Abstract
The presence of foreign nucleic acids in the cytosol is a marker of infection. Cells have sensors, also known as pattern recognition receptors (PRRs), in the cytosol that detect foreign nucleic acid and initiate an innate immune response. Recent studies have reported the condensation of multiple PRRs including PKR, NLRP6, and cGAS, with their nucleic acid activators into discrete nucleoprotein assemblies. Nucleic acid–protein condensates form due to multivalent interactions and can create high local concentrations of components. The formation of PRR‐containing condensates may alter the magnitude or timing of PRR activation. In addition, unique condensates form following RNase L activation or during paracrine signaling from virally infected cells that may play roles in antiviral defense. These observations suggest that condensate formation may be a conserved mechanism that cells use to regulate activation of the innate immune response and open an avenue for further investigation into the composition and function of these condensates. Here we review the nucleic acid–protein granules that are implicated in the innate immune response, discuss general consequences of condensate formation and signal transduction, as well as what outstanding questions remain.
Keywords: granule, innate immune response, RNP
Subject Categories: Immunology, Translation & Protein Quality
Parker and colleagues discuss how nucleic acids can affect nucleoprotein condensate formation and the function of pattern‐recognition receptors as well as other antiviral defense proteins.

Pattern recognition receptors detect foreign nucleic acid
The presence of cytosolic DNA or double‐stranded RNA (dsRNA) is a marker of infection and thus cells have evolved sensors, known as pattern recognition receptors (PRRs) that detect foreign nucleic acid and initiate an immune response (Takeuchi & Akira, 2010; Maelfait et al, 2020; Li & Wu, 2021; Okude et al, 2021). These sensors include cytoplasmic NOD‐like receptors (NLRs), cyclic GMP‐AMP synthase (cGAS), RIG‐I‐like receptors, oligoadenylate synthetases (OASs), and dsRNA‐activated protein kinase R (PKR; Hu et al, 2018; Li & Wu, 2021; Okude et al, 2021; Fig 1). DNA or RNA can also be recognized by toll‐like receptors, thereby activating signaling based on extracellular or endosomal nucleic acids (Kawasaki & Kawai, 2014). Upon activation, these PRRs trigger translational repression, RNA decay, interferon production, and cell death, which limits viral replication (Takeuchi & Akira, 2010; Gal‐Ben‐Ari et al, 2019; Okude et al, 2021).
Figure 1. Diagram depicting the numerous nucleic acid sensors in the cell.
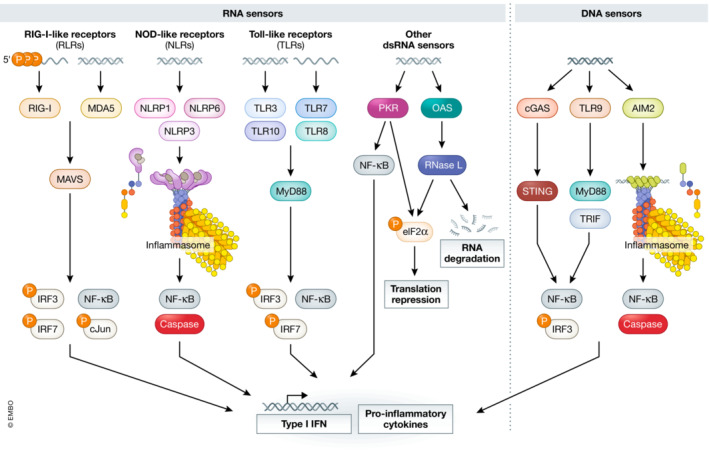
Mammalian cells express various sensors which detect foreign nucleic acids such as DNA, double‐stranded RNA (dsRNA), or modified RNAs. Upon detection of their nucleic acid activator, these sensors initiate innate immune responses which often result in the production of Type I interferon (IFN), pro‐inflammatory cytokines, translational repression, and cell death.
Recently, several studies have shown that many of the nucleic acid PRRs, including PKR, cGAS, NLRP6, and NLRP1 assemble into discrete cytosolic condensates with their cognate nucleic acid substrates (Du & Chen, 2018; Shen et al, 2021; Corbet et al, 2022; Zappa et al, 2022). Critical issues in each of these assemblies are as follows: (i) what molecular interactions drive condensate formation? (ii) what are the functional consequences of condensate formation? (iii) what is the required size of the condensates to exert a biological response? (iv) what additional factors enrich in these condensates? Two general principles emerge from this review. First, the formation of PRR‐nucleic acid condensates is similar to other condensate formation and driven by a diversity of multivalent interactions. Second, the concentration of PRRs into condensates with their nucleic acid activator is a method that cells may use to regulate the sensitivity or timing of a response to a PRR‐recognized nucleic acid under specific conditions.
Multiple pattern recognition receptors form condensates with their cognate nucleic acid
Recent studies have reported multiple PRRs, including PKR, NLRP6, and cGAS, form biological condensates with their nucleic acid activators, which we describe in this section.
NLRP6 droplets
Inflammasomes are protein complexes that form upon activation of specific PRRs and function to activate caspase‐1 and promote the secretion of pro‐inflammatory cytokines (Guo et al, 2015; Evavold & Kagan, 2019). Different inflammasomes form depending on the specific stimuli, but most are formed by members of the nucleotide binding and oligomerization domain NLR family (Saxena & Yeretssian, 2014; Guo et al, 2015). Inflammasome assembly begins with activation of a receptor (NLR or other receptor), often by binding to its nucleic acid substrate, which relieves autorepression of the NLR and allows for NLR oligomerization (Lamkanfi & Dixit, 2014; Lechtenberg et al, 2014). Oligomerization of the NLR results in the recruitment of both the adapter protein ASC and caspase‐1. This leads to the production of the inflammatory cytokines IL‐1β and IL‐18, which ultimately results in to cell death (Lamkanfi & Dixit, 2014; Guo et al, 2015).
A recent study identified the inflammasome protein NLRP6 as a dsRNA sensor that forms condensates to initiate an innate immune response (Shen et al, 2021; Fig 2). Specifically, both in vitro and in cells, the authors found that dsRNA induces condensation of NLRP6 into higher‐order assemblies which contain dsRNA. Shen et al identified a poly‐Lysine tract in NLRP6 which, when mutated, disrupts condensate formation, but not NLRP6 binding to dsRNA. Mutation of this poly‐Lysine tract also reduced the condensation of viral RNA into puncta and reduced inflammasome activation during murine hepatitis virus (MHV) infection. NLRP6‐dsRNA condensates also recruit DHX15 and ASC, which suggests these condensates can modulate the different signaling pathways activated by dsRNA‐induced NLRP6 activation (Shen et al, 2021). This suggests that NLRP6 condensation with dsRNA plays a role in the activation of the NLRP6 inflammasome during MHV infection.
Figure 2. Condensates of nucleic acid sensors with their nucleic acid activators.

Several distinct condensates formed by nucleic acid sensors together with their nucleic acid activators have been recently described. Here we summarize the known components and possible functions of each of these condensates.
A fuller understanding of NLRP6 condensation with dsRNA will require addressing some remaining issues. For example, NLRP6 forms condensates itself without the dsRNA, although dsRNA does enhance condensation. This suggests that NLRP6 condensation may be an inherent property of NLRP6 and is not strictly dependent on dsRNA. Moreover, the formation of NLRP6 condensates may be limited to certain cell types since NLRP6 condensation with dsRNA was not observed in A549 cells transfected with poly(I:C) (Corbet et al, 2022). Finally, as with all proteins that form condensates, it is difficult to distinguish between disruptions in the protein's function generally, and the protein's function specifically within a condensate. However, these observations do suggest that NLRP6's interaction with dsRNA in a higher‐order assembly is important for inflammasome activation, at least in some contexts.
dsRNA‐induced foci
A second type of PRR‐nucleic acid condensate contains dsRNA and the dsRNA‐activated kinase PKR, as well as other dsRNA‐binding proteins (Fig 2). PKR is a Ser/Thr kinase that is activated by binding and homodimerizing on dsRNA (Meurs et al, 1990; Nanduri et al, 2000; Ung et al, 2001; Lemaire et al, 2008). Upon activation, PKR phosphorylates the translation initiation factor eIF2α, inhibiting translation initiation (De Benedetti & Baglioni, 1984; Wek et al, 2006; Dalet et al, 2015). The inhibition of translation results in the formation of stress granules (Lu et al, 1995; Kedersha et al, 1999; Wek et al, 2006). Interestingly, several studies suggested that PKR localizes to stress granules and that this localization is important for the antiviral response (Onomoto et al, 2012; Yoo et al, 2014; Manivannan et al, 2020; preprint: Cadena et al, 2021).
However, two reports identify a new dsRNA‐induced RNA–protein condensate that contains PKR and is distinct from stress granules (Corbet et al, 2022; Zappa et al, 2022). These condensates have been termed dsRNA‐induced foci (dRIFs). dRIFs form after transfection with the dsRNA mimic poly(I:C), during measles virus infection, and during conditions of increased endogenous dsRNA from transposons (Corbet et al, 2022; Zappa et al, 2022). Several components of dRIFs have been identified, although a full description of their composition is lacking. dRIFs contain multiple molecules of dsRNA as demonstrated by the co‐localization of differentially tagged dsRNA molecules in the same foci (Corbet et al, 2022). dsRNA appears to act as a scaffold for dRIF formation since dRIFs form in response to increased endogenous or exogenous cytoplasmic dsRNA, they form in proportion to dsRNA concentration, and they are enhanced by longer dsRNAs (Corbet et al, 2022; Zappa et al, 2022). Consistent with dsRNA forming a scaffold for dRIFs, several other dsRNA‐binding proteins including ADAR1, NLRP1, PACT, and DHX9 also enrich in dRIFs (Corbet et al, 2022). However, not all dsRNA‐binding proteins enrich in dRIFs, including RIG‐I and NLRP3, suggesting that dRIF formation regulates distinct dsRNA/antiviral pathways. It also remains possible that some dsRNA binding proteins are not seen in dRIFs simply due to poor immunoreactivity of the antibodies utilized.
dsRNA binding is one manner by which proteins are recruited to dRIFs. This is, for example, demonstrated by the observation that the dsRNA‐binding domains of ADAR1 are necessary and sufficient to target ADAR1 to dRIFs (Corbet et al, 2022). Similarly, a mutation in PKR that reduces dsRNA binding shows decreased partitioning into dRIFs (Corbet et al, 2022). The recruitment of proteins to dRIFs may also be regulated by post‐translational modifications. For example, the autophosphorylation of PKR due to/upon its dimerization or oligomerization on dsRNA, reduces its affinity for dsRNA (Cole, 2007), suggesting that activated PKR might accumulate less in dRIFs. Consistent with this interpretation, inhibition of PKR kinase activity by mutations or chemical inhibitors leads to a greater enrichment of PKR in dRIFs (Corbet et al, 2022).
Since some dsRNA‐binding proteins, including ADAR1, PACT, Stau1, and PKR, contain multiple dsRNA‐binding domains, a simple model for dRIF formation is that such proteins bind and crosslink multiple molecules of dsRNA, condensing dsRNA and dsRNA‐binding proteins into larger foci (Fig 3). However, this model remains to be tested.
Figure 3. Model for dsRNA‐induced foci (dRIF) formation.

Cytosolic double‐stranded RNA (dsRNA) is bound by multivalent dsRNA‐binding proteins, resulting in the crosslinking of dsRNA–protein assemblies and condensation into dRIF.
Additional data suggest that protein–protein interactions will contribute to dRIF formation. The key observation is that mutations in PKR that limit PKR‐PKR interactions (Mayo et al, 2019) show reduced partitioning into PKR foci (Zappa et al, 2022). Furthermore, mutating PKR's ability to bind dsRNA reduces, but does not eliminate PKR localization to dRIFs, indicating that there are additional interactions that partition PKR into dRIFs (Corbet et al, 2022). Thus, one anticipates that a combination of protein–protein, protein–RNA, and RNA–RNA interactions contribute to the formation of dRIFs, as they do for other RNP assemblies (Van Treeck & Parker, 2018). Future analyses are needed to determine the differential contributions of these interactions for dRIF formation.
A currently unresolved issue is how dRIFs affect PKR signaling. Some observations suggest that dRIFs might promote PKR signaling by increasing the local concentration of PKR, thereby promoting autophosphorylation and PKR activation. In support of this possibility, dRIFs form prior to translational shutoff, and dRIFs localize near the sites where cells initiate translational repression as assessed by stress granule formation (Corbet et al, 2022). Alternatively, two observations have argued that dRIFs might serve as sinks for activated PKR, thereby reducing PKR signaling. First, PKR's substrate eIF2α is not enriched in dRIFs, implying that PKR in dRIFs would be unable to phosphorylate eIF2α (Zappa et al, 2022). However, other work suggests that eIF2α is not excluded from dRIFs and therefore may be available for phosphorylation by PKR found in dRIFs (Corbet et al, 2022). Second, point mutations reducing PKR‐PKR interactions and reduced PKR recruitment to dRIFs show enhanced phosphorylation of eIF2α prior to, and in response to, exogenous dsRNA (Zappa et al, 2022), suggesting that less PKR in dRIFs results in increased PKR signaling. It is possible that dRIFs may have a positive or negative impact on PKR function depending on the concentration of dsRNA, and future studies are needed to parse out how dRIF formation modulates PKR activation.
An interesting possibility is that concentration of dsRNA sensors in dRIFs may function to accelerate or attenuate the antiviral response to dsRNA. Numerous viruses target and inhibit PKR to promote their replication. By concentrating PKR in dRIFs, and potentially excluding viral inhibitors, dRIF formation may be a mechanism cells use to counter this viral inhibition (Davies et al, 1993; Lu et al, 1995; Li et al, 2006; Tu et al, 2012).
In an alternative view, dRIFs may function to dampen PKR signaling. Cells have feedback mechanisms to dampen unwarranted innate immune response activation. For example, GADD34 is induced by dsRNA via IRF3 and dephosphorylates p‐eIF2α to permit translation of antiviral mRNAs (Clavarino et al, 2012; Dalet et al, 2017). The RNA triphosphatase DUSP11 reduces both host and viral 5′‐triphosphorylated RNA to dampen RIG‐I sensitivity to avoid unwarranted immune activation and initiate hepatitis C viral RNA decay. However, these functions occur at the expense of reducing RIG‐I sensitivity to viruses (Burke et al, 2016; Burke & Sullivan, 2017; Kincaid et al, 2018; Choi et al, 2020; Choi & Sullivan, 2021), and may also affect PKR activation and/or dRIF recruitment (Nallagatla et al, 2007). Therefore, PRR condensates similarly may serve as an autoregulatory mechanism in response to high concentration of RNA substrates, whereby they negatively regulate the signaling pathways of specific PRRs.
Since dRIFs contain multiple antiviral dsRNA‐binding proteins in addition to PKR, including ADAR1 and NLRP1, dRIFs may also impact other aspects of the dsRNA response independent of PKR. Future studies are needed to determine the full protein composition of dRIFs and how dRIF formation affects the various facets of the dsRNA response. In addition, aberrant PKR activation and inflammation are found in patients with autoimmune disorders such as Aicardi‐Goutières Syndrome, as well as neurodegenerative diseases such as ALS caused by a repeat expansion mutation (Tian et al, 2000; Zu et al, 2020; Martinez et al, 2021; Maurano et al, 2021). An important area to explore in future work is thus also whether dRIFs or related dsRNA condensates are found in patient tissues that display aberrant PKR activation/inflammation. Their presence during disease may indicate that they either promote disease or are actively serving to negatively regulate aberrant stress responses. Thus, pharmacological modulation of these structures may serve as a potential therapeutic strategy to regulate PKR activation that contribute to neurodegeneration.
cGAS liquid droplets
Cytosolic DNA is a marker of infection and elicits an immune response. The PRR cGAS is activated by direct binding to cytosolic DNA and produces the secondary messenger cyclic GMP‐AMP (cGAMP; Sun et al, 2013), which then signals activation of the innate immune response by binding the STING protein (Wu et al, 2013).
A recent report found that cGAS and DNA form condensates that facilitate activation of cGAS both in vitro and in cells (Du & Chen, 2018; Fig 2). These droplets also concentrate Zn2+ ions, which promote cGAS activity. Similar to dRIFs, cGAS droplets were dependent on DNA length, likely due to the increased valency of longer DNA. Mutating cGAS to reduce its valency, but not its binding to DNA, reduced both cGAS droplet formation and cGAMP production, a readout of cGAS activation. This observation argues that droplet formation enhances cGAS activity. This report adds to the growing list of observations suggesting that innate immune sensors form condensates with their nucleic acid activators to modulate the immune response.
Interestingly, the stress granule protein G3BP1 has been shown to interact directly with cGAS to promote its condensation and activation (Hu et al, 2019; Liu et al, 2019; Zhao et al, 2022). G3BP1 reportedly binds directly to cGAS, stimulating its DNA binding, similar to G3BP1 promoting RIG‐I targeting to viral RNA (Kim et al, 2019). This potentially links cGAS droplets to stress granules, another RNP granule discussed below.
Do stress granules affect the innate immune response?
Stress granules form in the cytosol due to condensation of non‐translating RNAs with RNA‐binding proteins when translation initiation is inhibited due to stress (Fig 4; Kedersha et al, 1999; Protter & Parker, 2016; Khong et al, 2017). Stresses such as oxidative stress, heat shock, osmotic stress, and viral infection can all trigger stress granule formation (Kedersha & Anderson, 2007; Protter & Parker, 2016). While the exact function of stress granules is yet to be determined, it has been suggested that they may play roles in RNA storage, cell survival, cell signaling, and cell cycle progression (Kedersha et al, 2005, 2013; Anderson & Kedersha, 2008; Eiermann et al, 2020; Mateju & Chao, 2022).
Figure 4. Stress granule‐like assemblies during viral infection.

(A) In cells lacking RNase L (RNase L(−)), cytosolic double‐stranded RNA (dsRNA) triggers protein kinase R (PKR) activation and phosphorylation of eIF2α, resulting in translational repression and stress granule formation. (B) In cells competent for RNase L (RNase L(+)), cytosolic dsRNA triggers PKR activation and phosphorylation of eIF2α as well as RNase L activation and mRNA degradation, resulting in translational repression and RNase L‐body formation. (C) Novel stress granule‐like paracrine granules were observed to form due to paracrine signaling from infected cells to bystander cells, which correlates with increased antiviral defense.
Three types of observations have suggested that stress granules may have a role in the antiviral response (for in depth reviews, see Eiermann et al, 2020; Mateju & Chao, 2022). First, multiple studies have reported the recruitment of PRRs, including PKR, MDA‐5, RIG‐I, and OAS to stress granules (Onomoto et al, 2012; Langereis et al, 2013; Reineke & Lloyd, 2014; Yoo et al, 2014; Zhang et al, 2014; Reineke et al, 2015; Beauclair et al, 2020; preprint: Cadena et al, 2021). These reports propose that interactions between stress granule proteins modulate the activation of immune sensors to regulate the immune response. It remains to be determined why PKR is seen in stress granules during some stress responses and accumulates in dRIFs in others.
A second set of observations connecting stress granules to antiviral functions is that several viruses, including influenza A virus, poliovirus, Zika virus, and SARS‐Cov‐2, inhibit stress granule formation by multiple mechanisms, including inhibition of PKR, or inhibition of G3BP1 function by cleavage or competing binding (Li et al, 2006; White et al, 2007; Dougherty et al, 2015; Bonenfant et al, 2019; Gao et al, 2021). The viral inhibition of G3BP1 and PKR has been used to infer that stress granules in some manner interfere with viral lifecycles.
Finally, G3BP1 KD reduces PKR phosphorylation in herpes simplex virus 1 (HSV‐1) infection (Burgess & Mohr, 2018), suggesting that stress granules, or G3BP1, may promote PKR activation, a model similarly proposed by Richard Lloyd's lab (Reineke et al, 2015). An unresolved issue here is whether G3BP1 has direct antiviral activities independent of stress granules, or whether it is the formation of stress granules themselves that leads to antiviral outputs.
Novel RNP granules and their possible roles in the innate immune response
RNP granules related to, but distinct from, stress granules form in cells when cellular RNA is degraded by RNase L activation, which occurs during some viral infections (Burke et al, 2019, 2020). Cytoplasmic dsRNA activates OASs, which produce 2′‐5′ oligoadenylates that activate RNase L (Silverman, 2007; Lohöfener et al, 2015; Li et al, 2016; Hu et al, 2018). Upon activation, RNase L degrades bulk cytoplasmic mRNA, leading to bulk translational reduction and formation of small RNP granules termed RNase L‐bodies (RLBs; Fig 4; Burke et al, 2019, 2020). While RLBs share various components with stress granules, they are distinct assemblies as assessed by their biogenesis, composition, and biophysical properties. RLB formation requires RNase L activation and when PKR, but not RNase L, is activated in response to dsRNA, cells will form canonical stress granules (Fig 4). However, dsRNA‐induced stress granules are dependent on PKR, while RLBs are not, and whether stress granules or RLBs will form upon detection of dsRNA is dependent on the level of RNase L expression, which varies between cell lines (Burke et al, 2020). Future studies are required to determine whether RLB formation has an antiviral function or whether it is simply a consequence of cleaved RNA fragments allowing for condensation of RNA‐binding proteins and possibly RNase L cleavage products.
It was recently reported that paracrine signaling induces stress granule‐like assemblies, termed paracrine granules, to form uninfected cells during feline calicivirus infection (Iadevaia et al, 2022). Paracrine granules are distinct from stress granules in that they are independent of both G3BP1/2 and phosphorylation of eIF2α, and have a distinct protein and RNA composition from stress granules (Fig 4). Treating cells with supernatant from infected cells to induce paracrine granules prior to infection with FCV resulted in reduced viral replication, suggesting that paracrine granules may have a role in the antiviral response and may prime cells for infection, allowing cells to initiate an immune response before the virus is detected inside the cell. An important future question is understanding how paracrine granules assemble and promote antiviral activity.
General consequences of condensate formation and signal transduction
The formation of condensates concentrating the components of signaling pathways occurs repeatedly in multiple biological contexts. For example, NLRP6 inflammasome formation (Shen et al, 2021), T‐cell receptor activation through the LAT‐Grb2‐SOS pathway (Su et al, 2016; Huang et al, 2019), and the Nephrin‐Nck‐N‐WASP signaling pathway (Li et al, 2012; Su et al, 2016; Case et al, 2019) all utilize condensate formation during signal transduction (reviewed in Jaqaman & Ditlev, 2021; Martin & Mittag, 2019; Su et al, 2021).
In principle, condensate formation can alter the output of a signaling pathway in multiple manners. Most simply, for signaling molecules that self‐activate when brought into proximity, such as PKR (Kostura & Mathews, 1989; Nanduri et al, 2000; Lemaire et al, 2005, 2008; Mayo et al, 2019), the formation of a condensate could contribute to enhanced activation. A good example of this is the enhanced activation of cGAS by condensate formation (Du & Chen, 2018). Second, under conditions where the condensate recruits and retains downstream effectors, coupled with a slow activation rate, the condensate can increase the signal‐to‐noise essentially by kinetic proofreading (Fig 5A; Case et al, 2019; Martin & Mittag, 2019). Third, condensate formation could prolong the signal transduction period if negative regulators of signaling are excluded from the condensate. For example, if phosphatases that dephosphorylate activated PKR are excluded from dRIFs, the formation of dRIFs will promote prolonged PKR signaling (Fig 5B). Fourth, condensate formation could limit signal transduction if key effectors of the signaling pathway are excluded from the condensate, thus sequestering sensors from their effectors, as has been proposed for eIF2α being excluded from dRIFs (Fig 5C; Zappa et al, 2022). However, a sequestration model requires that a substantial amount of an effector must be sequestered in the granule to affect signaling initiated outside of the granule, though the degree of this parameter may be modulated by substrate concentration. Fifth, condensate formation could limit signal transduction of negative regulators of the sensor are enriched in the condensate (Fig 5D).
Figure 5. Possible consequences of condensate formation for signaling.

Condensates may enhance or inhibit signaling by condensate components, depending on several factors including enrichment or depletion of activators or repressors of signaling components within the condensate.
Finally, condensates could also serve to integrate multiple signaling pathways in response to a given stimulus, such as dsRNA. By concentrating multiple signaling proteins within a singular condensate, the output of multiple signaling pathways may be modulated by increasing or decreasing condensate formation. An understanding of how a given PRR‐nucleic acid condensate affects signaling will require developing methods to specifically limit condensate formation and then examining how the response is altered under a diversity of biological conditions.
Conclusion and outstanding questions
Increasing reports have shown that nucleic acid sensors are prone to form condensates together with their ligands. Nucleic acid molecules are ideal molecules to assemble condensates since even short viral DNAs or RNAs are typically 1,000s of bases in length, thereby having the potential to be highly multivalent for assembly. These observations suggest a role for condensates in regulating the function of nucleic acid sensors. Numerous outstanding questions regarding these condensates remain.
What is the composition of these condensates other than the few components identified, and how does partitioning of proteins or nucleic acids into these condensates affect signaling? Does altering condensate formation alter immune function? One possible function of dRIFs formed by numerous weak, multivalent interactions is that they may still be able to form and recruit PKR despite virus‐mediated inhibition of PKR dimerization. Many viral proteins, such as influenza A virus NS1 protein, bind viral dsRNA to inhibit activation of PRRs, including PKR and OAS/RNase L (Hatada & Fukuda, 1992; Min & Krug, 2006). Whether these proteins directly prevent dRIF formation has not been addressed. Condensates may also function to integrate multiple responses to a given stimulus, such as dsRNA or cytoplasmic DNA, by concentrating sensors which feed into different signaling pathways in the same assembly.
A second outstanding question is how the properties of the nucleic acid will affect the formation of such condensates. One anticipates that the longer DNA or dsRNA will promote cGAS or dRIF assemblies. However, shorter DNAs or dsRNAs may also alter the composition of condensates by changing the ratio of end binding versus internally binding proteins. It also remains to be seen if the presence of internal bulges and/or loops will affect condensate formation, composition, or function. Moreover, given how modifications such as pseudoU or m6A can alter recognition and activation of innate immune responses (Karikó et al, 2005, 2008; Anderson et al, 2011; Chen et al, 2019), one anticipates that modifications of DNA or RNA may alter the assembly and possible function of such innate immune sensing condensates.
A continual challenge in studying the function of RNP granules is separating the function of the granule components inside the granule from their functions outside of the granule. Mutations which alter a protein's recruitment to an RNP granule may also affect the protein's function outside of the granule. For example, depleting G3BP1/2 prevents stress granule formation in many contexts (Tourrière et al, 2003; Kedersha et al, 2016; Guillén‐Boixet et al, 2020; Yang et al, 2020); however, all stress granule‐independent functions of G3BP1/2 are also lost. Additional methods of limiting granules, such as knockout of different proteins that regulate granules, will be needed to distinguish the specific function of granules from the roles of their constitutive nucleic‐acid‐binding proteins that are unrelated to granule formation.
Author contributions
Giulia A Corbet: Conceptualization; writing – original draft; writing – review and editing. James M Burke: Conceptualization; writing – original draft; writing – review and editing. Roy Parker: Conceptualization; writing – original draft; writing – review and editing.
Disclosure and competing interests statement
Roy Parker is a founder and consultant for Faze Medicines.
The EMBO Journal (2023) 42: e111870
References
- Anderson P, Kedersha N (2008) Stress granules: the Tao of RNA triage. Trends Biochem Sci 33: 141–150 [DOI] [PubMed] [Google Scholar]
- Anderson BR, Muramatsu H, Jha BK, Silverman RH, Weissman D, Karikó K (2011) Nucleoside modifications in RNA limit activation of 2′‐5′‐oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res 39: 9329–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauclair G, Streicher F, Chazal M, Bruni D, Lesage S, Gracias S, Bourgeau S, Sinigaglia L, Fujita T, Meurs EF et al (2020) Retinoic acid inducible gene I and protein kinase R, but not stress granules, mediate the proinflammatory response to yellow fever virus. J Virol 94: e00403‐20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenfant G, Williams N, Netzband R, Schwarz MC, Evans MJ, Pager CT (2019) Zika virus subverts stress granules to promote and restrict viral gene expression. J Virol 93: e00520‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HM, Mohr I (2018) Defining the role of stress granules in innate immune suppression by the herpes simplex virus 1 endoribonuclease VHS. J Virol 92: e00829‐18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Sullivan CS (2017) DUSP11 – an RNA phosphatase that regulates host and viral non‐coding RNAs in mammalian cells. RNA Biol 14: 1457–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Kincaid RP, Nottingham RM, Lambowitz AM, Sullivan CS (2016) DUSP11 activity on triphosphorylated transcripts promotes Argonaute association with noncanonical viral microRNAs and regulates steady‐state levels of cellular noncoding RNAs. Genes Dev 30: 2076–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Moon SL, Matheny T, Parker R (2019) RNase L reprograms translation by widespread mRNA turnover escaped by antiviral mRNAs. Mol Cell 75: 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Lester ET, Tauber D, Parker R (2020) RNase L promotes the formation of unique ribonucleoprotein granules distinct from stress granules. J Biol Chem 295: 1426–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena C, Paget M, Wang H‐T, Kim E, Ahmad S, Zhang Q, Koo B, Lyons SM, Ivanov P, Mu X et al (2021) Stress granules are shock absorbers that prevent excessive innate immune responses to dsRNA. BioRxiv 2021 10.1101/2021.04.26.441141 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Zhang X, Ditlev JA, Rosen MK (2019) Stoichiometry controls activity of phase‐separated clusters of Actin signaling proteins. Science 363: 1093–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Chen R, Ahmad S, Verma R, Kasturi S, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B et al (2019) N6‐methyladenosine modification controls circular RNA immunity. Mol Cell 76: 96–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Sullivan CS (2021) DUSP11 and triphosphate RNA balance during virus infection. PLoS Pathog 17: e1009145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Burke JM, Szymanik KH, Nepal U, Battenhouse A, Lau JT, Stark A, Lam V, Sullivan CS (2020) DUSP11‐mediated control of 5′‐triphosphate RNA regulates RIG‐I sensitivity. Genes Dev 34: 1697–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavarino G, Cláudio N, Couderc T, Dalet A, Judith D, Camosseto V, Schmidt EK, Wenger T, Lecuit M, Gatti E et al (2012) Induction of GADD34 is necessary for dsRNA‐dependent interferon‐β production and participates in the control of chikungunya virus infection. PLoS Pathog 8: e1002708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JL (2007) Activation of PKR: an open and shut case? Trends Biochem Sci 32: 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet GA, Burke JM, Bublitz GR, Tay JW, Parker R (2022) dsRNA‐induced condensation of antiviral proteins modulates PKR activity. Proc Natl Acad Sci USA 119: e2204235119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalet A, Gatti E, Pierre P (2015) Integration of PKR‐dependent translation inhibition with innate immunity is required for a coordinated anti‐viral response. FEBS Lett 589: 1539–1545 [DOI] [PubMed] [Google Scholar]
- Dalet A, Argüello RJ, Combes A, Spinelli L, Jaeger S, Fallet M, Vu Manh T, Mendes A, Perego J, Reverendo M et al (2017) Protein synthesis inhibition and GADD34 control IFN‐β heterogeneous expression in response to dsRNA. EMBO J 36: 761–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MV, Chang HW, Jacobs BL, Kaufman RJ (1993) The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double‐stranded RNA‐dependent protein kinase by different mechanisms. J Virol 67: 1688–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A, Baglioni C (1984) Inhibition of mRNA binding to ribosomes by localized activation of dsRNA‐dependent protein kinase. Nature 311: 79–81 [DOI] [PubMed] [Google Scholar]
- Dougherty JD, Tsai W‐C, Lloyd RE (2015) Multiple poliovirus proteins repress cytoplasmic RNA granules. Viruses 7: 6127–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Chen ZJ (2018) DNA‐induced liquid phase condensation of cGAS activates innate immune signaling. Science 361: 704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiermann N, Haneke K, Sun Z, Stoecklin G, Ruggieri A (2020) Dance with the devil: Stress granules and signaling in antiviral responses. Viruses 12: 984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, Kagan JC (2019) Inflammasomes: threat‐assessment organelles of the innate immune system. Immunity 51: 609–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal‐Ben‐Ari S, Barrera I, Ehrlich M, Rosenblum K (2019) PKR: a kinase to remember. Front Mol Neurosci 11: 480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Gong X, Fang S, Weng W, Wang H, Chu H, Sun Y, Meng C, Tan L, Song C et al (2021) Inhibition of anti‐viral stress granule formation by coronavirus endoribonuclease nsp15 ensures efficient virus replication. PLoS Pathog 17: e1008690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén‐Boixet J, Kopach A, Holehouse AS, Wittmann S, Jahnel M, Schlüßler R, Kim K, Trussina IREA, Wang J, Mateju D et al (2020) RNA‐induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181: 346–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JP‐Y (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21: 677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada E, Fukuda R (1992) Binding of influenza a virus NS1 protein to dsRNA in vitro. J Gen Virol 73: 3325–3329 [DOI] [PubMed] [Google Scholar]
- Hu J, Wang X, Xing Y, Rong E, Ning M, Smith J, Huang Y (2018) Origin and development of oligoadenylate synthetase immune system. BMC Evol Biol 18: 201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sun H, Yin L, Li J, Mei S, Xu F, Wu C, Liu X, Zhao F, Zhang D et al (2019) PKR‐dependent cytosolic cGAS foci are necessary for intracellular DNA sensing. Sci Signal 12: eaav7934 [DOI] [PubMed] [Google Scholar]
- Huang WYC, Alvarez S, Kondo Y, Lee YK, Chung JK, Lam HYM, Biswas KH, Kuriyan J, Groves JT (2019) A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363: 1098–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadevaia V, Burke JM, Eke L, Moller‐Levet C, Parker R, Locker N (2022) Novel stress granules‐like structures are induced via a paracrine mechanism during viral infection. J Cell Sci 135: jcs259194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaqaman K, Ditlev JA (2021) Biomolecular condensates in membrane receptor signaling. Curr Opin Cell Biol 69: 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K, Buckstein M, Ni H, Weissman D (2005) Suppression of RNA recognition by toll‐like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23: 165–175 [DOI] [PubMed] [Google Scholar]
- Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D (2008) Incorporation of Pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 16: 1833–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T (2014) Toll‐like receptor signaling pathways. Front Immunol 5: 461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Anderson P (2007) Mammalian stress granules and processing bodies. Methods Enzymol 431: 61–81 [DOI] [PubMed] [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P (1999) RNA‐binding proteins Tia‐1 and Tiar link the phosphorylation of Eif‐2α to the assembly of mammalian stress granules. J Cell Biol 147: 1431–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke‐Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P (2005) Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol 169: 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Ivanov P, Anderson P (2013) Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci 38: 494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, Thomas M, Lieberman J, McInerney GM, Ivanov P et al (2016) G3BP–Caprin1–USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol 212: 845–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R (2017) The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol Cell 68: 808–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS‐Y, Sze L, Lam K‐P (2019) The stress granule protein G3BP1 binds viral dsRNA and RIG‐I to enhance interferon‐β response. J Biol Chem 294: 6430–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid RP, Lam VL, Chirayil RP, Randall G, Sullivan CS (2018) RNA triphosphatase DUSP11 enables exonuclease XRN‐mediated restriction of hepatitis C virus. Proc Natl Acad Sci USA 115: 8197–8202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostura M, Mathews MB (1989) Purification and activation of the double‐stranded RNA‐dependent eIF‐2 kinase DAI. Mol Cell Biol 9: 1576–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157: 1013–1022 [DOI] [PubMed] [Google Scholar]
- Langereis MA, Feng Q, van Kuppeveld FJ (2013) MDA5 localizes to stress granules, but this localization is not required for the induction of type I interferon. J Virol 87: 6314–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtenberg BC, Mace PD, Riedl SJ (2014) Structural mechanisms in NLR inflammasome signaling. Curr Opin Struct Biol 29: 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire PA, Lary J, Cole JL (2005) Mechanism of PKR activation: dimerization and kinase activation in the absence of double‐stranded RNA. J Mol Biol 345: 81–90 [DOI] [PubMed] [Google Scholar]
- Lemaire PA, Anderson E, Lary J, Cole JL (2008) Mechanism of PKR activation by dsRNA. J Mol Biol 381: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wu M (2021) Pattern recognition receptors in health and diseases. Signal Transduct Target Ther 6: 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Min J‐Y, Krug RM, Sen GC (2006) Binding of the influenza a virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double‐stranded RNA. Virology 349: 13–21 [DOI] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng H‐C, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF et al (2012) Phase transitions in the assembly of multi‐valent signaling proteins. Nature 483: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Banerjee S, Wang Y, Goldstein SA, Dong B, Gaughan C, Silverman RH, Weiss SR (2016) Activation of RNase L is dependent on OAS3 expression during infection with diverse human viruses. Proc Natl Acad Sci USA 113: 2241–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z‐S, Cai H, Xue W, Wang M, Xia T, Li W‐J, Xing J‐Q, Zhao M, Huang Y‐J, Chen S et al (2019) G3BP1 promotes DNA binding and activation of cGAS. Nat Immunol 20: 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohöfener J, Steinke N, Kay‐Fedorov P, Baruch P, Nikulin A, Tishchenko S, Manstein DJ, Fedorov R (2015) The activation mechanism of 2′‐5′‐oligoadenylate synthetase gives new insights into OAS/cGAS triggers of innate immunity. Structure 23: 851–862 [DOI] [PubMed] [Google Scholar]
- Lu Y, Wambach M, Katze MG, Krug RM (1995) Binding of the influenza virus NS1 protein to double‐stranded RNA inhibits the activation of the protein kinase that phosphorylates the eIF‐2 translation initiation factor. Virology 214: 222–228 [DOI] [PubMed] [Google Scholar]
- Maelfait J, Liverpool L, Rehwinkel J (2020) Nucleic acid sensors and programmed cell death. J Mol Biol 432: 552–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivannan P, Siddiqui MA, Malathi K (2020) RNase L amplifies interferon signaling by inducing protein kinase R‐mediated antiviral stress granules. J Virol 94: e00205‐20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EW, Mittag T (2019) Dwelling at membranes promotes decisive signaling. Science 363: 1036–1037 [DOI] [PubMed] [Google Scholar]
- Martinez NW, Gómez FE, Matus S (2021) The potential role of protein kinase R as a regulator of age‐related neurodegeneration. Front Aging Neurosci 13: 638208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateju D, Chao JA (2022) Stress granules: regulators or by‐products? FEBS J 289: 363–373 [DOI] [PubMed] [Google Scholar]
- Maurano M, Snyder JM, Connelly C, Henao‐Mejia J, Sidrauski C, Stetson DB (2021) Protein kinase R and the integrated stress response drive immunopathology caused by mutations in the RNA deaminase ADAR1. Immunity 54: 1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo CB, Erlandsen H, Mouser DJ, Feinstein AG, Robinson VL, May ER, Cole JL (2019) Structural basis of protein kinase R autophosphorylation. Biochemistry 58: 2967–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs E, Chong K, Galabru J, Thomas NSB, Kerr IM, Williams BRG, Hovanessian AG (1990) Molecular cloning and characterization of the human double‐stranded RNA‐activated protein kinase induced by interferon. Cell 62: 379–390 [DOI] [PubMed] [Google Scholar]
- Min J‐Y, Krug RM (2006) The primary function of RNA binding by the influenza a virus NS1 protein in infected cells: inhibiting the 2′‐5′ oligo (a) synthetase/RNase L pathway. Proc Natl Acad Sci USA 103: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE, Bevilacqua PC (2007) 5'-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 318: 1455–1458. 10.1126/science.1147347 [DOI] [PubMed] [Google Scholar]
- Nanduri S, Rahman F, Williams BRG, Qin J (2000) A dynamically tuned double‐stranded RNA binding mechanism for the activation of antiviral kinase PKR. EMBO J 19: 5567–5574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okude H, Ori D, Kawai T (2021) Signaling through nucleic acid sensors and their roles in inflammatory diseases. Front Immunol 11: 625833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onomoto K, Jogi M, Yoo J‐S, Narita R, Morimoto S, Takemura A, Sambhara S, Kawaguchi A, Osari S, Nagata K et al (2012) Critical role of an antiviral stress granule containing RIG‐I and PKR in viral detection and innate immunity. PLoS ONE 7: e43031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protter DSW, Parker R (2016) Principles and properties of stress granules. Trends Cell Biol 26: 668–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke LC, Lloyd RE (2014) The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses. J Virol 89: 2575–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke LC, Kedersha N, Langereis MA, van Kuppeveld FJM, Lloyd RE (2015) Stress granules regulate double‐stranded RNA‐dependent protein kinase activation through a complex containing G3BP1 and Caprin1. MBio 6: e02486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena M, Yeretssian G (2014) NOD‐like receptors: master regulators of inflammation and cancer. Front Immunol 5: 327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Li R, Negro R, Cheng J, Vora SM, Fu T‐M, Wang A, He K, Andreeva L, Gao P et al (2021) Phase separation drives RNA virus‐induced activation of the NLRP6 inflammasome. Cell 184: 5759–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH (2007) Viral encounters with 2′,5′‐oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol 81: 12720–12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, Vale RD (2016) Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352: 595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Mehta S, Zhang J (2021) Liquid‐liquid phase separation: orchestrating cell signaling through time and space. Mol Cell 81: 4137–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP‐AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140: 805–820 [DOI] [PubMed] [Google Scholar]
- Tian B, White RJ, Xia T, Welle S, Turner DH, Mathews MB, Thornton CA (2000) Expanded CUG repeat RNAs form hairpins that activate the double‐stranded RNA‐dependent protein kinase PKR. RNA 6: 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J (2003) The RasGAP‐associated endoribonuclease G3BP assembles stress granules. J Cell Biol 160: 823–831 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tu Y‐C, Yu C‐Y, Liang J‐J, Lin E, Liao C‐L, Lin Y‐L (2012) Blocking double‐stranded RNA‐activated protein kinase PKR by Japanese encephalitis virus nonstructural protein 2A. J Virol 86: 10347–10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung TL, Cao C, Lu J, Ozato K, Dever TE (2001) Heterologous dimerization domains functionally substitute for the double‐stranded RNA binding domains of the kinase PKR. EMBO J 20: 3728–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Treeck B, Parker R (2018) Emerging roles for intermolecular RNA‐RNA interactions in RNP assemblies. Cell 174: 791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang H‐Y, Anthony TG (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11 [DOI] [PubMed] [Google Scholar]
- White JP, Cardenas AM, Marissen WE, Lloyd RE (2007) Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2: 295–305 [DOI] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ (2013) Cyclic GMP‐AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339: 826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Mathieu C, Kolaitis R‐M, Zhang P, Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q et al (2020) G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181: 325–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J‐S, Takahasi K, Ng CS, Ouda R, Onomoto K, Yoneyama M, Lai JC, Lattmann S, Nagamine Y, Matsui T et al (2014) DHX36 enhances RIG‐I signaling by facilitating PKR‐mediated antiviral stress granule formation. PLoS Pathog 10: e1004012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa F, Muniozguren NL, Ponce‐Rojas JC, Acosta‐Alvear D (2022) Signaling by the integrated stress response kinase PKR is fine‐tuned by dynamic clustering. J Cell Biol 221: e202111100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li Y, Xia J, He J, Pu J, Xie J, Wu S, Feng L, Huang X, Zhang P (2014) IPS‐1 plays an essential role in dsRNA‐induced stress granule formation by interacting with PKR and promoting its activation. J Cell Sci 127: 2471–2482 [DOI] [PubMed] [Google Scholar]
- Zhao M, Xia T, Xing J‐Q, Yin L‐H, Li X‐W, Pan J, Liu J‐Y, Li W‐H, Li T (2022) The stress granule protein G3BP1 promotes pre‐condensation of cGAS to allow rapid responses to DNA. EMBO Rep 23: e53166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Guo S, Bardhi O, Ryskamp DA, Li J, Tusi SK, Engelbrecht A, Klippel K, Chakrabarty P, Nguyen L et al (2020) Metformin inhibits RAN translation through PKR pathway and mitigates disease in C9orf72 ALS/FTD mice. Proc Natl Acad Sci USA 117: 18591–18599 [DOI] [PMC free article] [PubMed] [Google Scholar]


