Abstract
Childhood obesity has become a global health problem. Previous studies showed that childhood obesity is associated with brain structural differences relative to controls. However, few studies have been performed with longitudinal evaluations of brain structural developmental trajectories in childhood obesity. We employed voxel-based morphometry (VBM) analysis to assess gray matter (GM) volume at baseline and 2-year follow-up in 258 obese children (OB) and 265 normal weight children (NW), recruited as part of the National Institutes of Health Adolescent Brain and Cognitive Development study. Significant group × time effects on GM volume were observed in the prefrontal lobe, thalamus, right precentral gyrus, caudate, and parahippocampal gyrus/amygdala. OB compared with NW had greater reductions in GM volume in these regions over the 2-year period. Body mass index (BMI) was negatively correlated with GM volume in prefrontal lobe and with matrix reasoning ability at baseline and 2-year follow-up. In OB, Picture Test was positively correlated with GM volume in the left orbital region of the inferior frontal gyrus (OFCinf_L) at baseline and was negatively correlated with reductions in OFCinf_L volume (2-year follow-up vs. baseline). These findings indicate that childhood obesity is associated with GM volume reduction in regions involved with reward evaluation, executive function, and cognitive performance.
Keywords: ABCD, childhood obesity, executive function, gray matter volume, prefrontal lobe
Introduction
Overweight and obesity in children and adolescents has become a serious global health problem (Gonzalez-Alvarez et al. 2020). The World Health Organization (WHO) reported that over 340 million children and adolescents aged 5–19 were overweight or obese in 2016 (WHO Obesity and Overweight 2021, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight). Childhood obesity not only leads to musculoskeletal, respiratory, and digestive diseases, but also results in psychological problems such as depression, social marginalization, and lack of self-confidence (Pont et al. 2017; Sarokhani et al. 2020). Thus, mental and physical health problems associated with childhood obesity continue into adulthood, increasing the risk of ill health throughout the lifespan (Singh et al. 2008).
A number of studies reported that childhood obesity is associated with executive dysfunction (Maayan et al. 2011; Reinert et al. 2013; Liang et al. 2014; Yau et al. 2014; Laurent et al. 2020; Ronan et al. 2020), including inhibitory-control, attention, working memory, and reward sensitivity. Children with higher BMI had poorer cognitive performance than their lean counterparts (Liang et al. 2014; Laurent et al. 2020; Ronan et al. 2020), which had a large impact on food-intake, decision-making, response inhibition, and reward evaluation (Gluck et al. 2017). Functional magnetic resonance imaging studies demonstrated differences in activation in the dorsolateral prefrontal cortex in both adults and children with obesity (Le et al. 2006; Davids et al. 2010; Reinert et al. 2013). The orbitofrontal cortex (OFC) is also closely linked to obesity, and its activity was involved in decision making and emotional modulation (Rudebeck and Rich 2018). Differences in OFC activation during food cue exposure were observed between children/adolescents with obesity and normal weight (Batterink et al. 2010; Bruce et al. 2010; Stice et al. 2010).
Recently, a growing number of structural magnetic resonance imaging (sMRI) studies showed structural alterations in regions involved in executive function in obese children (Maayan et al. 2011; Saute et al. 2018; Laurent et al. 2020; Ronan et al. 2020). However, differences in sample size, statistical testing, sample population characteristics, and MRI metrics have led to mixed results. sMRI studies reported that obese children performed worse in working memory tasks than lean children and had lower cortical volume (Maayan et al. 2011) and cortical thickness (Saute et al. 2018) in the OFC, which was associated with BMI and visceral fat, respectively. Several recent studies based on the Adolescent Brain and Cognitive Development (ABCD) dataset reported that higher BMI was associated with lower prefrontal cortical thickness and executive function (Laurent et al. 2020; Ronan et al. 2020), and the decreased cortical thickness mediated the relationship between childhood obesity and impaired executive function. BMI also partially mediated the relationship between area deprivation index (ADI) and both cortical volume and executive function (Dennis et al. 2022). An increasing number of studies have highlighted the important role of the reward system including nucleus accumbens, caudate, putamen, and thalamus and emotion system including amygdala and hippocampus in weight regulation (Holsen et al. 2005; Holsen et al. 2006; Stoeckel et al. 2008; Stoeckel et al. 2009; Orsi et al. 2011). Other studies have demonstrated that higher BMI was associated with lower gray matter (GM) volume in the hippocampus, amygdala, caudate, and thalamus compared with normal weight controls (Orsi et al. 2011; Kirouac 2015). However, these studies were conducted cross-sectionally; few studies have been performed longitudinal evaluations of brain developmental trajectories in obese children.
The current study aimed to explore brain structural changes in obese children longitudinally by using the ABCD dataset, which includes sMRI data of both baseline and 2-year follow-up. Voxel-based morphometry (VBM) analysis was employed to assess differences in GM volume between 258 obese children (OB) and 265 normal weight children (NW). We hypothesized that the development of childhood obesity would be associated with differential developmental trajectories of GM volume in regions involved with executive function and reward processing.
Materials and methods
Participants
The current longitudinal study used data from the ABCD study (release 3.0; https://abcdstudy.org/), which was designed to prospectively examine the impact of childhood experiences on brain development and evaluate how these experiences are associated with social, emotional, and physical health and the development of risky behaviors and substance use. More than eleven thousand 9–10 years old children were recruited at 21 US sites and will be followed over the course of 10 years (Jernigan et al. 2018). Children are extensively assessed with measures of mental health, cognitive function, and social, cultural, and physical environments (Laurent et al. 2020). The assessments included structural and functional MRI using a standardized multi-site protocol (Casey et al. 2018). Analyses were conducted on data from the ABCD study (release 3.0), which included 11 787 children at baseline, 11 158 children at 1-year follow-up and 6546 children at 2-year follow-up (Supplementary Fig. 1). We excluded children with a current or past diagnosis of attention deficit/hyperactivity disorder, type 1 or type 2 diabetes, lead exposure, muscular dystrophy, history of traumatic brain injury, gestational age younger than 28 weeks and a BMI <10 or >45. The BMI percentiles for age and sex were used to classify individuals as underweight (i.e. < 5%), within acceptable limits (i.e. 5–85%), overweight (i.e. 85–95%), and obese (i.e. ≥95%) (CDC Growth Charts 2018, https://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm). Subjects who were obese at both baseline and 2-year follow-up were included in the OB group (n = 258, female 48.4%). Similarly, age-, gender-, race/ethnicity-, parental education-, household income-, site-matched NW subjects were included (n = 265, female 47.2%, Table 1).
Table 1.
Demographic and clinical information of obese (OB) and normal weight (NW) participants.
| OB, N = 258 (mean ± SE) | NW, n = 265 (mean ± SE) | OB 1 vs. NW1 | OB 2 vs. NW2 | Time × Group effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2-Year | P value | Baseline | 2-Year | P value | P value | P value | P value | |
| Age (months) | 119.5 ± 0.5 | 143.2 ± 0.5 | <0.001 | 119.1 ± 0.4 | 142.8 ± 0.4 | <0.001 | 0.456 | 0.501 | 0.819 |
| Gender (F/M) | 125/133 | 125/133 | N/A | 125/140 | 125/140 | N/A | 0.770a | 0.770a | N/A |
| BMI (kg/m 2 ) | 26.5 ± 0.2 | 30.0 ± 0.3 | <0.001 | 16.8 ± 0.1 | 18.1 ± 0.1 | <0.001 | <0.001 | <0.001 | <0.001 |
| ICV (cm 3 ) | 1495.0 ± 10.3 | 1521.1 ± 10.4 | <0.001 | 1483.1 ± 9.1 | 1513.7 ± 9.3 | <0.001 | 0.387 | 0.594 | 0.148 |
| Race/Ethnicity | |||||||||
| White | 39.1% | 39.1% | N/A | 38.9% | 38.9% | N/A | 0.809a | 0.809a | N/A |
| Black | 15.5% | 15.5% | 19.2% | 19.2% | |||||
| Hispanic | 32.2% | 32.2% | 29.8% | 29.8% | |||||
| Asian | 3.5% | 3.5% | 3.8% | 3.8% | |||||
| Other | 9.7% | 9.7% | 8.3% | 8.3% | |||||
| Household income, $ | |||||||||
| <50 K | 47.7% | 47.7% | N/A | 45.7% | 45.7% | N/A | 0.511a | 0.511a | N/A |
| 50 K–100 K | 48.4% | 48.4% | 48.3% | 48.3% | |||||
| >100 K | 3.9% | 3.9% | 6.0% | 6.0% | |||||
| Parental education | |||||||||
| <GED | 24.8% | 18.2% | N/A | 23.8% | 23.8% | N/A | 0.880a | 0.880a | N/A |
| college | 39.9% | 39.9% | 38.1% | 38.1% | |||||
| Bachelor | 24.4% | 24.4% | 24.9% | 24.9% | |||||
| Postgraduate | 10.9% | 10.9% | 13.2% | 13.2% | |||||
| ADI | 99.33 | N/A | N/A | 95.66 | N/A | N/A | 0.056 | N/A | N/A |
| Site | N/A | N/A | N/A | N/A | N/A | N/A | 0.803a | 0.803a | N/A |
Note: a: χ2 test.
OB1, OB_Baseline; NW1, NW_Baseline; OB2, OB_2-Year; NW2, NW_2-Year.
MRI acquisition
Subjects received an MRI scan at baseline, and an identical MRI scan was conducted at the 2-year follow-up. sMRI data were acquired using a protocol optimized for 3 T scanners (including Siemens, General Electric, and Philips). Whole-brain coverage was achieved using isotropic voxel resolution of 1 × 1 × 1 mm3, 256 × 256 matrix, repetition time of 2400–2500 ms, echo time of 2–2.9 ms, flip angle of 8°, inversion time of 1060 ms, 176–225 sections, field of view of 256 × 240 to 256, field of view phase of 93.75–100%, and parallel imaging of 1.5 × 2.2. Due to different parameters, the total acquisition time ranged from 5 min 38 s to 7 min 12 s (Casey et al. 2018).
Executive functions
All subjects were required to complete the National Institutes of Health (NIH) toolbox Cognitive measures (including baseline and 2-year follow-up) (Luciana et al. 2018), Cash Choice Task (CCT, including baseline) (Wulfert et al. 2002), Game of Dice Task (GDT, including 2-year follow-up) (Brand et al. 2005), and Matrix Reasoning Task (including baseline). Flanker Inhibitory-control and Attention (Flanker), Pattern Comparison Processing Speed (Pattern), Picture Sequence Memory (Picture), Oral Reading Recognition (Reading), Picture Vocabulary (Picvocab) and Crystallized Composite (Cryst) NIH toolbox Cognitive measures were included in the analysis. The raw scores were corrected for age to yield a final age-corrected score.
Voxel-based morphometry
T1 structural images were analyzed with Matlab 2012a (MathWorks Inc., Natick, MA) using VBM toolbox (http://dbm.neuro.uni-jena.de/vbm/download/) and Statistical Parametric Mapping (SPM12, https://www.fil.ion.ucl.ac.uk/spm/). In the current longitudinal study, preprocessing steps containing realignment, bias-correction, tissue classification, and spatial normalization were performed for imaging data of the two time points simultaneously. The GM images were smoothed by convolution with an isotropic Gaussian kernel (Full width at half maximum [FWHM] = 8 mm).
In addition, image quality check was performed visually (option "Display slices") and quantitatively (option "Check sample homogeneity") with tools available in the VBM toolbox. A total of 22 subjects (including 12 NW subjects and 10 OB subjects) were removed from the group analyses.
Statistical parametric mapping
SPM12 was used to perform voxel-wise analysis on GM images. Repeated-measures analysis of variance (ANOVA) was employed to assess time × group effects on GM volume while regressing out ADI. Clusters showing significant differences in GM volume after whole-brain false discovery rate (FDR) corrected P < 0.05 at the minimum cluster size of 20 voxel level were identified as regions of interest.
Time effects on GM volume were corrected for multiple comparisons using family wise error (FWE) corrections at the voxel level (PFWE < 0.001) with a minimum cluster size of 100 voxels. Group effects on GM volume were corrected for multiple comparisons using FWE corrections at the voxel level (PFWE < 0.01) with a minimum cluster size of 100 voxels.
Statistical analysis
Demographic information and executive function were analyzed by using SPSS (Statistical Package for Social Sciences, Release 22.0, IBM). Chi-square test was employed to evaluate differences in gender, race/ethnicity (including White, Black, Hispanic, Asian, and other), household income (including < $50 K, $50 K–$100 K and >$100 K), parental education (including <general educational development [GED], college, Bachelor, and postgraduate) and research site. Two-sample t-test was employed to examine differences in BMI, age (in months), intracranial volume (ICV), ADI, and executive function between OB and NW. Paired t-test was employed to examine differences in BMI, age, and ICV between baseline and 2-year follow-up. Repeated-measures ANOVA was employed to calculate interaction effects on age, BMI, ICV, and cognitive measures.
Associations between BMI, executive function, and GM volume
Partial correlations were performed to examine associations between GM volume, BMI, and executive function while controlling for ADI. Bonferroni corrections for multiple comparisons were set at P < 0.00177 (0.05/30) to control for 10 regions (Table 3), 2 cognitive measures (Picture Sequence Memory test and Matrix Reasoning total scaled score), and BMI.
Table 3.
Repeated measures ANOVA analysis on GM volumes between OB and NW group at baseline and 2-year follow-up. (voxel level-corrected, PFDR < 0.05).
| Region | Cluster size | MNI | Peak T - value | OB 1 vs. OB2 | NW 1 vs. NW2 | OB 1 vs. NW1 | OB 2 vs. NW2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | T | P | T | P | T | P | T | P | |||
| IPL_R | 976 | 56 | −42 | 52 | 4.99 | 9.324 | <0.001 | 1.896 | 0.059 | −0.215 | 0.830 | −4.044 | <0.001 |
| OFCsup/mid_R | 2922 | 15 | 69 | −3 | 4.89 | 8.126 | <0.001 | 1.561 | 0.120 | −0.879 | 0.380 | −5.197 | <0.001 |
| SFGmed_R | 9 | 65 | 12 | 4.52 | 8.864 | <0.001 | 2.137 | 0.033 | −2.537 | 0.011 | −6.669 | <0.001 | |
| SFGdor/med_L OFCsup/mid_L | −22 | 66 | 3 | 4.51 | 9.696 | <0.001 | 3.211 | 0.001 | −2.274 | 0.023 | −5.973 | <0.001 | |
| THA_R | 158 | 15 | −16 | 9 | 4.29 | 10.878 | <0.001 | 5.981 | <0.001 | −3.376 | <0.001 | −6.464 | <0.001 |
| PreCG_R | 79 | 41 | −12 | 58 | 3.58 | 9.619 | <0.001 | 4.929 | <0.001 | −0.683 | 0.495 | −3.241 | 0.001 |
| THA_L | 31 | −14 | −16 | 13 | 3.53 | 9.541 | <0.001 | 4.541 | <0.001 | −3.634 | <0.001 | −6.827 | <0.001 |
| OFCinf_L | 20 | −26 | 18 | −23 | 3.47 | 10.297 | <0.001 | 3.912 | <0.001 | −1.417 | 0.157 | −4.257 | <0.001 |
| CAU_R | 124 | 18 | 12 | 16 | 3.37 | 11.780 | <0.001 | 4.808 | <0.001 | −2.831 | 0.005 | −5.082 | <0.001 |
| PHG/AMYG_R | 82 | 21 | 0 | −24 | 3.36 | 5.311 | <0.001 | 0.453 | 0.651 | −1.588 | 0.113 | −4.435 | <0.001 |
Abbreviation: IPL_R, Right Inferior parietal lobule; OFCsup/mid_R, Right Orbital part of superior and middle frontal gyrus; SFGmed_R, Right Superior medial frontal gyrus; SFGdor/med_L, Left Superior medial and dorsolateral frontal gyrus; OFCsup/mid_L, Left Orbital part of superior and middle frontal gyrus; THA_R, Right Thalamus; PreCG_R, Right Precentral gyrus; THA_L, Left Thalamus; OFCinf_L, Left Orbital part of inferior frontal gyrus; CAU_R, Right Caudate; PHG/AMYG_R, Right Parahippocampal gyrus/Amygdala.
Results
Demographic characteristics
There were no significant group differences in gender, age, ICV, race/ethnicity, household income, highest educational level of caregiver, ADI, and research site between OB and NW (P > 0.05, Table 1), as expected. There were no time × group effects on age, ICV (Table 1), or cognitive measures (Table 2). There was a significant interaction effect on BMI (P < 0.001, Table 1), such that the OB group had significantly greater BMI increases than NW at 2-year follow-up compared with baseline. There were significant group effects on the Picture Sequence Memory test (at baseline and 2-year follow-up) and in the Matrix Reasoning total scaled score (at baseline), such that OB relative to NW had significantly lower scores on both tests (Table 2). There were no significant group differences in other cognitive tasks (P > 0.05, Table 2).
Table 2.
Executive function of OB and NW group.
| OB, N = 258 (Mean ± SE) | NW, N = 265 (Mean ± SE) | Group effect | Time × Group effect | ||||
|---|---|---|---|---|---|---|---|
| T value | P value | F value | P value | ||||
| NIH toolbox cognitive measurement age-corrected | |||||||
| Flanker Test | Baseline | 93.87 ± 0.83 | 94.68 ± 0.86 | −0.682 | 0.496 | 0.109 | 0.742 |
| 2-Year | 94.96 ± 0.88 | 95.33 ± 0.80 | −0.311 | 0.756 | |||
| Pattern Test | Baseline | 90.34 ± 1.37 | 91.94 ± 1.35 | −0.837 | 0.403 | 0.109 | 0.741 |
| 2-Year | 104.21 ± 1.25 | 106.44 ± 1.22 | −1.277 | 0.202 | |||
| Picture Test | Baseline | 98.21 ± 0.952 | 100.97 ± 0.93 | −2.074 | 0.039 | 0.660 | 0.417 |
| 2-Year | 102.10 ± 0.99 | 106.01 ± 0.90 | −2.932 | 0.004 | |||
| Reading Test | Baseline | 98.47 ± 1.03 | 99.49 ± 0.97 | −0.724 | 0.469 | 1.228 | 0.268 |
| 2-Year | 97.77 ± 0.97 | 99.96 ± 0.93 | −1.631 | 0.103 | |||
| Picvocab Test | Baseline | 104.35 ± 1.06 | 103.90 ± 0.95 | 0.318 | 0.750 | 1.214 | 0.271 |
| 2-Year | 99.78 ± 0.89 | 100.53 ± 0.93 | −0.581 | 0.562 | |||
| Cryst Test | Baseline | 101.77 ± 1.05 | 102.11 ± 0.97 | −0.236 | 0.813 | 2.052 | 0.153 |
| 2-Year | 98.78 ± 0.97 | 100.47 ± 0.94 | −1.246 | 0.213 | |||
| CCT | Baseline | 1.63 ± 0.03 | 1.62 ± 0.03 | 0.179a | 0.914a | N/A | N/A |
| GDT | 2-Year | 8.36 ± 0.33 | 8.12 ± 0.30 | 0.539 | 0.590 | N/A | N/A |
| Matrix Reasoning Total Scaled Score | Baseline | 9.19 ± 0.17 | 9.87 ± 0.18 | −2.800 | 0.005 | N/A | N/A |
Note: a: χ2 test.
Abbreviation: OB, obese children; NW, normal-weight children; Flanker, Flanker inhibitory control and attention; Pattern, pattern comparison processing speed; Picture, picture sequence memory; Reading, oral reading recognition; Picvocab, picture vocabulary; Cryst, crystallized composite.
Alterations in GM volume
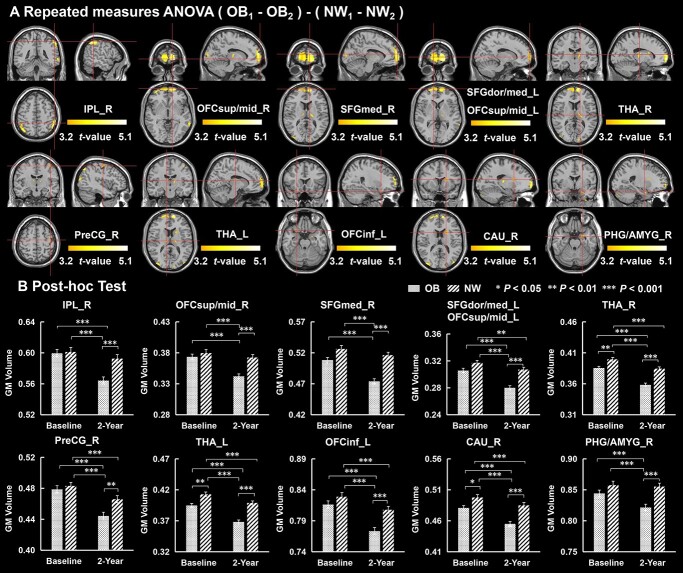
Significant group × time interaction effects on GM volume were observed in the right inferior parietal lobule (IPL_R), superior medial frontal gyrus (SFGmed), superior dorsolateral frontal gyrus (SFGdor), orbital part of superior and middle frontal gyrus (OFCsup/mid), OFCinf_L, THA, PreCG_R, CAU_R, and PHG/AMYG_R (PFDR < 0.05, Fig. 1A). Post hoc tests showed that compared with NW, OB had significantly greater decreases in GM volume in the IPL_R, SFGmed, SFGdor, OFCsup/mid, OFCinf_L, THA, PreCG_R, CAU_R, and PHG/AMYG_R at 2-year follow-up compared with baseline (Fig. 1B, Table 3).
Fig. 1.
Repeated measures ANOVA analysis for GM volume at baseline and 2-year follow-up between OB and NW group (voxel level-corrected, PFDR < 0.05). (A) Compared with NW, there were significant decreases in GM volume in the IPL_R, OFCsup/mid, SFGmed, SFGdor_L, PreCG_R, OFCinf_L THA, CAU_R and PHG/AMYG_R at 2-year follow-up compared with baseline in OB group. ADI was regressed out as a covariate. (B) Post-hoc tests for GM volume. IPL_R, right inferior parietal lobule; OFCsup/mid_R, right orbital part of superior and middle frontal gyrus; SFGmed_R, right superior medial frontal gyrus; SFGdor/med_L, left superior medial and dorsolateral frontal gyrus; OFCsup/mid_L, left orbital part of superior and middle frontal gyrus; THA_R, right thalamus; PreCG_R, right precentral gyrus; THA_L, left thalamus; OFCinf_L, left orbital part of inferior frontal gyrus; CAU_R, right caudate; PHG/AMYG_R, right Parahippocampal gyrus/amygdala.
Significant time effects on GM volume were observed across the whole brain (PFWE < 0.001). Compared with baseline, children had significantly lower whole brain GM volume at 2-year follow-up (Supplementary Fig. 2).
Significant group effects on GM volume were observed in the superior temporal gyrus (STG), middle cingulate cortex (MCC), right insula (INS_R), OFCinf_R, THA, left triangular part of inferior frontal gyrus (IFGtri_L), AMYG_R, right supplementary motor area (SMA_R), left precuneus (PCUN_L), and calcarine fissure (CAL) (PFWE < 0.01). Compared with NW, OB had significantly lower GM volume in the STG, MCC, INS_R, OFCinf_R, THA, IFGtri_L, AMYG_R, SMA_R, PCUN_L, and CAL (Supplementary Fig. 3, Supplementary Table 1).
Correlation between BMI, executive function, and GM volume
Across individuals in the OB group, BMI was negatively correlated with GM volume in THA_R (r = −0.2151, P = 0.0005) at baseline, and with GM volume in the THA_R (r = −0.2802, P < 0.0001) and THA_L (r = −0.2186, P = 0.0004; Fig. 2) at the 2-year follow-up, which all survived the correction for multiple comparisons (P < 0.00177).
Fig. 2.
Correlation analysis between BMI and GM volume in the THA_R at baseline and 2-year follow-up, and correlation analysis between BMI and GM volume in the THA_L at 2-year follow-up in OB. THA_R, right thalamus; THA_L, left thalamus.
In OB, there was a negative correlation between GM volume in the prefrontal lobe (OFCsup/mid_R, SFGmed_R, SFGdor/med_L, OFCsup/mid_L, OFCinf_L), CAU_R, and PHG/AMYG_R with BMI (Supplementary Figs. 4 and 5). GM volume in OFCinf_L was positively correlated with the Picture Sequence Memory test score at baseline (r = 0.137, P = 0.028, Supplementary Fig. 4), and the reduction in GM volume in OFCinf_L (2-year follow-up vs. baseline) was negatively correlated with Picture Sequence Memory Test score (at baseline, r = −0.133, P = 0.032, Supplementary Fig. 4) in OB. Matrix reasoning total scaled score (at baseline) was positively correlated with GM volume in OFCsup/mid_R at 2-year follow-up (r = 0.130, P = 0.046, Supplementary Fig. 4) and negatively correlated with BMI in OB at baseline (r = −0.162, P = 0.012) and at 2-year follow-up (r = −0.133, P = 0.041, Supplementary Fig. 5). However, these correlations did not survive correction for multiple comparisons.
Discussion
The current study compared changes in GM volume at baseline and 2-year follow-up between OB and NW children using the ABCD study dataset and explored their associations with BMI and executive functions. Results showed significant group × time effects on GM volume in IPL, OFC, SFG, CAU, PHG, AMYG, and THA. Compared with NW, OB had significantly decreased GM volume in IPL, OFC, SFG, CAU, PHG, AMYG, and THA at 2-year follow-up compared with baseline. BMI was negatively correlated with GM volume in THA_R at both time points and with GM volume in THA_L at 2-year follow-up. These findings highlight that childhood obesity is associated with GM volume reductions in regions involved with reward evaluation, executive function, and emotion, and that high BMI negatively affects brain development.
A number of studies showed decreases in GM volume across cortical regions in healthy adolescents throughout adolescence, with the largest decreases occurring in the prefrontal and parietal cortices (Tamnes et al. 2017), which are important components of the executive-control network and whose dysfunctions are closely related to obesity (Krafft et al. 2014; Kim et al. 2019; Ding et al. 2020), food preferences, eating disorders (Park et al. 2018; Lee et al. 2020), and cognitive functions (Seeley et al. 2007). Our data showed that OB compared with NW had significantly greater decreases in GM volume in the prefrontal lobe (OFCsup/mid_R, SFGmed_R, SFGdor/med_L, OFCsup/mid_L, OFCinf_L) and IPL_R at 2-year follow-up compared with baseline. Obesity is associated with accelerated reduction in GM volume in the IPL_R and prefrontal lobe (2-year follow-up vs. baseline), regions that are known to contribute to dysfunction of inhibitory-control and eating disorders. A functional magnetic resonance imaging (fMRI) study reported that children with higher BMI had less activation in the prefrontal cortex in response to unhealthy food (van Meer et al. 2016). Continuous changes in GM volume in these brain regions may reflect the children's eating habits. Our data documented a negative correlation between GM volume in the prefrontal lobe and BMI, which suggests detrimental structural changes in the prefrontal cortex (PFC) may lead to ensuing behavioral changes such as impairments in self-regulation that exacerbate weight gain. The GM volume in OFCinf_L was positively correlated with scores in the Picture Sequence Memory Test score at baseline, whereas volume reductions at 2-year follow-up (compared with baseline) were negatively correlated with the scores in Picture Sequence Memory Test at baseline. An fMRI study reported that obese compared with lean children had greater activation in the OFC when exposed to food cues (Bruce et al. 2010). The Picture Sequence Memory Test assess episodic memory, which plays an important role in food intake and weight regulation. High BMI was associated with worse episodic memory (Higgs and Spetter 2018). Several sMRI studies demonstrated that obese children had worse working memory performance than normal weight children and had lower cortical volume and cortical thickness in the OFC (Maayan et al. 2011; Saute et al. 2018; Laurent et al. 2020; Ronan et al. 2020). Cortical thickness in the OFC mediated the association between BMI and working memory (Laurent et al. 2020; Ronan et al. 2020). Episodic memory and working memory impairments contribute to problems with appetite control and weight gain (Higgs and Spetter 2018). The relationship between GM volume in the OFC and Picture Sequence Memory Test suggests obesity may affect GM volume development in children and impair episodic memory and working memory, which may further contribute to overeating and weight gain. We also found that Matrix Reasoning total scaled score (at baseline) was positively correlated with GM volume in OFCsup/mid_R at 2-year follow-up and negatively correlated with BMI in OB at baseline. Previous studies also reported a positive correlation between GM volume in the OFC and matrix reasoning ability in adolescents and young adults (Laurent et al. 2020; Ronan et al. 2020). The OFC is an important component of reward processing, which may regulate motivational pathways for food approach. The link between decreased GM volume in the OFC and reduced Matrix Reasoning ability in obese children suggests that lower reasoning ability might contribute to increased BMI by affecting food choices (Freidl et al. 2013). Although we did not observe the correlation between GM volume in SFG and executive function in the current study, prior studies found that GM volume in SFG was positively correlated with cognitive function, especially working memory and inhibitory control (du Boisgueheneuc et al. 2006; Taylor et al. 2020). In summary, we speculate that obesity leads to accelerated reductions in GM volume in IPL_R, SFG and OFC, which may account for the dysfunction of executive-control, episodic memory, and working memory.
The brain structures related to reward processing, particularly the amygdala (Kim, Luo, et al. 2020b), hippocampus, caudate, thalamus, play an important role in food intake control and body weight regulation (Areias and Prada 2015; Kirouac 2015; Kim, Shim, et al. 2020a). Previous sMRI studies revealed that obese individuals had reduced GM volume in the hippocampus, amygdala, caudate, and thalamus compared with normal weight individuals (Shott et al. 2015; Nouwen et al. 2017). Obese individuals have abnormal structural connectivity and lower fiber integrity of the reward network (including amygdala, caudate), which are associated with increased food intake (Marques-Iturria et al. 2015). In the present study OB showed significantly lower GM volume in THA_L/R, CAU_R, and PHG/AMYG_R at baseline and 2-year follow-up, compared with NW. These group differences GM volumes increased over the 2-year period, which was not reported in previous studies. Obese children with higher BMI had lower GM volume in the THA. These findings indicated that persistently reduced GM volume in these regions may contribute to weight gain in children. Since these regions have known roles in control of food intake and reward processing, we speculate that children who are obese for a long time may lead to dysfunction of the reward system. In addition, BMI (at 2-year follow-up) was negatively correlated with GM volume in PHG/AMYG_R (at 2-year follow-up), and with reduced GM volume in the CAU_R (2-year follow-up vs. baseline). Hippocampal-dependent learning and memory mechanisms involve food intake and body weight regulation (Kanoski and Grill 2017). The AMYG is an important region for food intake regulation and emotions such as anxiety and fear (Areias and Prada 2015). The CAU is an important component of reward system, including food-related reward processing. Previous studies reported that OB had reduced GM volume in these regions (Shott et al. 2015; Nouwen et al. 2017; Huang et al. 2019). In obese individuals, hippocampus and amygdala showed greater activation than normal weight individuals when exposed to food cues and greater resting-state activity (Li et al. 2021). In addition, the hippocampus and amygdala are also involved in emotional processing. Damage to the hippocampus and amygdala contribute to overeating. Previous studies showed that the hippocampus and amygdala are strongly involved in emotional eating in obese individuals, such as using overeating to counteract negative emotions (Wang et al. 2006; Konttinen 2020). However, due to the lack of eating behavior measurements, in the current study we could not determine the relationship between structural changes and motivation toward obtaining food rewards.
Limitations
There are some limitations of the current study. Firstly, at the time of this study, the ABCD dataset (release 3.0) has released only imaging data at 2-year follow-up for 6546 children. Due to the strict matching of children's gender, age, race/ethnicity, household income, parental education, sites distribution and ICV, the sample size is relatively small, which limits the generalizability of our findings. Secondly, we evaluated obese children at two time points but longer follow-up studies are needed to determine how childhood obesity influences brain structural developmental trajectories throughout adolescence. Finally, our findings are currently limited to sMRI studies. In the future, we will conduct multimodal MRI to evaluate brain developmental trajectories in obese children.
Conclusion
The current study revealed that compared with NW, OB had sustained reductions in GM volume in the prefrontal lobe, THA, PreCG_R, CAU_R, and PHG/AMYG_R at 2-year follow-up compared with baseline, which are regions involved in food intake control, reward processing, executive-control, episodic memory, and working memory. The findings indicate the impact of obesity on brain structural developmental trajectories in children.
Author contribution
Conceptualization: Gene-Jack Wang, Yi Zhang; Data acquisition, Fukun Jiang, Weibin Ji, Feifei Wu, Yaqi Zhang, Wenchao Zhang; Data analysis: Fukun Jiang, Guanya Li, Yang Hu; Writing-Original Draft: Fukun Jiang, Yi Zhang, Gene-Jack Wang; Writing-Review and Editing: XinBo Gao, Peter Manza, Dardo Tomasi, and Nora D. Volkow.
Supplementary Material
Acknowledgments
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the National Institute of Mental Health (NIMH) Data Archive (NDA). This is a multisite, longitudinal study designed to recruit >10 000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the National Institutes of Health (NIH) or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from NIMH Data Archive Digital Object Identifier (http://dx.doi.org/10.15154/1519007).
Contributor Information
Fukun Jiang, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Guanya Li, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Weibin Ji, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Yaqi Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Feifei Wu, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Yang Hu, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Wenchao Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Peter Manza, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, United States.
Dardo Tomasi, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, United States.
Nora D Volkow, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, United States.
Xinbo Gao, Chongqing Key Laboratory of Image Cognition, Chongqing University of Posts and Telecommunications, Chongqing 400065, China; Guangyang Bay Laboratory, Chongqing Institute for Brain and Intelligence, Chongqing 400064, China.
Gene-Jack Wang, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, United States.
Yi Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University and Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, Xi'an, Shaanxi 710126, China; International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment and Xi'an Key Laboratory of Intelligent Sensing and Regulation of Trans-Scale Life Information, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82172023); Natural Science Basic Research Program of Shaanxi (grant numbers 2022JC-44, 2022JQ-622); and support in part from the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism (grant number Y1AA3009 to PM, DT, NDV, GJW).
Conflict of interest statement: The authors declare no biomedical financial interests or potential conflicts of interest.
References
- Areias MF, Prada PO. Mechanisms of insulin resistance in the amygdala: influences on food intake. Behav Brain Res. 2015:282:209–217. [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. NeuroImage. 2010:52:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. Decision-making deficits of korsakoff patients in a new gambling task with explicit rules: associations with executive functions. Neuropsychology. 2005:19:267–277. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010:34:1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018:32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, Hamm A, Lotze M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes. 2010:34:94–104. [DOI] [PubMed] [Google Scholar]
- Dennis E, Manza P, Volkow ND. Socioeconomic status, BMI, and brain development in children. Transl Psychiatry. 2022:12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Ji G, Li G, Zhang W, Hu Y, Liu L, Wang Y, Hu C, von Deneen KM, Han Y, et al. Altered interactions among resting-state networks in individuals with obesity. Obesity (Silver Spring). 2020:28:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006:129:3315–3328. [DOI] [PubMed] [Google Scholar]
- Freidl EK, Sysko R, Devlin MJ, Zitsman JL, Kaplan SC, Walsh BT. School and cognitive functioning problems in adolescent bariatric surgery candidates. Surg Obes Relat Dis. 2013:9:991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck ME, Viswanath P, Stinson EJ. Obesity, appetite, and the prefrontal cortex. Curr Obes Rep. 2017:6:380–388. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvarez MA, Lazaro-Alquezar A, Simon-Fernandez MB. Global trends in child obesity: are figures converging? Int J Environ Res Public Health. 2020:17:9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Growth Charts . 2018. https://www.cdc.gov/growthcharts/clinical_charts.htm (last accessed 2021 December 3).
- Higgs S, Spetter MS. Cognitive control of eating: the role of memory in appetite and weight gain. Curr Obes Rep. 2018:7:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. NeuroImage. 2005:27:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring). 2006:14:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li X, Jackson T, Chen S, Meng J, Qiu J, Chen H. Interaction effect of sex and body mass index on gray matter volume. Front Hum Neurosci. 2019:13:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown SA, ABCD consortium coordinators . Introduction. Dev Cogn Neurosci. 2018:32:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Grill HJ. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry. 2017:81:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park BY, Byeon K, Park H, Kim Y, Eun YM, Chung JH. The effects of high-frequency repetitive transcranial magnetic stimulation on resting-state functional connectivity in obese adults. Diabetes Obes Metab. 2019:21:1956–1966. [DOI] [PubMed] [Google Scholar]
- Kim AY, Shim JH, Choi HJ, Baek HM. Comparison of volumetric and shape changes of subcortical structures based on 3-dimensional image between obesity and normal-weighted subjects using 3.0 T MRI. J Clin Neurosci. 2020a:73:280–287. [DOI] [PubMed] [Google Scholar]
- Kim MS, Luo S, Azad A, Campbell CE, Felix K, Cabeen RP, Belcher BR, Kim R, Serrano-Gonzalez M, Herting MM. Prefrontal cortex and amygdala subregion morphology are associated with obesity and dietary self-control in children and adolescents. Front Hum Neurosci. 2020b:14:563415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015:56:315–329. [DOI] [PubMed] [Google Scholar]
- Konttinen H. Emotional eating and obesity in adults: the role of depression, sleep and genes. Proc Nutr Soc. 2020:79:283–289. [DOI] [PubMed] [Google Scholar]
- Krafft CE, Schaeffer DJ, Schwarz NF, Chi L, Weinberger AL, Pierce JE, Rodrigue AL, Allison JD, Yanasak NE, Liu T, et al. Improved frontoparietal white matter integrity in overweight children is associated with attendance at an after-school exercise program. Dev Neurosci. 2014:36:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent JS, Watts R, Adise S, Allgaier N, Chaarani B, Garavan H, Potter A, Mackey S. Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatr. 2020:174:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006:84:725–731. [DOI] [PubMed] [Google Scholar]
- Lee H, Park BY, Byeon K, Won JH, Kim M, Kim SH, Park H. Multivariate association between brain function and eating disorders using sparse canonical correlation analysis. PLoS One. 2020:15:e0237511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GY, Hu Y, Zhang WC, Ding YY, Wang YY, Wang J, He Y, Lv GG, von Deneen KM, Zhao Y, et al. Resting activity of the hippocampus and amygdala in obese individuals predicts their response to food cues. Addict Biol. 2021:26:e12974. [DOI] [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes. 2014:38:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, Banich MT. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018:32:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring). 2011:19:1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Iturria I, Scholtens LH, Garolera M, Pueyo R, Garcia-Garcia I, Gonzalez-Tartiere P, Segura B, Junque C, Sender-Palacios MJ, Vernet-Vernet M, et al. Affected connectivity organization of the reward system structure in obesity. NeuroImage. 2015:111:100–106. [DOI] [PubMed] [Google Scholar]
- Nouwen A, Chambers A, Chechlacz M, Higgs S, Blissett J, Barrett TG, Allen HA. Microstructural abnormalities in white and gray matter in obese adolescents with and without type 2 diabetes. Neuroimage Clin. 2017:16:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi G, Perlaki G, Kovacs N, Aradi M, Papp Z, Karadi K, Szalay C, Karadi Z, Lenard L, Tenyi T, et al. Body weight and the reward system: the volume of the right amygdala may be associated with body mass index in young overweight men. Brain Imaging Behav. 2011:5:149–157. [DOI] [PubMed] [Google Scholar]
- Park BY, Lee MJ, Kim M, Kim SH, Park H. Structural and functional brain connectivity changes between people with abdominal and non-abdominal obesity and their association with behaviors of eating disorders. Front Neurosci. 2018:12:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont SJ, Puhl R, Cook SR, Slusser W, Section on Obesity, Obesity Society . Stigma experienced by children and adolescents with obesity. Pediatrics. 2017:14:e20173034. [DOI] [PubMed] [Google Scholar]
- Reinert KR, Po'e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes. 2013:2013:820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cereb Cortex. 2020:30:2519–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Rich EL. Orbitofrontal cortex. Curr Biol. 2018:28:R1083–R1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarokhani D, Sarokhani M, Hasanpour Dehkordi A, Ghanei Gheshlagh R, Fakhri M. Prevalence of obesity and overweight in Iranian students: a systematic review and meta-analysis. J Pediatr Endocrinol Metab. 2020:33:453–468. [DOI] [PubMed] [Google Scholar]
- Saute RL, Soder RB, Alves Filho JO, Baldisserotto M, Franco AR. Increased brain cortical thickness associated with visceral fat in adolescents. Pediatr Obes. 2018:13:74–77. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007:27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shott ME, Cornier MA, Mittal VA, Pryor TL, Orr JM, Brown MS, Frank GK. Orbitofrontal cortex volume and brain reward response in obesity. Int J Obes. 2015:39:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008:9:474–488. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010:50:1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008:41:636–647. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain Res Bull. 2009:79:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, Guroglu B, Raznahan A, Sowell ER, Crone EA, et al. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017:37:3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RL, Cooper SR, Jackson JJ, Barch DM. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open. 2020:3:e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meer F, van der Laan LN, Charbonnier L, Viergever MA, Adan RA, Smeets PA, Consortium IF. Developmental differences in the brain response to unhealthy food cues: an fMRI study of children and adults. Am J Clin Nutr. 2016:104:1515–1522. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Wong CT, Tomasi D, Thanos PK, Fowler JS. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006:103:15641–15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Obesity and overweight. 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (last accessed 2021 December 30).
- Wulfert E, Block JA, Santa Ana E, Rodriguez ML, Colsman M. Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. J Pers. 2002:70:533–552. [DOI] [PubMed] [Google Scholar]
- Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring). 2014:22:1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.