Abstract
STUDY QUESTION
Do twins conceived through assisted reproductive treatments (ART) grow differently from naturally conceived (NC) twins in early life?
SUMMARY ANSWER
Assessments at 6–8 weeks old and at school entry show that ART twins conceived from frozen embryo transfer (FET) grow faster than both NC twins and ART twins conceived from fresh embryo transfer (ET).
WHAT IS KNOWN ALREADY
Singletons born from fresh ET grow more slowly in utero and in the first few weeks of life but then show postnatal catch-up growth by school age, compared to NC and FET babies. Evidence on early child growth of ART twins relative to NC twins is inconsistent; most studies are small and do not distinguish FET from fresh ET cycles.
STUDY DESIGN, SIZE, DURATION
This cohort study included 13 528 live-born twin babies conceived by ART (fresh ET: 2792, FET: 556) and NC (10 180) between 1991 and 2009 in Scotland. The data were obtained by linking Human Fertilisation and Embryology Authority ART register data to the Scottish Morbidity Record (SMR02) and Scottish child health programme datasets. Outcome data were collected at birth, 6–8 weeks (first assessment), and school entry (4–7 years old) assessments. The primary outcome was growth, measured by weight at the three assessment points. Secondary outcomes were length (at birth and 6–8 weeks) or height (at school entry), BMI, occipital circumference, gestational age at birth, newborn intensive care unit admission, and growth rates (between birth and 6–8 weeks and between 6–8 weeks and school entry).
PARTICIPANTS/MATERIALS, SETTING, METHODS
All twins in the linked dataset (born between 1991 and 2009) with growth data were included in the analysis. To determine outcome differences between fresh ET, FET, and NC twins, linear mixed models (or analogous logistic regression models) were used to explore the outcomes of interest. All models were adjusted for available confounders: gestational age/child age, gender, maternal age and smoking, Scottish Index of Multiple Deprivation, year of treatment, parity, ICSI, and ET stage.
MAIN RESULTS AND THE ROLE OF CHANCE
In the primary birth weight models, the average birth weight of fresh ET twins was lower [–35 g; 95% CI: (−53, −16)g] than NC controls, while FET twins were heavier [71 g; 95% CI (33, 110) g] than NC controls and heavier [106 g; 95% CI (65, 146) g] than fresh ET twins. However, the difference between FET and NC twins was not significant when considering only full-term twins (≥37 weeks gestation) [26 g; 95% CI (–30, 82) g], while it was significantly higher in preterm twins [126 g; 95% CI (73, 179) g]. Growth rates did not differ significantly for the three groups from birth to 6–8 weeks. However, FET twins grew significantly faster from 6 to 8 weeks than NC (by 2.2 g/week) and fresh ET twins (by 2.1 g/week). By school entry, FET twins were 614 g [95% CI (158, 1070) g] and 581 g [95% CI (100, 1063) g] heavier than NC and fresh ET twins, respectively. Length/height and occipital frontal circumference did not differ significantly at any time point.
LIMITATIONS, REASONS FOR CAUTION
Although the differences between ART and NC reflect the true ART effects, these effects are likely to be mediated partly through the different prevalence of mono/dizygotic twins in the two groups. We could not explore the mediating effect of zygosity due to the unavailability of data. The confounding variables included in the study were limited to those available in the datasets.
WIDER IMPLICATIONS OF THE FINDINGS
Live-born twins from FET cycles are heavier at birth, grow faster than their fresh ET and NC counterparts, and are still heavier at school entry. This differs from that observed in singletons from the same cohort, where babies in the three conception groups had similar weights by school entry age. The results are reassuring on known differences in FET versus fresh ET and NC twin outcomes. However, FET twins grow faster and are consistently larger, and more ART twins depict catch-up growth. These may lead to an increased risk profile for non-communicable diseases in later life. As such, these twin outcomes require careful evaluation using more recent and comprehensive cohorts.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by the EU H2020 Marie Sklodowska‐Curie Innovative Training Networks (ITN) grant Dohartnet (H2020‐MSCA‐ITN‐2018-812660). The authors have no competing interests to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: assisted reproduction, birth weight, child growth, twins, catch-up growth, catch-down growth
Introduction
ART utilization has increased steadily since 1978 and now accounts for over 10 million babies born worldwide (ESHRE ART Fact Sheet, 2022). Even though most ART babies are born healthy, ART treatments are linked with a higher incidence of poor birth outcomes, including low birth weight (LBW), congenital abnormalities, preterm births (PTB), small for gestational age (<10th birth weight centile of babies with same gestational age), and in some cases perinatal mortality (Kamphuis et al., 2014; Sunde et al., 2016; Qin et al., 2017; Cavoretto et al., 2018; Maheshwari et al., 2018; Castillo et al., 2019; Wennerholm and Bergh, 2020; Xiong et al., 2022). These poor perinatal outcomes are partly the result of restricted intrauterine growth leading to altered growth trajectories, which may predispose babies to adverse later life outcomes (Barker, 1997).
Specific ART procedures, particularly frozen embryo transfer (FET) and fresh embryo transfer (ET), have been linked to differences in outcomes within the ART cohort. Several studies have reported that singleton fresh ET babies are smaller at birth, while FET babies are larger than their naturally conceived (NC) counterparts (Maheshwari et al., 2018; Castillo et al., 2019; Laval et al., 2020; Terho et al., 2021a). Some smaller ART growth studies have reported inconsistent differences in growth for fresh ET, FET, and NC babies (Ludwig et al., 2006; Sakka et al., 2010; Yeung et al., 2016; Magnus et al., 2021; Terho et al., 2021b). Two recent large cohort studies demonstrated that small fresh ET singletons show catch-up growth and weigh the same as FET and NC singletons by ∼5 years old or adolescence (Hann et al., 2018; Turner et al., 2020).
Most research on ART-related child growth outcomes has considered only singletons, and data on the development of ART twins are limited. ART is linked to increased rates of multiple births, mainly from the transfer of two or more embryos and a higher incidence of monozygotic twinning relative to NC pregnancies (Vitthala et al., 2009; Hviid et al., 2018; Ikemoto et al., 2018). These twins grow differently in utero, have a higher risk for adverse perinatal outcomes, and show different growth patterns in early life compared to their singleton counterparts (Vitthala et al., 2009; Geisler et al., 2014; Murray et al., 2019; Hiersch et al., 2020).
Despite the known risks when comparing twins to singletons, it is unclear whether twin pregnancies following ART are associated with higher risks of complications compared to NC twin pregnancies. Studies on ART twin growth are few and some report conflicting growth patterns between ART and NC twins in infancy (Koivurova et al., 2003; Lee et al., 2010; van Beijsterveldt et al., 2011; Yeung et al., 2016; Zhang et al., 2021). Other studies have not established significant differences in adverse perinatal outcomes between ART and NC twins (Andrijasevic et al., 2014; Geisler et al., 2014; Pourali et al., 2016; Chen et al., 2019; Murray et al., 2019; Pavoković et al., 2021). However, most of these studies are based on small samples and also do not distinguish FET from fresh ET babies, even though the impact of FET is now well documented in ART.
Although targeted policies such as elective single embryo transfer (eSET) have reduced twin rates to ∼10% in the UK and slightly higher elsewhere (Roberts et al., 2010; El-Toukhy et al., 2018), there is still a higher incidence of twin births following ART than NC. In addition, there is a generation of twins born before eSET; hence there is a need to understand how these babies grow and develop to understand risks in current and future twin generations. We hypothesize that similar to the singleton cohort (Hann et al., 2018), ART twins in this cohort may grow differently from their NC counterparts, translating into an increased risk profile for non-communicable diseases in later life. Therefore, this study aimed to compare the child growth outcomes for ART (fresh ET and FET cycles) relative to NC twins from birth to school entry age.
Materials and methods
Dataset
Data on ARTs between 1991 and 2009 from the Human Fertilisation and Embryology Authority (HFEA) register was linked to the Scottish Morbidity Record (SMR02) and Scottish child health programme datasets held by NHS Scotland (NHS NSS). The HFEA ART Register holds comprehensive records of all women who have used ART since August 1991. The SMR02 contains routinely collected birth records on obstetric outcomes from a woman’s period of care in an obstetric clinic. The Scottish child health programme collects child growth data from routine screening programmes. In an earlier study, Hann et al. (2018) first linked the HFEA and SMR02 datasets using probabilistic matching based on maternal names and maternal and child dates of birth, linking maternal ART treatment characteristics to birth outcomes. The individual child-level birth data were then linked to the Scottish child health programme dataset using individual child Community Health Index numbers. The study matched each individual baby conceived by ART to approximately four NC babies based on the mother’s age and child’s gender, resulting in 8791 ART and 35 100 NC babies (singleton and multiple births) born between 1991 and 2009. The growth data were collected at 6–8 weeks and school entry assessments (4–7 years old). Ethical approval was obtained before accessing and analysing this dataset.
Inclusion–exclusion criteria
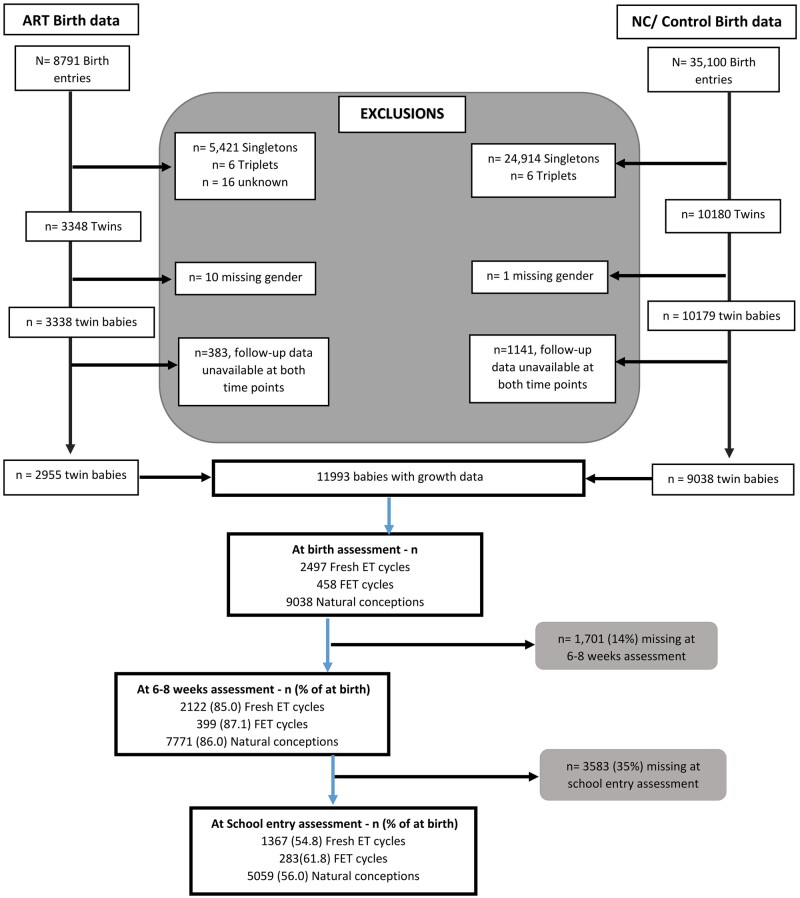
We included only twin-baby entries and excluded all singletons and triplets for the current study. We also excluded babies with missing gender and babies without growth data at either the 6–8 weeks and school entry assessments (n = 1142 (11%) NC and n = 393 (12%) ART). We thus extracted data on 11 993 twin babies for the current study based on the inclusion and exclusion criteria summarized in Fig. 1. In some cases, only one twin was available in the final dataset, as the corresponding twin was excluded. The resultant twin dataset comprised 2955 ART and 9038 NC babies. Data were thus available for ∼86% (n = 10 292) at 6–8 weeks and 56% (n = 6709) at school entry assessments. Lower numbers at 6–8 weeks and school entry assessments were assumed to be random dropouts. A sensitivity analysis of missing data/dropouts revealed that missingness was not associated with any available patient characteristics.
Figure 1.
Extraction of eligible twins sample. Flow chart showing exclusions made to ART (FET and fresh ET) and NC growth datasets to extract the resultant twins’ datasets. ET: embryo transfer; FET: frozen embryo transfer; NC: natural conception.
Outcome variables
The primary outcome was growth as measured by weight (g) at the three assessment points after adjustment for age at assessment (gestation at birth). Secondary outcomes were length (measured while lying down at birth and 6–8 weeks) or height (measured while standing at school entry), occipital circumference (OFC) (mm) at birth and 6–8 weeks assessment, BMI (kg/m2) at school entry, gestational age at birth (weeks), newborn intensive care unit (NICU) admission, and growth rates. Average growth rates were defined as the average change in weight per week (between birth and 6–8 week measurements, and between 6–8 weeks and school entry measurements). We further explored growth rates for discordant twins as a sensitivity analysis. Twin growth discordance was defined as an inter-twin birth weight discordance > 20%: (larger twin birth weight − smaller twin birth weight)/larger twin birth weight × 100% (Miller et al., 2012).
In addition, we explored whether babies with lower or higher birth weights (smaller or larger babies) tended to the median, i.e. catch-up or catch-down growth. We transformed birth weight and school entry weight into deciles of age-standardized z-scores using tables of age and gender-specific L (skew), M (median), and S (coefficient of variation) values (Cole, 2012). A baby was categorized as smaller or larger at birth if they fell into the 1st–3rd or 8th–10th deciles, respectively. We defined catch-up or catch-down growth as a baby moving at least one decile up or down on the standardized growth charts from birth to school entry age.
Data analysis
Descriptive statistics are presented as counts (percentages) or mean values (±standard deviation). We modelled the outcomes (weight, length, OFC, BMI, gestation, and growth rate) using separate multivariable linear mixed models at each of the three time points (birth, 6–8 weeks, and school entry) with a random effect term (twin-set) to account for correlation within twin pairs or shared parental characteristics. NICU admission was analysed using analogous logistic regression models. We utilized the intraclass correlation coefficient (ICC) to report the correlation in outcomes within a twin set.
For analyses comparing ART (fresh ET and FET cycles) and NC babies, we adjusted for baby gender, age (gestational age at birth, child age at 6–8 weeks, and school entry), deprivation index (assessed from the quintiles of the 2012 Scottish Index of Multiple Deprivation) (The Scottish Government, 2020), smoking status (self-reported), year of delivery, maternal age, and feeding mode (breast, bottle, both) at 10 days after birth (for child growth outcomes at 6–8 weeks and school entry). Gestational age at birth was fitted as a restricted cubic spline with four degrees of freedom, and maternal age was fitted using a quadratic function. Sensitivity analyses indicated that the selected functional forms adequately captured the age–outcome relationships; additional degrees of freedom for the splines did not improve model fit as assessed using AIC. Similarly, interaction terms between conception groups and gender did not significantly improve the fit. For within ART analyses, we additionally adjusted for the use of ICSI, parity, infertility causes, and IVF treatment centre. As sensitivity analyses, we further explored the weight outcome for twins born at different gestational ages (extremely preterm (GA < 28), preterm (GA < 37), term babies (GA ≥ 37)) and growth rates for discordant twins.
All statistical analyses were performed in Stata (version 16; Stata Corporation, College Station, TX, USA), and 95% confidence intervals for the estimates are presented.
Results
Data summaries
The primary and secondary outcome variables are presented in Table I. Other summary statistics (characteristics of mothers, babies, and ART treatment information) are presented in Supplementary Table SI. Overall, the proportion of infants within particular subgroups of characteristics did not vary substantively across conception groups or at different assessment points (Supplementary Table SII).
Table I.
Summaries of outcome variables at the assessment points by conception type.
| Birth outcomes | Naturally conceived n = 9038 | ART fresh ET n = 2497 | ART frozen ET n= 458 |
|---|---|---|---|
| Raw birth weight (g)a | 2399 [571] | 2395 [572] | 2575 [510] |
| Raw birth weightb | |||
| <2500 g | 4767 (52.7) | 1322 (52.9) | 197 (43.0) |
| ≥2500 g and ≤4000 g | 4252 (47.1) | 1173 (47.0) | 261 (57.0) |
| >4000 g | 7 (0.1) | 2 (0.1) | 0 (0.0) |
| Crown–heel length (cm)a | 47.2 [3.4] | 47.4 [3.2] | 47.9 [3.1] |
| 4034 (44.6) | 1154 (46.2] | 195 (42.4) | |
| OFC (cm)a | 32.90 [1.73] | 33.0 [1.74] | 33.30 [1.46] |
| 6082 (67.3) | 1683 (67.4) | 322 (70.1) | |
| NICU admissionb | |||
| No | 5353 (59.2) | 1437 (57.6) | 318 (69.4) |
| Yes | 3592 (39.7) | 1031 (41.3) | 135 (29.5) |
| Gestation (weeks)a | 35.7 [2.7] | 35.7 [2.8] | 36.1 [2.3] |
| 9036 (99.2) | 2497 (100.0) | 458 (100.0) | |
| Gestation (weeks)b | |||
| Full term (gestation ≥ 37) | 4393 (48.6) | 1279 (51.2) | 254 (55.5) |
| Preterm (gestation < 37) | 4643 (51.4) | 1218 (48.8) | 204 (44.5) |
| 6–8 week assessmenta | 7771 (86.0) | 2122 (85.0) | 399 (87.1) |
| Weight (g) | 4464 [817] | 4422 [937] | 4538 [770] |
| 7771 (86.0) | 2122 (85.0) | 399 (87.1) | |
| OFC (cm) | 37.95 [2.19] | 38.08 [2.48] | 38.09 [1.53] |
| 7664 (84.8) | 2098 (84.0) | 399 (83.1) | |
| Length (cm) | 54.4 [3.3] | 54.4 [3.4] | 55.0 [3.0] |
| 7649 (84.6) | 2089 (83.7) | 396 (86.5) | |
| School entry assessmenta | 5059 (56.0) | 1367 (54.8) | 283 (61.8) |
| Weight (g) | 19 806 [3047] | 19 939 [2906] | 20 203 [3284] |
| 5059 (56.0) | 1367 (54.8) | 283 (61.8) | |
| Height (cm) | 112.3 [5.4] | 113.1 [4.9] | 113.1 [6.0] |
| 5059 (56.0) | 1367 (54.8) | 283 (61.8) | |
| Body mass index (kg/m2) | 16.1 [24.3] | 15.5 [1.6] | 15.8 [1.7] |
| 5059 (56.0) | 1367 (54.8) | 283 (61.8) |
Continuous variables are presented as mean [standard deviation] and cell count (percentage of the sample at birth).
Categorical variables are presented as cell count (column percentage). ET: embryo transfer; OFC: occipital frontal circumference; NICU: neonatal intensive care unit.
Child weight at all assessment points: ART versus NC
The average unadjusted birthweight was 2399 g, 2395 g, and 2575 g for NC, fresh ET, and FET twins, respectively (Table I). Compared to NC twins, birth weight was significantly lower in fresh ET twins [−35 g; 95% CI (−53, −16)] and higher in FET twins [71 g; 95% CI (33, 110)] in the fully adjusted model (Table II). Additional sensitivity analyses showed that the difference between FET and NC twins was not significant when considering only full-term babies (GA ≥ 37 weeks) [26 g; 95% CI (−30, 82)], while it was significantly higher in preterm babies (GA < 37 weeks) [126 g; 95% CI (73, 179)]. In contrast, differences between NC and fresh ET babies persisted for both preterm and full-term babies. At 6–8 weeks, fresh ET twins were still smaller than NC twins [−59 g; 95% CI (−103, −16)], while FET twins though slightly heavier, did not differ significantly from NC twins (Table II and Supplementary Table SIII). FET twins were 614 g and 581 g heavier than NC and fresh ET twins at school entry, respectively.
Table II.
Weight at the three assessment points for ART versus NC twins.
|
Unadjusted
BW N = 11 982 |
Gestation-adjusted BW N = 11 946 | Weight at 6–8 weeks N = 9798 | Weight at school entry N = 5814 | |
|---|---|---|---|---|
|
|
||||
| Effect size [95% CI] (g) | ||||
| Type of conception | ||||
| ART—fresh ET vs NC | –9 [–42 to 25] | –35 [–53 to –16] | –59 [–103 to –16] | 32 [–203 to 268] |
| ART—frozen ET vs NC | 196 [127–266] | 71 [33–110] | 58 [–30 to 147] | 614 [158–1070] |
| Frozen ET vs fresh ET | 205 [131–279] | 105 [65–146] | 118 [25–211] | 581 [100–1063] |
| ICCa | 0.76 [0.75–0.77] | 0.38 [0.36–0.41] | 0.41 [0.38–0.43] | 0.51 [0.49–0.53] |
Intraclass correlation coefficient. Multiple linear regression results for models (except unadjusted birthweight) adjusted for child gender and age (gestation at birth, child age at 6–8 weeks and school assessment), maternal age, Scottish Index of Multiple Deprivation, smoking status, feeding mode (at 6–8 weeks and school assessment) and year of birth. Full model results are presented in Supplementary Table SIII. ET: embryo transfer; BW: Birthweight; NC: natural conception.
The ICC values show that outcomes for babies born to the same mother (twin sets and the very few sibling twin sets) were highly correlated, suggesting that some proportion of variance in twin sets was associated with shared maternal/paternal factors. Further analysis also revealed that only 17% (n = 2038 babies) of the twins in the study were discordant twins (highly divergent from one another).
Child weight at all assessment points and ART factors: FET versus fresh ET
Within the ART cohort, we explored whether other ART factors (infertility diagnosis, treatment centre, ICSI, and parity) accounted for the observed associations between FET and fresh ET outcomes. Adjusting for extra ART factors (Table III), FET twins were consistently heavier at birth [104 g; 95% CI (63, 145)] and school entry assessment [by 601 g; 95% CI (119, 1083)] than fresh ET twins. None of the other explored ART factors significantly affected the outcome estimates (Table III).
Table III.
Effect of patient and ART treatment factors on the twin weights at the three assessment points for fresh and frozen ET twins.
| Birth weight N = 2892 | Weight at 6–8 weeks N = 2360 | Weight at school entry N = 1429 | |
|---|---|---|---|
|
|
|||
| Effect size [95% CI] (g) | |||
| Embryo transfer type | |||
| ART—fresh cyclea | Ref | Ref | Ref |
| ART—frozen cycle | 104 [63–145] | 95 [–12 to 202] | 601 [119–1083] |
| ICSI | |||
| Yesa | Ref | Ref | Ref |
| No | 16 [–27 to 59] | –58 [–171 to 55] | –163 [–717 to 391] |
| Infertility causeb | |||
| Endometrial | 17 [–39 to 74] | –62 [–209 to 86] | –25 [–708 to 1658] |
| Male factor | 31 [–17 to 80] | 28 [–104 to 160] | 263 [–388 to 912] |
| Ovulatory | 15 [–41 to 71] | –57 [–208 to 94] | –282 [–1019 to 456] |
| Fallopian tube | 22 [–29 to 72] | 20 [–116 to 156] | 264 [–360 to 889] |
| Unknown | 36 [–16 to 88] | 8 [–131 to 148] | 219 [–443 to 881] |
| Previous births | |||
| Nonea | Ref | Ref | Ref |
| 1 | 38 [–15 to 90] | 87 [–50 to 224] | 150 [–482 to 782] |
| 2+ | 397 [19 to 776] | 225 [–691 to 1142] | – |
| ICCc | 0.26 [0.21–0.31] | 0.29 [0.23 to 0.35] | 0.39 [0.33 to 0.46] |
Reference group.
Patients can have multiple infertility causes.
Intraclass correlation coefficient. Multiple linear regression results for weight (fresh ET vs frozen ET) at the three time points, adjusted for the variables shown and additionally for child gender, age (gestation at birth, child age at 6–8weeks, and school entry), Scottish Index of Multiple Deprivation, maternal age, year of delivery, smoking, treatment centre, feeding mode (6–8 weeks and school entry only). Effect estimate for 2+ previous births is not available at school entry due to the unavailability of data. ET: embryo transfer.
Secondary birth and growth outcomes
In the adjusted model, gestation was slightly longer in FET twins by 0.32 weeks (∼3 days) (Supplementary Table SIV). These results correspond to the higher rates of PTB observed in fresh (49%) and NC (51%) twins compared to FET (44%) twins. Babies conceived from fresh ET were 23% more likely, while FET babes were 26% less likely to be admitted to the NICU. OFC and crown–heel length did not differ significantly between NC babies and babies born from FET or fresh transfers.
At the 6–8 weeks assessment, OFC did not differ significantly between ART and NC twins, while FET twins were longer than NC twins [by 0.5 cm; 95% CI (0.15, 0.85)] and fresh ET twins [by 0.61 cm; 95% CI (0.24, 0.97)] (Supplementary Table SV). At school entry assessment, FET twins were slightly taller than NC twins [1.02 cm; 95% CI (0.25, 1.80)], while BMI did not differ significantly between ART and NC twins.
Considering fresh ET versus FET babies and adjusting for ART factors, FET babies were slightly taller at 6–8 weeks [by 0.72 cm; 95% CI (0.32, 1.10)] and school entry [by 0.87 cm; 95% CI (0.04, 1.70)]. OFC at birth was 0.22 cm longer [95% CI (0.01, 0.42)] in FET twins, while there were no differences in OFC at 6–8 weeks and BMI at school entry between fresh and FET twins (Supplementary Table SVI).
Growth rates
Average growth rates did not differ significantly between NC and ART twins from birth to 6–8 weeks. From 6–8 weeks to school entry, FET twins grew significantly faster than their NC counterparts [by 2.17 g/week; 95% CI (0.54, 3.82)] (Table IV). A sensitivity analysis indicated that the difference in growth rates was mainly confined to term babies [2.8g/week; 95% CI (0.74, 4.88)] compared to preterm babies [1.2 g/week; 95% CI (−1.44, 2.83)]. No differences were observed when comparing fresh ET and NC twins.
Table IV.
Growth rates of twin births from fresh or frozen ET and NC twins between birth and 6–8 weeks, and 6–8 weeks and primary school entry (4–7 years).
| Birth to 6–8 weeks (grams per week) |
6–8 weeks to school entry (grams per week) |
|||
|---|---|---|---|---|
| Unadjusted (N = 10 254) | Adjusted (N = 10 231) | Unadjusted (N = 5675) | Adjusted (N = 5663) | |
| Type of conception | ||||
| Ref | Ref | |||
| ART—fresh ET vs NC | –5.03 [–9.50 to –0.60] | –3.60 [–8.21 to 1.01] | 0.14 [–0.69 to 0.97] | 0.11 [–0.76 to 1.03] |
| ART—frozen ET vs NC | 2.50 [–6.86 to 11.85] | 3.11 [–6.23 to 12.41] | 2.07 [0.45–3.69] | 2.17 [0.54–3.82] |
| Frozen vs fresh ET | 7.52 [–2.41 to 17.46] | 6.71 [–3.10 to 16.51] | 1.93 [0.20–3.66] | 2.05 [0.32–3.79] |
| ICCa | 0.22 [0.20–0.25] | 0.20 [0.17–0.23] | 0.54 [0.52–0.57] | 0.54 [0.51–0.57] |
Intraclass correlation coefficient. Linear regression models for growth rates between birth and 6–8 weeks, and 6–8 weeks and primary school entry (average weekly growth rates), adjusting for type of conception, gender, Scottish Index of Multiple Deprivation, maternal age and smoking status during pregnancy, feeding mode, and the year of delivery.
A total of 2038 (17%) babies were categorized as birthweight-discordant twins, of which 1503 (17%), 453 (18%), and 82 (18%) were NC, fresh ET, and FET, respectively. Overall, growth rates between 6–8 weeks and school entry did not differ when comparing smaller to larger twins from discordant twin sets, while smaller twins grew slightly faster between birth and 6–8 weeks assessment [by 80.1 g/week; 95% CI (71.6, 88.6)] (Supplementary Table SVII).
Adjusting for additional ART variables, FET twins grew faster than fresh ET twins between 6–8 weeks and school entry [2.09 g/week; 95% CI (0.43 3.74)]. Though still higher in FET twins, the growth rates did not differ significantly from birth to 6–8 weeks [5.8 g/week; 95% CI (−5.7, 17.2)] (Supplementary Table SVII).
Catch-up/catch-down growth
Table V summarizes and compares babies showing catch-up or catch-down growth between ART and NC babies. With marginal statistical significance, slightly more fresh ET babies [43%, OR: 1.32 (1.01, 1.73)] showed catch-up growth from birth to school entry compared to FET babies (37%). The catch-up growth proportions did not significantly differ when comparing NC to fresh ET or FET babies, even though fresh ET babies were more likely [63%, OR: 1.31 (1.08, 1.57)] to depict catch-up growth than NC babies (57%) when considering the smaller babies. There were no differences in catch-down growth between NC and fresh ET or FET babies. However, slightly fewer fresh ET babies [OR: 0.71 (0.53, 0.97)] showed catch-down growth than FET babies (27% FET vs 21% fresh). A sensitivity analysis looking at growth rates for babies categorized as smaller or larger (in Table V) revealed that smaller babies grew slightly slower [−2.47 g/week; 95% CI (−2.96, −1.97)] between 6–8 weeks and school entry, while larger babies grew slightly faster [by 2.32 g/week; 95% CI (1.61, 3.02)] (Supplementary Figures S1 and S2).
Table V.
Comparisons of proportions of catch-up or catch-down growth between birth and school entry for twins conceived by NC, fresh ET, or frozen ET.
| Catch-up growth |
Catch-down growth |
|||||||
|---|---|---|---|---|---|---|---|---|
| All babies (n = 6707) |
Smaller babies (n = 3109) |
All babies |
Larger babies (n = 974) |
|||||
| n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | |
| NCa | 2119 (42%) | — | 1360 (57%) | — | 1165 (23%) | — | 438 (61%) | — |
| Fresh ET | 595 (43%) | 1.06 (0.94–1.20) | 400 (63%) | 1.31 (1.08–1.57) | 292 (21%) | 0.91 (0.78–1.05) | 101 (56%) | 0.83 (0.59–1.17) |
| Frozen ET | 105 (37%) | 0.82 (0.63–1.05) | 64 (58%) | 1.07 (0.72–1.63) | 78 (27%) | 1.27 (0.96–1.67) | 39 (55%) | 0.79 (0.47–1.34) |
| Frozenb vs fresh | — | 1.32 (1.01–1.73) | — | 1.21 (0.78–1.87) | — | 0.71 (0.53–0.97) | — | 1.05 (0.58–1.89) |
Reference group for raw odds ratios comparing NC with fresh ET twins, NC with frozen ET twins.
Reference group comparing Frozen ET with Fresh ET twins. Catch-up or catch-down growth is defined as a baby crossing one decile upwards or downwards on the age/gender-adjusted growth charts between birth and school entry. Smaller babies are defined as those in the 1st–3rd deciles at birth, while larger babies are those in the 8th–10th deciles. Proportions are presented for all twins experiencing catch-up growth for smaller babies or catch-down growth for larger babies. NC: natural conception; ET: embryo transfer; OR: odds ratio.
Discussion
The results from our large national cohort study suggest that newborn twins conceived by fresh ET have a significantly lower birth weight than NC twins, whereas FET twins are heavier, consistent with singleton studies (Hann et al., 2018; Maheshwari et al., 2018; Laval et al., 2020; Terho et al., 2021b). Contrary to what was observed in singletons from the same cohort (Hann et al., 2018), FET twins grow faster than NC and fresh ET twins after the first assessment, and by school entry, FET twins are heavier than NC and fresh ET twins. Of the ART treatment factors explored, only embryo transfer type (fresh ET/FET) significantly impacted the studied outcomes. The use of ICSI, infertility diagnosis, and previous pregnancies did not pose any strong confounding effects in the within-ART analyses.
Compared to NC twins, FET twins have a slightly longer average gestation (by ∼3 days) than fresh ET twins. This may partly explain why FET twins have a higher unadjusted BW; however, FET twins remain significantly heaver even following gestation length adjustment. In addition, sensitivity analyses revealed that birth weight differences between FET and NC twins were only observed in preterm babies (gestation < 37 weeks), suggestive of possible differences in embryo growth emerging after 37 weeks gestation, where FET twins presumably grew more slowly. The mechanisms behind these suggested foetal growth differences remain unclear. However, some researchers linked the differences to varying epigenetic changes and differences in gene expression in placentas and children from pregnancies originating after FET, fresh ET, and NC (Estill et al., 2016; Lee et al., 2019). Others have linked oestradiol and progesterone concentrations following ovarian stimulation with an increased risk of LBW in fresh ET babies (Järvelä et al., 2014).
Growth rates between birth and 6–8 weeks did not differ significantly between ART and NC twins. At school entry, FET twins were heavier than NC and fresh ET twins, which is consistent with higher growth rates observed in the FET group after 6–8 weeks. One study (Yeung et al., 2016) reported similar results of slightly rapid infant weight gain (adjusted OR: 1.08; 95% CI: 1.00, 1.16) among twins conceived by ART only up to 9 months. Afterwards, no significant differences in growth through to 3 years of age were observed between the ART and NC groups. Contrary to our weight findings, a study (van Beijsterveldt et al., 2011) investigating the growth of ART twins from birth to 12 years of age reported similar weight and height growth patterns between IVF and NC twins. Similarly, an earlier Finish study of 100 IVF twins matched to control twins until age 3 years found no evidence of differences in growth measures between IVF and NC twins (Koivurova et al., 2003), consistent with a study comparing 157 ART to 549 NC twins from birth and up to age 18 months in Taiwan (Lee et al., 2010). It is noteworthy that the above studies were small, and the ART groups did not distinguish fresh ET from FET cycles, which could potentially mask the differences as the literature is now clear and consistent on differences in outcomes between these two ART groups. A sensitivity analysis of our data comparing the two groups (ART vs NC twins) could only detect minor differences in birthweight whilst masking the reported differences between NC and ART (FET) cycles at school entry. Additionally, our results show that the effects differ in magnitude and often direction between fresh ET and FET twins. Based on these results, it is clear that we would have reported similar findings to the above studies if we did not account for the use of FET.
Unlike in singletons (Hann et al., 2018), where fresh ET infants notably catch up more, nearly half of the babies in each of the three groups depict catch-up growth by school entry. The results further suggest that slightly more FET twins show catch-up growth. In contrast, larger fresh ET and NC twins had catch-down growth, probably further explaining why we observed higher weight averages in the FET group at school entry. It is well-documented from historical studies that twins are generally smaller than singletons at birth (Wilson, 1979; Chaudhari et al., 1997; Buckler and Green, 2004). In most cases, they experience restricted intrauterine growth starting from ∼27 weeks gestation (Hiersch et al., 2020), often corrected postnatal by altered/accelerated growth trajectories, evidenced by ∼40% of all babies in our study experiencing catch-up growth. A study comparing growth in NC singletons and twins suggested fast weight gain in twins immediately after birth which slowed down after 2 years old (Chaudhari et al., 1997; Buckler and Green, 2004), consistent with other historical studies that reported catch-up growth by 8 years old (Wilson, 1979; Morley et al., 1989). The differences between fresh ET and NC singletons in Hann's et al. (2018) study can be attributed to fresh ET babies, on average, being smaller than NC babies, hence the observed catch-up. However, it is unclear why FET twins appear to grow faster than the smaller NC and fresh ET twins. Perhaps the generally small size of twins implies that all twin babies, regardless of conception type, are susceptible to some form of catch-up in growth or grow faster after birth. It can be argued that the observed growth patterns in both ART and NC twin babies are a compensatory process where the infants attempt to reach their genetic growth potential after a period of prenatal growth restraint. The evidence from NC twin studies suggests that twins slow down in growth after ∼2 years old and slowly catch up to their singleton counterparts by 8 years old. It is possible that by school entry age (5–7 years old), both NC and ART twins in this cohort are still catching up in growth; hence, the differences observed in growth may be attributed to known growth patterns in twins and not only ART factors. Perhaps a more extended period would provide information about whether twins in the three conception groups reach similar weights at a later age.
We are aware of complexities associated with twin pregnancies, such as differences in twin pregnancy outcomes due to zygosity–chorionicity. Buckler and Green (2008) reported that dizygotic twins had slightly better outcomes than monozygotic twins. Therefore, some of the observed differences between ART and NC twins in the current study may be associated with single ET ART pregnancies resulting in more monozygotic twins than NC pregnancies (Sobek et al., 2016). Although it would be informative to explore the impact of zygosity on the outcomes, our primary comparisons of ART and NC twins remain valid. As a sensitivity analysis, we replicated the analyses on a subset of girl–boy twin sets that were assumed to be dizygotic. The differences in child outcomes and growth rate estimates among conception groups were similar to what is reported in the main analysis. In addition, we acknowledge the use of singleton growth references for centile calculations whose application in twins has been disputed; hence, we cautiously interpret the results that focus on smaller versus larger twin catch-up or catch-down comparisons. We are also mindful that gestation and feeding mode can be considered mediators instead of confounders. However, we treated feeding mode as more of a maternal characteristic (certain groups of mothers are likely to breastfeed or not) and a decision likely to have been taken before birth or even conception. We adjusted for gestation length as child growth (size for age) needs to be adjusted for time to grow (gestational age in utero). In the main, we were interested in the effect of ART on outcomes and hence adjusted for gestation and feeding, which are known to be associated with these outcomes; and this is in line with most studies on ART growth.
Mainly, these data include babies born before the introduction of twin reduction policies and changes in recent treatment trends; thus, outcomes in more recent data may differ. In addition, the introduction of consent for disclosure allowing the use of HFEA data for similar linkage studies may lead to serious biases due to low and potentially non-random consent rates in the post-2009 cohort. However, our study is one of the few large national follow-up ART twin cohorts with a reasonable sample size to distinguish fresh ET from FET cycles in the analysis. Even though we acknowledge that more comprehensive data on confounders would be preferred, we managed to adjust for some important confounding factors; hence, we believe that the results are informative.
The results of our study are somewhat reassuring. However, the observed small differences at the child level between ART and NC twins do translate into small but not insignificant population-level differences. In addition, these minor differences in birth outcomes (suggestive of altered foetal growth) and growth outcomes (suggestive of catch-up/down growth) after birth could still potentially lead to a higher risk of cardiovascular diseases (Ceelen et al., 2009) and metabolic diseases, including obesity and Type 2 diabetes in later life (Ceelen et al., 2009; Kerkhof and Hokken-Koelega, 2012; Martin et al., 2017). The higher proportion of twins showing catch-up growth implies that these risks may be more pronounced in twins. Since twin rates are higher in ART, it implies more adverse outcomes; hence, it is reasonable to advance twinning preventative measures in ART.
Conclusion
This UK national cohort study is one of the few large ART twin growth studies showing differences in birth weight and growth trajectories between ART (separating fresh ET from FET cycles) and NC twins between birth and school entry age (4–7 years old). We have linked the ART treatment (fresh ET/FET) to the weight outcome and have demonstrated that compared to fresh ET and NC twins, FET twins differ significantly in birth weight, grow faster, and remain heavier by school entry age. Treatments are constantly changing, and this cohort ends in 2009; hence there is a need to replicate these in more recent cohorts to capture changes in ART practice. Future research should explore more recent ART cohorts with more extended follow-up periods and collect more detailed data for more comprehensive adjustments for confounding.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the considerable and vital roles of both the Human Fertilisation and Embryology Authority and NHS Public Health Scotland in conducting this research. In particular, Lizzie Nicholson guided the use of the Safe Haven as a data storage and analysis platform, monitored study outputs, and replied to numerous and varied queries relating to all aspects of the study conduct and logistics.
Contributor Information
Fiskani J M Kondowe, Division of Population Health, Health Services Research & Primary Care, School of Health Sciences, Faculty of Biology, Medicine and Health, Centre for Biostatistics, The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Peter Clayton, Division of Developmental Biology and Medicine, Child Health & Paediatric Endocrinology, School of Medical Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Sciences Centre, Manchester, UK.
Matthew Gittins, Division of Population Health, Health Services Research & Primary Care, School of Health Sciences, Faculty of Biology, Medicine and Health, Centre for Biostatistics, The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Stephen W D’Souza, Division of Developmental Biology and Medicine, Maternal & Fetal Health Research Centre, School of Medical Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Sciences Centre, Manchester, UK.
Daniel R Brison, Division of Developmental Biology and Medicine, Maternal & Fetal Health Research Centre, School of Medical Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester Academic Health Sciences Centre, Manchester, UK; Department of Reproductive Medicine, Old St Mary’s Hospital, Manchester University NHS. Foundation Trust, Manchester Academic Health Sciences Centre, Manchester, UK.
Stephen A Roberts, Division of Population Health, Health Services Research & Primary Care, School of Health Sciences, Faculty of Biology, Medicine and Health, Centre for Biostatistics, The University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article were provided by the Human Fertilisation and Embryology Authority and NHS Public Health Scotland under licence/by permission. The data are not publicly accessible, but may be accessed after ethics approvals.
Authors’ roles
F.J.M.K. performed all the data manipulation and cleaning, designed and conducted the formal analyses, and drafted the manuscript. Expert methodological advice was provided by S.A.R. and M.G., with expert clinical input from D.R.B., P.C., and S.W.D.S. D.R.B. and S.A.R. obtained the funding and conceptualized the study. S.A.R., D.R.B., M.G., and P.C. co-supervised the project and reviewed the analysis. All authors contributed to the preparation and critical review of the manuscript.
Funding
This study was funded by the EU H2020 Marie Sklodowska‐Curie Innovative Training Networks (ITN) grant Dohartnet (H2020‐MSCA‐ITN‐2018-812660).
Conflict of interest
The authors have no competing interests.
References
- Andrijasevic S, Dotlic J, Aksam S, Micic J, Terzic M.. Impact of conception method on twin pregnancy course and outcome. Geburtshilfe Frauenheilkd 2014;74:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Intra-uterine programming of the adult cardiovascular system. Curr Opin Nephrol Hypertens 1997;6:106–110. [DOI] [PubMed] [Google Scholar]
- Buckler JMH, Green M.. A comparison of the early growth of twins and singletons. Ann Hum Biol 2004;31:311–332. [DOI] [PubMed] [Google Scholar]
- Buckler JMH, Green M.. The growth of twins between the ages of 2 and 9 years. Ann Hum Biol 2008;35:75–92. [DOI] [PubMed] [Google Scholar]
- Castillo CM, Horne G, Fitzgerald CT, Johnstone ED, Brison DR, Roberts SA.. The impact of IVF on birthweight from 1991 to 2015: a cross-sectional study. Hum Reprod 2019;34:920–931. [DOI] [PubMed] [Google Scholar]
- Cavoretto P, Candiani M, Giorgione V, Inversetti A, Abu-Saba MM, Tiberio F, Sigismondi C, Farina A.. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound Obstet Gynecol 2018;51:43–53. [DOI] [PubMed] [Google Scholar]
- Ceelen M, Van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JPW, Spreeuwenberg M, Van Leeuwen FE, Delemarre-van de Waal HA.. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod 2009;24:2788–2795. [DOI] [PubMed] [Google Scholar]
- Chaudhari S, Bhalerao MR, Vaidya U, Pandit A, Nene U.. Growth and development of twins compared with singletons at ages one and four years. Indian Pediatr 1997;34:1081–1086. [PubMed] [Google Scholar]
- Chen H, Wan Y, Xi H, Su W, Cheng J, Zhu C, Lv J, Wu X, Zhao J.. Obstetric and perinatal outcomes of dizygotic twin pregnancies resulting from in vitro fertilization versus spontaneous conception: a retrospective study. PeerJ 2019;7:e6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ. The development of growth references and growth charts. Ann Hum Biol 2012;39:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Toukhy T, Bhattacharya S, Akande VA.. Multiple pregnancies following assisted conception: scientific impact paper No. 22. BJOG 2018;125:e12–e18. [DOI] [PubMed] [Google Scholar]
- ESHRE ART Fact Sheet. European Society of Human Reproduction and Embryology. 2022. https://www.icmartivf.org/reports-publications (12 December 2022, date last accessed).
- Estill MS, Bolnick JM, Waterland RA, Bolnick AD, Diamond MP, Krawetz SA.. Assisted reproductive technology alters deoxyribonucleic acid methylation profiles in bloodspots of newborn infants. Fertil Steril 2016;106:629–639.e10. [DOI] [PubMed] [Google Scholar]
- Geisler ME, O’Mahony A, Meaney S, Waterstone JJ, O’Donoghue K.. Obstetric and perinatal outcomes of twin pregnancies conceived following IVF/ICSI treatment compared with spontaneously conceived twin pregnancies. Eur J Obstet Gynecol Reprod Biol 2014;181:78–83. [DOI] [PubMed] [Google Scholar]
- Hann M, Roberts SA, D’Souza SW, Clayton P, Macklon N, Brison DR.. The growth of assisted reproductive treatment-conceived children from birth to 5 years: a national cohort study. BMC Med 2018;16:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiersch L, Okby R, Freeman H, Rosen H, Nevo O, Barrett J, Melamed N.. Differences in fetal growth patterns between twins and singletons. J Matern Fetal Neonatal Med 2020;33:2546–2555. [DOI] [PubMed] [Google Scholar]
- Hviid KVR, Malchau SS, Pinborg A, Nielsen HS.. Determinants of monozygotic twinning in ART: a systematic review and a meta-analysis. Hum Reprod Update 2018;24:468–483. [DOI] [PubMed] [Google Scholar]
- Ikemoto Y, Kuroda K, Ochiai A, Yamashita S, Ikuma S, Nojiri S, Itakura A, Takeda S.. Prevalence and risk factors of zygotic splitting after 937 848 single embryo transfer cycles. Hum Reprod 2018;33:1984–1991. [DOI] [PubMed] [Google Scholar]
- Järvelä IY, Pelkonen S, Uimari O, Mäkikallio K, Puukka K, Ruokonen A, Tekay A, Martikainen H.. Controlled ovarian hyperstimulation leads to high progesterone and estradiol levels during early pregnancy. Hum Reprod 2014;29:2393–2401. [DOI] [PubMed] [Google Scholar]
- Kamphuis EI, Bhattacharya S, Van Der Veen F, Mol BWJ, Templeton A;. Evidence Based IVF Group. Are we overusing IVF? BMJ 2014;348:g252. [DOI] [PubMed] [Google Scholar]
- Kerkhof GF, Hokken-Koelega ACS.. Rate of neonatal weight gain and effects on adult metabolic health. Nat Rev Endocrinol 2012;8:689–692. [DOI] [PubMed] [Google Scholar]
- Koivurova S, Hartikainen AL, Sovio U, Gissler M, Hemminki E, Järvelin MR.. Growth, psychomotor development and morbidity up to 3 years of age in children born after IVF. Hum Reprod 2003;18:2328–2336. [DOI] [PubMed] [Google Scholar]
- Laval M, Garlantézec R, Guivarc'h-Levêque A.. Birthweight difference of singletons conceived through in vitro fertilization with frozen versus fresh embryo transfer: an analysis of 5406 embryo transfers in a retrospective study 2013–2018. J Gynecol Obstet Hum Reprod 2020;49:101644. [DOI] [PubMed] [Google Scholar]
- Lee B, Koeppel A, Wang E, Gonzalez T, Sun T, Kroener L, Lin Y, Joshi N, Ghadiali T, Turner S. et al. Differential gene expression during placentation in pregnancies conceived with different fertility treatments compared with spontaneous pregnancies. Fertil Steril 2019;111:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee MY, Chiang TL, Lee MS, Lee MC.. Child growth from birth to 18 months old born after assisted reproductive technology—results of a national birth cohort study. Int J Nurs Stud 2010;47:1159–1166. [DOI] [PubMed] [Google Scholar]
- Ludwig AK, Sutcliffe AG, Diedrich K, Ludwig M.. Post-neonatal health and development of children born after assisted reproduction: a systematic review of controlled studies. Eur J Obstet Gynecol Reprod Biol 2006;127:3–25. [DOI] [PubMed] [Google Scholar]
- Magnus MC, Wilcox AJ, Fadum EA, Gjessing HK, Opdahl S, Juliusson PB, Romundstad LB, Håberg SE.. Growth in children conceived by ART. Hum Reprod 2021;36:1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Raja EA, Shetty A, Hamilton M, Bhattacharya S.. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? 2018;24:35–58. [DOI] [PubMed] [Google Scholar]
- Martin A, Connelly A, Bland RM, Reilly JJ.. Health impact of catch-up growth in low-birth weight infants: systematic review, evidence appraisal, and meta-analysis. Matern Child Nutr 2017;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, , ChauhanSP, , Abuhamad AZ.. Discordant twins: diagnosis, evaluation and management. Am J Obstet Gynecol 2012;206:10–20. [DOI] [PubMed] [Google Scholar]
- Morley R, Cole TJ, Powell R, Lucas A.. Growth and development in premature twins. Arch Dis Child 1989;64:1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SR, Bhattacharya S, Stock SJ, Pell JP, Norman JE.. Gestational age at delivery of twins and perinatal outcomes: a cohort study in Aberdeen, Scotland. Wellcome Open Res 2019;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavoković D, Gašparović VE, Tupek T, Gregorić A, Luetić AT.. Perinatal outcome in twins. A hospital based comparative study at a single third-level care centre in Croatia. Clin Exp Obstet Gynecol 2021;48:631. [Google Scholar]
- Pourali L, Ayati S, Jelodar S, Zarifian A, Andalibi MSS.. Obstetrics and perinatal outcomes of dichorionic twin pregnancy following art compared with spontaneous pregnancy. Int J Reprod Biomed 2016;14:317–322. [PMC free article] [PubMed] [Google Scholar]
- Qin JB, Sheng XQ, Wu D, Gao SY, You YP, Yang TB, Wang H.. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Arch Gynecol Obstet 2017;295:285–301. [DOI] [PubMed] [Google Scholar]
- Roberts S, McGowan L, Hirst W, Brison D, Vail A, Lieberman B.. Towards single embryo transfer? Modelling clinical outcomes of potential treatment choices using multiple data sources: Predictive models and patient perspectives. Health Technol Assess 2010;14:1–237. [DOI] [PubMed] [Google Scholar]
- Sakka SD, Loutradis D, Kanaka-Gantenbein C, Margeli A, Papastamataki M, Papassotiriou I, Chrousos GP.. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil Steril 2010;94:1693–1699. [DOI] [PubMed] [Google Scholar]
- Sobek A, Prochazka M, Klaskova E, Lubusky M, Pilka R.. High incidence of monozygotic twinning in infertility treatment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016;160:358–362. [DOI] [PubMed] [Google Scholar]
- Sunde A, Brison D, Dumoulin J, Harper J, Lundin K, Magli MC, Van Den Abbeel E, Veiga A.. Time to take human embryo culture seriously. Hum Reprod 2016;31:2174–2182. [DOI] [PubMed] [Google Scholar]
- Terho AM, Pelkonen S, Opdahl S, Romundstad LB, Bergh C, Wennerholm UB, Henningsen AA, Pinborg A, Gissler M, Tiitinen A.. High birth weight and large-for-gestational-age in singletons born after frozen compared to fresh embryo transfer, by gestational week: a Nordic register study from the CoNARTaS group. Hum Reprod 2021a;36:1083–1092. [DOI] [PubMed] [Google Scholar]
- Terho AM, Pelkonen S, Toikkanen R, Koivurova S, Salo J, Nuojua-Huttunen S, Pokka T, Gissler M, Tiitinen A, Martikainen H.. Childhood growth of term singletons born after frozen compared with fresh embryo transfer. Reprod Biomed Online 2021b;43:719–726. [DOI] [PubMed] [Google Scholar]
- The Scottish Government. The Scottish Index of Multiple Deprivation. 2020. https://www.isdscotland.org/Products-and-Services/GPDSupport/Deprivation/SIMD/ (1 June 2020, date last accessed). [Google Scholar]
- Turner S, Maclean E, Dick S, Aucott L, Maheshwari A.. Is conception by in vitro fertilization associated with altered antenatal and postnatal growth trajectories? Fertil Steril 2020;114:1216–1224. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, Bartels M, Boomsma DI.. Comparison of naturally conceived and IVF-DZ twins in the Netherlands Twin Registry: a developmental study. J Pregnancy 2011;2011:517614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitthala S, Gelbaya TA, Brison DR, Fitzgerald CT, Nardo LG.. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod Update 2009;15:45–55. [DOI] [PubMed] [Google Scholar]
- Wennerholm UB, Bergh C.. Perinatal outcome in children born after assisted reproductive technologies. Ups J Med Sci 2020;125:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS. Twin growth: Initial deficit, recovery, and trends in concordance from birth to nine years. Ann Hum Biol 1979;6:205–220. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Zang X, Xie T, Yang C, Jiang X, Chen M.. Additional adverse perinatal outcomes with no effect on neonatal mortality and birth defects in pregnancies conceived by assisted reproductive technology. Front Pediatr 2022;10:809259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EH, Sundaram R, Bell EM, Druschel C, Kus C, Xie Y, Louis GMB.. Infertility treatment and children’s longitudinal growth between birth and 3 years of age. Hum Reprod 2016;31:1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong X, Gao W, Lv J, Yu C, Wang S, Huang T, Sun D, Liao C, Pang Z. et al. A comparison of preterm birth rate and growth from birth to 18 years old between in vitro fertilization and spontaneous conception of twins. Twin Res Hum Genet 2021;24:228–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the Human Fertilisation and Embryology Authority and NHS Public Health Scotland under licence/by permission. The data are not publicly accessible, but may be accessed after ethics approvals.