Abstract
Background
The clinical and molecular characteristics of three patients with previously unreported SERPINA1 mutations associated with severe alpha-1 antitrypsin deficiency (AATD) are described. The pathophysiology of the chronic obstructive pulmonary disease (COPD) present in these patients was characterized through clinical, biochemical, and genetic examinations.
Case presentations
Case 1: A 73-year-old male with bilateral centri-to panlobular emphysema and multiple increasing ventrobasal bullae and incomplete fissures, COPD (Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade III B), progressive dyspnea on exertion (DOE), AAT level of 0.1–0.2 g/L. Genetic testing revealed a unique SERPINA1 mutation: Pi*Z/c.1072C > T. This allele was designated PiQ0Heidelberg II. Case 2: A 47-year-old male with severely heterogenous centri-to panlobular emphysema concentrated in the lower lobes, COPD GOLD IV D with progressive DOE, AAT <0.1 g/L. He also had a unique Pi*Z/c.10del mutation in SERPINA1. This allele was named PiQ0Heidelberg III. Case 3: A 58-year-old female with basally accentuated panlobular emphysema, GOLD II B COPD, progressive DOE. AAT 0.1 g/L. Genetic analysis revealed Pi*Z/c.-5+1G > A and c.-472G > A mutations in SERPINA1. This variant allele was named PiQ0Heidelberg IV.
Conclusions
Each of these patients had a unique and previously unreported SERPINA1 mutation. In two cases, AATD and a history of smoking led to severe lung disease. In the third case, timely diagnosis, and institution of AAT replacement stabilized lung function. Wider screening of COPD patients for AATD could lead to faster diagnosis and earlier treatment of AATD patients with AATD which could slow or prevent progression of their disease.
Keywords: Chronic obstructive pulmonary disease, Emphysema, SERPINA1, Alpha-1 antitrypsin deficiency
Highlights
-
•
Three patients with COPD were found to have unique variants of SERPINA1 resulting in severe alpha-1 antitrypsin deficiency.
-
•
A patient with less severe disease at diagnosis was treated with AAT augmentation and achieved stable pulmonary function.
-
•
More research is warranted to assess whether earlier diagnosis of and therapy for AAT deficiency can improve outcomes.
1. Introduction
Alpha-1antitrypsin (AAT) is a 52-KDa glycoprotein that is the predominant protease inhibitor in serum. AAT also functions in the lungs to offset the activity of elastase enzymes, primarily neutrophil elastase. For individuals with AAT deficiency (AATD), an uncommon inherited condition, the equilibrium between AAT and elastases is distorted and unchecked elastase activity can lead to damage to lung tissue and organ dysfunction [1]. Another common pathological result of AATD is hepatic dysfunction generally due to protein accumulation in the hepatocytes [2,3].
The SERPINA1 gene encodes for AAT. Most people have the M-allele of SERPINA1 which produces normal serum levels of AAT. The most common pathogenic alleles associated with AATD are the S-allele and the Z-allele which produce a 40% and 90% reduction in serum AAT, respectively [4]. In addition to the common alleles (S and Z) responsible for AATD, more than 150 rare mutations of the SERPINA1 gene have been identified to date [5,6]. Around 40% of these mutations have been associated with AATD [7]. SERPINA1 alleles associated with AATD are classified as deficient variants when there is a significant reduction in plasma AAT levels, or null variants (Q0) when AAT is undetectable in plasma. The lack of AAT in the plasma of individuals with null variants can be due to transcription defects (e.g., premature stop codons or splicing abnormalities leading to truncated protein synthesis and premature degradation), incorrect protein conformation or transport defects [4,8,9]. Individuals with these null variants have a high risk for lung disease but minimal risk for liver disease [4,10,11].
In this article, we describe the clinical and molecular characteristics of three patients each with a distinct and previously undescribed SERPINA1 mutation associated with severe AATD. These patients were seen for chronic obstructive pulmonary disease (COPD) and underwent clinical, biochemical, and genetic workups to fully characterize the pathophysiology of their lung disease.
2. Clinical presentations
Informed consent (written) was obtained from these patients to allow their de-identified information to be used in this article. Approval from the University of Heidelberg Ethics Committee was not requested since the university does not require ethics committee approval for the reporting of individual patient cases. This study was completed in compliance with the Helsinki Declaration (2013 Revision) and all applicable local and national regulations.
The three patients described in these case reports were referred to the Special Outpatient Department of the Thoraxklinik Heidelberg for treatment of their COPD (Table 1). As part of their diagnostic workup, the patients underwent measurement of their AAT serum level (by turbidimetry: Photometer, ADVIA Chemistry XPT, Siemens, Heidelberg), genotyping (Progenika A1AT Genotyping Kit, Progenika Biopharma, S.A., Derio, Spain and Luminex 200, Luminex, Austin, TX, USA) and isoelectric focusing (IEF) (Hydrasis 2 Scan Focusing, Sebia, Fulda, Germany) at the AAT Lab, University Hospital, Marburg. If there were conflicting results between the genotyping by polymerase chain reaction (PCR) and IEF, or if there was an indication of a rare mutation, the seven exons of SERPINA1 gene were analyzed by next generation sequencing (Progenika Biopharma, S.A.)
Table 1.
Summary of three patients with null mutations in the SERPINA1 gene and alpha-1 antitrypsin deficiency.
| Gender | Case 1 |
Case 2 |
Case 3 |
|---|---|---|---|
| male | male | female | |
| Age at diagnosis (yr) | 73 | 47 | 58 |
| Initial diagnosis of AAT deficiency | 03/2015 | 06/2016 | 11/2016 |
| AAT Level [g/L] | 0.1–0.2 | <0.1 | 0.1 |
| Mutation | Pi*Z/c.1072C > T | Pi*Z/c.10del | Pi*Z/c.-5+1G > A and c.-472G > A |
| Allele | PiQ0Heidelberg II | PiQ0Heidelberg III | PiQ0Heidelberg IV |
| Pulmonary emphysema | Bilateral centri- to panlobular pulmonary emphysema with multiple increasing bullae ventrobasal, Incomplete fissures | Severely heterogeneous centri- to panlobular pulmonary emphysema with lower lobe emphasis | Basally accentuated panlobular (apical mixed-shaped) pulmonary emphysema |
| Onset of symptoms | Progressive DOE after bronchopulmonary infection −1996, Increased after a bilateral pulmonary embolism - 2008, Progressive DOE since 2015 | Progressive DOE Since 09/2015 | Progressive DOE Since 10/2016 |
| Further Diagnoses | COPD Gold III B, 3-vessel-coronary heart disease | COPD Gold IV D | COPD Gold II B, Obstructive sleep apnea, Obesity grade II |
| Smoking History(cumulative) | 4 pack-years until 1993 | 40 pack-years until 2019 | 25 pack-years until 2000 |
AAT = alpha-1 antitrypsin; DOE = dyspnea on exertion; COPD = chronic obstructive pulmonary disease.
All three patients received lung CT scans using a 64-channel multidetector CT scanner (Siemans Somatom Definition AS, Siemans Healthineers). The scanning parameters were 120 kVp, gantry rotation time 0.33 seconds (pitch 1.5), 0.6 mm ST with an increment of 0.7 mm (reconstructed ST 1 mm). An in-house developed computer software was used (YACTA) for fully automatic detection and quantification of emphysema on thin section CTs of the chest. It combines different segmentation algorithms and threshold-based techniques. In addition to providing thorough statistical analyses, YACTA also provides visualization of the calculated area affected by emphysema using color overlays on the original CT images.
The three cases described here include two males and one female with the following ages at diagnosis: 47, 58 and 73 years. All three presented with COPD with moderate to very severe airway obstruction (Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades II-IV). Serum AAT levels were very low (0.1–0.2 g/L). PCR testing revealed P1*MZ genotypes for all three patients and IEF showed only the Z-band. These tests suggested the presence of rare Q0 (null) mutations. DNA Sequencing of the SERPINA1 gene (7 exons) was conducted which identified three mutations which had not been previously described: Pi*Zc/1072C > T, Pi*Z/c.10del, and Pi*Z/c.-5+1G > A and c.-472G > A. These alleles were named PiQ0Heidelberg II, PiQ0Heidelberg III, and PiQ0Heidelberg IV, respectively. The PiQ0Heidelberg I variant was previously identified [12] and clinically characterized [13].
3. Case 1 - PiQ0Heidelberg II
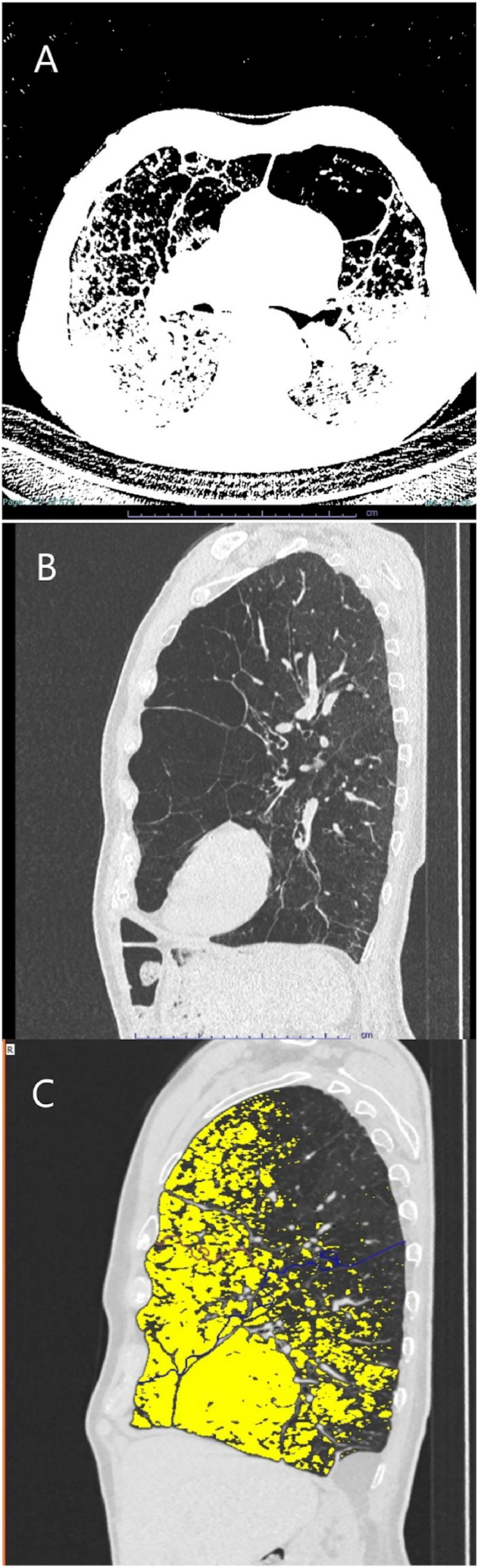
At his first visit, this 73-year-old male reported that dyspnea on exertion was first observed in 1996 after a bronchopulmonary infection. The patient has a 4 pack-year smoking history ending in 1993. Dyspnea increased after bilateral pulmonary embolisms in 2008. In March of 2015, the patient was diagnosed late with centrilobular to panlobular pulmonary emphysema and AATD (Fig. 1). The patient's COPD was GOLD IIB with the following lung function values: forced expiratory volume in 1 s (FEV1): 1.8 L (55% of predicted), residual volume (RV): 6.2 L (226% of predicted), diffusing capacity of lung for carbon monoxide in a single breath (DLCO SB): 50% of predicted. This patient was on supplemental oxygen, as needed, from 2015 and switched to long-term oxygen therapy at 2 L/min at a later time (exact date unknown). In 2021, progressive emphysema and worsening lung function was observed with an FEV1 of 1.2 L (37%) and a residual volume of 7.0 L (235%). This case of ventrally emphasized emphysema without a clear target and incomplete fissures was not amenable to surgical or endoscopic lung volume reduction.
Fig. 1.
CT scans and YACTA analysis of Case 1 - PiQ0Heidelberg II.A. A transverse view of the patient's lungs showing ventrally focused panlobular emphysema and deformed bronchopathy. B. A sagittal CT of the patient's lung again showing ventrally focused panlobular emphysema. C. YACTA analysis of the patient's sagittal CT scan showing panlobular emphysema (yellow) with an overall emphysema index of the lung of approximately 38%. Courtesy: Oliver Weinheimer, University Hospital Heidelberg. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Liver enzymes were not significantly elevated in this patient. No further hepatic studies were conducted.
The genotyping on this patient (heterogeneous polymorphism) was initially misinterpreted and no AAT augmentation therapy was initiated. Due to the incongruence of severe AATD and suspected heterogeneous polymorphism, DNA sequencing of the SERPINA1 gene was performed. Sequencing revealed a previously undescribed mutation, Pi*Zc/1072C > T. This allele was named PiQ0Heidelberg II.
In further treatment of this patient, several hospitalizations were necessary due to cardiac and pulmonary disease. Subsequently, the patient decided against substitution therapy.
4. Case 2 - PiQ0Heidelberg III
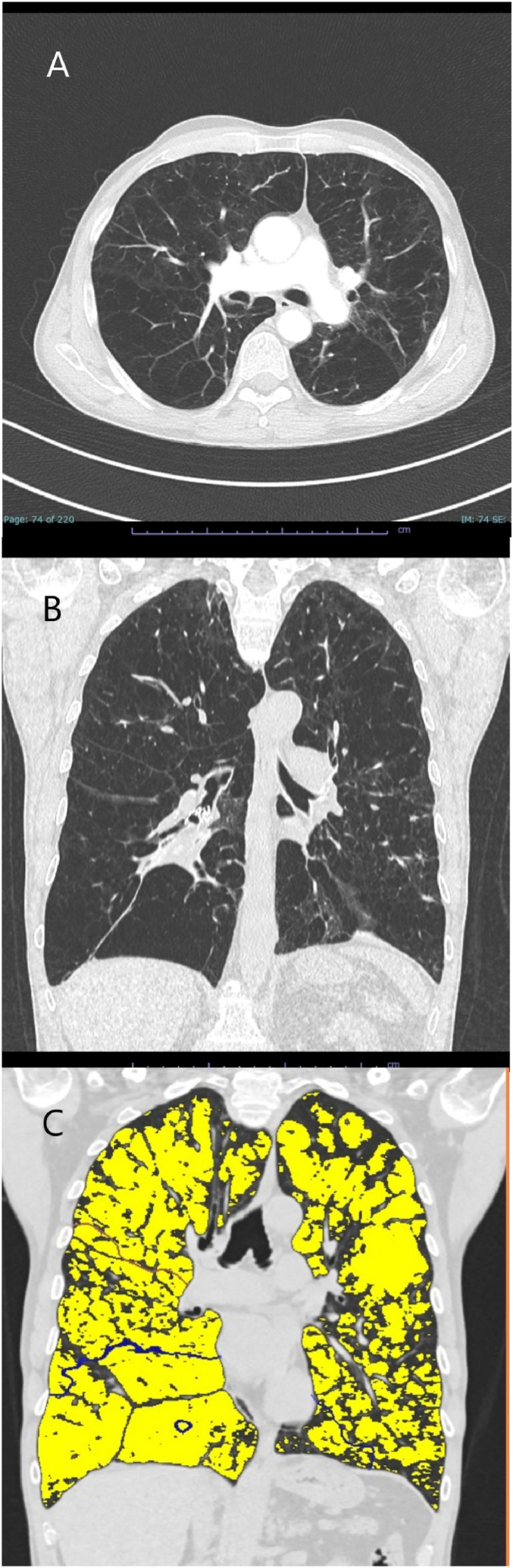
This 47-year-old male patient has suffered from progressive dyspnea on exertion since March 2015. They were diagnosed with severe AATD in 2016. The patient had a 40 pack-year history of smoking (until 2019) and advanced COPD (GOLD IV D) with severe pulmonary emphysema with emphasis on the lower lobes (Fig. 2). Initial lung function tests at clinic: FEV1 0.7 L (21%) and RV 5.4 L (259%). The patient has rejected long-term oxygen therapy. Endoscopic lung volume reduction was performed in December 2019 with placement of valves in the right lower lobe. At the 180-day follow-up, an improvement in clinical symptoms and lung function was noted with an increase in FEV1 to 0.9 L (27%) and a decrease in residual volume to 3.7 L (177%). In addition, an increased volume reduction of the right lower lobe was observed by computed tomography, however atelectasis could not be achieved. Liver transaminases were not significantly elevated in this patient and no additional hepatic testing was conducted.
Fig. 2.
CT scans and YACTA analysis of Case 2 - PiQ0Heidelberg III.A. A transverse view of the patient's lungs showing panlobular emphysema with deforming bronchopathy. Valves have been inserted in the right lower lobe with downstream dystelectic bundling. B. A coronal CT of the patient's lung showing severe panlobular emphysema especially in the right lower lobe. C. YACTA analysis of the patient's coronal CT scan showing severe panlobular emphysema (yellow) with an overall emphysema index of the lung of approximately 57%. Courtesy: Oliver Weinheimer, University Hospital Heidelberg. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Due to persistent smoking, initiation of AAT substitution therapy was delayed and was planned to start in September 2022. DNA sequencing of the SERPINA1 gene for this patient revealed a null mutation, PiZ/c.1072C > T. This allele has been named PiQ0HeidelbergIII.
5. Case 3 - PiQ0Heidelberg IV
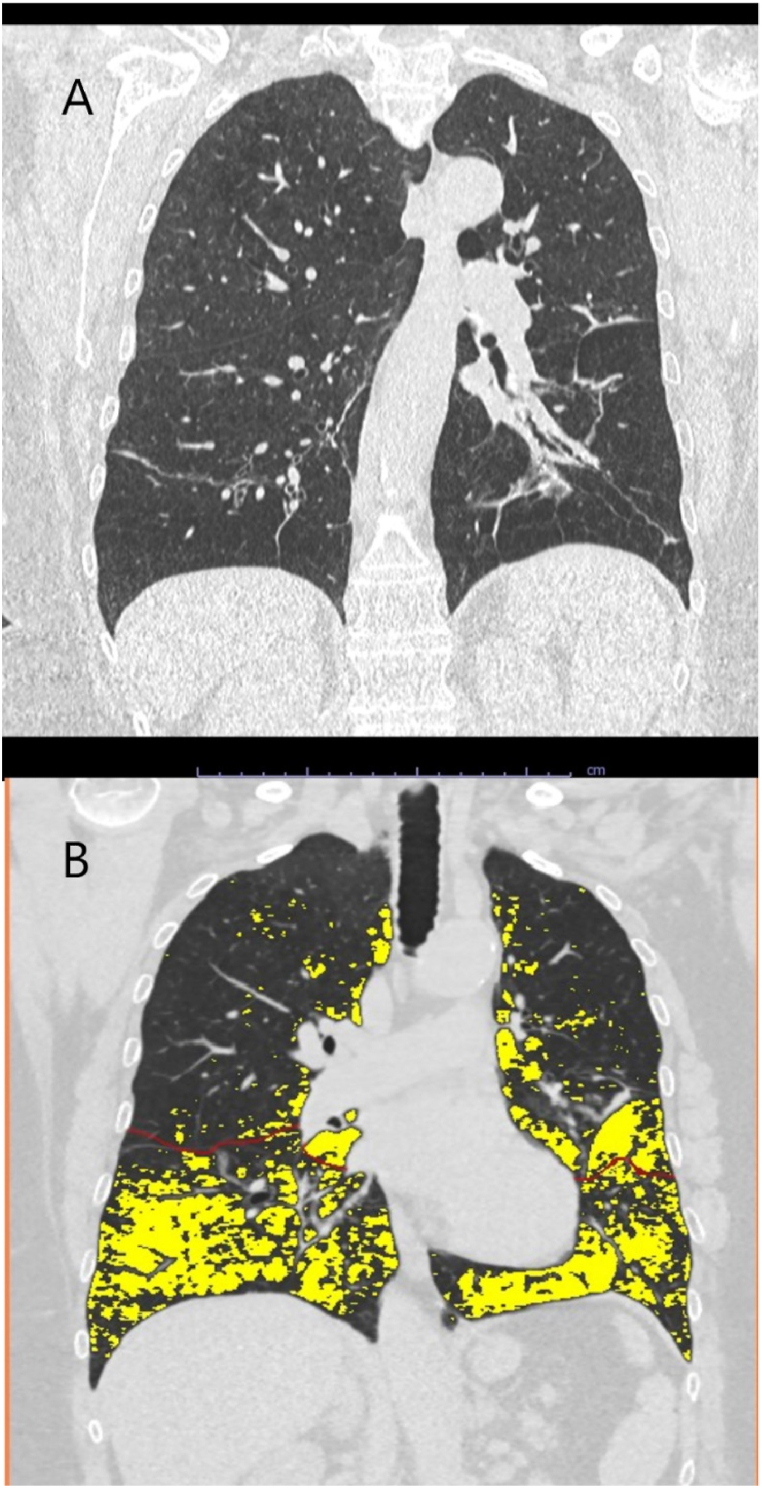
AATD was first detected in this 58-year-old female patient in November 2016. At the time of diagnosis, the patient's COPD was GOLD II B (Fig. 3) with the following lung function tests: FEV1: 1.6 L (63%), RV 4.2 L (210%), and DLCO SB: 57%. The patient reported a smoking history of 25 pack-years ending in 2000.
Fig. 3.
CT scan and YACTA analysis of Case 3 - PiQ0Heidelberg IV.A. A coronal view of the patient's lungs showing basal-focused panlobular emphysema and deforming bronchopathy with squamous atelectasis. B. YACTA analysis of the patient's coronal CT scan showing severe panlobular emphysema (yellow) with an overall emphysema index of the lung of approximately 19%. Courtesy: Oliver Weinheimer, University Hospital Heidelberg. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Liver transaminases were not significantly elevated in this patient but a liver elastography test was performed (Fibroscan®, Echosens, Paris, France). A normal value of 4.4 kPa was found (upper limit of normal 7.1 kPa). There was no evidence of major damage to the liver parenchyma, but the patient's controlled attenuation parameter (CAP) value was slightly elevated consistent with incipient steatosis hepatitis.
AAT augmentation therapy was initiated in this patient in December 2018. Stabilization of the patient's condition and lung function was achieved. DNA sequencing of the SERPINA1 gene in this patient reveal a novel null mutation, Pi*Z/c.-5+G > A and c.-472G > A. This allele was termed PiQ0Heidelberg IV.
6. Discussion and conclusions
In summary, severe AAT deficiency in addition to a history of smoking led to severe airway obstruction and advanced emphysema in patients with PiQ0Heidelberg II and PiQ0Heidelberg III mutations. In contrast, clinical and pulmonary function stabilization was achieved in PiQ0Heidelberg IV with timely initiation of substitution therapy.
Since both chronic obstructive pulmonary disease and AATD produce similar clinical symptoms such as dyspnea, cough and sputum production, diagnosis of AATD is frequently delayed. Therefore, early screening of COPD patients and identification of AATD is essential to help prevent the progression of emphysema through augmentation therapy. Very little clinical data is available on the rare SERPINA1 variants. Therefore, it is advisable to evaluate patients with rare SERPINA1 variants individually based on their serum AAT levels and clinical symptoms. Counseling the patient on treatment options and/or providing a referral to a center specializing in the treatment of AATD can be helpful in making the most effective treatment decision.
Ethics approval and consent to participate
Approval from the University of Heidelberg Ethics Committee was not requested since the university does not require ethics committee approval for the reporting of individual patient cases. Written informed consent was obtained from the patients in these case presentations.
Consent for publication
Written informed consent was obtained from the patients to allow their de-identified information to be used in this article.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
Publication of these case reports was supported by Grifols, S.A., Barcelona, Spain.
Authors contributions
All authors met the criteria for authorship established by the International Committee of Medical Journal Editors (ICMJE). PH and FCT compiled the patient cases and wrote the first draft of the manuscript. PH, FCT, JB, KS, MAP, JCS, and FH were involved in the clinical diagnosis and treatment of the patients as well as in the revision of the manuscript. KS assessed the CT scans and the findings of the YACTA analyses. MV and TG were responsible for the laboratory analyses at the Marburg University laboratory and the and interpretation of the genotyping findings. All authors read and approved the final manuscript.
Declaration of competing interest
PH, MV, TG, EL, JB, KS, KB, MAP, JCS and FH have no conflicts of interest. FCT reported personal fees from GlaxoSmithKline, Novartis and CSL Behring, outside the submitted work.
Acknowledgements
Michael K. James, PhD, CMPP (Grifols) is acknowledged for medical writing assistance.
List of abbreviations
- AAT
alpha-1 antitrypsin
- AATD
alpha-1 antitrypsin deficiency
- COPD
chronic obstructive pulmonary disease
- DLCO SB
diffusing capacity of lung for carbon monoxide in a single breath
- DOE
dyspnea on exertion
- FEV1
forced expiratory volume in one second
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- IEF
isoelectric focusing
- PCR
polymerase chain reaction
- RV
residual volume
References
- 1.Bashir A., Shah N.N., Hazari Y.M., Habib M., Bashir S., Hilal N., et al. Novel variants of SERPIN1A gene: interplay between alpha1-antitrypsin deficiency and chronic obstructive pulmonary disease. Respir. Med. 2016;117:139–149. doi: 10.1016/j.rmed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Gooptu B., Dickens J.A., Lomas D.A. The molecular and cellular pathology of alpha(1)-antitrypsin deficiency. Trends Mol. Med. 2014;20(2):116–127. doi: 10.1016/j.molmed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society and European Respiratory Society American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2003;168(7):818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 4.Dickens J.A., Lomas D.A. Why has it been so difficult to prove the efficacy of alpha-1-antitrypsin replacement therapy? Insights from the study of disease pathogenesis. Drug Des. Dev. Ther. 2011;5:391–405. doi: 10.2147/DDDT.S14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiello M., Frizzelli A., Marchi L., Ferrarotti I., Piloni D., Pela G., et al. Clinical manifestations of a new alpha-1 antitrypsin genetic variant: Q0parma. Respirol Case Rep. 2022;10(5) doi: 10.1002/rcr2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacopuzzi E., Laffranchi M., Berardelli R., Ravasio V., Ferrarotti I., Gooptu B., et al. Real-world clinical applicability of pathogenicity predictors assessed on SERPINA1 mutations in alpha-1-antitrypsin deficiency. Hum. Mutat. 2018;39(9):1203–1213. doi: 10.1002/humu.23562. [DOI] [PubMed] [Google Scholar]
- 7.Silva D., Oliveira M.J., Guimaraes M., Lima R., Gomes S., Seixas S. Alpha-1-antitrypsin (SERPINA1) mutation spectrum: three novel variants and haplotype characterization of rare deficiency alleles identified in Portugal. Respir. Med. 2016;116:8–18. doi: 10.1016/j.rmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Poller W., Merklein F., Schneider-Rasp S., Haack A., Fechner H., Wang H., et al. Molecular characterisation of the defective alpha 1-antitrypsin alleles PI mwurzburg (Pro369Ser), mheerlen (Pro369Leu), and Q0lisbon (Thr68Ile) Eur. J. Hum. Genet. 1999;7(3):321–331. doi: 10.1038/sj.ejhg.5200304. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.H., Brantly M. Molecular mechanisms of alpha1-antitrypsin null alleles. Respir. Med. 2000;94(Suppl C):S7–S11. doi: 10.1053/rmed.2000.0851. [DOI] [PubMed] [Google Scholar]
- 10.Renoux C., Odou M.F., Tosato G., Teoli J., Abbou N., Lombard C., et al. Description of 22 new alpha-1 antitrypsin genetic variants. Orphanet J. Rare Dis. 2018;13(1):161. doi: 10.1186/s13023-018-0897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santangelo S., Scarlata S., Poeta M.L., Bialas A.J., Paone G., Incalzi R.A. Alpha-1 antitrypsin deficiency: current perspective from genetics to diagnosis and therapeutic approaches. Curr. Med. Chem. 2017;24(1):65–90. doi: 10.2174/0929867324666161118125827. [DOI] [PubMed] [Google Scholar]
- 12.Graham R.P., Dina M.A., Howe S.C., Butz M.L., Willkomm K.S., Murray D.L., et al. SERPINA1 full-gene sequencing identifies rare mutations not detected in targeted mutation analysis. J. Mol. Diagn. 2015;17(6):689–694. doi: 10.1016/j.jmoldx.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Presotto M.A., Veith M., Trinkmann F., Schlamp K., Polke M., Eberhardt R., et al. Clinical characterization of a novel alpha1-antitrypsin null variant: PiQ0Heidelberg. Respir Med Case Rep. 2022;35 doi: 10.1016/j.rmcr.2021.101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.