Abstract
Increasing evidence indicates that inflammatory cytokines are involved in the pathogenesis of recurrent aphthous stomatitis (RAS). A wide range of over ten cytokines in peripheral blood of RAS patients have been investigated in different studies. Yet, which of the ones are the most prominent indicators contributed for the process of RAS are uncertain. Herein, a total of 16 eligible case–control studies including 1051 cases of RAS and 616 health controls were summarized. The bubble chart analysis showed that the most prominent cytokines for RAS were interleukin (IL)-6 (646 cases, 308 controls), TNF-α (498 cases, 298 controls), and IL-2 (371 cases, 264 controls). On the other hand, 9 studies on cytokines as therapeutic indicators of RAS were identified. The effect of levamisole and thalidomide on cytokines mainly were IL-6, TNF-α, and IL-8. Collectively, an optimum panel of IL-6, TNF-α, and IL-2 maybe serve as the potential significant indicators for RAS investigations.
Keywords: Bubble analysis, Cytokines, Interleukin, Peripheral blood, Recurrent aphthous stomatitis, Serum
Introduction
Recurrent aphthous stomatitis (RAS) is the most common oral mucosal disease, and can affect up to 25% of the general population.1 The clinical symptoms of RAS involvement can severely influence the patients’ speaking, swallowing, and eating.1 Hence, RAS represents an important public health concern due to its high prevalence, associated pain, and impact on the quality of life of affected individuals. It is reported that some predisposing risk factors including genetic susceptibilities, local trauma, psychologic stress, viral and bacterial infections, hormonal disorders, nutritional deficiencies, hematological deficiencies, smoking, and immune-alteration factors have been proposed as causative agents.1
Although the precise etiology and pathogenesis of RAS remain unclear, evidence indicates that RAS occurs through epithelial destruction mediated by a cellular immune response.2 Based on this principle, the aphthous process is accepted to be initiated by stimulation of the mucosal keratinocytes by unknown antigens, leading to T-lymphocyte stimulation and the secretion of various cytokines. Subsequently, the cytokines are believed to be released into peripheral blood, and then play a major role in T cell-mediated immunologic dysregulation of RAS.1,2 Since interleukin (IL)-6 as a diagnostic and therapeutic indicator for RAS was studied by Chiang C.P. group from Taiwan in 2003,3 over 10 cytokines were reported with a great variation in different studies on this disease.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17
However, which of the ones are the most prominent indicators contributed for the process of RAS are uncertain, partly due to lack of the summary analysis on this issue. For instance, some investigators selected only 1 cytokine and some choose 10 cytokines in a study. Besides, the investigators in 2 studies utilized entirely different cytokines in the similar study design.5,6 Therefore, we, to begin with, summarize these publications focused on peripheral blood cytokines in RAS patients; and then attempt to identify an optimum panel of cytokines by a visual analysis. This might provide the pertinent evidence for the investigators to choose rational cytokines into their study design and avoid potential unnecessary research.
Materials and methods
In order to identify the relevant papers on cytokines in peripheral blood of RAS patients, literature searches were performed in Scopus, Web of Science, and PubMed. We searched electronic databases without any restriction following the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Key search terms were: aphthous stomatitis, aphthous ulcerations, cytokine, interleukin, blood, plasma, serum, sera, and their equivalents. To identify more results, hand search (checking references of included primary articles and relevant reviews) performed manually. All the observational association studies about the distributional difference of peripheral blood cytokines between RAS patients and healthy controls published up to Jun 10, 2022 have been included in this study. Studies with not available full text and lack of peripheral blood cytokine data, other type of studies such as case reports or conference papers were excluded. Two independent authors (H.S. and W.L.) screened the titles, abstracts, and full text of all publications, and subsequently analyzed the case–control studies.
A descriptive analysis was performed on the parameters of included studies. The data on the number of RAS patients, the number of healthy controls, and the number of the studies with significant results was extracted to carried out a bubble chart analysis. A bubble chart used to visualize and interpret the performance of cytokines in RAS. The bubble chart for each cytokine shows the number of patients/healthy subjects and the number of the studies by the vertical/horizontal axis and bubble sizes. All the bubbles constitute a dashboard where the numbers of the subjects and studies in each theme bubble could be viewed by hovering over each data point. Excel Visual Basic for Applications (Microsoft 365, Seattle, WA, USA) was used to programme a module to plot the bubble chart.
Results
Cytokines as diagnostic indicators
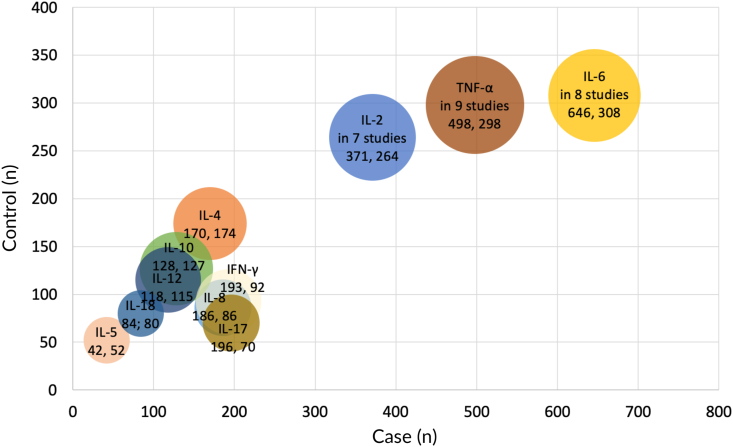
A total of 72 potentially relevant papers was screened, and 56 were excluded with some reasons. As presented in Supplementary Figure S1, 16 eligible articles on over ten cytokines, such as IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-17, IL-18, TNF-a, and IFN-γ, were identified for detailed evaluation from the search of the published literature (Table 1). These eligible case–control studies including 1051 cases of RAS and 616 health controls were identified. Serum was the most sample type used in 12 of 16 studies, followed by peripheral blood mononuclear cells and plasma. Enzyme-linked immunosorbent assay was the most method used in 10 of 16 studies to detect the quantitative concentration of cytokines. As shown in Fig. 1, the bubble chart analysis reveals that most prominent cytokines in peripheral blood of RAS patients were IL-6, TNF-α, and IL-2. IL-6 was reported by 8 studies including 646 cases of RAS and 308 controls. TNF-α was confirmed by 9 studies including 498 cases and 298 controls. IL-2 was verified by 7 studies including 371 cases and 264 controls.
Table 1.
Characteristics of included studies on peripheral blood cytokines in the pathogenesis of patients with recurrent aphthous stomatitis.
| Author, year | Country/Region | Cases |
Control |
Matched factors | Sample | Detection method | Cytokine detected | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (mean ± SD, y) | F/M | n | Age (mean ± SD, y) | F/M | |||||||
| Shen et al., 20212 | China | 127 | 43.7 ± 12.9 | 55/72 | 20 | 44.2 ± 13.3 | 10/10 | Age, gender | Serum | |||
| Han et al., 20214 | China | 80 | 44.4 ± 6.1 | 40/40 | 80 | 44.3 ± 5.8 | 40/40 | Age, gender | Plasma | ELISA | IL-1β, IL-2, TNF-α | |
| Lu et al., 20205 | China | 60 | 40.1 ± 2.8 | 30/30 | 60 | 40.3 ± 2.7 | 30/30 | Age, gender | Plasma | ELISA | IL-6, IL-18 | |
| Mimura et al., 20176 | Brazil | 45 | NA | 16/29 | 30 | 37.4 | 16/14 | Age, gender, ethnicity | Serum | MAP Human Cytokine/Chemokine Magnetic Bead | IL-4, IL-6, IL-10, IL-12p70, IL-17A, TNF-α | |
| Ozyurt et al., 20147 | Turkey | 24 | 34.4 ± 10.6 | 13/11 | 20 | 37.0 ± 11.1 | 11/9 | Age, gender | Serum | ELISA | IL-1, IL-13, IL-17, IL-18, IFN-γ, α-enolase | |
| Avci et al., 20148 | Turkey | 25 | NA | NA | 25 | NA | NA | Age, gender | Serum | ELISA | IL-2, IL-10, IL-12, TNF-a | |
| Gupta et al., 20149 | India | 30 | 27.8 ± 10.3 | 11/19 | 20 | 27.3 ± 9.1 | 8/12 | Age, gender | Serum | ELISA | IL-8 | |
| Pekiner et al., 201210 | Turkey | 30 | 37.0 ± 13.8 | 18/12 | 15 | 30.3 ± 9.0 | 6/9 | Age, gender | Serum | Flow cytometry | IL-2, IL-6 | |
| Borra et al., 200911 | Brazil | 17 | 31 ± 8.5 | 10/7 | 17 | 33 ± 10.6 | 10/7 | Age, gender | Serum | ELISA | sCD14, TNF-a | |
| Albanidou et al., 200712 | Greece | 32 | 35.4 ± 12.0 | 18/14 | 40 | 31.7 ± 13.1 | 22/18 | Age, gender | PBMC | ELISA | IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, TNF-α, IFN-γ | |
| Sun et al., 200613 | China, Taiwan | 146 | 39.4 | 80/66 | 54 | NA | 30/24 | Gender | Serum | ELISA | TNF-α | |
| Lin et al., 200514 | China, Taiwan | 67 | NA | NA | 72 | NA | NA | NA | Serum | ELISA | IL-2, IL-4, GM-CSF, sFas, FasL | |
| Lewkowicz et al., 200515 | Poland | 10 | 38.2 ± 13.0 | 4/6 | 12 | NA | NA | Age, gender | PBMC | Flow cytometry | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, TGF-β1, TNF-α, IFN-γ | |
| Sun et al., 200416 | China, Taiwan | 146 | 39.4 | 80/66 | 54 | NA | 30/24 | Gender | Serum | Immulite assay | IL-6, IL-8 | |
| Aridogan et al., 200317 | Turkey | 16 | 36.8 ± 13.1 | 8/8 | 20 | 33.2 ± 11.4 | 11/9 | Age, gender | Serum | ELISA | IL-4, IL-10, IL-12, IL-13, TNF-α | |
| Sun et al., 20033 | China, Taiwan | 196 | 38 | 107/90 | 77 | NA | 44/33 | Gender | Serum | Immulite assay | IL-6 | |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; F, female; M, male; NA, not available; PBMC, peripheral blood mononuclear cells; IFN, interferon; IL, interleukin; TGF, transforming growth factor; TNF, tumor necrosis factor.
Figure 1.
Bubble chart graphically represents the weighted value of the articles on recurrent aphthous stomatitis (RAS) by distinct cytokines. The number in the bubbles represents the sample size of case and control, respectively. The bubble diameter is proportionate to the number of articles included.
Cytokines as therapeutic indicators
On the other hand, 9 studies focused on cytokines in peripheral blood as indicators for monitoring therapeutic response of RAS were identified (Table 2). There are 2 randomized controlled trials and 7 comparative studies on this issue. Among cytokines in peripheral blood with significant difference after treatment, the levels of IL-2, IL-4, IL-6, IL-8, IL-18, TNF-α, and IFN-γ were decreased after different systemic therapies, and the levels of IL-1, IL-6, and TNF-α were decreased after a topical treatment. Among these therapies, there were 5 studies on levamisole and thalidomide, which are highly effective at minimizing RAS symptoms and reducing the rate of ulcer recurrence. As systemic immunomodulators, levamisole and thalidomide are accepted to be second in line to systemic corticosteroids for RAS treatment. The effect of levamisole and thalidomide on blood cytokines mainly were IL-6, TNF-α, and IL-8.
Table 2.
Characteristics of included studies on peripheral blood cytokines in the treatment of patients with recurrent aphthous stomatitis.
| Author, year | Country /Region |
Study design | Case (n) | Treatment | Sample | Detection method | Cytokine detected | Cytokine with significant difference after treatment |
|---|---|---|---|---|---|---|---|---|
| Elamrousy et al., 202118 | Egypt | Randomized controlled | 20 | Topical camel whey protein | Serum | Flow cytometry | IL-1, IL-6, TNF-α | IL-1, IL-6, TNF-α |
| Han et al., 20214 | China | Comparative study | 80 | Not reported | Plasma | ELISA | IL-1β, IL-2, TNF-α | IL-1β, IL-2, TNF-α |
| Lu et al., 20205 | China | Comparative study | 60 | Systemic thalidomide | Plasma | ELISA | IL-6, IL-18 | IL-6, IL-18 |
| Mimura et al., 20176 | Brazil | Randomized controlled | 22 | Systemic symbiotic | Serum | MAP Human Cytokine/Chemokine Magnetic Bead | IL-4, IL-6, IL-10, IL-12p70, IL-17A, TNF-α | IL-4, TNF-α |
| Gupta et al., 20149 | India | Comparative study | 30 | Systemic levamisole | Serum | ELISA | IL-8 | IL-8 |
| Sun et al., 200613 | China, Taiwan | Comparative study | 55 | Systemic levamisole | Serum | ELISA | TNF-α | TNF-α |
| Sun et al., 200519 | China, Taiwan | Comparative study | 19 | Systemic Tien-Hsien liquid | Serum | ELISA | IL-2, IL-6, IL-10, TNF-α, IFN-γ | IL-2, IL-6, TNF-α, IFN-γ |
| Sun et al., 200416 | China, Taiwan | Comparative study | 82 | Systemic levamisole | Serum | Immulite assay | IL-6, IL-8 | IL-6, IL-8 |
| Sun et al., 20033 | China, Taiwan | Comparative study | 45 | Systemic levamisole and Chinese medicinal herbs | Serum | Immulite assay | IL-6 | IL-6 |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IFN, interferon; IL, interleukin; TGF, transforming growth factor; TNF, tumor necrosis factor.
Discussion
Increasing evidence indicates that T cell-mediated immune responses are involved in the pathogenesis of RAS, in which cytokines secreted by T helper (Th) cells have been shown to play a major role.1,2 Thus, research investigating the correlation between inflammation-related cytokines and RAS activity is important for elucidating the pathogenesis of this disease. It is proposed that T-cell mediated immune responses may contribute to a loss of immunological tolerance in the oral mucosa, favoring the occurrence of inflammatory reactions, and the appearance of oral ulcerations.2 The overall Th cell-related cytokine networks consist of Th1 (IL-1β, IL-2, IL-12p70, TNF-α, and IFN-γ), Th2 (IL-4, IL-5, IL-6, and IL-10), Th17 (IL-17), and cell-death signals (Fas and FasL). A wide range of over ten cytokines in peripheral blood of RAS patients have been investigated in different studies.
Currently, the evaluation of RAS disease severity and therapeutic response mainly rely on the subjective indicators, such as clinical presentation, score of disease activity, and visual analog scale for symptoms. Admittedly, the objective laboratory indicators like cytokines are needed to be investigated and established in clinical practice. To begin with, increasing evidence indicates that inflammatory cytokines are involved in the pathogenesis of RAS. Increased levels of cytokines in peripheral blood of RAS patients compared to control was observed before treatment. Next, decreased levels of blood cytokines in RAS patients was observed after treatment, as expected. Thus, detection of blood cytokines has the potential in monitoring therapeutic response of RAS.18,19
Although blood cytokines may be promising minimal invasive biomarkers for monitoring therapeutic response of RAS, there still existed some obvious limitations. First, limited studies reported blood cytokines in RAS treatment, and a great mass of the studies had small sample size. Secondly, different therapies should be prescribed based on the patient's severity of RAS and personal circumstances. The level of the cytokine in RAS patients received different therapies might be varied based on personal inflammatory microenvironment. Thirdly, it is a challenge to make narrowing down of accurate cytokines because the results of the previous studies are varied. For instance, various cytokines adopted diverse cutoff values for the aberrant levels of quantification.
To obtain an optimum proposal panel of cytokines, we use a bubble chart analysis method to summarize the most common cytokines using the existing literature on this issue. Based on the data analysis, we propose that a panel of IL-6, TNF-α, and IL-2 maybe serve as the most prominent indicators for RAS investigations. They may be used as molecular markers to monitor the treatment response during the aphthous active period, and predict the recurrence of RAS during the aphthous stationary phase. This panel serves as a reference for investigators to select the appropriate cytokines in their studies.
In summary, the panel of IL-6, TNF-α, and IL-2 as significant indicators for RAS patients are required to evaluate and verify in more studies with a larger sample size. Furthermore, more clinical trials with a large sample size should be carried out to further confirm the roles of the various cytokines during different therapies in diagnosis and monitoring of RAS.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This work was supported by National Natural Science Foundation of China (82205200, 82174041).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2022.10.013.
Contributor Information
Wei Liu, Email: liuweb@hotmail.com.
Huan Shi, Email: shihuan1312@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chiang C.P., Chang J.Y., Wang Y.P., Wu Y.H., Wu Y.C., Sun A. Recurrent aphthous stomatitis - etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J Formos Med Assoc. 2019;118:1279–1289. doi: 10.1016/j.jfma.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Shen C., Ye W., Gong L., Lv K., Gao B., Yao H. Serum interleukin-6, interleukin-17A, and tumor necrosis factor-alpha in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2021;50:418–423. doi: 10.1111/jop.13158. [DOI] [PubMed] [Google Scholar]
- 3.Sun A., Chia J.S., Chang Y.F., Chiang C.P. Levamisole and Chinese medicinal herbs can modulate the serum interleukin-6 level in patients with recurrent aphthous ulcerations. J Oral Pathol Med. 2003;32:206–214. doi: 10.1034/j.1600-0714.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 4.Han Y., Wang L., Li Q., Chen H., Ma X. LncRNA NEAT1 is upregulated in recurrent aphthous stomatitis (RAS) and has predictive values. BMC Oral Health. 2021;21:673. doi: 10.1186/s12903-021-01909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J., Zhang N., Wu C. LncRNA CASC 2 is upregulated in aphthous stomatitis and predicts the recurrence. BMC Oral Health. 2020;20:12. doi: 10.1186/s12903-019-0993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimura M.A.M., Borra R.C., Hirata C.H.W., de Oliveira Penido N. Immune response of patients with recurrent aphthous stomatitis challenged with a symbiotic. J Oral Pathol Med. 2017;46:821–828. doi: 10.1111/jop.12621. [DOI] [PubMed] [Google Scholar]
- 7.Ozyurt K., Celik A., Sayarlıoglu M., et al. Serum Th1, Th2 and Th17 cytokine profiles and alpha-enolase levels in recurrent aphthous stomatitis. J Oral Pathol Med. 2014;43:691–695. doi: 10.1111/jop.12182. [DOI] [PubMed] [Google Scholar]
- 8.Avci E., Akarslan Z.Z., Erten H., Coskun-Cevher S. Oxidative stress and cellular immunity in patients with recurrent aphthous ulcers. Braz J Med Biol Res. 2014;47:355–360. doi: 10.1590/1414-431X20143714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta P., Ashok L., Naik S.R. Assessment of serum interleukin-8 as a sensitive serological marker in monitoring the therapeutic effect of levamisole in recurrent aphthous ulcers: a randomized control study. Indian J Dent Res. 2014;25:284–289. doi: 10.4103/0970-9290.138293. [DOI] [PubMed] [Google Scholar]
- 10.Pekiner F.N., Aytugar E., Demirel G.Y., Borahan M.O. Interleukin-2, interleukin-6 and T regulatory cells in peripheral blood of patients with Behçet's disease and recurrent aphthous ulcerations. J Oral Pathol Med. 2012;41:73–79. doi: 10.1111/j.1600-0714.2011.01061.x. [DOI] [PubMed] [Google Scholar]
- 11.Borra R.C., de Mesquita Barros F., de Andrade Lotufo M., Villanova F.E., Andrade P.M. Toll-like receptor activity in recurrent aphthous ulceration. J Oral Pathol Med. 2009;38:289–298. doi: 10.1111/j.1600-0714.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- 12.Albanidou-Farmaki E., Markopoulos A.K., Kalogerakou F., Antoniades D.Z. Detection, enumeration and characterization of T helper cells secreting type 1 and type 2 cytokines in patients with recurrent aphthous stomatitis. Tohoku J Exp Med. 2007;212:101–105. doi: 10.1620/tjem.212.101. [DOI] [PubMed] [Google Scholar]
- 13.Sun A., Wang J.T., Chia J.S., Chiang C.P. Levamisole can modulate the serum tumor necrosis factor-alpha level in patients with recurrent aphthous ulcerations. J Oral Pathol Med. 2006;35:111–116. doi: 10.1111/j.1600-0714.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin S.S., Chou M.Y., Ho C.C., et al. Study of the viral infections and cytokines associated with recurrent aphthous ulceration. Microb Infect. 2005;7:635–644. doi: 10.1016/j.micinf.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Lewkowicz N., Lewkowicz P., Banasik M., Kurnatowska A., Tchórzewski H. Predominance of Type 1 cytokines and decreased number of CD4(+) CD25(+high) T regulatory cells in peripheral blood of patients with recurrent aphthous ulcerations. Immunol Lett. 2005;99:57–62. doi: 10.1016/j.imlet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Sun A., Chang Y.F., Chia J.S., Chiang C.P. Serum interleukin-8 level is a more sensitive marker than serum interleukin-6 level in monitoring the disease activity of recurrent aphthous ulcerations. J Oral Pathol Med. 2004;33:133–139. [PubMed] [Google Scholar]
- 17.Aridogan B.C., Yildirim M., Baysal V., Inaloz H.S., Baz K., Kaya S. Serum levels of IL-4, IL-10, IL-12, IL-13 and IFN-gamma in Behçet's disease. J Dermatol. 2003;30:602–607. doi: 10.1111/j.1346-8138.2003.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 18.Elamrousy W.A., Mortada A., Shoukheba M. Evaluation of novel topical camel whey protein gel for the treatment of recurrent aphthous stomatitis: randomized clinical study. J Int Soc Prev Community Dent. 2021;11:574–581. doi: 10.4103/jispcd.JISPCD_172_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun A., Chia J.S., Wang W.B., Chiang C.P. Tien-Hsien liquid" can modulate antigen-stimulated cytokine production by T-cells isolated from patients with recurrent aphthous ulcerations. Am J Chin Med. 2005;33:559–571. doi: 10.1142/S0192415X05003168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.