Abstract
STUDY QUESTION
Is preimplantation genetic testing (PGT) associated with adverse perinatal outcome and early childhood health?
SUMMARY ANSWER
Children born after PGT had comparable perinatal outcomes to children born after IVF/ICSI and comparable findings regarding early childhood health.
WHAT IS KNOWN ALREADY
PGT is offered to couples affected by monogenic disorders (PGT-M) or inherited chromosomal aberrations (PGT-SR), limiting the risk of transferring the disorder to the offspring. PGT, an invasive technique, requires genetic analysis of one or up to ten cells from the embryo and is combined with IVF or ICSI. Several studies, most of them small, have shown comparable results after PGT and IVF/ICSI concerning perinatal outcome. Only a few studies with limited samples have been published on PGT and childhood health.
STUDY DESIGN, SIZE, DURATION
We performed a register-based study including all singletons born after PGT (n = 390) in Sweden during 1 January 1996–30 September 2019. Singletons born after PGT were compared with all singletons born after IVF/ICSI (n = 61 060) born during the same period of time and with a matched sample of singletons (n = 42 034) born after spontaneous conception selected from the Medical Birth Register. Perinatal outcomes, early childhood health, and maternal outcomes were compared between pregnancies after PGT and IVF/ICSI as well as between pregnancies after PGT and spontaneous conception. Primary outcomes were preterm birth (PTB) and low birthweight (LBW) whereas childhood morbidity was the secondary outcome.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Data on women who went through PGT and gave birth were obtained from the local databases at the two PGT centres in Sweden, whereas data on IVF treatment for the IVF/ICSI group were obtained from the national IVF registers. These data were then cross-linked to national health registers; the Medical Birth Register, the Patient Register, and the Cause of Death Register. Logistic multivariable regression analysis and Cox proportional hazards models were performed with adjustment for relevant confounders.
MAIN RESULTS AND THE ROLE OF CHANCE
The mean follow-up time was 4.6 years for children born after PGT and 5.1 years for children born after spontaneous conception, whereas the mean follow-up time was 9.0 years for children born after IVF/ICSI. For perinatal outcomes, PTB occurred in 7.7% of children after PGT and in 7.3% of children after IVF/ICSI, whereas the rates were 4.9% and 5.2% for LBW (adjusted odds ratio (AOR) 1.22, 95% CI 0.82–1.81 and AOR 1.17, 95% CI 0.71–1.91, respectively). No differences were observed for birth defects. In comparison to spontaneous conception, children born after PGT had a higher risk for PTB (AOR 1.73, 95% CI 1.17–2.58). Regarding early childhood health, the absolute risk of asthma was 38/390 (9.7%) in children born after PGT and 6980/61 060 (11.4%) in children born after in IVF/ICSI, whereas the corresponding numbers were 34/390 (8.7%) and 7505/61 060 (12.3%) for allergic disorders. Following Cox proportional hazards models, no significant differences were found for these outcomes. Sepsis, hypothyroidism, attention deficit hyperactivity disorder, autism spectrum disorders, mental retardation, cerebral palsy, and epilepsy were diagnosed in a maximum of three PGT children. No PGT children died during the follow-up period. Regarding maternal outcomes, the rates of placenta praevia and caesarean delivery were significantly higher after PGT in comparison to spontaneous conception (AOR 6.46, 95% CI 3.38–12.37 and AOR 1.52, 95% CI 1.20–1.92, respectively), whereas no differences were seen comparing pregnancies after PGT and IVF/ICSI.
LIMITATIONS, REASONS FOR CAUTION
The rather small sample size of children born after PGT made it impossible to adjust for all relevant confounders including fertilization method and culture duration. Moreover, the follow-up time was short for most of the children especially in the PGT group, probably lowering the absolute number of diagnoses in early childhood.
WIDER IMPLICATIONS OF THE FINDINGS
The results are reassuring and indicate that the embryo biopsy itself has no adverse effect on the perinatal, early childhood, or maternal outcomes. Although the results are comparable to IVF/ICSI also regarding early childhood outcome, they should be taken with caution due to the low number of children with diagnoses and short follow-up time. Long-term follow-up studies on children born after PGT are scarce and should be conducted considering the invasiveness of the technique.
STUDY FUNDING/COMPETING INTEREST(S)
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (LUA/ALF 70940), the Board of National Specialised Medical Care at Sahlgrenska University Hospital and Hjalmar Svensson Research Foundation. There are no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: preimplantation genetic testing, perinatal outcome, child health, in vitro fertilization, intracytoplasmic sperm injection
Introduction
Preimplantation genetic testing (PGT) is an invasive technique in ART, combining both IVF or ICSI and embryo biopsy. PGT is today offered to couples affected by monogenic disorders (PGT-M) and inherited structural chromosomal rearrangements (PGT-SR), yet, a parallel technique, a method to screen for aneuploid embryos (PGT-A) in order to increase live birth rate, can also be used. The first child after PGT was born in 1990 in UK (Handyside et al., 1990) and since 1999, the European Society of Human Reproduction and Embryology (ESHRE) PGT Consortium presents annual reports on PGT data from more than 60 centres. These reports are, however, mainly descriptive and with a lot of missing data (van Montfoort et al., 2021). In USA, where PGT is not legally regulated, the technique was used in 43.8% out of 330 773 ART cycles in 2019, majority of which are PGT-A cycles (cdc.gov).
Several cohort studies, most of them small, have investigated perinatal outcome after PGT. In a recent meta-analysis on singletons born after PGT/PGT-A, no difference in regards of preterm birth (PTB <37 weeks, 8 studies including 2283 PGT singletons) or low birthweight (LBW <2500 g, 7 studies including 1938 PGT singletons) was seen in comparison to singletons after IVF/ICSI (adjusted odds ratio (AOR) 1.01, 95% CI 0.84–1.22 and AOR 0.95, 95% CI 0.71–1.28, respectively) (Zheng et al., 2021). Moreover, another meta-analysis showed comparable results for singletons after PGT/PGT-A and IVF/ICSI in mean gestational age and mean birth weight (Hou et al., 2021). The rate of birth defects seems to be comparable in children born after PGT/PGT-A and IVF/ICSI as shown in the two recent meta-analyses (Hou et al., 2021; Zheng et al., 2021).
In a recent Australian cohort study investigating child health in 155 children after PGT/PGT-A and 303 children after IVF, twins included, no differences could be seen between the groups. Outcomes included asthma, allergies, attention and behavioural issues, depression, and epilepsy (Lewis et al., 2021). In studies from Belgium and the Netherlands, including between 43 and 103 PGT offspring, investigating growth, body composition, blood pressure, neurological, motor, and cognitive development, no differences were seen when children born after PGT were compared to children born after IVF/ICSI and spontaneous conception (Desmyttere et al., 2009; Belva et al., 2018; Heijligers et al., 2018, 2019; Kuiper et al., 2018). In addition, a systematic review including 13 small studies on children follow-up concluded that children up to 9 years of age after PGT and PGT-A do not differ from children born after IVF/ICSI in terms of cognitive, behavioural, psychomotor, or growth development (Natsuaki and Dimler, 2018). However, no meta-analysis was conducted due to the low number of studies for each outcome. The major drawback of studies on childhood outcome is an unclear selection of controls, the methods of data collection by self-reporting questionnaires to the parents and high non-participation rates.
For maternal outcomes, publications are scarce. In an American study on 155 singleton pregnancies after PGT/PGT-A and 150 after IVF, the rate of pre-eclampsia was significantly higher in pregnancies after PGT/PGT-A. However, the rates of pregnancy-induced hypertension, placenta praevia, and postpartum haemorrhage were comparable between the groups (Zhang et al., 2019).
The aim of this study was to investigate perinatal outcomes, childhood health, and maternal outcomes after PGT-M and PGT-SR in Sweden compared to IVF/ICSI and spontaneous conception in a complete national cohort of PGT singletons and with adequate controls.
Materials and methods
Study groups
PGT is allowed in Sweden since 1994, initially only for genetic disorders leading to death in early childhood. The first live-born child after PGT in Sweden was born in 1997 (Hanson et al., 2001) and PGT is currently performed at two centres in Sweden, Karolinska University Hospital, Stockholm and Sahlgrenska University Hospital, Gothenburg. PGT is regulated by Swedish law and today offered to couples affected by monogenic disorders (PGT-M) and inherited structural rearrangements (PGT-SR).
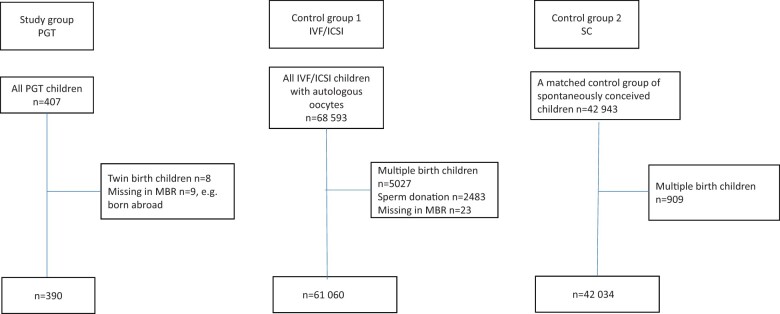
In this population-based registry study, all singletons born during 1 January 1996–30 September 2019 after PGT were included. Since PGT-A is not allowed in clinical practice in Sweden, and has been performed only in clinical trials with three live-births (Hardarson et al., 2008), these children were excluded. Four sets of PGT twins were excluded. For comparisons between children after PGT and IVF/ICSI, all singletons born after IVF/ICSI in Sweden during the same period were included and identified from national IVF registers. Only pregnancies with autologous gametes were included. For comparisons between children after PGT and spontaneous conception, we aimed for 100 spontaneous controls per PGT child. Yet, when excluding multiples in the PGT and spontaneous conception group and children born abroad in the PGT group, we ended up with 42 034 spontaneously conceived singletons as controls (Fig. 1). The controls were identified from the Medical Birth Register (MBR) and matched for year of birth of child, maternal age ±5 years, parity, and for delivery hospital or nearest delivery hospital.
Figure 1.
Flow-chart of patient selection and exclusion for the study group and the control groups. PGT, preimplantation genetic testing; MBR, medical birth register; SC, spontaneous conception.
Embryo biopsy and PGT
During embryo biopsy one or two (Day 3 biopsy), or five to ten (Day 5 or Day 6 biopsy), cells are removed from the Day 3 to Day 6 embryo and analysed for the specific disorder using polymerase chain reaction (PCR)-based techniques, fluorescence in situ hybridization (FISH), array-comparative genome hybridization (aCGH), or next generation sequencing (NGS). One, or in rare cases two, unaffected embryos are transferred to the uterine cavity in a fresh or frozen-thawed cycle reducing the likelihood for a child to inherit the specific disorder.
Data sources
The local databases at the Gothenburg and Stockholm centres doing the procedure were used to identify the women who went through PGT and gave birth. The local databases provided data on indication for PGT (International Statistical Classification of Diseases and Related Health Problems (ICD) code if possible), dates for oocyte aspiration and embryo transfer, number of oocytes retrieved, fertilization method (IVF or ICSI), techniques used for zona opening (laser or acid), embryo stage at biopsy (cleavage stage or blastocyst), method for genetic analysis (PCR, FISH, aCGH, or NGS), fresh or frozen cycle, cryopreservation method (slow-freeze or vitrification), and number of embryos transferred.
Children born after IVF/ICSI were identified through the IVF registers used in Sweden. Until 2007, data for women given birth after IVF was stored in a data file at the National Board of Health and Welfare, called MBR-IVF in this study. From 2007, this data has been collected at the National Quality Register for Assisted Reproduction, Q-IVF (Q-IVF). The Q-IVF collects data on treatment characteristics and pregnancy results from all public and private clinics (n = 19). The patients are informed about the Q-IVF and may choose not to have their data included, although this is very rare giving a coverage rate of close to 100%. The IVF registers include information on dates for oocyte aspiration and embryo transfer, fertilization method (IVF or ICSI), culture duration, fresh or frozen transfer, number of embryos transferred, and date of birth. Moreover, the Q-IVF includes information on number of oocytes retrieved and cryopreservation method (slow-freeze or vitrification).
The MBR, established in the early 1970s, covers nearly all deliveries in Sweden and provides data on maternal characteristics (i.e. age, parity, BMI, smoking habits, all recorded at first antenatal visit), data on delivery, and neonatal outcomes including mode of delivery, child’s sex, gestational age, birth weight, Apgar score, birth defects, and live birth or stillbirth based on standardized medical records filled in at the antenatal units as well as delivery clinics. Birth defects registered at birth were retrieved from the MBR and defects registered during the first year of life from the National Patient Register (NPR). From 1987, the NPR includes all in-patient care in Sweden. Following 2001, the register also covers out-patient visits including day surgery and psychiatric care from both private and public caregivers. However, primary care is not yet covered. The register is based on ICD-codes and in this study NPR provided data on diagnoses in early childhood in addition to birth defects during the first year of life. In Sweden, ICD-9 codes were used through 1996 and ICD-10 codes since 1997. The Cause of Death Register was used for information on infant and childhood mortality during the follow-up period. The MBR, the NPR, and the Cause of Death Register are hosted by the National Board of Health and Welfare and all proven to be of good quality (Cnattingius et al., 1990; Brooke et al., 2017; https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-swedish-medical-birth-register, Ludvigsson et al., 2011). According to Swedish regulations individual patients cannot withhold or withdraw their medical data from the health registers hosted by the National Board of Health and Welfare.
The unique personal identification number (PIN), given to every citizen living in Sweden, enabled individual level data linkage between mothers, children, and registers.
Data on deliveries included all live born children and stillbirths. In Sweden, the definition of stillbirth has changed during the study period. Before 1 July 2008, only stillbirths ≥28 completed gestational weeks were registered as stillbirths and from 1 July 2008, stillbirths ≥22 completed gestational weeks were defined as stillbirths and thus included.
Outcomes
Primary outcomes were PTB (<37 weeks) and LBW (<2500 g).
As secondary outcomes, we assessed early childhood health. We examined the rate of asthma, allergic disorders, sepsis, hypothyroidism, attention deficit hyperactivity disorder (ADHD), autism spectrum disorders (ASD), affective disorders, schizophrenia, mental retardation, cerebral palsy, epilepsy, and mortality from birth through end of follow-up in the study, i.e. 30 September 2019. A child could have more than one diagnosis. The first date of diagnosis in each disease group was considered in the analysis.
Tertiary outcomes consisted of both perinatal and maternal outcomes. Perinatal outcomes were infant sex, post-term birth (≥42 weeks), very preterm birth (VPTB) (<32 weeks), very low birth weight (VLBW) (<1500 g), macrosomia (≥4500 g), small for gestational age (SGA, <−2 standard deviation (SD)), large for gestational age (LGA, >+2SD) (SGA and LGA defined according to Marsal et al., 1996), Apgar score <4 at 5 min, perinatal mortality (stillbirth and death in the first week of life), neonatal mortality (death <28 days postpartum), and infant mortality (death <1 year). Any birth defects included ICD-9 codes 740–759 A–X and ICD-10 codes beginning with Q. Major birth defects were defined according to the EUROCAT classification system and analysed at birth and up to 1 year of age (www.eurocat-network.eu/content/Stat-Mon-Report-2012.pdf).
Gestational age was determined according to the day of embryo transfer and number of days in culture for IVF pregnancies including PGT pregnancies and by the second trimester ultrasound for spontaneous conception pregnancies.
Regarding maternal outcomes the rates of HDP (pregnancy-induced hypertension and pre-eclampsia/eclampsia), placenta praevia, placental abruption, and postpartum haemorrhage (>1000 ml) were assessed according to ICD-codes found in the MBR. Additionally, the rate of caesarean delivery was assessed according to checkboxes as well as ICD and procedure codes at the standardized medical records used for registration in MBR.
ICD-9 and ICD-10 codes for early childhood and maternal outcomes are listed in Supplementary Table SI.
Statistical methods
Demographic data are given as number (n) and percentages for categorical variables and mean and SD or median and interquartile range (IQR) for continuous variables. Logistic regression analysis was performed for dichotomous variables and adjustment made for year of birth of child (continuous variable), maternal age (continuous variable), and parity (0 or ≥1). Additionally, for PTB, LBW, major birth defects registered up to 1 year of age, HDP, PPH, and Caesarean delivery the number of events were regarded high enough to also be adjusted for BMI. When pregnancies after PGT and IVF/ICSI were compared, adjustment was moreover made for fresh or frozen transfer. Missing values were not imputed. Crude and adjusted ORs with 95% CI were calculated for each perinatal and maternal outcome and a 95% CI not involving 1 was considered statistically significant. For early childhood morbidity, hazard ratios (HRs) with 95% CI were calculated using Cox proportional hazards models. We used age as the time scale and included each child’s time at risk computed from the date of birth until whichever event occurred first: diagnosis, death or end of follow-up, i.e. 30 September 2019. Adjustment was made for year of birth of child (continuous variable), maternal age (continuous variable), parity (0 or ≥1), and smoking at first antenatal visit (yes/no). To ensure anonymity for the participants, the exact numbers for some outcomes are not reported due to few events (<3) in the PGT group. A number of <10 events in any group was considered too small to calculate a stable estimate. The statistical analyses were conducted using SPSS version 28.0 and STATA version 16.
Power calculation
With 390 children in the smallest group, it is possible to detect a difference in PTB of 4.2%, from 7.3% in the IVF/ICSI group to 11.5% in the PGT group (α = 0.05, β = 0.80).
Ethical approval
Permission from the Ethics Committee was given (the Regional Ethical Committee in Sweden, at the University of Gothenburg) Dnr 086/18.
Results
The study included 390 singletons born after PGT, 61 060 singletons born after IVF/ICSI, and 42 034 singletons born after spontaneous conception (Fig. 1). The mean follow-up time was 4.6 (±4.1) years for children born after PGT, 9.0 (±6.2) years for children born after IVF/ICSI, and 5.1 (±3.9) years for children born after spontaneous conception. Demographic data are presented in Table I. The rate of primiparas was 40.0% for PGT mothers and 67.8% for IVF/ICSI mothers whereas 0.8% of the PGT mothers smoked compared to 2.5% and 3.4% of the IVF/ICSI and spontaneous conception mothers, respectively. Moreover, ICSI was used in 86.4% of PGT treatments whereas the percentage was 46.1% in the IVF/ICSI group. Data on indications and specific procedures for PGT pregnancies are presented in Table II. The most common indications for PGT were reciprocal translocations (n = 75), Huntington disease (n = 25), and Robertsonian translocation (n = 20).
Table I.
Maternal and cycle characteristics in singleton pregnancies after preimplantation genetic testing (PGT), IVF/ICSI, and spontaneous conception in Sweden 1996–2019.
| PGT | IVF/ICSI | Spontaneous conception | |
|---|---|---|---|
| Deliveries, n | 390 | 61 060 | 42 034 |
| Year of birth of child, n (%) | |||
| 1996–2003 | 7 (1.8) | 10 188 (16.7) | 659 (1.6) |
| 2004–2011 | 68 (17.4) | 22 395 (36.7) | 7651 (18.2) |
| 2012–2019 | 315 (80.8) | 28 477 (46.6) | 33 724 (80.2) |
| Maternal age, mean ± SD | 33.2 ± 3.5 | 33.8 ± 4.2 | 32.2 ± 4.0 |
| Primiparous, n (%) | 156 (40.0) | 41 426 (67.8) | 16 110 (38.3) |
| Smoking at first antenatal visit, n (%) | 3 (0.8) | 1547 (2.5) | 1426 (3.4) |
| Missing, n (%) | 22 (5.6) | 3537 (5.8) | 2107 (5.0) |
| BMI, mean ± SD | 24.1 (3.9) | 24.4 (4.0) | 24.43 (4.4) |
| Missing, n (%) | 24 (6.2) | 5251 (8.6) | 2374 (5.6) |
| Years of infertility, median (Q1–Q3) | 2 (1–3) | 3 (2–4) | 1 (1–2) |
| Duration of infertility missing, n (%) | 175 (44.9) | 13 321 (21.8) | 38 703 (92.1) |
| Fertilization method, n (%) | |||
| IVF | 53 (13.6) | 32 929 (53.9) | NA |
| ICSI | 337 (86.4) | 28 131 (46.1) | NA |
| Culture duration at embryo transfer, n (%) | |||
| Day 2–3 | 8 (2.1) | 36 077 (59.1) | NA |
| Day 4 | 185 (47.4) | 197 (0.3) | |
| Day 5–6 | 195 (50.0) | 15 433 (25.3) | NA |
| Day 7 | – | 3 (0.3) | |
| Missing | 2 (0.5) | 9350 (15.3) | NA |
| Cycle type, n (%) | |||
| Fresh transfer | 258 (66.2) | 43 065 (70.5) | NA |
| Frozen transfer | 132 (33.8) | 17 995 (29.5) | NA |
| Freezing technique | |||
| Vitrification | 98 (74.2) | 10 256 (57.0) | NA |
| Slow-freezing | 34 (25.8) | 7736 (43.0) | NA |
| Missing | 0 (0.0) | 3 (0.0) | NA |
| Number of embryos transferred, n (%) | |||
| 1 | 335 (85.9) | 43 864 (71.8) | NA |
| ≥2 | 50 (12.8) | 9125 (14.9) | NA |
| Missing | 5 (1.3) | 8071 (13.2) | NA |
Q1–Q3, first and third quartiles; NA, not applicable.
Table II.
Indications and procedures for singleton pregnancies after preimplantation genetic testing, (PGT) in Sweden 1996–2019.
| Deliveries, n | 390 |
|---|---|
| Indication for treatment, n (%) | |
| Monogenic disorders | 259 (66.4) |
| X-linked disorders | 18 (4.6) |
| Chromosomal aberrations | 113 (29.0) |
| Biopsy stage n, (%) | |
| Cleavage stage | 193 (49.5) |
| Blastocyst stage | 195 (50.0) |
| Missing | 2 (0.5) |
| Zona opening, n (%) | |
| Laser | 334 (85.6) |
| Acid | 47 (12.1) |
| Missing | 9 (2.3) |
| Genetic analyses, n (%) | |
| PCR | 269 (69.0) |
| FISH | 76 (19.5) |
| Array-CGH | 43 (11.0) |
| NGS | 2 (0.5) |
FISH, fluorescence in situ hybridization; CGH, comparative genome hybridization; NGS, next generation sequencing.
Perinatal outcomes
Perinatal outcomes are summarized in Table III.
Table III.
Perinatal outcome in singleton pregnancies after preimplantation genetic testing (PGT), IVF/ICSI, and spontaneous conception (SC) in Sweden 1996–2019.
| Crude odds ratio (95% CI) | Adjusted odds ratioa (95% CI) | Crude odds ratio (95% CI) | Adjusted odds ratioa (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| PGT | IVF/ICSI | Spontaneous conception | PGT versus IVF/ICSI | PGT versus IVF/ICSI | PGT versus SC | PGT versus SC | |
| Deliveries, n | 390 | 61 060 | 42 034 | ||||
| Male gender, n (%) | 182 (46.7) | 31 391 (51.4) | 21 492 (51.1) | 0.83 (0.68–1.01) | 0.83 (0.68–1.01) | 0.84 (0.69–1.02) | 0.83 (0.68–1.02) |
| Gestational age, mean (days ±SD) | 277 ± 15.0 | 277 ± 15.1 | 279 ± 13.1 | – | – | – | – |
| Post-term birth ≥42 weeks, n (%) | 24 (6.2) | 3978 (6.5) | 2834 (6.7) | 0.94 (0.62–1.42) | 1.20 (0.79–1.83) | 0.91 (0.60–1.37) | 0.88 (0.58–1.33) |
| Pre-term birth <37 weeks, n (%) | 30 (7.7) | 4434 (7.3) | 1926 (4.6) | 1.06 (0.73–1.55) | 1.22 (0.82–1.81) | 1.74 (1.19–2.53) | 1.73 (1.17–2.58) |
| Very pre-term birth <32 weeks, n (%) | 3 (0.8) | 834 (1.4) | 361 (0.9) | 0.56 (0.18–1.75) | NAc | 0.90 (0.29–2.80) | NAc |
| Birth weight, mean (grams ±SD) | 3472 ± 601 | 3466 ± 613 | 3538 ± 564 | – | – | – | – |
| <2500 g, n (%) | 19 (4.9) | 3145 (5.2) | 1327 (3.2) | 0.94 (0.59–1.49) | 1.17 (0.71–1.91) | 1.57 (0.99–2.50) | 1.52 (0.93–2.49) |
| <1500 g, n (%) | 4 (1.0) | 719 (1.2) | 326 (0.8) | 0.87 (0.32–2.33) | NAc | 1.33 (0.49–3.57) | NAc |
| ≥4500 g, n (%) | 13 (3.3) | 1996 (3.3) | 1450 (3.4) | 1.02 (0.59–1.77) | 0.96 (0.55–1.68) | 0.96 (0.55–1.68) | 0.97 (0.56–1.69) |
| SGA<−2 SD, n (%) | 16 (4.1) | 2685 (4.4) | 1309 (3.1) | 0.93 (0.56–1.54) | 1.20 (0.72–1.98) | 1.33 (0.81–2.20) | 1.29 (0.78–2.14) |
| LGA>+2 SD, n (%) | 18 (4.6) | 2694 (4.4) | 1835 (4.4) | 1.05 (0.65–1.68) | 0.87 (0.54–1.40) | 1.06 (0.66–1.71) | 1.07 (0.67–1.73) |
| Apgar score <4 at 5 min, n (%) | <3 (<0.8)b | 337 (0.6) | 251 (0.6) | NAc | NAc | NAc | NAc |
| Perinatal mortality, n (%) | <3 (<0.8)b | 354 (0.6) | 235 (0.6) | NAc | NAc | NAc | NAc |
| Neonatal mortality, n (%) | 0 (0.0) | 114 (0.2) | 55 (0.1) | NAc | NAc | NAc | NAc |
| Infant mortality, n (%) | 0 (0.0) | 169 (0.3) | 80 (0.2) | NAc | NAc | NAc | NAc |
| Any birth defects registered at birth, n (%) | 18 (4.6) | 2556 (4.2) | 1538 (3.7) | 1.11 (0.69–1.78) | 1.20 (0.75–1.93) | 1.27 (0.79–2.05) | 1.26 (0.78–2.03) |
| Major birth defects registered at birth, n (%) | 7 (1.8) | 1594 (2.6) | 854 (2.0) | 0.68 (0.32–1.44) | NAc | 0.88 (0.42–1.87) | NAc |
| Major birth defects registered up to 1 year of age, n (%) | 21 (5.4) | 3149 (5.2) | 2028 (4.8) | 1.05 (0.67–1.63) | 0.96 (0.60–1.53) | 1.12 (0.72–1.75) | 1.05 (0.66–1.68) |
NA, not applicable; SGA/LGA, small/large for gestational age, defined according to Marsal et al. (1996).
Any birth defects included ICD-9 codes 740–759 A–X and ICD-10 codes beginning with Q. Major birth defects according to EUROCAT (www.eurocat network.eu/content/Stat Mon Report).
Bold values indicate a statistically significant result.
Adjustment was made for maternal age, parity, year of birth of child, and for PGT versus IVF/ICSI also for fresh or frozen transfer. Adjustment was also made for BMI in the analysis of PTB, LBW, and major birth defects registered up to 1 year of age.
The exact numbers are not reported to ensure anonymity of the participants.
Not applicable. Numbers too small (<10 in the PGT group) to calculate a stable estimate.
PGT versus IVF/ICSI
PTB occurred in 30/390 (7.7%) of pregnancies after PGT and in 4434/61 060 (7.3%) of pregnancies after IVF/ICSI, whereas the corresponding numbers for LBW were 19/390 (4.9%) and 3145/61 060 (5.2%), respectively. After adjustment for relevant confounders, there were no significant differences for PTB and LBW when comparing PGT and IVF/ICSI (AOR 1.22, 95% CI 0.82–1.81 and AOR 1.17, 95% CI 0.71–1.91, respectively). Neither was any significant differences observed for post-term birth, macrosomia, SGA, or LGA. Regarding major birth defects registered at birth the absolute numbers were 7/390 (1.8%) for children born after PGT and 1594/61 060 (2.6%) for children born after IVF/ICSI (AOR 0.77, 95% CI 0.36–1.62). Correspondingly, at follow-up to 1 year of age the numbers were 21/390 (5.4%) and 3149/61 060 (5.2%) (AOR 0.96, 95% CI 0.60–1.53).
PGT versus spontaneous conception
PTB occurred in 30/390 (7.7%) of pregnancies after PGT and in 1926/42 034 (4.6%) of pregnancies following spontaneous conception (AOR 1.73, 95% CI 1.17–2.58). For LBW, the risks were 19/390 (4.9%) and 1327/42 034 (3.2%), respectively (AOR 1.52, 95% CI 0.93–2.49). Neither was any significant differences found for post-term birth, macrosomia, SGA, LGA, or major birth defects registered at birth and up to 1 year of age.
Early childhood outcome
Childhood morbidity is summarized in Table IV.
Table IV.
Early childhood outcome in singleton pregnancies after preimplantation genetic testing (PGT), IVF/ICSI, and spontaneous conception (SC) for in Sweden 1996–2019.
| Crude hazard ratio (95% CI) | Adjusted hazard ratioa (95% CI) | Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| PGT | IVF/ICSI | SC | PGT versus IVF/ICSI | PGT versus IVF/ICSI | PGT versus SC | PGT versus SC | |
| Deliveries, n | 390 | 61 060 | 42 034 | – | – | – | – |
| Follow-up time in years, mean (SD) | 4.62 ± 4.1 | 9.05 ± 6.2 | 5.12 ± 3.9 | – | – | – | – |
| Asthma | 38 (9.7) | 6980 (11.4) | 4016 (9.6) | 1.25 (0.91–1.72) | 1.03 (0.73–1.44) | 1.14 (0.83–1.57) | 1.11 (0.79–1.55) |
| Allergic disorders | 34 (8.7) | 7505 (12.3) | 4451 (10.6) | 1.08 (0.77–1.51) | 0.97 (0.69–1.38) | 0.89 (0.64–1.25) | 0.88 (0.62–1.24) |
| Sepsis | <3 (<0.8)b | 134 (0.2) | 64 (0.2) | NAc | NAc | NAc | NAc |
| Hypothyroidism | <3 (<0.8)b | 165 (0.3) | 56 (0.1) | NAc | NAc | NAc | NAc |
| ADHD | 3 (0.8) | 1649 (2.7) | 363 (0.9) | 1.08 (0.35–3.34) | NAc | 0.95 (0.31–2.97) | NAc |
| ASD | <3 (<0.8)b | 1012 (1.7) | 301 (0.7) | NAc | NAc | NAc | NAc |
| Affective disorders | 0 (0.0) | 641 (1.0) | 77 (0.2) | NAc | NAc | NAc | NAc |
| Schizophrenia | 0 (0.0) | 20 (0.0) | 4 (0.0) | NAc | NAc | NAc | NAc |
| Mental retardation | <3 (<0.8)b | 343 (0.6) | 112 (0.3) | NAc | NAc | NAc | NAc |
| Cerebral palsy | <3 (<0.8)b | 128 (0.2) | 56 (0.1) | NAc | NAc | NAc | NAc |
| Epilepsy | <3 (<0.8)b | 486 (0.8) | 171 (0.4) | NAc | NAc | NAc | NAc |
| Mortality d | 0 (0.0) | 220 (0.4) | 89 (0.2) | NAc | NAc | NAc | NAc |
NA, not applicable; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorders.
Early childhood outcomes included asthma (ICD-9 codes 493A–B, 493X, ICD-10 codes J45.0, J46.9), allergic disorders (ICD-9 codes 372, 477, 558, 691, 708A, 995A–B, ICD-10 codes H10.1, J30, K52.2, L20, L50.0, T78.0–78.9), sepsis (ICD-9 codes 038, ICD-10 codes A40–41), hypothyroidism (ICD-9 codes 243, 244A–D, 244W, 244X, ICD-10 codes E03.0, E03.1, E03.8, E03.9), ADHD (ICD-9 codes 314, ICD-10 codes F90, F98.9), ASD (ICD-9 codes 299, ICD-10 codes F84.0, F84.1, F84.5, F 84.8–84.9), affective disorders (ICD-9 codes 296, 300E, 311, ICD-10 codes F30–39), schizophrenia (ICD-9 codes 295, 297, 298B, 298C, 298E, 298W, 298X, 299, ICD-10 codes F20–29), mental retardation (ICD-codes 317–319, ICD-10 codes F70–F79), cerebral palsy (ICD-9 code 343, ICD-10 code G80), epilepsy (ICD-9 code 345, ICD-10 codes G40–41).
Adjustment made for year of birth of child, maternal age, parity, and smoking at first antenatal visit.
The exact numbers are not reported to ensure anonymity of the participants.
Not applicable. Numbers too small (<10 in the PGT group) to calculate a stable estimate.
From birth through end of follow-up in the study, 30 September 2019.
PGT versus IVF/ICSI
In total, 38/390 (9.7%) children born after PGT and 6980/61 060 (11.4%) children born after IVF/ICSI were diagnosed with asthma (adjusted HR (AHR) 1.03, 95% CI 0.73–1.44). For allergic disorders, the corresponding numbers were 34/390 (8.7%) and 7505/61 060 (12.3%) (AHR 0.97, 95% CI 0.69–1.38). Sepsis, hypothyroidism, ADHD, ASD, mental retardation, cerebral palsy, and epilepsy were diagnosed in a maximum of three children born after PGT. There were no children with affective disorders or schizophrenia in the PGT group. None of the singletons born after PGT died during the follow-up period.
PGT versus spontaneous conception
In total, 38/390 (9.7%) children born after PGT and 4016/42 034 (9.6%) children born following spontaneous conception were diagnosed with asthma (AHR 1.11, 95% CI 0.79–1.55). For allergic disorders, the corresponding numbers were 34/390 (8.7%) and 4451/42 034 (10.6%) (AHR 0.88, 95% CI 0.62–1.24).
For sepsis, hypothyroidism, ADHD, ASD, mental retardation, cerebral palsy, and epilepsy the number of events was <3 in the PGT group.
Maternal outcome
Maternal outcomes are summarized in Table V.
Table V.
Maternal outcome in singleton pregnancies after preimplantation genetic testing (PGT), IVF/ICSI, and spontaneous conception (SC) in Sweden 1996–2019.
| Crude odds ratio (95% CI) | Adjusted odds ratioa (95% CI) | Crude odds ratio (95% CI) | Adjusted odds ratioa (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| PGT | IVF/ICSI | Spontaneous conception | PGT versus IVF/ICSI | PGT versus IVF/ICSI | PGT versus SC | PGT versus SC | |
| Deliveries, n | 390 | 61 060 | 42 034 | ||||
| HDP, n (%) | 17 (4.4) | 3643 (6.0) | 1986 (4.7) | 0.72 (0.44–1.17) | 0.98 (0.60–1.61) | 0.92 (0.56–1.50) | 0.98 (0.60–1.61) |
| PE, n (%) | 8 (2.1) | 2562 (4.2) | 1191 (2.8) | 0.48 (0.24–0.96) | NAc | 0.72 (0.36–1.45) | NAc |
| Placenta praevia, n (%) | 10 (2.6) | 1119 (1.8) | 156 (0.4) | 1.41 (0.75–2.65) | 1.21 (0.64–2.28) | 7.06 (3.70–13.50) | 6.46 (3.38–12.37) |
| Placental abruption, n (%) | <3 (<0.8)b | 437 (0.7) | 157 (0.4) | NAc | NAc | NAc | NAc |
| PPH, n (%) | 27 (6.9) | 4509 (7.4) | 2540 (6.0) | 0.93 (0.63–1.38) | 1.02 (0.68–1.52) | 1.16 (0.78–1.71) | 1.17 (0.78–1.74) |
HDP, hypertensive disorders in pregnancy; PE, pre-eclampsia; PPH, postpartum haemorrhage.
Maternal outcomes included hypertensive disorders in pregnancy (ICD-9 codes 642A–B, 642D, 642X, 642E–H, ICD-10 codes O13–O15), placenta praevia (ICD-9 codes 641A–B, ICD-10 code O44), placental abruption (ICD-9 code 641C, ICD-10 code O45), postpartum haemorrhage (ICD-9 codes 666A–D, ICD-10 code O67.8 and O72).
Bold values indicate a statistically significant result.
Adjustment was made for maternal age, parity, year of birth of child, and for PGT versus IVF/ICSI also for fresh or frozen transfer. Adjustment was also made for BMI in the analysis of HDP, PPH, and Caesarean delivery.
The exact numbers are not reported to ensure anonymity of the participants.
Not applicable. Numbers too small (<10 in the PGT group) to calculate a stable estimate.
PGT versus IVF/ICSI
Placenta praevia occurred in 10/390 (2.6%) and 1119/61 060 (1.8%) and HDP in 17/390 (4.4%) and 3643/61 060 (6.0%) of pregnancies after PGT and IVF/ICSI, respectively. After adjustment neither of these differences reached statistical significance. Neither were there any significant differences between the groups regarding postpartum haemorrhage or rate of Caesarean delivery.
PGT versus spontaneous conception
The risk of placenta praevia was significantly higher in pregnancies after PGT, 10/390 (2.6%), in comparison to pregnancies after spontaneous conception, 156/42 024 (0.4%) (AOR 6.46, 95% CI 3.38–12.37). The rate of caesarean delivery was significantly higher after PGT in comparison to spontaneous conception, 26.9% and 18.6%, respectively (AOR 1.52, 95% CI 1.20–1.92). Regarding HDP and postpartum haemorrhage, there were no significant differences.
Discussion
In this register-based study including a complete national cohort of 390 singletons born after PGT, there were no significant differences in the rate of PTB and LBW when comparing singletons born after PGT and IVF/ICSI. Neither were there any significant differences for other perinatal outcomes, including birth defects, when comparing the groups. Moreover, we found comparable results for asthma and allergic disorders when comparing singletons after PGT to singletons after IVF/ICSI and spontaneous conception. For sepsis, hypothyroidism, ADHD, ASD, mental retardation, cerebral palsy, and epilepsy only few cases were reported in the PGT group and thus insufficient data to draw any firm conclusions. During the follow-up period there were no deaths among PGT offspring. In addition, maternal outcomes were comparable in pregnancies after PGT in comparison to IVF/ICSI, yet adverse outcomes in terms of higher rates of PTB, placenta praevia, and rates of caesarean delivery compared to spontaneous conception.
For perinatal outcomes, the results are reassuring. Our results are consistent with previously published research on singletons (Liebaers et al., 2010; Hasson et al., 2017; Sunkara et al., 2017; Zhang et al., 2019; Zheng et al., 2021) showing comparable results in pregnancies after PGT and IVF/ICSI. Moreover, no significant difference in major birth defects could be found when comparing PGT to IVF/ICSI or spontaneous conception, findings also in line with other studies (Desmyttere et al., 2012; Bay et al., 2016; Hasson et al., 2017; Sharpe et al., 2018; Zhang et al., 2019; Zheng et al., 2021). In the present study, the rate of any birth defects in singletons after PGT was 4.6% at birth in accordance with the latest reported numbers from the PGT Consortium (De Rycke et al., 2017) presenting a 5% risk of any birth defect. In our study, there is a favour for female gender after PGT both compared to IVF/ICSI and spontaneous conception even though this did not reach significant levels. Similar results have been shown in other studies (Bay et al., 2016; Sharpe et al., 2018). The reason for this is not clear but could partly be due to the selection of female foetuses in X-linked disorders. To date, there are no reported misdiagnoses in Sweden (C. Hanson and E. S. Lundberg, personal communication). According to the latest report from the PGT Consortium, neither were any misdiagnoses noted for years 2013–2015 (Coonen et al., 2020). Throughout the years the rate of misdiagnoses has declined, possibly due to quality improvement in laboratory settings but an unwillingness to report misdiagnosis should also be considered (De Rycke et al., 2017). In Sweden, regarding the accuracy of the genetic analysis (Harton et al., 2011) confirmatory tests have not been routinely performed except for in the early days of PGT.
Some studies have included both PGT-M, PGT-SR, and PGT-A cycles which might influence the outcome (Liebaers et al., 2010; Desmyttere et al., 2012; Zhang et al., 2019; Hou et al., 2021; Zheng et al., 2021). PGT-A is usually performed in subfertile couples while PGT-M and PGT-SR are used to prevent a disease or disorder to be inherited and the parents are usually not subfertile. Subfertility per se is well known to be associated with poorer perinatal and maternal outcomes (Pinborg et al., 2013; Berntsen et al., 2019). In a study from Israel, comparing 158 PGT singletons, no PGT-A children included, and 158 IVF/ICSI singletons, there was a lower risk of LBW in children born after PGT (Eldar-Geva et al., 2014). Yet, in our study, neither including PGT-A pregnancies, we did not observe a lower risk of LBW after PGT. The discrepancy in results in comparison to the Israeli study could possibly be explained by the difference in absolute risks of LBW after IVF/ICSI, 5.2% in our study compared to 12% in the study by Eldar-Geva. In our study, 55.1% of the PGT parents had reported involuntary childlessness. This variable is collected from the MBR. During the first antenatal visit at the maternity unit the parents are routinely asked whether they have suffered from involuntary childlessness and for how long. Possibly, because of the need of embryo biopsy and the associated IVF/ICSI, PGT parents consider themselves suffering from involuntary childlessness even if they are not suffering from subfertility per se.
Regarding outcome in early childhood in this study, the risk of asthma and allergic disorders after PGT was similar as opposed to IVF/ICSI and spontaneous conception. This agrees with an Australian cohort study on 155 PGT/PGT-A children, twins included, with a follow-up of 6 years (Lewis et al., 2021). There are, however, some previous studies, including a recent meta-analysis, showing an association between asthma/allergy and ART, findings that could be attributable to the higher incidence of PTB as shown for asthma in the study by Källén and co-workers, where restricting the analysis to term births made the difference disappear (Ericson et al., 2002; Källén et al., 2005; Wijs et al., 2021). For ASD and ADHD, a maximum of three PGT children in our study had been diagnosed with these conditions during the follow-up and hence, no statistical analyses were made. The Australian study (Lewis et al., 2021) also found similar risks in PGT and IVF children. There are some conflicting results concerning ART and the risk of ASD and ADHD. A recent meta-analysis found a higher risk of ASD after ART in comparison to spontaneous conception, however no such differences could be seen when restricting the analysis to singletons (Liu et al., 2017). For ADHD, in comparison to spontaneous conception, earlier studies have observed a higher risk for children born after ART (Källén et al., 2011; Svahn et al., 2015) while a recent Nordic registry study, including more than 116 000 ART singletons, found comparable results in the two groups both for ASD (AHR 1.07, 95% CI 0.98–1.16) and ADHD (AHR 1.05, 95% CI 0.99–1.12) (Rönö et al., 2022). Regarding cerebral palsy, there were few cases following PGT in the present study. We have not found any other studies examining this outcome after PGT. Earlier studies have shown a higher risk cerebral palsy after ART in comparison to spontaneous conception (Stromberg et al., 2002; Källén et al., 2010; Hvidtjorn et al., 2011; Wang et al., 2021) mainly associated with multiple births and PTB. In a recent large registry study from the Nordic countries, a decline in risk of cerebral palsy for ART children over the past two decades was, however, observed, in parallel with a substantial decrease of multiple births. For singletons born during years 2007–2010 and 2011–2014, no significant difference in the risk of cerebral palsy could be seen when comparing children born after ART and spontaneous conception (Spangmose et al., 2021). Yet, the diagnosis cerebral palsy is usually not given before the age of 4 years and thus, the short follow-up time in our study might underestimate the risks.
With respect to maternal outcomes after PGT compared with IVF/ICSI, evidence is still limited. We found no differences in the rate of HDP or pre-eclampsia in pregnancies after PGT as opposed to pregnancies after IVF/ICSI. In a recent study from USA, adjusting for several confounders including prior history of hypertensive disease and mode of conception, a 3-folded risk of pre-eclampsia was found in the PGT group compared to IVF/ICSI. When restricting the analysis to singletons the risk of preeclampsia persisted (AOR 2.95, 95% CI 0.98–8.92, P = 0.04). The rate of pregnancy-induced hypertension was not significantly different (Zhang et al., 2019). However, it is not clear how the selection of participants was performed. In total, 505 women with a viable gestation in early pregnancy consented to participation during the 6-year study period while the total number of eligible women is not presented and neither if any demographic differences existed between participants and non-participants. Two meta-analyses have shown a 30–50% higher risk of HDP after PGT/PGT-A in comparison to IVF/ICSI (Hou et al., 2021; Zheng et al., 2021). Yet, in the study from Hou et al. (2021), including also multiples, restricting the analysis to only frozen cycles made the difference in HDP disappear, whereas the study from Zheng et al. (2021), included only singleton pregnancies, but analysed fresh and frozen cycles together. Both frozen embryo cycles and multiple pregnancies have been associated with an increased risk of HDP (Opdahl et al., 2015; Maheshwari et al., 2018; Ginström Ernstad et al., 2019; von Versen-Hoynck et al., 2019; Eapen et al., 2020). In our study, only singleton pregnancies were included and adjustment was made for fresh or frozen transfer. The risk of placenta praevia after PGT was comparable to that of IVF/ICSI pregnancies, consistent with other studies (Bay et al., 2016; Hou et al., 2021; Zheng et al., 2021). The finding of a 6-folded increase in risk for placenta praevia in our study when comparing PGT to spontaneous conception is in accordance with a Danish study reporting on a 9-fold risk (Bay et al., 2016). In our study, around 50% of biopsies were performed at the cleavage stage and 50% at the blastocyst stage whereas all biopsies were performed on cleavage stage embryos in the Danish study. Laser was the main method used for zona opening in our study and all placenta praevia (n = 10) in the PGT group were observed following the use of laser. In the Danish study, the risk of placenta praevia after PGT was only significantly increased following laser and not after use of acid (Bay et al., 2016), findings that warrants further research. Conflicting results have been reported concerning placenta praevia and blastocyst transfer. Nordic studies (Ginström Ernstad et al., 2016; Spangmose et al., 2020) have found a 2-fold increase of placenta praevia in pregnancies after blastocyst transfer compared to cleavage stage while studies from USA and UK (Maheshwari et al., 2013; Ishihara et al., 2014) have not confirmed such an association. Additionally, in comparison to spontaneous pregnancies, an almost 4-fold risk after ART has been seen in meta-analyses (Qin et al., 2016; Vermey et al., 2019). The aetiology of placenta praevia remains uncertain but, along with blastocyst transfer, and ART treatment, also advanced maternal age, smoking, multiple pregnancies, underlying reason for infertility, male sex, and previous caesarean delivery have been shown to affect the risk, confounders not always adjusted for in studies (Faiz and Ananth, 2003; Rombauts et al., 2014; Karami et al., 2018).
The main strengths of our study are the inclusion of a cohort representing all singletons born after PGT in Sweden since the birth of the first child and as long as information on perinatal and maternal outcome are available, i.e. through 30 September 2019, as well as large control groups. Moreover, early childhood outcomes for specific diagnoses were included. Cross-linking several registers with high coverage rates on an individual level enables population-based studies and minimizes the risk of selection bias. A limitation is a rather small sample size of PGT children which makes it impossible to adjust for several confounders including fertilization method and culture duration. In addition, for the diagnoses in early childhood, the follow-up time is short for many diseases and considerably shorter for the PGT children in comparison to the IVF/ICSI children. Moreover, information on diagnoses in primary care is lacking. Thus, drawing firm conclusions of early childhood outcome cannot be done.
Conclusions
We conclude that, perinatal and maternal outcomes after PGT-M and PGT-SR are comparable to pregnancies after IVF/ICSI suggesting that the embryo biopsy per se does not affect the outcome. Data on early childhood health in singletons born after PGT are so far also comparable to singletons conceived through IVF/ICSI and spontaneous conception. PGT offers a valuable option to parents affected by genetic disorders to have unaffected children. However, considering the invasiveness of the technique and the rather short follow-up time, additional studies of children born after PGT are of great importance.
Supplementary Material
Acknowledgements
We would like to thank all the IVF-clinics in Sweden for reporting data on ART procedures throughout the years. Moreover, the authors want to thank Lena Hyberg for collecting data on PGT procedures at Karolinska University Hospital.
Contributor Information
Erica Ginström Ernstad, Department of Obstetrics and Gynaecology, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, East Hospital, Sahlgrenska University Hospital, Gothenburg, Sweden.
Charles Hanson, Department of Obstetrics and Gynaecology, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Reproductive Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden.
Kjell Wånggren, Division of Obstetrics and Gynaecology, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden.
Ann Thurin-Kjellberg, Department of Obstetrics and Gynaecology, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Reproductive Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden.
Cecilia Hulthe Söderberg, Department of Clinical Genetics, Sahlgrenska University Hospital, Gothenburg, Sweden.
Elisabeth Syk Lundberg, Department of Clinical Genetics, Karolinska University Hospital, Stockholm, Sweden; Department of Molecular Medicine and Surgery, Centre for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden.
Max Petzold, School of Public Health and Community Medicine, Institute of Medicine, University of Gothenburg, Sweden.
Ulla-Britt Wennerholm, Department of Obstetrics and Gynaecology, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, East Hospital, Sahlgrenska University Hospital, Gothenburg, Sweden.
Christina Bergh, Department of Obstetrics and Gynaecology, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Reproductive Medicine, Sahlgrenska University Hospital, Gothenburg, Sweden.
Data availability
Data cannot be shared publicly due to restrictions by law. For using sensitive health data used in this study, permissions from both the Ethics Committee as well as register keeping authorities is mandatory. The corresponding author can be contacted for questions and advice on the process.
Authors’ roles
All authors participated in the study design and interpretation of the data. E.G.E. analysed the data and M.P. contributed to statistical analyses. E.G.E. drafted the first manuscript and all co-revised it. The manuscript was finalized by E.G.E. and approved by all authors.
Funding
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (LUA/ALF 70940), the Board of National Specialised Medical Care at Sahlgrenska University Hospital and Hjalmar Svensson Research Foundation. The funders had no role in study design, data collection, interpretation of data, or the submission process.
Conflict of interest
None reported.
References
- Bay B, Ingerslev HJ, Lemmen JG, Degn B, Rasmussen IA, Kesmodel US.. Preimplantation genetic diagnosis: a national multicenter obstetric and neonatal follow-up study. Fertil Steril 2016;106:1363–1369.e1. [DOI] [PubMed] [Google Scholar]
- Belva F, Roelants M, Kluijfhout S, Winter C, D, Schrijver F, Desmyttere S, De Rycke M, Tournaye H, Liebaers I, Bonduelle M.. Body composition and blood pressure in 6-year-old singletons born after pre-implantation genetic testing for monogenic and structural chromosomal aberrations: a matched cohort study. Hum Reprod Open 2018;2018:hoy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen S, Söderström-Anttila V, Wennerholm U-B, Laivuori H, Loft A, Oldereid NB, Romundstad LB, Bergh C, Pinborg A.. The health of children conceived by ART: ‘the chicken or the egg?’. Hum Reprod Update 2019;25:137–158. [DOI] [PubMed] [Google Scholar]
- Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R.. The Swedish cause of death register. Eur J Epidemiol 2017;32:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). cdc.gov/art/pdf/2015-report/ART-2015-National-Summary-Report.pdf (23 December 2022, date last accessed).
- Cnattingius S, Ericson A, Gunnarskog J, Kallen B.. A quality study of a medical birth registry. Scand J Soc Med 1990;18:143–148. [DOI] [PubMed] [Google Scholar]
- Coonen E, van Montfoort A, Carvalho F, Kokkali G, Moutou C, Rubio C, De Rycke M, Goossens V.. ESHRE PGT Consortium data collection XVI-XVIII: cycles from 2013 to 2015. Hum Reprod Open 2020;2020:hoaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C.. ESHRE PGD Consortium data collection XIV-XV: cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013. Hum Reprod 2017;32:1974–1994. [DOI] [PubMed] [Google Scholar]
- Desmyttere S, Bonduelle M, Nekkebroeck J, Roelants M, Liebaers I, De Schepper J.. Growth and health outcome of 102 2-year-old children conceived after preimplantation genetic diagnosis or screening. Early Hum Dev 2009;85:755–759. [DOI] [PubMed] [Google Scholar]
- Desmyttere S, De Rycke M, Staessen C, Liebaers I, De Schrijver F, Verpoest W, Haentjens P, Bonduelle M.. Neonatal follow-up of 995 consecutively born children after embryo biopsy for PGD. Hum Reprod 2012;27:288–293. [DOI] [PubMed] [Google Scholar]
- Eapen A, Ryan GL, Ten Eyck P, Van Voorhis BJ.. Current evidence supporting a goal of singletons: a review of maternal and perinatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril 2020;114:690–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Geva T, Srebnik N, Altarescu G, Varshaver I, Brooks B, Levy-Lahad E, Bromiker R, Schimmel MS.. Neonatal outcome after preimplantation genetic diagnosis. Fertil Steril 2014;102:1016–1021. [DOI] [PubMed] [Google Scholar]
- Ericson A, Nygren KG, Olausson PO, Källén B.. Hospital care utilization of infants born after IVF. Hum Reprod 2002;17:929–932. [DOI] [PubMed] [Google Scholar]
- European Network of Publication-Based Registries for the Epidemiological Surveillance of Congenital Anomalies (EUROCAT). www.eurocat-network.eu/content/Stat-Mon-Report-2012.pdf (23 December 2022, date last accessed).
- Faiz AS, Ananth CV.. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med 2003;13:175–190. [DOI] [PubMed] [Google Scholar]
- Ginström Ernstad E, Bergh C, Khatibi A, Källén KBM, Westlander G, Nilsson S, Wennerholm U-B.. Neonatal and maternal outcome after blastocyst transfer: a population-based registry study. Am J Obstet Gynecol 2016;214:378.e1. [DOI] [PubMed] [Google Scholar]
- Ginström Ernstad E, Wennerholm U-B, Khatibi A, Petzold M, Bergh C.. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol 2019;221:126.e121–e126. [DOI] [PubMed] [Google Scholar]
- Handyside AH, Kontogianni EH, Hardy K, Winston RM.. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 1990;344:768–770. [DOI] [PubMed] [Google Scholar]
- Hanson C, Jakobsson AH, Sjögren A, Lundin K, Nilsson L, Wahlström J, Hardarson T, Stevic J, Darnfors C, Janson PO et al Preimplantation genetic diagnosis (PGD): the Gothenburg experience. Acta Obstet Gynecol Scand 2001;80:331–336. [PubMed] [Google Scholar]
- Hardarson T, Hanson C, Lundin K, Hillensjo T, Nilsson L, Stevic J, Reismer E, Borg K, Wikland M, Bergh C.. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod 2008;23:2806–2812. [DOI] [PubMed] [Google Scholar]
- Harton GL, De Rycke M, Fiorentino F, Moutou C, SenGupta S, Traeger-Synodinos J, Harper JC; European Society for Human Reproduction and Embryology (ESHRE) PGD Consortium. ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum Reprod 2011;26:33–40. [DOI] [PubMed] [Google Scholar]
- Hasson J, Limoni D, Malcov M, Frumkin T, Amir H, Shavit T, Bay B, Many A, Almog B.. Obstetric and neonatal outcomes of pregnancies conceived after preimplantation genetic diagnosis: cohort study and meta-analysis. Reprod Biomed Online 2017;35:208–218. [DOI] [PubMed] [Google Scholar]
- Heijligers M, Peeters A, van Montfoort A, Nijsten J, Janssen E, Gunnewiek FK, de Rooy R, van Golde R, Coonen E, Meijer-Hoogeveen M et al Growth, health, and motor development of 5-year-old children born after preimplantation genetic diagnosis. Fertil Steril 2019;111:1151–1158. [DOI] [PubMed] [Google Scholar]
- Heijligers M, Verheijden LMM, Jonkman LM, van der Sangen M, Meijer-Hoogeveen M, Arens Y, van der Hoeven MA, de Die-Smulders CEM.. The cognitive and socio-emotional development of 5-year-old children born after PGD. Hum Reprod 2018;33:2150–2157. [DOI] [PubMed] [Google Scholar]
- Hou W, Shi G, Ma Y, Liu Y, Lu M, Fan X, Sun Y.. Impact of preimplantation genetic testing on obstetric and neonatal outcomes: a systematic review and meta-analysis. Fertil Steril 2021;116:990–1000. [DOI] [PubMed] [Google Scholar]
- Hvidtjorn D, Grove J, Schendel D, Schieve LA, Svaerke C, Ernst E, Thorsen P.. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health 2011;65:497–502. [DOI] [PubMed] [Google Scholar]
- Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD.. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril 2014;101:128–133. [DOI] [PubMed] [Google Scholar]
- Karami M, Jenabi E, Fereidooni B.. The association of placenta previa and assisted reproductive techniques: a meta-analysis. J Matern Fetal Neonatal Med 2018;31:1940–1947. [DOI] [PubMed] [Google Scholar]
- Kuiper D, Bennema A, Bastide-van Gemert S, Seggers J, Schendelaar P, Mastenbroek S, Hoek A, Heineman MJ, Roseboom TJ, Kok JH et al Developmental outcome of 9-year-old children born after PGS: follow-up of a randomized trial. Hum Reprod 2018;33:147–155. [DOI] [PubMed] [Google Scholar]
- Källén AJ, Finnstrom OO, Lindam AP, Nilsson EM, Nygren KG, Otterblad Olausson PM.. Is there an increased risk for drug treated attention deficit/hyperactivity disorder in children born after in vitro fertilization? Eur J Paediatr Neurol 2011;15:247–253. [DOI] [PubMed] [Google Scholar]
- Källén AJ, Finnström OO, Lindam AP, Nilsson EM, Nygren KG, Olausson PM.. Cerebral palsy in children born after in vitro fertilization. Is the risk decreasing? Eur J Paediatr Neurol 2010;14:526–530. [DOI] [PubMed] [Google Scholar]
- Källén B, Finnström O, Nygren K-G, Olausson PO.. In vitro fertilization in Sweden: child morbidity including cancer risk. Fertil Steril 2005;84:605–610. [DOI] [PubMed] [Google Scholar]
- Lewis S, Amor DJ, Glynn A, Wilton L, Halliday J.. Child health after preimplantation genetic testing. Reprod Biomed Online 2021;42:609–619. [DOI] [PubMed] [Google Scholar]
- Liebaers I, Desmyttere S, Verpoest W, De Rycke M, Staessen C, Sermon K, Devroey P, Haentjens P, Bonduelle M.. Report on a consecutive series of 581 children born after blastomere biopsy for preimplantation genetic diagnosis. Hum Reprod 2010;25:275–282. [DOI] [PubMed] [Google Scholar]
- Liu L, Gao J, He X, Cai Y, Wang L, Fan X.. Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: a meta-analysis. Sci Rep 2017;7:46207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO.. External review and validation of the Swedish National Inpatient Register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Kalampokas T, Davidson J, Bhattacharya S.. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of blastocyst-stage versus cleavage-stage embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril 2013;100:1615–1621.e10. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S.. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018;24:35–58. [DOI] [PubMed] [Google Scholar]
- Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B.. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848. [DOI] [PubMed] [Google Scholar]
- National Registry of Assisted Reproduction. Q-IVF. http://www.ucr.uu.se/qivf/ (23 2022, date last accessed).
- Natsuaki MN, Dimler LM.. Pregnancy and child developmental outcomes after preimplantation genetic screening: a meta-analytic and systematic review. World J Pediatr 2018;14:555–569. [DOI] [PubMed] [Google Scholar]
- Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, Wennerholm UB, Gissler M, Skjærven R, Romundstad LB.. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod 2015;30:1724–1731. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, Nygren KG, Hazekamp J, Bergh C.. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- Qin J, Liu X, Sheng X, Wang H, Gao S.. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril 2016;105:73–85.e6. [DOI] [PubMed] [Google Scholar]
- Rombauts L, Motteram C, Berkowitz E, Fernando S.. Risk of placenta praevia is linked to endometrial thickness in a retrospective cohort study of 4537 singleton assisted reproduction technology births. Hum Reprod 2014;29:2787–2793. [DOI] [PubMed] [Google Scholar]
- Rönö K, Rissanen E, Bergh C, Wennerholm U-B, Opdahl S, Romundstad LB, Henningsen A-KA, Spangmose AL, Pinborg A, Gissler M et al The neurodevelopmental morbidity of children born after assisted reproductive technology: a Nordic register study from the Committee of Nordic Assisted Reproductive Technology and Safety group. Fertil Steril 2022;117:1026–1037. [DOI] [PubMed] [Google Scholar]
- Sharpe A, Avery P, Choudhary M.. Reproductive outcome following pre-implantation genetic diagnosis (PGD) in the UK. Hum Fertil (Camb) 2018;21:120–127. [DOI] [PubMed] [Google Scholar]
- Spangmose AL, Christensen LH, Henningsen AA, Forman J, Opdahl S, Romundstad LB, Himmelmann K, Bergh C, Wennerholm UB, Tiitinen A et al Cerebral palsy in ART children has declined substantially over time: a Nordic study from the CoNARTaS group. Hum Reprod 2021;36:2358–2370. [DOI] [PubMed] [Google Scholar]
- Spangmose AL, Ginström Ernstad E, Malchau S, Forman J, Tiitinen A, Gissler M, Opdahl S, Romundstad LB, Bergh C, Wennerholm UB et al Obstetric and perinatal risks in 4601 singletons and 884 twins conceived after fresh blastocyst transfers: a Nordic study from the CoNARTaS group. Hum Reprod 2020;35:805–815. [DOI] [PubMed] [Google Scholar]
- Stromberg B, Dahlquist G, Ericson A, Finnstrom O, Koster M, Stjernqvist K.. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet 2002;359:461–465. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Antonisamy B, Selliah HY, Kamath MS.. Pre-term birth and low birth weight following preimplantation genetic diagnosis: analysis of 88 010 singleton live births following PGD and IVF cycles. Hum Reprod 2017;32:432–438. [DOI] [PubMed] [Google Scholar]
- Svahn MF, Hargreave M, Nielsen TSS, Plessen KJ, Jensen SM, Kjaer SK, Jensen A.. Mental disorders in childhood and young adulthood among children born to women with fertility problems. Hum Reprod 2015;30:2129–2137. [DOI] [PubMed] [Google Scholar]
- Swedish National Board of Health and Welfare. The Swedish Medical Birth Register: A Summary of Content and Quality. https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-medical-birth-register/ (23 December 2022, date last accessed).
- van Montfoort A, Carvalho F, Coonen E, Kokkali G, Moutou C, Rubio C, Goossens V, De Rycke M.. ESHRE PGT Consortium data collection XIX-XX: PGT analyses from 2016 to 2017(†). Hum Reprod Open 2021;2021:hoab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermey BG, Buchanan A, Chambers GM, Kolibianakis EM, Bosdou J, Chapman MG, Venetis CA.. Are singleton pregnancies after assisted reproduction technology (ART) associated with a higher risk of placental anomalies compared with non-ART singleton pregnancies? A systematic review and meta-analysis. BJOG 2019;126:209–218. [DOI] [PubMed] [Google Scholar]
- von Versen-Hoynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, Williams S, Rhoton-Vlasak R, Nichols A, Fleischmann WW et al Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019;73:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FF, Yu T, Chen XL, Luo R, Mu DZ.. Cerebral palsy in children born after assisted reproductive technology: a meta-analysis. World J Pediatr 2021;17:364–374. [DOI] [PubMed] [Google Scholar]
- Wijs LA, Fusco MR, Doherty DA, Keelan JA, Hart RJ.. Asthma and allergies in offspring conceived by ART: a systematic review and meta-analysis. Hum Reprod Update 2021;28:132–148. [DOI] [PubMed] [Google Scholar]
- Zhang WY, von Versen-Hoynck F, Kapphahn KI, Fleischmann RR, Zhao Q, Baker VL.. Maternal and neonatal outcomes associated with trophectoderm biopsy. Fertil Steril 2019;112:283–290.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Yang C, Yang S, Sun S, Mu M, Rao M, Zu R, Yan J, Ren B, Yang R et al Obstetric and neonatal outcomes of pregnancies resulting from preimplantation genetic testing: a systematic review and meta-analysis. Hum Reprod Update 2021;27:989–1012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly due to restrictions by law. For using sensitive health data used in this study, permissions from both the Ethics Committee as well as register keeping authorities is mandatory. The corresponding author can be contacted for questions and advice on the process.