Abstract
BEST family is a class of Ca2+-activated Cl-channels evolutionary well conserved from bacteria to human. The human BEST paralogs (BEST1-BEST4) share significant amino acid sequence homology in the N-terminal region, which forms the transmembrane helicases and contains the direct calcium-binding site, Ca2+-clasp. But the cytosolic C-terminal region is less conserved in the paralogs. Interestingly, this domain-specific sequence conservation is also found in the BEST1 orthologs. However, the functional role of the C-terminal region in the BEST channels is still poorly understood. Thus, we aimed to understand the functional role of the C-terminal region in the human and mouse BEST1 channels by using electrophysiological recordings. We found that the calcium-dependent activation of BEST1 channels can be modulated by the C-terminal region. The C-terminal deletion hBEST1 reduced the Ca2+-dependent current activation and the hBEST1-mBEST1 chimera showed a significantly reduced calcium sensitivity to hBEST1 in the HEK293 cells. And the C-terminal domain could regulate cellular expression and plasma membrane targeting of BEST1 channels. Our results can provide a basis for understanding the C-terminal roles in the structure-function of BEST family proteins.
Keywords: Bestrophin, Ca2+-dependent activation, Functional modulation, Surface expression, Whole-cell recording
INTRODUCTION
Bestrophin (BEST) channels are a class of calcium-activated Cl-channels, which are activated by a cytoplasmic [Ca2+] increase (1). The BEST1 channel was initially identified by the linkage analysis of the eye disease, Bestrophin vitelliform macular dystrophy (BVMD). Thereafter four BEST channel paralogs (BEST1 to BEST4) have been identified in the human genome and the BEST channels have been found in virtually all living organisms from bacteria to humans (1, 2). Although the mutations in the BEST1 channel lead to retinopathy, the pathophysiological role of the BEST1 channel is still not clear (1, 3). Interestingly, a series of seminal works from Justin C. Lee’s group showed that BEST1 channels, expressed in the mouse astrocytes, secrete gliotransmitters including glutamate, GABA, and D-serine, which can modulate neuronal activities in the tripartite synapse (4-6).
Human BEST channel paralogs (hBEST1-hBEST4) share ∼60% sequence identity in the N-terminal ∼370 amino acids but retain 6-19% sequence identity in the remaining C-terminal region. Similarly, the distinct sequence identities between human and mouse BEST1 ortholog are also present (7). Although four human paralogs share significant sequence identity in the N-terminal region, the electrophysiological recordings showed different current-voltage (I-V) characteristics of BEST paralogs: The hBEST1 produces instantaneous slightly outward-rectifying current, the hBEST2 shows linear I-V relationship, the hBEST3 has time-dependently activated current with strong inward rectification, and the hBEST4 produces linear I-V curve with time-dependent inactivation (7, 8). These functional diversities of BEST paralogs imply that the marginally conserved C-terminal domain could modulate the BEST paralogs differentially. Indeed, the electrophysiological studies suggested that the autoinhibitory domain is localized in the cytosolic C-terminal domain of BEST3 (AID) and the AID was also present in the BEST2, but its action could be antagonized by the facilitatory domain (9, 10).
Structural studies have revealed the three-dimensional architectures of several BEST channel homologs including chicken BEST1 (cBEST1) (11, 12), bovine BEST2 (bBEST2) (13), hBEST1, and hBEST2 (14). The structures showed that the functional BESTs are formed by the homo-pentameric assembly. Also, several important regions of the BEST channels were revealed based on the structures. First, the BEST structures showed that the conserved acid patch forms the direct calcium-binding site (Ca2+-clasp). Second, the anion conduction pore in BEST channels has two physical constriction zones. The neck is formed by the pore-lining hydrophobic residues (76Ile, 80Phe, 84Phe) and the aperture is formed by the nonpolar residue (205Ile in hBEST1). Third, the auto-inhibitory segment (AS, residue 346-378 in hBEST1) wraps the periphery of the BEST channel cytosolic domain in the closed state, but the detachment of AS may facilitate channel opening (14). However, none of the BEST channel structures visualized the C-terminal domain beyond amino acid residue 379. The structures of cBEST1 and bBEST2 were determined by using the C-terminal truncated proteins (cBEST1-405, cBEST11-345, or bBEST21-406; the numbers indicate the amino acid residues used in the structural studies) for the ease of structural determination (11-13). However, the full-length hBEST1 and hBEST2 structures also failed to resolve the C-terminal structure (14), which might be due to the disordered C-terminal domain in the cryo-EM conditions or intrinsically disordered nature. Thus, the structural role of the cytosolic C-terminal domain remains elusive.
In this study, we aimed to understand the functional roles of the BEST1 channel’s C-terminal domain based on the electrophysiological characterizations of human and mouse orthologs. We found that the expression and Ca2+-dependent activation of hBEST1 differ from those of mBEST1 despite having identical calcium-binding sites. Interestingly, the deletion of C-terminal 218 residues in hBEST1 significantly reduced Ca2+-dependent current activation though total and surface expressions were not significantly reduced. The replacement of the hBEST1 C-terminal region with mBEST1 (hBEST1-mBEST1 chimeric channel) resulted in a 15-fold reduced calcium sensitivity to hBEST1. However, the functionality of mBEST1-hBEST1 chimera was barely observed. These results indicate that the calcium-dependent activation of BEST1 channels could be determined by the direct calcium binding to the Ca2+-clasp in the N-terminal region as well as the modulatory function of the C-terminal region. Also, the C-terminal domain could regulate the cellular expression and plasma membrane targeting of BEST1 channels.
RESULTS
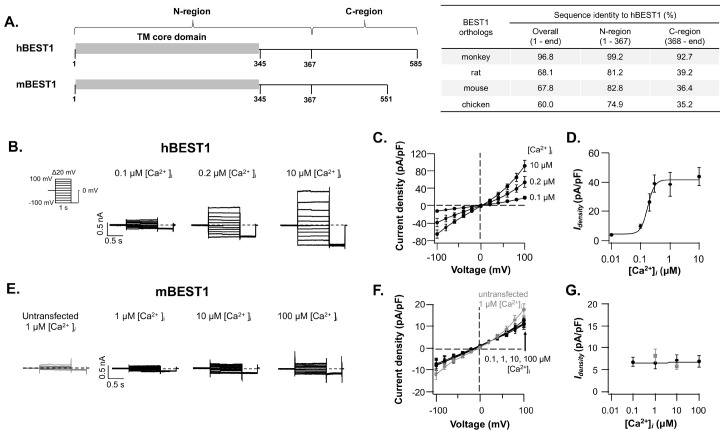
The amino acid sequence identities among BEST1 channel orthologs are categorized into two regions: The highly homologous N-region (residues 1-367), which shares 74.9-99.2% identities, and the marginally homologous C-region (residue 368-end) having 35.2-92.7% identities (Fig. 1A and Supplementary Fig. 1). Are these marginal conserved and structurally disordered C-region simply tethered to the BEST1 channel core? However, the previous study suggested that the facilitatory domain (residue 405-454) has an active role in the functioning of the mouse BEST2 channel (9). Also, several hBEST1 mutations in the C-region are known to be linked to retinopathy (15). These pathogenic hBEST1 variants imply that the C-region is not merely attached to the BEST1 channel core. Thus, we wondered what could be the functional role of the C-region in BEST1 channel activation.
Fig. 1.
Ca2+-dependent activation of hBEST1 and mBEST1. (A) Schematic drawing of BEST1 channels domain architecture (left) and amino acid sequence identities of BEST1 orthologs to hBEST1 (right). The UniProt accession numbers of BEST1 orthologs are O76090 (human), Q6UY87 (monkey), Q6AYG9 (rat), O88870 (mouse), and E1C3A0 (chicken). Representative whole-cell current traces of hBEST1 (B) and mBEST1 (E) activated by indicated [Ca2+]i. The currents were evoked by 1-s step pulses from −100 mV to +100 mV with 20 mV increments (B, left). The representative current of untransfected cell was drawn in gray (E, left). The dashed lines in the current traces indicate the zero-current levels throughout the remaining figures. The current-voltage (Idensity-V) relationships of hBEST1 (C) and mBEST1 (F) in the various [Ca2+]i. Symbols represent the steady state mean current ± standard error of the mean (s.e.m.) (n = 3-15). The Idensity-V relationship of untransfected cells in 1 mM [Ca2+]i is in gray. The [Ca2+]i-dependent activation curves of hBEST1 (D) and mBEST1 (G) at +60 mV. Gray symbols represent the Idensity of untransfected cells. Dose-response current increases of hBEST1 were fitted with Hill equation.
Ca2+-dependent activation and expression of hBEST1 and mBEST1 are different
The activities of hBEST1 and mBEST1 were examined by using whole-cell patch clamp experiments with various [Ca2+]i. To avoid any unexpected effect on the electrophysiological characteristics of BEST1 channels, we have used the BEST1 channel coding sequences without any protein tag. The hBEST1 channels expressed in HEK293T cells generated slightly outward rectifying Cl-currents in response to the intracellular calcium (Fig. 1B). The hBEST1 channels were activated at 100 nM [Ca2+]i and reached the maximal activation at 300 nM [Ca2+]i with the half-maximal activation [Ca2+]i (EC50) of ∼174 nM and the Hill coefficient of ∼3.7 (5-15 observations for each [Ca2+]i ). But the mBEST1 transfected cells did not show any [Ca2+]i-dependent current even in the presence of 100 mM [Ca2+]i, which was more than 500-fold excess of hBEST1’s EC50 (Fig. 1E-G). The current densities at +60 mV were 38.3 ± 8.0 pA/pF for hBEST1 (1 mM [Ca2+]i, n = 15) and 6.9 ± 1.4 pA/pF for mBEST1 (100 mM [Ca2+]i, n = 3). The current density level of mBEST1 transfected cells was indistinguishable from the untransfected cells.
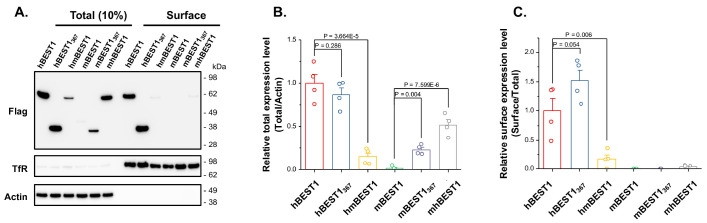
To investigate whether the low current density of the mBEST1 transfected cells was due to the low protein expression level, reduced membrane surface expression, or functional impairment of the mBEST1 channel, the surface biotinylated HEK293T cells expressing each BEST1 construct were subjected to the Western blot analysis to quantify the total expression levels and cell surface targeted fractions as previously described (Fig. 2) (16). Apparently, we used BEST1 genes in-frame fused with C-terminal Flag tag to detect both hBEST1 and mBEST1 proteins simultaneously. Surprisingly, the total and surface expressions of mBEST1 were not detectable (Fig. 2). However, the C-region (residue 368-551) with deleted mBEST1 (mBEST1367) was expressed in HEK293T cells and the expression level was 22.5 ± 2.9% (n = 4) of hBEST1, albeit the surface expression was not detectable (Fig. 2). To test whether these distinct expression levels of the hBEST1 and mBEST1 can be replicated in the murine cell lines, the expression of hBEST1 and mBEST1 tagged with either N-terminal or C-terminal protein tag (Flag, BRIL-twin strep, or BRIL-3X Flag) was examined in CHO-K1 cells. The BRIL tag (∼10 kDa) originated from the thermostabilized mutant apocytochrome b562RIL, which has been widely used for stabilizing various membrane proteins including the GPCR (17). The expression level of N-terminal Flag-tagged hBEST1 (Flag-hBEST1) was identified to be significantly lower than other C-terminal tagged or untagged hBEST1. However, the expression level of hBEST1-Flag driven by either CMV or CAG promoter was not significantly different (Supplementary Fig. 2A, B). Interestingly, the mBEST1 with a C-terminal BRIL-3X Flag tag demonstrated excellent expression; whereas the N- or C-terminal Flag-tagged mBEST1 did not show any expression in the CHO-K1 cells. These results indicate that the C-region can regulate the expression and membrane targeting of BEST1 channels and the expression of mBEST1 can be stabilized with a large C-terminal tag.
Fig. 2.
Total and cell surface expression of wildtype, C-terminal truncated, and chimeric BEST1 channels. (A) Representative western blot images of total protein expression and cell surface expression of various BEST1 constructs with C-terminal Flag tag expressed in HEK293T cells. Quantification of total protein expression level normalized to the density of actin (B) and relative surface expression level to the total expression level (C). Data (mean ± s.e.m.) were produced by four independent transfections. Empty circles in the bar graphs indicate the values from the independent observations. One-Way ANOVA test was used for comparisons, and the appropriate P-values are indicated in each graph.
The C-terminal domain regulates the Ca2+-dependent gating of hBEST1
To investigate the role of the C-region in determining the [Ca2+]i-sensitivity of BEST1 channels, we examined the effect of the C-region deletions on the hBEST1 currents (Fig. 3A). First, the functional consequences of hBEST1345 (hBEST11-345) mutant, which encodes only transmembrane core domain (14), were accessed by the electrophysiological recordings. The hBEST1345 showed robust [Ca2+]i-dependent currents with the EC50 of ∼470 nM (3-8 observations), which differs less than an order of magnitude (∼2.5-fold decrease) to wildtype hBEST1. The current density of hBEST1345 was slightly larger than wildtype hBEST1 (Fig. 3B, C, green). However, the cooperativity of [Ca2+]i-dependency became shallower: the Hill coefficient for the [Ca2+]i-dependent gating of hBEST1345 is ∼1.5 (Fig. 3D, green).
Fig. 3.
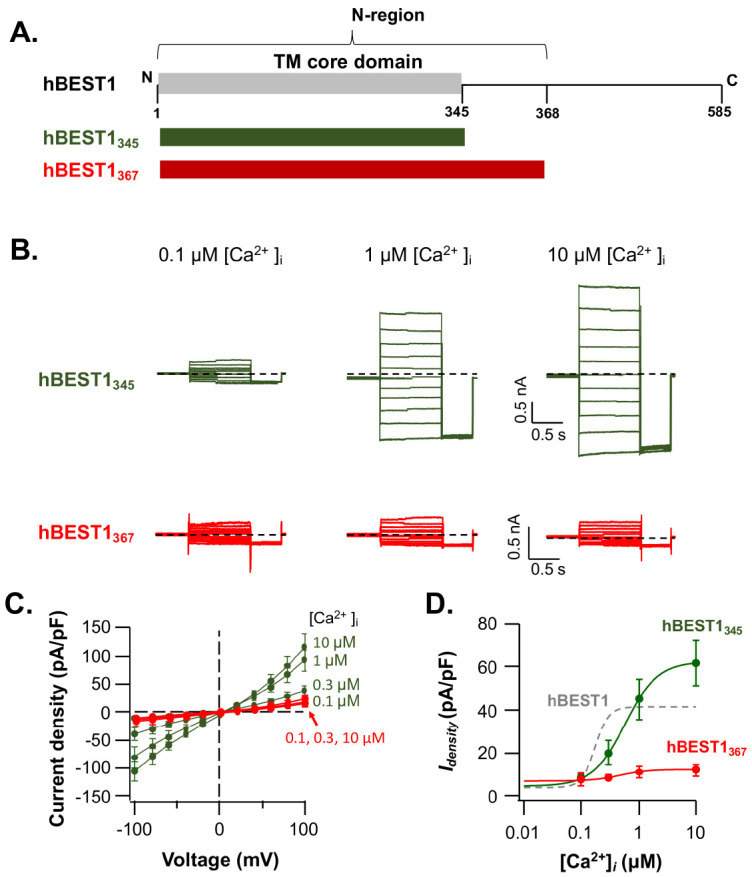
Ionic currents through the C-terminal truncated hBEST1 channels. (A) schematic drawing of the C-terminal truncated hBEST1345 and hBEST1367. (B) Representative whole-cell current traces of hBEST1345 (green) and hBEST1367 (red) activated by indicated [Ca2+]i. (C) The Idensity-V relationships of hBEST1345 (green) and hBEST1367 (red) in the various [Ca2+]i. Symbols represent the mean ± s.e.m. (n = 3-8). (D) the [Ca2+]i-dependent activation curves of hBEST1345 (green) and hBEST1367 (red) at +60 mV. Dose-response current increases of hBEST1 were fitted with Hill equation. Dashed gray curve represents the fitted [Ca2+]i-response of wildtype hBEST1 (as in Fig. 1D).
Another truncated mutant hBEST1367 (hBEST11-367), which spans the highly conserved N-region (Fig. 2A, Fig. 1A, and Supplementary Fig. 1), resulted in a very low level of [Ca2+]i-dependent currents with the maximum current density of 12.0 ± 2.6 pA/pF at +60 mV in the presence of 10 mM [Ca2+]i. The EC50 and Hill coefficient were ∼470 nM and ∼1.8, respectively (3-4 observations). However, the total protein expression and surface expression level of hBEST1367 were not significantly different from those of wildtype hBEST1 (Fig. 2). These results suggested that the C-region (residue 368-585) deletion of hBEST1 could perturb the [Ca2+]i-dependent gating of hBEST1 without affecting protein expression and membrane targeting.
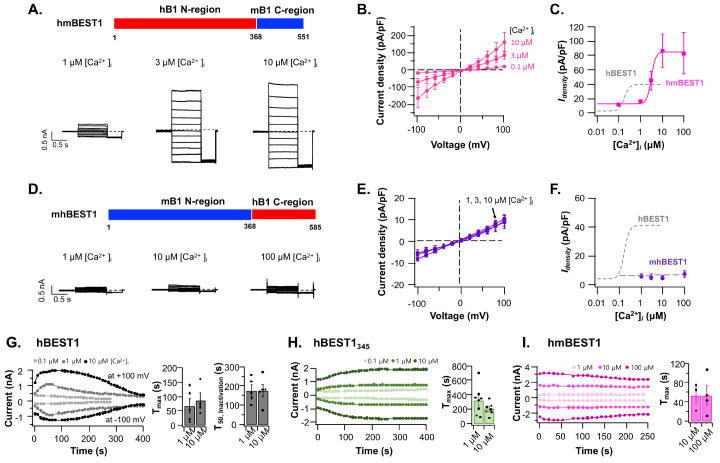
The C-region swapped mutant, hmBEST1 has an altered Ca2+-sensitivity
Next, we examined the electrophysiological activities of C-domain swap constructs of hmBEST1 (hBEST11-367-mBEST1368-551) and mhBEST1 (mBEST11-367-hBEST1368-585) (Fig. 4). The hmBEST1 showed ∼2-fold increased current density (86.7 ± 23.6 pA/pF at +60 mV in the presence of 10 mM [Ca2+]i) to wildtype hBEST1, but the mhBEST1 chimera did not evoke the [Ca2+]i-dependent currents (Fig. 4A-F). The hmBEST1 channels were activated by [Ca2+]i with the EC50 of ∼3.1 mM and the Hill coefficient of ∼4.3 (3-5 observations for each [Ca2+]i) (Fig. 4C). Interestingly, the Ca2+-sensitivity of hmBEST1 was reduced by ∼18-fold compared to wildtype hBEST1 without significantly changing the [Ca2+] cooperativity.
Fig. 4.
Ca2+-dependent activation of C-terminal swapped hBEST1 and mBEST1. Schematic drawing of the domain-swapped hmBEST1 (hBEST11-367-mBEST1368-551) (A) and mhBEST1 (mBEST11-367-hBEST1368-585) channels (D). Representative whole-cell current traces of hmBEST1 (A) and mhBEST1 (D) activated with indicated [Ca2+]i. The current density-voltage (Idensity-V) relationships and the [Ca2+]i-dependent activation curves of hmBEST1 (B, C, respectively) and mhBEST1 (E, F, respectively) in the various [Ca2+]i. Dashed gray curve represents the fitted [Ca2+]i-response of wildtype hBEST1 (as in Fig. 1D). Run-up and run-down time courses of hBEST1 (G), hBEST1345, and (H) hmBEST1 channels. The time zero represents the time point of first voltage stimulation immediately after making whole-cell configuration. The currents were evoked by the 1-s stimulation of +100 mV followed by 1-s stimulation of −100 mV in every 10 s. Each symbol represents steady state current at +100 mV (outward current) or −100 mV (inward current) in the presence of the indicated [Ca2+]i. The Tmax is the time for reaching the maximum current value at +100 mV (G-I) and the T50, inactivation is the time for reaching the 50% value of maximum current from the maximum level (G). The bar graphs represent the mean ± s.e.m. (n = 3-8). Black circles in the bar graphs indicate the values from the independent observations.
The C-terminal region regulates the Ca2+-dependent run-up and run-down of BEST1 channels
The effect of the C-terminal region on the time-dependent whole-cell current generations was examined. The hBEST1 currents reached the maximum level in 67.1 ± 25.0 s at +100 mV in 10 mM [Ca2+]i (n = 5) and then began to be inactivated with the half-maximum time (T50) of 176.1 ± 28.4 s (n = 5) (Fig. 4G). However, the hBEST1345 took more time for full activation (201.3 ± 32.1 s at +100 mV in 10 mM [Ca2+]i (n = 7) and the current level persisted without run-down (Fig. 4H). Previously, the C-terminal deletion mutant hBEST1380 (hBEST11-380) showed a similar whole-cell current kinetics to hBEST1345, but other deletion mutants, hBEST1350, hBEST1360, and hBEST1370 were essentially nonfunctional (18). The hmBEST1 chimera showed accelerated run-up (53.5 ± 16.3 s at +100 mV in the presence of 10 mM [Ca2+]i (n = 3) and slowed run-down (Fig. 4I). These results implied that the BEST1 C-terminal region (residue 346-end) can regulate both the Ca2+-dependent run-up and run-down of BEST1 channel.
As seen in Fig. 2, the total expression levels of hmBEST1 and mhBEST1 were 14.4 ± 4.6% (n = 4) and 51.5 ± 6.0% (n = 4) of hBEST1 level, respectively. And the relative surface expression levels were 17.0 ± 7.2% (n = 4) and 4.7 ± 0.8% (n = 4) of hBEST1 level, respectively. The results of increased current density but the reduced surface expression of hmBEST1 to wildtype hBEST1 were somewhat counterintuitive. Though we do not have direct experimental evidence for making an explanation, the C-region may have an inhibitory function for BEST1 channel opening in a species-specific manner. It is hypothesized that the removed run-down of hBEST1345 and the slowed run-down of hmBEST1 chimera partly support the idea (Fig. 4H, I). These results supported the hypothesis that the C-region could regulate the total and membrane surface expression of BEST1 channels as well as modulate channel functions.
DISCUSSION
In summary, we found that BEST1 orthologs, C-terminal deletions, and C-region swapped BEST1 sense intracellular calcium with different EC50 values and cooperativities. And these differences could be attributed to the modulatory effect of the marginally homologous C-region. Also, we found that the C-regions can regulate the expression and membrane targeting of BEST1 channels. We believe that these data provide insight to understand the functional role of marginally conserved C-regions among the BEST1 homologs on the Ca2+-dependent gating.
The low-level current density of hBEST1367 is well-matched to the previous work of the Hartzell group, where the C-truncated hBEST1 channels (residues 1-350, 1-360, and 1-370) were essentially non-functional (Fig. 3) (18). However, the recent work done in the Yang laboratory showed that the hBEST11-367 and hBEST21-368 channels could produce larger current densities than parental wildtype channels even in the absence of Ca2+ (14). One plausible explanation for this obvious discrepancy may come from the different compositions of pipette (intracellular) solution during whole-cell patch-clamp recordings, especially in the presence or absence of ATP. Since it had been suggested that ATP can activate the BEST channels through direct binding to the cytosolic loop (residue 199-203 in hBEST1) (19), we did not include ATP in our pipette solution to rule out any effect to activate BEST1 channels other than [Ca2+]i. However, we do not have any experimental data for supporting this possibility at this moment. Another possibility might be due to the presence of the C-terminal tag used in the previous study (14), in which all the C-terminally truncated hBEST1 and hBEST2 constructs were fused with the C-terminal YFP protein. It will be interesting to compare the functional characteristics of BEST1 channels with various protein tags.
In the previous study, the mBEST1 was expressed in the TRex-293 cells with a tetracycline-inducible expression system of pcDNA4/TO/c-myc-HIS vector, where the mBEST1 cDNA was in-frame fused with the C-terminal c-myc and 6X His tags in tandem (∼20 amino acids long) (20). Counterintuitively, the mBEST1 with N- or C-terminal Flag-tag (8 amino acids long) was not expressed in the HEK293 and CHO-K1 cells (Fig. 2 and Supplementary Fig. 2C). However, the mBEST1 with a large C-terminal tandem tag (BRIL-3X Flag) was effectively expressed in CHO-K1 cells (Supplementary Fig. 2C). This result indicates that the presence of large C-terminal tags may stabilize the expression of mBEST1 in the heterologous expression systems.
How does the C-region regulate the Ca2+-sensitivity on the BEST1 channel? The previous work showed that the deletion or neutralization mutations of a potential Ca2+-binding site (380EDEED384) in the C-region did not have any effect on the Ca2+-dependent activation. And the C-truncated mutant hBEST1390 had a wild-type-like activity (18). However, the D312G mutant hBEST1 was ∼20-fold less sensitive to Ca2+ with the EC50 of 2.7 mM and lost cooperative Ca2+-binding (18). Thus, the reduced Ca2+-sensitivity in hmBEST1 is likely due to the failure of modulation by the mBEST1’s C-region, though we do not have any experimental evidence for ruling out the existence of another Ca2+-binding site(s) in the BEST1’s C-region. Nonetheless, our results indicated that the calcium-dependent activation of BEST1 channels is a result of the concerted action of the direct calcium binding to Ca2+-clasp in the N-region and the modulatory action of the C-region.
Our results showed that the mBEST1 was not expressed in the HEK293T cells, but the C-region deletion mutant mBEST1367 and the chimeric mhBEST1 were expressed though their membrane localizations were largely impaired. In addition, the expression level and surface targeting of hBEST1 with mBEST’s C-region (hmBEST1) were also reduced (Fig. 2). Thus, it could be understood that the C-region of mBEST1 somehow hinders the expression and the N-region is important to membrane targeting of BEST1. It will be interesting to explore whether BEST1’s domain itself or any auxiliary partner regulates this intriguing ortholog-dependent expression.
MATERIALS AND METHODS
The cells, culture media, antibodies, and biochemical reagents used in this work are listed in the supplementary information (Supplementary Table 1).
Methods for the plasmid DNA construction, cell culture, transfection, electrophysiological recording, cell surface biotinylation, and western blot analysis are described in the supplementary information (Supplementary Methods).
Funding Statement
ACKNOWLEDGEMENTS We thank the members of the Lim laboratory for their timely help throughout the study. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) grants (2021R1A2C1004884 to H.-H.L.) and the Brain Research Program of the NRF (2020M3E5D9079 to H.-H.L.) funded by the Ministry of Science and ICT, Republic of Korea, and the KBRI basic research program through Korea Brain Research Institute funded by the Ministry of Science and ICT (22-BR-01-02 to H.-H.L.).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- 2.Hagen AR, Barabote RD, Saier MH. The bestrophin family of anion channels: identification of prokaryotic homologues. Mol Membr Biol. 2005;22:291–302. doi: 10.1080/09687860500129711. [DOI] [PubMed] [Google Scholar]
- 3.Owji AP, Kittredge A, Zhang Y, Yang T. Structure and function of the bestrophin family of calcium-activated chloride channels. Channels (Austin) 2021;15:604–623. doi: 10.1080/19336950.2021.1981625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo DH, Han KS, Shim JW, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151:25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Jo S, Yarishkin O, Hwang YJ, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh W, Park M, Chun YE, et al. Astrocytes render memory flexible by releasing D-serine and regulating NMDA receptor tone in the hippocampus. Biol Psychiatry. 2022;91:740–752. doi: 10.1016/j.biopsych.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Tsunenari T, Sun H, Williams J, et al. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem. 2003;278:41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu Z, Cui Y, Hartzell C. A short motif in the C-terminus of mouse bestrophin 3 [corrected] inhibits its activation as a Cl channel. FEBS Lett. 2006;580:2141–2146. doi: 10.1016/j.febslet.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Qu ZQ, Yu K, Cui YY, Ying C, Hartzell C. Activation of bestrophin Cl-channels is regulated by C-terminal domains. J Biol Chem. 2007;282:17460–17467. doi: 10.1074/jbc.M701043200. [DOI] [PubMed] [Google Scholar]
- 11.Kane Dickson V, Pedi L, Long SB. Structure and insights into the function of a Ca2+-activated Cl− channel. Nature. 2014;516:213–218. doi: 10.1038/nature13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller AN, Vaisey G, Long SB. Molecular mechanisms of gating in the calcium-activated chloride channel bestrophin. Elife. 2019;8:e43231. doi: 10.7554/eLife.43231.c74c26aa33974173a05a64992ef3c233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owji AP, Zhao Q, Ji C, et al. Structural and functional characterization of the bestrophin-2 anion channel. Nat Struct Mol Biol. 2020;27:382–391. doi: 10.1038/s41594-020-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owji AP, Wang J, Kittredge A, et al. Structures and gating mechanisms of human bestrophin anion channels. Nat Commun. 2022;13:3836. doi: 10.1038/s41467-022-31437-7.f20545733ff64a9199865a3c3234ef9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonyan V, Chumakov K, Dingerdissen H, et al. High-performance integrated virtual environment (HIVE): a robust infrastructure for next-generation sequence data analysis. Database (Oxford) 2016;2016:baw022. doi: 10.1093/database/baw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang J, Park K, Lee GY, et al. Transmembrane topology and oligomeric nature of an astrocytic membrane protein, MLC1. Open Biol. 2021;11:210103. doi: 10.1098/rsob.210103.38f4b72b65bd4330b9ced569bc801818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun E, Thompson AA, Liu W, et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure. 2012;20:967–976. doi: 10.1016/j.str.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Q, Prussia A, Yu K, Cui YY, Hartzell HC. Regulation of bestrophin Cl channels by calcium: role of the C terminus. J Gen Physiol. 2008;132:681–692. doi: 10.1085/jgp.200810056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Kittredge A, Ward N, Ji C, Chen S, Yang T. ATP activates bestrophin ion channels through direct interaction. Nat Commun. 2018;9:3126. doi: 10.1038/s41467-018-05616-4.d2fbb4e9fd0d4e02a648786cc5e5354a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Driscoll KE, Leblanc N, Hatton WJ, Britton FC. Functional properties of murine bestrophin 1 channel. Biochem Biophys Res Commun. 2009;384:476–481. doi: 10.1016/j.bbrc.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.