Abstract
Background/purpose
Diabetes mellitus (DM) is a chronic metabolic disorder that affects millions of people worldwide. A growing evidence suggests that hyperglycemia in DM causes a pre-aging and pro-inflammatory condition known as inflammaging, which increases periodontitis susceptibility. Bromelain has been demonstrated to have anti-inflammatory and anti-aging properties in variety of tissues, but its effects on diabetic periodontitis remain unclear. Thus, the aim of this study is to investigate the its Bromelain's impact in diabetic periodontitis in terms of inflammation and senescence activity.
Materials and methods
We assessed the wound healing capacity, production of pro-inflammatory cytokines Interleukin (IL)-6 and IL-8 and senescence marker p16 in human gingival fibroblasts (HGFs) in response to Advanced glycation end-products (AGEs) stimulant, with or without Bromelain treatment. The expression of p65, p-ERK, and p-p38 were also examined to elucidate whether Bromelain's anti-inflammaging activity is mediated through NF-κB and MAPK/ERK signaling pathway.
Results
Bromelain concentrations ranging from 2.5 to 20 g/mL had no adverse effect on HGF cell proliferation. Bromelain improved wound healing in HGFs with AGEs stimulation. In addition, Bromelain suppressed the production of pro-inflammatory cytokines IL-6 and IL-8 in HGFs elicited by AGEs. Meanwhile, Bromelain treatment also inhibited the senescence activity and expression of p16 in AGEs-stimulated HGFs. Western blot analysis indicated that the upregulation of p-ERK, p-p38 and p65 induced by AGEs were inhibited by Bromelain in HGFs.
Conclusion
These data suggest that excessive AGEs in the gingiva may lead to the accumulation of pro-inflammatory cytokines and marked senescence activity. Bromelain application may be helpful in enhancing wound healing by suppressing inflammaging via downregulation of NF-κB and MAPK/ERK signaling pathways in DM individuals with periodontal disease.
Keywords: Advanced glycation end products, Bromelain, Diabetic periodontitis
Introduction
According to the report from World Health Organization (WHO), diabetes mellitus (DM) is a chronic, metabolic disease characterized by elevated blood glucose level and if left uncontrolled, can give rise to multiple health complications. The last estimates indicated that the global DM prevalence in 20–79 year-olds was 10.5%, and will increase to 12.2% in 2045.1
DM are associated with elevated systemic inflammation, and the hyperglycaemia can stimulate the activation of inflammation, oxidative stress and apoptosis pathways.2 Periodontitis is a chronic inflammatory disease of the underlying supporting tissues of teeth, mainly characterized by the loss of the connective tissue and alveolar bone resorption, and would eventually lead to tooth loss if left unresolved.3 In the past few years, several studies attempted exploring the link between periodontitis and other diseases. For instance, periodontal severity was associated with the inflammatory disease psoriasis.4 Neurofibromatosis (NF) type 1 patient has a higher prevalence of age-dependent oral complications, which might be mediated by following the NF-induced systemically inflammation.5 Some studies have revealed that the host immune response in DM causes excessive inflammation and periodontal tissue destruction.6,7 Hyperglycaemia in DM is known to accumulate advanced glycation end-products (AGEs),8 which is a complex compound formed through the glycation of amino acids, lipids, and DNA molecules.9 A growing evidence suggests that accumulation of AGEs contributes to a low grade chronic inflammatory and pre-aging state termed as inflammaging, which leads to an increased susceptibility to periodontitis.10 Several mechanisms, including up-regulation of inflammatory processes and cellular senescence, have been discovered in the involvement of AGEs in inflammaging.11 AGEs have the ability to enhance host inflammatory response in DM individuals and predispose them to diabetic periodontitis (DP). Studies have shown increased expression of periodontal interleukin (IL)-612 and systemic IL-813 in the patients who is systemically healthy but periodontitis. In fact, individuals with DP have shown greater alveolar bone loss and a worse prognosis following standard therapies than those without DM.6 The cellular inflammatory gene expression was mainly controlled by nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPK) signaling pathways, such as the extracellular signal-related kinase (ERK1/2) and the p38 MAPK pathway.14 AGEs interact with their receptors (RAGE) to increase cellular oxidant stress and aggravate the production of pro-inflammatory cytokines.10,11,15,16 It has been demonstrated that AGEs affected extracellular matrix (ECM) composition in human glomerular cells16 by inducing reactive oxygen species (ROS) production and ERK and NF-κB pathways activation. A previous study revealed that AGEs/RAGE up-regulated IL-6 and ICAM-1 expression in human gingival fibroblasts (HGFs) through the activation of MAPK and NF-κB pathways.15 These findings indicate that AGEs can aggravate periodontitis by increasing the pro-inflammatory cytokines and ROS in HGFs.
As mentioned, AGEs is well acknowledged for its role in stimulating cellular senescence, which is an irreversible cell cycle halt that promotes activation of inflammatory processes, which could result in tissue malfunction and a deterioration in regenerative capacity.17 The mechanism underlying AGEs-induced senescence is through the induction of p21, which is a potent cyclin-dependent kinase (CKI). It was thought that during the engagement of AGEs to RAGE, endoplasmic reticulum stress is sustained,18 leading to the activation of p21 signaling. p21 contributes to the inhibition of cell cycle, and thus would give rise to premature aging or senescence.19 On the other hand, these senescent cells can express and secrete variety of modulators of inflammatory status such as cytokines, chemokines and bioactive lipids, which is collectively termed as the senescence-associated secretory phenotype (SASP).20 Periodontal cells in DM mice models have been shown to exhibit increased cellular senescence and SASP secretion.21 At the cellular level, the accumulation of cell senescence coupled with a hyper-inflammatory status could greatly contribute to the perpetuation of inflammaging and contribute to DP progression.22 As a result, therapeutic approach aimed at reducing the influence of inflammaging on periodontal disease in diabetic individuals would be crucial.
Bromelain is a proteolytic enzyme derived from the pineapple plant Ananas comosus. It has been utilized as an adjuvant treatment in various chronic inflammatory illnesses23 owing to its anti-inflammatory and anti-oxidative properties. Its administration has been proven to lower serum pro-inflammatory IL-6 levels in diabetic individuals.24 Bromelain has also been demonstrated to have an anti-hyperglycemic action in diabetic rats and to slow the course of ligature-induced periodontitis.25 Thus, we examined whether Bromelain can reduce inflammation and cell senescence in HGFs with diabetic condition in-vitro. Moreover, Bromelain's anti-inflammaging activity was examined whether it is mediated through NF-κB and MAPK/ERK signalling pathway.
Materials and methods
Cell culture
All procedures were conducted out in accordance with the approved guidelines from the Institutional Review Board at the Chung Shan Medical University Hospital. HGFs from two healthy individuals were selected from the crown lengthening procedure using an explant technique as previously described.26 Cell cultures between the third and eighth passages were used in this study. All experiments have been done in two individual HGFs. Advanced Glycation End-Products (AGEs)-BSA was purchased from BioVision (Milpitas, CA, USA) and Bromelain was purchased from Sigma Chemical Co (St. Louis, MO, USA). To test the effect of Bromelain, HGFs were exposed to advanced glycation end-products (AGEs)-BSA simultaneously with Bromelain at indicated concentration for a period of 24 h in the following experiments.
Cell viability assay
HGFs were seeded in 96-well plates (Corning Inc., Rochester, NY, USA) at a density of 10,000 cells/well for 24 h incubation. After cells were well-adhesion, Bromelain was directly added at indicated concentrations (2.5, 5, 10, 20, and 40 μg/mL) for another 24 h incubation. Cells viability was determined using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay according to manufacturer's instructions. The 570-nm absorbance of the untreated cells (0 μg/mL Bromelain) was set as 100%, and data were presented as percentage compared to of control.
Wound healing assay
Cells were seeded into a 12-well culture dish to reach ∼80% confluence, and then we scratched the monolayer with a sterile 200 μL pipette tip across the center of the well to create a denuded area. Cells were allowed to grow for an additional 48 h and stain cells with crystal violet. The microscopic images of scratched area were taken with at 0 and 24 h26
Western blot
Western blot analysis was followed the previously described protocol.26 The primary antibodies against cellular senescence marker p16 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) marker, NF-κB signaling marker (p65) (Cell Signaling Technology, Beverly, MA, USA), and ERK signaling marker (p-ERK, ERK, p-p38, and p38) (Cell Signaling Technology, Beverly, MA, USA) were used.
ELISA analysis
Using the ELISA kits (R&D Systems, Minneapolis, MN) with manufacturer's instruction, the concentration of IL-6 and IL-8 was measured at the absorbance with a 450 nm filter by a microplate reader (MRX, Dynatech Laboratories, Chantilly, VA, USA). Each HGF sample was analyzed in triplicate.
Senescence activity detection
Cellular senescence was determined by measuring the activity of senescence-associated-β-gal (SA-β-Gal) with a Cellular Senescence Assay kit as described previously.26 SA-β-Gal positive cells were observed by microscopy, and over 100 cells were counted in five independent fields.
Statistical analysis
Three replicates of each experiment were performed. Statistical analysis was carried out by one-way analysis of variance (ANOVA). Tests of differences in the treatments were analyzed by Duncan's test and a value of P < 0.05 was considered statistically significant.
Results
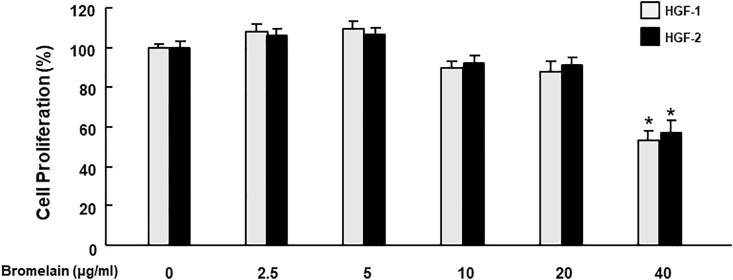
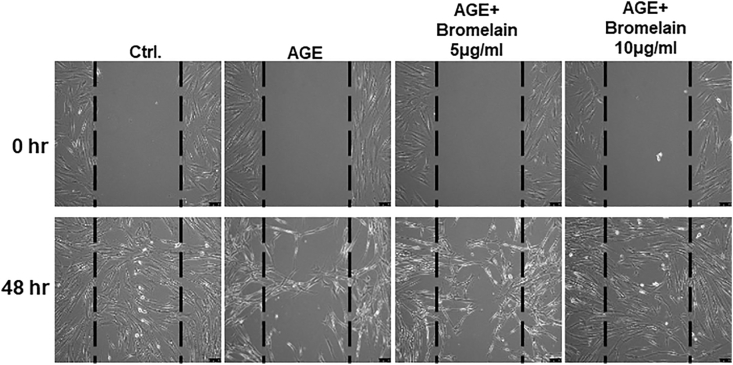
Firstly, we revealed that Bromelain of 2.5–20 μg/mL had no significant effect on the cell proliferation rate in HGFs, while 40 μg/mL showed less proliferation (Fig. 1). In the following experiments, HGFs were exposed to advanced glycation end-products (AGEs)-BSA (200 μg/mL) to mimic the diabetic periodontal environment, Bromelain was simultaneously applied to examine its therapeutic effect. Fibroblasts play a critical role in wound-healing; they rapidly migrate into the injury site in response to the cytokines released by injured tissue and then build the connective tissue to support the wound repair.27 The impaired healing machinery caused by DM has been linked to the AGEs-induced inflammation and senescence.28 In DP individuals, the uncontrolled hyperglycemia shows the impaired wound healing of periodontal tissue.29 Therefore, we carried out a wound healing scratch test of fibroblasts to evaluate their ability that migrate and close the wound gap. We found that presence of AGEs deteriorated the cell's wound healing ability. On the contrary, treatment of Bromelain attenuated the AGE's deterioration in a dose dependant manner (Fig. 2).
Figure 1.
Effects of Bromelain on the cell proliferation rate in HGFs. Bromelain ranging from 2.5 to 20 μg/mL did not significantly affect the cell proliferation rate in HGFs. Data represent the mean ± SD. ∗P < 0.05 compared to the group with 0 μg/mL bromelain.
Figure 2.
Effects of Bromelain on the wound healing ability in the AGEs-treated HGFs. AGEs-stimulated HGFs has significantly impaired wound healing and this was reversed with the administration of Bromelain (0–10 μg/mL) in a dose-dependent manner. Data represent the mean ± SD.
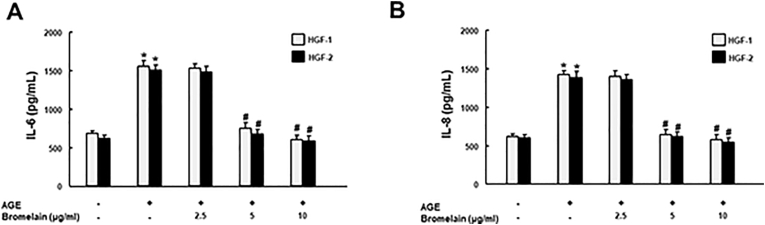
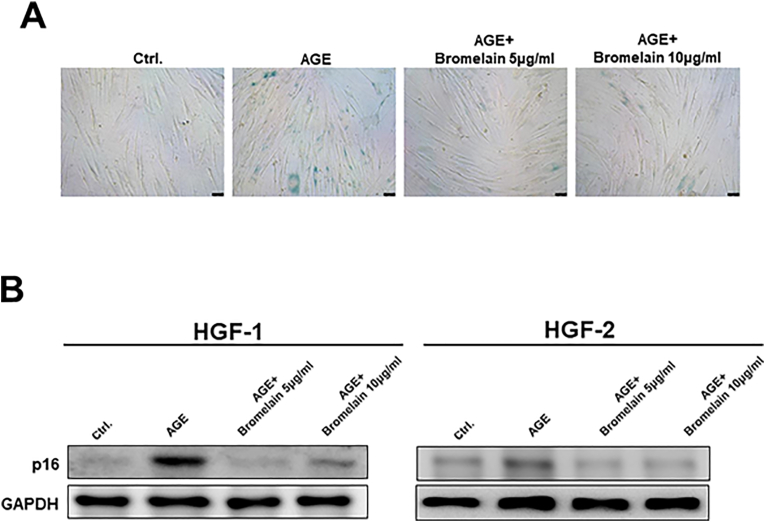
To explore the core mechanisms behind the improved wound healing, anti-inflammatory and anti-aging properties of Bromelain were probed into. We examined IL-6 and IL-8 secretion in the AGEs-treated HGFs with and without various doses of Bromelain. Bromelain was demonstrated to suppress AGEs-elicited IL-6 and IL-8, indicating its anti-inflammatory potential (Fig. 3). Besides that, cellular senescence activity in the AGEs-stimulated HGFs was assessed utilizing senescence markers, SA-β-Gal staining (Fig. 4A) and p16 (Fig. 4B). As shown, AGEs markedly enhanced the senescence activity in cells, but the addition of Bromelain counteracted this phenomenon.
Figure 3.
Effects of Bromelain on the production of IL-6 and IL-8 in the AGEs-treated HGFs. The AGEs-elicited secretions of IL-6 (A) and IL-8 (B) in HGFs were downregulated in response to Bromelain treatment (0, 2.5, 5, and 10 μg/mL) in a dose-dependent manner. Data represent the mean ± SD. ∗P < 0.05 compared to control group; #P < 0.05 compared to AGEs only group.
Figure 4.
Effects of Bromelain on the cell senescence activity in the AGEs-treated HGFs. The cell senescence activity was up-regulated after stimulated with AGEs, measured by the increased expression of β-galactosidase (SA-β-Gal) staining and p16. Bromelain treatment (5–10 μg/mL) then reversed the increased cellular senescence. Data represent the mean ± SD.
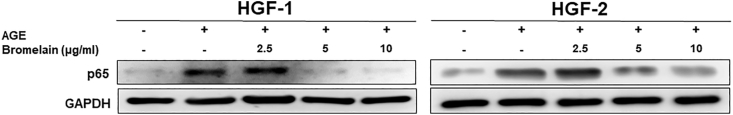
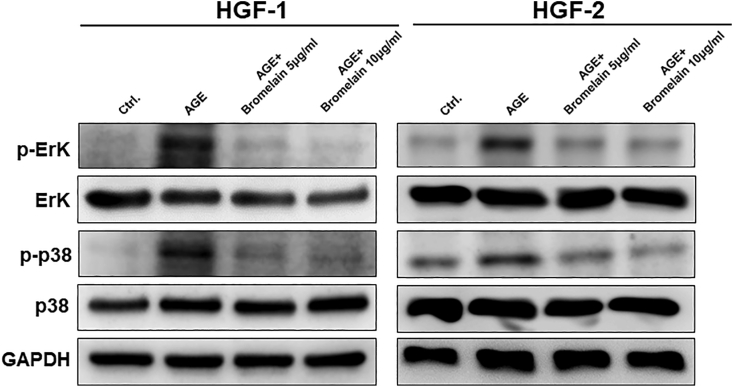
To elucidate how Bromelain exerts its anti-inflammaging effect, we assessed the expression of protein levels associated with NF-κB and MAPK/ERK signaling pathways. We measured the phosphorylation activities of p65 (Fig. 5), ERK and p38 (Fig. 6) and observed that the greater concentrations of Bromelain were able to inhibit the activation of NF-κB (Fig. 5) and MAPK/ERK signaling pathways (Fig. 6). Our findings suggested that Bromelain's anti-inflammaging activities were mediated via modulation of the NF-κB and MAPK/ERK signaling pathways.
Figure 5.
Effects of Bromelain on the NF-κB pathway in the AGEs-stimulated HGFs. Western blot was utilized to measure the expression of p65, a subunit of the NF-κB pathway and it was found that the up-regulation of p65 induced by AGEs was suppressed by Bromelain application.
Figure 6.
Effects of Bromelain on the MAPK pathway in the AGEs-stimulated HGFs. Western blot was utilized to measure the expression of pERK, ERK, p-p38, and p38. Phosphorylated-ERK and -p38 were up-regulated by treatment with AGEs and suppressed by Bromelain application.
Discussion
Diabetes mellitus is a metabolic disease characterized by chronic hyperglycemia. A growing evidence suggests that hyperglycemia in DM causes a pre-aging and pro-inflammatory condition known as inflammaging, which increases periodontitis susceptibility.10 The local inflammatory reaction in the periodontal tissues in response to oral pathogenic bacteria are attributed to DM's hyperinflammatory response coupled with delayed wound healing.30 In this study, addition of bromelain dose-dependently promoted wound healing in AGEs-stimulated HGFs. This compound's ability to improve wound healing has been demonstrated not only in gingival tissues,31 but also in epithelium,32 and diabetes-induced wounds.33
AGEs in DM has been acknowledged to contribute to the pathogenesis of diabetic periodontitis (DP) via inflammaging.11,34 In this work, AGEs build up was found to up-regulate IL-6 and IL-8 as well as cellular senescence (shown in Figure 3, Figure 4 respectively), which are consistent to earlier findings.15,21,34 When Bromelain was introduced as an intervention, the greater dosage averted the inflammaging state triggered by AGEs. This anti-inflammatory trait of bromelain has been proven in in-vitro studies of inflamed human dental pulp cells in response to LPS stimuli.35 Inflammatory cytokines such as IL-6 are engaged in the immune responses and are responsible for the course of acute-phase inflammation36 whereas IL-8 aids in the recruitment and activation of neutrophils to acute inflammation sites.37
Next, AGEs in this study was shown to upregulate NF-κB signaling and treatment of cells with Bromelain inhibited nuclear translocation of p65, which are in line with previous reports in other cell types.38 Activation of NF-κB signaling is known as the key inducer of inflammaging as it promotes inflammatory cytokines secretion, cellular senescence and senescence-associated secretory phenotype (SASP).39 Moreover, pro-inflammatory mediators affect NF-κB activation via positive feedback, resulting in a loop cycle that constantly aggravates inflammatory damage.
The MAPK p38 and ERK are important modulators of the pro-inflammatory responses and strong inducers of SASP40 in periodontal cell models.41,42 In agreement to this, the present study discovered that AGEs stimulated the phosphorylation of these protein kinases. Bromelain, on the other hand, was revealed to have a substantial suppressive impact on the MAPK/ERK pathway. Bromelain has been found to have anti-inflammatory quality in studies involving LPS-induced inflammation of human dental pulp cells35 which were linked to NF-κB and the MAPK/ERK and p38 pathway. The use of Bromelain has been observed to disrupt these pathways in a variety of cell types.35,38
Taken together, this study demonstrated that Bromelain may have therapeutic potential in diabetic periodontitis models against AGE-induced inflammaging. We demonstrated that Bromelain was able to promote wound healing in human gingival fibroblasts in in-vitro by inhibiting IL-6 and IL-8 as well as cellular senescence via down-regulation of NF-κB and MAPK pathways. Still further research will be needed, bromelain application may be beneficial to DM patients with periodontitis.
Declaration of competing interest
None declared.
Acknowledgments
This study was funded by Chung Shan Medical University Hospital (CSH-2022-C-016) in Taiwan.
Contributor Information
Taichen Lin, Email: taichenlin23@gmail.com.
Cheng-Chia Yu, Email: ccyu@csmu.edu.tw.
References
- 1.Sun H., Saeedi P., Karuranga S., et al. Idf diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 3.Könönen E., Gursoy M., Gursoy U.K. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med. 2019;8:1135. doi: 10.3390/jcm8081135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu K.J., Tu C.C., Hu J.X., et al. Severity of periodontitis and salivary interleukin-1beta are associated with psoriasis involvement. J Formos Med Assoc. 2022;121:1908–1916. doi: 10.1016/j.jfma.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Thota E., Veeravalli J.J., Manchala S.K., et al. Age-dependent oral manifestations of neurofibromatosis type 1: a case-control study. Orphanet J Rare Dis. 2022;17:93. doi: 10.1186/s13023-022-02223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J.H., Lee D.E., Gunawardhana K.S., et al. Effect of the interaction between periodontitis and type 1 diabetes mellitus on alveolar bone, mandibular condyle and tibia. Acta Odontol Scand. 2014;72:265–273. doi: 10.3109/00016357.2013.822551. [DOI] [PubMed] [Google Scholar]
- 7.Duarte P.M., Bezerra J.P., Miranda T.S., Feres M., Chambrone L., Shaddox L.M. Local levels of inflammatory mediators in uncontrolled type 2 diabetic subjects with chronic periodontitis. J Clin Periodontol. 2014;41:11–18. doi: 10.1111/jcpe.12179. [DOI] [PubMed] [Google Scholar]
- 8.Singh V.P., Bali A., Singh N., Jaggi A.S. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negre-Salvayre A., Salvayre R., Augé N., Pamplona R., Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxidants Redox Signal. 2009;11:3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P., Wang Q., Nie L., et al. Hyperglycemia-induced inflamm-aging accelerates gingival senescence via nlrc4 phosphorylation. J Biol Chem. 2019;294:18807–18819. doi: 10.1074/jbc.RA119.010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 12.Ross J.H., Hardy D.C., Schuyler C.A., Slate E.H., Mize T.W., Huang Y. Expression of periodontal interleukin-6 protein is increased across patients with neither periodontal disease nor diabetes, patients with periodontal disease alone and patients with both diseases. J Periodontal Res. 2010;45:688–694. doi: 10.1111/j.1600-0765.2010.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borilova Linhartova P., Kavrikova D., Tomandlova M., et al. Differences in interleukin-8 plasma levels between diabetic patients and healthy individuals independently on their periodontal status. Int J Mol Sci. 2018;19:3214. doi: 10.3390/ijms19103214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A., Mahendra J., Mahendra L., et al. Synergistic effect of biphasic calcium phosphate and platelet-rich fibrin attenuate markers for inflammation and osteoclast differentiation by suppressing nf-kappab/mapk signaling pathway in chronic periodontitis. Molecules. 2021;26:6578. doi: 10.3390/molecules26216578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonaka K., Kajiura Y., Bando M., et al. Advanced glycation end-products increase il-6 and icam-1 expression via rage, mapk and nf-kappab pathways in human gingival fibroblasts. J Periodontal Res. 2018;53:334–344. doi: 10.1111/jre.12518. [DOI] [PubMed] [Google Scholar]
- 16.Berrou J., Tostivint I., Verrecchia F., et al. Advanced glycation end products regulate extracellular matrix protein and protease expression by human glomerular mesangial cells. Int J Mol Med. 2009;23:513–520. doi: 10.3892/ijmm_00000159. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Piperi C., Adamopoulos C., Dalagiorgou G., Diamanti-Kandarakis E., Papavassiliou A.G. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012;97:2231–2242. doi: 10.1210/jc.2011-3408. [DOI] [PubMed] [Google Scholar]
- 19.Liu J., Huang K., Cai G.-Y., et al. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal. 2014;26:110–121. doi: 10.1016/j.cellsig.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Lopes-Paciencia S., Saint-Germain E., Rowell M.C., Ruiz A.F., Kalegari P., Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. doi: 10.1016/j.cyto.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Qin Z.Y., Gu X., Chen Y.L., et al. Toll-like receptor 4 activates the nlrp3 inflammasome pathway and periodontal inflammaging by inhibiting bmi-1 expression. Int J Mol Med. 2021;47:137–150. doi: 10.3892/ijmm.2020.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivieri F., Recchioni R., Marcheselli F., et al. Cellular senescence in cardiovascular diseases: potential age-related mechanisms and implications for treatment. Curr Pharmaceut Des. 2013;19:1710–1719. [PubMed] [Google Scholar]
- 23.Harats M., Haik J., Cleary M., Vashurin I., Aviv U., Kornhaber R. A retrospective review of an off-label bromelain-based selective enzymatic debridement (nexobrid®) in the treatment of deep, partial, and full thickness burns and hard to heal wounds. Isr Med Assoc J. 2020;22:83–88. [PubMed] [Google Scholar]
- 24.Paczek L., Kropiewnicka E.H., Bartlomiejczyk I., Gradowska L., Heidland A., Wood G. Systemic proteolytic enzyme treatment diminishes urinary interleukin 6 in diabetic patients. Nephron. 2000;84:194–195. doi: 10.1159/000045573. [DOI] [PubMed] [Google Scholar]
- 25.Alves E.H.P., Carvalho A.D.S., Silva F.R.P., et al. Bromelain reduces the non-alcoholic fatty liver disease and periodontal damages caused by ligature-induced periodontitis. Oral Dis. 2020;26:1793–1802. doi: 10.1111/odi.13476. [DOI] [PubMed] [Google Scholar]
- 26.Lin C.Y., Liao Y.W., Hsieh P.L., et al. Lncrna gas5-as1 inhibits myofibroblasts activities in oral submucous fibrosis. J Formos Med Assoc. 2018;117:727–733. doi: 10.1016/j.jfma.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Kawasumi A., Sagawa N., Hayashi S., Yokoyama H., Tamura K. Wound healing in mammals and amphibians: toward limb regeneration in mammals. Curr Top Microbiol Immunol. 2013;367:33–49. doi: 10.1007/82_2012_305. [DOI] [PubMed] [Google Scholar]
- 28.Berlanga-Acosta J.A., Guillen-Nieto G.E., Rodriguez-Rodriguez N., et al. Cellular senescence as the pathogenic hub of diabetes-related wound chronicity. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.573032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faria-Almeida R., Navarro A., Bascones A. Clinical and metabolic changes after conventional treatment of type 2 diabetic patients with chronic periodontitis. J Periodontol. 2006;77:591–598. doi: 10.1902/jop.2006.050084. [DOI] [PubMed] [Google Scholar]
- 30.Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 31.Soheilifar S., Bidgoli M., Hooshyarfard A., Shahbazi A., Vahdatinia F., Khoshkhooie F. Effect of oral bromelain on wound healing, pain, and bleeding at donor site following free gingival grafting: a clinical trial. J Dent. 2018;15:309–316. [PMC free article] [PubMed] [Google Scholar]
- 32.Hasannasab M., Nourmohammadi J., Dehghan M.M., Ghaee A. Immobilization of bromelain and zno nanoparticles on silk fibroin nanofibers as an antibacterial and anti-inflammatory burn dressing. Int J Pharm. 2021;610 doi: 10.1016/j.ijpharm.2021.121227. [DOI] [PubMed] [Google Scholar]
- 33.Fathi A.N., Sakhaie M.H., Babaei S., Babaei S., Slimabad F., Babaei S. Use of bromelain in cutaneous wound healing in streptozocin-induced diabetic rats: an experimental model. J Wound Care. 2020;29:488–495. doi: 10.12968/jowc.2020.29.9.488. [DOI] [PubMed] [Google Scholar]
- 34.Chiu H.C., Fu M.M., Yang T.S., et al. Effect of high glucose, porphyromonas gingivalis lipopolysaccharide and advanced glycation end-products on production of interleukin-6/-8 by gingival fibroblasts. J Periodontal Res. 2017;52:268–276. doi: 10.1111/jre.12391. [DOI] [PubMed] [Google Scholar]
- 35.Hong J.H., Kim M.R., Lee B.N., et al. Anti-inflammatory and mineralization effects of bromelain on lipopolysaccharide-induced inflammation of human dental pulp cells. Medicina. 2021;57:591. doi: 10.3390/medicina57060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 38.Bhui K., Tyagi S., Srivastava A.K., et al. Bromelain inhibits nuclear factor kappa-b translocation, driving human epidermoid carcinoma a431 and melanoma a375 cells through g(2)/m arrest to apoptosis. Mol Carcinog. 2012;51:231–243. doi: 10.1002/mc.20769. [DOI] [PubMed] [Google Scholar]
- 39.Salminen A., Kauppinen A., Kaarniranta K. Emerging role of nf-κb signaling in the induction of senescence-associated secretory phenotype (sasp) Cell Signal. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Freund A., Patil C.K., Campisi J. P38mapk is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aquino-Martinez R., Eckhardt B.A., Rowsey J.L., et al. Senescent cells exacerbate chronic inflammation and contribute to periodontal disease progression in old mice. J Periodontol. 2021;92:1483–1495. doi: 10.1002/JPER.20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H.K., Park H.R., Sul K.H., Chung H.Y., Chung J. Induction of rantes and ccr5 through nf-kappab activation via mapk pathway in aged rat gingival tissues. Biotechnol Lett. 2006;28:17–23. doi: 10.1007/s10529-005-4681-6. [DOI] [PubMed] [Google Scholar]