary antibiofilm coating with long-term activity. ACS Nano. 2018;12:11881. doi: 10.1021/acsnano.8b05512. [DOI] [PubMed] [Google Scholar]
- 219.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir. 2001;17:5605. doi: 10.1021/la010384m. [DOI] [PubMed] [Google Scholar]
- 220.Chapman RG, Ostuni E, Takayama S, Holmlin RE, Yan L, Whitesides GM. Surveying for surfaces that resist the adsorption of proteins. J Am Chem Soc. 2000;122:8303. doi: 10.1021/ja000774f. [DOI] [Google Scholar]
- 221.Jokinen V, Kankuri E, Hoshian S, Franssila S, Ras RHA. Superhydrophobic blood-repellent surfaces. Adv Mater. 2018;30:1705104. doi: 10.1002/adma.201705104. [DOI] [PubMed] [Google Scholar]
- 222.Wu JG, Chen JH, Liu KT, Luo SC. Engineering antifouling conducting polymers for modern biomedical applications. ACS Appl Mater Interfaces. 2019;11:21294. doi: 10.1021/acsami.9b04924. [DOI] [PubMed] [Google Scholar]
- 223.Bekard IB, Asimakis P, Bertolini J, Dunstan DE. The effects of shear flow on protein structure and function. Biopolymers. 2011;95:733. doi: 10.1002/bip.21646. [DOI] [PubMed] [Google Scholar]
- 224.Wang R, Xie Y, Xiang T, Sun SD, Zhao CS. Direct catechol conjugation of mussel-inspired biomacromolecule coatings to polymeric membranes with antifouling properties, anticoagulant activity and cytocompatibility. J Mate Chem B. 2017;5:3035. doi: 10.1039/C6TB03329F. [DOI] [PubMed] [Google Scholar]
- 225.Zou W, Qin H, Shi WB, Sun SD, Zhao CS. Surface modification of poly(ether sulfone) membrane with a synthesized negatively charged copolymer. Langmuir. 2014;30:13622. doi: 10.1021/la502343c. [DOI] [PubMed] [Google Scholar]
- 226.Wang C, Wang R, Xu Y, Zhang M, Yang F, Sun SD, Zhao CS. A facile way to prepare anti-fouling and blood-compatible polyethersulfone membrane via blending with heparin-mimicking polyurethanes. Mater Sci Eng C-Mater Biol Appl. 2017;78:1035. doi: 10.1016/j.msec.2017.04.123. [DOI] [PubMed] [Google Scholar]
- 227.Xie BW, Zhang RR, Zhang H, Xu AX, Deng Y, Lv YL, Deng F, Wei SC. Decoration of heparin and bovine serum albumin on polysulfone membrane assisted via polydopamine strategy for hemodialysis. J Biomater Sci-Polym Ed. 2016;27:880. doi: 10.1080/09205063.2016.1169479. [DOI] [PubMed] [Google Scholar]
- 228.Luo X, Han CZ, Yang P, Zhao A, Zou D, Jiang L, Gao PY, Yin BL, Huang N. The co-deposition coating of collagen iv and laminin on hyaluronic acid pattern for better biocompatibility on cardiovascular biomaterials. Colloid Surf B-Biointerfaces. 2020;196:111307. doi: 10.1016/j.colsurfb.2020.111307. [DOI] [PubMed] [Google Scholar]
- 229.Deng JC, Yuan SH, Li X, Wang KB, Xie LX, Li N, Wang J, Huang N. Heparin/DNA aptamer co-assembled multifunctional catecholamine coating for epc capture and improved hemocompatibility of vascular devices. Mater Sci Eng C-Mater Biol Appl. 2017;79:305. doi: 10.1016/j.msec.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 230.Jen MC, Serrano MC, van Lith R, Ameer GA. Polymer-based nitric oxide therapies: recent insights for biomedical applications. Adv Funct Mater. 2012;22:239. doi: 10.1002/adfm.201101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Riccio DA, Schoenfisch MH. Nitric oxide release: Part i. Macromol scaffolds Chem Soc Rev. 2012;41:3731. doi: 10.1039/c2cs15272j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Du H, Li C, Luan YF, Liu Q, Yang WK, Yu Q, Li D, Brash JL, Chen H. An antithrombotic hydrogel with thrombin-responsive fibrinolytic activity: Breaking down the clot as it forms. Mater Horizons. 2016;3:556. doi: 10.1039/C6MH00307A. [DOI] [Google Scholar]
- 233.Song X, Xu T, Yang L, Li YP, Yang Y, Jin LQ, Zhang J, Zhong R, Sun SD, Zhao WF, Zhao CS. Self-anticoagulant nanocomposite spheres for the removal of bilirubin from whole blood: a step toward a wearable artificial liver. Biomacromol. 2020;21:1762. doi: 10.1021/acs.biomac.9b01686. [DOI] [PubMed] [Google Scholar]
- 234.Zhao LJ, Ma F, Yu Y, Li YP, Wang Y, He LJ, Zhou ML, Tian XJ, Jing R, Li L, Huang C, Bai M, Sun SR. Regional citrate anticoagulation versus no-anticoagulation for continuous venovenous hemofiltration in acute severe hypernatremia patients with increased bleeding risk: a retrospective cohort study. Blood Purif. 2020;49:44. doi: 10.1159/000502937. [DOI] [PubMed] [Google Scholar]
- 235.Marlu R, Malvezzi P, Seyve L, Jouve T, Maurizi J, Defendi F, Carron PL, Christophe M, Le Gouellec A, Polack B, Rostaing L. Effect of double-filtration plasmapheresis for antibody-mediated rejection on hemostasis parameters and thrombin generation. Thromb Res. 2018;166:113. doi: 10.1016/j.thromres.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 236.Calzavarini S, Baeriswyl V, Quarroz C, Heinis C, Angelillo-Scherrer A. Fxii618, a new factor xii inhibitor: validation for thrombin generation assay. J Thromb Haemost. 2015;13:300. [Google Scholar]
- 237.Harvey RA, Kim HC, Pincus J, Trooskin SZ, Wilcox JN, Greco RS. Binding of tissue plasminogen-activator to vasular grafts. Thromb Haemost. 1989;61:131. doi: 10.1055/s-0038-1646541. [DOI] [PubMed] [Google Scholar]
- 238.Garcia A, Gander JW, Gross ER, Reichstein A, Sheth SS, Stolar CJ, Middlesworth W. The use of recombinant tissue-type plasminogen activator in a newborn with an intracardiac thrombus developed during extracorporeal membrane oxygenation. J Pediatr Surg. 2021;2011:46. doi: 10.1016/j.jpedsurg.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 239.Ji MB, Chen XR, Luo JQ, Wan YH. Improved blood compatibility of polysulfone membrane by anticoagulant protein immobilization. Colloid Surf B-Biointerfaces. 2019;175:586. doi: 10.1016/j.colsurfb.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 240.Ma L, Qin H, Cheng C, Xia Y, He C, Nie CX, Wang LR, Zhao CS. Mussel-inspired self-coating at macro-interface with improved biocompatibility and bioactivity via dopamine grafted heparin-like polymers and heparin. J Mater Chem B. 2014;2:363. doi: 10.1039/C3TB21388A. [DOI] [PubMed] [Google Scholar]
- 241.Goushki MN, Mousavi SA, Abdekhodaie MJ, Sadeghi M. Free radical graft polymerization of 2-hydroxyethyl methacrylate and acrylic acid on the polysulfone membrane surface through circulation of reaction media to improve its performance and hemocompatibility properties. J Membr Sci. 2018;564:762. doi: 10.1016/j.memsci.2018.07.071. [DOI] [Google Scholar]
- 242.Alenazi NA, Alamry KA, Hussein MA, Elfaky MA, Asiri AM. Enhanced antifouling and anticoagulant properties of grafted biomolecule polyethersulfone membranes. Polym Adv Technol. 2019;30:1493. doi: 10.1002/pat.4581. [DOI] [Google Scholar]
- 243.Maas C, Renne T. Coagulation factor xii in thrombosis and inflammation. Blood. 1903;2018:131. doi: 10.1182/blood-2017-04-569111. [DOI] [PubMed] [Google Scholar]
- 244.Las AG, Pitakjakpipop H, Rahn MS, Siedlecki CA, Vogler EA. Enzymes produced by autoactivation of blood factor xii in buffer a contribution from the hematology at biomaterial interfaces research group. Biomaterials. 2015;37:1. doi: 10.1016/j.biomaterials.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 245.Pixley RA, Cassello A, Delacadena RA, Kaufman N, Colman RW. Effect of heparin on the activation of factor-xii and the contact system in plasma. Thromb Haemost. 1991;66:540. doi: 10.1055/s-0038-1646456. [DOI] [PubMed] [Google Scholar]
- 246.Golas A, Parhi P, Dimachkie ZO, Siedlecki CA, Vogler EA. Surface-energy dependent contact activation of blood factor xii. Biomaterials. 2010;31:1068. doi: 10.1016/j.biomaterials.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yu K, Lai BFL, Foley JH, Krisinger MJ, Conway EM, Kizhakkedathu JN. Modulation of complement activation and amplification on nanoparticle surfaces by glycopolymer conformation and chemistry. ACS Nano. 2014;8:7687. doi: 10.1021/nn504186b. [DOI] [PubMed] [Google Scholar]
- 248.Andersson J, Larsson R, Richter R, Ekdahl KN, Nilsson B. Binding of a model regulator of complement activation (rca) to a biomaterial surface: Surface-bound factor h inhibits complement activation. Biomaterials. 2001;22:2435. doi: 10.1016/S0142-9612(00)00431-2. [DOI] [PubMed] [Google Scholar]
- 249.Wu YQ, Qu HC, Sfyroera G, Tzekou A, Kay BK, Nilsson B, Ekdahl KN, Ricklin D, Lambris JD. Protection of nonself surfaces from complement attack by factor h-binding peptides: Implications for therapeutic medicine. J Immunol. 2011;186:4269. doi: 10.4049/jimmunol.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.D'Arrigo G, Baggetta R, Tripepi G, Galli F, Bolignano D. Effects of vitamin e-coated versus conventional membranes in chronic hemodialysis patients: a systematic review and meta-analysis. Blood Purif. 2017;43:101. doi: 10.1159/000453444. [DOI] [PubMed] [Google Scholar]
- 251.Piroddi M, Pilolli F, Aritomi M, Galli F. Vitamin e as a functional and biocompatibility modifier of synthetic hemodialyzer membranes: an overview of the literature on vitamin e-modified hemodialyzer membranes. Am J Nephrol. 2012;35:559. doi: 10.1159/000338807. [DOI] [PubMed] [Google Scholar]
- 252.Mahlicli FY, Sen Y, Mutlu M, Altinkaya SA. Immobilization of superoxide dismutase/catalase onto polysulfone membranes to suppress hemodialysis-induced oxidative stress: A comparison of two immobilization methods. J Membr Sci. 2015;479:175. doi: 10.1016/j.memsci.2014.12.025. [DOI] [Google Scholar]
- 253.Wei QY, Feng SM, Zhang ZZ, Liu LL, Wu LL. A high-protein retained pes hemodialysis membrane with tannic acid as a multifunctional modifier. Colloid Surf B-Biointerfaces. 2022;220:112921. doi: 10.1016/j.colsurfb.2022.112921. [DOI] [PubMed] [Google Scholar]
- 254.Chen Q, Kou MS, He Y, Zhao YP, Chen L. Constructing hierarchical surface structure of hemodialysis membranes to intervene in oxidative stress through michael addition reaction between tannic acid and petox brushes. J Membr Sci. 2022;657:120700. doi: 10.1016/j.memsci.2022.120700. [DOI] [Google Scholar]
- 255.Chen Q, He Y, Zhao Y, Chen L. Tannic acid and poly(n-acryloyl morpholine) layer-by-layer built hemodialysis membrane surface for intervening oxidative stress integrated with high biocompatibility and dialysis performance. J Membr Sci. 2021;621:118896. doi: 10.1016/j.memsci.2020.118896. [DOI] [Google Scholar]
- 256.Qi X, Yang N, Luo Y, Jia X, Zhao J, Feng X, Chen L, Zhao Y. Resveratrol as a plant type antioxidant modifier for polysulfone membranes to improve hemodialysis-induced oxidative stress. Mater Sci Eng C. 2021;123:111953. doi: 10.1016/j.msec.2021.111953. [DOI] [PubMed] [Google Scholar]
- 257.Kohlová M, Rocha S, Gomes Amorim C, de Nova AA, Santos-Silva A, Solich P, da Silva B, Montenegro MC. Doping polysulfone membrane with alpha-tocopherol and alpha-lipoic acid for suppressing oxidative stress induced by hemodialysis treatment. Macromol Biosci. 2020;20:e2000046. doi: 10.1002/mabi.202000046. [DOI] [PubMed] [Google Scholar]
- 258.Pannuzzo M, Esposito S, Wu LP, Key J, Aryal S, Celia C, di Marzio L, Moghimi SM, Decuzzi P. Overcoming nanoparticle-mediated complement activation by surface peg pairing. Nano Lett. 2020;20:4312. doi: 10.1021/acs.nanolett.0c01011. [DOI] [PubMed] [Google Scholar]
- 259.Sanfins E, Augustsson C, Dahlback B, Linse S, Cedervall T. Size-dependent effects of nanoparticles on enzymes in the blood coagulation cascade. Nano Lett. 2014;14:4736. doi: 10.1021/nl501863u. [DOI] [PubMed] [Google Scholar]
- 260.Hao F, Liu QS, Chen X, Zhao XC, Zhou QF, Liao CY, Jiang GB. Exploring the heterogeneity of nanoparticles in their interactions with plasma coagulation factor xii. ACS Nano. 1990;2019:13. doi: 10.1021/acsnano.8b08471. [DOI] [PubMed] [Google Scholar]
- 261.Vu VP, Gifford GB, Chen FF, Benasutti H, Wang GK, Groman EV, Scheinman R, Saba L, Moghimi SM, Simberg D. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat Nanotechnol. 2019;14:260. doi: 10.1038/s41565-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pascual M, Swinford RD, TolkoffRubin N. Acute renal failure: role of dialysis membrane biocompatibility. Annu Rev Med. 1997;48:467. doi: 10.1146/annurev.med.48.1.467. [DOI] [PubMed] [Google Scholar]
- 263.Zheng X, Ni CJ, Xiao WW, Yu GP, Li YJ. In vitro hemocompatibility and hemodialysis performance of hydrophilic ionic liquid grafted polyethersulfone hollow fiber membranes. Sep Purif Technol. 2022;298:121464. doi: 10.1016/j.seppur.2022.121464. [DOI] [Google Scholar]
- 264.Rose II, Kather M, Roth H, Duenkelberg H, Rein L, Klimosch SN, Schmolz M, Wessling M. Single-step chitosan functionalized membranes for heparinization. J Membr Sci. 2022;655:120567. doi: 10.1016/j.memsci.2022.120567. [DOI] [Google Scholar]
- 265.Fu X, Lei T, Li SJ, Liu YF, Peng J, Ning JP. Construction of novel antiplatelet modified polyethersulfone membrane and study into its blood compatibility. Biomater Adv. 2022;135:112659. doi: 10.1016/j.msec.2022.112659. [DOI] [PubMed] [Google Scholar]
- 266.Sharifloo MM, Ghaee A, Salimi E, Sadatnia B, Mansourpour Z. Hemocompatibility and antifouling properties of pegma grafted polyethersulfone/aminated halloysite nanotubes mixed matrix membrane. Int J Polym Mater Polym Biomat. 2019;68:762. doi: 10.1080/00914037.2018.1493688. [DOI] [Google Scholar]
- 267.Yu XF, Zhu YD, Cheng C, Zhang TH, Wang XF, Hsiao BS. Novel thin-film nanofibrous composite membranes containing directional toxin transport nanochannels for efficient and safe hemodialysis application. J Membr Sci. 2019;582:151. doi: 10.1016/j.memsci.2019.04.006. [DOI] [Google Scholar]
- 268.Wang JX, Qiu M, He CJ. A zwitterionic polymer/pes membrane for enhanced antifouling performance and promoting hemocompatibility. J Membr Sci. 2020;606:118119. doi: 10.1016/j.memsci.2020.118119. [DOI] [Google Scholar]
- 269.Liu CN, Wang WY, Li YL, Cui FY, Xie CC, Zhu LY, Shan BJ. Pmwcnt/pvdf ultrafiltration membranes with enhanced antifouling properties intensified by electric field for efficient blood purification. J Membr Sci. 2019;576:48. doi: 10.1016/j.memsci.2019.01.015. [DOI] [Google Scholar]
- 270.Salimi E, Ghaee A, Ismail AF, Karimi M. Anti-thrombogenicity and permeability of polyethersulfone hollow fiber membrane with sulfonated alginate toward blood purification. Int J Biol Macromol. 2018;116:364. doi: 10.1016/j.ijbiomac.2018.04.137. [DOI] [PubMed] [Google Scholar]
- 271.Hoseinpour V, Ghaee A, Vatanpour V, Ghaemi N. Surface modification of pes membrane via aminolysis and immobilization of carboxymethylcellulose and sulphated carboxymethylcellulose for hemodialysis. Carbohydr Polym. 2018;188:37. doi: 10.1016/j.carbpol.2018.01.106. [DOI] [PubMed] [Google Scholar]
- 272.Zhao R, Li YM, Li X, Li YZ, Sun BL, Chao S, Wang C. Facile hydrothermal synthesis of branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane as a highly efficient and reusable bilirubin adsorbent in hemoperfusion. J Colloid Interface Sci. 2018;514:675. doi: 10.1016/j.jcis.2017.12.059. [DOI] [PubMed] [Google Scholar]
- 273.Modi A, Verma SK, Bellare J. Graphene oxide-doping improves the biocompatibility and separation performance of polyethersulfone hollow fiber membranes for bioartificial kidney application. J Colloid Interface Sci. 2018;514:750. doi: 10.1016/j.jcis.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 274.Wang C, Xu Y, Sun SD, Zhao CS. Post-functionalization of carboxylic polyethersulfone composite membranes. Compos Sci Technol. 2018;156:48. doi: 10.1016/j.compscitech.2017.12.014. [DOI] [Google Scholar]
- 275.Liu TM, Xu JJ, Qiu YR. A novel kind of polysulfone material with excellent biocompatibility modified by the sulfonated hydroxypropyl chitosan. Mater Sci Eng C-Mater Biol Appl. 2017;79:570. doi: 10.1016/j.msec.2017.05.103. [DOI] [PubMed] [Google Scholar]
- 276.Zhu LJ, Song HM, Wang JR, Xue LX. Polysulfone hemodiafiltration membranes with enhanced anti-fouling and hemocompatibility modified by poly(vinyl pyrrolidone) via in situ cross-linked polymerization. Mater Sci Eng C-Mater Biol Appl. 2017;74:159. doi: 10.1016/j.msec.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 277.Yu XF, Shen LD, Zhu YD, Li X, Yang Y, Wang XF, Zhu MF, Hsiao BS. High performance thin-film nanofibrous composite hemodialysis membranes with efficient middle-molecule uremic toxin removal. J Membr Sci. 2017;523:173. doi: 10.1016/j.memsci.2016.09.057. [DOI] [Google Scholar]